Abstract
COVID-19, the syndrome caused by the infection with SARS-CoV-2 coronavirus, is characterized, in its severe form, by interstitial diffuse pneumonitis and acute respiratory distress syndrome (ARDS). ARDS and systemic manifestations of COVID-19 are mainly due to an exaggerated immune response triggered by the viral infection. Cytokine release syndrome (CRS), an inflammatory syndrome characterized by elevated levels of circulating cytokines, and endothelial dysfunction are systemic manifestations of COVID-19. CRS is also an adverse event of immunotherapy (IMTX), the treatment of diseases using drugs, cells, and antibodies to stimulate or suppress the immune system. Graft-versus-host disease complications after an allogeneic stem cell transplant, toxicity after the infusion of chimeric antigen receptor-T cell therapy and monoclonal antibodies can all lead to CRS. It is hypothesized that anti-inflammatory drugs used for treatment of CRS in IMTX may be useful in reducing the mortality in COVID-19, whereas IMTX itself may help in ameliorating effects of SARS-CoV-2 infection. In this paper, we focused on the potential shared mechanisms and differences between COVID-19 and IMTX-related toxicities. We performed a systematic review of the clinical trials testing anti-inflammatory therapies and of the data published from prospective trials. Preliminary evidence suggests there might be a benefit in targeting the cytokines involved in the pathogenesis of COVID-19, especially by inhibiting the interleukin-6 pathway. Many other approaches based on novel drugs and cell therapies are currently under investigation and may lead to a reduction in hospitalization and mortality due to COVID-19.
Keywords: immunotherapy, COVID-19, inflammation mediators
Introduction
COVID-19 is a recent global public health catastrophe with substantial mortality and morbidity across the globe, caused by a novel beta coronavirus popularly known as SARS-CoV-2. SARS-CoV-2 has infected over 144 million people and caused approximately 3 million deaths globally, as of April 22, 2021.1 The most prominent clinical manifestation of SARS-CoV-2 infection is acute respiratory distress syndrome (ARDS), which is also the primary cause of admission to intensive care units (ICUs). The viral replication and the inflammatory events occurring within the lung are also thought to be critical for initiating many other extrapulmonary manifestations of COVID-19. SARS-CoV-2 RNA has been isolated from many organs and virtually all body fluids.2 COVID-19 is often characterized by extrapulmonary involvement and signs of systemic inflammation, potentially leading to multiorgan failure and death.3 4 Interestingly, even after SARS-CoV-2 is controlled or cleared, patients remain in the hospital with inflammatory cytokines elevated and with elevated inflammatory cytokines and ongoing pulmonary damage.4
Immunotherapy (IMTX), defined here as any treatment using drugs, immune cells or antibodies to stimulate or suppress the immune system, is an emerging field in cancer therapy and infectious diseases.5 6 IMTX has produced impressive response rates in select patients with relapsed and refractory cancers; however, the toxicity profile of some of these approaches, such as chimeric antigen receptor-T cells (CAR-T cells), still represents a major limitation in their widespread use.7 A potentially fatal complication after IMTX is a condition referred to as ‘cytokine storm’ or ‘cytokine release syndrome’ (CRS), characterized by fever, hypotension, and respiratory failure in the presence of elevated cytokine and inflammatory markers.8 Many drugs have been successful in the treatment of CRS after IMTX, and many serologic markers are currently available to confirm the diagnosis and to monitor the therapeutic response. Systemic manifestations of COVID-19 and toxicity following IMTX may share similar pathophysiologic mechanisms. Therefore, the management of IMTX-related toxicities could be used as a paradigm for treating COVID-19 complications, and IMTX may have a potential role in the treatment of SARS-CoV-2 infection. In this review, we will compare these two clinical scenarios and potential opportunities to leverage IMTX in treating patients with COVID-19.
Pathways of inflammation in COVID-19 infection and immunotherapy: parallels and differences
SARS-CoV-2 triggers severe inflammation initiated in the lung
SARS-CoV-2 recognizes the protein ACE2 expressed on the surface of the epithelial cells of the respiratory tract. The viral protein that mediates the adhesion and the recognition of ACE2 is the spike protein.9 After initial replication of the virus in the upper respiratory tract, viral replication can spread to the lower respiratory tract and cause pneumonia and ARDS. The majority of patients hospitalized for COVID-19 infections present with signs of pulmonary disease, including pneumonia and ARDS.10 Early signs and symptoms of lung involvement in SARS-CoV-2 infection are fever, tachypnea, low oxygen saturation, shortness of breath, and dry cough.11 Other symptoms include dysphagia and coryza.12 A substantial proportion of patients, ranging from 20% to 33%, require admission to an ICU.13 ARDS is the most frequent cause of admission to ICU and the major cause of mortality.14 Diffuse alveolar damage is the most common histologic finding; the blood–air interface is damaged, resulting in inflammation and thickening of the mucosa during the acute phases of infection. Furthermore, microvascular thrombosis and pleural effusion, two common postmortem findings, contribute to the COVID-19 acute respiratory syndrome.15 Similar pathology is seen in patients receiving monoclonal antibodies (mAbs) targeting the tumor microenvironment by engaging the receptors PD-1 and CTLA4, the so called ‘checkpoint inhibitors’ (CPIs),16 to revert T cell anergy induced by cancer cells. A rare but significant side effect of CPI, especially nivolumab, is severe inflammatory interstitial pneumonitis showing clinical and radiologic features resembling lung involvement in COVID-19.17 The exact mechanism of CPI-related pneumonitis is unclear, but it has been suggested that pulmonary dendritic cells and macrophages are immunologically regulated by PD-1 positive T cells.18 The similarity between CPI-related pneumonitis and pulmonary manifestations of COVID-19 could be explained by a common pathway involving PD-1 and the toll-like receptors (TLRs): in fact, the stimulation of TLRs on CD8+ T lymphocytes decreases the expression of PD-1,19 and the SARS-CoV-2 spike protein has been demonstrated to bind to TLR and stimulate the production of inflammatory cytokines.20
The role of endothelial dysfunction in COVID-19 and toxicities after immunotherapy
From the initial stages, COVID-19 is accompanied by a coagulopathy characterized by elevated levels of D-dimer and fibrinogen, with minimal changes in prothrombin time, activated partial thromboplastin time, and platelet counts.21 22 D-dimer levels at admission and their increase throughout the hospitalization were both correlated with higher mortality.12 23 24 Thrombosis is recognized as a major cause of morbidity and mortality among patients with COVID-19 as it occurs at all levels of the circulatory system22 25 26 and in intravenous catheters and extracorporeal circuits.27 The complement cascade is also activated and contributes to microvascular damage and thrombosis.28 29 The rates of thromboembolic complications in critically ill patients with COVID-19 were shown to be higher than in other patients hospitalized in ICU for other causes,30 despite the use of prophylactic anticoagulation. SARS-CoV-2 infection outside of the respiratory system seems to follow the abundance of ACE2 beyond the respiratory tract; tropism to renal, myocardial, and gastrointestinal tissues have been described,31 likely because endothelial cells in artery and veins throughout the body express ACE2 on their surface.32 The invasion of lung endothelial cells by SARS-CoV-2 represents a crucial point in the worsening of pneumonitis and the transmission to other organs. The resulting endothelialitis can trigger excessive thrombin production in multiple organs and inhibit fibrinolysis; the complement pathway is also activated, leading to the deposition of microthrombi and microvascular dysfunction.33 As a result, the histopathologic findings of this process are neutrophil extracellular traps, fibrin deposits and/or microthrombi.34 Inflammation itself can be responsible for the procoagulative status in COVID-19 as many proinflammatory cytokines are able to activate the coagulation system. In vivo, high levels of tumor necrosis factor (TNF-α), interleukin (IL)-6 and IL-1 were detected in patients with acute inflammatory conditions35 together with a hypercoagulable status, sometimes evolving into diffuse intravascular coagulation.36 Data from a preliminary study demonstrate that SARS-CoV-2 may directly promote platelet adhesion and aggregation.37 Therefore, a parallel can be drawn between endothelial involvement in COVID-19 and post-CAR-T toxicity. Hay et al reported increased levels of circulating endothelial-derived factors, such as von Willebrand and angiopoietin-2, as a sign of endothelial activation, and they also found a correlation with subsequent development of CRS.38
Systemic inflammation and CRS in COVID-19 and immunotherapy
The propagation of the infection and the hyperinflammatory response observed in many individuals affected by COVID-19 depend on the interplay between SARS-CoV-2 and cytokine production. During the first phases of infection, SARS-CoV-2 may antagonize the host’s interferon (IFN) response39 and induce downregulation of the expression of major histocompatibility complex class I molecules on the surface of many cells.40 This promotes the escape of the virus from immune recognition and delays its clearance. Lymphopenia is a common finding in COVID-19 and may also contribute to uncontrolled viral replication.31 The accumulation of infected cells across different tissues and the hyperstimulation of components of the innate immune system may trigger inappropriate production of cytokines, leading to CRS and causing further activation of the coagulation and complement cascades. CRS may, in some cases of COVID-19, evolve into hemophagocytic lymphohistiocytosis (HLH), a severe multisystemic inflammatory syndrome characterized by a specific hyperactivation of macrophages, high levels of circulating cytokines, hyperbilirubinemia, and hemolysis, which is associated with high mortality.10 41
CRS is a frequent complication of many immunotherapy approaches. Hematopoietic stem-cell transplantation (HSCT) from familiar haploidentical donors can be complicated by CRS, and CRS may occur in up to 90% of patients receiving stem cells from peripheral blood.42 The transplant-related inflammatory syndrome, graft-versus-host disease (GVHD), occurs when donor-derived T cells (the graft) recognize the recipient (the host) as foreign.43 GVHD presents clinically as acute and chronic (cGVHD) with an estimated incidence of 30%–50% and 40%, respectively.43 44 Both forms can be localized or can involve multiple systems and lead to CRS. HLH can be a rare and fatal complication of HSCT, potentially related to GVHD, triggered by other mechanisms such as persistent Epstein-Barr virus reactivation.45
CAR-T cells, engineered to recognize tumor antigens through a fusion protein comprised of an antibody-like moiety and T cell-signaling domains,46 are frequently associated with CRS. In most clinical trials of CAR-T cell therapy for acute B-cell leukemia, the most common severe toxicity was CRS with an overall incidence of 70% and high-grade CRS reported in 15% of patients.47 In non-Hodgkin’s lymphomas, CRS seemed to occur at a more variable rate (24%–95%).48 Fever is the most common initial manifestation of CRS; others may include hypotension, cardiac involvement, dyspnea, hypoxia, and other organ dysfunction.8 Although post-CAR-T cell toxicities can resolve spontaneously, most require treatment with anti-inflammatory drugs, and severe cases require intensive care.
Similarly to CAR-T cells, mAbs49 and bispecific antibodies, such as blinatumomab, can cause CRS.50
Circulating cytokines as markers of disease severity and response to therapies: similarities and differences between COVID-19 and immunotherapy
Recent meta-analyses identified pathways of inflammatory cytokines and chemokines, including IL-6, IL-1b, and TNF-α, that were significantly elevated in patients with severe COVID-19 and led to CRS.51 IL-6 plays an important role in autoimmune diseases and is recognized as a crucial proinflammatory cytokine.52 IL-6 exerts its effects on cells expressing the functional IL-6 receptor (IL-6R), such as T cells, B cells, monocytes, endothelial cells, and hepatocytes.53 IL-6 is essential for recovery from multiple viral infections, but overproduction of IL-6 may be detrimental to viral clearance and survival. The main source of IL-6 during COVID-19 remains elusive; on binding to IL-6R on the surface of target cells, the transduction of the intracellular signal leads to the phosphorylation of the JAK/STAT3 axis.54 A number of publications reported an association between increased IL-6 in peripheral blood and a severe response to SARS-CoV-2, defined as ARDS, CRS, and extrapulmonary damage.55 Post-CAR-T CRS shows remarkably high serum concentrations of many inflammatory cytokines, especially IL-6. In CAR-T-related CRS, the release of IL-1 seems to precede that of IL-6; therefore, targeting IL-1 might mitigate or prevent CRS.56 IL-6 production was also exhibited after treatment of B cell malignanices with mAbs (rituximab)54 and the bispecific antibody blinatumomab.57
In a cohort of 69 patients hospitalized with SARS-CoV-2 infection, serum IL-6 concentrations did show a correlation with the severity of the pulmonary disease whereas other cytokines such as IL-2 and IL-4 did not.58 A large meta-analysis of 25 COVID-19 studies reports an estimated mean for IL-6 concentrations of 36.7 pg/mL which is nearly a 100-fold difference when compared with four studies on CAR-T cell-induced CRS showing a mean IL-6 of 3110.5 pg/mL.51 A major limitation of the study may be due to the heterogeneity in the assays used for measuring IL-6 and of the timepoints at which the samples were collected, even though biological differences between COVID-19 and post-CAR-T toxicities are present. First, in patients with COVID-19, IL-6 production is related either directly or indirectly to the viral infection, even if no direct correlation with the viral load has been confirmed, whereas there is no underlying viral replication in patients experiencing CRS after CAR-T cells. Furthermore, IL-6 peaks are transient in CAR-T patients, especially if controlled by therapy, whereas IL-6 levels remain high for a long time in patients with COVID-19.4 Thus, IL-6 may represent both a biomarker and a potential therapeutic target for patients hospitalized with COVID-19, which is an attractive concept in the absence of alternative direct-acting antiviral strategies. Proposed clinical cut-off values for IL-6 in this setting have started to emerge, although the sample size of the already published studies is still relatively small.59
Other markers including TNF-α, and IFN-γ, and the soluble IL-2 receptor were comparatively lower in COVID-19 than in CAR-T toxicity.51 Despite their relatively lower serum levels, a recent study showed a potential benefit in early blockage of TNF-α/IFN-γ axis in COVID-19.60 In another study, combined TNF-α and IL-6 levels at the moment of hospitalization for COVID-19 were proposed as an early predictive marker of mortality.4 Ferritin, a marker of macrophage activation, increases substantially in patients with severe CAR-T-related CRS and was also found to be elevated in COVID-19.61
In summary, despite the differences in the pathophysiology and in the mean serum levels of some cytokines, COVID-19 shares the elevation of IL-6 with CRS due to CAR-T infusions and, in general, shows an inflammatory profile more similar to IMTX-related CRS than to systemic inflammation of other causes.
Immune-related effects of COVID-19 and immunotherapy on the central nervous system and other organs
Similar to other infections with respiratory coronaviruses, COVID-19 has shown multiple neurological manifestations.62 In an analysis of 214 patients with severe COVID-19, the incidence of neurologic symptoms was approximately 30%.63 Various non-specific mild neurological symptoms were reported in up to 40% of hospitalized patients with COVID-19.64 65 While direct invasion of the central nervous system (CNS) and microthrombosis play a role in the neurologic complications, immune dysfunction and increased cytokine production can also contribute to CNS damage.66–68 The suggested mechanisms related to CNS damage in COVID-19 have also been recognized as a typical immune-mediated complication of CAR-T cells and is referred to as immune effector cell-associated neurotoxicity syndrome (ICANS), which occurs in up to 65% of patients.69 70 In ICANS, cytokines cause an alteration of the blood–brain barrier.71 Most cases of neurotoxicity and CRS are reversible with targeted treatment and supportive care. Rituximab, an anti-CD20 antibody used in the treatment of B cell lymphomas, can cause posterior reversible encephalopathy, a rare clinical condition manifesting with headache, altered level of consciousness, convulsions, visual loss, and reversible cerebral edema visible on brain MRI.49
Finally, similar to many other viruses, systemic manifestations of COVID-19 due to involvement of the gastrointestinal system, liver, kidneys, heart, endocrine system, and the skin are reported relatively frequently.36 72–76 COVID-19 can also cause a systemic inflammatory syndrome similar to some autoimmune diseases.77 Although a limited proportion of all patients with COVID-19 worldwide are under the age of 15 years, several reports from European and US groups have reported cases of children with a severe inflammatory syndrome similar to Kawasaki disease or toxic shock syndrome.78
What we have learned from immunotherapy that can be used for treating COVID-19
A detailed description of the methods that we used for performing this search is outlined in online supplemental 1. Table 1 shows a list of the treatments and targets currently under investigation for COVID-19.
Table 1.
Shared mechanisms of action between COVID-19 and immunotherapy, and potential common therapeutic strategies
| Molecular target | Role in immunotherapy | Role in COVID-19 | Available drugs | Trials in COVID-19 | Results in COVID-19 | Comments |
| IL-6 | Elevation during CRS following CAR-T and mAb infusion.119 | Elevated levels correlate with severity of disease55 58 | Tocilizumab Sarilumab128 |
Active trials: 38 Completed: 8 Active trials: 9 Completed: 3 |
Recommended in recently hospitalized patients with severe respiratory disease in combination with Dex.123–125 127 No clear evidence in patients requiring hospitalization.128 The SARICOR Study is testing whether the administration of sarilumab (an IL-6 receptor inhibitor) in hospitalized patients with COVID-19, pulmonary infiltrates and a high IL-6 or D-dimer serum level could reduce the progression of ARDS.145 |
Clear cut-off of IL-6 levels still have to be determined.51 The NIH COVID-19 Treatment Guideline Panel recommends tocilizumab+Dex to recently hospitalized patients (not in the ICU) with rapidly increasing oxygen needs and who have significantly increased markers of inflammation. |
| JAK/STAT intracellular signaling | Approved for steroid-refractory GVHD.131 | JAK kinases are activated after stimulation of IL receptors | Baricitinib Ruxolitinib |
Active trials: 9 Completed: 1 Active trials: 16 Completed: 1 |
Better outcome for hospitalized patients in association with remdesivir compared with remdesivir alone.134 Apparent efficacy at reducing progression of ARDS and need for respiratory support.132 |
Now recommended for hospitalized patients in combination with remdesivir Potential toxic effects on the bone marrow |
| IL-1 | In CAR-T-related CRS, the release of IL-1 seems to precede that of IL-6.56 | IL-1b was significantly elevated in patients with severe COVID-19146 | Anakinra | Active trials: 14 Completed: 2 |
Evaluating the efficacy in reducing the number of patients requiring mechanical ventilation | IL-1 may precede the production of IL-6 in COVID-19, as it seems to happen during CAR-T toxicity |
| IFN-γ | Blocking IFN is approved for familiar HLH, not approved for post-IMTX CRS. | Potential benefit in early blockage of TNF-α/IFN-γ axis in COVID-1960 | Emapalumab | Active trials: 1 Completed: 0 |
Comparing anakinra to emapalumab which demonstrated efficacy in pediatric HLH137 | Data in COVID-19 are insufficient to draw a conclusion |
| TNF-α | Largely used in the treatment of severe forms of autoimmune diseases. | TNF-α is elevated in early phases of disease51 | Infliximab | Active trials: 1 Completed: 1 |
An anti-TNF-α mAb used in autoimmune diseases has shown effectiveness in treating patients with COVID-19138 | Potential high risk of bacterial superinfection |
| Plasmin | Dysregulated in SOS/VOD and DIC for all causes.141 | Hypercoagulation is secondary to endothelialitis and ACE2-dependent hypofibrinolysis33 | Defibrotide | Active trials: 4 Completed: 0 |
No results from small observations or trials so far | Potential benefit in association with other anticoagulants |
| C5 protein of the complement | Hyperactivated during TA-TMA and HUS.147 | Both C3 and C5 inhibitors showed a robust anti-inflammatory response, reflected by a steep decline in IL-6 levels, marked lung function improvement, and resolution of SARS-CoV-2-associated ARDS144 | Eculizumab | Active trials: 1 Completed: 0 |
Strong anti-inflammatory response in a small trial144 | Potential benefit in patients with consumption of complement factors and signs of microthrombosis and kidney injury. Contraindicated if there is bacterial infection |
| All pro-inflammatory cytokines | CRS and ICANS are associated with remarkably high serum concentrations of many inflammatory cytokines. | Many proinflammatory cytokines are produced after SARS-CoV-2 infection. They cause tissue damage, promote NETs, and activate the coagulation and complement systems. | Dexamethasone (Dex) | Active trials: 21 Completed: 2 |
At the time of writing, Dex is the only drug that has shown a reduction in mortality due to COVID-19116 | Current recommendation for Dex in COVID-19 is restricted to patients experiencing initial ARDS and signs of CRS |
| Mesenchymal cells (MSC) | Active trials: 49 Completed: 5 |
First trials show capacity of MSC to attenuate lung and systemic inflammation96 148 | Some limit due to the time of manufacturing, but potential use of third-party sources for off-the-shelf production | |||
| Spike protein of SARS-CoV-2 | No recognized role of ACE2 receptor in IMTX. | It is the protein that mediated the entry of the viral particles into the cells149 | mAbs | Active trials: 33 Completed: 1 |
Chi et al111 isolated, from convalescent patients with COVID-19, 3 mAbs displaying neutralization against SARS-CoV-2 | Utility for treating patients until a vaccine will be available, and then to transfer adoptive immunity to immunosuppressed patients |
| Anti-spike CAR-T cells | Active trials: 0 Completed: 0 |
No results from anti-spike CAR-T so far | CAR-T cells are very successful in the treatment of hematologic tumors,150 but there remain many complications, including severe CRS and ICANS, that have discouraged their use in COVID-19 | |||
| Anti-spike NK cells | Active trials: 6 Completed: 0 |
NK and CAR-NK cells seem to control viral replication without increased risk of CRS75 | Compared with CAR-T, the use of wild-type NK and NK-CAR cells seems to be safer even if there is no definitive answer about their efficacy | |||
| Anti-spike TCR-engineered cells | Active trials: 0 Completed: 0 |
No data available | TCR-engineered T cells have the same limitations of CAR-T for the treatment of active disease. However, as anti-viral mAbs, they might be useful in the future to confer adoptive cell-mediated immunity against SARS-CoV-2 in immunocompromised patients.151 |
ARDS, acute respiratory distress syndrome; CAR-T, chimeric antigen receptor-T cell; CRS, cytokine release syndrome; DIC, diffuse intravascular coagulation; GVHD, graft-versus-host disease; HLH, hemophagocytic lymphohistiocytosis; HUS, hemolytic uremic syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; ICU, intensive care unit; IFN-γ, interferon γ; IL, interleukin; IMTX, immunotherapy; mAb, monoclonal antibody; NETs, neutrophil extracellular traps; NIH, National Institutes of Health; NK, natural killer; SOS/VOD, sinusoidal obstruction syndrome/veno-occlusive disease; TA-TMA, transplant-associated thrombotic microangiopathy; TCR, T cell receptor; TNF-α, tumor necrosis factor-α.
jitc-2021-002392supp001.pdf (110.1KB, pdf)
Cellular immunotherapy for COVID-19
Cellular immunotherapy may be used in treating COVID-19 by either targeting virus-infected cells or modulating the inflammatory pathways leading to CRS. However, no studies have reported treatment with SARS-CoV-2-specific T cells to date. T cell clones specifically recognizing SARS-CoV-2 were identified from convalescent patients and their use was proposed for the prevention or early treatment of SARS-CoV-2 infection in immunocompromised patients with blood disorders or after bone marrow transplantation.79 Banks of allogeneic, third-party ‘off-the-shelf’ T cell products could be considered for timely administration of CAR-T cells, but much remains unknown about safety and efficacy of this approach at this time, with primary concens for triggering or worsening CRS or ICANS. An alternative technique to redirect the immune system toward antigens of interest involves the use of T cells carrying an engineered tumor-recognizing T cell receptor (TCR). TCR-engineered T cells have shown efficacy in solid tumors as melanoma, esophageal cancer, synovial sarcoma, and Wilms tumor,80 with encouraging results in acute myeloid leukemia.81 In the majority of clinical trials, the infusion of ex vivo expanded TCR-modified cells was well tolerated.82 However, there are some data to suggest TCR-engineered cells may cause adverse on-target, off-tumor effects, as well as off-target effects. In the field of antiviral therapy, TCR-engineered T cells can be used to target viral antigens, such as for cytomegalovirus, and offer an exciting approach to improve the treatment or prevention of viral infections in high-risk settings such as T cell-depleted HSCT and delayed immune reconstitution.83 So far, TCR-engineered T cells against SARS-CoV-2 antigens have not been investigated and may share some of the limitations as CAR-T cells.
Natural killer (NK) cells, an important component of the innate immune system, play a pivotal role in immune surveillance84 and can also be used as adoptive IMTX. Haploidentical and third party-expanded NK cells have been used for relapsed and refractory myeloid leukemia, either as post-HSCT infusion or as isolated therapy, showing good response rates and very low rates of toxic effects.85 As an alternative to CAR-T cells, NK cells engineered to express a CAR (CAR-NK) are candidate effectors for cancer treatment. NK cells can be generated off the shelf from an allogeneic source, such as cord blood, to be safely administered without the need for full human leukocyte antigen matching and eliminating the need to produce a unique CAR product for each patient.86 An in vitro study indicates ‘off-the-shelf’ generated anti-SARS-CoV-2 CAR-NK cells exhibit a high efficacy at targeting and killing cells infected with SARS-CoV-2.87 One phase II/III trial testing the in vivo potential of these cells is open and recruiting in China. Other trials are studying the effects of cryopreserved, allogeneic, off-the-shelf, non-transgenic NK cells as a means of restoring the depressed NK activity observed during COVID-19.88 Treatment with NK cells derived from human placental CD34+ cells and culture-expanded (CYNK-001) are also being investigated in a clinical trial (ClinicalTrials.gov Identifier: NCT04365101).
Mesenchymal stromal cells (MSCs) are a heterogeneous population of stromal cells found throughout the body that migrate to specific tissues in the context of remodeling and regeneration. They reportedly play an immunomodulatory role in tumor microenvironment, also contributing to local tumor aggressiveness in some hematological malignancies.89–91 Due to their anti-inflammatory effects and their interaction with macrophages, many trials use MSC to treat aggressive GVHD,92 but in some cases MSCs were also enginereed to hyperexpress cytokines as IFN-γ and inoculated within tumor tissue.93 The administration of systemic third-party MSC in patients with steroid refractory acute and chronic GVHD showed response rates up to 70%.94 Cell therapy with MSCs is an appealing approach to target many inflammatory patterns altogether. Results from two small trials in patients with COVID-19 showed clinical and radiological improvement after MSC infusion with no toxic effects.95 96 Another trial showed that exosomes isolated from MSC and infused intravenously can help in mitigating severe manifestations of COVID-19.97 Forty-nine clinical trials are testing the infusion of MSC derived from autologous or allogeneic adipose tissue, dental pulp, bone marrow, or from cord blood Wharton’s jelly for the treatment of COVID-19.
Antibody-based immunotherapy to target the virus–cell interaction
Neutralizing antibodies can block the transmission of the SARS-CoV-2 and its spread throughout the body. Targeting the SARS-CoV-2 spike protein seems to be the most efficient strategy in preventing virus–cell interactions. Several vaccines encoding the stabilized SARS-CoV-2 spike protein recently showed promising efficacy in terms of inducing a protein-specific antibody response with an acceptable rate of adverse events.98–102 Nonetheless, strategies to rapidly block the infection are still needed for patients who are currently affected by COVID-19 and for the patients who will not respond to the vaccine in the future. Plasma pooled from volunteers who recovered from the SARS-CoV-2 infection was reported to be effective in some studies,103 including in a recent randomized trial showing efficacy if given within the first 72 hours from the onset of symptoms.104 However, contrasting evidence has emerged from other publications, including a prospective trial on more than 300 patients, that failed to demonstrate superiority of convalescent plasma over placebo.105 The major limitations of this approach are the availability of donors, safety concerns for allergic reactions, and the use of blood-derived products. Another limitation is that by the time the patients are sick, neutralizing antibodies are already present,106 and the value of adoptively transferred immunoglobulins becomes more limited; conversely, non-neutralizing antibodies produced by B cells may enhance SARS-CoV-2 infection through antibody-dependent enhancement, further exacerbating organ damage.107 Approaches using polyclonal intravenous immunoglobulins (IVIGs) collected from the general population, which are beneficial in some autoimmune conditions,108 did not show clear evidence of benefit for ameliorating ARDS or mitigating CRS in patients with COVID-19.109 Data from several clinical trials will clarify soon whether IVIG can be considered for hospitalized patients. IVIG might be contemplated for the treatment of the rare Guillain-Barre syndrome during the course of COVID-19.110
The synthesis of anti-SARS-CoV-2 mAbs on a large scale would be more cost-effective and sustainable compared with donor-derived antibodies. Identifying the right viral epitope to target is the first step for a successful strategy. Chi et al isolated, from convalescent patients with COVID-19, three mAbs displaying neutralization against SARS-CoV-2.111 Several other synthetic mAbs are already being tested in clinical trials all around the world. One being investigated in two trials is the combination of two mAbs, REGN10987 and REGN10933, called ‘REGN-COV2’, which showed efficacy and good toxicity profile in non-human primates and hamsters.112 The trial has stopped the enrollment of patients hospitalized with severe COVID-19 for lack of efficacy, but the Food and Drug Administration (FDA) has approved the use of REGN-COV2 cocktail for adult patients with recently diagnosed mild to moderate COVID-19 at high risk of progression.
Targeting the cytokine-mediated immune dysfunction in COVID-19: corticosteroids, mAbs, and selective inhibitors borrowed from immunotherapy
Corticosteroids such as dexamethasone are potent anti-inflammatory drugs that are broadly used in the treatment of immune-mediated diseases, including autoimmune diseases, GVHD, CAR-T toxicity, toxic effects of mAbs and sepsis.113 114 Since the early days of the SARS-CoV-2 pandemic, many groups have published reports and results of prospective trials using dexamethasone in patients with COVID-19. Many of these trials showed an advantage of using corticosteroids; among them, the RECOVERY trial, a controlled open-label trial comparing oral and intravenous dexamethasone (at a dose of 6 mg once daily) for up to 10 days versus usual care alone. The RECOVERY trial showed a lower 28-day mortality among patients of the dexamethasone group who were receiving either invasive mechanical ventilation or oxygen at randomization, but not among those receiving no respiratory support.115 A meta-analysis of seven randomized trials on 1703 critically ill patients with COVID-19 confirmed the role of dexamethasone in reducing the mortality at day 28.116 At the time of writing, dexamethasone is the only drug that has shown a reduction in mortality due to COVID-19. There are still unanswered questions as to why the dosage and timing of administration are crucial to the outcome, especially in severe cases. In fact, administering glucocorticoids too early may favor the viral replication, but prolonged administration might delay viral clearance. For this reason, the current recommendation for dexamethasone in COVID-19 is restricted to patients experiencing initial ARDS and signs of CRS. Since dexamethasone has been shown to be effective in reducing IL-6,4 an early measure of serum IL-6 on patients’ admission to the hospital might be used in identifying patients at higher risk of CRS and given dexamethasone immediately. Currently, 21 clinical trials are geared towards addressing those questions.
The ability of mAbs to block cytokines, TNF-α and ILs is of particular interest when treating chronic inflammatory conditions such as autoimmune diseases, transplant rejection, and GVHD.117 IL-6 and its receptor, IL-6R, are druggable targets of interest in CRS of any origin. The anti-IL-6R mAb, tocilizumab, was developed two decades ago for the treatment of severe autoimmune diseases.118 CAR-T and mAbs can cause CRS by inducing a massive release of IL-6, and tocilizumab is now the standard of care in the management of severe CRS after CD19-specific CAR-T cells.119 In COVID-19, real-world experience with tocilizumab has shown that a substantial proportion of patients with severe pneumonia and fever improved clinically and radiologically. Increased laboratory parameters such as C reactive protein in patients with COVID-19 appear to decrease significantly with tocilizumab, and lymphocyte levels also return to normal.120–122 Worldwide, as of March 22, 2021, 38 clinical trials evaluating the effectiveness and safety of tocilizumab for treating patients with COVID-19 pneumonia are open, and 8 studies have been completed. In October 2020, the results of three prospective trials were published. A randomized North American study failed to demonstrate any superiority of tocilizumab over placebo in terms of required intubation, clinical respiratory improvement, and survival.123 Another multicenter trial from Italy showed similar results.124 Interestingly, a French group reported a reduction in the probability of non-invasive ventilation, mechanical ventilation, or death by day 14, but no difference on day 28 mortality.125 A large multicenter non-randomized cohort study showed higher overall survival with tocilizumab given in the first 2 days of ICU admission,126 but recent results from a phase 3 randomized controlled trial did not show superiority of tocilizumab over a placebo group.127 Another anti IL-6 antibody that has been proposed is sarilumab; despite some positive preliminary evidence, a phase 3 trial has failed to demonstrate a benefit of the drug over a placebo in a large cohort of hospitalized patients requiring oxygen.128 Based on all the published data, the National Institutes of Health COVID-19 Treatment Guideline Panel currently recommends the early use of tocilizumab in combination with corticosteroids for certain hospitalized patients who exhibit rapid respiratory decompensation, unless contraindications such as use of immunosuppressive drugs, severe neutropenia, and active bacterial or fungal infection are present.
Another interesting target to block the inflammatory signals might be the downstream pathway of IL-6R as the JAK kinases are phosphorylated after IL-6 binds to its receptor, and they are responsible for translating the signal.129 Ruxolitinib, a JAK inhibitor, is used in chronic myeloid neoplasms130 and in the treatment of steroid-refractory cGVHD.131 In a retrospective study and case series, ruxolitinib was shown to reduce the progression of ARDS and need of respiratory assistance in COVID-19.132 Sixteen trials using ruxolitinib are currently ongoing, with one phase 3 randomized clinical trial completed. Baricitinib, another JAK inhibitor, is currently investigated in nine trials. The results of a large prospective trial on 1000 patients were recently published, and when compared with remdesivir alone,133 the association of baricitinib+remdesivir showed efficacy in reducing recovery time and accelerating improvement in clinical status among patients with COVID-19, most notably among those receiving high-flow oxygen or non-invasive ventilation.134
Anakinra is an antagonist of the IL-1R approved for the treatment of severe rheumatoid arthritis.135 Clinical trials showed efficacy of anakinra in treating neurotoxicity (ICANS) after CAR-T cell infusion.56 Huet et al reported lower rates of ICU admissions, respiratory support, and death in 52 consecutive patients who received anakinra when compared with a historical cohort.136 Currently, 14 clinical trials are evaluating the efficacy of anakinra in reducing the number of patients requiring mechanical ventilation. One of them is comparing anakinra with emapalumab, an anti-IFN-γ mAb that demonstrated efficacy in pediatric HLH.137
Alternative targets: other cytokines, coagulation, and complement
Infliximab,138 an anti-TNF-α mAb used in auotimmune diseases, has shown effectiveness in treating patients with COVID-19. Surprisingly, only three clinical trials are targeting TNF-α in the COVID-19 setting. Few trials are also testing a mAb against IL-8; other authors have suggested a role for the IL-33-ST2 axis in the chain of events leading to severe COVID-19,139 but there are no clinical trials currently targeting these molecules. There are also no trials combining anti-IL-6 and anti-TNF-α, despite both cytokines being independently associated with mortality. These two cytokines have different pathways, and the anti-inflammatory effect might be more potent if they are targeted in combination. Other trials are testing the benefit of direct administration of cytokines such as IL-7 with the aim of modulating the inflammation and increasing CD4+ and CD8+ counts, based on encouraging results from a Belgian pilot trial.140
Four trials are investigating the role of defibrotide, the only approved drug for the treatment of sinusoidal obstruction syndrome/veno-occlusive disease, which is a syndrome characterized by endothelial dysfunction occurring after HSCT.141 However, there are no preliminary data available about the utility of this approach. Targeting the complement with eculizumab, an anti-C5 mAb used in the treatment of hemolytic uremic syndrome and paroxysmal nocturnal hemoglobinuria,142 143 and with the anti-C3 AMY-101144 may be an effective alternative strategy, as suggested by preliminary results.
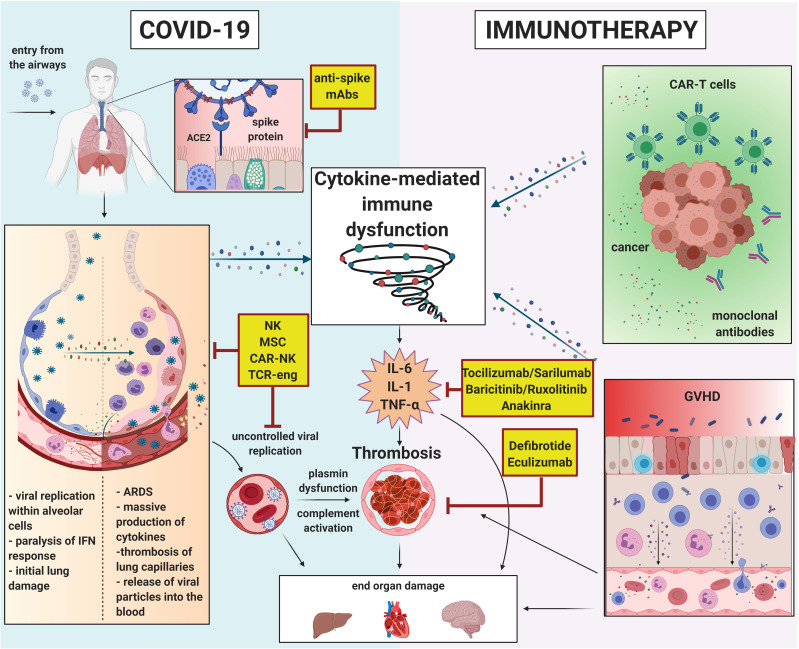
A summary of all the above-described mechanisms is outlined in figure 1.
Figure 1.
Pathways of immune dysfunction in COVID-19 and immunotherapy-related adverse events. ARDS, acute respiratory distress syndrome; CAR-T, chimeric antigen receptor-T cell; GVHD, graft-versus-host disease; IFN, interferon; IL, interleukin; mAbs, monoclonal antibodies; MSC, mesenchymal stromal cell; NK, natural killer; TCR, T cell receptor; TNF-α, tumor necrosis factor-α. Created with BioRender.com.
Final considerations
COVID-19 is an inflammatory disease caused by a viral infection. The ultimate strategy to stop the SARS-CoV-2 pandemic is a vaccine campaign covering the majority of the population worldwide. A variety of vaccines have been recently approved in different countries and are increasing in availability to high-risk and low-risk individuals. Until then, social distancing measures will remain the most efficient way to limit the spread of the disease and protect the most sensitive individuals. Immunotherapeutic approaches to modulate the immune system may help to treat patients with COVID-19 by mitigating viral replication and stemming the cascade of inflammatory events induced by SARS-CoV-2.
To date, a few immunomodulating therapies have been successful in the treatment of COVID-19. Corticosteroids have demonstrated efficacy in reducing the mortality in patients with COVID-19 with ARDS, and the FDA approved the use of the REGN-COV2 mAb cocktail for patients with recent infection, mild symptoms, and high risk of progression. Baricitinib, an anti-JAK agent, is now recommended in association with remdesivir for hospitalized patients. Tocilizumab in combination with dexamethasone has recently received recommendation for some group of patients with rapidly evolving respiratory disease. Other anti-IL-6 agents, such as sarilumab, have shown promising preliminary results, as well as ruxolitinib and other anti-inflammatory mAbs such as anakinra. Cell therapy and other inhibitors, such as defibrotide and eculizumab, have potential for benefit and are being tested in many ongoing clinical trials. Stratification of patients based on values of the cytokines that appear more involved in the underlying COVID-19 inflammatory process might expand our knowledge on the before-mentioned drugs in specific subgroups. The rapid development of several anti-SARS-CoV-2 vaccines, and the volume of clinical trials testing anti-inflammatory drugs for COVID-19 based on what we have learned from immunotherapy, highlight the capability of modern medicine to produce high-quality evidence and offer effective strategies in the middle of a pandemic.
Footnotes
FM and JAH contributed equally.
Contributors: LI, AC, FM and JAH conceived the structure of the manuscript. LI and LAT wrote the manuscript with the support of AC, SG, FM and JAH, and drew the table. LAT performed the search on clinicaltrial.gov. LI drew the figure. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: JAH is a consultant for Gilead Sciences, Amplyx, Allovir, Allogene therapeutics, CRISPR therapeutics, Takeda; and provides research support from Takeda, Allovir, Karius, Gilead Sciences. SG reports research current or past funding from Bristol-Myers Squibb, Genentech, Immune Design, Agenus, Janssen R&D, Pfizer, Takeda, and Regeneron; and past consultancy and/or advisory roles for Merck, Neon Therapeutics and OncoMed.
Provenance and peer review: Commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20:533–4. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng KI, Feng G, Liu WY. Extrapulmonary complications of COVID-19: a multisystem disease? J Med Virol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen HR, O'Connell C, Nadim MK, et al. Extrapulmonary manifestations of severe acute respiratory syndrome coronavirus-2 infection. J Med Virol 2021;93:2645–53. 10.1002/jmv.26595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Valle DM, Kim-Schulze S, Huang H-H, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 2020;26:1636–43. 10.1038/s41591-020-1051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol 2020;20:651–68. 10.1038/s41577-020-0306-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zumla A, Rao M, Wallis RS, et al. Host-directed therapies for infectious diseases: current status, recent progress, and future prospects. Lancet Infect Dis 2016;16:e47–63. 10.1016/S1473-3099(16)00078-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroschinsky F, Stölzel F, von Bonin S, et al. New drugs, new toxicities: severe side effects of modern targeted and immunotherapy of cancer and their management. Critical Care 2017;21:89. 10.1186/s13054-017-1678-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thakar MS, Kearl TJ, Malarkannan S. Controlling cytokine release syndrome to harness the full potential of CAR-Based cellular therapy. Front Oncol 2019;9:1529. 10.3389/fonc.2019.01529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A 2020;117:11727–34. 10.1073/pnas.2003138117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quan C, Li C, Ma H, et al. Immunopathogenesis of coronavirus-induced acute respiratory distress syndrome (ARDS): potential infection-associated hemophagocytic lymphohistiocytosis. Clin Microbiol Rev 2020;34. 10.1128/CMR.00074-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020;324:782–93. 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 12.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abate SM, Ahmed Ali S, Mantfardo B, et al. Rate of intensive care unit admission and outcomes among patients with coronavirus: a systematic review and meta-analysis. PLoS One 2020;15:e0235653. 10.1371/journal.pone.0235653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine 2020;8:475–81. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular Endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med Overseas Ed 2020;383:120–8. 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darvin P, Toor SM, Sasidharan Nair V, et al. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 2018;50:1–11. 10.1038/s12276-018-0191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koyama N, Iwase O, Nakashima E, et al. High incidence and early onset of nivolumab-induced pneumonitis: four case reports and literature review. BMC Pulm Med 2018;18:23. 10.1186/s12890-018-0592-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Zugazagoitia J, Ahmed FS, et al. Immune cell PD-L1 colocalizes with macrophages and is associated with outcome in PD-1 pathway blockade therapy. Clin Cancer Res 2020;26:970–7. 10.1158/1078-0432.CCR-19-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zahm CD, Colluru VT, McIlwain SJ, et al. TLR Stimulation during T-cell Activation Lowers PD-1 Expression on CD8 + T Cells. Cancer Immunol Res 2018;6:1364–74. 10.1158/2326-6066.CIR-18-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirato K, Kizaki T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via Toll-like receptor 4 signaling in murine and human macrophages. Heliyon 2021;7:e06187. 10.1016/j.heliyon.2021.e06187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamel MH, Yin W, Zavaro C, et al. Hyperthrombotic milieu in COVID-19 patients. Cells 2020;9:2392. 10.3390/cells9112392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazzaroni MG, Piantoni S, Masneri S, et al. Coagulation dysfunction in COVID-19: the interplay between inflammation, viral infection and the coagulation system. Blood Rev 2021;46:100745. 10.1016/j.blre.2020.100745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gayam V, Chobufo MD, Merghani MA, et al. Clinical characteristics and predictors of mortality in African-Americans with COVID-19 from an inner-city community teaching hospital in New York. J Med Virol 2021;93:812–9. 10.1002/jmv.26306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu H-H, Qin C, Chen M, et al. D-Dimer level is associated with the severity of COVID-19. Thromb Res 2020;195:219–25. 10.1016/j.thromres.2020.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020;135:2033–40. 10.1182/blood.2020006000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elgendy IY, Kolte D, Mansour MK. Incidence, predictors, and outcomes of thrombotic events in hospitalized patients with viral pneumonia. Am J Cardiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joly BS, Siguret V, Veyradier A. Understanding pathophysiology of hemostasis disorders in critically ill patients with COVID-19. Intensive Care Med 2020;46:1603–6. 10.1007/s00134-020-06088-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song WC, FitzGerald GA. COVID-19, microangiopathy, hemostatic activation, and complement. J Clin Invest 2020;130:3950–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghebrehiwet B, Peerschke EI. Complement and coagulation: key triggers of COVID-19–induced multiorgan pathology. J Clin Invest 2020;130:5674–6. 10.1172/JCI142780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poissy J, Goutay J, Caplan M. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation 2020;142:184–6. [DOI] [PubMed] [Google Scholar]
- 31.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020;26:1017–32. 10.1038/s41591-020-0968-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamming I, Timens W, Bulthuis MLC, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–7. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karmouty-Quintana H, Thandavarayan RA, Keller SP, et al. Emerging mechanisms of pulmonary vasoconstriction in SARS-CoV-2-Induced acute respiratory distress syndrome (ARDS) and potential therapeutic targets. Int J Mol Sci 2020;21:8081. 10.3390/ijms21218081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radermecker C, Detrembleur N, Guiot J, et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J Exp Med 2020;217. 10.1084/jem.20201012. [Epub ahead of print: 07 Dec 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karki R, Sharma BR, Tuladhar S, et al. COVID-19 cytokines and the hyperactive immune response: synergism of TNF-α and IFN-γ in triggering inflammation, tissue damage, and death. bioRxiv 2020. 10.1101/2020.10.29.361048. [Epub ahead of print: 29 Oct 2020]. [DOI] [Google Scholar]
- 36.AlSamman M, Caggiula A, Ganguli S, et al. Non-Respiratory presentations of COVID-19, a clinical review. Am J Emerg Med 2020;38:2444–54. 10.1016/j.ajem.2020.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S, Liu Y, Wang X, et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol 2020;13:120. 10.1186/s13045-020-00954-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hay KA, Hanafi L-A, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 2017;130:2295–306. 10.1182/blood-2017-06-793141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sa Ribero M, Jouvenet N, Dreux M, et al. Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathog 2020;16:e1008737. 10.1371/journal.ppat.1008737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flower TG, Buffalo CZ, Hooy RM, et al. Structure of SARS-CoV-2 ORF8, a rapidly evolving coronavirus protein implicated in immune evasion. bioRxiv 2020. 10.1101/2020.08.27.270637. [Epub ahead of print: 27 Aug 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lima R, Filho CC, Ferreira Filho CM. Hemophagocytic syndrome and COVID-19. Respir Med Case Rep 2020;31:101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imus PH, Blackford AL, Bettinotti M, et al. Severe cytokine release syndrome after haploidentical peripheral blood stem cell transplantation. Biology of Blood and Marrow Transplantation 2019;25:2431–7. 10.1016/j.bbmt.2019.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeiser R. Advances in understanding the pathogenesis of graft-versus-host disease. Br J Haematol 2019;187:563–72. 10.1111/bjh.16190 [DOI] [PubMed] [Google Scholar]
- 44.Flowers MED, Martin PJ. How we treat chronic graft-versus-host disease. Blood 2015;125:606–15. 10.1182/blood-2014-08-551994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandler RD, Carter S, Kaur H, et al. Haemophagocytic lymphohistiocytosis (HLH) following allogeneic haematopoietic stem cell transplantation (HSCT)-time to reappraise with modern diagnostic and treatment strategies? Bone Marrow Transplant 2020;55:307–16. 10.1038/s41409-019-0637-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cirillo M, Tan P, Sturm M, et al. Cellular immunotherapy for hematologic malignancies: beyond bone marrow transplantation. Biology of Blood and Marrow Transplantation 2018;24:433–42. 10.1016/j.bbmt.2017.10.035 [DOI] [PubMed] [Google Scholar]
- 47.Brudno JN, Kochenderfer JN. Recent advances in car T-cell toxicity: mechanisms, manifestations and management. Blood Rev 2019;34:45–55. 10.1016/j.blre.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frey N, Porter D. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biology of Blood and Marrow Transplantation 2019;25:e123–7. 10.1016/j.bbmt.2018.12.756 [DOI] [PubMed] [Google Scholar]
- 49.Hansel TT, Kropshofer H, Singer T, et al. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov 2010;9:325–38. 10.1038/nrd3003 [DOI] [PubMed] [Google Scholar]
- 50.Jain T, Litzow MR. No free rides: management of toxicities of novel immunotherapies in all, including financial. Hematology 2018;2018:25–34. 10.1182/asheducation-2018.1.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. The Lancet Respiratory Medicine 2020;8:1233–44. 10.1016/S2213-2600(20)30404-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitsuyama K, Toyonaga A, Sasaki E, et al. Soluble interleukin-6 receptors in inflammatory bowel disease: relation to circulating interleukin-6. Gut 1995;36:45–9. 10.1136/gut.36.1.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choy EH, De Benedetti F, Takeuchi T, et al. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol 2020;16:335–45. 10.1038/s41584-020-0419-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hunter CA, Jones SA. Il-6 as a keystone cytokine in health and disease. Nat Immunol 2015;16:448–57. 10.1038/ni.3153 [DOI] [PubMed] [Google Scholar]
- 55.Copaescu A, Smibert O, Gibson A, et al. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J Allergy Clin Immunol 2020;146:518–34. 10.1016/j.jaci.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strati P, Neelapu SS. Chimeric antigen Receptor-Engineered T cell therapy in lymphoma. Curr Oncol Rep 2019;21:38. 10.1007/s11912-019-0789-z [DOI] [PubMed] [Google Scholar]
- 57.Klinger M, Brandl C, Zugmaier G, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell–engaging CD19/CD3-bispecific bite antibody blinatumomab. Blood 2012;119:6226–33. 10.1182/blood-2012-01-400515 [DOI] [PubMed] [Google Scholar]
- 58.Zhang P, Shi L, Xu J, et al. Elevated interleukin-6 and adverse outcomes in COVID-19 patients: a meta-analysis based on adjusted effect estimates. Immunogenetics 2020;72:431–7. 10.1007/s00251-020-01179-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen LYC, Hoiland RL, Stukas S, et al. Confronting the controversy: interleukin-6 and the COVID-19 cytokine storm syndrome. Eur Respir J 2020;56:2003006. 10.1183/13993003.03006-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karki R, Sharma BR, Tuladhar S, et al. COVID-19 cytokines and the hyperactive immune response: synergism of TNF-α and IFN-γ in triggering inflammation, tissue damage, and death. bioRxiv 2020. 10.1101/2020.10.29.361048. [Epub ahead of print: 29 Oct 2020]. 10.1101/2020.10.29.361048 [DOI] [Google Scholar]
- 61.Cheng L, Li H, Li L, et al. Ferritin in the coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. J Clin Lab Anal 2020;34:e23618. 10.1002/jcla.23618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ellul MA, Benjamin L, Singh B. Neurological associations of COVID-19. Lancet Neurol 2020;19:767–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683–90. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Divani AA, Andalib S, Biller J, et al. Central nervous system manifestations associated with COVID-19. Curr Neurol Neurosci Rep 2020;20:60. 10.1007/s11910-020-01079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry 2020;7:875–82. 10.1016/S2215-0366(20)30287-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Achar A, Ghosh C. COVID-19-Associated neurological disorders: the potential route of CNS invasion and blood-brain barrier relevance. Cells 2020;9:2360. 10.3390/cells9112360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Najjar S, Najjar A, Chong DJ, et al. Central nervous system complications associated with SARS-CoV-2 infection: integrative concepts of pathophysiology and case reports. J Neuroinflammation 2020;17:231. 10.1186/s12974-020-01896-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nikbakht F, Mohammadkhanizadeh A, Mohammadi E. How does the COVID-19 cause seizure and epilepsy in patients? the potential mechanisms. Mult Scler Relat Disord 2020;46:102535. 10.1016/j.msard.2020.102535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirayama AV, Turtle CJ. Toxicities of CD19 CAR‐T cell immunotherapy. Am J Hematol 2019;94:S42–9. 10.1002/ajh.25445 [DOI] [PubMed] [Google Scholar]
- 70.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nat Rev Clin Oncol 2018;15:47–62. 10.1038/nrclinonc.2017.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gust J, Hay KA, Hanafi L-A, et al. Endothelial activation and Blood–Brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov 2017;7:1404–19. 10.1158/2159-8290.CD-17-0698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kullar R, Patel AP, Saab S. Hepatic injury in patients with COVID-19. J Clin Gastroenterol 2020;54:841–9. 10.1097/MCG.0000000000001432 [DOI] [PubMed] [Google Scholar]
- 73.Barbosa da Luz B, de Oliveira NMT, França Dos Santos IW. An overview of the gut side of the SARS-CoV-2 infection. Intestinal research 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ye L, Yang Z, Liu J, et al. Digestive system manifestations and clinical significance of coronavirus disease 2019: a systematic literature review. J Gastroenterol Hepatol 2020. 10.1111/jgh.15323. [Epub ahead of print: 05 Nov 2020]. 10.1111/jgh.15323 [DOI] [PubMed] [Google Scholar]
- 75.van Eeden C, Khan L, Osman MS, et al. Natural killer cell dysfunction and its role in COVID-19. Int J Mol Sci 2020;21:6351. 10.3390/ijms21176351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lundholm MD, Poku C, Emanuele N, et al. SARS-CoV-2 (COVID-19) and the endocrine system. J Endocr Soc 2020;4:bvaa144. 10.1210/jendso/bvaa144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Icenogle T. COVID-19: infection or autoimmunity. Frontiers in immunology 2055;2020:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaushik A, Gupta S, Sood M, et al. A systematic review of multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection. Pediatr Infect Dis J 2020;39:e340–6. 10.1097/INF.0000000000002888 [DOI] [PubMed] [Google Scholar]
- 79.Keller MD, Harris KM, Jensen-Wachspress MA, et al. SARS-CoV-2-specific T cells are rapidly expanded for therapeutic use and target conserved regions of the membrane protein. Blood 2020;136:2905–17. 10.1182/blood.2020008488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao L, Cao YJ. Engineered T cell therapy for cancer in the clinic. Front Immunol 2019;10:2250. 10.3389/fimmu.2019.02250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chapuis AG, Egan DN, Bar M, et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat Med 2019;25:1064–72. 10.1038/s41591-019-0472-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li D, Li X, Zhou W-L, et al. Genetically engineered T cells for cancer immunotherapy. Sig Transduct Target Ther 2019;4:35. 10.1038/s41392-019-0070-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wagner EK, Qerqez AN, Stevens CA, et al. Human cytomegalovirus-specific T-cell receptor engineered for high affinity and soluble expression using mammalian cell display. Journal of Biological Chemistry 2019;294:5790–804. 10.1074/jbc.RA118.007187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caligiuri MA. Human natural killer cells. Blood 2008;112:461–9. 10.1182/blood-2007-09-077438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee DA, Denman CJ, Rondon G, et al. Haploidentical natural killer cells infused before allogeneic stem cell transplantation for myeloid malignancies: a phase I trial. Biol Blood Marrow Transplant 2016;22:1290–8. 10.1016/j.bbmt.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu E, Marin D, Banerjee P, et al. Use of CAR-Transduced natural killer cells in CD19-Positive lymphoid tumors. N Engl J Med 2020;382:545–53. 10.1056/NEJMoa1910607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma M, Badeti S, Geng K, et al. Efficacy of targeting SARS-CoV-2 by CAR-NK cells. bioRxiv 2020. 10.1101/2020.08.11.247320. [Epub ahead of print: 12 Aug 2020]. [DOI] [Google Scholar]
- 88.Li M, Guo W, Dong Y, et al. Elevated Exhaustion Levels of NK and CD8+ T Cells as Indicators for Progression and Prognosis of COVID-19 Disease. Front Immunol 2020;11:580237. 10.3389/fimmu.2020.580237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pacini S, Montali M, Mazziotta F, et al. Mesangiogenic progenitor cells are forced toward the angiogenic fate, in multiple myeloma. Oncotarget 2019;10:6781–90. 10.18632/oncotarget.27285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rambaldi B, Diral E, Donsante S. Heterogeneity of the bone marrow niche in patients with myeloproliferative neoplasms: ActivinA secretion by mesenchymal stromal cells correlates with the degree of marrow fibrosis. Ann Hematol 2020. [DOI] [PubMed] [Google Scholar]
- 91.Pittenger MF, Discher DE, Péault BM, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med 2019;4:22. 10.1038/s41536-019-0083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blazar BR, MacDonald KPA, Hill GR. Immune regulatory cell infusion for graft-versus-host disease prevention and therapy. Blood 2018;131:2651–60. 10.1182/blood-2017-11-785865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sartoris S, Mazzocco M, Tinelli M, et al. Efficacy assessment of Interferon-Alpha–Engineered mesenchymal stromal cells in a mouse plasmacytoma model. Stem Cells Dev 2011;20:709–19. 10.1089/scd.2010.0095 [DOI] [PubMed] [Google Scholar]
- 94.Sánchez-Guijo F, Caballero-Velázquez T, López-Villar O, et al. Sequential Third-Party Mesenchymal Stromal Cell Therapy for Refractory Acute Graft-versus-Host Disease. Biology of Blood and Marrow Transplantation 2014;20:1580–5. 10.1016/j.bbmt.2014.06.015 [DOI] [PubMed] [Google Scholar]
- 95.Shu L, Niu C, Li R, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther 2020;11:361. 10.1186/s13287-020-01875-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meng F, Xu R, Wang S, et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduction and Targeted Therapy 2020;5:172. 10.1038/s41392-020-00286-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sengupta V, Sengupta S, Lazo A, et al. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev 2020;29:747–54. 10.1089/scd.2020.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anderson EJ, Rouphael NG, Widge AT. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baden LR, El Sahly HM, Essink B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New England Journal of Medicine 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99–111. 10.1016/S0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1–2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med Overseas Ed 2021. 10.1056/NEJMoa2034201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu STH, Lin H-M, Baine I, et al. Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat Med 2020;26:1708–13. 10.1038/s41591-020-1088-9 [DOI] [PubMed] [Google Scholar]
- 104.Libster R, Pérez Marc G, Wappner D, et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med 2021;384:610–8. 10.1056/NEJMoa2033700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med 2021;384:619–29. 10.1056/NEJMoa2031304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Crawford KHD, Dingens AS, Eguia R. Dynamics of neutralizing antibody titers in the months after SARS-CoV-2 infection. J Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tay MZ, Poh CM, Rénia L, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020;20:363–74. 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lo MS, Newburger JW. Role of intravenous immunoglobulin in the treatment of Kawasaki disease. Int J Rheum Dis 2018;21:64–9. 10.1111/1756-185X.13220 [DOI] [PubMed] [Google Scholar]
- 109.Pei L, Zhang S, Huang L, et al. Antiviral agents, glucocorticoids, antibiotics, and intravenous immunoglobulin in 1142 patients with coronavirus disease 2019: a systematic review and meta-analysis. Pol Arch Intern Med 2020;130:726–33. 10.20452/pamw.15543 [DOI] [PubMed] [Google Scholar]
- 110.Carrillo-Larco RM, Altez-Fernandez C, Ravaglia S, et al. COVID-19 and Guillain-Barre syndrome: a systematic review of case reports. Wellcome Open Res 2020;5:107. 10.12688/wellcomeopenres.15987.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chi X, Yan R, Zhang J, et al. A neutralizing human antibody binds to the N-terminal domain of the spike protein of SARS-CoV-2. Science 2020;369:650–5. 10.1126/science.abc6952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baum A, Ajithdoss D, Copin R, et al. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science 2020;370:1110–5. 10.1126/science.abe2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martin PJ, Rizzo JD, Wingard JR, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of blood and marrow transplantation. Biol Blood Marrow Transplant 2012;18:1150–63. 10.1016/j.bbmt.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cooper AS. Corticosteroids for treating sepsis in children and adults. Crit Care Nurse 2020;40:83–4. 10.4037/ccn2020588 [DOI] [PubMed] [Google Scholar]
- 115.Horby P, Lim WS. et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sterne JAC, Diaz J, Villar J, et al. Corticosteroid therapy for critically ill patients with COVID-19: a structured summary of a study protocol for a prospective meta-analysis of randomized trials. Trials 2020;21:734. 10.1186/s13063-020-04641-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bacigalupo A, Angelucci E, Raiola AM, et al. Treatment of steroid resistant acute graft versus host disease with an anti-CD26 monoclonal antibody-Begelomab. Bone Marrow Transplant 2020;55:1580–7. 10.1038/s41409-020-0855-z [DOI] [PubMed] [Google Scholar]
- 118.Scott LJ. Tocilizumab: a review in rheumatoid arthritis. Drugs 2017;77:1865–79. 10.1007/s40265-017-0829-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–48. 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Luo P, Liu Y, Qiu L, et al. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol 2020;92:814–8. 10.1002/jmv.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020;117:10970–5. 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol 2020;2:e474–84. 10.1016/S2665-9913(20)30173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med Overseas Ed 2020;383:2333–44. 10.1056/NEJMoa2028836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med 2021;181:24–31. 10.1001/jamainternmed.2020.6615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hermine O, Mariette X, Tharaux P-L, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med 2021;181:32. 10.1001/jamainternmed.2020.6820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med 2021;181:41. 10.1001/jamainternmed.2020.6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med 2021. 10.1056/NEJMoa2028700. [Epub ahead of print: 25 Feb 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lescure F-X, Honda H, Fowler RA, et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2021. 10.1016/S2213-2600(21)00099-0. [Epub ahead of print: 04 Mar 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol 2012;30:1005–14. 10.1200/JCO.2010.31.8907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Campbell PJ, Baxter EJ, Beer PA, et al. Mutation of JAK2 in the myeloproliferative disorders: timing, clonality studies, cytogenetic associations, and role in leukemic transformation. Blood 2006;108:3548–55. 10.1182/blood-2005-12-013748 [DOI] [PubMed] [Google Scholar]
- 131.Zeiser R, von Bubnoff N, Butler J, et al. Ruxolitinib for Glucocorticoid-Refractory acute graft-versus-host disease. N Engl J Med 2020;382:1800–10. 10.1056/NEJMoa1917635 [DOI] [PubMed] [Google Scholar]
- 132.La Rosée F, Bremer HC, Gehrke I, et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia 2020;34:1805–15. 10.1038/s41375-020-0891-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med 2020;383:1813–26. 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N Engl J Med 2021;384:795–807. 10.1056/NEJMoa2031994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mertens M, Singh JA. Anakinra for rheumatoid arthritis: a systematic review. J Rheumatol 2009;36:1118–25. 10.3899/jrheum.090074 [DOI] [PubMed] [Google Scholar]
- 136.Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol 2020;2:e393–400. 10.1016/S2665-9913(20)30164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Locatelli F, Jordan MB, Allen C, et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med 2020;382:1811–22. 10.1056/NEJMoa1911326 [DOI] [PubMed] [Google Scholar]
- 138.Stallmach A, Kortgen A, Gonnert F, et al. Infliximab against severe COVID-19-induced cytokine storm syndrome with organ failure-a cautionary case series. Crit Care 2020;24:444. 10.1186/s13054-020-03158-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zizzo G, Cohen PL. Imperfect storm: is interleukin-33 the Achilles heel of COVID-19? Lancet Rheumatol 2020;2:e779–90. 10.1016/S2665-9913(20)30340-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Laterre PF, François B, Collienne C, et al. Association of interleukin 7 immunotherapy with lymphocyte counts among patients with severe coronavirus disease 2019 (COVID-19). JAMA Netw Open 2020;3:e2016485. 10.1001/jamanetworkopen.2020.16485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cairo MS, Cooke KR, Lazarus HM, et al. Modified diagnostic criteria, grading classification and newly elucidated pathophysiology of hepatic SOS/VOD after haematopoietic cell transplantation. Br J Haematol 2020;190:822–36. 10.1111/bjh.16557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Diurno F, Numis FG, Porta G, et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci 2020;24:4040–7. 10.26355/eurrev_202004_20875 [DOI] [PubMed] [Google Scholar]
- 143.Laurence J, Mulvey JJ, Seshadri M, et al. Anti-Complement C5 therapy with eculizumab in three cases of critical COVID-19. Clin Immunol 2020;219:108555. 10.1016/j.clim.2020.108555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mastellos DC, Pires da Silva BGP, Fonseca BAL, et al. Complement C3 vs C5 inhibition in severe COVID-19: early clinical findings reveal differential biological efficacy. Clin Immunol 2020;220:108598. 10.1016/j.clim.2020.108598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.León López R, Fernández SC, Limia Pérez L, et al. Efficacy and safety of early treatment with sarilumab in hospitalised adults with COVID-19 presenting cytokine release syndrome (SARICOR study): protocol of a phase II, open-label, randomised, multicentre, controlled clinical trial. BMJ Open 2020;10:e039951. 10.1136/bmjopen-2020-039951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mulchandani R, Lyngdoh T, Kakkar AK. Deciphering the COVID-19 cytokine storm: systematic review and meta-analysis. Eur J Clin Invest 2021;51:e13429. 10.1111/eci.13429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jodele S, Davies SM, Lane A, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood 2014;124:645–53. 10.1182/blood-2014-03-564997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis 2020;11:216–28. 10.14336/AD.2020.0228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A 2020;117:11727–34. 10.1073/pnas.2003138117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pan J, Yang JF, Deng BP, et al. High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia 2017;31:2587–93. 10.1038/leu.2017.145 [DOI] [PubMed] [Google Scholar]
- 151.Hossain NM, Chapuis AG, Walter RB. T-Cell Receptor-Engineered cells for the treatment of hematologic malignancies. Curr Hematol Malig Rep 2016;11:311–7. 10.1007/s11899-016-0327-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-002392supp001.pdf (110.1KB, pdf)