Background:
Patients with heart failure (HF) and reduced ejection fraction will experience multiple hospitalizations for heart failure during the course of their disease. We assessed the efficacy of dapagliflozin on reducing the rate of total (ie, first and repeat) hospitalizations for heart failure in the DAPA-HF trial (Dapagliflozin and Prevention of Adverse-Outcomes in Heart Failure).
Methods:
The total number of HF hospitalizations and cardiovascular deaths was examined by using the proportional-rates approach of Lei-Wei-Yang-Ying and a joint frailty model for each of recurrent HF hospitalizations and time to cardiovascular death. Variables associated with the risk of recurrent hospitalizations were explored in a multivariable Lei-Wei-Yang-Ying model.
Results:
Of 2371 participants randomly assigned to placebo, 318 experienced 469 hospitalizations for HF; of 2373 assigned to dapagliflozin, 230 patients experienced 340 admissions. In a multivariable model, factors associated with a higher risk of recurrent HF hospitalizations included higher heart rate, higher N-terminal pro-B-type natriuretic peptide, and New York Heart Association class. In the Lei-Wei-Yang-Ying model, the rate ratio for the effect of dapagliflozin on recurrent HF hospitalizations or cardiovascular death was 0.75 (95% CI, 0.65–0.88), P=0.0002. In the joint frailty model, the rate ratio for total HF hospitalizations was 0.71 (95% CI, 0.61–0.82), P<0.0001, whereas, for cardiovascular death, the hazard ratio was 0.81 (95% CI, 0.67–0.98), P=0.0282.
Conclusions:
Dapagliflozin reduced the risk of total (first and repeat) HF hospitalizations and cardiovascular death. Time-to-first event analysis underestimated the benefit of dapagliflozin in HF and reduced ejection fraction.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03036124.
Keywords: 2-(3-(4-ethoxybenzyl)-4-chlorophenyl)-6-hydroxymethyltetrahydro-2H-pyran-3,4,5-triol; heart failure; recurrence; sodium-glucose transporter 2 inhibitors
Clinical Perspective.
What Is New?
In the placebo-controlled DAPA-HF trial (Dapagliflozin and Prevention of Adverse-Outcomes in Heart Failure), dapagliflozin reduced the risk of total hospitalizations in patients with heart failure with reduced ejection fraction, even when the concomitant reduction in cardiovascular death was accounted for.
Factors associated with more hospitalizations were male sex, higher heart rate, N-terminal pro-B-type natriuretic peptide, New York Heart Association class, type 2 diabetes, and longer duration of heart failure with fewer hospitalizations in those with higher systolic blood pressure and higher ejection fraction.
What Are the Clinical Implications?
Patients who are at risk of multiple hospitalizations can be identified from easily measured characteristics.
Dapagliflozin reduced the burden of heart failure including total hospitalization as well as death.
Although it is recognized that the natural history of heart failure is characterized by repeated hospitalizations and that their impact at a personal, health care system, and societal level are also well understood, these recurrent events are usually not accounted for when evaluating the effect of treatment. Even recent clinical trials underpinning treatment recommendations in guidelines examined the efficacy of therapy according to time-to-first event analysis, based on a single event per participant, ignoring further events after a first hospitalization.1–3 Given the significance of repeat hospitalizations, it is important to examine whether a new treatment for heart failure reduces not only first, but also total admissions. It is common to evaluate the effect of therapy on repeat events (relapses or recurrences) in other areas of medicine, including neurology (eg, epilepsy, migraine, multiple sclerosis), pulmonology (eg, asthma, chronic obstructive pulmonary disease), gerontology (eg, falls), and psychiatry (eg, schizophrenia). This approach has also been used in coronary artery disease of late,4–7 but has not routinely been applied in heart failure. In part, this relates to the uncertainty about which statistical methods are most appropriate when death, as well as the repeating nonfatal event, is common.8,9 Such methods need to account for not only the correlation of nonfatal events with each other, but also the association between nonfatal events and death (which precludes further hospitalization), especially if the treatment under investigation reduces death. Recently, joint frailty models that account for within-subject event dependence and provide separate estimates for rate ratios for repeat events (hospitalizations) and the hazard ratio for the terminal event (death) have emerged as an attractive option.9
In the DAPA-HF trial (Dapagliflozin and Prevention of Adverse-Outcomes in Heart Failure), the sodium-glucose cotransporter 2 inhibitor, dapagliflozin, was shown to reduce the risk of cardiovascular death and a first episode of worsening heart failure (HF) in patients with heart failure and reduced ejection fraction (HFrEF).3 In this analysis of DAPA-HF, we describe the efficacy of dapagliflozin on the predefined secondary end point of total failure hospitalizations (first and recurrent) and cardiovascular death by using a joint frailty model.
Methods
In DAPA-HF, patients with HFrEF, with and without type 2 diabetes, were randomly assigned in a double-blind, placebo-controlled, event-driven trial.3,10,11 Dapagliflozin, a sodium-glucose cotransporter 2 inhibitor, at a dose of 10 mg once daily, in addition to standard care, was compared with matching placebo. The design, baseline characteristics, and primary results have been published previously.3,10,11 The Ethics Committee of the 410 participating institutions in 20 countries approved the protocol; all patients gave written informed consent. The corresponding author had full access to all the trial data and takes responsibility for its integrity and the data analysis. The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Patients
Patients were enrolled if they were in New York Heart Association (NYHA) functional class II to IV, had a left ventricular ejection fraction ≤40%, and an elevated N-terminal pro-B-type natriuretic peptide level. Investigators were also asked to ensure individually optimized use of guideline-recommended pharmacological and device therapy. The main exclusions to enrollment were a history of type 1 diabetes, symptomatic hypotension/systolic blood pressure <95 mm Hg, and estimated glomerular filtration rate <30 mL·min–1·1.73 m–2.
HF Hospitalizations
The primary outcome of DAPA-HF was the composite of worsening HF (HF hospitalization or urgent visit for HF requiring intravenous therapy) or cardiovascular death, whichever occurred first. In this analysis, we explore the predefined secondary end point of total (first and recurrent) hospitalizations for HF and cardiovascular death. Hospitalizations occurring on the day of death were not counted in this analysis.
In addition, we explored a post hoc outcome of recurrent worsening of HF (HF hospitalizations or urgent visit requiring intravenous therapy) and cardiovascular death. This composite, analyzed as time-to-first event, was the primary outcome of the trial, but this analysis examined total episodes of worsening HF (ie, first and subsequent episodes).
Statistical Analysis
Baseline characteristics were summarized as means (standard deviations), median (interquartile ranges), or percentages. Rates were calculated using the total number of hospitalizations and cardiovascular deaths divided by the patient years of follow-up and expressed as a rate per 100 patient-years. Recurrent events were examined by using 2 methods that were prespecified in the statistical analysis plan. The first method was by the semiparametric proportional-rates model described by Lin Wei Yang Ying (LWYY).12 This is an extension of the proportional-hazards model and is based on the gap-time approach that considers the times since the previous event, that is, the interevent time. The LWYY model uses robust standard errors to account for the interdependence of events within an individual. Subgroup estimates using the subgroups predefined for the primary efficacy analysis of the trial were also calculated by using the LWYY method and the interaction term was tested. In a post hoc analysis, an adjusted model was also constructed using variables previously known to be associated with outcomes in HFrEF and variables that differed at baseline according to the number of repeat hospitalizations. The second statistical method for the analysis of recurrent hospitalizations that was prespecified in the statistical analysis plan was the joint frailty model.9 This method allows for the within-subject association between HF hospitalizations and subsequent mortality and the competing risk of mortality on HF hospitalization to be accounted for. The advantage of this model is that it allows a distinct treatment effect to be estimated for each of the individual outcomes while taking account of the association between the 2 through a common frailty term, where “frailty” is a random term specific to a subject that accounts for some patients being at higher risk (large frailty) than others (who have a small frailty). A rate ratio for the effect of dapagliflozin on HF hospitalizations and hazard ratio for cardiovascular death are provided separately for the joint model. For both the LWYY and joint frailty models, individuals were censored at the end of follow-up or at death if this was a noncardiovascular death. Recurrent hospitalizations were plotted using nonparametric estimates of the marginal mean of the cumulative number of recurrent HF hospitalizations over time, allowing for death as the terminal event according to the approach of Ghosh and Lin.13 The number needed to treat (NNT) was calculated using a negative binomial model for comparison with the previous literature on NNT in recurrent events in HF.14 However, given that dapagliflozin reduced the risk of cardiovascular death, and the first HF hospitalization in the trial, as well, we calculated the NNT from the joint frailty model so that we could account for the competing risk of death when calculating the NNT, because the negative binomial method censors deaths and does not account for the interdependence on recurrent events in the analysis.15 In a sensitivity analysis, we examined the composite end point of all-cause death or total HF hospitalizations using the LWYY method to account for all potential causes of death including noncardiovascular deaths. All analyses were conducted using Stata version 16.1 and SAS version 9.4. A P value <0.05 was considered statistically significant.
Results
Among the 4744 participants randomly assigned in DAPA-HF, 548 patients experienced a total of 809 HF hospitalizations (Figure 1). Most patients hospitalized had 1 or 2 admissions during the median follow-up of 18.2 months (Q1–Q3, 14.2–21.5). The largest number of hospitalizations was 7. The number of hospitalizations was higher in the placebo group (469 admissions among 318 patients) than in the dapagliflozin group (340 admissions among 230 patients; Figure 2). There were 500 cardiovascular deaths (227 in the dapagliflozin group and 273 in the placebo group) and 105 noncardiovascular deaths (49 in the dapagliflozin group and 56 in the placebo group).
Figure 1.
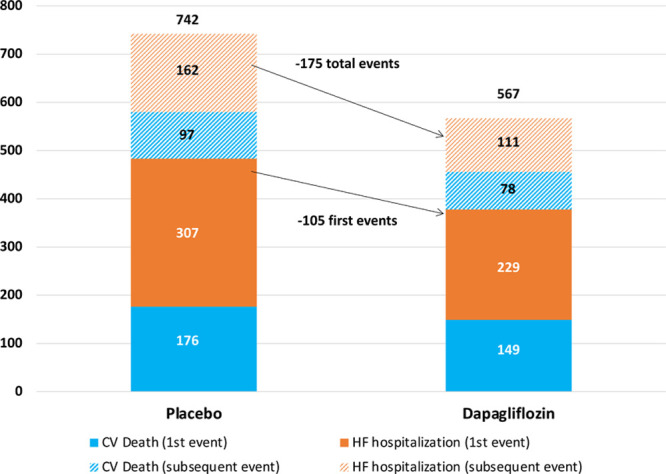
Number of extra cardiovascular (CV) deaths and heart failure (HF) hospitalizations in a recurrent-events analysis compared with time-to-first event analysis by treatment group.
Figure 2.
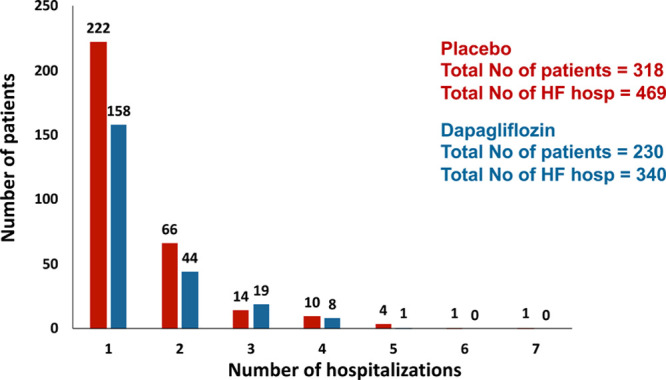
Number of patients with ≥1 hospitalization in the dapagliflozin and placebo groups. HF indicates heart failure.
Baseline Characteristics
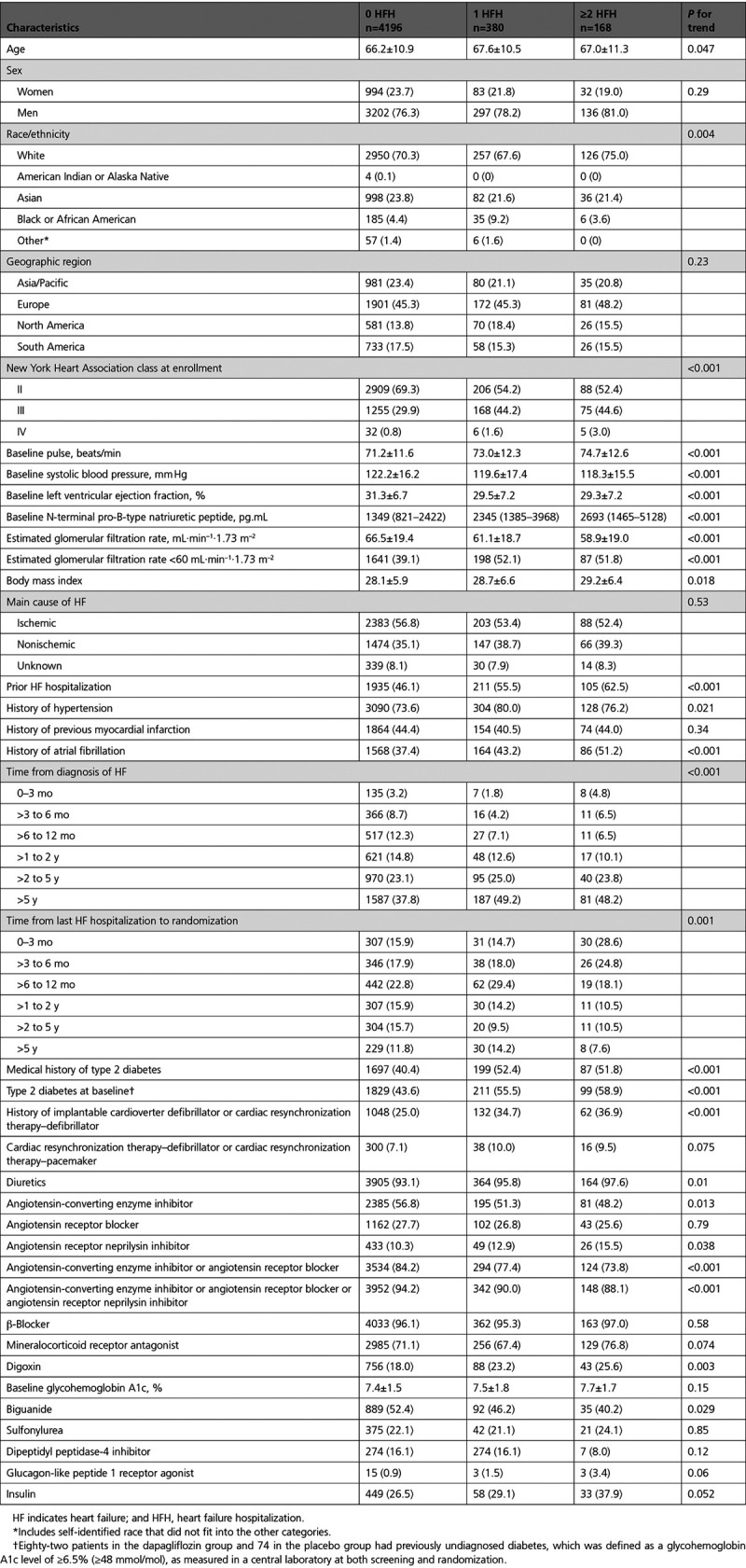
Compared with participants who were not hospitalized for HF, those admitted were older, more likely to be men, and White. There was no significant difference according to geographical region. Hospitalized patients had worse NYHA class, higher heart rate, higher body mass index, higher N-terminal pro-B-type natriuretic peptide, lower blood pressure, lower ejection fraction, and lower estimated glomerular filtration rate than those not hospitalized. Compared with patients not hospitalized, those admitted at least once were more likely to have a history of atrial fibrillation, hypertension, type 2 diabetes, and chronic kidney disease. There was no difference in history of myocardial infarction or ischemic origin between people hospitalized and not hospitalized. Hospitalized patients were more likely to have longer-duration HF and a previous hospital admission for HF. Among those with previous HF hospitalization, patients who were hospitalized during the trial had a more recent hospital admission than participants who were not admitted during the trial (who had a more remote previous admission).
Hospitalized patients were more likely to have an implantable cardioverter defibrillator and receive digoxin and less likely to be treated with an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, or angiotensin receptor neprilysin inhibitor.
The differences described between patients with and without a hospitalization during follow-up were generally more marked among individuals with ≥2 admissions, compared with 1 admission (Table).
Table.
Baseline Characteristics by Number of HF Hospitalizations
Predictors of Repeat Events
In a multivariable model, men were more likely to have a recurrent hospitalization for HF or cardiovascular death (Figure 3). Those in NYHA class III/IV were more likely to have a recurrent HF hospitalization than those in class II, as were patients with type 2 diabetes compared with those without type 2 diabetes. Patients with a longer duration of HF were more likely to have a recurrent HF hospitalization than those with a duration of <1 year. Higher heart rate and higher N-terminal pro-B-type natriuretic peptide also predicted a higher risk of recurrent HF hospitalization or cardiovascular death. Having had no HF hospitalization before randomization was associated with a lower risk of a recurrent hospitalization, as was better renal function and randomization to dapagliflozin.
Figure 3.
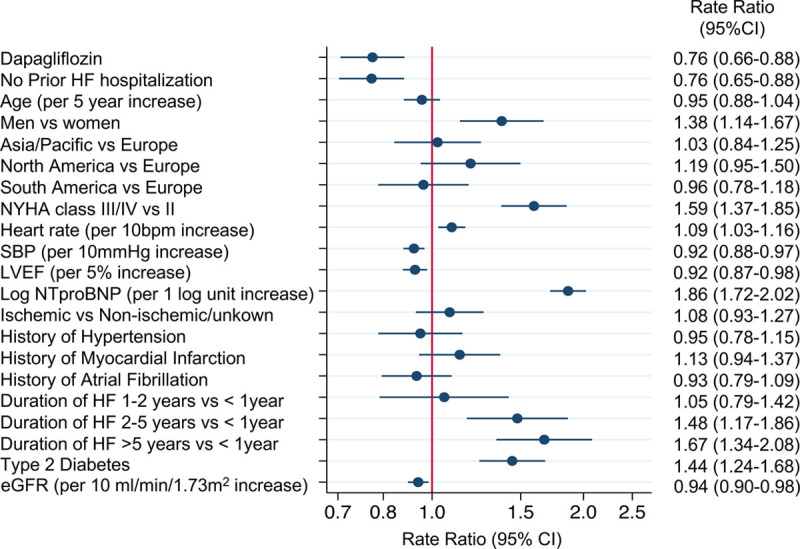
Variables associated with cardiovascular death or recurrent HF hospitalizations in a multivariable model using the Lin-Wei-Yang-Ying method to model recurrent events. eGFR indicates estimated glomerular filtration rate; HF, heart failure; LVEF, left ventricular ejection fraction; NTproBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; and SBP, systolic blood pressure.
Effect of Dapagliflozin on Total HF Hospitalizations and Cardiovascular Death
The rate of total (first and recurrent) HF hospitalizations and cardiovascular death was 21.6 per 100 patient-years in the placebo group and 16.3 per 100 patient-years in the dapagliflozin group. This compares with time-to-first event rates of 15.3 per 100 patient-years and 11.4 per 100 patient-years, respectively, for the same event composite.
The rate ratio for dapagliflozin versus placebo from the LWYY model was 0.75 (95% CI, 0.65–0.88), P=0.0002 (Figure 4), which was identical to the hazard ratio from time-to-first event analysis of HF hospitalization or cardiovascular death (Figure 5) suggesting no attenuation of the efficacy of dapagliflozin over recurrent events.
Figure 4.
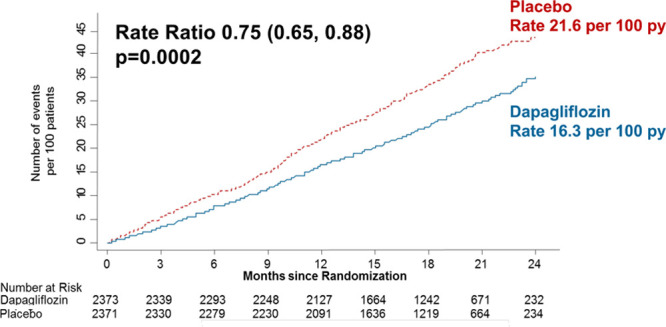
Cumulative incidence of total heart failure hospitalizations and cardiovascular death. py indicates person-years.
Figure 5.
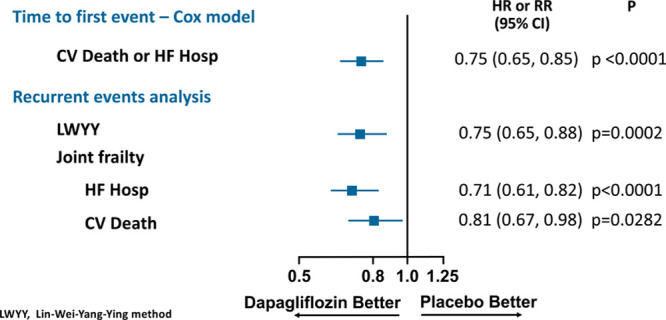
Effect of dapagliflozin on cardiovascular death or HF hospitalization using a time-to-first event Cox model, recurrent HF hospitalizations or cardiovascular death using the LWYY method and joint frailty model. CV indicates cardiovascular; HF, heart failure; and LWYY, Lin-Wei-Yang-Ying.
In the joint frailty model, the rate ratio for total HF hospitalizations was 0.71 (95% CI, 0.61–0.82), P<0.0001, whereas, for cardiovascular death, the hazard ratio was 0.81 (95% CI, 0.67–0.98), P=0.028. The corresponding hazard ratios from the time-to-first event analyses were 0.70 (0.59–0.83; P<0.0001) and 0.82 (0.69–0.98; P=0.029) for HF hospitalization and cardiovascular death, respectively. We calculated that the NNT based on the negative binomial model (where cardiovascular deaths were censored) was 23 patient-years needed to prevent 1 additional HF hospitalization. In the joint frailty model accounting for the competing risk of cardiovascular death and the joint frailty between recurrent HF hospitalizations and cardiovascular death, the NNT was 13 patient-years needed to prevent 1 additional HF hospitalization.
The exploratory post hoc outcome of worsening HF events (a HF hospitalization or urgent visit requiring intravenous therapy, both first and repeat) or cardiovascular death added a further 39 events in 33 patients to the 2-component composite of total HF hospitalizations or cardiovascular death. The rate ratio for the effect of dapagliflozin on this expanded, 3-component, composite outcome was 0.74 (95% CI, 0.64–0.86), P=0.0001. In a sensitivity analysis of all-cause death and total HF hospitalizations, the results were consistent rate ratio 0.76 (95% CI, 0.66–0.88), P=0.0002.
The efficacy of dapagliflozin did not differ by any of the predefined subgroups, except potentially for NYHA class (Figure 6). The statistical interaction was driven by a greater reduction in recurrent HF hospitalizations in those in NYHA class II, but it was not consistent with other markers of less advanced disease such as lower N-terminal pro-B-type natriuretic peptide levels.
Figure 6.
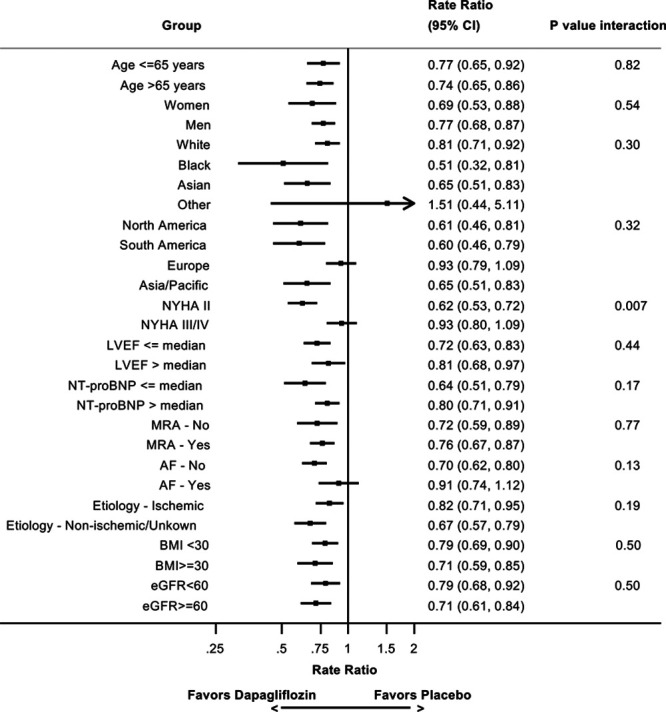
Effect of dapagliflozin compared with placebo on recurrent heart failure hospitalizations or cardiovascular death by subgroups as modeled by the Lin-Wei-Yang-Ying method. AF indicates atrial fibrillation; BMI, body mass index; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NTproBNP, N-terminal pro-B-type natriuretic peptide; and NYHA, New York Heart Association.
Discussion
Even during a relatively short median follow-up of 18.2 months, most HF hospitalizations in DAPA-HF were repeat admissions (429 of 809 total hospitalizations, 53%) that are ignored in conventional time-to-first event analysis. Both first and repeat hospitalizations were reduced by dapagliflozin, leading to a reduction in total admissions for HF.
The rationale for evaluating and preventing repeat admissions is well rehearsed. In addition to their personal impact, each hospitalization is prognostically important. First, the risk of death rises sharply when a patient is admitted to the hospital for decompensated HF. Second, in the days after discharge, patients with HFrEF are at a high risk of dying. Last, each successive hospitalization is associated with a greater risk of death in the longer term. In addition to their prognostic significance, hospitalizations exert a substantial burden on health care systems and society in general. Admissions for the treatment of HF account for a large proportion of all hospital bed-days in many health care systems and are the main driver of the cost of HF around the world. It is clearly desirable, therefore, to reduce repeat and first admissions, as well, although there is no guarantee that any given treatment will do this because of the development of tolerance (eg, with nitrates) or resistance (eg, through neurohumoral escape), for example, concepts not usually considered for cardiovascular therapy but familiar in the treatment of infection and cancer. The magnitude of benefit may also differ between the initial period early after starting therapy and during chronic treatment because of different mechanisms of action, a theoretical possibility relevant to sodium-glucose cotransporter 2 inhibitors which have an initial diuretic effect. From an effectiveness, economic, and system perspective, treatment discontinuation because of poor adherence or intolerance may also result in the attenuation of treatment effect over time that may be underestimated in time-to-first event analysis. Last, patients with recurrent admissions may simply be refractory to the treatment tested and continue to be hospitalized. These points emphasize that not only does time-to-first event analysis underestimate the morbidity/mortality burden of the disease, but it also may over- or underestimate the benefit of therapy.
In the case of dapagliflozin, the effect of treatment on hospitalizations and death was sustained over time, in keeping with its effects on patient-reported outcomes and physiological measures such as glycohemoglobin A1c and hematocrit. Consequently, in DAPA-HF, time-to-first event analysis underestimated the absolute benefit of therapy. Because the relative risk reduction with dapagliflozin on total events was identical to that on first events, the total number of hospitalizations prevented was larger when all events were accounted for. Specifically, when we accounted for the competing risk of cardiovascular death, the risk of which is also reduced by dapagliflozin therapy, only 13 patient-years of treatment were needed to prevent 1 hospitalization. As reported previously, the equivalent treatment time to prevent a first HF hospitalization was 35 patient-years. We also found that accounting for competing noncardiovascular deaths in a composite of all-cause death or HF hospitalizations did not change our findings given that most deaths were cardiovascular in origin and that dapagliflozin also reduced the risk of all-cause death. Very few other trials have examined patient-time–based treatment effects on total events. In an analysis of SHIFT (Systolic Heart Failure Treatment with the IF Inhibitor Ivabradine Trial) 14 patient-years were needed to prevent 1 additional HF hospitalization, although ivabradine had no effect on cardiovascular death in the trial, and the analysis was conducted using a negative binomial model that is suboptimal in the context of a therapy that also reduces cardiovascular death.14
The same considerations apply to the analysis of total HF hospitalizations plus cardiovascular death, where there were 1309 events, compared with 877 patients in the time-to-first event analysis, again demonstrating an almost 50% loss of events carrying information about the morbidity and mortality related to HFrEF when only the conventional time-to-first event analysis is used. The absolute risk reduction with dapagliflozin was greater for total HF hospitalizations plus cardiovascular death compared with this composite analyzed as time-to-first event. In general, the benefit of dapagliflozin on total HF hospitalizations plus cardiovascular death was also consistent in all prespecified subgroups.
The EMPEROR-Reduced trial (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure and a Reduced Ejection Fraction) recently showed that a different sodium-glucose cotransporter 2 inhibitor reduced the primary composite outcome of time-to-first occurrence of HF hospitalization or cardiovascular death in patients with HFrEF, with a hazard ratio of 0.75 (0.65–0.86), a treatment effect nearly identical to the effect of dapagliflozin on the same outcome (hazard ratio, 0.75; 0.65–0.85) in DAPA-HF. In EMPEROR-Reduced, a joint frailty model was used to estimate the effect of empagliflozin on total HF hospitalizations, giving a rate ratio of 0.70 (95% CI, 0.58–0.85; P<0.001); the hazard ratio for cardiovascular death was not reported because the reduction in cardiovascular deaths was not statistically significant.16 This compared with a rate ratio of 0.71 (95% CI, 0.61–0.82; P<0.001) in DAPA-HF, even after accounting for the reduction in cardiovascular death with dapagliflozin. A post hoc analysis of EMPORER-Reduced using the LWYY method of analyzing total HF hospitalizations or cardiovascular death gave a rate ratio of 0.76 (95% CI, 0.65–0.89).17 Our findings are also consistent with reports of the effects of cardiac resynchronization therapy and sacubitril/valsartan, eplerenone, ivabradine, and hydralazine-isosorbide dinitrate,18 although similar analyses have not been done for other evidence-based pharmacological therapy in HFrEF. In each of these reports, the relative risk reduction for total events and first events has been very similar, which appears to be different from 2 studies in HF with preserved ejection fraction where the competing risk of death from cardiovascular causes is much smaller, and the beneficial effect on total HF hospitalizations was larger than on first events.
Although we have advocated the use of recurrent events as an end point in clinical trials, and this has been accepted by both the US Food and Drug Administration and the European Medicines Agency,19,20 the decision to adopt such an approach may be a difficult choice for sponsors. For the reasons described above, there are several treatment- and patient-related reasons why the effect of therapy on second and subsequent events may be less than on first events. Drop-in use of study treatment or alternative effective therapies after a first hospitalization may also diminish the difference between the placebo and active treatment groups. Designing a trial with a recurrent events primary end point is also more difficult because factors such as heterogeneity of patient risk, not currently a part of routine power calculations, must be considered and sample size estimation is more complex.8 Nevertheless, we would still argue that analysis of recurrent events should at least be a prespecified secondary end point in view of the clinical importance of establishing the effect of therapy on total and not just first events.
Last, although the optimal statistical method for the analyses we have described is still open to discussion,21 we used 2 that have gained favor, a joint frailty model and the LWYY method, and found that their results were consistent. Moreover, the robustness and limitations of other methods in the setting of HF have been discussed elsewhere.9,22–24
Limitations
As with any clinical trial, the follow-up time was limited, and the effect of treatment on total events might be different over longer periods of observation. We were only able to study 2 types of events, and, although we would have also liked to investigate urgent visits for worsening HF requiring intravenous therapy, few of these events occurred in DAPA-HF.
Conclusions
In summary, in patients with HFrEF, compared with placebo, dapagliflozin reduced the risk of total (first and recurrent) HF hospitalizations and cardiovascular death. Recurrent hospitalizations remain a common problem in patients with HFrEF and are preventable.
Sources of Funding
The DAPA-HF trial (Dapagliflozin and Prevention of Adverse-Outcomes in Heart Failure) was funded by AstraZeneca. Drs Petrie and McMurray are supported by a British Heart Foundation Centre of Research Excellence Grant RE/18/6/34217.
Disclosures
Dr Jhund’s employer, the University of Glasgow, has been remunerated by Astra-Zeneca for working on the DAPA-HF trial (Dapagliflozin and Prevention of Adverse-Outcomes in Heart Failure) and the DELIVER trial (Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure) and speakers and advisory board fees from AstraZeneca. Speakers and advisory board fees from Novartis and advisory board fees and grants from Boehringer Ingelheim. Dr Ponikowski reports personal fees and other from AstraZeneca, Boehringer Ingelheim, Bayer, BMS, Cibiem, Novartis, and RenalGuard; personal fees from Pfizer, Servier, Respicardia, and Berlin-Chemie; other from Amgen; and grants, personal fees, and other from Vifor Pharma. Dr Docherty’s employer, the University of Glasgow, has been remunerated by AstraZeneca for working on the DAPA-HF trial. Drs Gasparyan, Bengtsson, Langkilde, and Sjostrand are employees of AstraZeneca. Dr Boehm received personal fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Servier, Medtronic, Vifor, and Novartis. Dr Chiang received honoraria for lectures from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Merck Sharp & Dohme, Novartis, Pfizer, and Sanofi. Dr Desai reports receiving personal fees from Abbott, Biofourmis, Boston Scientific, Boehringer Ingelheim, Merck, Regeneron, and Relypsa and grants and personal fees from AstraZeneca, Alnylam, and Novartis. Dr Howlett reports receiving grants and personal fees from AstraZeneca Canada and Boerhinger Ingelheim/Eli Lilly during the conduct of the study and grants and personal fees from Servier Canada, Novartis, Pfizer, and Bayer; personal fees from Otsuka, Alnylam, and Akcea; grants from Medtronic; and serving on the medical advisory board for Caridiol outside the submitted work. Dr Kitakaze reports receiving grants and personal fees from AstraZeneca during the conduct of the study and grants from the Japanese government, the Japan Heart Foundation, and the Japan Agency for Medical Research and Development; grants and personal fees from Asteras, Sanofi, Pfizer, Ono, Novartis, Tanabe-Mitubishi, and Takeda; and personal fees from Daiichi-Sankyo, Bayer, Bheringer, Kowa, Sawai, Merck Sharp & Dohme, Shionogi, Kureha, Japan Medical Data, Taisho-Toyama, and Toa Eiyo outside the submitted work. Dr Petrie reports receiving lecture fees from AstraZeneca and Eli Lilly during the conduct of the study and personal fees from Novo Nordisk, AstraZeneca, NAPP Pharmaceuticals, Takeda Pharmaceutical, Alnylam, Bayer, Resverlogix, and Cardiorentis, and grants and personal fees from Boehringer Ingelheim and Novartis. Dr Verma reports receiving personal fees from AstraZeneca, Sun Pharmaceuticals, and Toronto Knowledge Translation Working Group during the conduct of the study and grants and personal fees from Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, and Merck; grants from Bristol-Myers Squibb; and personal fees from Eli Lilly, Janssen, Novartis, Novo Nordisk, and Sanofi. Dr Inzucchi reports personal fees and nonfinancial support from AstraZeneca, Boehringer Ingelheim, Sanofi/Lexicon, Merck, VTV Therapeutics, and Abbott/Alere, and personal fees from AstraZeneca and Zafgen, as well. Dr Køber reports other support from AstraZeneca and personal fees from Novartis and Bristol-Myers Squibb as a speaker. Dr Kosiborod reports personal fees from AstraZeneca and Vifor Pharma; grants, personal fees, and other from AstraZeneca; grants and personal fees from Boehringer Ingelheim; and personal fees from Sanofi, Amgen, NovoNordisk, Merck (Diabetes), Janssen, Bayer, Applied Therapeutics, and Eli Lilly. Dr Martinez reports personal fees from AstraZeneca. Dr Sabatine reports grants from Bayer, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Pfizer, Poxel, Quark Pharmaceuticals, and Takeda; grants and personal fees from Amgen, AstraZeneca, Intarcia, Janssen Research and Development, The Medicines Company, MedImmune, Merck, and Novartis; and personal fees from Anthos Therapeutics, Bristol-Myers Squibb, CVS Caremark, Dal- Cor, Dyrnamix, Esperion, IFM Therapeutics, and Ionis. Dr Sabatine is a member of the TIMI Study Group, which has also received institutional research grant support through Brigham and Women’s Hospital from Abbott, Aralez, Roche, and Zora Biosciences. Biosciences. Dr Solomon has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, and has consulted for Abbott, Action Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boeringer-Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, Gilead, GSK, Ironwood, Lilly, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, and American Regent. Dr McMurray’s employer, the University of Glasgow, has been remunerated by AstraZeneca for working on the DAPA-HF and DELIVER trial, Cardiorentis, Amgen, Oxford University/Bayer, Theracos, Abbvie, Novartis, Glaxo Smith Kline, Vifor-Fresenius, Kidney Research UK, and Novartis, and other support from Bayer, DalCor, Pfizer, Merck, Bristol Myers, and Squibb, as well.
Footnotes
P. S. Jhund and P. Ponikowski contributed equally.
The full author list is available on page 1971.
Sources of Funding, see page 1971
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
Contributor Information
Piotr Ponikowski, Email: ppponikowski@gmail.com.
Kieran F. Docherty, Email: kieran.docherty@glasgow.ac.uk.
Samvel B. Gasparyan, Email: samvel.gasparyan@astrazeneca.com.
Michael Böhm, Email: Michael.Boehm@uks.eu.
Chern-En Chiang, Email: cechiang@vghtpe.gov.tw.
Akshay S. Desai, Email: adesai@bwh.harvard.edu.
Jonathon Howlett, Email: howlettjonathan@gmail.com.
Masafumi Kitakaze, Email: kitakaze@zf6.so-net.ne.jp.
Mark C. Petrie, Email: mark.petrie@glasgow.ac.uk.
Subodh Verma, Email: Subodh.Verma@unityhealth.to.
Olof Bengtsson, Email: Olof.Bengtsson@astrazeneca.com.
Anna-Maria Langkilde, Email: annamaria.langkilde@astrazeneca.com.
Mikaela Sjöstrand, Email: Mikaela.Sjostrand@astrazeneca.com.
Silvio E. Inzucchi, Email: silvio.inzucchi@yale.edu.
Mikhail N. Kosiborod, Email: mkosiborod@saint-lukes.org.
Felipe A. Martinez, Email: Dr.martinez@usa.net.
Marc S. Sabatine, Email: msabatine@partners.org.
Scott D. Solomon, Email: ssolomon@rics.bwh.harvard.edu.
John J.V. McMurray, Email: john.mcmurray@glasgow.ac.uk.
References
- 1.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. ; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 2.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa100949221073363 [Google Scholar]
- 3.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. ; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 4.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Jr, Juliano RA, Jiao L, Granowitz C, et al. ; REDUCE-IT Investigators. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol. 2019;73:2791–2802. doi: 10.1016/j.jacc.2019.02.032 [DOI] [PubMed] [Google Scholar]
- 5.Szarek M, White HD, Schwartz GG, Alings M, Bhatt DL, Bittner VA, Chiang CE, Diaz R, Edelberg JM, Goodman SG, et al. ; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab reduces total nonfatal cardiovascular and fatal events: the ODYSSEY OUTCOMES trial. J Am Coll Cardiol. 2019;73:387–396. doi: 10.1016/j.jacc.2018.10.039 [DOI] [PubMed] [Google Scholar]
- 6.Tikkanen MJ, Szarek M, Fayyad R, Holme I, Cater NB, Faergeman O, Kastelein JJ, Olsson AG, Larsen ML, Lindahl C, et al. ; IDEAL Investigators. Total cardiovascular disease burden: comparing intensive with moderate statin therapy insights from the IDEAL (Incremental Decrease in End Points Through Aggressive Lipid Lowering) trial. J Am Coll Cardiol. 2009;54:2353–2357. doi: 10.1016/j.jacc.2009.08.035 [DOI] [PubMed] [Google Scholar]
- 7.Murphy SA, Cannon CP, Wiviott SD, McCabe CH, Braunwald E. Reduction in recurrent cardiovascular events with intensive lipid-lowering statin therapy compared with moderate lipid-lowering statin therapy after acute coronary syndromes from the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22) trial. J Am Coll Cardiol. 2009;54:2358–2362. doi: 10.1016/j.jacc.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 8.Claggett B, Pocock S, Wei LJ, Pfeffer MA, McMurray JJV, Solomon SD. Comparison of time-to-first event and recurrent-event methods in randomized clinical trials. Circulation. 2018;138:570–577. doi: 10.1161/CIRCULATIONAHA.117.033065 [DOI] [PubMed] [Google Scholar]
- 9.Rogers JK, Yaroshinsky A, Pocock SJ, Stokar D, Pogoda J. Analysis of recurrent events with an associated informative dropout time: application of the joint frailty model. Stat Med. 2016;35:2195–2205. doi: 10.1002/sim.6853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, et al. ; DAPA-HF Committees and Investigators. A trial to evaluate the effect of the sodium-glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur J Heart Fail. 2019;21:665–675. doi: 10.1002/ejhf.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, et al. ; DAPA-HF Committees and Investigators. The Dapagliflozin And Prevention of Adverse-outcomes in Heart Failure (DAPA-HF) trial: baseline characteristics. Eur J Heart Fail. 2019;21:1402–1411. doi: 10.1002/ejhf.1548 [DOI] [PubMed] [Google Scholar]
- 12.Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Stat Soc Ser B. 2000;62:711–730. [Google Scholar]
- 13.Ghosh D, Lin DY. Nonparametric analysis of recurrent events and death. Biometrics. 2000;56:554–562. doi: 10.1111/j.0006-341x.2000.00554.x [DOI] [PubMed] [Google Scholar]
- 14.Rogers JK, Kielhorn A, Borer JS, Ford I, Pocock SJ. Effect of ivabradine on numbers needed to treat for the prevention of recurrent hospitalizations in heart failure patients. Curr Med Res Opin. 2015;31:1903–1909. doi: 10.1185/03007995.2015.1080155 [DOI] [PubMed] [Google Scholar]
- 15.Cook RJ. Number needed to treat for recurrent events. J Biom Biostat. 2013;4:1–6. doi:10.4172/2155-6180.1000167 [Google Scholar]
- 16.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. ; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 17.Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819–829. doi: 10.1016/S0140-6736(20)31824-9 [DOI] [PubMed] [Google Scholar]
- 18.Anand IS, Win S, Rector TS, Cohn JN, Taylor AL. Effect of fixed-dose combination of isosorbide dinitrate and hydralazine on all hospitalizations and on 30-day readmission rates in patients with heart failure: results from the African-American Heart Failure Trial. Circ Heart Fail. 2014;7:759–765. doi: 10.1161/CIRCHEARTFAILURE.114.001360 [DOI] [PubMed] [Google Scholar]
- 19.Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, et al. ; PARAGON-HF Investigators and Committees. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655 [DOI] [PubMed] [Google Scholar]
- 20.Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, et al. ; CHAMPION Trial Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3 [DOI] [PubMed] [Google Scholar]
- 21.Ozga AK, Kieser M, Rauch G. A systematic comparison of recurrent event models for application to composite endpoints. BMC Med Res Methodol. 2018;18:2. doi: 10.1186/s12874-017-0462-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers JK, Pocock SJ, McMurray JJ, Granger CB, Michelson EL, Östergren J, Pfeffer MA, Solomon SD, Swedberg K, Yusuf S. Analysing recurrent hospitalizations in heart failure: a review of statistical methodology, with application to CHARM-Preserved. Eur J Heart Fail. 2014;16:33–40. doi: 10.1002/ejhf.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen L, Jhund PS, Mogensen UM, Køber L, Claggett B, Rogers JK, McMurray JJV. Re-examination of the BEST trial using composite outcomes, including emergency department visits. JACC Heart Fail. 2017;5:591–599. doi: 10.1016/j.jchf.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 24.Mogensen UM, Gong J, Jhund PS, Shen L, Køber L, Desai AS, Lefkowitz MP, Packer M, Rouleau JL, Solomon SD, et al. Effect of sacubitril/valsartan on recurrent events in the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail. 2018;20:760–768. doi: 10.1002/ejhf.1139 [DOI] [PMC free article] [PubMed] [Google Scholar]


