ABSTRACT
Gastrointestinal basidiobolomycosis (GIB) is a rare fungal infection with limited geographic distribution. However, the incidence of GIB has shown an increasing trend because of globalization and frequent traveling. GIB is commonly seen to mimic gastrointestinal malignancy and other diseases such as intestinal tuberculosis and inflammatory bowel disease. Tissue diagnosis is considered to be the gold standard for differentiating these mycotic lesions from tuberculosis and malignancy with confirmation of species performed by culture or polymerase chain reaction. The diagnosis of GIB should be conjectured in patients with suspicion of malignancy, with an inconclusive biopsy. It seems prudent to proceed with radical excision of mass early because both colonic malignancy and GIB have high mortality if untreated.
INTRODUCTION
Basidiobolomycosis is a rare fungal infection caused by Basidiobolus ranarum belonging to order Entomophthorales of the class Zygomycetes.1 Usually, it infects the skin and the subcutaneous tissue, but it can also involve the gastrointestinal (GI) tract, lungs, kidneys, and other systems. Although gastrointestinal basidiobolomycosis (GIB) is extremely rare, with around 100 cases reported in the literature, it is on an increasing trend with most of cases reported in the last 2 decades.2 It produces invasive infection in both immunocompetent and immunosuppressed individuals, is resistant to amphotericin B, and usually mimics carcinoma of the GI tract.3–9 Because of its rarity and unfamiliarity to the clinician, it is usually misdiagnosed as carcinoma, inflammatory bowel disease (IBD), tuberculosis (TB), or other fungal infection such as mucormycosis.3–5,7,10–12 We report our experience with this rare entity along with a review of the literature regarding the preferred modality of treatment.
CASE REPORT
A 50-year-old man presented with mild periumbilical pain of 2 months duration. He was a known diabetic for the past 20 years, which was found to be poorly controlled with oral hypoglycemics for which he was put on insulin after admission. He also has undergone treatment for pulmonary TB 10 years back. His general and systemic examination did not reveal abnormalities. Laboratory examination showed hemoglobin of 11.8 g/dL and total leukocyte count of 10.52 × 103 cells per cubic millimeter, with raised absolute eosinophil count of 1,780 cells per cubic millimeter. The renal and liver function tests were normal.
Abdominal ultrasonography showed a space-occupying lesion in the right iliac fossa. Contrast-enhanced computed tomography revealed diffuse symmetric wall thickening of the cecum, the proximal ascending colon, the base of the appendix, and the terminal ileum. The lesion showed significant enhancement with a few focal nonenhancing areas (Figure 1). There were a few enlarged enhancing lymph nodes at the root of the mesentery and right common iliac vessels, the largest measuring 11 mm in diameter. Colonoscopy showed circumferential ulcers with thickened and edematous folds in the cecum and ascending colon, which were suspicious of malignancy (Figure 2). TB of the colon was kept as a differential, given the history of TB. However, the colonoscopic biopsy specimen failed to demonstrate any malignant tissue or tubercular granuloma. Further sections and use of special staining revealed fungal hyphae with tissue reaction, primarily suggestive of the mucormycosis in one of the slides (Figure 3).
Figure 1.
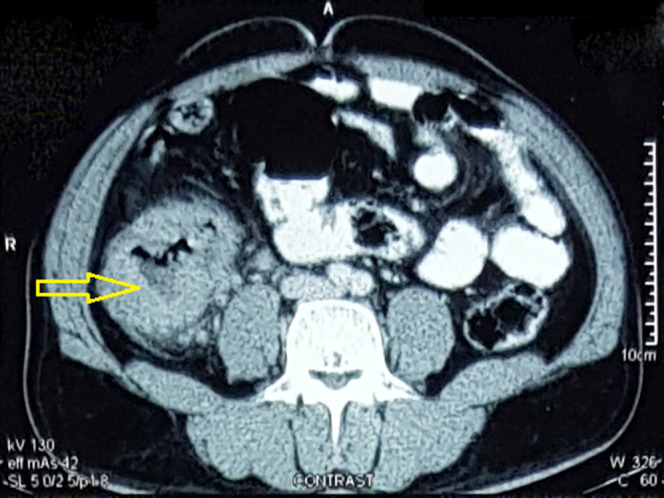
Contrast-enhanced computed tomography axial section showing diffuse symmetric wall thickening in the proximal ascending colon (marked with yellow arrow), few enlarged enhancing lymph nodes were also seen.
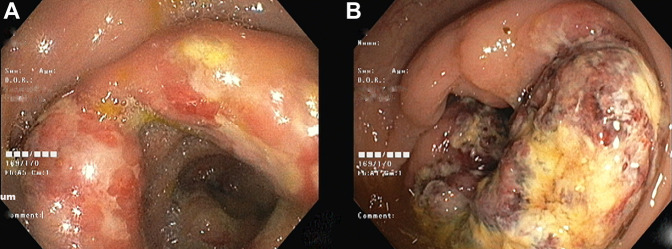
Figure 2.
(A) Initial colonoscopy circumferential ulcers with thickened and edematous folds seen in the cecum and ascending colon and (B) follow-up colonoscopy showing circumferential ulcero-nodular growth at ascending colon and cecum with luminal narrowing raising suspicion of rapidly progressing malignancy.
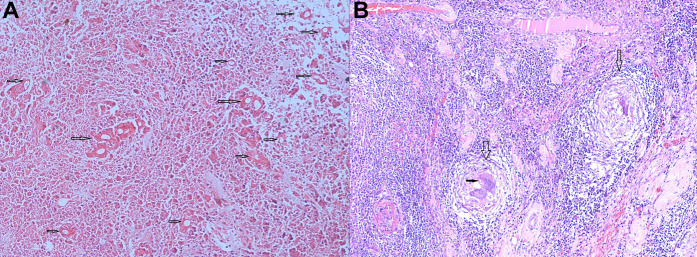
Figure 3.
Postoperative microscopy of the lesion showing (A) extensive coagulative necrosis of the mucosa with numerous broad, thin-walled, aseptate fungal hyphae surrounded by a prominent Splendore-Hoeppli phenomenon (marked with arrows) and (B) transmural dense chronic inflammatory infiltrates and multiple epithelioid cell granulomata with giant cells (marked with empty vertical arrows). Some giant cells are seen engulfing the fungal organism (marked with a solid horizontal arrow).
Based on this finding, liposomal amphotericin B was started at a dose of 5 mg/kg body weight. Amphotericin B was discontinued after 3 days of treatment because of derangement in the renal functions. Posaconazole could not be started because of the limited availability and unaffordability of the drug by the patient. A multidisciplinary meet, including surgeons, pathologists, gastroenterologists, and a mycologist, was held to decide about further management. Repeat colonoscopy was performed to access the response of antifungal. The lesion had increased exponentially in size along with ulceration, and there was critical narrowing beyond which scope was not negotiable. The colonoscopy image was highly suggestive of rapidly progressing malignancy (Figure 2). Surgical resection was planned. Intraoperatively, a mass was found in the cecum and the ascending colon. A right radical hemicolectomy was performed with palpably clear margins of 5 cm on either side. The resected specimen was sent for both histopathological examination and fungal culture.
The gross specimen revealed a circumferential symmetric ulcero-nodular lesion involving the distal 5 cm of the ileum, ileocecal valve, cecum, and appendix. It was extending until the proximal ascending colon and measured 9.5 × 5.0 × 4.0 cm in size. The cut section of the lesion was firm and yellow-colored and was seen invading the wall of the colon up to the muscularis propria. Two small pedunculated polyps were seen on the mucosal surface of the ascending colon, about 3 cm above the growth. Multiple enlarged mesenteric and mesocolic lymph nodes were also seen (Figure 4). The microscopic examination showed ulceration and transmural inflammation with the prominence of eosinophils. Areas of coagulative necrosis and multiple epithelioid cell granulomas centered around fungal filaments were noted. Some of the giant cells were found engulfing fungal hyphae. These were aseptate, broad branched with a sheath of bright eosinophilic material of Splendore-Hoeppli reaction (Figure 2). Special stain for acid-fast bacilli was negative, and there was no evidence of malignancy. The polyps in the ascending colon were found to be traditional serrated adenomas with low-grade dysplasia. Based on the morphology and tissue reaction, a diagnosis of invasive mycotic lesion was made. Calcofluor potassium hydroxide preparation of the tissue confirmed the presence of broad septate hyphae, and the culture showed the growth of yellowish waxy colonies with radial fissures within 4 days of inoculation. Lactophenol cotton blue mount of the colonies demonstrated broad aseptate hyphae, forcefully ejected conidia, and zygospores with conjugation beak (Figure 5). The fungus was identified as the B. ranarum. He was discharged on postoperative day 6 on oral itraconazole. The patient has taken itraconazole for 6 months and is asymptomatic at the end of 6 months of follow-up.
Figure 4.
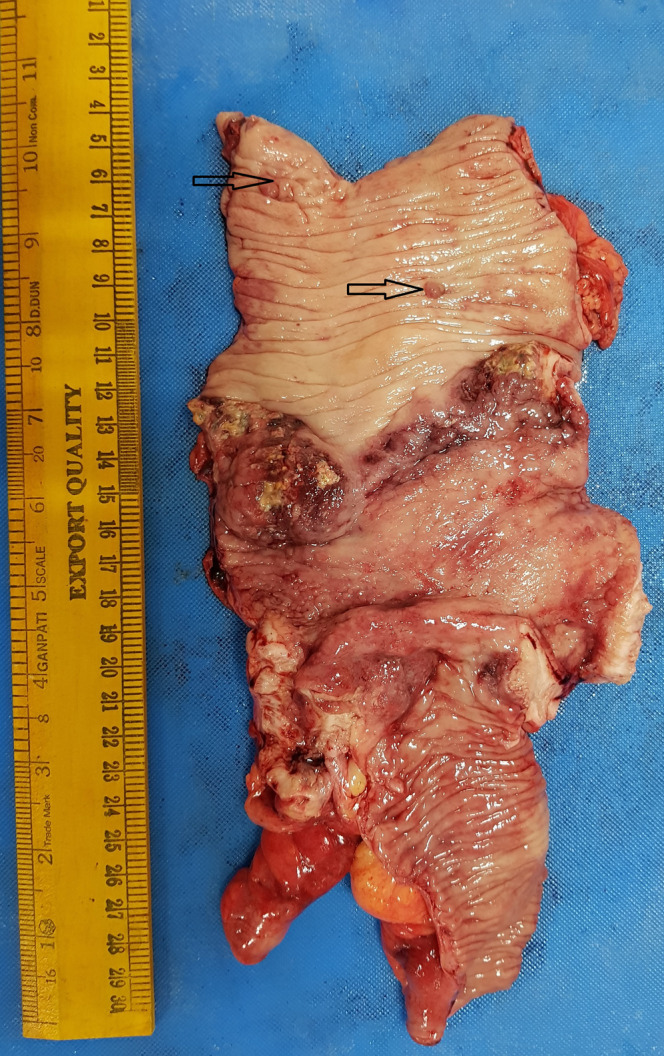
Gross specimen showing circumferential symmetric ulcero-nodular lesion from distal 5 cm of the ileum involving complete cecum and appendix extending until the proximal ascending colon. Two small pedunculated polyps are also seen (marked with black arrow).
Figure 5.
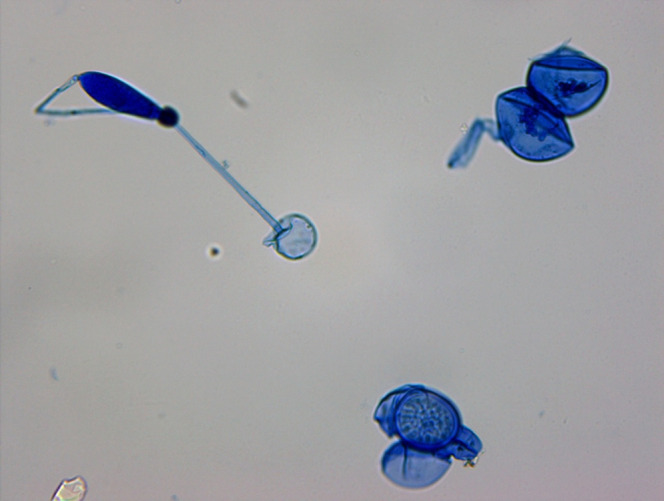
Lactophenol cotton blue mount of the colonies of Basidiobolus ranarum showing broad aseptate hyphae, forcefully ejected conidia, and zygospores with conjugation beak.
DISCUSSION
GIB is an unusual fungal infection of the GI tract caused by B. ranarum.1 Recently, this disease is on an increasing trend because of frequent travel and globalization.10 Clinical diagnosis of GIB is difficult because it presents with nonspecific clinical presentations such as fever, abdominal pain, or abdominal mass. The same features can be seen with infections of the GI tract or tumors.3–5,7,8,10–12 Holenarasipur et al reported 44 cases of GIB in 2012; most were from the United States (40%) and Saudi Arabia (25%). Of the 19 patients from the United States, 17 (89%) were from Arizona, whereas 1 was from a Utah town that borders Arizona. They proposed that the entry of pathogen might have occurred from the natural reservoirs of B. ranarum in the southwestern desert (ie, from soil and reptiles).6 Most of the cases occurs more common in patients from arid climates.6 Commonly noted risk factors associated with this condition are immunosuppression and diabetes, although it can affect the immunocompetent individuals.7,13,14 In addition, the male predilection of the disease has been noted in the literature.4 Uncontrolled diabetes with likely immunosuppression in our case might have acted as a potential risk factor.
The route of transmission (GIB) is unknown, but most of the researchers believe it is due to the ingestion of contaminated soil, animal faeces, or food.6,10,13 In addition, “toilet leaves” used for cleansing after a bowel movement might result in direct inoculation in the perineum.4,7,14,15 The GIB predominately involves the large bowel (82%), with the majority located in the right colon.6 Rarely, it can also affect the rectum and appendix.13,16,17 The clinical presentation of GIB is usually indolent. Most of the cases present with mild abdominal pain. Other symptoms could be fever, weight loss, constipation, and abdominal mass.6
Carcinoma of the colon was the most common provisional diagnosis in most published literature, with GIB only being diagnosed postoperatively (43%).6 Few cases have also presented like IBD with Saadah et al publishing fistulizing Crohn's disease-like presentation in a child with GIB.7,11 The Splendore-Hoeppli phenomenon is invariably present in cases of GIB, which is characterized by broad, septate, hyphae-like structures surrounded by an eosinophilic sheath.10,18 This, along with other pathological findings such as transmural granulomatous inflammation (histiocytes, giant cells, and lymphocytes) and eosinophilic microabscesses, helps to make a probable diagnosis of GIB.13,18 Histological mimics such as intestinal pythiosis and lagenidiosis can only be ruled out by the culture, which is the gold standard for the diagnosis.4,5
The preferred mode of treatment is surgery combined with antifungal therapy. The rapidity of the growth seen on colonoscopy forced us to embark on surgical resection.4,5,19 Radical resection should be performed with a wide margin to avoid the recurrence. The literature supports early surgical intervention to decrease mortality.4,6,8,10,13,14 B. ranarum is intrinsically resistant to amphotericin B. Long-term itraconazole therapy and follow-up are advised to prevent the recurrence. The disseminated disease should be treated with debridement and itraconazole.6,7,10,13
Diagnosis of GIB should be suspected in patients with abdominal lump and eosinophilia without conclusive evidence of malignancy.6,8,13,14,16,20 A rapidly growing mass can prohibit the surgeon from waiting for the fungal culture report to confirm the diagnosis. We suggest surgical intervention if there is high clinical suspicion of malignancy even when repeated biopsies suggest benign lesions and recommend holding a multidisciplinary team meeting to deal with this type of difficult situation. Surgical resection of the infected tissue coupled with prolonged treatment with itraconazole seems to be the best available clinical option. Early diagnosis and surgical resection are cornerstones of management to avoid dissemination because disseminated GIB has a high mortality rate.
DISCLOSURES
Author contributions: P. Kumar and Vikram VS wrote the article. V. Hallur and S. Samal edited the article. MI Chouhan, SJ Bhat, and TS Mishra revised the article for intellectual content. P. Kumar is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Vikram VS, Email: vikramvskumar@gmail.com.
Vinay Hallur, Email: vinay118@gmail.com.
Swagatika Samal, Email: swagatikavss@gmail.com.
Mohd Imran Chouhan, Email: chouhan.imran43@gmail.com.
Sunil Jee Bhat, Email: sunilbhat123.sb@gmail.com.
Tushar S. Mishra, Email: doctushra@gmail.com.
REFERENCES
- 1.Greer DL, Friedman L. Studies on the genus Basidiobolus with reclassification of the species pathogenic for man. Med Mycol. 1966;4(4):231–41. [PubMed] [Google Scholar]
- 2.Pezzani MD, Di Cristo V, Parravicini C, et al. Gastrointestinal basidiobolomycosis: An emerging mycosis difficult to diagnose but curable. Case report and review of the literature. Trav Med Infect Dis. 2019;31:101378. [DOI] [PubMed] [Google Scholar]
- 3.Taghipour Zahir S, Sharahjin NS, Kargar S. Basidiobolomycosis a mysterious fungal infection mimic small intestinal and colonic tumour with renal insufficiency and ominous outcome. Case Rep. 2013;2013:bcr2013200244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Shanafey S, AlRobean F, Hussain IB. Surgical management of gastrointestinal basidiobolomycosis in pediatric patients. J Pediatr Surg. 2012;47(5):949–51. [DOI] [PubMed] [Google Scholar]
- 5.Choonhakarn C, Inthraburan K. Concurrent subcutaneous and visceral basidiobolomycosis in a renal transplant patient. Clin Exp Dermatol. 2004;29(4):369–72. [DOI] [PubMed] [Google Scholar]
- 6.Vikram HR, Smilack JD, Leighton JA, Crowell MD, De Petris G. Emergence of gastrointestinal basidiobolomycosis in the United States, with a review of worldwide cases. Clin Infect Dis. 2012;54(12):1685–91. [DOI] [PubMed] [Google Scholar]
- 7.El-Shabrawi MHF, Kamal NM, Jouini R, Al-Harbi A, Voigt K, Al-Malki T. Gastrointestinal basidiobolomycosis: An emerging fungal infection causing bowel perforation in a child. J Med Microbiol. 2011;60(9):1395–402. [DOI] [PubMed] [Google Scholar]
- 8.Rabie ME, El Hakeem I, Al-Shraim M, Al Skini MS, Jamil S. Basidiobolomycosis of the colon masquerading as stenotic colon cancer. Case Rep Surg. 2011;2011:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geramizadeh B, Shekarkhar G. Gastrointestinal basidiobolomycosis, a rare and under-diagnosed fungal infection in immunocompetent hosts: A review article. Iran J Med Sci. 2015;40(2):8. [PMC free article] [PubMed] [Google Scholar]
- 10.Nemenqani D, Saif OA, Amra NK, Amr SS. Gastrointestinal basidiobolomycosis. Arch Pathol Lab Med. 2009;133:5. [DOI] [PubMed] [Google Scholar]
- 11.Saadah OI, Farouq MF, Daajani NA, Kamal JS, Ghanem AT. Gastrointestinal basidiobolomycosis in a child; an unusual fungal infection mimicking fistulising Crohn's disease. J Crohns Colitis. 2012;6(3):368–72. [DOI] [PubMed] [Google Scholar]
- 12.Zavasky D, Samowitz W, Loftus T, Segal H, Carroll K. Gastrointestinal zygomycotic infection caused by Basidiobolus ranarum: Case report and review. Clin Infect Dis. 1999;28(6):1244–8. [DOI] [PubMed] [Google Scholar]
- 13.El-Shabrawi MHF, Kamal NM. Gastrointestinal basidiobolomycosis in children: An overlooked emerging infection? J Med Microbiol. 2011;60(7):871–80. [DOI] [PubMed] [Google Scholar]
- 14.Geramizadeh B, Foroughi R, Keshtkar-Jahromi M, Malek-Hosseini S-A, Alborzi A. Gastrointestinal basidiobolomycosis, an emerging infection in the immunocompetent host: A report of 14 patients. J Med Microbiol. 2012;61(Pt_12):1770–4. [DOI] [PubMed] [Google Scholar]
- 15.Rose SR, Lindsley MD, Hurst SF, Paddock CD, Damodaran T, Bennett J. Gastrointestinal basidiobolomycosis treated with posaconazole. Med Mycol Case Rep. 2013;2:11–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan ZU, Prakash B, Kapoor MM, Madda JP, Chandy R. Basidiobolomycosis of the rectum masquerading as crohn's disease: Case report and review. Clin Infect Dis. 1998;26(2):521–3. [DOI] [PubMed] [Google Scholar]
- 17.Pandit V, Rhee P, Aziz H, Jehangir Q, Friese RS, Joseph B. Perforated appendicitis with gastrointestinal basidiobolomycosis: A rare finding. Surg Infect. 2014;15(3):339–42. [DOI] [PubMed] [Google Scholar]
- 18.Hussein MR, Musalam AO, Assiry MH, Eid RA, El Motawa A-M, Gamel A-M. Histological and ultrastructural features of gastrointestinal basidiobolomycosis. Mycol Res. 2007;111(8):926–30. [DOI] [PubMed] [Google Scholar]
- 19.Chakrabarti A, Chatterjee SS, Das A, et al. Invasive zygomycosis in India: Experience in a tertiary care hospital. Postgrad Med J. 2009;85(1009):573–81. [DOI] [PubMed] [Google Scholar]
- 20.Lyon GM, Smilack JD, Komatsu KK, et al. Gastrointestinal basidiobolomycosis in Arizona: Clinical and epidemiological characteristics and review of the literature. Clin Infect Dis. 2001;32(10):1448–55. [DOI] [PubMed] [Google Scholar]