Abstract
Background:
Cycloserine, or its structural analogue terizidone, has been associated with neuropsychiatric toxicity (psychosis, depression, and neuropathy). Prospective clinical data on the incidence of and risk factors for neuropsychiatric toxicity in TB patients treated with cycloserine are limited.
Methods:
A prospective evaluation of neuropsychiatric toxicity was performed using validated screening tools in patients with multidrug-resistant tuberculosis treated with terizidone. Cox proportional hazard modelling was performed to explore the effects of clinical variables and measures of cycloserine pharmacokinetics in plasma.
Results:
A total 144 participants were recruited: 86 were male and 58 were female; their median age was 35.7 years and 91 (63%) were HIV-infected. Fifty-five (38%) participants developed at least one neuropsychiatric event (30 cases per 100 person-months): 50 (35%) neuropathy, 14 (10%) depression, and 11 (8%) psychosis. Neuropathy was independently associated with cycloserine clearance ((adjusted hazard ratio 0.34 (aHR), P = 0.03)) and high-dose pyridoxine (200 mg vs 150 mg daily, aHR: 2.79, P = 0.01).
Conclusions:
A high incidence of early neuropsychiatric toxicity was observed in this cohort of patients treated with terizidone. Cycloserine clearance and higher doses of pyridoxine are associated with incident or worsening peripheral neuropathy.
Keywords: Cycloserine, Terizidone, Pyridoxine, Pharmacokinetics, Neuropathy, Neuropsychiatric
Introduction
D-4-amino-3-isoxazolidinone, or D-cycloserine (cycloserine), was first discovered and synthesized by Hidy et al. almost 70 years ago (Hidy et al., 1955). Neuropsychiatric toxicity, including depression and psychosis, was first reported with the earliest known use of cycloserine and in subsequent early treatment reports (Kendig et al., 1956; Murray, 1956). Cycloserine-associated peripheral neuropathy has been reported less frequently (Kendig et al., 1956; Desmeules et al., 1957; Conradie et al., 2014). Neuropsychiatric side-effects led to patients and pharmacologists giving cycloserine the moniker ‘psych-serine’ (Gumbo, 2018). The popular press has called cycloserine a “cure that also kills” (Emily Wise, 2013). Historically, this has impeded widespread inclusion of cycloserine, and its structural analogue terizidone, in treatment regimens for drug-resistant tuberculosis (TB) (Hwang et al., 2013). The World Health Organization (WHO) has recently included cycloserine or terizidone as a group B drug for long multidrug-resistant tuberculosis (MDR-TB) treatment regimens (WHO, 2019), after an individual participant data meta-analysis showed cycloserine to be more efficacious than some commonly used anti-TB drugs, including kanamycin and ethionamide (Ahmad et al., 2018). Terizidone, which is hydrolyzed pre-systematically to two molecules of cycloserine (Court et al., 2018; WHO, 2015), was previously considered to be associated with less neurotoxicity than cycloserine, but a recent review demonstrated no significant safety difference between the two drugs (Hwang et al., 2013).
There are limited systematically collected prospective data describing neuropsychiatric toxicity, including peripheral neuropathy, in patients treated for MDR-TB. Further, there are no existing data describing the association of cycloserine concentrations with incident or worsening neuropsychiatric events in TB patients. Studies reporting the effect of cycloserine concentrations on both microbial kill and resistance suppression have recently been published, including penetration of the drug into TB cavities and resistance arising therein (Deshpande et al., 2018; Dheda et al., 2018). Defining the relationship between cycloserine concentrations and neuropsychiatric toxicity, identifying exposure thresholds associated with specific neuropsychiatric events, and then comparing these thresholds to those associated with microbial kill and resistance suppression, will significantly contribute to dose optimization in the management of patients treated for MDR-TB.
A prospective observational study was conducted amongst hospitalized patients treated with a terizidone-containing regimen for rifampicin-resistant TB or MDR-TB, to determine risk factors, including cycloserine pharmacokinetic parameters, for neuropsychiatric toxicity. Serial measurements were performed using standardized tools to detect treatment-emergent (or worsening on treatment) psychiatric disorders and peripheral neuropathy.
Methods
Study design and patient recruitment
A prospective observational study was performed in patients treated for MDR-TB at Brooklyn Chest Hospital and DP Marais Hospital in Cape Town. Patients who were diagnosed with pulmonary rifampicin-resistant TB or MDR-TB were recruited between July 2015 and September 2017. Inclusion criteria included adults ≥18 years of age with confirmed pulmonary MDR-TB, initiated on standard MDR-TB treatment within the previous month. Critically ill patients and those unable to provide informed consent were excluded.
Treatment administered
During the study period, the standard regimen for MDR-TB in South Africa consisted of pyrazinamide, moxifloxacin, kanamycin, terizidone, and either ethionamide or isoniazid (depending on the presence of katG and inhA mutations identified by line-probe assay in the pre-treatment sputum culture, indicating high-level resistance to isoniazid or low-level resistance to isoniazid and resistance to ethionamide, respectively) (Caminero et al., 2010). Ethambutol was added if the risk of ethambutol resistance was considered low. High-dose pyridoxine (150 or 200 mg daily) was included as prophylaxis for terizidone-related pyridoxine deficiency, and the dose of terizidone was adjusted for weight as per national guidelines during the study period (Anon, 2021a). The dose of terizidone administered to participants is the same terizidone dose currently recommended by the WHO (WHO, 2019). Dosing was adjusted for renal dysfunction at the discretion of the treating physician.
Clinical follow-up for adverse events monitoring
Neuropsychiatric toxicity was evaluated at recruitment and monthly to 12 weeks using validated tools. The Brief Peripheral Neuropathy Rating Screen (BPNS) for peripheral neuropathy (Mehta et al., 2010) was used to rate neuropathic symptoms (pain, paresthesia, and numbness) on a numeral rating scale from 0 to 11 points. Neuropathy (incident or worsening) was defined as an increase in BPNS symptom score after recruitment of ≥2 points for pain, numbness, or pins and needles (Mehta et al., 2010). A minimum of two serial neuropathy assessments per participant were therefore required for inclusion in the analysis. The BPNS objective clinical scores (i.e., ankle jerks and vibration sense) were not included in the analysis, as these assessments could not be reliably standardized due to a high turnover of study staff during the recruitment period.
The Kessler 10 scale (K10) and the Brief Psychiatric Rating Scale (BPRS) tool were also administered at recruitment and monthly to 12 weeks to screen for depression and psychosis, respectively (Andersen et al., 2011; Anon, 2021b). Participants who had a K10 or BPRS score on treatment of ≥20 or ≥32 were defined as having probable depression or psychosis, respectively; these cut-offs have previously been validated as predictive of these psychiatric events (Kessler, 2021; Etschel et al., 2005). Participants who had K10 and BPRS scores above the identified threshold at the time of recruitment were considered to have treatment-related depression or psychosis if their score had increased by ≥1 point on treatment. Where appropriate, adverse event severity was graded according to the Division of AIDS Classification (Division of AIDS, 2017).
Pharmacokinetics
Blood was drawn after a minimum of 1 week of therapy at six time-points over 10 h (pre-dose, and 2, 4, 6, 8, and 10 h post-dose). A subset of patients had three additional blood samples drawn at 12, 24, and 26 h post-dose. An additional subset of patients had two pharmacokinetic sampling occasions. Liquid chromatography tandem mass spectrometry was used to obtain the cycloserine concentrations in plasma using a validated assay at the Division of Clinical Pharmacology, University of Cape Town (Court et al., 2018). The lower limit of quantification for plasma cycloserine was 0.313 μg/mL and the top of the validated range was 40.0 μg/mL (Court et al., 2018). Cycloserine concentration–time data were interpreted using non-linear mixed-effects modelling, as described previously for this cohort dosed with terizidone (Chirehwa et al., 2020). The final model was used to generate the steady-state 24-h cycloserine area under the concentration time curve (AUC0–24), trough concentration (C24), peak concentration (Cmax), and clearance.
Statistical analyses
Stata v.15 (StataCorp, TX, USA) was used to perform the statistical analysis. Factors associated with key neuropsychiatric adverse events were explored, including psychosis and/or depression and/or peripheral neuropathy, using a Cox proportional hazards regression model. The following potential covariates were selected a priori and evaluated in univariate models: sex, age, HIV status, previous exposure to anti-TB drugs, history of alcohol and recreational drug use respectively, and key cycloserine measures including AUC0–24, Cmax, Cmax >35 μg/mL, C24, and drug clearance. For the analysis of peripheral neuropathy, the following additional factors were included, which were also identified a priori as potential causes of, or predisposing factors for neuropathy: height, presence of diabetes, pyridoxine dose (150 vs 200 mg daily), and history of isoniazid and/or ethionamide use since treatment initiation of the current MDR-TB episode. Isoniazid and efavirenz use were added in the models exploring factors associated with psychosis and/or depression. Covariates with a P-value of <0.2 in the univariate analysis were included in multivariate analyses for psychosis, depression, and neuropathy, respectively, and combined events, i.e., any psychiatric event (depression and/or psychosis) or any neuropsychiatric event (depression and/or psychosis and/or neuropathy). If more than one cycloserine pharmacokinetic measure had a P-value of <0.2 on univariate analysis, the pharmacokinetic measure with the strongest univariate association was included in the multivariate analysis. Incident or worsening neuropathy and depression and/or psychosis were evaluated over time using Kaplan–Meier failure analyses, and the two-sample Wilcoxon rank sum (Mann–Whitney) test was used to compare cycloserine AUC0–24, Cmax, C24, and clearance between participants who developed new or worsening peripheral neuropathy and those who did not. A P-value of <0.05 was considered as significant.
Results
The clinical characteristics of the 144 participants recruited into the study are shown in Table 1. Cycloserine pharmacokinetic data were available for 132 (92%) participants, of whom 20 had two pharmacokinetic sampling occasions and eight had three additional blood draws to 26 h post-dose. For one participant, only the pre-dose cycloserine concentration was available. The pharmacokinetic results have been reported before (Chirehwa et al., 2020). A one-compartment disposition model with absorption described by a transit compartment model and first-order elimination described the data well. Elimination of cycloserine was described using two pathways: renal and non-renal (Chirehwa et al., 2020). The renal pathway was driven by creatinine clearance, while the non-renal pathway included the effects of fat-free mass and smoking status. Other pharmacokinetic covariates included the effects of tablet crushing on the duration of absorption delay and fat-free mass on the volume of distribution (included via allometric scaling). Clearance varied between individuals, while bioavailability, absorption rate constant, and transit time varied between occasions.
Table 1.
Clinical and demographic characteristics of 144 patients on treatment with terizidone for multidrug-resistant tuberculosis.
| Characteristic | Number (%) or median (IQR) |
|---|---|
| Number | 144 |
| Sex | |
| Male | 86 (59.7%) |
| Female | 58 (40.3%) |
| Age, years | 35.7 (29.7–43.8) |
| BMI, kg/m2 | 17.2 (15.6–18.9) |
| HIV status | |
| Positive | 91 (63.2%) |
| Negative | 52 (36.1%) |
| Unknown | 1 (0.7%) |
| Diabetes | 10 (6.9%) |
| Terizidone dose | |
| 750 mg | 108 (81.8%) |
| 500 mg | 22 (16.7%) |
| 250 mg | 2 (1.5%) |
| Pyridoxine dose | |
| 200 mg | 12 (8.3%) |
| 150 mg | 121 (84.0%) |
| Unknown | 11 (7.6%) |
| Creatinine clearance, ml/min | 99.2 (78.8–119.6) |
| Alcohol | |
| Use prior to recruitment | 98 (68.1%) |
| Never used | 46 (31.9%) |
| Recreational drugs | |
| Use prior to recruitment | 74 (51.4%) |
| Never used | 70 (48.6%) |
| Cycloserine AUC0–24, μg/mL | 597.2 (425.7–762.7) |
| Cycloserine Cmax, μg/mL | 33.5 (24.6–40.4) |
| Cmax >35 μg/mL | 67(50.8%) |
| Cmax ≤35 μg/mL | 65 (49.2%) |
| Cycloserine trough concentration (C24), μg/mL | 16.8 (11.0–24.2) |
| Cycloserine clearance, l/h | 0.8 (0.6–1.1) |
IQR, interquartile range; BMI, body mass index.
The classification of key adverse events is shown in Table 2. Fifty-five of the 144 participants (38.2%) developed at least one new or worsening neuropsychiatric event (30 cases per 100 person-months), with peripheral neuropathy being the major contributor, affecting 50 (34.7%) participants (25 cases per 100 person-months). As a change in the BPNS score was used to define treatment-related peripheral neuropathy, it was not possible to grade neuropathy severity using the Division of AIDS Classification instrument (Division of AIDS, 2017). Fourteen participants who had only one neuropathy assessment at recruitment were excluded from the neuropathy time-to-event analysis. The median time to neuropathy was 42.5 days (interquartile range (IQR) 21–63 days); in comparison, the median time to depression and/or psychosis was 49.5 days (IQR 14–63 days). The median time to neuropathy in participants who were HIV-positive was 42 days (IQR 21–62 days) vs 48 days (21–70 days) in those who were HIV-negative.
Table 2.
Number of participants with neuropsychiatric adverse events out of 144 patients on treatment with terizidone.
| Any grade | Grade 3 or higher | |
|---|---|---|
| Psychosisa | 11 (7.6%) | 0 |
| Depressionb | 14 (9.7%) | 0 |
| Any neuropsychiatric event (depression or psychosis) | 21 (14.6%) | 0 |
| Peripheral neuropathyc | 50 (34.7%) | NA |
| Seizures | 3 (2.1%) | 1 |
NA, Not applicable Grading by Division of AIDS classification unless otherwise indicated.
Psychosis: Brief Psychiatric Rating Scale (BPRS) score ≥32 = any grade; score ≥55 = grade 3 or higher.
Depression: Kessler 10 scale (K10) score >20 = any grade; score >29 = grade 3 or higher.
Peripheral neuropathy: ≥2 symptom point increase on the Brief Peripheral Neuropathy Rating Screen (BPNS).
Figure 1 shows the time in days to neuropathy stratified by HIV status and Table 3 reports the covariates associated with neuropathy. Figure 2 shows the time to neuropathy in participants who were dosed with 200 mg pyridoxine daily as prophylaxis versus those who received 150 mg daily. The median time to neuropathy in participants dosed with pyridoxine 200 mg daily was 38.5 days (IQR 21–55.5 days) versus 43 days (IQR 22–63 days) in participants dosed with 150 mg daily. Table 3 shows that increasing cycloserine AUC0–24 was associated with the development of incident/worsening neuropathy in the univariate analysis, as were Cmax >35 μg/l, trough concentration, and total clearance. The significance of the association of cycloserine clearance with neuropathy remained significant (adjusted hazard ratio 0.31, P = 0.026) after adjusting for the effect of HIV and age. Pyridoxine dose was associated with incident or worsening neuropathy in both univariate and multivariate analyses (see Table 3). An analysis was performed to determine whether any of the covariates included in the final multivariate model modified the effect of cycloserine clearance on peripheral neuropathy, but no such modification was found. A comparison of the key cycloserine pharmacokinetic parameters in those who developed peripheral neuropathy versus those who did not is shown in Table 4.
Figure 1.
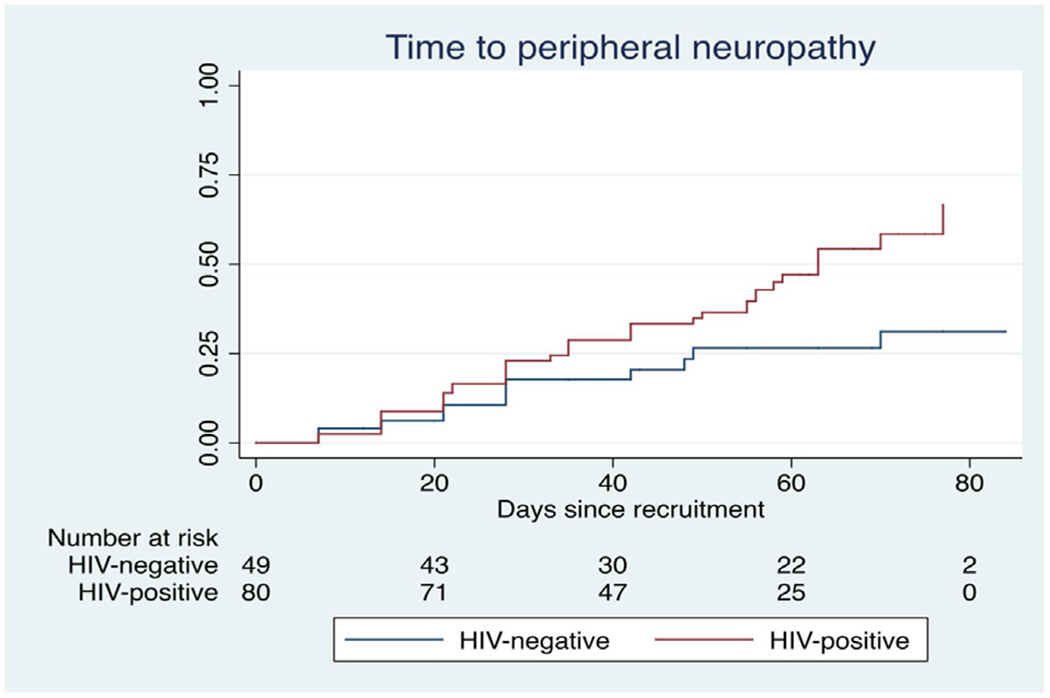
Time to incident or worsening peripheral neuropathy stratified by HIV status in patients treated with terizidone for multidrug-resistant tuberculosis.
Table 3.
Covariates associated with incident or worsening peripheral neuropathy in patients treated with terizidone for multidrug-resistant tuberculosis.
| HR (95% CI) | P-value | aHR (95% CI) | P-value | |
|---|---|---|---|---|
| HIV | 2.12 (1.10–4.07) | 0.025 | 1.67 (0.81–3.44) | 0.161 |
| Sex (male vs female) | 1.03 (0.59–1.81) | 0.912 | ||
| Age (per 1-year increase) | 1.02 (1.00–1.05) | 0.110 | 1.03 (1.00–1.07) | 0.036 |
| Diabetes | 1.29 (0.47–3.60) | 0.621 | ||
| Height (per 1-cm increase) | 1.00 (0.97–1.03) | 0.949 | ||
| Previous TB treatment | 1.59 (0.77–3.27) | 0.210 | ||
| History of alcohol use | 1.50 (0.81–2.71) | 0.200 | ||
| History of recreational drug use | 0.70 (0.40–1.23) | 0.217 | ||
| Ethionamide use, n = 120 | 1.41 (0.64–3.15) | 0.395 | ||
| Isoniazid use, n = 121 | 0.69 (0.33–1.41) | 0.305 | ||
| Ethionamide and isoniazid use, n = 101 | 0.98 (0.54–1.80) | 0.955 | ||
| Cycloserine AUC0–24 (per 100-unit increase) | 1.08 (1.00–1.17) | 0.037 | ||
| Cycloserine Cmax | 1.02 (1.00–1.04) | 0.020 | ||
| Cmax >35 μg/mL | 1.89 (1.04–3.44) | 0.035 | ||
| Trough concentration (C24) | 1.02 (1.00–1.05) | 0.044 | ||
| Clearance, l/h | 0.24 (0.09–0.62) | 0.003 | 0.31 (0.11–0.87) | 0.026 |
| Pyridoxine dose | ||||
| 150 mg (Ref.) | ||||
| 200 mg | 2.80 (1.29–6.08) | 0.009 | 2.79 (1.26–6.20) | 0.012 |
| Unknown | 1.26 (0.45–3.54) | 0.658 | 1.92 (0.54–6.86) | 0.314 |
HR, hazard ratio; aHR, adjusted hazard ratio; CI, confidence interval.
Figure 2.
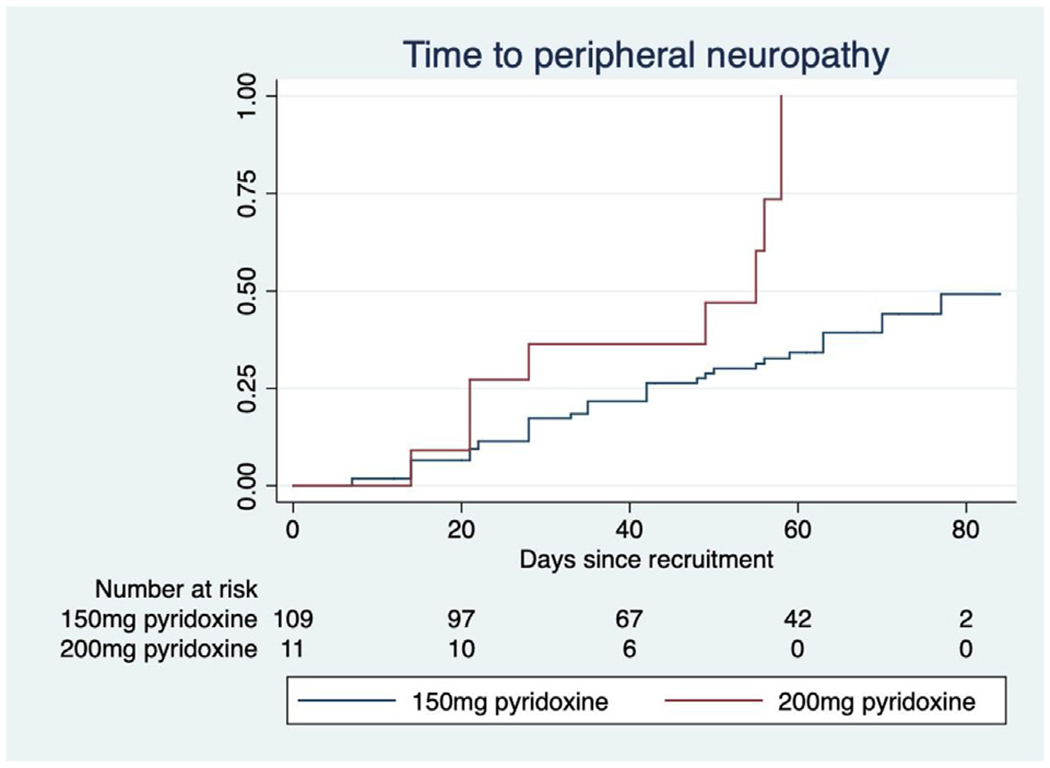
Time to incident or worsening peripheral neuropathy stratified by pyridoxine dose in patients treated with terizidone for multidrug-resistant tuberculosis.
Table 4.
Comparison of key pharmacokinetic measures in 132 participants with and without incident or worsening peripheral neuropathy treated with terizidone for multidrug-resistant tuberculosis.
| Neuropathy n = 47 |
No neuropathy n = 85 |
P-value | |
|---|---|---|---|
| AUC0–24, μg.ml/h | 651.7 (516.2–803.5) | 547.1 (395.3–726.0) | 0.010 |
| Cmax, μg/mL | 38.1 (32.5–50.9) | 33.5 (24.6–40.4) | 0.006 |
| C24, μg/mL | 20.0 (14.8–25.8) | 15.6 (9.7–22.8) | 0.009 |
| Clearance, l/h | 0.7 (0.5–0.9) | 0.9 (0.7–1.2) | 0.002 |
The median value is shown with the interquartile range in brackets.
Eleven of the 144 participants (7.6%) developed either incident or worsening psychosis (5 cases per 100 person-months) and 14 (9.7%) developed either incident or worsening depression (7 cases per 100 person-months). Altogether 21 (14.6%) of the 144 participants developed a psychiatric event (incident or worsening depression and/or incident or worsening psychosis), i.e. 10 cases per 100 person-months. Table 5 illustrates the relationship between the selected covariates and depression and/or psychosis, respectively. When exploring associations with individual psychiatric adverse events, none of the covariates explored, including HIV infection, age, and cycloserine measures, was associated with the incidence of new or worsening depression or psychosis.
Table 5.
Covariates associated with new or worsening depression and/or psychosis in 144 patients treated with terizidone for multidrug-resistant tuberculosis.
| HR (95% CI) | P-value | |
|---|---|---|
| Age | 1.02 (0.98–1.07) | 0.325 |
| Sex | 1.21 (0.48–3.07) | 0.692 |
| HIV | 1.09 (0.43–2.77) | 0.855 |
| Previous TB treatment | 0.78 (0.29–2.04) | 0.608 |
| History of alcohol use | 0.99 (0.38–2.63) | 0.999 |
| History of recreational drug use | 1.12 (0.46–2.76) | 0.804 |
| Isoniazid use | 0.78 (0.23–2.69) | 0.699 |
| Efavirenz use | 1.06 (0.43–2.65) | 0.895 |
| Cycloserine AUC0–24 (per 100 unit increase) | 0.86 (0.71–1.05) | 0.134 |
| Cycloserine Cmax | 0.97 (0.94–1.01) | 0.160 |
| Cmax >35 μg/mL | 1.00 (0.40–2.52) | 0.999 |
| Trough concentration (C24) | 0.96 (0.92–1.01) | 0.161 |
| Clearance, l/h | 1.22 (0.38–3.94) | 0.736 |
HR, hazard ratio; CI, confidence interval.
Discussion
High rates of different neuropsychiatric events were identified in participants treated with terizidone for MDR-TB. The neuropsychiatric incident or worsening rate of 30 cases per 100 person-months means that with the currently recommended dose of cycloserine, which is frequently co-administered with high-dose pyridoxine in MDR-TB treatment programmes to prevent neuropathy, a large proportion of patients will develop clinically important adverse events. The major contributor to the high rate of neuropsychiatric toxicity was neuropathy, with 25 cases per 100 person-months. Over a third of the participants in the study cohort developed new or worsening neuropathy during the first 12 weeks of MDR-TB treatment, which is higher than previously reported (Murray, 1956).
It was found that cycloserine exposure was associated with an 8% increase in the risk of developing peripheral neuropathy for each 100 μg.mL/h increase in cycloserine AUC0–24. Cycloserine Cmax and C24 were also significantly associated with neuropathy on univariate analysis, while cycloserine clearance was associated with neuropathy in both univariate and multivariate analyses. That cycloserine clearance retained its association in the multivariable model suggests that cycloserine pharmacokinetics are related to an increased risk of neuropathy independent of HIV and/or age, which might be associated with changes in bioavailability or distribution more than clearance. As all patients received high-dose pyridoxine, it was not possible to identify the risks of neuropathy associated with its use. However, patients prescribed the 200 mg daily dose had 2.78 times the risk of neuropathy compared to those on 150 mg daily (P = 0.012, in the adjusted analysis).
Cycloserine has infrequently been reported as a significant peripheral nerve toxin. Early cycloserine treatment reports either do not mention or report a low incidence of neuropathy (Kendig et al., 1956; Murray, 1956; Desmeules et al., 1957; Helmy, 1970; Storey and Mclean, 1957). More recently, cycloserine dosed as terizidone was shown to increase the incidence of neuropathy in MDR-TB patients, although the association was not statistically significant (Conradie et al., 2014). Conversely, reports of high-dose pyridoxine as a cause of peripheral neuropathy are emerging with increasing frequency. The effect appears to be dose-related, but the duration of treatment, even at lower doses, appears to be an important risk factor Dalton and Dalton, 1987; Ghavanini and Kimpinski, 2014; van Hunsel et al., 2018; Lheureux et al., 2005; Parry and Bredesen, 1985). It is plausible that the high dose of pyridoxine intended to prevent neuropathy, rather than cycloserine, is responsible for the high incidence of peripheral neuropathy observed. The highest pyridoxine dose may have been prescribed for patients at risk of neuropathy or for patients with established neuropathic pain at baseline, thereby explaining the association observed. The dose of pyridoxine required to prevent isoniazid-related neuropathy (6–50 mg/day) is significantly lower than the 150–200 mg routinely prescribed to prevent cycloserine-related neuropathy in the study cohort (Van Der Watt et al., 2011). High-dose pyridoxine (>25 mg/day) was not found to improve overall vitamin B6 status over the standard 25 mg/kg dosage in patients treated with isoniazid for drug-sensitive TB (Centner et al., 2014).
On univariate analysis, increasing age was significantly associated with neuropathy, and HIV infection doubled the hazard for neuropathy; both are well-established risk factors for neuropathy (Simpson et al., 2002; Mold et al., 2004). The exposure–toxicity relationship of cycloserine with neuropathy was enhanced by the finding of an association of cycloserine clearance with neuropathy, which was significant on both univariate and multivariate analysis. The relationship with clearance (which has renal and non-renal components) also suggests that procedures that improve cycloserine clearance, such as hemodialysis, could be explored for roles in managing severe neuropsychiatric adverse events. The management of new or worsening peripheral neuropathy in patients treated with cycloserine should also include optimization of the pyridoxine dose. Previous alcohol or recreational drug use was not found to be associated with neuropathy. Data were collected on alcohol use via patient self-report, specifically enquiring about the quantity of alcohol consumed in the months leading up to the MDR-TB diagnosis. Alcohol consumption quantified by patient self-report has been shown to underestimate alcohol intake (Feunekes et al., 1999); it is therefore possible that the lack of an observed association between alcohol use and neuropathy may have been due to under-reporting.
The mechanism of cycloserine-induced neuropathy is understood to be a combination of pyridoxine antagonism by cycloserine and increased renal elimination of pyridoxine (Donald, 2010). Supplemental pyridoxine is included in many programmatic MDR-TB treatment regimens. The finding that cycloserine clearance itself was associated with adverse events also suggests that direct accumulation of the drug could have a neurotoxic effect, independent of pyridoxine renal elimination. An analysis was also performed to determine whether the use of isoniazid and/or ethionamide, which cause neuropathy via a similar mechanism (Ghavanini and Kimpinski, 2014), were associated with incident neuropathy, but no such association was found.
It is currently unknown what threshold cycloserine concentration is associated with incident or worsening neuropsychiatric events in patients treated for MDR-TB. The typical peak concentration range of cycloserine in patients receiving a dose of 250 mg or 500 mg is 20–35 μg/mL (Alghamdi et al., 2019). An early study of cycloserine in the management of TB described psychotic symptoms in several patients with cycloserine concentrations >40 μg/mL (Holmes et al., 1959). In a more recent report, a case of psychosis in an MDR-TB patient treated with cycloserine was reported with cycloserine concentrations >35 μg/mL (Hung et al., 2014). In the present study, it was found that patients with a cycloserine Cmax >35 μg/mL were approximately twice as likely to develop peripheral neuropathy on univariate analysis (hazard ratio 1.89, 95% confidence interval 1.04–3.44; P = 0.035).
Psychosis and/or depression were not significantly associated with cycloserine exposure or any of the other covariates in this study. Although the study found a higher incidence of depression and psychosis than a pooled estimate of drug-related neuropsychiatric events in a recent review of neurotoxicity in patients treated with cycloserine or terizidone for MDR-TB (Hwang et al., 2013), the present study may have been underpowered to assess associations with cycloserine exposure. The relationship between cycloserine exposure and the incidence of any psychiatric adverse event was consequently explored by combining depression and psychosis, but no association was found (Table 5). No symptoms suggestive of possible psychosis or depression graded 3 or higher were observed (Table 2).
This study has several limitations. First, the participants in the cohort had multiple risk factors for neuropsychiatric toxicity (comorbidities, high-dose pyridoxine, and other drugs). Therefore, we cannot be certain that the neuropsychiatric toxicity observed was due to cycloserine exposure at all. Second, it was not possible to perform the neuropsychiatric assessments at treatment start, as most participants were referred to the TB hospitals from referral clinics or tertiary centers where MDR-TB treatment was initiated. Therefore, the onset of neuropathy and early psychiatric events occurring in the first weeks of treatment, before recruitment, may have been missed. The adverse event rate reported here is therefore likely to be an underestimate. Third, cycloserine exposure was assessed on one pharmacokinetic sampling occasion only, and therefore the possibility that changes in dose and/or exposure during the study period may have affected the observed incidence of neuropsychiatric toxicity cannot be excluded.
This study appears to be the first large prospective longitudinal study describing the association of cycloserine exposure with neuropsychiatric toxicity in patients treated for MDR-TB. The results of this study highlight the growing evidence that high-dose pyridoxine is toxic to peripheral nerves and, although the association should be confirmed, cycloserine should be considered as a cause of unexplained neuropathy in patients on treatment for MDR-TB. The relationship of neuropsychiatric adverse events with cycloserine concentrations and clearance provides clinicians with potential tools for use in managing patients with neuropsychiatric toxicity. Finally, the study findings support the role of therapeutic drug monitoring to lower cycloserine doses, and adjustment of dosing schedules for pyridoxine in patients treated for MDR-TB.
Acknowledgements
We would like to acknowledge the contributions of the patients who volunteered for the study.
Funding
This study was supported by a grant from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health(R01AI116155 to HM and TG). The University of Cape Town (UCT) Clinical Pharmacokinetic Laboratory is also supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health under award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) at UCT was provided by the National Institute of Allergy and Infectious Diseases (U01 AI068632), The Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Institute of Mental Health grant AI068632. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. GM is also supported by the National Research Foundation of South Africa (grant number 85810). HM is also supported by the Wellcome Trust (206379/Z/17/Z).
Footnotes
Ethical approval
The study protocol was reviewed and approved by the Human Research Ethics Committee at the University of Cape Town (HREC 065/2015) and registered at clinicaltrials.gov (NCT02727582). Written informed consent was taken from each participant in a language of their choice (English, Afrikaans or isiXhosa).
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahmad N, Ahuja SD, Akkerman OW, Alffenaar JWC, Anderson LF, Baghaei P, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet 2018;392 (10150):821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghamdi WA, Alsultan A, Al-Shaer MH, An G, Ahmed S, Alkabab Y, et al. Cycloserine population pharmacokinetics and pharmacodynamics in patients with tuberculosis. Antimicrob Agents Chemother 2019;63(5):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen LS, Grimsrud A, Myer L, Williams DR, Stein DJ, Seedat S. The psychometric properties of the K10 and K6 scales in screening for mood and anxiety disorders in the South African Stress and Health study. Int J Methods Psychiatr Res 2011;20(4):215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminero JA, Sotgiu G, Zumla A, Migliori GB. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis 2010;10 (9):621–9. [DOI] [PubMed] [Google Scholar]
- Centner CM, Carrara H, Harrison TB, Benatar M, Heckmann JM. Sensory polyneuropathy in human immunodeficiency virus-infected patients receiving tuberculosis treatment. Int J Tuberc Lung Dis 2014;18(1):27–33. [DOI] [PubMed] [Google Scholar]
- Chirehwa MT, Court R, de Kock M, Wiesner L, de Vries N, Harding J, et al. Population pharmacokinetics of cycloserine and pharmacokinetic/pharmacodynamic target attainment in multidrug-resistant tuberculosis patients dosed with terizidone. Antimicrob Agents Chemother 2020;64(11):e01381–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradie F, Mabiletsa T, Sefoka M, Mabaso S, Louw R, Evans D, et al. Prevalence and incidence of symmetrical symptomatic peripheral neuropathy in patients with multidrug-resistant TB. South African Med J 2014;104(1):24–6. [DOI] [PubMed] [Google Scholar]
- Court R, Wiesner L, Stewart A, de Vries N, Harding J, Maartens G, et al. Steady state pharmacokinetics of cycloserine in patients on terizidone for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2018;22(1):30–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton K, Dalton MJ. Characteristics of pyridoxine overdose neuropathy syndrome. Acta Neurol Scand 1987;76(July (1)):8–11. [DOI] [PubMed] [Google Scholar]
- Deshpande D, Alffenaar JWC, Köser CU, Dheda K, Chapagain ML, Simbar N, et al. D-Cycloserine Pharmacokinetics/Pharmacodynamics, Susceptibility, and dosing implications in multidrug-resistant tuberculosis: a Faustian deal. Clin Infect Dis 2018;67(Suppl 3):S308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmeules R, Dorval CH, Dion R, Montminy L, Cote A, Paradis G, et al. Considerations on cycloserine in the treatment of pulmonary tuberculosis. Laval Med 1957;24 (November (2)):157–64. [PubMed] [Google Scholar]
- Dheda K, Lenders L, Magombedze G, Srivastava S, Raj P, Arning E, et al. Drug-penetration gradients associated with acquired drug resistance in patients with tuberculosis. Am J Respir Crit Care Med 2018;198(9):1208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events. [Internet]; [cited 2020 Apr 7]. Available from:. 2017. https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf.
- Donald PR. Cerebrospinal fluid concentrations of antituberculosis agents in adults and children. Tuberculosis 2010;90(5):279–92. [DOI] [PubMed] [Google Scholar]
- Wise Emily. ‘Five of our patients have attempted to take their own lives’. The Guardian. [Internet]; Available from:. 2013. https://www.theguardian.com/global-development-professionals-network/2013/aug/02/depression-drug-resistant-tuberculosis-uzbekistan.
- Etschel E, Kane JM, Engel R, Leucht S, Kissling W, Hamann J. Clinical implications of brief psychiatric rating scale scores. Br J Psychiatry 2005;187(04):366–71. [DOI] [PubMed] [Google Scholar]
- Feunekes GIJ, Van’t Veer P, Van Staveren WA, Kok FJ. Alcohol intake assessment: the sober facts. Am J Epidemiol 1999;150(1):105–12. [DOI] [PubMed] [Google Scholar]
- Ghavanini AA, Kimpinski K. Revisiting the evidence for neuropathy caused by pyridoxine deficiency and excess. J Clin Neuromuscul Dis 2014;16(1):25–31. [DOI] [PubMed] [Google Scholar]
- Gumbo T Chemotherapy of tuberculosis, Mycobacterium avium complex disease, and leprosy. In: Brunton L, Chabner B, Knollmann B, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics, vol. 13. McGraw Hill Medical; 2018. [Google Scholar]
- Helmy B Side effects of cycloserine. Scand J Respir Dis Suppl 1970;71:220–5. [PubMed] [Google Scholar]
- Hidy PH, Hodge EB, Young VV, Harned RL, Brewer GA, Phillips WF, et al. Structure and reactions of cycloserine. J Am Chem Soc 1955;77(8):2345–6. [Google Scholar]
- Holmes CX, Martin GE, Fetterhoff KI. The role of the cycloserine (seromycin) blood level in the treatment of pulmonary tuberculosis and the prevention and control of cycloserine (seromycin) toxicity. Dis Chest 1959;36(6):591–3. [DOI] [PubMed] [Google Scholar]
- Hung WY, Yu MC, Chiang CY, Chang JH, Chiang CY, Chang CC, et al. Serum concentrations of cycloserine and outcome of multidrug-resistant tuberculosis in Northern Taiwan. Int J Tuberc Lung Dis 2014;18(5):601–6. [DOI] [PubMed] [Google Scholar]
- Hwang TJ, Wares DF, Jafarov A, Jakubowiak W, Nunn P, Keshavjee S. Safety of cycloserine and terizidone for the treatment of drug-resistant tuberculosis: a meta-analysis. Int J Tuberc Lung Dis 2013;17:1257–66. [DOI] [PubMed] [Google Scholar]
- Kendig I, Charen S, Lepine L. Psychological side effects induced by cycloserine in the treatment of pulmonary tuberculosis. Am Rev Tuberc 1956;73: 438–441.9. [DOI] [PubMed] [Google Scholar]
- Kessler R Kessler Psychological Distress Scale (K10) [Internet]; [cited 2020. April 15]. Available from: https://www.tac.vic.gov.au/files-to-move/media/upload/k10_english.pdf.
- Lheureux P, Penaloza A, Gris M. Pyridoxine in clinical toxicology: a review. Eur J Emerg Med 2005;12(April (2)):78–85. [DOI] [PubMed] [Google Scholar]
- Mehta SA, Ahmed A, Kariuki BW, Said S, Omasete F, Mendillo M, et al. Implementation of a validated peripheral neuropathy screening tool in patients receiving antiretroviral therapy in Mombasa, Kenya. Am J Trop Med Hyg 2010;83(3):565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold JW, Vesely SK, Keyl BA, Schenk JB, Roberts M. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older patients. J Am Board Fam Pract 2004;17(5):309–18. [DOI] [PubMed] [Google Scholar]
- Murray FJ. A pilot study of cycloserine toxicity: a United States Public Health Service cooperative clinical investigation. Am Rev Tuberc 1956;74: 196–209.8. [PubMed] [Google Scholar]
- Parry D, Bredesen D. Sensory neuropathy with low-dose pyridoxine. Neurology 1985;35(10). [DOI] [PubMed] [Google Scholar]
- Simpson DM, Haidich A, Schi G, Yiannoutsos CT, Geraci AP, Mcarthur JC, et al. Severity of HIV-associated neuropathy is associated with plasma HIV-1 RNA levels. AIDS 2002;(16):407–12. [DOI] [PubMed] [Google Scholar]
- Storey PB, Mclean RL. Some considerations of cycloserine toxicity. Am Rev Tuberc 1957;75(March (3)):514–6. [DOI] [PubMed] [Google Scholar]
- Van Der Watt JJ, Harrison TB, Benatar M, Heckmann JM. Polyneuropathy, anti-tuberculosis treatment and the role of pyridoxine in the HIV/AIDS era: a systematic review. Int J Tuberc Lung Dis 2011;15(6):722–8. [DOI] [PubMed] [Google Scholar]
- van Hunsel F, van de Koppel S, van Puijenbroek E, Kant A. Vitamin B6 in Health supplements and neuropathy: case series assessment of spontaneously reported cases. Drug Saf 2018;41(September (9)):859–69. [DOI] [PubMed] [Google Scholar]
- WHO. Notes on the Design of Bioequivalence Study: Terizidone. 2015. [Internet]; [cited 2020 Apr 15]. Available from: https://extranet.who.int/prequal/sites/default/files/documents/29BEterizidone_Oct2015_0.pdf.
- WHO. WHO consolidated guidelines on drug-resistant tuberculosis treatment. 2019. [Internet]; [cited 2020 Apr 15]. Available from: https://www.who.int/tb/publications/2019/consolidated-guidelines-drug-resistant-TB-treatment/en/. [PubMed]
- South African department of health. Management of drug resistant tuberculosis. 2013.. [Internet]; [cited 2020 Nov 23]. p. 42–50. Available from: https://www.health-e.org.za/wp-content/uploads/2014/06/MDR-TB-Clinical-Guidelines-Updated-Jan-2013.pdf.
- Brief Psychiatric Rating Scale [Internet]. [cited 2020. April 7]. Available from: https://www.sciencedirect.com/topics/medicine-and-dentistry/brief-psychiatric-rating-scale.


