Abstract
Introduction.
The published data showed the importance of metabolic control in preventing complications in metabolic syndrome (MS) and the role of nutritional medical therapy in glycemic control and in the control of dyslipidemia, hypertension, weight loss/normalization (in overweight or malnourished subjects).
Objectives.
This study follows the evolution of sarcopenic index (SI) and other clinical parameters (body mass index (BMI), homeostasis evaluation index (HOMA index)) correlated with MS after diet therapy or diet therapy combined with sports, in patients with MS.
Patients and methods.
Our research was conducted during 12 months, on 110 patients >18 years of age, with HOMA index>2, divided into three groups: control group (CG, N=20), diet therapy group (DTG, N=58), diet therapy and sports group (DTSG, N=32). HOMA index for insulin resistance was calculated as the product of resting plasma insulin (in microunits/milliliter) and plasma glucose (in millimoles/liter), divided by 22.5. SI was determined using BIA, as being the ratio between muscle mass and fat mass, measured in cm2/m2.
Results.
A significant decrease of BMI (p<0.05) in DTG (from 31.63 to 24.50) and DTSG (from 30.18 to 24.17) vs. CG was observed (Pearson coefficient r=0.281, p<0.001). Weight status changed significantly (p<0.05) in the high-risk patients. There was a significant decrease of HOMA index (p<0.05) in DTG (from 5.93 to 2.57), DTSG (from 3.93 to 2.23), and in CG an increase was observed (from 3.15 to 3.37).
Conclusion.
The best results in the prevention/ treatment of sarcopenia in MS patients were obtained for DTSG, which benefited from both the positive effect of diet and physical activity.
Keywords: sarcopenic index, HOMA index, metabolic syndrome, diet therapeutic intervention, obesity
Introduction
Sarcopenia is a much-discussed health issue recently, although there is little information in the current literature. Being a component of frailty syndrome, it indicates a progressive decline in health by reducing the quality and strength of muscle tissue (1). Muscle loss and intramuscular fat accumulation may be associated with metabolic syndrome (MS) through a complex interaction of factors, including nutritional intake, physical activity, body fat, pro-inflammatory cytokines, insulin resistance and mitochondrial dysfunction (2) as it is presented in Fig 1.
Figure 1.
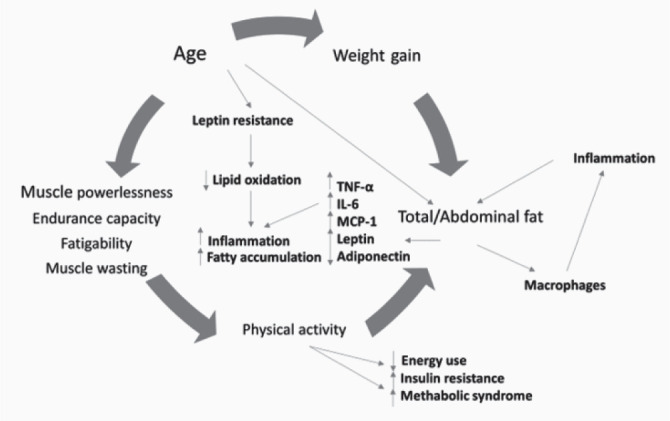
The relationship between muscle and adipose tissue. The mechanism that leads to the development of sarcopenia. (TNF-alpha: tumor necrosis factor-alpha; IL-6: interleukin-6; MCP1: monocyte chemoattractant protein-1).
Individuals with sedentary lifestyle and with varying degrees of obesity present a high risk of losing muscle mass (3). Especially for these subjects, monitoring the sarcopenic index (SI) provides important data related to the effects of diet therapy and physical activity (4). The evolution of the SI is monitored extremely quickly, using body composition analysis devices through a non-invasive, high-precision method.
Signs that characterize the MS (i.e. visceral obesity, insulin resistance, hyperglycemia, dyslipidemia, inflammation and hypertension) (5) favor the onset of type 2 diabetes (6, 7) and are associated with sarcopenic obesity, a form of sarcopenia. Physiological changes in the muscles, such as aging, also lead to a decrease in the SI (8). Normal aging results in a loss of muscle mass and muscle strength (sarcopenia). This is caused by a gradual loss of the regenerative capacity of new satellite muscle cells and a decrease in the action of growth factors, which normally maintain a healthy muscle mass (9).
In parallel with the loss of muscle mass due to aging, there is a redistribution of fat mass, mainly in the visceral compartment, but also fat deposits are observed in the skeletal muscle and liver (10). It has been observed that up to 30 years of age, skeletal muscle mass develops at an increasing rate. However, specialists have noticed a decrease in muscle mass and function after this age. If there are no other health problems that can cause sarcopenia and, implicitly, cachexia (for example, cancer), it has two reasons: inactive lifestyle and aging (11). Some internal and extreme factors such as systemic and muscular oxidative stress, inflammation and insulin resistance or adiposity, can cause metabolic disorders. All of these disorders can cause increased fat storage (12). These increases in fat mass (which are interconnected) promote catabolic processes, as well as a state of anabolic resistance to nutrients in skeletal muscles (13), that can be diminished by introducing regular physical activity and more importantly, by changing the diet. Numerous studies have been carried out in various research centers to determine the causes of skeletal muscle loss and strength in the elderly (14). It seems that the total elimination of muscle loss is not possible by any method (15).
Muscle damage can be caused by reduced nerve cells responsible for transmitting signals from the brain to the muscles, decreased ability of the body to metabolize proteins, inadequate consumption of nutrients, especially proteins, to support muscle mass, or dietary errors and decreased hormones levels (16). Typically, testosterone levels and insulin-like growth factor, the insulin-like growth factor-1 (IGF-1 hormone) affect muscle growth and muscle mass (17).
A recent study showed that currently 20% of the population is affected by sarcopenia, and this percentage is constantly increasing; as well, the increase in overall life expectancy is a relevant contribution to this trend. According to statistical data, by 2045 it can reach 63% of the population (18).
Fat and muscle (and lean tissue in general) have opposite effects on glucose sensitivity (19). Therefore, the ratio between the two masses, muscle mass (appendicular skeletal muscle mass) and fat mass, is a determining factor for metabolic health and is the basis for calculating the SI (20). In this study, the calculation of the SI was performed non-invasively, using the body bioelectric impedance analyzer (BIA) (21). Muscles use glucose, while fatty acids derived from excess adipose tissue inhibit the use of glucose; therefore, the danger is twofold (10).
Muscle atrophy occurs as a side effect of many clinical conditions, being influenced by food intake as well as in case of cachexia and catabolism of muscle tissue (22, 23).
Cancer, AIDS, heart failure, lung and kidney disease, severe burns and alcoholism are examples of conditions determining muscle loss (24). There are many other neurological disorders that affect muscle function and can eventually lead to muscle weakness (stroke, Parkinson’s disease, multiple sclerosis, myasthenia gravis, etc.) (25).
The aim of this research was to follow the evolution of some clinical parameters correlated with MS or with the insulin resistance (component of MS). Risk factors for insulin resistance are as follows: sedentarism, obesity, lifestyle, various health conditions, family history of diabetes and certain medications. A personalized diet therapy, adapted to existing disorders, was combined with testing allergic reactions of IgG type to food, managed according to the specialized provisions, in order to find the most suitable formula that improves the verified parameters. These parameters may indicate the influence of changing eating habits and the importance of physical activity in the evolution of MS.
Patients and Methods
Study design
The study was performed at SC Echo Laboratoare SRL, Oradea, Romania, on 110 individuals diagnosed with MS, who presented to the private nutrition and diet therapy office to assess nutritional status during 2018-2020.
The research was conducted in accordance with the Helsinki Declaration and International Ethical Guidelines for Good Clinical Practice and Biomedical Research for Human Subjects, and it was approved by the Ethical Committee of the Faculty of Medicine and Pharmacy of the University of Oradea (no. 12/01.04.2019). Each patient included in this study signed an informed consent at his first consultation.
Patients’ inclusion criteria in the study were considered as follows: age >18 years-old; high homeostasis evaluation (HOMA) index (>2); diagnostics of MS or insulin resistance; body composition (the value of visceral fat, dyslipidemia, BMI, fat mass); the presence of hypertension. Presence of medical complications (decompensated diabetes, grade 2 and 3 hypertension, heart failure, kidney failure, thrombosis, thrombophlebitis) and insulin-dependent diabetes was considered as exclusion criteria. The evolution of the SI in patients who underwent diet therapy or sports-associated diet therapy was compared to that of those who maintained the same lifestyle. The patients were divided into 3 groups using a selection method based on the following considerations:
-
-
their desire to do sports/physical exercises,
-
-
their preference to follow a nutritional therapy,
-
-
each subject’s medical history,
-
-
physical condition after the assessment of risks and benefits of therapeutic interventions for each patient.
The groups resulted were named as: diet therapy group DTG (n=58), diet therapy group and sports DTSG (n=32) and control group CG (20). The gender distribution of patients in research groups was approximately equal.
In all groups, the SI, followed by BIA, was monitored, depending on sex, age, body mass index (BMI) reflecting weight status) and HOMA index. The diet therapy was individualized for each participant included in the study, being applied in the DTSG group as a specific one (increasing the muscle mass, with the increase of the protein intake from 20-25% to 30-35%, without concomitant increase of the lipid intake). Carbohydrates were the staple food, accounting for 50-55% of the daily intake. The recommended proteins were 85% of vegetable origin and 15% of animal origin. The physical activity program included aerobic exercises, with a frequency of 5 times/week, of which, at least 20 minutes for cardiovascular stimulation. The protocol consisted of following the adherence to diet therapy and sports and the evolution of the parameters was evaluated each month, presenting the results obtained at 12 months, compared to the initial ones, in all three groups. BMI was noticed initial, at 6 months, and at the end of the study.
Clinical investigations
The clinical evaluation was performed with a bio-electric impedance body analyzer (Tanita MC780MA, Germany), using GMON 3.4.1. medical software, determining the evolution of the clinical parameters. BIA are devices accepted by the World Public Health Nutrition Association (WPHNA) that determine body composition with high accuracy. The limit of error is 0.1 kg (26). The initial assessment consisted of noninvasive analyses of weight, BMI, weight status, SI, visceral fat, presentation of laboratory results of HOMA index and C-reactive protein. SI, much discussed in the last decade, is all the more relevant as the population has a higher average age, which is associated with a high degree of sedentary lifestyle. The ratio between the two masses, muscle mass (appendicular skeletal muscle mass) and fat mass, is a determining factor for metabolic health, and is the basis for calculating SI. Until the improved appearance of medical equipment (bioelectric impedance devices) based on dual energy X-ray absorptiometry (DEXA), the determination of the SI was performed only by invasive analyses (blood sample), based on the formula:
| SI=(serum creatinine value / cystatin C value) ×100. |
Monthly measurements of the weight were most important in adjusting the diet according to body changes, but the evaluation of the results was done only at 6 and at 12 months, to reduce errors due to minor monthly changes.
The patient steps on the analyzer with both feet, barefoot, before physical activity and holds two electrodes in his hands. BIA evaluates the composition of the body by passing a current of very low intensity through the body, assessing the impedance differences caused by the fact that the fatty and weak tissues have different electrical properties. Information is obtained on weight, fat mass, muscle mass, bone mass, visceral fat, SI, basal metabolism, intracellular water, extracellular water, angle phase and the predominant location of both fat and muscle mass.
The use of the BIA method (which is non-invasive) allows the monitoring of body composition, easily and quickly. It provides information on numerous parameters (muscle mass, fat mass, visceral fat, extra- / intracellular water, bone mass and SI), being useful in people with uncomplicated MS. Body weight pursued by BMI alone is not enough, because the ratio between fat and muscle mass has a major influence on the evolution of SI, so SI is recognized as a good indicator for muscle mass increasing.
Regarding paraclinical tests, they involve checking blood parameters. In the morning, on an empty stomach, samples of venous blood are collected in sterile vacutainers, which are centrifuged. The obtained serum is separated and stored for one hour in the incubator (at a constant temperature of 37° Celsius), being then processed automatically by enzyme-linked immunosorbent assay (ELISA) method. The blood test results (which indicate also glucose, insulin and CRP levels) are printed and read.
Statistical analysis
Statistical analysis was performed using SPSS Statistics 22. All mean values of the parameters, standard deviations, frequency intervals and tests of statistical significance were calculated using the t-Student method. The distribution of the groups proved to be similar to the normal one, hypotheses involving numerical data being used. The Bravais-Pearson correlation coefficient was used to calculate an independent indicator of the units of measurement of the two variables. The value p <0.05 was assigned to statistical significance. ANOVA with a post-hoc analysis (Bonferroni) was used to analyze the differences between the groups, as an additional analysis of the subgroups.
Results
Demographic and clinical characteristics
At the initial evaluation, the three groups were relatively homogeneous in terms of clinical and demographic characteristics, without statistically significant differences (p>0.05) between the 3 groups, with a mean age of 37.49 (±14.12), the initial SI average 8.49 (±1.56) cm2/m2 and initial average BMI 30.63 (±7.55). The statistical data are presented in Table 1, screened for each group. BMI evolution is shown in Figure 2.
Figure 2.
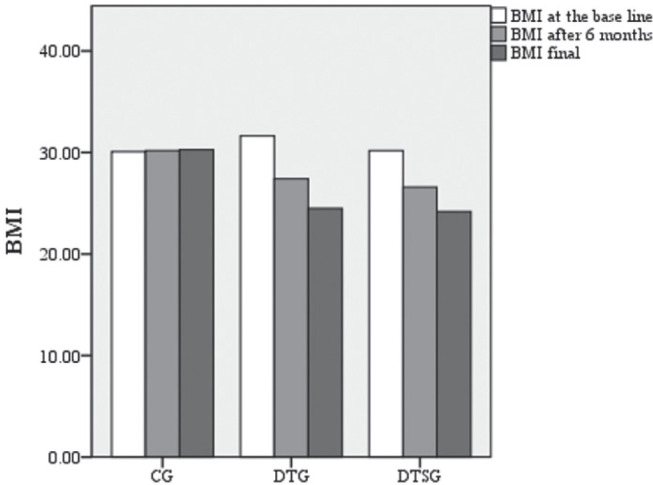
BMI at the base line, at 6 months, and at the end of the study in CG, DTG and DTSG.
Table 1.
Demographic and clinical characteristics of the patients at the base line
| Characteristics | GC (N=20) | DTG (N=58) | DTSG (N=32) | p value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Gender | |||||||
| Women | 9 | 45.00 | 24 | 41.38 | 17 | 53.13 | -0.06 |
| Men | 11 | 55 | 34 | 58.62 | 15 | 46.87 | |
| Total | 20 | 100 | 58 | 100 | 32 | 100 | |
| Age | |||||||
| <40 | 13 | 65 | 26 | 44.83 | 21 | 65.63 | -0.05 |
| 40-65 | 6 | 30 | 29 | 50 | 11 | 34.37 | |
| >65 | 1 | 5 | 3 | 5.17 | 0 | 0 | |
| Average age | 38.3484 (± 17.00) |
38.6552 (± 16.26) |
35.4688 (± 9.09) |
||||
| Area of provenience | |||||||
| Urban | 15 | 75 | 47 | 81.03 | 26 | 81.25 | -0.04 |
| Rural | 5 | 25 | 11 | 18.97 | 6 | 18.75 | |
| Total | 20 | 100 | 58 | 100 | 32 | 100 | |
| Clinical parameters | |||||||
| Initial average BMI |
30.09 (± 6.40) |
31.63 (± 7.44) |
30.18 (± 8.82) |
-0.01 | |||
| Initial average Sarcopenic Index | 8.70 (± 1.5344) |
8.14 (± 2.10) |
8.10 (± 1.75) |
-0.09 | |||
| Weight status | |||||||
| Underweight | 0 | 0 | 1 | 1.73 | 0 | 0 | 0.01 |
| Normal | 2 | 10 | 8 | 13.79 | 11 | 34.37 | |
| Overweight | 12 | 60 | 12 | 20.68 | 9 | 28.12 | |
| Obesity grade I | 2 | 10 | 20 | 34.49 | 5 | 15.63 | |
| Obesity grade II | 1 | 5 | 9 | 15.52 | 2 | 6.25 | |
| Obesity grade III | 3 | 15 | 8 | 13.79 | 5 | 15.63 | |
Legend: N: Number of patients.
Evolution of the body weight
To verify the effect of the type of intervention on BMI, the ANOVA statistical test was used for 3 independent groups; F=19.412 was obtained. So, in the post-test (after 1 year), BMI differs significantly (p<0.05) in all the three groups (Table 2). BONFERRONI Post Hoc tests indicate significant differences (p<0.001) between groups as follows: between CG and DTG (average CG=30.27, average DTG=24.50), between CG and DTSG p<0.001 (average CG=30.27, mean DTSG=24.17). There are no significant differences (p>0.05) between BMI at one year, recorded for DTG and DTSG. This can be explained by the fact that BMI can be kept at a similar level, with or without physical activity. Significance thresholds indicate the following directions: for CG there is a significant increase in BMI (p<0.004), for DTG the BMI decreases significantly (p=0.001), and for DTSG the BMI decreases significantly as well (t=0.54, p=0.01) (Table 2).
Table 2.
Differences in body mass index (BMI), both the sarcopenic and HOMA indexes, visceral fat and C reactive protein (CRP)
| Research variables | Group | N | Mean | Standard deviation | F | t | p value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | ||||||||||||
| Initial | GC | 20 | 30.09 | 6.40 | 19.41 | -3.28 | 0.01 | |||||
| Final | 30.27 | 6.44 | ||||||||||
| Initial | DTG | 58 | 31.63 | 7.44 | 10.54 | 0.01 | ||||||
| Final | 24.50 | 3.05 | ||||||||||
| Initial | DTSG | 32 | 30.18 | 8.82 | 5.16 | 0.01 | ||||||
| Final | 24.17 | 2.81 | ||||||||||
| Final | 8.57 | 1.22 | ||||||||||
| HOMA INDEX | ||||||||||||
| Initial | GC | 20 | 3.15 | 1.22 | 2.51 | -2.29 | 0.03 | |||||
| Final | 3.37 | 1.38 | ||||||||||
| Initial | DTG | 58 | 5.93 | 14.32 | 2.04 | 0.04 | ||||||
| Final | 2.57 | 2.01 | ||||||||||
| Initial | DTSG | 32 | 3.93 | 2.60 | 7.41 | 0.01 | ||||||
| Final | 2.23 | 1.58 | ||||||||||
| SARCOPENIC INDEX | ||||||||||||
| Initial | GC | 20 | 8.70 | 1.53 | 0.49 | -0.86 | 0.39 | |||||
| Final | 8.75 | 1.58 | ||||||||||
| Initial | DTG | 58 | 8.14 | 2.10 | -2.16 | 0.03 | ||||||
| Final | 8.36 | 1.73 | ||||||||||
| Initial | DTSG | 32 | 8.10 | 1.72 | -2.79 | 0.01 | ||||||
| 8.57 | 1.22 | |||||||||||
| VISCERAL FAT | ||||||||||||
| Initial | GC | 20 | 8.10 | 5.49 | 3.37 | 1.00 | 0.33 | |||||
| Final | 8.15 | 5.60 | ||||||||||
| Initial | DTG | 58 | 8.48 | 6.19 | 7.75 | 0.01 | ||||||
| Final | 6.84 | 5.13 | ||||||||||
| Initial | DTSG | 32 | 5.81 | 4.34 | 4.32 | 0.01 | ||||||
| Final | 4.75 | 3.70 | ||||||||||
| CRP | ||||||||||||
| Initial | GC | 20 | 0.45 | 0.51 | 0.58 | 1.00 | 1.00 | |||||
| Final | 0.45 | 0.51 | ||||||||||
| Initial | DTG | 58 | 0.50 | 0.50 | 4.05 | 0.01 | ||||||
| Final | 0.27 | 0.45 | ||||||||||
| Initial | DTSG | 32 | 0.43 | 0.48 | 2.39 | 0.02 | ||||||
| Final | 0.18 | 0.39 | ||||||||||
Legend: N: Number of patients; CG: Control group; DTG: Diet therapy group; DTSG: Diet therapy and sport group; F: ANOVA statistical test coefficient, for 3 or more independent groups; t: coefficient for the T-Student statistical test.
There is a significant increase (p<0.05) in BMI at CG, between the initial and final stage. In the case of DTG and DTSG groups, BMI values decrease significantly during the intervention, as can be seen from the analysis of means and significance thresholds.
Weight status was monitored throughout the study, being compared in Table 2, before and after dietary intervention, respectively diet therapy and sports. In this table it can be followed the evolution of the weight subgroups before and after the dietary intervention, respectively the percentage of all participants who changed their weight subgroup during the study. Significance thresholds indicate the following directions for weight status (obesity): for CG there is a significant increase in obesity (p>0.05), for DTG obesity decreases significantly (p=0.01), and DTSG obesity decreases less, but significantly (p=0.01), as can be seen from the data presented in Figure 3. The results show the effectiveness of diet therapy in terms of weight change.
Figure 3.
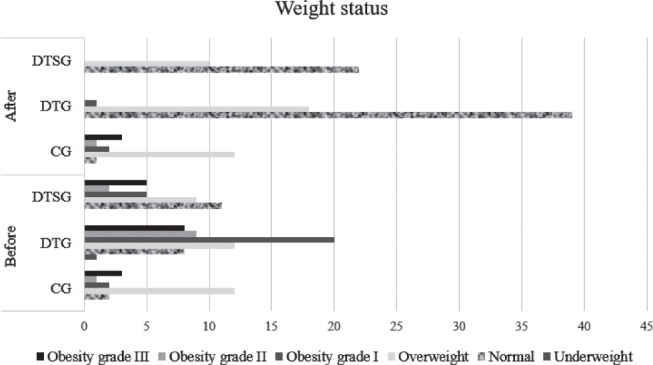
Descriptive and inferential statistics for comparing clinical and paraclinical indicators for all three researched groups, at the end of the intervention.
It is possible to follow the evolution of the number of participants included in the 6 subgroups of the weight status before and after the research period. Thus, a substantial increase can be observed regarding the number of subjects in the normal subgroup and a consistent decrease in the subgroups of obesity grade I, II and III. At the end of the research period, the subgroup with overweight patients comprises subjects from the subgroups with all three degrees of obesity (from the beginning of the research), patients who modified one, two or even all three weight status subgroups. For the relationship between the pre-test and post-test difference in weight status (obesity) according to sex, the following change was obtained: in DTG, men, those in the category of obesity grade III (4 patients), at the end of the study reached the category of overweight. From those in the category of initial obesity grade II (7 patients), 4 became normal weight and 3 overweight and of those with initial obesity grade I (5 patients), 4 reached the normal weight category and 1 overweight.
Figures 4 (A and B) show the evolution of the weight status in DTG and DTSG groups, in both genders. A slower decrease can be observed, fact that can be explained by the association with the increase in muscle mass, observed especially in men.
Figure 4.
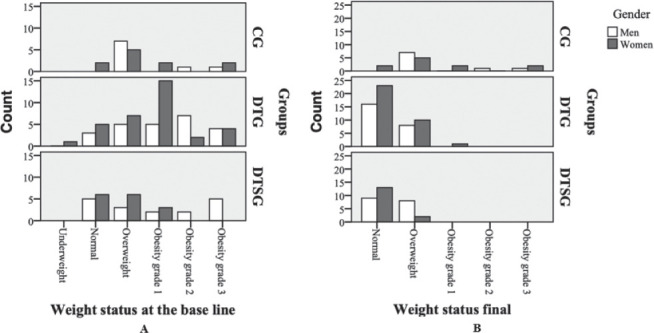
Graphical representation of the weight status in both genders: A.at the base line; B. final.
The evolution of the sarcopenic index
To verify the effect of the type of intervention on the SI, the ANOVA statistical test was used for all three independent groups. It was obtained F=0.495, in post-test (after 1 year) the SI does not differ significantly (p>0.05) in the three groups. The data were presented in Table 2. There is a difference, but statistically insignificant (p>0.05), between the 1st year when SI was recorded, for DTG and DTSG (p>0.05). Significance thresholds indicate the following directions: for CG there is no significant increase in the SI (p<0.397), for DTG the SI increases significantly (p=0.035), and for DTSG the SI increases significantly (p=0.009) as well. In the case of CG, the SI did not change significantly (p>0.05); instead, for DTG, there was an increase in the SI, but a smaller one, compared to the group that, in addition to diet therapy, also practiced physical exercises. These data are also depicted in the Table 2.
Evolution of metabolic parameters
Significance thresholds indicate the following directions: for the control group there is a significant increase in the HOMA index (p<0.033), for the diet therapy group the HOMA index decreases, but statistically insignificant (p=0.046), and for the diet therapy and physical exercises group the HOMA index decreases significantly (p=0.001).
The MS components modification including visceral fat and C reactive protein (CRP), where the significance thresholds indicate the following directions: for CG there is a slight increase, but without statistical significance of visceral fat (p=0.330), for DTG visceral fat decreases significantly (p=0.001) and for DTSG visceral fat decreases less, but with statistical significance (p=0.001) and the significance thresholds indicate the following directions for CRP: in CG there is no significant change (p>0.05), in DTG there is a significant decrease (p=0.001), and in DTSG, this parameter also decreases significantly (p=0.023). According to the results presented in Table 2, it was observed an improvement of the clinical parameters (i.e. BMI, weight status, SI and visceral fat), but also of the paraclinical parameters (HOMA index and CRP).
For the relationship between the pre-test and post-test difference at the SI and the HOMA index, a Pearson coefficient r=0.281 was obtained, p<0.001, which indicates a strong positive relationship. The smaller the difference in the SI, the smaller the difference in the HOMA index. This relationship, with statistical significance (p<0.05), results from the positive value of the Pearson coefficient and from the value less than 0.05 of the significance threshold.
The relationship between the pre-test and post-test difference at the SI and BMI determined a Pearson coefficient r=0.134, which indicates a relationship without statistical significance (p>0.05). Correlating an increase in the difference in the SI can be observed, related to the decrease in the BMI difference. This relation, without statistical significance, results from the positive value of the Pearson coefficient, the significance threshold being p=0.162. The Pearson coefficient, which shows the relationship of the pre-test and post-test difference for the SI and the weight status, is r=0.126, p>0.05, indicating the positive but statistically insignificant relationship (p=0.188). A directly proportional relationship cannot be observed between the two variables throughout the research period, the SI being directly proportional up to a point, after which an inversely proportional relationship is observed.
Discussion
The BMI values observed in the three groups reveal that in CG there is a significant increase (p<0.05), which is due to unchanged diet and increased body weight. In both DTG and DTSG groups, respectively, a significant decrease (p<0.05) in BMI was noted (smaller in DTSG), which shows the development of muscle mass. With the increase in muscle mass, the decrease in fat mass is not so substantial as to obtain a substantial decrease in BMI just by simple dietary therapy.
A study published in 2018 looked at the change in BMI in young people with and without physical activity, observing that a greater decrease in BMI was obtained in those who performed sports (which can be explained by the age of the participants and the lack of MS, elements indicated also in the present research) (27).
Another 2018 study, which included young people in South Africa, concluded that of all parameters, it is the most important to track the risk factor. In the control group, a slight increase in visceral fat was observed, as a consequence of the increase in fat mass. In the present study, in DTG, a more substantial decrease in visceral fat was noticed, compared to DTSG group, which can be associated with MS and special diet therapy to support physical activity. Thus, a slower decrease in fat can be observed, but at the same time, an increase in muscle mass and an improvement in the SI. It substantially reduces the risk of developing MS and contributes to improving the quality of life (28).
Sarcopenia and sarcopenic obesity have been associated with gastric carcinogenesis, according to a study published in 2019, on patients with MS (29). Therefore, it is important to follow several parameters, such as SI, weight status, and abdominal fat.
Because nutrition and diet therapy have not had, until now, an important place in monitoring the evolution of MS, specialized studies are of little interest in diet therapy. A review, published in 2019 (30), studied the effects of lifestyle change in patients with diabetes and MS. The results showed the importance of lifestyle change, with beneficial effects in improving the quality of life in subjects with diabetes or MS. In this paper, a significant decrease (p<0.05) in BMI for DTG compared to CG was obtained. By adjusting the intake of refined carbohydrates, excess proteins and lipids, statistically significant (p<0.05) results were obtained for this category. The reduction in BMI with an average value of 5.77 (from 30.27, in CG, to 24.5, in DTG) shows the evolution over a whole weight category, which means the reduction of fat and visceral fat, the reduction of muscle mass and the improvement of basal metabolism, lacking physical activity. BMI values in CG showed a slight increase, from 30.09 to 30.27, which demonstrates the preservation of wrong eating habits and a lifestyle that increases the risk of diseases associated with MS (31), without a balanced intake of macronutrients. Changes in BMI values at DTG, at the end of the study period, show a decrease of 7.13 units, which proves the importance of a proper and balanced diet in terms of protein, fat and carbohydrate intake. A study published in 2019 in Italy shows the importance of sleep in diet therapy (32) and another published research draws attention to the origin of lipids in diet therapy to achieve significant results (p<0.05) (33) in subjects with metabolic MS.
Rodriguez de Lima et al. followed a group of 533 obese adults with MS and concluded the link between abdominal adiposity and worsening of MS (34). They observed an inversely proportional link between muscle burning and the development of diseases associated with high health risk (dyslipidemia, cardiovascular disease). The present research revealed a decrease of 6.01 units in BMI at DTSG, less than at DTG. This is explained by increased muscle mass or toning of existing muscle mass, which prevented consistent muscle loss. Because of this, the comparative BMI reduction ratio between DTG and DTSG is 1.12 units more at DTG than at DTSG. The decrease by 6.10 units compared to CG in DTSG proved the effectiveness of these therapies.
According to some results published in 2007, 79% of the participants showed an improvement in muscle mass, irrelevant to the involvement of strict change in diet (35). In another 2018 study, which looked at the function and structure of skeletal muscle fibers (which are mainly affected by mitochondrial dynamics and morphology) it was shown that the inflammatory markers contribute to age-related muscle loss (36). More recently, another research concluded that the combination of diet and physical activity plays a decisive role in the prevention of sarcopenia (37).
In a review of nutritional influences on muscle aging, Welch (38) assessed established, less established and potential nutritional factors involved in the major biomechanics of age-related muscle loss (39). Western diets could have increased the risk of poor physical function through potential effects on the quality and composition of myofibers and increased pathophysiological processes involved in sarcopenia and functional decline, including fat infiltration, inflammation, oxidative stress and insulin resistance. Sarcopenia and sarcopenic obesity have been associated with gastric carcinogenesis, according to a study published in 2019, in patients with MS (29). That is why it is important to follow several parameters such as the SI, weight status, associated with other clinical and paraclinical parameters. This part still requires future studies to establish exactly the most conclusive link in the follow-up of MS.
In conclusion, physical activity increases muscle mass, which explains a smaller decrease in body weight, the muscles being heavier, for DTSG. Diet therapy and diet therapy associated with sports have been shown to be effective in weight loss, the best results being obtained at DTSG. In the case of DTG, patients lost both muscle mass and fat mass. Thus, the SI is higher for DTSG due to physical activity, being easily observable that sport has improved the results in this research group. The SI has a tendency to increase from a value of 5.06, and to decrease from 15.34 in adults, which indicates the instability of glycogen either due to sedentary lifestyle (in the case of low SI), or due to a high level of sugar (glycemia) in the blood and muscles. A greater decrease in sarcopenia can be observed in men than in female, the other parameters having similar tendencies for both sexes. A significant (p<0.05) improvement was obtained between the initial and final stages in most clinical parameters, in both DTG and DTSG groups. Endurance and cardio workouts, added to proper, healthy nutrition and aerobic exercise, are the main tools to prevent and treat sarcopenia in adults with MS. Dietary intervention has been shown to be effective in suppressing uncontrolled muscle mass loss, with or without fat accumulation. Physical activity included between daily activities also increased the value of SI.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Rubio-Ruiz ME, Guarner-Lans V, Pérez-Torres I, Soto ME. Mechanisms underlying metabolic syndrome-related sarcopenia and possible therapeutic measures. Int J Mol Sci. 2019;20(3):647. doi: 10.3390/ijms20030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, Heitmann BL, Kent-Smith L, Melchior JC, Pirlich M, Scharfetter H, Schols AM, Pichard C. Composition of the ESPEN Working Group. Bioelectrical impedance analysis-part I: review of principles and methods. Clin Nutr. 2004;23(5):1226–1243. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Zamboni M, Rubele S, Rossi AP. Sarcopenia and obesity. Curr Opin Clin Nutr Metab Care. 2019;22(1):13–19. doi: 10.1097/MCO.0000000000000519. [DOI] [PubMed] [Google Scholar]
- 4.Fonseca GWPD, Ribeiro de Souza F, dos Santos MR, Janieire de Nazaré Nunes Alves M. The role of exercise on sarcopenia. Cardiol Cardiovasc Med. 2020;4(5):540–562. [Google Scholar]
- 5.Kir S, Ekiz K, Alacam H, Turkel R, Koroglu E, Altintop BL. The association between pro and anti-inflamatory markers with the components of metabolic syndrome. Acta Endocrinol (Buchar). 2019;15(4):430–435. doi: 10.4183/aeb.2019.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaha DC, Vesa C, Uivarosan D, Bratu O, Fratila O, Mirela Tit D, Pantis C, Diaconu CC, Bungau S. Influence of inflammation and adipocyte biochemical markers on the components of metabolic syndrome. Exp Ther Med. 2020;20(1):121–128. doi: 10.3892/etm.2020.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vesa CM, Popa L, Popa AR, Rus M, Zaha AA, Bungau S, Tit DM, Corb Aron RA, Zaha DC. Current data regarding the relationship between type 2 diabetes mellitus and cardiovascular risk factors. Diagnostics. 2020;10(5):314. doi: 10.3390/diagnostics10050314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz-Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, Chen LK, Fielding RA, Martin FC, Michel JP, Sieber C, Stout JR, Studenski SA, Vellas B, Woo J, Zamboni M, Cederholm T. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748–759. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takemoto Y, Fukada SI. Molecular mechanism maintaining muscle satellite cells and the roles in sarcopenia. Clin Calcium. 2017;27(3):339–344. [PubMed] [Google Scholar]
- 10.Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, Nakagomi R, Kondo M, Nakatsuka T, Minami T, Sato M, Uchino K, Enooku K, Kondo Y, Asaoka Y, Tanaka Y, Ohtomo K, Shiina S, Koike K. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63(1):131–140. doi: 10.1016/j.jhep.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 11.von Haehling S, Ebner N, Anker SD. Oodles of opportunities: the Journal of Cachexia, Sarcopenia and Muscle in 2017. J Cachexia Sarcopenia Muscle. 2017;8(5):675–680. doi: 10.1002/jcsm.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mocan M, Vesa Ş, Suciu Ş, Blaga SN. Systemic markers of oxidative stress in relation to metabolic syndrome components. Clujul Med. 2013;86(3):227–234. [PMC free article] [PubMed] [Google Scholar]
- 13.Selberg O, Selberg D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur J Appl Physiol. 2002;86(6):509–516. doi: 10.1007/s00421-001-0570-4. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev. 2018;47:123–132. doi: 10.1016/j.arr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wanigatunga AA, Tudor-Locke C, Axtell RS, Glynn NW, King AC, McDermott MM, Fielding RA, Lu X, Pahor M, Manini TM. Effects of a long-term physical activity program on activity patterns in older adults. Med Sci Sports Exerc. 2017;49(11):2167–2175. doi: 10.1249/MSS.0000000000001340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alalwan TA. Phenotypes of sarcopenic obesity: Exploring the effects on peri-muscular fat, the obesity paradox, hormone-related responses and the clinical implications. Geriatrics (Basel). 2020;5(1):8. doi: 10.3390/geriatrics5010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morley JE. Hormones and sarcopenia. Curr Pharm Des. 2017;23(30):4484–4492. doi: 10.2174/1381612823666161123150032. [DOI] [PubMed] [Google Scholar]
- 18.Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev. 2013;41(3):169–173. doi: 10.1097/JES.0b013e318292f3d5. [DOI] [PubMed] [Google Scholar]
- 19.Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol. 2013;3(1):1–58. doi: 10.1002/cphy.c110062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narumi T, Watanabe T, Kadowaki S, Takahashi T, Yokoyama M, Kinoshita D, Honda Y, Funayama A, Nishiyama S, Takahashi H, Arimoto T, Shishido T, Miyamoto T, Kubota I. Sarcopenia evaluated by fat-free mass index is an important prognostic factor in patients with chronic heart failure. Eur J Intern Med. 2015;26(2):118–122. doi: 10.1016/j.ejim.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Bozkuş Y, Mousa U, Demir CC, Anil C, Kut A, Turhan Iyidir O, Gulsoy Kirnap N, Fırat S, Nar A, Tutuncu NB. Abdominal bioelectric impedance for follow-up of dieters: A prospective study. Acta Endocrinol (Buchar). 2019;15(2):145–152. doi: 10.4183/aeb.2019.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farhangi MA, Mesgari-Abbasi M, Shahabi P. Cardio-renal metabolic syndrome and pro-inflammatory factors: the differential effects of dietary carbohydrate and fat. Acta Endocrinol (Buchar). 2019;15(4):436–441. doi: 10.4183/aeb.2019.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roman G, Rusu A, Graur M, Creteanu G, Morosanu M, Radulian G, Amorin P, Timar R, Pircalaboiu L, Bala C. Dietary patterns and their association with obesity: A cross-sectional study. Acta Endocrinol (Buchar). 2019;5(1):86–95. doi: 10.4183/aeb.2019.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu R, Tando Y, Yokoyama A, Yanagimachi M. Skeletal muscle mass ratio as an index for sarcopenia in patients with type 2 diabetes. Topics Clin Nutr. 2019;34(3):209–217. [Google Scholar]
- 25.Koeberle A, Werz O. Multi-target approach for natural products in inflammation. Drug Discov Today. 2014;19(12):1871–1882. doi: 10.1016/j.drudis.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Body composition analyzer. MC-780-MA Instruction manual [ https://tanita.eu/media/wysiwyg/manuals/medical-approved-body-composition-monitors/mc-780-portable-instruction-manual.pdf] (accessed at 20 July 2019) [Google Scholar]
- 27.Agata K, Monyeki MA. Association between sport participation, body composition, physical fitness, and social correlates among adolescents: The PAHL Study. Int J Environ Res Public Health. 2018;15(12):2793. doi: 10.3390/ijerph15122793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lätt E, Jürimäe J, Harro J, Loit HM, Mäestu J. Low fitness is associated with metabolic risk independently of central adiposity in a cohort of 18-year-olds. Scand J Med Sci Sports. 2018;28(3):1084–1091. doi: 10.1111/sms.13002. [DOI] [PubMed] [Google Scholar]
- 29.Kim YM, Kim JH, Baik SJ, Chun J, Youn YH, Park H. Sarcopenia and sarcopenic obesity as novel risk factors for gastric carcinogenesis: A health checkup cohort study. Front Oncol. 2019;9:1249. doi: 10.3389/fonc.2019.01249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patterson E, Munn Z, Jennings C. Experiences of providers in delivering nutrition-focused lifestyle interventions for adults with obesity or metabolic syndrome in primary healthcare settings: a qualitative systematic review protocol. JBI Database System Rev Implement Rep. 2019;18(7):1573–1579. doi: 10.11124/JBISRIR-D-19-00182. [DOI] [PubMed] [Google Scholar]
- 31.Dimitrov BD, Bahchevanov KM, Atanassova PA, Mitkov MD, Massaldjieva RI, Chompalov KA, Hadzhipetrov GK. Metabolic syndrome severity score: range and associations with cardiovascular risk factors. Arch Med Sci Atheroscler Dis. 2016;1(1):e90–e97. doi: 10.5114/amsad.2016.62137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonanno L, Metro D, Papa M, Finzi G, Maviglia A, Sottile F, Corallo F, Manasseri L. Assessment of sleep and obesity in adults and children: Observational study. Medicine (Baltimore). 2019;98(46):e17642. doi: 10.1097/MD.0000000000017642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mamounis KJ, Shvedov NR, Margolies N, Yasrebi A, Roepke TA. The effects of dietary fatty acids in the physiological outcomes of maternal high-fat diet on offspring energy homeostasis in mice. J Dev Orig Health Dis. 2020;11(3):273–284. doi: 10.1017/S2040174419000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodrigues de Lima T, González-Chica DA, Santos Silva DA. Clusters of cardiovascular risk factors and its association with muscle strength in adults. J Sports Med Phys Fitness. 2020;60(3):479–485. doi: 10.23736/S0022-4707.19.10161-2. [DOI] [PubMed] [Google Scholar]
- 35.Barness LA, Opitz JM, Gilbert-Barness E. Obesity: genetic, molecular, and environmental aspects. Am J Med Genet A. 2007;143a(24):3016–3034. doi: 10.1002/ajmg.a.32035. [DOI] [PubMed] [Google Scholar]
- 36.Allison DB, Heshka S. Toward an empirically derived typology of obese persons. Int J Obes. 1991;15(11):741–754. [PubMed] [Google Scholar]
- 37.Granic A, Sayer AA, Robinson SM. Dietary patterns, skeletal muscle health, and sarcopenia in older adults. Nutrients. 2019;11(4):745. doi: 10.3390/nu11040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welch AA. Nutritional influences on age-related skeletal muscle loss. Proc Nutr Soc. 2014;73(1):16–33. doi: 10.1017/S0029665113003698. [DOI] [PubMed] [Google Scholar]
- 39.Kitazoe Y, Kishino H, Tanisawa K, Udaka K, Tanaka M. Renormalized basal metabolic rate describes the human aging process and longevity. Aging Cell. 2019;18(4):e12968. doi: 10.1111/acel.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]


