Abstract
Exosomes play an important role in intercellular communication by delivering microribonucleic acids (miRNAs) to recipient cells. Previous studies have demonstrated that multi-potent mesenchymal stromal cell (MSC)-derived exosomes improve functional recovery after experimental traumatic brain injury (TBI). This study was performed to determine efficacy of miR-17-92 cluster-enriched exosomes (Exo-17-92) harvested from human bone marrow MSCs transfected with a miR-17-92 cluster plasmid in enhancing tissue and neurological recovery compared with exosomes derived from MSCs transfected with an empty plasmid vector (Exo-empty) for treatment of TBI. Adult male rats underwent a unilateral moderate cortical contusion. Animals received a single intravenous injection of miR-17-92 cluster-enriched exosomes (100 μg/rat, approximately 3.75x1011 particles, Exo-17-92) or control exosomes (100 μg/rat, Exo-empty) or Vehicle (phosphate-buffered solution) one day after injury. A battery of neurological functional tests was performed weekly after TBI for five weeks. Spatial learning and memory were measured on days 31–35 after TBI using the Morris water maze test. All animals were sacrificed five weeks after injury. Their brains were processed for histopathological and immunohistochemical analyses of lesion volume, cell loss, angiogenesis, neurogenesis, and neuroinflammation. Compared with Vehicle, both Exo-17-92 and Exo-empty treatments significantly improved sensorimotor and cognitive function, reduced neuroinflammation and hippocampal neuronal cell loss, promoted angiogenesis and neurogenesis without altering the lesion volume. Moreover, Exo-17-92 treatment exhibited a significantly more robust therapeutic effect on improvement in functional recovery by reducing neuroinflammation and cell loss, enhancing angiogenesis and neurogenesis than did Exo-empty treatment. Exosomes enriched with miR-17-92 cluster have a significantly better effect on improving functional recovery after TBI compared with Exo-empty, likely by reducing neuroinflammation and enhancing endogenous angiogenesis and neurogenesis. Engineering specific miRNA in exosomes may provide a novel therapeutic strategy for management of unilateral moderate cortical contusion TBI.
Keywords: exosomes, functional outcome, microRNA, neuroinflammation, neuroplasticity, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a major public health problem worldwide.1 A TBI is a very complex injury, and currently there is no effective therapy for improving functional recovery identified from clinical TBI trials.2 Cell therapies have emerged as a potential management option for TBI.3–6 There are some concerns regarding the use of embryonic and fetal stem cells and induced pluripotent stem cells from somatic cells, particularly in terms of risks for immune rejection and tumorigenicity.7
Multi-potent mesenchymal stromal/stem cells (MSC) have emerged as a promising candidate for cell therapy.4–6,8 The MSCs are self-renewing stem/progenitor cells that exist in bone marrow, adipose tissue, blood, and many other organs.9 Previous studies show that some of the transplanted MSCs migrate into injured brain but only a fraction of them display neural cell-like markers.10,11 These data indicate that MSC treatment-induced functional recovery is not directly linked to neural differentiation of the transplanted MSCs.
Increasing evidence suggests that MSC-induced functional recovery is associated with their release of exosomes.12–14 Exosomes are a subtype of the extracellular vesicles that are delimited by a lipid bilayer and released from cells; they are small particles of endocytic origin with a diameter of 30–150 nm.15,16 Exosome cargo (e.g., ribonucleic acid [RNA], deoxyribonucleic acid [DNA], and proteins/enzymes) is protected from extracellular RNAase and other digestive enzymes and can be effectively transferred to recipient cells.13 In contrast to MSCs, nano-sized exosomes can easily cross the blood–brain barrier (BBB) and deliver their cargo to neural cells,17 without cell transplant-induced vascular obstructive effect and risks of tumorigenicity.18
The MSC-derived exosomes significantly improve functional recovery in rodents19,20 and pigs21 after TBI and in a monkey model of cortical injury.22 The mechanisms underlying the effects of MSC-derived exosomes on improvement of functional recovery after TBI remain elusive. However, the MSC-derived exosomes transfer their therapeutic factors, especially microRNAs (miRNAs), to recipient cells, and therein regulate gene expression, mediate biological function, and promote therapeutic response.13,23 The miRNAs are single-stranded and noncoding RNAs with approximately 22 nucleotides, typically by binding to the 3' untranslated region (3'-UTR) of the complementary mRNA sequence, resulting in translational repression and gene silencing.24,25
A single miRNA can bind to hundreds of different transcripts, and miRNAs may regulate up to 60% of the protein-coding genes in the human genome.26,27 Therefore, a miRNA transferred by exosomes can regulate multiple cellular signaling pathways in the recipient cell, and exosomal miRNAs are considered as an alternative mediator of intercellular communication.26
Our previous work demonstrates that MSC-derived exosomes enriched with the multi-functional miR-17-92 cluster enhance axonal growth of cultured cortical neurons28 and improve neurovascular remodeling and functional recovery in ischemic stroke rats.29 The present study was designed to determine the therapeutic effects of MSC-derived exosomes enriched with miR-17-92 cluster on amplifying brain repair and improving functional recovery in a clinically relevant model of TBI.
Methods
Animals
The use of young male Wistar rats (2–3 months, Charles River, Wilmington, MA) in this study was approved by the Henry Ford Health System Institutional Animal Care and Use Committee. The rigorous clinical trial design (randomization, placebo controlled, and blinding) to pre-clinical TBI with a clinically relevant treatment scheme has been employed.30,31 The persons who performed the experiments, collected data, and assessed outcome were blinded during the experiments (see Supplementary Data for details).
Generation and characterization of engineered exosomes carrying the elevated miR-17-92 cluster
Human MSCs (hMSCs) were expanded (Theradigm, Bethesda, MD). The method is essentially as described.32 Briefly, hMSCs at passage 5 were plated and cultured in α-Minimum Essential Media (α-MEM, Thermo Fisher Scientific, Waltham, MA).33 To generate tailored exosomes with elevated miR-17-92 cluster, hMSCs were transfected with a miR-17-92 cluster plasmid (pCAG-GFP-miR-17-92) or an empty vector according to our published protocol.28,34
The following primers were used for amplification: forward, 5′-CGGAATTCGTCAGGATAATGTCAAAGTGCTTACA; and reverse, CGGGTACCACCAAACTCAACAGGCCG, with EcoRI and KpnI at 5′ ends. A pCAG-green fluorescent protein (GFP)-miR-17-92 cluster vector was constructed by cloning the primary miR-17–92 cluster fragment into the EcoRI and KpnI sites in the pCAG-GFP vector (Addgene plasmid 11150).35 The pCAG-GFP-miR-17-92 vector (2 μg) was introduced into cultured hMSCs by electroporation using a Nucleofector kit (Mirus). An empty pCAG-GFP vector was used as a negative control.
For the exosome isolation, when the cells reached 60–80% confluence, conventional culture medium was replaced with an exosome-depleted fetal bovine serum-contained medium (EXO-FBS-250 A-1, System Biosciences, Mountain View, CA), and the hMSCs were cultured for an additional 48 h.19 The supernatant of cultured hMSCs was collected and passed through a 0.22 μm filter (Millipore, Temecula, CA) to remove dead cells and large growth debris. The filtered supernatant was run at 10,000 × g for 30 min. The supernatant was collected and run at 100,000 × g for 3 h to collect the exosomes, as described in our previous study.28 The supernatant was then collected as a negative control. The pellet was diluted with sterilized phosphate-buffered saline (PBS) for further characterization and experimental treatments.
Nanoparticle tracking analysis with the NanoSight instrument was used to measure numbers and sizes of particles in the exosome-enriched pellets according to the manufacturer's instructions (Merkel Technologies, Yehud, Israel). The size of exosomes was further verified by transmission electron microscopy (TEM, Philips EM208) image, as described in our previous study.14 Isolated exosomes were identified using Western blot analysis of exosomal markers (Alix and Hsp70). The exosomal protein concentration was measured with the micro Bicinchoninic Acid protocol (Pierce, Rockford, IL). Please see Methods in the Supplementary Data for details (Supplementary Fig. S1).
TBI model
A controlled cortical impact (CCI) model of TBI was employed in the present study.36 The CCI-TBI model is described in our previous study.37 In brief, rats were anesthetized with ketamine/xylazine (100/10 mg/kg) intraperitoneally (ip) and placed in a stereotactic frame. A 10-mm-diameter craniotomy was performed over the parietal cortex, and the dura mater was kept. THE CCI was delivered with a pneumatic piston (6-mm tip in diameter, 4 m/sec velocity, and 2.5 mm depth).19,37 This moderate TBI causes consistent cortical tissue loss (lesion cavity) and significant neuronal damage in the ipsilateral hippocampus with sensorimotor and cognitive deficits manifested.2,37
Animal groups
To determine the effect of exosomes enriched with the miR-17-92 cluster on TBI rats, we randomly divided TBI rats into one of the three groups: (1) TBI + Vehicle (PBS, n = 8), (2) TBI + Exo-empty (exosomes derived from human MSCs transfected with an empty vector, n = 8), and (3) TBI + Exo-17-92 (exosomes from human MSCs with enriched miR-17-92 cluster, n = 8). Exosomes (100 μg proteins, approximately 3.75 × 1011 particles, 0.5 mL in PBS/rat, n = 8) were administered via tail vein to rats starting at 24 h after TBI. Rats treated with Vehicle (0.5 mL PBS) were used as a treatment control (Vehicle group). Rats with sham surgery without TBI/treatment were used as a TBI control (Sham group).
The administration dose and time were chosen based on our previous dose-response and window study of exosomes in TBI rats.37 5-bromo-2'-deoxyuridine (BrdU, 100 mg/kg) was injected ip into rats starting one day after TBI, once daily for seven days, to label proliferating cells in the brain.37
Behavioral tests
Primary end-points included the Morris water maze (MWM) test performed at 31–35 days after TBI, and a battery of sensorimotor tests including modified neurological severity score (mNSS), footfault, and adhesive removal performed at one day and then weekly up to 35 days after TBI. These neurological outcome measurements were performed based on our published protocol.19,37 We employed a modified MWM test that uses the concept of probe trial because the hidden platform locations in the correct quadrant change for each trial.38 For details, please see Methods in the Supplementary Data.
Histology and immunostaining
Anesthetized animals were sacrificed for collecting of brains 35 days after TBI. Secondary end-points included neuronal cell loss, lesion volume, angiogenesis, neurogenesis, and neuroinflammation. In brief, brains after transcardial perfusion and fixation in paraformaldehyde were embedded in paraffin and a series of 6-μm-thick slides were prepared. The cortical lesion volume was measured according to our published protocol.37 In brief, lesion volume (mm3) was calculated by measuring the area of the lesion (mm2) and then by multiplying the sum of the lesioned areas obtained from each section by the distance between sections (mm) identified by comparing each hematoxylin and eosin section with the Rat Brain in Stereotaxic Coordinates by Paximos and Watson, and expressed as a percent of the contralateral (noninjured) hemisphere.37 Glial fibrillary acidic protein (GFAP, marker for astrocytes), CD 68- (marker for microglia/macrophages), and neuronal nuclei (NeuN, for neurons)-immunostainings were performed and counted according to our published methods32,37 and described in the Supplementary Data.
Immunofluorescent staining
Newly generated endothelial cells and newborn mature neurons 35 days after TBI were identified by double labeling for BrdU with endothelial barrier antigen (EBA) or neuronal nuclei (NeuN), respectively.19,37 The EBA+ endothelial cells, CD68+ microglia/macrophages, GFAP+ astrocytes, EBA+/BrdU+ cells were counted in the dentate gyrus (DG) and lesion boundary zone (LBZ).19 The LBZ is defined as the region of cortical tissue between the lesion cavity and the intact tissue.37 The NeuN/BrdU-colabeled cells in the subgranular zone and granular cell layer of the DG were counted for analysis of neurogenesis.
The images in the regions of interest were acquired at a magnification of either 200 or 400 under the microscope (Nikon, Eclipse 80i) using CoolSNAP color camera (Photometrics) and analyzed with MetaMorph image analysis system (Molecular Devices). In brief, five fields of view in the LBZ from the epicenter of the injury cavity (bregma −3.3 mm), and nine fields of view in the ipsilateral DG were counted in each section according to our published method.37 A Zeiss LSM 510 META confocal laser scanning microscope connected to a personal computer running the Zeiss LSM Image Software was employed to verify colocalization of BrdU with NeuN in the DG. For details, please see the Supplementary Data (Supplementary Fig. S2).
Cell counting and quantitation
In the present study, we did not use stereological quantification methods because immunoreactive positive cells in the present study are not homogenously distributed and the data of interest are relative difference and not absolute value. All counting was performed on a computer monitor to improve visualization and in one focal plane to avoid oversampling. The methods employed to count cells have been utilized in our previous studies.37 Please see detailed description of cell counting and quantitation in the Supplementary Data.
Statistical analysis
Data presented as the means with standard deviations were tested for normality. Ranked data or a nonparametric Kruskal-Wallis test was used to analyze data if they were not normally distributed. Data on repeated measurements of spatial performance and sensorimotor function were analyzed with analysis of variance (ANOVA). One-way ANOVA followed by post hoc Tukey tests was used to compare the differences in cell counting between the exosome-treated, Vehicle-treated and Sham groups. A p value <0.05 was considered significant.
Results
Identification of exosomes from hMSC culture medium
Using a qNano nanopore-based exosome detection system, we demonstrated that the size of hMSC-derived exosomes is consistent with that measured with the transmission electron microscopy (see Supplementary Data Fig. S1A). Western blots confirmed that exosomes contained high levels of exosome protein markers Alix and Hsp70 compared with the supernatant (See Supplementary Data Fig. S1B). There were no significant differences of exosomal characterization between Exo-17-92 and Exo-empty. The quantitative reverse transcription polymerase chain reaction (qRT-PCR) data show that levels of each member of miR-17-92 cluster (Supplementary Materials Fig. S1C, p < 0.05) were significantly higher in Exo-17-92 than in Exo-empty.
CCI-TBI model
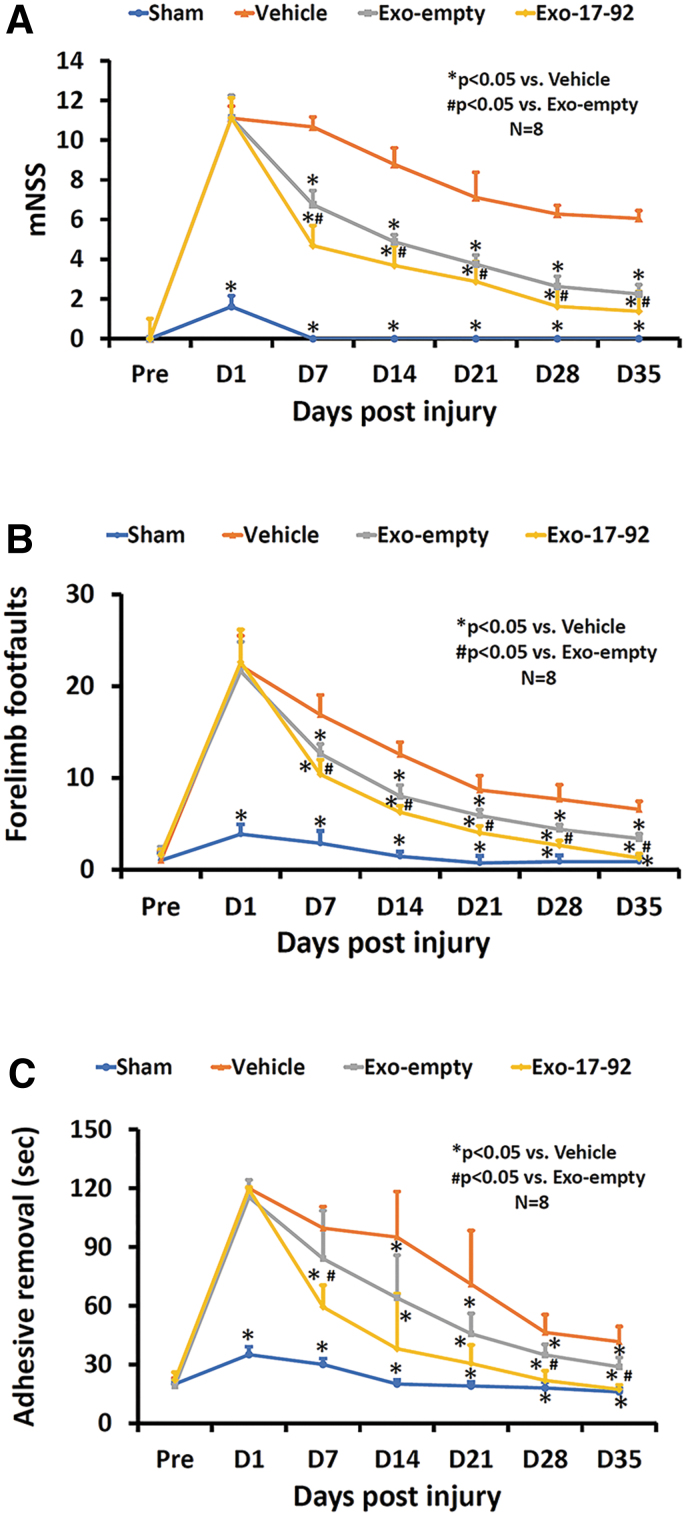
A total of 24 TBI animals and eight sham animals were included into the study. No animal died during the 35-day study period. Moderate TBI was induced in this study based on the CCI injury parameters and functional test results.37,39 No differences in the mNSS, adhesive removal, and footfault scores were detected 24 h post-injury among the three TBI groups (Fig. 1, p > 0.05). The lack of significant differences in these functional tests indicates that all TBI animals had equivalent severity injury before study treatments (Vehicle, Exo-empty, or Exo-17-92).
FIG. 1.
Treatment with exosomes derived from mesenchymal stem cells (MSCs) significantly improves neurological functional recovery measured by the modified neurological severity score (mNSS) (A), footfault test (B) and adhesive removal test (C) in rats after traumatic brain injury (TBI). Both Exo-empty and Exo-17-92 treatments administered intravenously 24 h after TBI significantly reduced sensorimotor deficits starting at seven days post-injury compared with Vehicle treatment. Compared with the Exo-empty, Exo-17-92 exhibited significantly better effects on functional recovery. Data represent mean ± standard deviation. N = 8/group. Color image is available online.
MiR-17-92 enriched exosomes improve sensorimotor functional recovery in rats after TBI
We generated hMSC-derived exosomes carrying elevated miR-17-92 cluster (Exo-17-92). We then treated TBI rats intravenously (iv) 24 h post-injury with Exo-17-92, or with control exosomes from MSCs transfected with an empty vector (Exo-empty), or with Vehicle (PBS). Compared with the Vehicle treatment, both Exo-empty and Exo-17-92 treatments initiated 24 h post-TBI significantly improved sensorimotor recovery measured using mNSS (Fig. 1A), right forelimb footfault (Fig. 1B), and adhesive removal (Fig. 1C) tests starting at day 7 post-TBI and lasting up to 35 days.
Importantly, at 35 days post-TBI, compared with Exo-empty treatment, Exo-17-92 significantly reduced the mNSS score (a decrease by 63% for Exo-empty vs. 77% for Exo-17-92, Fig. 1A, p < 0.05), the number of right forelimb footfault (a decrease by 48% for Exo-empty vs. 65% for Exo-17-92, Fig. 1B, p < 0.05), and adhesive removal time (a decrease by 21% for Exo-empty vs. 58% for Exo-17-92, Fig. 1C, p < 0.05).
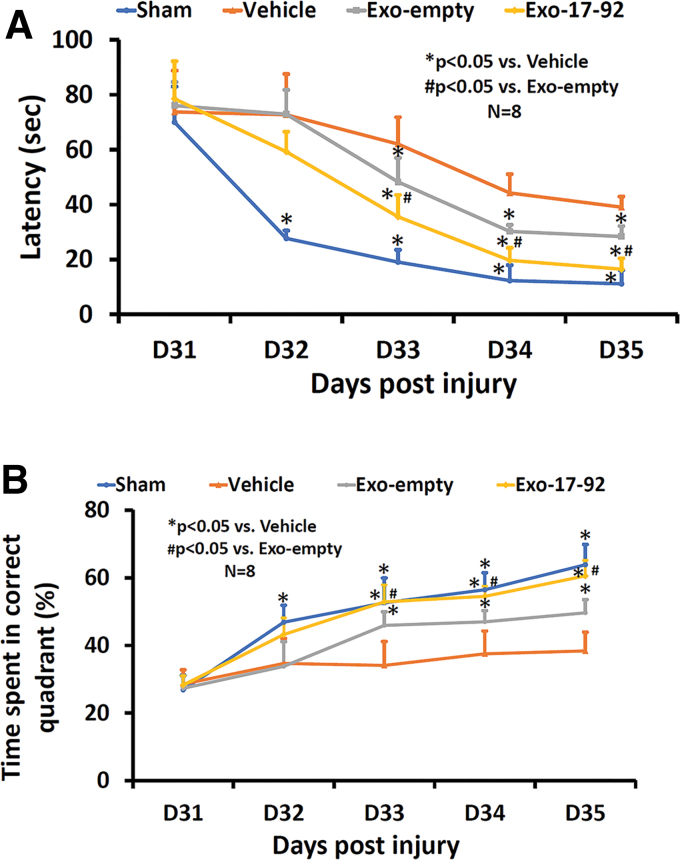
MiR-17-92 enriched exosomes improve spatial learning and memory
The MWM test is used for evaluating spatial learning and memory in rodents40 The MWM test demonstrated that TBI caused spatial learning deficits in rats after TBI. Compared with the Vehicle group, both exosome treatments significantly reduced the time (latency, Fig. 2A, p < 0.05) for animals to reach the hidden platform in the water maze and increased the % of time spent in the correct quadrant (Fig. 2B, p < 0.05), indicating that exosomes significantly improved spatial learning and memory in rats after TBI. Post hoc Tukey testing demonstrated that there was a statistically significant improved therapeutic effect on reducing latency and increasing % of time spent in the correct quadrant with Exo-17-92 treatment than with the Exo-empty treatment at day 33–35 (p < 0.05).
FIG. 2.
Treatment with exosomes derived from mesenchymal stem cells (MSCs) significantly improves spatial learning and memory in the Morris water maze (MWM) test measured by latency to find the hidden platform in the correct quadrant by rats after traumatic brain injury (TBI). Both Exo-empty and Exo-17-92 treatments administered intravenously 24 h after TBI reduced the latency to reach the hidden platform starting at 33 days (A) and increasing the percentage of time spent in the correct quadrant where the hidden platform was located (B) in the MWM testing compared with Vehicle treatment. Compared with the Exo-empty, Exo-17-92 exhibited significantly better effects on reducing the latency and increasing the percentage of time (A and B). Data represent mean ± standard deviation. N = 8/group. Color image is available online.
Collectively, our data demonstrate that both Exo-empty and Exo-17-92 treatments administered iv 24 h after TBI improved sensorimotor and cognitive functional outcomes at day 33–35. Compared with the Exo-empty treated rats, Exo-17-92 treated rats exhibited significantly improved effects on TBI functional recovery.
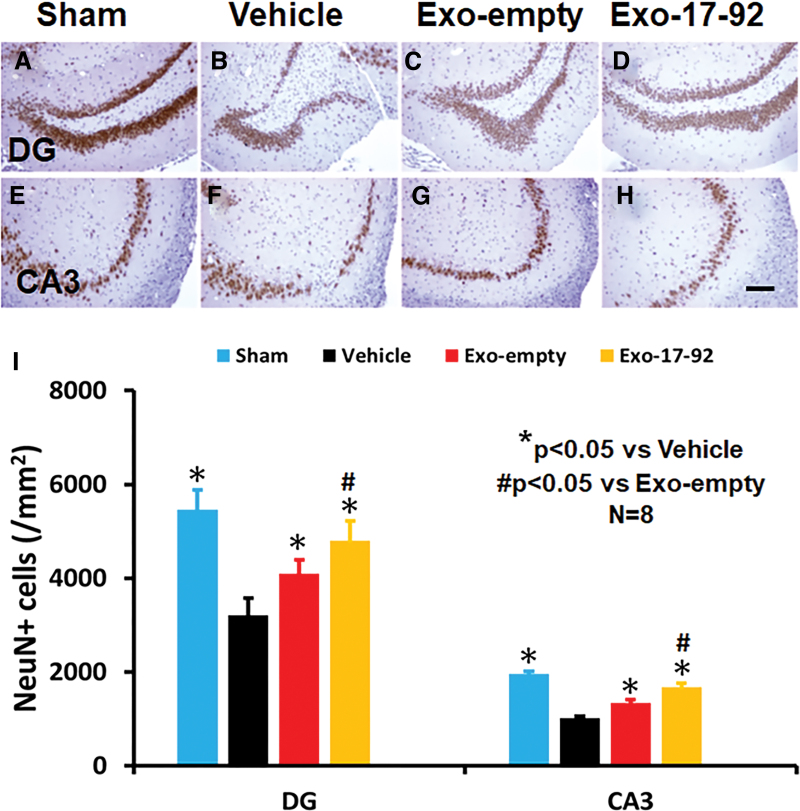
MiR-17-92 enriched exosomes reduce hippocampal neuronal cell loss in rats after TBI
Compared with the Vehicle treatment, exosome treatments significantly reduced the NeuN+ neuronal cell loss in the DG (Fig. 3A–D, F3,28 = 302.98, p < 0.05) and CA3 region (Fig. 3E–H, F3,28 = 51.94, p < 0.05) detected at day 35 post-injury. A statistically significant effect with exosomes on reducing neuronal cell loss was found in the DG (Fig. 3I, Exo-empty vs. Vehicle, Exo-17-92 vs. Vehicle, p < 0.05). There was a significant increase in neuronal cell number in the CA3 between the two exosome treatment groups with a better effect from Exo-17-92 treatment (Fig. 3I, Exo-17-92 v.s Exo-empty, p < 0.05).
FIG. 3.
Treatment with exosomes significantly reduces hippocampal neuronal cell loss after traumatic brain injury (TBI). Neuronal nuclei (NeuN) staining was performed for detection of mature neurons at day 35 after TBI in the dentate gyrus (DG) region (A–D) and CA3 region (E-–H). Both Exo-empty and Exo-17-92 treatments administered intravenously 24 h after TBI reduced the NeuN+ cell loss in the DG (C and D) and CA3 region (G and H) compared with Vehicle treatment (B and F). Compared with the Exo-empty (C and G), Exo-17-92 exhibited significantly better effects on reducing the neuronal cell loss in the DG region (D) and CA 3 region (H). Scale bars = 100 μm (H). Quantitative data shown in bar graph (I) represent mean ± SD. N = 8/group. Color image is available online.
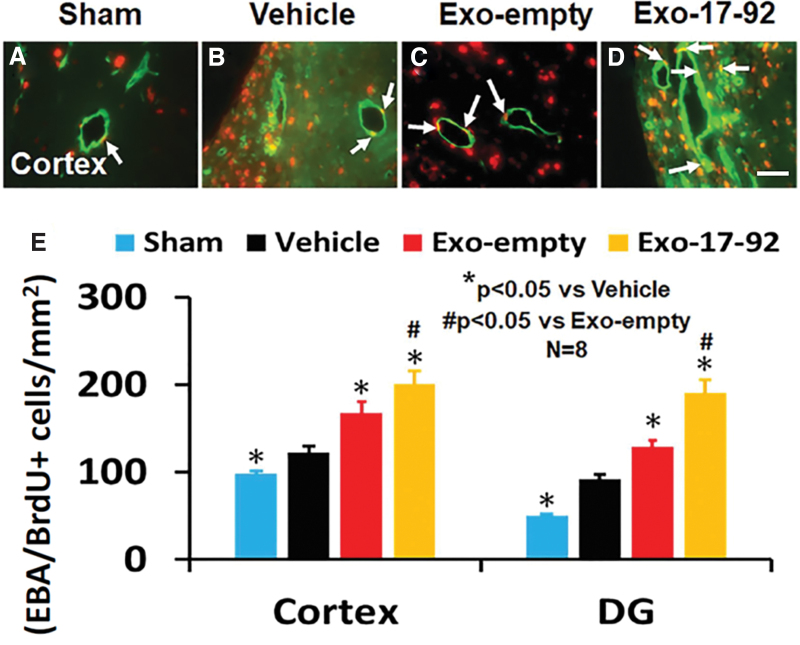
MiR-17-92 enriched exosomes increase angiogenesis in rats after TBI
Exosome treatments significantly increased angiogenesis identified by EBA/BrdU+ double labeling for newborn endothelial cells in the LBZ and DG compared with the Vehicle treatment (Fig. 4A–E, p < 0.05). A significant increase in angiogenesis in the LBZ (Fig. 4E, F3,28 = 137.99, p < 0.001) and DG (Fig. 4E, F3,28 = 138.90, p < 0.001) was detected in the Exo-17-92 group compared with the Exo-empty group.
FIG. 4.
Treatment with exosomes significantly increases angiogenesis after traumatic brain injury (TBI). 5-bromo-2'-deoxyuridine/endothelial barrier antigen (BrdU/EBA+) staining was performed for detection of newly generated endothelial cells at day 35 after TBI in the cortex and dentate gyrus (DG). Representative images (A–D) from the ipsilateral cortex show BrdU+ (red) /EBA+ (green) newborn endothelial cells (white arrows). Both Exo-empty (C) and Exo-17-92 (D) treatments administered intravenously 24 h after TBI promoted angiogenesis in the cortex compared with Vehicle treatment (B). Compared with the Exo-empty, Exo-17-92 exhibited significantly better effects on increasing angiogenesis in the cortex and DG (E). Scale bars = 100 μm (D). Quantitative data shown in bar graph (E) represent mean ± standard deviation. N = 8/group. Color image is available online.
MiR-17-92 enriched exosomes increase neurogenesis in the DG in rats after TBI
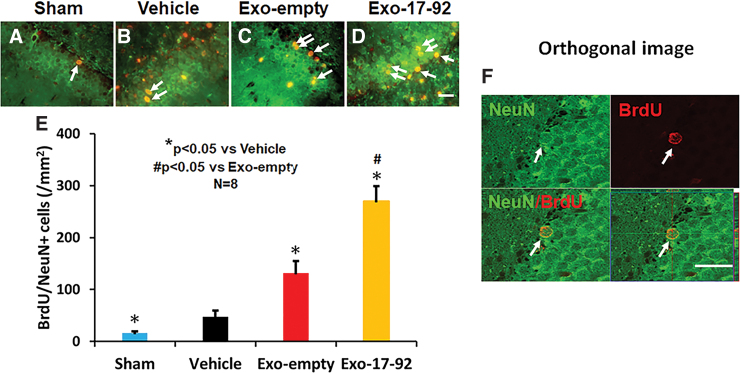
The TBI significantly increased the number of newborn mature neurons in the DG of the ipsilateral hemisphere compared with Sham controls measured at day 35 post-injury (Fig. 5A,B, F3,28 = 195.04, p < 0.001). Exosome treatments, however, significantly increased the number of newborn neurons compared with the Vehicle treatment (Fig. 5B,C, p < 0.01). A significant increase in newly generated neurons was detected by measuring the number of BrdU and NeuN positive cells in the Exo-17-92 group compared with the Exo-empty group (Fig. 5C,D, p < 0.05). Colocalization of BrdU with NeuN (Fig. 5A–E) was further verified using a Zeiss LSM 510 META confocal laser scanning microscope (Fig. 5F and Supplementary Fig. S3).
FIG. 5.
Treatment with exosomes significantly increases neurogenesis 35 days after traumatic brain injury (TBI). Double staining with 5-bromo-2'-deoxyuridine (BrdU, red)/neuronal nuclei (NeuN, green) was performed to identify newborn mature neurons in the dentate gyrus (DG, A–D, white arrows). Scale bar = 25 μm. Scale bars = 25 μm. Quantitative data shown in bar graphs (E) represent mean ± standard deviaion. N = 8/group. Colocalization between BrdU and NeuN in the DG was further verified using a Zeiss LSM 510 META confocal laser scanning microscope (F). Color image is available online.
MiR-17-92 enriched exosomes reduce brain inflammation in rats after TBI
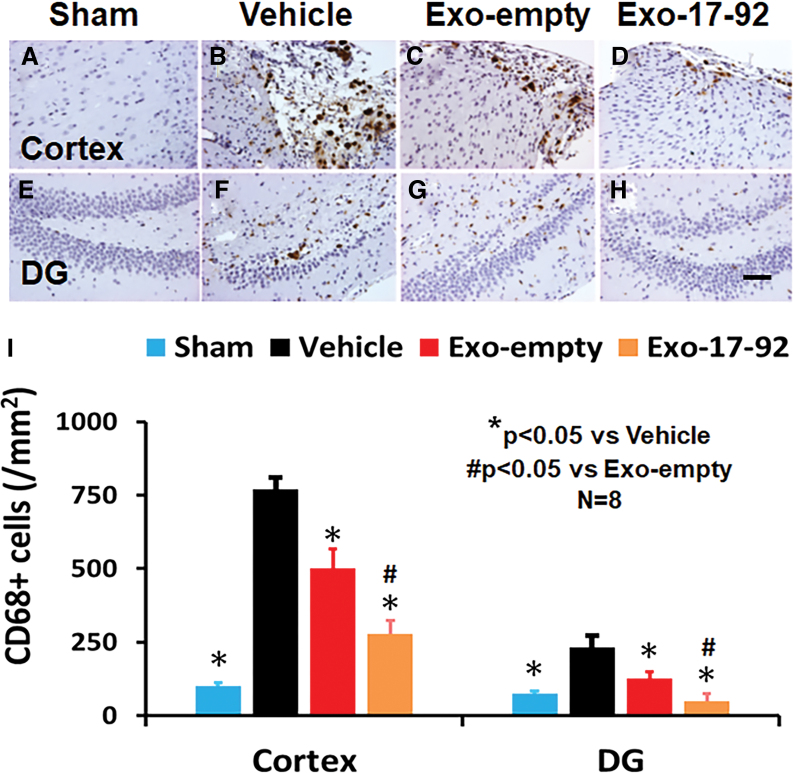
The TBI significantly increased the density of CD68+ macrophages/microglia in the LBZ (Fig. 6A,B, F3,28 = 388.85, p < 0.01) and DG (Fig. 6E,F, F3,28 = 73.07, p < 0.01) of the ipsilateral hemisphere compared with Sham controls. Exosome treatments significantly reduced the CD68+ cell density in the LBZ (Fig. 6C,D) and DG (Fig. 6G,H) compared with the Vehicle treatment (Fig. 6B–F, p < 0.01). Compared with the Exo-empty group, the Exo-17-92 group exhibited significantly reduced CD68+ cell number in the LBZ and DG region (Fig. 6I, p < 0.01).
FIG. 6.
Treatment with exosomes significantly reduces the number of activated microglia/macrophages after traumatic brain injury (TBI). CD68 staining was performed to identify activated microglia/macrophages in the cortex (A–D) and dentate gyrus (DG) region (E–H). Both Exo-empty (C and G) and Exo-17-92 (D and H) treatments administered intravenously 24 h after TBI reduced CD68+ microglia/macrophages compared with Vehicle treatment (B and F). Compared with the Exo-empty, Exo-17-92 exhibited significantly better effects on reducing the number of CD68+ cells. Scale bars = 100 μm (H). Quantitative data shown in bar graph (I) represent mean ± standard deviation. N = 8/group. Color image is available online.
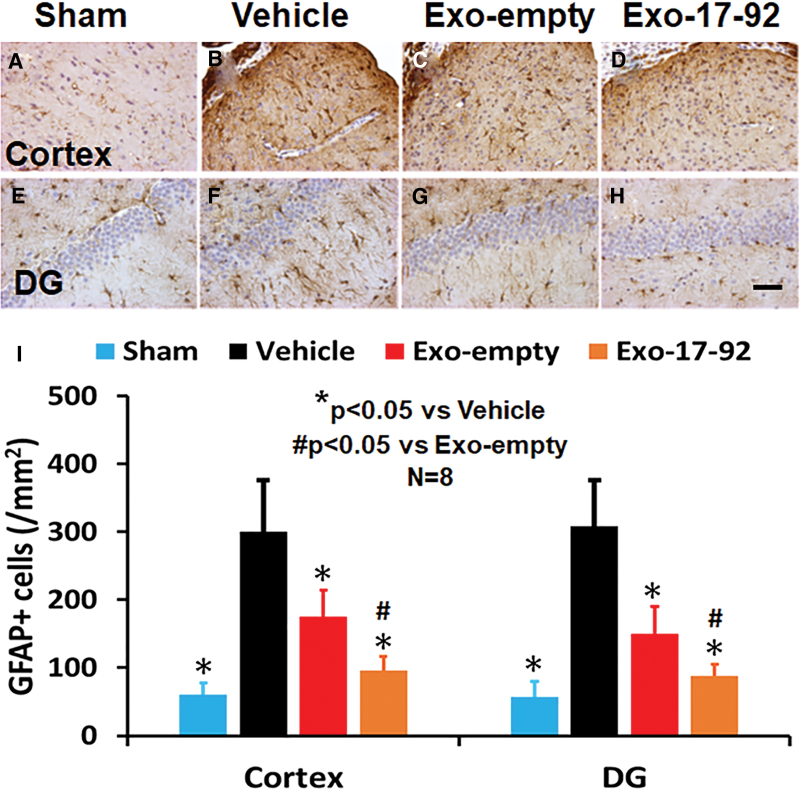
The TBI also significantly increased the number of GFAP+ astrocytes in the LBZ (Fig. 7A,B, F3,28 = 44.85, p < 0.001) and DG (Fig. 7E,F, F3,28 = 57.12, p < 0.001) compared with Sham controls (Fig. 7A, 7E). Exosome treatments significantly reduced the GFAP+ astrocyte density in the LBZ (Fig. 7A–C) and DG (Fig. 7G,H) compared with the Vehicle treatment (Fig. 7B, 7F, p < 0.01). The Exo-17-92 treatment group exhibited significantly reduced GFAP+ cell number compared with the Exo-empty treatment group (Fig. 7I, p < 0.05).
FIG. 7.
Treatment with exosomes significantly reduces the number of astrocytes after traumatic brain injury (TBI). Glial fibrillary acidic protein (GFAP) staining was performed to identify astrocytes in the cortex (A–D) and dentate gyrus (DG) region (E–H). Both Exo-empty (C and G) and Exo-17-92 (D and H) treatments administered intravenously 24 h after TBI reduced GFAP+ astrocytes compared with Vehicle treatment (B and F). Compared with the Exo-empty, Exo-17-92 exhibited significantly better effects on reducing the number of GFAP+ cells. Scale bars = 100 μm (H). Quantitative data shown in bar graph (I) represent mean ± standard deviation. N = 8/group. Color image is available online.
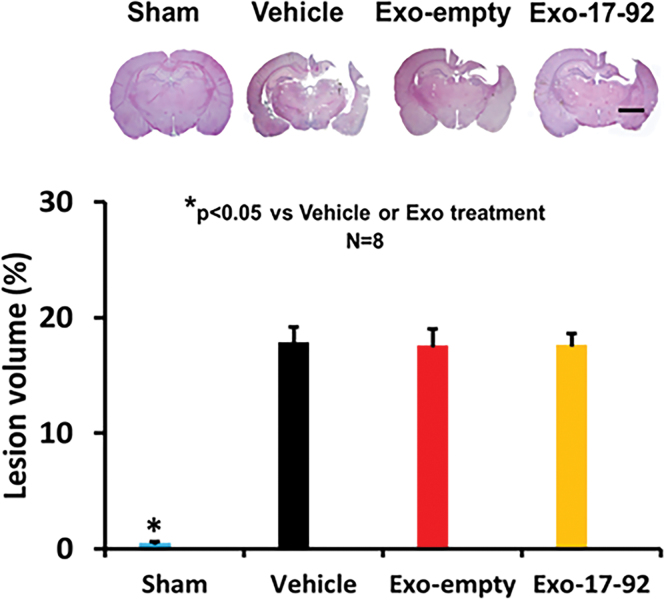
MiR-17-92 enriched exosomes do not alter the volume of total brain tissue loss in rats after TBI
No significant differences in the lesion volume were observed 35 days post-injury (Fig. 8, F2,21 = 0.89, p = 0.427) between exosome and Vehicle treatment groups, indicating that delayed (24 h post-injury) treatments with exosomes do not alter cortical lesion volume.
FIG. 8.
Treatment with exosomes does not alter the lesion size after traumatic brain injury (TBI). Exo-empty and Exo-17-92 treatments administered intravenously 24 h after TBI do not significantly alter the cortical lesion volume measured 35 days after TBI compared with Vehicle treatment. Scale bars = 3 mm. Quantitative data shown in bar graph represent mean ± standard deviation. N = 8/group. Color image is available online.
Discussion
In the present study, we generated human bone marrow MSC-derived exosomes carrying elevated miR-17-92 cluster (Exo-17-92) or control exosomes derived from MSCs transfected with empty vector (Exo-empty). Both Exo-empty and Exo-17-92 treatments administered iv 24 h in rats after moderate TBI improved sensorimotor and cognitive functional outcomes starting at seven days post-injury that persisted to at least 35 days post-injury compared with treatment control (Vehicle). More importantly, compared with the Exo-empty, TBI rats treated with Exo-17-92 exhibited significantly improved effects on both tissue and functional recovery.
Specifically, compared with the Vehicle treatment, iv administration of MSC-derived exosomes with or without enrichment of the miRNA-17-92 cluster significantly: (1) increases the number of BrdU+/NeuN+ newborn mature neurons in the DG (neurogenesis); (2) increases the number of BrdU+/EBA+ newborn endothelial cells in the LBZ and in the DG (angiogenesis); (3) reduces neuronal cell loss in the CA3 and in the DG (neuroprotection); and (4) reduces the number of CD68+ microglia/macrophages and GFAP+ astrocytes in the LBZ and DG (anti-neuroinflammation).
These beneficial effects, in concert, may contribute to significant improvement in sensorimotor and cognitive functional recovery. Further, the levels of neuroprotection and neurorestoration are significantly enhanced in the Exo-17-92 group compared with the Exo-empty group. These data suggest that exosomes engineered to carry select miRNAs may represent a novel approach for management of TBI.
Our data showed that post-traumatic iv administration of exosomes had no effects on lesion volume but reduced hippocampal neuronal cell loss. These findings agree with previous studies showing that delayed (24 h post-injury) treatment regimens confer functional improvement and hippocampal neural protection after TBI without significant reduction in cortical lesion volume.37,41,42 A recent study, however, demonstrated that early (one hour post-injury) iv administration of a single dose of exosomes was able to reduce brain lesion sizes in a swine model of severe TBI and hemorrhagic shock.43
It is known that CCI-induced moderate TBI causes focal cell death predominantly in the cerebral cortex while delayed cell death occurs in the perilesional regions including the hippocampus.44 It is likely that the delayed treatment with exosomes administered 24 h post-injury may not prevent severely damaged cells from death in the injured cortex while delayed neuronal loss in the hippocampus develops slower and provides a longer therapeutic window for treatment.
The MSCs are multi-potent stromal cells that can be isolated from virtually any adult tissue, self-renew, and differentiate into a variety of cell types including the osteogenic, adipogenic, chondrogenic, and neural lineages.45,46 Exogenously administered MSCs can migrate to injured sites of TBI.5,47 In addition to their soluble factors and immunomodulatory effects, therapeutic effects of MSCs for TBI may be attributed to their robust generation and release of exosomes.12 The use of MSC-derived exosomes, as an alternative to MSCs, confers several advantages including their ability to cross biological barriers and lack of complications that arise from stem cell-induced ectopic tumor formation, entrapment in lung microvasculature, and immune rejection.12,48,49
Intravenous administration of cell-free MSC-generated exosomes improves functional recovery after TBI in rats,19,20,32,50 pigs,21 and monkeys.22 The beneficial effects of exosome treatments for brain injury are attributed to: reduction of neuronal loss and neuroinflammation, enhancement in angiogenesis and neurogenesis,51 improvement in BBB integrity and neuroprotection and reduction in brain edema.52 The precise mechanisms of exosome therapeutic effects on functional recovery after TBI are not fully understood.
Exosomes contain complex molecular cargos.53,54 The benefit and potential strength of exosome treatment, as with stem cell therapy, result from targeting multiple injury and repair mechanisms mediated by delivery of exosomal complex cargos to recipient cells. Intravenously administered exosomes can incorporate into recipient parenchymal cells in rats with stroke55 and TBI.12
A single miRNA can regulate expression of many genes at the post-transcriptional level.56 Exosomal miRNAs play an important role in intercellular communication in neurological injuries.12,23,57 Exosome therapy may transfer miRNA to elicit a multi-targeted effect, rather than the traditional, single molecular pathway approach for treatment of TBI.12,13,23 Thus, exosomes provide a novel multi-factorial therapeutic approach capitalizing on the benefits of MSCs without having to administer the cells.
Accumulating studies have shown that engineered exosomes enriched with specific miRNAs play an important role in repairing damaged tissues including brain injury.12,13,23,58 For example, compared with naive MSC-exosomes, our previous studies show that engineered MSC-exosomes carrying elevated levels of the miR-17-92 cluster further enhance axonal growth in cultured cortical neurons,28 enhance axon-myelin remodeling and motor electrophysiological recovery after stroke,59 and increase neural plasticity and functional recovery after stroke in rats.29 Although TBI and stroke share similar pathophysiology including cell injury, BBB breakdown, apoptosis, neuroinflammation, white matter injury, and brain atrophy, the fact that these injuries arise from different types of primary insults leads to diverse cellular vulnerability patterns as well as a spectrum of injury processes.60
Ours is the first study demonstrating the therapeutic efficacy of miR-17-92 cluster-enriched MSC-derived exosomes in TBI. Our data in this study further confirm that selective manipulation of expression of miR-17-92 in the parent MSCs leads to an enhanced therapeutic efficacy of their harvested exosomes carrying an elevated level of miR-17-92 in a rat model of TBI.
Neuroinflammation is a key component of the secondary injury cascades after TBI.61–64 Pre-clinical studies indicate that post-traumatic persistent and progressive neuroinflammation is associated with neurodegeneration that may be treatable long after the original brain injury.64 In the present study, treatment with miR-17-92 cluster-enriched exosomes significantly reduced the number of GFAP+ astrocytes and CD68+ microglia/macrophages compared with the Exo-empty. Anti-inflammatory effects of exosomes are similar to those found after MSC therapy in animal models of stroke65,66 and TBI.67
Microglia constantly survey the surrounding parenchyma and respond rapidly to sustain neuronal health by releasing cytokines, free radicals, antioxidants, and neurotrophic factors.68
Astrocytes are actively involved in neurogenesis, synaptogenesis, angiogenesis, BBB repair, and glial scar formation after TBI.69 Astrocytes also play an important role in the brain waste clearance via the glymphatic system after TBI.70,71 Prolonged activation of astrocytes and microglia contribute to brain damage and functional deficits after TBI.64 Astrocytes and microglia play dual roles in mediating brain damage and repair where beneficial and the detrimental aspects of their cellular responses determine the extent of damage and subsequent repair.72
Our data also support that suppression of activated microglia/macrophages with exosome treatment contributes to increased angiogenesis and neurogenesis, and subsequent improvement in functional recovery after TBI. This is in agreement with a recent study that shows human adipose MSC-derived exosomes administered intracerebroventricularly 24 h post-injury suppress activation of microglia/macrophages, inhibit inflammation, and facilitate functional recovery in rats after TBI.73
Interestingly, we have also shown that exosomes harvested from miR-133b-overexpressing MSCs can stimulate secondary release of neurite-promoting exosomes from endogenous astrocytes, which is associated with improved neural plasticity and functional recovery after stroke.74 These findings suggest that treatment with select miRNA-enriched exosomes can promote additional exosome release from endogenous astrocytes (likely from other neural cells), which may amplify the therapeutic effects of exogenous exosome treatment.
New neurons are generated from neural stem/progenitor cells in mammals during adulthood and after select neurological disorders, and thus neurogenesis may be a potential target area for treatments.75 Angiogenesis and neurogenesis coordinately interact with each other in the developing and adult brain, during which they share a common post-transcriptional regulator, the miR-17-92 cluster.76 The generation of new vasculature facilitates highly coupled neurorestorative processes including neurogenesis and synaptogenesis, which in turn lead to improved functional recovery in TBI.77 The miR-17-92 cluster modulates proliferation of neural progenitor cells during development and during neurological disorders such as stroke, and the miR-17-92 cluster in endothelial cells regulates angiogenesis during embryonic stage and adulthood.34,78
Our recent study demonstrated that ablation of the miR-17-92 cluster in neural stem cells (NSC) significantly reduces the number of proliferating NSCs and neuroblasts and neuronal differentiation in the DG of the hippocampus and significantly impairs hippocampal-dependent learning and memory measured by social recognition memory, novel object recognition, and MWM tests.78 A highly significant correlation exists between reduction in newly generated neuroblasts in the DG and cognition deficits in miR-17-92 knockout mice.78
Our TBI data indicate that miR-17-92 cluster-enriched exosomes increase angiogenesis and neurogenesis in the DG that are associated with enhanced improvement in spatial learning and memory after brain injury, compared with Exo-empty. This is consistent with our previous study that the miR-17-92 cluster mediates adult NSC proliferation and survival in the subventricular zone after stroke.79
Our data indicate that increase of the miR-17-92 cluster amplifies endogenous neurogenesis in ischemic and traumatic injured brains. Our recent study demonstrates that miR-17-92 enriched exosomes derived from MSCs enhance axon-myelin remodeling and motor electrophysiological recovery after stroke.59 The roles of the miR-17-92 cluster in neuronal and vascular plasticity make miR-17-92 cluster-enriched exosomes a promising regenerative treatment to enhance brain tissue repair and functional recovery after brain injury.
Our previous proof-of-principle study demonstrated that CD63-GFP-tagged exosomes derived from MSCs were taken up by neurons and astrocytes of the injured brains measured 30 min after iv administration in rats with TBI.12 Although whether treatment with miR-17-92-enriched exosomes increases levels of miR-17-92 in the brain was not determined in the present study, our recent work demonstrated that iv administration of miR-17-92 cluster-enriched MSC exosomes enhanced neurofunctional recovery of stroke, which may be mediated in part via the activation of the PI3K/protein kinase B/mechanistic target of rapamycin/glycogen synthase kinase 3 beta signaling pathway induced by the downregulation of phosphatase and tensin homolog (PTEN).29,59 The PTEN is among the first validated miR-17-92 cluster targets.80,81
Emerging evidence has implicated miR-17-92 cluster as a master regulator of neurogenesis.81
Our recent study demonstrated that conditional knockout of the miR-17-92 cluster in neural stem cells (NSCs) significantly increases cytoskeleton-associated protein, Enigma homolog 1 (ENH1) and reduces its downstream transcription factor, inhibitor of differentiation 1 (ID1), as well as increases PTEN gene expression.78 These proteins are related to neuronal differentiation.78 The MiR-17-92 cluster knockdown mice have diminished hippocampal neurogenesis and impaired cognitive function.78 These data indicate that the miR-17-92 cluster in NSCs is critical for normal cognitive and behavioral function via targeting ENH1/ID1 and regulating neurogenesis in adult mice.
Further studies are warranted to investigate (1) whether miR-17-92 cluster-enriched exosomes regulate these signaling pathways for improving tissue and functional recovery in TBI; and (2) whether treatment with miR-17-92-enriched exosomes increases levels of miR-17-92 and decreases expression levels of their mRNA targets in the injured brain.
In this study, miR-17-92 cluster-enriched exosomes were generated from human MSCs transfected with a miR-17-92 cluster plasmid. Quantitative RT-PCR analysis of the harvested exosomes showed that levels of all individual six members (miR-17, 18a, 19a, 19b, 20a, 92) of the miR-17-92 cluster were increased several-fold within exosomes isolated from MSCs transfected with the miR-17-92 cluster (Exo-17-92) compared with their levels within exosomes from MSCs transfected with the empty vector (Exo-empty).
Cellular miRNA levels can also be increased by delivering miRNA mimics (agomir) or decreased by anti-miR (antagomir) to regulate native microRNA functions in recipient cells.26 Several miRNA mimics, including miR-23a, miR-27a, miR-21, miR-23b and miR-124-3p mimics, have been demonstrated to exert neuroprotection and anti-inflammation against brain damage and improve neurological functional recovery in animal models of TBI.26,82 Some antagomirs have also been found to exhibit protective effects against TBI. Suppression of select miRNAs such as miR-193a,83 miR-144,84 miR-155,85 miR-71186 with corresponding antagomirs improves neurological function recovery in animal models of TBI through antineuroinflammation, reduction in lesion volume, cell loss, and brain edema.
Regulation of miRNA is promising for management of TBI. Naked miRNA, agomir, and antagomir are vulnerable to nuclease degradation before reaching the target cells. Viral and nonviral vector systems are used for delivery of miRNA, agomir, and antagomir into the cells. There are safety concerns for bioactive viruses because of the immunogenicity and the risk of triggering an oncogenic transformation by viruses as well as their innate immunity and antigen specific adaptive immune responses leading to reduced efficiency of gene transfer.87
Exosomes have emerged as an effective nonviral delivery system for miRNAs. The miRNA and their mimics/inhibitors can be loaded by different methods including transfection or electroporation into isolated exosomes.87 Exosomes have unique advantages over other vectors for gene delivery including: (1) exosomes are a cell-free natural system for carrying miRNA to mediate intercellular communications, (2) exosomal membranes protect the miRNA from digestion by extracellular nuclease before they reach recipient cells, and (3) exosomes can cross the BBB and are rapidly taken up by target cells.12,13,23,64,88 These advantages make them a more efficient vehicle for gene delivery.
Our data demonstrate that empty exosomes as the negative control for miR-17-92-enriched exosomes also led to significant improvements in functional recovery, reduction in neuroinflammation and hippocampal neuronal cell loss, promotion in angiogenesis and neurogenesis compared with the saline treatment. These beneficial effects suggest that this control treatment possessed some therapeutic substances.
As shown in Supplementary Data Fig. S1C, low levels of miR-17-92 were detected in the Exo-empty although individual levels of miR-17-92-cluster in the Exo-17-92 were significantly higher (in the range of 2–11 times dependent on the individual miRNA member) than those in Exo-empty. The low levels of miR-17-92 cluster may contribute to the beneficial effects of Exo-empty, which are consistent with our recent studies demonstrating that compared with treatment controls, the empty exosomes significantly improved functional recovery in stroke rats while the miR-17-92-enriched exosomes further enhanced functional recovery.29,59 In addition, increasing evidence indicates that other miRNAs and cargoes (mRNAs, proteins, lipids) in exosomes also play an important role in the cell-cell communication and may contribute to therapeutic effects in the exosome treatment for TBI12,13,89 that we did not investigate in the present study.
The MiRNA-enriched exosomes from other cell sources have shown beneficial effects for management of TBI. For example, iv injection of miR-124-3p-enriched exosomes derived from microglia reveals that exosomal miR-124-3p is associated with a decrease in the modified mNSS score and improvement in MWM test results in mice with repetitive TBI via inhibiting neuronal autophagy.90 It is important to determine whether a combined approach of using exosomes with overexpression or deletion of the select miRNAs have additive or synergistic effects for management of TBI and other neurological disease. In addition to exosomes, MSCs also release other extracellular vesicles and soluble factors, which may contribute to therapeutic effects underlying MSC therapy and can be developed for management of TBI.53,54
In eukaryotic cells, miRNAs constitute a major regulatory gene family, and different cell types and tissues express different sets of miRNAs.91–93 Although the enriched miR-17-92 enhances the therapeutic benefit, we do not exclude a therapeutic contribution of other miRNAs, RNAs, proteins for management of TBI. In this study, a single dose of miR-17-92 cluster-enriched exosomes was administered 24 h in TBI rats. It is unknown, however, whether the administration of exosomes enriched with miR-17-92 cluster at the early or later stages or repeated treatments could result in a more effective tissue and functional recovery after TBI. Further investigation of these questions will make the application of these exosomes more translational in the management of TBI.
In the present study, we only evaluated therapeutic effects of exosomes in the moderate TBI model. We selected this moderate TBI rat model based on our previous studies that have demonstrated significant therapeutic effects of MSCs and naive exosomes for treatment of moderate TBI in rats.5,19,37 In addition to beneficial effects of exosome treatment in moderate TBI, our recent study further demonstrated that naïve exosomes derived from human MSCs had beneficial effects in a swine model of severe TBI and hemorrhagic shock.21
A recent study demonstrated that iv injected microglial exosomes enriched with miR-124-3p were taken up by neurons in injured brain, improved the cognitive outcome in mice after repetitive mild TBI.94 These findings indicate that both naïve exosomes and tailored exosomes overexpressing select cargos including miRNAs may hold a therapeutic potential for management of mild, moderate, and severe TBI.
Conclusions
We demonstrate that iv administration of exosomes engineered to enrich the miR-17-92 cluster significantly reduces neuroinflammation and neuronal cell loss, increases neurogenesis and angiogenesis, and significantly augments the therapeutic benefits on functional recovery for TBI compared with the Exo-empty. Contents in exosomes can be engineered to amplify neural plasticity and enhance functional recovery in TBI. The MSC-derived exosomes enriched with select miRNA may serve as an effective and possibly superior surrogate for stem cell therapy for neural injury or disease.
Supplementary Material
Acknowledgments
The authors thank Sutapa Santra and Qinge Lu for their technical assistance. All datasets presented in this study are included in the article/supplementary data.
Authors' Contributions
Conception and design: YX, MC; acquisition of data: YanZ, YiZ, HP, YX; analysis and interpretation of data: YanZ, YiZ, YX; drafting of the manuscript: YX; critical review of the manuscript: YX, MC, ZGZ, AM; review of final version of the manuscript and approval for submission: all authors.
Funding Information
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01NS100710 (YX). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Johnson, V.E., Stewart, W., Arena, J.D., and Smith, D.H. (2017). Traumatic brain injury as a trigger of neurodegeneration. Adv. Neurobiol. 15, 383–400 [DOI] [PubMed] [Google Scholar]
- 2. Xiong, Y., Mahmood, A., and Chopp, M. (2013). Animal models of traumatic brain injury. Nat. Rev. Neurosci. 14, 128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rolfe, A., and Sun, D. (2015). Stem cell therapy in brain trauma: implications for repair and regeneration of injured brain in experimental TBI models. In: Kobeissy, F.H., ed. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton, FL: CRC Press/Taylor and Francis [PubMed] [Google Scholar]
- 4. Mahmood, A., Lu, D., and Chopp, M. (2004). Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery 55, 1185–1193 [DOI] [PubMed] [Google Scholar]
- 5. Mahmood, A., Lu, D., and Chopp, M. (2004). Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J. Neurotrauma 21, 33–39 [DOI] [PubMed] [Google Scholar]
- 6. Lu, M., Chen, J., Lu, D., Yi, L., Mahmood, A., and Chopp, M. (2003). Global test statistics for treatment effect of stroke and traumatic brain injury in rats with administration of bone marrow stromal cells. J. Neurosci. Methods 128, 183–190 [DOI] [PubMed] [Google Scholar]
- 7. Sykova, E., and Forostyak, S. (2013). Stem cells in regenerative medicine. Laser Ther. 22, 87-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harding, J., and Mirochnitchenko, O. (2014). Preclinical studies for induced pluripotent stem cell-based therapeutics. J. Biol. Chem. 289, 4585–4593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ho, A.D., Wagner, W., and Franke, W. (2008). Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy 10, 320–330 [DOI] [PubMed] [Google Scholar]
- 10. Li, Y., Chen, J., Wang, L., Lu, M., and Chopp, M. (2001). Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology 56, 1666–1672 [DOI] [PubMed] [Google Scholar]
- 11. Lu, D., Mahmood, A., Wang, L., Li, Y., Lu, M., and Chopp, M. (2001). Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport 12, 559–563 [DOI] [PubMed] [Google Scholar]
- 12. Xiong, Y., Mahmood, A., and Chopp, M. (2017). Emerging potential of exosomes for treatment of traumatic brain injury. Neural. Regen. Res. 12, 19–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang, Z.G., Buller, B., and Chopp, M. (2019). Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 15, 193–203 [DOI] [PubMed] [Google Scholar]
- 14. Xin, H., Li, Y., Buller, B., Katakowski, M., Zhang, Y., Wang, X., Shang, X., Zhang, Z.G., and Chopp, M. (2012). Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30, 1556–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Russell, A.E., Sneider, A., Witwer, K.W., Bergese, P., Bhattacharyya, S.N., Cocks, A., Cocucci, E., Erdbrugger, U., Falcon-Perez, J.M., Freeman, D.W., Gallagher, T.M., Hu, S., Huang, Y., Jay, S.M., Kano, S.I., Lavieu, G., Leszczynska, A., Llorente, A.M., Lu, Q., Mahairaki, V., Muth, D.C., Noren Hooten, N., Ostrowski, M., Prada, I., Sahoo, S., Schoyen, T.H., Sheng, L., Tesch, D., Van Niel, G., Vandenbroucke, R.E., Verweij, F.J., Villar, A.V., Wauben, M., Wehman, A.M., Yin, H., Carter, D.R., and Vader, P. (2019). Biological membranes in EV biogenesis, stability, uptake, and cargo transfer: an ISEV position paper arising from the ISEV membranes and EVs workshop. J. Extracell. Vesicles 8, 1684862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thery, C., Zitvogel, L., and Amigorena, S. (2002). Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579 [DOI] [PubMed] [Google Scholar]
- 17. Kooijmans, S.A., Vader, P., van Dommelen, S.M., van Solinge, W.W., and Schiffelers, R.M. (2012). Exosome mimetics: a novel class of drug delivery systems. Int. J. Nanomedicine 7, 1525–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Melo, S.A., Sugimoto, H., O'Connell, J.T., Kato, N., Villanueva, A., Vidal, A., Qiu, L., Vitkin, E., Perelman, L.T., Melo, C.A., Lucci, A., Ivan, C., Calin, G.A., and Kalluri, R. (2014). Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 26, 707–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang, Y., Chopp, M., Meng, Y., Katakowski, M., Xin, H., Mahmood, A., and Xiong, Y. (2015). Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 122, 856–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim, D.K., Nishida, H., An, S.Y., Shetty, A.K., Bartosh, T.J., and Prockop, D.J. (2016). Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc. Natl. Acad. Sci. U. S. A. 113, 170–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williams, A.M., Dennahy, I.S., Bhatti, U.F., Halaweish, I., Xiong, Y., Chang, P., Nikolian, V.C., Chtraklin, K., Brown, J., Zhang, Y., Zhang, Z.G., Chopp, M., Buller, B., and Alam, H.B. (2019). Mesenchymal stem cell-derived exosomes provide neuroprotection and improve long-term neurologic outcomes in a swine model of traumatic brain injury and hemorrhagic shock. J. Neurotrauma 36, 54–60 [DOI] [PubMed] [Google Scholar]
- 22. Moore, T.L., Bowley, B.G.E., Pessina, M.A., Calderazzo, S.M., Medalla, M., Go, V., Zhang, Z.G., Chopp, M., Finklestein, S., Harbaugh, A.G., Rosene, D.L., and Buller, B. (2019). Mesenchymal derived exosomes enhance recovery of motor function in a monkey model of cortical injury. Restor. Neurol. Neurosci. 37, 347–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xin, H., Li, Y,. and Chopp, M. (2014). Exosomes/miRNAs as mediating cell-based therapy of stroke. Front. Cell. Neurosci. 8, 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huntzinger, E., and Izaurralde, E. (2011). Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat. Rev. Genet. 12, 99–110 [DOI] [PubMed] [Google Scholar]
- 25. Sakurai, F., Katayama, K., and Mizuguchi, H. (2011). MicroRNA-regulated transgene expression systems for gene therapy and virotherapy. Front. Biosci .(Landmark Ed) 16, 2389–2401 [DOI] [PubMed] [Google Scholar]
- 26. Sun, P., Liu, D.Z., Jickling, G.C., Sharp, F.R., and Yin, K.J. (2018). MicroRNA-based therapeutics in central nervous system injuries. J. Cereb. Blood Flow Metab. 38, 1125–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Friedman, R.C., Farh, K.K., Burge, C.B., and Bartel, D.P. (2009). Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19, 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang, Y., Chopp, M., Liu, X.S., Katakowski, M., Wang, X., Tian, X., Wu, D., and Zhang, Z.G. (2017). Exosomes derived from mesenchymal stromal cells promote axonal growth of cortical neurons. Mol. Neurobiol. 54, 2659–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xin, H., Katakowski, M., Wang, F., Qian, J.Y., Liu, X.S., Ali, M.M., Buller, B., Zhang, Z.G., and Chopp, M. (2017). MicroRNA cluster miR-17-92 Cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke 48, 747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang, Y., Chopp, M., Zhang, Y., Zhang, Z.G., Lu, M., Zhang, T., Wu, K.H., Zhang, L., Mahmood, A., and Xiong, Y. (2019). Randomized controlled trial of Cerebrolysin's effects on long-term histological outcomes and functional recovery in rats with moderate closed head injury. J. Neurosurg. 1–11 [DOI] [PubMed] [Google Scholar]
- 31. Zhang, L., Chopp, M., Lu, M., Zhang, T., Winter, S., Doppler, E., Meier, D., Chao, L., Eapen, A., Pabla, P., and Gang Zhang, Z. (2016). Cerebrolysin dose-dependently improves neurological outcome in rats after acute stroke: a prospective, randomized, blinded, and placebo-controlled study. Int. J. Stroke 11, 347–355 [DOI] [PubMed] [Google Scholar]
- 32. Zhang, Y., Chopp, M., Zhang, Z.G., Katakowski, M., Xin, H., Qu, C., Ali, M., Mahmood, A., and Xiong, Y. (2017). Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem. Int. 111, 69–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sotiropoulou, P.A., Perez, S.A., Salagianni, M., Baxevanis, C.N., and Papamichail, M. (2006). Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 24, 462–471 [DOI] [PubMed] [Google Scholar]
- 34. Zhang, Y., Ueno, Y., Liu, X.S., Buller, B., Wang, X., Chopp, M., and Zhang, Z.G. (2013). The MicroRNA-17-92 cluster enhances axonal outgrowth in embryonic cortical neurons. J. Neurosci. 33, 6885–6894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matsuda, T., and Cepko, C.L. (2004). Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. U. S. A. 101, 16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dixon, C.E., Clifton, G.L., Lighthall, J.W., Yaghmai, A.A., and Hayes, R.L. (1991). A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods 39, 253–262 [DOI] [PubMed] [Google Scholar]
- 37. Zhang, Y., Zhang, Y., Chopp, M., Zhang, Z.G., Mahmood, A., and Xiong, Y. (2020). Mesenchymal stem cell-derived exosomes improve functional recovery in rats after traumatic brain injury: a dose-response and therapeutic window study. Neurorehabil. Neural. Repair 34, 616–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Choi, S.H., Woodlee, M.T., Hong, J.J., and Schallert, T. (2006). A simple modification of the water maze test to enhance daily detection of spatial memory in rats and mice. J. Neurosci. Methods 156, 182–193 [DOI] [PubMed] [Google Scholar]
- 39. Dixon, C.E., Kochanek, P.M., Yan, H.Q., Schiding, J.K., Griffith, R.G., Baum, E., Marion, D.W., and DeKosky, S.T. (1999). One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. .J Neurotrauma 16, 109–122 [DOI] [PubMed] [Google Scholar]
- 40. Tucker, L.B., Velosky, A.G. and McCabe, J.T. (2018). Applications of the Morris water maze in translational traumatic brain injury research. Neurosci Biobehav Rev 88, 187-200 [DOI] [PubMed] [Google Scholar]
- 41. Kline, A.E., Massucci, J.L., Marion, D.W., and Dixon, C.E. (2002). Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J. Neurotrauma 19, 415–425 [DOI] [PubMed] [Google Scholar]
- 42. Xiong, Y., Mahmood, A., Meng, Y., Zhang, Y., Qu, C., Schallert, T., and Chopp, M. (2010). Delayed administration of erythropoietin reduces hippocampal cell loss, enhances angiogenesis and neurogenesis, and improves functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J. Neurosurg. 113, 598–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Williams, A.M., Wu, Z., Bhatti, U.F., Biesterveld, B.E., Kemp, M.T., Wakam, G.K., Vercruysse, C.A., Chtraklin, K., Siddiqui, A.Z., Pickell, Z., Dekker, S.E., Tian, Y., Liu, B., Li, Y., Buller, B., and Alam, H.B. (2020). Early single-dose exosome treatment improves neurologic outcomes in a 7-day swine model of traumatic brain injury and hemorrhagic shock. J. Trauma Acute Care Surg. 89, 388–396 [DOI] [PubMed] [Google Scholar]
- 44. Zhou, H., Chen, L., Gao, X., Luo, B., and Chen, J. (2012). Moderate traumatic brain injury triggers rapid necrotic death of immature neurons in the hippocampus. J. Neuropathol. Exp. Neurol. 71, 348–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hasan, A., Deeb, G., Rahal, R., Atwi, K., Mondello, S., Marei, H.E., Gali, A., and Sleiman, E. (2017). Mesenchymal stem cells in the treatment of traumatic brain injury. Front. Neurol. 8, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chopp, M., and Li, Y. (2002). Treatment of neural injury with marrow stromal cells. Lancet Neurol. 1, 92–100 [DOI] [PubMed] [Google Scholar]
- 47. Mahmood, A., Lu, D., Qu, C., Goussev, A., and Chopp, M. (2006). Long-term recovery after bone marrow stromal cell treatment of traumatic brain injury in rats. J. Neurosurg. 104, 272–277 [DOI] [PubMed] [Google Scholar]
- 48. Maumus, M., Rozier, P., Boulestreau, J., Jorgensen, C., and Noel, D. (2020). Mesenchymal stem cell-derived extracellular vesicles: opportunities and challenges for clinical translation. Front. Bioeng. Biotechnol. 8, 997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gowen, A., Shahjin, F., Chand, S., Odegaard, K.E., and Yelamanchili, S.V. (2020). Mesenchymal stem cell-derived extracellular vesicles: challenges in clinical applications. Front. Cell Dev. Biol. 8, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xin, H., Li, Y., Cui, Y., Yang, J.J., Zhang, Z.G., and Chopp, M. (2013). Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 33, 1711–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xiong, Y., Zhang, Y., Mahmood, A., and Chopp, M. (2015). Investigational agents for treatment of traumatic brain injury. Expert Opin. Investig. Drugs 24, 743–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Williams, A.M., Bhatti, U.F., Brown, J.F., Biesterveld, B.E., Kathawate, R.G., Graham, N.J., Chtraklin, K., Siddiqui, A.Z., Dekker, S.E., Andjelkovic, A., Higgins, G.A., Buller, B., and Alam, H.B. (2020). Early single-dose treatment with exosomes provides neuroprotection and improves blood-brain barrier integrity in swine model of traumatic brain injury and hemorrhagic shock. J. Trauma Acute Care Surg. 88, 207–218 [DOI] [PubMed] [Google Scholar]
- 53. Yu, B., Zhang, X., and Li, X. (2014). Exosomes derived from mesenchymal stem cells. Int .J. Mol. Sci. 15, 4142–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lai, R.C., Yeo, R.W., Tan, K.H., and Lim, S.K. (2013). Mesenchymal stem cell exosome ameliorates reperfusion injury through proteomic complementation. Regen. Med. 8, 197–209 [DOI] [PubMed] [Google Scholar]
- 55. Xin, H., Li, Y., Liu, Z., Wang, X., Shang, X., Cui, Y., Zhang, Z.G., and Chopp, M. (2013). MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 31, 2737–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lakshmipathy, U., and Hart, R.P. (2008). Concise review: MicroRNA expression in multipotent mesenchymal stromal cells. Stem Cells 26, 356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang, Z.G., and Chopp, M. (2015). Promoting brain remodeling to aid in stroke recovery. Trends Mol. Med. 21, 543–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tang, X., and Sun, C. (2020). The roles of MicroRNAs in neural regenerative medicine. Exp. Neurol. 332, 113394. [DOI] [PubMed] [Google Scholar]
- 59. Xin, H., Liu, Z., Buller, B., Li, Y., Golembieski, W., Gan, X., Wang, F., Lu, M., Ali, M.M., Zhang, Z.G., and Chopp, M. (2021). MiR-17-92 enriched exosomes derived from multipotent mesenchymal stromal cells enhance axon-myelin remodeling and motor electrophysiological recovery after stroke. J. Cereb. Blood Flow Metab. 41,1131–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bramlett, H.M., and Dietrich, W.D. (2004). Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J. Cereb. Blood Flow Metab. 24, 133–150 [DOI] [PubMed] [Google Scholar]
- 61. Sulhan, S., Lyon, K.A., Shapiro, L.A., and Huang, J.H. (2020). Neuroinflammation and blood-brain barrier disruption following traumatic brain injury: pathophysiology and potential therapeutic targets. J. Neurosci. Res. 98, 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schimmel, S.J., Acosta, S., and Lozano, D. (2017). Neuroinflammation in traumatic brain injury: a chronic response to an acute injury. Brain Circ. 3, 135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Simon, D.W., McGeachy, M.J., Bayir, H., Clark, R.S., Loane, D.J., and Kochanek, P.M. (2017). The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 13, 171–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xiong, Y., Mahmood, A., and Chopp, M. (2018). Current understanding of neuroinflammation after traumatic brain injury and cell-based therapeutic opportunities. Chin. J. Traumatol. 21, 137–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xin, H., Chopp, M., Shen, L.H., Zhang, R.L., Zhang, L., Zhang, Z.G., and Li, Y. (2013). Multipotent mesenchymal stromal cells decrease transforming growth factor beta1 expression in microglia/macrophages and down-regulate plasminogen activator inhibitor 1 expression in astrocytes after stroke. Neurosci. Lett. 542, 81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tsai, M.J., Tsai, S.K., Hu, B.R., Liou, D.Y., Huang, S.L., Huang, M.C., Huang, W.C., Cheng, H., and Huang, S.S. (2014). Recovery of neurological function of ischemic stroke by application of conditioned medium of bone marrow mesenchymal stem cells derived from normal and cerebral ischemia rats. J. Biomed. Sci. 21, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang, R., Liu, Y., Yan, K., Chen, L., Chen, X.R., Li, P., Chen, F.F., and Jiang, X.D. (2013). Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J. Neuroinflammation 10, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Woodcock, T., and Morganti-Kossmann, M.C. (2013). The role of markers of inflammation in traumatic brain injury. Front. Neurol. 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhou, Y., Shao, A., Yao, Y., Tu, S., Deng, Y., and Zhang, J. (2020). Dual roles of astrocytes in plasticity and reconstruction after traumatic brain injury. Cell Commun. Signal. 18, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li, L., Chopp, M., Ding, G., Davoodi-Bojd, E., Zhang, L., Li, Q., Zhang, Y., Xiong, Y., and Jiang, Q. (2020). MRI detection of impairment of glymphatic function in rat after mild traumatic brain injury. Brain Res. 1747, 147062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Plog, B.A., Dashnaw, M.L., Hitomi, E., Peng, W., Liao, Y., Lou, N., Deane, R., and Nedergaard, M. (2015). Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J. Neurosci. 35, 518–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Karve, I.P., Taylor, J.M., and Crack, P.J. (2016). The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol. 173, 692–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chen, Y., Li, J., Ma, B., Li, N., Wang, S., Sun, Z., Xue, C., Han, Q., Wei, J., and Zhao, R.C. (2020). MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging (Albany NY) 12, 18274–18296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xin, H., Wang, F., Li, Y., Lu, Q.E., Cheung, W.L., Zhang, Y., Zhang, Z.G. and Chopp, M. (2017). Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from microRNA 133b-overexpressing multipotent mesenchymal stromal cells. Cell Transplant. 26, 243–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Taupin, P. (2006). The therapeutic potential of adult neural stem cells. Curr. Opin. Mol. Ther. 8, 225-231 [PubMed] [Google Scholar]
- 76. Yang, P., Cai, L., Zhang, G., Bian, Z., and Han, G. (2017). The role of the miR-17-92 cluster in neurogenesis and angiogenesis in the central nervous system of adults. J. Neurosci. Res. 95, 1574–1581 [DOI] [PubMed] [Google Scholar]
- 77. Xiong, Y., Mahmood, A., and Chopp, M. (2010). Angiogenesis, neurogenesis and brain recovery of function following injury. Curr. Opin. Investig. Drugs 11, 298–308 [PMC free article] [PubMed] [Google Scholar]
- 78. Pan, W.L., Chopp, M., Fan, B., Zhang, R., Wang, X., Hu, J., Zhang, X.M., Zhang, Z.G., and Liu, X.S. (2019). Ablation of the microRNA-17-92 cluster in neural stem cells diminishes adult hippocampal neurogenesis and cognitive function. FASEB J. 33, 5257–5267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Liu, X.S., Chopp, M., Wang, X.L., Zhang, L., Hozeska-Solgot, A., Tang, T., Kassis, H., Zhang, R.L., Chen, C., Xu, J., and Zhang, Z.G. (2013). MicroRNA-17-92 cluster mediates the proliferation and survival of neural progenitor cells after stroke. J. Biol. Chem. 288, 12478–12488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mogilyansky, E., and Rigoutsos, I. (2013). The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 20, 1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Xia, X., Wang, Y., and Zheng, J.C. (2020). The microRNA-17 ∼ 92 family as a key regulator of neurogenesis and potential regenerative therapeutics of neurological disorders. Stem Cell Rev Rep. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pan, Y.B., Sun, Z.L., and Feng, D.F. (2017). The role of microRNA in traumatic brain injury. Neuroscience 367, 189–199 [DOI] [PubMed] [Google Scholar]
- 83. Si, L., Wang, H., and Wang, L. (2020). Suppression of miR-193a alleviates neuroinflammation and improves neurological function recovery after traumatic brain injury (TBI) in mice. Biochem. Biophys. Res. Commun. 523, 527–534 [DOI] [PubMed] [Google Scholar]
- 84. Sun, L., Zhao, M., Zhang, J., Liu, A., Ji, W., Li, Y., Yang, X., and Wu, Z. (2017). MiR-144 promotes beta-amyloid accumulation-induced cognitive impairments by targeting ADAM10 following traumatic brain injury. Oncotarget 8, 59181–59203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Henry, R.J., Doran, S.J., Barrett, J.P., Meadows, V.E., Sabirzhanov, B., Stoica, B.A., Loane, D.J., and Faden, A.I. (2019). Inhibition of miR-155 limits neuroinflammation and improves functional recovery after experimental traumatic brain injury in mice. Neurotherapeutics 16, 216–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sabirzhanov, B., Stoica, B.A., Zhao, Z., Loane, D.J., Wu, J., Dorsey, S.G., and Faden, A.I. (2016). miR-711 upregulation induces neuronal cell death after traumatic brain injury. Cell Death Differ. 23, 654–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wen, M.M. (2016). Getting miRNA therapeutics into the target cells for neurodegenerative diseases: a mini-review. Front. Mol. Neurosci. 9, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mathiyalagan, P., and Sahoo, S. (2017). Exosomes-based gene therapy for microRNA delivery. Methods Mol. Biol. 1521, 139–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yang, Y., Ye, Y., Su, X., He, J., Bai, W., and He, X. (2017). MSCs-derived exosomes and neuroinflammation, neurogenesis and therapy of traumatic brain injury. Front. Cell Neurosci. 11, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Li, D., Huang, S., Yin, Z., Zhu, J., Ge, X., Han, Z., Tan, J., Zhang, S., Zhao, J., Chen, F., Wang, H., and Lei, P. (2019). Increases in miR-124-3p in microglial exosomes confer neuroprotective effects by targeting FIP200-mediated neuronal autophagy following traumatic brain injury. Neurochem. Res. 44, 1903–1923 [DOI] [PubMed] [Google Scholar]
- 91. Ghosh, S., Garg, S., and Ghosh, S. (2020). Cell-derived exosome therapy: a novel approach to treat post-traumatic brain injury mediated neural injury. ACS Chem. Neurosci. 11, 2045–2047 [DOI] [PubMed] [Google Scholar]
- 92. Sun, M.K., Passaro, A.P., Latchoumane, C.F., Spellicy, S.E., Bowler, M., Goeden, M., Martin, W.J., Holmes, P.V., Stice, S.L., and Karumbaiah, L. (2020). Extracellular vesicles mediate neuroprotection and functional recovery after traumatic brain injury. J. Neurotrauma 37, 1358–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gao, W., Li, F., Liu, L., Xu, X., Zhang, B., Wu, Y., Yin, D., Zhou, S., Sun, D., Huang, Y., and Zhang, J. (2018). Endothelial colony-forming cell-derived exosomes restore blood-brain barrier continuity in mice subjected to traumatic brain injury. Exp. Neurol. 307, 99–108 [DOI] [PubMed] [Google Scholar]
- 94. Ge, X., Guo, M., Hu, T., Li, W., Huang, S., Yin, Z., Li, Y., Chen, F., Zhu, L., Kang, C., Jiang, R., Lei, P., and Zhang, J. (2020). Increased microglial exosomal miR-124-3p alleviates neurodegeneration and improves cognitive outcome after rmTBI. Mol. Ther. 28, 503–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.