Supplemental Digital Content is Available in the Text.
Key Words: doravirine, HIV-1, switch, long-term efficacy and safety, single-tablet regimen
Background:
In the primary analysis of the DRIVE-SHIFT trial, switching to doravirine/lamivudine/tenofovir disoproxil fumarate (DOR/3TC/TDF) maintained suppression of HIV-1 through week 48. Here, we present long-term efficacy and safety outcomes through week 144 of the DRIVE-SHIFT trial.
Methods:
This phase 3, randomized, open-label trial evaluated switching from a stable antiretroviral regimen to once-daily DOR/3TC/TDF in adults with HIV-1 suppressed for ≥6 months and no previous virologic failure. Participants switched at day 1 [immediate switch group (ISG); n = 447] or week 24 [delayed switch group (DSG); n = 209]. Nine ISG participants who completed week 48 but did not enter extension-1 were excluded from week 144 efficacy analyses.
Results:
At week 144, HIV-1 RNA <50 copies/mL was maintained in 80.1% of the ISG (351/438) and 83.7% of the DSG (175/209), while 2.7% (12/438) and 4.8% (10/209), respectively, had HIV-1 RNA ≥50 copies/mL (Food and Drug Administration Snapshot approach). Protocol-defined virologic failure after switch occurred in 2.1% of ISG (9/438) and 3.3% of DSG (7/209); no viral resistance to doravirine was detected in 4 participants with samples available. Reductions in fasting lipids were observed at 24 weeks after switch and maintained through week 144. The mean weight change from switch to week 144 was +1.4 kg for ISG and +1.2 kg for DSG. The most common adverse events were nasopharyngitis (16.2%), headache (12.3%), and diarrhea (9.1%). Overall, 4.1% discontinued because of adverse events, and no deaths occurred.
Conclusions:
These results confirm that switching to once-daily DOR/3TC/TDF is a generally well-tolerated option for maintaining viral suppression in adults considering a change in therapy.
Registration:
INTRODUCTION
Doravirine is a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI) with important attributes for the switch setting: a favorable resistance profile that is unique among NNRTIs,1 no food restrictions,2 and no interactions with statins, oral contraceptives, or gastric acid modifiers.3–5 We have previously reported the primary results of the DRIVE-SHIFT trial, which found that switching to a single-tablet regimen of doravirine 100 mg with lamivudine 300 mg and tenofovir 300 mg (DOR/3TC/TDF) was noninferior to continuing a stable antiretroviral regimen in virologically suppressed adults with HIV-1, with no viral resistance to doravirine, and a favorable safety profile over 48 weeks of treatment.6 Here, we present efficacy and safety results through week 144 of the DRIVE-SHIFT trial. Because weight gain and obesity have been increasing in people with HIV receiving antiretroviral therapy,7,8 we have included a post hoc analysis of changes in weight and body mass index (BMI) after switching to DOR/3TC/TDF.
METHODS
DRIVE-SHIFT (NCT02397096) is a phase 3, open-label, randomized controlled trial to evaluate switching from a stable antiretroviral regimen to DOR/3TC/TDF. Details of the study methodology have been previously reported, along with the primary and secondary end points through week 48.6 Participants who completed the week 48 visit were eligible to enter study extension-1 and continue receiving open-label DOR/3TC/TDF for an additional 96 weeks (up to week 144). Participants with protocol-defined virologic failure (PDVF) were discontinued from the trial, regardless of adherence to study therapy; PDVF was defined as 2 consecutive measurements of HIV-1 RNA ≥50 copies/mL at least 1 week apart.
All randomized participants who received at least 1 dose of DOR/3TC/TDF were included in the safety analyses; participants who completed the base study but did not continue to extension-1 were excluded from the efficacy analysis after week 48. Virologic outcomes were assessed with the Food and Drug Administration Snapshot approach (all missing data were counted as failures regardless of the reason) and the observed failure (OF) approach (values missing because of discontinuation for lack of efficacy were counted as failures). Mean changes in fasting lipids from the time of switch to 24 weeks after switch and to week 144 were summarized using the last-observation-carried-forward approach for missing data or data collected after changes in lipid-lowering therapy.
Weight change, percent weight change, and shift in BMI category after switch to DOR/3TC/TDF were examined in a post hoc analysis. Mean and median weight change, with 95% confidence interval, by time since switch to DOR/3TC/TDF were calculated, adjusting for baseline variables that have been associated with weight change, that is, weight at time of switch, race (Black or non-Black), ethnicity (Hispanic or others), sex, age, previous therapy class (cobicistat-boosted elvitegravir, NNRTI, or ritonavir/cobicistat-boosted PI), baseline CD4+ T-cell count, and log of baseline HIV-1 RNA. A repeated-measure mixed-effect model was used to estimate adjusted mean change, and a quantile regression model was used to estimate adjusted medians. For percent change in weight from time of switch to week 144, the proportion of participants in each of the following categories was calculated: ≤−10%, >−10% to ≤−5%, >−5% to ≤0%, >0% to <5%, ≥5% to <10%, and ≥10%. The proportion of participants with a shift in BMI category from time of switch to week 144 was calculated using standard BMI categories: underweight, BMI <18.5; normal weight, BMI ≥18.5 to <25; overweight, BMI ≥25 to <30; and obese, BMI ≥30.
RESULTS
A total of 656 participants switched to DOR/3TC/TDF during the base study. Most participants were men (84.5%) and White (76.8%); median age was 43 years (see Table, Supplemental Digital Content 1, http://links.lww.com/QAI/B625). The previous antiretroviral therapy regimen was a boosted PI in 70.4% of participants, an NNRTI in 24.1%, and boosted elvitegravir in 5.5%. Of the 609 participants who completed the base study, 600 entered extension-1. Sixty-four participants discontinued DOR/3TC/TDF before week 144 (see Trial Profile, Supplemental Digital Content 2, http://links.lww.com/QAI/B625). The most common reasons for early discontinuation were withdrawal by subject (n = 28) and adverse events (AEs, n = 12).
Efficacy
At week 144, virologic suppression (HIV-1 RNA <50 copies/mL) was maintained in 80.1% (351/438) of immediate switch group (ISG) participants and 83.7% (175/209) of delayed switch group (DSG) participants, while 2.7% (12/438) and 4.8% (10/209), respectively, had HIV-1 RNA ≥50 copies/mL. The remaining participants had no virologic data available at week 144 (Fig. 1), primarily because of treatment discontinuation for reasons other than AEs or death (ie, lost to follow-up, noncompliance with study drug, physician decision, pregnancy, and withdrawal by subject). By the OF approach, HIV-1 RNA <50 copies/mL was maintained in 96.7% of the ISG (351/363) and 94.6% of the DSG (175/185) at week 144.
FIGURE 1.
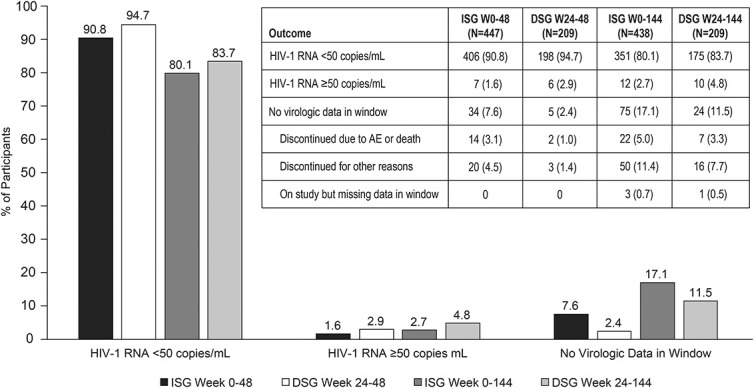
Virologic outcomes after switch to DOR/3TC/TDF (FDA Snapshot approach). Nine participants in the ISG who completed the base study (with HIV-1 RNA <40 copies/mL) but did not enter extension-1 were excluded from the week 144 analysis. ISG, immediate switch group. DSG, delayed switch group; FDA, Food and Drug Administration.
Sixteen participants met PDVF criteria after switching to DOR/3TC/TDF: 7 of 656 (1.1%) in the base study (weeks 0–48) and 9 of 647 (1.4%) in the extension (weeks 48–144). Ten participants had HIV-1 RNA <100 copies/mL at failure; only 5 participants had >200 copies/mL. At the final study visit, 8 participants with PDVF were resuppressed (7 with HIV-1 RNA <40 copies/mL) and 4 had low-level viremia (between 50 and 70 copies/mL). Four participants with PDVF, and 3 who discontinued without PDVF, had samples with sufficient HIV-1 RNA for resistance testing (>400 copies/mL); none developed genotypic or phenotypic resistance to doravirine, lamivudine, or tenofovir.
Safety
Drug-related AEs were less common during the 96-week extension (4.5%) than during the 48-week base study (19.7%). Serious AEs were more common during the extension (8.2%) than during the base study (4.0%), possibly because of the longer duration of the extension (96 vs 48 weeks). One serious AE that occurred during the extension (acute myocardial infarction) was considered drug related and resolved with no change in study therapy. No participants died during treatment with DOR/3TC/TDF or within 14 days of treatment discontinuation. Discontinuation due to an AE was less common during the extension (1.2%) than during the base study (2.9%). Of the 7 AEs that resulted in discontinuation from the extension, 5 were considered drug related (osteoporosis, headache, increased creatinine, increased hepatic enzyme, and hyperphosphaturia), and 2 were considered serious but not drug related (necrotizing fasciitis and acute kidney injury). The most commonly reported AEs of any causality were nasopharyngitis (16.2%), headache (12.3%), and diarrhea (9.1%); these events were rated as mild intensity by 73.6%, 74.1%, and 75.0% of participants, respectively.
Grade 3 or 4 laboratory changes were generally similar between the 96-week extension and the 48-week base study (see Table, Supplemental Digital Content 3, http://links.lww.com/QAI/B625). At week 144, the mean change from baseline in serum creatinine was 0.04 mg/dL in the ISG and DSG. Grade 3 or 4 changes in serum creatinine were rare, occurring in 1.2% (7/596) and 0.3% (2/596) of participants, respectively, during extension-1. Substantial reductions in fasting low-density lipoprotein cholesterol (LDL-C), non-high-density lipoprotein cholesterol (non-HDL-C), total cholesterol, and triglycerides were observed in both treatment groups at 24 weeks after switch and were maintained through week 144 (see Figure, Supplemental Digital Content 4, http://links.lww.com/QAI/B625). The mean change (95% confidence interval) in the total cholesterol to HDL-C ratio from time of switch to week 144 was −0.36 (−0.47 to −0.25) in the ISG and −0.03 (−0.48 to 0.42) in the DSG.
At 24 weeks after switch to DOR/3TC/TDF, the adjusted mean weight gain was 0.7 kg in the ISG and 0.5 kg in the DSG (Fig. 2A). Weight gain remained modest in both groups through week 144: the adjusted mean gain was 1.4 kg at ∼2.8 years after switch in the ISG and 1.2 kg at ∼2.3 years after switch in the DSG. Adjusted median weight changes over time (see Figure, Supplemental Digital Content 5, http://links.lww.com/QAI/B626) were consistent with the adjusted mean changes.
FIGURE 2.
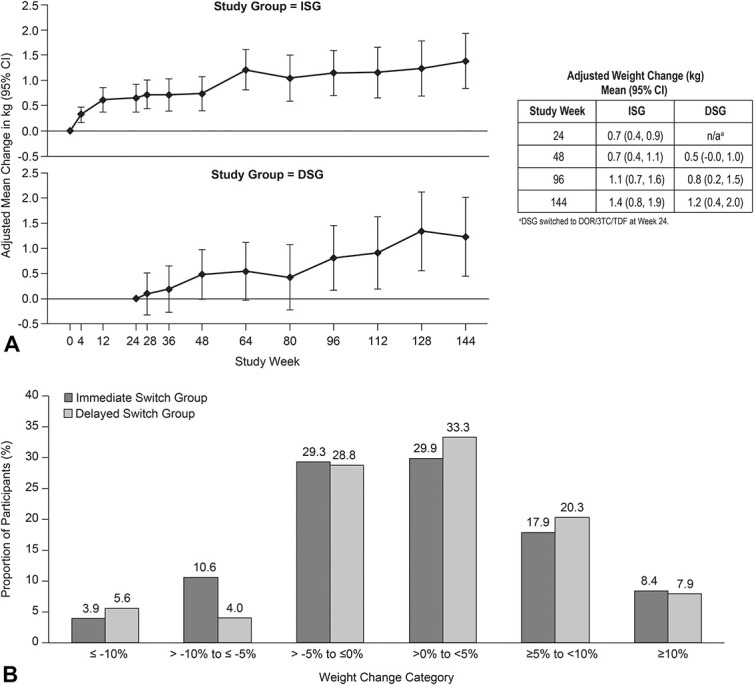
Weight change after switch to DOR/3TC/TDF. A, Mean change (95% CI), adjusted for weight at time of switch, race (Black/non-Black), ethnicity (Hispanic/others), sex, age, previous therapy class, baseline CD4+ T-cell count, and baseline HIV-1 RNA. B, Proportion of participants by percent change in weight at week 144. CI, confidence interval.
From time of switch to week 144, ∼40% of participants lost weight or had no change in weight, ∼30% gained less than 5%, ∼20% gained 5%–10%, and ∼8% gained 10% or more (Fig. 2B). Participants with <5% weight gain had larger decreases in total cholesterol, LDL-C, non-HDL-C, triglycerides, and the total cholesterol to HDL-C ratio, and smaller decreases in HDL-C than participants with ≥5% weight gain (see Table, Supplemental Digital Content 6, http://links.lww.com/QAI/B627).
In both treatment groups, the adjusted mean weight gain was not significantly different between men and women, Black and non-Black race, or Hispanic/Latino and other ethnicities (see Figure, Supplemental Digital Content 7, http://links.lww.com/QAI/B628). The adjusted mean weight gain from time of switch to week 144 was similar for participants who switched from a boosted PI regimen (ISG 1.4 kg, DSG 1.3 kg) and those who switched from an NNRTI regimen (ISG 1.5 kg, DSG 1.3 kg) but was slightly lower for those who switched from a boosted INSTI (elvitegravir) regimen (ISG 0.8 kg, DSG 0.6 kg). The latter group was relatively small, and most had also received tenofovir alafenamide in their previous regimen (17/20 in the ISG and 7/9 in the DSG).
At the time of switch to DOR/3TC/TDF, 2.2% of participants were underweight (BMI <18.5), 48.1% were normal weight (BMI ≥18.5 to <25), 33.0% were overweight (BMI ≥25 to <30), and 16.7% were obese (BMI ≥30). Most participants (82.6%) remained in the same BMI category at week 144 (see Figure, Supplemental Digital Content 8, http://links.lww.com/QAI/B629). Of the 445 participants who were not obese at time of switch, 18 (4.0%) became obese by week 144; most of these participants (16/18) were overweight when they switched to DOR/3TC/TDF. Of the 89 participants who were obese at time of switch, 8 (9.0%) were no longer obese by week 144.
DISCUSSION
This 144-week analysis of the DRIVE-SHIFT trial demonstrates the long-term efficacy of DOR/3TC/TDF in virologically suppressed adults who switched from a stable antiretroviral regimen that included a boosted PI, boosted elvitegravir, or an NNRTI. Very few participants (16/656, 2.4%) met criteria for PDVF after switching to DOR/3TC/TDF, and only 5 (<1%) participants had HIV-1 RNA >200 copies/mL at time of failure. At the final study visit, 75% of participants with PDVF were resuppressed or had very low-level viremia. No viral resistance to doravirine was identified; however, only a small number of participants (4 with PDVF and 3 who discontinued without PDVF) had samples with sufficient HIV-1 RNA for resistance testing.
No safety concerns related to long-term exposure to DOR/3TC/TDF were found in this study. DOR/3TC/TDF was generally well tolerated for up to 144 weeks, with no deaths and low rates of serious drug-related AEs and discontinuation due to drug-related AEs. Substantial reductions in fasting lipids occurred by 24 weeks after switching to DOR/3TC/TDF and were maintained over the next 2 years. The long-term safety profile of DOR/3TC/TDF in treatment-experienced adults who switched to this regimen is consistent with the safety profile in treatment-naive adults who received a doravirine-based regimen for up to 96 weeks.9–11 Weight gain over 144 weeks was modest: the mean change of 1.2–1.4 kg after more than 2 years on DOR/3TC/TDF was no higher than would be expected in an otherwise healthy population.12–14 Mean weight gain was not significantly different by sex, race, or ethnicity. More than 70% of participants experienced less than 5% weight gain, and 83% of participants remained in the same BMI category as at the time of switch. These findings are consistent with the minimal weight gain observed after initiation of a doravirine regimen in treatment-naive adults with HIV-1.15
Limitations of our study include lack of diversity in the study population, which was predominantly men (84%), White (77%), <65 years old (97%), and hepatitis B/C negative (96%). Additional research on DOR/3TC/TDF in women, other racial/ethnic groups, the elderly, and people with chronic hepatitis is needed. Similarly, only 5.5% of participants were previously on an integrase inhibitor (elvitegravir), which was always given with cobicistat and usually with tenofovir alafenamide, so we cannot draw conclusions regarding the effect on weight of switching from an integrase inhibitor to DOR/3TC/TDF.
In summary, virologic suppression was maintained for over 2 years in adults with HIV-1 who switched to DOR/3TC/TDF from a boosted PI, boosted elvitegravir, or an NNRTI. Rates of virologic failure were low, and no evidence of resistance to doravirine was found. Switching to DOR/3TC/TDF was associated with a favorable safety profile, an improved lipid profile, and minimal weight gain in participants who were treated for up to 144 weeks. These results confirm that switching to once-daily DOR/3TC/TDF is an effective and generally well-tolerated option for maintaining viral suppression in patients considering a change in therapy.
ACKNOWLEDGMENTS
The authors thank all the patients who participated in this study. The contributions of the investigators and their staff are also gratefully recognized. Primary investigators (by country): Argentina: W. Belloso, P.E. Cahn, I. Cassetti, and S.H. Lupo; Australia: J. McMahon, R. Finlayson, M. Bloch, and T. Read; Austria: A. Zoufaly, A. Rieger, and B. Haas; Belgium: L. Vandekerckhove, E. Florence, S. DeWit, I. Derdelinckx, and B. Vandercam; Canada: F. Smaill, S. Walmsley, B. Conway, and J. Szabo; Colombia: J. Onate Gutierrez; Denmark: J. Gerstoft, N. Weis, O. Larsen, and H. Nielsen; France: J.-M. Molina, F. Raffi, J. Reynes, and Y. Yazdanpanah; Germany: J. Rockstroh, M. Bickel, H. Jaeger, A. Baumgarten, J. Bogner, K. Arasteh, and W. Kern; Guatemala: E. Rojas Alvarado; Israel: Z. Sthoeger, M. Chowers, K. Riesenberg, E. Shahar, I. Levy, H. Elinav, and D. Turner; Italy: A. Antinori, A. Chirianni, G. Di Perri, A. Lazzarin, M. Andreoni, G. Migliorino, G. Angarano, G. Rizzardini, A. D'Arminio Monforte, and R. Cauda; Korea: S.W. Kim and J.Y. Choi; Mexico: J. Andrade, G. Reyes Teran, and B. Crabtree Ramirez; New Zealand: A. Pithie; Peru: R. Infante; Poland: A. Horban, W. Halota, and J. Gasiorowski; Puerto Rico: G. Ortiz-Lasanta; Russian Federation: E. Voronin, A. Yakovlev, S. Kizhlo, V. Pokrovsky, F. Nagimova, and I. Khaertynova; Spain: E. Negredo Puigmal, J. Mallolas, J. Arribas Lopez, R. Rubio Garcia, V. Estrada, J. Iribarren Loyarte, and F. Gutierrez Rodero; Switzerland: M. Cavassini, J. Fehr, and A. Rauch; United Kingdom: M. Nelson, C. Orkin, M. Gompels, I. Williams, A. Clarke, M. Johnson, G. Schembri, A. Ustianowski, and N. Fearnley; United States: D. Asmuth, K. Vigil, D. Berger, K. Casey, G. Crofoot, D. Cunningham, C. Dietz, D. Goldstein, J. Flamm, L. Gorgos, C. Hare, W. Henry, M. Jain, T. Jefferson, M. Johnson, D. Klein, P. Kumar, J. Lalezari, J. Sims, R. Novak, G. Pierone, D. Prelutsky, S. Schrader, D. Sweet, D. Riedel, O. Osiyemi, P. Tebas, W. Robbins, D. Parenti, W. Towner, R. Nahass, and J. Morales-Ramirez.
Footnotes
Funding for this research was provided by Merck Sharp & Dohme Corp. (MSD), a subsidiary of Merck & Co., Inc., Kenilworth, NJ. Medical writing and editorial assistance were provided by Kim Strohmaier, Carol Zecca, and Danielle Mancaruso, employees of MSD.
Parts of the data were presented at the 23rd International AIDS Conference, July 6–10, 2020, San Francisco, CA, and HIV Drug Therapy, October 5–8, 2020, Glasgow, Scotland.
P.K. is on advisory boards for ViiV, Janssen, Merck, and Theratechnologies; has received grants from Merck, ViiV, Gilead, and Theratechnologies; and owns stock in Johnson & Johnson, Gilead, Merck, Pfizer, and GSK. J.-M.M. has participated in advisory boards for Gilead, MSD, ViiV, and Sanofi. G.R. has received consulting/advisor fees from AbbVie, Gilead, MSD, and ViiV, and has board membership with AbbVie, Gilead, MSD, and ViiV. P.C. is an advisory board member for MSD and ViiV and has received research grants from AbbVie, MSD, and ViiV. H.W., Z.J.X., C.M., P.S., and W.G. are current employees of MSD. The remaining authors have no conflicts of interest to declare.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
Contributor Information
Princy Kumar, Email: kumarp@gunet.georgetown.edu.
Margaret Johnson, Email: margaret.johnson1@nhs.net.
Jean-Michel Molina, Email: jean-michel.molina@sls.aphp.fr.
Giuliano Rizzardini, Email: giuliano.rizzardini@asst-fbf-sacco.it.
Pedro Cahn, Email: pedro.cahn@huesped.org.ar.
Markus Bickel, Email: bickel@infektiologikum.de.
Hong Wan, Email: hong_wan@merck.com.
Zhi Jin Xu, Email: jin_xu@merck.com.
Cristiana Morais, Email: cristiana.morais@merck.com.
Peter Sklar, Email: peter.sklar@merck.com.
Wayne Greaves, Email: wayne.greaves@merck.com.
REFERENCES
- 1.Feng M, Wang D, Grobler JA, et al. In vitro resistance selection with doravirine (MK-1439), a novel nonnucleoside reverse transcriptase inhibitor with distinct mutation development pathways. Antimicrob Agents Chemother. 2015;59:590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behm MO, Yee KL, Liu R, et al. The effect of food on doravirine bioavailability: results from two pharmacokinetic studies in healthy subjects. Clin Drug Investig. 2017;37:571–579. [DOI] [PubMed] [Google Scholar]
- 3.Khalilieh S, Yee KL, Sanchez RI, et al. Results of a doravirine-atorvastatin drug-drug interaction study. Antimicrob Agents Chemother. 2017;61:e01364–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson MS, Kaufman D, Castronuovo P, et al. Effect of doravirine (MK-1439) on the pharmacokinetics of an oral contraceptive (ethinyl estradiol and levonorgestrel). Rev Antivir Ther Infect Dis. 2015;4:63–64. [Google Scholar]
- 5.Khalilieh SG, Yee KL, Sanchez RI, et al. A study to evaluate doravirine pharmacokinetics when co-administered with acid-reducing agents. J Clin Pharmacol. 2019;59:1093–1098. [DOI] [PubMed] [Google Scholar]
- 6.Johnson M, Kumar P, Molina JM, et al. Switching to doravirine/lamivudine/tenofovir disoproxil fumarate (DOR/3TC/TDF) maintains HIV-1 virologic suppression through 48 weeks: results of the DRIVE-SHIFT trial. J Acquir Immune Defic Syndr. 2019;81:463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koethe JR, Jenkins CA, Lau B, et al. Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses. 2016;32:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourgi K, Jenkins CA, Rebeiro PF, et al. Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc. 2020;23:e25484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molina JM, Squires K, Sax PE, et al. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVE-FORWARD): 96-week results of a randomised, double-blind, non-inferiority, phase 3 trial. Lancet HIV. 2020;7:e16–e26. [DOI] [PubMed] [Google Scholar]
- 10.Orkin C, Squires KE, Molina JM, et al. Doravirine/lamivudine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naïve adults with HIV-1 infection: week 96 results of the randomized, double-blind, phase 3 DRIVE-AHEAD noninferiority trial. Clin Infect Dis. 2020:ciaa822. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gatell JM, Morales-Ramirez JO, Hagins DP, et al. Doravirine dose selection and 96-week safety and efficacy versus efavirenz in antiretroviral therapy-naive adults with HIV-1 infection in a Phase IIb trial. Antivir Ther. 2019;24:425–435. [DOI] [PubMed] [Google Scholar]
- 12.Hill JO, Wyatt HR, Reed GW, et al. Obesity and the environment: where do we go from here? Science. 2003;299:853–855. [DOI] [PubMed] [Google Scholar]
- 13.Hutfless S, Maruthur NM, Wilson RF, et al. Strategies to prevent weight gain among adults. In: Comparative Effectiveness Review No. 97. AHRQ Publication No. 13-EHC029-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 14.Dutton GR, Kim Y, Jacobs DR, et al. 25-Year weight gain in a racially balanced sample of U.S. Adults: the CARDIA study. Obesity. 2016;24:1962–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orkin C, Elion R, Thompson M, et al. Effect of doravirine on body weight and body mass index in treatment naïve adults with HIV-1. AIDS. 2021;35:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]


