Abstract
Context.
Alimentary supplements may have beneficial effects on retinal microvasculature in diabetic patients.
Objective and Design.
State-of-the-art imaging techniques were used to assess retinal microcirculation in diabetic patients in an observational study before and after 3 months treatment with a multinutrient complex including resveratrol, vitamins D3, C, E, essential fatty acids, trace elements (zinc and copper) and macular pigments (lutein and zeaxanthin)-Resvega.
Subjects and Methods.
Fifteen subjects were included in this study. Adaptive optics ophthalmoscopy was used to measure the parameters of temporal retinal arterioles. Optical coherence tomography angiography was employed to assess foveal avascular zone and vessel densities of the superficial capillary plexus, deep capillary plexus and choricapillary plexus.
Results.
After 3 months of treatment, there was a statistically significant median decrease in wall-to-lumen ratio (p=0.0001). The same tendencies were noticed for wall thickness values (p=0.008) and wall cross sectional area values (p=0.001). On the other side, no significant changes were noticed concerning the OCTA parameters.
Conclusions.
Resvega seems to have a beneficial effect on the retinal arterioles in diabetic patients.
Keywords: diabetic retinopathy, resveratrol, adaptive optics ophthalmoscopy, optical coherence tomography angiography
Introduction
Diabetic retinopathy (DR) is a microvascular complication of diabetes mellitus (DM), representing a leading cause of vision loss and blindness worldwide (1). According to several studies, patients with DR commonly exhibit documented deficiencies related to vitamins, minerals and other micro- and macronutrients. Considering oxidative stress responsible, with different degrees of importance, in the onset and/or progression of several diseases, including diabetic retinopathy, antioxidants seem to play a critical role in keeping optimal cellular functions. Oxidative stress is induced by free radicals, as well as by nitric oxide inactivation and determines endothelial dysfunction, vascular inflammation, vascular smooth muscle cells proliferation, vasospasm and thrombosis (2-4). Due to a high metabolic rate, the human retina will be confronted with significant levels of oxidative stress, thus leading to microvascular damage (5).
Vitamins C and E limit the oxidative damage by increasing the nitric oxide level, improving the vascular endothelial function and reducing the arterial stiffness (5). It is proven that patients with DR display vitreous ascorbic acid levels which are 10 times lower than in the normal population (6). At high doses, both vitamin C and E may become pro-oxidants. Therefore it is important to co-administrate both molecules, due to vitamin’s C capacity to reverse the pro-oxidant state of vitamin E (3).
B vitamins, especially B2, B6, B9 and B12, represent sources of coenzymes which support a broad set of transformations known as One-carbon (1C) Metabolism, playing an important role in the conversion of homocysteine (HCY) to methionine (7). High serum levels of HCY stimulate the production of inflammatory molecules in human retinal pigment epithelium, such as cytokines (8). These may lead to retinal cells apoptosis, thus thinning the retinal nerve fiber layer (RNFL), and increase the severity of DR (9). Being known that vitamin D is involved in the pathogenesis and progression of diabetes mellitus, it has been lately noticed that, when reaching an optimal level, the fat-soluble, antirachitic compound might have a positive effect in reducing the risk and severity of DR (5). Vitamin D influences insulin secretion and resistance in patients with good glycemic control (10). Mutlu et al. (2016) has noticed that vitamin D deficiency is related to retinal microvascular damage (5). Gunger et al. (2015) observed that lower vitamin D levels display a good correlation with early thinning of the RNFL (10). Several studies have also proven that Vitamin D supplements may prevent intracellular reactive oxygen species formation, consequently reducing the expression of vascular endothelial growth factor (VEGF) (5).
Zinc (Zn) is an important nutrient that plays vital roles in the human body. Zinc deficiency is correlated with a longer duration of DM, an increased level of glycated hemoglobin (HbA1c) and microvascular complications (2). Zinc supplements may redress DR, by reducing VEGF expression and ischemic inflammation (11).
Lutein and Zeaxanthin are water-soluble carotenoids concentrated in the macula lutea, mainly found in green leafy vegetables as well as in other foods (5). Multiple studies show that lutein and zeaxanthin reduce the risk of chronic eye diseases, acting as antioxidants and exhibiting neuroprotective and anti-inflammatory properties (12). Despite their importance for vision, they can only be acquired from foods or supplements, because the human body does not synthesize them.
Omega 3 fatty acids (alpha-linolenic acid (ALA) – found in plant oil, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) – found in marine oil) are polyunsaturated fatty acids of great benefit for the human body, due to their antioxidant, anti-inflammatory and anti-angiogenic properties. A rich diet in omega 3 fatty acids might prevent DR progression in diabetic patients (13).
Resveratrol (3,4′,5- trihydroxystilbene) is a natural polyphenol recently attracting a lot of research attention due to its exciting pharmacological potential. It is a promising anti-cancer agent, showing chemopreventive and chemotherapeutic effects in vitro and in vivo (14). The NF-κB, Wnt, and PI3K/Akt/mTOR pathways regulate inflammation, immune response to infection, cellular response to stimuli, cell differentiation, growth and proliferation (15-18). Resveratrol has also been found to inhibit the expression of vascular cell adhesion molecules (VCAM), thus affecting the activity of vascular smooth muscle cells, and to prevent platelet aggregation and activation in vitro, with positive effects on various cardiovascular conditions (19).
A matter of concern is whether resveratrol can ameliorate glycemic control in humans, reflected by HbA1c plasmatic level. Bhatt et al. showed that 3-months daily resveratrol intake decreased HbA1c levels, systolic blood pressure, total cholesterol and total protein, improving overall glycemic control, current data inquiring resveratrol as a possible adjuvant for DM treatment (20).
Given the promising potential, there is a lot of variation in supplements created specifically for vision. For our research in the field, we have used Resvega (Thea Laboratories), with a daily recommended dose of 2 capsules/day encompassing: 606 mg omega-3 fatty acids (366 mg DHA, 172 mg EPA, ≤47.5 mg DPA), 60 mg trans-resveratrol, 120 mg vitamin C, 30 mg vitamin E, 5 μg vitamin D, 10 mg lutein, 2 mg zeaxanthin and minerals (1mg Cu, 12.5mg Zn).
Material and Methods
The study protocol of the present clinical investigation was designed in accordance with the Declaration of Helsinki and was formally approved by the local Ethics Commission. A written informed consent was signed by all subjects, after adequate explanations regarding the study’s objectives. All the investigations were conducted in the Retina Clinic, Bucharest, Romania.
Study participants
All subjects met the eligibility criteria, namely, adult age (>18 years old, Caucasians), confirmed diagnosis of type I or type II DM in compliance with the American Diabetes Association (21), no significant systemic medical history except for DM and arterial hypertension (10 subjects), transparent ocular media at the time of inclusion, refractive error less than 3 spherical diopters or 2.5 cylindrical diopters.
Following the ETDRS guidelines (22), the study group encompasses diabetic patients without retinopathy (4 eyes), diabetic patients with non-proliferative diabetic retinopathy (6 eyes) and diabetic patients with proliferative diabetic retinopathy (5 eyes).
Subjects examination
All subjects were extensively investigated before, one month and three months after daily intake of the nutritional supplement, in a prospective manner. Besides history taking, all patients underwent a comprehensive ophthalmic examination, encompassing best corrected visual acuity and intraocular pressure (IOP) assessment, together with the slit lamp eye examination (of both anterior and posterior segments). Subjects with pupillary diameters less than 4.5 mm received topical Phenylephrine 10% and Tropicamide 1% in order to obtain pharmacologic mydriasis.
Retinal microcirculation was evaluated using the rtx1TM adaptive optics (AO) flood illumination retinal camera (Imagine Eyes, Orsay, France). During the acquisition, the subjects were instructed to track the yellow cross of the instrument, whose position was decided by the investigator on the first visit and kept constant on the follow-up visits.
Retinal and choroidal microcirculations were further assessed using a swept-source OCT device (DRI OCT Triton, TOPCON Inc, Tokyo, Japan).
Axial length measurements were obtained via optical biometry (Aladdin, TOPCON Inc, Tokyo, Japan).
Image processing
The retinal vessels analysis following AO retinal camera acquisition was automatically performed by the manufacturer’s software (AO detect artery, Imagine Eyes, France) for each manually selected region of interest. The AO-related vascular parameters of interest are: vessel diameter (VD - the algebraic sum between the arterioles walls and the vessel lumen), lumen diameter (LD), mean wall thickness (WT), wall to lumen ratio (WLR - the ratio between the wall thickness and the lumen diameter) and cross sectional area of the vascular wall (WCSA - determined based on the lumen diameter and vessel diameter values). Three regions of interest were studied and a mean value was calculated for each parameter.
The OCTA scans were taken from 3 × 3 mm or 4.5 × 4.5 cubes, having a quality index over 40 and proper foveal centration. Based on automated layer segmentation performed by the OCT instrument software (IMAGEnet® 6, TOPCON Inc), en face images were generated for the superficial retinal layer (from 2.6 μm beneath the internal limiting membrane to 15.6 μm beneath the interface of the inner plexiform layer and inner nuclear layer), deep retinal layer (from 15.6 μm to 70.2 μm beneath the interface of the inner plexiform layer and inner nuclear layer) and for choriocapillaris (from the basement membrane to 10.4 μm beneath it). Projection artifacts were excluded using the same OCT instrument software.
The OCTA-related vascular parameters of interest are: foveal avascular zone area measured in the superficial capillary plexus (FAZ), vascular density along the superficial capillary plexus in the parafoveal area (SPD), vascular density along the deep capillary plexus in the parafoveal area (DPD) and vascular density along the choriocapillaris in the parafoveal area (CPD). Vascular density is defined as the percentage of a given area represented by vessels (23) and was calculated in the parafoveal area, representing the inner macula ETDRS subfield.
FAZ (mm2) is defined as the avascular area in the center of the fovea. The borders of the FAZ were manually drawn for each patient using ImageJ software (Version 1.8; National Institutes of Health, Bethesda, MD, USA) and FAZ was further adjusted for magnification effects, with reference to the report by Sampson et al. (24).
In order to quantify vascular densities, 8-bit grey scale en face images of the superficial retinal layer, deep retinal layer and choriocapillaries were binarized by an automated algorithm named “Mean”, using ImageJ software. The resulted white area was considered the vascular area, the pixel number was quantified and the layer’s vascular density was calculated by dividing the number of white pixels (vascular area) to the total number of pixels from the inner macula ETDRS subfield, using ImageJ software (Fig. 1).
Figure 1.
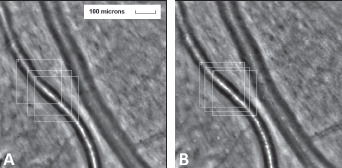
Retinal artery of a diabetic patient A. before and B. 3-months after daily Resvega intake. Employing AOdetect software, the mean WLR was calculated from the three selected regions of interest, for each time landmark. The obtained values are A. 0.36 and B. 0.29.
Statistical analysis
Descriptive statistics was done for all variables. The normality of all the parameters in the study was determined using Shapiro-Wilk’s test (p > 0.05). In order to establish whether the mean difference between paired observations was statistically significantly different from zero, the paired-samples t-test was used for normally distributed data. In case of non-normal distributions, variables were compared using the non-parametric Wilcoxon signed-rank test. In case of skewed (non-symmetric) distributions, sign test was conducted.
Data are expressed as medians, unless otherwise stated. P-values lower than 0.05 were considered statistically significant. Statistics was conducted using IBM SPSS Statistics software (Version 23; Armonk, NY: IBM Corp).
Results
Fifteen eyes (9 right eyes and 6 left eyes) from 15 diabetic patients (10 males and 5 females; 10 subjects with type II DM and 5 subjects with type I DM) were included into the current prospective observational study. All subjects were treated with oral hypoglycemic agents, 8 of them being insulin-dependent. The mean age of the participants was 55.93 ± 11.57 years, while the mean duration of diabetes was 17.93 ± 8.63 years (Table 1).
Table 1.
Characteristics of the study group (mean ± standard deviation 95%CI)
| Study group | |
|---|---|
| N | 15 |
| Sex (female/ male) | 5/ 10 |
| OD/ OS | 9/ 6 |
| Age (years) | 55.93 ± 11.57 |
| Axial length (mm) | 23.43 ± 0.90 |
| Duration of DM (years) | 17.93 ± 8.63 |
Nine out of 15 eyes have associated diabetic maculopathy. 7 out of 15 patients underwent pan-retinal photocoagulation (PRP), 7 subjects received anti-VEGF intravitreal injections and one patient underwent pars plana vitrectomy (PPV), all ophthalmic interventions dating more than 6 months before the inclusion into the current study. The best corrected visual acuity (BCVA) was 0.5 or better among all the study participants.
Retinal arterioles metrics are depicted in Tables 2 and 3. Considering the AO-related vascular parameters, VD did not significantly vary between the paired observations (z=-1.704, p=0.088), nor did LD (z=-0.398, p=0.691). There was a statistically significant median decrease in wall to lumen ratio (WLR decrease=-0.03, z=-3.419, p=0.0001) when subjects were treated for 3 months with Resvega (0.29), compared to the initial observation, before treatment (0.32). The same tendencies were noticed for WT values (z=-2.67, p=0.008) and WCSA values (z=-3.35, p=0.001).
Table 2.
Mean ± standard deviation and median of the retinal arterioles parameters values measured in the study group before and after 3 months of treatment with Resvega
| Study group | Vessel diameter (μm) | Lumen diameter (μm) | Wall thickness (μm) | Wall to lumen ratio | Cross sectional area of the vascular wall (μm2) |
|---|---|---|---|---|---|
| Before treatment | 101.38±32.21 101.46 |
77.44 ± 29.59 75.4 |
11.96 ± 3.02 12.01 |
0.34 ± 0.86 0.32 |
3550.55 ± 1599.76 3341.66 |
| After treatment | 98.67 ± 31.96 94.86 |
76.98 ± 29.47 73.96 |
10.64± 2.36 10.98 |
0.29 ± 0.78 0.29 |
3049.60± 1423.62 2664.36 |
Table 3.
Results of the paired samples analysis between the 2 paired observations in the study group
| Study group | Vessel diameter (Wilcoxon) | Lumen diameter (Wilcoxon) |
Mean wall thickness (Wilcoxon) | Wall to lumen ratio (Related-Samples Sign) |
Cross sectional area of the vascular wall (Wilcoxon) |
|---|---|---|---|---|---|
| Before treatment * After treatment (p value) |
0.088 | 0.691 | 0.008 | 0.0001 | 0.001 |
The OCTA parameters analysis (Tables 4 and 5) revealed no statistically significant differences between the paired observations in this study group. A Wilcoxon signed-rank test was conducted in order to check whether the treatment with Resvega had a significant impact on the foveal avascular zone area (z=-1.647, p=0.1) and deep capillary plexus density (z=-0.625, p=0.532), whereas a paired samples t-test was used to check whether there were statistically significant mean differences between the paired observations in the superficial capillary plexus density (t(14)=0.581, d=0.8, p=0.571) and in the choriocapillaris density (t(14)=0.271, d=0.07, p=0.79)(Figs 2-4).
Figure 2.
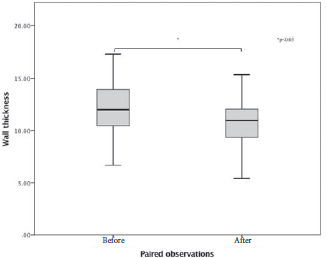
Box-plot of the values of wall thickness.
Figure 4.
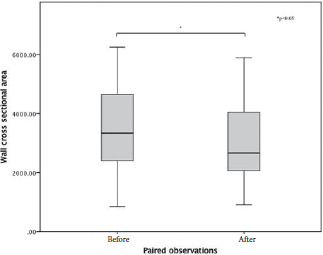
Box-plot of the values of wall cross sectional area.
Table 4.
Mean ± standard deviation and median of the OCTA parameters values measured in the study group before and after 3 months of treatment with Resvega
| Study group | Foveal avascular zone (mm2) | Superficial capillary plexus density | Deep capillary plexus density | Choriocapillary plexus density |
|---|---|---|---|---|
| Before treatment | 0.380 ± 0.11 0.386 | 32.83±1.32 32.61 | 36.20±11.74 41.17 | 36.81 ± 1.16 36.85 |
| After treatment | 0.346±0.10 0.365 | 33.02±1.35 32.84 | 38.57±6.68 40.8 | 36.91 ± 1.33 36.59 |
Table 5.
Results of the paired samples analysis of the OCTA parameters between the 2 paired observations in the study group
| Study group | FAZ (Wilcoxon) | Superficial capillary plexus density (t-test) | Deep capillary plexus density (Wilcoxon) | Choriocapillary plexus density (t-test) |
|---|---|---|---|---|
| Before treatment * After treatment (p value) | 0.1 | 0.571 | 0.532 | 0.79 |
Figure 3.
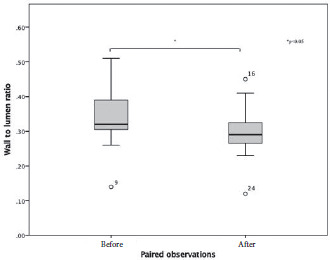
Box-plot of the values of wall to lumen ratio, with significant outliers plotted on the graph as points outside the box-plots.
Discussion
Diabetic retinopathy is a redoubtable complication of DM, whose pathogenesis is linked to oxidative stress and inflammation (25). There are divergent mechanisms leading to retinal changes and significant efforts have been made to prevent and treat them. There is a growing interest in studying the protective effects of polyphenols, omega-3-polyunsaturated fatty acids, vitamins as food supplements in diabetic retinopathy (26, 27).
The current investigation aimed to establish the effects of Resvega (Thea Laboratories) on retinal microvasculature in diabetic patients. AO fundus camera was used to determine the changes of retinal arterioles parameters in the study group, between paired observations. In addition, foveal avascular zone and vascular densities in three layers were assessed with the help of OCTA. Subjects that underwent laser pan-retinal photocoagulation, vitrectomy or intravitreal drug delivery less than 6 months before the starting point of the study were excluded, in order to preclude any interactions between the measurements and these therapies. Both type I and type II diabetic patients were selected, being all included in the same group, based on our previous findings showing that there are no significant differences in vascular parameters between type I and type II diabetic patients (28).
Adaptive optics ophthalmoscopy is an important imaging tool, used not only in photoreceptors’ assessment (29), but also for retinal vessels study at histological resolutions. After 3 months of food supplements administration, wall to lumen ratio, wall thickness and cross sectional area of the vascular wall had significantly decreased, when being compared to initial observations. High values of these parameters are correlated with lumen narrowing and wall thickening, being considered vascular remodelling processes determined by fibrosis and smooth muscle cells hypertrophy (30,31).
Furthermore, no significant changes were noticed with respect to the foveal avascular zone area and the superficial capillary plexus, deep capillary plexus and choriocapillaris densities. All these findings suggest that Resvega might not exhibit an early effect on macular microvascular flow and vessel density. Previous research has established that FAZ is an indicator of ischemia in DR and together with vascular densities is used for early detection of DR (32). Similar short-term effects on vessel density and FAZ area have been reported for intravitreal delivery of anti-VEGF agents (33,34). Anyway, several types of artifacts may influence the OCTA analysis, especially in pathologic eyes (35).
Our data suggest that Resvega supplementation in diabetic patients could have a positive effect on retinal microvasculature. Resveratrol was proved to lower insulin resistance, oxidative stress and concentration of pro-inflammatory cytokines, including VEGF, according to clinical trials on subjects with type II diabetes mellitus (36). However, the effect of resveratrol in DR was not clinically evaluated yet. A pilot study published by Chatziralli et al. showed the safety and efficacy of Resvega in diabetic patients with diabetic macular edema. Visual acuities and central retinal thickness were preserved after 4 months’ follow-up (37).
The present study had a small sample size, not extensive enough to draw a definite conclusion. Further investigations with larger sample sizes and longer follow-ups are needed to confirm our results.
In conclusion, Resvega seems to reverse the retinal microvascular changes in DR. Our findings are consistent with the results of in vivo and in vitro experiments and endorse it as a potential candidate for clinical trials in diabetic retinopathy. Moreover, state-of-the-art imaging tools (OCTA and AO ophthalmoscopy) are giving valuable information on retinal status, helping to refine new treatment options.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60(5):428–431. doi: 10.4103/0301-4738.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jurja S, Coman M, Hîncu MC. The ultraviolet influence upon soft eye tissues. Rom J Morphol Embryol. 2017;58(1):45–52. [PubMed] [Google Scholar]
- 3.Mozos I, Stoian D, Luca CT. Crosstalk between Vitamins A, B12, D, K, C, and E Status and Arterial Stiffness. Dis Markers. 2017;2017:8784971. doi: 10.1155/2017/8784971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Förstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch - Eur J Physiol. 2010;459:923–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- 5.Shi C, Wang P, Airen S, Brown C, Liu Z, Townsend JH, Wang J, Jiang H. Nutritional and medical food therapies for diabetic retinopathy. Eye Vis (Lond). 2020;18(7):33. doi: 10.1186/s40662-020-00199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashor AW, Siervo M, Lara J, Oggioni C, Afshar S, Mathers JC. Effect of vitamin C and vitamin E supplementation on endothelial function: a systematic review and meta-analysis of randomised controlled trials. Br J Nutr. 2015;113(8):1182–1194. doi: 10.1017/S0007114515000227. [DOI] [PubMed] [Google Scholar]
- 7.Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging. 2002;6(1):39–42. [PubMed] [Google Scholar]
- 8.Singh M, Tyagi SC. Homocysteine mediates transcriptional changes of the inflammatory pathway signature genes in human retinal pigment epithelial cells. Int J Ophthalmol. 2017;10(5):696–704. doi: 10.18240/ijo.2017.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srivastav K, Saxena S, Mahdi AA, Shukla RK, Meyer CH, Akduman L, Khanna VK. Increased serum level of homocysteine correlates with retinal nerve fiber layer thinning in diabetic retinopathy. Mol Vis. 2016;22:1352–1360. [PMC free article] [PubMed] [Google Scholar]
- 10.Long M, Wang C, Liu D. Glycated hemoglobin A1C and vitamin D and their association with diabetic retinopathy severity. Nutr Diabetes. 2017;7(6):e281. doi: 10.1038/nutd.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miao X, Sun W, Miao L, Fu Y, Wang Y, Su G, Liu Q. Zinc and diabetic retinopathy. J Diabetes Res. 2013;2013:425854. doi: 10.1155/2013/425854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neelam K, Goenadi CJ, Lun K, Yip CC, Au Eong KG. Putative protective role of lutein and zeaxanthin in diabetic retinopathy. Br J Ophthalmol. 2017;101(5):551–558. doi: 10.1136/bjophthalmol-2016-309814. [DOI] [PubMed] [Google Scholar]
- 13.Behl T, Kotwani A. Omega-3 fatty acids in prevention of diabetic retinopathy. J Pharm Pharmacol. 2017;69(8):946–954. doi: 10.1111/jphp.12744. [DOI] [PubMed] [Google Scholar]
- 14.Sarkar FH, Li Y, Wang Z, Kong D. Cellular signalling perturbation by natural products. Cell Signal. 2009;21:1541–1547. doi: 10.1016/j.cellsig.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kundu JK, Shin YK, Kim SH, Surh YJ. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-kappaB in mouse skin by blocking IkappaB kinase activity. Carcinogenesis. 2006;27:1465–1474. doi: 10.1093/carcin/bgi349. [DOI] [PubMed] [Google Scholar]
- 16.Park ES, Lim Y, Hong JT, Yoo HS, Lee CK, Pyo MY, Yun YP. Pterostilbene, a natural dimethylated analog of resveratrol, inhibits rat aortic vascular smooth muscle cell proliferation by blocking Akt-dependent pathway. Vascul Pharmacol. 2010;53(1-2):61–67. doi: 10.1016/j.vph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Bai Y, Mao QQ, Qin J, Zheng XY, Wang YB, Yang K, Shen HF, Xie LP. Resveratrol induces apoptosis and cell cycle arrest of human T24 bladder cancer cells in vitro and inhibits tumor growth in vivo. Cancer Sci. 2010;101(2):488–493. doi: 10.1111/j.1349-7006.2009.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parekh P, Motiwale L, Naik N, Rao KV. Downregulation of cyclin D1 is associated with decreased levels of p38 MAP kinases, Akt/PKB and Pak1 during chemopreventive effects of resveratrol in liver cancer cells. Exp Toxicol Pathol. 2011;63(1-2):167–173. doi: 10.1016/j.etp.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Higdon J, Drake VJ, Delage B. Resveratrol. Micronutrient Information Center. 2005 http://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/resveratrol#reference7. [Google Scholar]
- 20.Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res. 2012;32(7):537–541. doi: 10.1016/j.nutres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes - 2018. Diabetes Care. 2018;41(1):13–27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 22.Grading diabetic retinopathy from stereoscopic color fundus photographsan extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5):786–806. [PubMed] [Google Scholar]
- 23.Coscas F, Sellam A, Glacet-Bernard A, Jung C, Goudot M, Miere A, Souied EH. Normative data for vascular density in superficial and deep capillary plexuses of healthy adults assessed by optical coherence tomography angiography. Investig Ophthalmol Vis Sci. 2016;57(9):211–223. doi: 10.1167/iovs.15-18793. [DOI] [PubMed] [Google Scholar]
- 24.Sampson DM, Gong P, An D, Menghini M, Hansen A, Mackey DA, Sampson DD, Chen FK. Axial Length Variation Impacts on Superficial Retinal Vessel Density and Foveal Avascular Zone Area Measurements Using Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2017;58(7):3065–3072. doi: 10.1167/iovs.17-21551. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Liu H, Al-Shabrawey M, Caldwell RW, Caldwell RB. Inflammation and diabetic retinal microvascular complications. J Cardiovasc Dis Res. 2011;2(2):96–103. doi: 10.4103/0975-3583.83035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez ML, Pérez S, Mena-Mollá S, Desco MC, Ortega ÁL. Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxid Med Cell Longev. 2019;2019:4940825. doi: 10.1155/2019/4940825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N, Jr, Serhan CN, Smith LEH. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13(7):868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cristescu IE, Zagrean L, Balta F, Branisteanu DC. Retinal microcirculation investigation in type I and II diabetic patients without retinopathy using an adaptive optics retinal camera. Acta Endocrinol (Buchar). 2019;15(4):417–422. doi: 10.4183/aeb.2019.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cristescu IE, Baltă F, Zăgrean L. Cone photoreceptor density in type I diabetic patients measured with an adaptive optics retinal camera. Rom J Ophthalmol. 2019;63(2):153–160. [PMC free article] [PubMed] [Google Scholar]
- 30.Ritt M, Schmieder RE. Wall-to-lumen ratio of retinal arterioles as a tool to assess vascular changes. Hypertension. 2009;54(2):384–347. doi: 10.1161/HYPERTENSIONAHA.109.133025. [DOI] [PubMed] [Google Scholar]
- 31.Strain WD, Paldánius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 2018;17(1):57. doi: 10.1186/s12933-018-0703-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vujosevic S, Toma C, Villani E, Gatti V, Brambilla M, Muraca A, Ponziani MC, Aimaretti G, Nuzzo A, Nucci P, De Cilla’ S. Early Detection of Microvascular Changes in Patients with Diabetes Mellitus without and with Diabetic Retinopathy: Comparison between Different Swept-Source OCT-A Instruments. J Diabetes Res. 2019;2019:2547216. doi: 10.1155/2019/2547216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghasemi Falavarjani K, Iafe NA, Hubschman JP, Tsui I, Sadda SR, Sarraf D. Optical Coherence Tomography Angiography Analysis of the Foveal Avascular Zone and Macular Vessel Density After Anti-VEGF Therapy in Eyes With Diabetic Macular Edema and Retinal Vein Occlusion. Invest Ophthalmol Vis Sci. 2017;58(1):30–34. doi: 10.1167/iovs.16-20579. [DOI] [PubMed] [Google Scholar]
- 34.Sorour OA, Sabrosa AS, Yasin Alibhai A, Arya M, Ishibazawa A, Witkin AJ, Baumal CR, Duker JS, Waheed NK. Optical coherence tomography angiography analysis of macular vessel density before and after anti-VEGF therapy in eyes with diabetic retinopathy. Int Ophthalmol. 2019;39(10):2361–2371. doi: 10.1007/s10792-019-01076-x. [DOI] [PubMed] [Google Scholar]
- 35.Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina. 2015;35(11):2163–2180. doi: 10.1097/IAE.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toro MD, Nowomiejska K, Avitabile T, Rejdak R, Tripodi S, Porta A, Reibaldi M, Figus M, Posarelli C, Fiedorowicz M. Effect of Resveratrol on In Vitro and In Vivo Models of Diabetic Retinopathy A Systematic Review. Int J Mol Sci. 2019;20(14):3503. doi: 10.3390/ijms20143503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chatziralli I, Dimitriou E, Chatzirallis A, Aissopou E, Theodossiadis P. Efficacy and safety of Resvega in diabetic macular edema: preliminary results of a pilot study. Acta Ophthalmol. 2019;97:S263. [Google Scholar]


