Abstract
BACKGROUND:
Research suggests that children’s health and well-being are supported by core adaptive systems, including the autonomic nervous system (ANS). Despite evidence for the importance of adulthood ANS regulation in the development of disease, few studies have examined how early development may influence emerging ANS function. Therefore, we examined how infant adiposity gain during early infancy related to ANS regulation at 6-months.
METHODS:
Infant weight and length were abstracted from birth records and measured during the 6-month assessment in a low-income, racially/ethnically diverse sample (N = 60). WHO-standardized weight-for-length-gain change was calculated across the first 6 months of life. ANS reactivity was measured as the combined sympathetic (i.e., pre-ejection period) and parasympathetic (i.e., respiratory sinus arrhythmia) nervous system responses during the developmentally challenging Still Face Paradigm (SFP). ANS “classic reactivity” response was characterized by paired sympathetic activation and parasympathetic withdrawal.
RESULTS:
Lower weight-for-length gain in the first 6 months predicted classic reactivity during still face. However, greater weight-for-length gain predicted ‘classic reactivity’ during the reunion, when infants were expected to recover, suggesting autonomic dysregulation.
CONCLUSION:
These findings suggest an association between early life adiposity gain and the development of infant ANS regulation.
Understanding the development of a healthy child comes from recognizing societal, environmental, and biological factors that support health and wellness, including physiological regulation. The autonomic nervous system (ANS) is a core physiological system that supports our capacity to navigate an everchanging world and contributes to the modulation of energy expenditure and/or preservation [1, 2]. The ANS is co-regulated by two complementary inputs – the sympathetic excitatory system and the parasympathetic inhibitory system that work together to mobilize flexible stress reactivity (i.e., mobilization of energy to meet environmental demands) and recovery (i.e., restoration of homeostasis following mobilization). ANS regulation encompasses these adequate reactivity and/or recovery responses, however, responses may also be dysregulated, such as when reactivity and/or recovery processes may be inadequate to meet environmental demands or may meet immediate demands but increase potential risks for negative long-term physical or psychosocial outcomes. Cardiac measures of the ANS represent a moment-to-moment dynamic assessment of real-time regulation [3], and ANS regulation has been shown to predict a range of outcomes in infants and children including attachment to parent, occurrence of behavior problems, and psychopathology [4, 5]. However, despite existing theories and studies of adult populations supporting the importance of ANS regulation in the development of health risks and the pathogenesis of disease [6, 7]. very few studies have examined these associations across early development, before health problems manifest [4].
Bourgeoning research in adults suggests that ANS dysregulation may be one mechanism of disease development and pathological processes associated with immune problems, cardiovascular disease, cancer, and obesity [6–8]. Of these, childhood obesity is an increasing concern for younger populations because it is highly prevalent in the United States and a risk factor for poor physical and psychosocial health into adulthood [9]. Children with and without obesity exhibit differences in patterns of ANS regulation, but the majority of these studies are cross-sectional [10, 11]. Limited research has explored the development of these differences and whether early infant weight or adiposity gain may be associated with later ANS differences in early childhood [12–14].
Although only a few reports have examined the development of the ANS throughout infancy, findings suggest that early environments influence both structural development and functional regulation of these systems [15, 16]. Prenatal and early life stress is an especially salient predictor of differences in physiological reactivity and has been most consistently examined in previous research [17, 18]. However, preliminary research also suggests that physical systems, such as rapid early weight gain, may also influence ANS development [19], as well as increase risk for future obesity [20, 21]. Further, to our knowledge, no studies of infant weight gain or adiposity have examined the joint functioning of both sympathetic and parasympathetic branches to assess ANS coordination within individuals, despite longstanding acknowledgement of their complementary, integrated function.
The current study sought to address these gaps in the literature by examining if infant weight-for-length gain during the first 6 months is related to coordinated sympathetic and parasympathetic nervous system reactivity across periods of challenge and recovery in a racially- and ethnically- diverse sample of low-income children at increased risk for obesity. Importantly, given the consistent evidence that early life stress ‘programs’ physiological systems, particularly in this sample [22], we sought to examine whether weight-for-length gain would be associated with ANS regulation above and beyond the influence of early life stress [22]. The study leveraged the Still Face Paradigm (SFP), a gold standard experimental infant stress protocol, to identify infants who demonstrate a “typical” ANS stress response (sympathetic nervous system activation and parasympathetic withdrawal during the stressor) compared to infants with a dysregulated or dampened ANS response. We hypothesized that more normative infant weight-for-length gain across the first 6 months would be associated with a profile of typical ANS regulation during stress and recovery (i.e., reactivity during still face and recovery following still face). Further, we hypothesized that greater weight-for-length gain in the first 6 months would be associated with dysregulated ANS responses (e.g., no reactivity during still face and reactivity during recovery periods) following the still face.
Methods
Participants
The current sample leveraged a longitudinal cohort designed to explore the effects of prenatal influences such as maternal stress and weight gain on child health and development [22]. Participants were enrolled from a larger mindfulness intervention study of 215 pregnant women with obesity designed to prevent excessive weight gain during pregnancy [23]. Criteria for inclusion from the pregnancy study included that all women were: 1) English speaking, 2) between 18–45 years of age, 3) 8–23 weeks pregnant with singleton, 4) have a pre-pregnancy BMI of 25 – 40 kg/m2, and 6) incomes less than 500% of the Federal Poverty Level, which is considered lower-income given the cost of living in the area. Women were excluded if they 1) had medical conditions that were known to interfere with baseline body composition or gestational weight gain, 2) were currently taking medications related to weight loss, diabetes, antidepressants, antipsychotics, opiate drugs, or corticosteroids, and 3) have received gastric bypass surgery. All 202 mothers with live births were approached to participate in the subsequent longitudinal follow-up study and 162 mothers consented. The University of California San Francisco institutional review board approved all study protocols and written informed consent was collected from mothers before initiation of any data collection with mother or child.
Recruitment and further participant details for the original pregnancy study and the current infant study have been published previously [22, 23]. A total of 162 (80%) mothers and their infants were enrolled in the current study of children’s health and development. To retain highest sample size, participants were given the choice to participate in lab or in their home. Due to delays in funding, ANS measures were only collected in a subset of infants (N = 67), and a few infants (N = 7) did not tolerate the application of the electrodes, or other aspects of the protocol and thus had no or incomplete ANS data [22]. The current study sample included only infants who participated in data collection at 6 months (Mean Age = 6.4 months, SD = 0.51) with valid, complete ANS data (N= 60).
Infant Weight-for-Length Gain
Infant birth weight was abstracted from the birth record. Weight at six months was measured by trained research assistants using a SECA scale (model 383) and length was measured using the Infant/Child Height-Length ShorrBoard. Measurements were repeated twice, and a third measurement was obtained if the first two measurements were incongruent (e.g., weight difference > 0.2kg or length difference > 0.5cm). Then, an average of the congruent measurements was calculated for each infant. Weight measurements were used to derive age- and sex- specific weight according to the World Health Organization (WHO) 2006 growth charts [24]. These comparisons yielded a z-score of their normed weight-for-length. Weight-for-length change was then determined as the difference between the normed birthweight-for-length and the normed 6-month weight-for-length scores, providing a final continuous score of standardized weight-for-length change over the first 6 months. A weight-for-length change score of 0 would indicate that the infant gained the average amount of expected weight-for-length, relative to the WHO sample, while a positive or negative change score would represent more than the normed average and less than the normed average weight-for-length gain, respectively.
Autonomic Nervous System
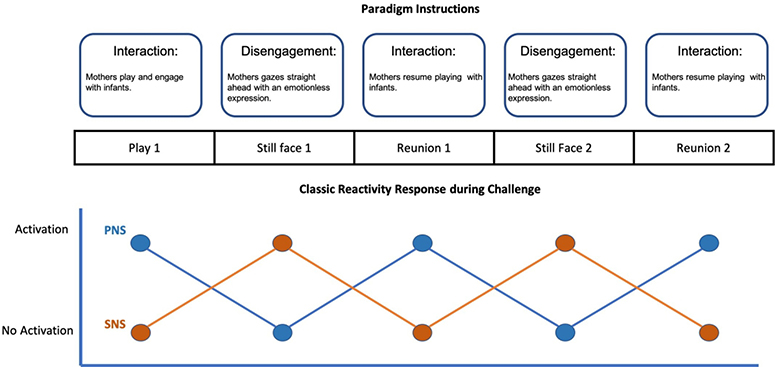
ANS regulation was measured during a standardized SFP (see https://youtu.be/apzXGEbZht0 for example). The SFP is a widely-used experimental paradigm for evaluating infant physiological, behavioral, and emotional regulation at 6-months of age, which has demonstrated validity [25] and reliability [26] across multiple samples [27]. The SFP is designed to elicit infant regulation in response to periods of parental interaction where play is encouraged as normal and periods of disengagement when the mother stares straight ahead with a neutral and unchanging expression. The current study utilized a 10-minute double SFP including five episodes: 2-min play (play1); 2-min still face challenge (SF 1); 2-min play (reunion 1); 2-min still face challenge (SF 2); and 2-min play (reunion 2), which is detailed in prior publications (see Figure 1) [22]. A subset of infants (N = 12) did not tolerate the second SF exposure, therefore, to maintain the largest possible sample size, SF reactivity and reunion scores were taken for the last completed SF and reunion episodes. A dichotomous variable indicating whether or not infants completed both SF challenges was included in statistical models to control for potential bias.
Figure 1.
Conceptual example of the Still Face Paradigm protocol and a classic reactivity response across all five episodesNote: This diagram is a conceptual example only and does not represent real data. There are individual differences in the patterns that were present in the current sample.
ANS regulation was collected continuously throughout the SFP using BioNex hardware and BioLab acquisition software version 3.0 (Mindware Technologies, Ltd., www.mindwaretech.com) via spot electrodes placed on the infants. The full ANS collection and scoring methods protocol have been previously described [28]. Parasympathetic nervous system reactivity was assessed using respiratory sinus arrhythmia (RSA; the naturally occurring variation in heart rate as a function of respiration). RSA was calculated using the interbeat intervals (IBI) detected from electrocardiogram (ECG) readings, respiration rates detected from impedance waveforms (e.g., dZ/dt), and a bandwidth range of 0.15 to 1.04 Hz for infants of 6 months of age [29]. Pre-ejection period (PEP), an indicator of sympathetic activity, is a systolic time interval representing the elapsed duration from the beginning of electrical stimulation until the ejection of blood from the left ventricle [30]. PEP data were extracted and scored using impedance technologies where the ECG and impedance waveforms were used to obtain PEP measures quantified as the time interval in milliseconds from the onset of the ECG Q-wave to the B point of the dZ/dt wave. RSA and PEP data were filtered, extracted, and then scored in 30-second intervals and cleaned by examining for artifacts, checking all outliers (>3SD), and deleting individual data files if more than 25% of the 30-second epochs were unscorable.
Separate PEP and RSA change scores were calculated for both the SFP challenge and the reunion episodes. “Reactivity” during the still face challenge was computed by subtracting the last completed still face by the baseline play 1 episode yielding two change variables, one for RSA and one for PEP. On the other hand, “recovery” during the reunion episode following the last still face was computed by subtracting the final reunion episode from the previous still face challenge yielding two change variables, one for RSA and one for PEP. Next, to assess joint ANS reactivity during still face, PEP and RSA change scores were combined. Previous research suggests that a “classic reactivity” response characterized by sympathetic activation (i.e., PEP shortening) and parasympathetic withdrawal (i.e., RSA decrease) is the most commonly studied response to a distressing challenge [31, 32]. Therefore, this study focused on understanding this response pattern only, and a dichotomous variable was calculated for those who demonstrated classic reactivity during the still face (i.e., negative PEP change score and negative RSA change score) versus all other types of responses. This process was repeated for recovery scores such that infants who had a paired ANS response of sympathetic activation and parasympathetic withdrawal during reunion compared to the last still face were classified as demonstrating a delayed ‘classic reactivity’ response versus all other types of dual-system responses.
Covariates
Gestational age, self-reported pre-pregnancy BMI (weight kg/height m2), birthweight, cigarette smoking during pregnancy, and infant sex were obtained via labor and delivery medical records. Participants reported total household income and household size at 6-months, which were used to calculate a continuous score of U.S. federal poverty level [33]. At the 6 month interview, mothers reported on their breastfeeding behavior and a dichotomized score was created to determine whether infants were exclusively breastfed from birth to 6-months-old or not [34]. During pregnancy and at the 6-month visit, mothers reported on their perceptions of stress in the previous month, using Cohen’s Perceived Stress Scale [PSS] [35]. Postnatally, they also retrospectively reported on their experiences of objective Stressful Life Events (SLE) that occurred during pregnancy [36]. Stress measures were maintained as continuous scores, and further detail about these measurements are presented in prior publications [22]. A variable indicating whether the infant completed the full SFP and another variable indicating whether or not mothers participated in the prenatal stress-management intervention conducted in the original study (which did not affect gestational weight gain but did reduce maternal perceptions of stress) [23] were also included in all models.
Data Analysis
Analyses were performed in SPSS version 26. Descriptive statistics were calculated for demographic characteristics of the sample. Preliminary independent samples t-tests exploring potential bias were used to assess if individuals differed significantly based on whether they completed one or both still face challenge episodes. Primary analyses examining the association between continuous infant weight-for-length gain and the dichotomous grouping of ANS reactivity utilized multivariate logistic regression testing the probability of observing the classic reactivity response during the still face challenge and reunion recovery episodes separately. Because of previous findings that prenatal stress was associated with infant ANS at 6-months in this sample [22, 27], comparisons of ANS regulation across the still face and reunion episodes included both prenatal and concurrent 6-month reports of stress. However, due to concerns about multicollinearity [22], separate models were conducted for the prenatal- and concurrent maternal stress variables, resulting in two models evaluating associations with still face ANS and two models evaluating associations with reunion ANS. Original models included all covariates, however, to preserve the most parsimonious model, covariates that did not meaningfully influence coefficients with p > 0.25 were deleted listwise [37].
Results
Figure 1 displays a conceptual example of anticipated infant responses across the SFP based off previous research. Table 1 presents demographic information of the whole sample, and the sample split by classic reactivity or other reactivity. On average, the current sample had more weight-for-length gain over the first 6-months compared to global WHO averages, (M = 1.19, SD = 1.77). Further, 17 (28.3%) infants in the current sample met clinical criteria for rapid infant weight gain [21]. Preliminary analyses found significant differences in RSA and PEP recovery scores during reunion for infants who completed one versus both still face episodes, confirming the need to include this as a covariate in all regression models to control for potential bias. Mother’s pre-pregnancy BMI, birthweight, intervention assignment, SLE, and poverty were not significant covariates in any logistic models (p-values = 0.42 – 0.87) and where therefore dropped from analyses. Final logistic models adjusted for the effects of either prenatal or concurrent perceived stress, breastfeeding, child sex, and whether children completed one or both still face challenges.
Table 1.
Descriptive statistics of demographics, family characteristics, and physiology split by classic reactivity versus other types of reactivity
| Reactivity during Still Face Calculation | Recovery during Reunion Calculation | |||||||||
|
| ||||||||||
| Total Sample (N = 60) | Classic Reactivity (N = 28) | Other (N = 32) | Delayed ‘Classic Reactivity’ (N = 22) | Other (N =34) | ||||||
|
|
||||||||||
| Dichotomous Variable | N | % | N | % | N | % | N | % | N | % |
|
| ||||||||||
| Child Sex female | 29 | 48.3 | 18 | 64.2 | 12 | 37.5 | 10 | 45.4 | 17 | 50.0 |
| Race/Ethnicity: Latinx | 20 | 33.3 | 10 | 35.7 | 10 | 31.2 | 6 | 27.2 | 12 | 35.2 |
| : Black/African American | 15 | 25.0 | 8 | 28.5 | 7 | 21.8 | 5 | 22.7 | 10 | 29.4 |
| : White | 7 | 11.6 | 2 | 7.1 | 5 | 15.6 | 5 | 22.7 | 2 | 5.8 |
| : Multiracial/other | 18 | 30.0 | 8 | 28.6 | 10 | 31.2 | 7 | 31.8 | 11 | 32.3 |
| Maternal Smoking during pregnancy | 2 | 3.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Breastfeeding exclusively at 6 months | 26 | 43.0 | 10 | 35.7 | 14 | 43.7 | 9 | 40.9 | 11 | 32.3 |
| Completed Both SFP | 40 | 66.6 | 18 | 64.2 | 23 | 71.8 | 11 | 50.0 | 29 | 85.3 |
| Assigned to Intervention | 20 | 33.3 | 10 | 35.7 | 10 | 31.2 | 5 | 22.7 | 11 | 32.3 |
| Continuous Variables | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
|
| ||||||||||
| Maternal Prenatal Perceived Stress | 1.87 | 0.66 | 19.46 | 6.43 | 17.48 | 5.85 | 19.98 | 5.25 | 17.56 | 6.49 |
| Maternal Prenatal Stressful Life Events Count | 2.57 | 2.07 | 3.04 | 1.89 | 2.5 | 2.67 | 2.86 | 2.65 | 2.74 | 2.28 |
| Maternal Concurrent Perceived Stress | 1.51 | 0.70 | 1.46 | 0.75 | 1.63 | 0.63 | 1.68 | 0.68 | 1.45 | 0.70 |
| Maternal Reported Household Income ($) | 26,143 | 20,941 | 27,555 | 21,364 | 23,200 | 20,763 | 28,213 | 22,053 | 23,082 | 18,965 |
| Maternal Pre-pregnancy BMI | 30.95 | 4.94 | 30.39 | 4.08 | 30.99 | 5.88 | 30.39 | 4.98 | 31.09 | 5.27 |
| Maternal Gestational Age (days) | 276.47 | 10.2 | 277.76 | 6.45 | 275.21 | 11.65 | 277.95 | 9.81 | 276.21 | 7.19 |
| Birth Weight (kg) | 3.32 | 0.46 | 3.35 | 0.43 | 3.29 | 0.43 | 3.21 | 0.41 | 3.40 | 0.46 |
| Weight-for-length Standardized Change | 1.19 | 1.77 | 0.97 | 1.87 | 1.35 | 1.54 | 1.56 | 1.97 | 1.02 | 1.53 |
| Still Face Reactivity PEP | −0.79 | 2.73 | −3.95 | 2.96 | 0.68 | 2.55 | 0.159 | 3.09 | −2.56 | 3.67 |
| Still Face Reactivity RSA | −0.56 | 1.11 | −1.46 | 1.15 | −0.082 | 0.95 | −0.55 | 1.35 | −0.93 | 1.24 |
| Recovery PEP | −0.83 | 2.55 | −.044 | 2.50 | −1.12 | 2.62 | −2.94 | 2.35 | 0.52 | 1.69 |
| Recovery RSA | −0.01 | 1.50 | 0.67 | 1.63 | −0.55 | 1.14 | −1.26 | 0.99 | 0.81 | 1.21 |
Adjusted logistic regression models across both the still face challenge and reunion episodes were each initially modeled twice to separately assess prenatal and concurrent stress. The beta coefficients across both models were similar, but prenatal stress was not statistically significant within its model, therefore, subsequent models controlled for only concurrent stress, and results for those models are shown in Table 2. The first logistic regression model showed that, for each one standard deviation of weight-for-length gain above the sample mean, infants were .52 times less likely to demonstrate classic reactivity to the still face challenge (OR = 0.52; 95% CI [0.27, 0.97]; p = .04). In other words, infants who gained on pace with WHO norms over the first 6 months were more likely to react to the still face with classic reactivity. Although not a main focus of the study, of note, higher levels of mother’s reported concurrent stress was associated with .38 lower odds of infants having a classic reactivity response to the still face (OR = 0.38; 95% CI [0.14, 0.99]; p = .04). No other covariates were significantly associated with classic reactivity.
Table 2.
Adjusted logistic regression of weight-for-length change and covariate predictors of classic reactivity response at 6 months
| Model by Dependent Variable | OR | 95% Confidence Interval |
|---|---|---|
| Reactivity to Still Face (N = 60) | ||
| Child Sex (Female = 1) | 3.17 | 0.94, 10.68 |
| Weight-for-length standardized Change (continuous) | 0.52* | 0.27, 0.97 |
| Breastfeeding exclusively at 6 months (Yes = 1) | 0.51 | 0.15, 1.68 |
| Completed both SF (Yes = 1) | 0.33 | 0.09, 1.25 |
| Perceived Stress_Concurrent (continuous) | 0.38* | 0.14, 0.99 |
| Recovery during Reunion (N = 56) | ||
| Child Sex (Female = 1) | 0.29 | 0.08, 1.70 |
| Weight-for-length standardized Change (continuous) | 2.75* | 1.14, 5.36 |
| Breastfeeding exclusively at 6 months (Yes = 1) | 2.15 | 0.42, 10.99 |
| Completed both SF (Yes = 1) | 0.33 | 0.03, 3.98 |
| Perceived Stress_Concurrent (continuous) | 2.10 | 0.57, 7.74 |
Note: Original models contained additional covariates that were removed listwise to create the most parsimonious model; intervention assignment, pre-pregnancy BMI, poverty, smoking during pregnancy, gestational age, birthweight, race/ethnicity; WFL = Weight-for-length.
p < .05.
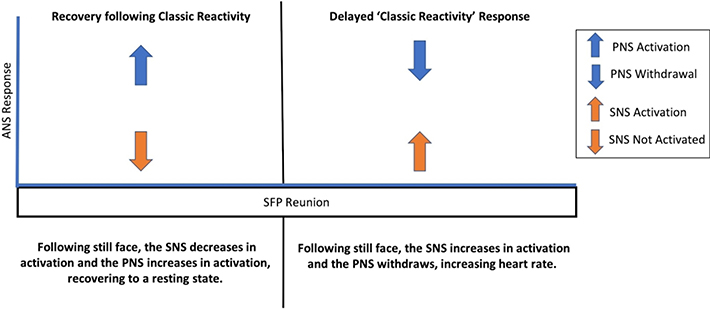
Second, examining the reunion episode following the still face challenge, infant weight-for-length gain was significantly associated with a ‘classic reactivity’ response such that infants with gains of one standard deviation above the sample mean over the first 6 months were 2.35 times more likely to evidence sympathetic activation and parasympathetic withdrawal during the reunion episode (OR =2.35; 95% CI [1.03, 5.36]; p = .03; Table 2). In other words, infants who gained more than expected based on WHO norms were more likely to react with ‘classic reactivity’ during the recovery period. Figure 2 presents a conceptual comparison of the expected recovery response versus the delayed ‘classic reactivity’ response. No covariates were significant predictors in this model.
Figure 2.
Conceptual comparison of the expected recovery during reunion versus a ‘classic reactivity’ response during the reunion episode of the Still Face Paradigm (SFP) protocol
Post-hoc explorations probing these two models revealed that only one infant who evidenced classic reactivity during the still face challenge also demonstrated ‘classic reactivity’ during the reunion episode. This confirmed that rather than prolonged classic reactivity (i.e., continuing to react in a classic reactivity pattern across both episodes), the infants who evidenced ‘classic reactivity’ during the intended recovery period (i.e., reunion episode) were exhibiting delayed ANS activation in both the sympathetic and parasympathetic branches.
Discussion
The present study found that, after adjusting for effects of maternal stress and key covariates, infant weight-for-length gain from birth to 6 months was associated with patterns of joint sympathetic and parasympathetic reactivity at 6 months, within a racially and ethnically diverse low-income sample of infants at increased risk for developing obesity [38]. In line with our hypotheses, less weight-for-length gain was associated with classic reactivity during the still face challenge and greater weight-for-length gain was associated with a dysregulated response of classic reactivity during reunion. Thus, results suggest that higher levels of weight-for-length gain in early life were associated with autonomic dysregulation at 6 months as measured using the gold standard SFP infant stress protocol. The current findings contribute new evidence supporting the view that early individual physical states [19], in addition to early adverse experiences [22], may influence developing autonomic nervous systems early in life in potentially impactful ways.
Importantly, the average weight-for-length change of the current sample is more than one standard deviation higher than global WHO averages and recommendations, putting those who gained more than one standard deviation into a clinically high-risk group for developing obesity [21]. Therefore, infants in our specific sample who gained one standard deviation less than our sample mean were more likely to have gained normative amounts of weight-for-length during their first 6-months of life. Follow up analyses (data not shown) confirmed that ANS differences were not associated with stunted linear length growth, further suggesting that the results shown here capture excessive weight gain. Given previous research that recognizes rapid and excessive infant weight gain as an especially salient predictor of later development of obesity [21], understanding the potential mechanism of ANS dysregulation is particularly important.
The SFP is a unique paradigm that enables the examination of behavioral, emotional, and physiological regulation in infants. In context of the parent-child relationship, unexpected disengagement from a primary caregiver causes distress that engages these regulatory systems and increases arousal. Classic reactivity is the autonomic response that is hypothesized to best support this type of infant distress [27], where infants show sympathetic activation and parasympathetic withdrawal, and has been associated with positive physical health outcomes in childhood [39]. Our findings suggest that infants who had weight-for-length gain closer to global WHO averages over the first 6 months of life were more likely to demonstrate a classic reactivity response during the still face challenge, suggesting that average weight-for-length gain in the first 6-month may facilitate programming of adequate stress responsivity. Conversely, following disengagement, infants should begin to decrease in arousal as their primary caregiver returns to normative playing behavior during reunion episodes, which is intended to provide emotional and physiological recovery from the stress of the challenge task. Therefore, ‘classic reactivity’ demonstrated during the reunion is potentially indicative of ANS dysregulation early in life. Our findings indicated that infants with greater than expected weight-for-length gain over the first 6-months were more likely to demonstrate this delayed or potentially displaced ‘classic reactivity’ response during reunion. Importantly, the quality of the parent-child relationship may also influence reactivity during this socio-emotional challenge. Evidence from studies using this paradigm suggests that infants of nurturant and responsive caregivers are upset by their disengagement because it is novel, and infants are soothed by their positive interaction during reunion [27], whereas infants of a less responsive caregiver may exhibit more disorganized reactions because care and nurturing are not consistent and/or expected [27]. Such differences in parent-child relationships or infant irritability could also influence the quality and quantity of feeding behavior, which may relate to relative weight-for-length gain in these infants.
Previous research examining the links between weight gain and ANS have done so primarily by examining correlates of group differences [10, 11], and very few have examined the development of these differences [14]. ANS dysregulation has been proposed as an explanatory mechanism of increased health risk and cardiovascular disease progression [40], and similar pathways may be evident in the development of obesity [41]. Understanding how early life influences may disrupt the development of these mechanistic pathways are important to fully understanding disease progression and develop interventions focused on prevention. High weight gain early in life may ‘program’ ANS regulatory processes in ways that contribute to potentially problematic adaptation to challenge and calibration of energy expenditure across development [14, 42]. Importantly, although the current findings suggest an association such that early gain contributes to ANS dysregulation, we were unable to test causation or possible multidirectionality of effects and recognize that the process is likely complex. This early dysregulation may subsequently contribute to maladaptive biological processes across systems that increase likelihood of obesity development. For example, studies in adulthood document links between the ANS and both plasticity of adipose tissue [43] and metabolic dysfunction [44] which may illustrate additional mechanisms by which ANS can contribute to obesity.
Although we observed that weight-for-length gain over the first 6 months was associated with ANS dysregulation at 6 months, the single time point of data available for each of the measures precludes us from identifying the directionality of the relationships. Weight change could be driving these associations, but other explanations are possible, such that infants have different ANS reactivity at birth, which leads to this increased weight-for-length gain. However, there is no known and reliable method of assessing ANS reactivity and recovery at birth, which prevents ascertainment of causal directionality. Further, infant sympathetic nervous system function is not easily measured—the current study represents one of a few known studies with PEP reactivity data in infancy. This study focuses on one of the earliest periods when ANS regulation can be reliably measured, and points to weight gain at a critical period early in life as associated with differences in ANS regulation. If these effects persist, it could set up a trajectory of higher risk for overeating and metabolic disease.
Limitations
Although the current study contributes new understanding of differences in ANS reactivity in early life that are relevant to young children’s health, there are a number of limitations that provide opportunities for future research. First, although our sample provides much-needed data on low-income women of diverse racial and ethnic backgrounds, this may limit the generalizability of our findings to other populations and our small sample warrants replication. Second, our inability to adjust for infant postnatal exposure to household cigarette smoking is also a limitation, although maternal cigarette smoking during pregnancy was very low in this sample (0.33%; consistent with low regional rates of smoking, in general), and its inclusion in analyses did not alter findings. Similarly, we do not have data on mother’s marijuana use pre- or postnatally. Third, reports suggest that using z-score in examinations of children’s height can occlude the observation of catch-up growth that occurs following early rearing in a deprivation context [45], although not directly relevant to the current study, this may be an important consideration of future work on older children in high-stress contexts. Importantly, we chose to focus on the most well understood ANS response of classic reactivity, however, by dichotomizing reactivity, we may be missing important individual differences captured in other ANS response patterns. Similarly, categorical split variables necessarily eliminate potentially important characteristics of ANS reactivity such as magnitude of response. Given our small sample size, this acts as a good first step in broadly understanding these associations but may be an oversimplification of the regulatory dynamics at play. Since we cannot definitively identify causal relationships between weight-for-length and ANS reactivity, this study is limited in its ability to predict ANS reactivity. Future studies need to replicate this study design and continue to follow infants into early childhood. Finally, ANS reactivity in infancy does not necessarily represent long term reactivity responses since research suggests regulatory systems are highly flexible during the first few years of life before stabilizing in later childhood [46]. Future longitudinal research should track developmental trajectories of these relations over time as well as timing and effectiveness of various interventions.
Conclusion
Overall, the current study results show that weight-for-length changes from birth to 6 months of life are associated with infant ANS regulation at 6 months, above and beyond other empirically documented influences, such as maternal pre- and postnatal stress and breastfeeding. If replicated, our findings may be especially relevant to policy and intervention work targeted at prevention of obesity in at risk children. In addition to identifying infants of mothers with obesity, implementing screenings to detect early adversity, accelerated early weight-for-length gain, and dysregulated physiological responses may detect compounding risks of infants developing future obesity. Further, highlighting physiological regulatory factors that stem from above average early gain may provide additional points of intervention [47]. Future research that employs longitudinal examinations from infancy through childhood is required to elucidate the directional effects of weight-for-length gain and ANS regulation as well as inform development of targeted interventions to support infants with potentially dysfunctional regulatory processes.
Impact:
Adiposity gain during early infancy was associated with Autonomic Nervous System regulation at 6-months
This study identifies early adiposity gain (greater than average infant weight-for-length gain) as a risk for ANS dysregulation
This research focuses on a critical developmental period of ANS plasticity
If confirmed, findings can be used to inform early intervention programs targeting obesity prevention and to promote self-regulation
Acknowledgements
The authors wish to acknowledge Kim Coleman-Phox, Marialma Gonzales-Cruz, Yurivia Cervantes, Katie Blackburn, Zoe Caron, and Jayme Congdon for their assistance with the SEED data collection. We are also thankful to the families for their generous participation in this research. We would also like to thank Drs. Elissa Epel, Nancy Adler, and Barbara Laraia, the principal investigators on the pregnancy study from which the current participant pool was drawn, for providing support for the offspring study and guidance on its design. Additionally, we thank Dr. Epel for her consultation and review of the current manuscript.
Funding Support: This work was supported by the National Heart, Lung, and Blood Institute (U01 HL097973; R01 HL116511-02), and the Lisa and John Pritzker Family Fund. Dr. Bush is the Lisa and John Pritzker Distinguished Professor in Developmental and Behavioral Health.
Footnotes
Disclosure statement: The authors have no conflicts of interest to disclose
Participant Consent: All participants provided written informed consent before initiation of any data collection.
References
- 1.Porges SW. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleveland clinic journal of medicine. 2009;76(Suppl 2):S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCorry LK. Physiology of the autonomic nervous system. American journal of pharmaceutical education. 2007;71(4):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thayer JF, Hansen AL, Johnsen BH. Noninvasive assessment of autonomic influences on the heart: Impedance cardiography and heart rate variability. In: Gallo LJLLC, editor. Handbook of physiological research methods in health psychology. Thousand Oaks, CA: Sage Publications; 2008. p. 183–209. [Google Scholar]
- 4.Hagan MJ, et al. Socioeconomic adversity, negativity in the parent child-relationship, and physiological reactivity: An examination of pathways and interactive processes affecting young children’s physical health. Psychosomatic medicine. 2016;78(9):998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Sheikh M, Erath SA. Family conflict, autonomic nervous system functioning, and child adaptation: State of the science and future directions. Development and Psychopathology. 2011;23(2):703–21. doi: 10.1017/S0954579411000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antoni MH, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nature reviews cancer. 2006;6(3):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abboud FM, Harwani SC, Chapleau MW. Autonomic neural regulation of the immune system: implications for hypertension and cardiovascular disease. Hypertension. 2012;59(4):755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bischoff SC, et al. Towards a multidisciplinary approach to understand and manage obesity and related diseases. Clinical nutrition. 2017;36(4):917–38. [DOI] [PubMed] [Google Scholar]
- 9.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics. 2018;141(3):e20173459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baum P, et al. Dysfunction of autonomic nervous system in childhood obesity: a cross-sectional study. PloS one. 2013;8(1):e54546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eyre E, Duncan MJ, Birch S, Fisher J. The influence of age and weight status on cardiac autonomic control in healthy children: a review. Autonomic neuroscience. 2014;186:8–21. [DOI] [PubMed] [Google Scholar]
- 12.Costa J, et al. Effects of weight changes in the autonomic nervous system: A systematic review and meta-analysis. Clinical nutrition. 2019;38(1):110–26. [DOI] [PubMed] [Google Scholar]
- 13.Straznicky NE, et al. Baseline sympathetic nervous system activity predicts dietary weight loss in obese metabolic syndrome subjects. The journal of clinical endocrinology & metabolism. 2012;97(2):605–13. [DOI] [PubMed] [Google Scholar]
- 14.Alkon A, et al. Latino children’s body mass index at 2–3.5 years predicts sympathetic nervous system activity at 5 years. Childhood obesity. 2014;10(3):214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein DJ, Harvey BH, Uys J, Daniels W. Suffer the children: The psychobiology of early adversity. CNS spectrums. 2005;10(8):612–5. [DOI] [PubMed] [Google Scholar]
- 16.Shonkoff JP, et al. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–e46. [DOI] [PubMed] [Google Scholar]
- 17.Shonkoff JP. Leveraging the biology of adversity to address the roots of disparities in health and development. Proceedings of the national academy of sciences. 2012;109(Supplement 2):17302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyce WT, Sokolowski MB, Robinson GE. Toward a new biology of social adversity. Proceedings of the national academy of sciences. 2012;109(Supplement 2):17143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Deutekom AW, et al. The association of birth weight and infant growth with childhood autonomic nervous system activity and its mediating effects on energy-balance-related behaviours—the ABCD study. International journal of epidemiology. 2016;45(4):1079–90. [DOI] [PubMed] [Google Scholar]
- 20.Roy SM, et al. Infant BMI or weight-for-length and obesity risk in early childhood. Pediatrics. 2016;137(5):e20153492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng M, et al. Rapid weight gain during infancy and subsequent adiposity: a systematic review and meta-analysis of evidence. Obesity reviews. 2018;19(3):321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bush NR, et al. Effects of pre-and postnatal maternal stress on infant temperament and autonomic nervous system reactivity and regulation in a diverse, low-income population. Development and psychopathology. 2017;29(5):1553–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epel E, et al. Effects of a Mindfulness-Based Intervention on Distress, Weight Gain, and Glucose Control for Pregnant Low-Income Women: A Quasi-Experimental Trial Using the ORBIT Model. International journal of behavioral medicine. 2019:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. 2006.
- 25.Mesman J, van IJzendoorn MH, Bakermans-Kranenburg MJ. The many faces of the Still-Face Paradigm: A review and meta-analysis. Developmental review. 2009;29(2):120–62. [Google Scholar]
- 26.Provenzi L, Olson KL, Montirosso R, Tronick E. Infants, mothers, and dyadic contributions to stability and prediction of social stress response at 6 months. Developmental psychology. 2016;52(1):1. [DOI] [PubMed] [Google Scholar]
- 27.Jones-Mason K, Alkon A, Coccia M, Bush NR. Autonomic nervous system functioning assessed during the still-face paradigm: A meta-analysis and systematic review of methods, approach and findings. Developmental review. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bush NR, Caron ZK, Blackburn KS, Alkon A. Measuring cardiac autonomic nervous system (ANS) activity in toddlers-Resting and developmental challenges. JoVE (Journal of Visualized Experiments). 2016;(108):e53652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bar-Haim Y, Marshall PJ, Fox NA. Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology. 2000;37(1):44–56. [DOI] [PubMed] [Google Scholar]
- 30.Berntson GG, Lozano DL, Chen YJ, Cacioppo JT. Where to Q in PEP. Psychophysiology. 2004;41(2):333–7. [DOI] [PubMed] [Google Scholar]
- 31.Pearson SR, et al. Autonomic reactivity and clinical severity in children with sickle cell disease. Clinical autonomic research. 2005;15(6):400–7. [DOI] [PubMed] [Google Scholar]
- 32.Salomon K, Matthews KA, Allen MT. Patterns of sympathetic and parasympathetic reactivity in a sample of children and adolescents. Psychophysiology. 2000;37(6):842–9. [PubMed] [Google Scholar]
- 33.Sebelius K Annual update of the HHS poverty guidelines. Federal register. 2011;76(13):3637–8. [Google Scholar]
- 34.Eidelman AI, Schanler RJ. Breastfeeding and the use of human milk. Pediatrics. 2012. [Google Scholar]
- 35.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of health and social behavior. 1983:385–96. [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Phase 5 Core Questionnaire—Pregnancy stressful life events. Pregnancy Risk Assessment Monitoring System (PRAMS). Washington, DC: 2005. [Google Scholar]
- 37.Agresti A Building and Applying Logistic Regression Models. Categorical data analysis 2002. p. 211–66. [Google Scholar]
- 38.Chandler-Laney PC, Bush NC. Maternal obesity, metabolic health, and prenatal programming of offspring obesity. Open obesity journal. 2011;3(1):42–50. [Google Scholar]
- 39.Treadwell MJ, Alkon A, Styles L, Boyce WT. Autonomic nervous system reactivity: children with and without sickle cell disease. Nursing research. 2011;60(3):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pagani M, Lucini D. Chronic fatigue syndrome: a hypothesis focusing on the autonomic nervous system. Clinical science. 1999;96(1):117–25. [PubMed] [Google Scholar]
- 41.Messina G, et al. Autonomic nervous system in the control of energy balance and body weight: personal contributions. Neurology research international. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Seminars in reproductive medicine. 2009;27(05):358–68. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pénicaud L, et al. The autonomic nervous system, adipose tissue plasticity, and energy balance. Nutrition. 2000;16(10):903–8. [DOI] [PubMed] [Google Scholar]
- 44.Tentolouris N, Argyrakopoulou G, Katsilambros N. Perturbed autonomic nervous system function in metabolic syndrome. Neuromolecular medicine. 2008;10(3):169–78. [DOI] [PubMed] [Google Scholar]
- 45.Leroy JL, Ruel M, Habicht J-P, Frongillo EA. Using height-for-age differences (HAD) instead of height-for-age z-scores (HAZ) for the meaningful measurement of population-level catch-up in linear growth in children less than 5 years of age. BMC pediatrics. 2015;15(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alkon A, Boyce WT, Davis NV, Eskenazi B. Developmental Changes in Autonomic Nervous System Resting and Reactivity Measures in Latino Children from 6 to 60 Months of Age. Journal of developmental & behavioral pediatrics. 2011;32(9):668–77. doi: 10.1097/DBP.0b013e3182331fa6. [DOI] [PubMed] [Google Scholar]
- 47.Blüher S, et al. Effect of a 1-year obesity intervention (KLAKS program) on preexisting autonomic nervous dysfunction in childhood obesity. Journal of child neurology. 2015;30(9):1174–81. [DOI] [PubMed] [Google Scholar]