Summary
Recent research found that enzymatic debridement clearly improves long-term scarring in burns. By reducing the spontaneous wound-healing period, scarring might be optimized. The latest publications show that wound healing can be accelerated by the application of platelet-rich fibrin (PRF). However to date no study that evaluates PRF treatment in burn wounds following enzymatic debridement has been published. We conducted a single-center prospective observational trial treating ten patients with partial thickness to deep dermal burns after enzymatic debridement with PRF. After wound treatment, the dressing remained untouched for five days. For wound healing, we compared different dressings and treatment options. Minimum pain and no signs of infection were observed during any of the treatments. Physicians were able to learn the manufacture of PRF quickly. For two early treatments, skin grafting was required. In one case, the dressing was removed too early. In a second case, the wait for spontaneous wound healing was not long enough. After a standardized treatment procedure was set, we found that results were clearly improving. Mean healing time of seven wounds treated with Suprathel® dressing was 18 days (min 9 days, max 21 days). PRF application might be useful to reduce healing time in partial thickness to deep dermal burn wounds that heal spontaneously after enzymatic debridement. Thus, scarring can be improved.
Keywords: platelet-rich fibrin (PRF), enzymatic debridement, partial thickness, deep dermal burn, reduction of healing time
Abstract
Les données récentes indiquent clairement que le débridement enzymatique (DE) diminue nettement les séquelles de brûlure, en accélérant leur cicatrisation (et l’on sait que la France est le seul pays d’Europe où cette technique est inutilisable, NDRLF). Les dernières publications montrent que cette cicatrisation peut être accélérée par l’utilisation locale de Fibrine Riche en Plaquettes (FRP). Cette technique n’a pas encore été évaluée couplée au DE. Nous avons évalué ce couplage auprès de 10 patients victimes de brûlures des 2èmes degrés intermédiaire et profond, le pansement étant laissé en place 5 jours après DE+PRP, plusieurs options ayant été essayées. La douleur restait minimale et aucune infection n’a été observée. Les praticiens ont facilement appris la préparation de FRP. Deux échecs ont été observés en début de série (nécessité de greffe). Dans un cas, le pansement a été enlevé trop précocement. Dans l’autre, la greffe a été décidée trop rapidement. La standardisation subséquente du protocole en a nettement amélioré les résultats. Le délai moyen de cicatrisation de 7 patients sous Suprathel® était de 18 j (9- 21). La FRP pourrait être utile à réduire le délai de cicatrisation des brûlures intermédiaires à profondes après débridement enzymatique et ainsi en limiter les séquelles.
Introduction
Post-burn scarring is one of the greatest unmet challenges in burn treatment.1 It has been found that more than 40% of patients following burn injury are not satisfied with their scars. In particular, facial scarring and scarring of the hands can stigmatize patients for life. Thus, approximately 15% of all burn patients cannot return to work, despite having completed rehabilitation. 2 Wound debridement is the initial step in the treatment of deep burn wounds.3 The long-term aesthetic outcome depends on the amount of vital tissue that can be preserved.4-8
Since the 1970s, tangential excision followed by skin grafting has been the gold standard in the debridement of deep dermal and full thickness burn wounds.9 However, results have not been satisfying regarding aesthetic and functional outcome.
In 2012 NexoBrid® (MediWound, Rüsselsheim, Germany), a new debridement agent based on a mixture of proteolytic enzymes, was introduced in the European market. Various studies found that this new debridement technique was much more selective than surgical tangential debridement.10-15 In a previous study on facial and hand burn injuries the selectivity of enzymatic debridement could be proven. By preserving vital tissue, the majority of these burn wounds healed spontaneously without skin grafting. 13,16 Thus, long term scar quality in partial thickness to deep dermal burn wounds was found to be improved compared to traditional surgical tangential debridement.17,18
Although debridement has been clearly improved by the use of NexoBrid®, severe burns of the face and hands still leave mild to moderate scarring, and the risk of hypertrophic scarring remains.6,18,19 Thus, there is need for further improvement of the treatment of severe burn wounds in general, with a special focus on wound healing after enzymatic debridement. Previous research showed that by reducing complications within the healing process and by accelerating the wound healing time, scar quality and the risk for pathological scarring can be clearly improved. 1 In the recent past it was shown by various studies that autologous platelet-rich fibrin (PRF) is a biomaterial suitable for improving and accelerating wound healing. PRF is commonly applied in dentistry for improving wound healing and bone regeneration. To the best of our knowledge, no study exists to date that investigates if PRF might also be suitable to improve wound healing in burn wounds following enzymatic debridement. The current study aims to answer this research question.
Materials and methods
The current study was reviewed and approved by the Ethical Review Committee of the University of Witten Herdecke, Germany (protocol no. 128/2017). All patients were willing to take part in the study and signed informed consent.
Patient selection
Ten patients with severe burn wounds, which were found suitable for enzymatic debridement, were included in the prospective observational study (Table I). Inclusion criteria were the following: (a) participants must be at least 18 years old, in good physical condition, both sexes, (b) enzymatic debridement was performed efficiently, (c) after enzymatic debridement wound depth was found to be partial thickness to full thickness (d) patients signed informed consent for application of PRF and participation in follow-up examinations up to 12 months after the initial treatment. Exclusion criteria were listed as (a) lack of consent and agreement to participate in the study and the required follow-up examinations, (b) pregnancy or nursing and (c) skin injuries caused by a long-term therapy with cortisone. Burn depth was evaluated by an independent physician, who was not informed about the study as described elsewhere.20,21 Wound healing was documented closely until wound closure was completed (defined as less than 5% remaining defect). Dressing changes, healing process, complications and adverse events were precisely stored in a database. All ten patients took part in the follow up examinations.
Material
Platelet-rich fibrin (PRF) is a fibrin-based biomaterial, which is produced from autologous patients’ blood by centrifugation. PRF contains thrombocytes and fibrinogen type one. If tissue is injured through a burn, thrombocytes are activated and migrate into the wound bed. They release chemokines, cytokines and growth factors, which are suitable to enhance the complex process of wound healing.22-25 It was shown that PRF promotes fibroblast proliferation, collagen synthesis and supports a sustained release of growth factors. The fibrin matrix plays an important role in the therapeutic potential of PRF.23 Firstly, the mesh structure captures cytokines and offers an extracellular matrix, which promotes angiogenesis. 26-28 By attracting neutrophils directly and via beta2 integrin receptors, fibrin supports the immune defense. Activated platelets are able to participate in adaptive immunity, interacting with antibodies.29 In addition, stem cells are trapped in the fibrin clot and can support defect regeneration.30,31 Furthermore, fibrin covers the wound and epithelial cells can easily migrate in the matrix. This ingrowth is enhanced by fibrin, fibrinogen and growth factors.23,29 In current clinical practice, PRF is not only used to stimulate the healing of chronic and infected wounds but also to promote bone regeneration.22,32
Treatment of the burn wound by PRF
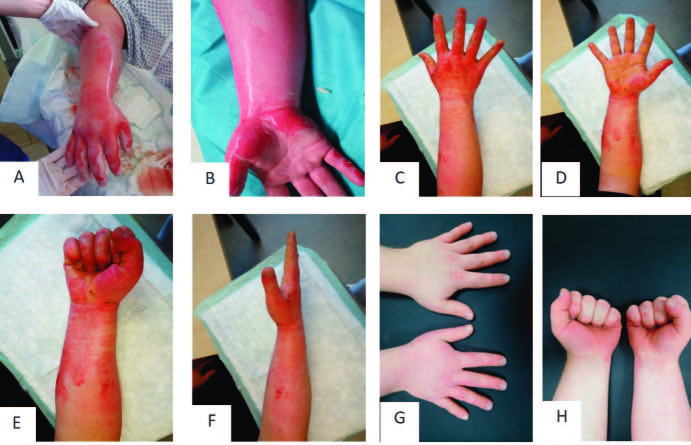
On the first day after admission, enzymatic debridement was performed as described previously (Fig. 1A-1C).6,9,33 After debridement was completed, patients were asked to take part in the study if they fulfilled the inclusion and exclusion criteria. Then, PRF was applied according to current treatment guidelines one day after enzymatic debridement (Fig. 2).6 The wound surface was rinsed with Prontosan ® (Polyhexanid) (B. Braun, Germany) disinfectant solution before treatment.
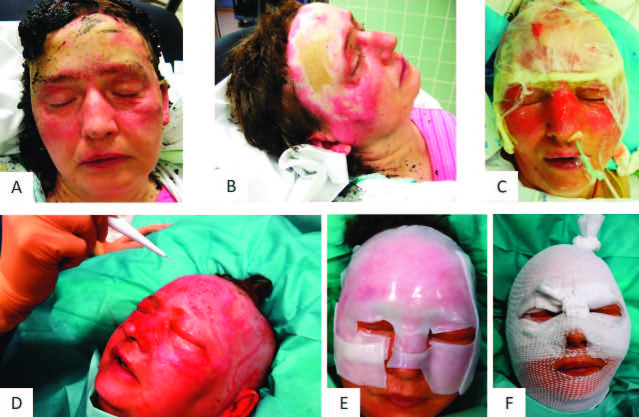
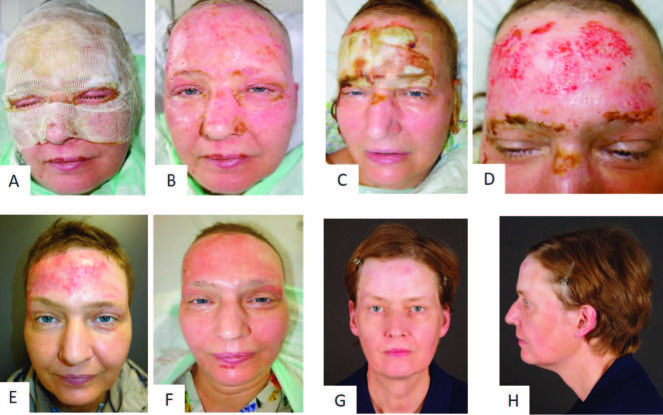
Fig. 1. Representative patient 6 with partial thickness to deep dermal facial burn treated with Vivostat™ PRF: (A) facial burn at arrival; (B) burn wound after mechanical cleaning; (C) application of enzymatic debridement; (D) application of Vivostat™ PRF; (E) covering the wound with Epicyte™ and a silicon layer; (F) second layer of cotton gauze and external head bandage.
Fig. 2. Vivostat™ PRF treatment: timeline of treatment procedure including enzymatic debridement.
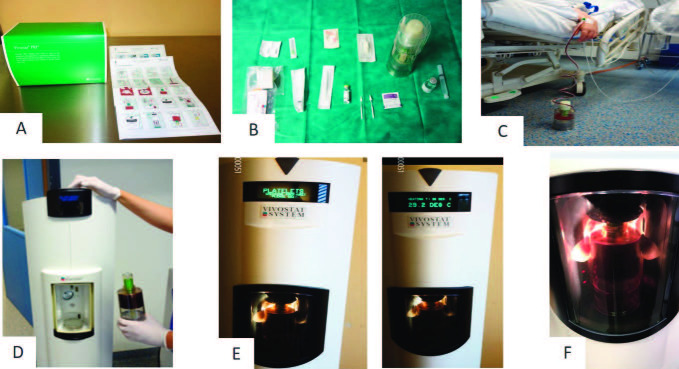
Autologous PRF was processed by the Vivostat® system (Vivostat A/S, Denmark). Treatment was described in detail by manufacturers’ instructions (Fig. 3A). All products for PRF production were included in the Vivostat® toolbox (Fig. 3B). Firstly, 120 ml of the patient’s whole blood was drawn in a collection chamber, which had been filled with a citrate solution beforehand (Fig. 3C). When the chamber was filled with blood, a delivery syringe filled with an acetate buffer was snapped in the chamber. After that, the collection chamber was positioned in a fully automated Vivostat® processor unit. By high centrifugation, the plasma was separated out and transferred to the reaction chamber. By a specific enzyme, fibrinogen was converted to fibrin 1. Fibrin 1 adhered during the rotation process to the wall of the chamber in a thin layer. It was then transferred back into the collection chamber. Then, PRF was mixed with the acid buffer in the reaction chamber and released into the delivery syringe. During the whole preparation process, temperature was kept constant at 36 degrees Celsius. PRF was prepared within approximately 25 minutes (Fig. 3D, 3E and 3F). Thus, the amount of PRF product was around 5-6 ml (Fig. 4). In order to start PRF to form a stable fibrin matrix on the wound surface, a solution with pH 10 was co-delivered during the application. Both solutions were sprayed simultaneously onto the wound surface (Fig. 1C, Fig. 4B and 4C). Finally, all wounds were covered by a wound dressing, a fatty and a cotton gauze and an elastic bandage.33 In the current study two different dressings were used to cover the wound surface. The first dressing was Suprathel® (PolyMedics Innovations GmbH, Germany), a synthetic, epidermal skin substitute that mimics the properties of natural epithelium. The second dressing was Epicyte hydro® (QRSKIN GmbH, Germany), a hydro-active wound dressing made of biotechnologically generated cellulose (Fig. 1E and 1F). Patients were asked to rate their pain on a 10-point Likert scale (1 = no pain) and 10 = maximum pain). Postoperative care and follow up examinations The first dressing change was performed five days after application. All patients were surveyed closely until wound closure was completed (defined as less than 5% remaining defect). A follow up examination was performed between 7 and 12 months after the initial treatment.
Fig. 3. Vivostat™ PRF treatment: (A) Vivostat™ toolbox and manufacturers’ instructions; (B) sterile table with content of Vivostat™ toolbox; (C) blood taken from patient in Vivostat™ container; (D) inserting Vivostat™ container in fully automated Vivostat® processor unit; (D, E, F) PRF is processed within approximately 25 minutes by centrifugation.
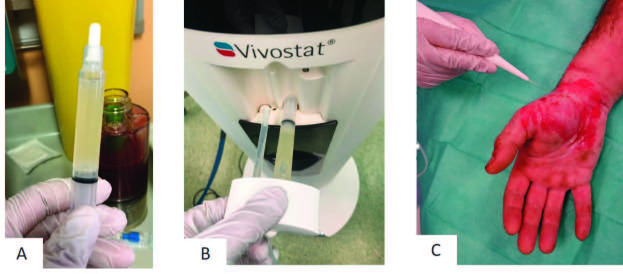
Fig. 4. Vivostat™ PRF application: (A) approximately 5-6ml PRF after centrifugation; (B) PRF and pH 10 solution are applied on the wound by Vivostat® applicator unit; (C) PRF and pH 10 solution are sprayed on the wound surface.
Results
Between June 2017 and October 2018, 10 partial thickness to deep dermal burn wounds were first debrided enzymatically and afterwards treated with PRF. Wounds were caused by flame (9 patients) and by hot tar (1 patient). Patients’ age ranged between 19 years and 73 years (average age 52 years). Two patients were female and 8 patients were male (Table I).
Two study physicians who quickly picked up both the handling of the device and the application of PRF performed all treatments. PRF was applied between the first day (six patients) and the second day (six patients) after debridement, and covered by a temporary dressing. Initially, PRF processing had to be repeated three times, because physicians had to understand the handling of the device. In one case (patient no. 3), due to poor vein conditions, not enough blood (120ml) could be taken to fill the preparation unit. Thus, PRF was not processed completely. The preparation process had to be repeated. When PRF was processed for patient no. 5, the physician forgot to add the delivery syringe filled with an acetate buffer to the collection chamber before positioning it in the processor unit. Thus, the system displayed an error. Unfortunately, the syringe could not be added at a later stage. Therefore, a second collection chamber had to be prepared and the process had to be restarted. During the PRF preparation for patient no. 4, the system displayed a sensor defect after the collection chamber was positioned in the chamber. Although the sensor was cleaned and the system was restarted, the process could not be finished. Finally, the treatment was repeated the next day. This time we made sure in advance that both the collection chamber and the chamber were completely clean (Table I).
Table I. Ten patients with partial thickness to deep dermal burns were treated first enzymatically. Burn wounds were treated with PRF the day after debridement.
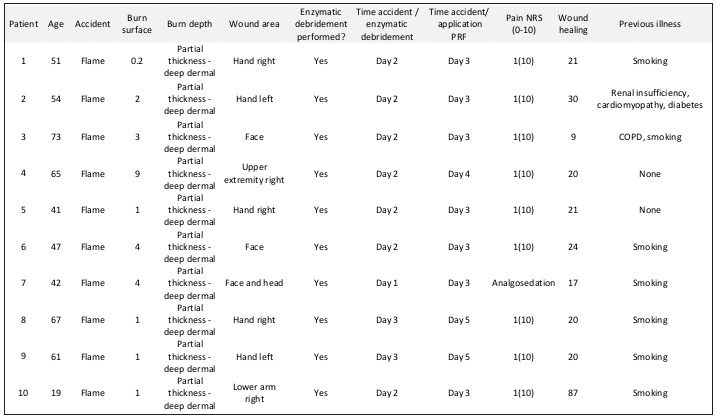
In sum, treatment areas were the face and head (three cases), hands (five cases) and upper extremity (two cases). The application of PRF was performed with minimal pain (1 out of 10/10-point Likert scale) under analgesia with Metamizole (1g intravenous). One treatment was performed under general anesthesia because the patient needed various additional surgical treatments. This patient remained intubated in the intensive care burn unit. Thus, PRF was applied under analgosedation the following day. This patient could not evaluate pain during the procedure.
During healing time, we found no signs of infection in any case. However due to different wound regimes, the course of healing and healing time was quite different among the ten patients.
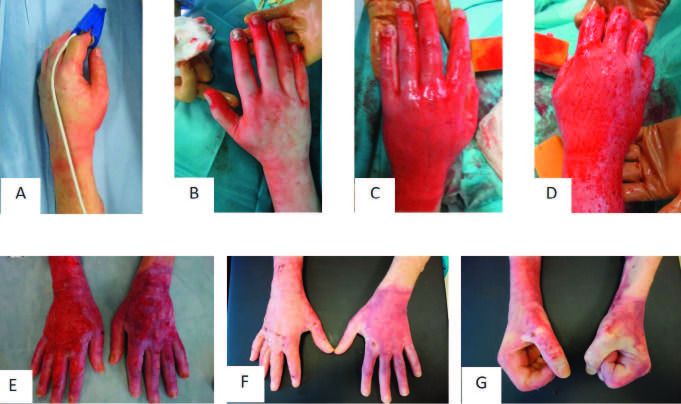
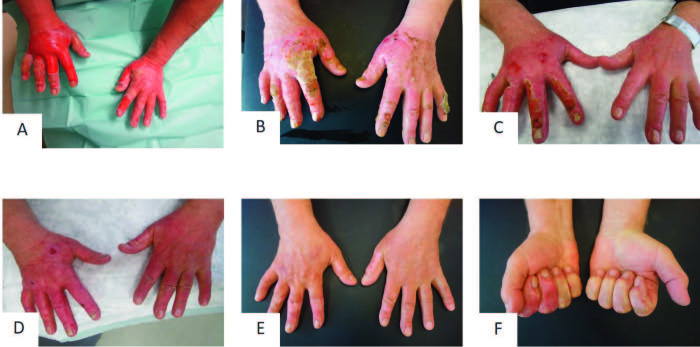
Patient 2 was admitted with a partial thickness to deep dermal burn on both dorsal hands of 2% TBSA (total body surface area) after flame burn (Fig. 5A and 5B). After enzymatic debridement on day 2, PRF was applied only on the left dorsal hand on day 3. Afterwards, both hands were covered with Epicyte hydro®. The patient was asked to keep the Epicyte hydro® dressing slightly moist by daily use of Prontosan® rinsing solution. Patient compliance was excellent but the patient complained about the great effort. Epicyte hydro® was changed every four to five days in our outpatient clinic. Due to insufficient wound healing on day 21 after initial treatment, the right dorsal hand was covered by skin grafts (Fig. 5E). The wound on the left hand was covered by Suprathel® dressing. Wound healing was completed at day 30 after initial treatment for both hands. After two months, the left hand was superior regarding functional and aesthetic outcome (Fig. 5F and 5G).
Fig. 5. Representative patient 2 with partial thickness to deep dermal burn of both hands, who was treated with Vivostat™ PRF on the left hand: (A) hand burn at arrival; (B) burn wound after mechanical cleaning; (C, D) result after enzymatic debridement; (E) wound healing at day 21 after application of Vivostat™ PRF and Epicyte hydro; (F) wound healing after spontaneous healing of the left dorsal hand and skin grafting of the right dorsal hand, (G) good function and aesthetic of the left hand after 2 months.
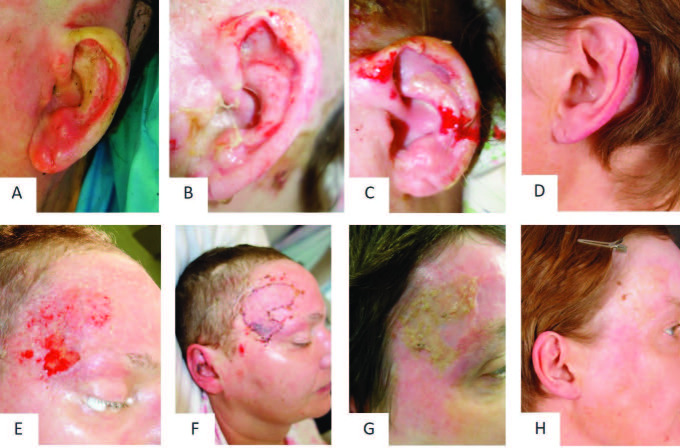
Patient 6 presented a partial thickness to deep dermal burn on the face and neck, with a total of 4% TBSA (Fig. 1A and 1B). Enzymatic debridement was successfully performed the day after admission. The next day, the wound was covered with Epicyte hydro® after PRF had been applied (Fig. 1C - 1F). The patient was asked to keep the wound bed moist by applying Prontosan® rinsing solution on a daily basis. First dressing change was performed five days after initial treatment (Fig. 6A and 6B). After 13 days, the dressing could be reduced to the forehead, the temples and both ears (Fig. 6C and 6D, Fig. 7A - C). As the wound healing was not completed at day 19 on the left ear and the left temple, we decided to perform skin grafting on the temple (Fig. 7F). On the left ear the cartilage was cut back, allowing a skin suture (Fig. 7C). The further healing process showed that the sheet graft on the temple led to an aesthetically unpleasant scar. In some areas the transplantation became detached and epithelialization started from beyond (Fig. 7G). For that reason, the graft was removed by ablative laser. Twelve months after burn injury, the aesthetic outcome of the burned areas on the face was satisfying, with a visible scar solely in the area of the skin graft (Fig. 7D and 7H).
Fig. 6. Wound healing in representative patient 6 with partial thickness to deep dermal facial burn after treatment with Vivostat™ PRF: (A) after removing external dressing at day 5; (B) after removing all dressings at day 5; (C) after removing external dressing at day 13; (D) wound healing at day 13; (E) scarring after 3 months; (F) scarring after 6 months; (G, H) scarring after 12 months.
Fig. 7. Detailed wound healing in ear and forehead of representative patient 6 with partial thickness to deep dermal facial burn after treatment with Vivostat™ PRF: (A) deep dermal burn of left ear at admission; (B) wound healing at the 15th day after enzymatic debridement and PRF treatment; (C) surgical excision of dried cartilage and secondary wound closure; (D) aesthetic outcome 12 months after initial treatment; (E) wound situation on right temple at day 19 after PRF treatment; (F) sheet graft transplantation; (G) scarring after 6 months; (H) outcome after treatment with laser ablation 12 months after initial treatment.
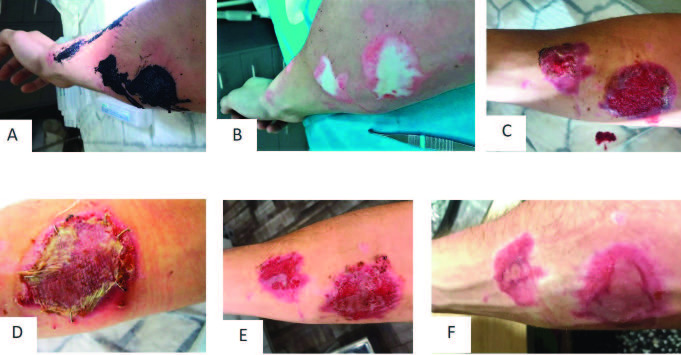
Patient 10 was admitted with a burn of 1% TBSA on the right arm caused by hot tar (Fig. 8A and 8B). After enzymatic debridement, PRF was applied the next day and the wound was covered with Suprathel®. Unfortunately, after hospital discharge an external physician removed the Suprathel® dressing before healing was completed. The wound bed became dry. Thus, surgical tangential necrectomy was necessary and the, then deeper, wound was covered with skin grafts (Fig. 8D). During the wound healing, the graft got lost partially and the remaining wound healed spontaneously (Fig. 8E and 8F). The entire treatment until wound closure took 83 days.
Fig. 8. Wound healing in the representative patient 10 with partial thickness to deep dermal burn of the right arm: (A) burn wound at arrival: (B) burn wound after mechanical cleaning; (C) reepithelialization under Suprathel® dressing; (D) wound healing after skin grafting; (E) wound healing after partial loss of skin graft and spontaneous reepithelialization; (F) aesthetic outcome 3 months after initial treatment.
The remaining seven wounds were covered with Suprathel® dressing after PRF application and healed without any complications (Fig. 9). After hand burns, patients were advised by a physiotherapist how to move the joints during healing time. The mean healing time in the last seven cases was 18 days (minimal healing time 9 days, maximum healing time 21 days). Two faces were found to heal faster (9 and 17 days) than the remaining wounds on the upper extremity (Table I). All patients were treated according to the standardized treatment protocol. However, two wounds were debrided on the second day after admission. Final wound treatment was postponed due to additional treatments of comorbidities in three cases (Fig. 2) while the wound bed was kept moist by Prontosan® application. Longterm functional and aesthetic outcome of the scars was good in all cases (Figs. 9 and 10).
Fig. 9. Wound healing in representative patient 8 with partial thickness to deep dermal burn of the right hand: (A) burn wound at arrival after mechanical cleaning; (B) reepithelialization under Suprathel® dressing at day 13; (C) wound healing at day 18; (D) wound healing at day 20; (E, F) aesthetic and functional outcome 7 months after initial treatment.
Fig. 10. hands, who was treated with Vivostat™ PRF on the left hand: (A) hand burn at arrival; (B) burn wound after mechanical cleaning; (C, D) result after enzymatic debridement; (E, F) wound healing at day 21 after application of Vivostat™ PRF and Epicyte hydro; (G) wound healing after spontaneous healing of the left dorsal hand and skin grafting of the right dorsal hand; (H) good function and aesthetic of the left hand after 2 months.
Discussion
It was found that PRF is suitable to improve wound healing in chronic wounds and can support bone generation.23,25,31,34,35 Studies that evaluate how PRF can affect healing of burn wounds following enzymatic debridement are missing. In an observational study design, we treated ten partial thickness to deep dermal burns with enzymatic debridement and PRF in an observational study design.
Scientific background of platelet-rich fibrin (PRF)
PRF is a concentrate of platelet-rich plasma protein derived from whole blood. By centrifugation, red blood cells are removed. Autologous platelets and leucocytes are present in a complex fibrin matrix to accelerate the healing of soft and hard tissue. Platelets are formed in the bone marrow from megakaryocytes. They have a lifespan of between eight and ten days and contain many proteins, platelet specific and non-platelet specific. Moreover, many molecules like collagen and thrombin insert into the platelet membrane.36 The main function of platelets is to contribute to haemostasis. A platelet plug is formed and the coagulation cascade is started. When the platelets are activated, they release 40 different growth factors, cytokines and chemokines. The interaction of these factors leads to angiogenesis, tissue regeneration and killing of wound bacteria, and allows the wound to heal in the long term.23,25,36-40
Handling of the preparation-unit and the Vivostat® device
Initially, we found that medical staff picked up the handling of the Vivostat® device quickly. The treatment is explained in detail in the manufacturers’ instructions. However, it is essential to remember that the pH4 acetate buffer has to be snapped into the collection chamber, before the chamber is inserted in the Vivostat® processor unit. If this important step is ignored, the preparation process will be interrupted at an early stage and cannot be started again. Furthermore, it is important to insert the chamber into the processor unit in the correct way. This means that the preparation-unit has to be placed in between metal spangling. Otherwise, the chamber will be twirled during centrifugation process and the processing will be stopped. In addition, the correct centrifugation program should be chosen. The user might choose between “Fibrin” and “Platelet” when starting the centrifugation program. In order to prepare PRF, “Platelet” is the right choice. When “Fibrin” is selected, the device prepares a fibrous tissue glue, which is only suitable for gluing the tissue together without improving wound healing. In order to prevent this selection mistake, a new type of preparation-unit is going to be introduced in the market in the near future.
The total amount of 120ml blood has to be collected from the patient. If the preparation-unit is inserted in the Vivostat® processor unit with a filling of less than 120ml, the preparation process will not start or it is interrupted. If the preparation-unit is not closed correctly after the collection process, blood will escape from the unit during the centrifugation process and the processing will be interrupted. For collecting 120ml blood from the patient, the hemoglobin level has to be checked in advance. In sum, inexperienced users have to make sure that the preparation-unit is used correctly following the manufacturer’s instructions, before starting the highly sensitive processing. In our study, three treatments were needed to overcome all problems mentioned above and to learn how to use the Vivostat® device correctly.
Enhancement of wound healing
After enzymatic debridement is completed, the wound is soaked overnight. The next day, PRF is applied. In order to guarantee an optimal wound healing, the wound surface has to stay moist until reepithelialization is completed. Within the first five to seven days after application, cytokines and growth factors are released continuously. Activation of thrombocytes happens step by step when they come into contact with the wound surface while the fibrin matrix is slowly reduced. Altogether, 40 different cytokines and growth factors are set free. Among them, human beta-defensin-2 (hBD-2) supports wound healing by stimulating keratinocyte migration and proliferation and by modulating angiogenesis.41,42
It was shown in various studies that by applying PRF, granulation can be clearly increased and even hard-to-heal wounds can achieve full healing or significant reduction in wound diameter.32,37,38,43,44 PRF is clearly superior to platelet-rich plasma (PRP), which is well known to support wound healing. It could be shown that in PRP platelet cytokines are released to quickly be built inside the fibrin matrix during polymerization. Furthermore, PRP is produced in a double centrifugation process and mixed with thrombin and calcium chloride.36 It was found that thrombin, which is suitable to activate fibrinogen type 1, has a negative effect on reepithelialization. Thus, thrombin reduces wound healing capacity. PRF prepared by the Vivostat® device does not contain thrombin. During the centrifugation process the plasma is divided from the resting blood elements. Instead of thrombin, the enzyme batroxobin is used to eliminate fibrinogen type 1. After becoming liquefied by adding a PH4 buffer, PRF can be stored over a maximum period of eight hours before it has to be activated. For activation, a PH 10 buffer is added and the solution can be applied on the wound surface.37,38,43,44
In our observational study we found proof that even problematic wounds healed well when PRF was applied. One more advantage is that by being fluid and easy to spray on the wound surface, PRF can even cover hard-to-reach and uneven wound surfaces like the interdigital space and body cavities. Moreover, various additional wound healing products and, for instance, stem cells or tranexamic acid, can be applied simultaneously. Most cytokines and rowth factors are set free between 24 and 72 hours after application of PRF.38 For that reason, no dressing change should be performed in the first five days after applying PRF to prevent PRF being removed from the wound surface before thrombocyte activation is completed.
Prevention of wound infection
The majority of chronic wounds are colonized by diverse bacterial species that prevent sufficient and uneventful wound healing.45 For that reason, a sufficient antimicrobial therapy is one important factor facilitating wound healing.22 More than 40 different cytokines and growth factors are released when thrombocytes are activated step by step. Among them, it was found that Vivostat PRF® can induce the antimicrobial peptides human beta-defensin-2 (hBD-2) and human beta-defensin-3 (hBD-3) in primary human keratinocytes in vitro and in vivo.46Antimicrobial activity can be found for hBD-2 against bacteria, yeast and viruses.47-49 The release of these proteins is increased more than 200-fold. Furthermore, platelet-released growth factors induce psoriasin in keratinocytes. Psoriasin is a multifunctional antimicrobial protein, which is expressed in keratinocytes. It acts as bacteriostatic and bactericidal, and it displays chemoattractant properties and regulates keratinocyte differentiation after skin injury.50-53 Psoriasin supports wound healing, angiogenesis, innate immunity and immune modulation.43,54 The maximum release is reached after 24 hours and lasts between five and seven days.38 In our study, we did not find any sign of infection, though healing time was prolonged due to spontaneous healing (minimum healing time was 9 days, maximum 87 days; Table I). By reducing infection rates, spontaneous healing is fast and scarring might be improved.55
Improvement of scar quality
Scar quality after severe burn injury can be optimized by preserving vital tissue during the initial surgical debridement and by making sure that the wound healing process is fast, without any complications.1,55 Enzymatic debridement was proven to preserve the maximum of vital tissue during the debridement process.6,17,18 Wound infection is among the main risks for scarring in deeper wounds due to prolonged healing time.1 As discussed before, PRF can both accelerate the wound healing process and reduce the risk of infection during this period.37,38,43,44 For that reason, it seems to be beneficial to apply PRF on the wound surface after enzymatic debridement. In the current observational study, we found that scar quality was good regarding function and aesthetics after seven and eight months (Fig. 9E and 9F, Fig. 7G and 7H) after spontaneous wound healing. However, we found that if we decided to perform skin grafting early, results after skin grafting were inferior compared to those wounds that healed spontaneously. Some transplants were even lost (Fig. 8D and 8E). In the current study, we found that Suprathel® dressing was superior to Epicyte hydro®. The reason was that the depth of burn wounds in this study was at least partial thickness to deep dermal. Therefore, they had to be kept moist during the whole healing period when Epicyte hydro® was chosen as a dressing. This required a high degree of patient motivation and was quite uncomfortable. However, wound healing was comparable to those wounds covered with Suprathel® dressing. One advantage of Epicyte hydro® could be soaking it in disinfecting solution before application. However, we did not find any wound infection, regardless which dressing we chose. In sum, more research is required to evaluate which dressing gives best results in combination with PRF on enzymatic debrided burn wounds. Suprathel® shows some disadvantages because it is an expensive dressing that creates an acidic wound environment and keeps the wound from drying out.56-58 However, these features are already fulfilled by the application of PRF. For that reason, it has to be questioned whether it is worth putting on such an expensive wound dressing in these cases or if an alternative dressing can be found.
Conclusion
The current observational trial indicates that the production process of PRF is convenient and can be picked up quickly by inexperienced users. The application is pain free and well tolerated by patients. First experiences offer evidence to suggest that wound healing is enhanced after enzymatic debridement by PRF application and the risk of infection is reduced. This might improve the aesthetic and functional outcome in the long term.
Limitations
This observational study also has limitations. On the one hand, only the first ten wounds, which we treated with PRF, were evaluated. Among them, we used two different dressings. Nevertheless, we intended to present our initial learning curve and main pitfalls of the treatment. We found that PRF application after enzymatic debridement in partial thickness to deep dermal burn wounds seems to be suitable to reduce healing time and might prevent infections. Furthermore, we presented a treatment al gorithm over three days. It has to be taken into consideration that PRF processing requires a physiological haemoglobin level. For spontaneous wound healing, patient compliance over a prolonged wound-healing period is needed. In a next step, treatment of partial thickness and deep dermal burns after enzymatic debridement and PRF application has to be evaluated on a larger scale in a prospective clinical study with special consideration to healing time and long-term scarring. This study is currently being performed by our group and will be presented in the near future.
Acknowledgments
Acknowledgements.The corresponding author has a consulting relationship with Vivostat®. No other research team members, writers or editors have any conflicts of interest, financial or intellectual. Vivostat® is funding an ongoing prospective clinical study dealing with burn treatment of faces and hands with PRF.
References
- 1.Finnerty CC, Jeschke MG, Branski LK, Barret JP. Hypertrophic scarring: the greatest unmet challenge after burn injury. The Lancet. 2016;388(10052):1427–1436. doi: 10.1016/S0140-6736(16)31406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Baar M, Essink-Bot M-L, Oen I, Dokter J. Functional outcome after burns: a review. Burns. 2006;32(1):1–9. doi: 10.1016/j.burns.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Ayello E, Baranoski S, Kerstein M, Cuddigan J. Wound care essentials: practice principles. Philadelphia: Lippincott, Williams & Wilkins; Wound debridement; pp. 117–126. [Google Scholar]
- 4.Hirche C, Citterio A, Hoeksema H. Eschar removal by bromelain based enzymatic debridement (Nexobrid®) in burns: a European consensus. Burns. 2017;43(8):1640–1653. doi: 10.1016/j.burns.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 5.Sander F, Rosenberg L. Verbrennungschirurgie”, Springer; 2016. Enzymatisches Débridement der Verbrennungswunde. pp. 99–104. [Google Scholar]
- 6.Schulz A, Perbix W, Shoham Y. Our initial learning curve in the enzymatic debridement of severely burned hands - management and pitfalls of initial treatments and our development of a post debridement wound treatment algorithm. Burns. 2017;43(2):326–336. doi: 10.1016/j.burns.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg L, Krieger Y, Bogdanov-Berezovsky A, Silberstein E. A novel rapid and selective enzymatic debridement agent for burn wound management: a multi-center RCT. Burns. 2014;40(3):4666–474. doi: 10.1016/j.burns.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg L, Lapid O, Bogdanov-Berezovsky A. Safety and efficacy of a proteolytic enzyme for enzymatic burn debridement: a preliminary report. Burns. 2004;30(8):843–850. doi: 10.1016/j.burns.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Janzekovic Z. A new concept in the early excision and immediate grafting of burns. J Trauma. 1970;10(12):1103–1108. [PubMed] [Google Scholar]
- 10.Choi M, Panthaki ZJ. Tangential excision of burn wounds. J Craniofac Surg. 2008;19(4):1056–1060. doi: 10.1097/SCS.0b013e318175f4f9. [DOI] [PubMed] [Google Scholar]
- 11.Hauben DJ, Mahler D. Histological investigation of burn eschar and the underlying recipient area in tangential early excision of burns. Burns. 1978;5(2):160–163. [Google Scholar]
- 12.Rosenberg L, Krieger Y, Bogdanov-Berezovski A, Silberstein E. A novel rapid and selective enzymatic debridement agent for burn wound management: a multi-center RCT. Burns. 2014;40(3):466–474. doi: 10.1016/j.burns.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg L, Krieger Y, Silberstein E. Selectivity of a bromelain based enzymatic debridement agent: a porcine study. Burns. 2012;38(7):1035–1040. doi: 10.1016/j.burns.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg L, Shoham Y, Krieger Y. Minimally invasive burn care: a review of seven clinical studies of rapid and selective debridement using a bromelain-based debriding enzyme (Nexobrid) Ann Burns Fire Disasters. 2015;28(4):264–274. [PMC free article] [PubMed] [Google Scholar]
- 15.Singer AJ, Taira BR, Anderson R, McClain SA, Rosenberg L. Reepithelialization of mid-dermal porcine burns after rapid enzymatic debridement with Debrase. J Burn Care Res. 2011;32(6):647–653. doi: 10.1097/BCR.0b013e31822dc467. [DOI] [PubMed] [Google Scholar]
- 16.Singer AJ, McClain SA, Taira BR, Rooney J. Rapid and selective enzymatic debridement of porcine comb burns with bromelain-derived Debrase: acute-phase preservation of noninjured tissue and zone of stasis. J Burn Care Res. 2010;31(2):304–309. doi: 10.1097/BCR.0b013e3181d0f4d4. [DOI] [PubMed] [Google Scholar]
- 17.Schulz A, Fuchs PC, Rothermundt I. Enzymatic debridement of deeply burned faces: healing and early scarring based on tissue preservation compared to traditional surgical debridement. Burns. 2017;43(6):1233–1243. doi: 10.1016/j.burns.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Schulz A, Shoham Y, Rosenberg L. Enzymatic versus traditional surgical debridement of severely burned hands: a comparison of selectivity, efficacy, healing time, and three-month scar quality. J Burn Care Res. 2017;38(4):e745–e755. doi: 10.1097/BCR.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 19.Schulz A, Fuchs PC, Rothermundt I. Enzymatic debridement of deeply burned faces: healing and early scarring based on tissue preservation compared to traditional surgical debridement. Burns. 2017;43(6):1233–1243. doi: 10.1016/j.burns.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Herndon DN. Total Burn Care: Expert Consult-Online. Elsevier Health Sciences. 2012 [Google Scholar]
- 21.Lehnhardt M, Hartmann B, Reichert B. Springer, Berlin, Heidelberg; 2016. Verbrennungschirurgie. [Google Scholar]
- 22.Bayer A, Lammel J, Lippross S. Platelet-released growth factors induce psoriasin in keratinocytes: implications for the cutaneous barrier. Annals of Anatomy -Anatomischer Anzeiger. 2017;213:25–32. doi: 10.1016/j.aanat.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Choukroun J, Diss A, Simonpieri A. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e56–e60. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Desai CB, Mahindra UR, Kini YK, Bakshi MK. Use of plateletrich fibrin over skin wounds: modified secondary intention healing. J Cutan Aesthet Surg. 2013;6(1):35–37. doi: 10.4103/0974-2077.110096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dohan DM, Choukroun J, Diss A. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: plateletrelated biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e45–e50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Van Hinsbergh VW, Collen A, Koolwijk P. Role of fibrin matrix in angiogenesis. Ann NY Acad Sci. 2001;936(1):426–437. doi: 10.1111/j.1749-6632.2001.tb03526.x. [DOI] [PubMed] [Google Scholar]
- 27.Gaultier F, Navarro G, Donsimoni J, Dohan DJI. Platelet concentrates. Part 3: clinical applications. Implantodontie. 2004;13(1):3–11. [Google Scholar]
- 28.Simonpieri A, Choukroun J, Girard M, Ouaknine T. Immediate post-extraction implantation: interest of the PRF. Implantodontie. 2004;13(177):89. [Google Scholar]
- 29.Loike JD, Sodeik B, Cao L. CD11c/CD18 on neutrophils recognizes a domain at the N terminus of the A alpha chain of fibrinogen. Proc Natl Acad Sci. 1991;88(3):1044–1048. doi: 10.1073/pnas.88.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bensaıd W, Triffitt J, Blanchat C, Oudina K. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24(14):2497–2502. doi: 10.1016/s0142-9612(02)00618-x. [DOI] [PubMed] [Google Scholar]
- 31.Yamada Y, Boo JS, Ozawa R. Bone regeneration following injection of mesenchymal stem cells and fibrin glue with a biodegradable scaffold. J Cranio Maxill Surg. 2003;31(1):27–33. doi: 10.1016/s1010-5182(02)00143-9. [DOI] [PubMed] [Google Scholar]
- 32.Steenvoorde P, Van Doorn L, Naves C, Oskam JJ. Use of autologous platelet-rich fibrin on hard-to-heal wounds. J Wound Care. 2008;17(2):60–63. doi: 10.12968/jowc.2008.17.2.28179. [DOI] [PubMed] [Google Scholar]
- 33.Schulz A, Perbix W, Shoham Y. Our initial learning curve in the enzymatic debridement of severely burned hands - management and pit falls of initial treatments and our development of a post debridement wound treatment algorithm. Burns. 2017;43(2):326–336. doi: 10.1016/j.burns.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Anitua E, Sánchez M, Nurden AT, Nurden P. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24(5):227–234. doi: 10.1016/j.tibtech.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Desai CB, Mahindra UR, Kini YK, Bakshi MK. Use of plateletrich fibrin over skin wounds: modified secondary intention healing. J Cutan Aesthet Surg. 2013;6(1):35. doi: 10.4103/0974-2077.110096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dohan DM, Choukroun J, Diss A. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e37–e44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Bayer A, Lammel J, Lippross S. Platelet-released growth factors induce psoriasin in keratinocytes: implications for the cutaneous barrier. Annals of Anatomy -Anatomischer Anzeiger. 2017;213:25–32. doi: 10.1016/j.aanat.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Bayer A, Lammel J, Rademacher F. Platelet-released growth factors induce the antimicrobial peptide human beta-defensin-2 in primary keratinocytes. Exp Dermatol. 2016;25(6):460–465. doi: 10.1111/exd.12966. [DOI] [PubMed] [Google Scholar]
- 39.Dohan DM, Choukroun J, Diss A. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e51–e55. doi: 10.1016/j.tripleo.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Velada JL, Hollingsbee DA, Menzies AR, Cornwell R, Dodd RA. Reproducibility of the mechanical properties of Vivostat® system patient-derived fibrin sealant. Biomaterials. 2002;23(10):2249–2254. doi: 10.1016/s0142-9612(01)00359-3. [DOI] [PubMed] [Google Scholar]
- 41.Suarez-Carmona M, Hubert P, Delvenne P, Herfs M. Defensins: “simple” antimicrobial peptides or broad-spectrum molecules? Cytokine Growth Factor Rev. 2015;26(3):361–370. doi: 10.1016/j.cytogfr.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Niyonsaba F, Ushio H, Nakano N. Antimicrobial peptides human β-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 2007;123(3):594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 43.Bayer A, Lammel J, Tohidnezhad M. The antimicrobial peptide human beta-defensin-3 is induced by platelet-released growth factors in primary keratinocytes. Mediators Inflamm. 2017 doi: 10.1155/2017/6157491. .https://doi.org/10.1155/2017/5671615 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bayer A, Tohidnezhad M, Lammel J. Platelet-released growth factors induce differentiation of primary keratinocytes. Mediators Inflamm. 2017 doi: 10.1155/2017/5671615. .https://doi.org/10.1155/2017/5671615 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jockenhöfer F, Chapot V, Stoffels‐Weindorf V. Bacterial spectrum colonizing chronic leg ulcers: a 10‐year comparison from a German wound care center. JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2014;12(12):1121–1127. doi: 10.1111/ddg.12540. [DOI] [PubMed] [Google Scholar]
- 46.Harder J, Bartels J, Christophers E, Schröder J. A peptide antibiotic from human skin. Nature. 19947;387(6636):861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 47.Crack L, Jones L, Malavige G, Patel V, Ogg GS. Human antimicrobial peptides LL‐37 and human β‐defensin‐2 reduce viral replication in keratinocytes infected with varicella zoster virus. Clin Exp Dermatol. 2012;37(5):534–543. doi: 10.1111/j.1365-2230.2012.04305.x. [DOI] [PubMed] [Google Scholar]
- 48.Rivas-Santiago B, Trujillo V, Montoya A. Expression of antimicrobial peptides in diabetic foot ulcer. J Dermatol Sci. 2012;65(1):19–26. doi: 10.1016/j.jdermsci.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 49.Laudien M, Dressel S, Harder J, Glaeser R. Differential expression pattern of antimicrobial peptides in nasal mucosa and secretion. Rhinology. 2011;49(1):107–111. doi: 10.4193/Rhino10.036. [DOI] [PubMed] [Google Scholar]
- 50.Lei H, Wang H, Zhang T, Chang L, Wu Y, Lai Y. TLR3 activation induces S100A7 to regulate keratinocyte differentiation after skin injury. Sci China Life Sci. 2017;60(2):157–167. doi: 10.1007/s11427-016-0027-2. [DOI] [PubMed] [Google Scholar]
- 51.Jinquan T, Vorum H, Larsen CG. Psoriasin: a novel chemotactic protein. J Invest Dermatol. 1996;107(1):5–10. doi: 10.1111/1523-1747.ep12294284. [DOI] [PubMed] [Google Scholar]
- 52.Gläser R, Harder J, Lange H, Bartels J. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nature. 2005;6(1):57. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- 53.Mildner M, Stichenwirth M, Abtin A. Psoriasin (S100A7) is a major Escherichia coli-cidal factor of the female genital tract. Mucosal Immunol. 2010;3(6):602. doi: 10.1038/mi.2010.37. [DOI] [PubMed] [Google Scholar]
- 54.Wittersheim M, Cordes J, Meyer-Hoffert U, Harder J, Hedderich J, Gläser R. Differential expression and in vivo secretion of the antimicrobial peptides psoriasin (S100A7), RN ase 7, human beta-defensin-2 and-3 in healthy human skin. Exp Dermatol. 2013;22(5):364–366. doi: 10.1111/exd.12133. [DOI] [PubMed] [Google Scholar]
- 55.Herndon DN. “Total burn care”. Elsevier Health Sciences. 2007 [Google Scholar]
- 56.Keck M, Selig HF, Lumenta DB, Kamolz LP. The use of Suprathel® in deep dermal burns: first results of a prospective study. Burns. 2012;38(3):388–395. doi: 10.1016/j.burns.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 57.Rahmanian-Schwarz A, Beiderwieden A, Willkomm LM, Amr A. A clinical evaluation of Biobrane® and Suprathel® in acute burns and reconstructive surgery. Burns. 2011;37(8):1343–1348. doi: 10.1016/j.burns.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 58.Schwarze H, Kuntscher M, Uhlig C. Suprathel®, a new skin substitute, in the management of partial-thickness burn wounds: results of a clinical study. Ann Plast Surg. 2008;60(2):181–185. doi: 10.1097/SAP.0b013e318056bbf6. [DOI] [PubMed] [Google Scholar]