Abstract
CREUTZFELDT–JAKOB DISEASE (CJD) IS THE FIRST major challenge that the blood system has faced since the completion of the Krever inquiry in 1997. We report the results of a detailed policy analysis comparing 2 CJD-related decisions: a 1995 recall of blood from a donor with classic CJD and the 1999 decision to defer donations from individuals with a 6-month travel history to the UK between 1980 and 1996 due to concerns related to variant CJD. Overall, we observed that decision-making improved significantly from 1995 to 1999. In 1998/99 the potential threat of variant CJD was identified at an early stage, and a systematic risk assessment process was initiated. Decision-making was consultative and involved consumers. However, the perception existed that further improvement could take place in the areas of transparency of process and interaction of organizations. We observed that the presence of a second operator had an important impact on decision-making in 1998/99.
The potential transmission by blood of Creutzfeldt–Jakob disease (CJD) has presented the Canadian blood system with several challenges. The response to these challenges provides insight into the blood system's decision-making process and its evolution since the Krever inquiry. We report the results of a detailed policy analysis that compares 2 CJD-related decisions: a 1995 recall of blood from a donor with classic CJD and the 1999 decision to defer donations from individuals with a 6-month travel history to the United Kingdom between 1980 and 1996 because of concerns related to variant CJD (vCJD). CJD is the first major challenge the blood system has faced since the completion of the Krever inquiry in 1997. We examined examples of the decision-making process before and after the blood system was changed in response to the inquiry's recommendations.
Methods
Our policy analysis consisted of 2 components: a literature review and semi-structured interviews with key informants. The literature review consisted of a systematic survey of the medical literature concerning the risk of transmission,1 a content analysis of newspaper articles reporting stories about CJD and blood transfusion, and a review of minutes of meetings of major decision-making organizations and of government documents obtained through access to information requests. We conducted 32 interviews with key informants, which were audiotaped and transcribed verbatim. Information from the interviews was coded to identify important concepts related to the policy process. Coding was verified, and the information was entered into a qualitative software program. The codes were collapsed into categories and the categories compared to develop themes to explain the decision-making process and identify the key policy determinants. We focused on the following domains of the policy process while developing themes: the organizational structure for decision-making, the information available to the decision-makers, the value systems of decision-makers and the key external factors that influenced decision-making. These domains are based on the components of the Sabatier-Lomas policy analysis framework.2 We also conducted sessions with the major stakeholders to obtain feedback on our findings.
Structure of the blood system (1995–1999)
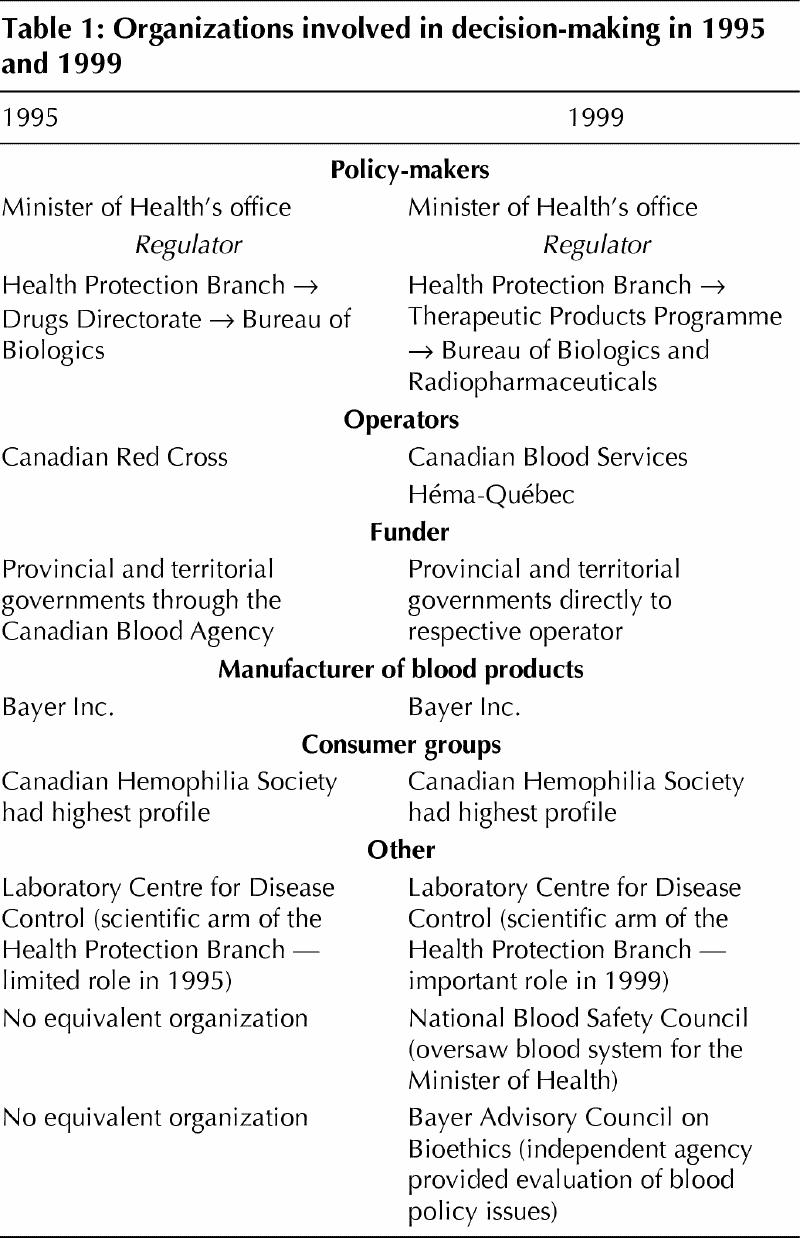
In 1995, the Drugs Directorate of Health Canada (part of the then Health Protection Branch), through its Bureau of Biologics, was the federal regulator of blood and set blood policy. The Red Cross, which was the national operator of the blood system, was responsible for collecting and distributing blood. The Red Cross received funding from the Canadian Blood Agency, whose responsibility was to direct, coordinate and finance aspects of the blood system consistent with the objectives of the provinces and territories. Bayer Inc. was the primary manufacturer of blood products for Canada and supplied blood products to the Red Cross.
Between 1995 and 1999, the Canadian blood system experienced considerable change. Based on the Krever inquiry's interim report, federal, provincial and territorial ministers proceeded to develop a plan to reform the blood system. Quebec opted out of these discussions.3 The new national blood authority became Canadian Blood Services (CBS), which began operations in September 1998. CBS received funding from the provinces and territories and, in turn, collected, tested and distributed blood products for all territories and provinces except Quebec. It combined the operating and financing roles of the Red Cross and the former Canadian Blood Agency. Quebec developed its own plan for a blood system following the recommendations of the Gélineau Committee. Its new provincial blood operator, Héma-Québec, also began operations in September 1998.4
Between 1995 and 1999, there were also important changes within the regulatory authority. In 1999, the Drugs Directorate was renamed the Therapeutic Products Programme. The Bureau of Biologics, which was now called the Bureau of Biologics and Radiopharmaceuticals (referred to as Bureau of Biologics in this article), continued to be responsible for the regulation of blood products. Both CBS and Héma-Québec were required to follow Health Canada regulations. The National Blood Safety Council was created in 1997, based on a recommendation of the Krever Commission. Its responsibilities were to oversee the decision-making process in the blood system and report directly to the Minister of Health. Bayer Inc. continued to be the national manufacturer of fractionated blood products (Table 1).
Table 1
Review of decisions
1995 recall of blood from a Vancouver donor
The issue of blood that was potentially infected with CJD became a public health concern on this continent in the United States in 1994. In that year, the American Red Cross and US blood manufacturers initiated 3 voluntary withdrawals of blood from donors who had subsequently developed CJD.5 In response to these withdrawals, the US Food and Drug Administration developed a policy on June 1995 to withdraw cellular product donations from individuals with CJD. One month later, on July 11, the issue of blood transmission of CJD came to the Canadian public's attention. On this date, a Canadian Press story was published in several papers recounting testimony by a US hematologist, Dr. Nathan Kobrinsky, at the Krever inquiry that CJD could be the next major threat to the blood supply.6 A Vancouver woman who read the article informed the Vancouver branch of the Red Cross that her father, a regular blood donor, had developed CJD. Within days of receiving this information, the Red Cross, in conjunction with Bayer Inc., voluntarily decided to withdraw blood products connected with this donor and informed the Bureau of Biologics of their decision.7 The Bureau supported the voluntary nature of the recall, although it did not have an official policy available to guide decision-making on this issue (for further information regarding knowledge about the risk of transmission of CJD by blood transfusion in 1995 and 1999, see the Appendix on the CMAJ Web site at www.cma.ca/cmaj/vol-165/issue-1/jakobappendix.htm).8 The immediate consequences of the recall were shortages of certain blood products.9 The total cost of the recall, the largest in Canadian history, was estimated at $11 million.10 On Oct. 20, 1995, the Health Protection Branch of Health Canada announced its official policy on CJD, supporting the blood recall and deferring donations from donors at risk of acquiring CJD.11
Discovery of vCJD
In 1996, investigators in the UK published the first case series describing a variant form of CJD.12 The investigators believed, and subsequent studies supported the fact, that this condition was related to bovine spongiform encephalopathy (BSE) or mad cow disease.13 Almost immediately, concerns arose that this condition could potentially be transmitted through blood transfusion. In February 1998, the UK decided to import plasma products due to a concern that an unknown portion of the British population could be harbouring vCJD.14 This decision stimulated discussion in the US and Canada about whether these countries should accept blood from individuals who had travelled to regions where BSE was occurring.
On Oct. 15, 1998, the potential threat of vCJD to the blood supply increased in public profile in Canada because of the release of the Bayer Advisory Council on Bioethics report on CJD, which received widespread media attention. One of the recommendations of this report was to not accept donations from individuals who had travelled to areas where BSE was occurring.15 On Oct. 27, 1998, an important scientific meeting at Health Canada discussed the Advisory Council's report and a separate report by Dr. Neil Cashman, which also recommended a deferral policy. There was a lack of agreement on the benefits of donor deferral, however, the potential risk to the blood supply was recognized. At the meeting, the Bureau of Biologics asked the operators to evaluate the impact a donor deferral would have on their respective supplies.16
1998 holding of blood from a Utah donor
As the blood system was determining how to address the issue of vCJD, it was also considering reversing its policy on classic CJD. During this time, the issue arose of a US blood donor from Utah who had developed CJD (Dec. 8, 1998). Some blood products manufactured from this donor's plasma were identified as being in the Canadian blood supply. Due to the young age of the donor, there was concern that he may have been suffering from a variant form of the condition, perhaps related to chronic wasting syndrome of elk (a transmissible spongiform encephalopathy similar to BSE and CJD). On Dec. 17, 1998, the blood products derived from this individual were placed on hold and were not to be issued for use.17 The regulator eventually obtained confirmation that the donor did not have a variant form of the condition and on Dec. 24, 1998, it removed the hold on his blood.18
1999 ban on blood from donors who had visited the UK
In March 1999, both operators had obtained the results of their surveys of the impact of donor deferral on the blood supply. Héma-Québec determined that excluding individuals who had spent 1 month in the UK between 1980 and 1996, the peak years of the BSE outbreak (a 1-month deferral policy), would reduce their blood supply by about 3%. The previous experience of the Red Cross had led the operators to believe that this was the maximum reduction of supply that the blood system could tolerate. Héma-Québec, in April, passed a resolution supporting a 1-month deferral policy.19 CBS was not prepared to proceed with a similar policy at that time, which would have reduced their blood supply by 10%, and was considering deferring donations based on a longer time spent in the UK. CBS also emphasized the potential impact a donor deferral policy would have on deterring future donors and decided to obtain further consultations on the issue. The Bureau of Biologics notified both agencies that they needed to work together to develop a unified proposal. It was the opinion of the federal Minister of Health's office that a single national standard would be in the best interests of the blood system, a position shared by the National Blood Safety Council. However, CBS and Héma-Québec were unable to present a unified proposal by the deadline of June 10, 1999.20 Health Canada developed its own regulation and presented a draft proposal to the operators that stated that individuals who had spent 6 months in the UK between 1980 and 1996 should be deferred from donating (a 6-month deferral policy). This proposal was based on a risk assessment model developed by the Laboratory Centre for Disease Control (LCDC) and the knowledge that the US would proceed with a similar policy.21 On August 17, Health Canada announced its policy regarding donor deferral.22 As operators can exceed the standard of the regulator on these matters, Héma-Québec chose to proceed with a 1-month policy.
Events following the 1999 blood ban
Both CBS and Héma-Québec implemented their respective donation deferral policies in advance of the regulator's deadline of February 2000.23 Due to concerns about reduced supply as a consequence of the deferral policy, both agencies introduced aggressive blood donor recruitment programs.24 In the year 2000, 2 new cases of vCJD were identified in France.25 Based on the LCDC risk assessment model, donor deferral was recommended. On Aug. 31, 2000, Health Canada announced that it would defer donations from individuals who had travelled to France for a period of 6 months between 1980 and 1996.26 Both Héma-Québec and CBS indicated they would follow this directive. The US did not implement a similar deferral policy at that time.
Comparison of decision-making leading to the 1995 recall and the 1999 donor deferral policy
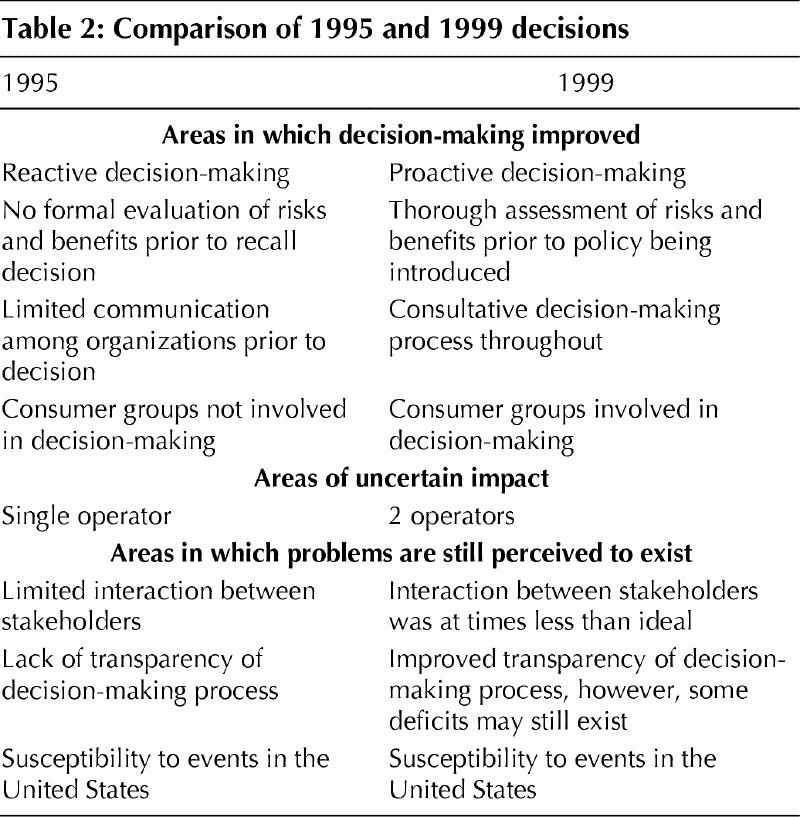
We observed that the 1999 decision to defer donations from individuals who had travelled to the UK demonstrated a significant improvement in decision-making over the 1995 recall of blood connected with a Vancouver donor and the 1998 holding of blood connected with a Utah donor. The potential threat of vCJD was identified at an early stage by the Bureau of Biologics, and a systematic risk assessment process was initiated by the LCDC. Decision-making was consultative and involved consumers. However, the perception exists that further improvement could take place in the areas of transparency of process and interaction of organizations (Table 2).
Table 2
Areas where there has been improvement in decision-making
Reactive versus proactive decision-making and the evaluation of risks and benefits of policy
According to interviews and documents, one of the main criticisms of the 1995 recall decision was that it was reactive. The Red Cross was forced to respond urgently to concerns that a donor had been diagnosed with CJD, due to the lack of a policy governing decision-making on the issue. In 1999, decision-making was proactive. The potential threat of vCJD to the blood supply was addressed by the Bureau of Biologics soon after the condition was described, and a policy development process was initiated.27 The proactive nature of decision-making contributed to another important improvement in the policy process in 1999. In 1995, the operator, the Canadian Red Cross, had to make the decision to withdraw blood products in the absence of a careful examination of the risk of transmission and the impact on the blood supply. In making the decision, the Red Cross primarily relied upon information on risk of transmission obtained from US decision-making bodies.28 The regulator, the Bureau of Biologics, did conduct a detailed evaluation of the issue of blood transmission of CJD; however, the results of this evaluation were not available until well after the recall decision had been made.29 In contrast, in 1999, the regulator and operators conducted a detailed, systematic assessment of the risk of transmission of vCJD and the potential impact of a donor deferral policy on the blood supply. This information was entered into a risk assessment model that guided the decision to choose a 6-month length of stay criterion. The information from this assessment and the model was also used to guide the decision to defer donors who had visited France.21
Consultative decision-making process
In 1995, prior to the decision to recall blood connected with a Vancouver donor, the important decision-making organizations examined the risk of CJD to the blood supply independently with little communication among them. In contrast, in 1999, the regulator involved the operators at an early stage in the decision-making process. In addition, the working relationship between the Bureau of Biologics and the LCDC was considerably more effective in 1999 than in 1995.
Involvement of consumers
There have been significant improvements in the involvement of groups representing consumers of blood products in the policy process from 1995 to 1999. In 1995, consumers were not involved in the decision-making process to any extent before the recall decision, although they were kept informed once the decision had been made. There was also no formal mechanism to involve consumers in decision-making. In contrast, in 1999, consumers were involved in several stages of decision-making by both the operators and the regulators. The operators also developed mechanisms to consult with blood donors.
Areas of uncertain impact on decision-making
The 2-operator system
One of the most significant differences in decision-making between the 1995 and 1999 decisions was the existence of a second operator. The presence of Héma-Québec introduced an important dynamic into the policy process that led to the donor deferral decision.
Héma-Québec significantly affected the policy process when it announced in April 1999 that it was prepared to introduce a 1-month donor deferral policy. CBS, at this time, had not completed its evaluation of the impact of a donor deferral. However, it recognized that it could not have as strict a donor deferral policy because a higher percentage of its donors had travelled to the UK. The possibility of having 2 different standards of safety for blood in Canada created concern on the part of the regulator and the federal Minister of Health's office. The regulator asked the 2 organizations to work together to develop a uniform policy. However, this initiative was unsuccessful.
It is important to recognize that CBS and Héma-Québec started the policy process on donor deferral from different positions. Both were newly created organizations; however, the initial development and management of CBS would be more challenging than for Héma-Québec due to CBS's considerably larger jurisdiction. CBS also had more concerns than Héma-Québec regarding the adequacy of its blood supply when the discussions about donor deferral began. Perhaps most importantly, CBS was aware that any decision on donor deferral would have a larger impact on its supply base than was the case for Héma-Québec.
Individuals whom we interviewed expressed differing views on the advantages and disadvantages of having Héma-Québec as a second operator in the policy process. Several of our informants indicated that they believed that Héma-Québec's rapid response and development of policy regarding donor deferral drove decision-making on this issue. The informants attributed Héma-Québec's speed and efficiency to both the structural advantage it had due to its smaller size as well as to the expertise of its personnel. However, other informants believed that there were political motivations for the introduction of what could be perceived as a “higher” standard by Héma-Québec, perhaps associated with an attempt to establish the autonomy of its organization. These informants also stated that this may have unnecessarily complicated the policy process. Héma-Québec asserted that it had no choice but to proceed with the policy it chose, because the agency had an obligation to provide the maximum protection to its recipients that its supply could withstand. Consumer representatives whom we interviewed believed that the second operator acted as an important check and balance in the system. Some stated that while they had initially supported a single operator system, following the donor deferral decision they now saw value in, and supported, the 2-operator system.
The 2-operator system serves as a useful case study of the strengths and weaknesses of decentralization of the blood system. One of the arguments in favour of decentralization of health and social policy sectors is that it allows for competition between regions to develop. This competition can lead to innovation and experimentation as competing systems attempt to produce “better” policy.30 The dynamic of Quebec and the rest of Canada produces a unique form of competition given the underlying political tension between these 2 regions. Social policy researchers have argued that this unique competitive environment has contributed to Canada's greater progress in social policy since the 1960s compared with the US.31 The primary disadvantage of decentralization of social policy areas is the potential for conflict between regions and difficulties in establishing national standards.32 From our analysis, it appears that a 2-operator system created a competitive environment that stimulated policy-oriented learning. However, at the same time, it also resulted in the development of conflict between the operators and, arguably, did not produce a single national standard.
Our evaluation of the 2-operator system took place soon after both operators had begun operations. As the operators become more familiar with each other, the impact on the policy process of a second operator could change.
Areas in which problems are still perceived to exist
Interaction of organizations
Although our analysis demonstrates that communication between organizations had improved since 1995, some difficulties may still exist. Many of our informants stated that, in the early stages of the decision-making process, there was a certain tension between the 2 operators as they learned to work with each other. The unfamiliarity of the working relationship led to problems of communication between the boards of the 2 organizations. Our informants also identified some difficulties in the relationship between the operators and the regulator. The failure of the operators to present a coordinated proposal on donor deferral to the regulator demonstrated some of the early communication difficulties. A lack of understanding of the roles and responsibilities of the National Blood Safety Council was also identified as being a problem early in the decision-making process. Many of these interactions have improved since the early stages of the policy to defer donors who had visited the UK. According to some of our informants, the key organizations interacted effectively to develop the policy to defer donors who had visited France.
Transparency and public consultation
Lack of transparency was one of the major criticisms of the blood system at the time when the blood supply was confronted with hepatitis C and HIV.33 Improving transparency has been a particularly important concern of the new blood system to re-establish public confidence. Although considerable progress has been made in this area, our analysis suggests that the perception continues to exist that the decision-making process may benefit from greater transparency. The blood system, in particular, came under criticism for transparency issues in 1998 surrounding the Utah donor issue. The regulator and the operators made an attempt at greater transparency of process in developing the policy to defer donors who had visited the UK by involving consumer groups throughout the policy process. Although some informants stated that these changes had improved transparency, we found that some consumers and members of the media thought that further progress could be made in this area.
One issue relating to transparency that emerged was whether consultation should take place with the general public and, if so, by what mechanism. The US Food and Drug Administration holds advisory board meetings, which are open to the public and at which voting on recommendations takes place. Our informants have both praised these meetings for their openness and education of the public and criticized them for leaving the blood system susceptible to having its agenda driven by interest groups. In Canada, the National Blood Safety Council periodically holds public forums on blood issues, however, no voting takes place at these meetings. CBS is currently responding to a task force's recommendations on how the operator can enhance public participation.34
The influence of events in the US on Canadian decision-making
Events in the US were a key factor that influenced the decision-making process in all 3 CJD-related decisions that we examined. One of the main reasons why Canadian policy on blood is so heavily influenced by US policy is that Canada is not self-sufficient in fractionated products. Consequently, Canada must rely upon plasma products derived from US donors, emphasizing the importance of the 2 countries' sharing similar safety standards. It should be noted, however, that in 2000 the US and Canada did not share the same policy on how to handle the issue of donations from individuals who had travelled to France, with Canada choosing to proceed with a donor deferral policy and the US initially deciding not to. The American Red Cross has since instituted a similar policy to Canada's.
Conclusion
Our analysis demonstrates a significant evolution in the mechanism by which decision-making in the blood system occurs, and we have outlined some of the important aspects of the current state of decision-making. The blood system will continue to face difficult challenges from CJD as BSE emerges in other European countries and new information becomes available on the risk of transmission of vCJD by blood. To handle these challenges effectively, the blood system will have to continue to develop its approach to policy-making.
Footnotes
This article has been peer reviewed.
Acknowledgements: The authors would like to acknowledge the participation of the key informants and decision-making organizations in this policy analysis. All existing key organizations were accessible and cooperative, which reflected to us their commitment to further understanding the decision-making process. We would also like to acknowledge the contributions of Dr. Cathy Code, Elaine Parker and Laura MacDougall to this project.
Dr. Hébert is an Ontario Ministry of Health Career Scientist, Ottawa, Ont. Dr. Graham is a Canadian Institutes of Health Research Scholar, Ottawa, Ont. Dr. Laupacis is a Senior Scientist with the Canadian Institutes of Health Research, Toronto, Ont. This project was funded by a grant from the Canadian Institutes of Health Research. The statements expressed in this article are solely those of the authors and do not reflect the views of the institutions they represent.
Competing interests: None declared.
Correspondence to: Dr. Kumanan Wilson, ENG-254, Toronto General Hospital, University Health Network, 200 Elizabeth St., Toronto ON M5G 2C4; fax 416 595-5826; kumanan.wilson@uhn.on.ca
References
- 1.Wilson K, Code C, Ricketts M. The risk of acquiring Creutzfeldt-Jakob disease from blood transfusions: a systematic review of case-control studies BMJ 2000; 321:17-9. [DOI] [PMC free article] [PubMed]
- 2.Lomas J. Connecting research and policy. Can J Policy Res 2000;1:140-4.
- 3.The new Canadian Blood Services and the new blood system. Ottawa: Health Canada; 1997 Oct. Available: www.hc-sc.gc.ca/english/archives/96-97/nba-e.htm (accessed 2001 June 11).
- 4.Quebec Committee on the Supply, Management and Distribution of Blood. The Quebec blood supply system. Quebec: The Committee, 1996 Nov.
- 5.Miles Inc. initiates voluntary market withdrawal of Prolastin® products [press release]. Miles Inc.; 1994 Nov 17.
- 6.D. McDougall. Brain virus could be latest threat to blood. Ottawa Citizen 1995 July 11; Sect A:3.
- 7.Minutes of the Canadian Red Cross Society: meeting to discuss Creutzfeldt-Jakob disease. Ottawa; 1995 July 12.
- 8.Minutes of the Bureau of Biologics: meeting of Crisis Management Team re Creutzfeldt-Jakob disease in Canadian donor. Ottawa: Health Canada; 1995 July 12.
- 9.Memorandum from Dr. Maung T. Aye, National Director, Blood Services of the Canadian Red Cross Society, to Medical Directors, Blood Banks, re 25% albumin withdrawal. 1995 July 20.
- 10.Minutes of telephone conversation between the Canadian Red Cross Society and the Bureau of Biologics to discuss CJD. 1995 July 14.
- 11.Policy on Creutzfeldt-Jakob disease [letter]. Ottawa: Health Canada; 1995 Oct 20.
- 12.Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, Alperovitch A, et al. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 1996; 347: 921-5. [DOI] [PubMed]
- 13.Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, et al. Transmissions to mice indicate that “new variant” CJD is caused by the BSE agent. Nature 1997;389(6650):498-501. [DOI] [PubMed]
- 14.Committee on Safety and Medicines completes review of blood products [press release on CJD/ BSE]. London (UK): Department of Health; 1998 May 13.
- 15.Trevor-Deutsch B, Connell G, Dickens BM, Kaufman F, Knoppers BM, Pringle D, et al. Creutzfeldt-Jakob disease, blood and blood products: a bioethics framework [working paper]. Ottawa: Bayer Advisory Council on Bioethics; 1998 Oct 15. Available: www.bayer-bioethics.org/bioFrames.html (accessed 2001 June 6).
- 16.Meeting on Creutzfeldt-Jakob disease and the Canadian blood system: final summary report. Ottawa: Health Canada; 1998 Oct 27.
- 17.Health Canada. Health Canada advises Canadian Blood Services to hold certain blood products [press release]. Ottawa: Health Canada; 1998 Dec 18. Available: www.mad-cow.org/00/archive_frame.html (search December 98 archive) (accessed 2001 June 12).
- 18.Health Canada. Health Canada advises blood agencies on disposition of blood products [press release]. Ottawa: Health Canada; 1998 Dec 24. Available: www .hc -sc .gc.ca/english/archives/releases/1998/98_106e.htm (accessed 2001 June 6).
- 19.Resolution that forms part of the minutes of the Héma-Québec board of directors' meeting held on April 7, 1999: new variant of Creutzfeldt-Jakob disease. Ville Saint-Laurent (Que): Héma-Québec; 1999. Available: www.hema-quebec.qc.ca/E/gestsang/fb3b7.htm (accessed 2001 June 6).
- 20.Memorandum to the Minister from Dr. Doug Kennedy, Bureau of Biologics and Radiopharmaceuticals, re variant CJD and the Canadian Blood System. Ottawa: Health Canada; 1999 June 25. Doc no 99-020193.
- 21.El Saadany S, Giulivi A. Comprehensive risk assessment for vCJD in France and other countries for Canadians and the Canadian blood supply. Ottawa: Division of Blood-borne Pathogens, Health Canada; 2000 June 26.
- 22.Therapeutic Products Programme, Health Canada. Directive 99-02: donor exclusion to address theoretical risk of transmission of variant CJD through the use of commercial blood products. Ottawa: Health Canada; 1999 Aug 17. Available: www.hc-sc.gc.ca/hpb-dgps/therapeut/htmleng/btox.html (accessed 2001 June 6).
- 23.Canadian Blood Services to defer donors who have spent six months or more in U.K. [press release]. 1999 Aug 17. Available: www.bloodservices.ca/english/home_english.html (see news releases) (accessed 2001 June 11).
- 24.A report to Canadians 1999/2000. Ottawa: Canadian Blood Services. Available: www.bloodservices.ca/english/pub/ar_2000/pdf/ar2000_toc.pdf (accessed 2001 June 6).
- 25.Dorozynski A. France prepares for more cases of vCJD. BMJ 2000;321:1241. [PMC free article] [PubMed]
- 26.Health Canada. Health Canada issues precautionary directive for deferral of blood and plasma donors who have spent extended periods of time in France [press release]. Ottawa: Health Canada; 2000 Aug 31. Available: www.hc-sc.gc.ca/english/archives/releases/2000/2000_85e.htm (accessed 2001 June 11).
- 27.Minutes of the expert advisory committee on blood regulation held April 23, 1998: item 5. Ottawa: Health Canada; 1998. Available: www.hc-sc.gc.ca/hpb-dgps/therapeut/zfiles/english/advcomm/eac/blood/minutes/98-04-23_e.html (accessed 2001 June 6).
- 28.Letter from Dr. Maung T Aye, National Director, Canadian Red Cross Society, to Dr. Keith Bailey, Director Bureau of Biologics, re FDA releasing memorandum regarding CJD. 1995 Sept 1.
- 29.Bureau of Drug Research, Health Canada. Status of CJD knowledge and research priorities (Bureau of Drug Research). Summary of meeting. Ottawa: Health Canada; 1995 July 24.
- 30.Courchene TJ. ACCESS: a convention on the Canadian economic and social systems. In: Assessing ACCESS. Towards a new social union. Proceedings of the symposium on the Courchene proposal; 1996 Oct 31-Nov 1; Kingston (Ont). Kingston (Ont): Institute of Intergovernmental Relations, Queen's University; 1997. p. 77-112.
- 31.Richards J. Reducing the muddle in the middle: three propositions for running the welfare state. In: Lazar H, editor. Canada: the state of the federation 1997. Non-constitutional renewal. Kingston: Institute of Intergovernmental Relations, Queens University; 1998.
- 32.Kennett SA. The Courchene proposal. Securing the social union: commentary on the decentralized approach. Kingston: Institute of Intergovernmental Relations, Queens University; 1998. Research paper no 34.
- 33.Picard A. The gift of death: confronting Canada's tainted-blood tragedy. Toronto: Harper Collins Canada; 1998.
- 34.Leiss W, Plumptre T, Segal H. Final Report of the Task Force on Public Participation, Canadian Blood Services. To the Board of Directors of Canadian Blood Services. 2000 Nov 1. Available: www .bloodservices .ca /english /participation/tfpp-report_e.pdf (accessed 2001 June 6).


