Summary
The aim of the study was to evaluate the application of different types of skin allograft as a skin substitute for coverage of major deep burn wounds, and their effect on the clinical outcomes and mortality of burned patients. This prospective study was conducted on 36 patients admitted to the Burn Unit from August 2016 to November 2019. The number and percentage of patients that needed auto-grafting after surgical intervention was 9 (100%) in Group I (allograft coverage not available), 13 (86.66%) in Group II (allograft source was from unrelated patients) and 8 (66.7%) in Group III (allograft from a first-degree relative). Patient survival was 55.6% in Group I, 86.7% in Group II and 91.7% in Group III. There was significant difference between the groups regarding time to complete healing, with P1 = 0.034* and P2 < 0.0001*. Human skin allograft harvested from living first-degree relatives is freshly donated at maximum viability and does not require complex preparation or preservation. It shows prolongation of graft survival that helps to improve general condition, decrease microbial wound contamination, improve vascularization and prepare the wound bed with healthy granulation tissue. This promotes wound healing and subsequent autograft take, and decreases mortality rate among burned patients.
Keywords: allograft, burn, major, impact, mortality
Abstract
Cet étude a pour but d’évaluer différents types d’allogreffes et de substituts cutanés utiliser pour couvrir des brûlures étendues profondes et leurs conséquences sur l’évolution et la mortalité des patients. Cette étude a été réalisée auprès de 36 patients hospitalisés entre août 2016 et novembre 2019. Neuf patients (groupe II) ont été autogreffés d’emblée, faute de disponibilité d’allogreffes ; 13 (86,6%) patients ayant reçu une allogreffe non familiale (groupe II) et 8 (66,7%) de ceux ayant reçu une allogreffe familiale ont reçu ensuite une autogreffe. Les taux de survie ont été de 55,6% dans le groupe I, 86,7% dans le groupe II et 91,7% dans le groupe III. Les différences de délai de cicatrisation étaient significatives avec P1 = 0,034 et P2< 0,0001. L’allogreffe prélevée sur un proche vivant a une viabilité élevée et ne nécessite pas de préparation ni de conservation. Ceci prolonge la durée de couverture ce qui permet d’améliorer l’état général, de diminuer la contamination bactérienne, d’améliorer la vascularisation, de préparer ainsi au mieux l’autogreffe et, in fine, d’accélérer la cicatrisation et diminuer la mortalité.
Introduction
Burn injury represents a major aetiology of trauma to the human body, and may lead to death and disability.1 Major burns cause systemic physiological derangements, including hypovolaemia, hypothermia, metabolic and immune system disturbances, and increase susceptibility to infection. Thus, re-establishment of the skin barrier is mandatory to normalize the physiological state.2
The mortality rate of burn injuries can be diminished with early debridement and skin grafting, but insuf ficient donor site and the patient’s unsuitable general condition for surgical intervention is an important obstacle for skin autografting.3
The use of “skin substitutes” for treating wounds dates back to 1880, when Joseph Gamgee described an absorbent dressing made of cotton wool sandwiched between layers of gauze.4
Skin allografts, also called homografts, are tissues that are harvested from a donor site of the same species with different genetic components.5 The use of cadaveric skin allografts as biological coverage or skin substitute in burn management dates back to World War II and is currently being practiced in many major burn centers all over the world.6
Human skin allograft application for wound coverage has been widely used and is one of the most beneficial and widely used dressings for deep burn injury worldwide.7 It improves the granulation tissue and becomes incorporated into the recipient tissue bed.8
The aim of the study was to evaluate the application of skin allograft as a skin substitute used for coverage of major deep burn wounds, and its effect on the clinical outcome of the patients.
Patients and methods
This prospective study was conducted on 36 patients who were admitted to the burn unit from August 2016 to November 2019. All patients were subjected to complete history taking as well as burn causations, examination, and investigations. Informed consent was taken from all patients or a family representative, and from all donors of skin allograft, for the surgical intervention and photography.
Burn depth was assessed and determined clinically 24 hours after injury. Inclusion criteria included: major deep burn more than 25% TBSA with limited donor site for autograft coverage. Exclusion criteria included burn less than 25% TBSA and superficial burn that is suspected to heal with conservative treatment.
Patients were divided into three groups according to the availability of different types of skin allograft, as follows: Group I included 9 patients with mean age 4.75 (years) and mean burn percentage of 37.42% TBSA, in whom burn debridement was done without allograft coverage as it was not available. Group II included 15 patients with mean age 7.50 (years) and mean burn percentage of 28.68% TBSA, in whom allograft source was discarded skin of body contouring operations (abdominoplasty, reduction mammoplasty or body lifting) from unrelated patients. Group III included 12 patients with mean age 6.44 (years) and mean burn percentage of 33.55% TBSA, in whom allograft was harvested from a firstdegree relative (mother, father, brother or sister).
Preoperative preparation
All the burned patients received resuscitation treatment until stabilization of their general condition, and treatment for electrolyte imbalance. Regular full laboratory and radiological investigations and anesthesia consultation were carried out. Also, full laboratory investigations were carried out for all donors, including virology profile (HIV, hepatitis B and hepatitis C viral infection).
Packed RBCs and fresh frozen plasma were also prepared for both donor and recipient patients. Antibiotics were given one hour before operation.
Allograft preparation
Allograft from an unrelated source. Excised skin of patients who underwent a body contouring operation with excision of excess discarded skin from a belt lipectomy, breast reduction or body lift operation was used. It can be taken by dermatome after applying traction for both ends of the excised segment, meshering by 3:1 plates and wrapping with vaseline gauze soaked in antiseptic solution ready for burn coverage.
Allograft from a first-degree relative donor. The donor of the skin allograft for all patients in Group III was a living first-degree relative (father, mother, brother or sister) as they share approximately 50% of their genes.
The preferred characteristics for choosing the donor were as follows: sharing the same blood group with the recipient patient, young age, male, and free from chronic or infectious disease. The thigh was the preferred donor site and the harvesting of split thickness skin graft was done using dermatome followed by meshering through 3:1 plates and soaking in antiseptic solution ready for immediate burn coverage.
Operative procedures for the burned patients
General anesthesia was used. The wound was sterilized with antiseptic solution.
The surgical intervention
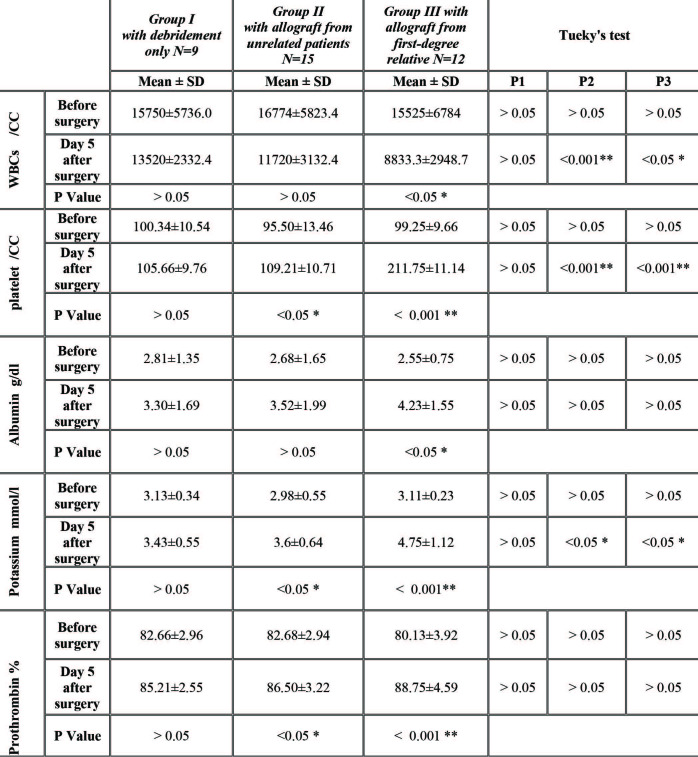
The burn areas were infiltrated with a solution of 100 mL 0.9% normal saline, 10 mL lidocaine (1%), 20 mL ropivacaine (7.5mg/mL), and epinephrine (1:250,000), as this helps to decrease bleeding, operative time and postoperative pain. Debridement of the burn eschar and necrotic tissue was done through tangential excision to a level of petechial bleeding that indicates a healthy wound bed. For wounds with granulation tissues, the superficial granulation tissues were excised deep to the fibrous plate. The excision level should suffice so as not to leave eschar and necrotic tissue remnants (Fig. 1).
Fig. 1. Male patient, 3 years old, with scald burn 22% TBSA and deep dermal in depth admitted to the ICU. A) Preoperative photo at third day of burn shows burned area prepared for early excision and coverage; B) Escharectomy through tangential excision was done to level of petechial bleeding indicating healthy bed for coverage; C) Necrotic unhealthy tissues removed through escharectomy.
Control of bleeding was managed by bipolar cautery of bleeding points then all burned areas were washed with saline hydrogen peroxide solution followed by antiseptic saline solution. Immediate coverage with the prepared skin allograft was done with fixation to the prepared bed, vaseline gauze soaked in antiseptic solution (inner layer) with bulky dressing coverage (external layer), and tie-over sutures to maintain stability and decrease shearing effect to the skin graft. Splinting of the involved joints was done in the extension position with slab to avoid contracture.
Post-operative evaluation and follow up
Dressing and water bath were done at the third and fifth postoperative day; external dressing was changed leaving the gauze (inner layer) attached to the burnt area and washing with saline antiseptic solution. The inner layer dressing did not need to be changed unless there was evidence of exudation or sign of local infection. The same dressing was repeated at the seventh day with removal of the inner gauze layer, and daily dressing with water bath. To follow up the effect of each surgical intervention on clinical outcome, the following parameters were observed and documented:
Laboratory changes occurring in plasma levels before and at the fifth day from intervention, including: WBCs (white blood cells), PLT (platelets), ALB (albumin), K (potassium) and PT (prothrombin concentration).
The need for auto-grafting
Time needed for complete healing
The number of patients that survived
Statistical analysis
The data were collected from history, examination, investigations and follow up of clinical outcome. They were entered and analyzed using Microsoft Excel software, then imported into Statistical Package for the Social Sciences (SPSS version 20.0) for analysis, as follows:
Description of quantitative variables as mean, standard deviation and range.
Description of qualitative variables as number and percentage.
P value estimated as: P value >0.05 insignificant, P<0.05* significant and P<0.001** highly significant
Pre-present comparison before and after the surgery. P1 represents comparison between Groups I and II, P2 represents comparison between Groups I and III, and P3 represents comparison between Groups II and III.
Results
Laboratory parameter data
The plasma concentration of the following laboratory parameters (WBCs, PLT, ALB, K and PT) one day before surgical intervention was investigated and compared for all patients in Groups I, II and III. P1, P2 and P3 > 0.05 (non-significant), as shown in Table I, denotes no significant statistical difference between the different parameters. Statistically significant differences were noted between the comparable parameters that were investigated at the fifth day after surgical intervention for all patients in the three groups, as shown in Table I:
The mean values of WBCs and Albumin; pvalue P> 0.05* (significant) in Group III.
The mean values of platelets, potassium and prothrombin concentration; p-value P>0.05* (significant) in Group II, P>0.001** (highly significant) in Group III.
Table I. Laboratory changes in plasma concentration of white blood cells, platelets, albumin, potassium and prothrombin before and at the fifth day of application of allograft to the prepared burn wounds.
There was great improvement in albumin level at the fifth day from allograft application in patients in Group III compared to the other two groups of patients, as there was more prolongation of graft survival and less rejection immunological reactions, thus a decrease in loss of electrolytes and significant proteins.
Local allograft rejection data
This was the primary clinical outcome measure that was assessed through clinical evaluation for each patient in all the studied groups, which appeared in the form of:
color changes at the bed of the wound (brown, grey, blue or black)
edema at the affected part of the body
dry sloughy appearance of the dead part of the allograft
local infection in the form of discharge, bad odor and necrotic tissues
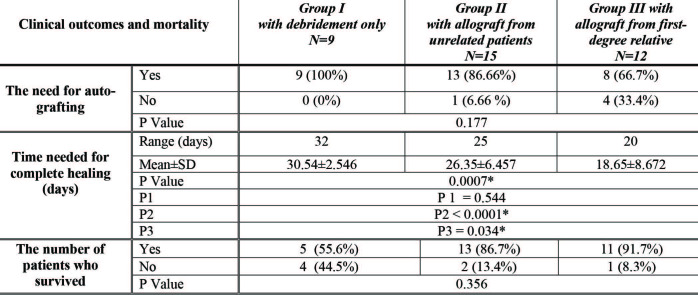
graft loss was the end local sign of rejection (as shown in Fig. 2).
Fig. 2. Local signs of allograft rejection as follows: A) Cyanosis at wound bed and color changes observed ten days after coverage of the burn areas; B) Erythema, edema, dry sloughy appearance and partial allograft loss.
Postoperative follow up
The number and percentage of the patients that needed auto-grafting after surgical intervention was 9 (100%) in Group I, 13 (86.66%) in Group II and 8 (66.7%) in Group III. Patient survival was 55.6% in Group I, 86.7% in Group II and 91.7% in Group III. There was significant difference between the groups regarding time to complete healing, with P1 = 0.034* and P2 < 0.0001* (Table II).
Table II. Patient outcome in the studied groups as regard the need for auto-grafting, time needed for complete healing and survival of patients.
With parts or most of the allograft, epithelialization with wound healing began to appear in the granulated raw area, resulting in a decrease in the surface area that needed subsequent autografting, and facilitating its take.
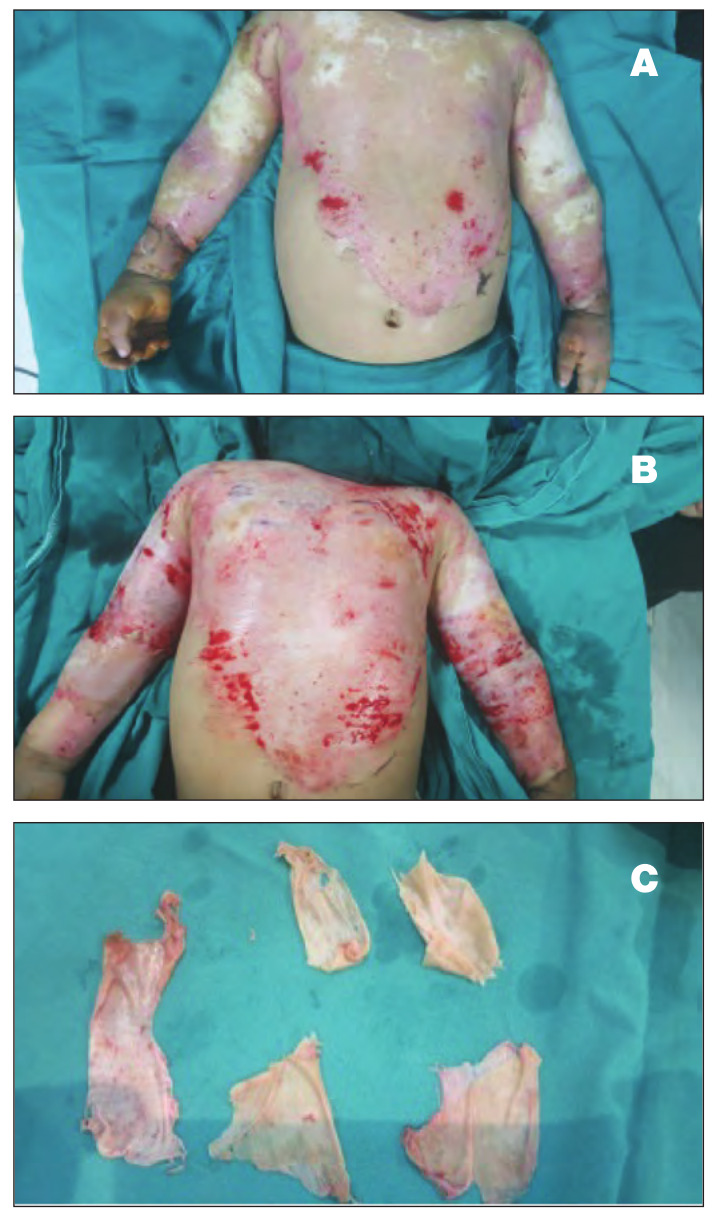
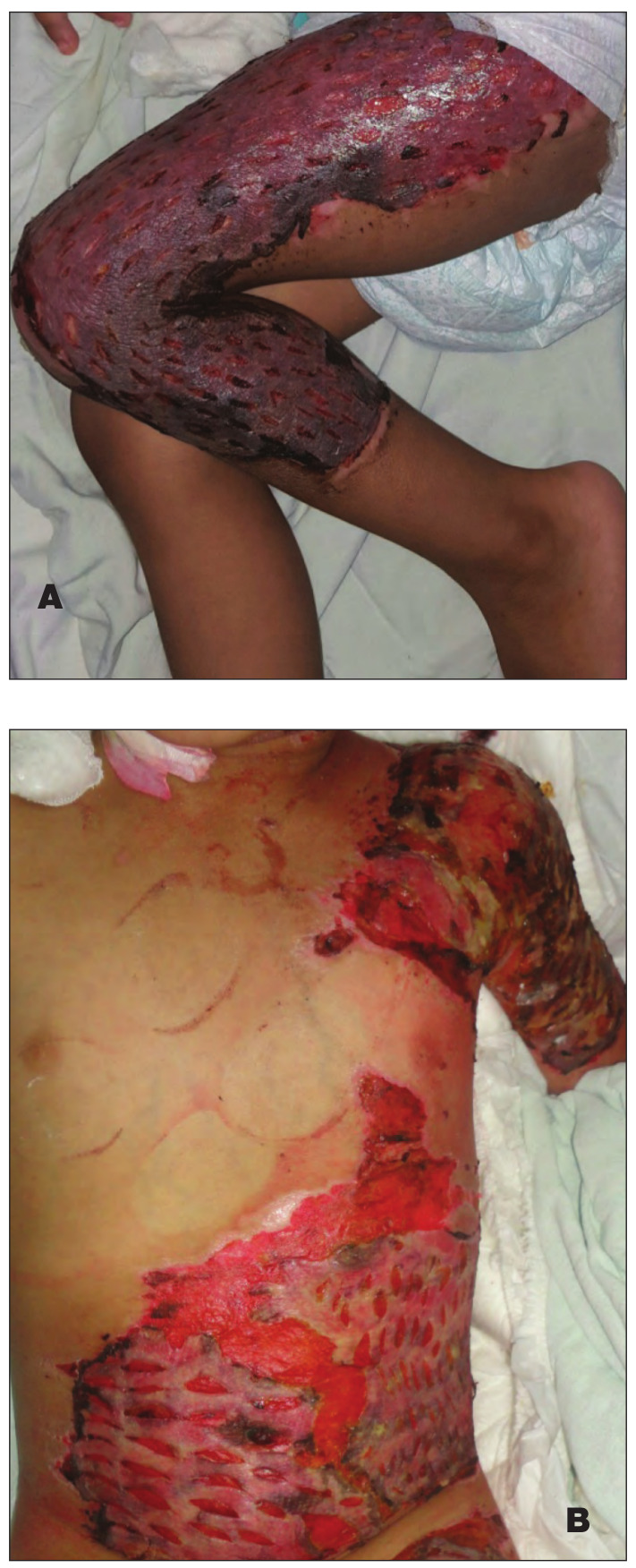
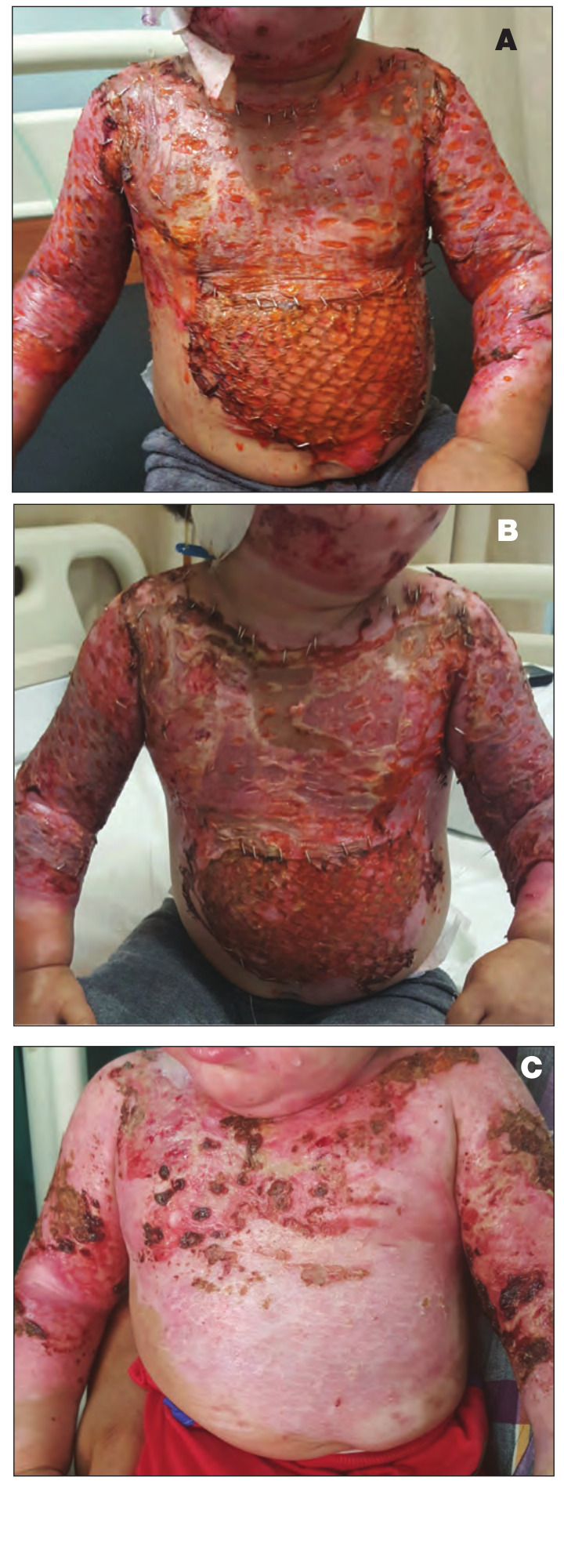
The most important observation in this study is that three patients with deep second-degree burns in Group III, in whom the burn area was covered by a skin allograft that was harvested from first-degree relative donors, showed complete healing of the burn wound without the need for autografting as there was more prolongation in the allograft survival period (Fig. 3).
Fig. 3. Male patient, 5 years old, with 25% deep dermal scald burn. A) Coverage of burn wound by split thickness allograft that was harvested from father (first-degree relative); B) Delayed and minimal signs of rejection, epithelialization with wound healing begins to appear in the granulated raw area 15 days after allograft coverage: C) Compete healing of all the burned area was observed 25 days after allograft coverage without the need for autografting.
Discussion
The approach to the management of burn injury depends on the percentage of total body surface area affected and burn depth. Superficial partial-thickness burns usually heal without the need for skin grafting. On the other hand, deep partial and full-thickness burns necessitate early tangential excision and skin grafting coverage to minimize wound infection and mortality.
There are restrictions for immediate autografting after tangential excision, such as limited donor site and poor general condition of the patient. The excised burn wound could be prepared for subsequent autograft by application of allograft as a temporary coverage with the concept of wound bed preparation.
Skin allograft provides a barrier function that limits desiccation of the wound, fluid loss and bacterial contamination, and protects underlying viable tissue. The main lines of attack on the allograft problem are host immune response and possibility of disease transmission.
In severely injured patients and limited donor sites, skin allograft is considered to be a reliable skin substitute for the coverage of excised wounds.9 Skin substitutes are used to restore the functional and aesthetic qualities of the skin, help prevent wound infection and maintain a moist wound environment.10 As cadaveric skin banking is not available in many countries, especially developing countries, human living skin allograft is considered to be a good skin substitute with low costs. It depends on donation from a relative or the availability of excised skin from body contouring operations like belt lipectomy or breast reduction in which the skin is usually discarded.
There are different types of allograft: fresh, cryo and glycerol preserved. In this study, the fresh allograft was used rather than the other types because of its fresh donation, viability and lower contamination. Cell viability of cryo and glycerol preserved is destroyed through the processing, and another problem is an increase in the possibility of infection. Microbial wound contamination is believed to be decreased after application of viable human cadaver allograft, which leads to improved vascularization of the allograft and subsequent autograft take; also, the fresh skin graft seems to be preferable compared to freeze dried skin.11 The survival of the skin allograft depends on the state of its viability, fresh donation and immunological relationship to the recipient.12
Pomahac et al. concluded that fresh allografts show less signs of immunologic rejection and in some critically ill patients represent an alternative method for permanent coverage.13 Abbott and Hembree concluded that freeze dried allografts do not seem to behave like freshly donated allogenic transplants.14 In their article, Bravo et al. noted that before cryopreservation, cadaver skin retains approximately 50% to 60% of the viability value of fresh living-donor skin. Immediately after cryopreservation these values decrease to approximately 40% to 50%.7
Skin taken from a live donor provides a ready source of skin substitute that does not require complex preparation or preservation, and can be used immediately after harvesting.15 The closer the skin donor is related to the recipient patient, the less the possibility of rejection.16
Phipps and Clarke said in their study that there are some specific advantages in skin that is derived from a parent rather than from an unrelated donor; the skin is freshly donated at maximum viability, avoiding the need for storage facilities, and the parent feels a significant participation in their child’s lifesaving.17
According to our study, there were statistically significant results as regards improvement in general condition and laboratory parameters after application of allograft that was harvested from a living first-degree relative donor for recipient burned patients in Group III. There was a significant improvement in plasma levels of WBCs, platelets, albumin, potassium and prothrombin concentration at the fifth day after burn coverage, as shown in Table I. Also, there was more prolongation in the graft survival period, a decrease in the percentage of completed rejected areas and a decrease in the percentage of patients developing local wound infection.
Chiu and Shah noticed that deep burn allografts or xenografts may become integrated.18 In patients in Group III, once the allograft had fixed to the prepared wound beds, it exhibited the features of revascularization in the first five days of application as part of the ‘take’ process, as occurs with autografting.
The skin is the most antigenic organ in the body, so the main obstacle to the skin allograft is body immune rejection and rapid sloughing of the graft after its application. First-degree relative donors share approximately 50% of the recipient’s genes with close HLA matching, thus allograft antigenicity is decreased and the ability of the recipient immune system to develop graft rejection allows the prolongation of allograft survival and hence facilitates closure of the wound. Allograft is the only temporary coverage; even if dermis may become incorporated, epidermis will eventually get rejected and patients will need subsequent autogonous epidermis. The prolongation of allograft survival with delayed appearance of rejection promotes granulation tissue formation, epithelialization and decreases the area that needs further autografting. Also, it helps to prepare the wound bed with healthy granulation tissue that improves subsequent autograft take and wound healing.
Among the most important observations in this study on the application of allograft, even with sloughing of the allograft there were many benefits from this procedure, including a decrease in the need for intravenous fluids, electrolyte replacement, analgesics, antibiotics and immunosuppressive drugs. Also, there were fewer episodes of tachycardia, tachypnea and fever, with a highly significant effect on general condition.
Conclusion
Human skin allograft is a good skin substitute for temporary coverage of major burn wounds with insuf ficient donor site for autografting. The closer the donor to the recipient patient, the less the possibility of graft rejection. Allograft harvested from a living first-degree relative is freshly donated at maximum viability and does not require complex preparation or preservation; also it shows prolongation of graft survival that helps to improve general condition, decreases microbial wound contamination, improves vascularization, and prepares the wound bed with healthy granulation tissue, resulting in promoting wound healing, improving subsequent autograft take and decreasing mortality rate among burned patients.
Acknowledgments
Funding.None
Conflict of interest.The authors declare no conflict of interest
References
- 1.Paggiaro AO, Bastianelli R, Carvalho VF, Isaac C, Gemperli R. Is allograft skin the gold-standard for burn skin substitute? A systematic literature review and meta-analysis. J Plast Reconstr Aesthet Surg. 2019;72(8):1245–1253. doi: 10.1016/j.bjps.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Khoo TL, Halim AS, Saad AZ, Dorai AA. The application of glycerol-preserved skin allograft in the treatment of burn injuries: an analysis based on indications. Burns. 2012;36(6):897–904. doi: 10.1016/j.burns.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Shakespeare PG. The role of skin substitutes in the treatment of burn in juries. Clin Dermatol. 2005;23(4):413–418. doi: 10.1016/j.clindermatol.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Horch RE, Jeschke MG, Spilker G, Herndon DN, Kopp J. Treatment of second-degree facial burns with allografts - preliminaryresults. Burns. 2005;31:597–602. doi: 10.1016/j.burns.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Hermans MH. Preservation methods of allografts and their lack of influence on clinical results in partial thickness burns. Burns. 2011;37:873–881. doi: 10.1016/j.burns.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Vloemans AFPM, Middelkop E, Kreis RW. A historical appraisal of the use of cryopreserved and glycerol-preserved allograft skin in the treatment of partial thickness burns. Burns. 2002;28:S16–S20. doi: 10.1016/s0305-4179(02)00087-6. [DOI] [PubMed] [Google Scholar]
- 7.Bravo D, Rigley TH, Gibran N. Effect of storage and preservation methods on viability in transplantable human skin allografts. Burns. 2000;26:367–378. doi: 10.1016/s0305-4179(99)00169-2. [DOI] [PubMed] [Google Scholar]
- 8.Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20:493–508. doi: 10.1097/01.ASW.0000288217.83128.f3. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher JL, Caterson EJ, Hale RG, Cancio LC. Characterization of skin allograft use in thermal injury. J Burn Care Res. 2013;34(1):168–175. doi: 10.1097/BCR.0b013e318270000f. [DOI] [PubMed] [Google Scholar]
- 10.Haddad AG, Giatsidis G, Orgill DP, Halvorson EG. Skin substitutes and bioscaffolds: temporary and permanent coverage. Clin Plast Surg. 2017;44(3):627–6634. doi: 10.1016/j.cps.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Atiyeh BS, Hayek SN, Gunn SW. New technologies for burn wound closure and healing - review of the literature. Burns. 2005;31:944–956. doi: 10.1016/j.burns.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 12.Burd A, Chiu T. Allogenic skin in the treatment of burns. Clin Dermatol. 2005;23:376–387. doi: 10.1016/j.clindermatol.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Pomahac B, Garcia JA, Lazar AJ, Tilney N, Orgill DP. The skin allograft revisited: a potentially permanent wound coverage option in the critically ill patient. Plast Reconstr Surg. 2009;123(6):1755–1758. doi: 10.1097/PRS.0b013e3181a65b1b. [DOI] [PubMed] [Google Scholar]
- 14.Abbott WM, Hembree JS. Absence of antigenicity in freezedried skin allograft. Cryobiology. 1970;6(5):414–416. doi: 10.1016/s0011-2240(70)80099-2. [DOI] [PubMed] [Google Scholar]
- 15.Saidi S. Live skin allograft in the management of severe burns. Annals of African Surgery. 2016;13()2:77–80. [Google Scholar]
- 16.Suthanthiran M. Clinical application of molecular biology: a study of allograft rejection with polymerase chain reaction. Am J Med Sci. 1997;313(5):264–267. doi: 10.1097/00000441-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Phipps AR, Clarke JA. The use of intermingled autograft and parental allograft skin in the treatment of major burns in children. Br J Plast Surg. 1991;44(8):608–611. doi: 10.1016/0007-1226(91)90100-x. [DOI] [PubMed] [Google Scholar]
- 18.Chiu T, Shah M. Porcine xenograft dressing for facial burns: beware of the mesh imprint. Burns. 2002;28(3):279–282. doi: 10.1016/s0305-4179(02)00009-8. [DOI] [PubMed] [Google Scholar]