Abstract
Simple Summary
Advances in melanoma treatment include v-Raf murine sarcoma viral oncogene homolog B (BRAF) inhibitors that target the predominant oncogenic mutation found in malignant melanoma. Despite initial success of the BRAF inhibitor (BRAFi) therapies, resistance and secondary cancer often occur. Mechanisms of resistance and secondary cancer rely on upregulation of pro-survival pathways that circumvent senescence. The repeated identification of a cellular senescent phenotype throughout melanoma progression demonstrates the contribution of senescent cells in resistance and secondary cancer development. Incorporating senotherapeutics in melanoma treatment may offer a novel approach for potentially improving clinical outcome.
Abstract
BRAF is the most common gene mutated in malignant melanoma, and predominately it is a missense mutation of codon 600 in the kinase domain. This oncogenic BRAF missense mutation results in constitutive activation of the mitogen-activate protein kinase (MAPK) pro-survival pathway. Several BRAF inhibitors (BRAFi) have been developed to specifically inhibit BRAFV600 mutations that improve melanoma survival, but resistance and secondary cancer often occur. Causal mechanisms of BRAFi-induced secondary cancer and resistance have been identified through upregulation of MAPK and alternate pro-survival pathways. In addition, overriding of cellular senescence is observed throughout the progression of disease from benign nevi to malignant melanoma. In this review, we discuss melanoma BRAF mutations, the genetic mechanism of BRAFi resistance, and the evidence supporting the role of senescent cells in melanoma disease progression, drug resistance and secondary cancer. We further highlight the potential benefit of targeting senescent cells with senotherapeutics as adjuvant therapy in combating melanoma.
Keywords: melanoma, BRAF mutation, BRAF inhibitors, secondary cancer, resistance, senescence, senotherapeutics
1. Introduction
Melanoma is a cancer originating from melanocytes, the pigment producing cells in the skin [1]. In the United States alone, there will be an estimated 106,110 new cases and 7180 deaths from melanoma in 2021. Melanoma represents 5.6% of all new cancer cases in the US, and the rate of new cases has been increasing over the past 40 years. The risk of melanoma increases with age, but sex differences have been reported with women having a higher risk under age 50 and men having a higher risk over age 50 [2,3]. Other common risk factors for melanoma include ultraviolet (UV) light exposure, nevi (moles), fair skin, having prior melanoma or other skin cancer, family history of melanoma, or having a compromised immune system. Most nevi will never develop into melanoma; however, people with many nevi (greater than 50) or with congenital melanocytic nevi or dysplastic nevi are also at higher risk. In rare cases, increased risk of melanoma can be caused by an inherited DNA repair deficiency disorder called xeroderma pigmentosum [3,4].
Melanoma accounts for only 4% of all diagnosed skin cancers, but it is the most lethal type of skin cancer, resulting in 80% of skin cancer deaths [4]. The stage of melanoma diagnosis can determine both the survival rate and course of treatment [5]. Currently, the first treatment strategy for all stages of melanoma is surgical resection, which is highly curative for early stages of the disease. Melanoma is associated with a 5-year relative survival rate of 93.3%, but for distant metastatic disease the 5 year relative survival is only 29.8% [3]. If cancer cells have spread to the lymph nodes, then adjuvant or targeted therapy would be considered. New melanoma adjuvant treatments include immunotherapy with programmed cell death protein 1 (PD-1), programmed death ligand-1 (PD-L1), or cytotoxic T-lymphocyte associated protein 4 (CTLA4) inhibitors or targeted drug therapies with BRAF, MAPK/ERK kinase 1 (MEK), or stem cell factor receptor (c-KIT) inhibitors [6,7,8]. For advanced metastatic melanoma, other options include radiation and genotoxic chemotherapy [3], but they are typically less effective than the newer targeted treatments.
Despite melanoma having a high passenger mutation load due to ultraviolet (UV) mutagenesis, several cancer driving mutations have been identified. The three most prevalent genes mutated in melanoma are BRAF, neuroblastoma RAS viral oncogene homolog (NRAS), and neurofibromatosis 1(NF1), and all participate in the mitogen-activated protein kinase (MAPK) signaling cascade that regulates cell proliferation [9,10,11,12]. In fact, constitutive activating mutations in BRAF are the most common oncogenic mutations, present in 40–60% of all melanoma cases [11,13,14]. Additionally, NRAS mutations are found in 15–30% of melanoma patients [13,14,15], and NF1 mutations are found in 12–18% of all melanomas [11,12]. Several other well know cancer genes that have been implicated in melanoma including phosphatase and tensin homolog (PTEN), tumor protein p53 (TP53), cyclin-dependent kinase inhibitor 2A (CDKN2A), and mitogen-activated protein kinase kinase 1 (MAP2K1) [11]. Familial melanoma studies identified the CDKN2A locus, which encodes for protein p16INK4a and p19ARF, and their loss of expression is common in melanoma [16,17]. Also, amplification of microphthalmia-associated transcription factor (MITF) occurs in 15% of melanomas, activating mutation of the receptor tyrosine kinase, KIT proto-oncogene (KIT) (~20–25% of melanomas), and germline melanocortin 1 receptor (MC1R) variants are lineage-specific casual alterations in melanoma [18,19,20]. Furthermore, novel driver mutations were identified in Rac family small GFPase 1 (RAC1), AT-rich interactive domain-containing protein 2 (ARID2), protein phosphatase 6 catalytic subunit (PPP6C), and serine/threonine-protein kinase 19 (STK19) through genome-wide studies of a large number of melanomas [11,21]. Knowledge of the patient-specific mutations help guide treatment options with new targeted therapies.
Senescence has long been known as a double-edged sword in cancer biology. It is essential for cancer prevention and therapy effectiveness, but can also pave the way for resistance [22,23,24]. Senescence is a permanent cell cycle arrest induced by a variety of cell stressors including aging, genotoxic stress, or tissue injury. These cellular stressors lead to permanent activation of the DNA damage response (DDR) through ataxia telangiectasia mutated (ATM). The activated DDR results in the stabilization of tumor suppressor protein 53 (p53) and its transcriptional targets cyclin-dependent kinase inhibitor 1 (p21CIP1) and cyclin-dependent kinase inhibitor 2A (p16INK4a), resulting in cell cycle arrest through inhibition of cyclin dependent kinases which prevent the phosphorylation of retinoblastoma (RB) and entrance into S phase of the cell cycle [25,26,27,28]. Senescent cells are characterized by increased cell size, senescent-associated beta-galactosidase (SA-β-gal) activity [29], upregulation of anti-apoptotic pathways [30,31], decreased lamin B1 [32], and a senescence-associated secretory phenotype (SASP) [33]. The SASP is initiated by nuclear factor kappa B (NF-kB) signaling and is composed of proinflammatory cytokines, matrix metalloproteinases (MMPs) and growth factors [33,34]. These markers of senescence are routinely used to identify senescent cells; however, individually, none can confirm senescence.
In this review, we summarize the current understanding of BRAF mutations in melanoma. We explore the specific BRAFV600E mutation and discuss additional gene mutations often found in conjunction with BRAF mutations that lead to melanoma. We discuss current treatment with BRAF inhibitors (BRAFi) with secondary cancer side effects and resistance development. Finally, we evaluate the role of senescence in melanoma progression and treatment and propose intentionally targeting senescent cells as a novel treatment strategy to prevent secondary cancer and drug resistance.
2. BRAF Oncogene, Common Mutation BRAFV600E
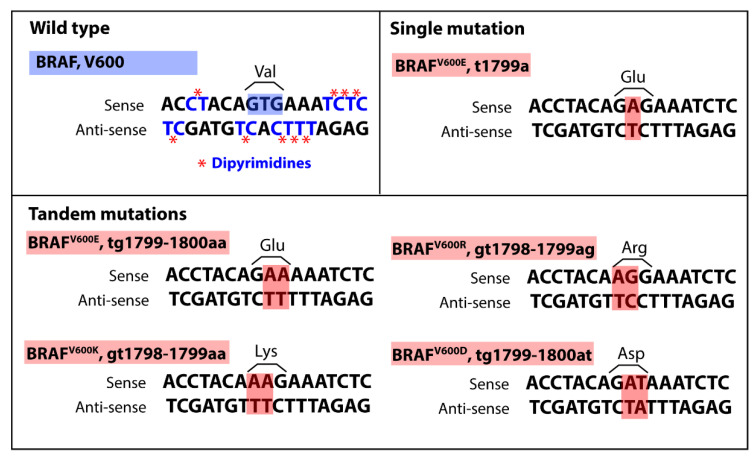
BRAF is a cytoplasmic serine-threonine kinase in the MAPK pathway. Not only is BRAF the most common gene mutated in melanoma, but mutations in the kinase domain occur in over 60% of malignant melanomas. The predominate BRAF mutation site is valine 600, and 80–90% of the time it is the missense mutation V600E [35]. However, other missense mutations at this site are also found in melanoma including V600K (10–20%), V600R (5%), V600D (<5%) [14]. The amino acid change from valine to glutamic acid is a phosphomimetic mutation and results in constitute activation of BRAF and the MAPK pathway. This missense mutation is associated with younger age of onset of melanoma and more aggressive disease [14,36,37]. The BRAFV600E mutation is often found in benign and dysplastic nevi and these nevi typically remain in growth arrest for decades without advancing to malignancy [15,38]. These nevi express p16INK4a and have increased senescence-associated acidic beta-galactosidase (SA-β-gal) activity, characteristic of senescent cells in permanent growth arrest. This senescence phenotype found in BRAFV600E nevi is presumed to be caused by the oncogene-induced senescence (OIS) induced by a robust activation of the DNA damage response due to hyper-replication [39,40,41]. Therefore, due to OIS, the BRAFV600E mutation alone is not sufficient for malignancy and is often accompanied by additional driving mutation(s) [21,42,43]. Studies have found mutations in PTEN, a negative regulator of the phosphoinositide 3-kinase (PI3K) pathway, to have an occurrence as high as 40% with BRAF mutations [11]. Other common gene mutations implicated with BRAF mutations in melanoma include CDKN2A, tumor protein p53 (TP53) and telomerase reverse transcriptase (TERT) [11,44]. Melanoma has a high mutational burden compared to most other solid tumors and these mutations are primarily consistent with UV mutagenesis. The typical UV mutation signature is a cytidine to thymine transition occurring at dipyrimidine sites [11]. Interestingly, it is a thymine to adenine transversion mutation that results in the BRAFV600E mutation [45]. The BRAFV600E mutation site is directly adjacent to several dipyrimidines, and BRAF tandem mutations in melanoma are relatively common [46,47] (Figure 1). Thus, this cancer driving mutation could be caused by an error-prone polymerase producing a mutation near, but not at sites of UV-induced cyclobutene pyrimidine dimer DNA adducts [45,46,47,48]. The strong selective advantage of the mutation can then drive its common occurrence. Understanding that these BRAF mutations are likely driven by UV mutagenesis and often result in OIS is important for the prevention and treatment of melanoma.
Figure 1.
Common BRAF codon 600 missense mutations found in melanocytic lesions. Wild typeBRAF encoding valine at codon 600 with dipyrimidines highlighted in blue with *. Dipyrimidines are common sites of UV mutagenesis resulting in C-T transitions and are prevalent surrounding BRAF codon 600. The Single mutation BRAFV600E T-A transversion mutation is the most common mutation in melanoma and is not typical of UV mutagenesis. Tandem mutations are commonly found in BRAF at codon 600 and include BRAFV600E, BRAFV600R, BRAFV600K, and BRAFV600D. All the Tandem mutations except BRAFV600D contain one C-T transition at a dipyrimidine site within the tandem mutation. The surrounding dipyrimidine sites and common tandem mutations highlight the possibility of UV mutagenesis and error-prone polymerase incorporation of incorrect bases at BRAF codon 600. BRAF, v-Raf murine sarcoma viral oncogene homolog B.
Determining the presence of a BRAF mutation has become a standard of care for advanced melanoma and can help direct treatment with targeted therapies [7,14]. BRAF mutant melanoma is often more clinically aggressive and occurs in younger patients, therefore early identification is essential for optimal disease management [14]. Additionally, detection of BRAFV600E mutations in circulating tumor-derived DNA (ctDNA) in patient peripheral blood is correlated with tumor burden [49,50,51]. In some cases, increasing ctDNA BRAFV600E can be used as a biomarker of melanoma disease progression post therapy [49,50,51]. Furthermore, combining ctDNA BRAFV600E detection with other markers of disease progression such as lactate dehydrogenase (LDH) or matrix metalloproteinase-9 (MMP-9) can add prognostic value [49,50]. However, additional advancements in identifying early signs of disease progression are necessary for improved outcomes in BRAFV600E mutant melanoma.
The BrafV600E mutation was evaluated in mice and embryonic expression resulted in embryonic lethality. When the BrafV600E mutation was induced in melanocytes, highly pigmented lesions occurred within 3–4 weeks, however, these nevi were non-malignant with a senescent phenotype [52]. Similar to humans, the Braf oncogene induces melanocyte senescence in mice and can transform immortalized murine melanocytes [53,54]. Additionally, BrafV600E has been found with other driving mutations, such as Pten. Mice with Pten knockout alone do not develop melanoma, but when combined with BrafV600E (BrafV600E/Pten−/− mice) there is rapid emergence of malignant lesions [52].
3. BRAF Inhibitors, Resistance and Secondary Cancer
Several BRAF inhibitors (BRAFi) that specifically target the BRAFV600 mutant protein have been developed through structure-based drug design and including vemurafenib, dabrafenib and encorafenib. These drugs improve progression free survival (PFS) in Phase 3 randomized clinical trials in patients with metastatic melanoma and BRAFV600 mutations. When compared to the standard of care chemotherapy, dacarbazine, the overall survival (OS) with vemurafenib was significantly increased from 9.7 months to 13.6 months and PFS increased from 1.6 months to 6.9 months [55]. Similarly, dabrafenib compared to dacarbazine treatment increased PFS in unresectable stage III melanoma from 2.7 to 5.1 months [56]. When the BRAFi, encorafenib was compared to vemurafenib with stage III and IV melanoma patients, the patients on either BRAF inhibitor had comparable PFS [57]. While initially very effective, melanoma resistance quickly develops to BRAFis, limiting their effectiveness.
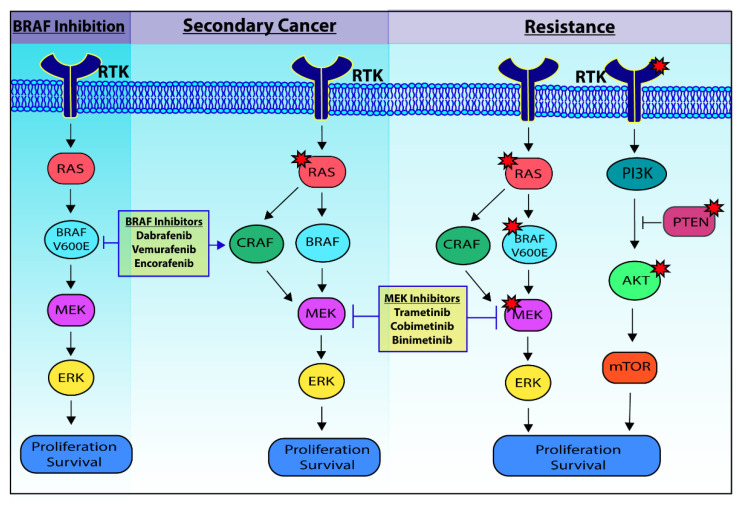
Genetic alterations providing resistance to BRAFi are found in the majority of resistant tumors (Figure 2) [58,59,60,61,62,63,64]. The most common genetic changes for resistance result in reactivation of the MAPK pathway. These occur through BRAF amplification, BRAF splice variants, or additional mutations in BRAF, RAS or MEK [58,59,62]. Loss of CDKN2A was also commonly found in BRAFi resistant melanomas and is linked to the MAPK pathway as an inhibitor of the downstream effectors cyclin D and cyclin-dependent kinase 4 (CDK4) [59,64]. The phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/ATK/mTOR) pathway was implicated as the second major pathway upregulated in BRAFi resistance. Several mutations in this pathway including loss of PTEN or upregulation of AKT or receptor tyrosine kinases (RTKs) are commonly found in resistant melanoma [43,52,58,62,65,66,67]. Overall, most BRAFi resistant melanomas have reactivated the MAPK pathway or upregulated the PI3K/AKT/mTOR pathway through specific mutations. However, altered epigenetic regulation and evasion of the immune system also occur to promote tumor proliferation and survival.
Figure 2.
BRAF inhibition and genetic mechanisms of secondary cancer and resistance. BRAF Inhibition: BRAFV600E results in constitutive activation of the pro-survival MAPK pathway. BRAF inhibitors target the oncogenic BRAF mutation and initially are highly effective. Secondary Cancer: BRAF inhibitors can paradoxically activate the MAPK pathway in cells with wild type BRAF and oncogenic RAS mutations by signaling through CRAF, resulting in cSCC development. The occurrence of cSCC is reduced when MEK inhibitors are used to further restrict the activation of the MAPK pathway. Resistance to both BRAF inhibition and MEK inhibition predominately develop through RAS, BRAFV600E, MEK, RTK, PTEN, or AKT mutations indicated by red stars. These mutations either reactivate the MAPK pathway or upregulate the PI3K/AKT/mTOR pro-survival pathway. cSCC, cutaneous squamous cell carcinoma; RTK, receptor tyrosine kinase; MAPK, mitogen activated protein kinase; BRAF, v-Raf murine sarcoma viral oncogene homolog B; ERK, extracellular signal-regulated kinase; MEK, MAPK/ERK Kinase 1; RAS, rat sarcoma; AKT, protein kinase B; PI3K, phosphoinositide 3-kinase; mTOR, mammalian target of rapamycin; PTEN, phosphatase and tensin homolog.
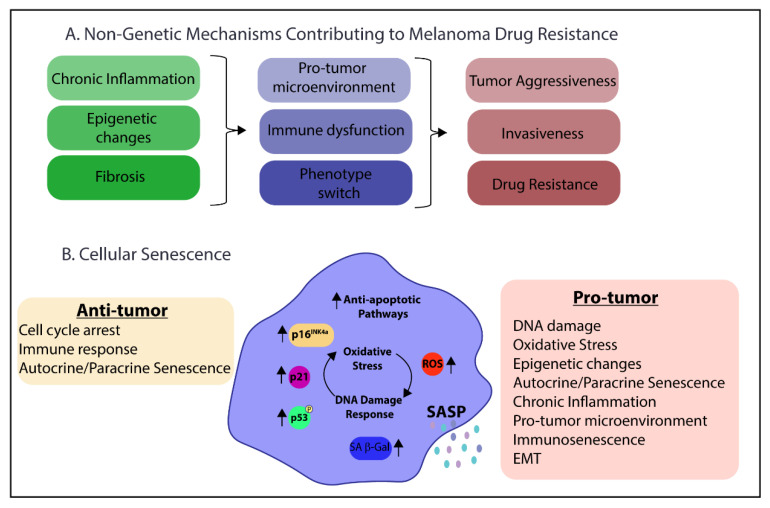
Several non-genetic mechanisms also contribute to melanoma progression and resistance including phenotypic switching, tumor microenvironment, inflammation, and epigenetic changes (Figure 3A). Rambow et al. investigated phenotypic switching associated with minimal residual disease (MRD) and drug resistance in melanoma [68]. The authors used patient-derived xenographs from BRAFV600 mutated tumors and subsequently treated the mice with BRAFi/MEKi. They found 60% of resistant cells had mutations in MAPK or PI3K/AKT pathways that helped drive drug resistance. However, these mutations were not observed at the MRD phase, indicating drug tolerance is also driven by epigenetic and environmental cues. Retinoid X receptors (RXR) signaling was identified as driving treatment resistance, in particular RXRγ [68]. While knockdown of RXR receptors is associated with senescence, primarily through RARα and RARβ, the role of RXRγ in senescence is not clearly defined [69].
Figure 3.
Parallels between (A) Non-genetic mechanisms contributing to melanoma drug resistance and (B) Cellular senescence pro-tumor characteristics. p16INK4a: cyclin-dependent kinase inhibitor 2A; p21: cyclin-dependent kinase inhibitor 1A; p53: tumor protein p53; ROS: reactive oxygen species, SA- β-gal: senescence-associated beta-galactosidase; SASP: senescence associated secretory phenotype; EMT: epithelial to mesenchymal transition.
Microphthalmia-associated transcription factor (MITF) has also been used in melanoma as an indicator of melanoma cell phenotype switching. The emergence of a drug resistant and invasive phenotype is associated with low expression of MITF, and in general, MITF is downregulated as disease progresses [68,70,71]. However, some heterogeneity in MITF expression is observed in MRD [68]. Decreased expression of MITF is also associated with senescence and increased genomic instability and the emergence of aggressive metastatic cells [72,73]. Proinflammatory cytokines released after treatment such as transforming growth factor beta (TGFβ), platelet-derived growth factor (PDGF), tumor necrosis factor alpha (TNFα), interleukin-1b (IL-1β), and interleukin-6 (IL-6) that can activate myofibroblast and contribute to chronic inflammation, fibrosis, and a pro-tumor microenvironment [71,74]. Hepatocyte growth factor secretion by cancer associated fibroblast (CAFs) can also activate receptor tyrosine kinases (RTKs) and the MAPK and PI3K/AKT pathways driving drug resistance [75]. All of these cytokines associated with creating the proinflammatory and pro-tumor environment are also SASP factors that could be secreted by senescent cells in the tumor microenvironment [76,77,78].
High mobility group box protein 1 (HMGB1) has also been implicated in melanoma progression with higher expression correlating with poor survival [79]. However, excretion of HMGB1 is also associated with a proinflammatory and anti-tumor response through activation of dendritic cells and induction of T-cell activation after BRAFi and MEKi treatment [80]. HMGB1 is a damage-associated molecular pattern (DAMP) that is also secreted by senescent fibroblasts induced by several different types of cellular stress including OIS, protease inhibitor, and ionizing radiation (IR) [77].
Immune escape is a common occurrence for patients treated with BRAFi. Immune escape and metastatic disease are associated with increased CEACAM1, an intercellular adhesion protein that has immune modulation functions [81,82,83]. Furthermore, CEACAM1 expression is required for senescence maintenance [84] and detection of high levels of CEACAM1 in melanoma is associated with oxidative stress, immune dysfunction and metastatic disease [81,82,83,85]. BRAFi treatment initially downregulates CEACAM1, but its expression is restored with the development of drug resistance [85], indicating a contribution of senescent cells to drug resistance.
Finally, epigenetic factors contribute to melanoma progression and drug resistance. One example is enhancer of zeste homologue 2 (EZH2), which is a histone methyltransferase that can trimethylate lysine 27 in histone 3 (H3K21me3) and repress transcription. In melanoma, EZH2 expression is associated with poor prognosis [86]. Additionally, depletion of EZH2 can activate p21CIP1 and result in senescence [87]. Furthermore, alterations in expression of histone deacetylases (HDACs) and histone acetyltransferases can result in epigenetic changes leading to BRAFi resistance. Some resistant melanomas were found to downregulate HDACs (Sirtuin 2 and Sirtuin 6) and histone acetyltransferase (HAT1), or to upregulate HDAC8 [58]. Similarly, components of the SASP are altered with HDAC inhibition to senescent fibroblasts, promoting tumor growth [88]. Collectively, these studies establish parallels between the non-genetic mechanisms of resistance in melanoma and some of the pro-tumor characteristics of senescence cells (Figure 3A,B).
One of the most concerning side effects of BRAFi is non-melanoma skin lesions, including cutaneous squamous cell carcinoma (cSCC), which occur in 15–20% of patients. Although cSCC is another form of skin cancer, it arises from keratinocytes instead of melanocytes. Importantly, BRAFi treatment of cells with wild type BRAF leads unexpectedly to activation of the MAPK pathway, and this upregulation is fundamental for cSCC development [89,90,91]. This paradoxical activation of the MAPK pathway with BRAFi is often found in keratinocytes with existing RAS mutations. In fact, RAS mutations are found in up to 60% of BRAFi therapy-induced secondary cSCC [61,91,92]. Oncogenic RAS with BRAFi has been found to activate the MAPK pathway by signally through CRAF, an analog of BRAF [61,90,91]. While the BRAFi have very low target inhibition of wild type BRAF, this activation of CRAF can result in increased proliferation and tumorigenesis in RAS mutant cells (Figure 2) [90,93]. Additionally, there have been reports of melanoma patients treated with BRAFi that have developed non-cutaneous RAS driven cancers including leukemia and colon cancer [94,95,96]. Another potential driving mechanism for secondary cSCC is human papilloma virus (HPV) infection [97,98]. Multiple variations of beta-HPV have been found in cSCC that were induced by BRAFi treatment [99]. The concern over paradoxical activation of the MAPK pathway, secondary cancer development and resistance with BRAFi led to combination therapy testing.
4. Addressing Secondary Cancer and Resistance to BRAF Inhibitors
The use of MEK inhibitors (MEKi) in combination with BRAFi was to prevent the paradoxical and reactivation of the MAPK pathway that leads to resistance and cSCC development [58,93,100,101]. The addition of MEKi were successful in reducing the incidence of cSCC and increasing PFS, but resistance routinely still develops. The combination treatments are Vemurafenib with Cobimetinib [102], Dabrafenib with Trametinib [103], and Encorafenib with Binimetinib [57]. While the MEK inhibitors reduce secondary cSCC, the two drugs target the same molecular pathway, increasing the chances of resistance and secondary cancer to occur through alternative pathways (Figure 2).
5. Senescence in Tumorigenesis and Melanoma
Senescence is typically seen as a repressor of tumorigenesis by halting growth in premalignant cells [104]. However, when senescence cells are not cleared by immunosurveillance, a pro-inflammatory environment can emerge that drives aging pathologies, including cancer [22,104]. For example, while transient senescence is associated with efficient wound healing, persistent senescent cells are associated with chronic wounds [105,106]. With increased age, there is an increase in senescent cells and an increase risk of cancer. The correlation of cancer with senescent cell burden was demonstrated using a genetic mouse model for inducible elimination of p16Ink4a-positive senescent cells. The continuous elimination of senescent cells in adult mice delayed the onset of age-associated cancer [107]. Furthermore, there is even evidence indicating the necessity of overriding senescence as a requirement for malignancy and increased cancer aggressiveness [60,108,109,110,111].
There are several characteristics of senescent cells that can contribute to tumorigenesis (Figure 3B). First, senescent cells are in a state of chronic DNA damage response (DDR) with an increase in DNA damage foci [112,113]. This chronic DDR leads to mitochondrial dysfunction and increased reactive oxygen species (ROS) that create a feedback loop to help maintain the DDR [114,115]. Persistent DNA damage and ROS production can precipitate intracellular changes that promote malignant transformation. Second, the senescence-associated secretory phenotype (SASP) is a mixture of cytokines, chemokines, growth factors, and proteases secreted from senescent cells and is credited with creating a pro-inflammatory and pro-tumor microenvironment [33]. The SASP has both paracrine and autocrine effects in maintaining senescence and spreading senescence to neighboring cells. Additionally, the SASP can induce both epithelial to mesenchymal transition (EMT) and reprogramming of cancer cells to a more stem-like phenotype [33,116,117]. Immunosenescence refers to age-related dysfunction of the immune system caused at least in part by immune cell senescence and low-grade chronic inflammation due to SASP (inflammaging). The SASP of the tumor microenvironment can drive both inflammaging and immunosenescence, resulting in reduced clearance and accumulation of senescent cells and tumor escape from immunosurveillance [74,118,119,120,121,122]. This impairment of the immune system also results in compromised immune surveillance that is vital for tumor progression and metastasis.
There is evidence of senescence or a senescence-like phenotype throughout the progression of BRAF mutant melanoma. A senescence phenotype is observed in pre-neoplastic cells where benign nevi express characteristic senescence markers with increased SA-β-gal activity, increased expression of p16INK4a, decreased lamin B1, and these markers decrease as the tumor progresses [39,123,124]. Additionally, BRAFV600E melanoma cells have increased senescence characteristics with increased SA-β-gal activity and expression of SASP factors, including IL-8 and TGFβ, compared to wild type BRAF melanoma cells. Moreover, therapy induced senescence (TIS) has been observed in vitro with Vemurafenib treatment resulting in increased SA-β-gal activity in both sensitive and resistance BRAFV600E mutant melanoma cells [60]. Of further interest, the secondary cSCC from BRAFi-treated melanoma patients stains strongly for p16INK4a [125,126]. Additionally, a senescent phenotype is often observed in HPV infected cells, HPV positive tumors, and from RAS oncogene expression [97,98,99,124,127], which are commonly found in BRAFi-induced secondary cSCC. Furthermore, BRAFi treatment initially increases the antitumor immunogenicity of melanoma, but when resistance develops the tumors revert to a low immunogenic state. This lower immunogenic state of the BRAF-resistant cells has fewer tumor infiltrating T-cells and NK cells and they are less effective at recognizing and killing the cancer cells [58,128], which are all characteristics of immunosenescence. Taken together, these studies demonstrate the potential role of senescence within melanocytes and the skin microenvironment that are influencing melanoma progression and secondary cancer occurrence.
6. Future Therapy Directions
While targeted therapies like BRAFi are highly effective therapeutics at first, melanoma resistance routinely develops. Cross-talk between pro-survival pathways is well-established and upregulation of alternate pathways, primarily PI3K/AKT/mTOR pathway, is consistently seen in resistance to BRAFi [129]. In addition, branched evolution of resistance with multiple pathways of acquired resistance have been found within the same melanoma patient [59]. Furthermore, BRAFi resistant BRAFV600E melanoma cells can even become dependent on the BRAF inhibitor for proliferation [60]. All these examples highlight the necessity for adjuvant treatments that can target several anti-apoptotic pathways to prevent drug resistance, such as senotherapeutics.
Senotherapeutics are a class of drugs that targets senescent cells and include senolytics and senomorphics. Senolytics selectively kill senescent cells and senomorphics suppress the SASP without inducing apoptosis. However, the distinction between these two drug classifications can sometimes be cell-type specific with the same drug capable of having senolytic function on one cell type and senomorphic on another [130]. Senolytics have been shown to combat aging and improve healthspan by reducing frailty and attenuating common age-associated morbidities [130,131,132,133]. Now senolytics are being considered and used in combination therapy for cancers [119,134,135,136]. Senotherapeutics could aid in the elimination of senescent cells after therapy-induced senescence (TIS) to reduce the emergence of drug resistance and cancer recurrence [134,135]. Several senotherapeutics have the added benefit of targeting multiple anti-apoptotic pathways that are employed by senescent cells to stay alive [131]. In addition, several senotherapeutics are flavonoids found in many fruits and vegetables and, therefore, expected to have lower toxicity than chemotherapy drugs that target proliferating cells. In theory, senotherapy could aid the immune system in clearing BRAFi-induced senescent cancer cells, reducing the possibility of acquiring additional adaptations for resistance and secondary cancers.
Several senotherapeutics have been tested with melanoma cells in vitro with encouraging results and some clinical trials with melanoma patients have been conducted (Table 1). One senolytic being researched with melanoma is dasatinib, a broad-specificity receptor tyrosine kinase (RTK) inhibitor. EGFR is a RTK that is commonly upregulated as a mechanism of resistance to BRAFi [66], therefore, dasatinib has both senolytic activity [133] and targets RTKs in BRAFi resistance. Importantly, dasatinib inhibits the proliferation and invasion of even BRAFi-resistant melanoma cells [67]. Another senolytic, fisetin is a flavonoid found to extend the health and lifespan of both progeroid and aged wild-type mice [132]. Fisetin has been tested in melanoma cells in an in vitro 3-D model system and found to promote tumor regression [137]. Fisetin has inhibitory effects on the PI3K/AKT/mTOR pathway and demonstrated efficiency at inhibiting multiple cancers and specifically melanoma [138,139]. Quercetin is another flavonoid and senolytic that has been found to work synergistically with dasatinib to combat age-associated frailty and extend healthspan in pre-clinical models [130]. Quercetin inhibits the growth, invasiveness, and metastatic potential of melanoma cell lines [140]. Additionally, quercetin can be metabolized by tyrosinase, which is expressed in melanocytes, into additional anti-cancer compounds, potentially increasing its potency specifically for melanoma [141,142]. Finally, piperlongumine, a natural extract from the Piper Longum pepper plant induces apoptosis of melanoma cells [143]. The benefits of the highlighted senolytic drugs in melanoma treatment is two-fold. First, senolytics could aid clearance of senescent tumor cells to prevent resistant cells from emerging. Second, they target senescent cell anti-apoptotic pathways that are involved in secondary cancer and development of drug resistance [131]. Intermittent dosing of BRAFi has demonstrated improved efficacy against some therapy resistant melanoma [144], and these resistant tumors could potential benefit from alternating BRAFi treatment with a senotherapy recently termed the “one-two punch” [145]. This “one-two punch” treatment strategy suggests treating with a tumor targeted therapy to induce senescence and following with a senolytic to help clear the senescent cells [145]. Some of the long-term effects of chemotherapy treatment on cancer survivors include increased incident of age-associated diseases linked to senescence such as cardiovascular disease, neurodegeneration, and secondary cancer. Thus, the use of senotherapeutics as part of cancer management could improve cancer treatment effectiveness and the healthspan of cancer survivors.
Table 1.
Clinical trials and other studies on applying senotherapeutics to melanoma. HSP: heat shock protein; Bcl-2/Bcl-xL: B-cell lymphoma 2/B-cell lymphoma extra-large; HDAC: histone deacetylase; OXR1: oxidation resistance 1; BET: bromodomain and extraterminal domain; PI3K: phosphoinositide 3-kinase; AKT: protein kinase B; mTOR: mammalian target of rapamycin; MIC: melanoma initiating cells This table is not an all-inclusive list of studies with senotherapeutics for melanoma, and a more extensive list of senotherapeutics used in additional cancers can be found in Prasanna, et al. [145].
| Drug | Mechanisms of Action | Treatment | Developmental Stage |
|---|---|---|---|
| Alvespimycin | HSP inhibitor | Alvespimycin hydrochloride |
Clinical Trail: Phase 1; NCT00089362; Metastatic or unresectable solid tumors including melanoma |
| Alvespimycin hydrochloride | Phase 1; NCT00248521; Adult solid tumor including melanoma [146] | ||
| Alvespimycin hydrochloride |
Discovery Phase: human melanoma cell line [147] |
||
| Tanespimycin | HSP inhibitor | Tanespimycin |
Clinical Trail: Phase 2; NCT00087386; Recurrent or phase III, IV melanoma |
| Tanespimycin | Phase 1; NCT00004065; Refractory advanced solid tumors including melanoma or hematologic cancer | ||
| Tanespimycin | Phase 2; NCT00104897; Metastatic malignant melanoma [148] | ||
| Tanespimycin | Phase 2; Metastatic Melanoma [149] | ||
| Tanespimycin and Sorafenib | Phase 1; Melanoma, renal cancer and colorectal cancer [150] | ||
| Digoxin | Na+/K+ ATPase inhibitor |
Trametinib and Digoxin |
Clinical Trail: Phase 1; NCT02138292; Unresectable or metastatic BRAF wild-type melanoma [151] |
| Vemurafenib and Digoxin | Phase 1; NCT01765569; Advanced BRAFV600 mutant melanoma | ||
| Navitoclax (ABT-263) |
Bcl-2/Bcl-xL inhibitor | Dabrafenib, trametinib, and navitoclax |
Clinical Trail: Phase 1/2; NCT01989585; BRAF mutant melanoma or unresectable or metastatic solid tumors |
| Novitoclax and selumetinib |
Discovery Phase: Melanoma cell lines [152] |
||
| Dasatinib | Pan receptor tyrosine kinase inhibitor |
Dasatinib |
Clinical Trail: Phase 2; NCT00700882; Melanoma (Skin) [153] |
| Dendritic cell Vaccines + Dasatinib | Phase 2; NCT01876212; Metastatic melanoma | ||
| Dasatinib and Dacarbazine | Phase 1/2; NCT00597038; Metastatic Melanoma | ||
| Dasatinib | Phase 2; NCT00436605; Unresectable stage III melanoma or stage IV melanoma | ||
| Dasatinib | Phase 1; Advanced melanoma [154] | ||
| Dasatinib and Dacarbazine | Phase 1; Metastatic melanoma [155] | ||
| Dasatinib |
Discovery Phase: Melanoma cell lines [67] |
||
| Panobinostat (LBH589) | Pan HDAC inhibitor | Panobinostat |
Clinical Trail: Phase 1; NCT01065467; Metastatic Melanoma |
| Panobinostat and Ipilimumab | Phase 1; NCT02032810; Unresectable stage III/IV melanoma | ||
| Temozolomide, Decitabine, Panobinostat | Phase 1/2; NCT00925132; Metastatic Melanoma [156] | ||
| Panobinostat (LBH589) | Phase 1; metastatic melanoma [157] | ||
| Curcumin analog, EF24 | Promote degradation of anti-apoptotic Bcl-2 proteins | EF24 |
Discovery Phase: Malignant melanoma cell lines [158] |
| Piperlongumine | Targets OXR1 | Piperlongumine |
Discovery Phase: Human melanoma cell [143] |
| Ouabain | Na+/K+ ATPase inhibitor |
Ouabain |
Discovery Phase: Malignant melanoma cell lines [159,160] |
| ABT-737 | Bcl-2/Bcl-xL inhibitor | ABT-737 and PLX4720 |
Discovery Phase: Human melanoma cell lines and primary melanoma cell culture [161] |
| ABT-737 and GSI (γ-Secretase Inhibitor) | Non-MIC (bulk of melanoma) and MICs [162] | ||
| JQ1 | BET inhibitor | JQ1 and vemurafenib |
Discovery Phase: BRAF mutant vemurafenib-resistant melanoma cells [163] |
| Quercetin | Activates estrogen receptors and inhibits PI3 kinase | Quercetin |
Discovery Phase: Melanoma cell lines [140,141,142,164,165] |
| Fisetin | Blocks PI3K/AKT/mTOR pathway | Fisetin |
Discovery Phase: Melanoma cell lines [137,138,166,167,168] |
7. Conclusions
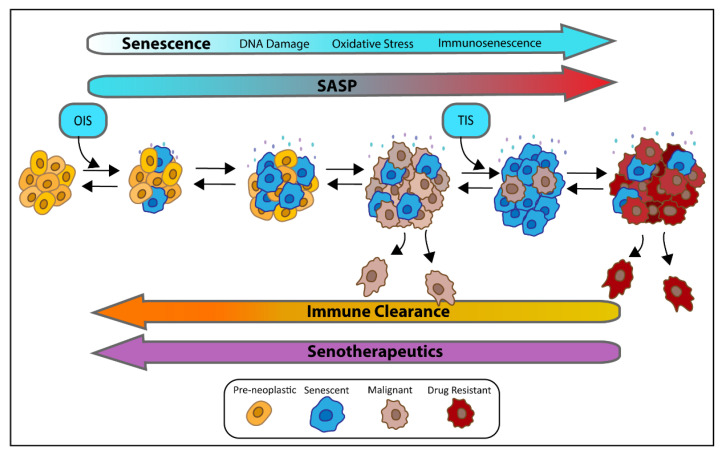
Early-stage melanoma is curable, but once metastases have occurred, the chance of survival diminishes significantly. Several targeted therapies have been developed toward oncogenic BRAF in malignant melanoma that significantly improve PFS, but resistance often develops. Throughout the progression of disease and treatment of BRAFV600E driven melanoma, senescence induction and escape are recurring themes from cancer initiation to secondary skin cancers (cSCC), and drug resistance (Figure 4). The cancer cells must escape OIS induced by the BRAFV600E mutation in stable nevi to become malignant. The BRAFi therapy-induced senescence (TIS) in the cancer cells, and the development of secondary cSCCs with BRAF inhibitor treatment relies on upregulation of the MAPK pathway or alternative pathways in keratinocytes that often have senescence driving alterations such has RAS oncogene activation or HPV infection. Finally, the emergence of resistance occurs through upregulation of anti-apoptotic pathways like the MAPK pathway and AKT/PI3K/mTOR to override senescence induction. This repeated failure of senescence maintenance to control disease progression represents an opportunity for combining the use of senotherapeutics in melanoma treatment regimens for BRAFV600E mutated cancer. Particularly considering the evidence of cancer cells that escape from senescence can be primed as more stem-like, aggressive, and drug resistant. Several senotherapeutics have been tested on melanoma cells and show promising results, but further in-vivo studies are necessary to confirm these results and advance senotherapeutics for melanoma treatment.
Figure 4.
The role of senescent cells in cancer progression and resistance. Pre-neoplastic lesions with BRAFV600E or RAS mutations can acquire senescent cells through oncogenic induced senescence (OSI). The OIS cells can spread senescence to nearby cells through their senescent associated secretory phenotype (SASP). If these senescent cells are not cleared by the immune system, the SASP can produce a pro-inflammatory and pro-tumor microenvironment with increased DNA damage, oxidative stress, and immunosenescence resulting in malignant transformation. Therapy induced senescence (TIS) by BRAFi is initially beneficial in combating malignant melanoma, but can lead to increased senescence, drug resistance, and aggressive, stem-like tumor phenotype. Senotherapeutics can reverse all phases of disease progression by targeting pro-survival pathways, reducing SASPs and/or by aiding the immune system in clearing senescent cells.
Author Contributions
The review was conceived by E.L.T. and L.J.N. and the first draft was written by E.L.T. and J.J.H., which was subsequently edited by L.J.N. All authors have read and agreed to the published version of the manuscript.
Funding
E.L.T. was funded by the Children’s Cancer Research Fund Emerging Scientist Award and by NIA Postdoctoral Training Grant T32 AG029796. L.J.N. was funded by U01 ES029603.
Conflicts of Interest
L.J.N. is a co-founder of NRTK Biosciences, a start-up biotechnology company developing senolytic drugs.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shain A.H., Bastian B.C. From melanocytes to melanomas. Nat. Rev. Cancer. 2016;16:345–358. doi: 10.1038/nrc.2016.37. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society Cancer Facts & Figures 2020. [(accessed on 16 April 2021)]; Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html.
- 3.National Cancer Institute Melanoma of the Skin—Cancer Stat Facts. [(accessed on 16 April 2021)]; Available online: https://seer.cancer.gov/statfacts/html/melan.html.
- 4.Miller A.J., Mihm M.C. Melanoma. N. Engl. J. Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 5.Berwick M., Erdei E., Hay J. Melanoma Epidemiology and Public Health. Dermatol. Clin. 2009;27:205–214. doi: 10.1016/j.det.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leonardi G.C., Candido S., Falzone L., Spandidos D.A., Libra M. Cutaneous melanoma and the immunotherapy revolution (Review) Int. J. Oncol. 2020;57:609–618. doi: 10.3892/ijo.2020.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel H., Yacoub N., Mishra R., White A., Yuan L., Alanazi S., Garrett J.T. Current Advances in the Treatment of BRAF-Mutant Melanoma. Cancers. 2020;12:482. doi: 10.3390/cancers12020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falzone L., Salomone S., Libra M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018;9:1300. doi: 10.3389/fphar.2018.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehnert J.M., Kluger H.M. Driver Mutations in Melanoma: Lessons Learned From Bench-to-Bedside Studies. Curr. Oncol. Rep. 2012;14:449–457. doi: 10.1007/s11912-012-0249-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo Y., Zhang Z., Liu J., Li L., Xu X., Yao X., Dai Z., Wang X., Yang S., Wu H., et al. Characterizations of Gene Alterations in Melanoma Patients from Chinese Population. BioMed Res. Int. 2020;2020:6096814. doi: 10.1155/2020/6096814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodis E., Watson I.R., Kryukov G.V., Arold S.T., Imielinski M., Theurillat J.-P., Nickerson E., Auclair D., Li L., Place C., et al. A Landscape of Driver Mutations in Melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiuru M., Busam K.J. The NF1 gene in tumor syndromes and melanoma. Lab. Investig. 2017;97:146–157. doi: 10.1038/labinvest.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burd C.E., Liu W., Huynh M.V., Waqas M.A., Gillahan J.E., Clark K.S., Fu K., Martin B.L., Jeck W.R., Souroullas G.P., et al. Mutation-Specific RAS Oncogenicity Explains NRAS Codon 61 Selection in Melanoma. Cancer Discov. 2014;4:1418–1429. doi: 10.1158/2159-8290.CD-14-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng L., Lopez-Beltran A., Massari F., MacLennan G.T., Montironi R. Molecular testing for BRAF mutations to inform melanoma treatment decisions: A move toward precision medicine. Mod. Pathol. 2018;31:24–38. doi: 10.1038/modpathol.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y., Sheikh M.S. Melanoma: Molecular Pathogenesis and Therapeutic Management. Mol. Cell. Pharmacol. 2014;6:228. [PMC free article] [PubMed] [Google Scholar]
- 16.Chin L. The genetics of malignant melanoma: Lessons from mouse and man. Nat. Rev. Cancer. 2003;3:559–570. doi: 10.1038/nrc1145. [DOI] [PubMed] [Google Scholar]
- 17.Yang G., Rajadurai A., Tsao H. Recurrent Patterns of Dual RB and p53 Pathway Inactivation in Melanoma. J. Investig. Dermatol. 2005;125:1242–1251. doi: 10.1111/j.0022-202X.2005.23931.x. [DOI] [PubMed] [Google Scholar]
- 18.Flaherty K.T., Hodi F.S., Fisher D.E. From genes to drugs: Targeted strategies for melanoma. Nat. Rev. Cancer. 2012;12:349–361. doi: 10.1038/nrc3218. [DOI] [PubMed] [Google Scholar]
- 19.Garraway L.A., Widlund H.R., Rubin M.A., Getz G., Berger A.J., Ramaswamy S., Beroukhim R., Milner D.A., Granter S.R., Du J., et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436:117–122. doi: 10.1038/nature03664. [DOI] [PubMed] [Google Scholar]
- 20.Carvajal R., Antonescu C., Wolchok J., Chapman P., Roman R., Teitcher J., Panageas K., Busam K., Chmielowski B., Lutzky J., et al. KIT as a Therapeutic Target in Metastatic Melanoma. JAMA. 2011;305:2327–2334. doi: 10.1001/jama.2011.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krauthammer M., Kong Y., Ha B.H., Evans P., Bacchiocchi A., McCusker J.P., Cheng E., Davis M.J., Goh G., Choi M., et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat. Genet. 2012;44:1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campisi J. Aging, Cellular Senescence, and Cancer. Annu. Rev. Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S., Lee A.J.-S. Cellular senescence: A promising strategy for cancer therapy. BMB Rep. 2019;52:35–41. doi: 10.5483/BMBRep.2019.52.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyld L., Bellantuono I., Tchkonia T., Morgan J., Turner O., Foss F., George J., Danson S., Kirkland J.L. Senescence and Cancer: A Review of Clinical Implications of Senescence and Senotherapies. Cancers. 2020;12:2134. doi: 10.3390/cancers12082134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alcorta D.A., Xiong Y., Phelps D., Hannon G., Beach D., Barrett J.C. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc. Natl. Acad. Sci. USA. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beauséjour C.M., Krtolica A., Galimi F., Narita M., Lowe S.W., Yaswen P., Campisi J. Reversal of human cellular senescence: Roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi A., Ohtani N., Yamakoshi K., Iida S.-I., Tahara H., Nakayama K., Nakayama K.I., Ide T., Saya H., Hara E. Mitogenic signalling and the p16INK4a–Rb pathway cooperate to enforce irreversible cellular senescence. Nature. 2006;8:1291–1297. doi: 10.1038/ncb1491. [DOI] [PubMed] [Google Scholar]
- 28.Yousefzadeh M., Henpita C., Vyas R., Soto-Palma C., Robbins P., Niedernhofer L. DNA damage—how and why we age? eLife. 2021;10 doi: 10.7554/eLife.62852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimri G.P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E.E., Linskens M., Rubelj I., Pereira-Smith O., et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryu S.J., Oh Y.S., Park S.C. Failure of stress-induced downregulation of Bcl-2 contributes to apoptosis resistance in senescent human diploid fibroblasts. Cell Death Differ. 2007;14:1020–1028. doi: 10.1038/sj.cdd.4402091. [DOI] [PubMed] [Google Scholar]
- 31.Chen W., Kang J., Xia J., Li Y., Yang B., Chen B., Sun W., Song X., Xiang W., Wang X., et al. p53-related apoptosis resistance and tumor suppression activity in UVB-induced premature senescent human skin fibroblasts. Int. J. Mol. Med. 2008;21:645–653. doi: 10.3892/ijmm.21.5.645. [DOI] [PubMed] [Google Scholar]
- 32.Freund A., Laberge R.-M., DeMaria M., Campisi J. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell. 2012;23:2066–2075. doi: 10.1091/mbc.e11-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coppé J.-P., Patil C.K., Rodier F., Sun Y., Muñoz D.P., Goldstein J.N., Nelson P.S., Desprez P.-Y., Campisi J. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor. PLoS Biol. 2008;6:e301. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salminen A., Kauppinen A., Kaarniranta K. Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP) Cell. Signal. 2012;24:835–845. doi: 10.1016/j.cellsig.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M.J., Bottomley W., et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 36.Hugdahl E., Kalvenes M.B., Puntervoll H.E., Ladstein R.G., A Akslen L. BRAF-V600E expression in primary nodular melanoma is associated with aggressive tumour features and reduced survival. Br. J. Cancer. 2016;114:801–808. doi: 10.1038/bjc.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long G.V., Menzies A.M., Nagrial A.M., Haydu L.E., Hamilton A.L., Mann G.J., Hughes T.M., Thompson J.F., Scolyer R.A., Kefford R.F. Prognostic and Clinicopathologic Associations of Oncogenic BRAF in Metastatic Melanoma. J. Clin. Oncol. 2011;29:1239–1246. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 38.Pollock P.M., Harper U.L., Hansen K.S., Yudt L.M., Stark M.S., Robbins C.M., Moses T.Y., Hostetter G., Wagner U., Kakareka J.W., et al. High frequency of BRAF mutations in nevi. Nat. Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 39.Michaloglou C., Vredeveld L.C.W., Soengas M.S., Denoyelle C., Kuilman T., Van Der Horst C.M.A.M., Majoor D.M., Shay J.W., Mooi W.J., Peeper D.S. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 40.Bennett D.C. Human melanocyte senescence and melanoma susceptibility genes. Oncogene. 2003;22:3063–3069. doi: 10.1038/sj.onc.1206446. [DOI] [PubMed] [Google Scholar]
- 41.Di Micco R., Fumagalli M., Cicalese A., Piccinin S., Gasparini P., Luise C., Schurra C., Garre’ M., Nuciforo P.G., Bensimon A., et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 42.Vredeveld L.C., Possik P.A., Smit M.A., Meissl K., Michaloglou C., Horlings H.M., Ajouaou A., Kortman P.C., Dankort D., McMahon M., et al. Abrogation of BRAFV600E-induced senescence by PI3K pathway activation contributes to melanomagenesis. Genes Dev. 2012;26:1055–1069. doi: 10.1101/gad.187252.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nogueira C., Kim K.-H., Sung H., Paraiso K.H.T., Dannenberg J.-H., Bosenberg M., Chin L., Kim M. Cooperative interactions of PTEN deficiency and RAS activation in melanoma metastasis. Oncogene. 2010;29:6222–6232. doi: 10.1038/onc.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang T., Dutton-Regester K., Brown K.M., Hayward N.K. The genomic landscape of cutaneous melanoma. Pigment. Cell Melanoma Res. 2016;29:266–283. doi: 10.1111/pcmr.12459. [DOI] [PubMed] [Google Scholar]
- 45.Brash D.E. UV Signature Mutations. Photochem. Photobiol. 2015;91:15–26. doi: 10.1111/php.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas N.E., Alexander A., Edmiston S.N., Parrish E., Millikan R.C., Berwick M., Groben P., Ollila D.W., Mattingly D., Conway K. Tandem BRAF Mutations in Primary Invasive Melanomas. J. Investig. Dermatol. 2004;122:1245–1250. doi: 10.1111/j.0022-202X.2004.22523.x. [DOI] [PubMed] [Google Scholar]
- 47.Thomas N.E., Berwick M., Cordeiro-Stone M. Could BRAF Mutations in Melanocytic Lesions Arise from DNA Damage Induced by Ultraviolet Radiation? J. Investig. Dermatol. 2006;126:1693–1696. doi: 10.1038/sj.jid.5700458. [DOI] [PubMed] [Google Scholar]
- 48.Rastogi R.P., Richa, Kumar A., Tyagi M.B., Sinha R.P. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J. Nucleic Acids. 2010;2010:592980. doi: 10.4061/2010/592980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang H., Kong Y., Si L., Cui C., Sheng X., Chi Z., Dai J., Yu S., Ma M., Wu X., et al. Clinical significance of BRAFV600E mutation in circulating tumor DNA in Chinese patients with melanoma. Oncol. Lett. 2017;15:1839–1844. doi: 10.3892/ol.2017.7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salemi R., Falzone L., Madonna G., Polesel J., Cinà D., Mallardo D., Ascierto P.A., Libra M., Candido S. MMP-9 as a Candidate Marker of Response to BRAF Inhibitors in Melanoma Patients With BRAFV600E Mutation Detected in Circulating-Free DNA. Front. Pharmacol. 2018;9:856. doi: 10.3389/fphar.2018.00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashida A., Sakaizawa K., Mikoshiba A., Uhara H., Okuyama R. Quantitative analysis of the BRAF V600E mutation in circulating tumor-derived DNA in melanoma patients using competitive allele-specific TaqMan PCR. Int. J. Clin. Oncol. 2016;21:981–988. doi: 10.1007/s10147-016-0976-y. [DOI] [PubMed] [Google Scholar]
- 52.Dankort D., Curley D.P., Cartlidge R.A., Nelson B., Karnezis A.N., Jr W.E.D., You M.J., Depinho R.A., McMahon M., Bosenberg M. BrafV600E cooperates with Pten loss to induce metastatic melanoma. Nat. Genet. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dhomen N., Reis-Filho J.S., Dias S.D.R., Hayward R., Savage K., Delmas V., LaRue L., Pritchard C., Marais R. Oncogenic Braf Induces Melanocyte Senescence and Melanoma in Mice. Cancer Cell. 2009;15:294–303. doi: 10.1016/j.ccr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 54.Wellbrock C., Ogilvie L., Hedley D., Karasarides M., Martin J., Niculescu-Duvaz D., Springer C.J., Marais R. V599EB-RAF is an oncogene in melanocytes. Cancer Res. 2004;64:2338–2342. doi: 10.1158/0008-5472.CAN-03-3433. [DOI] [PubMed] [Google Scholar]
- 55.McArthur G.A., Chapman P.B., Robert C., Larkin J., Haanen J.B., Dummer R., Ribas A., Hogg D., Hamid O., Ascierto P.A., et al. Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma (BRIM-3): Extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323–332. doi: 10.1016/S1470-2045(14)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hauschild A., Grob J.-J., Demidov L.V., Jouary T., Gutzmer R., Millward M., Rutkowski P., Blank C.U., Miller W.H., Kaempgen E., et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 57.Dummer R., Ascierto P.A., Gogas H.J., Arance A., Mandala M., Liszkay G., Garbe C., Schadendorf D., Krajsova I., Gutzmer R., et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF -mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603–615. doi: 10.1016/S1470-2045(18)30142-6. [DOI] [PubMed] [Google Scholar]
- 58.Proietti I., Skroza N., Bernardini N., Tolino E., Balduzzi V., Marchesiello A., Michelini S., Volpe S., Mambrin A., Mangino G., et al. Mechanisms of Acquired BRAF Inhibitor Resistance in Melanoma: A Systematic Review. Cancers. 2020;12:2801. doi: 10.3390/cancers12102801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi H., Hugo W., Kong X., Hong A., Koya R.C., Moriceau G., Chodon T., Guo R., Johnson D.B., Dahlman K.B., et al. Acquired Resistance and Clonal Evolution in Melanoma during BRAF Inhibitor Therapy. Cancer Discov. 2014;4:80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krayem M., Najem A., Journe F., Morandini R., Sales F., Awada A., Ghanem G.E. Acquired resistance to BRAFi reverses senescence-like phenotype in mutant BRAF melanoma. Oncotarget. 2018;9:31888–31903. doi: 10.18632/oncotarget.25879. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Clynick B., Tabone T., Fuller K., Erber W., Meehan K., Millward M., Wood B.A., Harvey N.T., Fuller K. Mutational Analysis of BRAF Inhibitor–Associated Squamoproliferative Lesions. J. Mol. Diagn. 2015;17:644–651. doi: 10.1016/j.jmoldx.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 62.Sullivan R.J., Flaherty K.T. Resistance to BRAF-targeted therapy in melanoma. Eur. J. Cancer. 2013;49:1297–1304. doi: 10.1016/j.ejca.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 63.Devji T., Levine O., Neupane B., Beyene J., Xie F. Systemic Therapy for Previously Untreated Advanced BRAF-Mutated Melanoma. JAMA Oncol. 2017;3:366–373. doi: 10.1001/jamaoncol.2016.4877. [DOI] [PubMed] [Google Scholar]
- 64.Nathanson K.L., Martin A.-M., Wubbenhorst B., Greshock J., Letrero R., D’Andrea K., O’Day S., Infante J.R., Falchook G.S., Arkenau H.-T., et al. Tumor Genetic Analyses of Patients with Metastatic Melanoma Treated with the BRAF Inhibitor Dabrafenib (GSK2118436) Clin. Cancer Res. 2013;19:4868–4878. doi: 10.1158/1078-0432.CCR-13-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paraiso K.H., Xiang Y., Rebecca V.W., Abel E.V., Chen Y.A., Munko A.C., Wood E., Fedorenko I.V., Sondak V.K., Anderson A.R., et al. PTEN Loss Confers BRAF Inhibitor Resistance to Melanoma Cells through the Suppression of BIM Expression. Cancer Res. 2011;71:2750–2760. doi: 10.1158/0008-5472.CAN-10-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gross A., Niemetz-Rahn A., Nonnenmacher A., Tucholski J., Keilholz U., Fusi A. Expression and activity of EGFR in human cutaneous melanoma cell lines and influence of vemurafenib on the EGFR pathway. Target. Oncol. 2014;10:77–84. doi: 10.1007/s11523-014-0318-9. [DOI] [PubMed] [Google Scholar]
- 67.Girotti M.R., Pedersen M., Sanchez-Laorden B., Viros A., Turajlic S., Niculescu-Duvaz D., Zambon A., Sinclair J., Hayes A., Gore M., et al. Inhibiting EGF Receptor or SRC Family Kinase Signaling Overcomes BRAF Inhibitor Resistance in Melanoma. Cancer Discov. 2013;3:158–167. doi: 10.1158/2159-8290.CD-12-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rambow F., Rogiers A., Marin-Bejar O., Aibar S., Femel J., Dewaele M., Karras P., Brown D., Chang Y.H., Debiec-Rychter M., et al. Toward Minimal Residual Disease-Directed Therapy in Melanoma. Cell. 2018;174:843–855.e19. doi: 10.1016/j.cell.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 69.Martin N., Ma X., Bernard D. Regulation of cellular senescence by retinoid X receptors and their partners. Mech. Ageing Dev. 2019;183:111131. doi: 10.1016/j.mad.2019.111131. [DOI] [PubMed] [Google Scholar]
- 70.Hoek K.S., Goding C.R. Cancer stem cells versus phenotype-switching in melanoma. Pigment. Cell Melanoma Res. 2010;23:746–759. doi: 10.1111/j.1755-148X.2010.00757.x. [DOI] [PubMed] [Google Scholar]
- 71.Diazzi S., Tartare-Deckert S., Deckert M. Bad Neighborhood: Fibrotic Stroma as a New Player in Melanoma Resistance to Targeted Therapies. Cancers. 2020;12:1364. doi: 10.3390/cancers12061364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giuliano S., Cheli Y., Ohanna M., Bonet C., Beuret L., Bille K., Loubat A., Hofman V., Hofman P., Ponzio G., et al. Microphthalmia-Associated Transcription Factor Controls the DNA Damage Response and a Lineage-Specific Senescence Program in Melanomas. Cancer Res. 2010;70:3813–3822. doi: 10.1158/0008-5472.CAN-09-2913. [DOI] [PubMed] [Google Scholar]
- 73.Strub T., Giuliano S., Ye T., Bonet C., Keime C., Kobi D., Le Gras S., Cormont M., Ballotti R., Bertolotto C., et al. Essential role of microphthalmia transcription factor for DNA replication, mitosis and genomic stability in melanoma. Oncogene. 2011;30:2319–2332. doi: 10.1038/onc.2010.612. [DOI] [PubMed] [Google Scholar]
- 74.Fane M., Weeraratna A.T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer. 2020;20:89–106. doi: 10.1038/s41568-019-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Straussman R., Morikawa T., Shee K., Barzily-Rokni M., Qian Z.R., Du J., Davis A., Mongare M.M., Gould J., Frederick D.T., et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tominaga K., Suzuki H.I. TGF-β Signaling in Cellular Senescence and Aging-Related Pathology. Int. J. Mol. Sci. 2019;20:5002. doi: 10.3390/ijms20205002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Basisty N., Kale A., Jeon O.H., Kuehnemann C., Payne T., Rao C., Holtz A., Shah S., Sharma V., Ferrucci L., et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020;18:e3000599. doi: 10.1371/journal.pbio.3000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schafer M.J., Zhang X., Kumar A., Atkinson E.J., Zhu Y., Jachim S., Mazula D.L., Brown A.K., Berning M., Aversa Z., et al. The senescence-associated secretome as an indicator of age and medical risk. JCI Insight. 2020;5:5. doi: 10.1172/jci.insight.133668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Q., Li J., Wen T., Zeng W., Peng C., Yan S., Tan J., Yang K., Liu S., Guo A., et al. Overexpression of HMGB1 in melanoma predicts patient survival and suppression of HMGB1 induces cell cycle arrest and senescence in association with p21 (Waf1/Cip1) up-regulation via a p53-independent, Sp1-dependent pathway. Oncotarget. 2014;5:6387–6403. doi: 10.18632/oncotarget.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Erkes D.A., Cai W., Sanchez I.M., Purwin T.J., Rogers C., Field C.O., Berger A.C., Hartsough E.J., Rodeck U., Alnemri E.S., et al. Mutant BRAF and MEK Inhibitors Regulate the Tumor Immune Microenvironment via Pyroptosis. Cancer Discov. 2020;10:254–269. doi: 10.1158/2159-8290.CD-19-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thies A., Moll I., Berger J., Wagener C., Brümmer J., Schulze H.-J., Brunner G., Schumacher U. CEACAM1 Expression in Cutaneous Malignant Melanoma Predicts the Development of Metastatic Disease. J. Clin. Oncol. 2002;20:2530–2536. doi: 10.1200/JCO.2002.05.033. [DOI] [PubMed] [Google Scholar]
- 82.Wicklein D., Otto B., Suling A., Elies E., Lüers G., Lange T., Feldhaus S., Maar H., Schröder-Schwarz J., Brunner G., et al. CEACAM1 promotes melanoma metastasis and is involved in the regulation of the EMT associated gene network in melanoma cells. Sci. Rep. 2018;8:11893. doi: 10.1038/s41598-018-30338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Helfrich I., Singer B.B. Size Matters: The Functional Role of the CEACAM1 Isoform Signature and Its Impact for NK Cell-Mediated Killing in Melanoma. Cancers. 2019;11:356. doi: 10.3390/cancers11030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sappino A.-P., Buser R., Seguin Q., Fernet M., Lesne L., Gumypause F., Reith W., Favaudon V., Mandriota S.J. The CEACAM1 tumor suppressor is an ATM and p53-regulated gene required for the induction of cellular senescence by DNA damage. Oncogenesis. 2012;1:e7. doi: 10.1038/oncsis.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kfir-Elirachman K., Ortenberg R., Vizel B., Besser M.J., Barshack I., Schachter J., Nemlich Y., Markel G. Regulation of CEACAM1 Protein Expression by the Transcription Factor ETS-1 in BRAF-Mutant Human Metastatic Melanoma Cells. Neoplasia. 2018;20:401–409. doi: 10.1016/j.neo.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bachmann I.M., Halvorsen O.J., Collett K., Stefansson I.M., Straume O., Haukaas S.A., Salvesen H.B., Otte A.P., Akslen L.A. EZH2 Expression Is Associated With High Proliferation Rate and Aggressive Tumor Subgroups in Cutaneous Melanoma and Cancers of the Endometrium, Prostate, and Breast. J. Clin. Oncol. 2006;24:268–273. doi: 10.1200/JCO.2005.01.5180. [DOI] [PubMed] [Google Scholar]
- 87.Fan T., Jiang S., Chung N., Alikhan A., Ni C., Lee C.-C.R., Hornyak T.J. EZH2-Dependent Suppression of a Cellular Senescence Phenotype in Melanoma Cells by Inhibition of p21/CDKN1A Expression. Mol. Cancer Res. 2011;9:418–429. doi: 10.1158/1541-7786.MCR-10-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pazolli E., Alspach E., Milczarek A., Prior J., Piwnica-Worms D., Stewart S.A. Chromatin Remodeling Underlies the Senescence-Associated Secretory Phenotype of Tumor Stromal Fibroblasts That Supports Cancer Progression. Cancer Res. 2012;72:2251–2261. doi: 10.1158/0008-5472.CAN-11-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hatzivassiliou G., Song K., Yen I., Brandhuber B.J., Anderson D.J., Alvarado R., Ludlam M.J.C., Stokoe D., Gloor S.L., Vigers G., et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 90.Poulikakos P.I., Zhang C., Bollag G., Shokat K.M., Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heidorn S.J., Milagre C., Whittaker S., Nourry A., Niculescu-Duvas I., Dhomen N., Hussain J., Reis-Filho J.S., Springer C.J., Pritchard C., et al. Kinase-Dead BRAF and Oncogenic RAS Cooperate to Drive Tumor Progression through CRAF. Cell. 2010;140:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su F., Viros A., Milagre C., Trunzer K., Bollag G., Spleiss O., Reis-Filho J.S., Kong X., Koya R.C., Flaherty K.T., et al. RASMutations in Cutaneous Squamous-Cell Carcinomas in Patients Treated with BRAF Inhibitors. N. Engl. J. Med. 2012;366:207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doma E., Rupp C., Varga A., Kern F., Riegler B., Baccarini M. Skin Tumorigenesis Stimulated by Raf Inhibitors Relies Upon Raf Functions That Are Dependent and Independent of ERK. Cancer Res. 2013;73:6926–6937. doi: 10.1158/0008-5472.CAN-13-0748. [DOI] [PubMed] [Google Scholar]
- 94.Abdel-Wahab O., Klimek V.M., Gaskell A.A., Viale A., Cheng D., Kim E., Rampal R., Bluth M., Harding J.J., Callahan M.K., et al. Efficacy of Intermittent Combined RAF and MEK Inhibition in a Patient with Concurrent BRAF- and NRAS-Mutant Malignancies. Cancer Discov. 2014;4:538–545. doi: 10.1158/2159-8290.CD-13-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Callahan M.K., Rampal R., Harding J.J., Klimek V.M., Chung Y.R., Merghoub T., Wolchok J.D., Solit D.B., Rosen N., Abdel-Wahab O., et al. Progression of RAS-Mutant Leukemia during RAF Inhibitor Treatment. N. Engl. J. Med. 2012;367:2316–2321. doi: 10.1056/NEJMoa1208958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Andrews M.C., Behren A., Chionh F., Mariadason J., Vella L.J., Do H., Dobrovic A., Tebbutt N., Cebon J. BRAF Inhibitor–Driven Tumor Proliferation in a KRAS-Mutated Colon Carcinoma Is Not Overcome by MEK1/2 Inhibition. J. Clin. Oncol. 2013;31:e448–e451. doi: 10.1200/JCO.2013.50.4118. [DOI] [PubMed] [Google Scholar]
- 97.Wu J., Cohen D., Rady P., Tyring S. BRAF inhibitor-associated cutaneous squamous cell carcinoma: New mechanistic insight, emerging evidence for viral involvement and perspectives on clinical management. Br. J. Dermatol. 2017;177:914–923. doi: 10.1111/bjd.15348. [DOI] [PubMed] [Google Scholar]
- 98.Purdie K.J., Proby C.M., Rizvi H., Griffin H., Doorbar J., Sommerlad M., Feltkamp M.C., Van Der Meijden E., Inman G.J., South A.P., et al. The Role of Human Papillomaviruses and Polyomaviruses in BRAF-Inhibitor Induced Cutaneous Squamous Cell Carcinoma and Benign Squamoproliferative Lesions. Front. Microbiol. 2018;9:1806. doi: 10.3389/fmicb.2018.01806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cohen D.N., Lawson S.K., Shaver A.C., Du L., Nguyen H.P., He Q., Johnson D.B., Lumbang W.A., Moody B.R., Prescott J.L., et al. Contribution of Beta-HPV Infection and UV Damage to Rapid-Onset Cutaneous Squamous Cell Carcinoma during BRAF-Inhibition Therapy. Clin. Cancer Res. 2015;21:2624–2634. doi: 10.1158/1078-0432.CCR-14-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paraiso K.H.T., Fedorenko I.V., Cantini L.P., Munko A.C., Hall M., Sondak V.K., Messina J.L., Flaherty K.T., Smalley K.S.M. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br. J. Cancer. 2010;102:1724–1730. doi: 10.1038/sj.bjc.6605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Welsh S.J., Corrie P.G. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther. Adv. Med Oncol. 2015;7:122–136. doi: 10.1177/1758834014566428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Larkin J., Ascierto P.A., Dréno B., Atkinson V., Liszkay G., Maio M., Mandalà M., Demidov L., Stroyakovskiy D., Thomas L., et al. Combined Vemurafenib and Cobimetinib in BRAF-Mutated Melanoma. N. Engl. J. Med. 2014;371:1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 103.Flaherty K.T., Infante J.R., Daud A., Gonzalez R., Kefford R.F., Sosman J., Hamid O., Schuchter L., Cebon J., Ibrahim N., et al. Combined BRAF and MEK Inhibition in Melanoma with BRAF V600 Mutations. N. Engl. J. Med. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Campisi J. CANCER: Suppressing Cancer: The Importance of Being Senescent. Science. 2005;309:886–887. doi: 10.1126/science.1116801. [DOI] [PubMed] [Google Scholar]
- 105.DeMaria M., Ohtani N., Youssef S.A., Rodier F., Toussaint W., Mitchell J.R., Laberge R.-M., Vijg J., Van Steeg H., Dollé M.E., et al. An Essential Role for Senescent Cells in Optimal Wound Healing through Secretion of PDGF-AA. Dev. Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Resnik S.R., Egger A., Abujamra B.A., Jozic I. Clinical Implications of Cellular Senescence on Wound Healing. Curr. Dermatol. Rep. 2020;9:286–297. doi: 10.1007/s13671-020-00320-3. [DOI] [Google Scholar]
- 107.Baker D.J., Childs B.G., Durik M., Wijers M.E., Sieben C.J., Zhong J., Saltness R.A., Jeganathan K.B., Verzosa G.C., Pezeshki A., et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Collado M., Gil J., Efeyan A., Guerra C., Schuhmacher A.J., Barradas M., Benguría A., Zaballos A., Flores J.M., Barbacid M., et al. Senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 109.Vernot J.P. Senescence-Associated Pro-inflammatory Cytokines and Tumor Cell Plasticity. Front. Mol. Biosci. 2020;7 doi: 10.3389/fmolb.2020.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Milanovic M., Fan D.N.Y., Belenki D., Däbritz J.H.M., Zhao Z., Yu Y., Dörr J.R., Dimitrova L., Lenze D., Barbosa I.A.M., et al. Senescence-associated reprogramming promotes cancer stemness. Nature. 2018;553:96–100. doi: 10.1038/nature25167. [DOI] [PubMed] [Google Scholar]
- 111.Milanovic M., Yu Y., Schmitt C.A. The Senescence–Stemness Alliance—A Cancer-Hijacked Regeneration Principle. Trends Cell Biol. 2018;28:1049–1061. doi: 10.1016/j.tcb.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 112.von Zglinicki T., Saretzki G., Ladhoff J., di Fagagna F.D., Jackson S. Human cell senescence as a DNA damage response. Mech. Ageing Dev. 2005;126:111–117. doi: 10.1016/j.mad.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 113.Hewitt G.M., Jurk D., Marques F.D., Correia-Melo C., Hardy T.L.D., Gackowska A., Anderson R., Taschuk M.T., Mann J., Passos J.F. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 2012;3:708. doi: 10.1038/ncomms1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Passos J.F., Nelson G., Wang C., Richter T., Simillion C., Proctor C.J., Miwa S., Olijslagers S., Hallinan J., Wipat A., et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 2010;6:347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Passos J.F., Saretzki G., Ahmed S., Nelson G., Richter T., Peters H., Wappler I., Birket M.J., Harold G., Schaeuble K., et al. Mitochondrial Dysfunction Accounts for the Stochastic Heterogeneity in Telomere-Dependent Senescence. PLoS Biol. 2007;5:e110. doi: 10.1371/journal.pbio.0050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Korkaya H., Liu S., Wicha M.S. Regulation of Cancer Stem Cells by Cytokine Networks: Attacking Cancer’s Inflammatory Roots: Figure 1. Clin. Cancer Res. 2011;17:6125–6129. doi: 10.1158/1078-0432.CCR-10-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Laberge R.-M., Awad P., Campisi J., Desprez P.-Y. Epithelial-Mesenchymal Transition Induced by Senescent Fibroblasts. Cancer Microenviron. 2011;5:39–44. doi: 10.1007/s12307-011-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kaur A., Ecker B.L., Douglass S.M., Kugel C.H., Webster M.R., Almeida F.V., Somasundaram R., Hayden J., Ban E., Ahmadzadeh H., et al. Remodeling of the Collagen Matrix in Aging Skin Promotes Melanoma Metastasis and Affects Immune Cell Motility. Cancer Discov. 2019;9:64–81. doi: 10.1158/2159-8290.CD-18-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Prieto L.I., Baker D.J. Cellular Senescence and the Immune System in Cancer. Gerontology. 2019;65:505–512. doi: 10.1159/000500683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lian J., Yue Y., Yu W., Zhang Y. Immunosenescence: A key player in cancer development. J. Hematol. Oncol. 2020;13:1–18. doi: 10.1186/s13045-020-00986-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Su D.-M., Aw D., Palmer D.B. Immunosenescence: A product of the environment? Curr. Opin. Immunol. 2013;25:498–503. doi: 10.1016/j.coi.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 122.Battram A.M., Bachiller M., Martín-Antonio B. Senescence in the Development and Response to Cancer with Immunotherapy: A Double-Edged Sword. Int. J. Mol. Sci. 2020;21:4346. doi: 10.3390/ijms21124346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gray-Schopfer V.C., Cheong S.C., Chong H., Chow J., Moss T., Abdel-Malek Z.A., Marais R., Wynford-Thomas D., Bennett D.C. Cellular senescence in naevi and immortalisation in melanoma: A role for p16? Br. J. Cancer. 2006;95:496–505. doi: 10.1038/sj.bjc.6603283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Suram A., Kaplunov J., Patel P.L., Ruan H., Cerutti A., Boccardi V., Fumagalli M., Di Micco R., Mirani N., Gurung R.L., et al. Oncogene-induced telomere dysfunction enforces cellular senescence in human cancer precursor lesions. EMBO J. 2012;31:2839–2851. doi: 10.1038/emboj.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boussemart L., Routier E., Mateus C., Opletalova K., Sebille G., Kamsu-Kom N., Thomas M., Vagner S., Favre M., Tomasic G., et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: A study of 42 patients. Ann. Oncol. 2013;24:1691–1697. doi: 10.1093/annonc/mdt015. [DOI] [PubMed] [Google Scholar]
- 126.Anforth R., Blumetti T., Kefford R., Sharma R., Scolyer R., Kossard S., Long G., Fernandez-Peñas P. Cutaneous manifestations of dabrafenib (GSK2118436): A selective inhibitor of mutant BRAF in patients with metastatic melanoma. Br. J. Dermatol. 2012;167:1153–1160. doi: 10.1111/j.1365-2133.2012.11155.x. [DOI] [PubMed] [Google Scholar]
- 127.Wells S.I., Francis D.A., Karpova A.Y., Dowhanick J.J., Benson J.D., Howley P.M. Papillomavirus E2 induces senescence in HPV-positive cells via pRB- and p21CIP-dependent pathways. EMBO J. 2000;19:5762–5771. doi: 10.1093/emboj/19.21.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bellmann L., Cappellano G., Schachtl-Riess J.F., Prokopi A., Seretis A., Ortner D., Tripp C.H., Brinckerhoff C.E., Mullins D.W., Stoitzner P. A TLR7 agonist strengthens T and NK cell function during BRAF-targeted therapy in a preclinical melanoma model. Int. J. Cancer. 2019;146:1409–1420. doi: 10.1002/ijc.32777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tang H., Xue G. Major Physiological Signaling Pathways in the Regulation of Cell Proliferation and Survival. Handb. Exp. Pharmacol. 2018;249:13–30. doi: 10.1007/164_2017_4. [DOI] [PubMed] [Google Scholar]
- 130.Zhu Y., Tchkonia T., Pirtskhalava T., Gower A.C., Ding H., Giorgadze N., Palmer A.K., Ikeno Y., Hubbard G.B., Lenburg M.E., et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kirkland J.L., Tchkonia T., Zhu Y., Niedernhofer L.J., Robbins P.D. The Clinical Potential of Senolytic Drugs. J. Am. Geriatr. Soc. 2017;65:2297–2301. doi: 10.1111/jgs.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yousefzadeh M.J., Zhu Y., McGowan S.J., Angelini L., Fuhrmann-Stroissnigg H., Xu M., Ling Y.Y., Melos K.I., Pirtskhalava T., Inman C.L., et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. doi: 10.1016/j.ebiom.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xu M., Pirtskhalava T., Farr J.N., Weigand B.M., Palmer A.K., Weivoda M.M., Inman C.L., Ogrodnik M.B., Hachfeld C.M., Fraser D.G., et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018;24:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mavrogonatou E., Pratsinis H., Kletsas D. The role of senescence in cancer development. Semin. Cancer Biol. 2020;62:182–191. doi: 10.1016/j.semcancer.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 135.Short S., Fielder E., Miwa S., von Zglinicki T. Senolytics and senostatics as adjuvant tumour therapy. EBioMedicine. 2019;41:683–692. doi: 10.1016/j.ebiom.2019.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Niedernhofer L.J., Robbins P.D. Senotherapeutics for healthy ageing. Nat. Rev. Drug Discov. 2018;17:377. doi: 10.1038/nrd.2018.44. [DOI] [PubMed] [Google Scholar]
- 137.Pal H.C., Sharma S., Strickland L.R., Katiyar S.K., Ballestas M.E., Athar M., Elmets C.A., Afaq F. Fisetin Inhibits Human Melanoma Cell Invasion through Promotion of Mesenchymal to Epithelial Transition and by Targeting MAPK and NFκB Signaling Pathways. PLoS ONE. 2014;9:e86338. doi: 10.1371/journal.pone.0086338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Syed D., Adhami V., Khan M., Mukhtar H. Inhibition of Akt/mTOR Signaling by the Dietary Flavonoid Fisetin. Anti-Cancer Agents Med. Chem. 2013;13:995–1001. doi: 10.2174/18715206113139990129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chamcheu J.C., Esnault S., Adhami V.M., Noll A.L., Banang-Mbeumi S., Roy T., Singh S.S., Huang S., Kousoulas K.G., Mukhtar H. Fisetin, a 3,7,3’,4’-Tetrahydroxyflavone Inhibits the PI3K/Akt/mTOR and MAPK Pathways and Ameliorates Psoriasis Pathology in 2D and 3D Organotypic Human Inflammatory Skin Models. Cells. 2019;8:1089. doi: 10.3390/cells8091089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Caltagirone S., Rossi C., Poggi A., Ranelletti F.O., Natali P.G., Brunetti M., Aiello F.B., Piantelli M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int. J. Cancer. 2000;87:595–600. doi: 10.1002/1097-0215(20000815)87:4<595::AID-IJC21>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 141.Harris Z., Donovan M.G., Branco G.M., Limesand K.H., Burd R. Quercetin as an Emerging Anti-Melanoma Agent: A Four-Focus Area Therapeutic Development Strategy. Front. Nutr. 2016;3 doi: 10.3389/fnut.2016.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thangasamy T., Sittadjody S., Lanza-Jacoby S., Wachsberger P.R., Limesand K.H., Burd R. Quercetin Selectively Inhibits Bioreduction and Enhances Apoptosis in Melanoma Cells That Overexpress Tyrosinase. Nutr. Cancer. 2007;59:258–268. doi: 10.1080/01635580701499545. [DOI] [PubMed] [Google Scholar]
- 143.Song X., Gao T., Lei Q., Zhang L., Yao Y., Xiong J. Piperlongumine Induces Apoptosis in Human Melanoma Cells Via Reactive Oxygen Species Mediated Mitochondria Disruption. Nutr. Cancer. 2018;70:502–511. doi: 10.1080/01635581.2018.1445769. [DOI] [PubMed] [Google Scholar]
- 144.Das Thakur M., Salangsang F., Landman A.S., Sellers W.R., Pryer N.K., Levesque M.P., Dummer R., McMahon M., Stuart D.D. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature. 2013;494:251–255. doi: 10.1038/nature11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Prasanna P.G., Citrin D.E., Hildesheim J., Ahmed M.M., Venkatachalam S., Riscuta G., Xi D., Zheng G., van Deursen J., Goronzy J., et al. Therapy-Induced Senescence: Opportunities to Improve Anti-Cancer Therapy. J. Natl. Cancer Inst. 2021 doi: 10.1093/jnci/djab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pacey S., Wilson R.H., Walton M., Eatock M.M., Hardcastle A., Zetterlund A., Arkenau H.-T., Moreno-Farre J., Banerji U., Roels B., et al. A Phase I Study of the Heat Shock Protein 90 Inhibitor Alvespimycin (17-DMAG) Given Intravenously to Patients with Advanced Solid Tumors. Clin. Cancer Res. 2011;17:1561–1570. doi: 10.1158/1078-0432.CCR-10-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shin M.K., Jeong K.-H., Choi H., Ahn H.-J., Lee M.-H. Heat shock protein 90 inhibitor enhances apoptosis by inhibiting the AKT pathway in thermal-stimulated SK-MEL-2 human melanoma cell line. J. Dermatol. Sci. 2018;90:357–360. doi: 10.1016/j.jdermsci.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 148.Pacey S., Gore M., Chao D., Banerji U., Larkin J., Sarker S., Owen K., Asad Y., Raynaud F., Walton M., et al. A Phase II trial of 17-allylamino, 17-demethoxygeldanamycin (17-AAG, tanespimycin) in patients with metastatic melanoma. Investig. New Drugs. 2010;30:341–349. doi: 10.1007/s10637-010-9493-4. [DOI] [PubMed] [Google Scholar]
- 149.Solit D.B., Osman I., Polsky D., Panageas K.S., Daud A., Goydos J.S., Teitcher J., Wolchok J.D., Germino F.J., Krown S.E., et al. Phase II Trial of 17-Allylamino-17-Demethoxygeldanamycin in Patients with Metastatic Melanoma. Clin. Cancer Res. 2008;14:8302–8307. doi: 10.1158/1078-0432.CCR-08-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vaishampayan U.N., Burger A.M., Sausville E.A., Heilbrun L.K., Li J., Horiba M.N., Egorin M.J., Ivy P., Pacey S., Lorusso P.M. Safety, Efficacy, Pharmacokinetics, and Pharmacodynamics of the Combination of Sorafenib and Tanespimycin. Clin. Cancer Res. 2010;16:3795–3804. doi: 10.1158/1078-0432.CCR-10-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Frankel A.E., Eskiocak U., Gill J.G., Yuan S., Ramesh V., Froehlich T.W., Ahn C., Morrison S.J. Digoxin Plus Trametinib Therapy Achieves Disease Control in BRAF Wild-Type Metastatic Melanoma Patients. Neoplasia. 2017;19:255–260. doi: 10.1016/j.neo.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sale M.J., Cook S.J. The BH3 mimetic ABT-263 synergizes with the MEK1/2 inhibitor selumetinib/AZD6244 to promote BIM-dependent tumour cell death and inhibit acquired resistance. Biochem. J. 2013;450:285–294. doi: 10.1042/BJ20121212. [DOI] [PubMed] [Google Scholar]
- 153.Kalinsky K., Lee S., Np K.M.R., Lawrence D.P., Iafrarte A.J., Borger D.R., Margolin K.A., Leitao M.M., Jr., Tarhini A.A., Koon H.B., et al. A phase 2 trial of dasatinib in patients with locally advanced or stage IV mucosal, acral, or vulvovaginal melanoma: A trial of the ECOG-ACRIN Cancer Research Group (E2607) Cancer. 2017;123:2688–2697. doi: 10.1002/cncr.30663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kluger H.M., Dudek A.Z., McCann C., Ritacco J., Southard N., Jilaveanu L.B., Molinaro A., Sznol M. A phase 2 trial of dasatinib in advanced melanoma. Cancer. 2011;117:2202–2208. doi: 10.1002/cncr.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Algazi A.P., Weber J.S., Andrews S.C., Urbas P., Munster P.N., DeConti R.C., Hwang J., Sondak V.K., Messina J.L., McCalmont T., et al. Phase I clinical trial of the Src inhibitor dasatinib with dacarbazine in metastatic melanoma. Br. J. Cancer. 2011;106:85–91. doi: 10.1038/bjc.2011.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xia C., Leon-Ferre R., Laux D., Deutsch J., Smith B.J., Frees M., Milhem M. Treatment of resistant metastatic melanoma using sequential epigenetic therapy (decitabine and panobinostat) combined with chemotherapy (temozolomide) Cancer Chemother. Pharmacol. 2014;74:691–697. doi: 10.1007/s00280-014-2501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ibrahim N., Buchbinder E.I., Granter S.R., Rodig S.J., Giobbie-Hurder A., Becerra C., Tsiaras A., Gjini E., Fisher D.E., Hodi F.S. A phase I trial of panobinostat (LBH 589) in patients with metastatic melanoma. Cancer Med. 2016;5:3041–3050. doi: 10.1002/cam4.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.He Y., Junling Z., Zhang J., Yang Y., Qian Y.-W., Zhou D. The curcumin analog EF24 is highly active against chemotherapy-resistant melanoma cells. Curr. Cancer Drug Targets. 2021;21:1. doi: 10.2174/1568009621666210303092921. [DOI] [PubMed] [Google Scholar]
- 159.Da Silva J.M.C., Campos M.L.A., Teixeira M.P., Faustino R.D.S., Aleixo R.C., Cavalcante F.J.P., Gomes L.R.O., De Albuquerque L.Z., Azevedo A.D.N., Cabral V.R., et al. Ouabain pre-treatment modulates B and T lymphocytes and improves survival of melanoma-bearing animals. Int. Immunopharmacol. 2020;86:106772. doi: 10.1016/j.intimp.2020.106772. [DOI] [PubMed] [Google Scholar]
- 160.Wang L., Cai W., Han B., Zhang J., Yu B., Chen M. Ouabain Exhibited Strong Anticancer Effects in Melanoma Cells via Induction of Apoptosis, G2/M Phase Arrest, and Migration Inhibition. OncoTargets Ther. 2021;14:1261–1273. doi: 10.2147/OTT.S283548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wroblewski D., Mijatov B., Mohana-Kumaran N., Lai F., Gallagher S.J., Haass N.K., Zhang X.D., Hersey P. The BH3-mimetic ABT-737 sensitizes human melanoma cells to apoptosis induced by selective BRAF inhibitors but does not reverse acquired resistance. Carcinogenesis. 2012;34:237–247. doi: 10.1093/carcin/bgs330. [DOI] [PubMed] [Google Scholar]
- 162.Mukherjee N., Almeida A., Partyka K.A., Lu Y., Schwan J.V., Lambert K., Rogers M., Robinson W.A., Robinson S.E., Applegate A.J., et al. Combining a GSI and BCL-2 inhibitor to overcome melanoma’s resistance to current treatments. Oncotarget. 2016;7:84594–84607. doi: 10.18632/oncotarget.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao B., Cheng X., Zhou X. The BET-bromodomain inhibitor JQ1 mitigates vemurafenib drug resistance in melanoma. Melanoma Res. 2018;28:521–526. doi: 10.1097/CMR.0000000000000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim S.-H., Yoo E.-S., Woo J.-S., Han S.-H., Lee J.-H., Jung S.-H., Kim H.-J., Jung J.-Y. Antitumor and apoptotic effects of quercetin on human melanoma cells involving JNK/P38 MAPK signaling activation. Eur. J. Pharmacol. 2019;860:172568. doi: 10.1016/j.ejphar.2019.172568. [DOI] [PubMed] [Google Scholar]
- 165.Soll F., Ternent C., Berry I.M., Kumari D., Moore T.C. Quercetin Inhibits Proliferation and Induces Apoptosis of B16 Melanoma Cells In Vitro. ASSAY Drug Dev. Technol. 2020;18:261–268. doi: 10.1089/adt.2020.993. [DOI] [PubMed] [Google Scholar]
- 166.Wang K., Hu D.-N., Lin H.-W., Yang W.-E., Hsieh Y.-H., Chien H.-W., Yang S.-F. Fisetin induces apoptosis through mitochondrial apoptosis pathway in human uveal melanoma cells. Environ. Toxicol. 2018;33:527–534. doi: 10.1002/tox.22538. [DOI] [PubMed] [Google Scholar]
- 167.Sechi M., Lall R.K., Afolabi S.O., Singh A., Joshi D.C., Chiu S.-Y., Mukhtar H., Syed D.N. Fisetin targets YB-1/RSK axis independent of its effect on ERK signaling: Insights from in vitro and in vivo melanoma models. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-018-33879-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liu-Smith F., Meyskens F.L. Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol. Nutr. Food Res. 2016;60:1264–1274. doi: 10.1002/mnfr.201500822. [DOI] [PMC free article] [PubMed] [Google Scholar]