Abstract
What makes fast, cumulative cultural evolution work? Where did it come from? Why is it the sole preserve of humans? We set out a self-assembly hypothesis: cultural evolution evolved culturally. We present an evolutionary account that shows this hypothesis to be coherent, plausible, and worthy of further investigation. It has the following steps: (0) in common with other animals, early hominins had significant capacity for social learning; (1) knowledge and skills learned by offspring from their parents began to spread because bearers had more offspring, a process we call CS1 (or Cultural Selection 1); (2) CS1 shaped attentional learning biases; (3) these attentional biases were augmented by explicit learning biases (judgements about what should be copied from whom). Explicit learning biases enabled (4) the high-fidelity, exclusive copying required for fast cultural accumulation of knowledge and skills by a process we call CS2 (or Cultural Selection 2) and (5) the emergence of cognitive processes such as imitation, mindreading and metacognition—‘cognitive gadgets' specialized for cultural learning. This self-assembly hypothesis is consistent with archaeological evidence that the stone tools used by early hominins were not dependent on fast, cumulative cultural evolution, and suggests new priorities for research on ‘animal culture'.
This article is part of the theme issue ‘Foundations of cultural evolution’.
Keywords: cognitive gadgets, cultural evolution, evolution of cognition, learning bias, social learning strategy, metacognition
1. Introduction
How did human cultural evolution get off the ground? It would be an exaggeration to say there is nothing at all like it elsewhere in the natural world. Cetaceans [1–4], great apes [5,6] and other animals [7–11] also pass skills down the generations through social learning, leading to stable differences between sub-populations. These differences are often called ‘traditions' [2,10]. Young bottlenose dolphins learn foraging techniques from their parents [12]; young chimpanzees learn from older group members how to extract ants and termites from their mounds with sticks [13]. But human cultural evolution (table 1) is different. It is cumulative: small improvements to skills and technologies accumulate, resulting in products of such complexity that no one individual could possibly have designed them alone, without any learning from others [14–17]. Examples include canoes, spears, fire control, detoxification of bitter manioc and, more recently, steam engines, computers and satellites. Moreover, the accumulation is fast: improvements spread rapidly through populations, no longer tied to the timescale of biological generations. What makes fast, cumulative cultural evolution work? Where did it come from? And why is it the sole preserve of humans?
Table 1.
Glossary.
| attentional bias | a social learning bias mediated by domain-general attentional processes |
| asocial learning | learning without the assistance of other agents, also known as ‘individual learning' |
| cognitive gadget | a domain-specific cognitive process shaped by cultural evolution |
| conformist bias | a social learning bias in favour of variants that are currently prevalent among available models |
| cultural evolution | change in the frequencies of cultural variants over time |
| cultural fitness | the number of learners an individual model succeeds in attracting, weighted by the model's degree of influence over the learner |
| cultural inheritance | the transmission down the generations of cultural variants, leading to persisting cultural differences between populations |
| cultural learning | social learning mediated by cognitive mechanisms that are specialized for promoting cultural inheritance |
| Cultural Selection 1 (CS1) | a Darwinian process in which cultural variants spread because they cause their bearers to have more biological offspring, and because offspring learn from their parents |
| Cultural Selection 2 (CS2) | a Darwinian process in which cultural variants spread because they cause their bearers to attract more learners, giving them higher cultural fitness |
| cultural variant | anything that can be learned socially (e.g. psychological processes, artefacts, skills, habits, customs, rituals, ideas, beliefs and values) |
| cumulative cultural evolution | cultural evolution in which small improvements to existing cultural variants spread through populations, gradually leading to complex adaptive products that no single individual could have designed from scratch |
| domain-general cognitive mechanism | a cognitive mechanism that works in the same way across a broad range of tasks and contexts, e.g. social and non-social |
| domain-specific cognitive mechanism | a cognitive mechanism that works in one task or context and less efficiently or not at all in others, e.g. social or non-social |
| explicit bias | a social learning bias mediated by a domain-specific psychological rule such as copy the majority |
| genetic assimilation | a process whereby environmentally induced phenotypic variation acquires a genetic basis |
| genetic evolution | change in the frequencies of genes over time |
| high-fidelity transmission | the learning of a cultural variant with sufficiently few errors that even small, unobvious improvements will be retained |
| horizontal transmission | the learning of a cultural variant by a member of one generation from another member of the same generation |
| oblique transmission | the learning of a cultural variant by a member of a younger generation from a member of an older generation who is not a biological parent |
| payoff bias | a social learning bias in the favour of learning cultural variants that effectively yield rewards |
| prestige bias | a social learning bias in favour of learning from models who are already successful in attracting learners and have markers of that success |
| social learning | learning assisted by observation of, or interaction with, other agents |
| social learning bias | any mechanism that biases an individual's social learning away from one cultural variant and towards another. Also known as ‘social learning strategy’, ‘learning bias’ and ‘transmission bias’ |
| vertical transmission | the learning of a cultural variant from a parent by its biological offspring |
There is an emerging consensus that fast cultural accumulation draws on a suite of cognitive mechanisms including selective social learning, imitation, language, mindreading (or theory-of-mind) for teaching, metacognition and normative cognition, and that humans have evolved uniquely sophisticated versions of these mechanisms [18,19]. But what explains the origin of these mechanisms?
A popular, influential type of answer appeals to gene–culture coevolution. This occurs when the genetic composition of a population responds to changes in the cultural environment, leading to yet further changes in the cultural environment, and so on. The idea is often illustrated by the case of lactose tolerance: genes for lactose tolerance followed the spread of dairy farming, and enabled yet more dairy farming. Boyd & Richerson [20] proposed that some of the cognitive mechanisms involved in human cultural evolution—including the mechanisms mediating conformist bias—evolved, like lactose tolerance, by gene–culture coevolution. This has remained a central tenet of the ‘California school' of cultural evolution that Boyd, Richerson, Henrich and their collaborators have built over the past 30 years.
While gene–culture coevolution has received a great deal of attention, much less has been given to its purely cultural counterpart. We call this (borrowing a term from Muthukrishna & Henrich [21]) culture–culture coevolution. As we define it here, culture–culture coevolution occurs when a culturally inherited cognitive mechanism evolves in response to a cultural environment, altering that environment and enabling further evolution of the cognitive mechanism, without any underlying genetic change.
Our aim here is to explain the basic idea of culture–culture coevolution and to argue that it is worthy of sustained empirical investigation. Indeed, when it comes to explaining the origins of cumulative culture, we think it may even provide a better explanation than gene–culture coevolution.
2. Cultural selection, fast and slow
At the core of our hypothesis about the origins of cumulative culture is a distinction between two types of cultural selection (CS). A Darwinian process is one that relies on blind variation and selective retention (electronic supplementary material, S1). In genetic evolution, ‘selective retention' always involves the differential survival and/or reproduction of individuals. But in cultural evolution, there are two different types of ‘selective retention' operating at different timescales.
The first, slower type is natural selection on culturally inherited variation [16,22]. We will call this Cultural Selection 1 or CS1. CS1 is closely analogous to natural selection on genetic variation. When offspring spend a long time learning practical skills and ecological knowledge from their parents, valuable skills and knowledge will tend to spread through the population for the simple reason that their bearers will tend to have more offspring. Just as in a traditional process of natural selection, change is driven by differences in the number of biological offspring an organism produces. However, unlike in a traditional process of natural selection, the differences in reproductive output are caused by inherited differences in cultural variants, not genes, and they are transmitted from parents to offspring through social learning rather than genetic inheritance.
The second, faster type of Darwinian process occurs when individuals compete with each other for learners, so that individuals who recruit more learners can be said to have higher cultural fitness [23]. We will call this Cultural Selection 2, or CS2. Roughly, an agent's cultural fitness, with respect to a specific cultural trait, is the number of learners to whom it transmits its variant of that trait through social learning [22–25]. When learners are choosing from a wide range of potential models, and the models are competing for learners, there is potential for fast accumulation of small improvements, because the process need not be tied to the timescale of biological reproduction. We think CS2 lies at the heart of ‘cumulative culture' as we know it today. Small improvements to existing techniques spread not because they necessarily increase anyone's reproductive success, but because they allow models to attract more learners.
Although we have set out the CS1/CS2 distinction at the individual level, the same distinction can be drawn at the group level. In other words, the CS1/CS2 distinction cross-cuts the distinction between individual selection and group selection. In the group-level version of CS1, change is driven by biological fitness differences between groups, and these differences are due to culturally inherited variation in group-wide patterns of behaviour. In the group-level version of CS2, change is driven by cultural fitness differences between groups—differences in their ability to attract new migrants who will learn their ways. This group-level version of CS2 will be important later on.
The CS1/CS2 distinction allows us to pose our question like this: at some point in human evolution, CS1 began to be supplemented by CS2, allowing fast cultural accumulation. Was this transition driven by gene–culture coevolution, or did CS1 itself assemble the mechanisms that made CS2 possible? If the latter is the case, then the mechanisms that enable cumulative culture were products of culture–culture coevolution. Cumulative culture was, in this specific sense, ‘self-assembling’.
3. A self-assembly hypothesis
According to our self-assembly hypothesis, the role of genetic evolution, though important, was limited. Genetic evolution driven by increasing climatic variability and environmental change gave us larger brains [26], longer childhoods [27] and more powerful domain-general cognitive resources. Associative learning, working memory and inhibitory control were all dialled up, leading, eventually, to a distinctive capacity for slow, deliberative, explicit cognition that made heavy use of working memory [28–31]. These genetically based upgrades to domain-general cognitive resources enabled early hominins to learn more efficiently the information they needed to hunt and gather in a changing and variable environment. But, on our hypothesis, these genetic changes were not responsible for shaping specialized cognitive mechanisms for accelerating cultural evolution. Cognitive mechanisms like imitation, mindreading and normative cognition emerged later via cultural evolutionary processes. Relatively simple cognition got fast cultural evolution off the ground. Learners were initially driven by attentional processes to copy better models and small improvements in technique; they did not make explicit comparisons between models or consciously recognize improvements. They had ‘competence without comprehension' [32].
Our hypothesis emphasizes the importance of culture–culture coevolution. We do not assume that specialized cognitive mechanisms such as imitation have been genetically assimilated to any substantial degree. This is because we think the present-day evidence from developmental psychology and cognitive neuroscience points towards these mechanisms being culturally inherited now [33]. To the extent that this is plausible, it is important to have viable evolutionary hypotheses that do not rely on genetic assimilation.
Our hypothesis has a basic platform—a starting point shared with other great apes—and five steps from that basic platform to a fast, cumulative culture (figure 1).
Figure 1.
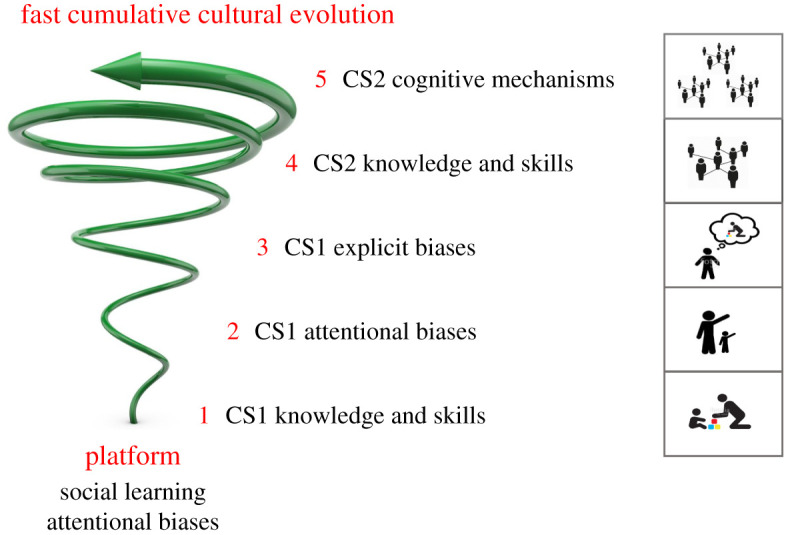
The self-assembly hypothesis. Culture–culture coevolution produces fast cumulative culture in five steps. CS1 initiates the coevolutionary process. First knowledge and skills (Step 1), then attentional social learning biases (Step 2), then explicit social learning biases (Step 3), are socially inherited from biological parents and spread through the population because their bearers tend to have more offspring. Subsequently, CS2 also contributes. Enhanced knowledge and skills (Step 4) and cognitive mechanisms (Step 5) are socially inherited from unrelated individuals and spread through the population because some models are more successful than others in competition for learners. The five-step process not only produces fast cumulative culture, but is itself cumulative: each step augments, rather than replaces, the previous step (spiral arrow). The schematic figures on the right represent typical social interactions in each step. See text for details. (Online version in colour.)
(a). Step 0: The basic platform
The human lineage, at the time of its divergence from the other great apes about 6 million years ago, would have possessed a significant capacity for social learning. Like a wide range of animal species alive today, our earliest human ancestors could learn by observing the behaviour of other agents that some places and objects are worth exploring (stimulus enhancement [34,35]), while others are either dangerous or rewarding (observational conditioning [36,37]). They could also learn socially what the outcomes of actions are likely to be (observational learning [38,39]): prodding a hive releases bees and digging in certain areas reveals tubers [40,41].
These social learning capacities were once thought to depend on specialized cognitive processes, but recent evidence indicates that they are based on the same associative mechanisms as those involved in asocial learning [38,42–47]. These mechanisms are powerful but, in the form present among our earliest ancestors, they do not allow high-fidelity copying of fine bodily movement and technique.
Early hominins would also have possessed structural and attentional social learning biases. A structural bias is a tendency to learn from some models rather than others owing to social structure and geography [48–50]. Juveniles would have learned most from their mothers, and to a lesser extent from other members of their immediate residential group, simply because they spent more time in close proximity to these agents than to others. An attentional bias is a tendency to learn from some models rather than others because they are particularly attention-grabbing [30,51]. These attention-grabbing actors or actions might be intrinsically salient (large, noisy, pungent) [52], close to salient rewards (e.g. close to food [53,54]) or previously associated with reward (e.g. their actions tend to produce or release food [55]).
Structural and attentional biases, like the social learning they modulate, are based on evolutionarily ancient, domain-general cognitive mechanisms [56,57]. The social learning biases found in fish, birds and small mammals [48,58] can be explained by attentional processes that bias social learning in the same way as asocial learning, and that evolved long before hominins appeared on the scene [57]. Nonetheless, we should not underestimate the power of attentional biases. They can produce patterns that, at the population level, fit the description of what cultural evolution theorists call conformist bias, prestige bias and payoff bias [16,59,60]. For example, suppose a child learns to associate a particular adult's behaviour with subsequent food rewards and, as a result, attends preferentially to that adult's behaviour. They will end up displaying a simple form of payoff-biased learning without needing to make any explicit judgements about whose behaviour leads to payoffs [55].
(b). Step 1: Cultural Selection 1 of knowledge and skills
A capacity for social learning, and for simple learning biases, can be found in a wide range of species and is clearly not sufficient for fast, cumulative culture. What made hominins different?
For early hominins, learned knowledge and skills were crucial for survival, and, with the extension of childhood, juveniles had more opportunity to acquire them vertically, primarily from their mothers [61]. Consequently, socially learned knowledge and skills began to spread through populations for the simple reason that their bearers tended to have greater reproductive fitness. Individuals who learned, for example, a more effective foraging technique from their mothers had better nutrition and more viable offspring and passed the technique on to each of those descendants. CS1 of knowledge and skills became increasingly powerful and important.
This does not mean that CS1 is uniquely human. In fact, CS1 appears to be occurring now among bottlenose dolphins. Some wild dolphins in Shark Bay, Western Australia, use marine sponges, worn on their closed rostrum (beak), to probe the sea floor for food [62]. There is evidence that this foraging technique is transmitted vertically through social learning from mothers to their female offspring [12], and that mothers who forage in this way have more offspring than mothers who do not [63]. If it is confirmed that the technique is spreading by selection on cultural variation, these dolphins could be an excellent model system for studying the very earliest stages of hominin cultural evolution.
(c). Step 2: Cultural Selection 1 of attentional learning biases
CS1 can be cumulative, but the accumulation will be slow (electronic supplementary material, S2). Since it relies on differential biological reproduction, CS1 occurs on broadly the same timescale as genetic evolution (although the supply of variation may differ: new cultural innovations may appear more or less frequently than genetic mutations). What it provides is a process that can drive the evolution of new cognitive mechanisms that accelerate cultural evolution. In the first accelerating innovation, learned attentional biases—cognitive rather than physical skills—became subject to CS1.
CS1 acting on knowledge and physical skills (Step 1) produced an increasing number of valuable, socially learnable traits in human populations: knowledge and skills that it would be advantageous for juveniles to learn from models other than their own parents. Ancient attentional biases (Step 0) were available to guide juveniles when they were learning from unrelated adults and peers (oblique transmission and horizontal transmission), but their choice of models became yet more adaptive when they began to socially learn attentional biases from their parents. For example, they no longer had to learn for themselves, by trial-and-error, that it is beneficial to attend more to females for foraging skills and males for hunting skills (if indeed it was), or to go to elders rather than peers for rare ecological information. Instead, they could learn these biases by tracking the social attention of their parents in different task contexts.
There is evidence that contemporary humans can socially learn attentional biases. Specifically, we can learn to attend more to some agents than others by following the gaze of third parties [64,65]. For example, when given choices of foods to consume and objects to manipulate, 3- and 4-year-old children are more likely to learn from an adult to whom they have seen other adults attending than from an adult who has been ignored [64]. Nonhuman primates have attentional biases that influence their choice of models [66,67] but, as far as we are aware, no one has asked whether nonhuman animals can socially learn attentional biases. For example, there is evidence that vervet monkeys attend more to female than male models when learning how to open a box to obtain food [66] but the origin of this attentional bias is not known. Does each vervet learn through its own efforts that the behaviour of females is more likely than the behaviour of males to have a desirable outcome, or can young vervets learn to attend more closely to females by watching the watching-behaviour of adults?
The existing evidence leaves a great deal of uncertainty as to when the ability to socially learn attentional biases first evolved. We conjecture that it was in place in Homo erectus well before 700 000 years ago, when the next steps in our account begin. It may have evolved much earlier. Although we suspect that socially learned attentional biases have been far more important for hominins than for other species, we do not rule out the possibility that some other animals do learn attentional biases from their parents, and that these learned attentional biases have been shaped by CS1.
Research on ‘shared attention' and ‘joint attention’ in contemporary children often assumes that these phenomena require an understanding of others' psychological states [68]. By contrast, Step 2 in our self-assembly model does not require mindreading. For social learning of attentional bias, learners need to track models looking-behaviour, not their mental states.
In so far as attentional biases are transmitted from parents to offspring, they can be targets of CS1. Parents with valuable attentional biases will have greater reproductive success, and the biases will spread.
(d). Step 3: Cultural Selection 1 of explicit learning biases
We come now to the steps that we propose to be unique to the hominin lineage. As noted above, we agree with the received wisdom that human cognitive evolution involved genetically based upgrades to domain-general cognitive resources, leading to a capacity for slow, deliberative, explicit cognition that made heavy use of working memory. The basic logic of our self-assembly hypothesis does not depend on any specific assumptions regarding when this happened. We suspect, however, that Late Acheulean lithic technology is a sign that a form of explicit cognition was in place by around 700 000 years ago, the time of Homo heidelbergensis. Neuroimaging studies suggest that, in modern humans, success in Late Acheulean toolmaking techniques relies on working memory [69–72]. We cannot be sure that H. heidelbergensis would have learned and executed Acheulean toolmaking skills in the same way we do, but it is a reasonable conjecture.
Once explicit cognition is present, it enables a new type of learning bias on which CS1 can get to work—explicit learning biases. Explicit learning biases are internally represented rules of thumb. They may include highly specific rules such as: ‘find individual X, Y or Z to observe the manufacture of cleavers, and individual A, B or C to observe the manufacture of handaxes'. Some rules may refer learners to specific models (X, Y or Z), while others may refer learners to a general type of model (such as ‘learn flint knapping from the person who makes the most symmetric shapes'). Explicit learning biases require a capacity for conceptual representation (e.g. possession of concepts referring to specific types of person, tool and shapes), but it is not clear that they require language. Language could have come along later.
Attentional biases, of the kind that were important in Steps 1 and 2, are limited in their specificity and accuracy. They lead learners to better than average models from the pool of nearby options, but they will struggle to lead learners to the best models in a sizeable social group. By relating cranial volume to social network size, Gamble, Gowlett and Dunbar estimate that H. heidelbergensis lived in social networks of 100–150 recognized individuals [73,74]. A more recent estimate, incorporating archaeological data alongside cranial volume, revised this downwards to 60–120 [75]. But even a group size of 60 is enough to create a problem. As group size grows, it becomes increasingly difficult for a juvenile to learn who the best experts are at specific skills purely by tracking the gaze of their parents. Juveniles might learn to attend to elders when uncertain about an important ecological fact, but they will not reliably find their way to the most knowledgeable elder. The problem is that they are not making explicit judgements about whom should, and who should not, be copied in particular domains. It is simply that their attention is reliably drawn to some models rather than others by relatively low-level, domain-general psychological processes. Because learners are not reliably able to find the most technically accomplished models in large groups, in Steps 1 and 2 we do not yet have conditions in which small improvements to an established technique can spread reliably and rapidly.
In Step 3, explicit learning biases allow juveniles to reliably access the best models available and to dedicate their learning efforts to those specific models. A learner who can reliably find the most technically accomplished models will gain biological fitness benefits from doing so, as long as their superior technique can be learned. This provides a selection pressure in favour of explicit learning biases, since they can overcome the limitations of attentional learning biases.
Although genetic changes of some kind will have been involved in the origin of explicit cognition, there is no reason to think that any further genetic changes are required for explicit learning biases, once explicit cognition in general exists. Explicit cognition brings with it a versatile capacity to represent rules. Rules about learning (such as copy the majority) can be learned, just like straightforwardly technical rules (such as strike flint against pyrite to make a spark). If the learning rules are passed down the generations from parents to offspring, CS1 will be able to shape them, gradually making them increasingly adaptive. We envisage this refinement of explicit learning biases through CS1 as a gradual process taking place over hundreds of thousands of years, beginning around 700 000 years ago.
There is evidence that explicit social learning biases—judgements about whom to copy—are crucial to fast cultural accumulation [51,76,77]. They are used, in addition to attentional learning biases, by adult humans alive today [78–80], but as yet there is no evidence that they are used by children younger than 4- or 5-year olds [81] or by nonhuman animals [57].
(e). Step 4: Cultural Selection 2 of knowledge and skills
With accurate, specific, explicit learning biases, assembled by CS1, comes the possibility of fast, cumulative cultural evolution of knowledge and skills. An explicit rule can be highly task-specific: it can pertain to the manufacture of a particular tool, for example, and may identify a correlate of success that is very specific to that task, such as the symmetry of the end-product. This task-specificity means high-quality models can be reliably identified, leading to greater exclusivity. A learner equipped with a model selection strategy that reliably picks out high-quality models for particular task-specific skills has more to gain from investing effort in copying the specific technique of a specific individual, rather than hedging their bets and learning from as many different models as possible [51,82].
High-fidelity copying of small improvements is the type of copying that allows fast accumulation. Because learners are very discriminating—they choose only the best models in the relevant task domain, and learn exclusively or nearly exclusively from them—small improvements in technique can spread reliably and rapidly without being explicitly recognized by the learner as improvements. They spread because they make a substantial difference to the cultural fitness of their bearers: their success in attracting learners who are selecting their models using explicit learning biases. If a small improvement to an existing technique promotes the correlate of success tracked by the learning bias (e.g. the symmetry of the end-product), learners will gravitate towards the innovator. Small improvements to existing techniques will be retained in the population and will spread widely, creating many new sites for new small improvements, which will in turn be retained and copied.
This is a process with a Darwinian character, but one in which change is driven not by differences in reproductive success but by differences between models in the number of learners they attract. This is CS2: cultural change driven by culturally inherited differences in cultural fitness. CS2 is the engine of fast, cumulative cultural change. We do not think there was a single, special moment when CS2 ‘took off’, and we do not think that explicit learning biases were the only crucial ingredient. Life history mattered: as juvenile development lengthened, juveniles were able to invest more and more time in learning knowledge and skills, increasing the opportunities for CS2. Demography also mattered: in large, richly interconnected populations, there were more heads in which innovation could occur, more heads in which small improvements could be retained, and more to gain from seeking out the best models for particular skills [83]. We envisage CS2 becoming gradually more important over time, as explicit learning biases become increasingly task-specific and exclusive, as the juvenile period of human life history becomes increasingly long, and as populations become larger and more richly interconnected. Levallois prepared core techniques may be an archaeological signature of cumulative CS2 [19].
(f). Step 5: Cultural Selection 2 of cognitive mechanisms
CS2 is not limited to the selection of small improvements to knowledge and skills. Small improvements to cognitive mechanisms that accelerate cultural accumulation, including imitation, mindreading and metacognition, can also increase in frequency.
How might this work? The idea is that the cognitive mechanisms of cultural learning are themselves learned skills, or ‘cognitive gadgets' (electronic supplementary material, S3; [30]), and learners are selective in whom they learn these skills from. Let us focus on the case of imitation—copying the topography of body movements. Imitation involves learning associations between how movements feel from the inside and how they look from the outside, solving the correspondence problem. An adult can help a child to develop imitation in various ways: by copying their own movements (i.e. by imitating them) in their sight, by giving them opportunities to practise copying bodily movements with appropriate feedback, and by seeding the child's environment with reflective surfaces that allow the child to see what they are doing [84].
In contrast with some other skills, a child will not learn imitation from a single model. More plausibly, the child will learn it gradually from a wide range of adults and peers in their residential group. Because of this, we think a process of model selection operating at the group level may have been particularly important. Provided migration between residential groups is possible (and, in contemporary hunter–gatherers, it is common), the migrating parent can choose the group in which they live and can therefore choose the child's set of models. The parent will choose using learned explicit rules, such as move where people look healthiest, which they can put into practice at large seasonal aggregations of many residential groups [85]. The parent's choice will be influenced by, among other factors, the imitative abilities of the group, in so far as groups with better imitative abilities will be more cooperative and have better ecological knowledge and skills. Groups with better imitative abilities will attract more migrants and therefore more learners. This is a form of CS2, since it relies on model selection, but the model selection involves evaluations of whole groups rather than individuals. Moreover, since imitation is transmitted in part through models imitating learners, we predict that models who are better imitators will also be better at transmitting their superior imitative abilities to those who spend time around them—a positive feedback loop.
In short, groups whose members have better cognitive mechanisms for high-fidelity, selective copying will attract more migrants and grow in size at the expense of others. As groups get larger, there are more and more heads in which a new, small innovation might occur. This has the potential to further accelerate the CS2 of knowledge and skills at the level of individuals within those groups, making the group as a whole yet more attractive to new migrants—another positive feedback loop.
4. Conclusion
The self-assembly hypothesis offers three new answers to the question: Why is cumulative culture uniquely human? They are not mutually exclusive, and each answer is empirically testable.
First, it is possible that CS1 of knowledge and skills (Step 1) has rarely evolved because few species have had a social structure that gives juveniles the opportunity to learn a lot from their parents with high fidelity [48,86]. This would be supported by evidence—perhaps modelled on recent work with dolphins [2,4,12,63]—showing that CS1 of knowledge and skills is more common in species where juveniles spend most of their time with biological relatives of the previous generation.
Second, it may be difficult to transition from CS1 of knowledge and skills to CS1 of attentional biases (Step 2) because variants required to track parental attention are rarely available. In humans, attention tracking is enhanced by inborn tendencies to orient to faces [87] and voices [88] and possibly by reduced scleral coloration [89]. A priority for future research is to find out which extant species are capable of socially learning attentional biases (for example, to learn copy females by observing the orienting-behaviour of third parties [66]), and whether, in human and nonhuman populations, such biases can be vertically inherited in a way that enhances biological fitness.
Third, there may be a roadblock at the emergence of explicit social learning biases (Step 3). These require explicit cognition of a kind that may or may not be dependent on language [51]. To find out, we need research with nonhuman animals that does not merely document social learning biases, but is designed to distinguish biases mediated by implicit (attentional) from explicit cognition [57].
The self-assembly hypothesis suggests that fast, cumulative culture emerged slowly by means of CS1. In this respect, it is consistent with archaeological evidence that the stone tools used by early hominins (Oldowan and Acheulean) were not dependent on fast, cumulative culture [70,90,91].
The search for animal culture [9,11] has been dominated by the idea that patterns of geographical variation in socially learned behaviour, and in particular the diffusion of qualitative novelty, are the most important things to investigate [3,7,8,46]. There has been interesting developmental work on opportunities for social learning in free-living primates, e.g. [92,93], but relatively little attention has been given to vertical cultural inheritance: How much do offspring learn from their parents? Are small improvements retained or not? The self-assembly hypothesis suggests that these questions matter, because the roots of fast cumulative culture are more likely to be found in species that, owing to their social structure, have significant potential for high-fidelity vertical cultural inheritance under free-living conditions. This would be evident in family-specific behaviours: techniques passed down the generations of a family group and varying between families, with small improvements being retained.
We have argued that the self-assembly hypothesis is coherent, plausible, and empirically testable. It contrasts with a standard gene–culture coevolution story by giving a central role to culture–culture coevolution, with a fairly circumscribed role for genetic change. This type of account is favoured by evidence that cultural learning is culturally inherited today (electronic supplementary material, S3) and can be tested further through empirical research on the behaviour of human and nonhuman animals.
Let us now return to the questions we started with. What makes fast, cumulative cultural evolution work? Our answer is CS2: models varying in the number of learners they attract, with small, unobvious improvements being copied faithfully and more often, allowing those improvements to spread rapidly through populations. Where did it come from? We suggest that CS2 came from CS1: a simpler, slower process in which knowledge and skills, then attentional biases, then explicit biases were inherited by juveniles predominantly from their biological parents. Why is cumulative cultural evolution the sole preserve of humans? Because there are several points along the path from a platform of basic social learning capacities to CS2 where the conditions have to be just right. Other species, throughout the animal kingdom, may be at various points on the path, but only one has made it to the end.
Acknowledgements
We are grateful to Christine Caldwell, Russell Gray, Kevin Laland, Michael Muthukrishna, Kim Sterelny, Claudio Tennie, Tobias Uller and two anonymous reviewers for their comments and advice. J.B. thanks an audience at the ‘Evolution Evolving' conference (University of Cambridge, April 2019) for their questions and feedback.
Data accessibility
This article has no additional data.
Authors' contributions
J.B. and C.H. contributed equally to the reading, thinking and writing of this article.
Competing interests
We declare we have no competing interests.
Funding
J.B.'s research was funded by a Philip Leverhulme Prize from the Leverhulme Trust.
References
- 1.Whitehead H, Rendell L. 2014. The cultural lives of whales and dolphins. Chicago, IL: University of Chicago Press. [Google Scholar]
- 2.Sargeant BL, Mann J. 2009. Developmental evidence for foraging traditions in wild bottlenose dolphins. Anim. Behav. 78, 715-721. ( 10.1016/j.anbehav.2009.05.037) [DOI] [Google Scholar]
- 3.Allen J, Weinrich M, Hoppitt W, Rendell L. 2013. Network-based diffusion analysis reveals cultural transmission of lobtail feeding in humpback whales. Science 340, 485-488. ( 10.1126/science.1231976) [DOI] [PubMed] [Google Scholar]
- 4.Krützen M, Kreicker S, MacLeod CD, Learmonth J, Kopps AM, Walsham P, Allen SJ. 2014. Cultural transmission of tool use by Indo-Pacific bottlenose dolphins (Tursiops sp.) provides access to a novel foraging niche. Proc. R. Soc. B 281, 20140374. ( 10.1098/rspb.2014.0374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiten A, Spiteri A, Horner V, Bonnie KE, Lambeth SP, Schapiro SJ, De Waal FB. 2007. Transmission of multiple traditions within and between chimpanzee groups. Curr. Biol. 17, 1038-1043. ( 10.1016/j.cub.2007.05.031) [DOI] [PubMed] [Google Scholar]
- 6.Boesch C, Tomasello M. 1998. Chimpanzee and human cultures. Curr. Anthropol. 39, 591-614. ( 10.1086/204785) [DOI] [Google Scholar]
- 7.Aplin LM, Farine DR, Morand-Ferron J, Cockburn A, Thornton A, Sheldon BC. 2015. Experimentally induced innovations lead to persistent culture via conformity in wild birds. Nature 518, 538. ( 10.1038/nature13998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whiten A, Caldwell CA, Mesoudi A. 2016. Cultural diffusion in humans and other animals. Curr. Opin. Psychol. 8, 15-21. ( 10.1016/j.copsyc.2015.09.002) [DOI] [PubMed] [Google Scholar]
- 9.Laland KN, Galef BG. 2009. The question of animal culture. Cambridge, MA: Harvard University Press. [Google Scholar]
- 10.Avital E, Jablonka E. 2000. Animal traditions: behavioural inheritance in evolution. Cambridge, UK: Cambridge University Press. [DOI] [PubMed] [Google Scholar]
- 11.Dean LG, Vale GL, Laland KN, Flynn E, Kendal RL. 2014. Human cumulative culture: a comparative perspective. Biol. Rev. 89, 284-301. ( 10.1111/brv.12053) [DOI] [PubMed] [Google Scholar]
- 12.Krützen M, Mann J, Heithaus MR, Connor RC, Bejder L, Sherwin WB. 2005. Cultural transmission of tool use in bottlenose dolphins. Proc. Natl Acad. Sci. USA 102, 8939-8943. ( 10.1073/pnas.0500232102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humle T. 2010. How are army ants shedding new light on culture in chimpanzees? In The mind of the chimpanzee: ecological and experimental perspectives (eds Lonsdorff EV, Ross SR, Matsuzawa T), pp. 116-127. Chicago, IL: University of Chicago Press. [Google Scholar]
- 14.Sterelny K. 2012. The evolved apprentice: how evolution made humans unique. Cambridge, MA: MIT Press. [Google Scholar]
- 15.Henrich J. 2016. The secret of our success: how culture is driving human evolution, domesticating our species, and making us smarter. Princeton, NJ: Princeton University Press. [Google Scholar]
- 16.Richerson PJ, Boyd R. 2005. Not by genes alone: how culture transformed human evolution. Chicago, IL: University of Chicago Press. [Google Scholar]
- 17.Boyd R. 2017. A different kind of animal: how culture transformed our species. Princeton, NJ: Princeton University Press. [Google Scholar]
- 18.Acerbi A, Tennie C, Nunn CL. 2011. Modeling imitation and emulation in constrained search spaces. Learn. Behav. 39, 104-114. ( 10.3758/s13420-010-0009-z) [DOI] [PubMed] [Google Scholar]
- 19.van Schaik CP, Pradhan GR, Tennie C. 2019. Teaching and curiosity: sequential drivers of cumulative cultural evolution in the hominin lineage. Behav. Ecol. Sociobiol. 73, 2. ( 10.1007/s00265-018-2610-7) [DOI] [Google Scholar]
- 20.Boyd R, Richerson PJ. 1985. Culture and the evolutionary process. Chicago, IL: University of Chicago Press. [Google Scholar]
- 21.Muthukrishna M, Henrich J. 2016. Innovation in the collective brain. Phil. Trans. R. Soc. B 371, 20150192. ( 10.1098/rstb.2015.0192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavalli-Sforza LL, Feldman MW. 1981. Cultural transmission and evolution: a quantitative approach. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- 23.El Mouden C, André JB, Morin O, Nettle D. 2014. Cultural transmission and the evolution of human behaviour: a general approach based on the Price equation. J. Evol. Biol. 27, 231-241. ( 10.1111/jeb.12296) [DOI] [PubMed] [Google Scholar]
- 24.Avital E, Jablonka E, Lachmann M. 1998. Adopting adoption. Anim. Behav. 55, 1451-1459. ( 10.1006/anbe.1998.0729) [DOI] [PubMed] [Google Scholar]
- 25.Birch J. 2017. The philosophy of social evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 26.González-Forero M, Gardner A. 2018. Inference of ecological and social drivers of human brain-size evolution. Nature 557, 554. ( 10.1038/s41586-018-0127-x) [DOI] [PubMed] [Google Scholar]
- 27.Hill K, Kaplan H. 1999. Life history traits in humans: theory and empirical studies. Annu. Rev. Anthropol. 28, 397-430. ( 10.1146/annurev.anthro.28.1.397) [DOI] [PubMed] [Google Scholar]
- 28.Anderson ML, Finlay BL. 2014. Allocating structure to function: the strong links between neuroplasticity and natural selection. Front. Hum. Neurosci. 7, 918. ( 10.3389/fnhum.2013.00918) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burkart JM, Schubiger MN, van Schaik CP. 2017. The evolution of general intelligence. Behav. Brain Sci. 40, e195. ( 10.1017/S0140525X16000959) [DOI] [PubMed] [Google Scholar]
- 30.Heyes C. 2018. Cognitive gadgets: the cultural evolution of thinking. Cambridge, MA: Harvard University Press. [Google Scholar]
- 31.Reader SM, Hager Y, Laland KN. 2011. The evolution of primate general and cultural intelligence. Phil. Trans. R. Soc. B 366, 1017-1027. ( 10.1098/rstb.2010.0342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dennett DC. 2017. From bacteria to Bach and back: the evolution of minds. New York, NY: WW Norton & Company. [Google Scholar]
- 33.Heyes C, Chater N, Dwyer DM. 2020. Sinking in: the peripheral Baldwinisation of human cognition. Trends Cogn Sci. 24, 884-899. ( 10.1016/j.tics.2020.08.006) [DOI] [PubMed] [Google Scholar]
- 34.Heyes C, Ray E, Mitchell C, Nokes T. 2000. Stimulus enhancement: controls for social facilitation and local enhancement. Learn. Motiv. 31, 83-98. ( 10.1006/lmot.1999.1041) [DOI] [Google Scholar]
- 35.Spence KW. 1937. Experimental studies of learning and the higher mental processes in infra-human primates. Psychol. Bull. 34, 806-850. ( 10.1037/h0061498) [DOI] [Google Scholar]
- 36.Mineka S, Davidson M, Cook M, Keir R. 1984. Observational conditioning of snake fear in rhesus monkeys. J. Abnorm. Psychol. 93, 355-372. ( 10.1037/0021-843X.93.4.355) [DOI] [PubMed] [Google Scholar]
- 37.Haaker J, Golkar A, Selbing I, Olsson A. 2017. Assessment of social transmission of threats in humans using observational fear conditioning. Nat. Protoc. 12, 1378. ( 10.1038/nprot.2017.027) [DOI] [PubMed] [Google Scholar]
- 38.Heyes CM. 1994. Social learning in animals: categories and mechanisms. Biol. Rev. 69, 207-231. ( 10.1111/j.1469-185X.1994.tb01506.x) [DOI] [PubMed] [Google Scholar]
- 39.Hill MR, Boorman ED, Fried I. 2016. Observational learning computations in neurons of the human anterior cingulate cortex. Nat. Commun. 7, 12722. ( 10.1038/ncomms12722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bryson JJ. 2009. Representations underlying social learning and cultural evolution. Interact. Stud. 10, 77-100. ( 10.1075/is.10.1.06bry) [DOI] [Google Scholar]
- 41.Whiten A, Ham R. 1992. On the nature and evolution of imitation in the animal kingdom: reappraisal of a century of research. Adv. Stud. Behav. 21, 239-283. ( 10.1016/S0065-3454(08)60146-1) [DOI] [Google Scholar]
- 42.Alem S, Perry CJ, Zhu X, Loukola OJ, Ingraham T, Søvik E, Chittka L. 2016. Associative mechanisms allow for social learning and cultural transmission of string pulling in an insect. PLoS Biol. 14, e1002564. ( 10.1371/journal.pbio.1002564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carcea I, Froemke RC. 2019. Biological mechanisms for observational learning. Curr. Opin. Neurobiol. 54, 178-185. ( 10.1016/j.conb.2018.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Happé F, Cook JL, Bird G. 2017. The structure of social cognition: in(ter)dependence of sociocognitive processes. Annu. Rev. Psychol. 68, 243-267. ( 10.1146/annurev-psych-010416-044046) [DOI] [PubMed] [Google Scholar]
- 45.Heyes C. 2012. What's social about social learning? J. Comp. Psychol. 126, 193-202. ( 10.1037/a0025180) [DOI] [PubMed] [Google Scholar]
- 46.Hoppitt W, Laland KN. 2013. Social learning: an introduction to mechanisms, methods, and models. Princeton, NJ: Princeton University Press. [Google Scholar]
- 47.Joiner J, Piva M, Turrin C, Chang SW. 2017. Social learning through prediction error in the brain. NPJ Sci. Learn. 2, 8. ( 10.1038/s41539-017-0009-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coussi-Korbel S, Fragaszy DM. 1995. On the relation between social dynamics and social learning. Anim. Behav. 50, 1441-1453. ( 10.1016/0003-3472(95)80001-8) [DOI] [Google Scholar]
- 49.Kulahci IG, Ghazanfar AA, Rubenstein DI. 2018. Knowledgeable lemurs become more central in social networks. Curr. Biol. 28, e1302. ( 10.1016/j.cub.2018.02.079) [DOI] [PubMed] [Google Scholar]
- 50.Claidiere N, Messer EJ, Hoppitt W, Whiten A. 2013. Diffusion dynamics of socially learned foraging techniques in squirrel monkeys. Curr. Biol. 23, 1251-1255. ( 10.1016/j.cub.2013.05.036) [DOI] [PubMed] [Google Scholar]
- 51.Heyes C. 2016. Who knows? Metacognitive social learning strategies. Trends Cogn. Sci. 20, 204-213. ( 10.1016/j.tics.2015.12.007) [DOI] [PubMed] [Google Scholar]
- 52.Kendal R, Hopper LM, Whiten A, Brosnan SF, Lambeth SP, Schapiro SJ, Hoppitt W. 2015. Chimpanzees copy dominant and knowledgeable individuals: implications for cultural diversity. Evol. Hum. Behav. 36, 65-72. ( 10.1016/j.evolhumbehav.2014.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Griffiths O, Le Pelley ME. 2019. The outcome predictability bias is evident in overt attention. J. Exp. Psychol. Anim. Learn. Cogn. 45, 290. ( 10.1037/xan0000210) [DOI] [PubMed] [Google Scholar]
- 54.Tankelevitch L, Spaak E, Rushworth MFS, Stokes MG. 2020. Previously reward-associated stimuli capture spatial attention in the absence of changes in the corresponding sensory representations as measured with MEG. J. Neurosci. 40, 5033-5050. ( 10.1523/JNEUROSCI.1172-19.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Le Pelley ME, Mitchell CJ, Beesley T, George DN, Wills AJ. 2016. Attention and associative learning in humans: an integrative review. Psychol. Bull. 142, 1111-1140. ( 10.1037/bul0000064) [DOI] [PubMed] [Google Scholar]
- 56.Alon Y, Arad G, Pine DS, Bar-Haim Y. 2019. Statistical learning as a predictor of attention bias modification outcome: a preliminary study among socially anxious patients. Behav. Res. Ther. 112, 36-41. ( 10.1016/j.brat.2018.11.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heyes C, Pearce JM. 2015. Not-so-social learning strategies. Proc. R. Soc. B 282, 20141709. ( 10.1098/rspb.2014.1709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kendal RL, Boogert NJ, Rendell L, Laland KN, Webster M, Jones PL. 2018. Social learning strategies: bridge-building between fields. Trends Cogn. Sci. 22, 651-665. ( 10.1016/j.tics.2018.04.003) [DOI] [PubMed] [Google Scholar]
- 59.Henrich J, McElreath R. 2003. The evolution of cultural evolution. Evol. Anthropol. 12, 123-135. ( 10.1002/evan.10110) [DOI] [Google Scholar]
- 60.Henrich J. 2004. Cultural group selection, coevolutionary processes and large-scale cooperation. J. Econ. Behav. Org. 53, 3-35. ( 10.1016/S0167-2681(03)00094-5) [DOI] [Google Scholar]
- 61.van de Waal E, Bshary R, Whiten A. 2014. Wild vervet monkey infants acquire the food-processing variants of their mothers. Anim. Behav. 90, 41-45. ( 10.1016/j.anbehav.2014.01.015) [DOI] [Google Scholar]
- 62.Smolker R, Richards A, Connor R, Mann J, Berggren P. 1997. Sponge carrying by dolphins (Delphinidae, Tursiops sp.): a foraging specialization involving tool use? Ethology 103, 454-465. ( 10.1111/j.1439-0310.1997.tb00160.x) [DOI] [Google Scholar]
- 63.Wild S, Krützen M, Rankin RW, Hoppitt WJ, Gerber L, Allen SJ. 2019. Long-term decline in survival and reproduction of dolphins following a marine heatwave. Curr. Biol. 29, R239-R240. ( 10.1016/j.cub.2019.02.047) [DOI] [PubMed] [Google Scholar]
- 64.Chudek M, Heller S, Birch S, Henrich J. 2012. Prestige-biased cultural learning: bystander's differential attention to potential models influences children's learning. Evol. Hum. Behav. 33, 46-56. ( 10.1016/j.evolhumbehav.2011.05.005) [DOI] [Google Scholar]
- 65.Dalmaso M, Pavan G, Castelli L, Galfano G. 2011. Social status gates social attention in humans. Biol. Lett. 8, 450-452. ( 10.1098/rsbl.2011.0881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van de Waal E, Renevey N, Favre CM, Bshary R. 2010. Selective attention to philopatric models causes directed social learning in wild vervet monkeys. Proc. R. Soc. B 277, 2105-2111. ( 10.1098/rspb.2009.2260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grampp M, Sueur C, van de Waal E, Botting J. 2019. Social attention biases in juvenile wild vervet monkeys: implications for socialisation and social learning processes. Primates 60, 261-275. ( 10.1007/s10329-019-00721-4) [DOI] [PubMed] [Google Scholar]
- 68.Liszkowski U, Carpenter M, Henning A, Striano T, Tomasello M. 2004. Twelve-month-olds point to share attention and interest. Dev. Sci. 7, 297-307. ( 10.1111/j.1467-7687.2004.00349.x) [DOI] [PubMed] [Google Scholar]
- 69.Stout D, Hecht E, Khreisheh N, Bradley B, Chaminade T. 2015. Cognitive demands of Lower Paleolithic toolmaking. PLoS ONE 10, e0121804. ( 10.1371/journal.pone.0121804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stout D, Hecht EE. 2017. Evolutionary neuroscience of cumulative culture. Proc. Natl Acad. Sci. USA 114, 7861-7868. ( 10.1073/pnas.1620738114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Putt SS, Wijeakumar S, Franciscus RG, Spencer JP. 2017. The functional brain networks that underlie Early Stone Age tool manufacture. Nat. Hum. Behav. 1, 102. ( 10.10.1038/s41562-017-0102) [DOI] [Google Scholar]
- 72.Pargeter J, Khreisheh N, Stout D. 2019. Understanding stone tool-making skill acquisition: experimental methods and evolutionary implications. J. Hum. Evol. 133, 146-166. ( 10.1016/j.jhevol.2019.05.010) [DOI] [PubMed] [Google Scholar]
- 73.Gowlett J, Gamble C, Dunbar R. 2012. Human evolution and the archaeology of the social brain. Curr. Anthropol. 53, 693-722. ( 10.1086/667994) [DOI] [Google Scholar]
- 74.Gamble C, Gowlett J, Dunbar R. 2011. The social brain and the shape of the Palaeolithic. Cambr. Archaeol. J. 21, 115-136. ( 10.1017/S0959774311000072) [DOI] [Google Scholar]
- 75.Grove M. 2010. The archaeology of group size. In Social brain, distributed mind (eds Dunbar R, Gamble C, Gowlett J), p. 391. Oxford, UK: Oxford University Press. ( 10.5871/bacad/9780197264522.003.0019) [DOI] [Google Scholar]
- 76.Dunstone J, Caldwell CA. 2018. Cumulative culture and explicit metacognition: a review of theories, evidence and key predictions. Palgrave Commun. 4, 145. ( 10.1057/s41599-018-0200-y) [DOI] [Google Scholar]
- 77.Hawkins RXD, Goodman ND, Goldstone RL. 2019. The emergence of social norms and conventions. Trends Cogn. Sci. 23, 158-169. ( 10.1016/j.tics.2018.11.003) [DOI] [PubMed] [Google Scholar]
- 78.Diaconescu AO, Mathys C, Weber LA, Daunizeau J, Kasper L, Lomakina EI, Fehr E, Stephan KE. 2014. Inferring on the intentions of others by hierarchical Bayesian learning. PLoS Comput. Biol. 10, e1003810. ( 10.1371/journal.pcbi.1003810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morgan T, Rendell L, Ehn M, Hoppitt W, Laland K. 2011. The evolutionary basis of human social learning. Proc. R. Soc. B 279, 653-662. ( 10.1098/rspb.2011.1172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wisdom TN, Song X, Goldstone RL. 2013. Social learning strategies in networked groups. Cogn. Sci. 37, 1383-1425. ( 10.1111/cogs.12052) [DOI] [PubMed] [Google Scholar]
- 81.Heyes C. 2017. When does social learning become cultural learning? Dev. Sci. 20, e12350. ( 10.1111/desc.12350) [DOI] [PubMed] [Google Scholar]
- 82.Heyes C. 2016. Blackboxing: social learning strategies and cultural evolution. Phil. Trans. R. Soc. B 371, 20150369. ( 10.1098/rstb.2015.0369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.French JC. 2016. Demography and the Palaeolithic archaeological record. J. Archaeol. Method Theory 23, 150-199. ( 10.1007/s10816-014-9237-4) [DOI] [Google Scholar]
- 84.Heyes C. 2016. Homo imitans? Seven reasons why imitation couldn't possibly be associative. Phil. Trans. R. Soc. B 371, 20150069. ( 10.1098/rstb.2015.0069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Binford L. 2001. Constructing frames of reference: an analytical method for archaeological theory building using ethnographic and environmental data sets. Berkeley, CA: University of California Press. [Google Scholar]
- 86.De Waal FBM, Bonnie KE. 2009. In tune with others: the social side of primate culture. In The question of animal culture (eds Laland KN, Galef BG), pp. 19-39. Cambridge, MA: Harvard University Press. [Google Scholar]
- 87.Johnson MH, Senju A, Tomalski P. 2015. The two-process theory of face processing: modifications based on two decades of data from infants and adults. Neurosci. Biobehav. Rev. 50, 169-179. ( 10.1016/j.neubiorev.2014.10.009) [DOI] [PubMed] [Google Scholar]
- 88.Vouloumanos A, Werker JF. 2007. Listening to language at birth: evidence for a bias for speech in neonates. Dev. Sci. 10, 159-164. ( 10.1111/j.1467-7687.2007.00549.x) [DOI] [PubMed] [Google Scholar]
- 89.Perea-García JO, Kret ME, Monteiro A, Hobaiter C. 2019. Scleral pigmentation leads to conspicuous, not cryptic, eye morphology in chimpanzees. Proc. Natl Acad. Sci. USA 116, 19 248-19 250. ( 10.1073/pnas.1911410116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tennie C, Premo LS, Braun DR, McPherron SP. 2017. Early stone tools and cultural transmission: resetting the null hypothesis. Curr. Anthropol. 58, 652-672. ( 10.1086/693846) [DOI] [Google Scholar]
- 91.Stout D, Khreisheh N. 2015. Skill learning and human brain evolution: an experimental approach. Cambr. Archaeol. J. 25, 867-875. ( 10.1017/S0959774315000359) [DOI] [Google Scholar]
- 92.Biro D, Inoue-Nakamura N, Tonooka R, Yamakoshi G, Sousa C, Matsuzawa T. 2003. Cultural innovation and transmission of tool use in wild chimpanzees: evidence from field experiments. Anim. Cogn. 6, 213-223. ( 10.1007/s10071-003-0183-x) [DOI] [PubMed] [Google Scholar]
- 93.Biro D, Sousa C, Matsuzawa T. 2006. Ontogeny and cultural propagation of tool use by wild chimpanzees at Bossou, Guinea: case studies in nut cracking and leaf folding. In Cognitive development in chimpanzees (eds Matsuzawa T, Tomonaga M, Tanaka M), pp. 476-508. Tokyo, Japan: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.


