Supplemental Digital Content is available in the text.
Keywords: aortic valve, aortic valve stenosis, disease outbreaks, registries, survival
Background:
Aortic stenosis requires timely treatment with either surgical aortic valve replacement (SAVR) or transcatheter aortic valve replacement (TAVR). This study aimed to investigate the indirect impact of coronavirus disease 2019 (COVID-19) on national SAVR and TAVR activity and outcomes.
Methods:
The UK TAVR Registry and the National Adult Cardiac Surgery Audit were used to identify all TAVR and SAVR procedures in England, between January 2017 and November 2020. The number of isolated aortic valve replacement (AVR), AVR+coronary artery bypass graft surgery, AVR+other surgery, and TAVR procedures per month was calculated. Separate negative binomial regression models were fit to monthly procedural counts, with functions of time as covariates, to estimate the expected change in activity during COVID-19.
Results:
We included 15 142 TAVR cases, 13 357 isolated AVR cases, 8550 AVR+coronary artery bypass graft cases, and 6773 AVR+other cases. Before March 2020 (UK lockdown), monthly TAVR activity was rising, with a slight decrease in the SAVR activity during 2019. We observed a rapid and significant drop in TAVR and SAVR activity during the COVID-19 pandemic, especially for elective cases. Cumulatively, over the period March to November 2020, we estimated an expected 4989 (95% CI, 4020–5959) cases of aortic stenosis who have not received treatment.
Conclusions:
This study has demonstrated a significant decrease in TAVR and SAVR activity in England following the COVID-19 outbreak. This situation should be monitored closely, to ensure that monthly activity rapidly returns to expected levels. There is potential for significant backlog in the near-to-medium term and potential for increased mortality in this population.
What Is Known
The coronavirus disease 2019 (COVID-19) pandemic has resulted in widespread changes in operational activity of health services.
The impact, from a national perspective, of COVID-19 on activity and outcomes of surgical and transcatheter aortic valve replacement is unknown.
What the Study Adds
We show that there has been a significant decrease in transcatheter aortic valve replacement and surgical aortic valve replacement activity during COVID-19.
Cumulatively, over the period March to November 2020, we estimated an expected 4989 (95% CI, 4020–5959) cases of severe aortic stenosis who have not received treatment.
There is potential for significant backlog in the near-to-medium term and potential for increased mortality in this population.
The coronavirus disease 2019 (COVID-19)1 presents a global health crisis and has resulted in significant excess mortality.2,3 Many countries have imposed restrictions based on social distancing and movement (ie, lockdowns), with the aim of mitigating and managing the spread of COVID-19. A UK-wide lockdown was initiated on March 23, 2020, with a second national lockdown imposed in England at beginning of November 2020.
The lockdown restrictions, and the pandemic, have resulted in widespread changes in operational activity of health services. Many health care systems have faced significant pressure on services, particularly within critical care.4,5 This has necessitated the need for restructuring of resources to meet those needs. Simultaneously, COVID-19 has influenced the ways in which people interact with health services. Previous studies have illustrated that there have been decreases in admissions and diagnosis of health conditions including acute coronary syndromes,6–10 stroke,11,12 and cancer.13 It is crucial to understand the consequences of this on public health and on future planning, especially for conditions requiring timely health care interventions, such as severe symptomatic aortic stenosis.
Aortic stenosis is the most common valvular heart disease where the onset of symptoms is associated with rapid deterioration. Thus, timely treatment by either surgical aortic valve replacement (SAVR) or transcatheter aortic valve replacement (TAVR) is key. SAVR has been the default treatment strategy for symptomatic aortic stenosis, although TAVR has emerged as an effective option across operative risk strata.14–18 While the activity and outcomes for aortic valve replacements (AVRs) have been studied in historic cohorts,19 there is a lack of data in contemporary practice, especially surrounding the impact of COVID-19 from a national perspective. A survey of members of the European Association of Percutaneous Coronary Intervention indicated that 51% had reported cessation of TAVR, and 89% reported at least decreased volumes.20 Furthermore, early in the COVID-19 pandemic, preliminary work characterized patients whose SAVR/TAVR procedures were deferred and their outcomes.21 The impact of COVID-19 on AVR activity from a national perspective is unclear.
The aim of this study was to investigate the activity and postprocedural outcomes of all AVRs in contemporary practice, from a national perspective, and to investigate the indirect impact of COVID-19 on activity and outcomes. The intention is to estimate the effect of reduced activity on projected backlog of cases.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the National Institute for Cardiovascular Outcomes Research (NICOR). The analytical code used for this study is available upon reasonable request, for the purposes of reproducibility.
UK TAVR Registry
The UK TAVR registry collects data for every TAVR procedure undertaken within the UK.22 Data collection occurs prospectively at each contributing center and is submitted to the NICOR. Data collection is mandated for all centers licensed to undertake TAVR procedures. We extracted data from NICOR on all TAVR procedures undertaken in England between January 2017 and November 2020.
UK National Adult Cardiac Surgery Audit
The NICOR National Adult Cardiac Surgery Audit contains data on all major heart operations undertaken in the United Kingdom.23 Each center is responsible for prospective data collection and submission of this to NICOR. We extracted all SAVRs undertaken in England between January 2017 and November 2020. We defined SAVR to be any procedure recorded as being a valve replacement, where the aortic valve implant type was recorded as being mechanical, biological, homograft, or autograft replacement. We further categorized SAVRs into (1) isolated AVR, (2) AVR with coronary artery bypass graft (CABG), or (3) AVR with other surgery (AVR+other). Here, other surgery was any mitral valve procedure, tricuspid valve procedure, pulmonary valve procedure, major aortic surgery, or other cardiothoracic procedures.
Data Flows During COVID-19
The British Cardiovascular Intervention Society and the Society for Cardiothoracic Surgery have made significant efforts to maintain data flows during COVID-19. NICOR has provided weekly uploads of data throughout the pandemic. Thus, at the time of analysis, we had updated data until the end of November 2020. Nonetheless, to reflect the possibility that some individual centers might have stopped submitting data to NICOR during the pandemic or have delays in submitting data, we define a set of rapid-data-submitting centers to be those that submitted at least 1 case (of either TAVR or SAVR) in November 2020 (latest month of our analysis). We perform sensitivity analysis (of the analyses described below) on this subset of centers. Similarly, we also considered sensitivity analyses focusing on the subset of centers that submitted at least 1 case (of either TAVR or SAVR) across every month in 2020.
Outcomes
The primary outcome was presentation and treatment of aortic stenosis with AVR. As secondary outcomes, we considered 30-day mortality and postprocedural length of stay (LOS). Mortality information was provided by linking the UK TAVR registry and the National Adult Cardiac Surgery Audit with the office for national statistics civil registration of deaths dataset. Linkage was made based on each patient’s NHS number. We defined postprocedural LOS to be the number of days between the TAVR/SAVR procedure and hospital discharge.
Statistical Analysis
We excluded any cases in which the age of the patient at the time of the procedure was under 18 years. Additionally, we excluded cases where the NHS number was missing or with missing procedure urgency. Finally, we removed any duplicate cases in either datasets, identified using NHS number, age, sex, admission date, and date/time of the procedure.
In all analyses, we stratified by procedure type (ie, isolated AVR, AVR+CABG, AVR+other, or TAVR). We made no formal comparisons between these procedural types, since there are several confounding factors surrounding the decision-making between TAVR/SAVR (many of which are not captured in the dataset).
Cases performed between February 1 and November 30, 2020, were defined into a during–COVID-19 group, with any case performed in these same months across the preceding years being defined into a pre–COVID-19 group. The February 1, 2020, was chosen since the first COVID-19 case reported in England was on January 28, 2020.
We report patient baseline characteristics for each procedure type, as whole cohorts and across the during COVID-19 and pre–COVID-19 groups. Continuous variables were reported using the mean with SDs. Categorical variables were presented as frequencies of occurrence with relative percentages. Comparisons between continuous variables were made using t tests/ANOVA, while comparisons between categorical variables were made using the χ2 test. Predicted procedural risk was quantified using the UK TAVR clinical prediction model24 for all TAVR procedures and the Logistic EuroSCORE clinical prediction model25 for all SAVR procedures. For the purposes of calculating the risk predictions, missing values in any predictor variables were set to risk factor absent, representing a plausible missingness process in the registries.19,24,26
The number of procedures per month was calculated across the full study period, separately for each procedure type. Percentage increase or decrease in monthly activity was calculated for each during–COVID-19 month, against the respective pre–COVID-19 months. We fitted negative binomial models to the number of TAVR/SAVR procedures performed per month between January 2017 and December 2019, using time as covariate, which was modeled continuously to capture trends in outcome and as a factor variable of month to capture seasonality. This model was used to estimate the expected number of TAVR/SAVR procedures per month in 2020, to compare with the observed activity level.
For each of the 4 procedural types, we compared mortality up to 30 days, across the during–COVID-19 and pre–COVID-19 groups by fitting a Cox proportional hazards model, with the COVID-19 group as a covariate, adjusting for the linear predictor of either the UK TAVR prediction model24 or the Logistic EuroSCORE,25 as appropriate. Differences in postprocedural LOS between the during–COVID-19 and pre–COVID-19 groups were also investigated by fitting a Cox proportional hazards model. The proportional hazards assumption was checked by examining the Schoenfeld residuals.
All analyses were performed using R, version 4.0.0,27 along with the tidyverse suite of packages,28 and the survival package.29,30
Ethics Approval
In the efforts to understand the impact of the COVID-19 pandemic on cardiology services, extraordinary government permission was obtained to evaluate anonymized records from these databases through an agreement with NHS Digital. This work was endorsed by (1) Scientific Advisory Group for Emergencies (a body responsible for ensuring timely and coordinated scientific advice is made available to decision makers to support UK cross-government decisions in the Cabinet Office Briefing Room), (2) NHS England—a public body of the Department of Health and Social Care, and (3) NHS Improvement, responsible for overseeing NHS trusts. NICOR, which houses the British Cardiovascular Intervention Society registry, has support under section 251 of the NHS Act 2006 to use patient information for approved medical research without informed consent. For this rapid NHS evaluation, health data analysis was enabled under Section 254 of the Health and Social Care Act 2012.
Results
The UK TAVR Registry included 15 741 procedures across the study period, of which we included 15 142. The National Adult Cardiac Surgery Audit registry included 108 881 cases during the study period, of which we included 28 680 SAVR procedures, comprised of 13 357 (46.6%) isolated AVR, 8550 (29.8%) AVR+CABG, and 6773 (23.6%) AVR+other cases.
Table 1 shows the baseline characteristics of each procedural group. The mean age of isolated AVR, AVR+CABG, AVR+other, and TAVR was 67.7, 72.2, 62.9, and 81.3, respectively. Across all surgical groups, the majority of cases were male. The mean Logistic EuroSCORE was 7.50%, 10.7%, and 14.1% for isolated AVR, AVR+CABG, and AVR+other, respectively, while the mean UK TAVR prediction model was 3.11% (Table 1).
Table 1.
Baseline Characteristics of the Surgical Aortic Valve Replacement and TAVR Cases Included in This Analysis
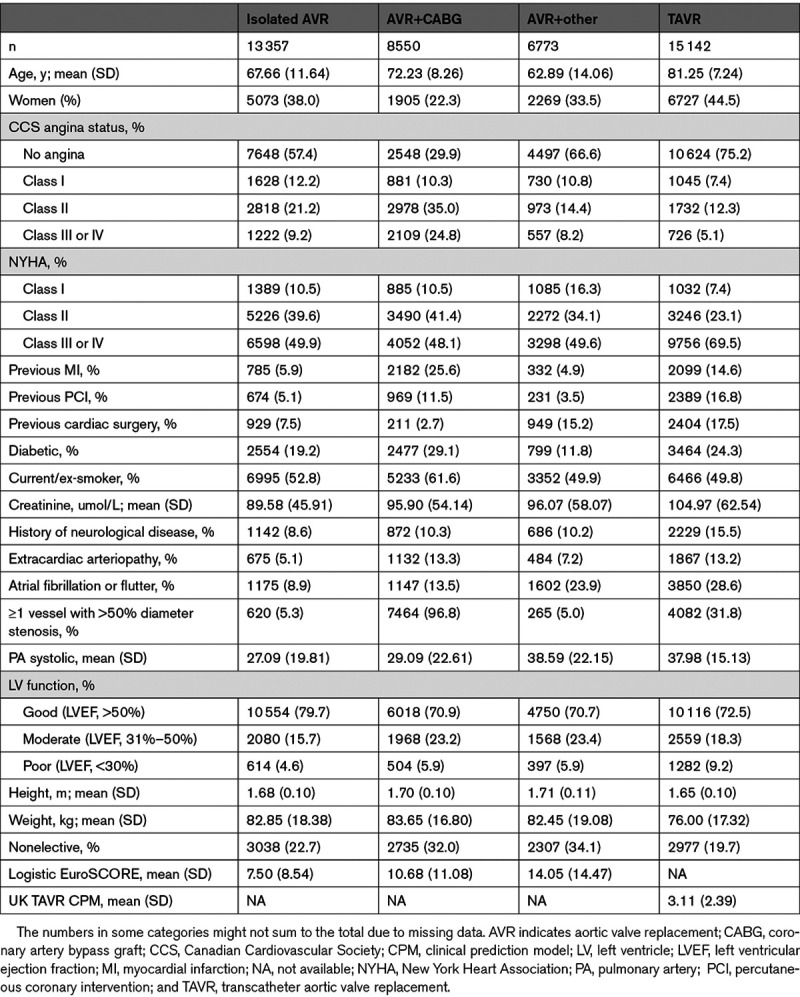
TAVR and SAVR Activity
There has been an increase in the number of TAVR procedures performed per month between January 2017 and December 2019, with the majority of procedures being elective (Figure 1). While the number of monthly AVR+CABG and AVR+other procedures remained relatively stable pre-2020, there was a slight decrease in the number of elective isolated AVR cases per month in 2019. The average number of elective isolated AVR cases per month was 250 in 2017 and 252 in 2018, while the monthly activity in 2019 decreased from 226 cases in January to 173 cases by December (Figure 1). After March 1, 2020, there was a significant drop in activity across all AVR procedures compared with historic levels (Figure 2). There was a slight recovery in AVR activity in May to August 2020.
Figure 1.
Temporal plot of the number of transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement procedures per month, stratified according to procedure urgency. The vertical dotted line denotes March 23, 2020 (date of UK lockdown), with March 1 denoted by the vertical dashed line. AVR indicates aortic valve replacement; and CABG, coronary artery bypass graft.
Figure 2.
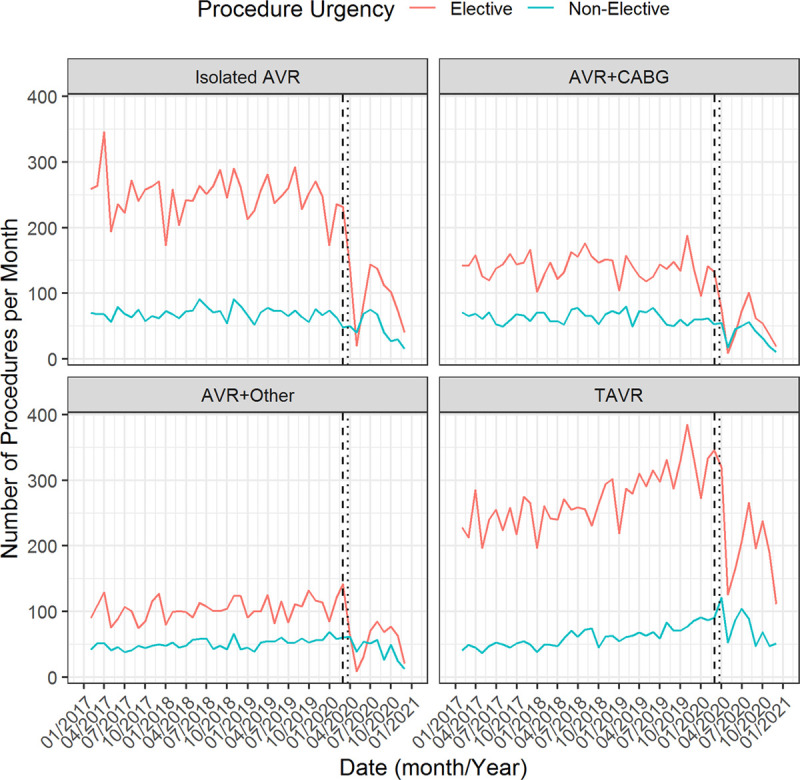
Number of transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (AVR) procedures per month compared to historic levels. A, Temporal plot of the number of TAVR and surgical aortic valve replacement procedures per month during 2020, compared with monthly averages (minimum and maximum shown by shaded region) across all other years in the dataset. B, Percentage change between the mean monthly activity in 2017 to 2019 and the monthly activity in 2020; negative percentage change denotes increase in 2020 over historic levels. AVR indicates aortic valve replacement; and CABG, coronary artery bypass graft.
Importantly, similar findings were found in the subgroup of rapid-data-submitting centers (Figure I in the Data Supplement). In particular, in this subset of centers, the observed rapid drop in cases during March and April 2020 persisted. Interestingly, activity in these rapid-data-submitting centers has actually returned (at least approximately) to expected levels in September to November 2020 (Figure I in the Data Supplement). This indicates that levels of AVR activity have started to recover following the initial rapid drop caused by the first national lockdown.
The number of elective SAVR procedures was below 150 cases per month between March to June 2020 for each of isolated AVR, AVR+CABG, and AVR+other (Figure 1). In contrast, there remained >100 elective TAVR cases per month after March 2020. The percentage change in monthly activity between 2020 and historic levels was lower for TAVR than SAVR with a maximum percentage difference of 86.5%, 80.7%, 83.4%, and 55.7%, for isolated AVR, AVR+CABG, AVR+other, and TAVR, respectively (Figure 2B).
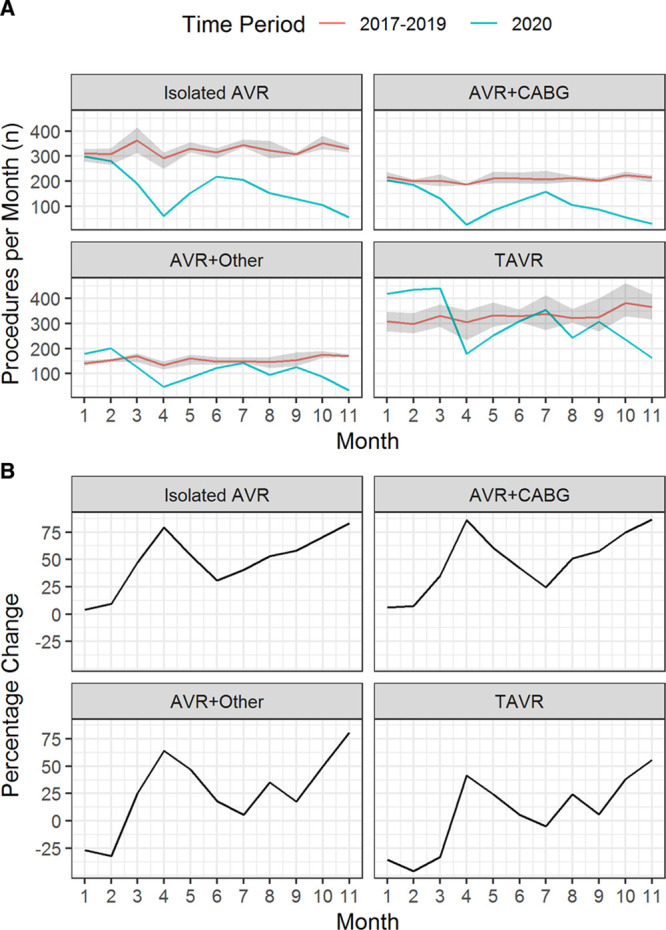
Figure 3 shows the expected (from the negative binomial model) and actual monthly AVR activity during 2020. For the first few months after lockdown, the estimated difference (95% CI) in the number of TAVR cases per month compared with those expected based on historic trends was −2 (−40 to 35) in March 2020, −229 (−264 to −193) in April 2020, −191 (−229 to −154) in May 2020, and −129 (−166 to −92) in June 2020 (Figure 3B). The estimated decrease in isolated AVR activity was −171 (−201 to −140), −231 (−257 to −205), −177 (−205 to −148) and −96 (−124 to −69), across March to June 2020, respectively. Similar observations were made for AVR+CABG and AVR+other cases (Figure 3B). Cumulatively, over the period March to November 2020, this amounts to an estimated expected drop of 4989 (95% CI, 4020–5959) cases of severe aortic stenosis in England, of which 1683 (95% CI, 1429–1937) were for isolated AVR, 1038 (95% CI, 848–1229) were for AVR+CABG, 703 (95% CI, 519–887) were for AVR+other, and 1565 (95% CI, 1223–1906) were for TAVR.
Figure 3.
Predicted vs expected number of transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (AVR) procedures per month. A, Temporal plot of the observed and expected number of TAVR/surgical aortic valve replacement (SAVR) procedures per month. B, The difference between the observed and expected number of TAVR/SAVR procedures per month. In both plots, the expected monthly count is estimated from a negative binomial model fitted to the 2017 to 2019 data. The vertical dotted line denotes March 23, 2020 (date of UK lockdown), with March 1 denoted by the vertical dashed line. AVR indicates aortic valve replacement; and CABG, coronary artery bypass graft.
Evolution of Patient Demographics and Procedural Risk
Table 2 shows patient baseline characteristics of isolated AVR cases across the pre–COVID-19 and during–COVID-19 groups. For isolated AVR, the mean age was significantly lower in the during–COVID-19 group than the pre–COVID-19 group (P<0.001), and there was a significantly higher Canadian Cardiovascular Society (CCS) angina status (P=0.002), NYHA class (P<0.001) and mean pulmonary artery (PA) systolic pressure (P<0.001). For TAVR cases, the mean age, proportion of current/ex-smokers, and mean creatinine were significantly lower in the during–COVID-19 group compared with the pre–COVID-19 group (Table 3). There was a lower proportion of TAVR cases with previous myocardial infarction (P<0.001), previous cardiac surgery (P<0.001), and extracardiac arteriopathy (P=0.001) in the during–COVID-19 group. Similar findings were found for AVR+CABG (Table I in the Data Supplement) and AVR+other (Table II in the Data Supplement). We also explored differences in baseline characteristics between pre–COVID-19 and during–COVID-19 groups, restricting to just 2019 and 2020 data (to look at changes in most contemporary practice); the findings were quantitively similar (Tables III through VI in the Data Supplement).
Table 2.
Baseline Characteristics of the Isolated Aortic Valve Replacement Cases Included in the Pre–COVID-19 and During–COVID-19 Groups, as Defined in the Methods Section
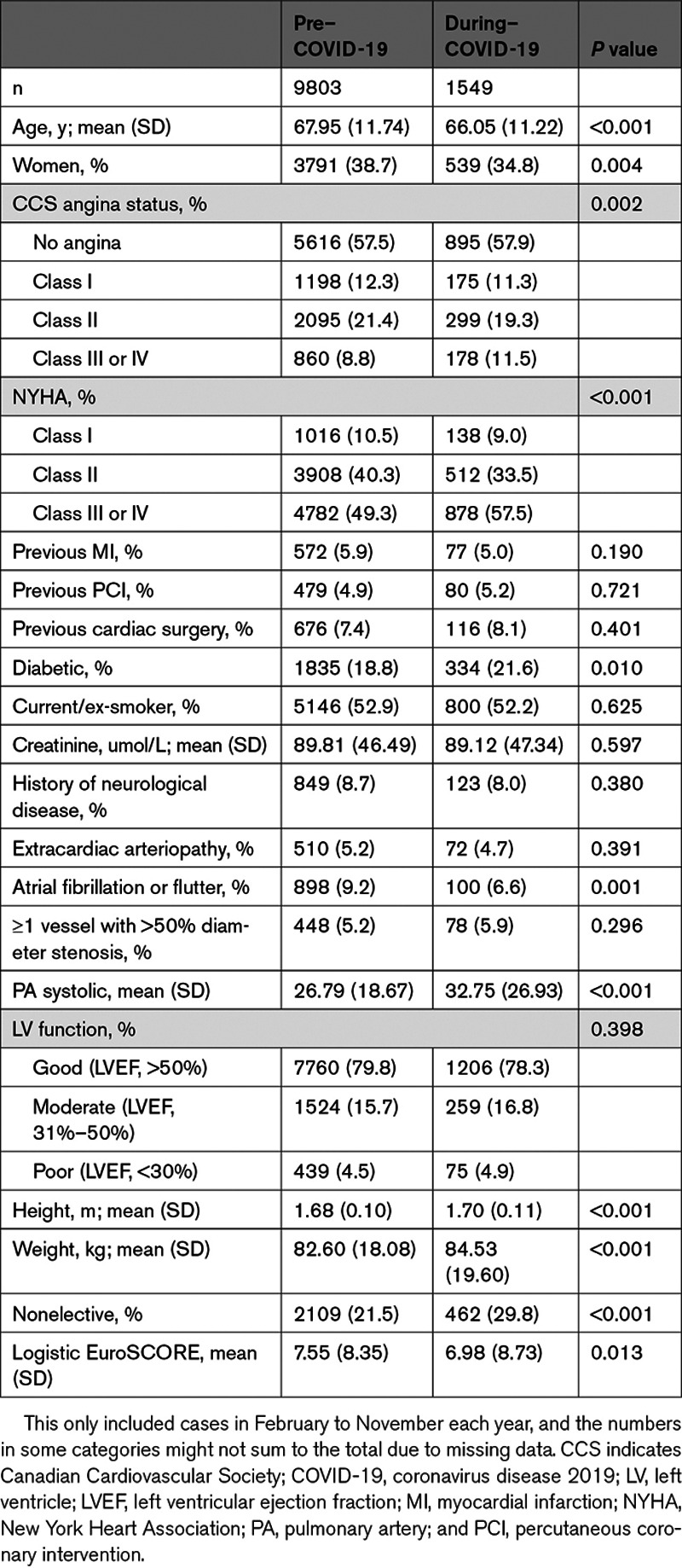
Table 3.
Baseline Characteristics of the TAVR Cases Included in Pre–COVID-19 and During-COVID-19 Groups, as Defined in the Methods Section
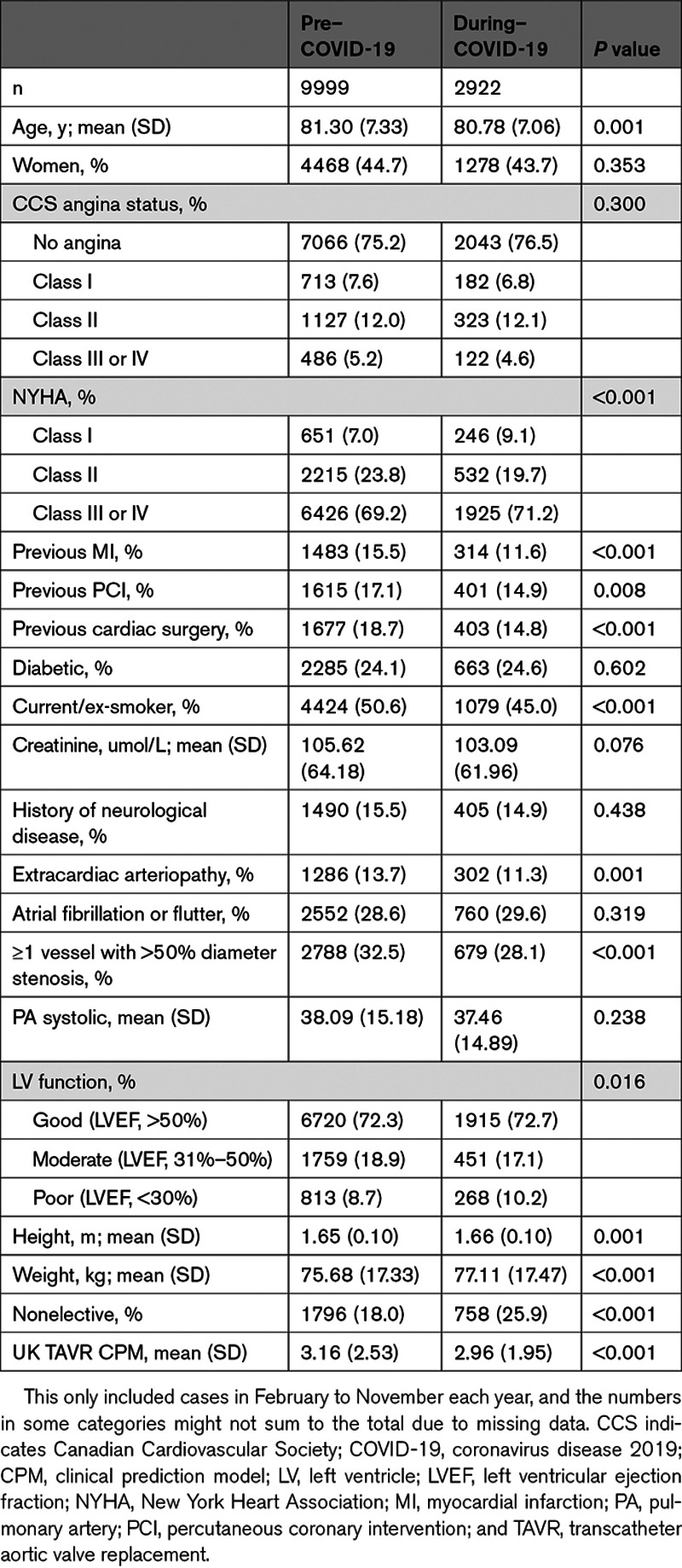
Overall surgical AVR procedural risk, as estimated by the Logistic EuroSCORE, has remained relatively stable over time (Figure II in the Data Supplement). While the mean UK TAVR prediction model was significantly lower in the during–COVID-19 group compared with the pre–COVID-19 group (P<0.001; Table 3), this was largely driven by 2017 cases (Figure II in the Data Supplement). Indeed, upon comparing cases in February to November 2019 with corresponding months in 2020, we found that there was no significant difference in the UK TAVR clinical prediction model between February and November 2020, compared with corresponding months in 2019 (Table VI in the Data Supplement).
Between 2017 and December 2019, there has been a steady increase in the monthly TAVR activity in the lowest quantiles of risk strata, while the monthly activity in the highest quantiles of risk strata has remained relatively stable (Figure III in the Data Supplement). In contrast, the monthly activity of surgical AVR has been gradually decreasing through time across all quantiles of risk strata (Figures IV through VI in the Data Supplement).
Outcomes
The overall Kaplan-Meier estimates of 30-day survival were 98.5% for isolated AVR, 95.8% for AVR+CABG, 94.8%, for AVR+other, and 97.5% for TAVR. For isolated AVR, AVR+other, and TAVR, we found no significant difference in mortality hazards up to 30 days post-procedure between the pre–COVID-19 group and the during–COVID-19 group (Table 4). In contrast, mortality hazards up to 30 days post-procedure were significantly higher in patients undergoing AVR+CABG during–COVID-19 compared with the pre–COVID-19 group (hazard ratio, 1.41 [95% CI, 1.05–1.89]).
Table 4.
Multivariable Adjusted Mortality Hazard Ratios (95% CI) of COVID-19 Period (During Versus Pre) for up to 30-Day Mortality
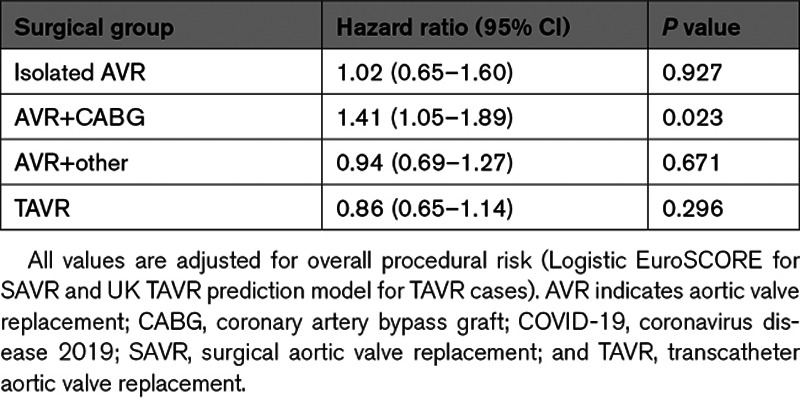
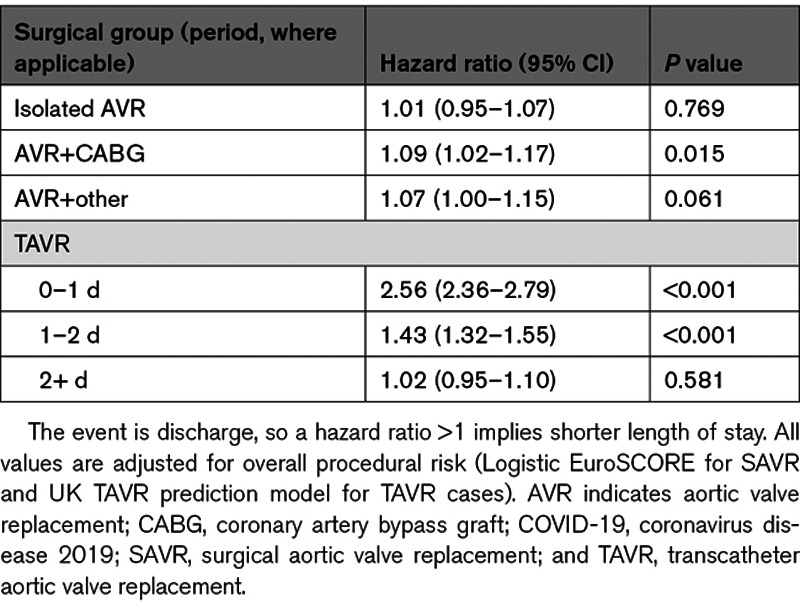
The median LOS following TAVR was 3 days (interquartile range, 2–5 days) in the pre–COVID-19 group and 2 days (interquartile range, 1–3 days) in the during–COVID-19 group. The median (interquartile range) LOS in the pre–COVID-19 group for isolated AVR, AVR+CABG, and AVR+other was 7 (5–9) days, 8 (6–12) days and 9 (6–15) days, respectively, with these being 6 (5–9) days, 7 (6–11) days, and 8 (6–14) days in the during–COVID-19 group. For AVR+CABG procedures performed in the during–COVID-19 period, the adjusted hazard ratio (95% CI) for early discharge was 1.09 (1.02–1.17), showing significantly shorter LOS (Table 5). TAVR patients in the during–COVID-19 group were also significantly more likely for early discharge, up to 2 days post-TAVR (Table 5).
Table 5.
Multivariable Adjusted Hazard Ratios (95% CI) for Discharge Across COVID-19 Period (During Versus Pre)
Discussion
This study is the first to investigate activity and outcomes following all AVR procedures in contemporary practice, including the potential indirect impacts of the COVID-19 pandemic. We observed a rapid decrease in TAVR and SAVR activity during the COVID-19 pandemic. Over the period March to November 2020, this decline in activity accounts for an estimated 4989 patients with aortic stenosis left untreated by AVR intervention. This will have major implications on this cohort of patients whose untreated mortality is high.
The treatment of severe aortic stenosis has evolved from SAVR being the default treatment modality to TAVR now being an evidence-based option at all surgical risk categories.14–18 We observed changes in TAVR and SAVR activity, with steadily increasing TAVR activity and corresponding slight decreases in elective isolated AVR cases, up to 2019. This supports previous findings in this area.19 Although TAVR is currently only approved for inoperable or high-risk cases in the United Kingdom, the evidence of equivalence in low-risk cases is accumulating.18,31 This may partially explain our finding of a steadily increasing proportion of TAVR cases within the lowest quantile of risk, before 2020. The observed decrease in SAVR activity before COVID-19 also provides evidence that the clinical envelope for TAVR has expanded into lower risk cases within real-world contemporary practice.
Inevitably, the initiation of national lockdown in the United Kingdom was associated with a significant reduction in the monthly number of AVR procedures being performed. We found a relatively smaller fall in TAVR than SAVR. One potential explanation is that TAVR has a low probability of requiring stay in an intensive treatment unit, which is important given constraints during the pandemic. Indeed, during the pandemic, patients with severe symptomatic aortic stenosis were recommended to be treated by TAVR where appropriate.32 However, given that the UK TAVR registry and the National Adult Cardiac Surgery Audit dataset do not contain information on the decision-making behind the SAVR or TAVR choice, we are not able to investigate this directly. Importantly, we observed evidence that monthly activity levels across SAVR and TAVR are returning to expected levels toward the end of the study period (September to November 2020), particularly in centers that rapidly submit data to NICOR. Nonetheless, there will inevitably remain a backlog of cases incurred by the first national lockdown in the United Kingdom.
The observed reduction in AVR activity was largely driven by elective cases. A possible explanation is that the UK government’s response to the pandemic was to recommend cancelation of elective procedures.33 This was made to allow a restructuring of hospital services, thereby allowing more staff and resource to deal with the increased admissions due to COVID-19. Another hypothesis for this observed reduction in elective cases could be secondary to patients being less active during COVID-19 and hence less AS-related symptoms resulting in nondiagnosis of AS or lack of urgent need for intervention. Interestingly, we observed that monthly activity for TAVR and SAVR had a slight recovery in May and June 2020. We were unable to investigate the reasons for this, but previous studies have made similar observations.6 Again, one could speculate that this relates to decreasing demands on health care systems as the pandemic evolved, with an aim to resume elective activity once the peak of the pandemic had passed. Given that England has entered a second national lockdown in November 2020, it will remain to be seen if there is another drop in elective AVR activity. Elective cases were being encouraged to continue through the second lockdown.
Nevertheless, despite restructure of health care services nationally during COVID-19, overall procedural risk has remained relatively constant through time. In many ways, some individual baseline characteristics of those undergoing SAVR during COVID-19 were lower risk than pre-COVID patients. There was inevitably an element of careful selection in patients eligible for SAVR during the initial lockdown, particularly for elective cases that were advised to be canceled. This might partially explain these findings, due to the complex (multivariable) interactions between procedural risk and individual baseline characteristics. Nonetheless, it is important to note that overall procedural risk for SAVR (quantified by the EuroScore) and TAVR (quantified by the UK TAVR clinical prediction model) was not significantly different between the pre–COVID-19 (2019 months) and during–COVID-19 groups.
While the observed temporal changes in activity are perhaps unsurprising, these findings raise important implications for health care resource planning in the near-to-medium term. Namely, the results suggest that there will likely be significant increased future demand for TAVR and SAVR. This will lead to an inevitable increase in waiting times34 and associated adverse impacts on outcomes.35 Recommendations for how to manage this challenge are emerging.34,36 It was not possible for us to forecast future demand for AVR since we do not have information on patients who are candidates for AVR but who have not currently undergone the procedure. However, based on the available data, we estimated that, cumulatively, between March and November 2020, there were 4989 (95% CI, 4020–5959) cases of severe aortic stenosis who have not received treatment in England. Previous studies have shown that, under normal circumstances, the median wait time for TAVR is 80 days.37 Thus, assuming these figures apply to AVR generally, we postulate that ≈2495 patients will remain untreated by 80 days (3742 if procedures are made at 50% capacity), even without considering the additional cases over this period. While such figures are an approximation, they do suggest that, on a national-level, strategies will be required to mitigate this large backlog of cases, to reduce avoidable deaths in patients with severe symptomatic aortic stenosis.
Indeed, it remains unclear what effect the reduction in the number of procedures per month has had on outcomes for patients with aortic stenosis who would otherwise have been treated with AVR during the initial lockdown period (March to June 2020). Previous studies have estimated that the risk of death while waiting for intervention for severe aortic stenosis in routine clinical practice is between 2% and 14%.38 This means that of the estimated 4989 currently delayed cases, there will be between 99 and 698 deaths while waiting for intervention. Any additional delays due to the backlog will lead to increased mortality. Of course, these are approximate figures and do not account for excess mortality due to COVID-19.39 Similarly, they are dependent on the estimated mortality proportion while waiting for AVR, and we note that this estimate varies across the literature. For example, other studies have reported mortality proportions while waiting for AVR in the high-risk TAVR era of 3.7%, 8.0%,and 9.6%, at 1, 6, and 12 months, respectively, with SAVR and 3.8%, 23.3%, and 27.5%, respectively, with TAVR.40 In the intermediate-risk TAVR era, waiting-time mortality proportions have been estimated at 2% at 80 days.37 Such figures can give further indications of the expected deaths while waiting for intervention.
Several limitations should be noted. First, we make no statistical comparisons between isolated AVR, AVR+CABG, AVR+other, or TAVR groups. Any such comparisons would be subject to confounding by indication. This means that we were not able to investigate changes in patient-level propensity to undergo SAVR versus TAVR through the COVID-19 period. Second, while we used the Logistic EuroSCORE to summarize overall SAVR procedural risk, this model is known to overpredict mortality risk. However, this model is commonly used for benchmarking in national cardiovascular registries, and we use the model in the same capacity here. Third, this analysis is limited to procedures in England; however, given that COVID-19 has caused changes in health care utilization globally, one might expect similar findings in other health care settings. Finally, some delays in data reporting during the pandemic might contribute to some of the results; however, significant efforts have been made to maintain data flows with weekly uploads of data. Additionally, we undertook a sensitivity analysis of rapid-data-submitting centers, which indicated quantitively similar results to the main analysis, particularly regarding the drastic decrease in activity following the first UK lockdown. Further work should explore whether activity is returning in later months, as suggested by this sensitivity analysis.
In conclusion, this study has demonstrated a significant drop in TAVR and SAVR activity following the COVID-19 outbreak in the United Kingdom. The case mix of patients who have undergone AVR during the COVID-19 period was similar to the case mix seen in the pre–COVID-19 period. There was evidence that activity is starting to return to expected levels by the end of study follow-up. Nonetheless, there will be a backlog of cases caused by the initial lockdown period, suggesting that there will be a sharp rise in demand for AVR intervention in the near-to-medium term, with the potential for an upturn in mortality in patients waiting to be treated.
Acknowledgments
We acknowledge Chris Roebuck, Tom Denwood, Tony Burton, and Courtney Stephenson and data support staff at NHS Digital for providing and creating secure environment for data hosting and analytical support. We also acknowledge Anil Gunesh and Julian Hains from the National Institute of Cardiovascular Outcomes Research for data transfer into the secure environment. Dr Martin and M.A. Mamas conceived and designed the study. Dr Martin performed the analysis and wrote the first draft of the manuscript in collaboration with M.A. Mamas. All authors contributed to reviewing and revising the manuscript critically for scientific content, helped interpret the results, and approved the final version of the paper.
Sources of Funding
None.
Disclosures
None.
Supplemental Materials
Figures I–VI
Tables I–VI
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AVR
- aortic valve replacement
- CABG
- coronary artery bypass graft
- COVID-19
- coronavirus disease 2019
- LOS
- length of stay
- NICOR
- National Institute for Cardiovascular Outcomes Research
- SAVR
- surgical aortic valve replacement
- TAVR
- transcatheter aortic valve replacement
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCINTERVENTIONS.120.010413.
This manuscript was sent to Herbert D. Aronow, MD, MPH, Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 542.
Contributor Information
Nick Curzen, Email: nick.curzen@uhs.nhs.uk.
Andrew T. Goodwin, Email: andrew.goodwin1@nhs.net.
James Nolan, Email: james.nolan@nhs.net.
Lognathen Balacumaraswami, Email: lognathen.balacumaraswami@uhnm.nhs.uk.
Peter F. Ludman, Email: Peter.Ludman@uhb.nhs.uk.
Evangelos Kontopantelis, Email: E.Kontopantelis@manchester.ac.uk.
Jianhua Wu, Email: j.h.wu@leeds.ac.uk.
Chris P. Gale, Email: c.p.gale@leeds.ac.uk.
References
- 1.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee A, Pasea L, Harris S, Gonzalez-Izquierdo A, Torralbo A, Shallcross L, Noursadeghi M, Pillay D, Sebire N, Holmes C, et al. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet. 2020;395:1715–1725. doi: 10.1016/S0140-6736(20)30854-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Department of Health and Social Care. CMO for England Announces First Death of Patient With Covid-19. 2020. Accessed November 18, 2020. https://www.gov.uk/government/news/cmo-for-england-announces-first-death-of-patient-with-covid-19
- 4.Arabi YM, Murthy S, Webb S. COVID-19: a novel coronavirus and a novel challenge for critical care. Intensive Care Med. 2020;46:833–836. doi: 10.1007/s00134-020-05955-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–1546. doi:10.1001/jama.2020.4031 [DOI] [PubMed] [Google Scholar]
- 6.Mafham MM, Spata E, Goldacre R, Gair D, Curnow P, Bray M, Hollings S, Roebuck C, Gale CP, Mamas MA, et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396:381–389. doi: 10.1016/S0140-6736(20)31356-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852–1853. doi: 10.1093/eurheartj/ehaa314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Rosa S, Spaccarotella C, Basso C, Calabrò MP, Curcio A, Filardi PP, Mancone M, Mercuro G, Muscoli S, Nodari S, et al. ; Società Italiana di Cardiologia and the CCU Academy Investigators Group. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J. 2020;41:2083–2088. doi: 10.1093/eurheartj/ehaa409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon MD, McNulty EJ, Rana JS, Leong TK, Lee C, Sung SH, Ambrosy AP, Sidney S, Go AS. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383:691–693. doi: 10.1056/NEJMc2015630 [DOI] [PubMed] [Google Scholar]
- 10.Garcia S, Albaghdadi MS, Meraj PM, Schmidt C, Garberich R, Jaffer FA, Dixon S, Rade JJ, Tannenbaum M, Chambers J, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871–2872. doi: 10.1016/j.jacc.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao J, Li H, Kung D, Fisher M, Shen Y, Liu R. Impact of the COVID-19 epidemic on stroke care and potential solutions. Stroke. 2020;51:1996–2001. doi: 10.1161/STROKEAHA.120.030225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kristoffersen ES, Jahr SH, Thommessen B, Rønning OM. Effect of COVID-19 pandemic on stroke admission rates in a Norwegian population. Acta Neurol Scand. 2020;142:632–636. doi: 10.1111/ane.13307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, Rachet B, Aggarwal A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. ; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]
- 15.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. ; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510 [DOI] [PubMed] [Google Scholar]
- 16.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, et al. ; PARTNER 2 Investigators. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 17.Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. ; PARTNER 3 Investigators. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052 [DOI] [PubMed] [Google Scholar]
- 18.Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, et al. ; Evolut Low Risk Trial Investigators. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–1715. doi: 10.1056/NEJMoa1816885 [DOI] [PubMed] [Google Scholar]
- 19.Grant SW, Hickey GL, Ludman P, Moat N, Cunningham D, de Belder M, Blackman DJ, Hildick-Smith D, Uppal R, Kendall S, et al. Activity and outcomes for aortic valve implantations performed in England and Wales since the introduction of transcatheter aortic valve implantation. Eur J Cardiothorac Surg. 2016;49:1164–1173. doi: 10.1093/ejcts/ezv270 [DOI] [PubMed] [Google Scholar]
- 20.Roffi M, Capodanno D, Windecker S, Baumbach A, Dudek D. Impact of the COVID-19 pandemic on interventional cardiology practice: results of the EAPCI survey. EuroIntervention. 2010;16:247–250. doi:10.4244/EIJ-D-20-00528 [DOI] [PubMed] [Google Scholar]
- 21.Ryffel C, Lanz J, Corpataux N, Reusser N, Stortecky S, Windecker S, Pilgrim T. Mortality, stroke, and hospitalization associated with deferred vs expedited aortic valve replacement in patients referred for symptomatic severe aortic stenosis during the COVID-19 pandemic. JAMA Netw Open. 2020;3:e2020402. doi: 10.1001/jamanetworkopen.2020.20402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludman PF. UK TAVI registry. Heart. 2019;105:s2–s5. doi:10.1136/heartjnl-2018-313510 [DOI] [PubMed] [Google Scholar]
- 23.NICOR. National Cardiac Audit Programme. 2019. Accessed November 18, 2020. http://www.nicor.org.uk/national-cardiac-audit-programme/
- 24.Martin GP, Sperrin M, Ludman PF, de Belder MA, Redwood SR, Townend JN, Gunning M, Moat NE, Banning AP, Buchan I, et al. Novel United Kingdom prognostic model for 30-day mortality following transcatheter aortic valve implantation. Heart. 2018;104:1109–1116. doi: 10.1136/heartjnl-2017-312489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roques F, Michel P, Goldstone AR, Nashef SA. The Logistic EuroSCORE. Eur Heart J. 2003;24:881–882. doi: 10.1016/s0195-668x(02)00799-6 [DOI] [PubMed] [Google Scholar]
- 26.Hickey GL, Grant SW, Cosgriff R, Dimarakis I, Pagano D, Kappetein AP, Bridgewater B. Clinical registries: governance, management, analysis and applications. Eur J Cardiothorac Surg. 2013;44:605–614. doi: 10.1093/ejcts/ezt018 [DOI] [PubMed] [Google Scholar]
- 27.R Core Team. R: A Language and Environment for Statistical Computing. 2020. R Foundation for Statistical Computing [Google Scholar]
- 28.Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, Grolemund G, Hayes A, Henry L, Hester J, et al. Welcome to the tidyverse. JOSS. 2019;4:1686. doi:10.21105/joss.01686 [Google Scholar]
- 29.Therneau TM. A package for survival analysis in r. 2020. Accessed April 28, 2020. https://cran.r-project.org/web/packages/survival/vignettes/survival.pdf
- 30.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox model. 2000. Springer [Google Scholar]
- 31.Coylewright M, Forrest JK, McCabe JM, Nazif TM. TAVR in low-risk patients. JACC. 2020;75:1208–1211. doi:10.1016/j.jacc.2019.12.057 [DOI] [PubMed] [Google Scholar]
- 32.NHS England. Clinical Guide for the Management of Cardiology Patients During the Coronavirus Pandemic. 2020. Accessed November 18, 2020. https://www.nice.org.uk/covid-19/specialty-guides
- 33.Stevens S, Pritchard A. NHS England and NHS Improvement. Important and Urgent – Next Steps on NHS Response to Covid-19. 2020. Accessed November 18, 2020. https://www.england.nhs.uk/coronavirus/publication/next-steps-on-nhs-response-to-covid-19-letter-from-simon-stevens-and-amanda-pritchard
- 34.Lauck S, Forman J, Borregaard B, Sathananthan J, Achtem L, McCalmont G, Muir D, Hawkey MC, Smith A, Højberg Kirk B, et al. Facilitating transcatheter aortic valve implantation in the era of COVID-19: recommendations for programmes. Eur J Cardiovasc Nurs. 2020;19:537–544. doi: 10.1177/1474515120934057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elbaz-Greener G, Yarranton B, Qiu F, Wood DA, Webb JG, Fremes SE, Radhakrishnan S, Wijeysundera HC. Association between wait time for transcatheter aortic valve replacement and early postprocedural outcomes. J Am Heart Assoc. 2019;8:e010407. doi:10.1161/JAHA.118.010407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung CJ, Nazif TM, Wolbinski M, Hakemi E, Lebehn M, Brandwein R, Rezende CP, Doolittle J, Rabbani L, Uriel N, et al. Restructuring structural heart disease practice during the Covid-19 pandemic. J Am Coll Cardiol. 2020;75:2974–2983. doi:10.1016/j.jacc.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elbaz-Greener G, Masih S, Fang J, Ko DT, Lauck SB, Webb JG, Nallamothu BK, Wijeysundera HC. Temporal trends and clinical consequences of wait times for transcatheter aortic valve replacement: a population-based study. Circulation. 2018;138:483–493. doi: 10.1161/CIRCULATIONAHA.117.033432 [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharyya S, Lloyd G. Mortality whilst waiting for intervention in symptomatic severe aortic stenosis. Eur Heart J Qual Care Clin Outcomes. 2019;6:89–90. doi:10.1093/ehjqcco/qcz043 [DOI] [PubMed] [Google Scholar]
- 39.Mohamed MO, Gale CP, Kontopantelis E, Doran T, de Belder M, Asaria M, Luscher T, Wu J, Rashid M, Stephenson C, et al. Sex differences in mortality rates and underlying conditions for COVID-19 deaths in England and wales. Mayo Clin Proc. 2020;95:2110–2124. doi: 10.1016/j.mayocp.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malaisrie SC, McDonald E, Kruse J, Li Z, McGee EC, Jr, Abicht TO, Russell H, McCarthy PM, Andrei AC. Mortality while waiting for aortic valve replacement. Ann Thorac Surg. 2014;98:1564–1570. discussion 1570. doi: 10.1016/j.athoracsur.2014.06.040 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


