Abstract
Objective:
No standardized system is currently used to report the presence or severity of parenchymal and ductal features of chronic pancreatitis (CP) on CT scan. We report a modification to the previously proposed Cambridge classification to serve this purpose.
Methods:
Contrast-enhanced CT scans of 158 well-phenotyped patients with CP enrolled in the North American Pancreatitis Studies (NAPS2) during 2000–2014 from the University of Pittsburgh were retrospectively reviewed by a subspecialty trained abdominal radiologist. Presence and severity (score scale 0–4) of pancreatic duct (PD) dilation, obstruction and contour irregularity, pancreatic calcifications, atrophy and extent of pancreatic involvement were recorded to grade the morphological severity of CP and stratify patients into distinct morphologic patterns. Findings were also correlated with clinical features.
Results:
Pancreatic atrophy, calcifications, PD dilation and PD irregularity were observed in 80%, 68%, 65%, 58% cases, respectively. An obstructive stone or PD stricture was present in 63%, and 86% had diffuse pancreatic involvement. Using these features, CP was noted to be moderate or severe in 61%, and classified morphologically as obstructive with/without calcifications, calcific but non-obstructive and non-calcific/non-obstructive in 65%, 20%, 15%, respectively. Functional abnormalities but not the presence of pain generally correlated with imaging findings.
Conclusion:
A structured scoring system can provide qualitative and quantitative assessment of imaging findings in CP and an opportunity for adoption into clinical practice and research for initial evaluation and longitudinal follow-up. Our findings need validation in a prospective cohort before widespread adoption.
Introduction
Chronic pancreatitis (CP) is an inflammatory, usually progressive disease of the pancreas, characterized by abdominal pain, episode(s) of acute pancreatitis (AP), loss of exocrine and/or endocrine function, and in a subset development of pancreatic ducal adenocarcinoma [1]. CP has a profound effect on quality of life [2]. The clinical course of CP is highly variable. There is a growing recognition that AP, recurrent acute pancreatitis (RAP) and CP represent a disease continuum [3]. Knowledge of the risk and determinants of transition from earlier stages of disease to established CP, and among patients with established CP is of importance, as it may provide an opportunity for interventions to prevent or slow disease progression and limit its associated morbidity [1].
CP in clinical practice is typically diagnosed by the presence of moderate-severe changes in pancreatic ductal system and/or parenchyma on cross-sectional imaging [4,5] Cambridge classification was proposed over 30 years ago as a measure to define the severity of CP on endoscopic retrograde cholangiopancreatography (ERCP), and have subsequently been adapted for MRI/MRCP [6–8]. ERCP is no longer used to diagnose CP. Corresponding changes on Cambridge classification were also proposed for CT scan [6].
Although recent guidelines have endorsed the use of Cambridge classification in CP it is infrequently used in clinical practice [8]. Moreover, several limitations affect its utility for cross-sectional comparison of different stages of CP, and for longitudinal assessment of changes in individual patients. For example, difficulty in interpretation of certain findings, such as heterogenous parenchyma, gland enlargement, increased echogenicity of duct walls and irregular contour of the gland; no specific findings are suggested for moderate category on CT scan; atrophy is not included in the classification; location of stricture or obstruction of the pancreatic duct (PD) do not carry any weight; and parenchymal calcification and the severity of calcifications are not included. Moreover, since initially proposed, many technical advances have occurred in CT scan and MRI/MRCP that are not incorporated in the classification [5,9–11].
A standardized approach to assess CP-related changes on imaging studies, such as contrast enhanced CT scan (CECT) and MRI/MRCP with contrast and secretin, at initial assessment and during longitudinal follow-up, will be of great value for clinical care and research purposes. While MRI is better at ductal assessment, particularly with use of secretin, and can also provide valuable information linked to pancreatic exocrine function [12–14]. However, CT scan is less expensive, more widely available, allows for quick image acquisition, and more importantly, is far superior to MRI in detecting calcifications, which are important pathologic changes in CP [5].
Herein we report a modification to the Cambridge classification to systematically document the presence and severity of parenchymal and ductal features of CP using CT scan. In this pilot study, we tested this system for its utility in classifying the morphological severity and major patterns of imaging findings on CT scan in over 150 well-phenotyped patients with CP enrolled in the North American Pancreatitis Studies (NAPS2) from University of Pittsburgh Medical Center (UPMC). Pilot data generated in this study formed the basis for the proposal of radiology standards for reporting of findings on cross-sectional imaging in patients with chronic pancreatitis by the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer (CPDPC) [15].
Methods
Study population
NAPS2 are a series of three studies (original NAPS2, NAPS2-CV, NAPS2-AS) which prospectively ascertained 1,195 CP, 568 RAP patients and 1,107 controls from 27 US centers from 2000 to 2014. Detailed methodology of the NAPS2 studies has been published [16–18]. For inclusion as a CP patient, presence of definitive changes on cross-sectional imaging (primarily CT scan, MRI/MRCP, ERCP, EUS [≥5 criteria or calcifications]) or histology were required, based on the Cambridge definition of CP. Detailed information was collected on demographics, personal and family history, risk factor exposure, disease phenotype, quality of life, disability and treatments received through structured questionnaires completed by patients with assistance of a research coordinator and the enrolling physician. Study subjects provided informed consent prior to enrollment, and the study was approved by the Institutional Review Board of each participating institution.
For the present study, we included 158 out of 293 CP patients in the NAPS2 cohort enrolled from the Presbyterian-Montefiore hospitals of UPMC who had CECT scans of the abdomen performed either during 12 months prior to or within 3 months after the enrollment date and had images available for review (Supplementary Fig. 1).
Imaging technique
All patients were imaged in portal venous phase except for one who had imaging in delayed venous phase. Some patients additionally had unenhanced (n = 39) and/or pancreatic parenchymal phase (n = 43) of imaging. CT scans in all but two patients were performed on multidetector helical CT scanners with 4–64 detector rows (General Electric Medical Systems). Details of one patient who had CECT performed at another hospital were unknown. One patient had imaging on a 16–detector row helical CT scanner (Somatom Sensation; Siemens Medical Solutions). Contiguous 5 mm or thinner axial sections were displayed in all but 1 patient who had 7 mm thick axial sections. In 143 patients, non-ionic intravenous contrast material (ioversol [Optiray 350/320; Mallinckrodt Imaging] or Isovue-370/350/300 [iopamidol injection 76%; Bracco Diagnostics Inc]) at a volume of 50–125 mL was used. Details of administered intravenous contrast was not available in the remaining 15 patients.
Image analysis and criteria
CECT scan images were reviewed by a subspecialty trained abdominal radiologist with over 10 years of experience (AD), who was blinded to clinical information. Images were analyzed for presence and severity of parenchymal and ductal changes as outlined in Table 1. The grading of individual features was based on analysis of relevant literature. For example, there is no consensus for a cut-off value for identifying PD dilation but up to 3–3.5 mm has been considered normal PD caliber in several publications [19–22]. Similarly, there are no widely accepted criteria for identifying pancreatic atrophy, which is a marker for CP and known to be associated with decreased endocrine and exocrine pancreatic function [8,23,24] (Fig. 1). Heuck et al. noted that normal pancreatic thickness ranged from 19.1 mm ( ±2.1 mm) in young adults to 14.4 mm ( ±2.7 mm) in older individuals in 8th decade [25]. Balci et al. found that mean pancreatic thickness in their study population was 21.8 mm in the subset with normal endoscopic pancreatic function testing [14]. Presence and degree of pancreatic calcifications correlate with severity of CP and are shown to be independent of gland atrophy and pancreatic ductal changes [26–28] (Fig. 2). However, punctate calcifications can sometimes be senescent and unrelated to CP [26]. Scoring ranged from 0 to 3 for all parameters except for pancreatic ductal contour and extent of pancreatic involvement which were given a weighted score ranging from 0, 2 and 4. Weighted score was given to ductal irregularity as it represents presence of periductal fibrosis, which is not only a cardinal sign of CP but can also potentially lead to significant disease progression from the associated strictures and stasis within the PD leading to stone formation and ductal obstruction with resultant upstream dilation and surrounding parenchymal atrophy [24,29]. Weighted scoring was also given to distribution of findings or the extent of pancreatic involvement to reflect the relationship of the volume of pancreas affected with a higher stage of disease or worse outcome. Score 0, rather than 1, was assigned to those with involvement of less than 30% of pancreas given the low likelihood of affecting pancreatic exocrine or endocrine function. We however, acknowledge the arbitrary nature of the weighted score assignment which was based on expert opinion. Pancreatic enhancement characteristics were not taken into account for scoring as not all patients had a triphasic pancreatic protocol CT scan.
Table 1.
Scoring for parenchymal and ductal changes of Chronic Pancreatitis.
| Feature | Score | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Pancreatic Duct Caliber | Normal | Dilated to <3.5 mm | 3.5–7 mm | >7 mm | NA |
| Pancreatic Duct Contour | Smooth | NA | dMildly irregular | NA | eMod to markedly irregular |
| Pancreatic Duct Strictures/Intraductal Obstructing Calculusa | None | In the tail | In the body | In the head | NA |
| Pancreatic Parenchymal Calcificationsb | None | <7 punctate foci | ≥7 punctate foci <50; <7 coarse foci | Innumerable (≥50) punctate foci; ≥7 coarse foci | NA |
| Pancreatic Parenchymal Atrophyc | None | >14mm < 21 mm | 7–14 mm | <7 mm | NA |
| Distribution of Findings (only atrophy and/or calcifications) | Focal, ≤30% of pancreas | NA | segmental, >30% but <70% of pancreas | NA | ≥70% of pancreas |
Either obstructing stricture or obstructing intraductal calculus should get score but not both.
Punctate calcification - <3 mm in size; Coarse calcification- ≥3 mm in size.
Thickness of pancreas measured at the level of the left border of adjacent vertebral body; measure thickness of pancreas upstream to PD obstruction if there is focal/segmental involvement of pancreatic body/tail.
Subtle subjective contour irregularity without significant ductal narrowing.
Distinct irregularity of pancreatic duct with discrete focal areas of narrowing and dilation.
Fig. 1.
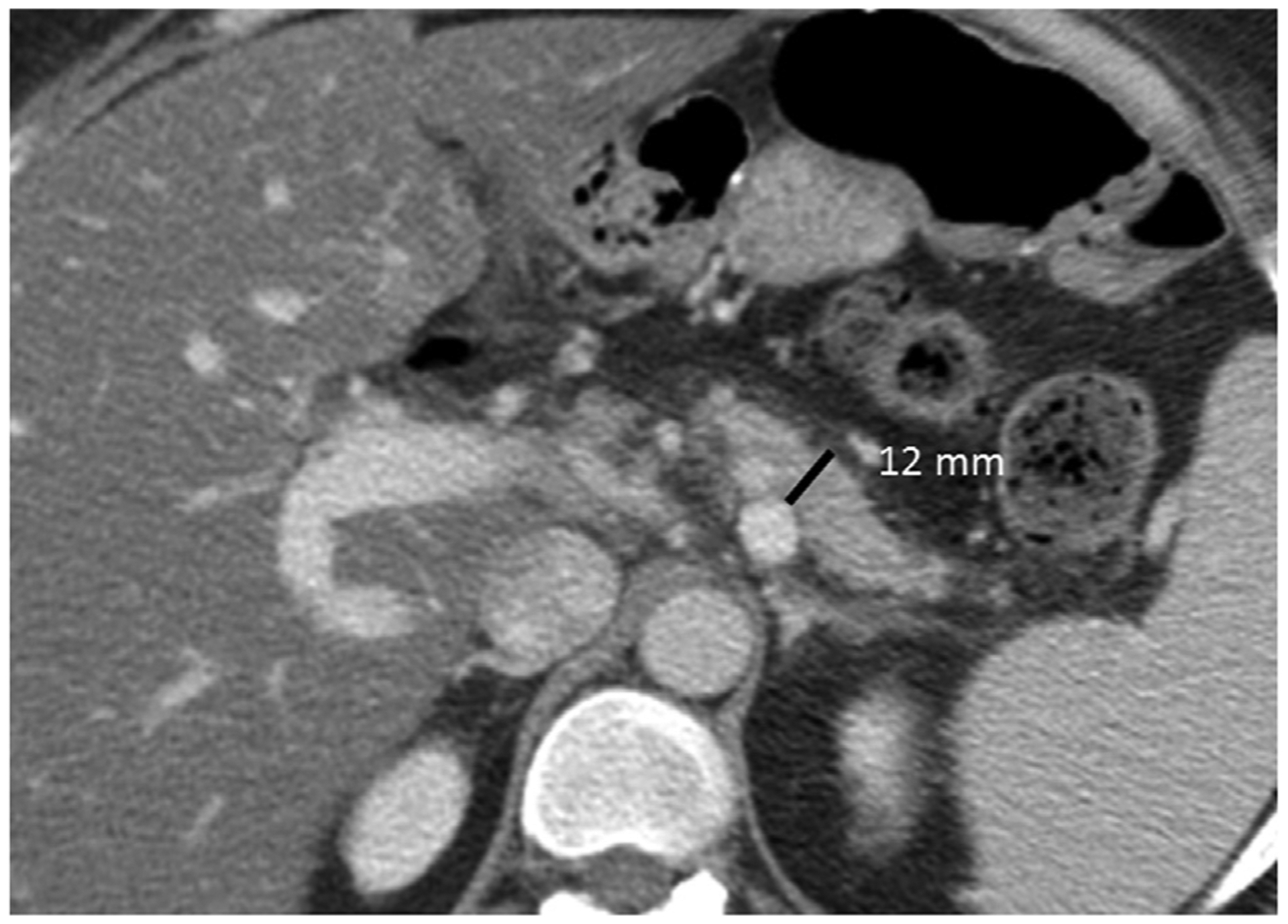
34-year-old-African American woman without endocrine insufficiency but with exocrine insufficiency, diagnosed with idiopathic CP 4 years earlier when she presented with steatorrhea. Axial CECT demonstrates parenchymal thickness of 12 mm corresponding to moderate atrophy. Note the lack of pancreatic ductal dilation and pancreatic calcifications compatible with non-calcific and non-obstructive morphological subtype of CP.
Fig. 2.
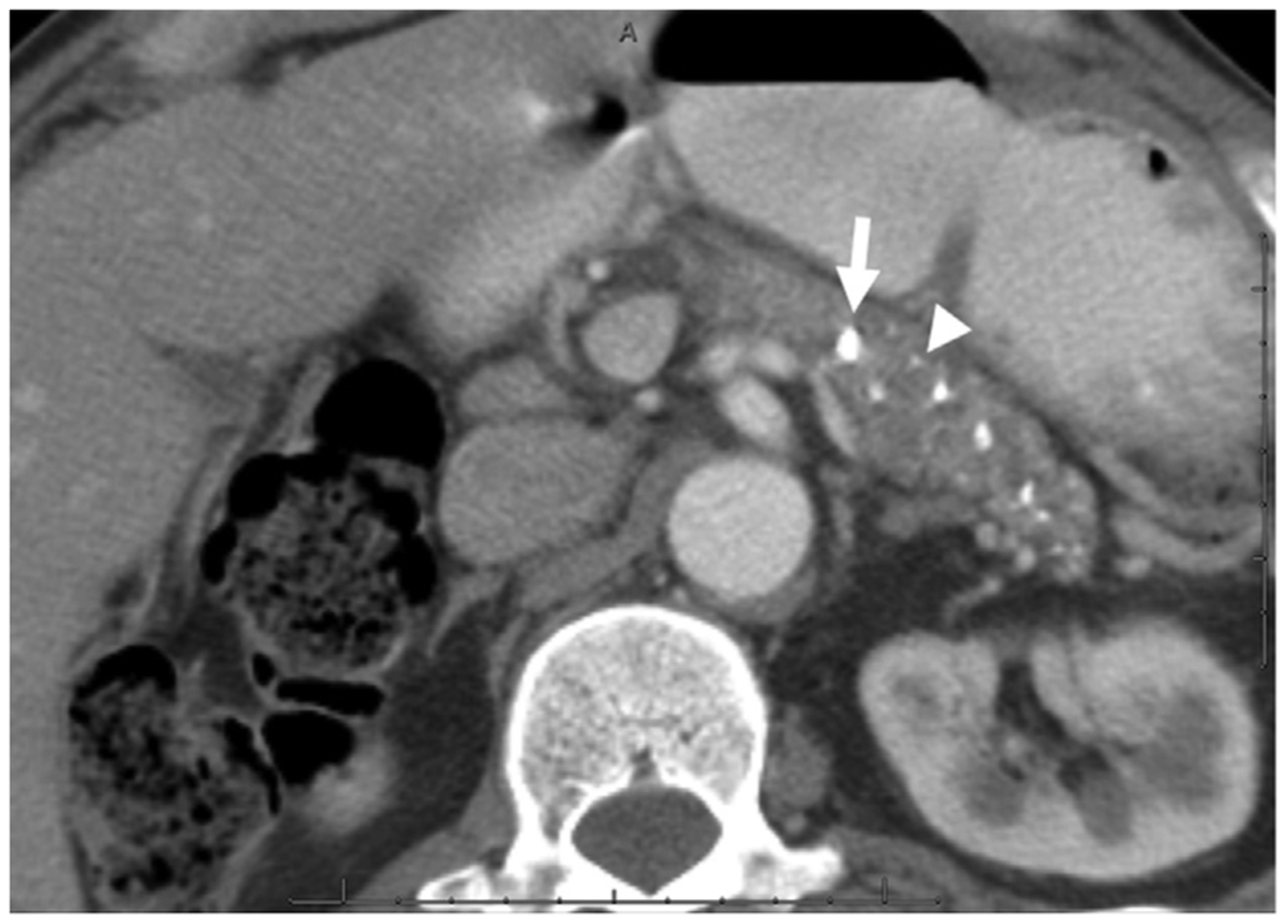
53-year-old-African American man without endocrine or exocrine insufficiency but with constant mild-moderate abdominal pain, diagnosed with alcoholic CP 19 years earlier by CT scan. Axial CECT image demonstrates multiple punctate (arrow head) and coarse (arrow) calcifications in the pancreatic body and tail without pancreatic ductal dilation compatible with calcific but non-obstructive morphological subtype of CP.
Morphological severity was graded based on a combination of features including main ductal dilation and contour irregularity, pancreatic calcifications and parenchymal atrophy (Table 2). Finally, we stratified patients into three major patterns of radiographic changes - non-calcific and non-obstructive CP (only manifestation being parenchymal atrophy with normal/non-dilated pancreatic duct and without pancreatic calcifications); calcific but non-obstructive CP (pancreatic calcifications which are not in the main duct and without pancreatic ductal obstruction/dilation); and obstructive CP with or without calcifications (main pancreatic ductal obstruction from a stricture or intraductal calculus, with or without additional pancreatic calcifications).
Table 2.
Grading of morphological severity of chronic pancreatitis.
| Major Features | Minor Features | |
|---|---|---|
| Pancreatic Atrophy | Severe parenchymal atrophy (<7 mm) | Mild to moderate parenchymal atrophy (7 - < 21 mm) |
| Pancreatic Calcifications | -≥ 7 coarse foci of pancreatic calcifications | - <7 coarse foci of calcifications |
| - Innumerable (≥50) punctate foci | - < 50 punctate calcific foci | |
| Pancreatic Duct features | -Obstructing Intraductal calculus | - PD dilation ≥4 mm |
| -mod to severe PD irregularity | - Mild PD irregularity | |
| - PD stricture | ||
| - Nonobstructing intraductal calculus |
Severe CP - 1 major feature or ≥4 minor features.
Moderate CP - 3 minor features.
Mild CP - 2 minor features or 1 minor feature of pancreatic calcifications.
Equivocal CP - 1 minor feature other than pancreatic calcifications.
Statistical analyses
Descriptive analyses are presented as proportions for categorical data. We evaluated the prevalence of individual findings, morphological severity grade and major morphological categories of CP in all patients and within subgroups based on etiology (alcohol yes/no), pain experience (presence, severity [mild-moderate or severe] and temporal nature [intermittent or constant]), and a diagnosis of exocrine and/or endocrine insufficiency. Pearson chi-square test and the Cochran-Armitage trend test were used to study the association between morphological severity of CP, atrophy severity, morphology and patient characteristics such as prevalence of abdominal pain, exocrine insufficiency and endocrine insufficiency. Data analysis was performed using SAS/STAT 9.4 (Statistical Analytic Systems). P values less than .05 were considered statistically significant.
Results
Demographics, etiology, pain, diabetes, and exocrine insufficiency
Of the 158 patients who formed the final study cohort, 52% were male and 82% were white. The mean age at the time of enrollment was 50.1 ± 15.2 years. Etiology included alcohol (54%), idiopathic (20%), genetic (11%), and other causes (15%). Abdominal pain was the most common symptom (135/157, 86%) – pain was reported to be severe by 56% and mild to moderate by 30% patients; the temporal nature of pain was reported as constant by 50% and intermittent by 36%. Endocrine and exocrine insufficiency was present in 34% and 36% patients, respectively.
Imaging findings
The distribution of individual imaging findings is shown in Table 3. Overall, the prevalence of pancreatic atrophy, calcifications, pancreatic duct dilatation, pancreatic duct irregularity (Fig. 3) was 80%, 68%, 65%, and 58%, respectively. An obstructive stone or pancreatic ductal stricture was present in 63%, and 86% had diffuse involvement of the pancreas. No significant difference was noted in the distribution of the individual imaging findings based on the etiology of CP. The aggregate CT scores based on the parenchymal and ductal features ranged from 0 to 20 with median score of 12 (IQR 6, 17).
Table 3.
Distribution of individual imaging findings of CP.
| Score | Pancreatic duct caliber* | Pancreatic duct contour# | Pancreatic Calcifications* | Pancreatic Atrophy* | Extent of pancreatic involvement# | PD stricture or obstructing PD calculus* |
|---|---|---|---|---|---|---|
| 0 | 55 (35) | 67 (42) | 51 (32) | 32 (20) | 22 (14) | 59 (37) |
| 1 | 18 (11) | N/A | 13 (8) | 61 (39) | N/A | 3 (2) |
| 2 | 51 (32) | 23 (15) | 36 (23) | 47 (30) | 4 (3) | 14 (9) |
| 3 | 34 (22) | N/A | 58 (37) | 17 (11) | N/A | 82 (52) |
| 4 | N/A | 68 (43) | N/A | N/A | 132 (84) | N/A |
Data shown as – n (%).
Possible score options are 0–3.
Possible score options are 0, 2 and 4.
Fig. 3.
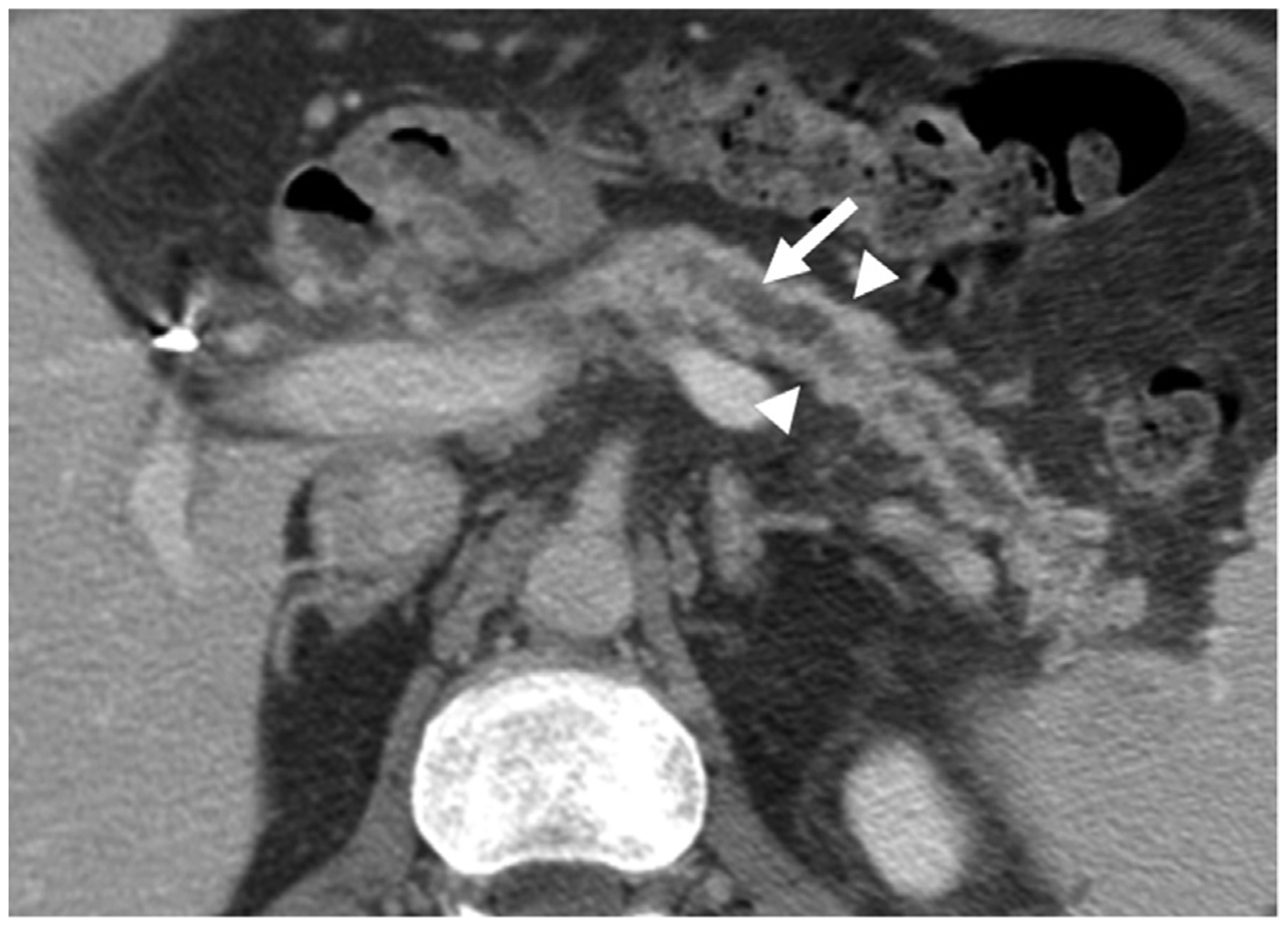
69-year-old -Caucasian man with endocrine insufficiency and episodes of severe abdominal pain diagnosed with idiopathic CP by ERCP. Axial CECT image demonstrates dilated, markedly irregular pancreatic duct (arrow) and moderate parenchymal atrophy (arrow heads) secondary to a stricture in the pancreatic head (not shown) compatible with obstructive morphological subtype of CP.
Morphological severity and major morphological patterns
The distribution of morphological severity of CP is shown in Fig. 4a - severe CP was the most common, present in 58%, followed by mild (22%) and equivocal (13%) changes. 6 patients (4%) had normal appearance on CT scan, but were diagnosed with CP based on EUS (n = 3), ERCP (n = 2), or secretin-MRCP (n = 1).The morphological pattern in the majority of patients was of obstructive CP with or without calcifications (65%), followed by calcific but non-obstructive CP (20%) and non-calcific and non-obstructive CP (15%) (Fig. 4b).
Fig. 4.
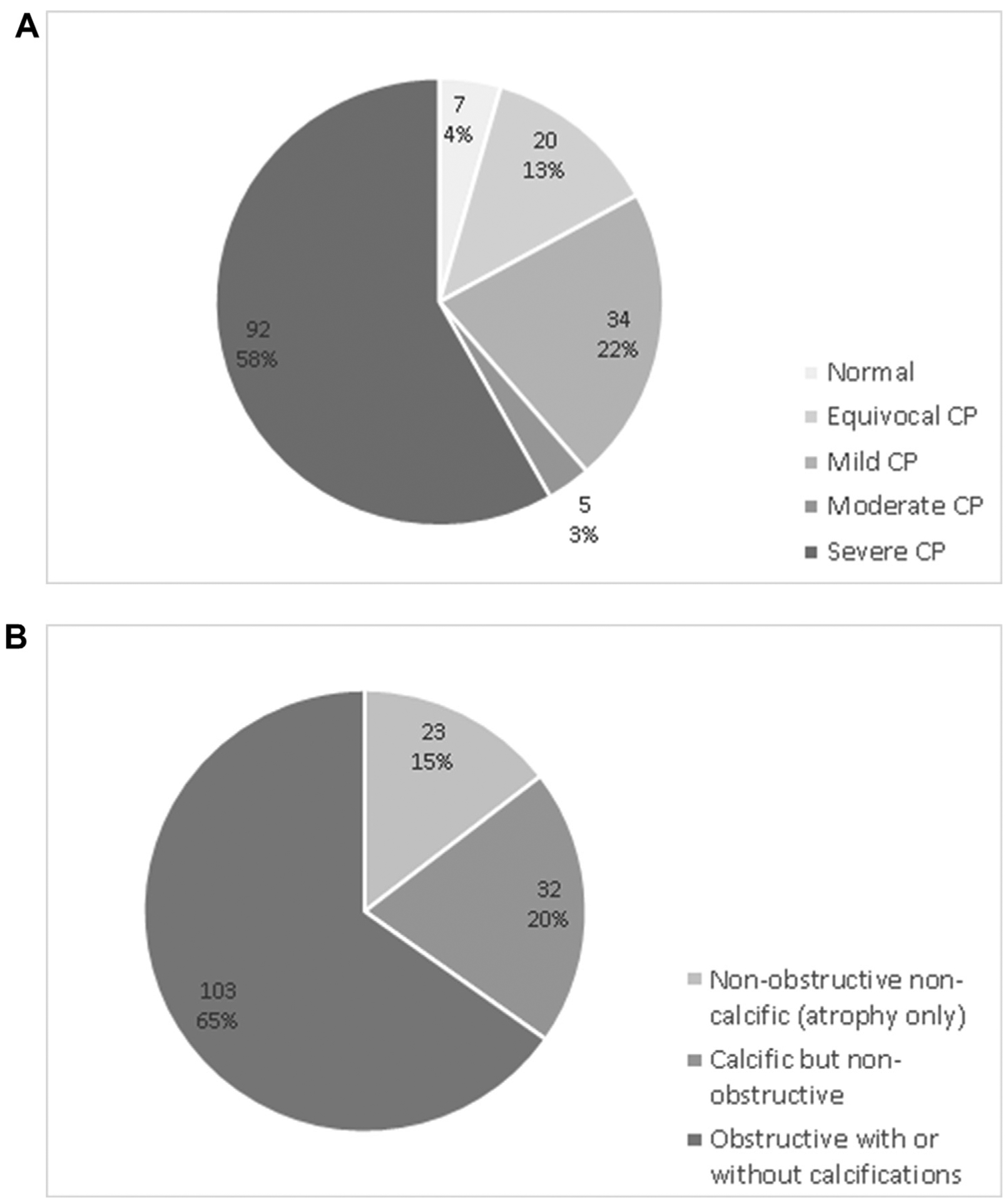
Distribution of morphological severity (4a) and morphologic categories (4b) in patients with Chronic Pancreatitis (CP).
Correlation of imaging findings with pain, endocrine and exocrine insufficiency
Correlation between pain, exocrine and endocrine insufficiency with CT findings is shown in Table 4. The prevalence of pain did not statistically differ based on severity or morphological subtype of CP. Interestingly, the prevalence of pain was somewhat lower in the presence of severe atrophy (71% when compared with 84–89% in those who had no or mild to moderate atrophy).
Table 4.
Prevalence of abdominal pain, endocrine insufficiency and exocrine insufficiency based on severity, pancreatic parenchymal atrophy and morphologic categories in patients with CP.
| Prevalence of pain | Endocrine insufficiency | Exocrine insufficiency | ||
|---|---|---|---|---|
| Severity of CP | Normal | 83% (5/6) | 33% (2/6) | 17% (1/6) |
| Equivocal | 85% (17/20) | 15% (3/20) | 45% (9/20) | |
| Mild | 91% (31/34) | 35% (12/34) | 29% (10/34) | |
| Moderate | 100% (5/5) | 40% (2/5) | 40% (2/5) | |
| Severe | 84% (77/92) | 38% (35/92) | 37% (34/92) | |
| Severity of pancreatic atrophy | None | 84% (26/31) | 32% (10/31) | 23% (7/31) |
| Mild | 89% (54/61) | 25% (15/61) | 26% (16/61) | |
| Moderate | 89% (42/47) | 38% (18/47) | 47% (22/47) | |
| Severe | 71% (12/17) | 59% (10/17) | 59% (10/17) | |
| Morphological categories of CP | Non-obstructive non-calcific | 82% (18/22) | 23% (5/22) | 41% (9/22) |
| Calcific but non-obstructive | 91% (29/32) | 38% (12/32) | 34% (11/32) | |
| Obstructive CP with or without calcifications | 85% (88/103) | 36% (37/103) | 35% (36/103) |
Endocrine insufficiency did not statistically differ based on morphological severity of CP. The prevalence of endocrine insufficiency was greater in the presence of severe pancreatic atrophy (59%) when compared with patients who had no or only mild-moderate atrophy (31%) (chi-square p = 0.06, trend test p = 0.03). Endocrine insufficiency was infrequent in non-obstructive non-calcific CP (22%) but increased to 38% in those with calcific non-obstructive CP and 36% with obstructive CP.
The prevalence of exocrine insufficiency was infrequent (17%) in patients without any morphological changes of CP when compared to patients with more severe CP (29%–45%), but such a relationship was not statistically significant (chi-square p = 0.09). Exocrine insufficiency was more frequent in patients with severe pancreatic atrophy (59%) when compared with those with no, mild, or moderate atrophy (32%) (chi-square p = 0.01, trend test p = 0.001). The prevalence of exocrine insufficiency was similar in the three morphological subtypes of CP.
Discussion
In this manuscript, we propose modifications to the Cambridge classification for CT scan to make it more relevant for adoption into clinical practice and research based on a systematic review of over 150 CT scans in a well-characterized cohort of patients with CP. We correlate findings on morphological severity and morphologic patterns with clinical symptoms and functional derangements of CP. These data formed the basis of the recently proposed reporting standards for CP on cross-sectional imaging by the CPDPC [30] which was validated by Razek et al. [31].
CP has varied appearances on CT ranging from normal to severe changes including marked pancreatic ductal dilation/irregularity, pancreatic calcifications and atrophy. However, the lack of an accepted reporting standard affects clinicians’ ability to accurately assess current stage of disease and temporal changes, or to compare results across research studies. We chose CT in this study since it is the most common cross-sectional modality used in clinical practice and has high accuracy in detecting key parenchymal and ductal features of CP [20,32–34]. Standards for reporting of MRI and MRCP were also included in the recent CPDPC report which will need further study.
As noted previously, Cambridge classification when adopted for CT scan, has several limitations. In the modifications, we excluded subjective and vaguely defined features such as gland heterogeneity and irregular head/body contour. We include ductal as well as parenchymal features (including pancreatic atrophy) and assign a severity score to each category. Furthermore, a combination of ductal and parenchymal findings is used to define morphological severity (equivocal, mild, moderate and severe) and morphological subtypes (non-calcific and non-obstructive, calcific but non-obstructive, obstructive with or without calcifications). This allows for a more objective stratification of the radiological stage of the disease and provides an opportunity to assess longitudinal changes in individual patients. However, the radiological features of CP may not correlate with clinical or functional parameters of CP. Our approach to scoring severity of imaging features of CP is also congruent with the recently published international guidelines for imaging and severity scoring of CP [35].
About two-thirds of patients (61%) were classified as having morphologically moderate-severe CP. In the remaining patients, a firm diagnosis of CP for inclusion in the NAPS2 study was established on other studies (such as MRI/MRCP, ERCP, EUS or histology). Interestingly, the proportion of patients with obstructive morphology (about two-thirds) in our analysis is much greater than our previous report [18]. This discrepancy is explained by the lack of systematic review of cross-sectional imaging studies by a dedicated on-site radiologist as part of the NAPS2 protocol. However, the primary finding of that study, i.e. poor correlation of pain symptoms with imaging findings was replicated in our current study. It should be noted that proportion of patients in different morphological severity categories seen in our cohort may not be generalizable and could potentially vary in based on etiology, duration of disease and patient demographics such as race, ethnicity and gender. The severity scoring for CP should be used with caution in the elderly population as ductal dilation and irregularity as well as pancreatic calcifications and volume loss may be seen in this age group without CP [36,37].
Presence and distribution of parenchymal and ductal findings allow for generation of a CT score (range 0–20). Though arbitrary, the scoring cut-offs were chosen to allow for increasing score, proportionate to worsening features of CP. For example, if 3 punctate pancreatic calcifications are present, a score of 1 is assigned, but presence of 3 coarse calcifications would correspond to a score of 2. Generally, higher aggregate CT scores correlated with the morphological severity of CP but is also important to note that a low score may not always indicate less severity. For example, in absence of ductal dilation or parenchymal atrophy, severe CP can be present in a patient with 7 or more coarse focal parenchymal calcifications involving less than 30% of pancreas with an aggregate score of only 3. Thus, presence of individual finding(s) and the aggregate CT score provide an opportunity to provide cross-sectional information as well as evolution of changes during longitudinal follow-up in individual patients.
We correlated patient’s self-reported pain experience, and physician-reported diagnosis of endocrine and exocrine insufficiency with findings on CT scan. Similar to previous reports, there was poor correlation between presence, severity and temporal nature of pain with severity of CP [18]. Inflammatory mass in the pancreatic head, which has been shown to be associated with pain in some studies, was seen infrequently in our cohort [38]. In general, there was a positive relationship between the presence of endocrine and exocrine insufficiency with increasing pancreatic atrophy, but not with disease severity. These data are consistent with published literature that although there is progressive increase in functional consequences of pancreatic destruction, a subset of patients may have preserved pancreatic function even after significant loss of pancreatic parenchyma [39].
Our proposed scoring system is not without limitations. Assessment of PD contour on CT scan is inherently subjective. However, it merits inclusion in grading CP as PD contour irregularity is a cardinal sign of CP. Obtaining reproducible thickness of pancreatic body is technically challenging. But variability can be minimized by consistently measuring along left lateral border of adjacent vertebral body. CT scans of patients were performed in different hospitals and hence, the technique is not uniform. However, only CECT scans were included in the study and evaluation of major pancreatic features assessed in this study was not affected. Although our analysis included a limited number of patients from a single center and we did not include traditional “gold standard” pathologic features of pancreatic biopsy or surrogate markers of ERCP to confirm the identity of morphologic findings on CT scans, all patients had clinical features of CP and detailed phenotyping by expert physicians at a tertiary care center with a specific interest in the management of patients with pancreatic diseases. The inclusion-exclusion criteria of NAPS2 studies did not consider patients with atrophy or minimal change pancreatitis that could be considered CP using the new Mechanistic Definition of CP [1]. We are confident that all patients that were enrolled had CP but recognize that some patients with early CP or atypical presentations or features would have been excluded. A control group of patients without chronic pancreatitis was not included in this study but we plan to evaluate such a cohort in future. Poor sensitivity of CT for detecting ductal irregularity and strictures is a limitation that can affect both the severity scoring and morphological severity grading of CP. However, these features have been included as they are an important component of CP and when evident, can contribute to the diagnosis.
In conclusion, systematic review and reporting of ductal and parenchymal features of CP on CT scan is feasible. We propose a CT scoring system for CP based on presence and distribution of pancreatic ductal and parenchymal features, and stratification of CP into morphological severity grades and morphological subtypes. The simplicity of the scoring system and near universal availability of CT provides an opportunity for eventual adoption into clinical practice for initial evaluation and longitudinal follow up of patients with CP. Future studies should empirically evaluate the proposed system in patients with different stages of CP, to evaluate the utility in the longitudinal follow-up of patients, and determine modifications to the interpretation or scoring of individual findings or their weighting to provide a more robust assessment.
Supplementary Material
Acknowledgements
We would like to acknowledge Dr. Temel Tirkes for reviewing the manuscript and offering his feedback.
Research reported in this publication was supported by the National Cancer Institute (NCI) and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under award number: U01DK108306 (DCW, DY), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under award numbers: NIH DK061451 (DCW), DK077906 (DY). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
Adam Slivka receives research funding from Boston Scientific. Georgios I Papachristou receives research funding from Abbvie and is a consultant with GlaxoSmithKline. David C Whitcomb is a consultant for Abbott, Regeneron and Ariel Precision Medicine, is a cofounder of Ariel Precision Medicine and has equity in Ariel Precision Medicine.
Financial support
No grants were received for this study.
Footnotes
Declaration of competing interest
None.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.pan.2019.09.013.
References
- [1].Whitcomb DC, Frulloni L, Garg P, Greer JB, Schneider A, Yadav D, et al. Chronic pancreatitis: an international draft consensus proposal for a new mechanistic definition. Pancreatology 2016;16(2):218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Amann ST, Yadav D, Barmada MM, O’Connell M, Kennard ED, Anderson M, et al. Physical and mental quality of life (QOL) in chronic pancreatitis (CP): a case-control study from the NAPS2 cohort. Pancreas 2013;42(2):293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013;144(6):1252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kim DH, Pickhardt PJ. Radiologic assessment of acute and chronic pancreatitis. Surg Clin N Am 2007;87(6):1341–58. [DOI] [PubMed] [Google Scholar]
- [5].Perez-Johnston R, Sainani NI, Sahani DV. Imaging of chronic pancreatitis (including groove and autoimmune pancreatitis). Radiol Clin N Am 2012;50(3):447–66. [DOI] [PubMed] [Google Scholar]
- [6].Sarner M, Cotton P. Classification of pancreatitis. Gut 1984;25(7):756–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schneider A, Löhr JM, Singer MV. The M-ANNHEIM classification of chronic pancreatitis: introduction of a unifying classification system based on a review of previous classifications of the disease. J Gastroenterol 2007;42(2):101–19. [DOI] [PubMed] [Google Scholar]
- [8].Conwell DL, Lee LS, Yadav D, Longnecker DS, Miller FH, Mortele KJ, et al. American pancreatic association practice guidelines in chronic pancreatitis: evidence-based report on diagnostic guidelines. Pancreas 2014;43(8):1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Heyn C, Sue-Chue-Lam D, Jhaveri K, Haider MA. MRI of the pancreas: problem solving tool. J Magn Reson Imaging 2012;36(5):1037–51. [DOI] [PubMed] [Google Scholar]
- [10].Tirkes T, Menias CO, Sandrasegaran K. MR imaging techniques for pancreas. Radiol Clin N Am 2012;50(3):379–93. [DOI] [PubMed] [Google Scholar]
- [11].Issa Y, Kempeneers M, van Santvoort H, Bollen T, Bipat S, Boermeester M. Diagnostic performance of imaging modalities in chronic pancreatitis: a systematic review and meta-analysis. Eur Radiol 2017:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tirkes T, Sandrasegaran K, Sanyal R, Sherman S, Schmidt CM, Cote GA, et al. Secretin-enhanced MR cholangiopancreatography: spectrum of findings. RadioGraphics 2013;33(7):1889–906. [DOI] [PubMed] [Google Scholar]
- [13].Fukukura Y, Fujiyoshi F, Sasaki M, Nakajo M. Pancreatic duct: morphologic evaluation with MR cholangiopancreatography after secretin stimulation 1. Radiology 2002;222(3):674–80. [DOI] [PubMed] [Google Scholar]
- [14].Balci NC, Smith A, Momtahen AJ, Alkaade S, Fattahi R, Tariq S, et al. MRI and SMRCP findings in patients with suspected chronic pancreatitis: correlation with endoscopic pancreatic function testing (ePFT). J Magn Reson Imaging 2010;31(3):601–6. [DOI] [PubMed] [Google Scholar]
- [15].Tirkes T, Shah ZK, Takahashi N, Grajo JR, Chang ST, Venkatesh SK, et al. Reporting standards for chronic pancreatitis by using CT, MRI, and MR cholangiopancreatography: the Consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. Radiology 2019;290(1):207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Whitcomb DC, Yadav D, Adam S, Hawes RH, Brand RE, Anderson MA, et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2). Pancreatology 2008;8(4–5):520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wilcox CM, Sandhu BS, Singh V, Gelrud A, Abberbock JN, Sherman S, et al. Racial differences in the clinical profile, causes, and outcome of chronic pancreatitis. Am J Gastroenterol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wilcox CM, Yadav D, Ye T, Gardner TB, Gelrud A, Sandhu BS, et al. Chronic pancreatitis pain pattern and severity are independent of abdominal imaging findings. Clin Gastroenterol Hepatol 2015;13(3):552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ladas S, Tassios P, Giorgiotis K, Rokkas T, Theodosiou P, Raptis S. Pancreatic duct width: its significance as a diagnostic criterion for pancreatic disease. Hepato-Gastroenterology 1993;40(1):52–5. [PubMed] [Google Scholar]
- [20].Luetmer PH, Stephens DH, Ward EM. Chronic pancreatitis: reassessment with current CT. Radiology 1989;171(2):353–7. [DOI] [PubMed] [Google Scholar]
- [21].Edge MD, Hoteit M, Patel AP, Wang X, Baumgarten DA, Cai Q. Clinical significance of main pancreatic duct dilation on computed tomography: single and double duct dilation. World J Gastroenterol: WJG 2007;13(11):1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mortelé KJ, Rocha TC, Streeter JL, Taylor AJ. Multimodality imaging of pancreatic and biliary congenital anomalies. RadioGraphics 2006;26(3): 715–31. [DOI] [PubMed] [Google Scholar]
- [23].Goda K, Sasaki E, Nagata K, Fukai M, Ohsawa N, Hahafusa T. Pancreatic volume in type 1 und type 2 diabetes mellitus. Acta Diabetol 2001;38(3):145–9. [DOI] [PubMed] [Google Scholar]
- [24].Klöppel G, Maillet B. Chronic pancreatitis: evolution of the disease. Hepato-Gastroenterology 1991;38(5):408–12. [PubMed] [Google Scholar]
- [25].Heuck A, Maubach PA, Reiser M, Feuerbach S, Allgayer B, Lukas P, et al. Age-related morphology of the normal pancreas on computed tomography. Gastrointest Radiol 1987;12(1):18–22. [DOI] [PubMed] [Google Scholar]
- [26].Lesniak RJ, Hohenwalter MD, Taylor AJ. Spectrum of causes of pancreatic calcifications. Am J Roentgenol 2002;178(1):79–86. [DOI] [PubMed] [Google Scholar]
- [27].Scuro LA, Cavallini G, Benini L, Brocco G, Bovo P, Riela A, et al. Pancreatic calcifications in patients with chronic pancreatitis. Int J Pancreatol 1990;6(2): 139–50. [PubMed] [Google Scholar]
- [28].Andersen PL, Madzak A, Olesen SS, Drewes AM, Frøkjaer JB. Quantification of parenchymal calcifications in chronic pancreatitis: relation to atrophy, ductal changes, fibrosis and clinical parameters. Scand J Gastroenterol 2017:1–7. [DOI] [PubMed] [Google Scholar]
- [29].Stevens T, Conwell DL, Zuccaro G. Pathogenesis of chronic pancreatitis: an evidence-based review of past theories and recent developments. Am J Gastroenterol 2004;99(11):2256–70. [DOI] [PubMed] [Google Scholar]
- [30].Tirkes T, Shah ZK, Takahashi N, Grajo JR, Chang ST, Venkatesh SK, et al. Reporting standards for chronic pancreatitis by using CT, MRI, and MR cholangiopancreatography: the Consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. Radiology 2018:181353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Razek AAKA, Elfar E, Abubacker S. Interobserver agreement of computed tomography reporting standards for chronic pancreatitis. Abdominal Radiology 2019:1–7. [DOI] [PubMed] [Google Scholar]
- [32].Ferrucci JT Jr, Wittenberg J, Black EB, Kirkpatrick RH, Hall DA. Computed body tomography in chronic pancreatitis. Radiology 1979;130(1):175–82. [DOI] [PubMed] [Google Scholar]
- [33].Anderson SW, Soto JA. Pancreatic duct evaluation: accuracy of portal venous phase 64 MDCT. Abdom Imag 2009;34(1):55. [DOI] [PubMed] [Google Scholar]
- [34].Arikawa S, Uchida M, Kunou Y, Kaida H, Uozumi J, Hayabuchi N, et al. Assessment of chronic pancreatitis: use of whole pancreas perfusion with 256-slice computed tomography. Pancreas 2012;41(4):535–40. [DOI] [PubMed] [Google Scholar]
- [35].Frøkjær JB, Akisik F, Farooq A, Akpinar B, Dasyam A, Drewes AM, et al. Guidelines for the diagnostic cross sectional imaging and severity scoring of chronic pancreatitis. Pancreatology 2018;18(7):764–73. [DOI] [PubMed] [Google Scholar]
- [36].Ross SO, Forsmark CE. Pancreatic and biliary disorders in the elderly. Gastroenterol Clin 2001;30(2):531–45. [DOI] [PubMed] [Google Scholar]
- [37].Sato T, Ito K, Tamada T, Sone T, Noda Y, Higaki A, et al. Age-related changes in normal adult pancreas: MR imaging evaluation. Eur J Radiol 2012;81(9): 2093–8. [DOI] [PubMed] [Google Scholar]
- [38].Bachmann K, Melling N, Groteluschen R, Fleischauer A, Reeh M, Ghadban T, et al. Morphologic factors predict pain relief following pancreatic head resection in chronic pancreatitis description of the chronic pancreatitis pain relief (CPPR) score. Ann Surg 2019. [DOI] [PubMed] [Google Scholar]
- [39].Sainani NI, Kadiyala V, Mortele K, Lee L, Suleiman S, Rosenblum J, et al. Evaluation of qualitative magnetic resonance imaging features for diagnosis of chronic pancreatitis. Pancreas 2015;44(8):1280–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


