Introduction
Broncholithiasis and bronchoesophageal fistulae (BEF) are rare events, with broncholithiasis occurring in 0.1%−0.2% of respiratory disease patients,1 and acquired fistulae rarer still. Management can have potentially life-threatening complications, including major artery and esophageal lacerations. Furthermore, bronchoesophageal fistulae, if stented, are commonly stented from the esophagus.2 We present a case of bronchoesophageal fistula that was repaired by a combination of fibrin sealant and a covered stent, which shows a novel management approach.
Case presentation
A 78 year old Caucasian male with a past medical history significant for COPD on 2L home oxygen, GERD, myelodysplastic syndrome, prostate cancer, and recently diagnosed left lower lobe lung cancer status post radiation treatment presented to the emergency room at an outside hospital (OSH) with two week history of worsening dysphagia, and dyspnea in the context of recurrent right lower lobe (RLL) pneumonia (Fig 1a). Pulmonary examination revealed crackles in the RLL. He was initially treated at the OSH with piperacillin-tazobactam and levofloxacin concurrently for pneumonia and exacerbation of chronic obstructive pulmonary disease (COPD).
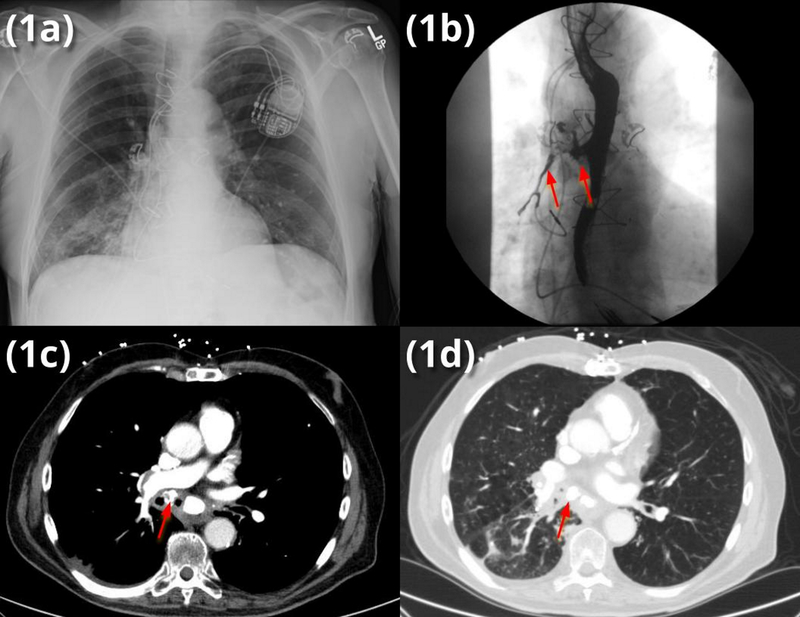
Figure 1.
(a) admission chest x-ray (CXR) indicating right lower lobe (RLL) pneumonia without evidence of broncholith on CXR
(b) modified barium swallow (MBS) demonstrating barium aspiration into the right mainstem bronchus (RMSB)
(c) mediastinal windows showing broncholith (arrow)
(d) lung windows showing both broncholith and post-obstructive RLL infiltrate
Due to his dysphagia, a fluoroscopic barium swallow was performed and indicated bronchoesophageal fistula was present (Fig 1b). Computed tomography of the chest revealed a partially-obstructing broncholith in the right mainstem bronchus (RMSB) (Fig 1c–d), originating from a calcified right hilar lymph node large enough to erode into the RMSB and esophagus, creating a BEF.
He was transferred to our hospital, where flexible bronchoscopy (Olympus Medical BF-1TH190 flexible bronchoscope) confirmed an embedded broncholith (which had partially eroded through the airway wall) that almost completely occluded the bronchus intermedius, complicated by post-obstructive mucus in the RLL.
He met with Thoracic Surgery to discuss operative management, however, given his own goals of care, he decided to forgo surgical intervention for the fistula and instead pursue a less invasive palliative intervention. A plan was made to perform a rigid bronchoscopy (Lymol Medical, Elite X Class rigid bronchoscope BT 2101) for broncholith removal and fistula occlusion to palliate his symptoms prior to hospice discharge.
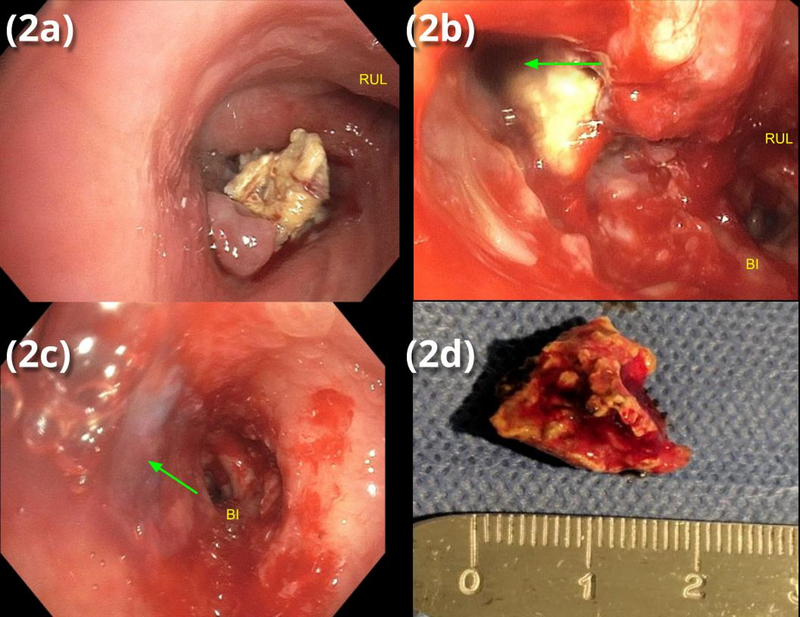
The broncholith was too large (>12mm) to be removed through the rigid bronchoscope barrel; optical forceps were used to destroy the broncholith and extract smaller fragments. The largest fragment was successfully removed using cryotherapy (Fig 2a, c) via a flexible cryoprobe (Erbokryo Flexible Cryoprobe #20416–032, cryogen nitrous oxide) and removed en bloc with the rigid bronchoscope. The abundant secretions adherent to the broncholith aided adhesion of the fragment to the cryoprobe. After broncholith removal, postobstructive purulent material was irrigated and aspirated from the right middle lobe (RML) and RLL, revealing a large (2cm) defect in the medial wall of the RMSB where the broncholith had eroded into the airway. Fibrin sealant (Ethicon Evicel) was then instilled via a dual lumen catheter through the bronchoscope to fill the fistula tract and the bronchial wall defect (Fig 2b, d). A hybrid, silicone-covered stent (Thoracent Bonastent) 12×30mm was deployed in the RMSB to keep the fibrin sealant in the tract and prevent it from dislodging, further occluding the fistula.
Figure 2.
(a) endoscopic view of RMSB with occlusion of BI by broncholith
(b) endoscopic view of RMSB after partial broncholith extraction with fistula (arrow)
(c) fibrin glue (arrow) occluding fistula
(d) extracted broncholith fragment
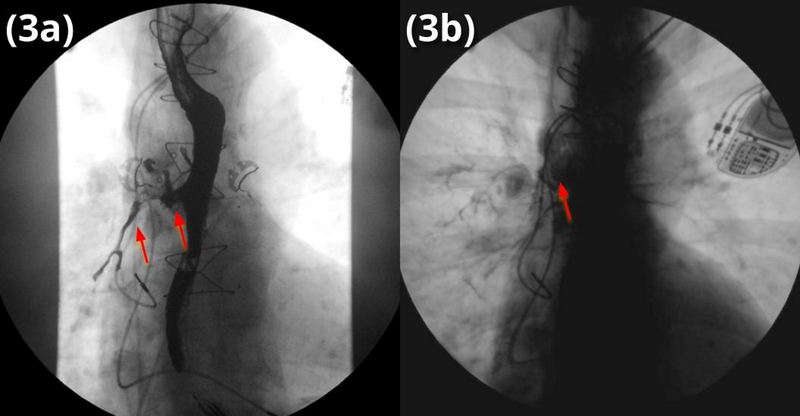
The combination of the fibrin sealant with stent was effective as his symptoms immediately improved with complete resolution of cough and resumption of oral intake prior to being discharged to home hospice on POD#3. Repeat barium swallow on POD#2confirmed fistula resolution (Fig 3b). As he desired home hospice, he did not return for any outpatient visits. As far as we are aware, the fibrin sealant and stent remained in place until his passing.
Figure 3.
(a) MBS demonstrating barium aspiration into the RMSB preprocedure
(b) MBS demonstrating no barium aspiration into the RMSB postprocedure
Discussion
Broncholithiasis occurs when a broncholith, or calcified material, - causes irritation, obstruction, or erosion in the airway. This calcification often obstructs and causes atelectasis, inflammation, and mucoid impaction, resulting in cough, wheezing, and fever, and rarely causes BEF. This condition most commonly results from the erosion and extrusion of a calcified lymph node (LN) into the bronchial lumen. Rarer causes include mechanical ventilation and surgical complications.
Thoracic nodal calcification generally results from long-standing lymphadenitis secondary to fungal or mycobacterial infections, most commonly Histoplasma capsulatum in the United States, but also including tuberculosis, coccidiomycosis, and cryptococcosis. Airway anatomy and lymph node distributions result in a preference for the proximal RML bronchus, the bronchus intermedius, and the anterior right upper lobe bronchus. Symptomatic broncholithasis requires removal with surgical or bronchoscopic intervention. Fistulae are almost always considered a symptomatic complication, as they often convey significant morbidity and mortality.
BEF and tracheosophageal fistulae (TEF), are connections between bronchi or trachea and esophagus.5 Acquired BEF/TEF are rare and commonly result from locally advanced malignancy or tracheosophageal fistulae, from invasive ventilation complications via cuff-related injuries.5–8 Other causes include broncholithiasis,9 trauma,10 ingestion,11 and surgical complications from esophagectomy or laryngectomy.10 In most cases, inflammation of the tissue between esophagus and airway is a critical step in tissue erosion and fistula formation.12,13
Regardless of the etiology of the fistula, the sequelae remain unchanged - respiratory tract contamination with significant pulmonary compromise and inadequate nutrition. Most patients present symptomatically, with intractable cough, increased secretions, pneumonia, and evidence of gastric aspiration.7 Patients are commonly diagnosed clinically or with the detection of gastric contents in the respiratory tract and, if on positive pressure ventilation, a dilated abdomen. For confirmatory studies, barium swallows are useful for radiographic visualization as the fistula may not be visible on CT scans. Bronchoscopy and/or esophagoscopy are often needed to confirm by direct visualization and to plan for repair.
Fistula repair techniques
Management of airway fistulas, including BEF and TEF, is difficult. Grillo et al fundamentally changed management when he described that the fistula and diseased airway both required repair.12 Since the most common insult is from endotracheal tube cuff, the lesion is often circumferential. The fundamentals of treatment include preventing further contamination of the respiratory tract and improving the nutrition status.
In most cases, treatment is palliative, most commonly utilizing a covered airway or esophageal stents.2,14,15 Stenting and/or other endoscopic interventions are designed to occlude the fistula and eliminate or reduce leakage. One of the most common complications includes stent migration, resulting in incomplete fistula occlusion.16 The idea of double stenting (i.e. placing two stents, one in the airway and one in the esophagus) has been suggested. Unfortunately, the two stents may rub against each other and lead to worsening erosion of the membranes.10 A less common solution utilizes fibrin sealant for fistula occlusion. Most case reports appear to be predominantly in children or in post operative surgical site dehiscence, where presumably airway stents may either be unavailable or be more likely to migrate.17–20 A major problem with fibrin sealant is that it typically does not adhere to the fistula tract due to moisture and secretions and frequently dislodges.
Surgical interventions offer more definitive treatment and commonly use soft tissue interposition flaps and two-layer techniques, often with simultaneous tracheal resection and reconstruction. Although these techniques are 90% effective, they carry over 50% morbidity.10
Conclusion
This is the first description of a minimally invasive technique to occlude a BEF using the synergy between fibrin sealant and a covered stent. Although both fibrin glue and stenting has been described as a method to occlude fistulas, the combination of the stent to cover and keep the fibrin glue in place within the fistula is novel and worth further investigation. Prior reports have described the individual interventions with mixed results, such as non-durable fistula occlusion secondary to stent migration. This combination of fibrin sealant and stenting may offer an alternative to provide better occlusion of the fistula, preventing passage of foreign material into the tracheobronchial tree and possibly allowing patients to resume oral intake, especially in situations requiring non-operative management.
Acknowledgments
Source of funding:
AIW is partially funded by NIGMS 2T32GM095442.
Footnotes
Conflicts of interest:
AIW is a cofounder of Ataia Medical
References
- 1.Chujo M, Yamashita S-I, Kawano Y, Miyawaki M, Imakiire T, Kawahara K. Left sleeve Basal segmentectomy for broncholithiasis. Ann Thorac Cardiovasc Surg. 2008;14(2):101–104. [PubMed] [Google Scholar]
- 2.Shin JH, Song H-Y, Ko G-Y, Lim J-O, Yoon H-K, Sung K-B. Esophagorespiratory fistula: long-term results of palliative treatment with covered expandable metallic stents in 61 patients. Radiology. 2004;232(1):252–259. [DOI] [PubMed] [Google Scholar]
- 3.Krishnan S, Kniese CM, Mankins M, Heitkamp DE, Sheski FD, Kesler KA. Management of broncholithiasis. J Thorac Dis. 2018;10(Suppl 28):S3419–S3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardillo G, Carbone L, Carleo F, et al. The Rationale for Treatment of Postresectional Bronchopleural Fistula: Analysis of 52 Patients. The Annals of Thoracic Surgery. 2015;100(1):251–257. doi: 10.1016/j.athoracsur.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 5.Debourdeau A, Gonzalez J-M, Dutau H, Benezech A, Barthet M. Endoscopic treatment of nonmalignant tracheoesophageal and bronchoesophageal fistula: results and prognostic factors for its success. Surg Endosc. 2019;33(2):549–556. [DOI] [PubMed] [Google Scholar]
- 6.Flege JB Jr. Tracheoesophageal fistula caused by cuffed tracheostomy tube. Ann Surg. 1967;166(1):153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reed MF, Mathisen DJ. Tracheoesophageal fistula. Chest Surg Clin N Am. 2003;13(2):271–289. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez AN, Diaz-Jimenez JP. Malignant respiratory-digestive fistulas. Curr Opin Pulm Med. 2010;16(4):329–333. [DOI] [PubMed] [Google Scholar]
- 9.Seo JB, Song K-S, Lee JS, et al. Broncholithiasis: review of the causes with radiologic-pathologic correlation. Radiographics. 2002;22 Spec No:S199-S213. [DOI] [PubMed] [Google Scholar]
- 10.Muniappan A, Wain JC, Wright CD, et al. Surgical treatment of nonmalignant tracheoesophageal fistula: a thirty-five year experience. Ann Thorac Surg. 2013;95(4):1141–1146. [DOI] [PubMed] [Google Scholar]
- 11.Gudovsky LM, Koroleva NS, Biryukov YB, Chernousov AF, Perelman MI. Tracheoesophageal fistulas. The Annals of Thoracic Surgery. 1993;55(4):868–875. doi: 10.1016/0003-4975(93)90108-t [DOI] [PubMed] [Google Scholar]
- 12.Grillo HC, Moncure AC, McEnany MT. Repair of inflammatory tracheoesophageal fistula. Ann Thorac Surg. 1976;22(2):112–119. [DOI] [PubMed] [Google Scholar]
- 13.Cooper JD, Grillo HC. The Evolution of Tracheal Injury Due to Ventilatory Assistance Through Cuffed Tubes. Annals of Surgery. 1969;169(3):334–348. doi: 10.1097/00000658-196903000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spaander MCW, Baron TH, Siersema PD, et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2016;48(10):939–948. [DOI] [PubMed] [Google Scholar]
- 15.Ke M, Wu X, Zeng J. The treatment strategy for tracheoesophageal fistula. J Thorac Dis. 2015;7(Suppl 4):S389–S397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ke M, Wu X, Zeng J. The treatment strategy for tracheoesophageal fistula. J Thorac Dis. 2015;7(Suppl 4):S389–S397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonelli M, Cicconetti F, Vivino G, Gasparetto A. Closure of a tracheoesophageal fistula by bronchoscopic application of fibrin glue and decontamination of the oral cavity. Chest. 1991;100(2):578–579. [DOI] [PubMed] [Google Scholar]
- 18.Hoelzer DJ, Luft JD. Successful long-term endoscopic closure of a recurrent tracheoesophageal fistula with fibrin glue in a child. Int J Pediatr Otorhinolaryngol. 1999;48(3):259–263. [DOI] [PubMed] [Google Scholar]
- 19.Richter GT, Ryckman F, Brown RL, Rutter MJ. Endoscopic management of recurrent tracheoesophageal fistula. J Pediatr Surg. 2008;43(1):238–245. [DOI] [PubMed] [Google Scholar]
- 20.Berkowitz DM. Endoscopic Management of Bronchopleural Fistulas. In: Ernst A, Herth FJF, eds. Principles and Practice of Interventional Pulmonology. New York, NY: Springer New York; 2013:435–448. [Google Scholar]