Abstract
Context.
Neoangiogenesis and lymphangio-genesis are essential for the growth of tumor and progression of malignancy.
Objective.
The study examined the significance of VEGF-C expression in comparison to classical prognostic factors in differentiated thyroid carcinoma (DTC), as well as an independent prognostic marker in DTC.
Design.
The study included 81 patients with DTC allocated in two groups according to the type of cancer (follicular versus papillary) and then compared to expression of VEGF-C and clinicopathological features.
Methods.
Expression of VEGF-C was identified with anti-VEGF-C antibody using tris-EDTA buffer Antigen Retrieval Protocol. Each specimen was scored with a semi-quantitative score system (H-score).
Results.
The analysis of T staging system showed a linear correlation between the size of a tumor, expression of VEGF-C and recurrence of a disease, with a statistical significance (p < 0.0001). There was a clear and significant correlation between VEGF-C expression and T stage in patients with papillary carcinoma (p = 0.0294). Analysis of invasion of a surgical margin demonstrated significant positivity in patients with papillary thyroid cancers who expressed VEGF-C (p = 0.0207) indicating the worse prognosis of a disease. Also a statistically significant correlation was between VEGF-C and extrathyroid extension, indicating the worse prognosis (p = 0.0133) in papillary cancers. The level of VEGF-C expression was statistically significant in patients with papillary thyroid cancer (p = 0.039).
Conclusions.
This study undoubtedly demonstrates that VEGF-C expression is an evident negative prognostic factor in patients with papillary thyroid carcinoma, along with the classic prognostic factors, such as a larger tumor size, tumor margin involvement, extrathyroid extension, i.e. local aggressiveness.
Keywords: thyroid cancer, angiogenesis, VEGF-C thyroid
Introduction
Neoangiogenesis (1) and lymphangiogenesis (2) are essential for the growth of tumor and progression of malignancy. However, it is not a simple linear process, but it encompasses multiple growth factors and signal transduction systems (3). Vascular endothelial growth factor (VEGF) is a cytokine that promotes the growth of blood and lymph vessels, i.e. it stimulates angiogenesis and lymphangiogenesis. All members of VEGF family activate the signal transduction system by binding to the thryrosine kinase receptors on the cell surface, causing its dimerization and transphosphorylation. However, members of the group differ in place, time and extent of activation of signal transduction. VEGF-A binds to VEGFR-1 and VEGFR-2 and is crucial for the development of circulatory system. In contrast, VEGF-C and VEGF-D bind to VEGFR-2 and VEGFR-3 and mediate lymphangiogenesis. It seems that VEGF-related regulation of angiogenesis as well as intrathyroid alterations of VEGF expression can be found in many other thyroid diseases. Since the thyroid gland is well vascularized and it has an ability to increase the blood flow in pathological states, it can serve as an ideal model for studying an integrative control of angiogenesis (4). It has been reported that increased expression of vascular endothelial growth factor is one of the features of differentiated tumors of the thyroid gland. Increased expression of VEGF has been implicated in growth, progression and invasiveness of tumors as well as in decreased recurrence-free survival in patients. Patients with papillary thyroid carcinoma, that expressed diffuse intensive immunohistochemical staining on VEGF in biopsy specimens, had higher rates of local recidive and distant metastases (5). VEGF-C plays a role in tumor progression in different human malignant tumors such as the breast (6), colon (7), gastric cancers (8), neuroblastoma (9), including papillary cancer of thyroid gland (10). Vascular endothelial growth factor C might stimulate lymphangiogenesis by binding to VEGFR 3 receptor, whereas it affects angiogenesis via VEGFR 2 (11). Binding of VEGF-C and VEGF-D to VEGFR-2 triggers several signaling pathways, (12) causing dysregulation of genes involved in proliferation and migration of endothelial cells and their survival as well as vascular permeability. Papillary thyroid carcinoma demonstrates a higher expression of VEGF-C comparing to other malignant tumors of thyroid gland, while its expression is generally higher in the malignant tissue in comparison to the normal thyroid tissue in the same patient (13).
It seems that early lymphangiogenic events might regulate angiogenesis through modification of VEGF expression in patients with papillary thyroid carcinoma, so that increased expression VEGF-C leads to local progression of primary tumors. In the further advancement of the disease, with the appearance of metastatic dissemination, there is a (sudden) decrease of VEGF-C expression with a concomitant increase in VEGF-A expression, which is the prerequisite for progression of metastatic disease.
In this study, we examined the significance of VEGF-C expression in comparison to classical prognostic factors in differentiated thyroid carcinoma, and their local presentation as well as their significance as an independent prognostic marker in differentiated carcinoma of the thyroid gland.
Methods
Patients and tissue specimens
This study includes 81 patients with differentiated thyroid carcinoma. Patients were allocated in two groups according to the type of cancer (follicular versus papillary). There were 42 patients with papillary carcinoma, while 39 patients were diagnosed with follicular carcinoma. All of the patients were treated and controlled at the Department of Nuclear Medicine and Endocrinology, University Clinical Center Sarajevo. All of the patients were followed up for at least five years, until death or until June 1st 2016. Patient’s follow-up included regular clinical check-ups along with the analysis of hormonal status, thyroglobulin (Tg), thyroglobulin antibody (TgAb), diagnostic WBS (whole body scan), cervical ultrasound and, when indicated, additional examinations: biopsy, CT scan, MRI and PET/CT scan. Medical records were used as a source for epidemiological and clinical data. All of the patients were diagnosed with differentiated thyroid gland cancer (follicular or papillary) on the basis of pathohistological examination and tissue specimens were archived at the Department of Pathology, University Clinical Center Sarajevo or at the Institute of Pathology, Medical Faculty, University of Sarajevo. Additional re-evaluation and immunohistochemical analysis were performed at the Department of Pathology, University Clinical Center Sarajevo. All relevant clinical and pathological data were used: gender, age at onset of the disease, pathohistological findings (type of tumor, TNM (assessed according to the 7th edition of Tumor, Node and Metastasis Classification proposed by the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC)), surgical margin, extrathyroid extension, vascular invasion, and multicentricity), progression-free survival (PFS). Progression-free survival is defined as the length of time from the onset of the disease (time of diagnosis) to the first signs of progression of the disease (presence of a local recidive or distant metastases). We confirmed the progression of the disease by measuring the increase of the level of serum Tg or by diagnostic WBS, along with the above-mentioned diagnostic examinations when indicated.
Immunohistochemistry
Expression of VEGF-C was identified with an adequate immunohistochemical analysis. Immunohistochemical staining was performed on 4 μm-thick formalin-fixed and paraffin-embedded tissue section of primary thyroid cancer.
We used mouse monoclonal anti-VEGF-C antibody (Clone C-1, manufactured by Santa Cruz Biotechnology, CA, USA), diluted at 1:50. Tris-EDTA Buffer Antigen Retrieval Protocol was used to unmask epitopes in formalin-fixed and paraffin embedded tissue sections. pH Tris EDTA epitope retrieval was 6, while primary antibody was incubated at room temperature overnight.
Review and VEGF immunostaining scoring
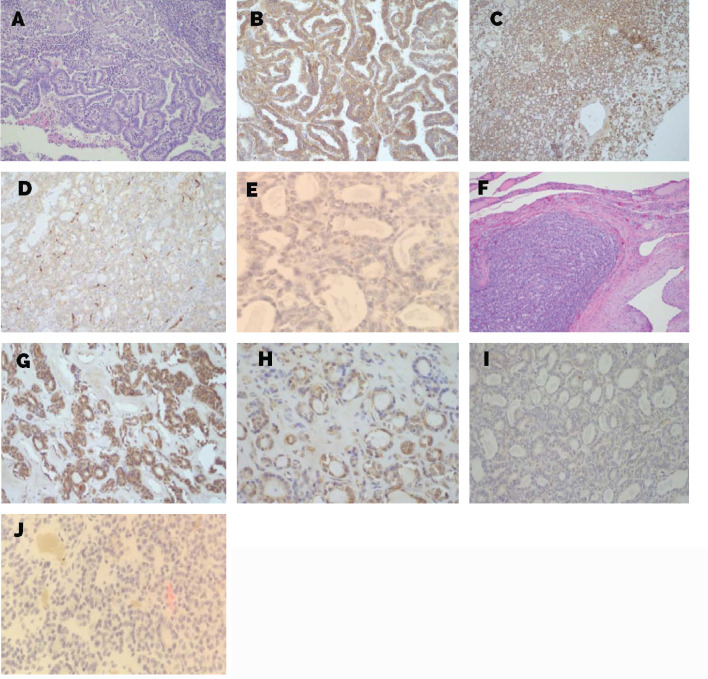
In order to assess the intensity of immunohistochemical staining (level of expression) with anti-VEGF, we introduced a semi-quantitative score system (H-score) designed by Klein et al. (13-15). Each specimen was scored according to the intensity of immunostaining: 0 = no staining; 1 if<30% of thyreocytes stained; 2 if 30–60% of thyreocytes stained; 3 if>60% of thyreocytes stained. For the purpose of statistical analysis, all patients were allocated in two groups: patients without VEGF expression (0 = no staining) and patients demonstrating VEGF expression (immunostaining present in intensity 1-3), as shown on Figure 5.
Figure 5.
Levels of expression of VEGF C. A-E. Thyroid tissue section from patients with papillary thyroid cancer (PTC): (A)Hematoxylin and eosin staining (H&E) (200x),immunohistochemical staining (level of expression) with anti-VEGF-C; (B) level of expression 3 (200x); (C) level of expression 2 (100x); (D) level of expression 1 (200x); (E) no staining (400x); F-J. Thyroid tissue section from patients with follicular thyroid cancer (FTC):(F) H&E (100x) immunohistochemical staining (level of expression) with anti-VEGF-C; (G) level of expression 3 (200x); (H) level of expression 2 (400x); (I) level of expression 1 (200x); (J) no staining (400x).
Statistical analysis
All of the calculations were performed in MedCalc® software ver. 14.8.1. (MedCalc Software, Mariakerke, Belgium). Kolmogorov-Smirnov test was used to assess the deviation of data in comparison to the normal distribution. In order to compare clinical-pathological prognostic factors between groups of patients with papillary and follicular carcinoma, Student T-test was used. For a comparison of clinical-pathological factors between patient groups as well as for a comparison of findings of immunohistochemical staining on VEGF between patient groups, Mann Whitney test was used, while χ2 test was used for the comparison of the frequency of individual factors between patient groups. To assess the effects of observed factors on five-year survival, Cox proportional-hazards regression analysis was employed. For all of the above-mentioned statistical analyses, the level of statistical significance was p < 0.05.
Ethical considerations
This study was presented to and approved by Institutional Ethical Board (Ethics Committee) University Clinical Center Sarajevo. Research is in compliance with WMA Declaration of Helsinki.
Results
Relationship between expression patterns of VEGF C in comparison with clinicopathological features
This study included a total of 81 patients with differentiated thyroid carcinoma allocated in two groups: 42 patients with papillary and 39 with follicular carcinoma.
Clinicopathological features of patients enrolled in this study are presented in Table 1.
Table 1.
Clinicopathological features of patients in the study
| Clinicopathological parameters | Papillary thyroid cancer | Follicular thyroid cancer |
|---|---|---|
| Sex | ||
| Male | 6 | 9 |
| Female | 36 | 30 |
| Age (average in years) | 45.7857 | 57.8462 |
| T stage | ||
| T1a | 13 | / |
| T1b | 7 | 5 |
| T2 | 13 | 18 |
| T3 | 8 | 15 |
| T4 | 1 | / |
| Margin | ||
| Positive | 13 | 1 |
| Negative | 29 | 38 |
| Vascular invasion | ||
| Positive | 12 | 8 |
| Negative | 30 | 31 |
| Extrathyroid extension | ||
| Positive | 9 | 0 |
| Negative | 33 | 39 |
| Multicentricity | ||
| Positive | 10 | 0 |
| Negative | 32 | 39 |
| VEGF | ||
| 0 | 3 | 7 |
| 1 | 11 | 2 |
| 2 | 14 | 18 |
| 3 | 14 | 12 |
Patients were predominantly females (81.5%), while there were 18.5% males in our sample (p < 0.0001). In terms of progression-free survival (PFS), male patients (67.4 months) had a shorter period to disease progression, i.e. they had worse prognosis comparing to female patients (94.1 months) with p < 0.0182 (Table 2). When we compared PFS in both sexes in patients with papillary and folicular thyroid cancer we had a statistical significance in papillary thyroid cancer ( p= 0.0058) but no in follicular thyroid cancer p= 0.23) as shown on Table 3.
Table 2.
Progression and PFS in total samples in patients with papillary and folicular thyroid cancer
| Progression | Papillary thyroid cancer | Follicular thyroid cancer |
|---|---|---|
| Yes | 12 | 7 |
| No | 30 | 32 |
| PFS (average in months) | 79.2619 | 99.8718 |
| P value | 0.0182 |
Table 3.
PFS based on sex in patients with papillary and folicular thyroid cancer
| PFS | Papillary thyroid cancer | Follicular thyroid cancer |
|---|---|---|
| Males (average in months) | 38.0 | 87.0 |
| Females (average in months) | 86.1 | 103.7 |
| P value | 0.0058 | 0.23 |
We did not find any statistical correlation between expression of VEGF-C and gender of a patient in patients with papillary and follicular thyroid cancer.
Correlation of papillary cancer with T stage, surgical margin and extrathyroid extension
Analysis of T staging system showed a linear correlation between the T stage, expression of VEGF-C and recurrence of a disease, with a statistical significance of p < 0.0001. Patients with greater T stage had a shorter period to disease progression. There was a clear and significant correlation between VEGF-C expression and T stage in patients with papillary carcinoma (p = 0.0294) (Fig. 1). That means the higher is the T stage the more intensive is expression of VEGF-C, which is in a direct correlation with the progression of a disease.
Figure 1.
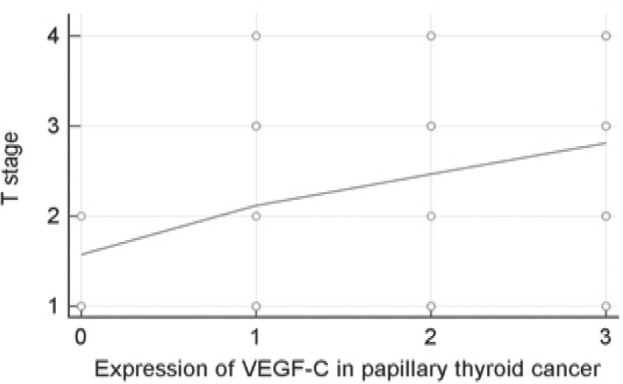
Scatter diagram - correlation between VEGF C and T stage in patients with papillary thyroid cancer.
Analysis of positive surgical margin demon-strated a statistical significance in patients with papillary thyroid cancers who expressed VEGF-C (Fig. 2). There is a statistically significant correlation between positivity of a surgical margin and progression of disease, so that patients with a positive margin had worse prognosis (p = 0.0386). In addition, there is a direct statistically significant correlation between expression of VEGF-C and surgical margin positivity in patients with papillary thyroid carcinoma, indicating the worse prognosis of a disease (p = 0.0207). We have not found any statistically significant correlation between VEGF-C and tumor multicentricity in patients with papillary carcinoma.
Figure 2.
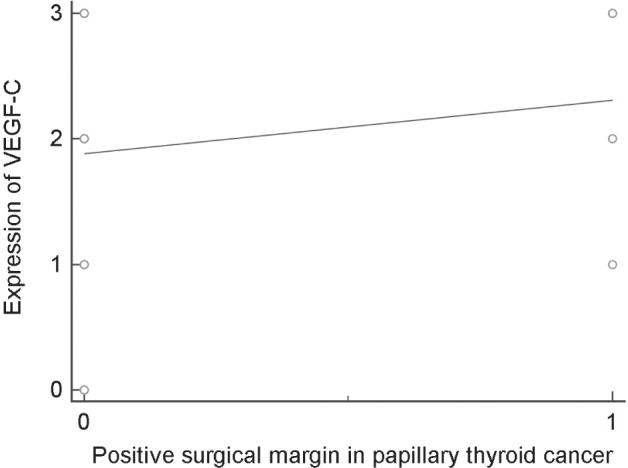
Scatter diagram – correlation between positive surgical margin and expression of VEGF in patients with papillary carcinoma of the thyroid gland.
The presence of a gross extrathyroid extension was identified only in patients with papillary thyroid cancer. It has been confirmed that these patients have worse prognosis, i.e. shorter progression-free survival (PFS) (p=0.0016). There was also a statistically significant correlation between VEGF-C and gross extrathyroid extension, indicating the worse prognosis (p = 0.0133) (Fig. 3).
Figure 3.
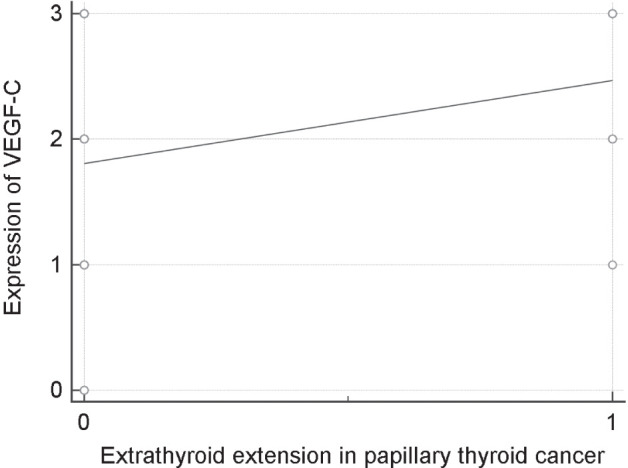
Scatter diagram: correlation between presence of gross extrathyroid extension and expression of VEGF-C in patients with papillary thyroid carcinoma.
Correlation of follicular cancer with vascular invasion
This study has not identified any statistically significant difference in vascular invasion between two groups (p = 0.401). However, there was a statistically significant correlation between vascular invasion and VEGF-C in patients with follicular carcinoma (p = 0.0369) (Fig. 4).
Figure 4.
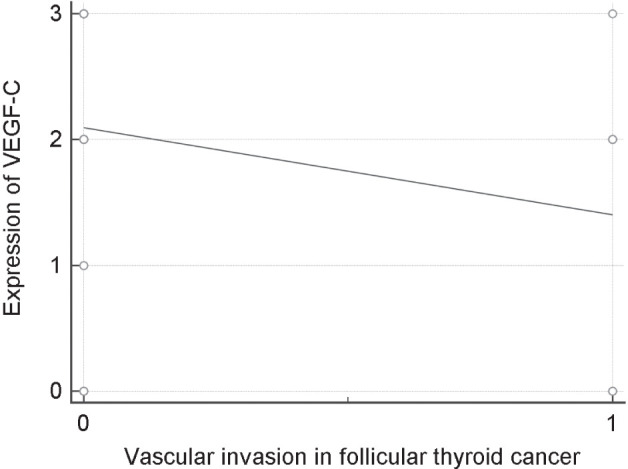
Scatter diagram: correlation between vascular invasion and expression of VEGF-C in patients with follicular thyroid cancer.
Distribution of expression levels for VEGF C
The levels of expression 1, 2 and 3 (Fig. 5) were equally distributed in patients with papillary thyroid cancer: 1 (26.19%), 2 (33.33%), and 3 (33.33%). In contrast, level 2 of VEGF expression was predominant in patients with follicular carcinoma (46.15%), followed by the level of expression 3 (30.77%).
Analyzing VEGF-C expression, we found a statistically significant difference in frequency level between the group of patients with papillary and the group of patients with follicular carcinoma in favour of papillary thyroid cancer (χ2 = 8.39, p = 0.039). There was no statistically significant difference between the level of expression of VEGF-C and progression-free survival (PFS) in groups of patients with papillary and follicular carcinoma (Mann-Whitney U=812.50, p = 0.9486). Furthermore, when we allocated patients in two groups: 0 (no expression of VEGF) and 1 (level of expression 1-3), there was no statistically significant difference in frequency of VEGF (0 and 1) between the patients’ groups (χ2= 2.15, p = 0.142) (Fig. 6).
Figure 6.
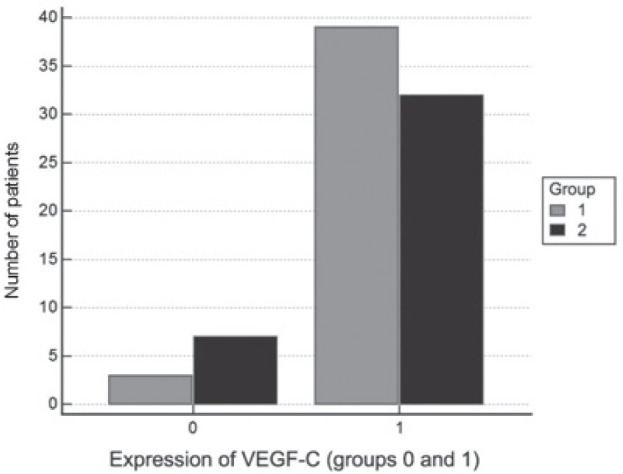
Comparison of frequency of VEGF (0 and 1) between groups of patients with papillary thyroid carcinoma (group 1) and follicular thyroid carcinoma (group 2).
However, a comparison in progression free survival (PFS) between patients with papillary and follicular thyroid carcinoma showed a statistically significant difference. A significantly higher PFS value was in the group of patients with follicular thyroid carcinoma (T= 2.389, P= 0.0193)(Fig. 7).
Figure 7.
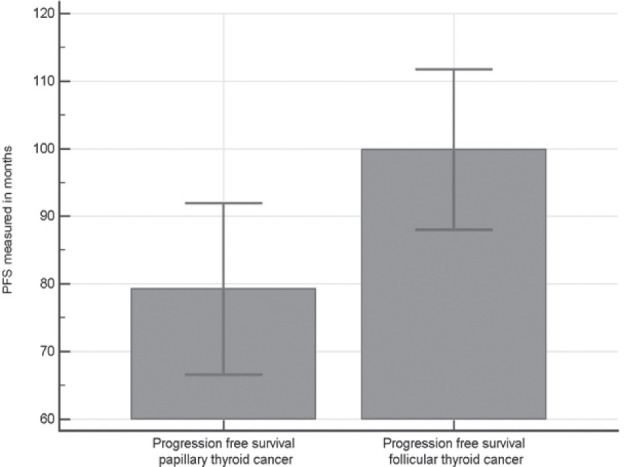
Comparison of progression free survival between groups of patients with papillary thyroid carcinoma and follicular thyroid carcinoma.
Our study did not provide clear statistical evidence comparing VEGF levels and PFS in papillary and follicular thyroid carcinoma, but it should be stated that the difference in PFS between these two groups (papillary and follicular) showed a statistically significant lower PFS in papillary thyroid cancer which is in concordance with the greater local aggressiveness of these tumors in our study.
Discussion
VEGF is an important stimulator of angiogenesis as well as of lymphogenesis. In this way, it affects proliferation and dissemination of different types of tumors. However, VEGF expression in thyroid tumors might be related to some of the clinicopathological features of patients.
The effect of VEGF on tumor growth has been explained by paracrine mechanisms or as the consequence of activation of angiogenesis. It was believed that tumor cells produce VEGF, but they cannot respond to its stimulation directly, because they lack VEGF receptors on the cell surface. In contrast, endothelial cells that participate in angiogenesis express a large number of VEGF receptors, but are not able to produce this growth factor. However, it has been reported recently that many different tumor cells are capable of VEGF receptor expression, so that VEGF can also act as a direct autocrine tumor growth factor (16). Vascular endothelial growth factor C can stimulate lymphangiogenesis by binding to VEGFR-3 and angiogenesis by binding to VEGFR-2. (11). It seems that VEGFR-2 serves as a mediator in all cellular responses to VEGF. In humans, VEGF-C is coded by VEGFR-C gene, located on the chromosome 4q34. Expression of VEGF A and C correlates with pathohistological parameters as well as with metastatic status in cancers of thyroid gland. There is a significant correlation between expression of these markers of neoangiogenesis and their interaction in progression of malignant thyroid disease.
In a study published by Salajegheh et al., that included 136 patients with thyroid gland tumors (123 diagnosed with papillary carcinoma and 13 with poorly differentiated thyroid carcinomas), it has been shown that the level of expression of VEGF A and C was more prominent in patients with poorly differentiated thyroid carcinomas, while 51% of patients with papillary carcinomas demonstrated the expression of these two markers of neoangiogenesis. Authors found a positive correlation between expression of VEGF and more aggressive tumor phenotypes of differentiated thyroid cancers that could be correlated with our study in sense of local aggressiveness of papillary thyroid tumors (17, 18). Papillary thyroid carcinoma shows a higher level of VEGF-C expression, comparing to other types of malignant tumors of thyroid gland (p < 0.0005, ANOVA). Comparison of VEGF-C expression between malignant thyroid tumors and normal thyroid tissue also showed an increased level of VEGF-C expression in papillary carcinoma of the thyroid gland (1.10 +/- 0.41 vs. 0.70 +/- 0.13; p = 0.001) (19).
Most of the metastatic tumors do not demonstrate intensive expression of VEGF-C (p = 0.0002)(20). Similarly, expression of VEGF-A in metastatic disease is stronger than the expression of VEGF-C. VEGF-C was predominantly expressed in non-metastatic lesions, although it was not found to be statistically significant.
Nowadays, it is widely accepted that early lymphangiogenic events regulate angiogenesis via modification of VEGF-C expression in a population of patients with thyroid carcinoma. Increase in VEGF-C expression in early stages of tumors is in correlation with their local invasion. At the same time, it is the prerequisite for an increase in VEGF-A expression which leads to a decrease of VEGF C, that correlates with the development of metastatic disease.
Common features of local progression in thyroid carcinomas make it a useful model for studying of process of angiogenesis and lymphangiogenesis in malignant tumors.
Šelemetjev et al. provide a significant insight in to lymphatic expansion and local invasion of papillary thyroid carcinoma in co-expression with MMP-9. The study proved a correlation between high levels of VEGF-C and active MMP-9 with lymphatic dissemination and local invasiveness, suggesting their potential value as a predictive biomarker of local aggressiveness (especially with the pT status and the level of tumor infiltration) in papillary thyroid carcinoma (10). Therefore, there is a clear parallel with our study, which demonstrates the significance of VEGF C in local aggressiveness of papillary thyroid carcinoma.
In conclusion, increased expression of VEGF-C represents one of the important carcinogenic processes in differentiated carcinomas of the thyroid gland. We have shown that VEGF-C expression is significantly correlated with pathological markers of tumor aggressiveness and it is a correlation to risk of progression.
Our study undoubtedly demonstrates that VEGF-C expression is an evident negative prognostic factor in patients with papillary thyroid carcinoma, along with the classic prognostic factors ( larger T stage, tumor margin involvement, extrathyroid extension), i.e. local aggressiveness.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006;126(10):2167–2177. doi: 10.1038/sj.jid.5700464. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Oliver G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev. 2010;24(19):2115–2126. doi: 10.1101/gad.1955910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka K, Sonoo H, Kurebayashi J, Nomura T, Ohkubo S, Yamamoto Y, Yamamoto S. Inhibition of infiltration and angiogenesis by thrombospondin-1 in papillary thyroid carcinoma. Clin Cancer Res. 2002;8(5):1125–1131. [PubMed] [Google Scholar]
- 4.Sprindzuk MV. Angiogenesis in Malignant Thyroid Tumors. World J Oncol. 2010;1(6):222. doi: 10.4021/wjon263e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu XM, Lo CY, Chan WF, Lam KY, Leung P, Luk JM. Increased expression of vascular endothelial growth factor C in papillary thyroid carcinoma correlates with cervical lymph node metastases. Clin Cancer Res. 2005;15;11(22):8063–8069. doi: 10.1158/1078-0432.CCR-05-0646. [DOI] [PubMed] [Google Scholar]
- 6.Kurebayashi J, Otsuki T, Kunisue H, Mikami Y, Tanaka K, Yamamoto S, Sonoo H. Expression of vascular endothelial growth factor (VEGF) family members in breast cancer. Jpn J Cancer Res. 1999;90:977–981. doi: 10.1111/j.1349-7006.1999.tb00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akagi K, Ikeda Y, Miyazaki M, Abe T, Kinoshita J, Maehara Y, Suqimachi K. Vascular endothelial growth factor-C (VEGF-C) expression in human colorectal cancer tissues. Br J Cancer. 2000;83(7):887–891. doi: 10.1054/bjoc.2000.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yonemura Y, Endo Y, Fujita H, Fushida S, Ninomiya I, Bandou E, Taniguchi K, Miwa K, Ohoyama S, Sugiyama K, Sasaki T. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res. 1999;5:1823–1829. [PubMed] [Google Scholar]
- 9.Eggert A, Ikegaki N, Kwiatkowski J, Zhao H, Brodeur GM, Himelstein BP. High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin Cancer Res. 2000;6:1900–1908. [PubMed] [Google Scholar]
- 10.Šelemetjev S, Đorić I, Paunović I, Tatić S, Cvejić D. Coexpressed High Levels of VEGF-C and Active MMP-9 Are Associated With Lymphatic Spreading and Local Invasiveness of Papillary Thyroid Carcinoma. Am J Clin Pathol. 2016;146(5):594–602. doi: 10.1093/ajcp/aqw184. [DOI] [PubMed] [Google Scholar]
- 11.Ali I, Chouhan VS, Dangi SS, Gupta M, Tandiya U, Hyder E, Yadav VP, Panda RP, Babitha V, Nagar V, Sonwane A, Khan FA, Das BC, Singh G, Bag S, Sarkar M. Expression and localization of locally produced growth factors regulating lymphangiogenesis during different stages of the estrous cycle in corpus luteum of buffalo (Bubalus bubalis).Theriogenology. 2014;81(3):428–436. doi: 10.1016/j.theriogenology.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312(5):549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Klein M, Picard E, Vignaud JM, Marie B, Bresler L, Toussaint B, Weryha G, Duprez A, Leclère J. Vascular endothelial growth factor gene and protein: strong expression in thyroiditis and thyroid carcinoma. J Endocrinol. 1999;161(1):41–49. doi: 10.1677/joe.0.1610041. [DOI] [PubMed] [Google Scholar]
- 14.Klein M, Vignaud JM, Hennequin V, Toussaint B, Bresler L, Plénat F, Leclère J, Duprez A, Weryha G. Increased expression of the vascular endothelial growth factor is a pejorative prognosis marker in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2001;86(2):656–658. doi: 10.1210/jcem.86.2.7226. [DOI] [PubMed] [Google Scholar]
- 15.Jebreel A, England J, Bedford K, Murphy J, Karsai L, Atkin S. Vascular endothelial growth factor (VEGF), VEGF receptors expression and microvascular density in benign and malignant thyroid diseases. Int J Exp Pathol. 2007;88(4):271–277. doi: 10.1111/j.1365-2613.2007.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudge JS, Holash J, Hylton D, Russell M, Jiang S, Leidich R, Papadopoulos N, Pyles EA, Torri A, Wiegand SJ, Thurston G, Stahl N, Yancopoulos GD. VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade. Proc Natl Acad Sci USA. 2007;104(47):18363–18370. doi: 10.1073/pnas.0708865104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salajegheh A, Pakneshan S, Rahman A, Dolan-Evans E, Zang S, Kwong E, Gopalan V, Lo CY, Smith RA, Larn AK. Co-regulatory potential of vascular endothelial growth factor – A and vascular growth factor – C in thyroid carcinoma. Hum Pathol. 2013;44(10):2204–2212. doi: 10.1016/j.humpath.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Rahman O. Targeting vascular endothelial growth factor (VEGF) pathway in iodine-refractory differentiated thyroid carcinoma (DTC): from bench to bedside. Crit Rev Oncol Hematol. 2015;94(1):45–54. doi: 10.1016/j.critrevonc.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Hung CJ, Ginzinger DG, Zarnegar R, Kanauchi H, Wong MG, Kebebew E, Clark OH, Duh QY. Expression of vascular endothelial growth factor-C in benign and malignant thyroid tumors. J Clin Endocrinol Metab. 2003;88(8):3694–3699. doi: 10.1210/jc.2003-030080. [DOI] [PubMed] [Google Scholar]
- 20.Ariana A, Smith RA, Pakneshan S, Gopalan V, KY Lam A. VEGF-A and VEGF-C: potential coregulators in angiogenic event in thyroid cancer. Cancer Research. 73(8):381. S1. [Google Scholar]