Summary
Burn patients, especially children, experience many problems during their hospitalization. Because of their unique physiologic and altered pharmacokinetic profile, children receive more off-label prescribing than adults. The aim of this study was to analyze the incidence of off-label prescribing in burned children. This was a retrospective observational study conducted in the Dr. Soetomo General Hospital from December 2019 to March 2020. Data were collected from the medical records of burned children hospitalized over a 3-year period, from January 2017 to December 2019. Burn patients under 18 years old who received at least one prescribing medication were enrolled in this study. Twenty-six burned children met the inclusion criteria. A total of 215 medications were prescribed during this study and 35% of them were classified as off-label. The term off-label for age range was the highest among the off-label prescribing medicines, as much as 53%, with 30% classified for an unapproved indication, 15% for an unapproved dosage, and 2% for an unapproved dosage form. The prevalence of off-label prescribing was higher in children from 2 to 12 years old than in adolescents. Analgesics were the therapeutic classes most often prescribed as off-label in burned children. Most burned children are administered off-label medication with uncertain evidence. Further research is needed in this population to focus on several medications with high risk potential.
Keywords: children, burns, off-label
Abstract
Les brûlés, particulièrement les enfants, ont une hospitalisation émaillée d’incidents. En raison de leurs profils physiologique et pharmacocinétique spécifique, les enfants ont plus souvent des prescriptions hors AMM que les adultes, que cette étude a pour but d’analyser. Il s’agit d’une étude rétrospective observationnelle réalisée à l’hôpital général Dr Soetomo entre décembre 2017 et mars 2020 en revoyant les dossiers des 26 enfants (< 18 ans) hospitalisés pour brûlure. Deux cent quinze prescriptions ont été retrouvées, dont 35% hors AMM. C’est la non prise en compte de l’âge qui était la cause la plus fréquente de sortie de l’AMM (53%), suivie de 30% d’utilisation hors indication, 15% de posologie inappropriée et 2% d’utilisation non adaptée à la galénique. L’utilisation hors AMM était plus fréquente entre 2 et 12 ans. Les analgésiques était la classe la plus fréquemment utilisée hors AMM. L’utilisation hors AMM n’est pas fondée sur des preuves solides et doit être plus largement explorée, en particulier concernant les médicaments les plus potentiellement dangereux.
Introduction
Burn injury is a condition characterized by tissue damage or loss due to fire, heat, chemicals, electrical contact or radiation. Burn is a major health problem all over the world because of high morbidity and mortality, complex wound healing and long-term debilitating effects. More than a half of burns in children occur accidentally.1 Several studies have stated that burn injuries in children are the third most deadly injury.2 Burns are related to the risk of death in children. Those who survive will suffer from disabilities and psychological trauma as a result of pain and prolonged treatment in hospital.3
Burn patients during treatment in the hospital receive multiple medications such as antibiotics, analgesics, fluid resuscitation, inhibitor of gastric acid secretion, and many more. The multiple medications lead more likely to off-label use. Unfortunately, more than half of the medicines used in burned children do not yet have established data related to their efficacy and safety. More than 75% of medicines approved by the Food and Medicine Administration (FDA) lack dosing, safety and efficiency information for use in children. As a consequence, physicians often prescribe off-label medicines, guided by clinical judgment as well as by several case reports with low quality evidence. Information about dosing, effectiveness and safety of medicines in adults cannot be easily extrapolated to children. It is known that physiological changes in the pediatric age affect the pharmacokinetic and pharmacodynamic properties of several medicines and the dose needs to be adjusted based on their age.
‘Off-label’ is the administration of an authorized marketing medicine that is used for a condition, at a dose, age, indication and route of administration that is not listed in the FDA marketing authorization or in its product characteristics.4 Children are at risk of receiving off-label medicines because of the limitation of clinical trials, incomplete pharmacokinetic and pharmacodynamic data and side effects of a medicine. The limitations of clinical trials related to the effectiveness and safety of a medicine for children are due to ethical issues.5 Therefore the administration of medicines to children is often based on data for adults.
In Indonesia, no study has evaluated the medicines to be used in children with burn injury. To the best of our knowledge, this is the first study in Indonesia that evaluates the off-label use of medicines in burned children. Based on this background, the aim of this study was to analyze the use of off-label prescribing in burned children.
Materials and methods
This was an observational retrospective study of burned children who were admitted to the burn center of Dr. Soetomo Hospital. Data of burned children were taken from medical records over a 3-year period, from January 2017 to December 2019. This study took place from January to March, 2020. Data including sex, age, cause of burns, TBSA, depth of injuries, medicine name, dosage and route of administration were recorded for further analysis. To analyze “off-label use”, the British National Formulary (BNF) for Children edition 2018-2019,6 Medicine Information Handbook (DIH),7 and patient information leaflets were used in this study. The inclusion criteria were children and adolescents with burn injury aged less than 18 years old, who received at least one off-label prescribing medication during hospitalization. Descriptive statistics were used for the demography of burned children as well as the frequency of off-label prescribing. In order to analyze the correlation between total body surface area (TBSA) and length of stay (LoS) as well as LoS and frequency of off-label prescribing, the Pearson correlation was used. P value less than 0,05 was considered statistically significant.
Results
Out of a total 26 cases of burned children over the 3-year period, only 24 patients were found to have received off-label prescribing medications. The most common depth of burn was second-degree burn (IIAB) more than 20% TBSA, in 50% of total patients. The distribution of patients by demographic characteristics is shown in Table I. Sixteen burned patients (67%) were male, with an age range of 1 to 17 years old. The largest group in this study consisted of children aged 2 to 12 years old, accounting for 58% of the burned child patients, followed by infants (21%), and adolescents (21%), as shown in Table II.
Table I. Demographic characteristics of the 24 burned children who received off-label prescribing medications.

Table II. Off-label prescribing by age group (n=24).
As many as 215 medications were prescribed for 24 burned children during this study, with an average of 8.9 prescriptions per patient. Of these 215 medicines, 35% of them were written off-label. Eighteen patients (75%) of the 24 burned children received at least one off-label prescribing medication. Twentyfour patients received a mean 2.87 ± 1.89 off-label prescribing medicines, ranging from 1 to 6. Overall, analgesics were the highest off-label medicines (40%), followed by medicine classes on immune function (27%), antibiotics (10%), and medicines that inhibit gastric acid (10%). To analyze the correlation between TBSA and LoS, as well as LoS and off-label prescribing medicines, a Pearson correlation was used. There was a significant positive correlation between TBSA and length of stay (LoS). This means the higher the TBSA, the longer the hospital stay, as shown in Table III. Another finding was that there was a significant correlation between length of stay and off-label prescribing medications (p value=0.008, r=0.527). This indicates that the longer the days of hospitalization, the higher the number of off-label prescribing medicines.
Table III. Correlation between TBSA and LoS (n=24).
Based on the classification of off-label, the term off-label for an unapproved age range was highest among the off-label prescribing medicines, with more than 50%, followed by an unapproved indication, an unapproved dosage and an unapproved dosage form, as shown in Fig. 1.
Fig. 1. Classification of off-label in burned children.

The most common off-label prescribing medicines in this study were metamizole 23 (32%), followed by probiotics preparations 11 (15%), and zinc sulfate 9 (12%). Metamizole was the most written off-label medicine for an unapproved age range in burned children, as shown in Table IV.
Table IV. Off-label for unapproved age range in burned children.
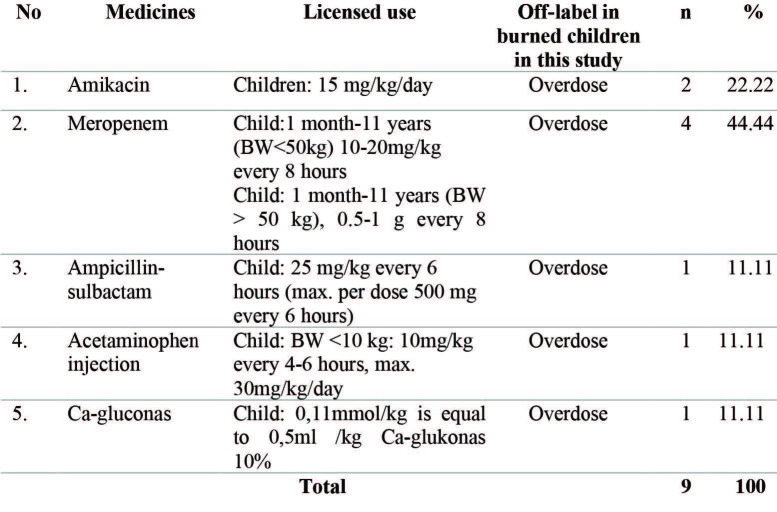
Meropenem was the most common medicine (44.44%), followed by amikacin (22.22%) in offlabel for an unapproved dose, as shown in Table V. This means that the antibiotic class was the highest among the off-labels for an unapproved dose in burned children.
Table V. Off-label for unapproved dose in burned children.
Probiotic preparations were most commonly used in off-label for an unapproved indication, followed by zinc sulfate, as shown in Table VI.
Table VI. Off-label for unapproved indication in burned children.
In children, the oral liquid preparation is the most suitable to be administered due to it being easy to swallow, and with a better taste than solid dosage forms such as tablets or capsules. In the classification of off-label for an unapproved dosage form, only potassium chloride was included, as shown in Table VII.
Table VII. Off-label for unapproved dosage form in burned children.
Discussion
Pediatric is an age group that needs special consideration in medicine administration. A child is not a “mini adult”, therefore the dosage should be given carefully based on body weight or body surface area. Small errors in the dosage and duration of treatment can lead to harmful effects. Interestingly, we found a significant positive correlation between the percentage of TBSA and LoS. Our study was similar to a study by Romero et al., that reported there was a strong and significant positive correlation between %TBSA and LoS (r=0.76). It showed that LoS increased as burn severity worsened.8 The greater the percentage of TBSA, the more frequent debridement surgery will be performed. A study by Bourgi et al. reported that in pediatric burn patients, LoS was significantly increased with age, TBSA, sepsis, escharotomy and cause of burn. The number of surgeries, burn excisions, as well as skin grafting and blood transfusion were significantly associated with LoS in burned children.9 We also found a significant correlation between LoS and off-label prescribing medicines. It showed that the longer the days of hospitalization, the higher the incidence of off-label prescribing medicines. In hospitalized patients, many medicines were administered based on the patient’s condition. However, many of these medicines do not yet have effectiveness data for use in children, so the use of off-label medicines increased.
Accordingly, the overall off-label prescribing medicines in our study was 35% in 24 burned children who met the inclusion criteria. Our findings were similar to the study by Hiltraud et al., which reported that the percentage of off-label use was 30%.10 A higher finding of off-label use was reported by Tefera et al., who showed that the prevalence of off-label use was 75.8%.11 It might lack pediatric product labelling, which is the most common reason to use off-label medicines in pediatric patients in most studies.12
The rate of off-label prescribing medicines in our study was higher in males than females. This was because, based on demographic data, there was a higher percentage of males than females. Metamizole, or dipyrone, was the highest percentage of offlabel prescribing medicines in our study. In several countries, such as the USA, Sweden, the UK, and other countries in Europe, metamizole, or dipyrone, was banned because harmful for agranulocytosis. However it is still available in Indonesia as a prescription medicine both in oral and parenteral preparations. The risk of agranulocytosis is higher with increasing duration of administration, and disappears after 10 days from the last dose. In our study, burned children were administered metamizole as an analgesic to control pain due to burns and after surgery (debridement). It is supposed that metamizole has minimal side effects in the gastrointestinal tract and does not affect renal function like the other NSAIDs. Metamizole is contraindicated in children less than 7 years old.13 Information about metamizole relating to age, dose, dosage form, and routes of administration to be used in children both as an analgesic and antipyretic was unclear.14
It is recommended to manage burn pain in children with acetaminophen with a dose of 10-15 mg/kg every 6 hours unless contraindicated. Besides that, NSAIDs such as ibuprofen 10 mg/kg every 8 hours over 6 months of age, or diclofenac 1-2 mg/kg twice daily over 5 years old should be added if pain control is not successful.15 In order to manage acute pain in burns, a multidisciplinary approach should be taken. Pain management, especially in burns, not only focuses on controlling the somatic component that triggers pain, but also involves comprehensive areas physically, emotionally and psychologically as well as pathophysiologically of pain.16 The second analgesic that was often used as an off-label prescribing medicine in our study was ketorolac. Ketorolac has a potent analgesic effect due to strong inhibition of cyclooxygenase-1 (COX-1) to reduce pain due to burns and pain after surgery as well. Ketorolac must be used carefully in children because of harmful side effects such as GI bleeding. A systematic review reported that the use of ketorolac in children was not supported by good evidence or very low-quality evidence and its safety was not established. 17 A pharmaco-vigilance study reported the adverse events of ketorolac in children under 16 years old: the most prevalent adverse event was gastrointestinal disorders (25%), followed by allergic and anaphylactic reaction (14.6%). The duration of parenteral ketorolac was limited to two days and oral administration of ketorolac should be continued up to seven days. The recommended dose of intravenous ketorolac in children was 0.5mg/kg as an initial dose, followed by 1.0mg/kg every 6 hours as the subsequent dose with a maximum daily dose of 90 mg.18 In terms of using ketorolac in children as an analgesic, the physician must consider the duration and the dose to avoid undesirable effects.
In our study, sucralfate and omeprazole were used to prevent GI ulcers due to burns. A study by Choi et al. reported that in burn patients, total body surface area more than 20% and presence of epigastric pain were significantly associated with GI ulcers.19 Patients with severe burns with TBSA more than 20% were susceptible to burn shock, leading to hypoperfusion to GIT, which causes mucosal disruption and atrophy.20
It has been argued that the use of proton pump inhibitors, especially for a long period, was associated with the risk of respiratory tract infection as well as pneumonia. Gastric acid serves as a protective barrier to pathogenic bacteria that are ingested by mouth. Proton pump inhibitors increase the pH of gastric acid and therefore increase the colonization of gastric bacteria. The pathogens move from the GIT into the lungs and cause pneumonia.21 Several studies reported community-acquired pneumonia (CAP) or respiratory infection as adverse effects of PPI in children.22,23
Meropenem was the antibiotic most prescribed as an off-label medicine in our study. Meropenem was used to treat systemic infection related to burns. Meropenem has one of the broadest spectra of antibiotic activity because of its stability against the most extended spectrum beta- lactamase. Furthermore, its stability increases the potency of meropenem against many antibiotic-resistant bacteria commonly responsible for life-threatening systemic infections among young children.24
Meropenem is currently approved by the Food and Drug Administration (FDA) to be used in children over 3 months of age to treat infections such as bacterial meningitis and/or complicated intra-abdominal infections; however, the use of meropenem in infants under three months is considered as off-label due to inadequate data of dosing regimentation, efficacy and safety. There is a substantial off-label use of meropenem in infants under three months of age.25 Meropenem is well tolerated in children. A study by Linden et al. reported that in children who received meropenem in a dose 10-40 mg/kg three times daily, the incidence of seizures was 0.37%, lower than imipenem/cilastatin at 0.43%.26 This is because meropenem has less affinity to receptors of gamma-aminobutyric acid than imipenem. We know that gamma-aminobutyric acid is responsible for adverse neurotoxicity effects.27,28
Another category of off-label prescribing medicines in our study was off-label for an unapproved indication: the probiotic preparation and zinc sulfate were the highest percentage in this classification. Probiotics are microorganisms that live in the host body in an adequate amount that will provide health benefits to the host. Probiotics have an impact on intestinal microbiota or enhance immune function. Probiotic bacteria (Lactobacillus and Bifidobacterium) produce lactic acid resulting in a decrease in pH of the GIT that inhibits pathogenic bacteria. Several studies have reported the beneficial effects of probiotics in burn patients. The administration of systemic antibiotics to treat infection or sepsis in burn patients for a long time leads to an imbalance of gut bacteria, and the use of probiotics seems beneficial to reduce and shorten the incidence of diarrhea. A study by El-Ghazali et al. showed that the use of probiotics in children with burns significantly reduced diarrhea frequency compared to the control group (without probiotics).29 The use of probiotics was considered safe with minimal adverse effects. The study by Mayes et al. showed that in the administration of probiotics in acutely burned pediatric patients, the incidence of flatulence was significantly higher than in the control group.30 In our previous study on adult burn patients, we found that the administration of both mono and multi strain probiotics significantly increased IgA level and reduced IL-6 circulation level without any side effects.31
Several studies have shown that the plasma level of zinc is significantly decreased after burns due to excessive excretion of zinc in the urine. Deficiency of zinc is associated with prolonging of wound healing. A study by Voruganti et al. reported that in burn patients, zinc concentration was higher in wound exudates than in plasma. This means a lower plasma zinc concentration because of high loss through wound exudates.32 A study by Coutris et al. showed that in the administration of zinc sulfate 50 mg daily, in 83% of 23 patients the serum level of zinc increased to normal level. Unfortunately, in our study the serum level of zinc was not measured, whether the serum concentration of zinc was low or not. Another consideration to use zinc supplementation is the side effects of GIT, including nausea, vomiting and gastrointestinal discomfort. Although zinc sulfate is relatively safe, the side effects were gastrointestinal disturbances. Nausea and vomiting occurred in 4 out of 26 patients, but zinc supplementation was still continued. 33 The administration of dispersible zinc in children in a dose of 20 mg had a 14% increased risk of vomiting at the first dose. This vomiting is light, transient, and occurred 10 minutes after the administration of zinc.34
In our study, KCl was administered in an oral solution to correct hypokalemia. This was because KCl oral solution is not available in our country. KCl oral solution was prepared by dissolving KCl into distilled water. The adverse effects of KCl are related to the route of administration. Oral formulations of KCl were most commonly correlated with GI discomforts, such as vomiting and diarrhea. Tablet and capsule dosage forms of KCl may cause GI disturbances with prolonged exposure to GI surfaces.35 The limitations of our study were its small sample size and we did not evaluate the possible adverse effects due to the use of off-label medicines in burned children. Further study is needed with a large number of patients to clarify the possible undesirable effects of off-label prescribing medicines in burned children.
Conclusion
It can be concluded that the term off-label for an unapproved age was the highest among the off-label prescribing medicines, as much as 53%, with 30% classified as for an unapproved indication, 15% for an unapproved dosage, and 2% for an unapproved dosage form. Most burned children are administered off-label medication with uncertain evidence. Clinicians and pharmacists should collaborate with each other to consider the use of off-label medicine in burned children by weighing the risks and benefits of these medicines.
Acknowledgments
Conflict of interest.No conflict of interest to declare replacement in clinical practice: a contemporary review by the National Council on Potassium in Clinical Practice
References
- 1.Torabian S, Saba MS. Epidemiology of pediatric burn injuries in Hamadan, Iran. Burns. 2009;35(8):1147–1151. doi: 10.1016/j.burns.2009.06.194. [DOI] [PubMed] [Google Scholar]
- 2.Al-Zacko SM, Zubeer HG, Mohammad AS. Burned children in Mosul: an epidemiological study. Ann Burns Fire Disasters. 2014;27(2):70–75. [PMC free article] [PubMed] [Google Scholar]
- 3.Kai-Yang L, Zhao-fan X, Luo-Man Z. Epidemiology of burned children requiring hospitalization in China: a literature review of retrospective studies. Pediatrics. 2008;122(1):132–142. doi: 10.1542/peds.2007-1567. [DOI] [PubMed] [Google Scholar]
- 4.Bavdekar SB, Sadawarte PA, Gogtay NJ. Off-label medicine use in a Pediatric Intensive Care Unit. Indian J Pediatr. 2009;76:1113–1118. doi: 10.1007/s12098-009-0238-3. [DOI] [PubMed] [Google Scholar]
- 5.Frattarelli DA GJ, Green TP. Off-label medicines in children. Pediatrics. 2014;133:563–567. doi: 10.1542/peds.2013-4060. [DOI] [PubMed] [Google Scholar]
- 6.British Medical Association and Royal Pharmaceutical Society of Great Britain. London: 2019. British National Formulary (BNF) for Children, 78th Edition. [Google Scholar]
- 7.Lacy CF. Lexi-Comp Inc & Apha North American. American Pharmaceutical Association; 2009. Medicine Information Handbook. [Google Scholar]
- 8.Mendez-Romero D, Clark AT, Christie A. Weight changes and patterns of weight measurement in hospitalized burn patients: a contemporary analysis. Burns & Trauma. 2018;6(30):1–7. doi: 10.1186/s41038-018-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourgi J, Yaacoob E, Berberi M. Factors affecting length of stay among pediatric and adult patients admitted to the Lebanese burn centre: a retrospective study. Ann Burn Fire Disasters. 2019;32(3):216–221. [PMC free article] [PubMed] [Google Scholar]
- 10.Hitraud K. Off-label medicine use in children and adolescents: results of a population-based study in Germany. BMC Public Health. 2013;13:631–631. doi: 10.1186/1471-2458-13-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tefera YG, Gebresillassie BM, Mekuria AB. Off-label medicine use in hospitalized children: a prospective observational study at Gondar University Referral Hospital, Northwestern Ethiopia. Pharma Res Per. 2017;5(2):1–6. doi: 10.1002/prp2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimland E, Odlind V. Off-label medicine use in pediatric patients. Clin Pharmacol Ther. 2012;91(5):796–801. doi: 10.1038/clpt.2012.26. [DOI] [PubMed] [Google Scholar]
- 13.Nikolova I, Petkova V, Tencheva J. Metamizole: A review profile of a well-known “forgotten” medicine. Part II: clinical profile. Biotechnol & Biotechnol Eq. 2012;27(2):3605–3615. [Google Scholar]
- 14.De Leeuw TG, Dirckx M. The use of dipyrone (metamizol) as an analgesic in children: What is the evidence? A review. Pediatric Anesthesia. 2017;27(12):1193–1201. doi: 10.1111/pan.13257. [DOI] [PubMed] [Google Scholar]
- 15.Marret E, Kurdi O, Zufferey P. Effects of nonsteroidal, anti-inflammatory medicines on patient-controlled analgesia morphine side effect: meta analysis of randomized, controlled trials. Anesthesiology. 2005;102:1249–1260. doi: 10.1097/00000542-200506000-00027. [DOI] [PubMed] [Google Scholar]
- 16.Jerez C, Elena L, Ribero G. Management of acute pain in extensive burn injury: nonsystemic review of the literature. Rev Col Anest. 2018;46(1):49–54. [Google Scholar]
- 17.McNicol ED, Rowe E, Cooper TE. Ketorolac for postoperative pain in children. Cochrane Database of Systematic Reviews. 2018;7 doi: 10.1002/14651858.CD012294.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forest JB, Heitlinger EL, Revell S. Ketorolac for postoperative pain management in children. Medicine Safety. 1997;16(5):309–329. doi: 10.2165/00002018-199716050-00003. [DOI] [PubMed] [Google Scholar]
- 19.Choi YH, Lee JH, Shin JJ. A revised risk analysis of stress ulcers in burn patients receiving ulcer prohylaxis. Clin Exp Emerg Med. 2015;2(4):250–255. doi: 10.15441/ceem.15.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herndon DN. Total burn care. 4th. Philadelphia, PA: Saunders; 2012. [Google Scholar]
- 21.Craven DE, Steger KA. Epidemiology of nosocomial pneumonias: new perspectives on an old disease. Chest. 1995;108(2suppl):1S–16S. doi: 10.1378/chest.108.2_supplement.1s. [DOI] [PubMed] [Google Scholar]
- 22.Sultan N, Nazareno J, Gregor J. Association between proton pump inhibitors and respiratory infections: a systematic review and meta-analysis of clinical trials. Can J Gastroenterol. 2008;22:761–766. doi: 10.1155/2008/821385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canani RB, Cirillo P, Roggero P. Therapy with gastric acid inhibitors increases the risk of acute gastroenteritis and communityacquired pneumonia in children. Pediatrics. 2006;117:817–820. doi: 10.1542/peds.2005-1655. [DOI] [PubMed] [Google Scholar]
- 24.Pfaller MA, Jones RN. A review of the in vitro activity of meropenem and comparative antimicrobial agents tested against 30,254 aerobic and anaerobic pathogens isolated world wide. Diagn Microbiol Infect Dis. 1997;28:157–163. doi: 10.1016/s0732-8893(97)00065-5. [DOI] [PubMed] [Google Scholar]
- 25.Clark RH, Bloom BT, Spitzer AR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117:1979–1987. doi: 10.1542/peds.2005-1707. [DOI] [PubMed] [Google Scholar]
- 26.Linden P. Safety profile of meropenem: an updated review of over 6000 patients treated with meropenem. Medicine Saf. 2007;30:657–668. doi: 10.2165/00002018-200730080-00002. [DOI] [PubMed] [Google Scholar]
- 27.Bradley JS, Faulkner KL, Klaugman KP. Effectiveness, safety, and tolerability of meropenem as empiric antibiotic therapy in hospitalized pediatric patients. Pediatr Infect Dis. 1996;15:749–757. doi: 10.1097/00006454-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 28.Jin C, Jung I, Ku HJ. Low convulsive activity of a new carbapenem antibiotic, DK-35C, as compared with existing congeners. Toxicology. 1999;138:59–67. doi: 10.1016/s0300-483x(99)00078-5. [DOI] [PubMed] [Google Scholar]
- 29.El-Ghazely MH, Mahmoud WH, Atia MA. Effect of probiotic administration in the therapy of pediatric thermal burn. Ann Burns Fire Disasters. 2016;29(4):268–272. [PMC free article] [PubMed] [Google Scholar]
- 30.Mayes T, Gootschlich MM, James LE. Clinical safety and efficacy of probiotic administration following burn injury. J Burn Care Res. 2015;36(1):92–99. doi: 10.1097/BCR.0000000000000139. [DOI] [PubMed] [Google Scholar]
- 31.Saputro ID, Putra ON, Pebrianton H, Suharjono Effects of probiotic administration on IgA and IL-6 level in severe burn patients: a randomized trial. Ann Burns Fire Disasters. 2019;32(1):70–76. [PMC free article] [PubMed] [Google Scholar]
- 32.Voruganti VS, Klein GL, Hong-Xing Lu. Impaired zinc and copper status in children with burn injuries: need to reassess nutritional requirement. Burns. 2005;31:711–716. doi: 10.1016/j.burns.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 33.Caldis-Countris N, Gawaziuk JP, Logsetty S. Zinc supplementation in burn patients. J Burn Care Res. 2012;33(5):678–682. doi: 10.1097/BCR.0b013e31824799a3. [DOI] [PubMed] [Google Scholar]
- 34.Charles P, Larson SK, Azharul Islam Khan R. Zinc treatment to under-five children: applications to improve child survival and reduce burden of disease. J Health Popul Nutr. 2008;26(3):356–365. doi: 10.3329/jhpn.v26i3.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.John JN, Kowey PR, Whelton PK. New guidelines for potassium replacement in clinical practice: a contemporary review by the National Council on Potassium in Clinical Practice. Arch Intern Med. 2000;160(16):2429–2436. doi: 10.1001/archinte.160.16.2429. [DOI] [PubMed] [Google Scholar]