Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disease for which no effective treatment exists at present. Previous research has found that exercise reduces the risk of AD. Since the apolipoprotein E (APOE) ε4 allele increases the risk of AD and is associated with faster disease progression than the other isoforms, we aimed to highlight the impact of exercise on AD pathology in APOE ε4 carriers. This review focuses on the effect of exercise on cognitive function, dementia risk, amyloid-β (Aβ) metabolism, lipid metabolism, neuroinflammation, neurotrophic factors and vascularization in APOE ε4 carriers. We searched the literature in the PubMed electronic database using the following search terms: physical activity, exercise, aerobic fitness, training, sport, APOE4, Alzheimer’s disease, AD and dementia. By cross-referencing, additional publications were identified. Selected studies required older adults to take part in an exercise intervention or to make use of self-reported physical activity questionnaires. All included studies were written and published in English between 2000 and 2020. From these studies, we conclude that exercise is a non-pharmacological treatment option for high-risk APOE ε4 carriers to ameliorate the AD pathological processes including reducing Aβ load, protecting against hippocampal atrophy, improving cognitive function, stabilizing cholesterol levels and lowering pro-inflammatory signals. Variation in study design related to age, cognitive outcomes and the type of intervention explained the differences in study outcomes. However, exercise seems to be effective in delaying the onset of AD and may improve the quality of life of AD patients.
Keywords: Alzheimer’s disease, APOE4 genotype, Exercise intervention, Mild cognitive impairment, Physical activity
Key Summary Points
| Why carry out this study? |
| Alzheimer’s disease is a major cause of death and disability and constitutes a large economic burden. |
| ApoE-ε4 is a strong genetic risk factor for Alzheimer’s disease. |
| This review investigated whether this genetic risk could be modified by environmental factors, specifically physical activity or exercise. |
| What was learned from the study? |
| Several studies have examined interactions and associations between exercise and ApoE-ε4 on mechanisms that contribute to the risk of Alzheimer’s disease. |
| Exercise may mitigate the negative effects of ApoE-ε4 on amyloid-beta 42 metabolism, neuroinflammation, neurotrophic factors, cerebral blood vessels and cognitive function, and thereby reduce the risk of Alzheimer’s disease. |
Digital Features
This article is published with digital features to facilitate understanding of the article. You can access the digital features on the article’s associated Figshare page. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13322291.
Introduction
Alzheimer’s Disease (AD) is a progressive neurodegenerative disease affecting cognitive function. This cognitive decline manifests as deficits including memory loss, communication problems, agnosia, apraxia and executive function impairment [1]. AD is the most common form of dementia. Quality of life is impaired in patients with dementia due to the reduced ability of daily-life functioning. The best known molecular mechanisms behind AD are amyloid plaque deposition and hyperphosphorylated tau proteins forming neurofibrillary tangles in the brain. Neuronal connections within the brain are disrupted by these protein aggregates, leading to regional atrophy. The blood circulation system may have a protective role in preventing aggregation by clearance of these proteins. However, the clearance of protein aggregates is deficient in AD due to cellular, molecular and genetic imbalances.
AD can be divided into an early-onset (age < 60) and the more common late-onset form. Genetic, clinical and lifestyle-related risk factors play a role in the development of AD. Early-onset AD is mainly caused by genetic variations in the APP gene encoding amyloid-β precursor protein, or PSEN1 and PSEN2 genes encoding presenilins I and II, respectively. These three genes have been found to play an important role in amyloid-β (Aβ) production. Cleavages of APP via β-secretase and γ-secretase secrete Aβ of several lengths, of which the most common forms are the 40- and 42-amino acid Aβ (Aβ40 and Aβ42, respectively). The latter isoform has a more hydrophobic structure, making it more susceptible to deposition in the brain. Both PSEN1 and PSEN2 proteins constitute the catalytic part of the γ-secretase complex [2]. Mutations in PSEN affect the Aβ42/40 ratio, resulting in elevated Aβ42 [3].
The late-onset form of AD is also genetically determined, albeit to a lesser extent. Genome-wide association studies have revealed the important role of the apolipoprotein E (APOE) gene encoding the ApoE protein on chromosome 19 in late-onset AD [4]. The ApoE protein is involved in the transport and metabolism of lipids, and it exists in three common isoforms: ApoE2, ApoE3 and ApoE4, encoded by APOE ε2, ε3 and ε4, respectively. They vary at amino acid residues 112 or 158, which results in significant differences in their functioning [5]. The APOE ε2 allele is protective against AD, whereas the ε3 allele has a neutral effect [6]. The risk of late-onset AD increases with the presence of one or two APOE ε4 alleles [7]. Furthermore, the age of onset will decrease in a dose-dependent relation with the APOE ε4 allele [8]. Besides the risk of AD, APOE ε4 homozygotes also demonstrate neuropathological characteristics of the disease such as increased Aβ deposition [4]. Previous studies have reported Aβ accumulation in an isoform-dependent fashion as ApoE4 > ApoE3 > ApoE2 [9, 10]. Since APOE ε4 carriers have lower cerebrospinal fluid (CSF) and plasma ApoE protein levels [10–12], the ApoE protein is suggested to be involved in preventing the accumulation of Aβ plaques [13, 14]. The ApoE4 protein is implicated in many other processes that may influence AD, including lipid metabolism, tau phosphorylation, cholesterol homeostasis, mitochondrial function, neuroinflammation, vascular function and synaptic plasticity [14].
To date, pharmacological agents only provide symptomatic treatment by targeting, for example, the synaptic dysfunction, Aβ accumulation and phosphorylation signalling pathways [15], and the efficiency can be increased by combining therapeutics [16]. However, reversing a multimodal disease such as AD still remains a challenge. The risk of AD is also influenced by lifestyle factors such as blood pressure, smoking, physical activity and cholesterol [17]. Much research is focused on non-pharmacological approaches including exercise. Previous studies have found positive effects of exercise on cognition in AD [18, 19]. Burns et al. found cardiorespiratory fitness to be protective against brain atrophy in early AD patients [20]. Furthermore, aerobic exercise in early AD was associated with reduced hippocampal atrophy and improved memory performance [21]. It was suggested that lifestyle intervention in APOE ε4 carriers may modify dementia risk, since the risk of dementia in these individuals was found to increase with adverse lifestyle factors such as physical inactivity [22]. Previous systematic reviews have focused on exercise and AD, but the association with the APOE4 genotype has received limited attention. The objective of this review is to highlight the modulatory role of exercise on AD pathology in APOE ε4 carriers. We will review the effect on several mechanisms contributing to AD including cognitive function, Aβ metabolism, lipid metabolism, neuroinflammation, neurotrophic factors and vascularization. We will discuss both human and animal studies. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by either of the authors.
Physical Activity in APOE ε4 Carriers
Cognitive Function
Cognitive decline in AD dementia affects most daily life activities. Thus, a growing need arises to ameliorate progressive cognitive symptoms. There is some evidence supporting the effectiveness of exercise as therapy (Table 1). Jensen et al. found that AD patients carrying the APOE ε4 gene and who were engaged in an exercise intervention showed an improvement of neuropsychiatric symptoms and stabilized cognition as assessed by the symbol digit modalities test (SDMT) in comparison to non-carriers and controls [23]. The exercise intervention consisted of supervised aerobic exercise three times a week for 16 weeks. Results showed that these carriers improved the physical, cognitive and neuropsychiatric outcomes compared to non-carriers. Similarly, APOE ε4 mice engaged in aerobic exercise demonstrated improved cognition and hippocampal function [24]. Cognition in these transgenic mice was tested by object and place recognition tests. A study by Etnier et al. supported the protective effect of aerobic exercise on cognition in cognitively normal older women carrying the APOE4 ε4 allele [25], based on different cognitive tests including an auditory verbal learning test, the complex figure test and the Wisconsin Card Sorting Test. Lautenschlager et al. studied the effect of a 24-week home-based physical activity intervention on cognitive function in adults with subjective memory impairment [26]. Physical activity was assessed with a survey in combination with data obtained from a pedometer to avoid bias. This study demonstrated that exercise improved cognitive function in adults aged 50 years or older, of whom 30% were carriers of the APOE ε4 allele. Additional analysis revealed that APOE ε4 non-carriers in the intervention group scored better on the Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) test compared to carriers and controls, as expected. On the contrary, a recent study by Sanders et al. showed that a 24-week aerobic exercise program did not modify cognitive function in APOE ε4 carriers with dementia [27]. Methods used to quantify the physical activity in this study were highly variable among patients with dementia. Therefore, the association between physical activity and cognitive function in APOE ε4 carriers could be absent.
Table 1.
Studies about physical activity in APOE ε4 carriers
| Reference | Species | Study population | Number of APOE ε4 carriers | Age, range or mean (SD) | Assessment of exercise | Follow-up period | Outcome measures | Major study outcome |
|---|---|---|---|---|---|---|---|---|
| Schuit et al. (2001) [37] | Human | 347 male elderly | 79: | 74.6 (4.3) years | Self-reported questionnaire | 3 years | Cognitive testing: MMSE | Maximal 1 h PA per day group showed a greater risk of cognitive decline, which was stronger in APOE ε4 carriers |
| 8 homozygotes | 3 categories: ≤ 30 min/day, 31–60 min/day and > 60 min/day | |||||||
| 71 heterozygotes | First two categories were taken together as maximal 1 h PA per day | |||||||
| Bernstein et al. (2002) [66] | Human | 1708 adults | 320 | 35–72 years | Self-reported physical activity frequency questionnaire about total and activity-specific energy expenditures | NA | Lipid profile: HDL cholesterol, LDL cholesterol, triglycerides | Enhanced HDL cholesterol in APOE ε4 carriers due to high-intensity exercise. An increase in high-intensity exercise was associated with a decrease in triglycerides only in male APOE ε4 carriers |
| Participants were grouped according to age | Categorized according to intensity | |||||||
| Podewils et al. (2005) [39] | Human | 3375 cognitively healthy older adults | 813 | 65 years or older | Interview with a modified Minnesota Leisure Time Activity Questionnaire about the frequency and duration of activities over the previous 2 weeks | 5.4 years | Dementia risk | Participants with more than 4 activities in the previous 2 weeks had a lower risk of dementia. This association was absent in carriers of the APOE ε4 allele |
| Categorized in the number of activities during 2 weeks | ||||||||
| Rovio et al. (2005) [35] | Human | 1251 participants for dementia analyses | Dementia analyses: 438 | At midlife: 39–64 years, mean age 50.6 (6) | Self-reported questionnaire based on frequency | 21 years | Dementia and AD risk late in life | Midlife PA reduced the late-life risk of dementia and AD in especially APOE ε4 carriers |
| 1239 participants for AD analyses | AD analyses: 433 | At re-examination: 65–79 years, mean age 71.6 (4.1) |
Active group: leisure-time PA at least twice a week Sedentary group: leisure-time PA less than twice a week |
|||||
| Etnier et al. (2007) [25] | Human | 94 cognitively intact older women |
35: 27 heterozygotes, 8 homozygotes |
51–81 years Mean age: 62 (7.39) |
Maximal aerobic fitness test (graded exercise test) | NA | Cognitive function: auditory verbal learning test (AVLT), the complex figure test (CFT) and the Wisconsin Card Sorting Test (WCST) | Aerobic fitness was associated with better memory performance in APOE ε4 homozygotes |
| Deeny et al. (2008) [32] | Human |
Sternberg test: 54 middle-aged adults MEG testing: 23 middle-aged adults |
Sternberg test: 16 MEG testing: 9 |
50–70 years | Interview with Yale Physical Activity Survey for Older Adults (records minutes and intensity of weekly PA); behavioural Sternberg testing; MEG Sternberg testing | NA | Behavioural Sternberg testing, MEG Sternberg testing | High PA was related to faster reaction time in executive functions of the working memory in APOE ε4 carriers. Sedentary carriers showed lower right temporal lobe activation |
| Nichol et al. (2009) [24] | Mouse | 16 ε3 and 15 ε4 targeted replacement mice | 15 | 10–12 months |
Aerobic exercise intervention: 1 min, twice a week for 6 weeks Two groups: exercise (in-cage running wheels) and sedentary group |
6 weeks | Cognitive function: object recognition, radial-arm water maze and place recognition testing; BDNF levels |
APOE ε4 mice engaged in aerobic exercise improved cognition and hippocampal function Increased hippocampal BDNF levels due to exercise in both groups |
| Chang et al. (2010) [36] | Human | 4761 non-demented and 184 demented participants | 583 |
Midlife examination: mean age 51 Late-life examination: mean age 76 |
Interview 3 groups: no midlife PA, ≤ 5 h PA and ≥ 5 h PA |
26 years | Cognitive tests: digit symbol substitution test, figure comparison, modified Stroop test, California Verbal Learning Test, immediate and delayed recall, digits backwards, short CANTAB Spatial Working Memory Test, Stroop test part III | Faster speed of processing and better memory and executive functions in both midlife PA groups. Midlife PA was associated with reduced risk for dementia in APOE ε4 non-carriers |
| Sanders et al. (2010) [27] | Human | 69 patients with dementia |
30: 3 homozygotes, 27 heterozygotes |
82.3 (7) years | Exercise intervention: walking and lower limb strength training program with low- and high-intensity training, each 6 weeks: three times/week for 12 weeks | 36 weeks | Physical function; cognitive function: neuropsychological tests and MMSE | No effect of exercise on physical or cognitive function in APOE ε4 carriers |
| Seip et al. (2011) [67] | Human | 166 participants | 54 | 18–70 years | Exercise intervention: aerobic training of moderate to vigorous intensity for 6 months. 40 min per training and 4 days/week | 6 months | Lipid profile: total glycerides, HDL cholesterol, LDL cholesterol, total cholesterol | Exercise did not modify HDL or LDL cholesterol in APOE ε4 carriers |
| Smith et al. (2011) [28] | Human | 68 cognitively intact older adults |
34: 2 homozygotes, 32 heterozygotes |
65–85 years |
Self-reported questionnaire Stanford Brief Activity Survey based on frequency and intensity Low-PA group: two or fewer low-intensity physical activities per week High-PA group: moderate to heavy physical activities at least 3 times a week |
NA | Neuropsychological testing: MMSE, Mattis Dementia Rating Scale 2 (DRS-2), RAVLT, Geriatric Depression Scale (GDS), Lawton Activities of Daily Living (ADLs); name discrimination task; semantic memory activation | More memory-related brain activation in highly active old adults carrying the APOE ε4 allele |
| Woodard et al. (2012) [30] | Human | 78 healthy, cognitively intact older adults |
26: 1 homozygote, 25 heterozygotes |
65–88 years Mean age: 73.1 (4.9) |
Stanford Brief Activity Survey Low-PA group: two or fewer low-intensity physical activities per week High-PA group: moderate to heavy physical activities at least 3 times a week |
18 months | Neuropsychological testing: MMSE, DRS-2, RAVLT Trials 1–5, RAVLT Delayed Recall; hippocampal volume | High PA was associated with reduced probability of cognitive decline in APOE ε4 carriers |
| Deeny et al. (2012) [33] | Human | 18 healthy female old adults | 9 | 56.53 (7.36) years | Maximal fitness test; the fitness level was based on the VO2max | NA | Cognitive tests: MMSE, Beck Depression Inventory, California Verbal Learning Test, Trail Making Test, category and verbal fluency; FDG-PET scan, Sternberg testing | Highly active ε4 carriers showed greater glucose uptake in the temporal lobe and low fit carriers in the frontal and parietal lobes |
| Head et al. (2012) [47] | Human |
201 cognitively intact adults: 163 for PiB-PET analysis 165 for cerebrospinal fluid (CSF) sample analysis |
PiB-PET sample: 52 CSF sample: 56 |
45–88 years |
Questionnaire administered by telephone Low-exercise and high-exercise engagement groups (high group: at or above 30 min of moderate exercise 5 days/week) |
NA | CSF Aβ42 samples and in vivo amyloid imaging via PET with [11C]PiB |
Sedentary APOE ε4 carriers showed more amyloid deposition The association of exercise with CSF Aβ42 did not differ between carriers and non-carriers |
| Brown et al. (2013) [49] | Human | 546 cognitively intact older adults | 148 |
60–95 years Mean age 69.6 (6.8) |
International Physical Activity Questionnaire (IPAQ) measured habitual physical activity Categorized into tertiles from low to high activity |
Serum insulin, glucose, cholesterol and plasma Aβ measurements; cerebral amyloid imaging by [11C]PiB-PET |
High PA was associated with increased HDL, lower insulin, triglycerides and Aβ1–42/1–40 ratio High PA reduced plasma Aβ1–42/1–40 ratio in non-carriers Low PA was associated with higher brain amyloid burden in APOE ε4 carriers |
|
| Braskie et al. (2014) [72] | Human | 43 cognitively intact older adults and 39 AD patients |
23: 2 homozygotes, 21 heterozygotes |
Cognitively intact older adults: 79.3 (4.8) AD patients: 81.9 (5.1) |
Modified Minnesota Leisure Time Physical Activity Questionnaire about frequency and duration of activities over the previous 2 weeks | 9 years | Regional brain volumes, inflammatory marker TNFα serum levels | High physical activity and low serum levels of TNFα showed independently greater total brain volume |
| Luck et al. (2014) [38] | Human | 2492 general practice patients without dementia | 514 |
75 and older Mean age 81.1 (3.5) |
Interview Active group: participating in an activity every day or several times a week |
4.5 years (follow-up every 1.5 years) | Cognitive testing: MMSE | Low fit APOE ε4 carriers had a higher risk for dementia and AD and a shorter survival time without clinical symptoms |
| Smith et al. (2014) [31] | Human | 97 healthy, cognitively intact older adults |
22: 2 homozygotes, 20 heterozygotes |
65–89 years |
Self-reported questionnaire based on frequency and intensity Stanford Brief Activity Survey Low-PA group: two or fewer low-intensity physical activities per week High PA group: moderate to heavy physical activities at least 3 times a week |
18 months | Neurobehavioural testing: MMSE, Mattis Dementia Rating Scale 2 (DRS-2), Geriatric Depression Scale (GDS), Lawton Activities of Daily Living (ADL); hippocampal volume | A decrease of 3% in hippocampal volume in the high-risk/low-PA group |
| Tan et al. (2016) [40] | Human | 3714 cognitively intact older adults | 796 |
60 years or older Mean age 70 (7) |
Interview about the number of activities in a day. PA index was calculated and categorized into 5 quartiles | 10 years | Risk of incident all-cause dementia and AD, total cerebral brain volume and hippocampal volume |
Low-PA group showed a higher risk of incident dementia; this effect was limited to non-carriers Higher PA index was related to greater hippocampal volume |
| Allard et al. (2017) [81] | Human | 22 elderly African Americans with mild cognitive impairment |
9: 2 homozygotes, 7 heterozygotes |
55–89 years Mean age 72.0 (7.2) |
Exercise intervention: stretch and aerobic exercise groups. Supervised training 3 days a week After 4–6 weeks the aerobic group walked 45–60 min during the weekend |
6 months | Serum BDNF levels |
No differences in BDNF levels between stretch and aerobic exercise groups, but a smaller change in APOE ε4 carriers BDNF levels were upregulated due to aerobic exercise only in APOE ε4 non-carriers |
| Fenesi et al. (2017) [41] | Human | 1646 older adults | 376 |
65 years or older Mean age 76.4 (6.8) |
Interview about the type and frequency of PA PA was categorized as exercisers and non-exercisers |
5 years | Dementia risk (odds ratio of developing dementia) | PA was effective in reducing the risk of dementia in non-carriers |
| Corlier et al. (2018) [74] | Human | 335 cognitively intact older adults |
57: 4 homozygotes, 53 heterozygotes |
65 years and older Mean age 77.3 (3.4) |
Self-reported questionnaire about PA in the previous 2 weeks | 9 years | Structural MRI analysis: cortical thickness of cortical regions and hippocampal volume; peripheral inflammation by serum CRP levels |
High CRP levels were associated with thinner cortex PA was positively correlated with hippocampal volume, but did not have effect on CRP levels Lower CRP levels and thinner cortex were observed in APOE ε4 carriers |
| Lee et al. (2018) [64] | Human | 28 ischemic stroke patients divided into APOE ε3 and ε4 groups | 15 | APOE ε3: 56.8 (10.65) | Exercise intervention: 5 days a week for 6 months. 3 days aerobic exercise of 45 min and 2 days resistance training | 6 months | Cognition: MMSE; lipid profile: HDL cholesterol, LDL cholesterol, total cholesterol, triglycerides |
Exercise increased HDL cholesterol in both groups A larger decrease in LDL cholesterol, total cholesterol and triglycerides due to exercise in APOE ε4 carriers |
| Jensen et al. (2019) [23] | Human | 199 patients with mild AD |
144: 54 homozygotes 90 heterozygotes |
50–90 years | Supervised aerobic exercise intervention: 1 h, three times a week for 16 weeks | 16 weeks | Cognition: symbol digit modalities test (SDMT), neuropsychiatric symptoms: NPI and physical performance | APOE ε4 carriers engaged in exercise improved cognitive, neuropsychiatric and physical performance |
| Piccarducci et al. (2019) [52] | Human | 42 healthy participants | 16 |
20–70 years Mean age 39.5 |
Habits questionnaire Non-active group: < 150 min per week of physical activity |
NA | Oxidative status and membrane composition of erythrocytes, Aβ levels | Aβ accumulation in erythrocytes decreases with physical activity in both APOE ε4 carriers and non-carriers, but carriers had higher levels of Aβ |
| Jeon et al. (2020) [48] | Human | 287 non-demented older adults | 66 |
55–90 years Mean age 71.91 (6.64) |
Lifetime Total Physical Activity questionnaire via an interview about frequency and intensity of leisure activities | NA | Cerebral Aβ accumulation by [11C]PiB-PET, AD-signature cerebral glucose metabolism by [18F]FDG-PET hippocampal volume | Midlife PA moderated the effect of Aβ accumulation in APOE4 on cerebral glucose metabolism. At low PA, high Aβ accumulation was associated with low cerebral glucose metabolism |
| Stringa et al. (2020) [34] | Human | 7176 older adults over three cohorts | 1863 | 55 years and older | Self-reported questionnaires(LAPAQ, interview, Zutphen Physical Activity questionnaire) | 9 years | Cognitive functioning: MMSE | PA was not associated with cognitive decline in APOE ε4 carriers |
| 3 categories: low, moderate and high PA |
AD Alzheimer’s disease, MEG magnetoencephalography, PiB Pittsburgh compound B, PA physical activity, LAPAQ LASA (Longitudinal Aging Study Amsterdam) Physical Activity Questionnaire, MMSE Mini-Mental State Examination, Aβ amyloid beta, HDL high-density lipoprotein, LDL low-density lipoprotein, BDNF brain-derived neurotrophic factor, RAVLT Rey Auditory Verbal Learning Test, NPI Neuropsychiatric Inventory, TNFα tumor necrosis factor alpha, IL-1β interleukin-1β, CRP C-reactive protein
The majority of studies assessed the physical activity by self-reported questionnaires (Table 1). Smith et al. found that high physical activity promoted cortical semantic processing activity on the name discrimination task among APOE ε4 carriers who were older cognitively intact adults [28]. Greater cortical semantic activation may protect older adults against future cognitive decline [29]. A follow-up study revealed that reduced predicted probability of cognitive decline in APOE ε4 carriers was associated with high physical activity [30]. Furthermore, APOE ε4 carriers with low physical activity in daily life had decreased hippocampal volume after 18 months, whereas the high physical activity group remained stable over time [31]. Physical activity could have a protective effect against hippocampal atrophy in APOE ε4 carriers, which in turn may reduce cognitive decline. A magnetoencephalography (MEG) study of participants aged between 50 and 70 found that highly active APOE ε4 carriers had a faster processing speed in working memory executive function [32]. In addition, carriers with low levels of physically activity showed decreased right temporal lobe activation. Another study on working memory revealed higher glucose uptake in core regions involved in working memory such as frontal, parietal, temporal and cerebellar regions in highly active ε4 carriers with a mean age of 63.67 ± 6.37 years [33]. However, a recent multi-cohort study by Stringa et al., consisting of older adults with a mean age of 67.8 years at baseline, indicated that physical activity did not moderate cognitive decline in APOE ε4 carriers [34]. For each cohort, a different self-reported questionnaire was used to assess the physical activity, which was classified into three categories including inactive, light and moderate–high activity based on walking, cycling, diverse sports and hobbies. Cognitive function was assessed with the Mini-Mental State Examination (MMSE).
AD Dementia Risk
Midlife or late-life exercise influences dementia risk. Some studies examined the effect of mid-life exercise on dementia risk in adults aged younger than 65. APOE ε4 carriers showed a significant inverse association between midlife physical activity and dementia risk compared to non-carriers [35], while in the AGES-Reykjavik study, the association between midlife physical activity and late-life cognition was strongest in non-carriers [36]. The combination of physical activity and the presence of an APOE ε4 allele may modify the risk of developing AD dementia later in life, but more replication is required. On the contrary, late-life physical activity seems to be effective in reducing dementia risk. The beneficial effect of late-life physical activity on the risk of cognitive decline was more pronounced in APOE ε4 carriers [37]. In addition, physical activity in late life may be beneficial in APOE ε4 carriers by reducing the risk of AD dementia and delaying its onset [38].
One of the studies that did not find any significant interaction between physical activity and the APOE4 genotype is the Cardiovascular Health Cognition Study, wherein a significant association between physical activity and dementia was observed in non-carriers, but not in APOE ε4 carriers, after a follow-up period of approximately 5 years [39]. However, only 28 APOE ε4 carriers showed cognitive decline in this period, making the interaction with physical activity subject to variability. In addition, the Framingham study showed increased risk of dementia in sedentary non-carriers aged 60 years and older, suggesting an inverse association between physical activity and dementia risk [40]. A study of 1646 older adults followed over a 5-year period reported that physical exercise was effective in preventing dementia in non-carriers [41]. No significant differences were observed between active and non-active APOE ε4 carriers. Several findings have been reported about the association between midlife or late-life exercise and AD dementia risk in APOE ε4 carriers. Exercise seems to modify AD dementia risk, but more replication is required to infer whether this is characteristic of APOE ε4 carriers.
Aβ Metabolism
Aggregation of Aβ peptides in the brain is one of the pathological hallmarks of AD. The most abundant forms are Aβ40 and Aβ42, with the latter known to be deposited more easily because of its hydrophobic and fibrillogenic composition [42]. In the healthy central nervous system, Aβ is rapidly produced and cleared, with a production rate of 7.6% per hour and a clearance rate of 8.3% per hour [43]. Due to molecular, cellular and genetic imbalances, the clearance of Aβ via the blood circulation system is deficient in late-onset AD. Mawuenyega et al. found impaired clearance of both Aβ40 and Aβ42 in the central nervous system of late-onset AD patients compared with cognitively healthy controls, whereas the production rates did not differ [44]. The effectiveness of Aβ clearance depends on APOE isoforms, decreasing from APOE2 to APOE3 to APOE4 [45]. This defect in clearance could lead to Aβ plaques that aggregate in the cortex. Transgenic AD mice showed reduced Aβ plaques and amyloid angiopathy if they performed aerobic exercise in their enriched housing [46]. This raises the question of whether exercise is associated with cerebral Aβ deposition in APOE ε4 carriers. Head et al. suggested that APOE ε4 carriers with low exercise levels might have increased risk for cerebral Aβ deposition [47]. A recent study by Jeon et al. investigated whether APOE ε4 status combined with high midlife physical activity could modify Aβ retention [48]. APOE ε4 carriers with a mean age of 72 years in a low physical activity state had higher Aβ accumulation, and this was absent in highly active carriers. Furthermore, a study of 546 cognitively healthy elderly individuals with an average age of 69.6 ± 6.8 years revealed decreased cerebral amyloid load in physically active APOE ε4 carriers, whereas high physical activity was associated with a reduced plasma Aβ42/40 ratio only in non-carriers [49].
A difference between Aβ in the brain and the periphery related to the ApoE4 protein seems plausible. The APOE ε4 allele alters the permeability of the blood–brain barrier, which may result in increased levels of Aβ in the peripheral system of carriers compared to non-carriers [50]. This could be one explanation why reduced plasma Aβ levels were observed only in highly physically active non-carriers. Aβ fibrils in the periphery have an increased affinity for binding erythrocytes that can take up these fibrils, resulting in oxidative stress [51]. Piccarducci et al. confirmed that APOE ε4 carriers have higher levels of Aβ in erythrocytes than non-carriers [52]. The Aβ levels in erythrocytes decreased with physical activity in both carriers and non-carriers, but the decrease in APOE ε4 in non-carriers was significantly greater than that in carriers. Although the reduction in Aβ accumulation in erythrocytes related to physical activity seems to be independent of the APOE4 genotype, physical activity may have a beneficial role in relieving oxidative stress in APOE ε4 carriers.
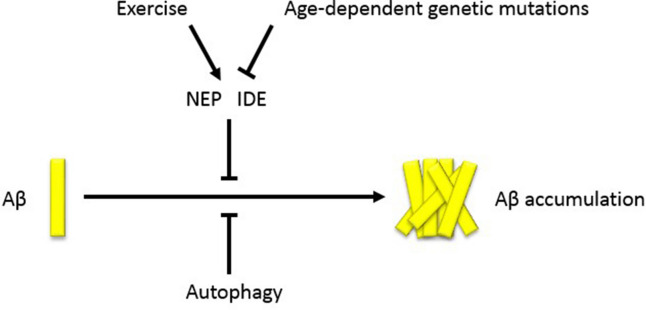
Neprilysin (NEP) and insulin-degrading enzyme (IDE) are important enzymes in maintaining the Aβ levels in the brain. NEP, a zinc metalloendopeptidase, is involved in the degradation of Aβ in monomeric and oligomeric forms in the brain [53]. IDE, also a zinc metalloendopeptidase, has the ability to degrade extracellular Aβ in the brain and to clear cytoplasmic products of APP [54]. Studies have shown that these enzymes are downregulated due to age-dependent genetic mutations which could lead to AD by disrupting cerebral Aβ clearance (Fig. 1) [54–56]. Moore et al. used AD transgenic mice with elevated Aβ levels to show the effect of exercise on Aβ metabolism [57]. They investigated reduced levels of both soluble Aβ40 and Aβ42 in the cortex and hippocampus by exercise in a dose-dependent manner. Exercise may be beneficial for APOE ε4 carriers, since the ApoE4 protein can enhance Aβ40 and Aβ42 aggregation compared to the other two isoforms [58]. NEP and IDE expression were also increased in the exercise groups in a dose-dependent fashion, providing further evidence that exercise may upregulate these Aβ degradation enzymes, leading to Aβ clearance (Fig. 1).
Fig. 1.
The influence of exercise on Aβ accumulation. In the healthy brain, Aβ levels are maintained by autophagy and Aβ-degrading enzymes NEP and IDE. Age-dependent genetic mutations may affect NEP and IDE, leading to Aβ accumulation. Exercise engagement may promote Aβ clearance indirectly by upregulating the Aβ-degrading enzymes. Aβ amyloid-β, NEP neprilysin, IDE insulin-degrading enzyme
Lipid Metabolism
Lipids are crucial molecules in the human body. The central nervous system has the second highest lipid content after the adipose tissue [59]. Signal transduction in the brain depends on lipids as well, since axons are surrounded by a myelin sheath consisting of lipids. In addition, the ApoE protein is involved in lipid transport by regulating lipoprotein concentrations. ApoE proteins are attached to the surface of lipoproteins filled with lipids, enabling the binding to receptors on tissue or cells. In the central nervous system, ApoE released by astrocytes is needed to transport cholesterol inside lipoproteins to neuronal cells [6, 52]. This process starts with the binding of ApoE to high-density lipoprotein (HDL) filled with cholesterol. ApoE has high affinity for binding to the low-density lipoprotein (LDL) receptor family at cell surfaces mediating endocytosis, where HDL is degraded and cholesterol is released [60]. Cholesterol is crucial for axonal growth and formation and remodelling of synapses, which are important for learning, memory formation and neuronal repair [6]. Previous studies found lower ApoE levels in the serum, brain and cerebrospinal fluid in both APOE ε4-carrying AD patients and cognitively normal persons [61]. A reduction in ApoE can affect cholesterol transport in the brain. In AD, brain cholesterol levels are commonly reduced in hippocampal neurons and cortical areas. Furthermore, cholesterol transport to hippocampal neurons occurs in an isoform-dependent manner, where the ApoE3 protein is more efficient than ApoE4 [62]. Animal studies have shown consistent results of decreased brain cholesterol in homozygous ε4 mice [63].
Cholesterol can be transported by either HDL or LDL in serum; the latter enhances the risk of atherosclerosis, leading to cardiovascular disease, stroke and dementia [64]. It has been widely demonstrated that APOE ε4 carriers show lower HDL and higher LDL cholesterol serum levels [52, 65]. ApoE4-knock-in mice also showed increased serum LDL cholesterol [63]. Evidence was found that physical activity may modify the altered lipid profile; for instance, physical activity in older adults was positively associated with HDL cholesterol [49]. Bernstein et al. (2002) found that high levels of physical activity may reduce the atherosclerosis risk of the APOE4 genotype on the lipid profile [66]. Furthermore, highly active APOE ε4 carriers consisting of men and women between the ages of 35 and 74 years showed enhanced HDL cholesterol. Due to the large age range, the groups were divided into < 55 and 50+ years, where the association between high physical activity and the APOE4 genotype was more pronounced among the 50+ group. Lee et al. (2018) suggested that exercise training may decrease LDL cholesterol levels and total cholesterol in APOE ε4-carrying ischemic stroke patients [64]. In particular, in relatively young participants, no change in HDL or LDL cholesterol level was observed after physical training in APOE ε4 carriers [67]. These results indicate that age is an important factor in modifying cholesterol levels in APOE ε4 carriers by exercise. High physical activity reduces the high-risk LDL cholesterol in older APOE ε4 carriers compared to their younger counterparts. Taken together, these observations build a strong case supporting the association between exercise and lipid profiles of APOE ε4 carriers.
Neuroinflammation
Neuroinflammation is involved in the neurodegenerative process in AD. Microglia and astrocytes are the central innate immune cells of the central nervous system. Microglia can be activated by the presence of Aβ to induce phagocytosis of Aβ plaques. However, activated microglia are not able to clear Aβ aggregation, leading to inflammation and thereby recruitment of more microglia, which can lead to cytotoxicity [68]. Upon activation, microglia release cytokines to regulate neuroinflammation, but these pro-inflammatory cytokines can induce damage to surrounding neurons and even cause neurodegeneration as occurs in later stages of AD. ApoE is involved in microglia suppression, which has a protective effect on neuroinflammation by inducing anti-inflammatory cytokines. ApoE-deficient mice showed enhanced levels of pro-inflammatory cytokines including tumor necrosis factor alpha (TNFα), interleukin-1β (IL-1β) and interleukin 6 (IL-6) mRNA by stimulation with lipopolysaccharide [69]. These pro-inflammatory cytokines were significantly increased, especially in APOE4 mice [70]. Administration of an ApoE-mimetic peptide derived from the receptor-binding region of the ApoE holoprotein counteracts this effect, showing its protective role in neuroinflammation. The innate immune response seems to be influenced by specific APOE isoforms. Soto et al. demonstrated evidence to support ApoE as a key player in neuroinflammation [71]. Aged mice engaged in exercise showed a reduction in microglia activation and improved neurovascular functioning and behaviour, whereas ApoE-deficient mice did not benefit from the intervention, suggesting the important role of ApoE in neurovascular functioning. Another animal study revealed that exercise in an enriched housing environment was effective in downregulating pro-inflammatory genes and upregulating anti-inflammatory genes in transgenic AD mice, which may reduce Aβ deposition in the brain [46]. Braskie et al. studied the relation between physical activity, inflammation and risk of AD in cognitively healthy older adults and AD patients [72]. They did not find an association between physical activity and TNFα, but lower TNFα and high physical activity levels were both independently associated with reduced regional brain atrophy. On the contrary, the animal study by Nichol et al. showed that exercise in aged AD mice reduced pro-inflammatory cytokines such as TNFα and IL-1β, thereby activating an adaptive response around Aβ plaques leading to decreased Aβ [73].
During inflammation, C-reactive protein (CRP) is mainly upregulated in serum. At the same time, pro-inflammatory cytokines are released. The study by Corlier et al. (2018) followed cognitively healthy older adults for 9 years [74]. Over time, CRP levels were enhanced, which was associated with greater cortical thinning. Physical activity was not related to CRP levels, which could be due to bias in the self-reports. The APOE4 genotype showed lower CRP levels and thinner cortex, which is contradictory in terms of inflammation. Therefore, Corlier et al. suggested that other mechanisms could be responsible for the enhanced AD risk in APOE4 carriers. The low CRP in APOE ε4 carriers was also supported by a previous study [75]. It was suggested that the low CRP levels in ε4 carriers are not associated with neuroinflammation, due to contradictory findings of increased inflammation in these carriers and low CRP levels. It remains unclear, but a possible explanation may be the production of cholesterol by the mevalonate pathway of the liver, because this pathway is known to be downregulated in APOE ε4 carriers, leading to low levels of CRP [75].
Neurotrophic Factors
We previously mentioned that exercise can reduce pro-inflammatory cytokines. Exercise is also indirectly involved in growth factor stimulation. Insulin-like growth factor 1 (IGF-1) and brain-derived neurotrophic factor (BDNF) are growth factors involved in hippocampal function, synaptic functioning and learning. Exercise may upregulate both growth factors in the brain and periphery directly by increasing the concentrations or indirectly by reducing pro-inflammatory cytokines, which could alter IGF-1 and BDNF signalling in neurons [76]. IGF-1 has a protective role in AD because of its ability to decrease Aβ deposition. This growth factor is involved in upregulating the transport of Aβ carrier proteins to the brain to increase the clearance of Aβ plaques [77]. In this way, cognitive functioning is improved. A recent study by Galle et al. found low IGF-1 levels in homozygous APOE ε4 carriers showing increased risk of dementia [77]. Besides the role of IGF-1 in AD, BDNF is important in maintaining the memory function. The APOE ε4 allele reduces BDNF expression, which may induce synaptic loss in the hippocampus, leading to cognitive deficits [78]. Aerobic exercise was found to compensate the hippocampal volume loss in late adulthood that was associated with increased plasma levels of BDNF [79]. In addition, plasma BDNF levels were increased in AD patients due to aerobic exercise [80]. Conversely, BDNF levels were upregulated due to aerobic exercise only in APOE ε4 non-carriers and not in carriers [81]. However, this study was restricted to one ethnic group with a higher risk of AD. An animal model showed increased hippocampal BDNF levels upon exercise in both APOE ε4 and APOE ε3 transgenic mice [24]. Studies assessing the effects of exercise on neurotrophic factors in APOE e4 carriers are sparse, but the available evidence may support a relation between the two, suggesting more replication is needed.
Vascularization
Vascular risk factors including hypertension, hypercholesterolemia, atrial fibrillation and smoking can affect the incidence and progression of AD [82]. For example, high systolic blood pressure in APOE ε4 carriers may lead to cognitive decline [83, 84]. Caselli et al. noted that any of the cerebrovascular risk factors including hypercholesterolemia, hypertension, smoking and diabetes mellitus may potentiate age-dependent memory deficits in APOE ε4 homozygotes [85]. These vascular risk factors induce vascular oxidative stress by downregulation of vascular nitric oxide (NO) and endothelial NO synthase (eNOS) pathways, which can impair vascular reactivity in the form of hypercontractility. Cerebral blood flow (CBF) will decrease due to impaired vasodilation, which mediates oxidative stress and promotes neuronal degeneration. In addition, Aβ plays a role in this process by inhibiting eNOS activity. This is supported by the finding that hypertensive mice showed increased permeability of the blood–brain barrier in the cortex and hippocampus and Aβ deposition [86]. A human study demonstrated that hypertensive APOE ε4 carriers aged 49–89 years had greater cortical amyloid burden as quantified by PET imaging compared to hypertensive non-carriers, normotensive carriers and non-carriers [87]. Zhang et al. found decreased eNOS activity, increased Aβ in the hippocampus and cortex, and decreased cognition in hypertensive mice [88]. Engagement in exercise led to improved eNOS levels, cognition and AD pathology. In addition, long-term exercise increased IGF-1 levels in these hypertensive mice, which is important in attenuating Aβ clearance.
Reduced CBF is another hallmark of AD. Older adults carrying the APOE ε4 allele showed lower CBF than non-carriers, along with reduced memory [89]. Adult APOE4-mice had reduced CBF in regions susceptible to AD pathology compared to wild-type mice and younger mice with the APOE ε4 allele, suggesting an age-dependent interaction [90]. Since APOE ε4 carriers have lower levels of the ApoE protein, a complete knock-out of the ApoE protein resulted in lower CBF compared to wild-type mice already at a young age, demonstrating the importance of the ApoE protein. It has been shown that exercise is effective in increasing CBF. Aged mice showed increased CBF in the hippocampus with walking [91]. Burdette et al. reported increased hippocampal CBF with a 16-week exercise program in older adults at risk for cognitive decline and with subjective cognitive impairment [92]. Furthermore, greater hippocampal connectivity was observed in the exercise group. Conversely, a 16-week aerobic program was not sufficient to increase CBF in AD patients [93].
Vascular endothelial-derived growth factor (VEGF) is another important factor involved in vascularization. VEGF is lower in AD [94], and especially in APOE ε4 carriers, compared to the other isoforms, which may lead to vascular impairment. By increasing the VEGF levels using a viral vector, the AD-related pathology in APOE ε4-carrying mice was reversed [95]. Exercise may increase the VEGF plasma levels to improve vascularization [76]. Increased VEGF expression was found to induce angiogenesis in aged mice through exercise engagement [96]. Studies about the association between VEGF levels in APOE ε4 carriers and exercise are sparse.
Discussion
This review focused on both human and animal studies that highlighted the role of exercise in APOE ε4 carriers with respect to the risk of AD dementia for the following domains: cognitive function, Aβ metabolism, lipid metabolism, neuroinflammation and vascularization. Exercise may modify the association between the APOE ε4 allele and AD-related pathology (Table 1).
The APOE ε4 allele increases the risk of AD. Carriers of this allele were shown to have lower levels of the ApoE protein, which is needed in many processes for proper functioning. Due to this lack of the ApoE protein in APOE ε4 carriers, cholesterol transport is affected, which may lead to higher LDL and lower HDL cholesterol transport and increased AD pathology. Through engagement in exercise, the risk of atherosclerosis may be lowered. Together with deficits in cholesterol transport, neuroinflammation may be induced by lack of ApoE. Since ApoE is involved in suppression of microglia to reduce pro-inflammatory cytokines, lower levels of ApoE could reverse this process. Upregulation of pro-inflammatory cytokines, as shown in APOE ε4 carriers, may downregulate IGF-1 and thereby increase Aβ pathology. In addition, BDNF levels may inhibit the induction of hippocampal volume loss. Lower levels of both IGF-1 and BDNF were observed in APOE ε4 carriers. Exercise is known to be effective in enhancing the levels of these growth factors, but the exact effect in APOE ε4 carriers remains unknown. However, our findings suggest that exercise may play a role in reducing pro-inflammatory cytokines and upregulating IGF-1 and BDNF in APOE ε4 carriers, because of the lower levels of the ApoE protein.
The majority of studies showed that Aβ metabolism could be improved by exercise. Exercise was found to reduce the cerebral amyloid load in APOE ε4 carriers. We observed that carriers with low physical activity levels had increased risk of Aβ deposition. In contrast, enzymes involved in the clearance of Aβ were upregulated by exercise. Improvement of Aβ metabolism may reduce cognitive deficits in AD. Low IGF-1 levels in APOE ε4 carriers were found to play a role in impaired clearance of Aβ deposition. Since exercise may ameliorate Aβ metabolism in APOE ε4 carriers and can increase IGF-1 levels in general, we suggest a relation of exercise with IGF-1 levels on the APOE ε4 allele.
In terms of cognitive function, exercise seems to be effective in reducing cognitive decline in APOE ε4 carriers. Exercise interventions improved cognition and hippocampal function in both human and animal studies [23–25]. Cognitive function was assessed mainly by neuropsychological tests. For example, Jensen et al. used the SDMT neuropsychological test, which is sensitive to very early changes in determining dysfunctional aspects of executive function in AD [23]. Intervention studies with contradictory findings related to cognitive function in APOE ε4 carriers were limited by participant age or variability in cognitive tests. A portion of the studies assessed physical activity by survey, increasing the subjectivity of these outcomes. Most of the studies found positive associations between cognition in the APOE ε4 genotype and physical activity. High physical activity may induce increased semantic processing activity, protection against hippocampal atrophy and faster speed of processing of the working memory. Physical activity might delay the onset of AD by acting on memory processes in the hippocampus of APOE ε4 carriers.
The findings as to whether physical activity can ameliorate cognitive decline in APOE ε4 carriers were heterogeneous. Multiple factors were shown to play a key role, including the type of cognitive test, the type of exercise, the use of exercise intervention versus self-reported questionnaires, the follow-up time, the age of participants and the course of dementia. We noted that most of the studies with contradictory results used less sensitive outcome variables, such as clinical diagnosis of dementia or MMSE scores, and these kinds of tests are not sensitive to preclinical cognitive ageing [32]. Furthermore, the type of exercise intervention is crucial, because of the differences between aerobic and anaerobic exercise. Aerobic exercise was proven to be more effective in AD pathology by reducing amyloid angiopathy in transgenic mice [46]. Exercise interventions also avoid the risk of bias, which is more prominent in self-reported assessment of physical activity. Participant age and the course of dementia are crucial factors in determining the outcomes, because dementia occurs mostly in the elderly and progresses with age: the older the participant, the greater the neural pathology accumulation and the greater the further decline in dementia. Some studies showed differences between midlife and late-life exercise, suggesting that age plays a crucial role. There are still questions as to whether a threshold related to age exists in the protective effect of exercise on neural pathology accumulation in APOE ε4 carriers [41]. However, since the APOE ε4 allele causes deleterious effects in neural protection and repair mechanisms, these carriers may be more influenced by lifestyle-related modifications [35].
Conclusion
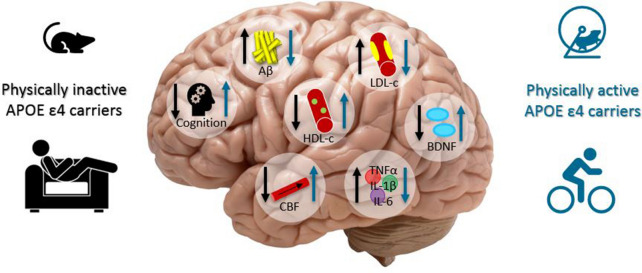
Exercise could be a potential non-pharmacological therapeutic or prevention option for reducing the risk of AD in APOE ε4 carriers (Fig. 2). By preventing or slowing neurodegeneration, exercise could increase the quality of life of AD patients. In the future, further research targeting midlife exercise will expand our knowledge about early prevention and therapeutic opportunities to delay the onset of AD in APOE ε4 carriers.
Fig. 2.
Graphical summary of the results. Physically inactive APOE ε4 carriers may have lower cognitive functioning, CBF, HDL cholesterol, BDNF and higher Aβ accumulation, LDL cholesterol and neuroinflammation factors such as TNF- α, IL-1β and IL-6 compared to physically active APOE ε4 carriers. Physical activity may be involved in the reduction of Aβ accumulation, LDL cholesterol, neuroinflammation factors such as TNF- α, IL-1β and IL-6 and in the increase of cognitive functioning, HDL cholesterol, CBF and BDNF in APOE ε4 carriers. Aβ amyloid-β, HDL-c high density lipoprotein cholesterol, LDL-c low density lipoprotein cholesterol, CBF cerebral blood flow, BDNF brain-derived neurotrophic factor, TNFα tumor necrose factor α, IL-1β interleukin-1β, IL-6 interleukin-6
Acknowledgements
The authors would like to thank the anonymous referees and the editor of Cardiology and Therapy for constructive and pertinent comments.
Funding
This publication received no funding or sponsorship. No Rapid Service Fee was received by the journal for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Sevilay Tokgöz and Jurgen A.H.R. Claassen have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Duong S, Patel T, Chang F. Dementia: what pharmacists need to know. Can Pharm J (Ott) 2017;150(2):118–129. doi: 10.1177/1715163517690745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanoiselée H-M, Nicolas G, Wallon D, Rovelet-Lecrux A, Lacour M, Rousseau S, et al. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: A GENETIC screening study of familial and sporadic cases. PLoS Med. 2017;14(3):e1002270-e. doi: 10.1371/journal.pmed.1002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kabir MT, Uddin MS, Setu JR, Ashraf GM, Bin-Jumah MN, Abdel-Daim MM. Exploring the role of PSEN mutations in the pathogenesis of Alzheimer's disease. Neurotox Res. 2020;38(4):833–49. doi: 10.1007/s12640-020-00232-x. [DOI] [PubMed] [Google Scholar]
- 4.Naj AC, Schellenberg GD, Alzheimer's Disease Genetics C Genomic variants, genes, and pathways of Alzheimer's disease: An overview. Am J Med Genet B Neuropsychiatr Genet. 2017;174(1):5–26. doi: 10.1002/ajmg.b.32499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mamun AA, Uddin MS, Bin Bashar MF, Zaman S, Begum Y, Bulbul IJ, et al. Molecular insight into the therapeutic promise of targeting APOE4 for Alzheimer's disease. Oxid Med Cell Longev. 2020;2020:5086250. doi: 10.1155/2020/5086250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7(2):180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 8.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 9.Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2000;97(6):2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tai LM, Bilousova T, Jungbauer L, Roeske SK, Youmans KL, Yu C, et al. Levels of soluble apolipoprotein E/amyloid-beta (Abeta) complex are reduced and oligomeric Abeta increased with APOE4 and Alzheimer disease in a transgenic mouse model and human samples. J Biol Chem. 2013;288(8):5914–5926. doi: 10.1074/jbc.M112.442103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruchaga C, Kauwe JS, Nowotny P, Bales K, Pickering EH, Mayo K, et al. Cerebrospinal fluid APOE levels: an endophenotype for genetic studies for Alzheimer's disease. Hum Mol Genet. 2012;21(20):4558–4571. doi: 10.1093/hmg/dds296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Morillo E, Hansson O, Atagi Y, Bu G, Minthon L, Diamandis EP, et al. Total apolipoprotein E levels and specific isoform composition in cerebrospinal fluid and plasma from Alzheimer's disease patients and controls. Acta Neuropathol. 2014;127(5):633–643. doi: 10.1007/s00401-014-1266-2. [DOI] [PubMed] [Google Scholar]
- 13.Kanekiyo T, Xu H, Bu G. ApoE and Abeta in Alzheimer's disease: accidental encounters or partners? Neuron. 2014;81(4):740–754. doi: 10.1016/j.neuron.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safieh M, Korczyn AD, Michaelson DM. ApoE4: an emerging therapeutic target for Alzheimer's disease. BMC Med. 2019;17(1):64. doi: 10.1186/s12916-019-1299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uddin MS, Kabir MT, Jeandet P, Mathew B, Ashraf GM, Perveen A, et al. Novel anti-Alzheimer's therapeutic molecules targeting amyloid precursor protein processing. Oxid Med Cell Longev. 2020;2020:7039138. doi: 10.1155/2020/7039138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabir MT, Uddin MS, Mamun AA, Jeandet P, Aleya L, Mansouri RA, et al. Combination drug therapy for the management of Alzheimer's disease. Int J Mol Sci. 2020;21(9):3272. doi: 10.3390/ijms21093272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samieri C, Perier MC, Gaye B, Proust-Lima C, Helmer C, Dartigues JF, et al. Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA. 2018;320(7):657–664. doi: 10.1001/jama.2018.11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui MY, Lin Y, Sheng JY, Zhang X, Cui RJ. Exercise Intervention Associated with Cognitive Improvement in Alzheimer's Disease. Neural Plast. 2018;2018:9234105. doi: 10.1155/2018/9234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du Z, Li Y, Li J, Zhou C, Li F, Yang X. Physical activity can improve cognition in patients with Alzheimer's disease: a systematic review and meta-analysis of randomized controlled trials. Clin Interv Aging. 2018;13:1593–1603. doi: 10.2147/CIA.S169565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71(3):210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris JK, Vidoni ED, Johnson DK, Van Sciver A, Mahnken JD, Honea RA, et al. Aerobic exercise for Alzheimer's disease: a randomized controlled pilot trial. PLoS ONE. 2017;12(2):e0170547. doi: 10.1371/journal.pone.0170547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kivipelto M, Rovio S, Ngandu T, Kåreholt I, Eskelinen M, Winblad B, et al. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med. 2008;12(6b):2762–2771. doi: 10.1111/j.1582-4934.2008.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen CS, Simonsen AH, Siersma V, Beyer N, Frederiksen KS, Gottrup H, et al. Patients with Alzheimer's disease who carry the APOE epsilon4 allele benefit more from physical exercise. Alzheimers Dement (N Y) 2019;5:99–106. doi: 10.1016/j.trci.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5(4):287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Etnier JL, Caselli RJ, Reiman EM, Alexander GE, Sibley BA, Tessier D, et al. Cognitive performance in older women relative to ApoE-epsilon4 genotype and aerobic fitness. Med Sci Sports Exerc. 2007;39(1):199–207. doi: 10.1249/01.mss.0000239399.85955.5e. [DOI] [PubMed] [Google Scholar]
- 26.Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer Disease: a randomized trial. JAMA. 2008;300(9):1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 27.Sanders LMJ, Hortobágyi T, Karssemeijer EGA, Van der Zee EA, Scherder EJA, van Heuvelen MJG. Effects of low- and high-intensity physical exercise on physical and cognitive function in older persons with dementia: a randomized controlled trial. Alzheimers Res Ther. 2020;12(1):28. doi: 10.1186/s13195-020-00597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith JC, Nielson KA, Woodard JL, Seidenberg M, Durgerian S, Antuono P, et al. Interactive effects of physical activity and APOE-ε4 on BOLD semantic memory activation in healthy elders. NeuroImage. 2011;54(1):635–644. doi: 10.1016/j.neuroimage.2010.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodard JL, Seidenberg M, Nielson KA, Smith JC, Antuono P, Durgerian S, et al. Prediction of cognitive decline in healthy older adults using fMRI. J Alzheimer's Dis. 2010;21:871–885. doi: 10.3233/JAD-2010-091693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodard JL, Sugarman MA, Nielson KA, Smith JC, Seidenberg M, Durgerian S, et al. Lifestyle and genetic contributions to cognitive decline and hippocampal structure and function in healthy aging. Curr Alzheimer Res. 2012;9(4):436–446. doi: 10.2174/156720512800492477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith JC, Nielson KA, Woodard JL, Seidenberg M, Durgerian S, Hazlett KE, et al. Physical activity reduces hippocampal atrophy in elders at genetic risk for Alzheimer's disease. Front Aging Neurosci. 2014;6:61. doi: 10.3389/fnagi.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deeny SP, Poeppel D, Zimmerman JB, Roth SM, Brandauer J, Witkowski S, et al. Exercise, APOE, and working memory: MEG and behavioral evidence for benefit of exercise in epsilon4 carriers. Biol Psychol. 2008;78(2):179–187. doi: 10.1016/j.biopsycho.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deeny SP, Winchester J, Nichol K, Roth SM, Wu JC, Dick M, et al. Cardiovascular fitness is associated with altered cortical glucose metabolism during working memory in ε4 carriers. Alzheimer's Dement. 2012;8(4):352–356. doi: 10.1016/j.jalz.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stringa N, van Schoor NM, Milaneschi Y, Ikram MA, Del Panta V, Koolhaas CM, et al. Physical activity as moderator of the association between APOE and cognitive decline in older adults: results from three longitudinal cohort studies. J Gerontol A Biol Sci Med Sci. 2020;75(10):1880–6. doi: 10.1093/gerona/glaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rovio S, Kåreholt I, Helkala E-L, Viitanen M, Winblad B, Tuomilehto J, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 2005;4(11):705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 36.Chang M, Jonsson PV, Snaedal J, Bjornsson S, Saczynski JS, Aspelund T, et al. The effect of midlife physical activity on cognitive function among older adults: AGES—Reykjavik Study. J Gerontol Ser A. 2010;65A(12):1369–1374. doi: 10.1093/gerona/glq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33(5):772–777. doi: 10.1097/00005768-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Luck T, Riedel-Heller SG, Luppa M, Wiese B, Köhler M, Jessen F, et al. Apolipoprotein E epsilon 4 genotype and a physically active lifestyle in late life: analysis of gene-environment interaction for the risk of dementia and Alzheimer's disease dementia. Psychol Med. 2014;44(6):1319–1329. doi: 10.1017/S0033291713001918. [DOI] [PubMed] [Google Scholar]
- 39.Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161(7):639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 40.Tan ZS, Spartano NL, Beiser AS, DeCarli C, Auerbach SH, Vasan RS, et al. Physical activity, brain volume, and dementia risk: the Framingham Study. J Gerontol Ser A. 2016;72(6):789–795. doi: 10.1093/gerona/glw130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fenesi B, Fang H, Kovacevic A, Oremus M, Raina P, Heisz JJ. Physical exercise moderates the relationship of apolipoprotein E (APOE) genotype and dementia risk: a population-based study. J Alzheimer's Dis. 2017;56:297–303. doi: 10.3233/JAD-160424. [DOI] [PubMed] [Google Scholar]
- 42.Murphy MP, LeVine H., 3rd Alzheimer's disease and the amyloid-beta peptide. J Alzheimers Dis. 2010;19(1):311–323. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-β synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12(7):856–861. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, et al. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science (New York, NY) 2010;330(6012):1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karran E, Mercken M, Strooper BD. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat Rev Drug Discovery. 2011;10(9):698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 46.Ambrée O, Leimer U, Herring A, Görtz N, Sachser N, Heneka MT, et al. Reduction of amyloid angiopathy and Abeta plaque burden after enriched housing in TgCRND8 mice: involvement of multiple pathways. Am J Pathol. 2006;169(2):544–552. doi: 10.2353/ajpath.2006.051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Head D, Bugg JM, Goate AM, Fagan AM, Mintun MA, Benzinger T, et al. Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch Neurol. 2012;69(5):636–643. doi: 10.1001/archneurol.2011.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeon SY, Byun MS, Yi D, Lee JH, Ko K, Sohn BK, et al. Midlife lifestyle activities moderate APOE ε4 effect on in vivo Alzheimer's disease pathologies. Front Aging Neurosci. 2020;12:42. doi: 10.3389/fnagi.2020.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown BM, Peiffer JJ, Taddei K, Lui JK, Laws SM, Gupta VB, et al. Physical activity and amyloid-β plasma and brain levels: results from the Australian Imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry. 2013;18(8):875–881. doi: 10.1038/mp.2012.107. [DOI] [PubMed] [Google Scholar]
- 50.Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485(7399):512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jayakumar R, Kusiak JW, Chrest FJ, Demehin AA, Murali J, Wersto RP, et al. Red cell perturbations by amyloid β-protein. Biochim Biophys Acta (BBA) Gener Subjects. 2003;1622(1):20–28. doi: 10.1016/s0304-4165(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 52.Piccarducci R, Daniele S, Fusi J, Chico L, Baldacci F, Siciliano G, et al. Impact of ApoE polymorphism and physical activity on plasma antioxidant capability and erythrocyte membranes. Antioxidants (Basel) 2019;8(11):538. doi: 10.3390/antiox8110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Amouri SS, Zhu H, Yu J, Marr R, Verma IM, Kindy MS. Neprilysin: an enzyme candidate to slow the progression of Alzheimer's disease. Am J Pathol. 2008;172(5):1342–1354. doi: 10.2353/ajpath.2008.070620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ebrahimi K, Majdi A, Baghaiee B, Hosseini SH, Sadigh-Eteghad S. Physical activity and beta-amyloid pathology in Alzheimer's disease: A sound mind in a sound body. EXCLI J. 2017;16:959–972. doi: 10.17179/excli2017-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miners JS, Van Helmond Z, Chalmers K, Wilcock G, Love S, Kehoe PG. Decreased expression and activity of neprilysin in Alzheimer disease are associated with cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2006;65(10):1012–1021. doi: 10.1097/01.jnen.0000240463.87886.9a. [DOI] [PubMed] [Google Scholar]
- 56.Del Campo M, Stargardt A, Veerhuis R, Reits E, Teunissen CE. Accumulation of BRI2-BRICHOS ectodomain correlates with a decreased clearance of Aβ by insulin degrading enzyme (IDE) in Alzheimer's disease. Neurosci Lett. 2015;589:47–51. doi: 10.1016/j.neulet.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 57.Moore KM, Girens RE, Larson SK, Jones MR, Restivo JL, Holtzman DM, et al. A spectrum of exercise training reduces soluble Aβ in a dose-dependent manner in a mouse model of Alzheimer's disease. Neurobiology of Disease. 2016;85:218–224. doi: 10.1016/j.nbd.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 58.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63(3):287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu T-B, Zhang Z, Luo P, Wang S-S, Peng Y, Chu S-F, et al. Lipid metabolism in Alzheimer’s disease. Brain Res Bull. 2019;144:68–74. doi: 10.1016/j.brainresbull.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 60.Poirier J, Miron J, Picard C, Gormley P, Théroux L, Breitner J, et al. Apolipoprotein E and lipid homeostasis in the etiology and treatment of sporadic Alzheimer's disease. Neurobiol Aging. 2014;35 Suppl 2(Suppl 2):S3–S10. doi: 10.1016/j.neurobiolaging.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poirier J. Apolipoprotein E, cholesterol transport and synthesis in sporadic Alzheimer's disease. Neurobiol Aging. 2005;26(3):355–361. doi: 10.1016/j.neurobiolaging.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 62.Rapp A, Gmeiner B, Hüttinger M. Implication of apoE isoforms in cholesterol metabolism by primary rat hippocampal neurons and astrocytes. Biochimie. 2006;88(5):473–483. doi: 10.1016/j.biochi.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 63.Hamanaka H, Katoh-Fukui Y, Suzuki K, Kobayashi M, Suzuki R, Motegi Y, et al. Altered cholesterol metabolism in human apolipoprotein E4 knock-in mice. Hum Mol Genet. 2000;9(3):353–361. doi: 10.1093/hmg/9.3.353. [DOI] [PubMed] [Google Scholar]
- 64.Lee J-H, Hong S-M, Shin Y-A. Effects of exercise training on stroke risk factors, homocysteine concentration, and cognitive function according the APOE genotype in stroke patients. J Exerc Rehabil. 2018;14(2):267–274. doi: 10.12965/jer.1836108.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fuzikawa AK, Peixoto SV, Taufer M, Moriguchi EH, Lima-Costa MF. Association of ApoE polymorphisms with prevalent hypertension in 1406 older adults: the Bambuí Health Aging Study (BHAS) Braz J Med Biol Res. 2008;41(2):89–94. doi: 10.1590/s0100-879x2008000200002. [DOI] [PubMed] [Google Scholar]
- 66.Bernstein MS, Costanza MC, James RW, Morris MA, Cambien F, Raoux S, et al. Physical activity may modulate effects of ApoE genotype on lipid profile. Arterioscler Thromb Vasc Biol. 2002;22(1):133–140. doi: 10.1161/hq0102.101819. [DOI] [PubMed] [Google Scholar]
- 67.Seip RL, Zoeller RF, Angelopoulos TJ, Salonia J, Bilbie C, Moyna NM, et al. Interactive effects of APOE haplotype, sex, and exercise on postheparin plasma lipase activities. J Appl Physiol (1985). 2011;110(4):1021–1028. doi: 10.1152/japplphysiol.00287.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kloske CM, Wilcock DM. The important interface between apolipoprotein e and neuroinflammation in Alzheimer’s disease. Front Immunol. 2020;11(754). [DOI] [PMC free article] [PubMed]
- 69.Lynch JR, Morgan D, Mance J, Matthew WD, Laskowitz DT. Apolipoprotein E modulates glial activation and the endogenous central nervous system inflammatory response. J Neuroimmunol. 2001;114(1):107–113. doi: 10.1016/s0165-5728(00)00459-8. [DOI] [PubMed] [Google Scholar]
- 70.Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, et al. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem. 2003;278(49):48529–48533. doi: 10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]
- 71.Soto I, Graham LC, Richter HJ, Simeone SN, Radell JE, Grabowska W, et al. APOE Stabilization by Exercise Prevents Aging Neurovascular Dysfunction and Complement Induction. PLoS Biol. 2015;13(10):e1002279-e. [DOI] [PMC free article] [PubMed]
- 72.Braskie MN, Boyle CP, Rajagopalan P, Gutman BA, Toga AW, Raji CA, et al. Physical activity, inflammation, and volume of the aging brain. Neuroscience. 2014;273:199–209. doi: 10.1016/j.neuroscience.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG, Cotman CW. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J Neuroinflammation. 2008;5:13. doi: 10.1186/1742-2094-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corlier F, Hafzalla G, Faskowitz J, Kuller LH, Becker JT, Lopez OL, et al. Systemic inflammation as a predictor of brain aging: Contributions of physical activity, metabolic risk, and genetic risk. NeuroImage. 2018;172:118–129. doi: 10.1016/j.neuroimage.2017.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yun Y-W, Kweon S-S, Choi J-S, Rhee J-A, Lee Y-H, Nam H-S, et al. APOE Polymorphism is associated with c-reactive protein levels but not with white blood cell count: Dong-gu Study and Namwon Study. J Korean Med Sci. 2015;30(7):860–865. doi: 10.3346/jkms.2015.30.7.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 77.Galle SA, van der Spek A, Drent ML, Brugts MP, Scherder EJA, Janssen JAMJL, et al. Revisiting the role of insulin-like growth factor-I receptor stimulating activity and the apolipoprotein E in Alzheimer’s disease. Front Aging Neurosci. 2019;11(20). [DOI] [PMC free article] [PubMed]
- 78.Sen A, Nelson TJ, Alkon DL. ApoE4 and Aβ Oligomers Reduce BDNF Expression via HDAC Nuclear Translocation. J Neurosci. 2015;35(19):7538–7551. doi: 10.1523/JNEUROSCI.0260-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coelho FGDM, Vital TM, Stein AM, Arantes FJ, Rueda AV, Camarini R, et al. Acute aerobic exercise increases brain-derived neurotrophic factor levels in elderly with Alzheimer's Disease. J Alzheimer's Dis. 2014;39:401–408. doi: 10.3233/JAD-131073. [DOI] [PubMed] [Google Scholar]
- 81.Allard JS, Ntekim O, Johnson SP, Ngwa JS, Bond V, Pinder D, et al. APOEε4 impacts up-regulation of brain-derived neurotrophic factor after a six-month stretch and aerobic exercise intervention in mild cognitively impaired elderly African Americans: a pilot study. Exp Gerontol. 2017;87(Pt A):129–136. doi: 10.1016/j.exger.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lange-Asschenfeldt C, Kojda G. Alzheimer’s disease, cerebrovascular dysfunction and the benefits of exercise: From vessels to neurons. Exp Gerontol. 2008;43(6):499–504. doi: 10.1016/j.exger.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 83.Zade D, Beiser A, McGlinchey R, Au R, Seshadri S, Palumbo C, et al. Interactive effects of apolipoprotein E type 4 genotype and cerebrovascular risk on neuropsychological performance and structural brain changes. J Stroke Cerebrovasc Dis. 2010;19(4):261–268. doi: 10.1016/j.jstrokecerebrovasdis.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bender AR, Raz N. Age-related differences in memory and executive functions in healthy APOE ɛ4 carriers: the contribution of individual differences in prefrontal volumes and systolic blood pressure. Neuropsychologia. 2012;50(5):704–714. doi: 10.1016/j.neuropsychologia.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Caselli RJ, Dueck AC, Locke DEC, Sabbagh MN, Ahern GL, Rapcsak SZ, et al. Cerebrovascular risk factors and preclinical memory decline in healthy APOE ε4 homozygotes. Neurology. 2011;76(12):1078–1084. doi: 10.1212/WNL.0b013e318211c3ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gentile MT, Poulet R, Pardo AD, Cifelli G, Maffei A, Vecchione C, et al. β-Amyloid deposition in brain is enhanced in mouse models of arterial hypertension. Neurobiol Aging. 2009;30(2):222–228. doi: 10.1016/j.neurobiolaging.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 87.Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Sr, Diaz-Arrastia R, Park DC. Risk factors for β-amyloid deposition in healthy aging: vascular and genetic effects. JAMA Neurol. 2013;70(5):600–606. doi: 10.1001/jamaneurol.2013.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang L, Zheng H, Luo J, Li L, Pan X, Jiang T, et al. Inhibition of endothelial nitric oxide synthase reverses the effect of exercise on improving cognitive function in hypertensive rats. Hypertens Res. 2018;41(6):414–425. doi: 10.1038/s41440-018-0033-5. [DOI] [PubMed] [Google Scholar]
- 89.Filippini N, Ebmeier KP, MacIntosh BJ, Trachtenberg AJ, Frisoni GB, Wilcock GK, et al. Differential effects of the APOE genotype on brain function across the lifespan. NeuroImage. 2011;54(1):602–610. doi: 10.1016/j.neuroimage.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 90.Zerbi V, Wiesmann M, Emmerzaal TL, Jansen D, Van Beek M, Mutsaers MPC, et al. Resting-state functional connectivity changes in aging apoE4 and apoE-KO mice. J Neurosci. 2014;34(42):13963–13975. doi: 10.1523/JNEUROSCI.0684-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Uchida S, Suzuki A, Kagitani F, Hotta H. Responses of acetylcholine release and regional blood flow in the hippocampus during walking in aged rats. J Physiol Sci. 2006;56(3):253–257. doi: 10.2170/physiolsci.SC001706. [DOI] [PubMed] [Google Scholar]
- 92.Burdette JH, Laurienti PJ, Espeland MA, Morgan A, Telesford Q, Vechlekar CD, et al. Using network science to evaluate exercise-associated brain changes in older adults. Front Aging Neurosci. 2010;2:23. doi: 10.3389/fnagi.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van der Kleij LA, Petersen ET, Siebner HR, Hendrikse J, Frederiksen KS, Sobol NA, et al. The effect of physical exercise on cerebral blood flow in Alzheimer's disease. Neuroimage Clin. 2018;20:650–654. doi: 10.1016/j.nicl.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mateo I, Llorca J, Infante J, Rodríguez-Rodríguez E, Fernández-Viadero C, Peña N, et al. Low serum VEGF levels are associated with Alzheimer's disease. Acta Neurol Scand. 2007;116(1):56–58. doi: 10.1111/j.1600-0404.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- 95.Salomon-Zimri S, Glat MJ, Barhum Y, Luz I, Boehm-Cagan A, Liraz O, et al. Reversal of ApoE4-driven brain pathology by vascular endothelial growth factor treatment. J Alzheimers Dis. 2016;53(4):1443–58. doi: 10.3233/JAD-160182. [DOI] [PubMed] [Google Scholar]
- 96.Yun-Hong D, Jie L, Yandong Z, Jose AR, Justin CC, Yuchuan D. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr Neurovasc Res. 2006;3(1):15–23. doi: 10.2174/156720206775541787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.