Supplemental Digital Content is available in the text.
Keywords: cardiovascular diseases, COVID-19, health status disparities, race factors
Background:
The coronavirus disease 2019 (COVID-19) pandemic has exposed longstanding racial and ethnic inequities in health risks and outcomes in the United States. We aimed to identify racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19.
Methods:
The American Heart Association COVID-19 Cardiovascular Disease Registry is a retrospective observational registry capturing consecutive patients hospitalized with COVID-19. We present data on the first 7868 patients by race/ethnicity treated at 88 hospitals across the United States between January 17, 2020, and July 22, 2020. The primary outcome was in-hospital mortality. Secondary outcomes included major adverse cardiovascular events (death, myocardial infarction, stroke, heart failure) and COVID-19 cardiorespiratory ordinal severity score (worst to best: death, cardiac arrest, mechanical ventilation with mechanical circulatory support, mechanical ventilation with vasopressors/inotrope support, mechanical ventilation without hemodynamic support, and hospitalization alone. Multivariable logistic regression analyses were performed to assess the relationship between race/ethnicity and each outcome adjusting for differences in sociodemographic, clinical, and presentation features, and accounting for clustering by hospital.
Results:
Among 7868 patients hospitalized with COVID-19, 33.0% were Hispanic, 25.5% were non-Hispanic Black, 6.3% were Asian, and 35.2% were non-Hispanic White. Hispanic and Black patients were younger than non-Hispanic White and Asian patients and were more likely to be uninsured. Black patients had the highest prevalence of obesity, hypertension, and diabetes. Black patients also had the highest rates of mechanical ventilation (23.2%) and renal replacement therapy (6.6%) but the lowest rates of remdesivir use (6.1%). Overall mortality was 18.4% with 53% of all deaths occurring in Black and Hispanic patients. The adjusted odds ratios for mortality were 0.93 (95% CI, 0.76–1.14) for Black patients, 0.90 (95% CI, 0.73–1.11) for Hispanic patients, and 1.31 (95% CI, 0.96–1.80) for Asian patients compared with non-Hispanic White patients. The median odds ratio across hospitals was 1.99 (95% CI, 1.74–2.48). Results were similar for major adverse cardiovascular events. Asian patients had the highest COVID-19 cardiorespiratory severity at presentation (adjusted odds ratio, 1.48 [95% CI, 1.16–1.90]).
Conclusions:
Although in-hospital mortality and major adverse cardiovascular events did not differ by race/ethnicity after adjustment, Black and Hispanic patients bore a greater burden of mortality and morbidity because of their disproportionate representation among COVID-19 hospitalizations.
Clinical Perspective.
What Is New?
In a large national registry of coronavirus disease 2019 (COVID-19) hospitalizations, Black and Hispanic patients accounted for >50% of the hospitalizations and were significantly younger than non-Hispanic White patients.
After adjusting for sociodemographic and clinical characteristics, mortality and major cardiovascular or cerebrovascular adverse events did not differ by race/ethnicity in hospitalized patients.
Asian patients had the highest risk of cardiorespiratory disease severity at presentation.
What Are the Clinical Implications?
Although race/ethnicity was not independently associated with worse in-hospital mortality or major adverse cardiovascular events, Black and Hispanic patients bore a greater burden of mortality and morbidity because of their disproportionate representation among COVID-19 hospitalizations.
Interventions to reduce disparities in COVID-19 should focus upstream from hospitalizations.
Editorial, see p 2343
The coronavirus disease 2019 (COVID-19) pandemic has exposed longstanding racial/ethnic health inequities in the United States. In preliminary, mostly single-center or regional studies, Black and Hispanic individuals were shown to bear a disproportionate rate of COVID-19 infection, hospitalizations, and adverse outcomes.1–4 The reasons for these disparities are complex and include the pervasive effects of structural racism that result in conditions that increase the risk of transmission of COVID-19, such as being overrepresented in the essential workforce, living in multigenerational households, and limited access to regular testing.5–7 Historically marginalized populations also have a higher prevalence of underlying comorbidities that may increase disease severity and adverse outcomes.8
The relationship between COVID-19 and cardiovascular disease (CVD) complications has not been fully characterized. However, retrospective single-center studies have found an association between underlying CVD and increased mortality.6,9,10 In part because of the affinity of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for the angiotensin-converting enzyme 2 receptor and its tendency to cause coagulopathy, COVID-19 is postulated to have important effects on the vasculature beyond that of other traditional respiratory viruses.11–13 The observed cardiovascular manifestations are broad and include myocardial injury, heart failure, and thrombotic complications.14,15 Differences by race/ethnicity of these CVD complications have not been previously described.
The American Heart Association (AHA) COVID-19 CVD Registry leveraged a preexisting quality improvement registry infrastructure to rapidly and systematically collect data that are inclusive of cardiovascular outcomes associated with COVID-19 across diverse hospitals and patient populations in the United States. Using these national data, we aimed to comprehensively characterize racial/ethnic differences in risk factors, presentation, patterns of care, cardiorespiratory disease severity, and mortality for patients hospitalized with COVID-19. We hypothesized that Black and Hispanic patients would be overrepresented in COVID-19 hospitalizations and experience worse in-hospital outcomes.
Methods
Study Population
Development and deployment of the AHA COVID-19 CVD Registry have been described previously.16 In brief, the AHA COVID-19 CVD Registry is powered by the Get With The Guidelines quality improvement program and was made available as a free quality improvement tool to all US hospitals caring for adults with active COVID-19. To minimize data collection bias, hospitals retrospectively abstracted all consecutive patients who were ≥18 years of age and hospitalized with COVID-19 as a diagnosis. Data abstraction was not considered complete until the patient was discharged from the hospital, with complete data on admission and discharge dates, age, sex, and medical history assessment. For all hospitals, participation in this quality improvement registry was approved or review was waived by an institutional review board.
Data Collection
The AHA COVID-19 CVD registry contains >200 data elements including patient demographics, insurance status, hospital admission, and disposition dates, presenting symptoms and admission clinical features. Because of its CVD focus, the registry includes detailed information on several CVD complications including myocardial infarction and new heart failure. Medication use during the hospitalization includes the use of therapies either as part of a clinical trial or routine care. The case report form is available at https://www.heart.org/-/media/files/professional/quality-improvement/covid-19-cvd-registry/ahacovidcvdcrf428-fillable-pdf.pdf?la=en. The Get With The Guidelines programs are provided by the AHA. IQVIA (Parsippany, NJ) serves as the data collection and coordination center. Deidentified data from the AHA COVID-19 CVD Registry are available through the AHA’s Precision Medicine Platform. Requests to access the data should be made through the AHA COVID-19 CVD Registry after submitting a proposal: https://www.heart.org/en/professional/quality-improvement/covid-19-cvd-registry/covid-19-cvd-registry-research-opportunities.
Race/ethnicity of study participants was extracted from the electronic health records at each site. For this study, race/ethnicity was categorized using hierarchical, mutually exclusive categories as follows: Hispanic for all races; non-Hispanic Black, Asian/Pacific Islander, non-Hispanic White, and American Indian/Alaska Native. Median household income, educational attainment, and employment status were extracted from the 2018 American Community Survey 5-year estimates based on patient zip code. We also estimated the racial/ethnic demographic composition of the geographic area served by each hospital in the registry based on the hospital’s zip code. Hospital characteristics, including region, number of beds, rural location, and academic/teaching status, were obtained from hospital self-report, supplemented by American Hospital Association survey data.
Outcomes
The primary outcome was in-hospital all-cause mortality. Secondary outcomes included major adverse cardiovascular events (MACEs) and COVID-19 cardiorespiratory disease severity. For this study, MACE was defined as a composite of death, myocardial infarction, stroke, new-onset heart failure, or cardiogenic shock. A cardiorespiratory-specific COVID-19 disease severity scale, adapted from the World Health Organization’s ordinal outcomes scale,17 ranked the following outcomes from worst to best: in-hospital death, in-hospital cardiac arrest, mechanical ventilation with mechanical circulatory support (intra-aortic balloon pump, percutaneous ventricular assist devices, extracorporeal membrane oxygenation), mechanical ventilation with vasopressors/inotrope, mechanical ventilation without hemodynamic support, and hospitalization without any of the preceding.
Statistical Analyses
Among 8590 patients with completed records in the registry, 688 (8%) had no race or ethnicity documented. Patient characteristics were compared between those with and without documented race/ethnicity with standardized differences and are shown in Table I in the Data Supplement.
Baseline clinical characteristics, admission features, and in-hospital treatments for each racial/ethnic group were reported as percentages for categorical variables and median and interquartile range for continuous variables. Because of the small number of the American Indian/Alaskan Native patients (n=34), this group was not included in the tables. For laboratory results, rates of measurement, and measured values, as well, were presented.
Weekly unadjusted in-hospital mortality rates were calculated as naive case fatality ratios (number who died that week divided by the sum of those who died or were discharged to home or a nonacute health care facility). Patients who were discharged to hospice, who were discharged to another hospital, or who had an unknown discharge disposition were excluded from case fatality ratio calculations. To ensure stable assessments of mortality, weekly hospitalization and mortality rates were calculated from March 2020 to May 2020 because prior time periods had denominators <20 patients and some sites reported ongoing data abstraction for more recent hospitalizations.
We performed sequential multivariable logistic regression analyses to test the strength of the association between race/ethnicity and each clinical outcome after incrementally adjusting for sociodemographic and patient characteristics. The base unadjusted model included race/ethnicity alone. Model 1 adjusted for age only. Model 2 also adjusted for other sociodemographic factors, including sex, insurance status, household income, employment status, and education level. Model 3 added medical history, including coronary artery disease, heart failure, cerebrovascular disease, atrial fibrillation/flutter, chronic kidney disease, diabetes, hypertension, smoking/e-cigarette use, cancer, pulmonary disease, immune disorder, pulmonary embolism/deep venous thrombosis, and obesity (body mass index ≥30 kg/m2). Model 4 added clinical features at presentation, including days from symptom onset to hospital arrival, on- versus off-hour presentation (on-hour defined as hospital arrival between 8 am and 5 pm on Mondays through Fridays), fever (temperature >38 °C) on admission, tachycardia (heart rate >100 beats/min) on admission, hypotension (systolic blood pressure <90 mm Hg) on admission, hypoxia on admission (oxygen saturation <94% or need for supplemental oxygen on admission), and pulmonary infiltrate present on chest radiograph or computed tomogram. All models included a random intercept for hospital to account for within- and across-hospital variability. We also reported the median odds of each clinical outcome across hospitals to capture the random-effect variance or variation in log-odds attributable to between-hospital differences. The same models were fit for the primary outcome of in-hospital mortality, and the secondary outcomes of MACE and COVID-19 cardiorespiratory disease severity, as well; the latter was fit with an ordinal logistic model. Sensitivity analyses for each outcome used a nonhierarchical generalized estimating equation modeling approach adjusting for the covariates in model 4, while considering within-hospital clustering.
All continuous variables were accounted for using restricted cubic splines. Missing insurance status values for patients ≥65 years of age were imputed to Medicare; otherwise, multiple imputation methods were used with 10 data sets. Variable missing rates were <3.5% for all adjustment variables, with the exception of obesity (10.6%), symptom onset to hospital arrival (13.1%), off-hour presentation (8.0%), and hypoxia (O2 saturation <94% or requiring supplemental oxygen [8.4%]). Statistical analyses were performed using SAS software (version 9.4, SAS Institute), and 2-tailed P values <0.05 were considered significant for all statistical tests.
Results
From January 17, 2020, to July 22, 2020, 8950 unique patients were hospitalized with COVID-19 at 88 hospitals in the AHA COVID-19 CVD Registry. Weekly hospitalization rates by race/ethnicity showed little change over time (Figure I in the Data Supplement). Participating hospitals were located across 76 zip codes with the following median racial/ethnic demographics: non-Hispanic White 59.3%, Black 10.6%, Hispanic (all races) 9%, and Asian 4.7%. These hospitals had a median size of 415 beds (interquartile range, 265–550), 36.4% were in the Northeast, 34.1% in the South, 13.6% in the West, and 13.6% in the Midwest. The majority (81.8%) were teaching hospitals.
Among hospitalized patients, 33.0% were Hispanic, 25.5% were non-Hispanic Black, 6.3% were Asian, and 35.2% were non-Hispanic White; fewer than half were women (45%). The ratio of patients treated at each site by race/ethnicity compared with the demographic characteristics of each site’s zip code are shown in Figure II in the Data Supplement. Hospitalized Hispanic and Black patients were substantially younger than non-Hispanic White patients (median age 57 and 60 years of age versus 69, respectively; Table 1). Hispanic patients were more than twice as likely to be uninsured than other groups and had the lowest educational attainment. Black patients had the lowest median household income and highest unemployment rate.
Table 1.
Baseline and In-Hospital Characteristics of Patients Hospitalized With COVID-19, by Race/Ethnicity
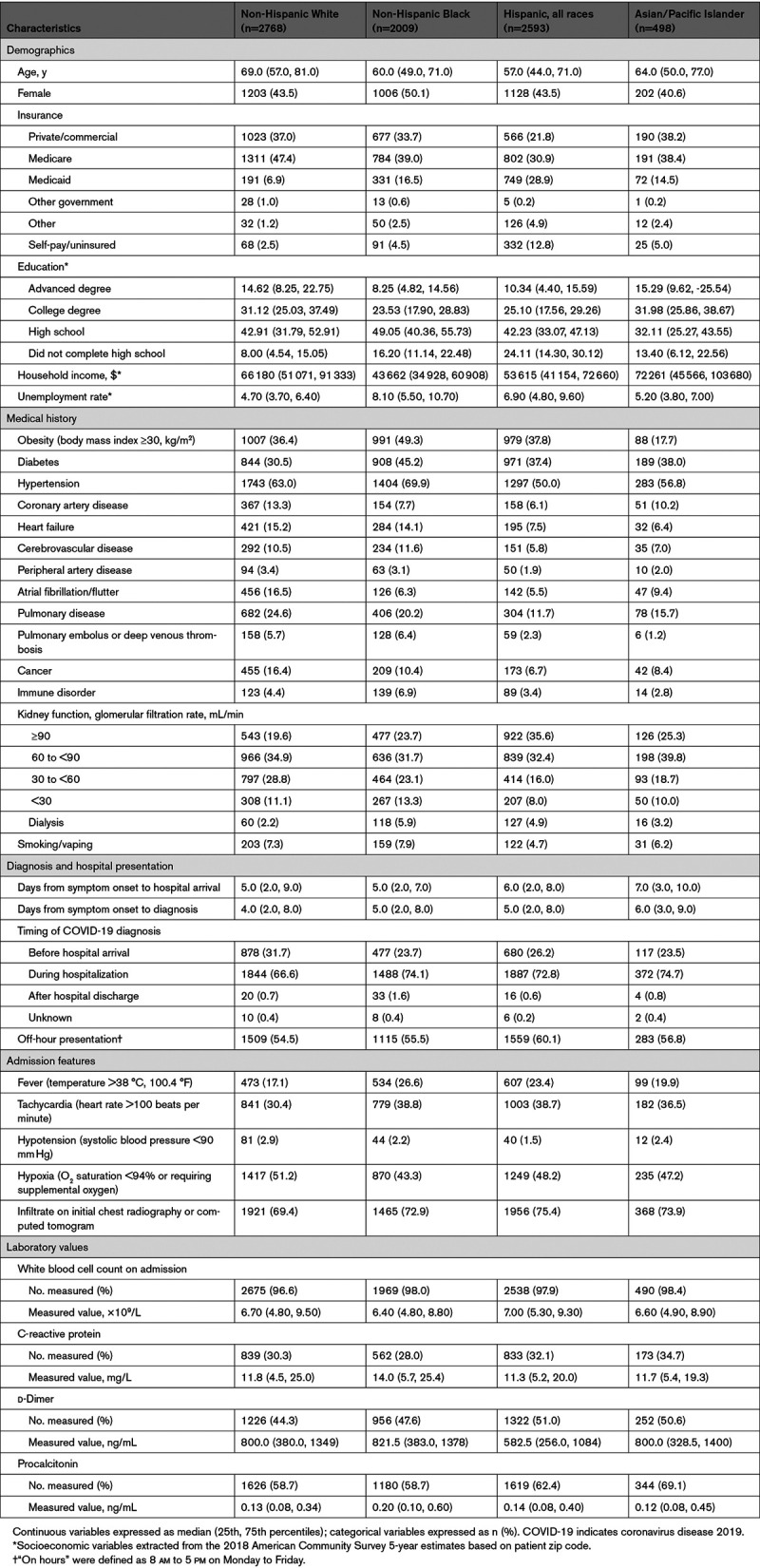
Almost half of hospitalized Black patients were obese (49.3%), compared with just 17.7% of hospitalized Asian patients. Whereas Black patients had the highest prevalence of obesity, hypertension, diabetes, prior cerebrovascular disease, and advanced kidney disease on admission, non-Hispanic White patients had the highest prevalence of prior coronary artery disease and pulmonary disease (Table 1) in comparison with other racial/ethnic groups. Asian patients had the longest time from symptom onset to hospital arrival (7 days) compared with other racial/ethnic groups. The timing of COVID-19 diagnosis was 71.1% during the hospitalization with fewer non-White patients diagnosed before hospital arrival. Hispanic patients were most likely to present during off hours (60.1%). Black and Hispanic patients were more likely to present with fever and tachycardia, but less likely to be hypoxic on admission compared with non-Hispanic White patients. Admission laboratory values did not differ meaningfully across racial/ethnic groups (Table 1). Laboratory measurements, such as d-dimer and procalcitonin levels, were more frequently assessed in non-White patients. Among patients with measurements, Black patients had the highest C-reactive protein and procalcitonin levels.
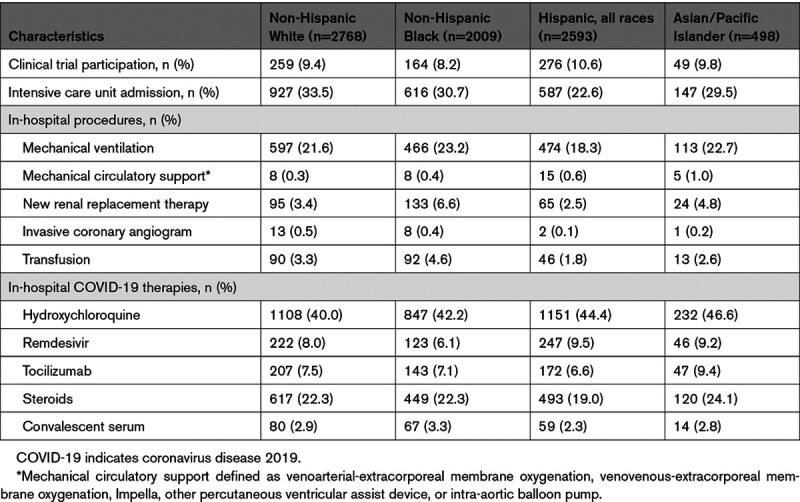
One in five patients required mechanical ventilation; the lowest likelihood was among Hispanic patients (18.3%), who also had the lowest likelihood of treatment in an intensive care unit (22.6%). Black patients had the highest observed rates of mechanical ventilation, new renal replacement therapy, and transfusion (Table 2). Only 9.5% of patients were enrolled in a COVID-19–related trial; the lowest enrollment was among Black patients. The most frequently administered COVID-19–specific therapy was hydroxychloroquine (42.4%), with the highest use in Asian patients. In contrast, remdesivir was used to treat 8.1% of patients during this study time period, with the lowest rate of use in Black patients.
Table 2.
In-Hospital Treatment of Patients Hospitalized With COVID-19, by Race/Ethnicity
During the study period, there was a total of 1447 deaths (18.4%), of which 768 (53.1% of all deaths) occurred in Black or Hispanic patients (Table 3). In-hospital deaths were observed in 17.6% of Black, 16.0% of Hispanic, and 19.3% of Asian patients compared with 21.1% of non-Hispanic White patients (P<0.001). For patients <70 years of age, mortality was highest among Black patients and lowest in Asian patients (Table 3). Case fatality ratios had an early peak, then appeared to slowly decline Figure A, P for trend=0.049) with similar trends for Black and Hispanic patients (Figure B). In sequential modeling, racial/ethnic differences in mortality became nonsignificant after adjusting for differences in age at presentation (Table 4). In the final multivariable model, additionally adjusting for sociodemographic, clinical, and presentation characteristic differences among groups, the adjusted odds ratios (ORs) for mortality were 0.93 (95% CI, 0.76–1.14) for Black patients, 0.90 (95% CI, 0.73–1.11) for Hispanic patients, and 1.31 (95% CI, 0.96–1.80) for Asian patients compared with non-Hispanic White patients. Sequential model results stratified by age group are shown in Table II in the Data Supplement. Sensitivity analyses using nonhierarchical generalized estimating equations showed similar results (Table III in the Data Supplement). The median OR across hospitals was 1.99 (95% CI, 1.74–2.48) in the final adjustment model, suggesting that the odds of in-hospital death for a patient was 99% higher at 1 hospital than for a similar patient treated at another hospital.
Table 3.
In-Hospital Outcomes of Patients Hospitalized With COVID-19, by Race/Ethnicity
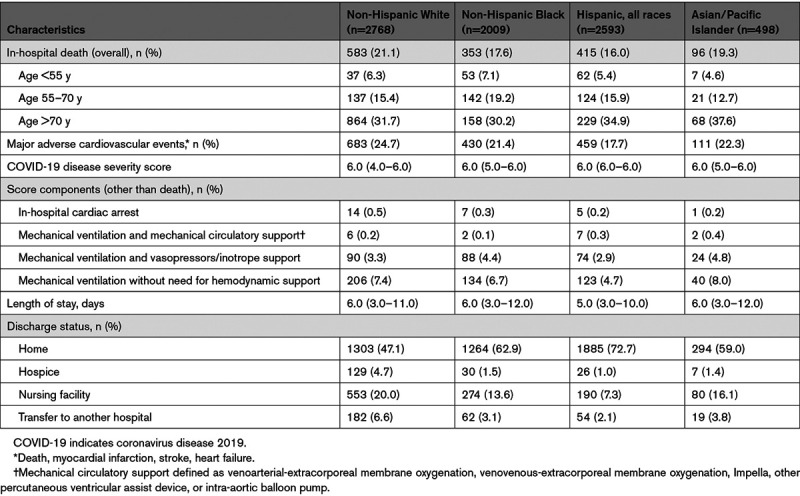
Table 4.
Odds Ratios of Clinical Outcomes Among 7868 Patients Hospitalized for COVID-19, by Race/Ethnicity
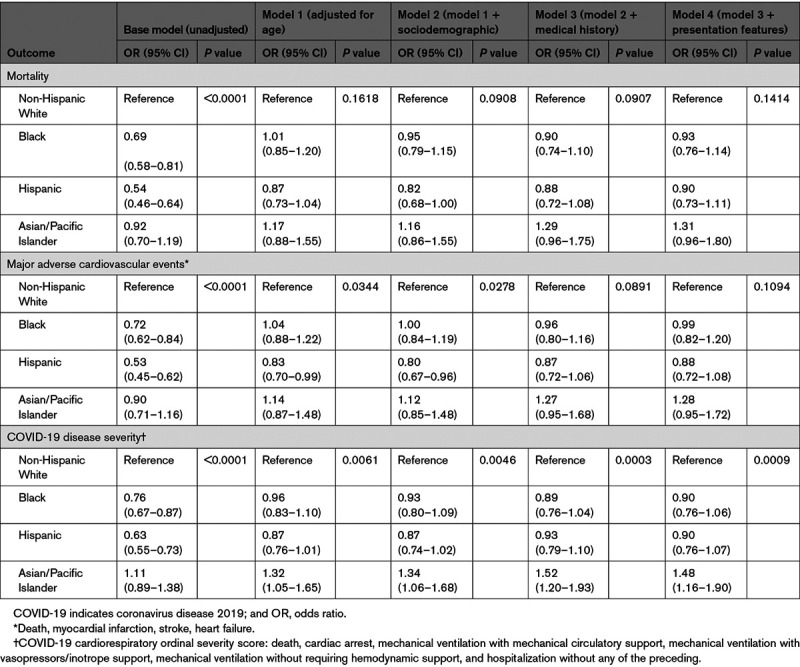
Figure.
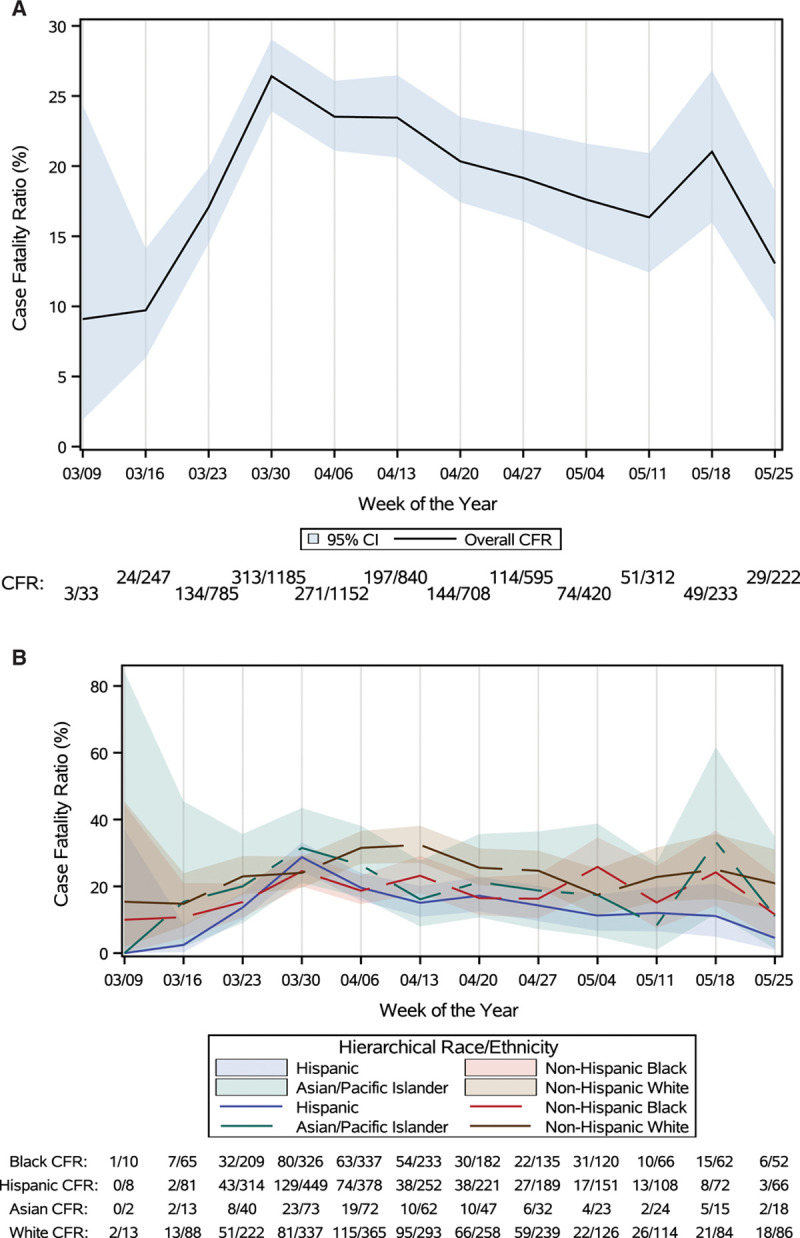
Weekly case fatality ratio in the overall population (A) and by race/ethnicity (B). A and B show the temporal trends in coronavirus disease 2019 (COVID-19) hospitalizations and case fatalities across registry sites. The change in the trend of case fatalities from March to May is trending to nonsignificance (P=0.0492). CFR indicates case fatality ratio.
Similar results were seen for the secondary outcome of MACE that occurred in 21.4% of patients (Table 3). Whereas observed unadjusted MACE rates were lower in Black (21.4%) and Hispanic (17.7%) patients than in non-Hispanic White patients (24.7%, P<0.001), these differences were attenuated and no longer different after full adjustment. In the fully adjusted models, the adjusted ORs for MACE were 0.99 (95% CI, 0.82–1.20) for Black patients, 0.88 (95% CI, 0.72–1.08) for Hispanic patients, and 1.28 (95% CI, 0.95–1.72) for Asian patients compared with non-Hispanic White patients. The median OR across hospitals was 1.92 (95% CI, 1.69–2.36) in the final model.
For the secondary outcome of cardiorespiratory COVID-19 disease severity, Black and Hispanic patients had lower unadjusted probabilities of experiencing more severe disease, but these differences were attenuated after multivariable adjustment. After adjusting for sociodemographic characteristics, Asian patients had a higher probability of experiencing greater COVID-19 cardiorespiratory disease severity than non-Hispanic White patients, and this relationship persisted after further adjusting for medical history and presentation characteristics (adjusted OR, 1.48 [95% CI. 1.16–1.90]).
Median length of stay was shortest among Hispanic patients, and non-Hispanic White patients were more likely to be discharged to a nursing facility compared with other patients (Table 3).
Discussion
In a large study of patients hospitalized for COVID-19 across the United States, we report several noteworthy findings. First, Hispanic and Black patients comprised more than half of patients hospitalized with COVID-19; these patients were more often uninsured, had lower socioeconomic status, and had longer delays from symptom onset to COVID-19 diagnosis. Second, hospitalized Hispanic and Black patients were substantially younger than non-Hispanic White and Asian patients. Third, Black patients had the highest prevalence of obesity, hypertension, and diabetes, and they had the highest rates of mechanical ventilation and renal replacement therapy initiation during their hospitalization. Last, observed differences between racial/ethnic groups were no longer significant after adjusting for age and further attenuated after accounting for sociodemographic characteristics, clinical comorbidities, and presenting features. Overall mortality was high at 18.4%, with Black and Hispanic patients representing more than half of all in-hospital deaths. Similar results were seen for the outcomes of MACE and cardiorespiratory COVID-19 disease severity. Hospitalized Asian patients had the highest risk-adjusted cardiorespiratory disease severity at presentation.
Our study supports state and administrative national reports that Black and Hispanic individuals are more likely to be hospitalized with severe acute respiratory syndrome coronavirus 2 infection and therefore bear a disproportionate burden of COVID-19 mortality.1,18–20 In census data, Black and Hispanic individuals constituted 13% and 19% of the US population in 2019,21 respectively, but they comprised 26% and 33% of hospitalized COVID-19 patients in our study. The representation of Black and Hispanic patients was even higher than the expected racial/ethnic composition of each of the zip codes where the participating hospital sites where located and other AHA Get With The Guidelines registries.22,23 Despite being almost 10 years younger than non-Hispanic White patients, Black patients had the highest rates of diabetes, hypertension, and obesity, all of which have been associated with adverse COVID-19 outcomes.24 Hispanic patients in our study, in contrast, did not have more comorbidities than other racial/ethnic groups. This observation may be explained by their younger age and relative lack of health insurance, which may delay diagnosis of underlying chronic disease. Black and Hispanic individuals are also overrepresented in the essential workforce,25 which increases the potential for viral exposure and community transmission. The observed longer time to diagnosis from symptom onset for non-White patient groups may represent inequities in testing access. Structural and social factors such as discrimination, multigenerational households, and living in crowded, urban areas also increase the chance of viral transmission.20,26,27
Almost one-third of patients required intensive care unit admission and one-fifth required mechanical ventilation. Black patients had the highest observed rate of mechanical ventilation, whereas Hispanic patients had the lowest. Black patients were also more likely to require initiation of renal replacement therapy during their hospitalization. Asian patients had the worst cardiorespiratory disease severity with second highest rates of mechanical ventilatory and circulatory support. This may be explained by the longer time to hospital arrival and diagnosis from symptom onset, older age, and a high burden of comorbidities. Other studies have shown that health services are underused among Asian immigrant populations, which may be influenced by acculturation and experiences of discrimination, in particular, during the COVID-19 pandemic.28,29
During this earlier phase of the pandemic, hydroxychloroquine use rates were >40% across all racial/ethnic groups. Remdesivir use rates were low overall and were lower among Black patients despite greater need for mechanical ventilation. This may be explained by more advanced kidney disease and lower rates of clinical trial participation seen in this population. It will be imperative to carefully evaluate and ensure that emerging evidence-based therapies for COVID-19 are delivered equitably across diverse populations.
The overall rate of MACE in our study was 21.4%, driven largely by the high mortality rate. The 18% mortality rate observed in our study is consistent with other US studies from early in the pandemic.30,31 Mortality rates appeared to decline over time, because hospitals were better equipped to deal with the pandemic and new therapeutic options became available. The improving trends were consistent among racial/ethnic groups. Contrary to our hypothesis, we found no differences in mortality by race/ethnicity in fully adjusted models. Sensitivity analyses showed similar associations for Black and Hispanic patients and outcomes, regardless of whether modeling accounted for across-hospital and within-hospital effects. Similar to our study, other risk-adjusted single-center reports found no association between race/ethnicity and in-hospital survival. In a large cohort of patients cared for by an integrated-delivery health system in Louisiana, Black race was not independently associated with higher in-hospital mortality.1 Kabarriti et al32 also found no association between race/ethnicity and survival in a large urban medical center in New York, although this study also included patients who were not hospitalized. The neutral association of race/ethnicity with hospital outcomes must be viewed in the context of the marked overrepresentation of Black race and Hispanic ethnicity among consecutive hospitalized patients captured in this registry. Despite similar comparative outcomes, Black and Hispanic patients comprised more than half of the in-hospital COVID-19 mortality and morbidity.
Our study also provided evidence that the hospital at which the patient was treated contributed significantly to the variation in the odds of poor outcomes. Our results suggest that where a patient was treated explained more of the variation in odds of mortality than race/ethnicity in and of itself. Early in a new pandemic, we expected significant interhospital practice variation in the absence of solid treatment evidence. We also observed significant variation in the proportion of Black and Hispanic patients with COVID-19 treated at each hospital. Our findings of similar risk-adjusted outcomes by race/ethnicity suggests that racial/ethnic differences in age, demographics, and clinical characteristics at hospital presentation all contribute to the differences in observed clinical outcomes. We still need to address the important disparities contributing to higher COVID-19 burden upstream of hospitalization, and potential interhospital care differences, as well, that might be related to the proportion of non-White patients served at each hospital.
Our study should be interpreted in the context of several limitations. First, this study describes racial/ethnic differences in treatment and outcomes among patients hospitalized with COVID-19, not all patients with COVID-19. Patients may have died or experienced nonfatal events without seeking hospital-based medical care, and racial/ethnic disparities in care-seeking behavior have been described previously.33,34 Second, the 88 hospitals enrolled in this registry overrepresent academic hospitals in urban communities with concentrations in certain geographic regions such as the Northeast and South, areas that were initially hard hit by the pandemic. It is possible that patients treated at these institutions (that volunteered to be part of a quality improvement registry) receive different care and have different outcomes than those treated at other hospitals. Third, race/ethnicity was not recorded in 8% of individuals. Fourth, sociodemographic factors were extracted from the American Community Survey based on patient zip code data and do not fully account for individual patient factors. Fifth, because this was an observational quality improvement registry, not all patients underwent all laboratory and testing procedures, and clinical outcomes were not independently validated. Last, as with all ongoing registries, incomplete and nonconsecutive data entry may result in a biased study sample, but we expect this to be nondifferential by race/ethnicity.
Conclusion
Hispanic and Black patients comprised more than half of the patients hospitalized with COVID-19. Although substantially younger than non-Hispanic White patients, Hispanic and Black patients more often had lower socioeconomic status and differences in COVID-19 diagnosis and presentation characteristics. After adjustment for demographic and clinical characteristics, no racial/ethnic differences were observed for in-hospital mortality and MACE. Asian patients had the highest risk of cardiorespiratory COVID-19 disease severity. However, overall mortality was high at 18.4%, and Black and Hispanic patients bore a greater burden of the mortality and morbidity because of their disproportionate representation among COVID-19 hospitalizations.
Sources of Funding
This study was funded by the American Heart Association (AHA). AHA’s suite of Registries is funded by multiple industry sponsors. The AHA COVID-19 CVD Registry is supported in part by The Gordon and Betty Moore Foundation. Dr Rodriguez was funded by a career development award from the National Heart, Lung, and Blood Institute (K01 HL 144607) and the American Heart Association/Robert Wood Johnson Harold Amos Medical Faculty Development Program. Dr Abdalla receives support through 18AMFDP34380732 from the American Heart Association and from the NIH/NHLBI (K23 HL141682-01A1 and R01HL146636-01A1).
Disclosures
Dr Rodriguez has received consulting fees from Novartis, Janssen, NovoNordisk, and HealthPals unrelated to this work. Dr de Lemos has received income from Eli Lilly, Novo Nordisc, Regeneron, and Amgen for participating in data monitoring committees or steering committees unrelated to this work. Dr Morrow reports grants from Anthos Therapeutics, Daiichi Sankyo, Eisai, GlaxoSmithKline, Medicines Company, Siemens, Takeda, and Zora Biosciences; grants and personal fees from AstraZeneca, Merck & Co, Novartis, Pfizer, Quark, Regeneron, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work; and Dr Morrow is a member of the TIMI Study Group that has received institutional research grant support through Brigham and Women’s Hospital from Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc, BRAHMS, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Regeneron, Roche, Siemens, Takeda, The Medicines Company, and Zora Biosciences. Dr Elkind receives study drug in kind from the BMS-Pfizer Alliance for Eliquis and ancillary support but no personal compensation from Roche for an National Institutes of Health–funded trial of stroke prevention (outside the submitted work), and receives royalties from UpToDate for chapters on stroke, including on COVID-19. Dr Elkind serves as an Officer of the American Heart Association. Dr Wang reports research grants to Duke Clinical Research Institute from Abbott, AstraZeneca, Bristol Myers Squibb, Boston Scientific, Cryolife, Chiesi, Merck, Portola, and Regeneron, and consulting honoraria from AstraZeneca, Bristol Myers, and Novartis, as well. Drs Das and Solomon, D. Holmes, and Drs Matsouaka and Abdalla have no disclosures.
Supplemental Materials
Data Supplement Tables I–III
Data Supplement Figures I and II
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AHA
- American Heart Association
- COVID-19
- coronavirus disease 2019
- MACE
- major adverse cardiovascular events
- OR
- odds ratio
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
This work was presented as an abstract at the American Heart Association Scientific Sessions, November 13 to 17, 2020.
This manuscript was sent to Dr Erin Michos, Guest Editor, for review by expert referees, editorial decision, and final disposition.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.120.052278.
For Sources of Funding and Disclosures, see page 2341.
Contributor Information
Nicole Solomon, Email: nicole.solomon@duke.edu.
James A. de Lemos, Email: james.delemos@utsouthwestern.edu.
Sandeep R. Das, Email: sandeep.das@utsouthwestern.edu.
David A. Morrow, Email: dmorrow@partners.org.
Steven M. Bradley, Email: steven.bradley@allina.com.
Mitchell S.V. Elkind, Email: mse13@columbia.edu.
Joseph H. Williams, Email: Joseph.williams@heart.org.
DaJuanicia Holmes, Email: dajuanicia.holmes@duke.edu.
Roland A. Matsouaka, Email: roland.matsouaka@duke.edu.
Divya Gupta, Email: dgupta2@emory.edu.
Ty J. Gluckman, Email: tygluckman@gmail.com.
Marwah Abdalla, Email: ma2947@cumc.columbia.edu.
Michelle A. Albert, Email: michelle.albert@ucsf.edu.
Clyde W. Yancy, Email: cyancy@nm.org.
Tracy Y. Wang, Email: tracy.wang@duke.edu.
References
- 1.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382:2534–2543. doi: 10.1056/NEJMsa2011686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yancy CW. COVID-19 and African Americans. JAMA. 2020;323:1891–1892. doi: 10.1001/jama.2020.6548 [DOI] [PubMed] [Google Scholar]
- 3.Martinez DA, Hinson JS, Klein EY, Irvin NA, Saheed M, Page KR, Levin SR. SARS-CoV-2 positivity rate for Latinos in the Baltimore-Washington, DC Region. JAMA. 2020;324:392–395. doi: 10.1001/jama.2020.11374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukherjee V, Toth AT, Fenianos M, Martell S, Karpel HC, Postelnicu R, Bhatt A, Deshwal H, Kreiger-Benson E, Brill K, et al. Clinical outcomes in critically ill coronavirus disease 2019 patients: a unique New York City public hospital experience. Crit Care Explor. 2020;2:e0188. doi: 10.1097/CCE.0000000000000188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wadhera RK, Wadhera P, Gaba P, Figueroa JF, Joynt Maddox KE, Yeh RW, Shen C. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA. 2020;323:2192–2195. doi: 10.1001/jama. 2020.7197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figueroa JF, Wadhera RK, Lee D, Yeh RW, Sommers BD. Community-level factors associated with racial and ethnic disparities in COVID-19 rates in Massachusetts. Health Aff (Millwood). 2020;39:1984–1992. doi: 10.1377/hlthaff.2020.01040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alsan M, Stantcheva S, Yang D, Cutler D. Disparities in coronavirus 2019 reported incidence, knowledge, and behavior among US adults. JAMA Netw Open. 2020;3:e2012403. doi: 10.1001/jamanetworkopen.2020.12403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR. 0000000000000757 [DOI] [PubMed] [Google Scholar]
- 9.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, Bonanomi E, Cabrini L, Carlesso E, Castelli G, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020. doi: 10.1001/jamainternmed.2020.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonow RO, O’Gara PT, Yancy CW. Cardiology and COVID-19. JAMA. 2020;324:1131–1132. doi: 10.1001/jama.2020.15088 [DOI] [PubMed] [Google Scholar]
- 12.Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, Muenchhoff M, Hellmuth JC, Ledderose S, Schulz H, et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colling ME, Kanthi Y. COVID-19-associated coagulopathy: an exploration of mechanisms. Vasc Med. 2020;25:471–478. doi: 10.1177/1358863X20932640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, et al. ; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fried JA, Ramasubbu K, Bhatt R, Topkara VK, Clerkin KJ, Horn E, Rabbani L, Brodie D, Jain SS, Kirtane AJ, et al. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alger HM, Rutan C, Williams JH, IV, Walchok JG, Bolles M, Hall JL, Bradley SM, Elkind MSV, Rodriguez F, Wang TY, et al. American Heart Association COVID-19 CVD Registry powered by Get With The Guidelines. Circ Cardiovasc Qual Outcomes. 2020;13:e006967. doi: 10.1161/CIRCOUTCOMES.120.006967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO R&D Blueprint. Novel Coronavirus. COVID-19 Therapeutic Trial Synopsis [draft]. February 18, 2020. World Health Organization; Accessed on September 10, 2020. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf?ua=1 [Google Scholar]
- 18.Killerby ME, Link-Gelles R, Haight SC, Schrodt CA, England L, Gomes DJ, Shamout M, Pettrone K, O’Laughlin K, Kimball A, et al. ; CDC COVID-19 Response Clinical Team. Characteristics associated with hospitalization among patients with COVID-19 - Metropolitan Atlanta, Georgia, March–April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:790–794. doi: 10.15585/mmwr.mm6925e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu HE, Ashe EM, Silverstein M, Hofman M, Lange SJ, Razzaghi H, Mishuris RG, Davidoff R, Parker EM, Penman-Aguilar A, et al. Race/ethnicity, underlying medical conditions, homelessness, and hospitalization status of adult patients with COVID-19 at an Urban Safety-Net Medical Center - Boston, Massachusetts, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:864–869. doi: 10.15585/mmwr.mm6927a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore JT, Ricaldi JN, Rose CE, Fuld J, Parise M, Kang GJ, Driscoll AK, Norris T, Wilson N, Rainisch G, et al. ; COVID-19 State, Tribal, Local, and Territorial Response Team. Disparities in incidence of COVID-19 among underrepresented racial/ethnic groups in counties identified as hotspots during June 5–18, 2020 – 22 states, February–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1122–1126. doi: 10.15585/mmwr.mm6933e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.United States Census Bureau. QuickFacts. United States: Accessed on September 30, 2020. https://www.census.gov/quickfacts/fact/table/US/RHI225219 [Google Scholar]
- 22.Powell-Wiley TM, Ngwa J, Kebede S, Lu D, Schulte PJ, Bhatt DL, Yancy C, Fonarow GC, Albert MA. Impact of body mass index on heart failure by race/ethnicity from the Get With The Guidelines-Heart Failure (GWTG-HF) Registry. JACC Heart Fail. 2018;6:233–242. doi: 10.1016/j. jchf.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Man S, Xian Y, Holmes DN, Matsouaka RA, Saver JL, Smith EE, Bhatt DL, Schwamm LH, Fonarow GC. association between thrombolytic door-to-needle time and 1-year mortality and readmission in patients with acute ischemic stroke. JAMA. 2020;323:2170–2184. doi: 10.1001/jama.2020.5697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Lancet. The plight of essential workers during the COVID-19 pandemic. Lancet. 2020;395:1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453–1463. doi: 10.1016/S0140- 6736(17)30569-X [DOI] [PubMed] [Google Scholar]
- 27.Selden TM, Berdahl TA. COVID-19 and racial/ethnic disparities in health risk, employment, and household composition. Health Aff (Millwood). 2020;39:1624–1632. doi: 10.1377/hlthaff.2020.00897 [DOI] [PubMed] [Google Scholar]
- 28.Salant T, Lauderdale DS. Measuring culture: a critical review of acculturation and health in Asian immigrant populations. Soc Sci Med. 2003;57:71–90. doi: 10.1016/s0277-9536(02)00300-3 [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Gee GC, Bahiru E, Yang EH, Hsu JJ. Asian-Americans and Pacific Islanders in COVID-19: emerging disparities amid discrimination. J Gen Intern Med. 2020. doi: 10.1007/s11606-020-06264-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, et al. ; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suleyman G, Fadel RA, Malette KM, Hammond C, Abdulla H, Entz A, Demertzis Z, Hanna Z, Failla A, Dagher C, et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw Open. 2020;3:e2012270. doi: 10.1001/jamanetworkopen.2020.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kabarriti R, Brodin NP, Maron MI, Guha C, Kalnicki S, Garg MK, Racine AD. Association of race and ethnicity with comorbidities and survival among patients with COVID-19 at an urban medical center in New York. JAMA Netw Open. 2020;3:e2019795. doi: 10.1001/jamanetworkopen.2020.19795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maranda MJ, Deen D, Elshafey S, Herrera M, Gold MR. Response to a patient activation intervention among Spanish-speaking patients at a community health center in New York City. J Health Care Poor Underserved. 2014;25:591–604. doi: 10.1353/hpu.2014.0110 [DOI] [PubMed] [Google Scholar]
- 34.Hibbard JH, Greene J, Becker ER, Roblin D, Painter MW, Perez DJ, Burbank-Schmitt E, Tusler M. Racial/ethnic disparities and consumer activation in health. Health Aff (Millwood). 2008;27:1442–1453. doi: 10.1377/hlthaff.27.5.1442 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


