Abstract
Excessive alcohol drinking has been shown to modify brain circuitry to predispose individuals for future alcohol abuse. Previous studies have implicated the central nucleus of the amygdala (CeA) as an important site for mediating the somatic symptoms of withdrawal and for regulating alcohol intake. In addition, recent work has established a role for both the Kappa Opioid Receptor (KOR) and its endogenous ligand dynorphin in mediating these processes. However, it is unclear whether these effects are due to dynorphin or KOR arising from within the CeA itself or other input brain regions. To directly examine the role of preprodynorphin (PDYN) and KOR expression in CeA neurons, we performed region-specific conditional knockout of these genes and assessed the effects on the Drinking in the Dark (DID) and Intermittent Access (IA) paradigms. Conditional gene knockout resulted in sex-specific responses wherein PDYN knockout decreased alcohol drinking in both male and female mice, whereas KOR knockout decreased drinking in males only. We also found that neither PDYN nor KOR knockout protected against anxiety caused by alcohol drinking. Lastly, a history of alcohol drinking did not alter synaptic transmission in PDYN neurons in the CeA of either sex, but excitability of PDYN neurons was increased in male mice only. Taken together, our findings indicate that PDYN and KOR signaling in the CeA plays an important role in regulating excessive alcohol consumption and highlight the need for future studies to examine how this is mediated through downstream effector regions.
Introduction
Excessive alcohol drinking is a serious public health problem and was estimated to cost the United States over $249 billion in 2010 (1). The majority of these expenses could be attributed to binge drinking, a form of alcohol consumption wherein individuals imbibe large amounts of alcohol within a short period of time and reach blood alcohol concentrations above 0.08% (80 mg/dL) (NIAAA). Notably, individuals who engage in frequent binge drinking have a higher risk of later developing Alcohol Use Disorder (AUD) (2,3). Because binge-alcohol consumption has been shown to engage the neural circuitry involved in the addiction cycle (4), understanding the adaptations that occur following repeated episodes of binge drinking may provide important insights into the ways in which excessive alcohol drinking contributes to the development of AUDs. Specifically, work from our lab has found that both the central nucleus of the amygdala (CeA) and the Bed Nucleus of the Stria Terminalis (BNST), components of the extended amygdala (5), are involved in binge-like alcohol consumption (6,7). These regions are important for regulating negative affective states, which are thought to underlie the negative reinforcing properties of alcohol (8).
Abundant within these brain regions is the kappa opioid receptor (KOR) and its endogenous ligand dynorphin encoded by the Pdyn gene (9,10). Activation of KOR results in dysphoria in human subjects (11), and recently it has been shown that there is reduced KOR occupancy in the amygdala of patients with an AUD (12). In animal models, systemic treatment with KOR antagonists reduces ethanol self-administration (13), stress-enhanced drinking (14), and ethanol dependence-induced increases in anxiety (15). Additionally site-specific KOR antagonism in the CeA decreases ethanol self-administration in dependent animals (16) and alcohol consumption in a model of binge-like drinking (17). Anderson et al. (2018) also found that chemogenetic inhibition of preprodynorphin (PDYN) neurons in the CeA reduced binge-like drinking, suggesting that the source of dynorphin release may be within the CeA. However, one limitation of these experiments is that PDYN neurons co-express a variety of other neuropeptides such as corticotropin releasing factor, somatostatin, and neurotensin (18). As the CeA is comprised of GABAergic medium spiny neurons that make many recurrent local connections (19), it is unclear whether the results of the chemogenetic inhibition experiment are due directly to release of dynorphin, GABA or some other co-expressed neuropeptide.
To examine more closely how this system may work to regulate ethanol consumption, we performed dual in situ hybridization of Pdyn and Oprk1 mRNA (the Oprk1 gene encodes KOR protein) and found that they are expressed in separate, relatively non-overlapping neuronal populations in the CeA. We then separately knocked out Pdyn and Oprk1 and found that these manipulations resulted in reductions in alcohol drinking in a sex-specific manner. Finally, we performed slice electrophysiology recordings from mice that underwent binge-like drinking and found that a history of alcohol drinking resulted in sex-specific alterations in PDYN neuron excitability with no effect on synaptic transmission.
Materials and Methods
Subjects
Experiments were performed on adult male and female mice between 8–10 weeks of age at the beginning of the experiments. PdynIRES-Cre and Gt(ROSA26)SorloxSTOPlox-L10-GFP (20), Oprk1lox/lox conditional-knockout mice (21) were generated as described and bred in house at UNC. Additionally, Pdynlox/lox mice were generated as described in the supplemental experimental procedures and were subsequently transferred and bred in house at UNC. All mice were group housed in colony rooms on a 12:12 hr light-dark cycle (lights on at 7 a.m.) with ad libitum access to rodent chow and water. All animal procedures were performed in accordance with the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill.
Stereotaxic Surgeries
Mice were anesthetized with 4% isoflurane and placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA, USA) and maintained under 1–2% isoflurane for the duration of the surgery. The skull was exposed and burr holes were made with a drill over the coordinates of the Central Amygdala (M/L +/− 2.90, A/P −1.20, D/V −4.70). A Hamilton Syringe (Hamilton Company, Reno, NV) was used to deliver 200 nL of an adeno associated virus encoding ere recombinase with a translationally fused green fluorescent protein (AAV5-Camk2a-Cre:GFP) or control vector (AAY5-Camk2a-eGFP) into the CeA. The fused Cre:GFP variant is nuclear-restricted resulting in a more punctate pattern of labeling compared to the cytosolic GFP control vector despite being matched in terms of injection volume. The virus was infused at a rate of 0.1 μL per minute and left in place for at least 5 minutes to ensure diffusion.
Histology
Following the completion of behavioral experiments, mice were deeply anesthetized with tribromoethanol (250 mg/kg, intraperitoneal) and transcardially perfused with 4% paraformaldehyde in PBS. Brains were sliced for histology and placements were verified using procedures described in the supplemental experimental methods.
In Situ Hybridization
Mice were anesthetized with isoflurane and rapidly decapitated. Brains were dissected and flash frozen on dry ice for 15 min and stored at −80°C until sectioned for in situ hybridization (ISH). Brain slices (16 μm) containing the CeA were obtained on a Leica CM 3050S cryostat (Leica Biosystems, Nussloch, Germany) at −20°C and were mounted directly onto microscope slides. Slides were stored at −80°C until tissue was processed for ISH. RNAscope ISH was conducted following the manufacturer’s protocol as described in the supplementary experimental methods.
qPCR on Tissue Punches
Mice were anesthetized with isoflurane and rapidly decapitated. The brain was dissected out and 1-mm coronal sections were made using a brain block. Slices were flash frozen on dry ice and tissue punches containing bilateral samples from the CeA were taken and stored at −80°C until future use. Total RNA from tissue punches was extracted using Direct-zol RNA miniprep kit (Zymo Research, Irvine, CA, USA). Reverse transcription and qPCR were performed using Superscript II and Applied Biosystems Taqman Assay on a StepOnePlus Real Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). The following TaqMan assay probes were purchased from Invitrogen (USA): ACTB Mm00607939_s1, Oprk1 Mm01230885_m1, Pdyn Mm00457573_m1. Three technical replicates were averaged together to compute mean cycle threshold (Ct) values for the housekeeping gene and experimental genes of interest. The Ct value for β-actin was then subtracted from the Ct values for Oprk1 and Pdyn to compute normalized ΔCt values for each sample.
Alcohol and Tastant Drinking Procedures
All alcohol and tastant drinking experiments were conducted with mice single housed in a reverse light cycle space (lights off at 7am, lights on at 7pm). Drinking in the Dark (DID) was carried out as described (4,6,22). Briefly, on days 1–3 beginning 3 hr into the dark cycle, water bottles were removed from all cages and replaced with a bottle containing 20% (v/v) ethanol solution. Mice had 2 hr of access to ethanol, after which the ethanol bottles were removed from cages and water bottles were replaced. The same procedure was followed on day 4 except that ethanol access was extended to 4 hr. Following completion of DID experiments, cage tops were replaced with lids designed for 2-bottle drinking. Following 3 days of acclimation to bottles containing only water, animals began Intermittent Access to Ethanol (IA) as described previously (23) and in the supplemental experimental methods.
Ethanol-naïve animals underwent drinking of aversive or palatable solutions using the similar procedures as the drinkers. However, the animals always had access to one bottle containing water and the other containing the tastant solution of interest. The concentrations of quinine (0.3, 0.6, and 0.9 mM), saccharin (0.33% and 0.66%), and sucrose (1%) were chosen based on values previously used to characterize global PDYN and KOR knockout mice (24,25). The bottle choice assay began with the lowest concentration of tastant in the series (0.3 mM quinine or 0.33% saccharin), which was then increased over the course of the next 6 days. The order of presentation of each solution type (palatable or aversive) was counterbalanced across cohorts within an experiment.
Elevated Plus Maze
Elevated Plus Maze (EPM)testing was conducted 8 hrs after the last DID binge session (approximately 10pm or 3 hr in the light cycle). Testing in ethanol naive animals was performed at the same time of day with respect to the light/dark cycle. Time in the open arm served as the primary measure of anxiety-like behavior and was quantified as described in the supplemental experimental procedures.
Slice Electrophysiology
Mice underwent three cycles of DID with access to water or ethanol as described above. To ensure that differences between groups were driven by a history of sufficiently intoxicating levels of ethanol consumption, only mice that consumed cumulatively greater than 20g/Kg were included in the analysis. Twenty-four hours after the final binge session, mice were sacrificed for slice electrophysiology recordings. Whole-cell electrophysiology experiments were performed similar to those published previously (4,26). Briefly, 300-μm coronal slices containing the CeA were prepared on a vibratome (Leica VT1200, Leica, Wetzlar, Germany) from mice rapidly decapitated under isoflurane. The brains were removed and placed in ice-cold modified high sucrose artificial cerebrospinal fluid (aCSF) containing the following (in mM): 194 sucrose, 20 NaCl, 4.4 KCl, 2 CaCl2, 1 MgCl2, 1.2 NaH2PO4, 10.0 glucose, and 26.0 NaHC03. Slices were then transferred to normal aCSF maintained at approximately 30°C (Warner Instruments, Hamden, Connecticut) containing the following (in mM): 124 NaCl, 4.4 KCl, 2 CaCl2, 1.2 MgSO4, 1 NaH2PO4, 10.0 glucose, and 26.0 NaHCO3. Slices were placed in a holding chamber where they were allowed to rest for at least one hour. Slices were continuously bubbled with a 95% O2 / 5% CO2 mixture throughout slicing and experiments. Thin-walled borosilicate glass capillary recording electrodes (3–6 MΩ) were pulled on a Flaming-Brown micropipette puller (Sutter Instruments, Novato, CA). Following rupture of the cell membrane, cells were allowed to equilibrate to the intracellular recording solutions for at least 5 minutes before any experiments were conducted. Excitability and synaptic transmission experiments were performed as described in the supplemental experimental methods.
Sample size and replication
The target number of samples in each group was determined based on numbers reported in published studies. No statistical methods were used to predetermine sample size. Sample sizes for each in experiment are listed in the figure legends whereby N denotes the number of mice used and n denotes the total number of cells or images analyzed. Histology and gene expression experiments were conducted in a single cohort and were not replicated (Figure 1). Findings from genetic knockout studies on alcohol drinking were replicated across two cohorts (Figures 2–3). Results from electrophysiology experiments were replicated from three separate cohorts of mice that underwent alcohol drinking (Figure 4–5).
Figure 1-. PDYN and KOR expression in CeA neurons is unaltered by a history of ethanol drinking.
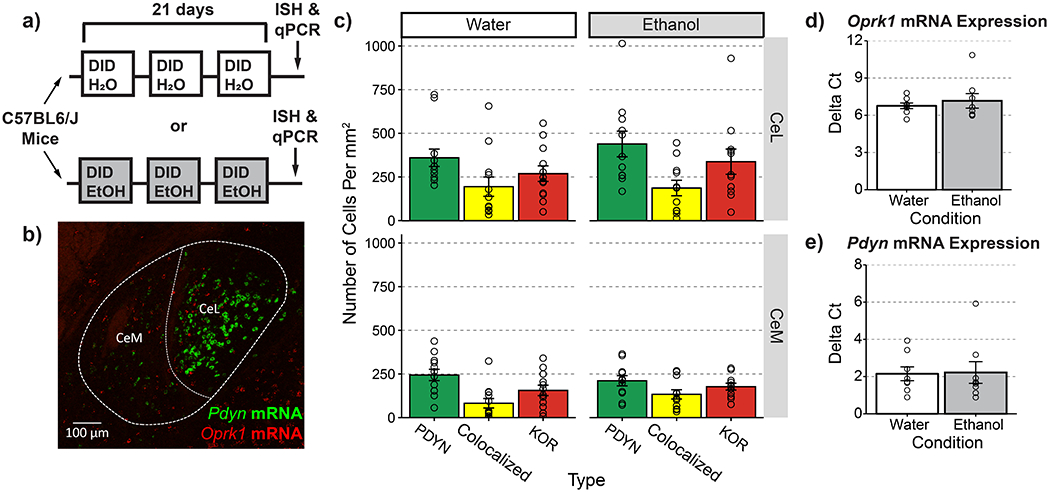
(a) Timeline for experimental procedures. (b) Representative image of Pdyn and Oprk1 mRNA expression in the CeA (20x objective, NA=0.8) (c) Quantification of PDYN+ and KOR+ cell counts following experimental manipulations. Ethanol treatment did not affect the proportion of PDYN+, KOR+, and colocalized neurons X2(2)=1.494, p=0.474 (d) Ethanol treatment did not affect Oprk1 gene expression t(9.04)=0.64, p= 0.534 or (e) Pdyn gene expression t(11,899)=0.108, p=0.916. (c) n=12 images from N=3 ethanol or N= 2 water drinking mice. Individual dots reflect counts within a single image which were not pooled within subjects (d-e) punches from n=8 water and n=8 ethanol drinking animals
Figure 2-. Knockout of KOR in CeA decreases ethanol consumption in male, but not female mice.
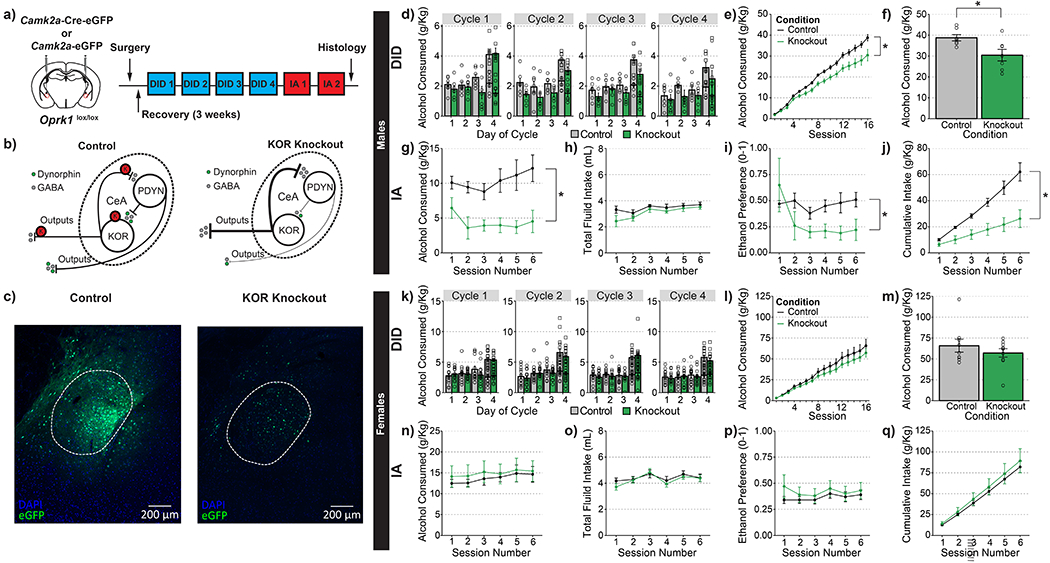
(a) Timeline for experimental procedures. (b) Hypothesized actions of KOR knockout in CeA. (c) Representative images of control virus expression (left) and knockout virus (right). (d-j) Ethanol consumption in male animals. (d) Group averages for individual sessions over the course of four weeks of DID. Consumption at both the 2-hr and 4-hr time points are plotted separately on day 4. (e) Cumulative averages by group for four weeks of DID; knockout of KOR in CeA significantly decreased alcohol consumption in male animals, main effect of condition F(1,12)=7.100, p=0.021; condition X cycle interaction F(3,36)=0.324, p=0.808. (f) Cumulative totals from individual mice. (g) KOR knockout resulted in a significant reduction in the amount of alcohol consumed during IA; main effect of genotype: F(1,12)=13.628, p=0.003. (h) KOR knockout did not affect total fluid intake during IA; main effect of genotype: F(1,12)=1.652, p=0.223 (i) KOR knockout resulted in a significant reduction in ethanol preference; F(1,12)=6.616, p=0.024. (j) KOR knockout significantly reduced the cumulative amount of ethanol consumed during IA; main effect of genotype: F(1,12)=13.694, p<0.001. (k-q) Ethanol consumption in female mice. (k) Group averages for individual sessions over the course of four weeks of DID. Consumption at both the 2-hr and 4-hr time points are plotted separately on day 4. (l) Cumulative averages by group for four weeks of DID; KOR knockout in CeA did not significantly alter alcohol consumption in female animals, main effect of condition F(1,17)=0.909, p=0.353; cycle X condition interaction F(3,51)=0.614, p=0.609. (m) Cumulative totals from individual mice. (n) KOR knockout did not alter the amount of alcohol consumed during IA; main effect of genotype: F(1,17)=0.196, p=0.663. (o) KOR knockout did not affect total fluid intake during IA; main effect of genotype: F(1,17)=0.232, p=0.636. (p) KOR knockout did not alter ethanol preference; main effect of genotype: F(1,17)=0.512, p=0.484. (q) KOR knockout did not affect cumulative ethanol consumption during IA; main effect of genotype: F(1,17)= 0.263, p=0.614. (d,k) round dots indicate values at 2-hr time points and square dots indicate values at 4-hr time points (d-j) n=8 control males and n=7 knockout males (k-q) n=9 control females and n=10 knockout females
Figure 3-. PDYN knockout in CeA decreases ethanol consumption in male and female mice.
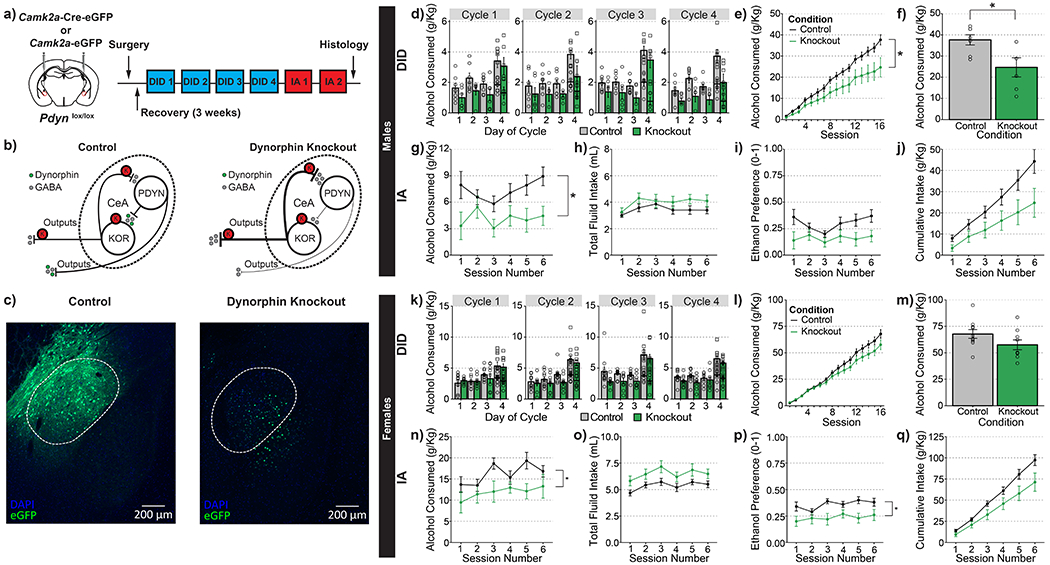
(a) Timeline for experimental procedures. (b) Hypothesized actions of PDYN knockout mice. (c) Representative images of control virus expression (left) and knockout virus (right). (d-j) Ethanol consumption in male animals. (d) Group averages for individual sessions over the course of 4 wk of DID; consumption at both 2-hr and 4-hr time points are plotted separately on day 4. (e) Cumulative averages by group for 4 wk of DID. PDYN knockout in CeA significantly decreased alcohol consumption in male animals; main effect of genotype: F(1,11)=6.899, p=0.024; genotype X cycle interaction F(3,33)=0.753, p=0.528. (f) Cumulative totals from individual mice. (g) PDYN knockout resulted in a significant reduction in the amount of alcohol consumed during IA; main effect of genotype: F(1,11)=4.860, p=0.0497. (h) PDYN knockout did not affect total fluid intake during IA; main effect of genotype: F(1,11)=1.485, p=0.248. (i) PDYN knockout did not affect ethanol preference; main effect of genotype: F(1,11)=3.788, p=0.078. (j) PDYN knockout did not significantly reduce the cumulative amount of ethanol consumed during IA F(1,11)=3.943, p=0.073. (k-q) Ethanol consumption in female animals. (k) Group averages for individual sessions over the course of 4 wk of DID. Consumption at both the 2-hr and 4-hr points are plotted separately on day 4. (l) Cumulative averages by group for 4 wk of DID; knockout of PDYN in CeA did not significantly alter alcohol consumption in female animals; main effect of genotype: F(1,17)=2.707, p=0.118; genotype X cycle interaction F(3,51)=1.643, p=0.191. (m) Cumulative totals from individual mice. (n) PDYN knockout significantly reduced the amount of alcohol consumed during IA; main effect of genotype: F(1,17)=4.490, p=0.049. (o) PDYN knockout did not affect total fluid intake during IA; main effect of genotype: F(1,17)=4.450, p=0.501. (p) PDYN knockout significantly reduced ethanol preference; main effect of genotype: F(1,17)=6.766, p=0.019. (q) PDYN knockout did not affect cumulative ethanol consumption during IA; main effect of genotype: F(1,17)=4.135, p=0.579 (d,k) round dots indicate values at 2-hr time points and square dots indicate values at 4-hr time points (d-j) n=7 control males and n=6 knockout males. (k-q) n=10 control females and n=9 knockout females
Figure 4-. Ethanol Drinking Does Not Alter Synaptic Transmission Onto CeA PDYN Neurons.
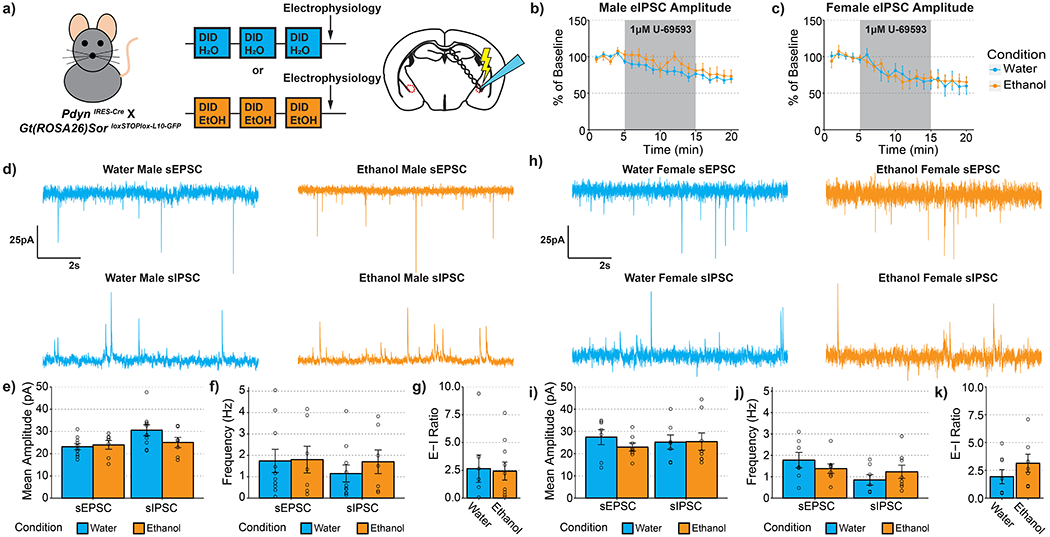
(a) Experimental timeline. (b) U69593 inhibition of eIPSCs is unaltered by a history of alcohol drinking in males t(9.391)=0.687, p=0.508. (c) U69593 inhibition of eIPSCs is also unaltered by a history of alcohol drinking in females t(14.880)=0.054, p=0.957. (d-g) Synaptic transmission in male animals. (d) Representative traces of excitatory and inhibitory events onto CeA dynorphin neurons. (e) Ethanol drinking did not alter the amplitude of excitatory t(11.152)=0.334, p=0.745 or inhibitory transmission t(14.704)=1.644, p=0.121. (f) Ethanol drinking did not alter the frequency of excitatory transmission t(13.390)=0.075, p=0.941 or inhibitory transmission t(11.767)=0.815, p=0.431. (g) synaptic drive was also unaltered after alcohol drinking t(10.982)=0.153, p=0.881. (h-k) Synaptic transmission in female animals. (h) Representative traces of excitatory and inhibitory events onto CeA dynorphin neurons. (i) Ethanol drinking did not alter the amplitude of excitatory transmission t(9.164)= 1.170, p=0.272 or inhibitory transmission t(12.847)=0.046, p=0.964. (j) Ethanol drinking did not alter the frequency of excitatory transmission t(10.198)=0.966, p=0.356 or inhibitory transmission t(12.731)=0.950, p=0.359. (k) synaptic drive was also unaltered after alcohol drinking t(11.806)=1.182, p=0.260. (b) Water male n=9 cells, N=6 mice, ethanol male n=7 cells, n=4 mice; (c) water female n=7 cells, N=5 mice, ethanol female n=6 cells, n=4 mice; (d-g) water male n=10 cells, n=6 mice, ethanol male n=9 cells, n=4 mice; (h-k) water female n=7 cells, n=4 mice, ethanol female n=8 cells, n=5 mice
Figure 5-. Ethanol Drinking Alters the Excitability of PDYN Neurons in A Sex-Specific Manner.
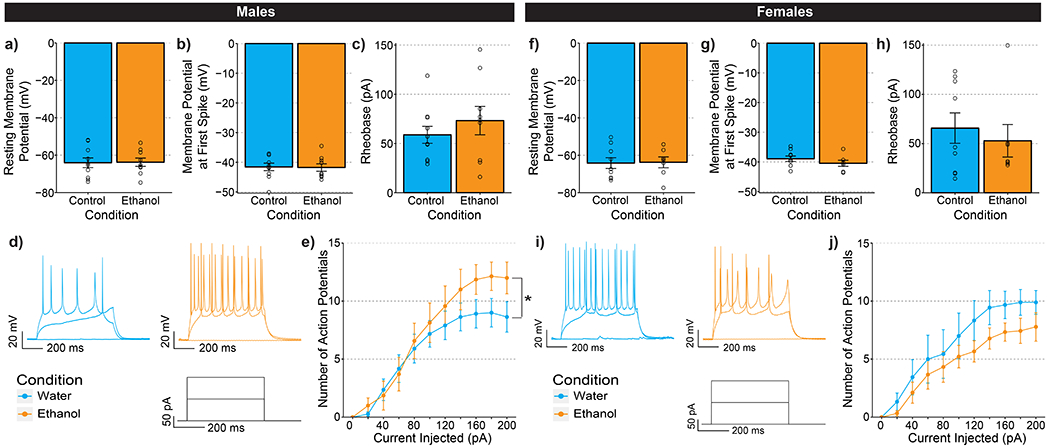
(a-e) Data collected from male mice. (a)There was no effect of ethanol drinking on resting membrane potential t(16.833)=0.076, p=0.940. (b) Ethanol drinking did not change action potential threshold t(16.947)=0.111, p=0.912. (c) There was no significant difference in the amount of current injected needed to elicit an action potential t(13.279)=0.860, p=0.405. (d) Representative traces of PDYN-neuron firing in CeA. (e) There was a significant interaction between ethanol drinking and the number of action potentials fired in the VI plot F(10,160)=2.004, p=0.036. (f-j) Data collected from female mice. (f) There was no effect of ethanol drinking on resting membrane potential t(13.681)=0.101, p=0.920. (g) Action potential threshold t(13.248)=1.120, p=0.283 or (h) rheobase t(13.386)=0.568, p=0.579. (i) Representative traces of CeA PDYN-neuron firing. (j) Ethanol drinking resulted in a nonsignificant decrease in the number of action potentials fired in the VI plot F(1,16)=2.523, p=0.132. (a-e) Water males: n=10 cells from n=6 mice; ethanol male: n=9 cells from N=6 mice; (f-j) water females: n=8 cells from n=5 mice; ethanol female n=9 from n=5 mice
Data Analysis and Statistics
Data are displayed as means ± SEM. For changes in the proportion of overlapping PDYN and KOR neurons after alcohol drinking, groups were compared with a χ2 test. Changes in mRNA expression were compared with a two-sample t test with Welch’s correction. Alcohol consumption and preference were analyzed as within subjects repeated measures ANOVA. Measures of anxiety-like behavior in the elevated plus maze were compared with a two-way (alcohol treatment X gene knockout) factorial ANOVA. Changes in synaptic transmission and excitability were assessed with either a repeated measures ANOVA or a two-sample t test with Welch’s correction where appropriate. Results of statistical tests examining homogeneity of variances can be found in Table 1. In some instances, we found that the data to be analyzed by the repeated measures ANOVA violated Mauchly’s test of sphericity. In these cases, degrees of freedom and p-values reported for ANOVAs reflect Greenhouse-Geisser correction for non-sphericity of the data. All analyses were performed with R statistical software version 3.3.2.
Results
PDYN- and KOR-Expressing Neurons Form Separate Populations in the CeA and The Expression of Which is Not Altered Following Alcohol Drinking
Many studies have implicated the CeA as an important locus for regulating both dependent and non-dependent forms of alcohol drinking (4,27,28). Despite evidence showing that both PDYN and KOR are expressed in the CeA, the circuit mechanism underlying this effect remains unclear. To assess the expression of PDYN and KOR, and how it may be affected by a history of alcohol drinking, we exposed C57BL/6J mice to 3 cycles of Drinking in the Dark (DID). Eight hours after the final binge session, animals were sacrificed for in situ hybridization and qPCR experiments (Figure 1a). Interestingly, we found that Pdyn and Oprk1 mRNA were expressed on relatively distinct populations of neurons (Degree of co-localization: Lateral subdivision (CeL) Mean=22.8% ± 3.9%; Medial subdivision (CeM) Mean=18.5% ± 2.6%), and that the proportion of PDYN+, KOR+, and colocalized neurons was unaltered by ethanol drinking (Figure 1b-c). Additionally, Pdyn and Oprk1 mRNA levels were unchanged following DID (Figure 1d-e). These findings suggest that although PDYN and KOR may be involved in the regulation of alcohol consumption, their gene expression levels are unaffected by a history of alcohol drinking.
Knockout of KOR in CeA Reduces Alcohol Consumption and Preference in a Sex-Specific Manner
Previous studies employing pharmacological approaches have shown that KOR antagonism in the CeA can decrease alcohol intake (16,17). However, it remains unclear whether this is mediated by KOR expression on CeA neurons or on presynaptic terminals from other brain regions. To establish a causal role for KOR signaling in CeA neurons, we performed a conditional knockout of KOR in young adult male and female mice. Oprk1lox/lox mice (21) were infused with an AAV encoding Cre recombinase fused to eGFP or eGFP only as a control (Figure 2a-c). Alcohol drinking experiments began three weeks after virus injection, a time point we have previously demonstrated is sufficient for AAV-Cre driven knockout of KOR (26). As predicted by the findings of Anderson et al. (2018), CeA KOR knockout resulted in a significant reduction in the amount of alcohol consumed across 4 wk of DID in male mice (Figure 2d-f).
Because global KOR knockout has been shown to alter fluid intake (25), we examined whether the differences in DID observed were due to overall reductions in fluid intake. Immediately following completion of DID, experimental bottles were replaced for Intermittent Access to Ethanol (IA) so that changes in water consumption and ethanol preference could also be measured. Notably, reductions in alcohol drinking in KOR-knockout mice were observed throughout the duration of IA experiments (Figure 2g). Additionally, knockout mice had significantly lower ethanol preference, but no significant difference in the total amount of fluid consumed (Figure 2h-j).
While systemic antagonism of KOR has generally resulted in reductions in drinking in male animals (29), some experiments with female animals revealed that KOR antagonism does not alter their alcohol consumption (30). Additionally, KOR activation is less aversive to female animals (31) suggesting that there may be sex differences in KOR function. Interestingly, KOR knockout in the CeA of female mice did not result in reduced alcohol consumption in drinking in DID in contrast to that observed with their male littermates (Figure 2k-m). Additionally, there were no significant differences in total fluid consumption or ethanol preference between wild-type and knockout animals during IA (Figure 2n-q). Thus, the reduction in alcohol drinking by global KOR-knockout females appears to be mediated outside the CeA (25,32).
To assess whether KOR knockout was directly responsible for the reductions in alcohol drinking observed between groups, we performed correlations of the degree of KOR knockout with the cumulative alcohol of mice across four weeks of DID. Using the density of GFP+ nuclei in the CeA as an indirect measure of KOR knockout, we found that a greater degree of KOR knockout was significantly correlated with reductions in alcohol drinking in male mice (Figure S1a). Notably, as female KOR-knockout mice did not exhibit the same KOR-mediated reductions in alcohol drinking as male mice, there was also no significant correlation between levels of GFP+ expression and alcohol drinking (Figure S1b). The Oprk1lox/lox line of mice has been validated in other brain regions (21,26). We confirmed the efficacy of KOR inactivation in the CeA by measuring the effect of agonists to affect eIPSCs (33,34). Knockout of KOR in the CeA suppressed the ability of U-69593 to inhibit eIPSCs (Figure S1c).
Knockout of KOR in CeA Does Not Alter the Palatability of Appetitive or Aversive Tastants
One explanation for the reductions in alcohol drinking observed in the male KOR-knockout mice is that the experimental manipulation renders the taste of ethanol more aversive to knockout animals. If that were the case, the changes observed would be due to alterations in taste perception rather than the pharmacological actions of ethanol. Contrary to this possibility though, we did not observe any significant differences in either sex in their preference other tastants including quinine, sucrose, or saccharin at any of the concentrations tested (Figure S2). As ethanol is a source of calories it is possible that the reductions in ethanol consumption are secondary to changes in overall metabolic intake. However, we did not observe any effects of KOR knockout on binge consumption of 10% sucrose, a caloric reinforcer (Figure S2d,g).
Knockout of PDYN in CeA Reduces Alcohol Consumption and Preference in a Sex-General Manner
One question arising from the previous experiment is where is the source of the endogenous dynorphin that mediates the reduction of alcohol drinking observed in male mice. Given the large adjacent population of PDYN+ neurons, one likely possibility is that it could come from locally released PDYN. To selectively manipulate PDYN expression in a conditional manner, we generated homozygous Pdynlox/lox mice to allow inactivation of Pdyn expression in the CeA using Cre recombinase as described in Figure S3a and Experimental Methods section. Validation by in situ hybridization showed that viral expression of Cre recombinase resulted in a significant reduction in the number of PDYN+ nuclei (Figure S3b-f), confirming that the Pdynlox/lox line is an effective tool for manipulating Pdyn gene expression in the CeA.
To directly examine the effects of dynorphin signaling on alcohol-drinking behavior, we performed conditional knockout of PDYN in the CeA following the same timeline as that used in the KOR-knockout experiments (Figure 3a). As PDYN knockout would have the same net effect on the proposed circuit as the KOR knockout (Figure 3b), we hypothesized there would be a similar reduction in alcohol drinking by male mice. In concordance with this prediction, we observed that PDYN knockout in the CeA resulted in a significant reduction in ethanol consumption across four weeks of DID in male mice (Figure 3d-f). Additionally, this reduction in alcohol drinking was observed throughout the two weeks of IA and did not significantly alter overall fluid intake (Figure 3g,h). One notable divergence from the KOR knockout experiments is that PDYN knockout did not significantly reduce ethanol preference (Figure 3i). However, any difference may be obscured by a floor effect, as the Pdynlox/+ control animals had considerably lower preference than Oprk1lox/+ control animals.
We also tested whether knockout of PDYN in CeA would result in similar reductions in female mice. We observed that inactivation of PDYN in the CeA lowered, but did not significantly reduce, ethanol consumption during DID (Figure 3k-m). However, PDYN-knockout females did significantly reduce their alcohol consumption during IA (Figure 3n) and reduced ethanol preference without affecting overall fluid intake (Figure 3o,p). Thus, it appears that PDYN in the CeA plays a role in regulating ethanol consumption in female mice.
PDYN Knockout in CeA Does Not Alter the Palatability of Appetitive or Aversive Tastants
It has been demonstrated that global PDYN-knockout mice have significantly altered fluid intake, saccharin preference, quinine preference, and sucrose consumption compared to littermate controls (24). To assess the effects of PDYN knockout in the CeA on each of these parameters, ethanol-naïve mice underwent tastant drinking experiments following the same timeline as KOR-knockout experiments (Figure S4a). In contrast to what was observed in the PDYN global-knockout animals, PDYN knockout in the CeA did not alter quinine, saccharin, or sucrose preference in either sex (Figure S4 b,c). Additionally, there were no differences in either sex on the binge consumption of a 10% sucrose solution suggesting that the effects of the PDYN knockout are due to a general reduction in caloric drive.
Neither KOR nor PDYN Knockout Protects Mice Against Alcohol Drinking-Induced Increases in Anxiety
Ethanol dependence has been shown to increase anxiety-like behavior in the elevated-plus maze (EPM), which can be reversed by systemic treatment with a KOR antagonist (15). If PDYN or KOR knockout is protective against the negative-reinforcing properties of ethanol, then these manipulations should produce a detectable change in anxiety-like behavior during acute withdrawal. Because CeA KOR knockout decreased ethanol consumption, we examined the effects of CeA KOR knockout in both ethanol-naïve animals and those that underwent DID (Figure S5a). We observed a main effect of alcohol drinking whereby a history of alcohol use increased anxiety-like behavior in the EPM in male mice (Figure S5bc). However, there was no main effect or interaction with genotype, indicating that KOR knockout does not alter anxiety in the basal state or during withdrawal. Similar effects were observed in female mice, as there was a main effect of alcohol drinking on open-arm entries, but no main effect or interaction with genotype (Figure S5f). With PDYN-knockout, we did not observe any effects of ethanol drinking on open-arm time, entries, or locomotion in either male or female mice (Figure S5 h-m).
A History of Ethanol Drinking Does Not Alter Synaptic Transmission onto CeA PDYN Neurons
A primary role ascribed to activation of KOR signaling in the CeA is that it reduces presynaptic GABA release (33,34). To assess whether KOR modulation of inhibitory synaptic transmission was altered after a history of binge-like, alcohol drinking, we performed electrophysiology recordings in slices from the CeA of PdynIRES-Cre :: Gt(ROSA26)SorloxSTOPlox-L10-GFP mice that have fluorescent ribosomes only in PDYN neurons (see Experimental Methods). The mice underwent three cycles of DID with access to 20% ethanol or water (Figure 4a). As reported previously, we found that the KOR agonist U-69593 (1 μM) reduced electrically evoked inhibitory post synaptic current (eIPSC) amplitude in mice of both sexes (Figure 4b-c). However, the degree of inhibition was unaltered following a history of alcohol drinking in mice of either sex.
It has been shown that a history of alcohol exposure can alter the balance of excitatory and inhibitory transmission in the CeA (35–37). Thus, we assessed whether binge-like alcohol drinking alters spontaneous synaptic transmission onto PDYN neurons in the CeA. We did not observe any effect of alcohol drinking on either the frequency, amplitude or decay kinetics of excitatory or inhibitory transmission in male mice (Figure 4 d-g; Table 3 & 4) or female mice (Figure 4 h-k). These experiments suggest that the protective effects of PDYN/KOR knockout are not mediated by altering the balance of excitatory and inhibitory transmission in the CeA.
Ethanol Drinking Results in Sex-Specific Changes in Intrinsic Excitability of PDYN Neurons in the CeA
Alcohol exposure has been shown to result in cell-type specific changes in the excitability of CeA neurons (38). To assess whether alcohol drinking may alter cell intrinsic excitability, we examined whether a history of alcohol drinking altered the firing properties of PDYN neurons in CeA. In these experiments, the reporter mice underwent DID with 20% ethanol or water as described above. In male mice, ethanol drinking did not alter the resting membrane potential, action-potential threshold, or rheobase of PDYN neurons (Figure 5a-c). However, ethanol drinkers fired significantly more action potentials in response to increasing steps of depolarizing current (Figure 5d-e). In female mice, resting membrane potential, action-potential threshold, and rheobase were similarly unaltered after alcohol exposure (Figure 5f-h). There was also a small, but insignificant trend towards reduced number of action potentials fired in a voltage versus current plot (Figure 5j). These experiments demonstrate that a history of alcohol drinking changes the excitability of PDYN neurons in opposing directions in male and female mice, which may partially explain the divergent results seen with manipulations in vivo.
Discussion
Despite its high prevalence, there remain only three FDA approved pharmacotherapies available to treat AUD (39). One of these therapies, naltrexone, works as a non-specific opioid antagonist. Recently, there has been an interest in developing KOR-specific antagonists to treat AUD as well as other psychiatric conditions (40–42). Understanding how KOR signaling works in the amygdala is critically important with respect to AUD, as a recent study found reduced KOR occupancy in the amygdala in alcoholics, consistent with tonic in vivo engagement of DYN/KOR signaling (12). The present study sought to address this by mechanistically dissecting how PDYN and KOR signaling in the CeA contribute to excessive alcohol intake. We observed that PDYN and KOR form spatially adjacent, but mostly distinct populations suggesting that the balance of these two cell types may control the balance of different outputs. We then performed separate knockouts of PDYN and KOR and found that while knockout of PDYN decreased alcohol consumption in both sexes, knockout of KOR resulted in reductions of alcohol intake in male mice only. Finally, we found sex-specific reductions in PDYN neuron excitability in female mice following alcohol drinking.
There are several important nuances to our findings. First, it is important to consider the time course over which the manipulations had an effect. For both CeA KOR knockout and PDYN knockout in male mice, there was a main effect of gene knockout in both DID and IA suggesting that these manipulations were relatively stable over the course of the experiment. However, there was a significant week by knockout interaction during DID of male KOR knockout mice wherein reductions in alcohol drinking did not become more pronounced until later weeks. This may be consistent with the hypothesized role of the CeA being involved in mediating the negative reinforcing properties of alcohol (8) and may thus require multiple sessions of alcohol drinking to engage CeA circuitry.
Next, the mechanism of protective action of PDYN or KOR knockout is likely different between sexes. The fact that KOR knockout in CeA did not affect alcohol drinking in female mice may not be surprising given that KOR activation has been shown to be less aversive to female animals (31). However, it should be noted that female KOR global knockout animals showed reduced ethanol consumption and preference comparable to their male counterparts (25). In the present study, the results in female mice were not entirely negative; PDYN knockout produced a significant reduction in ethanol consumption and preference in IA. Interestingly, this effect was not present during DID suggesting that either (1) higher levels of alcohol drinking are required to engage PDYN signaling in females or (2) the previous history of alcohol drinking was required in order to observe these effects. Because the design of the present study using sequential DID and IA experiments was not able to discern between these two possibilities, this may be an interesting avenue for future research.
Notably, the effects of CeA KOR knockout on alcohol drinking in females were negative and suggests that the effects of PDYN knockout on IA are mediated outside the CeA. One possibility is that PDYN neurons could be sending projections to other brain regions that express KOR, including the BNST, and terminal release of dynorphin in these regions could drive changes in behavior independent of KOR expressed in the CeA. Another possibility is that divergent results between sexes are attributable to differences in gonadal hormones as it has been shown that estradiol can attenuate Gβγ-mediated KOR signaling in female mice (43). The present study did not assess the stage of the estrous cycle in female mice, however because the time course of the drinking experiments (6 weeks) is much longer than the mouse estrous cycle, any variations related to estrous status should have been apparent. Finally, it is possible that the effects of PDYN knockout in females may be mediated by opioid peptides other than dynorphin. As Pdyn also encodes leu-enkephalin and β-neoendorphin, the reductions in alcohol drinking seen in female CeA PDYN knockout mice may instead be mediated by one of these other peptides.
The two sexes were also divergent at the level of CeA PDYN neuron plasticity. We observed that a history of ethanol drinking increased the excitability of PDYN neurons in male mice but exhibited a trend towards decreased excitability in female mice. In the present study, we did not observe any effects of DID on spontaneous excitatory and inhibitory transmission in the CeA of either sex. This was surprising given the findings that Chronic Intermittent Ethanol vapor (CIE) alters spontaneous synaptic transmission in the CeA (35,38). However, because CIE results in higher sustained blood ethanol levels, it is possible that this form of plasticity may not be observed after DID. Given that we find that there was increased PDYN neuron excitability in males, this suggests that this may be a cell intrinsic adaptation. To examine whether these changes in excitability may be due to KOR regulation of synaptic transmission, we examined the effects of KOR agonism on evoked inhibitory transmission after a history of DID. Consistent with previous results, we found that KOR activation reduces eIPSC amplitude in male mice and demonstrates for the first time that KOR activation similarly decreases eIPSC amplitude in female mice as well. However, we did not find any effects of ethanol drinking on KOR inhibition of GABAergic transmission in either sex. Future studies should examine whether a history of binge-like drinking results in alterations in endogenous PDYN tone.
Another important finding was that neither PDYN nor KOR knockout protected against ethanol withdrawal-induced increases in anxiety. While ethanol vapor exposure and ethanol liquid diet have been shown to increase anxiety-like behavior in rodent models (15,35,44,45), increased anxiety after DID typically has not been observed (46). However, these studies assessed anxiety using the EPM and open-field paradigms. Lee et al. (47) have shown that increased anxiety-like behavior can be observed after DID in the light-dark assay suggesting that changes in anxiety may not be revealed by all assays. Another possibility is that the differences observed may be due to the timing of when the testing took place relative to the last ethanol exposure session. We performed EPM testing 8 hr after the last binge session based on the finding that withdrawal symptoms peak around 6–10 hr after ethanol (48). Because testing in the study by Cox et al. (2013) was conducted 24 hr after the last binge session, it is possible that their experiments may have missed transient changes in anxiety like behavior. Additionally, there was no significant main effect of PDYN or KOR knockout or interaction with ethanol treatment on anxiety-like behavior suggesting that the reductions in alcohol drinking were mediated without concomitant changes in anxiety levels. This does not preclude a role for KOR-driven anxiety in other regions as it has recently been shown that KOR antagonism in the BNST can reduce negative affective states induced by ethanol withdrawal (49).
One limitation of our study is that we did not examine the outputs of CeA PDYN and KOR neurons. It has been demonstrated that optogenetic inhibition of Corticotropin Releasing Factor (CRF)-expressing neurons in the CeA that project to the BNST reduces ethanol vapor-induced increases in ethanol self-administration (50). Given the high overlap of PDYN and CRF expression in the CeA (18), PDYN projections to the BNST are a likely output that mediates these effects. Additionally, because we hypothesize the balance of excitation between these two populations is important for regulating alcohol intake, it would be informative to trace the outputs of CeA KOR neurons. Identifying any common or divergent outputs of these two populations would provide further insight into what behavioral processes may be affected. As Oprk1 is also expressed in the Basolateral Amygdala (BLA), our injections may have resulted in a small degree of KOR knockout in the BLA. However, we did not observe the BLA KOR-mediated reductions in anxiety in these subjects (Figure S5b) suggesting that these effects are mediated by CeA KORs.
The major advancement of the present study was to identify the effects of PDYN or KOR signaling in the CeA in regulating alcohol drinking. Here we find that they do so in a sex-specific manner without affecting general taste preference or anxiety-like behavior. These results contribute to our growing appreciation that the specific effects of KOR signaling in different brain regions may diverge from effects seen with global manipulations. Indeed, PDYN or KOR activation in the NAc has led to both appetitive and aversive responses based on anatomical sub-region that was targeted (51,52). Additionally, because KOR may be differentially expressed on different cell types within a brain region and shape output (53), it is important to examine the effects of KOR activation in the projection-specific circuits. For example, KOR inhibition of GABAergic projections to the BNST are mediated through ERK1/2, whereas KOR inhibition of glutamatergic BLA inputs are mediated by p38/MAP Kinase (26,54). These differences can then be exploited therapeutically with biased agonists (55–57) and could then be used to shift the balance of inputs from one brain region to another. Taken together, our findings support the continued search for KOR therapeutics to treat AUD. Moreover, they suggest that KOR antagonism as a treatment modality may exhibit sex differences if excessive alcohol consumption is driven via amygdala KOR signaling.
Supplementary Material
Acknowledgments
We would like to thank Christina Catavero for assistance with histology. We would also like to thank Maria Luisa Torruella-Suarez and Zoe McElligott for thoughtful discussion.
Footnotes
Conflict of Interests
The authors have no conflict of interests to disclose.
References
- 1.Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. 2010 National and State Costs of Excessive Alcohol Consumption. Am J Prev Med. 2015Nov;49(5):e73–9. [DOI] [PubMed] [Google Scholar]
- 2.Jennison KM. The Short-Term Effects and Unintended Long-Term Consequences of Binge Drinking in College: A 10-Year Follow-Up Study. Am J Drug Alcohol Abuse. 2004Jan 1;30(3):659–84. [DOI] [PubMed] [Google Scholar]
- 3.McCarty CA, Ebel BE, Garrison MM, DiGiuseppe DL, Christakis DA, Rivara FP. Continuity of Binge and Harmful Drinking From Late Adolescence to Early Adulthood. Pediatrics. 2004Sep 1;114(3):714–9. [DOI] [PubMed] [Google Scholar]
- 4.Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR, et al. Corticotropin Releasing Factor Signaling in the Central Amygdala is Recruited During Binge-Like Ethanol Consumption in C57BL/6J Mice. J Neurosci. 2012Mar 7;32(10):3405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988Oct 1;27(1):1–39. [DOI] [PubMed] [Google Scholar]
- 6.Pleil KE, Rinker JA, Lowery-Gionta EG, Mazzone CM, McCall NM, Kendra AM, et al. NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat Neurosci. 2015Apr;18(4):545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinker JA, Marshall SA, Mazzone CM, Lowery-Gionta EG, Gulati V, Pleil KE, et al. Extended Amygdala to Ventral Tegmental Area Corticotropin-Releasing Factor Circuit Controls Binge Ethanol Intake. Biol Psychiatry. 2017Jun 1;81(11):930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the “dark side” of drug addiction. Nat Neurosci. 2005Nov;8(11):1442–4. [DOI] [PubMed] [Google Scholar]
- 9.Lin S, Boey D, Lee N, Schwarzer C, Sainsbury A, Herzog H. Distribution of prodynorphin mRNA and its interaction with the NPY system in the mouse brain. Neuropeptides. 2006Apr 1 ;40(2):115–23. [DOI] [PubMed] [Google Scholar]
- 10.Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995Jan 1; 18(1):22–9. [DOI] [PubMed] [Google Scholar]
- 11.Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986Aug 15;233(4765):774–6. [DOI] [PubMed] [Google Scholar]
- 12.Vijay A, Cavallo D, Goldberg A, Laat B de, Nabulsi N, Huang Y, et al. PET imaging reveals lower kappa opioid receptor availability in alcoholics but no effect of age. Neuropsychopharmacology. 2018Dec;43(13):2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker BM, Koob GF. Pharmacological Evidence for a Motivational Role of κ-Opioid Systems in Ethanol Dependence. Neuropsychopharmacology. 2007May 2;33(3):643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson RI, Lopez MF, Becker HC. Stress-Induced Enhancement of Ethanol Intake in C57BL/6J Mice with a History of Chronic Ethanol Exposure: Involvement of Kappa Opioid Receptors. Front Cell Neurosci [Internet]. 2016Feb 23; 10. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4763044/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valdez GR, Harshberger E. Kappa opioid regulation of anxiety-like behavior during acute ethanol withdrawal. Pharmacol Biochem Behav. 2012Jul;102(1):44–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG, et al. The One-Two Punch of Alcoholism: Role of Central Amygdala Dynorphins/Kappa-Opioid Receptors. Biol Psychiatry. 2014May 15;75(10):774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson RI, Lopez MF, Griffin WC, Haun HL, Bloodgood DW, Pati D, et al. Dynorphin-kappa opioid receptor activity in the central amygdala modulates binge-like alcohol drinking in mice. Neuropsychopharmacology. 2018Dec 11;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S. Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors. Neuron. 2017Mar 22;93(6): 1464–1479.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, Blasio A, et al. A Transgenic Rat for Investigating the Anatomy and Function of Corticotrophin Releasing Factor Circuits. Neuroendocr Sci. 2015;487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014Mar 13;507(7491):238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chefer VI, Bäckman CM, Gigante ED, Shippenberg TS. Kappa Opioid Receptors on Dopaminergic Neurons Are Necessary for Kappa-Mediated Place Aversion. Neuropsychopharmacology. 2013Dec;38(13):2623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005Jan 31 ;84(1):53–63. [DOI] [PubMed] [Google Scholar]
- 23.Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent Escalation of Alcohol Drinking in C57BL/6J Mice With Intermittent Access to 20% Ethanol. Alcohol Clin Exp Res. 2011Nov 1;35(11):1938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blednov YA, Walker D, Martinez M, Harris RA. Reduced alcohol consumption in mice lacking preprodynorphin. Alcohol. 2006Oct;40(2):73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovacs KM, Szakall I, O’Brien D, Wang R, Vinod KY, Saito M, et al. Decreased Oral Self-Administration of Alcohol In κ-Opioid Receptor Knock-Out Mice. Alcohol Clin Exp Res. 2005May 1;29(5):730–8. [DOI] [PubMed] [Google Scholar]
- 26.Crowley NA, Bloodgood DW, Hardaway JA, Kendra AM, McCall JG, Al-Hasani R, et al. Dynorphin Controls the Gain of an Amygdalar Anxiety Circuit. Cell Rep [Internet], 2016Mar 17[cited 2016 Mar 17];0(0). Available from: http://www.cell.com/article/S2211124716302042/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-Releasing Factor within the Central Nucleus of the Amygdala Mediates Enhanced Ethanol Self-Administration in Withdrawn, Ethanol-Dependent Rats. J Neurosci. 2006Nov 1;26(44): 11324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sparrow AM, Lowery-Gionta EG, Pleil KE, Li C, Sprow GM, Cox BR, et al. Central Neuropeptide Y Modulates Binge-Like Ethanol Drinking in C57BL/6J Mice via Y1 and Y2 Receptors. Neuropsychopharmacology. 2012May;37(6):1409–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson RI, Becker HC. Role of the Dynorphin/Kappa Opioid Receptor System in the Motivational Effects of Ethanol. Alcohol Clin Exp Res. 2017;41(8):1402–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y, Crowley RS, Ben K, Prisinzano TE, Kreek MJ. Synergistic blockade of alcohol escalation drinking in mice by a combination of novel kappa opioid receptor agonist Mesyl Salvinorin B and naltrexone. Brain Res. 2017May 1;1662:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell SE, Rachlin AB, Smith KL, Muschamp J, Berry L, Zhao Z, et al. Sex differences in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol Psychiatry. 2014Aug 1;76(3):213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van’t Veer A, Smith KL, Cohen BM, Carlezon WA, Bechtholt AJ. Kappa-opioid receptors differentially regulate low and high levels of ethanol intake in female mice. Brain Behav. 2016Sep 1;6(9):n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilpin NW, Roberto M, Koob GF, Schweitzer P. Kappa opioid receptor activation decreases inhibitory transmission and antagonizes alcohol effects in rat central amygdala. Neuropharmacology. 2014Feb;77:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang-Park M, Kieffer BL, Roberts AJ, Siggins GR, Moore SD. κ-Opioid Receptors in the Central Amygdala Regulate Ethanol Actions at Presynaptic GABAergic Sites. J Pharmacol Exp Ther. 2013Jul 1;346(1):130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pleil KE, Lowery-Gionta EG, Crowley NA, Li C, Marcinkiewcz CA, Rose JH, et al. Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology. 2015Dec 1;99:735–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA Release in the Central Amygdala of Ethanol-Dependent Rats. J Neurosci. 2004Nov 10;24(45): 10159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and Chronic Ethanol Alter Glutamatergic Transmission in Rat Central Amygdala: an In Vitro and In Vivo Analysis. J Neurosci. 2004Feb 18;24(7): 1594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herman MA, Contet C, Roberto M. A Functional Switch in Tonic GABA Currents Alters the Output of Central Amygdala Corticotropin Releasing Factor Receptor-1 Neurons Following Chronic Ethanol Exposure. J Neurosci. 2016Oct 19;36(42):10729–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heilig M, Egli M. Pharmacological treatment of alcohol dependence: Target symptoms and target mechanisms. Pharmacol Ther. 2006Sep 1; 111(3):855–76. [DOI] [PubMed] [Google Scholar]
- 40.Carroll FI, Carlezon WA. Development of κ Opioid Receptor Antagonists. J Med Chem. 2013Mar 28;56(6):2178–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chavkin C, Koob GF. Dynorphin, Dysphoria, and Dependence: the Stress of Addiction. Neuropsychopharmacol N Y. 2016Jan;41(1):373–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crowley NA, Kash TL. Kappa opioid receptor signaling in the brain: Circuitry and implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2015Oct 1;62:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abraham AD, Schattauer SS, Reichard KL, Cohen JH, Fontaine HM, Song AJ, et al. Estrogen Regulation of GRK2 Inactivates Kappa Opioid Receptor Signaling Mediating Analgesia, But Not Aversion. J Neurosci. 2018Sep 12;38(37):8031–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Overstreet DH, Knapp DJ, Breese GR. Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from ethanol. Pharmacol Biochem Behav. 2004Jul;78(3):459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rose JH, Karkhanis AN, Chen R, Gioia D, Lopez MF, Becker HC, et al. Supersensitive Kappa Opioid Receptors Promotes Ethanol Withdrawal-Related Behaviors and Reduce Dopamine Signaling in the Nucleus Accumbens. Int J Neuropsychopharmacol [Internet]. 2016May 1[cited 2017 Jul 13];19(5). Available from: https://academic.oup.com/ijnp/article/19/5/pyv127/2910094/Supersensitive-Kappa-Opioid-Receptors-Promotes [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cox BR, Olney JJ, Lowery-Gionta EG, Sprow GM, Rinker JA, Navarro M, et al. Repeated Cycles of Binge-Like Ethanol Drinking in Male C57BL/6J Mice Augments Subsequent Voluntary Ethanol Intake But Not Other Dependence-Like Phenotypes. Alcohol Clin Exp Res. 2013Oct;37(10):1688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee KM, Coelho MA, Class MA, Szumlinski KK. mGlu5-dependent modulation of anxiety during early withdrawal from binge-drinking in adult and adolescent male mice. Drug Alcohol Depend. 2018Mar 1;184:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Becker HC, Hale RL. Repeated Episodes of Ethanol Withdrawal Potentiate the Severity of Subsequent Withdrawal Seizures: An Animal Model of Alcohol Withdrawal “Kindling.” Alcohol Clin Exp Res. 1993. Feb 1;17(1):94–8. [DOI] [PubMed] [Google Scholar]
- 49.Erikson CM, Wei G, Walker BM. Maladaptive behavioral regulation in alcohol dependence: Role of kappa-opioid receptors in the bed nucleus of the stria terminalis. Neuropharmacology. 2018Sep 15;140:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guglielmo G de, Kallupi M, Pomrenze MB, Crawford E, Simpson S, Schweitzer P, et al. Inactivation of a CRF-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats. Nat Commun. 2019Mar 18;10(1): 1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, et al. Distinct Subpopulations of Nucleus Accumbens Dynorphin Neurons Drive Aversion and Reward. Neuron. 2015Sep 2;87(5):1063–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castro DC, Berridge KC. Opioid Hedonic Hotspot in Nucleus Accumbens Shell: Mu, Delta, and Kappa Maps for Enhancement of Sweetness “Liking” and “Wanting.” J Neurosci. 2014. Mar 19;34(12):4239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tejeda HA, Wu J, Kornspun AR, Pignatelli M, Kashtelyan V, Krashes MJ, et al. Pathway-and Cell-Specific Kappa-Opioid Receptor Modulation of Excitation-Inhibition Balance Differentially Gates D1 and D2 Accumbens Neuron Activity. Neuron. 2017Jan 4;93(1):147–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li C, Pleil KE, Stamatakis AM, Busan S, Vong L, Lowell BB, et al. Presynaptic Inhibition of Gamma-Aminobutyric Acid Release in the Bed Nucleus of the Stria Terminalis by Kappa Opioid Receptor Signaling. Biol Psychiatry. 2012Apr 15;71(8):725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bohn LM, Aubé J. Seeking (and Finding) Biased Ligands of the Kappa Opioid Receptor. ACS Med Chem Lett. 2017Jul 13;8(7):694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, et al. Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci Signal. 2016Nov 29;9(456):ra117–ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ho J-H, Stahl EL, Schmid CL, Scarry SM, Aubé J, Bohn LM. G protein signaling–biased agonism at the κ-opioid receptor is maintained in striatal neurons. Sci Signal. 2018Aug 7;11(542):eaar4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


