Abstract
Background:
The outcomes of deep vein thrombosis (DVT) in children with May-Thurner Syndrome (MTS) remain unclear.
Objectives:
This systematic review and patient-level meta-analysis aims to describe the outcomes of children with MTS presenting with DVT.
Methods:
A systematic review of the published literature was performed. Data related to patients <18 years diagnosed with MTS and DVT was extracted. Risk of bias was assessed using the Murad criteria. Outcomes included vessel patency post-treatment, DVT recurrence, and post-thrombotic syndrome (PTS). Predictive and explanatory models were developed for these outcomes.
Results:
In total, 109 cases were identified (age range 4–17 years; 77 females) in 28 studies; 75% of patients had ≥1 additional risk factor for DVT. PTS was seen in 61% of patients, DVT recurrence in 38%, and complete vessel patency post-treatment in 65%. The models developed to predict and explain PTS performed poorly overall. Recurrent thrombosis (adjusted for age and patency) predicted PTS (odds ratio [OR] 3.36, 95% confidence interval [CI] 1.28–8.82). DVT management strategies (adjusted for age and DVT characteristics) predicted vessel patency (OR 2.10, 95% CI 1.43–3.08). Lack of complete vessel patency (adjusted for age and thrombophilia) predicted recurrent DVT (OR 2.70, 95% CI 1.09–6.67). Sensitivity analyses showed the same direction of effects for all outcomes.
Conclusions:
PTS and DVT recurrence occur frequently in pediatric MTS. PTS prediction is complex and it was not possible to identify early predictors to guide clinical practice. Use of imaging-guided therapy and thrombus burden predicted venous patency, and lack of patency predicted DVT recurrence.
Keywords: anticoagulants, iliac vein compression, iliac vein lesion, post-thrombotic syndrome, post-phlebitic syndrome
1 |. INTRODUCTION
May-Thurner Syndrome (MTS) is defined as the venous signs and symptoms caused by external compression of the left common iliac vein by the overriding right common iliac artery.1 First described by Virchow in 1851, who remarked on the propensity of iliofemoral deep venous thrombosis (DVT) to occur on the left side,2 MTS is a variant of the iliac vein obstruction syndrome.1 The condition was subsequently named after May and Thurner, who described the occurrence of venous spurs due to chronic compression of the left common iliac vein in the 1950s.3,4
Given that a certain degree of compression of the left common iliac vein is likely normal in the general population, the presence of venous symptoms is critical to MTS diagnosis. Thrombosis may or may not be present, but its presence commonly leads to therapeutic intervention due to the risk of pulmonary embolism,5 progression of thrombosis, and post-thrombotic syndrome (PTS) in the long term.
Despite increased recognition of this entity in pediatrics, the management and outcomes of cases presenting with DVT remain uncertain. It is clear that there is significant variation in therapy selection relative to physician and institutional experience and that the choice of therapy will also vary relative to patient-specific factors. This systematic review and individual patient meta-analysis aims to describe the outcomes (PTS, DVT recurrence, vessel patency) of children aged <18 years with MTS presenting with DVT.
2 |. METHODS
2.1 |. Eligibility criteria
Studies were included in this systematic review if they reported cases of pediatric MTS with ipsilateral DVT in patients aged <18 years.
2.2 |. Search strategy
A systematic search of literature published in Medline (1946 to September 2017), Evidence Based Medicine Reviews (EBMR)—Cochrane Central Register of Controlled Trials (until September 2017), and Embase (1947 to 2017 Week 41) was performed with the assistance of a research librarian (protocol not registered). The search strategies for each database are shown in Appendix S1 in supporting information. No publication year or language restrictions were applied to the search. Abstracts and titles were first screened by one of the authors (NC) to identify relevant publications. Full reports of manuscripts identified through this search were independently reviewed by two authors (NC, LB) to determine eligibility.
2.3 |. Data abstraction
Data of individual patients in the included papers were abstracted by two authors (NC, LA) and comprised patient sex and age at the time of DVT diagnosis, DVT risk factors (oral contraceptives, overweight/obesity, thrombophilia as per local criteria), clinical presentation, duration of symptoms prior to DVT diagnosis, localization, affected venous segments, degree of occlusion, DVT treatment, and treatment-related complications. Bleeding was classified as per current guidelines by the ISTH.6 Thrombophilia was classified as major, minor, or none, as previously described.7
The main outcomes were PTS occurrence, vessel patency after treatment, and DVT recurrence. Due to the expected heterogeneity of criteria used to define PTS in each case, and according to the current definition, PTS was considered to be present when patients were reported to have any signs or symptoms secondary to DVT and its sequelae.1 The tool or criteria used for PTS assessment, PTS severity, and time of PTS assessment and of DVT recurrence were extracted.
When manuscripts reported aggregated data and in cases of missing data, the authors were contacted to obtain disaggregated or missing values using a standardized data collection form. Data were checked for consistency.
Ethics review board approval was not sought as cases were published and, therefore, freely available in the public domain. Missing data were shared according to ethics review board approval at the institution of the authors of each individual study.
2.4 |. Risk of bias assessment
The criteria developed by Murad et al. were used for evaluation of methodology and risk of bias of case series and case reports, with focus on the domains of patient selection, ascertainment of outcome and exposure, and reporting.8 management, a relevant confounder of this direct association, was not available.
2.5 |. Data analysis
Data were described using medians, ranges, 25th–75th percentile, or percentages, as appropriate. Logistic regression was used to predict the occurrence of the outcomes. In accordance with modern regression modeling strategies,9 predictors included in the models were prespecified. Candidate predictors were chosen on the basis of clinical relevance, quality of measurement, and the moment in time at which they were available (at diagnosis or follow-up). Their hypothesized relationships are depicted in Appendix S2 in supporting information.
A first explanatory model included known prognostic factors for PTS that have been more consistently reported in the literature.10,11 Predictors for this model included patient age at the time of DVT, DVT resolution at the end of treatment, and DVT recurrence. A second predictive model consisted of early prognostic factors available at the time of DVT diagnosis and initial management. The objective for this second model was to determine whether those early factors could help guide clinicians on the initial management of these patients. Prognostic factors for the second model consisted of three domains: (1) patient age, (2) characteristics of the index DVT (degree of occlusion of index DVT, extension of the initial DVT, DVT localization [iliofemoral vs. femoropopliteal]), and (3) DVT treatment-related factors (image-guided vs. non—image-guided DVT therapy, including catheter-directed, mechanical and pharmaco-mechanical thrombolysis, venous stent placement in the acute setting, venous angioplasty), which was the main exposure. Extension of DVT was measured as the absolute number of affected venous segments and as the percentage of compromised venous bed from inferior vena cava (IVC) to calf. Percentage of affected venous bed was determined by estimating the length of individual affected venous segments in relation to the total size of the lower limb vascular bed.12 In order to accommodate the number of predictors that the data were able to support, these variables were clustered into domains for variable reduction.9 Cluster summary scores were developed using additive, best scoring, and multiplicative weights (Appendix S3 in supporting information). Because linear relationships of continuous predictors are rare, the relationship between age and outcomes was visualized using spike histograms and was fitted using splines if plots suggested a non-linear relationship.
A model to predict vessel patency after treatment was built using patient age, DVT characteristics, and DVT treatment (both as defined above) as potential predictors. A model to predict DVT recurrence was built using vessel patency, thrombophilia (major, minor, none), and age as hypothesized predictors. Last, the potential direct role of stents on recurrence by relieving venous compression, besides their effect through vessel patency, was explored. The analysis was exploratory only because long-term anticoagulation
Sensitivity analyses including only studies that reported unselected MTS cases or cases selected based on clinical presentation (i.e., excluding cases selected due to their treatment strategy) were conducted.
Predictive mean matching was used to handle missing data on the predictor variables. Bootstrapping was used to estimate the optimism-corrected discriminative ability and calibration of the model (internal validation). The c-index was used to assess discrimination. Absolute prediction accuracy was assessed by means of the calibration slope. Analyses were carried out in R version 4.0.0 using the Hmisc and rms packages.
3 |. RESULTS
A total of 1803 articles were identified and screened for relevance (Figure 1), 45 of which reported cases of pediatric MTS. Ultimately, 28 articles describing 105 cases that met our eligibility criteria were included in the analysis (Appendix S4 in supporting information). Four additional cases were provided by the authors of one of the identified studies, for a total of 109 cases. MTS was diagnosed using venography in 63 patients (63/109, 58%), computed tomography venography (CTV) or magnetic resonance venography (MRV) in 26 patients (26/109, 24%) and combination of CTV or MRV and venography in 19 patients (19/109, 17%).
FIGURE 1.

Flowchart of study selection
Median follow up time in the cohort, as per time of PTS assessment, was 20 months (25th–75th percentile: 13–36 months).
Risk of bias assessment showed that 13/28 (46%) articles comprised a representative sample of all cases diagnosed at the institution or cases selected based on clinical presentation, whereas the remaining studies reported MTS cases selected according to treatment strategy or for unknown reason. In terms of outcome ascertainment, it was not possible to determine the criteria or instrument used for PTS assessment in 11/103 (11%) cases with reported PTS. Researchers used local criteria for PTS diagnosis in 11/103 (11%) cases, and the modified Villalta score, Villalta score, or the Manco-Johnson instrument in the remaining cases (64/103 [62%]; 12/103 [11%]; 5/103 [5%], respectively). Ninety-three of 103 (90%) cases reporting the outcome patency described the imaging modality used for assessment. As to overall reporting, only three articles (four cases) did not provide enough data for analyses and the authors were unable to provide the missing data.
No relevant issues were identified when checking individual patient data.
3.1 |. Demographics
Median age of patients at the time of DVT diagnosis was 16 years (range 4–17 years); 77 patients (77/109, 71%) were female. Characteristics of the included patients are shown in Table 1.
TABLE 1.
Characteristics of included patients and frequency of main outcomes.
| Variable | Distribution |
|---|---|
| Age at DVT diagnosis, median (25th–75th percentile) | 16 years (14–16 years) |
| Male:female ratio | 1:2.4 |
| Clinical findings, n (%) | |
| Limb swelling | 96/109 (88%) |
| Limb pain | 93/109 (85%) |
| Skin cyanosis/discoloration | 14/109 (13%) |
| Paresthesia | 5/109 (5%) |
| Difficulty ambulating | 4/109 (4%) |
| Median duration of symptoms prior to DVT diagnosis, median (25th–75th percentile) | 4.5 days (2–7 days) |
| Thrombophilia, n (%) | |
| None | 49/103 (48%) |
| Minor, total number of patients with ≥1 trait | 24/103 (23%) |
| Major, total number of patients with ≥1 trait | 30/103 (29%) |
| Minor, specific traits | |
| 1. Heterozygous FVL | 22/103 (21%) |
| 2. High FVIII levels | 8/103 (8%) |
| 3. PTG mutation | 2/103 (2%) |
| Major, specific traits | |
| 1. Antiphospholipid syndrome | 13/103 (13%) |
| 2. Antithrombin deficiency | 7/103 (7%) |
| 3. Protein S deficiency | 4/103 (4%) |
| 4. Protein C deficiency | 4/103 (4%) |
| 5. Homozygous FVL | 2/103 (2%) |
| 6. Double heterozygous FVL/PTG mutation | 1/103 (1%) |
| Female patients on oral contraceptives, n (%) | 48/73 (66%) |
| Overweight or obese patients, n (%) | 26/107 (24%) |
| Occlusion of one or more venous segments, n (%) | 86/92 (93%) |
| Ilio-femoral DVT, n (%) | 88/96 (89%) |
| Percentage of vascular bed affected, median (25th–75th percentile) | 70% (48%–71%) |
| Number of affected venous segments, median (25th–75th percentile) | 5 segments (3–5 segments) |
| DVT management, n (%) | |
| Non-interventional therapy | 26/109 (24%) |
| Interventional therapy | 83/109 (76%) |
| Stent placement within 1 month of DVT diagnosis | 56/67 (84%) |
| Stent placement >1 month after DVT diagnosis | 11/67 (16%) |
| Angioplasty | 64/108 (59%) |
| PTS, n (%) | |
| Any PTS | 63/103(61%) |
| Moderate to severe PTS | 13/103 (13%) |
| Patency, n (%) | 67/103 (65%) |
| Recurrence, n (%) | 41/108 (38%) |
Abbreviations: DVT, deep vein thrombosis; FVIII, factor VIII; FVL, factor V Leiden; PTG, prothrombin gene mutation; PTS, post-thrombotic syndrome.
3.2 |. Clinical symptoms
Lower limb swelling and pain were the most common clinical findings. The frequency of the most commonly reported clinical findings is shown in Table 1. Sixteen children (16/109, 15%) had non-lower extremity symptoms including abdominopelvic/inguinal or back pain (11/109, 10%), fever/nausea/diarrhea (3/109, 3%), and chest pain (3/109, 3%). Two of the three patients with chest pain had pulmonary embolism. Associated pulmonary embolism was diagnosed in 19 patients (19/109, 17%).
3.3 |. Presence of additional risk factors for DVT
Eighty-two patients (82/109, 75%) had ≥1 additional risk factor for DVT (45 patients had one, 30 patients had two, and 7 patients had three risk factors). Thrombophilia was diagnosed in 54/103 (52%) patients with complete data on this variable. Its frequency is shown in Table 1. Twelve patients (12/103, 12%) had two or more thrombophilia traits.
The frequency of females on oral contraceptive agents temporally related to the DVT diagnosis and of obesity or overweight at the time of DVT diagnosis is shown in Table 1.
3.4 |. Characteristics of thrombosis
All thromboses affected the left lower limb; four events (4/109, 4%) were bilateral. Data on affected venous segments were available in 96 cases (96/109, 88%). Characteristics of the DVT are shown in Table 1.
3.5 |. DVT management
As shown in Table 1, approximately one quarter of patients were treated with anticoagulation only, including heparin, low molecular weight heparin, warfarin, and rivaroxaban. The remaining patients underwent interventional techniques for DVT treatment. One patient (1/109, 1%) underwent surgical thrombectomy only; of note, this was the earliest reported case, published in 1969. Fifty patients (50/109, 46%) underwent pharmaco-mechanical thrombolysis only, 20 patients (20/109, 18%) underwent catheter-directed thrombolysis only, and 2 patients (2/109, 2%) underwent mechanical thrombectomy only. The remaining 10 patients (10/109, 9%) underwent combined techniques, typically involving catheter-directed thrombolysis in combination with either pharmaco-mechanical or mechanical thrombolysis. One patient underwent systemic thrombolysis to treat pulmonary embolism followed by pharmaco-mechanical thrombolysis for DVT treatment. Sixteen patients (16/83, 19%) were reported to have had an IVC filter placed during the procedures.
Sixty-four of the 108 patients (64/108, 59%) with complete data underwent angioplasty. Sixty-seven of the 107 patients (67/107, 62%) with complete data underwent stent placement. The frequency of angioplasty and stent placement is described in Table 1. Eighty-four patients (84/108, 78%) underwent either stent placement or angioplasty for management of the iliac compression within one month of DVT diagnosis, and 36 (36/108, 33%) underwent both, typically at the same time.
Twelve of 78 patients (15%) who underwent image-guided therapy and whose data on bleeding complications was complete had minor bleeding reported; 3/78 (4%) had a major bleeding episode with a ≥2 g/L hemoglobin drop or requiring packed red blood cell transfusion. Conversely, 1/23 (4%) patients managed with anticoagulation only and whose data were complete had minor bleeding events; no major bleeding events were reported. No deaths were reported in the entire cohort.
3.6 |. Outcomes
3.6.1 |. PTS
Data on PTS were available in 103 patients. As shown in Table 1, nearly two thirds of patients had ≥1 clinical finding compatible with PTS on follow-up. Overall, PTS was seen in 46 out of 78 patients (46/78, 59%) treated with interventional modalities for DVT management, and 17 out of 25 patients (17/25, 68%) treated with anticoagulation alone (p = .57). Results of the logistic regression models for this outcome are shown in Table 2 and Figure 2. Age was fitted as a restricted cubic spline with three knots for the models. The first model, which included previously published predictors (age, recurrence, patency), showed an association between PTS and recurrence, adjusted for age and patency. Internal validation showed that the model had low discriminative power, and the calibration curve showed moderate underestimation of actual PTS probabilities on the lower end of the prediction and overestimation on the upper end (Figure 2). Partial effect plots for each adjusted predictor on this model are also shown in Figure 2. The sensitivity analysis for this model, which included 61 unselected cases or cases selected based on their clinical presentation but not their treatment strategy, is shown in Appendix S5 in supporting information. Predictors showed the same direction of relationship seen in the model developed in the original dataset.
TABLE 2.
Regression model coefficients and performance.
| OR (95% CI) | Optimism corrected c statistics | Optimism corrected calibration slope | |
|---|---|---|---|
| Outcome post-thrombotic syndrome, previously published predictors (omnibus likelihood ratio χ2 11.13; 4DF; p = .02) | |||
| Age (10 vs. 14 years) | 1.78 (0.69–4.55) | 0.64 | 0.73 |
| Recurrence (yes vs. no) | 3.36(1.28–8.82) | ||
| Patency (no vs. yes) | 1.35 (0.54–3.33) | ||
| Outcome post-thrombotic syndrome, early predictors (omnibus likelihood ratio χ2 4.76; 4DF; p = .31) | |||
| Age (10 vs. 14 years) | 1.58 (0.65–3.86) | 0.54 | 0.52 |
| Index DVT scorea | 1.12 (0.44–2.82) | ||
| DVT treatment scoreb | 0.83 (0.61–1.13) | ||
| Outcome patency (omnibus likelihood ratio χ2 19.71; 4DF; p = .0007) | |||
| Age (10 vs. 14 years) | 0.41 (0.13–1.29) | 0.71 | 0.74 |
| Index DVT scorea | 0.31 (0.10–0.98) | ||
| DVT treatment scoreb | 2.10 (1.43–3.08) | ||
| Outcome recurrent DVT (omnibus likelihood ratio χ2 10.63; 4DF; p = .02) | |||
| Age in years | 0.83 (0.70–1.00) | 0.66 | 0.76 |
| Thrombophilia (major vs. none) | 2.24 (0.84–5.99) | ||
| Thrombophilia (minor vs. none) | 0.92 (0.31–2.78) | ||
| Patency (no vs. yes) | 2.70 (1.09–6.67) | ||
Abbreviations: CI, confidence interval; DF, degrees of freedom; DVT, deep vein thrombosis; OR odds ratio.
Index DVT score: Degree of occlusion of index DVT (occlusive = 1) + DVT localization (ilio-femoral = 1) + percentage of extension of the initial DVT (lower inferior vena Cava to calf = 1, see Appendix S3.1 for description of score development).
DVT treatment score: Interventional vs. non-interventional therapy (interventional = 1) + angioplasty (yes = 1) + (stent placement in acute setting [yes = 1]*2). See Appendix S3.2 for description of score development.
FIGURE 2.
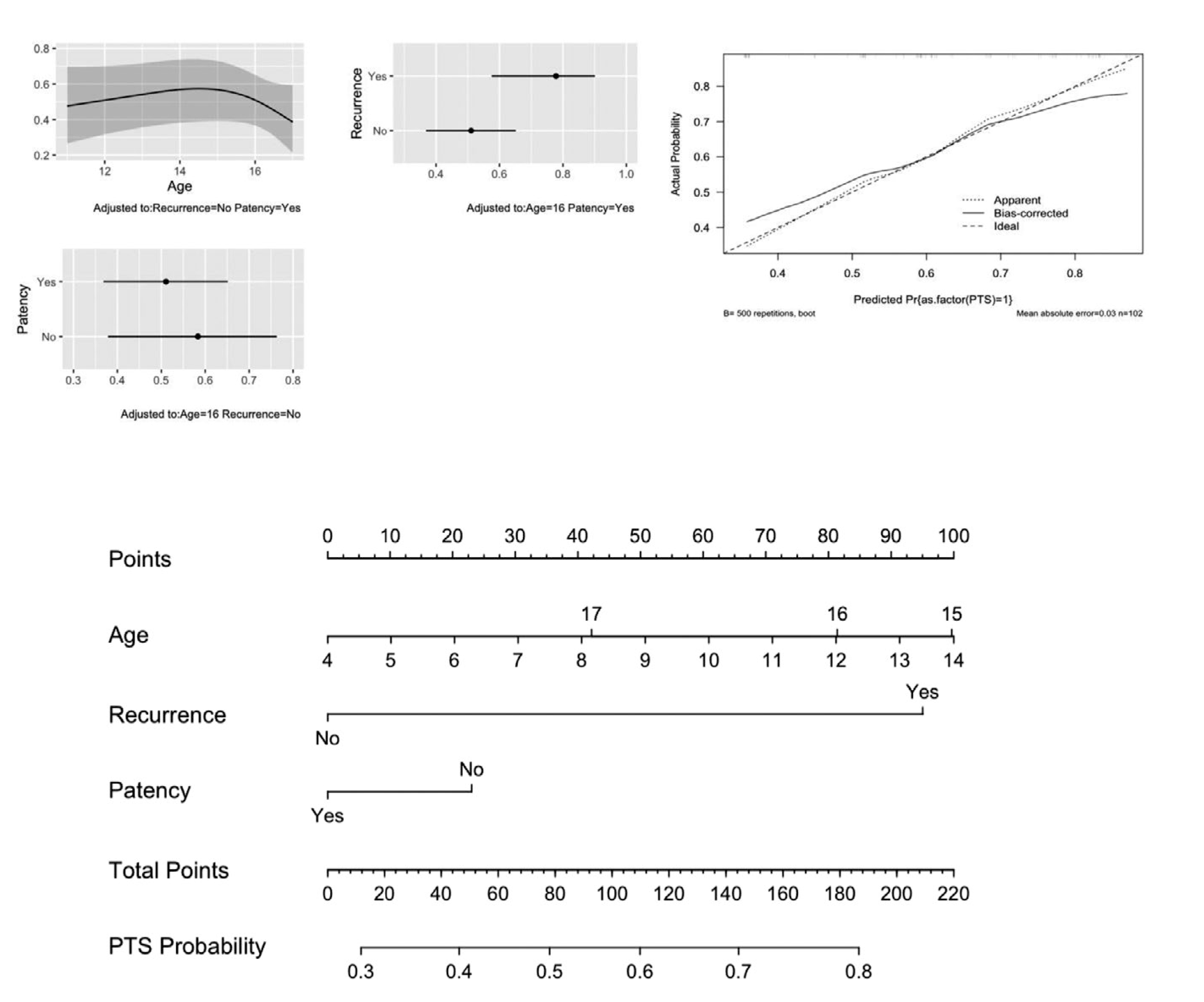
Outcome post-thrombotic syndrome (PTS), previously published predictors, partial effect plots, calibration curve, and nomogram
The second model, which was built using PTS predictors known ex ante (at the time of DVT presentation, including age, characteristics of the DVT or index DVT score, and treatment or DVT treatment score), performed poorly (Table 2) and it was not possible to predict PTS using factors known at the time of DVT diagnosis. The index DVT score and DVT treatment score are shown in Table 2 and in Appendix S3.1 and S3.2.
3.6.2 |. Patency
Data on patency were available in 103 patients. Two thirds of patients were reported to have complete patency on follow-up, as shown in Table 1. Patency was seen in 55 out of 79 patients (55/79, 70%) treated with interventional modalities for DVT management, and in 12 out of 24 patients (12/24, 50%) treated with anticoagulation alone (P = .13). Results of the regression model that included age, index DVT score, and DVT treatment score are shown in Table 2 and Figure 3. The index DVT score and DVT treatment score are described in Table 2 and in Appendix S3.1 and S3.2. Age was fitted as a restricted cubic spline with three knots for this model. Patients with higher DVT treatment scores had higher odds of patency on follow-up, adjusted for index DVT score and patient age at the time of DVT, as compared to patients with lower DVT treatment scores. Similarly, patients with lower index DVT scores (lower thrombus burden) had higher odds of patency than patients with higher scores, adjusted for DVT treatment and patient’s age. Model discrimination was acceptable (Table 2) and calibration slope also showed acceptable prediction accuracy, with moderate underestimation and overestimation of actual probabilities, as shown in Figure 3. Partial effect plots for each adjusted predictor and a nomogram to assist with model interpretation are shown in Figure 3. Sensitivity analysis is shown in Appendix S5. Predictors showed similar coefficients to those of the original model.
FIGURE 3.

Outcome patency, partial effect plots, calibration curve, and nomogram. DVT, deep vein thrombosis
3.6.3 |. Recurrent thrombosis
Data on this outcome were reported in 108 patients. More than one third of them had recurrent thrombosis, as described in Table 1. Median time to recurrence was 135 days (25th-75th percentile, 11–752 days). Results of the regression model that includes the predictors age, thrombophilia, and patency are shown in Table 2 and Figure 4. Age was kept linear in this model. Model discrimination was low (Table 2) and the calibration slope showed underestimation and overestimation of actual probability of recurrence on the lower and upper end of predicted probabilities, respectively (Figure 4). Sensitivity analysis is shown in Appendix S5; predictor coefficients in this model also showed a similar direction to that seen in the model developed in the original dataset. The exploratory model including stents is shown in Appendix S6 in supporting information. This model had better prediction accuracy.
FIGURE 4.

Outcome recurrence, partial effect plots, calibration curve, and nomogram
4 |. DISCUSSION
Pediatric MTS is now universally recognized and published globally. The number of published cases rose from 15 recorded until 2010, to 90 between 2010 and 2017, likely due to the increased use of image-guided techniques for treatment of acute DVT in children.
MTS was diagnosed mainly among teenage girls with most cases presenting with acute symptoms of limb pain and swelling. While it is interesting to note that the initial report of Cockett and Thomas, who coined the term “iliac compression syndrome,” reported an almost equal male-to-female ratio,13 we now recognize MTS as a syndrome predominantly affecting female patients, likely influenced by concomitant risk factors, including oral contraceptive use. The effects of oral contraceptives were not known at the time of Cockett and Thomas’s initial report as they had just been introduced to the market.14 In addition, population studies report that, overall, DVT is more frequent among teenage girls.15,16 Additional risk factors for DVT were seen in 75% of patients, and included thrombophilia in more than 50% of tested patients, exposure to oral contraceptives in more than 50% of female patients, and classification as obese or overweight in 24% of patients, highlighting the multifactorial etiology of thrombotic events, particularly in children.17,18
A minority of patients were treated with anticoagulation only, while most underwent pharmaco-mechanical thrombolysis, alone or in combination with catheter-directed thrombolysis. This contrasts with current pediatric guidelines, which suggest the use of anticoagulation only to treat children with DVT,19 recommending thrombolysis for limb- or life-threatening thrombosis.20 However, the American Society of Hematology expert panel acknowledged very low certainty of the evidence supporting this recommendation and considered that the extent and clinical impact of the thrombotic event were important when evaluating risks and benefits of thrombolysis. Of note, pediatric guidelines do not address MTS in particular.
Our first model investigated previously published predictors of PTS. The model was developed to test potential relationships among PTS predictors using existing knowledge, as shown in Appendix S2.21 In this model, DVT recurrence, adjusted for patency and age, predicted PTS. DVT recurrence is one of the earliest, most consistent, and the strongest overall predictor of PTS reported in adults to date.22–24 In an attempt to predict PTS at a time that is appropriate to make decisions regarding treatment, the second model included variables available at the time of DVT presentation (ex ante) that are relatively easy to measure. Treatment modality was the exposure of interest. Characteristics of the index DVT, which could confound treatment selection, were also included. This strategy did not yield a useful predictive model. Many factors can explain the difficulty in predicting and explaining PTS found in this study. First, the relatively small sample size precludes the development of a more complex model that would be required to explain a very complex biological phenomenon that is PTS. Second, even if known or hypothesized to be relevant, most predictors and even the outcome can be difficult to measure and standardize, which adds to variability among studies and hinders our understanding of the occurrence of PTS. This is demonstrated by the fact that a total of 12 pediatric studies that aimed to investigate PTS predictors in children identified 15 different variables,25–36 and only two of these variables were reported in more than one study. The difficulty in predicting and understanding PTS is not unique to pediatrics. At least 20 different PTS predictors were reported in adult patients.11 These predictors were assessed in different ways in the different studies, and, unsurprisingly, their strength and consistency of association to the outcome varied.11
In addition, we found that a combination of image-guided management (catheter-directed or pharmaco-mechanical thrombolysis, stenting, or angioplasty), adjusted for DVT characteristics and patient’s age, led to improved vessel patency. This effect of treatment on patency was evident even after excluding studies that selected patients by treatment strategy. This finding is in line with data from randomized controlled trials (RCT) in adults, which support the association between thrombolysis and vessel patency.37
RCTs in adult patients also showed that change in thrombus burden predicted DVT recurrence,38 which is deemed a strong predictor of PTS in adults, as mentioned above. Similarly, persistent venous obstruction was also associated with PTS in adult patients.39–41 These observations led to the “open vein hypothesis,” according to which early removal of DVT was thought to result in improved long-term DVT outcomes due to preservation of vein valve function and prevention of venous obstruction.42,43 However, RCTs investigating the efficacy of catheter-directed and pharmaco-mechanical thrombolysis to prevent PTS in adults with lower limb DVT through early thrombus removal, including the CaVenT,44,45 ATTRACT,46,47 and CAVA48 trials, showed that the benefit of this treatment modality was far more modest than predicted, and only benefited selected patients. This could be due to the fact that vein wall damage may occur earlier than expected.49 These results help illustrate the complexity of measuring, characterizing, and predicting PTS. We were also unable to document a direct relationship between DVT treatment modality and PTS occurrence.
As shown in adults,38 we found that vessel patency after treatment, adjusted for thrombophilia and patient’s age, predicted DVT recurrence. However, this model also showed low predictive power, which may be due to its simplicity relative to the included number of predictors. The exploratory model including stent placement in the acute setting to predict DVT recurrence showed better predictive accuracy. In this model, stents did not appear to have a direct role and it is possible that their effect is mainly mediated by patency. However, residual confounding should be considered when interpreting both models, because predictors such as quality and length of anticoagulation treatment were not available in the present study.
Angioplasty and stent placement in adults with symptomatic MTS are expected to decrease the risk of long-term outcomes, including DVT recurrence and PTS.50 Registry data showed improved patency in adults with symptomatic stented iliac lesions compared to non-stented lesions,51 although non-stented patients may not have had stenosis.52 Nonetheless, current adult guidelines recommend the use of stents53 and/or angioplasty54 in flow-limiting persistent iliac obstructions.53
Although the indication for image-guided thrombus removal remains controversial, experts consider that selected adult patients with acute iliofemoral DVT and low risk of bleeding may benefit from this therapeutic modality,42,46,55 and this is reflected in the most recent guidelines for venous thrombosis management in adults.56
There are several limitations to this study, one of which is the variability in the assessment of predictors and outcomes. To overcome this problem, we chose variables that are easy to define and then contacted all authors to obtain specific data on those variables. Although the strategy did not allow creation of more complex models to explain and predict the outcomes, it provided a valuable insight into the management of DVT in pediatric MTS, a highly challenging task in view of the relatively low frequency of MTS in children. In addition, important variables such as length of treatment were not available for most cases and were not included in the models. Therefore, we did not focus our analyses on management following the acute DVT setting. Similarly, PTS definitions varied among studies. We therefore classified the presence of any sign or symptom attributed to PTS as true PTS, in line with the current definition.1 This definition resulted in considering PTS cases that may have been mild or not clinically relevant. The benefit of using image-guided therapies to prevent mild PTS is controversial given the potential occurrence of major bleeding events. However, the long-term impact of pediatric PTS remains to be clarified. It is not known whether minor clinical manifestations could interfere with a child’s development or cause health consequences throughout the patient’s lifespan. In addition, severity rating of PTS is radically different in children and adults. For example, severe limb pain is the most important clinical feature of pediatric PTS, and a child with severe limb pain would undoubtedly be deemed to have clinically important PTS.57 In contrast, severe limb pain as the only symptom is not sufficient to establish even a mild PTS diagnosis in adults.58
In conclusion, our systematic review and meta-analysis demonstrates the difficulty in characterizing and predicting PTS in children with MTS and DVT. Even though treatment modality was not helpful in predicting PTS, it predicted patency, and lack of patency in turn predicted DVT recurrence. This is in line with current data in adults. Thrombosis burden was also a predictor of patency. Image-guided management of pediatric MTS could be considered to improve patency and thus decrease recurrence in patients at low risk of bleeding.
Supplementary Material
Essentials.
Post-thrombotic syndrome and deep vein thrombosis (DVT) recurrence are frequent in children with May-Thurner Syndrome presenting with DVT.
Use of imaging-guided therapy and thrombus burden, adjusted for age, predicted venous patency.
Lack of patency after treatment, adjusted for age and thrombophilia, predicted DVT recurrence.
Major bleeding was seen in 4% of children undergoing imaging-guided therapy for DVT management.
ACKNOWLEDGMENTS
We thank Dr. L. Raffini, N. Goldenberg, A. Mousa, M. Olivieri, Y. De Bast, I. Baumgartner, Y. Diab, and C. Bemrich-Stolz for their help with the study data and Alanna Marson for her help in performing the literature search.
Footnotes
Manuscript handled by: David Lillicrap
CONFLICTS OF INTEREST
The authors have no conflicts to declare.
SUPPORTING INFORMATION Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Eklof B, Perrin M, Delis KT, et al. Updated terminology of chronic venous disorders: the VEIN-TERM transatlantic interdisciplinary consensus document. J Vasc Surg. 2009;49:498–501. [DOI] [PubMed] [Google Scholar]
- 2.Virchow R. Ueber die Erweiterung kleinerer Gefäfse: Hierzu Tab. IV. Arch Für Pathol Anat Physiol Für Klin Med. 1851;3(3):427–462. [Google Scholar]
- 3.May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8(5):419–427. [DOI] [PubMed] [Google Scholar]
- 4.May R, Thurner J. A vascular spur in the vena iliaca communis sinistra as a cause of predominantly left-sided thrombosis of the pelvic veins. Z Kreislaufforsch. 1956;45(23–24):912–922. [PubMed] [Google Scholar]
- 5.Plate G, Ohlin P, Eklöf B. Pulmonary embolism in acute iliofemoral venous thrombosis. Br J Surg. 1985;72(11):912–915. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell LG, Goldenberg NA, Male C, et al. Definition of clinical efficacy and safety outcomes for clinical trials in deep venous thrombosis and pulmonary embolism in children. J Thromb Haemost. 2011;9:1856–1858. [DOI] [PubMed] [Google Scholar]
- 7.Avila ML, Amiri N, Stanojevic S, et al. Can thrombophilia predict recurrent catheter-related deep vein thrombosis in children? Blood. 2018;131(24):2712–2719. [DOI] [PubMed] [Google Scholar]
- 8.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid-Based Med. 2018;23(2):60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis [Internet]. Cham: Springer International Publishing; 2015. [cited 2020 May 27]. (Springer Series in Statistics). Available from: 10.1007/978-3-319-19425-7 [DOI] [Google Scholar]
- 10.Engel ER, Nguyen ATH, Amankwah EK, et al. Predictors of postthrombotic syndrome in pediatric thrombosis: a systematic review and meta-analysis of the literature. J Thromb Haemost. 2020;18(10):2601–2612. [DOI] [PubMed] [Google Scholar]
- 11.Kahn SR, Comerota AJ, Cushman M, et al. The postthrombotic syndrome: evidence-based prevention, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2014;28(130):1636–1661. [DOI] [PubMed] [Google Scholar]
- 12.Ouriel K, Greenberg RK, Green RM, Massullo JM, Goines DR. A volumetric index for the quantification of deep venous thrombosis. J Vasc Surg. 1999;30(6):1060–1066. [DOI] [PubMed] [Google Scholar]
- 13.Cockett FB, Thomas ML. The iliac compression syndrome. Br J Surg. 1965;52(10):816–821. [DOI] [PubMed] [Google Scholar]
- 14.Christin-Maitre S. History of oral contraceptive drugs and their use worldwide. Best Pract Res Clin Endocrinol Metab. 2013;27(1):3–12. [DOI] [PubMed] [Google Scholar]
- 15.Sabapathy CA, Djouonang TN, Kahn SR, Platt RW, Tagalakis V. Incidence trends and mortality from childhood venous thromboembolism: a population-based cohort study. J Pediatr. 2016;172(175–180):e1. [DOI] [PubMed] [Google Scholar]
- 16.Stein PD, Kayali F, Olson RE. Incidence of venous thromboembolism in infants and children: data from the National Hospital Discharge Survey. J Pediatr. 2004;145:563–565. [DOI] [PubMed] [Google Scholar]
- 17.Rosendaal F. Venous thrombosis: a multicausal disease. The Lancet. 1999;353(9159):1167–1173. [DOI] [PubMed] [Google Scholar]
- 18.Rosendaal FR. Venous thrombosis: the role of genes, environment, and behavior. Hematology. 2005;2005(1):1–12. [DOI] [PubMed] [Google Scholar]
- 19.Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monagle P, Chan AK, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e737S–801S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shmueli G. To explain or to predict? Stat Sci. 2010;25(3):289–310. [Google Scholar]
- 22.Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;1(125):1–7. [DOI] [PubMed] [Google Scholar]
- 23.Bouman AC, Smits JJ, Ten Cate H, Ten Cate-Hoek AJ. Markers of coagulation, fibrinolysis and inflammation in relation to post-thrombotic syndrome. J Thromb Haemost. 2012;10:1532–1538. [DOI] [PubMed] [Google Scholar]
- 24.van Dongen CJ, Prandoni P, Frulla M, Marchiori A, Prins MH, Hutten BA. Relation between quality of anticoagulant treatment and the development of the postthrombotic syndrome. J Thromb Haemost. 2005;3:939–942. [DOI] [PubMed] [Google Scholar]
- 25.Goldenberg NA, Knapp-Clevenger R, Manco-Johnson MJ. Elevated plasma factor VIII and D-dimer levels as predictors of poor outcomes of thrombosis in children. N Engl J Med. 2004;9(351):1081–1088. [DOI] [PubMed] [Google Scholar]
- 26.Kreuz W, Stoll M, Junker R, et al. Familial elevated factor VIII in children with symptomatic venous thrombosis and post-thrombotic syndrome: results of a multicenter study. Arterioscler Thromb Vasc Biol. 2006;26:1901–1906. [DOI] [PubMed] [Google Scholar]
- 27.Kuhle S, Spavor M, Massicotte P, et al. Prevalence of post-thrombotic syndrome following asymptomatic thrombosis in survivors of acute lymphoblastic leukemia. J Thromb Haemost. 2008;6:589–594. [DOI] [PubMed] [Google Scholar]
- 28.Sharathkumar AA, Pipe SW. Post-thrombotic syndrome in children: a single center experience. J Pediatr Hematol Oncol. 2008;30:261–266. [DOI] [PubMed] [Google Scholar]
- 29.Tousovska K, Zapletal O, Skotakova J, Bukac J, Sterba J. Treatment of deep venous thrombosis with low molecular weight heparin in pediatric cancer patients: safety and efficacy. Blood Coagul Fibrinolysis. 2009;20(7):583–589. [DOI] [PubMed] [Google Scholar]
- 30.Creary S, Heiny M, Croop J, et al. Clinical course of postthrombotic syndrome in children with history of venous thromboembolism. Blood Coagul Fibrinolysis. 2012;23:39–44. [DOI] [PubMed] [Google Scholar]
- 31.Lyle CA, Gibson E, Lovejoy AE, Goldenberg NA. Acute prognostic factors for post-thrombotic syndrome in children with limb DVT: A Bi-institutional cohort study. Thromb Res. 2013;131(1):37–41. [DOI] [PubMed] [Google Scholar]
- 32.Avila ML, Duan L, Cipolla A, et al. Postthrombotic syndrome following upper extremity deep vein thrombosis in children. Blood. 2014;14(124):1166–1173. [DOI] [PubMed] [Google Scholar]
- 33.Kumar R, Rodriguez V, Matsumoto JMS, et al. Prevalence and risk factors for post thrombotic syndrome after deep vein thrombosis in children: a cohort study. Thromb Res. 2015;135(2):347–351. [DOI] [PubMed] [Google Scholar]
- 34.Avila ML, Pullenayegum E, Williams S, Yue N, Krol P, Brandão LR. Post-thrombotic syndrome and other outcomes of lower extremity deep vein thrombosis in children. Blood. 2016;128:1862–1869. [DOI] [PubMed] [Google Scholar]
- 35.Leeper CM, Vissa M, Cooper JD, Gaines BA. Venous thromboembolism in pediatric trauma patients: Ten-year experience and long-term follow-up in a tertiary care center: Leeper et al. Pediatr Blood Cancer. 2017;64(8):e26415. [DOI] [PubMed] [Google Scholar]
- 36.Manlhiot C, McCrindle BW, Williams S, et al. Characterization of post-thrombotic syndrome in children with cardiac disease. J Pediatr. 2019;207:42–48. [DOI] [PubMed] [Google Scholar]
- 37.Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Vascular Group, editor. Cochrane Database Syst Rev. 2016:1:CD002783. Available from: 10.1002/14651858.CD002783.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hull RD, Marder VJ, Mah AF, Biel RK, Brant RF. Quantitative assessment of thrombus burden predicts the outcome of treatment for venous thrombosis: a systematic review. Am J Med. 2005;118(5):456–464. [DOI] [PubMed] [Google Scholar]
- 39.Prandoni P, Frulla M, Sartor D, Concolato A, Girolami A. Vein abnormalities and the post-thrombotic syndrome. J Thromb Haemost. 2005;3:401–402. [DOI] [PubMed] [Google Scholar]
- 40.Vedovetto V, Dalla Valle F, Milan M, Pesavento R, Prandoni P. Residual vein thrombosis and trans-popliteal reflux in patients with and without the post-thrombotic syndrome. Thromb Haemost. 2013;110:854–855. [DOI] [PubMed] [Google Scholar]
- 41.Galanaud JP, Holcroft CA, Rodger MA, et al. Predictors of post-thrombotic syndrome in a population with a first deep vein thrombosis and no primary venous insufficiency. J Thromb Haemost. 2013;11:474–480. [DOI] [PubMed] [Google Scholar]
- 42.Thukral S, Vedantham S. Catheter-based therapies and other management strategies for deep vein thrombosis and post-thrombotic syndrome. J Clin Med. 2020;9(5):1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sista AK, Vedantham S, Kaufman JA, Madoff DC. Endovascular interventions for acute and chronic lower extremity deep venous disease: state of the art. Radiology. 2015;276(1):31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Enden T, Haig Y, Klow NE, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;7(379):31–38. [DOI] [PubMed] [Google Scholar]
- 45.Haig Y, Enden T, Grotta O, et al. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol. 2016;3:e64–71. [DOI] [PubMed] [Google Scholar]
- 46.Comerota AJ, Kearon C, Gu C-S, et al. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis: analysis from a stratified multicenter randomized trial. Circulation. 2019;139(9):1162–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vedantham S, Goldhaber SZ, Julian JA, et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Notten P, ten Cate-Hoek AJ, Arnoldussen CWKP, et al. Ultrasound-accelerated catheter-directed thrombolysis versus anticoagulation for the prevention of post-thrombotic syndrome (CAVA): a single-blind, multicentre, randomised trial. Lancet Haematol. 2020;7(1):e40–e49. [DOI] [PubMed] [Google Scholar]
- 49.Henke P, Sharma S, Wakefield T, Myers D, Obi A. Insights from experimental post-thrombotic syndrome and potential for novel therapies. Transl Res. 2020;225:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Padrnos JL, Garcia D. May-Thurner syndrome and thrombosis: a systematic review of antithrombotic use after endovascular stent placement. Res Pract Thromb Haemost. 2019;3(1):70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999;211(1):39–49. [DOI] [PubMed] [Google Scholar]
- 52.Birn J, Vedantham S. May-Thurner syndrome and other obstructive iliac vein lesions: Meaning, myth, and mystery. Vasc Med. 2015;20(1):74–83. [DOI] [PubMed] [Google Scholar]
- 53.Meissner MH, Gloviczki P, Comerota AJ, et al. Early thrombus removal strategies for acute deep venous thrombosis: Clinical Practice Guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2012;55(5):1449–1462. [DOI] [PubMed] [Google Scholar]
- 54.Vedantham S, Sista AK, Klein SJ, et al. quality improvement guidelines for the treatment of lower-extremity deep vein thrombosis with use of endovascular thrombus removal. J Vasc Interv Radiol. 2014;25(9):1317–1325. [DOI] [PubMed] [Google Scholar]
- 55.Chiasakul T, Cuker A. The case for catheter-directed thrombolysis in selected patients with acute proximal deep vein thrombosis. Blood Adv. 2018;2(14):1799–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ortel TL, Neumann I, Ageno W, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4(19):4693–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Avila ML, Brandão LR, Williams S, et al. Development of CAPTSure™ - a new index for the assessment of pediatric postthrombotic syndrome. J Thromb Haemost. 2016;14:2376–2385. [DOI] [PubMed] [Google Scholar]
- 58.Villalta S, Bagatella P, Piccioli A, Lensing AW, Prins MH, Prandoni P. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24:57a. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


