Abstract
With improved healthcare, the Down syndrome (DS) population is both growing and aging rapidly. However, with longevity comes a very high risk of Alzheimer’s disease (AD). The LIFE-DSR study (NCT04149197) is a longitudinal natural history study recruiting 270 adults with DS over the age of 25. The study is designed to characterize trajectories of change in DS-associated AD (DS-AD). The current study reports its cross-sectional analysis of the first 90 subjects enrolled. Plasma biomarkers phosphorylated tau protein (p-tau), neurofilament light chain (NfL), amyloid β peptides (Aβ1-40, Aβ1-42), and glial fibrillary acidic protein (GFAP) were undertaken with previously published methods. The clinical data from the baseline visit include demographics as well as the cognitive measures under the Severe Impairment Battery (SIB) and Down Syndrome Mental Status Examination (DS-MSE). Biomarker distributions are described with strong statistical associations observed with participant age. The biomarker data contributes to understanding DS-AD across the spectrum of disease. Collectively, the biomarker data show evidence of DS-AD progression beginning at approximately 40 years of age. Exploring these data across the full LIFE-DSR longitudinal study population will be an important resource in understanding the onset, progression, and clinical profiles of DS-AD pathophysiology.
Keywords: Down syndrome, Alzheimer’s disease, blood biomarkers, phosphorylated tau protein, amyloid β peptide, neurofilament light chain, glial fibrillary acidic protein
1. Introduction
With improved healthcare, the Down syndrome (DS) population is experiencing a rapid increase in longevity with a life expectancy of >55 years of age compared to just 25 years of age in the 1980s. It is estimated that there are 210,000 people in the United States and about 417,000 people in Europe living with DS. Furthermore, about 40% of the DS population in the United States is over the age of 30 years [1,2]. However, with this change in longevity comes a very high risk of Alzheimer’s disease (AD). It is estimated that the lifetime risk of AD is >90% [3], and it is the leading cause of death for adults with DS [4]. DS-associated AD (DS-AD) is a slow, progressive disease associated with many of the same symptoms commonly seen in sporadic AD such as cognitive decline, behavioral issues, and functional decline impacting activities of daily living [5,6].
Early-onset AD (EOAD) is defined as those who present with clinical signs of AD before the age of 65. People with EOAD represent only about 5% or less of the 5.8 million AD cases in the United States [7] and 50 million globally [8]. A well-studied sub-set of the EOAD population are those with a known autosomal dominant AD (AD-AD) mutation in amyloid precursor protein (APP) and presenilin 1 and 2 (PSEN1 and PSEN2) [9,10,11]. Individuals with DS represent the largest group of individuals with EOAD with a median age of diagnosis of AD dementia of approximately 55 years [12]. Yet people with DS remain under-studied and are often not included in the investigations seeking to generate prevalence estimates of EOAD.
DS-AD is likely driven by genetics through a gene-dose effect of APP, which is located on chromosome 21 and is overexpressed in DS [13]. Furthermore, DS-AD biomarkers follow an order of change like sporadic AD, progressing over 10–20 years before the onset of cognitive decline [14]. By their 40s, the vast majority of adults with DS develop neuropathology consistent with AD [15], while individuals with DS and partial trisomy 21 results in 2 copies of the APP gene serving as a notable exception. The latter support the hypothesis that an extra copy of the APP gene contributes to the risk of AD in people with DS [16]. There are other factors that might impact the age of onset. For example, there is evidence that people with DS who carry apolipoprotein E (APOE) ε4 exhibit increased risk of earlier symptom onset and an increased amyloid load [17]. There could be other risk factors including co-morbid disorders such as obesity or untreated sleep apnea, that may also contribute to an earlier age of onset, but more research is needed to clarify their potential role.
The neuropathology of DS-AD is known to be very similar to that found in sporadic or late-onset AD (LOAD) and AD-AD. A widely supported model for sporadic and autosomal dominant AD consists of early amyloid β (Aβ) deposition, followed by hyperphosphorylated tau protein (p-tau) accumulation that is subsequently followed by neurodegeneration [18]. In this model, a long preclinical phase with the presence of AD pathology, specifically Aβ deposition, occurs more than 15 years before an individual develops overt cognitive symptoms. With the characterization of amyloid biomarkers (A), tau biomarkers (T), and neurodegeneration markers (N), it is now possible to apply both the AT(N) framework [19] and the biologically based research diagnostic criteria for Alzheimer’s disease [20]. This predominant model for LOAD and AD-AD was recently adapted to DS-AD [21,22]. Support for this model in DS-AD was seen in a recent study of a cross-sectional examination of a large cohort of adults with DS. The data from the study showed that DS-AD follows a similar trajectory of changes to that described in AD-AD. The study assessed multiple AD biomarkers (including samples from blood and cerebrospinal fluid (CSF), positron emission tomography (PET), magnetic resonance imaging (MRI), and cognitive tests) in 388 participants with DS. Data showed that biomarkers reflecting AD pathogenesis in individuals with DS behave similarly to AD biomarkers in individuals with LOAD and AD-AD [23,24].
The utility of fluid biomarkers to define the stage and progression in DS-AD is emerging. Recent data from the multi-site Alzheimer’s Biomarker Consortium–Down Syndrome (ABC-DS) cohort (n = 44) showed that the CSF AD biomarker profiles are very similar to LOAD and AD-AD [25]. The study showed that individuals with AD dementia had low CSF Aβ1–42 and Aβ42/Aβ40 ratio, indicative of amyloid deposition; increased p-tau, denoting the presence of neurofibrillary tangles [26,27,28]; and increased total tau and neurofilament light chain (NfL), indicating neurodegeneration [25]. The ABC-DS study findings are consistent with the results from the Down Alzheimer Barcelona Neuroimaging Initiative (DABNI) cohort in Europe [29]. Taken together, there is directional support for this revised AD diagnostic framework being potentially applicable and valuable in DS, with further need to characterize the sequencing and evolution of these biomarkers in DS from the asymptomatic through dementia stages [19,20]. The arrival of plasma biomarkers, which mirror CSF, and PET imaging measures provides a very attractive and more applicable less invasive fluid biomarker.
Data from the DABNI study and the LonDownS (London Down Syndrome) Consortium both point to NfL as a prognostic biomarker [29,30]. CSF Aβ42/Aβ40 ratio and plasma NfL were the first biomarkers to show changes at 28–30 years of age [23]. Use of NfL as a biomarker of LOAD has been problematic as it is a general marker of neurodegeneration and is itself correlated with age. Because people with LOAD are older than 65 and often show evidence of multiple comorbidities, the interpretation of increased NfL can be difficult. In fact, there is evidence that multiple pathologies are present together with those characteristics of AD in approximately 66% of the LOAD population. Given that aging is associated with presence of several neurodegenerative and non-degenerative diseases that also increase NfL levels, the increases in NfL cannot be attributed specifically to AD [31]. In contrast, EOAD populations, such as DS-AD, are much less likely to harbor other degenerative disorders. Thus, in EOAD, NfL can be used to more confidently define the onset of AD. In fact, emerging data indicate that plasma NfL could be useful as a prognostic biomarker in the unique DS-AD population [32]. Glial fibrillary acidic protein (GFAP) is an intermediate filament protein found in the astroglial cytoskeleton and is another emerging marker of neurodegeneration. GFAP expression is key to astrocyte function and is upregulated in neurodegenerative diseases such as AD [33]. However, more research is needed on the use of plasma GFAP as a biomarker of DS-AD progression. It appears that our study is the first report of plasma GFAP data from DS individuals in the literature. A recent study with samples from three different cohorts that included LOAD and AD-AD participants showed that plasma p-tau 217 can distinguish AD from other neurodegenerative diseases [34]. The use of plasma p-tau 217 in DS may have some specificity in being able to further define the progression and clinical diagnosis of DS-AD.
Given the strong genetic risk of AD in DS and the biomarker evidence of a temporal sequence of pathology similar to that in LOAD, a close exploration of the natural history of DS-AD should enrich our understanding of all forms of AD and enhance knowledge of potential biomarkers and outcome measures for therapeutic interventional studies. The LIFE-DSR study [35] is a longitudinal natural history study recruiting 270 adults with DS over the age of 25. The LIFE-DSR study has been designed to better understand the factors that underlie symptoms, progression, and age-related changes in the clinical presentation of DS-AD. Its design aims at characterizing trajectories of change in DS-AD at 3 visits across 32 months (baseline, month 16, and month 32) and collects sociodemographic characteristics, medical history, physical exam, neuropsychiatric evaluation (not reported here), cognitive status assessments, and a blood draw for AD biomarkers including APOE ε4 analyses.
2. Experimental Section
Enrollment in the study started at the end of 2019. Paused in 2020 due to the COVID-19 pandemic, an opportunity was provided to conduct an exploratory analysis of the first 90 banked plasma samples to assess the cross-sectional distribution of baseline AD biomarkers p-tau 181, p-tau 217, NfL, Aβ1-40, Aβ1-42, and GFAP. Statistical associations between clinical measures, sociodemographic characteristics, and biomarkers were evaluated.
2.1. Cognitive Assessments
The Severe Impairment Battery (SIB) [36] assesses the skills of people with severe dementia utilizing 40 simple one-step commands and gestural cues, presented in a relatively naturalistic, conversational style. There are six subscales: attention, orientation, language, memory, visuospatial ability, and construction. Assessment of praxis, social interaction, and orientation to name is also included. The maximum score is 100, with higher score indicating less impairment.
Down Syndrome Mental Status Examination (DS-MSE) is a neuropsychological test battery measuring a broad range of skills including recall of personal information, orientation to season and day of the week, short-term memory, language, visuospatial construction, and praxis [37]. The maximum score is 87 using scoring method 2, with a higher score indicating less impairment [38].
2.2. Genetic Assays
Apolipoprotein E (APOE)
APOE genotypes for rs7412 and rs429358 were assayed from LIFE-DSR participant DNA by the National Centralized Repository for Alzheimer’s Disease and Related Dementias (NCRAD) to generate APOE ε2/ε3/ε4 alleles. Genotyping was done using a customized 96-SNP chip with the Fluidigm microfluidics-based DNA fingerprinting, from DNA samples banked at NCRAD. Fingerprint data were evaluated for call rate, heterozygosity ratio, subject sex, and concordance with any other available samples; only samples passing all quality control were used to generate APOE genotypes.
2.3. Biomarker Assays
2.3.1. Plasma P-tau Assays
Both p-tau181 and p-tau217 levels were measured in duplicate by electrochemiluminescence using a proprietary assay developed by Lilly Research Laboratories as previously described [25,26,27,34,39,40,41]. Briefly, samples were diluted 1:2 and 50 μL of diluted sample was used for each replicate. The assay was performed on a streptavidin small spot plate using the Meso Scale Discovery platform. P-tau181 used Biotinylated-AT270 (mIgG1) as the capture, and p-tau217 used Biotinylated-IBA493 (mIgG1) as the capture. In this study, both assays used SULFO-4G10-E2 (anti-tau monoclonal antibody developed by Lilly Research Laboratories) as the detector. Each assay was calibrated using a unique synthetic p-tau peptide.
2.3.2. NfL and 4-Plex Assays
Quanterix Simoa NfL and Neurology 4-Plex E (NfL, Aβ1-40, Aβ1-42, and GFAP) assays were allowed to equilibrate to room temperature before use. Plasma samples were thawed on wet ice and briefly vortexed prior to dilution and spun at 2000× g for 10 min at 4C. Prediluted calibrators were also thawed on wet ice prior to use along with provided control samples. Plasma samples as well as controls were diluted 1:4 in the provided sample diluent. Further, 200 μL of all calibrators, control samples, and plasma samples were loaded onto a 450 μL v-bottom plate and loaded onto the Simoa HD-X along with kit provided SBG, beads, detector, and RGP. Each assay was run according to the manufacturer assay definitions. Since NfL results were available from both single and multiplex assays and the correlation coefficient was very high (R2 = 0.92), single plex Simoa NfL data were used in all analyses [32].
2.4. Statistical Analyses
Spearman rank-based correlations were used to test monotonic associations between biomarker and clinical variables. Associations between biomarkers and age were further investigated using linear regression with restricted cubic spline fits on raw or z-transformed scales and Huber–White robust standard errors. A secondary analysis of the association between biomarkers and age considered APOE ε4 carrier status and sex as additive covariates. An alpha of 5% was used for statistical significance unless otherwise noted. Confidence intervals (CI) are 95% CIs. Data management, graphics, and statistical analyses were performed using Stata v16.1 and R v4.0.3 software (including tidyverse and psych packages).
3. Results
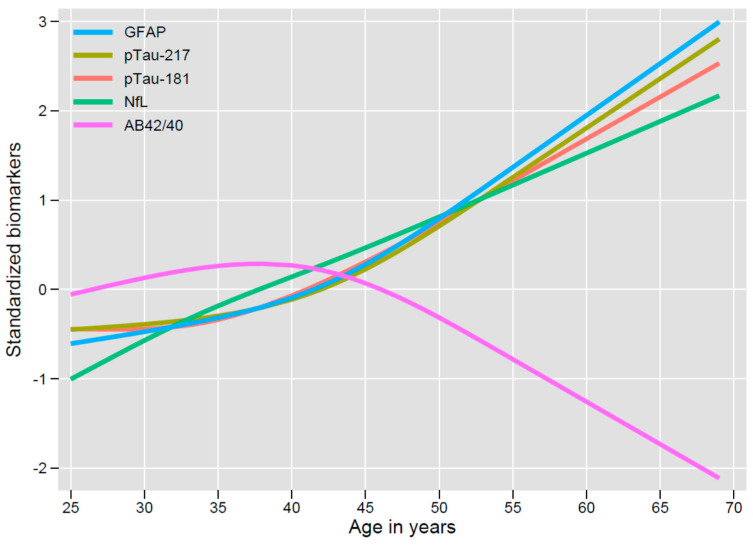
Among the first 90 LIFE-DSR study participants with plasma samples accessible for analysis, where demographics were available, the mean age was 38.3 years, and 46.5% were women. Baseline demographics, plasma biomarker values, and cognitive assessment scores are summarized in Table 1. Despite the relatively young age of the participants, age was strongly correlated with biomarker levels as illustrated in Figure 1. Decline in the Aβ42/Aβ40 ratio was observed starting with participants at about 45 years of age indicative of the formation of amyloid plaques.
Table 1.
Demographics and baseline biomarkers for first 90 subjects in Longitudinal Investigation for Enhancing Down Syndrome Research (LIFE-DSR) Study.
| Characteristic | Mean (SD) | Median (Q1, Q3) | Min, Max | N (%) |
|---|---|---|---|---|
| p-tau 217 pg/mL | 0.26 (0.38) | 0.14 (0.08, 0.25) | 0.01, 2.72 | 90 (100.00%) |
| p-tau 181 pg/mL | 1.22 (1.10) | 0.81 (0.59, 1.45) | 0.37, 7.40 | 90 (100.00%) |
| NfL pg/mL | 13.84 (7.19) | 11.90 (9.28, 18.06) | 5.13, 46.87 | 90 (100.00%) |
| GFAP pg/mL | 100.37 (57.02) | 84.74 (63.44, 123.65) | 36.19, 445.03 | 90 (100.00%) |
| Aβ1-40 pg/mL | 210.03 (52.36) | 215.82 (188.07, 234.87) | 66.32, 417.74 | 90 (100.00%) |
| Aβ1-42 pg/mL | 8.81 (2.42) | 8.90 (7.36, 10.24) | 2.22, 13.82 | 90 (100.00%) |
| Aβ42/Aβ40 ratio | 0.04 (0.01) | 0.04 (0.04, 0.05) | 0.01, 0.06 | 90 (100.00%) |
| DS-MSE Total Score 2 | 64.23 (11.25) | 65.00 (59.00, 72.00) | 30.00, 82.00 | 79 (87.78%) |
| SIB Total Score | 87.92 (14.04) | 93.00 (86.00, 96.50) | 14.00, 100.00 | 84 (93.33%) |
| Age (years) | 38.31 (9.47) | 37.00 (30.00, 45.00) | 25.00, 69.00 | 86 (95.56%) |
| Female | Male | |||
| Gender | 40 (46.51%) | 46 (53.49%) | ||
| Non-Carrier | Carrier | |||
| APOE ε4 | 67 (74.44%) | 23 (25.56%) |
Aβ1-40 = amyloid β peptide 1-40, Aβ1-42 = amyloid β peptide 1-42, APOE ε4 = apolipoprotein E ε4 allele, DS-MSE = Down Syndrome Mental Status Examination, GFAP = glial fibrillary acidic protein, Min = minimum, Max = maximum, N = number, NfL = neurofilament light, pg/mL = picogram/milliliter, p-tau 181 = phosphorylated tau at threonine-181, p-tau 217 = phosphorylated tau at threonine−217, Q1 = quartile 1, Q3 = quartile 3, SD = standard deviation, SIB = Severe Impairment Battery.
Figure 1.
Shown are the predictions from linear regressions of each biomarker on age, using restricted cubic splines with 4 knots. Biomarkers were z-transformed to standardize the Y-axis. Robust standard errors were used. F-tests for the association of each biomarker with age was statistically significant (GFAP: F(3, 82) = 14.05, p < 0.0001; p-tau 217: F(3, 82) = 9.91, p < 0.0001; p-tau 181: F(3, 82) = 10.14, p < 0.0001; NfL: F(3, 82) = 34.57, p < 0.0001; Aβ42/Aβ40 ratio: F(3, 82) = 4.71, p = 0.0044), but for the Aβ42/Aβ40 ratio, this is dependent on the oldest participant; see Supplementary Figures S2–S6. (Abbreviations: Aβ42/40 = amyloid β42/40 ratio, GFAP = glial fibrillary acidic protein, NfL = neurofilament light, p-tau 181 = phosphorylated tau at threonine − 181, p-tau 217 = phosphorylated tau at threonine−217).
As indicated in Figure 1, NfL values increased steadily with increasing age. In contrast, GFAP, p-tau217, and p-tau181 values were stable until the late forties at which point, they also demonstrated increases with age. Non-standardized restricted cubic spline fits for the plasma biomarkers are illustrated in Supplementary Figures S2–S6. The significant associations with age for p-tau, NfL, and GFAP were found to be robust after adjusting for APOE ε4 carrier status and sex (Supplementary Figure S7). The increase in p-tau181 and of p-tau217 by age 40 is evidence for hyperphosphorylated tau protein 10–15 years before the average age of symptom onset.
Spearman correlations between age, plasma biomarker values, and SIB and DS-MSE scores are summarized in Supplementary Figure S1. Baseline SIB and DS-MSE scores were positively correlated with each other.
Inter-plasma biomarker correlations provide further insight into the progression of DS-AD. As expected, Aβ42/40 ratio was negatively correlated with NfL and p-tau181 and strong correlations were seen between p-tau217 and p-tau181. The strong correlations seen between NfL and p-tau217 or p-tau181, respectively, and GFAP and p-tau217 or p-tau181 are similar to previous studies in LOAD where the order of biomarker changes has been investigated [42]. Spearman correlations with scatter plots are illustrated in Supplementary Figure S1. All statistically significant correlations remained after test multiplicity correction by Bonferroni’s method, except the weaker associations between Aβ42/Aβ40 and NfL or p-tau181.
4. Discussion
Plasma protein biomarkers provide an accessible tool for elucidating the molecular processes active in DS-AD. These emerging biomarkers may be useful for prognosis and diagnosis in DS-AD. In our interim analysis, we evaluated AD biomarkers of APP metabolism (Aβ1-40 and Aβ1-42), tau tangle pathology (p-tau 181 and p-tau 217), and neurodegeneration (NfL, GFAP) at a single timepoint and showed a strong association with age. These three classes of plasma biomarkers correspond with the AT(N) research framework to define AD [20]. From the modelling undertaken, the amyloid (A) biomarker measure of Aβ1-42/Aβ1-40 appears to change earliest with an initial increase in ratio followed inflection point in the mid-1940s and then progressive lowering thereafter. The tau (T) biomarkers both p-181 and p-217 tau have a progressively upwards trajectory that becomes apparent in the early to mid-1940s, while the neurodegenerative biomarkers of GFAP and NfL are also both informative. The NfL levels appear to increase progressively from the mid-1920s, while GFAP has its upward inflection point from the mid-1940s. In the initial AT(N) proposal, these neurodegenerative biomarkers were not included, however, their potential utility in DS was appreciated. The steady increase in NfL with increasing age may be particularly useful for assessing overall disease progression [32]. Overall, we searched for correlations between biomarkers and with age, APOE ε4 status and clinical assessments of cognition and function. As highlighted above, this interim analysis of plasma biomarkers showed a strong age association.
The use of plasma protein biomarkers, while encouraging, may have limitations. For example, the CSF Aβ42/Aβ40 ratio shows an initial decline at 28–30 years of age in DS [23]. Furthermore, evidence of amyloid deposition in people around the age of 30 is consistent with previous studies of DS brain pathology [15]. Why the Aβ42/Aβ40 measures in the CSF and plasma differ is not clear; however, one speculation is that the recently created plasma Aβ1-40 and Aβ1-42 assays lack sensitivity to detect Aβ deposition in people with DS before the age of 45.
Correlations were not observed between either the SIB or DS-MSE and the plasma biomarkers. This may be due to wide variability in cognition and function in people with DS. It may also speak to the need for more sensitive assessments designed specifically for DS-AD and validated for use in longitudinal studies. A recent longitudinal study showed an association with amyloid positron emission tomography (PET) imaging with cognitive decline as measured by the Cued Recall Test [43]. In addition, a novel composite scale has been developed with longitudinal data from multiple sites which also has the potential to detect early stages of DS-AD [44]. The LIFE-DSR study also plans to collect longitudinal cognitive and functional data and blood samples at 16 and 32 months with the hope of observing associations between plasma biomarker progression and cognitive and functional decline.
Our interim analysis has provided valuable data on the use of plasma biomarkers to help characterize DS-AD. While the number of participants was modest (n = 90) and the mean age is relatively young (38.3 years), the modeling of the biomarkers provides useful insights into the potential evolution of DS-AD. Remarkably, given that our analysis was cross-sectional, the biomarkers were exceptionally well behaved. They suggest that age alone predicts biomarker status and, therefore, the state of AD progression. Taken together, biomarker data are evidence that progression of DS-AD begins no later than the early forties i.e., 10–15 years before dementia is diagnosed. The next steps for the LIFE-DSR study include increasing the number of participants to 270 and gathering longitudinal data at 16 and 32 months. Longitudinal data from 270 participants should provide even greater insights into the progression of DS-AD and in so doing set the stage for clinical trials of potentially effective treatments for DS-AD and possibly for all forms of AD.
5. Patents
Subject matter relating to the assays, methods, reagents and/or compositions of matter set forth herein are subject to patents and/or patent applications of Eli Lilly and Company.
Acknowledgments
The authors wish to thank all the participants with Down syndrome, their families, and their caregivers for their support and dedication to this on-going research study.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10091907/s1, Figure S1: Histograms (diagonal), scatterplots (below diagonal), and Spearman correlations (above diagonal) for biomarkers and clinical data; Figure S2: Linear regression fit of untransformed GFAP on age, using restricted cubic splines with 4 knots and robust standard errors; Figure S3: Linear regression fit of untransformed p-tau 217 on age, using restricted cubic splines with 4 knots and robust standard errors; Figure S4: Linear regression fit of untransformed p-tau 181 on age, using restricted cubic splines with 4 knots and robust standard errors; Figure S5: Linear regression fit of untransformed NfL on age, using restricted cubic splines with 4 knots and robust standard errors; Figure S6: Linear regression fit of untransformed Aβ42/Aβ40 ratio on age, using restricted cubic splines with 4 knots and robust standard errors; Figure S7: Shown are the predictions from linear regressions of each biomarker on age, using restricted cubic splines with 4 knots, and adjusted for APOE e4 carrier status and sex using additive covariates; APOE e4 adjusted to non-carrier and sex adjusted to female.
Author Contributions
Conceptualization: J.A.H., J.L.D.; Methodology: D.C.A., J.L.D., N.K.P., J.A.Z.; Formal Analysis: D.C.A., J.L.D., J.A.Z.; Investigation: J.A.H.; Resources: H.H.; Data Curation: J.M., J.N.-J.; Writing–Original Draft Preparation: J.A.H., J.A.Z.; Writing–Review & Editing: All Authors; Visualization: D.C.A.; Project Administration: A.B., R.C., C.L.E., D.L.L., C.R., K.S., H.H.F., W.M., K.M.F., T.M.F., K.W.; Funding Acquisition: H.H. All authors have read and agreed to the published version of the manuscript.
Funding
The LIFE-DSR study is funded by the LuMind IDSC Foundation. Eli Lilly provided the analysis of the plasma biomarkers to LuMind IDSC as an in-kind contribution. Samples from the National Centralized Repository for Alzheimer’s Disease and Related Dementias (NCRAD), which receives government support under a cooperative agreement grant (U24 AG21886) awarded by the National Institute on Aging (NIA), were used in this study. We thank contributors who collected samples used in this study, as well as patients and their families, whose help and participation made this work possible.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the central Institutional Review Board of Advarra (Pro00031559, approved 14 February 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. In cases where the participant could not provide written informed consent, the participant’s legally authorized representative (LAR) provided consent prior to initiating any study-specific activities. The participant assented to the study procedures to ensure their awareness of their role in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the ongoing nature of the LIFE-DSR natural history study.
Conflicts of Interest
D.A., J.L.D., N.K.P. and J.Z. are full-time employees and minor shareholders of Eli Lilly and Company. Lilly is exploring commercialization opportunities for the Lilly p-tau217 blood test. The remaining authors declare no conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Antonarakis S., Skotko B.G., Rafii M.S., Strydom A., Pape S.E., Bianchi D.W., Sherman S.L., Reeves R.H. Down syndrome. Nat. Rev. Dis. Primers. 2020;6:9. doi: 10.1038/s41572-019-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Graaf G., Buckley F., Skotko B.G. Estimation of the number of people with Down syndrome in Europe. Eur. J. Hum. Genet. 2021;29:402–410. doi: 10.1038/s41431-020-00748-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCarron M., McCallion P., Reilly E., Dunne P., Carroll R., Mulryan N. A prospective 20-year longitudinal follow-up of dementia in persons with Down syndrome. J. Intellect. Disabil. Res. 2017;61:843–852. doi: 10.1111/jir.12390. [DOI] [PubMed] [Google Scholar]
- 4.Hithersay R., Startin C.M., Hamburg S., Mok K.Y., Hardy J., Fisher E.M.C., Tybulewicz V.L.J., Nizetic D., Strydom A. Association of Dementia With Mortality Among Adults With Down Syndrome Older Than 35 Years. JAMA Neurol. 2019;76:152–160. doi: 10.1001/jamaneurol.2018.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lott I.T., Doran E., Nguyen V.Q., Tournay A., Movsesyan N., Gillen D.L. Down syndrome and dementia: Seizures and cognitive decline. J. Alzheimers Dis. 2012;29:177–185. doi: 10.3233/JAD-2012-111613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rissman R.A., Mobley W.C. Implications for treatment: GABAA receptors in aging, Down syndrome and Alzheimer’s disease. J. Neurochem. 2011;117:613–622. doi: 10.1111/j.1471-4159.2011.07237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alzheimer’s Association Facts and Figures. [(accessed on 10 March 2020)];2020 Available online: https://www.alz.org/media/Documents/alzheimers-facts-and-figures.pdf.
- 8. [(accessed on 8 January 2018)]; Available online: https://www.alzheimers.net/resources/alzheimers-statistics/
- 9.Gomez W., Morales R., Maracaja-Coutinho V., Parra V., Nassif M. Down syndrome and Alzheimer’s disease: Common molecular traits beyond the amyloid precursor protein. Aging. 2020;12:1011–1033. doi: 10.18632/aging.102677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cacace R., Sleegers K., Van Broeckhoven C. Molecular genetics of early-onset Alzheimer’s disease revisited. Alzheimers Dement. 2016;12:733–748. doi: 10.1016/j.jalz.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Mills S.M., Mallmann J., Santacruz A.M., Fuqua A., Carril M., Aisen P.S., Althage M.C., Belyew S., Benzinger T.L., Brooks W.S., et al. Preclinical trials in autosomal dominant AD: Implementation of the DIAN-TU trial. Rev. Neurol. 2013;169:737–743. doi: 10.1016/j.neurol.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinai A., Mokrysz C., Bernal J., Bohnen I., Bonell S., Courtenay K., Dodd K., Gazizova D., Hassiotis A., Hillier R., et al. Predictors of Age of Diagnosis and Survival of Alzheimer’s Disease in Down Syndrome. J. Alzheimers Dis. 2018;61:717–728. doi: 10.3233/JAD-170624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballard C., Mobley W., Hardy J., Williams G., Corbett A. Dementia in Down’s syndrome. Lancet Neurol. 2016;15:622–636. doi: 10.1016/S1474-4422(16)00063-6. [DOI] [PubMed] [Google Scholar]
- 14.Zigman W.B., Devenny D.A., Krinsky-McHale S.J., Jenkins E.C., Urv T.K., Wegiel J., Schupf N., Silverman W. Alzheimer’s Disease in Adults with Down Syndrome. Int. Rev. Res. Ment. Retard. 2008;36:103–145. doi: 10.1016/S0074-7750(08)00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemere C.A., Blusztajn J.K., Yamaguchi H., Wisniewski T., Saido T.C., Selkoe D.J. Sequence of deposition of heterogeneous amyloid beta-peptides and APO E in Down syndrome: Implications for initial events in amyloid plaque formation. Neurobiol. Dis. 1996;3:16–32. doi: 10.1006/nbdi.1996.0003. [DOI] [PubMed] [Google Scholar]
- 16.Doran E., Keator D., Head E., Phelan M.J., Kim R., Totoiu M., Barrio J.R., Small G.W., Potkin S.G., Lott I.T. Down Syndrome, Partial Trisomy 21, and Absence of Alzheimer’s Disease: The Role of APP. J. Alzheimers Dis. 2017;56:459–470. doi: 10.3233/JAD-160836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C.C., Liu C.C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jack C.R., Knopman D.S., Jagust W.J., Shaw L.M., Aisen P.S., Weiner M.W., Petersen R.C., Trojanowski J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jack C.R., Bennett D.A., Blennow K., Carrillo M.C., Feldman H.H., Frisoni G.B., Hampel H., Jagust W.J., Johnson K.A., Knopman D.S., et al. An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jack C.R., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., Holtzman D.M., Jagust W., Jessen F., Karlawish J., et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rafii M.S., Ances B.M., Schupf N., Krinsky-McHale S.J., Mapstone M., Silverman W., Lott I., Klunk W., Head E., Christian B., et al. The AT(N) framework for Alzheimer’s disease in adults with Down syndrome. Alzheimers Dement. 2020;12:e12062. doi: 10.1002/dad2.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lott I.T., Head E. Dementia in Down syndrome: Unique insights for Alzheimer disease research. Nat. Rev. Neurol. 2019;15:135–147. doi: 10.1038/s41582-018-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortea J., Vilaplana E., Carmona-Iragui M., Benejam B., Videla L., Barroeta I., Fernández S., Altuna M., Pegueroles J., Montal V., et al. Clinical and biomarker changes of Alzheimer’s disease in adults with Down syndrome: A cross-sectional study. Lancet. 2020;395:1988–1997. doi: 10.1016/S0140-6736(20)30689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Head E., Ances B. Biomarkers in Down syndrome can help us understand Alzheimer’s disease. Lancet. 2020;395:1951–1953. doi: 10.1016/S0140-6736(20)30916-8. [DOI] [PubMed] [Google Scholar]
- 25.Henson R.L., Doran E., Christian B.T., Handen B.L., Klunk W.E., Lai F., Lee J.H., Rosas H.D., Schupf N., Zaman S.H., et al. Cerebrospinal fluid biomarkers of Alzheimer’s disease in a cohort of adults with Down syndrome. Alzheimers Dement. 2020;12:e12057. doi: 10.1002/dad2.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brickman A.M., Manly J.J., Honig L.S., Sanchez D., Reyes-Dumeyer D., Lantigua R.A., Lao P.J., Stern Y., Vonsattel J.P., Teich A.F., et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement. 2021:1–12. doi: 10.1002/alz.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thijssen E.H., La Joie R., Wolf A., Strom A., Wang P., Iaccarino L., Bourakova V., Cobigo Y., Heuer H., Spina S., et al. Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL) investigators. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med. 2020;26:387–397. doi: 10.1038/s41591-020-0762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janelidze S., Mattsson N., Palmqvist S., Smith R., Beach T.G., Serrano G.E., Chai X., Proctor N.K., Eichenlaub U., Zetterberg H., et al. Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 2020;26:379–386. doi: 10.1038/s41591-020-0755-1. [DOI] [PubMed] [Google Scholar]
- 29.Fortea J., Carmona-Iragui M., Benejam B., Fernández S., Videla L., Barroeta I., Alcolea D., Pegueroles J., Muñoz L., Belbin O., et al. Plasma and CSF biomarkers for the diagnosis of Alzheimer’s disease in adults with Down syndrome: A cross-sectional study. Lancet Neurol. 2018;17:860–869. doi: 10.1016/S1474-4422(18)30285-0. [DOI] [PubMed] [Google Scholar]
- 30.Strydom A., Heslegrave A., Startin C.M., Mok K.Y., Hardy J., Groet J., Nizetic D., Zetterberg H. LonDownS Consortium. Neurofilament light as a blood biomarker for neurodegeneration in Down syndrome. Alzheimers Res. Ther. 2018;10:39. doi: 10.1186/s13195-018-0367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabinovici G.D., Carrillo M.C., Forman M., DeSanti S., Miller D.S., Kozauer N., Petersen R.C., Randolph C., Knopman D.S., Smith E.E., et al. Multiple comorbid neuropathologies in the setting of Alzheimer’s disease neuropathology and implications for drug development. Alzheimers Dement. 2016;3:83–91. doi: 10.1016/j.trci.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shinomoto M., Kasai T., Tatebe H., Kondo M., Ohmichi T., Morimoto M., Chiyonobu T., Terada N., Allsop D., Yokota I., et al. Plasma neurofilament light chain: A potential prognostic biomarker of dementia in adult Down syndrome patients. PLoS ONE. 2019;14:e0211575. doi: 10.1371/journal.pone.0211575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verberk I.M.W., Thijssen E., Koelewijn J., Mauroo K., Vanbrabant J., de Wilde A., Zwan M.D., Verfaillie S.C.J., Ossenkoppele R., Barkhof F., et al. Combination of plasma amyloid beta(1–42/1–40) and glial fibrillary acidic protein strongly associates with cerebral amyloid pathology. Alzheimers Res. Ther. 2020;12:118. doi: 10.1186/s13195-020-00682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmqvist S., Janelidze S., Quiroz Y.T., Zetterberg H., Lopera F., Stomrud E., Su Y., Chen Y., Serrano G.E., Leuzy A., et al. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA. 2020;324:772–781. doi: 10.1001/jama.2020.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Longitudinal Investigation for Enhancing Down Syndrome Research (LIFE-DSR) Study. [(accessed on 4 November 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04149197.
- 36.Fish J. Severe Impairment Battery. In: Kreutzer J.S., DeLuca J., Caplan B., editors. Encyclopedia of Clinical Neuropsychology. Springer; New York, NY, USA: 2011. [Google Scholar]
- 37.Haxby J.V. Neuropsychological evaluation of adults with Down’s syndrome: Patterns of selective impairment in non-demented old adults. Pt 3J. Ment. Defic. Res. 1989;33:193–210. doi: 10.1111/j.1365-2788.1989.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 38.Krinsky-McHale S.J., Zigman W.B., Lee J.H., Schupf N., Pang D., Listwan T., Kovacs C., Silverman W. Promising outcome measures of early Alzheimer’s dementia in adults with Down syndrome. Alzheimers Dement. 2020;12:e12044. doi: 10.1002/dad2.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janelidze S., Berron D., Smith R., Strandberg O., Proctor N.K., Dage J.L., Stomrud E., Palmqvist S., Mattsson-Carlgren N., Hansson O. Associations of Plasma Phospho-Tau217 Levels With Tau Positron Emission Tomography in Early Alzheimer Disease. JAMA Neurol. 2021;78:149–156. doi: 10.1001/jamaneurol.2020.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janelidze S., Stomrud E., Smith R., Palmqvist S., Mattsson N., Airey D.C., Proctor N.K., Chai X., Shcherbinin S., Sims J.R., et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat. Commun. 2020;11:1683. doi: 10.1038/s41467-020-15436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mielke M.M., Hagen C.E., Xu J., Chai X., Vemuri P., Lowe V.J., Airey D.C., Knopman D.S., Roberts R.O., Machulda M.M., et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 2018;14:989–997. doi: 10.1016/j.jalz.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmqvist S., Insel P.S., Stomrud E., Janelidze S., Zetterberg H., Brix B., Eichenlaub U., Dage J.L., Chai X., Blennow K., et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer’s disease. EMBO Mol. Med. 2019;11:e11170. doi: 10.15252/emmm.201911170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartley S.L., Handen B.L., Devenny D., Tudorascu D., Piro-Gambetti B., Zammit M.D., Laymon C.M., Klunk W.E., Zaman S., Cohen A., et al. Cognitive indicators of transition to preclinical and prodromal stages of Alzheimer’s disease in Down syndrome. Alzheimers Dement. 2020;12:e12096. doi: 10.1002/dad2.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aschenbrenner A.J., Baksh R.A., Benejam B., Beresford-Webb J.A., Coppus A., Fortea J., Handen B.L., Hartley S., Head E., Jaeger J., et al. Markers of early changes in cognition across cohorts of adults with Down syndrome at risk of Alzheimer’s disease. Alzheimers Dement. 2021 doi: 10.1002/dad2.12184. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the ongoing nature of the LIFE-DSR natural history study.