Supplemental Digital Content is Available in the Text.
Key Words: 2-drug regimen, simplification, insulin resistance, metabolic syndrome
Background:
In TANGO, switching to dolutegravir/lamivudine was noninferior at 48 weeks to continuing 3-/4-drug tenofovir alafenamide–based regimens in virologically suppressed individuals with HIV-1. Antiretroviral agents have been associated with weight gain and metabolic complications.
Setting:
One hundred thirty-four centers; 10 countries.
Methods:
We assessed weight; fasting lipids, glucose, and insulin; and prevalence of insulin resistance and metabolic syndrome at baseline and week 48 in TANGO participant subgroups by boosting agent use in baseline regimens (boosted and unboosted).
Results:
In each treatment group, 74% of participants used boosted regimens at baseline. In boosted and unboosted subgroups, weight and fasting glucose changes at week 48 were small and similar between treatment groups. Overall and in the boosted subgroup, greater decreases from baseline were observed with dolutegravir/lamivudine in fasting total cholesterol (P < 0.001), low-density lipoprotein cholesterol (P < 0.001), triglycerides (P < 0.001), total cholesterol/high-density lipoprotein cholesterol ratio (overall, P = 0.017; boosted, P = 0.007), and insulin (boosted, P = 0.005). Prevalence of HOMA-IR ≥2 was significantly lower at week 48 with dolutegravir/lamivudine overall [adjusted odds ratio (aOR), 0.59; 95% confidence interval (CI), 0.40 to 0.87; P = 0.008] and in the boosted subgroup [aOR, 0.56; 95% CI, 0.36 to 0.88; P = 0.012] but not in the unboosted subgroup [aOR, 0.70; 95% CI, 0.31 to 1.58; P = 0.396]. Prevalence of metabolic syndrome at week 48 was low and consistent between treatment groups overall, with differences trending to favor dolutegravir/lamivudine in the unboosted subgroup [aOR, 0.41; 95% CI, 0.15 to 1.09; P = 0.075].
Conclusion:
Generally, switching from 3-/4-drug tenofovir alafenamide–based regimens to dolutegravir/lamivudine improved metabolic parameters, particularly when switching from boosted regimens. Because of smaller sample size in the unboosted subgroup, results warrant further investigation.
INTRODUCTION
Antiretroviral therapy (ART) has been associated with excess weight gain and metabolic complications in people living with HIV (PLWH).1,2 The integrase strand transfer inhibitors (INSTIs) dolutegravir and bictegravir and the nucleoside reverse transcriptase inhibitor tenofovir alafenamide have been associated with increased weight gain compared with other antiretroviral agents.3–8 In the ADVANCE trial, PLWH receiving dolutegravir with tenofovir alafenamide experienced greater weight gain vs those receiving dolutegravir or efavirenz plus tenofovir disoproxil fumarate/emtricitabine, with higher weight gain observed in women.5 Weight gain has been reported in PLWH who switched from tenofovir disoproxil fumarate–based to tenofovir alafenamide–based regimens4,8,9 and in HIV-negative individuals on tenofovir alafenamide–based preexposure prophylaxis.6 Tenofovir alafenamide has also been associated with lipid increases compared with tenofovir disoproxil fumarate,4,5,7 potentially linked to atherosclerotic cardiovascular disease risk.4 Boosted protease inhibitor–based regimens have been linked to metabolic complications, including weight gain, lipid abnormalities, and insulin resistance.10–13
Antiretroviral regimens containing 2 drugs (2DRs) rather than ≥3 have been investigated to reduce lifelong antiretroviral use and potentially limit long-term safety concerns.14,15 The randomized phase 3 TANGO study demonstrated that switching to a 2DR of dolutegravir/lamivudine is noninferior at 48 weeks to continuing 3-/4-drug tenofovir alafenamide–based regimens (3/4DRs) in maintaining virologic suppression in PLWH.16
Although weight gain is an important metabolic outcome, it is often assessed in isolation and may not provide a complete understanding of overall metabolic health. Therefore, in this post hoc analysis, we assessed broad metabolic health parameters, including weight; fasting lipids, glucose, and insulin; and prevalence of insulin resistance and metabolic syndrome at week 48.
METHODS
Study Design
TANGO (ClinicalTrials.gov, NCT03446573) was a phase 3, randomized, open-label, noninferiority study evaluating the efficacy and safety of switching to dolutegravir/lamivudine vs continuing tenofovir alafenamide–based 3/4DRs for the maintenance of virologic suppression in PLWH.16 Adults with HIV-1 infection and virologic suppression (HIV-1 RNA <50 copies/mL) for >6 months on a first-line tenofovir alafenamide–based 3/4DR (switch from tenofovir disoproxil fumarate without other ART changes ≥3 months before screening was allowed) were randomized in a 1:1 ratio (stratified by baseline third agent class) to switch to once-daily dolutegravir 50 mg/lamivudine 300 mg fixed-dose combination tablet or continue their current regimen. The primary outcome was the proportion of participants with HIV-1 RNA ≥50 copies per milliliter (US Food and Drug Administration Snapshot algorithm) at week 48 in the intention-to-treat–exposed (ITT-E) population.
Metabolic Health Assessments
Metabolic outcomes at week 48 were assessed overall and by baseline boosting status (boosted vs unboosted) and included change from baseline in weight; fasting lipids [total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), TC/HDL-C ratio, and triglycerides], glucose, HbA1c, and insulin; and prevalence of insulin resistance and metabolic syndrome. Insulin resistance was tested using the homeostatic model assessment of insulin resistance [HOMA-IR: fasting glucose (mmol/L) × fasting insulin (mU/L)/22.5] and defined as HOMA-IR ≥2.17–19 Metabolic syndrome, based on the new International Diabetes Federation definition, was defined as having obesity [body mass index (BMI) ≥30 kg/m2] and any 2 of the following20: raised triglycerides (≥1.7 mmol/L or treatment for dyslipidemia), reduced HDL-C (men, <1.03 mmol/L; women, <1.29 mmol/L; or treatment for dyslipidemia), raised blood pressure (systolic, ≥130 mm Hg; diastolic, ≥85 mm Hg; or treatment for hypertension), and raised fasting glucose (≥5.6 mmol/L or previously diagnosed type 2 diabetes). Week 48 blood pressure data were not available; baseline blood pressure, hypertension-related adverse events, or initiation of hypertension medication was used for week 48 metabolic syndrome assessment.
Statistical Analysis
Metabolic parameters were assessed in the safety population, which included participants who received ≥1 dose of randomized study treatment. Absolute and percent changes from baseline were based on adjusted geometric mean ratios (week 48 to baseline) in each treatment group calculated from a mixed-models repeated-measures model applied to nontransformed (absolute) or loge-transformed (percent) data adjusting for relevant baseline variables. Adjusted odds ratios (aORs) for the prevalence of insulin resistance and metabolic syndrome at week 48 between treatment groups by baseline boosting status were calculated using a logistic regression model adjusting for relevant baseline variables. In both mixed-models repeated-measures model and logistic regression analyses, testing for differences between treatment groups by baseline boosting status was achieved using treatment-by-baseline boosting status interaction terms. Baseline covariates are specified in Table 1 and Figure 1 legends. Two-sided P < 0.05 was considered statistically significant.
TABLE 1.
Change From Baseline in Metabolic Health Parameters at Week 48 With Dolutegravir/Lamivudine or Tenofovir Alafenamide–Based Regimens by Boosting Status of Baseline Antiretroviral Regimen
| Parameter | Boosted | Unboosted | |||
| 2DR (N = 272) | 3/4DR (N = 277) | 2DR (N = 97) | 3/4DR (N = 94) | ||
| Weight | |||||
| Baseline, n | 272 | 277 | 97 | 94 | |
| Median (range), kg | 77.0 (50.2 to 153.0) | 79.2 (47.6 to 138.0) | 84.0 (56.0 to 135.0) | 81.3 (49.4 to 141.0) | |
| Week 48, n | 252 | 259 | 91 | 84 | |
| Change from baseline, adjusted mean (SE), kg* | 0.81 (0.27) | 0.88 (0.25) | 0.81 (0.45) | 0.40 (0.44) | |
| Difference (95% CI); P value | −0.06 (−0.79 to 0.66); 0.861 | 0.41 (−0.82 to 1.64); 0.513 | |||
| Total cholesterol | |||||
| Baseline, n | 227 | 229 | 78 | 71 | |
| Median (range), mmol/L | 5.05 (2.65 to 8.25) | 4.90 (2.75 to 9.30) | 4.65 (2.90 to 7.05) | 4.45 (2.70 to 7.00) | |
| Week 48, n† | 202 | 203 | 73 | 60 | |
| Change from baseline, adjusted mean (95% CI), %‡ | −5.7 (−7.5 to −3.8) | 2.2 (0.6 to 3.9) | −0.8 (−4.1 to 2.5) | 2.1 (−0.9 to 5.3) | |
| Treatment ratio§ (95% CI); P value | 0.923 (0.899 to 0.947); <0.001 | 0.971 (0.928 to 1.015); 0.193 | |||
| LDL-C | |||||
| Baseline, n | 227 | 229 | 78 | 71 | |
| Median (range), mmol/L | 2.99 (1.02 to 5.11) | 2.95 (1.03 to 6.09) | 2.80 (1.03 to 5.23) | 2.57 (1.20 to 5.14) | |
| Week 48, n† | 202 | 203 | 73 | 60 | |
| Change from baseline, adjusted mean (95% CI), %‡ | −6.6 (−9.5 to −3.7) | 2.9 (0.01 to 5.8) | −2.0 (−6.9 to 3.2) | −0.3 (−5.2 to 4.9) | |
| Treatment ratio§ (95% CI); P value | 0.907 (0.871 to 0.946); <0.001 | 0.983 (0.915 to 1.056); 0.637 | |||
| HDL-C | |||||
| Baseline, n | 227 | 229 | 78 | 71 | |
| Median (range), mmol/L | 1.30 (0.60 to 4.20) | 1.35 (0.45 to 2.80) | 1.30 (0.55 to 2.35) | 1.25 (0.70 to 2.50) | |
| Week 48, n† | 202 | 203 | 73 | 60 | |
| Change from baseline, adjusted mean (95% CI), %‡ | −0.8 (−3.4 to 1.9) | 2.2 (0.0 to 4.5) | −2.3 (−6.6 to 2.2) | 0.1 (−3.9 to 4.2) | |
| Treatment ratio§ (95% CI); P value | 0.970 (0.937 to 1.005); 0.088 | 0.976 (0.919 to 1.037); 0.436 | |||
| Total cholesterol to HDL-C ratio | |||||
| Baseline, n | 227 | 229 | 78 | 71 | |
| Median (range) | 3.76 (1.54 to 10.0) | 3.70 (1.54 to 9.78) | 3.50 (2.17 to 8.00) | 3.45 (1.73 to 8.00) | |
| Week 48, n† | 202 | 203 | 73 | 60 | |
| Change from baseline, adjusted mean (95% CI), %‡ | −4.8 (−7.4 to −2.1) | 0.1 (−2.2 to 2.4) | 1.4 (−3.3 to 6.3) | 1.8 (−2.4 to 6.1) | |
| Treatment ratio§ (95% CI); P value | 0.951 (0.918 to 0.986); 0.007 | 0.996 (0.936 to 1.061); 0.907 | |||
| Triglycerides | |||||
| Baseline, n | 227 | 229 | 78 | 71 | |
| Median (range), mmol/L | 1.36 (0.44 to 9.84) | 1.24 (0.42 to 6.64) | 1.14 (0.48 to 2.96) | 1.14 (0.32 to 5.16) | |
| Week 48, n† | 202 | 203 | 73 | 60 | |
| Change from baseline, adjusted mean (95% CI), %‡ | −14.1 (−18.7 to −9.3) | 4.0 (−1.8 to 10.1) | −1.6 (−10.2 to 7.8) | 12.2 (1.0 to 24.6) | |
| Treatment ratio§ (95% CI); P value | 0.825 (0.763 to 0.894); <0.001 | 0.877 (0.764 to 1.008); 0.064 | |||
| Fasting glucose | |||||
| Baseline, n | 264 | 272 | 94 | 88 | |
| Median (range), mmol/L | 5.10 (2.9 to 10.0) | 5.10 (1.7 to 15.7) | 5.20 (3.9 to 6.6) | 5.20 (4.2 to 13.8) | |
| Week 48, n | 222 | 221 | 82 | 60 | |
| Change from baseline, adjusted mean (95% CI), %‡ | 2.3 (0.8 to 3.8) | 3.8 (2.3 to 5.4) | −0.2 (−2.5 to 2.2) | 2.1 (−0.9 to 5.2) | |
| Treatment ratio§ (95% CI); P value | 0.985 (0.964 to 1.006); 0.154 | 0.977 (0.941 to 1.015); 0.234 | |||
| Fasting insulin | |||||
| Baseline, n | 258 | 266 | 94 | 86 | |
| Median (range), pmol/L | 72.0 (11 to 582) | 72.0 (11 to 690) | 78.0 (11 to 558) | 66.0 (18 to 420) | |
| Week 48, n | 224 | 229 | 84 | 65 | |
| Change from baseline, adjusted mean (95% CI), %‡ | −11.6 (−17.3 to −5.4) | 0.9 (−5.4 to 7.5) | −7.3 (−17.0 to 3.6) | 3.7 (−8.1 to 17.0) | |
| Treatment ratio§ (95% CI); P value | 0.877 (0.799 to 0.962); 0.005 | 0.894 (0.760 to 1.051); 0.175 | |||
Estimated mean change from baseline at week 48 calculated from mixed-models repeated-measures model adjusting for the following: treatment, visit, baseline boosting status, CD4+ cell count (continuous), age (continuous), sex, weight at baseline (continuous), race, treatment-by-visit interaction, baseline value-by-visit interaction, treatment-by-boosting status interaction, boosting status-by-visit interaction, and boosting status-by-treatment-by-visit interaction, with visit as the repeated factor.
Lipid parameter data collected after introduction of a lipid-modifying agent were not used, and the last available fasting, on-treatment lipid value before initiation of a lipid-modifying agent was used per a last observation carried forward method.
Percent change from baseline based on adjusted geometric mean ratio (week 48 to baseline) in each group calculated from mixed-models repeated-measures model applied to change from baseline in loge-transformed data adjusting for the following: treatment, visit, baseline boosting status, CD4+ cell count (continuous), loge-transformed baseline value (continuous), treatment-by-visit interaction, baseline value-by-visit interaction, treatment-by-boosting status interaction, boosting status-by-visit interaction, and boosting status-by-treatment-by-visit interaction, with visit as the repeated factor. Percent changes from baseline in glucose and insulin were adjusted for the following additional factors: age (continuous), sex, baseline BMI (continuous), race (white, black, other), and baseline hypertension (yes, no). Percent change in glucose was also adjusted for baseline smoking status (previous, current, never).
Treatment ratio was calculated as week 48/baseline geometric mean ratio in the 2DR group divided by the same ratio in the 3/4DR group.
FIGURE 1.
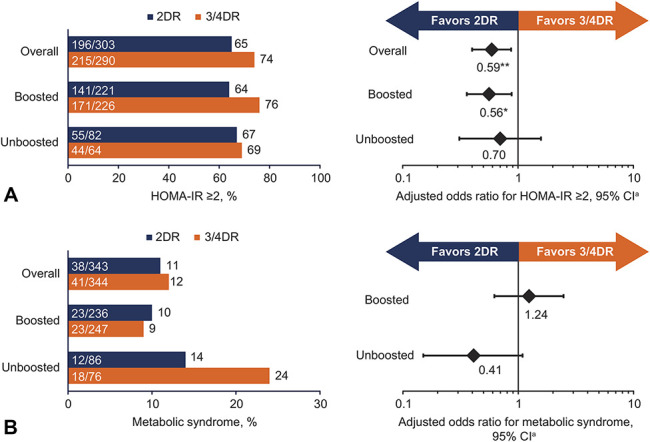
Odds of (A) insulin resistance and (B) metabolic syndrome at week 48 with dolutegravir/lamivudine or tenofovir alafenamide–based regimen by boosting status of baseline antiretroviral regimen. Includes participants with no missing baseline covariate data and week 48 metabolic data. Participants with evidence of diabetes at baseline were excluded from HOMA-IR analysis. aOdds ratios and 95% CIs were calculated using a logistic regression model. Insulin resistance was adjusted for treatment regimen (2DR vs 3/4DR), baseline boosting status (boosted vs unboosted), race (black, other vs white), sex (female vs male), baseline BMI (continuous), baseline CD4+ cell count (continuous), age (continuous), baseline hypertension (yes vs no), baseline HOMA-IR (continuous), and treatment-by-baseline boosting status interaction. Metabolic syndrome was adjusted for treatment regimen (2DR vs 3/4DR), baseline boosting status (boosted vs unboosted), sex (female vs male), baseline hypertension (yes vs no), baseline triglycerides (borderline high, high, very high vs normal), baseline HDL-C (low, high vs normal), baseline HOMA-IR (2 to <3, 3 to <4, ≥4 vs <2), and treatment-by-baseline boosting status interaction. *P = 0.012. **P = 0.008.
TANGO was performed in accordance with International Conference on Harmonization Good Clinical Practice, following the principles of the Declaration of Helsinki, with protocol approvals and informed consent obtained before participant screening.
RESULTS
Baseline Characteristics
There were 369 and 372 participants randomized to dolutegravir/lamivudine and tenofovir alafenamide–based regimens, respectively, who received ≥1 dose of study drug (ITT-E population).16 Most participants in the ITT-E population were male (92%) and white (79%); median age was 39 years. In the dolutegravir/lamivudine and tenofovir alafenamide–based regimen groups, respectively, median (range) duration of ART was 33.8 (7.1–201.2) months and 35.1 (7.0–160.8) months, and median (range) duration of tenofovir alafenamide use was 17.7 (3.6–73.7) months and 18.2 (3.9–71.2) months. Baseline ART regimens included INSTIs for 78% and 80% of participants in the dolutegravir/lamivudine and tenofovir alafenamide–based regimen groups, respectively. There were 272 participants (74%) and 277 participants (74%) in the dolutegravir/lamivudine and tenofovir alafenamide–based regimen groups, respectively, who used boosting agents at baseline; boosted regimens most commonly included cobicistat-boosted elvitegravir [2DR, n = 243 (66%); 3/4DR, n = 249 (67%)] and cobicistat- or ritonavir-boosted darunavir [2DR, n = 25 (7%); 3/4DR, n = 27 (7%)]. One participant included in the ITT-E population from the tenofovir alafenamide–based regimen group received a tenofovir disoproxil fumarate–based regimen and was excluded from the safety analyses.
Baseline metabolic characteristics of the safety population were generally balanced between treatment groups (see Table, Supplemental Digital Content 1, http://links.lww.com/QAI/B619). Baseline insulin resistance was observed in 69% and 68% of participants in the dolutegravir/lamivudine and tenofovir alafenamide–based regimen groups, respectively. Metabolic syndrome was observed in 10% of all participants at baseline. Of the 12 participants (3%) and 18 participants (5%) in the dolutegravir/lamivudine and tenofovir alafenamide–based regimen groups, respectively, with baseline diabetes, 1 in each group had type 1 diabetes. Proportions of participants exhibiting each symptom of metabolic syndrome in the dolutegravir/lamivudine and tenofovir alafenamide–based regimen groups were 17% and 21% for obesity, 31% and 26% for raised triglycerides, 17% and 19% for reduced HDL-C, 42% and 40% for raised blood pressure, and 24% and 24% for raised fasting glucose, respectively.
Metabolic Health Outcomes at Week 48
At week 48, adjusted mean (SE) change from baseline in weight was +0.81 (0.23) kg and +0.76 (0.22) kg with dolutegravir/lamivudine and tenofovir alafenamide–based regimens, respectively.16 Weight gain ≥5% and ≥10%, respectively, was observed in 20% and 3% of participants who switched to dolutegravir/lamivudine and 19% and 4% of those who continued tenofovir alafenamide–based regimens. Changes from baseline in weight were comparable between the treatment groups by baseline boosting status (Table 1).
Participants who switched to dolutegravir/lamivudine experienced greater adjusted mean (95% CI) decreases from baseline at week 48 vs those continuing tenofovir alafenamide–based regimens in TC [−4.5% (−6.1 to −2.8) vs +2.3% (0.8 to 3.8); P < 0.001], LDL-C [−5.5% (−8.0 to −2.9) vs +2.2% (−0.02 to 4.7); P < 0.001], TC/HDL-C ratio [−3.3% (−5.6 to −0.9) vs +0.5% (−1.5 to 2.5); P = 0.017], and triglycerides [−11.2% (−15.3 to −6.9) vs +6.0% (0.9 to 11.4); P < 0.001].16 In participants with boosted baseline regimens, statistically significantly greater decreases were observed with dolutegravir/lamivudine vs tenofovir alafenamide–based regimens in TC (P < 0.001), LDL-C (P < 0.001), TC/HDL-C ratio (P = 0.007), and triglycerides (P < 0.001; Table 1). Although not statistically significant, mean decrease from baseline in triglycerides for participants with unboosted baseline regimens was greater with dolutegravir/lamivudine vs tenofovir alafenamide–based regimens (P = 0.064; Table 1).
Changes in fasting glucose were small and comparable between treatment groups, regardless of baseline boosting status. Participants who switched to dolutegravir/lamivudine from boosted baseline regimens experienced significantly greater decreases in fasting insulin vs those who continued tenofovir alafenamide–based regimens (P = 0.005). In participants with unboosted baseline regimens, mean changes in insulin were −7.3% and +3.7% with dolutegravir/lamivudine and tenofovir alafenamide–based regimens, respectively (P = 0.175; Table 1). In the dolutegravir/lamivudine and tenofovir alafenamide–based regimen groups, respectively, fasting insulin ≥174 pmol/L at week 48 was observed in 3% (6/222) and 7% (15/218) of participants with boosted baseline regimens and 14% (12/83) and 10% (6/62) of those with unboosted baseline regimens, excluding participants with prestudy diabetes.
Insulin Resistance at Week 48
In the dolutegravir/lamivudine and tenofovir alafenamide–based regimen groups, respectively, change from baseline in adjusted geometric mean HOMA-IR was −9.7% and +4.5% (P = 0.001).16 Insulin resistance was observed in 196 of 303 participants (65%) and 215 of 290 participants (74%) in the dolutegravir/lamivudine and tenofovir alafenamide–based regimen groups, respectively [aOR (95% CI), 0.59 (0.40 to 0.87); P = 0.008].
In participants with boosted baseline regimens, odds of insulin resistance were significantly lower with dolutegravir/lamivudine vs tenofovir alafenamide–based regimens [aOR (95% CI), 0.56 (0.36 to 0.88); P = 0.012; Figure 1A]. Although not statistically significant, odds of insulin resistance trended lower with dolutegravir/lamivudine vs tenofovir alafenamide–based regimens in participants with unboosted baseline regimens [aOR (95% CI), 0.70 (0.31 to 1.58); P = 0.396; Figure 1A]. Other variables associated with statistically significant increases in odds of insulin resistance (independent of treatment) included baseline BMI [aOR per unit increase (95% CI), 1.10 (1.04 to 1.17); P = 0.001] and baseline HOMA-IR [1.88 (1.55 to 2.26); P < 0.001; see Figure, Supplemental Digital Content 2, http://links.lww.com/QAI/B619].
Metabolic Syndrome at Week 48
Metabolic syndrome was observed at week 48 in 38 of 343 participants (11%) and 41 of 344 participants (12%) in the dolutegravir/lamivudine and tenofovir alafenamide–based regimen groups, respectively. Proportions of participants exhibiting each symptom of metabolic syndrome in the dolutegravir/lamivudine and tenofovir alafenamide–based regimen groups at week 48 were 18% and 23% for obesity, 26% and 28% for raised triglycerides, 19% and 13% for reduced HDL-C, 44% and 43% for raised blood pressure, and 30% and 33% for raised fasting glucose, respectively. Odds of metabolic syndrome among participants with unboosted baseline regimens favored dolutegravir/lamivudine through week 48 [aOR (95% CI), 0.41 (0.15 to 1.09); P = 0.075] but were not statistically significant in either subgroup (Figure 1B). Factors associated with significantly higher odds of metabolic syndrome were female sex [vs male; aOR (95% CI), 3.90 (1.55 to 9.82); P = 0.004], hypertension [vs no hypertension; 5.25 (2.82 to 9.78); P < 0.001], high triglycerides [vs normal; 2.26 (1.07 to 4.75); P = 0.032], low HDL-C [vs normal; 4.30 (2.23 to 8.29); P < 0.001], and HOMA-IR ≥4 [vs <2; 8.32 (3.05 to 22.72); P < 0.001] at baseline (see Figure, Supplemental Digital Content 2, http://links.lww.com/QAI/B619).
DISCUSSION
This post hoc analysis of TANGO assessed the potential effects on metabolic health of switching to dolutegravir/lamivudine vs continuing a tenofovir alafenamide–based regimen after 48 weeks (for a more detailed discussion, see Author Video, Supplemental Digital Content 3, http://links.lww.com/QAI/B620). Small increases in weight were observed in both treatment groups, consistent with other reports of dolutegravir/lamivudine.21,22 Although weight gain is an important risk factor for the development of future endocrine or cardiovascular comorbidities, other factors should be considered when assessing overall metabolic health. Of note, two-thirds of participants who switched from tenofovir alafenamide to dolutegravir/lamivudine were on a cobicistat-boosted elvitegravir-containing regimen at baseline. Cobicistat-boosted elvitegravir has been associated with lower amounts of weight gain compared with other INSTIs or tenofovir alafenamide in ART-naive individuals.3 Therefore, potential weight changes may have been obscured by dolutegravir/lamivudine–related weight gain after the prior boosted regimen.
Results from this analysis demonstrate that participants who switched to dolutegravir/lamivudine generally experienced favorable changes from baseline across other metabolic parameters compared with those who continued tenofovir alafenamide–based regimens.
Metabolic benefits with dolutegravir/lamivudine were generally greater in participants switching from a boosted baseline regimen. Participants with boosted baseline regimens who switched to dolutegravir/lamivudine experienced statistically significant improvements in fasting lipids and fasting insulin vs those who continued tenofovir alafenamide–based regimens. In participants with boosted baseline regimens who switched to dolutegravir/lamivudine, significant decreases in fasting insulin were consistent with significantly lower odds of insulin resistance compared with those continuing tenofovir alafenamide–based regimens. Prevalence of metabolic syndrome at week 48 was low, and no significant difference in odds of metabolic syndrome was observed between the treatment groups. As the criteria used for metabolic syndrome were obesity (BMI ≥30 kg/m2) in combination with any 2 additional associated symptoms, individuals with multiple signs of metabolic syndrome but BMI <30 kg/m2 were not included in the prevalence or association analysis. This may have contributed to the low prevalence of metabolic syndrome in the analysis and the lack of significant difference between regimens at week 48.
Of note, TANGO participants were mostly white and male.16 In other studies, significantly more weight gain with ART has been observed among black female participants vs other groups.3,5 Our findings suggest that ART may impact overall metabolic health in a broader population of PLWH, warranting further investigation among demographic subgroups.
Limitations of the current post hoc analysis include the small sample size in the unboosted baseline regimen subgroup. Additionally, 48 weeks is relatively a short treatment duration considering the lifelong administration of ART. Extended follow-up time may be required to determine the long-term effects of switching to dolutegravir/lamivudine on metabolic health.
Although contemporary antiretroviral agents have favorable safety profiles compared with older agents, dyslipidemia and insulin resistance have been observed across antiretroviral classes and contribute to increased risk of type 2 diabetes and cardiovascular disease over time.23–25 Such metabolic complications associated with ART are increasingly important to consider when initiating or switching regimens, as PLWH have life expectancies similar to HIV-negative individuals and also encounter long-term environmental risk factors that affect the metabolic health of the general population.26 Findings from this analysis demonstrate that switching to dolutegravir/lamivudine may improve metabolic health of PLWH compared with continuing tenofovir alafenamide–based regimens and warrant further investigation in longer-term studies specifically designed to address these questions.
ACKNOWLEDGMENTS
This study was funded by ViiV Healthcare. The authors thank the study participants; their families and caregivers; investigators, and site staff who participated in the study; and the ViiV Healthcare, GlaxoSmithKline, Pharmaceutical Product Development, and Phastar study team members. Editorial assistance was provided under the direction of the authors by Sara Gibson, PhD, and Jennifer Rossi, MA, ELS, MedThink SciCom, and funded by ViiV Healthcare.
Footnotes
Presented in part at the 23rd International AIDS Conference; July 6–10, 2020; San Francisco and Oakland, CA, Virtual; Slides OAB0606.
J.v.W. (Global Medical), M.A.-K. (Clinical Science), B.J. (Global Patient Affairs), and B.W. (Medicines Development) are employees of ViiV Healthcare and own stock in GlaxoSmithKline (GSK). M.-C.N. (Clinical Development) and A.R.T. (Medical Director) were employees of ViiV Healthcare at the time of the study and may hold stock in GSK. J.S. has received honoraria for speaker and/or advisory roles from Janssen, ViiV Healthcare, Merck, and Gilead. S.S. has received honoraria from PPD/GSK for participation in the TANGO study and has received grants and/or personal fees for consultancy, lectures, and congress support from ViiV Healthcare, AbbVie, Gilead, and Janssen. M.W. serves on the Janssen HIV Prophylactic Vaccine Advisory Board. D.E.S. has received honoraria for speaker roles from Janssen and Gilead. J.W. is an employee of GSK and owns stock in GSK. F.A. has nothing to disclose. This study was funded by ViiV Healthcare.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
M.-C.N. and A.R.T. were employees of ViiV Healthcare at the time of the study.
Contributor Information
Mounir Ait-Khaled, Email: mounir.c.ait-khaled@gsk.com.
Jesus Santos, Email: med000854@gmail.com.
Stefan Scholten, Email: stefan.scholten@praxis-hohenstaufenring.de.
Michael Wohlfeiler, Email: michael.wohlfeiler@ahf.org.
Faïza Ajana, Email: fajana@ch-tourcoing.fr.
Bryn Jones, Email: bryn.c.jones@viivhealthcare.com.
Maria-Claudia Nascimento, Email: claudinhacacau100@gmail.com.
Allan R. Tenorio, Email: astenorio@gmail.com.
Don E. Smith, Email: Don.Smith@health.nsw.gov.au.
Jonathan Wright, Email: jonathan.j.wright@gsk.com.
Brian Wynne, Email: brian.r.wynne@viivhealthcare.com.
REFERENCES
- 1.Achhra AC, Mocroft A, Reiss P, et al. Short-term weight gain after antiretroviral therapy initiation and subsequent risk of cardiovascular disease and diabetes: the D:A:D study. HIV Med. 2016;17:255–268. [DOI] [PubMed] [Google Scholar]
- 2.Nansseu JR, Bigna JJ, Kaze AD, et al. Incidence and risk factors for prediabetes and diabetes mellitus among HIV-infected adults on antiretroviral therapy: a systematic review and meta-analysis. Epidemiology. 2018;29:431–441. [DOI] [PubMed] [Google Scholar]
- 3.Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2020;71:1379–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schafer JJ, Sassa KN, O'Connor JR, et al. Changes in body mass index and atherosclerotic disease risk score after switching from tenofovir disoproxil fumarate to tenofovir alafenamide. Open Forum Infect Dis. 2019;6:ofz414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381:803–815. [DOI] [PubMed] [Google Scholar]
- 6.Ogbuagu O. Longer-Term Safety of F/TAF and F/TDF for HIV PrEP: DISCOVER Trial Week-96 Results [OL-08]. Presented at: Conference on Retroviruses and Opportunistic Infections; 2020; Boston, MA, Virtual.
- 7.Huhn GD, Shamblaw DJ, Baril JG, et al. Atherosclerotic cardiovascular disease risk profile of tenofovir alafenamide versus tenofovir disoproxil fumarate. Open Forum Infect Dis. 2019;7:ofz472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mallon P, Brunet L, Hsu R, et al. Weight gain before and after switch from TDF to TAF [OAB0604]. Presented at: International AIDS Conference; 2020; Oakland, CA, Virtual. [DOI] [PMC free article] [PubMed]
- 9.Sax PE, Rockstroh JK, Luetkemeyer AF, et al. Switching to bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with HIV. Clin Infect Dis. 2020. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeJesus E, Rockstroh JK, Henry K, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet. 2012;379:2429–2438. [DOI] [PubMed] [Google Scholar]
- 11.Dirajlal-Fargo S, Moser C, Brown TT, et al. Changes in insulin resistance after initiation of raltegravir or protease inhibitors with tenofovir-emtricitabine: AIDS Clinical Trials Group A5260s. Open Forum Infect Dis. 2016;3:ofw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lake JE, Stanley TL, Apovian CM, et al. Practical review of recognition and management of obesity and lipohypertrophy in human immunodeficiency virus infection. Clin Infect Dis. 2017;64:1422–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randell PA, Jackson AG, Boffito M, et al. Effect of boosted fosamprenavir or lopinavir-based combinations on whole-body insulin sensitivity and lipids in treatment-naive HIV-type-1-positive men. Antivir Ther. 2010;15:1125–1132. [DOI] [PubMed] [Google Scholar]
- 14.Back D. 2-Drug regimens in HIV treatment: pharmacological considerations. Germs. 2017;7:113–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly SG, Nyaku AN, Taiwo BO. Two-drug treatment approaches in HIV: finally getting somewhere? Drugs. 2016;76:523–531. [DOI] [PubMed] [Google Scholar]
- 16.van Wyk J, Ajana F, Bisshop F, et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose two-drug regimen versus continuing a tenofovir alafenamide-based three- or four-drug regimen for maintenance of virologic suppression in adults with HIV-1: phase 3, randomized, non-inferiority TANGO study. Clin Infect Dis. 2020;71:1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim B, Choi HY, Kim W, et al. The cut-off values of surrogate measures for insulin resistance in the Korean population according to the Korean Genome and Epidemiology Study (KOGES). PLoS One. 2018;13:e0206994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee CH, Shih AZ, Woo YC, et al. Optimal cut-offs of homeostasis model assessment of insulin resistance (HOMA-IR) to identify dysglycemia and type 2 diabetes mellitus: a 15-year prospective study in Chinese. PLoS One. 2016;11:e0163424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghasemi A, Tohidi M, Derakhshan A, et al. Cut-off points of homeostasis model assessment of insulin resistance, beta-cell function, and fasting serum insulin to identify future type 2 diabetes: Tehran Lipid and Glucose Study. Acta Diabetol. 2015;52:905–915. [DOI] [PubMed] [Google Scholar]
- 20.International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Brussels, Belgium: International Diabetes Federation; 2006. [Google Scholar]
- 21.Ciccullo A, Dusina A, Lassandro AP, et al. No significant changes in body fat mass in virologically suppressed, HIV-positive patients switched to lamivudine–dolutegravir. AIDS. 2020;34:956–957. [DOI] [PubMed] [Google Scholar]
- 22.Taramasso L, Bonfanti P, Ricci E, et al. Factors associated with weight gain in people treated with dolutegravir. Open Forum Infect Dis. 2020;7:ofaa195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Cunha J, Ferreira Maselli LM, Bassi Stern AC, et al. Impact of antiretroviral therapy on lipid metabolism of human immunodeficiency virus-infected patients: old and new drugs. World J Virol. 2015;4:56–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Samaras K. The impact of weight gain during HIV treatment on risk of pre-diabetes, diabetes mellitus, cardiovascular disease, and mortality. Front Endocrinol (Lausanne). 2018;9:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srinivasa S, Grinspoon SK. Metabolic and body composition effects of newer antiretrovirals in HIV-infected patients. Eur J Endocrinol. 2014;170:R185–R202. [DOI] [PubMed] [Google Scholar]
- 26.Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4:e349–e356. [DOI] [PMC free article] [PubMed] [Google Scholar]


