Summary
It is known that patients suffering from neurological illnesses have an increased risk of burn injuries. These burns are often very severe and lead to poor outcomes. To date, only a few studies have evaluated the impact of pre-existing neurological illnesses on the outcome of burn injuries. None of them performed a regression analysis regarding specific influence on mortality. Between 1996 and 2016, 1475 patients were admitted to the BICU of a specialized German burn center: 26 had less than 1% TBSA burned and were excluded; 177 had pre-existing neurological disorders (group N). 87 patients with psychological disorders were excluded. 1185 patients without neurological or psychological disorders formed the control group. Length of hospital stay, TBSA and number of operations were analyzed using the chi-squared test and Mann–Whitney U-test. Additionally, mortality was evaluated using the logistic regression analysis adjusted for known outcome predictors. Mean age of the patients in the control group was 41.53 years with a BICU stay of 18 days, TBSA of 18.25% and mortality rate of 12.4%; 23.7% had inhalation injuries. Patients in group N had a mean age of 54.63 years, a BICU stay of 27 days, mean TBSA of 20.97%; 31.1% had inhalation injuries and mortality was 20.3%. Patients with neurological disorders were older and showed higher affected TBSA, higher rates of inhalation injury, mortality and affected TBSA, and a longer stay in the BICU compared to the control group. Nevertheless, pre-existing neurological disorders alone had no significant influence on mortality.
Keywords: burn injuries, neurological disorders, BICU, burn mortality
Abstract
Il est avéré que les patients souffrant de pathologie neurologique ont un risque plus élevé de brûlure. Elles sont souvent particulièrement graves et d’évolution défavorable mais la littérature à ce sujet reste pauvre et aucune étude n’a utilisé de régression logistique pour évaluer la corrélation pathologie neurologique- évolution d’une brûlure. Entre 1996 et 2016, 1 475 patients ont été hospitalisés en réanimation spécifique dans un CTB allemand. Vingt- six d’entre eux, brûlés sur moins de 1% SCT, n’ont pas été inclus dans l’étude, pas plus 87 patients psychiatriques si bien que 177 patients souffrant de pathologie neurologique (N) ont été comparés à 1 185 n’en souffrant pas (C). Les durées d’hospitalisation, la surface brûlée et le nombre d’interventions chirurgicales ont été analysée en utilisant C² ou Mann-Whitney. En outre, nous avons effectué une régression logistique étudiant la mortalité, en utilisant les facteurs connus de mortalité. Le groupe C avait 41,53 ans, souffrait de brûlures sur 18,25% SCT, avait inhalé des fumées dans 23,7% des cas, avait un taux de mortalité de 12,4% et restait 18 j en réanimation. Dans le groupe N, ces chiffres étaient respectivement de 54,63 ans, 20,97% SCT, 31,1% de fumées, 20,3% de mortalité et 27 jours en réa. Tous les chiffres étudiés étaient plus élevés dans N que dans C. Toutefois, l’existence de comorbidité neurologique n’apparaissait pas un critère indépendant de mortalité.
Introduction
To date, burn injuries have been frequently observed in Germany and worldwide, and prevention strategies are not always successful.1 It is challenging to prognosticate burn outcomes, especially for patients with neurological comorbidities, because of the unclear impact of these comorbidities.2 In particular, neurological disorders and psychiatric diseases can be a major reason for burns and can lead to treatment difficulties.3,4 Neurological illnesses such as paraplegia or polyneuropathy can lead to a decreased sensation of pain. Patients are unaware of heat contact, which may result in severe burns.5,6 In the case of diabetes mellitus, burn injuries are often aggravated by impaired wound healing.6 Furthermore, other neurological diseases like Morbus Parkinson, Multiple Sclerosis, and Morbus Alzheimer have often been reported to have complicated and prolonged treatment.3 Patients suffering from epilepsy are known to have a three-fold higher risk of burn accidents than healthy individuals due to the loss of protective reflexes.7,8
Nevertheless, to the best of our knowledge, to date only one study has compared the length of hospital stay and mortality of patients with pre-existing neurological illnesses to other burn patients.9 A few other studies concentrated either on mortality or on length of hospital stay.9-12 These studies are rather old and the impact of pre-existing neurological comorbidities is still poorly understood.
The aim of this study was to evaluate whether the duration of treatment of burn patients with neurological diseases is prolonged compared to a control group and if their outcomes differ. This is a very important topic because these impairments can have a large impact on morbidity with prolonged periods of rehabilitation.
Methods
A retrospective data analysis was performed for all patients admitted to the burn intensive care unit (BICU) at Cologne-Merheim Medical Center, University of Witten/Herdecke, between 1996 and 2016. With 10 BICU beds, the burn center of Cologne-Merheim is one of the biggest burn centers in Germany. According to the treatment guidelines of the German Society for Burn Treatment, the following burn categories should be treated at specialized centers: deep partial thickness burns of more than 10% TBSA; partial thickness burns of >20% TBSA; full thickness burns; burns of the hands, face and genitals; burns caused by electricity (including those due to lightning); chemical burns; inhalation injuries; burns combined with other injuries, which increase the severity of the burns.13
Clinical data of all patients admitted to the BICU were acquired, including preexisting illnesses, depth and extent of burns, inhalation injuries (diagnosed by bronchoscopy), number of operations, mortality, duration of stay in the burn intensive care unit, and length of total hospital stay (without rehabilitation). Severe burn was defined as burn trauma that needs to be treated in the BICU.
Preexisting neurological disorders were collected with the help of medical history, regardless of their extent. Patients who developed burn-related neurological disorders were not included.
Data collection was performed using the hospital electronic medical record database and evaluated retrospectively. Before patients were discharged from hospital, all data were checked by a senior burn surgeon who was managing the database.
In total, 1475 patients were admitted to the BICU during the study period. Patients with burns of less than 1% TBSA (n=26) were excluded. Additionally, all patients with self-inflicted burns (Group S, 87 patients) were not included in the control group since many studies have shown in the past that this patient cohort is very specific and generally has a poor outcome.14-18
All statistical analyses were performed using Microsoft Excel and IBM SPSS Statistics 22.0 software (IBM, New York, USA). The chisquared test and Mann–Whitney U-test were used to compare categorical and quantitative data. A p-value <0.05 was considered to be statistically significant.
Previously the study had been reviewed and approved by the Ethical Review Committee of the University of Witten/Herdecke, Germany, according to the declaration of Helsinki.
Results
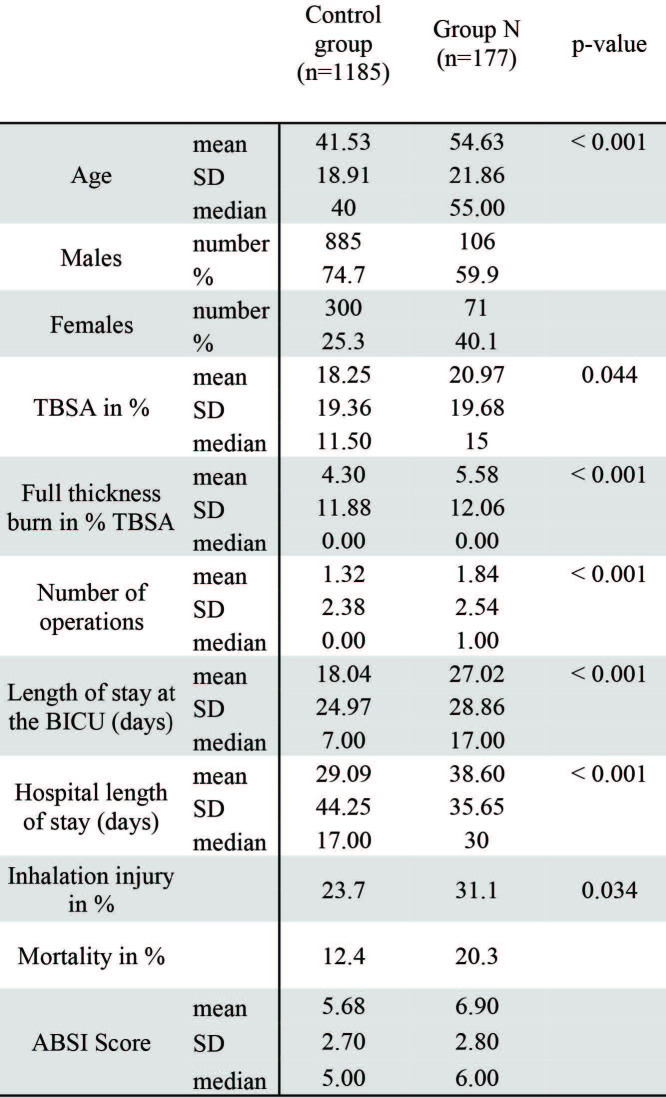
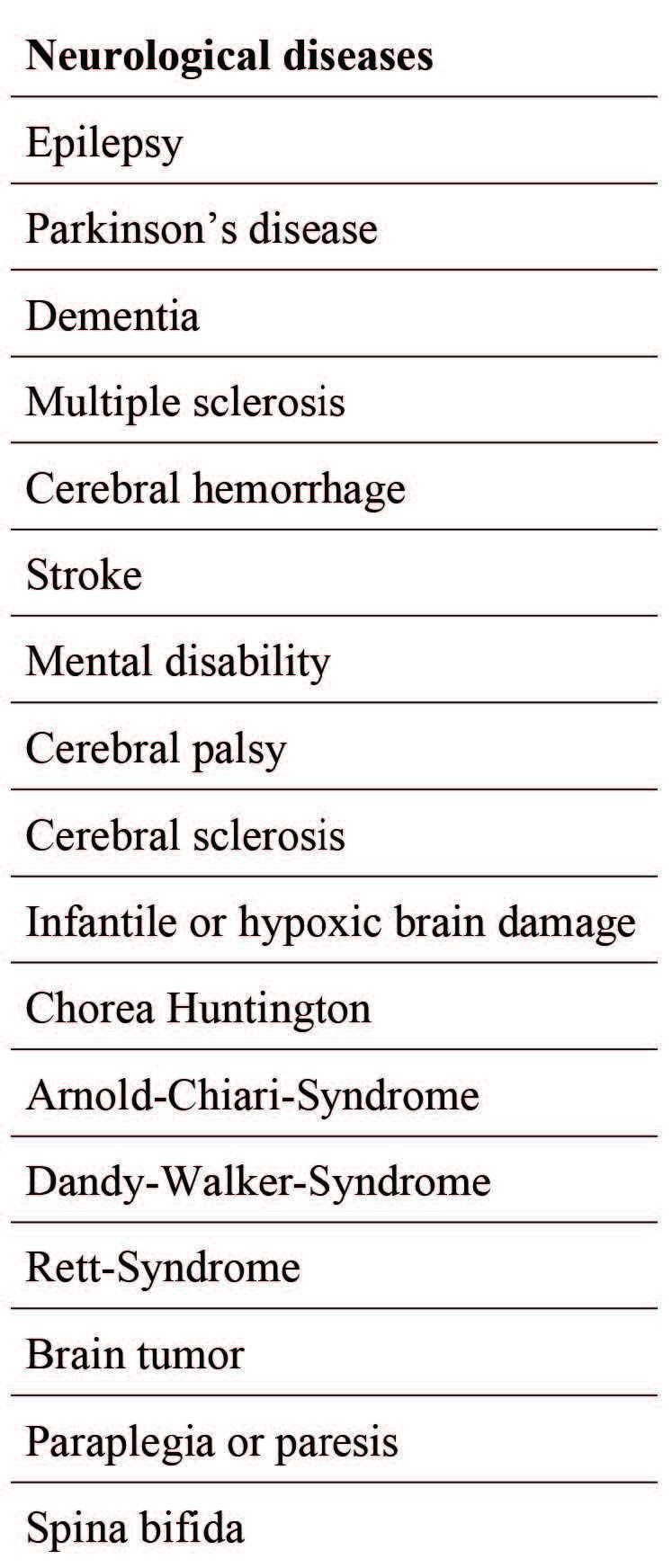
In total, between 1996 and 2016, 177 patients were identified with pre-existing neurological illnesses (Group N); the patient characteristics are listed in Table I.
Table I. Overview of included neurological diseases.
All the remaining patients comprised the control group C (n=1185). The mean age of all included patients was 43.17 years, the average extent of burn injuries (TBSA) was 19.95% with an average of 1.5 operations. The length of stay in the burn intensive care unit (BICU) was 20.39 days, with an additional 10.65 days in the peripheral ward. Most patients were male (72.7%) and 26.3% patients had accompanying inhalation injuries. Overall, 215 patients (14.8%) died during hospitalization (Table II).
Patients in group N had a significantly higher mean age (54.63 years) than those in group C, where the mean age was 41.53 years (p<0.001).
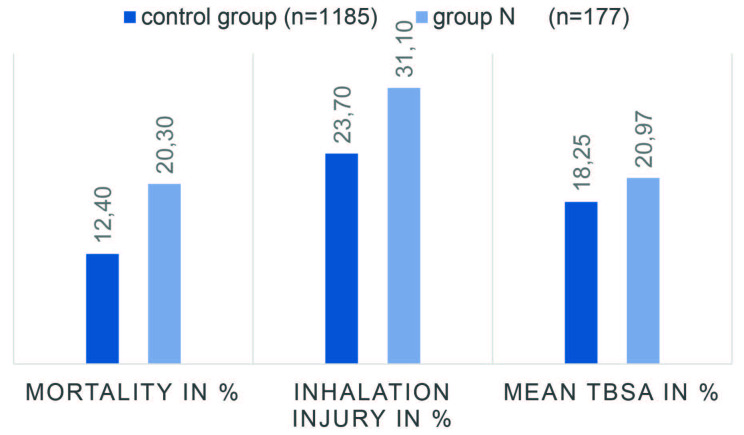
Patients in group N showed larger TBSA than those in group C, with an average of 20.97% compared to 18.25% (p = 0.044), respectively (Table II, Fig. 1). Additionally, patients in group N showed more scalding injuries than patients in the control group (27.1% vs. 17.5%; p < 0.001). No patient had an injury caused by electricity or chemicals. The women´s share in group N (40.1%) was significantly higher than in group C (25.3%) (p < 0.001). Number of operations in group C was significantly lower than in group N (Mean 1.32 vs. 1.84 operations, p < 0.001) (Table II). Furthermore, patients in group N had a longer duration of hospital stay (38.6 days) compared to group C (29.9 days) (p < 0.001). Inhalation trauma was noted in 23.7% of the patients in group C and in 31.1% of the patients in group N (p = 0.034). Altogether, 20.3% of group N patients died, whereas 12.4% of patients in the control group died (Table II, Fig. 1). Full thickness burns were noted in 31.1% of group C patients and 42.9% of group N patients (p = 0.002). Additionally, the Abbreviated Burn Severity Index (ABSI) score was 5.68 for group C and 6.90 for group N (p < 0.001).
Table II. Overview of included patients, sex, age, TBSA, number of operations, days of hospital and BICU stay and mortality.
Fig. 1. Overview of mortality, inhalation injury and mean TBSA in % of the two groups in direct comparison.
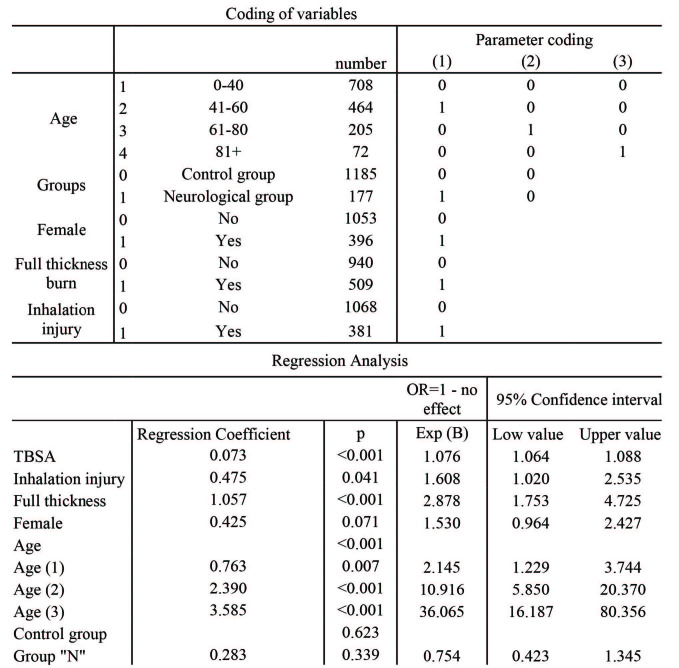
Multivariate regression analysis revealed that TBSA as well as age were significantly associated with mortality (Table III). Pre-existing neurological disorders had no significant influence on mortality. Instead, the fact that these patients are either very old and have large affected TBSA results in the high mortality rate (Table III).
Table III. Multi-variant analysis regarding the influence on mortality; pre-existing neurological illnesses had no significant influence in mortality (p<0.05).
Discussion
The aim of this study was to determine whether treatment of patients with neurological diseases is prolonged compared to the control group and if their mortality rates differ.
The mean age of all patients and mean stay at the BICU for all patients was 43.17 years and 8 days, respectively, which is in concordance with other studies.19-22
The average age of the patients with neurological illnesses was 54.63 years in this study, which was significantly higher than that of patients in the control group. This phenomenon is underlined by a Japanese study wherein patients with neurological disturbances had a higher average age than those of the control group.23 Overall, 65.1% of these patients were male. Different diseases show different distributions of age and sex. Studies which analyzed burn injuries of patients suffering from epilepsy had in mean younger and a higher number of female patients24-26 compared to studies which describe burn injuries of patients with dementia (79.9 years).27
Patients with neurological diseases showed a slightly higher TBSA of 20.97% compared to that of the control group with a TBSA of 18.25% (p=0.044). This phenomenon was also observed in other studies. Bozkurt et al. described patients with dementia, Morbus Parkinson and paralysis with a median TBSA of 16.3%.28 Aust et al. described epilepsy patients with burn injuries with a median TBSA of 16%.29 Similar findings were reported by Ramirez et al.. Interestingly they found no differences in the TBSA of patients with neurological disorders and the control group,30 although missing defensive reflexes in the former rendered them susceptible to deep burn injuries.24
Patients with neurological diseases had a higher number of operations than patients in the control group. This is in accordance with an American study wherein patients with neurological diseases also needed more operations than patients in the control group (1.71 vs. 1.08).31 Other studies only describe the total number of operations and show no data on the number of operations per patient.24
Patients with neurological disabilities showed longer lengths of hospital stay, especially in the BICU. In 2004, Alden et al. described similar results. 32 Although the affected TBSA was not significantly higher in their study, the patients with neurological disorders had significantly longer lengths of stay in the ICU than the patients in the control group.32 It can be concluded that neurological impairments can complicate the treatment of burn injuries and can elongate the duration of hospital stay.33-36 This is underlined by Thombs et al., who showed that length of hospital stay is directly linked to the number of comorbidities.12 For patients with neurological diseases, longer hospital stays also were reported.12
Interestingly inhalation injury was significantly more frequent in patients with neurological impairments than in the control group (p = 0.034), although a high number of patients with neurological disorders suffered from scalding (37.2%). Consequently, from the remaining 62.8% of all neurological patients, approximately every second patient suffered from an inhalation injury. Other publications dealing with neurological patients have not evaluated this aspect.7,9,37 Therefore, further investigations could lead to better understanding of this specific patient group.
Mortality rates associated with burns differ worldwide. Our study showed an overall mortality rate of 14.8%; in literature, we found differences even in industrial countries where a Dutch study (2015) talked about a mortality rate of 6% compared to a Chinese study (2012) which reported a mortality rate of 28%.38-41 Interestingly, patients with neurological impairments showed higher but not significantly higher mortality rates than those of the control group, which is supported by previous studies wherein patients with neurological disorders presented significantly higher mortality rates.3,12
There is only one study by Larson et al. from 1988 which focused solely on the impact of neurological comorbidities on burn injuries without performing a regression analysis.9 Larson et al. reported in their study that five out of 37 patients (13.5%) died. Compared to the mortality rate in group N of our study (20.3%) this is notably lower. Different studies analyzed various pre-illnesses. Germann et al. described a higher mortality (48%) in the group with neurological disorders vs. 30% in the “no neurology” group without logistic regression analysis.11 Barret et al. showed that 13% of their patients suffered from neurological disorders but could not show after separate analysis a significantly increased mortality.10 Thombs et al. described longer lengths of stay for patients with paralysis and dementia but without significantly elevated mortality.12 In summary these studies are 13 years old and more.
The presence of neurological illnesses did not result in higher mortality. Nevertheless, neurological illnesses may lead to a slower reaction time, which then again might result in a longer heat exposition, and in turn, larger and deeper burn injuries. In this context, age and TBSA had a significant influence on mortality. Our patients in group N were older and had larger TBSA than patients in the control group. Therefore, preventive strategies and educational efforts for patients with neurological illnesses are very important, particularly with regard to the greater difficulty in caring for them.
Limitations
The main limitation of this study is the retrospective, single center study design. The severity of the underlying neurological disorders is not recorded in the database.
Conclusion
Compared to the control group, patients with neurological disorders were older, had a higher rate of inhalation injury, higher mortality rate, higher affected TBSA and a longer stay in the BICU. However, the logistic regression analysis shows that pre-existing neurological disorders had no significant influence on mortality.
Nevertheless, the implication of this study is that patients with neurological illnesses could benefit from environmental improvements like reduced water temperature in the bathroom and designed prevention programs to prevent the occurrence of severe burns or any burn at all, especially since mortality rates did not decrease in the course of time. This could not only reduce personal risk, but also the social and economic costs.
Acknowledgments
Conflict of interest.None
Funding.None
Ethics approval.This is a retrospective study with already existing patient data, presented completely anonymized without the possibility to draw conclusions about anybody, thus no ethical approval is necessary according to the German law in NRW.
References
- 1.Harvey L, Mitchell R, Brodaty H, Draper B, Close J. Dementia: a risk factor for burns in the elderly. Burns. 2016;42(2):282–290. doi: 10.1016/j.burns.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Knowlin L, Stanford L, Moore D, Cairns B, Charles A. The measured effect magnitude of co-morbidities on burn injury mortality. Burns. 2016;42(7):1433–1438. doi: 10.1016/j.burns.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backstein R, Peters W, Neligan P. Burns in the disabled. Burns. 1993;43(5):192–197. doi: 10.1016/0305-4179(93)90147-z. [DOI] [PubMed] [Google Scholar]
- 4.Hudson A, Al Youha S, Samargandi OA, Paletz J. Pre-existing psychiatric disorder in the burn patient is associated with worse outcomes. Burns. 2017;43(5):973–982. doi: 10.1016/j.burns.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin C, Gittler M, Lee R. Burn from car seat heater in a man with paraplegia: case report. J Spinal Cord Med. 2011;34(3):332–334. doi: 10.1179/2045772311Y.0000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jose RM, Vidyadharan R, Roy DK, Erdmann M. Hot water bottles and diabetic patients - a cautionary tale. Br J Gen Pract. 2005;55(512)X:222–223. [PMC free article] [PubMed] [Google Scholar]
- 7.Ansari Z, Brown K, Carson N. Association of epilepsy and burns - a case control study. Aust Fam Physician. 2008;37(7):584–589. [PubMed] [Google Scholar]
- 8.Akhtar MS, Ahmad I, Khan AH, Fahud Khurram M, Haq A. Burn injury in epileptic patients: an experience in a tertiary institute. Ann Burns Fire Disasters. 2014;27(3):126–129. [PMC free article] [PubMed] [Google Scholar]
- 9.Larson CM, Braun S, Sullivan J, Saffle JR. Complications of burn care in patients with neurologic disorders. J Burn Care Rehabil. 1988;9(5):482–484. doi: 10.1097/00004630-198809000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Barret JP, Gomez P, Solano I, Gonzalez-Dorrego M, Crisol FJ. Epidemiology and mortality of adult burns in Catalonia. Burns. 1999;25(4):325–329. doi: 10.1016/s0305-4179(98)00190-9. [DOI] [PubMed] [Google Scholar]
- 11.Germann G, Barthold U, Lefering R, Raff T, Hartmann B. The impact of risk factors and pre-existing conditions on the mortality of burn patients and the precision of predictive admission-scoring systems. Burns. 1997;23(3):195–203. doi: 10.1016/s0305-4179(96)00112-x. [DOI] [PubMed] [Google Scholar]
- 12.Thombs BD, Singh VA, Halonen J, Diallo A, Milner SM. The effects of preexisting medical comorbidities on mortality and length of hospital stay in acute burn injury: evidence from a national sample of 31,338 adult patients. Ann Surg. 2007;245(4):629–634. doi: 10.1097/01.sla.0000250422.36168.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF). S2k Leitlinie: Behandlung thermischer Verletzungen des Erwachsenen 2018 [updated 07.12.2018 March 26, 2019] Available from: https://www.awmf.org/leitlinien/detail/ll/044-001.html . [Google Scholar]
- 14.James WA, Frierson RL, Balajepalli B, Lippmann SB. Suicide attempts by burning. J Ky Med Assoc. 2006;104(10):459–467. [PubMed] [Google Scholar]
- 15.Wallace KL, Pegg SP. Self-inflicted burn injuries: an 11-year retrospective study. J Burn Care Rehabil. 1999;20(2):89–90. [PubMed] [Google Scholar]
- 16.Nisavic M, Nejad SH, Beach SR. Intentional self-inflicted burn injuries: review of the literature. Psychosomatics. 2017;58(6):581–591. doi: 10.1016/j.psym.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Laloe V. Patterns of deliberate self-burning in various parts of the world. A review. Burns. 2004;30(3):207–215. doi: 10.1016/j.burns.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Hahn AP, Jochai D, Caufield-Noll CP, Hunt CA. Self-inflicted burns: a systematic review of the literature. J Burn Care Res. 2014;35(1):102–119. doi: 10.1097/BCR.0b013e31828b0a46. [DOI] [PubMed] [Google Scholar]
- 19.Burton KR, Sharma VK, Harrop R. A populationbased study of the epidemiology of acute adult burn injuries in the Calgary Health Region and factors associated with mortality and hospital length of stay from 1995 to 2004. Burns. 2009;35(4):572–579. doi: 10.1016/j.burns.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Cheng W, Yan-hua R, Fang-gang N, Wei-li D, Guo-an Z. Epidemiology of 1974 burn patients at a major burn center in Beijing: a nine-year study. J Burn Care Res. 2012;33(5):e228–e233. doi: 10.1097/BCR.0b013e3182479b13. [DOI] [PubMed] [Google Scholar]
- 21.Tang K, Jian L, Qin Z, Zhenjiang L, Gomez M, Beveridge M. Characteristics of burn patients at a major burn center in Shanghai. Burns. 2006;32(8):1037–1043. doi: 10.1016/j.burns.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Yao Z, Tan J, Zhou J, Li Y, Wu J. Epidemiology and outcome analysis of 6325 burn patients: a five-year retrospective study in a major burn center in Southwest China. Sci Rep. 2017;7:460–466. doi: 10.1038/srep46066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanagawa Y, Saitoh D, Sakamoto T, Okada Y. Unfavorable outcome of burn patients with neuropsychiatric disorders. TohokuJ Exp Med. 2005;205(3):241–245. doi: 10.1620/tjem.205.241. [DOI] [PubMed] [Google Scholar]
- 24.Josty IC, Narayanan V, Dickson WA. Burns in patients with epilepsy: changes in epidemiology and implications for burn treatment and prevention. Epilepsia. 2000;41(4):453–456. doi: 10.1111/j.1528-1157.2000.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 25.Rimmer RB, Bay RC, Foster KN, Jones MA. Thermal injury in patients with seizure disorders: an opportunity for prevention. J Burn Care Res. 2007;28(2):318–323. doi: 10.1097/BCR.0B013E318031A161. [DOI] [PubMed] [Google Scholar]
- 26.Jang YC, Lee JW, Han KW, Han TH. Burns in epilepsy: seven years of experience from the Hallym Burn Center in Korea. J Burn Care Res. 2006;27(6):877–881. doi: 10.1097/01.BCR.0000245649.38644.27. [DOI] [PubMed] [Google Scholar]
- 27.Alden NE, Rabbitts A, Yurt RW. Burn injury in patients with dementia: an impetus for prevention. J Burn Care Rehabil. 2005;26(3):267–271. [PubMed] [Google Scholar]
- 28.Bozkurt M, Kapi E, Gedik E, Kuvat SV. Impact of para-neurologic and para-mental premorbidities on burn injury patients. Ulus Travma Acil Cerrahi Derg. 2011;17(3):220–224. doi: 10.5505/tjtes.2011.82160. [DOI] [PubMed] [Google Scholar]
- 29.Aust M, Guggenheim M, Gohritz A, Kunzi W. Thermal trauma sustained during epileptic seizures - analysis of 33 cases. Handchir Mikrochir Plast Chir. 2008;40(6):372–376. doi: 10.1055/s-2008-1039002. [DOI] [PubMed] [Google Scholar]
- 30.Ramirez RJ, Behrends LG, Blakeney P, Herndon DN. Children with sensorimotor deficits: a special risk group. J Burn Care Rehabil. 1998;19(2):124–127. doi: 10.1097/00004630-199803000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Brezel BS, Kassenbrock JM, Stein JM. Burns in substance abusers and in neurologically and mentally impaired patients. J Burn Care Rehabil. 1988;9(2):169–171. doi: 10.1097/00004630-198803000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Alden NE, Rabbitts A, Rolls JA, Bessey PQ, Yurt RW. Burn injury in patients with early-onset neurological impairments: 2002 ABA paper. J Burn Care Rehabil. 2004;25(1):107–111. doi: 10.1097/01.BCR.0000105099.60083.F5. [DOI] [PubMed] [Google Scholar]
- 33.Boschini LP, Tyson AF, Samuel JC, Kendig CE. The role of seizure disorders in burn injury and outcome in Sub-Saharan Africa. J Burn Care Res. 2014;35(5):e406–e412. doi: 10.1097/BCR.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Othman D, Jones O. Burns patients with epilepsy or a learning disability have a greater length of stay in hospital than those patients with a history of drug or alcohol abuse. Burns. 2011;37(3):546–547. doi: 10.1016/j.burns.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Holmes EG, Jones SW, Laughon SL. A retrospective analysis of neurocognitive impairment in older patients with burn injuries. Psychosomatics. 2017;58(4):386–394. doi: 10.1016/j.psym.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Modjarrad K, McGwin G, Jr., Cross JM, Rue LW, 3rd. The descriptive epidemiology of intentional burns in the United States: an analysis of the National Burn Repository. Burns. 2007;33(7):828–832. doi: 10.1016/j.burns.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Unglaub F, Prueter C, Block F, Pallua N. Severe burn as a consequence of an epileptic seizure while showering. Nervenarzt. 2005;76(2):209–211. doi: 10.1007/s00115-004-1803-7. [DOI] [PubMed] [Google Scholar]
- 38.Buttemeyer R, Steen M, Henkel VDG, Germann G. Establishing a baseline for organisation and outcome in burn care-basic data compiled by German burn centres, 1991-2000. Burns. 2004;30(2):115–120. doi: 10.1016/j.burns.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Dokter J, Felix M, Krijnen P, Vloemans JF. Mortality and causes of death of Dutch burn patients during the period 2006-2011. Burns. 2015;41(2):235–240. doi: 10.1016/j.burns.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Lancerotto L, Sferrazza R, Amabile A, Azzena B. Burn care in relation to burn epidemiology in Italy. Burns. 2011;37(5):835–841. doi: 10.1016/j.burns.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Xie B, Xiao SC, Peng XD, Zhu SH. Epidemiology and outcome analysis of severe extensive burns: a 12-year summary of 103 cases in a burn center in China. J Burn Care Res. 2012;33(3):e127–e132. doi: 10.1097/BCR.0b013e3182335a5d. [DOI] [PubMed] [Google Scholar]