Abstract
The novel coronavirus disease 2019, otherwise known as COVID-19, is a global pandemic with primary respiratory manifestations in those who are symptomatic. It has spread to >187 countries with a rapidly growing number of affected patients. Underlying cardiovascular disease is associated with more severe manifestations of COVID-19 and higher rates of mortality. COVID-19 can have both primary (arrhythmias, myocardial infarction, and myocarditis) and secondary (myocardial injury/biomarker elevation and heart failure) cardiac involvement. In severe cases, profound circulatory failure can result. This review discusses the presentation and management of patients with severe cardiac complications of COVID-19 disease, with an emphasis on a Heart-Lung team approach in patient management. Furthermore, it focuses on the use of and indications for acute mechanical circulatory support in cardiogenic and/or mixed shock.
Keywords: acute coronary syndrome, cardiovascular diseases, coronavirus, heart failure, myocardial infarction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19), which is now present throughout the world and has infected >4 137 591 individuals as of May 30, 2020.1 On March 11, 2020, the World Health Organization declared the spread of COVID-19 a pandemic. Patients with preexisting comorbidities, particularly cardiovascular disease (CVD), seem to be at increased risk of severe illness. In addition to feared pulmonary complications like acute respiratory distress syndrome (ARDS), COVID-19 may lead to acute myocardial injury and dysfunction, which can precipitate or contribute to shock and multi-system organ failure.
SARS-CoV-2 is the seventh known coronavirus to infect humans. The virus is similar to SARS-CoV from the 2002 SARS outbreak and the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) from 2012 MERS outbreak.2,3 Although COVID-19 is reportedly less lethal than SARS and MERS, it has claimed more lives than both combined. The case fatality rate varies by country and age; the case fatality rate ranges from 0.25% to 5.7% and there have been >283 526 fatalities thus far.1,4,5 The current median R0, a quantitative representation of infectivity or basic reproductivity, of COVID-19 is estimated to be 5.7 (95% confidence interval, 3.8–8.9).6–9 The R0 is greater than SARS (R0: 2–4) and MERS (R0: 2.5–7.2), and almost quadruple that of seasonal influenza.10–12
New York City, the epicenter of the United States COVID-19 outbreak, has 178 766 cases, 44 812 hospitalizations, and 14 753 confirmed deaths as of May 10, 2020.13 A New York State antibody seroprevalence study of 15 000 patients demonstrated 12.3% of the population to be antibody positive.14 The true number of COVID-19 infections in New York is likely higher than the confirmed number as there is a known high rate of asymptomatic transmission of disease. The estimated asymptomatic proportion of those with disease is calculated to be between 13% and 41%.15–17 At our center, 215 asymptomatic obstetrics patients presenting for delivery were screened for SARS-CoV-2 and 29 (13.7%) were positive.18 In densely packed cities, this high rate of asymptomatic transmission specifically is a challenge to containment.
COVID-19 AND CARDIOVASCULAR DISEASE
CVD is a common comorbidity among patients with symptomatic COVID-19 infection as seen with SARS and MERS2; the co-existence of hypertension ranges from 35% to 57%, coronary artery disease 10% to 17% and congestive heart failure (CHF) 6% to 7%.19–22 Cardiovascular comorbidity prevalence is likely even higher amongst those that are critically ill; one case series found a 42.9% prevalence of preexisting CHF in those requiring intensive care unit (ICU) care.23 The explanation of this association is unclear and whether COVID-19 has a specific predisposition for patients with CVD has not been studied. Notably, patients with CVD appear to be at increased risk of severe manifestations of COVID-19 disease, and 30% to 35% of COVID-related deaths have underlying CVD.24,25 Chinese reports demonstrate an increased case fatality rate (10.5%) in those with CVD compared with a case fatality rate of 0.9% in those with no comorbidities.26
In this review, COVID-19 impact on the cardiovascular system will be divided into primary or secondary cardiac involvement; there is of course much overlap between the 2. Primary cardiac manifestations of COVID-19 disease include arrhythmias, acute coronary syndrome (ACS), and myocarditis. Secondary cardiac involvement is usually because of a systemic inflammatory syndrome and can manifest as acute myocardial injury/biomarker elevation and CHF. Secondary cardiac involvement is often accompanied by other evidence of end-organ damage. Last, we will review additional vascular complications of COVID-19 disease, and then discuss management strategies for patients who develop shock or have chronic CHF in the setting of COVID-19.
Primary Cardiac Involvement
Arrhythmias
While there is a paucity of literature detailing COVID-19-related arrhythmogenic complications, there are reports of ventricular tachycardia and ventricular fibrillation as late manifestations of COVID-19. An early case series from China reports a 16.7% incidence of arrhythmia but did not specify the cause or type.27 A later report found a 5.9% incidence of malignant arrhythmias, with a significantly greater incidence in those with evidence of myocardial injury (17.3% versus 1.5%, P<0.001).25 This perhaps suggests that myocardial injury may serve as a substrate for subsequent cardiac arrhythmias, and frequent arrhythmia should heighten suspicion for a myocardial inflammatory process. This phenomenon may, in part, account for the reported increase in out-of-hospital arrests noticed during the COVID-19 pandemic period.28 Notably, however, analysis of in-hospital arrests in patients with COVID-19 seem to rarely be from shockable rhythms (89.7% asystole, 4.4% pulseless electrical activity, and 5.9% shockable rhythm).29
Some of the current investigational drug therapies, such as hydroxychloroquine (with or without azithromycin), can adversely affect QTc duration and subsequently lead to arrhythmia.30–32 Concurrent treatment of hydroxychloroquine with azithromycin is associated with greater QTc prolongation and risk of QTc >500 ms.31,32 Those with a baseline QTc of ≥450 ms have a greater likelihood of developing a prolonged QTc (adjusted odds ratio, 7.11 [95% confidence interval, 1.75–28.87]).31 Therefore, baseline ECG assessment with repeat follow-up ECG after initiation of these medications should be routine even in the absence of an overt cardiac involvement. In some settings, telemetry monitors can be configured to continuously display QTc duration in an effort to minimize provider exposure. Additionally, the following precautions should be taken in those who are being administered hydroxychloroquine therapy: (1) dose reduction for patients with severe renal insufficiency (50% for creatinine clearance <10 mL/min), (2) aggressive electrolyte correction before use, and (3) avoidance or careful monitoring for congenital long-QT patients or those on multiple QT-prolonging medications.33,34 Recognition of bradycardia and discontinuation of medications that cause bradycardia is important as an episode of torsades de pointes is often preceded by bradycardia or a pause.34 Last, irregular RR intervals can affect QT measurement, potentially leading to an underestimation of the true interval length.
The risk of arrhythmia in patients with COVID-19 may be potentiated, at least in part, by the high incidence of hypokalemia.35 SARS-CoV-2 invades cells by binding to ACE (angiotensin-converting enzyme)-2 receptors, which can enhance urinary potassium excretion due to increased availability of angiotensin II.35 Treatment of arrhythmias should focus on addressing all reversible causes, especially electrolyte abnormalities, and follow standard guidelines for management of arrhythmias. In the setting of frequent and uncontrolled ventricular arrhythmia not responding to anti-arrhythmic therapy, transvenous pacemaker insertion, and mechanical circulatory support should be considered.
Acute Coronary Syndrome
The incidence of ACS increases in the setting of viral infection, likely due to inflammation-mediated plaque destabilization. The risk in the setting of COVID-19 infection is unknown, but other viruses are associated with a 3- to 10-fold increased risk.36–38
In the throes of this global crisis, many healthcare institutions in Europe, North America, and Asia have reexamined their protocols for management of ACS, taking into account the potential risks of healthcare worker exposure and personal protective equipment utilization. In the case of non–ST-segment–elevation myocardial infarction care, COVID-19 testing results can usually be obtained before further determination of the patient’s care plan. ST-segment–elevation myocardial infarction (STEMI) care is more nuanced. The limited ability of rapid COVID-19 testing in the United States is a challenge given the urgency of STEMI care. Additionally, there are multiple reports of STEMI mimics, such as myopericarditis. However, patients with COVID-19 may not present with classic angina symptoms. With these additional challenges, risk stratification, careful ECG examination and limited bedside transthoracic echocardiogram or point of care cardiac ultrasound may all be used to help guide clinical decision making.39,40 A case series from New York evaluated 18 COVID-19 patients with STEMI on ECG; of these patients, 6 (33%) presented with chest pain, 14 (78%) had focal ST-segment elevation, 6 (35%) had a regional wall motion abnormality on transthoracic echocardiogram, and 8 (44%) received a clinical diagnosis of myocardial infarction.41 A total of 9 (50%) patients underwent coronary angiography, of whom 6 (67%) showed obstructive disease.41 In response to the wide-spread severity of this pandemic in New York, at our institution, we developed a tiered STEMI care plan based on the crisis level of the hospital system. Given the potential STEMI mimickers as well as the risk of staff exposure during a catheterization laboratory procedure, a higher threshold for cardiac catheterization lab activation is preferred. In most cases, primary percutaneous coronary intervention is preferred; fibrinolysis with tenecteplase should be reserved for circumstances of extreme resource constraints, and if necessary, only used in carefully selected patients. Outside of the STEMI pathway, the decision to activate the cardiac catheterization lab is made on a case-by-case basis balancing multiple factors, such as infarct location, hemodynamic stability, and bleeding risk.40There are multiple additional considerations which may affect the clinical approach and management of ACS in patients with concomitant COVID-19, including infection control and staff exposure. This has been further addressed in a position paper by the American College of Cardiology and Society of Cardiovascular Angiography and Intervention.42 Protective measures to ensure the safety of staff is of paramount importance; as of April 9, 2020, there have been 9282 US cases of COVID-19 in healthcare personnel, of whom 723 (8%–10%) were hospitalized, 184 (2%–5%) required ICU admission, and 27 (0.3%–0.6%) have died.43
Myocarditis
The true prevalence of COVID-19 acute myocarditis is unknown. Several cases of clinically diagnosed myocarditis in patients with COVID-19 have been reported, but the number of autopsy and endomyocardial biopsy (EMB) proven cases is limited. One case from Italy had cardiovascular magnetic resonance imaging demonstrating diffuse myocardial edema and EMB while SARS-CoV-2 virus-negative, showed diffuse T-lymphocytic inflammatory infiltrates.44 Another case had an EMB that demonstrated localization of SARS-CoV-2 in cardiac macrophages, but not cardiomyocytes.45 These data confirm myocarditis as a cause of myocardial injury in patients with COVID-19; however, the true mechanism of myocarditis remains unknown. The utility of EMB and cardiovascular magnetic resonance imaging in management of these patients is limited. Additionally, EMB and cardiovascular magnetic resonance imaging may not be feasible in many centers from an infection control standpoint. Diagnosis should, therefore, rely on the integration of clinical, laboratory (cardiac biomarkers), electrocardiographic (ST-segment changes), and echocardiographic (wall motion abnormalities, ejection fraction, and pericardial effusion) data.46
Limited data are available to inform our management COVID-19 myocarditis patients, but multiple antiviral (eg, remdesivir, ribavirin, lopinavir, and hydroxychloroquine) and anti-inflammatory (eg, interferon, corticosteroids, tocilizumab, sarilumab, siltuximab, anakinra, intravenous immunoglobulin, and statin) therapies are under investigation.47 Hydroxychloroquine more recently has shown poor efficacy for treatment of COVID-19 disease in a well-designed retrospective study.48 Published cases of severe myocarditis have reported positive results with early use of immunoglobulin, corticosteroid, and anti-inflammatory therapy.49–51 In cases of refractory shock, mechanical circulatory support should be considered.
Secondary Cardiac Involvement
Acute Myocardial Injury
The cause of acute myocardial injury in COVID-19 disease is likely multifactorial with multiple overlapping mechanisms (Table 1).52,53 The presence of acute myocardial injury has been reported in approximately one-quarter of hospitalized patients, with higher cardiac troponin levels associated with more severe disease.22,25,54,55 One retrospective study noted that COVID-19 nonsurvivors demonstrated rapid elevation of troponin late in their course (median day 16), suggesting that local troponin assay in addition to CRP (C-reactive protein), D-dimer, NT-pro-brain natriuretic peptide can be used to track clinical course.54
Table 1.
Mechanisms of Myocardial Injury
| Hypothesized Mechanism of Injury | |
|---|---|
| Myocarditis | Systemic inflammatory response; direct myocardial cell injury via viral entry using ACE-2 receptor; T-cell–mediated immune response |
| Myocardial infarction | Plaque rupture (Type I MI); myocardial oxygen supply/demand mismatch (Type II MI) from increased cardiometabolic demand |
| Microangiopathy/cytokine storm | Cytokine-induced activation of microvasculature predisposing to vasomotor abnormalities; augmented thrombosis and other aspects of dysfunction |
| Arrhythmia | Hypoxia-mediated; coronary perfusion impairment; direct tissue injury; scar-mediated injury, inflammatory response; medication-induced; electrolyte abnormality. |
ACE indicates angiotensin-converting enzyme; and MI, myocardial infarction.
Mechanisms of direct myocardial injury include acute plaque rupture (as discussed in the myocardial infarction section) and viral invasion. The ACE-2 receptor, which is used by SARS-CoV-2 for cell entry, is widely expressed both in lungs, myocytes, and vascular endothelial cells.56 There are emerging histopathologic data of direct endothelial viral invasion with diffuse endothelial inflammation-causing COVID-19 endotheliitis.57 Endotheliitis is known to impair microcirculatory function, causing a tissue inflammatory response and organ ischemia.58 Additionally, there have been reports of patients without preexisting cardiac disease presenting with predominantly cardiac symptoms in the setting of COVID-19 infection, resembling a possible clinical picture of myocarditis.49,50
Indirect myocardial injury may occur from cytokine storm, microangiopathy, or myocardial oxygen supply/demand mismatch. The inflammatory response and cytokine storm characteristic of severe COVID-19 disease may potentiate myocardial stress and injury. This can impair cardiac function indirectly through peripheral effects on systemic vascular resistance, as well as catecholamine-driven effects, such as those seen with stress-induced cardiomyopathy.59 In limited case reports, significant myocardial dysfunction seems to correlate with severely elevated inflammatory markers suggesting the potential for an immune-mediated mechanism.60,61 Inappropriate activation of type 1 T-helper cells with a subsequent cell-mediated immune response and an exaggerated cytokine response could be an alternative mechanism of myocardial injury in patients with COVID-19.19 These findings are hypothesis generating for current and future clinical studies examining the use of anti-inflammatory medications in conjunction with antiviral therapies.
Heart Failure
The development of new CHF is not uncommon in those with COVID-19 disease. A retrospective study from Wuhan, China, reported CHF as a complication in 23% of patients overall (52% of nonsurvivors).54 A small US case series by Arentz et al23 identified 7 out of 21 critically ill patients (33%) developed cardiomyopathies during the course of their ICU stay. The exact cause of ventricular failure in COVID-19 remains unknown.
In ARDS, higher levels of serum brain natriuretic peptide have been shown to correlate with cardiogenic pulmonary edema.62 Interestingly, patients with COVID-19 may have high levels of brain natriuretic peptide in the absence of significant ventricular dysfunction.49,54 Still, the presence of elevated cardiac biomarkers, particularly troponin, should raise clinical suspicion for CHF. Interpretation of cardiac biomarkers does, however, present significant challenges as there are multiple mechanisms of cardiac injury. If CHF is suspected, a limited transthoracic echocardiogram or point of care cardiac ultrasound may be considered to assess biventricular function to tailor treatment.
Additional Vascular Complications of COVID-19
Patients with COVID-19 infection are at risk for venous and arterial thromboembolic events, especially in the setting of disseminated intravascular coagulation. A report from Italy described a 7.7% (28/362) incidence of at least one thromboembolic event for hospitalized patients with COVID-19.63 This rate has been reported to be as high as 31% in those requiring ICU-level care.64 Additionally, acute arterial thrombotic events other than ACS, such as cerebrovascular accident or systemic thrombosis, have been observed in patients with COVID-19 with no or few predisposing factors.63–65 Although the mechanism of coagulopathy is unclear, it is likely multifactorial with critical illness, inflammation, and endothelial dysfunction contributing to coagulopathy. It is evident that patients with COVID-19 develop some degree of abnormal coagulation parameters.66,67 Reports from China demonstrate that elevated levels of D-Dimer (>1 μg/L) and fibrin degradation products are strongly associated with in-hospital death.54,66
Retrospective studies have identified pulmonary embolism (PE) as the most common thrombotic event.63,64 Those with thrombotic complications have a higher risk of death and higher D-dimer levels.63,64 A prospective cohort study examining 12 post-mortem patient with COVID-19 autopsies found deep venous thrombosis in 58% (7/12) and PE in 33% (4/12).69 Thromboembolic events, particularly PE, may contribute to the rate of cardiac injury detected in severe COVID-19 disease as right ventricular strain may lead to elevation in cardiac biomarkers. Even in the absence of significant clot burden, PE may significantly impair right ventricular performance, especially in the setting of high right ventricular afterload that is characteristic of ARDS.70–73 In severe cases, cor pulmonale can develop, which may contribute to the mixed shock or sudden cardiac arrest observed in severe COVID-19 infection.
Venous and arterial thromboembolic prophylaxis should be maintained in all hospitalized patients with COVID-19 without high risk for bleeding complications. There should be a low threshold for therapeutic anticoagulation when thromboembolism is suspected. In the absence of renal impairment, low molecular weight heparin might be preferred since it does not require frequent lab monitoring and dose adjustments. There are multiple ongoing trials looking at the preferred anticoagulant and dosing strategy for thromboprophylaxis in patients with COVID-19 given the concern for increased coagulopathy in these patients (URL: https://www.clinicaltrials.gov. Unique identifiers: NCT04367831 and NCT04366960). In cases of documented massive PE with significant hemodynamic compromise, systemic thrombolytic should be considered in the absence of contraindications. The role of invasive treatment for PE with either catheter-directed thrombolytic therapies or surgical embolectomy remains uncertain. Furthermore, whether those who survive critical illness should be on prolonged thromboprophylaxis is a topic of clinical discussion and focus of future research.74
ACUTE CIRCULATORY FAILURE IN COVID-19
Shock and multi-system organ failure is a hallmark of severe COVID-19 infection. It appears that distributive or septic shock typically predominates, but many patients are at risk for mixed shock given the propensity for cardiac dysfunction in severe disease. Additionally, those with underlying CHF may progress to cardiogenic shock (CGS), either in isolation or in combination with vasodilatory shock. The COVID-19 crisis has mandated remodeling of the standard shock team approach and critical care delivery methods in many centers.75 At our center, we have embraced the Heart-Lung team approach for a comprehensive and timely evaluation of patients with circulatory shock. This focused multidisciplinary approach entails a dedicated specialist from different disciplines, including pulmonary critical care, cardiac intensive care, advanced heart failure, interventional cardiology, and cardiothoracic surgery, who can review the detailed clinical and hemodynamic information. Virtual assessment of at-risk patients facilitates early evaluation and transfer of patients with new or worsening cardiac dysfunction to a step-down unit with telemetry monitoring or cardiac ICU.75
It is evident that COVID-19 infected patients develop pronounced and diffuse bilateral pulmonary infiltrates with at times rapid deterioration to acute hypoxic respiratory failure.76 However, it is unknown whether cardiac dysfunction and elevated filling pressures co-exist and to what extent this contributes to worsening pulmonary function. In these ambiguous clinical scenarios, a pulmonary artery catheter could be extremely beneficial, if feasible. Whenever possible, placement should be performed at the bedside by an experienced operator in an effort to shorten duration of exposure time and improve the procedural success rate. If mixed shock is suspected in the setting of cardiac dysfunction, one should also maintain a high index of suspicion for myocarditis.50 The accuracy of noninvasive estimation of volume status and hemodynamic parameters, especially in the setting of ARDS has not been validated.77 The laboratory evidence of shock (renal function panel, liver function panel, and serum lactic acid) in addition to clinical assessments (urine output) play an important role, however, they tend to lag behind.78,79
In the setting of refractory hypoxemia and mixed/CGS, extracorporeal membrane oxygenation (ECMO) should be considered in centers with expertise. Veno-Venous ECMO may be used to treat refractory hypoxemia and has been used successfully in previous pandemics, mainly the influenza A (H1N1) ARDS outbreak in 2009.80,81 On April 7, 2020, the Food and Drug Administration issued a guidance document for ECMO use in patients with COVID-19 specifically. Although the dominant configuration in patients with COVID-19 is veno-venous ECMO, veno-arterial-venous ECMO may be considered in selected patients who develop severe refractory cardiogenic or mixed shock where there is a perceived high likelihood for reversibility. Isolated veno-arterial ECMO configuration in the absence of severe ARDS and refractory hypoxemia appears to be less frequent. To date, the experience with ECMO in patients with COVID-19 and CGS is limited and varies among tertiary care centers depending on level of expertise. Table 2 highlights the mechanical circulatory support modalities that have been used at our center during the pandemic period. A multidisciplinary Heart-Lung team in these difficult situations has shown favorable outcomes in available case reports.39 The Extracorporeal Life Support Organization has created a registry of patients with COVID-19 that have required ECMO support. The Extracorporeal Life Support Organization guidance document recommends immediate consideration of ECMO for patients without significant comorbidities who have a high risk of mortality despite optimal care otherwise.82 As of May 11, 2020, there are 802 confirmed COVID-19 cases recorded in the registry.
Table 2.
Device Strategies for COVID Patients With Circulatory Failure
| ARDS | Cause | Cases (n=8) | |
|---|---|---|---|
| IABP | + | Cardiomyopathy | 2 (25.0%) |
| VAV ECMO | + | Cardiomyopathy | 5 (62.5%) |
| IABP + VA ECMO | − | Acute coronary syndrome | 1 (12.5%) |
ARDS indicates acute respiratory distress syndrome; COVID, coronavirus; ECMO, extracorporeal membrane oxygenation; IABP, intraaortic balloon pump; and VAV, veno-arterial-venous.
Inotropes should be considered in patients with CGS or mixed shock in the setting of significant cardiac dysfunction, but if used patients should be closely monitored for atrial or ventricular tachyarrhythmias. Regarding primary cardiac support devices, an intraaortic balloon pump can be placed at the bedside without the need for fluoroscopy, thus avoiding excessive personnel exposure.83,84
Additionally, there is some evidence pointing to the efficacy of intraaortic balloon pump in chronic CHF patients who develop CGS, although few reports are available of intraaortic balloon pump use in patients with COVID-19.45,83,84 The use of percutaneous ventricular assist devices for CGS may also be considered through requires fluoroscopy and echocardiography guided adjustment. Percutaneous ventricular assist devices devices may be more feasible in the cases of patients with acute myocardial infarction associated CGS as these patients are in large taken to the cardiac catheterization lab. Invasive hemodynamic measurements in conjunction with laboratory and clinical assessment may help facilitate optimal device selection and determining timing of mechanical circulatory support weaning.85 Moreover, in patient’s requiring mechanical circulatory support, hemodynamic-driven patient has been shown to improve patient outcomes.86 The Figure highlights the key points for evaluation and management of patients with cardiogenic and mixed shock. For acute myocardial infarction associated CGS, primary percutaneous coronary intervention is preferred and further device management, if necessary, should follow standard protocol.
Figure. Evaluation and management of novel coronavirus disease 2019 (COVID-19) patients with cardiogenic or mixed circulatory shock.
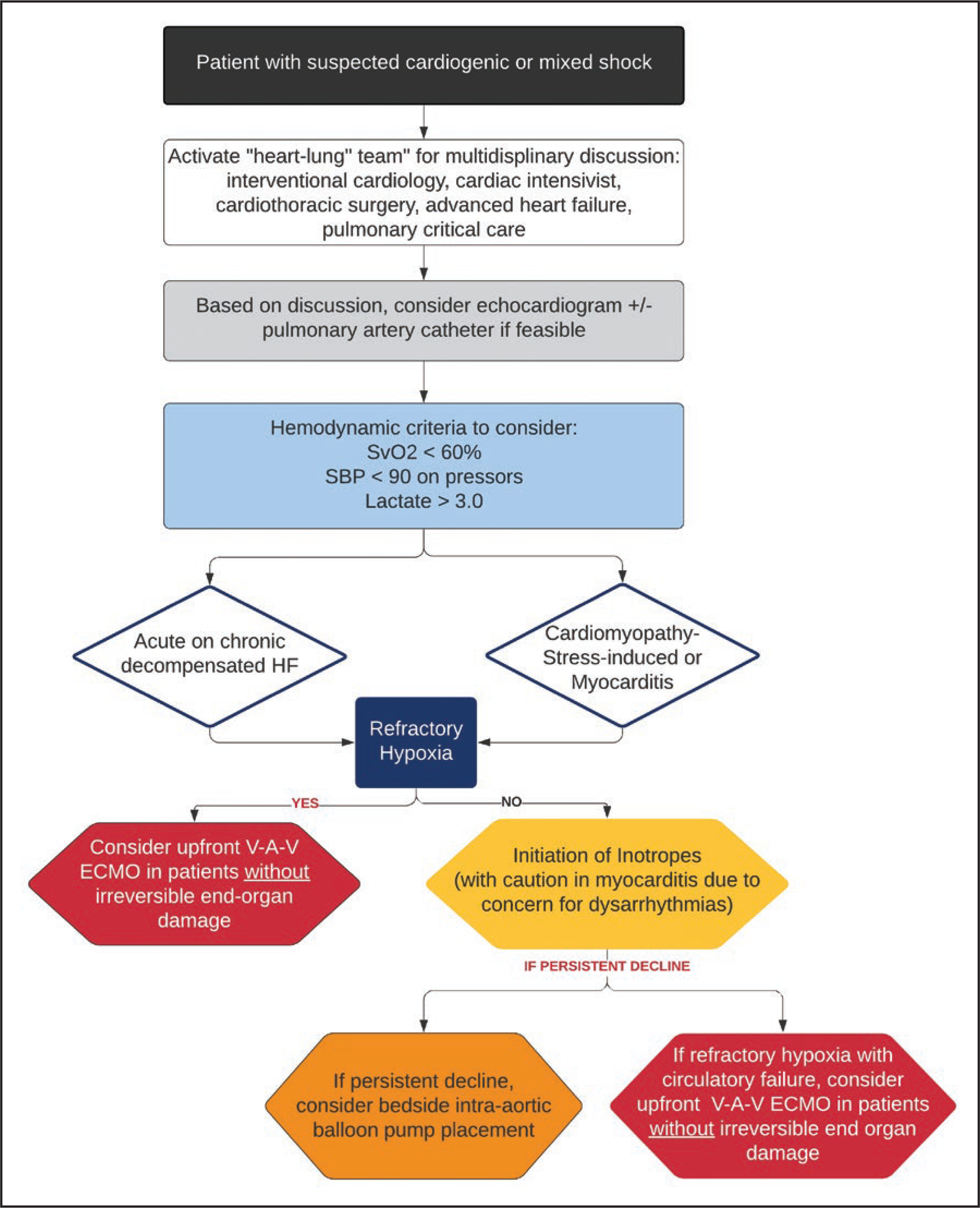
The approach to circulatory shock during the COVID-19 pandemic has mandated modification. In response to the predominance of refractory hypoxemia and frequent need for veno-venous extracorporeal membrane oxygenation (ECMO), we have adopted an upfront veno-arterial-venous (V-A-V) ECMO strategy in the setting of mixed severe acute respiratory distress syndrome and circulatory failure in appropriate candidates. HF indicates heart failure; SBP, systolic blood pressure; and SvO2, mixed venous oxygen saturation.
Heart transplantation in the era of COVID-19 is an active area of discussion. There are unique implications for donor evaluation, actively listed patients, immediate post-transplant care, and management of transplant recipients.87 Heart transplant centers will have to consider whether and in what circumstances patients with COVID-19 who develop acute circulatory failure should be eligible for heart transplantation.
CHRONIC CHF MANAGEMENT
Patients with chronic CHF are particularly vulnerable to, COVID-19. Indeed, respiratory illness is a contributing factor in over 50% of decompensated CHF admissions.88 To avoid exposing patients with CHF to the healthcare setting (where they may be exposed to SARS-CoV-2), professional societies have encouraged the use of televisits and remote monitoring for the provision of longitudinal chronic care.89,90
CHF patients with COVID-19 require close monitoring for any signs of cardiac decompensation. Limited cardiac and respiratory reserve in patients with CHF, in addition to conventionally indicated therapies for sepsis management such as intravenous fluid administration to maintain blood pressure, increases the risk for decompensation in the face of severe systemic stress.91 Acute systemic inflammation can stress a failing myocardium, leading to further depression in myocardial function. Furthermore, there is risk of drug-induced cardiac toxicity with some of the pharmacological agents being administered for treatment of COVID-19, such as antiviral therapies. Renal failure, which is common in severe COVID-19 disease, can also potentiate an exacerbation of underlying cardiomyopathy. Last, the overlap between the hallmark plain x-ray radiographic features of COVID-19 (bilateral airspace opacification) and pulmonary vascular congestion/edema may delay diagnosis and treatment of decompensated CHF. Meticulous attention to intravascular volume status, particularly in elderly patients, is critical. Similarly, use of medications that may alter the sodium and water homeostasis, such as NSAIDS, should be avoided.
There has been much controversy regarding the use of medications that cause renin-angiotensin-aldosterone system inhibition in patients with COVID-19, specifically ACE inhibitor and ARB (angiotensin receptor blocker), given that the virus uses the ACE-2 receptor for viral cell entry.92 The hypothesis that renin-angiotensin-aldosterone system inhibition may result in upregulation of ACE-2 receptors potentially leading to higher susceptibility to infection or more fatal disease has worried clinicians.93 To date, there has been no data to show a relationship between ACE-2 activity and mortality nor has there been correlation between ACE-2 expression and severity of infection. In fact, a retrospective study found that ACE inhibitor/ARB users have a lower all-cause mortality compared with nonusers (adjusted hazard ratio, 0.37 [95% confidence interval, 0.15–0.89], P=0.03).94 Furthermore, 2 recent studies found no association between ACE inhibitor and ARB use and COVID-19 positivity.95,96 Much more research is needed to clarify the role of renin-angiotensin-aldosterone system in COVID-19 pathogenesis, and many clinical trials are ongoing (URL: https://www.clinicaltrials.gov. Unique identifiers: NCT04287686, NCT04312009, and NCT04311177). The American College of Cardiology/American Heart Association/Heart Failure Society of America released a joint statement recommending against discontinuation of these agents in CHF patients with no evidence of COVID-19 infection unless otherwise advised by their physician; however, ACE inhibitor and ARB medications should be avoided in COVID-19 infected patients with progressive proinflammatory manifestations of disease, shock, or acute kidney injury.97
DIRECTION FOR FUTURE RESEARCH FOR ACUTE CARDIAC DISEASE IN COVID-19
To combat the growing COVID-19 pandemic, there are several important areas of research that must be addressed across the basic, clinical, and translational research domains. The predictors of increased morbidity and mortality must be better understood to identify high-risk patients earlier in their clinical course. Additionally, understanding the basic mechanisms of how COVID-19 causes direct myocardial injury will be important for development of novel therapeutic agents. Table 3 lists some priority research questions related to acute cardiac complications from COVID-19.
Table 3.
High Priority Research Questions
| Are current biomarkers sufficient or are there novel biomarkers that can be used for risk stratification for patients with COVID-19? |
| What is the prevalence of nonobstructive acute coronary syndrome in COVID-19 patients? |
| Are patients with COVID-19 at higher risk for thromboembolic disease? |
| What are the short and long-term outcomes among COVID-19 patients presenting with acute STEMI who are initially treated with thrombolytic therapy? |
| What is the optimal treatment regimen for patients with acute COVID-19 related myocarditis or cardiac dysfunction? |
| Is acute mechanical circulatory support as a bridge to recovery associated with improved outcomes among COVID-19 patients? |
| What are the predictors of cardiovascular morbidity and mortality among COVID-19 patients? |
| What are the long-term outcomes of patients who have recovered from COVID-19 related cardiovascular complications? |
COVID-19 indicates novel coronavirus disease 2019; and STEMI, ST-segment–elevation myocardial infarction.
SUMMARY
COVID-19 can have multiple impacts on the cardiovascular system. Acute cardiovascular complications pose significant challenges and require a multidisciplinary Heart-Lung team to assess the need for invasive hemodynamic monitoring and device therapy. In situations of suspected cardiogenic or mixed shock, further hemodynamic assessment may provide valuable information that can help guide therapy in these critically ill patients. There remain several questions for future investigation to better understand the mechanism of myocardial injury and determine which therapies improve long-term survival.
Sources of Funding
Dr Abdalla receives support through 18AMFDP34380732 from the American Heart Association and from the National Institutes of Health/National Heart, Lung, and Blood Institute (K23 HL141682-01A1 and R01HL146636-01A1).
Disclosures
Dr Karmpaliotis reports honoraria from Boston Scientific, Abbott Vascular, Abiomed; and equity in Saranas, Soundbite, Traverse Vascular. Dr Kirtane reports institutional funding to Columbia University and/or Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, Philips, and ReCor Medical. Personal: CME program honoraria and travel/meal reimbursements only. The other authors report no conflicts.
Contributor Information
Lauren S. Ranard, Division of Cardiology, Department of Medicine, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA.
Justin A. Fried, Division of Cardiology, Department of Medicine, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA.
Marwah Abdalla, Division of Cardiology, Department of Medicine, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA.
D. Edmund Anstey, Division of Cardiology, Department of Medicine, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA.
Raymond C. Givens, Division of Cardiology, Department of Medicine, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA.
Deepa Kumaraiah, Division of Cardiology, Department of Medicine, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA.
Susheel K. Kodali, Division of Cardiology, Department of Medicine, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA; Clinical Trials Center, Cardiovascular Research Foundation, New York, NY, USA.
Koji Takeda, Department of Surgery, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA.
Dimitrios Karmpaliotis, Division of Cardiology, Department of Medicine, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA; Clinical Trials Center, Cardiovascular Research Foundation, New York, NY, USA.
LeRoy E. Rabbani, Division of Cardiology, Department of Medicine, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA.
Gabriel Sayer, Division of Cardiology, Department of Medicine, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA.
Ajay J. Kirtane, Division of Cardiology, Department of Medicine, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA; Clinical Trials Center, Cardiovascular Research Foundation, New York, NY, USA.
Martin B. Leon, Division of Cardiology, Department of Medicine, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA; Clinical Trials Center, Cardiovascular Research Foundation, New York, NY, USA.
Allan Schwartz, Division of Cardiology, Department of Medicine, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA.
Nir Uriel, Division of Cardiology, Department of Medicine, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA.
Amirali Masoumi, Division of Cardiology, Department of Medicine, Columbia University Irving Medical Center/New York-Presbyterian Hospital, New York, NY, USA.
REFERENCES
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan JW, Ng CK, Chan YH, Mok TY, Lee S, Chu SY, Law WL, Lee MP, Li PC. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS). Thorax. 2003;58:686–689. doi: 10.1136/thorax.58.8.686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson N, Kvalsvig A, Barnard LT, Baker MG. Case-Fatality Risk Estimates for COVID-19 Calculated by Using a Lag Time for Fatality. Emerg Infect Dis 2020;26:1339–1441. doi: 10.3201/eid2606.200320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L and Favre G. Real estimates of mortality following COVID-19 infection [published online March 12, 2020]. Lancet Infect Dis doi: 10.1016/S1473-3099(20)30195-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imperial College London. Report 3: Transmissibility of 2019-nCoV. Imperial College London. 2020. https://www.imperial.ac.uk/media/imperial-college/medicine/mrc-gida/2020-01-25-COVID19-Report-3.pdf. Accessed May 11, 2020. [Google Scholar]
- 8.Zhang S, Diao M, Yu W, Pei L, Lin Z, Chen D. Estimation of the reproductive number of novel coronavirus (COVID-19) and the probable outbreak size on the Diamond Princess cruise ship: A data-driven analysis. Int J Infect Dis 2020;93:201–204. doi: 10.1016/j.ijid.2020.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanche S, Lin Y, Xu C, Romero-Severson E, Hengartner N and Ke R. High Contagiousness and Rapid Spread of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect Dis 2020;26. doi: 10.3201/eid2607.200282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JE, Jung S, Kim A, Park JE. MERS transmission and risk fac-tors: a systematic review. BMC Public Health. 2018;18:574. doi: 10.1186/s12889-018-5484-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coburn BJ, Wagner BG, Blower S. Modeling influenza epidemics and pandemics: insights into the future of swine flu (H1N1). BMC Med 2009;7:30. doi: 10.1186/1741-7015-7-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biggerstaff M, Cauchemez S, Reed C, Gambhir M, Finelli L. Estimates of the reproduction number for seasonal, pandemic, and zoonotic influenza: a systematic review of the literature. BMC Infect Dis 2014;14:480. doi: 10.1186/1471-2334-14-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.City of New York. COVID-19: Data. 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page. Accessed May 11, 2020.
- 14.New York State. Amid Ongoing COVID-19 Pandemic, Governor Cuomo Announces Results of Completed Antibody Testing Study of 15,000 People Showing 12.3 Percent of Population Has COVID-19 Antibodies. 2020. https://www.governor.ny.gov/news/amid-ongoing-covid-19-pandemic-governor-cuomo-announces-results-completed-antibody-testing. Accessed May 11, 2020.
- 15.Mizumoto K, Kagaya K, Zarebski A and Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill 2020; 25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishiura H, Kobayashi T, Miyama T, Suzuki A, Jung SM, Hayashi K, Kinoshita R, Yang Y, Yuan B, Akhmetzhanov AR, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). Int J Infect Dis 2020;94:154–155. doi: 10.1016/j.ijid.2020.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimball A, Hatfield KM, ARons M, James A, Taylor J, Spicer K, Bardossy AC, Oakley LP, Tanwar S, Chisty Z, et al. Asymptomatic and Presymptomatic SARS-CoV-2 Infections in Residents of a Long-Term Care Skilled Nursing Facility—King County, Washington, March 2020. MMWR Morb Mortal Wkyl Rep 2020;69:377–281. doi: 10.15585/mmwr.mm6913e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutton D, Fuchs K, D’Alton M and Goffman D. Universal Screening for SARS-CoV-2 in Women Admitted for Delivery. N Engl J Med 382(22):2163–2164. 2020. doi: 10.1056/NEJMc2009316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng YY, Ma YT, Zhang JY, Xie X. Reply to: ‘Interaction between RAAS inhibitors and ACE2 in the context of COVID-19’. Nat Rev Cardiol 2020;17:313–314. doi: 10.1038/s41569-020-0369-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR Jr, Nahid M and Ringel JB. Clinical Characteristics of Covid-19 in New York City. N Engl J Med 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell LF, Chernyak Y, Tobin K, Cerfolio RJ, Francois F and Horwitz LI. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. medRxiv. doi: 10.1101/2020.04.08.20057794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; Consortium atNC-R. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area [published online April 22, 2020]. JAMA. 2020. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M and Lee M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onder G, Rezza G and Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 25.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X and Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) [published online March 27, 2020]. JAMA Cardiol 2020: e201017. doi: 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Z and McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldi E, Sechi GM, Mare C, Canevari F, Brancaglione A, Primi R, Klersy C, Palo A, Contri E and Ronchi V. Out-of-Hospital Cardiac Arrest during the Covid-19 Outbreak in Italy [published online April 29, 2020]. N Engl J Med 2020. doi: 10.1056/NEJMc2010418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao F, Xu S, Ma X, Xu Z, Lyu J, Ng M, Cui H, Yu C, Zhang Q, Sun P, et al. In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation. 2020;151:18–23. doi: 10.1016/j.resuscitation.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roden DM, Harrington RA, Poppas A and Russo AM. Considerations for Drug Interactions on QTc in Exploratory COVID-19 (Coronavirus Disease 2019) Treatment [published online April 8, 2020]. Circulation. 2020. doi: 10.1161/CIRCULATIONAHA.120.047521 [DOI] [PubMed] [Google Scholar]
- 31.Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ and Gold HS. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19) [published online May 1, 2020]. JAMA Cardiol 2020; e201834. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bessière F, Roccia H, Delinière A, Charrière R, Chevalier P, Argaud L and Cour M. Assessment of QT Intervals in a Case Series of Patients With Coronavirus Disease 2019 (COVID-19) Infection Treated With Hydroxychloroquine Alone or in Combination With Azithromycin in an Intensive Care Unit [published online May 1, 2020]. JAMA Cardiol 2020; e201787. doi: 10.1001/jamacardio.2020.1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lakkireddy DR, Chung MK, Gopinathannair R, Patton KK, Gluckman TJ, Turagam M, Cheung J, Patel P, Sotomonte J and Lampert R. Guidance for cardiac electrophysiology during the coronavirus (COVID-19) pandemic from the Heart Rhythm Society COVID-19 task force; electrophysiology section of the American College of Cardiology; and the electrocardiography and arrhythmias Committee of the Council on clinical cardiology, American Heart Association [published online April 1, 2020]. Heart Rhythm 2020. doi: 10.1016/j.hrthm.2020.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayad RF, Assar MD, Simpson L, Garner JB, Schussler JM. Causes and management of drug-induced long QT syndrome. Proc (Bayl Univ Med Cent). 2010;23:250–255. doi: 10.1080/08998280.2010.11928628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen D, Li X, Song Q, Hu C, Su F, Dai J, Ye Y, Huang J, and Zhang X. Hypokalemia and Clinical Implications in Patients with Coronavirus Disease 2019 (COVID-19). medRxiv doi: 10.1101/2020.02.27.20028530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyle JJ. Association of coronary plaque rupture and atherosclerotic inflammation. J Pathol 1997;181:93–99. doi: [DOI] [PubMed] [Google Scholar]
- 37.Naghavi M, Wyde P, Litovsky S, Madjid M, Akhtar A, Naguib S, Siadaty MS, Sanati S, Casscells W. Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation. 2003;107:762–768. doi: 10.1161/01.cir.0000048190.68071.2b [DOI] [PubMed] [Google Scholar]
- 38.Gattone M, Iacoviello L, Colombo M, Castelnuovo AD, Soffiantino F, Gramoni A, Picco D, Benedetta M, Giannuzzi P. Chlamydia pneumoniae and cytomegalovirus seropositivity, inflammatory markers, and the risk of myocardial infarction at a young age. Am Heart J 2001;142:633–640. doi: 10.1067/mhj.2001.118118 [DOI] [PubMed] [Google Scholar]
- 39.Fried JA, Ramasubbu K, Bhatt R, Topkara VK, Clerkin KJ, Horn E, Rabbani L, Brodie D, Jain SS and Kirtane A. The Variety of Cardiovascular Presentations of COVID-19. Circulation. 2020;141(23):1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ranard LS, Ahmad Y, Masoumi A, Chuich T, Romney MS, Gavin N, Sayan OR, Kirtane AJ, Rabbani LE. Clinical Pathway for Management of Suspected or Positive Novel Coronavirus-19 Patients With ST-Segment Elevation Myocardial Infarction. Crit Pathw Cardiol 2020;19:49–54. doi: 10.1097/HPC.0000000000000223 [DOI] [PubMed] [Google Scholar]
- 41.Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, Ibrahim H, Friedman GH, Thompson C and Alviar CL. ST-segment elevation in patients with Covid-19—A case series [published online April 17, 2020]. N Engl J Med 2020. doi: 10.1056/NEJMc2009020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welt FGP, Shah PB, Aronow HD, Bortnick AE, Henry TD, Sherwood MW, Young MN, Davidson LJ, Kadavath S, Mahmud E, et al. ; American College of Cardiology’s Interventional Council and the Society for Cardiovascular Angiography and Interventions. Catheterization Laboratory Considerations During the Coronavirus (COVID-19) Pandemic: From the ACC’s Interventional Council and SCAI. J Am Coll Cardiol 2020;75:2372–2375. doi: 10.1016/j.jacc.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.CDC COVID-19 Response Team. Characteristics of Health Care Personnel with COVID-19 — United States, February 12–April 9, 2020. MMWR Morb Mortal Wkly Rep 2020;69:477–481. doi: 10.15585/mmwr.mm6915e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, De Cobelli F, Tresoldi M, Cappelletti AM, Basso C, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R and Bruno R. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 2020;22(5):911–915. doi: 10.1002/ejhf.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirkpatrick JN, Mitchell C, Taub C, Kort S, Hung J and Swaminathan M. ASE Statement on Protection of Patients and Echocardiography Service Providers During the 2019 Novel Coronavirus Outbreak. J Am Coll Cardiol 2020; S0735–1097(20)34815–4. doi: 10.1016/j.jacc.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hendren NS, Drazner MH, Bozkurt B and Cooper J, Leslie T. Descrip-tion and Proposed Management of the Acute COVID-19 Cardiovascular Syndrome [published online April 16, 2020]. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, Labella A, Manson D, Kubin C, Barr RG, et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19 [published online May 7, 2020]. N Engl J Med 2020. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu H, Ma F, Wei X and Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin [published online March 16, 2020]. Eur Heart J 2020. doi: 10.1093/eurheartj/ehaa190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D and Gavazzi E. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) [published online March 27, 2020]. JAMA Cardiol 2020. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Irabien-Ortiz A, Carreras-Mora J, Sionis A, Pamies J, Montiel J and Tauron M. Fulminant myocarditis due to COVID-19. Revista espanola de cardiologia 2020;73(6):503–504. doi: 10.1016/j.rec.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Bondi-Zoccai G, Brown TS, Nigoghossian CD, Zidar DA, Haythe J, et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the Coronavirus Disease 2019 (COVID-19) Pandemic. J Am Coll Cardiol 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, et al. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941 [DOI] [PubMed] [Google Scholar]
- 54.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; 295:1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lippi G, Lavie CJ and Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis [published online March 10, 2020]. Prog Cardiovasc Dis 2020. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 2020;46:586–590. doi: 10.1007/s00134-020-05985-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc [DOI] [PubMed] [Google Scholar]
- 59.Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of Takot-subo Syndrome. Circulation. 2017;135:2426–2441. doi: 10.1161/CIRCULATIONAHA.116.027121 [DOI] [PubMed] [Google Scholar]
- 60.Zhou Z-G, Xie S-M, Zhang J, Zheng F, Jiang D-X, Li K-Y, Zuo Q, Yan Y-S, Liu J-Y and Xie Y-L. Short-Term Moderate-Dose Corticosteroid Plus Immunoglobulin Effectively Reverses COVID-19 Patients Who Have Failed Low-Dose Therapy [published online March 8, 2020]. Preprints. 2020. [Google Scholar]
- 61.Zeng JH, Liu Y-X, Yuan J, Wang F-X, Wu W-B, Li J-X, Wang L-F, Gao H, Wang Y, Dong C-F, et al. First Case of COVID-19 Complicated with Fulminant Myocarditis: A Case Report and Insights [published online April 10, 2020]. Infection. 2020;12–5. doi: 10.1007/s15010-020-01424-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karmpaliotis D, Kirtane AJ, Ruisi CP, Polonsky T, Malhotra A, Talmor D, Kosmidou I, Jarolim P, de Lemos JA, Sabatine MS, et al. Diagnostic and prognostic utility of brain natriuretic Peptide in subjects admitted to the ICU with hypoxic respiratory failure due to noncardiogenic and cardiogenic pulmonary edema. Chest. 2007;131:964–71. doi: 10.1378/chest.06-1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt J-D, Sacco C and Alexia B. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thrombosis Res 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Res 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR and Yaeger KA. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med 2020; 382:e60. doi: 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang N, Li D, Wang X and Sun Z. Abnormal Coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18: 844–847. doi: 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan BE, Chong VCL, Chan SSW, Lim GH, Lim KGE, Tan GB, Mucheli SS, Kuperan P and Ong KH. Hematologic parameters in patients with COVID-19 infection. Am J Hematol 2020;95:E131–E134. doi: 10.1002/ajh.25774 [DOI] [PubMed] [Google Scholar]
- 68.Chen J, Wang X, Zhang S, Liu B, Wu X, Wang Y, Wang X, Yang M, Sun J and Xie Y. Findings of acute pulmonary embolism in COVID-19 patients [published online March 1, 2020]. Lancet Infect Dis 2020. [Google Scholar]
- 69.Wichmann D, Sperhake J-P, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study [published online May 6, 2020]. Ann Intern Med 2020. doi: 10.7326/m20-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zochios V, Parhar K, Tunnicliffe W, Roscoe A and Gao F. The right ventricle in ARDS. Chest 2017;152:181–193. doi: 10.1016/j.chest.2017.02.019 [DOI] [PubMed] [Google Scholar]
- 71.Zapol WM, Kobayashi K, Snider MT, Greene R, and Laver MB. Vascular obstruction causes pulmonary hypertension in severe acute respiratory failure. 1977;71(2 suppl):306–307. doi: 10.1378/chest.71.2_supplement.306 [DOI] [PubMed] [Google Scholar]
- 72.Zapol WM, Snider MT. Pulmonary hypertension in severe acute respiratory failure. N Engl J Med 1977;296:476–480. doi: 10.1056/NEJM197703032960903 [DOI] [PubMed] [Google Scholar]
- 73.Vieillard-Baron A, Naeije R, Haddad F, Bogaard HJ, Bull TM, Fletcher N, Lahm T, Magder S, Orde S, Schmidt G, et al. Diagnostic workup, etiologies and management of acute right ventricle failure: A state-of-the-art paper. Intensive Care Med 2018;44:774–790. doi: 10.1007/s00134-018-5172-2 [DOI] [PubMed] [Google Scholar]
- 74.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian CD, Ageno W, Madjid M, Guo Y, et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-up [published online April 17, 2020]. J Am Coll Cardiol 2020. doi: 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katz JN, Sinha SS, Alviar CL, Dudzinski DM, Gage A, Brusca SB, Flanagan MC, Welch T, Geller BJ and Miller PE. Disruptive Modifications to Cardiac Critical Care Delivery During the Covid-19 Pandemic: An International Perspective [published online April 15, 2020]. J Am Coll Cardiol 2020. doi: 10.1016/j.jacc.2020.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Scordilli M, Pinamonti B, Albani S, Gregorio C, Barbati G, Daneluzzi C, Korcova R, Perkan A, Fabris E, Geri P, et al. Reliability of noninvasive hemodynamic assessment with Doppler echocardiography: comparison with the invasive evaluation. J Cardiovasc Med (Hagerstown). 2019;20:682–690. doi: 10.2459/JCM.0000000000000841 [DOI] [PubMed] [Google Scholar]
- 78.Lehman LW, Saeed M, Moody G, Mark R. Hypotension as a Risk Fac-tor for Acute Kidney Injury in ICU Patients. Comput Cardiol (2010). 2010;37:1095–1098. [PMC free article] [PubMed] [Google Scholar]
- 79.Tarvasmäki T, Haapio M, Mebazaa A, Sionis A, Silva-Cardoso J, Tolppanen H, Lindholm MG, Pulkki K, Parissis J, Harjola VP, et al. ; CardShock Study Investigators. Acute kidney injury in cardiogenic shock: definitions, incidence, haemodynamic alterations, and mortality. Eur J Heart Fail 2018;20:572–581. doi: 10.1002/ejhf.958 [DOI] [PubMed] [Google Scholar]
- 80.Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P, Gattas D, Granger E, Herkes R, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302:1888–95. doi: 10.1001/jama.2009.1535 [DOI] [PubMed] [Google Scholar]
- 81.MacLaren G, Fisher D and Brodie D. Preparing for the Most Critically Ill Patients With COVID-19: The Potential Role of Extracorporeal Membrane Oxygenation. JAMA. 2020; 323: 1245–1246. doi: 10.1001/jama.2020.2342 [DOI] [PubMed] [Google Scholar]
- 82.Bartlett RH, Ogino MT, Brodie D, McMullan DM, Lorusso R, MacLaren G, Stead CM, Rycus P, Fraser JF, Belohlavek J, et al. Initial ELSO Guidance Document: ECMO for COVID-19 Patients with Severe Cardiopulmonary Failure. ASAIO J 2020;66:472–474. doi: 10.1097/MAT.0000000000001173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fried JA, Nair A, Takeda K, Clerkin K, Topkara VK, Masoumi A, Yuzefpolskaya M, Takayama H, Naka Y, Burkhoff D, et al. Clinical and hemodynamic effects of intra-aortic balloon pump therapy in chronic heart failure patients with cardiogenic shock. J Heart Lung Transplant 2018;37:1313–1321. doi: 10.1016/j.healun.2018.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Malick W, Fried JA, Masoumi A, Nair A, Zuver A, Huang A, Haythe J, Farr M, Rabbani L, Karmpaliotis D, et al. Comparison of the Hemodynamic Response to Intra-Aortic Balloon Counterpulsation in Patients With Cardiogenic Shock Resulting from Acute Myocardial Infarction Versus Acute Decompensated Heart Failure. Am J Cardiol 2019;124:1947–1953. doi: 10.1016/j.amjcard.2019.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saxena A, Garan AR, Kapur NK, O’Neill WW, Lindenfeld J, Pinney SP, Uriel N, Burkhoff D, Kern M. Value of Hemodynamic Monitoring in Patients With Cardiogenic Shock Undergoing Mechanical Circulatory Support. Circulation. 2020;141:1184–1197. doi: 10.1161/CIRCULATIONAHA.119.043080 [DOI] [PubMed] [Google Scholar]
- 86.Hernandez GA, Lemor A, Blumer V, Rueda CA, Zalawadiya S, Stevenson LW, Lindenfeld J. Trends in Utilization and Outcomes of Pulmonary Artery Catheterization in Heart Failure With and Without Cardiogenic Shock. J Card Fail 2019;25:364–371. doi: 10.1016/j.cardfail.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 87.DeFilippis EM, Farr MA and Givertz MM. Challenges in Heart Transplantation in the Era of COVID-19 [published online April 21, 2020]. Circulation. 2020. doi: 10.1161/CIRCULATIONAHA.120.047096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alon D, Stein GY, Korenfeld R, Fuchs S. Predictors and outcomes of infection-related hospital admissions of heart failure patients. PLoS One. 2013;8:e72476. doi: 10.1371/journal.pone.0072476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gorodeski EZ, Goyal P, Cox ZL, Thibodeau JT, Reay R, Rasmusson K, Rogers JG and Starling RC. Virtual Visits for Care of Patients with Heart Failure in the Era of COVID-19: A Statement from the Heart Failure Society of America [published online April 18, 2020]. J Card Fail 2020. doi: 10.1016/j.cardfail.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reza N, DeFilippis EM, Jessup M. Secondary Impact of the COVID-19 Pandemic on Patients With Heart Failure. Circ Heart Fail 2020;13:e007219. doi: 10.1161/CIRCHEARTFAILURE.120.007219 [DOI] [PubMed] [Google Scholar]
- 91.Dong N, Cai J, Zhou Y, Liu J and Li F. End-stage Heart Failure with COVID-19: Strong Evidence of Myocardial Injury by 2019-nCoV [published online April 7, 2020]. JACC Heart Fail doi: 10.1016/j.jchf.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sommerstein R, Kochen MM, Messerli FH and Gräni C. Coronavirus Disease 2019 (COVID-19): Do Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers Have a Biphasic Effect? J Am Heart Assoc 2020;9:e016509. doi: 10.1161/JAHA.120.016509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang P, Zhu L, Cai J, Lei F, Qin J-J, Xie J, Liu Y-M, Zhao Y-C, Huang X and Lin L. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020;126(12):1671–1681. doi: 10.1161/CIRCRESAHA.120.317134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, et al. Renin–Angiotensin–Aldosterone System Inhibitors and Risk of Covid-19 [published online May 1, 2020]. N Engl J Med 2020. doi: 10.1056/NEJMoa2008975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mehta N, Kalra A, Nowacki AS, Anjewierden S, Han Z, Bhat P, Carmona-Rubio AE, Jacob M, Procop GW, Harrington S, et al. Association of Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Testing Positive for Coronavirus Disease 2019 (COVID-19) [published online May 5, 2020]. JAMA Cardiol 2020. doi: 10.1001/jamacardio.2020.1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bozkurt B, Kovacs R and Harrington B. HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19. 2020. 26(5):370. doi: 10.1016/j.cardfail.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]


