Abstract
This scientometric analysis of 393 original papers published from January 2000 to June 2019 describes the development and use of bioinks for 3D bioprinting. The main trends for bioink applications and the primary considerations guiding the selection and design of current bioink components (i.e., cell types, hydrogels, and additives) were reviewed. The cost, availability, practicality, and basic biological considerations (e.g., cytocompatibility and cell attachment) are the most popular parameters guiding bioink use and development. Today, extrusion bioprinting is the most widely used bioprinting technique. The most reported use of bioinks is the generic characterization of bioink formulations or bioprinting technologies (32%), followed by cartilage bioprinting applications (16%). Similarly, the cell-type choice is mostly generic, as cells are typically used as models to assess bioink formulations or new bioprinting methodologies rather than to fabricate specific tissues. The cell-binding motif arginine-glycine-aspartate is the most common bioink additive. Many articles reported the development of advanced functional bioinks for specific biomedical applications; however, most bioinks remain the basic compositions that meet the simple criteria: Manufacturability and essential biological performance. Alginate and gelatin methacryloyl are the most popular hydrogels that meet these criteria. Our analysis suggests that present-day bioinks still represent a stage of emergence of bioprinting technology.
Keywords: Bioinks, Bioprinting, Scientometrics, Tissue engineering, Organ
1. Introduction
Bioprinting is a growing technology[1,2] that promises a future in which patients in need will have access to 3D-printed tissues and organs that can substitute those lost or damaged[3,4]. Bioprinting has already enabled the fabrication of small units of tissues and organs that recapitulate some functions of their native counterparts[5,6]. These mini-tissues and organoids have also proven useful as in vitro models for basic research and as testing platforms for drug screening, drug development, and personalized therapies.[5,7]
The goal of 3D bioprinting is to manufacture living volumetric constructs by depositing a material containing living cells (i.e., a bioink) in a layer-by-layer fashion[6,8,9]. A bioink is composed of living cells that may contain other elements, such as a water-rich polymer network and functional additives (i.e., molecules or particles) associated with the intended application[10]. The bioink is so central to the concept of bioprinting that it is the defining element that differentiates “bioprinting” from “3D printing.” The success of generating functional tissues relies heavily on the quality of the bioink.
Research groups around the globe have devoted their efforts to developing protocols and bioink-related technologies that are bringing us closer to the ambitious goal of bioprinting fully functional tissues and organs.
In this work, we describe the present landscape of bioink use and development, as reported in 393 original research papers published from January 2000 to June 2019. We also discuss the trends revealed by this scientometric analysis from a technical perspective. We start by presenting and discussing the most frequently reported applications in bioprinting and the most commonly used bioprinting techniques. We then describe the trends related to the three main components of the bioinks: Cells, hydrogels, and functional additives.
2. Information search methodology
We conducted document search using the Scopus database. Figure 1 presents the terms and search criteria used. We considered words used in the literature as synonyms of bioink and excluded terms that could lead to the inclusion of documents not related to bioprinting.
Figure 1.
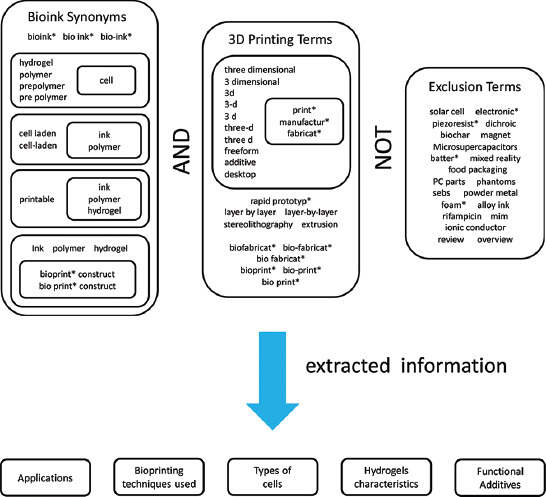
Schematic representation of the query terminology used for the data search. The asterisks (*) mark root words and indicate that all possible suffixes were covered in the query.
From this query, we obtained 529 manuscripts that include 457 original articles and 72 conference papers. We conducted a de-duplication process (i.e., discarding replicate items) followed by a one-by-one validation of these documents to confirm that all of them were related to bioprinting and contained information on the bioinks used. This process yielded 393 original articles which were analyzed in this study (Supplementary File 1 (277.1KB, pdf) ). The articles were examined to find the trends in the intended applications, the bioprinting technologies used and the design/composition of the bioinks employed (cell types, hydrogels, and functional additives).
3. Applications
Figure 2 shows the most reported applications revealed in the final pool of selected articles. The outcome of our analysis reveals the two main reasons that motivate the research and use of bioinks: (i) The development of bioprinting technology (generic) and (ii) clinical needs. Approximately one third of the analyzed papers focused on the development of new materials to formulate bioinks or the introduction of novel bioprinting strategies. This observation was expected as bioprinting is an innovative technology currently transitioning through an early development stage.
Figure 2.
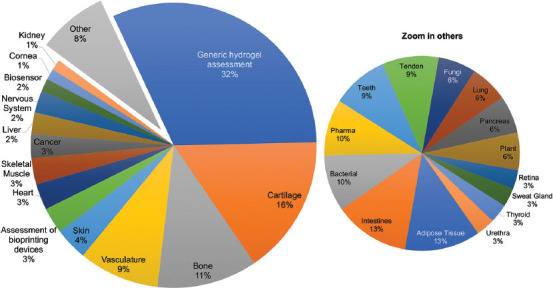
Most reported bioprinting applications.
The development of bioprinting strategies[11,12] and bioprinters with novel features has become a frequent endeavor of engineers in academia and industry[13]. The portfolio of bioprinting methods and bioprinters has greatly evolved in the last two decades. The original devices were less complex[14-16] and adapted to perform proof-of-principle experiments and demonstrate the deposition of drops containing living cells in a single layer (and their short-term survival). These have now evolved into sophisticated designs that enable multi-material and multi-cell type fabrication of multi-layered constructs in the size range of ~ cm3 at resolutions of ~ 10 μm[17].
The development of bioprinting devices and bioink formulations has also advanced in a parallel fashion during the past 20 years. A vast number of published papers about bioprinting have focused more on the development and characterization of bioink formulations[18,19] than on the use of bioprinting for a particular application aligned to clinical needs. Bioink characterization frequently involves an analysis of rheology, because rheology strongly influences the printability[20] of the bioink under different printing conditions (e.g., flow rates, printhead linear speed, temperature, and printing pressure)[18,21]. For instance, the interplay between the ink rheology and the printing parameters determines the fidelity and resolution of extrusion-based bioprinting[12,20,21]. In addition, an assessment of the biological performance of the bioink is practically mandatory in papers related to the development of bioinks. The evaluation of biological performance includes the determination of cell viability immediately after printing and over time, as well as the assessment of indicators of metabolic activity, proliferation, gene or protein expression, and/or differentiation[18].
When it comes to clinical needs, the most frequently reported bioprinted tissues are cartilage[22-24] and bone[25-27], representing 16% and 11% of the analyzed documents, respectively (Figure 2). The statistics reveal that bone- and cartilage-related medical interventions are indeed in great demand. Every year, around 500,000 bone graft procedures are performed in the United States, making bone second only to blood as the most transplanted tissue in that country.[28] More than 7500 cartilage repair interventions were reported to the American Board of Orthopedic Surgery from 2003 to 2015.[29] Moreover, the demand is expected to increase in upcoming years due to the growing prevalence of osteoarthritis disorders in the population.[30]
Cartilage bioprinting has experienced great progress during the last decade (Figure 3A)[31,32]. The recapitulation of the mechanical properties of cartilage is a highly challenging task because cartilage is a load-bearing tissue that is exposed to continuous and repeated friction and compression. The use of bioprinting approaches that employ multi-materials[33,34], multi-cell types[31], and multi-stages[34,35] has enabled the substantial progress in this particular front. Today, relatively complex and large (~ 1–10 cm3) bioprinted constructs of cartilage have been implanted in large animal models[33,35] with excellent results in terms of both integration and mechanical performance.
Figure 3.
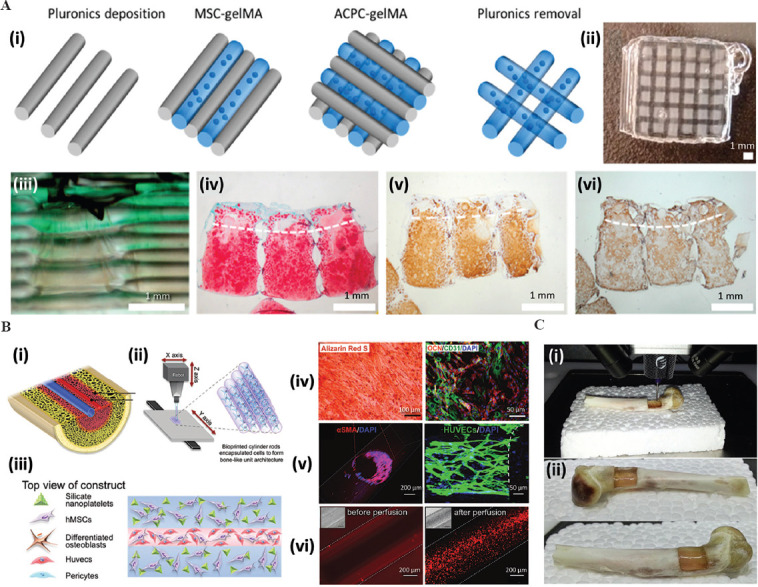
The application of bioprinting in cartilage and bone engineering. (A) Schematic representation of a (i) multilayered, multimaterial, and multi-cell-type bioprinted cartilage-like tissue; (ii) photograph of the actual construct after bioprinting; (iii) sideward view of the construct showing different composition in the layers. Histological micrographs showing the presence of (iv) glycosaminoglycans, (v) collagen type II, and (vi) collagen type 1 in matured constructs[31]. Adapted from Levato et al., with permission from Elsevier. Reprinted from Acta Biomater, 61, Levato R, Webb W R, Otto IA, et al., The bio in the ink: Cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells., 41–53, Copyright (2017), with permission from Elsevier. (B) Schematic representation of (i) a vascularized bone model, (ii) bioprinting method based on piling-up cell-laden hydrogel rods, and (iii) architectural design and composition of the bioprinted construct. Microscopy images showing the maturation of the vascularized bone model: (iv) Calcium deposition and expression of bone markers; (v) expression of endothelial markers morphology of an endothelial-like wall; (vi) perfusion through the vascular channel[37]. Reprinted (adapted) from Byambaa B, Annabi N, Yue K, et al., Adv Healthc Mater, 2020, 6(16): 1700015 with permission from Wiley. © 2017 WILEY-VCH Verlag GmbH Co. KGaA, Weinheim. (C). In situ bioprinting of bone: (i) Filling a bone defect and (ii) outcome after bioprinting. Adapted from Li et al., 2017[38]; licensed under Creative Commons Attribution 4.0 International License.
Bone tissue engineering has also greatly benefited from bioprinting[36]. Different bioprinting strategies have been explored to fabricate small vascularized bone-like fragments[37] (Figure 3B). Experimental evidence has shown that relatively large bone defects (Figure 3C) can be repaired in situ using osteoinductive bioinks and relatively portable extrusion bioprinters[38].
After bone and cartilage, vasculature bioprinting[39-41] follows closely, at 9% of the applications. This is hardly a surprise, as vasculature fabrication is critical for developing any tissue or organ larger than 400 μm in size[42,43]. Often, the rationale behind bioprinting vasculature is to provide an artificial blood-vessel network within a bioprinted construct to enable the perfusion of nutrients and gases and the removal of waste products[44].
Ultimately, the aim of bioprinted vasculature is to extend the survival and enable the proper functioning of thick bioprinted tissues[45,46]. The progress made on the front of vascularization fabrication using bioprinting has been spectacular in the last decade. Today, the fabrication of vascular networks is possible by combining permanent and fugitive inks, and several successful strategies have been well-documented in the literature[47,48]. The removal of the fugitive component yields a network of void conduits that can be endothelialized (i.e., cell-lined with endothelial cell monolayers) to develop perfusable and stable vascularization in cell-laden constructs[48] (Figure 4A). Furthermore, bioinks can be engineered with protease-degradable cross-linkers to allow cell remodeling. Therefore, the endothelial cells covering the main vascular channels may undergo angiogenic sprouting, guided by gradients of angiogenic factors (e.g., vascular endothelial growth factor [VEGF], phorbol-12-myristate-13-acetate, and sphingosine-1-phosphate) to form capillary vessels[49] (Figure 4B).
Figure 4.
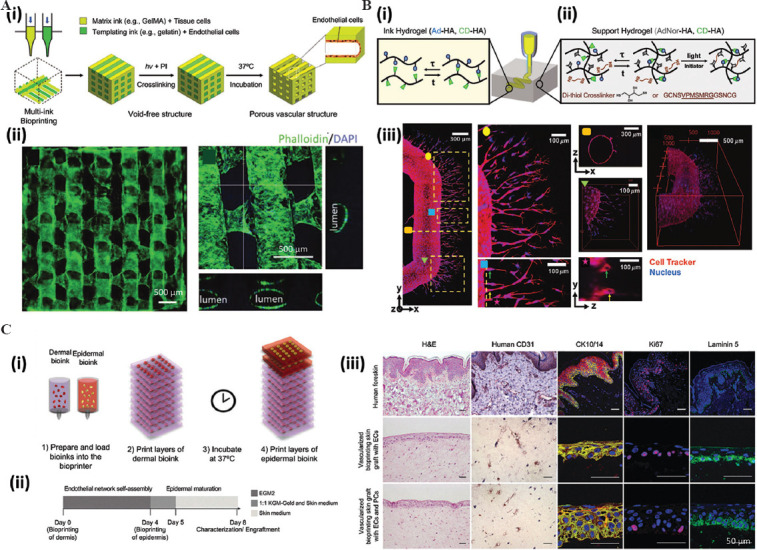
The application of bioprinting to fabricate vascularization and skin. (A) Schematic representation of the (i) bioprinting process of a multilayered vascularized construct. (ii) Fluorescence micrographs showing the endothelialization of the void spaces (created as a result of using permanent and sacrificial inks during the bioprinting process) and cross-sections showing a lumen. Adapted from Ouyang et al., 2020[48], licensed under Creative Commons Attribution 4.0 International License. (B) A strategy to promote angiogenesis based on the use of (i) a sacrificial ink that renders a channel and (ii) cell-degradable ink. (iii) Microscopy analysis showing the endothelialization of the channel and the angiogenic sprouting within the cell-degradable hydrogel. Magnifications show the presence of lumen structures (as small as ~10 μ) and the three-dimensional architecture[49]. Reprinted (adapted) from Song KH, Highley CB, Rouff A, et al., Adv Funct Mater, 2018, 28(31):1–10. At 2018 WILEY-VCH Verlag GmbH Co. KGaA, Weinheim. (C) Schematic representation of the (i) bioprinting design of a multilayered skin construct. (ii) Experimental program involving skin bioprinting, implantation, explantation, and analysis. (iii) Histological micrographs showing the multilayered architecture and dermal markers of expression in human skin (for reference), grafted bioprinted skin without placental pericytes (PCs), and grafted bioprinted skin with PCs in the dermal bioink. EC: Endothelial cells. Adapted from Baltazar et al., 2020, Tissue Engineering Part A, 26: 5-6[62]. The publisher for this copyrighted material is Mary Ann Liebert, Inc. publishers.
Skin[50,51] and muscle[52,53] bioprinting have been addressed in 4% and 3% of the total manuscripts, respectively. These tissues and organs are relatively simple in architecture and composition, and yet they present substantial challenges to the current state-of-the-art bioprinting platforms. Skin is a multi-layered tissue with different cell types accomplishing distinct tasks at each layer[54]. By contrast, muscle tissue has a fiber-like multi-scale structure[55], with cell alignment an additional characteristic that is vital for functional skeletal muscle. Some of these papers have presented simplified skin[56-58] or muscle models[59-61], with the aim of recreating only the most relevant features of these tissues.
Recent studies took steps forward and fabricated thick skin constructs that closely recapitulate the multi-layered architecture of human skin and demonstrated the importance of including endothelial cells and pericytes (in addition to keratinocytes and fibroblasts) to achieve integration and vascularization after implantation in animal models[62] (Figure 4C). Pigmented human skin has also been successfully bioprinted in proof-of-concept experiments[63]. Some outstanding challenges remain on this front; for example, the use of bioprinting to fabricate human skin with functional hair follicles remains to be demonstrated. However, the technological basis to develop skin capable of producing hair de novo is at hand[64]. Furthermore, recent reports have shown the feasibility of mimicking this multi-scale structure and alignment of muscle-like fibers using emerging bioprinting techniques[12,65-67].
In terms of social impact and clinical demands, the bioprinting of fully functional organs would solve the critical situation faced by one million patients worldwide each year, as estimated by the outage management system[68]. In the USA alone, 103,655 patients will be waiting for an organ by 2020; these include 91,790 patients in need of a kidney, 12,521 needing a liver, and 3504 needing a heart[69]. These are all highly complex organs, not only in terms of their architecture and cell type composition but also because of their size and function. Major organ fabrication through bioprinting (or through any other fabrication technique) remains a major unsolved challenge[70,71]. A recent contribution demonstrates the feasibility of 3D printing a full-size human heart made entirely of alginate (not yet including cells). To do this, the authors used Freeform Reversible Embedding of Suspended Hydrogels (FRESH), an emerging extrusion-based technique that enables the printing of practically any shape by injecting a hydrogel into a thermo-reversible support bath. However, FRESH (and any currently available bioprinting technique) has its limitation in its ability to fabricate full-size functional tissues. For example, printing this non-cellularized and non-structured heart took 4 days[71].
Kidney is the most in-demand organ, but it is also a very defiant organ to mimic due to its sophisticated physiological architecture of multiple different cell types. In addition, this organ is solid (not hollow), making the task of bioprinting an artificial kidney very challenging[72]. Not surprisingly, only 1% of the retrieved articles addressed the bioprinting of kidney tissues[73,74].
4. Bioinks for different bioprinting technologies
Scientists and engineers continue to develop bioprinting technologies with enhanced capabilities. Today, the most frequently reported bioprinting technologies are extrusion, droplet, and laser assisted bioprinting (Figure 5A). Extrusion-based bioprinting ejects the bioink by a pneumatic, piston, microfluidic, or screw mechanism to deposit filaments[75]. Droplet bioprinting deposits drops of the bioink under the control of a piezoelectric or thermal system[76]. Laser-assisted bioprinting involves a laser sensitive-substrate that contains the bioink at the bottom. Patterns of the bioink are transferred to a receiving surface underneath, assisted by laser pulses aimed onto the bioink-containing substrate[77] (Figure 5B).
Figure 5.
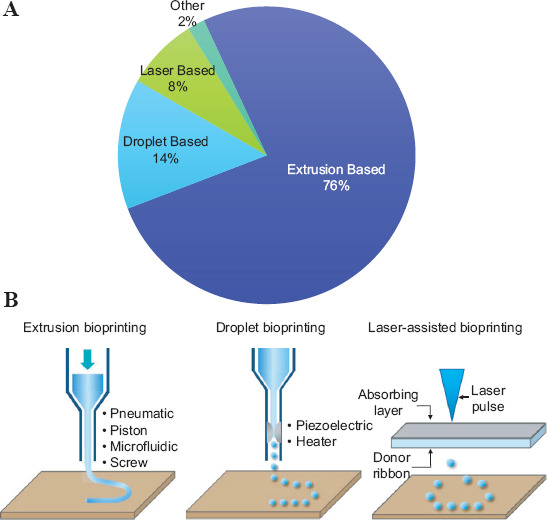
Bioprinting methods. (A) Pie chart showing the most frequently reported bioprinting technologies (B) Schematic representations showing the working principle of the three most frequently reported bioprinting methods. Adapted by permission from Springer Nature: Nature Biotechnology. Copyright 2014[6].
By far, extrusion bioprinting[75,78-80] is the simplest and most widely used form of bioprinting accounting for 76% of the reports analyzed in this study. Current trends (Figure 5) revealed that the major factors driving the selection of bioprinting technologies are cost and ease of use, which are, in fact, the main strengths of extrusion bioprinting. Droplet-based bioprinting[76,81-83] follows with a presence of 14%, and papers related to laser-assisted bioprinting[77] account for 8%. However, in terms of printing velocity, extrusion bioprinting is arguably the less attractive option among these three technologies.
The maximum reported printing speeds are ~150 mm/s for extrusion bioprinting, ~200 mm/s for inkjet bioprinting, and ~20 mm/s–~500 mm/s for laser-based bioprinting[84]. Droplet and laser-assisted bioprinting technologies[85-87] also show higher resolution and precision capabilities than the extrusion bioprinting[88]. However, the cost and required expertise for operation may be preventing the widespread use of droplet and laser-based methods[89,90].
Regardless of the method, the operation of any of these bioprinting platforms needs bioinks with a particular set of properties. Table 1 lists the important parameters for bioinks associated with each bioprinting technology. For all three techniques, the rheological properties of the bioinks are fundamental in determining the success of the bioprinting process. Several factors such as the cell density, cell type, the chemical nature of the hydrogel, and the hydrogel concentration, will directly influence the rheological properties of a bioink[91-94]. Other specific parameters must also be considered when designing a bioink for a particular bioprinting method; for instance, extrusion bioprinting needs shear-thinning materials (materials that behave as liquids while being extruded through a nozzle and then render solid-like and non-collapsing filaments when deposited on the printing bed)[95,96].
Table 1.
Key parameters and requirements for the most frequently used bioprinting technologies
Droplet bioprinting benefits from bioinks with low viscosities and low cell densities. These characteristics favor the deposition of small droplets and, consequently, high-resolution architectures. Surface tension is another important parameter to consider when designing a bioink for droplet bioprinting, as the gelation should occur after material deposition to avoid nozzle clogging[97]. By contrast, bioinks designed for laser bioprinting should give consideration on viscosity[99], cell density, and interaction with the receiving substrate (i.e., the surface tension and wettability).
Extrusion bioprinting requires several other innovations. Many of these are related to the ability to co-extrude two or more different materials concurrently (multimaterial bioprinting)[78,100-102]. For instance, these materials may contain different types of cells or different hydrogels to recapitulate the composition and architecture of a native tissue. In this context, a significant challenge is the selection/design of a compatible set of bioinks in terms of rheology, interfacial tension, and co-flowability, among other properties[103].
Novel embodiments of extrusion bioprinting have greatly pushed the limits of biofabrication in the last 5 years. For example, innovative bioprinting heads that use chaotic static mixers[102] to fabricate microstructured hydrogel filaments are enabling new applications, such as the facile fabrication of muscle-like fibers[12]. Extrusion-based bioprinters are also becoming more portable, and hand-held models promise to enable in situ and in vivo bioprinting for a wide range of scenarios, including wound healing, dentistry, and minor and major surgeries.[104-108]
Emergent bioprinting technologies have been powerful drivers of innovation in 3D biomanufacturing. For example, vat polymerization-based bioprinting brings unprecedented precision, and therefore resolution, to the bioprinting arena[77]. This enhanced resolution will push the limits of tissue engineering and create the need for development of new cell-friendly photoinitiators, novel materials, and additives for vat-bioprinting applications. Volumetric bioprinting, a recently developed 3D printing strategy inspired by optical tomography, is based on a programmed projection of 2D-planes of light in a 3D volume. This disruptive bioprinting strategy enables the fabrication of relatively large free-form constructs using photo-cross-linkable hydrogels[109].
As bioprinting technology evolves and matures, we expect to observe a more ad hoc selection of the bioprinting method based on specific clinical needs.
4.1. Most frequently used cells in bioinks
Arguably, living cells constitute the most important component of a bioink. Indeed, cells are a mandatory element if a bioink is to be considered as such[110]. The origin/source of the cells used to prepare a bioink is very important; for example, in implantable tissues, the selection of the cell type may determine the acceptance/rejection of the bioprinted construct by the recipient[6,111].
One emerging research line (the papers was not included in this scientometric analysis) illustrates the significant advances in the cell source-related technologies aimed at generating a safe and effective cell source for clinical use[112-114]. Nevertheless, the vast majority of the bioprinting studies conducted today use cells to develop proof-of-concept tissue constructs rather than functional tissues for transplantation[111,115]. As a general rule, the bioink design and the bioprinting conditions for tissue constructs, whether for transplantation or for ex-vivo applications, should favor cell viability for extended periods, cell proliferation, and the capability to develop into mature tissues (assessed, e.g., by protein expression and immunohistochemistry)[116-118]. Within this framework, cells can be purchased (i.e., from the American Type Culture Collection or other cell culture companies[56,119] or harvested from primary tissues[120,121]).
Figure 6 presents the most reported cell types used in bioink formulations. At first sight, no direct correlation is observed between the applications (Figure 2) and the cells used (Figure 6). Notably, the utilization of cells usually serves one of two purposes: Biofabrication of a specific tissue or organ, or assessment of the performance of a bioink material or a bioprinting technology (technology development). Stem cells (SCs) and induced pluripotent SC (iPSCs)[122,123] comprise the main group of cells used in bioinks. SCs are attractive candidates for bioprinting studies since they possess the ability to differentiate into different cell lineages when cultured using ad hoc inducing conditions.[117,124] Interestingly, the origin of the SCs and iPSCs varied significantly within the set of analyzed articles (Figure 6)[125-127]. The combination of bioprinting techniques and the use of SCs may, at least in concept, provide tissue engineers with great flexibility to fabricate any tissue.
Figure 6.
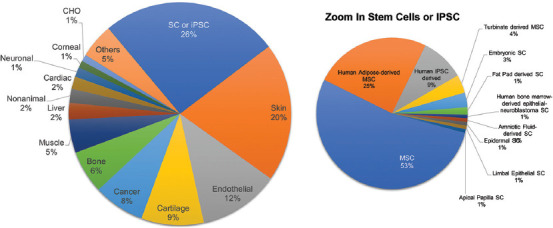
The most frequently reported cells used in bioinks.
The second most important cell type in our analysis corresponds to skin cells, accounting for 20% of the papers[128,129]. Counterintuitively, in terms of the frequency of applications, skin bioprinting only accounts for 4% of the papers (Figure 2). The extended use of skin cells (mostly fibroblasts) reflects that they are the most popular cellular model for assessing bioink formulations and bioprinting techniques[130,131].
Similarly, cancer cells are also frequently employed as models in evaluating technological bioprinting innovations[132,133] in addition to the bioprinting of in vitro 3D cancer models[79,134,135]. Any commercial cell lines that are current gold standard models for several tissues originated from cancerous tissues (i.e., the 3T3, BJ, C2C12, MCF7, and MCF10A cell lines)[136]. The other mammalian cell type presented in Figure 6 (~50%) are directly related with the tissue intended to bioprint. In the near future, we anticipate convergences between mature bioprinting technologies and the technologies required to generate safe and effective cell sources for clinical use.
Non-animal cells have also been used in bioink formulations. Bacterial bioprinting is an emerging field that is gaining momentum[137] and will enable exciting applications in the coming years. Recent papers illustrate the use of bioprinting techniques for the fabrication of bacterial biofilms with different functionalities[138-141] or the re-creation of complex bacterial communities[66,102,142].
Microalgae[143,144] have also had representation within the bioprinting literature. Recent experimental evidence shows that the symbiotic coexistence of microalgae and mammalian cells in thick bioprinted tissue constructs is a feasible alternative for enabling a sustainable supply of oxygen within the constructs.
4.2. Hydrogel formulations used in bioinks
Bioinks may consist solely of cells[94,145,146]. However, 13 of the analyzed documents used cell aggregates or cell spheroids as bioinks. Most bioinks are cell-laden hydrogels and the hydrogel matrix has a starring role in the functionality of the bioink which contributes to the success of the bioprinting technology. Hydrogels must provide the right environment for living cells while still exhibiting the physicochemical properties (i.e., rheology, stability, molecular integrity) needed to facilitate their processability or manipulation[147]. We identified 156 different hydrogels and 48 cross-linking methods within the analyzed literature. Figure 7 presents the top 10 most frequently reported hydrogels and cross-linking methods used for bioink formulations.
Figure 7.

The top 10 most frequently reported (A) hydrogels and (B) cross-linking methods for bioink formulations. Colored connectors relate hydrogels with their respective cross-linking methods.
The most popular combination used in bioink formulations is alginate (a carbohydrate extracted from brown algae) and its preferred cross-linking agent (calcium chloride; CaCl2)[148]. Alginate[149-151] is an anionic carbohydrate-based polymer that cross-links efficiently (easily and rapidly) in environments rich in divalent cations such as aqueous solutions of CaCl2 or calcium sulfate (CaSO4).[152] The ease and speed of this type of cross-linking make alginate a convenient working matrix.
Gelatin methacryloyl (GelMA)[120,153,154] comes in second place, followed by gelatin and collagen in third and fourth place, respectively. These polymers have a common origin, namely collagen from animal tissues including mammals[155,156], fish[157], and poultry[158]. As a group, they are the most prevalent type of hydrogels used in bioprinting applications. GelMA and gelatin are simpler versions of collagen, and both are friendlier materials to process and handle than collagen when formulating bioinks. They are also less costly; for instance, a gram of collagen from Sigma-Aldrich costs US$ 2360, while a gram of GelMA and gelatin costs US$ 206 and US$ 0.342, respectively. GelMA is a chemically modified gelatin that cross-links upon exposure to ultraviolet or visible light in the presence of a suitable photoinitiator, such as Irgacure[153,159], lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP)[160,161], or eosin Y[162,163], the end result is a solid hydrogel held together by covalent bonds[154]. In fact, Irgacure, LAP, and eosin Y appear in the top 10 most used cross-linking methods.
Gelatin[164], in contrast, forms physical hydrogels in response to low temperatures[165]. In this case, weak intermolecular forces between the gelatin chains, rather than covalent cross-links, hold the hydrogel together. Indeed, temperature occupies the third place in Figure 7B as a “cross-linking method” (in this case, no covalent or ionic bonds are involved, only weak molecular interactions).
A technological advantage of using GelMA instead of gelatin is that, while gelatin melts under incubation conditions (37°C), a photo-cross-linked GelMA construct remains stable[166].
However, neither gelatin nor GelMA conserves the tertiary and quaternary structure of collagen, which may represent a disadvantage for many applications. However, they both preserve the arginine-glycine-aspartate (RGD) domains (arginine, glycine, and aspartate) required for cell anchoring, which is a central attribute when designing cell scaffolds for tissue engineering[166], as promoting cell anchoring to the bioink matrix is a crucial step for developing artificial living tissues. This is not a particular functional characteristic of pristine alginate hydrogels, and this establishes the main difference between the two most popularly used hydrogels for bioinks. Arguably, the key reasons for using alginate, GelMA, and gelatin are practicality, availability, and cost-effectiveness.
Similarly, practicality, availability, and low cost lead to the frequent use of poly(ethylene glycol) diacrylate (PEGDA)[167] (a photo-cross-linkable synthetic polymer)[168] and agarose (a thermo-sensitive natural carbohydrate)[169] in bioink formulations. PEGDA and agarose also appear in the top-ten list of printable hydrogels. PEGDA hydrogels are amenable to chemical functionalization, making them very versatile materials for bioinks[168]. The synthetic nature of PEGDA also makes it very reliable in terms of batch-to-batch consistency, and it is an easily tunable material.
Fibrinogen is a protein amenable to enzymatic cross-linking (using thrombin) that renders a stable fibrin matrix[113,170]. This combination (matrix and cross-linker) contains cell-anchoring motifs similar to those provided by gelatin, collagen, and GelMA, making fibrinogen suitable for tissue engineering applications. Like GelMA, the high commercial cost of fibrinogen (~$205 USD/g) may limit its broad use[171,172]. Fibrinogen also has poor rheological properties and needs to be combined with shear-thinning materials when formulating extrusion bioinks[172].
Silk fibroin, an insect-produced protein, also holds a spot among the top 10 most used hydrogels for formulating bioinks. This is perhaps the most innovative material within this list[173,174]. Among its attributes, biocompatibility, strength, and rheological and mechanical tunability have made this material an attractive choice for bioinks.[175]
Hyaluronic acid methacrylate[176,177], a photo-cross-linkable version of hyaluronic acid, also holds a place in the top-ten list. This is not a surprise as hyaluronic acid is a major component of the extracellular matrix of cartilage and bone, the most bioprinted tissues today[177,178].
The use of enzymes, such as the microbial transglutaminase (MTGase)[179], tyrosinase[128], and genipin, are also among the top ten list of cross-linking methods. These enzymes are used to cross-link protein-based hydrogels. This analysis reveals that, when formulating a bioink, the most important criteria are related to the ease of use (practicality), cost, availability, and basic biological functionality (i.e., mainly the presence of cell-anchoring motifs).
The recent incorporation of recombinant proteins[18,180] into the portfolio of materials for bioink preparation will be a powerful enabler for customized and “smart” bioink engineering (at the molecular level).
4.3. Composite hydrogels in bioinks
Figure 7 presents the most frequently reported composite hydrogels used in bioink formulations: Hydrogel blends and hydrogels enhanced with functional additives. From the pool of 393 articles analyzed, 263 mention the use of composite or hybrid bioinks. We identified 102 materials combined in different ways in bioink formulations. A total of 213 documents reported the use of hydrogel blends (i.e., hybrid matrices containing more than one hydrogel) (Figure 8A), while 54 reported the use of functional additives (other than hydrogels) within the bioinks (Figure 8B). From the group of hydrogel blends, 170 of the 213 (80%) were composed of two hydrogels[181,182], 36 (17%) of three hydrogels[183,184], and 7 (3%) of four hydrogels[185,186] (Figure 8).
Figure 8.
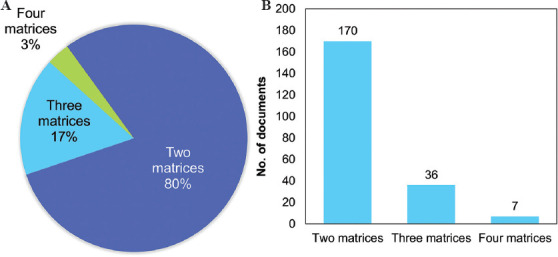
The most frequently reported hydrogel blends. (A) Distribution and (B) number of documents of hydrogel blends according to the number of matrices used.
Figure 9 shows the correlation maps corresponding to all hydrogel blends (Figure 9A), and 2–4 hydrogel blends (Figures 9B-D). Table 2 lists the most frequently used hydrogel blends, categorized by the number of matrices involved. Not surprisingly, the main components in all the correlation maps are alginate and GelMA, which is consistent with the top ten hydrogels reported in Figure 7A. Evident reasons for combining these two hydrogels are the facile and rapid cross-linking of alginate and the presence of cell-anchoring motifs in GelMA. GelMA hydrogels are somewhat recalcitrant to cross-linking immediately after extrusion from the bioprinting nozzle, and the use of alginate easily overcomes this challenge. Very often, alginate is used as a temporary template and GelMA as the permanent cell scaffolding.[187–190] A filament of an alginate-GelMA blend can be ionically cross-linked immediately after extrusion to preserve its 3D shape. GelMA can then be covalently cross-linked by exposed to light. Finally, the alginate matrix can be removed using a Ca2+-chelating agent, such as EDTA.[133] This methodology and minor variations of it were reported in 22 of the 393 analyzed manuscripts.
Figure 9.
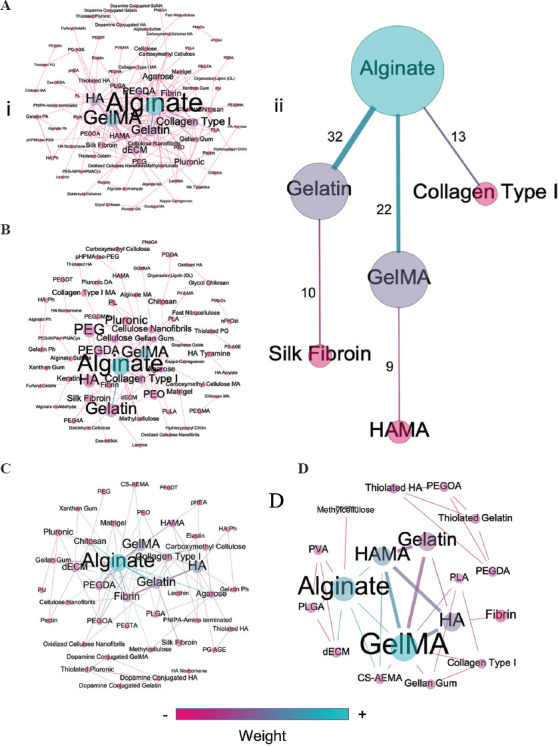
Correlation maps showing the interconnections of hydrogels for bioink formulations in (A) (i) all hydrogel blends, (ii) most frequent interconnections in all hydrogel blends; (B) two-hydrogel blends, (C) three-hydrogel blends, and (D) four-hydrogel blends.
Table 2.
The most frequently used hydrogel blends categorized by the number of matrices involved
| Ranking | Two hydrogels | Three hydrogels | Four hydrogels |
|---|---|---|---|
| 1° | Alginate Gelatin |
Fibrin Gelatin HA |
Gelatin GelMA HA HAMA |
| 2° | Alginate GelMA |
Alginate Chitosan Fibrin |
Alginate CS-AEMA GelMA HAMA |
| 3° | Alginate Collagen type 1 |
Alginate GelMA PEGDA |
PEGDA PEGOA Thiolated Gelatin Thiolated HA |
| 4° | Gelatin Silk Fibroin |
Alginate Carboxymethyl cellulose Collagen type 1 |
Collagen type 1 GelMA Gellan Gum PLA |
| 5° | Agarose Collagen type 1 |
Alginate Collagen type 1 Gelatin |
Alginate dECM PLGA PVA |
For the other composite hydrogels, manufacturability and biological functionality are also the main guidelines for bioink design. Other criteria are often considered, such as the use of reinforcers or sacrificial matrices that leave hollow spaces within the hydrogels to mimic vascular networks[191-193].
Bioink formulations may also include other functional additives, such as biomolecules[194,195], particles[151,196], and drugs[197,198] (Table 3). A larger version of these correlation maps is presented as supplementary file (Supplementary Figures S1-S4 (643.7KB, pdf) ). Incorporating RGD domains to promote cell adhesion is the most popular functionalization strategy in this category[199,200]. The use of growth factors[121,195], and especially VEGF[201-203], is also a frequent strategy for enhancing the biological performance of bioinks.
Table 3.
Functional additives used in bioinks
| Type of additive | Additive | Count | References |
|---|---|---|---|
| Particles | Silicate nanoparticles | 6 | [167,207,217–220] |
| Iron oxide nanoparticles | 3 | [82,151,221] | |
| Bioactive glass particles | 2 | [222,223] | |
| Other particles | 7 | [125,196,204–206,208,224] | |
| Biomolecules | RGD | 14 | [199,200,225–236] |
| VEGF | 6 | [37,201–203,217,220] | |
| BMP-2 | 5 | [125,237–240] | |
| TGF-b3 | 4 | [125,240–242] | |
| Drugs | Rifampin | 1 | [197] |
| Daptomycin | 1 | [197] | |
| Dimethyl-L-oxaloylglycine | 1 | [198] | |
| Naproxen | 1 | [243] | |
| Ibuprofen | 1 | [243] | |
| Atorvastatin | 1 | [244] | |
| Ropinirole HCl | 1 | [245] |
From the pool of articles analyzed, 18 incorporated nano- or micro-particles into their bioink formulations for different purposes (i.e., to enhance rheological properties, immobilize functional molecules, and/or provide chemical[125,204], electrical[205,206], and topological[207,208] cues). In this category, the most popular choice was silicate nanoparticles. These nanoparticles are versatile, commercially available in a wide range of sizes at a relatively low cost, cytocompatible, and amenable to functionalization with basic chemistry methods[209-216].
Bioink formulations can also be designed to function as controlled-release matrices for drugs[198,244]. From the pool analyzed, seven reports incorporated different drugs for different aims, from modeling a drug-delivery system[245] to conferring antibiotic[197] and anti-inflammatory[243] activities.
New ingredients have been recently added to the repertoire of bioink additives to provide relevant functionalities for hydrogel-based inks. Examples are the use of a flexuous filamentous plant virus[65] to enhance cell attachment and proliferation in the context of fabrication of muscle fibers; the incorporation of protease-degradable cross-linkers to enable cell remodeling[49] and oxygen-releasing agents to improve and prolong tissue viability[246]
5. Conclusions
This scientometric analysis of the last two decades of progress on the use and development of bioinks reveals some very clear trends. Most of the analyzed documents report the use of simple compositions that fulfill the basic requirements of manufacturability and indispensable biological performance (cytocompatibility and cell adhesion). This is consistent with the current stage of development of bioprinting technology. As with any emerging technology scenario, bioprinting is naturally evolving as users address challenges with increasing degrees of difficulty with the available resources. However, many of the analyzed documents already deal with the development of advanced bioinks. This is particularly evident in the papers published in the last 5 years.
In the future, we anticipate that the scientific reports will deal with a broader and even more specialized portfolio of bioprinting technologies, hydrogels, additives, and cell sources. We expect to witness an evolution in the field whereby the parameters that guide the bioink design are related to the clinical and market demands (more in line with biomedical/clinical needs instead of what we can accomplish now).
Bioprinting technology must attain a more advanced level before facing its most ambitious challenge: the printing of functional tissues and organs (i.e., kidneys, livers, brain organoids, and relevant-sized tumors) to fulfill the global transplant demands. However, at this early stage, bioprinting has already proven useful in fabricating 3D human biological models for research and development purposes. We envision that this new market of reliable biological models will trigger and amplify the development of bioprinting and advanced bioinks.
Conflicts of interest
The authors declare that they have no conflicts of interest
Acknowledgments
We thank MSc. Jessica Janelly Mancilla de la Cruz for her assistance in proofreading the manuscript.
Funding
This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT) and Tecnológico de Monterrey. S.C.P.G and B.E.P.B acknowledges funding received by CONACYT in the form of a Graduate Studies Scholarships. G.T.dS, M.M.A, and M.R.S gratefully acknowledge the Academic Scholarships provided by CONACyT as members of the National System of Researchers (Sistema Nacional de Investigadores).
References
- 1.Rodríguez-Salvador M, Rio-Belver RM, Garechana-Anacabe G. Scientometric and Patentometric Analyses to Determine the Knowledge Landscape in Innovative Technologies:The Case of 3D Bioprinting. PLoS One. 2017;12:e0180375. doi: 10.1371/journal.pone.0180375. https://doi.org/10.1371/journal.pone.0180375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodríguez-Salvador M, Villarreal-Garza D, Álvarez MM, et al. Analysis of the Knowledge Landscape of Three-dimensional Bioprinting in Latin America. Int J Bioprinting. 2019;5:16–25. doi: 10.18063/ijb.v5i2.2.240. https://doi.org/10.18063/ijb.v5i2.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng WL, Chua CK, Shen YF. Print Me An Organ!Why We Are Not There Yet. Prog Polym Sci. 2019;97:101145. https://doi.org/10.1016/j.progpolymsci.2019.101145. [Google Scholar]
- 4.Ravnic DJ, Leberfinger AN, Koduru SV, et al. Transplantation of Bioprinted Tissues and Organs:Technical and Clinical Challenges and Future Perspectives. Ann Surg. 2017;266:48–58. doi: 10.1097/SLA.0000000000002141. https://doi.org/10.1097/SLA.0000000000002141. [DOI] [PubMed] [Google Scholar]
- 5.Singh AV, Ansari MHD, Wang S, et al. The Adoption of Three-dimensional Additive Manufacturing from Biomedical Material Design to 3D Organ Printing. Appl Sci. 2019;9:811. https://doi.org/10.3390/app9040811. [Google Scholar]
- 6.Murphy SV, Atala A. 3D Bioprinting of Tissues and Organs. Nat Biotechnol. 2014;32:773–85. doi: 10.1038/nbt.2958. https://doi.org/10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 7.Fang Y, Eglen RM. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. 2017;22:456–72. doi: 10.1177/1087057117696795. https://doi.org/10.1177/10∁7117696795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.KačarevićŽ P, Rider PM, Alkildani S, et al. An Introduction to 3D Bioprinting:Possibilities, Challenges and Future Aspects. Materials (Basel) 2018;11:2199. doi: 10.3390/ma11112199. https://doi.org/10.3390/ma11112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mobaraki M, Ghaffari M, Yazdanpanah A, et al. Bioinks and Bioprinting: A Focused Review. Bioprinting. 2020;18:e00080. https://doi.org/10.1016/j.bprint.2020.e00080. [Google Scholar]
- 10.Ashammakhi N, Ahadian S, Xu C, et al. Bioinks and Bioprinting Technologies to Make Heterogeneous and Biomimetic Tissue Constructs. Mater Today Bio. 2019;1:100008. doi: 10.1016/j.mtbio.2019.100008. https://doi.org/10.1016/j.mtbio.2019.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mironov V, Kasyanov V, Markwald RR. Organ Printing:From Bioprinter to Organ Biofabrication Line. Curr Opin Biotechnol. 2011;22:667–73. doi: 10.1016/j.copbio.2011.02.006. https://doi.org/10.1016/j.copbio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Bolívar-Monsalve EJ, Ceballos-González CF, Borrayo-Montaño KI, et al. Continuous Chaotic Bioprinting of Skeletal Muscle-like Constructs. Bioprinting. 2021;21:e00125. doi: 10.1021/acsbiomaterials.0c01646. https://doi.org/10.1016/j.bprint.2020.e00125. [DOI] [PubMed] [Google Scholar]
- 13.Choudhury D, Anand S, Naing MW. The Arrival of Commercial Bioprinters-Towards 3D Bioprinting Revolution! Int J Bioprinting. 2018;4:1–20. doi: 10.18063/IJB.v4i2.139. https://doi.org/10.18063/IJB.v4i2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson WC, Boland T. Cell and Organ Printing 1:Protein and Cell Printers. Anat Rec Part A Discov Mol Cell Evol Biol. 2003;272:491–6. doi: 10.1002/ar.a.10057. https://doi.org/10.1002/ar.a.10057. [DOI] [PubMed] [Google Scholar]
- 15.Ringeisen BR, Kim H, Barron JA, et al. Laser Printing of Pluripotent Embryonal Carcinoma Cells. Tissue Eng. 2004;10:483–91. doi: 10.1089/107632704323061843. https://doi.org/10.1089/107632704323061843. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura M, Kobayashi A, Takagi F, et al. Biocompatible Inkjet Printing Technique for Designed Seeding of Individual Living Cells. Tissue Eng. 2005;11:1658–66. doi: 10.1089/ten.2005.11.1658. https://doi.org/0.1089/ten.2005.11.1658. [DOI] [PubMed] [Google Scholar]
- 17.Sun W, Starly B, Daly AC, et al. The Bioprinting Roadmap. Biofabrication. 2020;12:022002. doi: 10.1088/1758-5090/ab5158. https://doi.org/10.1088/1758-5090/ab5158. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Sani ES, Spencer AR, et al. Human-Recombinant-Elastin-Based Bioinks for 3D Bioprinting of Vascularized Soft Tissues. Adv Mater. 2020;32:1–10. doi: 10.1002/adma.202003915. https://doi.org/10.1002/adma.202003915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SH, Yeon YK, Lee JM, et al. Precisely Printable and Biocompatible Silk Fibroin Bioink for Digital Light Processing 3D Printing. Nat Commun. 2018;9:1620. doi: 10.1038/s41467-018-03759-y. https://doi.org/10.1038/s41467-018-03759-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Jin Y, Yin J, et al. Evaluation of Bioink Printability for Bioprinting Applications. Appl Phys Rev. 2018;5:041304. https://doi.org/10.1063/1.5053979. [Google Scholar]
- 21.Ruberu K, Senadeera M, Rana S, et al. Coupling Machine Learning with 3D Bioprinting to Fast Track Optimisation of Extrusion Printing. Appl Mater Today. 2021;22:100914. https://doi.org/10.1016/j.apmt.2020.100914. [Google Scholar]
- 22.Gu Y, Zhang L, Du X, et al. 2018 Reversible Physical Crosslinking Strategy with Optimal Temperature for 3D Bioprinting of Human Chondrocyte-laden Gelatin Methacryloyl Bioink. J Biomater Appl. 33:609–18. doi: 10.1177/0885328218805864. https://doi.org/10.1177/0885328218805864. [DOI] [PubMed] [Google Scholar]
- 23.Koo Y, Choi EJ, Lee J, et al. 3D Printed Cell-laden Collagen and Hybrid Scaffolds for In Vivo Articular Cartilage Tissue Regeneration. J Ind Eng Chem. 2018;66:343–55. http://doi.org/10.1016/j.jiec.2018.05.049. [Google Scholar]
- 24.Yang X, Lu Z, Wu H, et al. Collagen-alginate as Bioink for Three-Dimensional (3D) Cell Printing Based Cartilage Tissue Engineering. Mater Sci Eng C. 2018;83:195–201. doi: 10.1016/j.msec.2017.09.002. http://doi.org/10.1016/j.msec.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Shim JH, Lee JS, Kim JY, et al. Bioprinting of a Mechanically Enhanced Three-dimensional Dual Cell-laden Construct for Osteochondral Tissue Engineering Using a Multi-head Tissue/Organ Building System. J Micromech Microeng. 2012;22:85014. https://doi.org/10.1088/0960-1317/22/8/085014. [Google Scholar]
- 26.Lee JY, Koo Y, Kim G. Innovative Cryopreservation Process Using a Modified Core/Shell Cell-Printing with a Microfluidic System for Cell-Laden Scaffolds. ACS Appl Mater Interfaces. 2018;10:9257–68. doi: 10.1021/acsami.7b18360. https://doi.org/10.1021/acsami.7b18360. [DOI] [PubMed] [Google Scholar]
- 27.Kim YB, Lee H, Kim GH. Strategy to Achieve Highly Porous/Biocompatible Macroscale Cell Blocks, Using a Collagen/Genipin-bioink and an Optimal 3D Printing Process. ACS Appl Mater Interfaces. 2016;8:32230–40. doi: 10.1021/acsami.6b11669. https://doi.org/10.1021/acsami.6b11669. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin P, Li DJ, Auston DA, et al. Autograft, Allograft, and Bone Graft Substitutes:Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J Orthop Trauma. 2019;33:203–13. doi: 10.1097/BOT.0000000000001420. https://doi.org/10.1097/BOT.0000000000001420. [DOI] [PubMed] [Google Scholar]
- 29.Frank RM, Cotter EJ, Hannon CP, et al. Cartilage Restoration Surgery:Incidence Rates, Complications, and Trends as Reported by the American Board of Orthopaedic Surgery Part II Candidates. Arthrosc J Arthrosc Relat Surg. 2019;35:171–8. doi: 10.1016/j.arthro.2018.08.028. https://doi.org/10.1016/j.arthro.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 30.Cartilage Repair Market Size, Share. [Last accessed on 2020 Oct 20];Global Industry Report, 2025, n.d. viewed September 16, 2020. Available from: https://www.grandviewresearch.com/industry-analysis/cartilage-repair-regeneration-market .
- 31.Levato R, Webb WR, Otto IA, et al. The Bio in the Ink:Cartilage Regeneration with Bioprintable Hydrogels and Articular Cartilage-derived Progenitor Cells. Acta Biomater. 2017;61:41–53. doi: 10.1016/j.actbio.2017.08.005. https://doi.org/10.1016/j.actbio.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.You F, Eames BF, Chen X. Application of Extrusion-based Hydrogel Bioprinting for Cartilage Tissue Engineering. Int J Mol Sci. 2017;18:8–14. doi: 10.3390/ijms18071597. https://doi.org/10.3390/ijms18071597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi W, Sun M, Hu X, et al. Structurally and Functionally Optimized Silk-Fibroin-Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury In Vitro and In Vivo. Adv Mater. 2017;29:1–7. doi: 10.1002/adma.201701089. https://doi.org/10.1002/adma.201701089. [DOI] [PubMed] [Google Scholar]
- 34.Kilian D, Ahlfeld T, Akkineni AR, et al. 3D Bioprinting of Osteochondral Tissue Substitutes In Vitro-Chondrogenesis in Multi-Layered Mineralized Constructs. Sci Rep. 2020;10:1–17. doi: 10.1038/s41598-020-65050-9. https://doi.org/10.1038/s41598-020-65050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Huang H, Hao G, et al. 3D Printing Hydrogel Scaffolds with Nanohydroxyapatite Gradient to Effectively Repair Osteochondral Defects in Rats. Adv Funct Mater. 2021;31:1–13. https://doi.org/10.1002/adfm.202006697. [Google Scholar]
- 36.Ashammakhi N, Hasan A, Kaarela O, et al. Advancing Frontiers in Bone Bioprinting. Adv Healthc Mater. 2019;8:1801048. doi: 10.1002/adhm.201801048. https://doi.org/10.1002/adhm.201801048. [DOI] [PubMed] [Google Scholar]
- 37.Byambaa B, Annabi N, Yue K, et al. Bioprinted Osteogenic and Vasculogenic Patterns for Engineering 3D Bone Tissue. Adv Healthc Mater. 2017;6:1700015. doi: 10.1002/adhm.201700015. https://doi.org/10.1002/adhm.201700015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Yu F, Shi J, et al. In Situ Repair of Bone and Cartilage Defects Using 3D Scanning and 3D Printing. Sci Rep. 2017;7:1–12. doi: 10.1038/s41598-017-10060-3. https://doi.org/10.1038/s41598-017-10060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui X, Boland T. Human Microvasculature Fabrication Using Thermal Inkjet Printing Technology. Biomaterials. 2009;30:6221–7. doi: 10.1016/j.biomaterials.2009.07.056. http://doi.org/10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 40.Stratesteffen H, Köpf M, Kreimendahl F, et al. GelMA-collagen Blends Enable Drop-on-demand 3D Printablility and Promote Angiogenesis. Biofabrication. 2017;9:45002. doi: 10.1088/1758-5090/aa857c. https://doi.org/10.1088/1758-5090/aa857c. [DOI] [PubMed] [Google Scholar]
- 41.Jia W, Gungor-Ozkerim PS, Zhang YS, et al. Direct 3D Bioprinting of Perfusable Vascular Constructs Using a Blend Bioink. Biomaterials. 2016;106:58–68. doi: 10.1016/j.biomaterials.2016.07.038. https://doi.org/10.1016/j.biomaterials.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rouwkema J, Khademhosseini A. Vascularization and Angiogenesis in Tissue Engineering:Beyond Creating Static Networks. Trends Biotechnol. 2016;34:733–45. doi: 10.1016/j.tibtech.2016.03.002. https://doi.org/10.1016/j.tibtech.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Auger FA, Gibot L, Lacroix D. The Pivotal Role of Vascularization in Tissue Engineering. Annu Rev Biomed Eng. 2013;15:177–200. doi: 10.1146/annurev-bioeng-071812-152428. https://doi.org/10.1146/annurev-bioeng-071812-152428. [DOI] [PubMed] [Google Scholar]
- 44.Suntornnond R, Tan EYS, An J, et al. A Highly Printable and Biocompatible Hydrogel Composite for Direct Printing of Soft and Perfusable Vasculature-Like Structures. Sci Rep. 2017;7:1–11. doi: 10.1038/s41598-017-17198-0. https://doi.org/10.1038/s41598-017-17198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomasina C, Bodet T, Mota C, et al. Bioprinting Vasculature:Materials, Cells and Emergent Techniques. Materials (Basel) 2019;12:2701. doi: 10.3390/ma12172701. https://doi.org/10.3390/ma12172701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miri AK, Khalilpour A, Cecen B, et al. Multiscale Bioprinting of Vascularized Models. Biomaterials. 2019;198:204–16. doi: 10.1016/j.biomaterials.2018.08.006. https://doi.org/10.1016/j.biomaterials.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas A, Orellano I, Lam T, et al. Vascular Bioprinting with Enzymatically Degradable Bioinks Via Multi-material Projection-based Stereolithography. Acta Biomater. 2020;117:121–32. doi: 10.1016/j.actbio.2020.09.033. https://doi.org/10.1016/j.actbio.2020.09.033. [DOI] [PubMed] [Google Scholar]
- 48.Ouyang L, Armstrong JPK, Chen Q, et al. 2020,Void-Free 3D Bioprinting for In Situ Endothelialization and Microfluidic Perfusion. Adv Funct Mater. 30:1908349. doi: 10.1002/adfm.201908349. https://doi.org/10.1002/adfm.201908349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song KH, Highley CB, Rouff A, et al. Complex 3D-Printed Microchannels within Cell-Degradable Hydrogels. Adv Funct Mater. 2018;28:1–10. https://doi.org/10.1002/adfm.201801331. [Google Scholar]
- 50.Hakimi N, Cheng R, Leng L, et al. Handheld Skin Printer: In Situ Formation of Planar Biomaterials and Tissues. Lab Chip. 2018;18:1440–51. doi: 10.1039/c7lc01236e. https://doi.org/10.1039/C7LC01236E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shamloo A, Aghababaie Z, Afjoul H, et al. Fabrication and Evaluation of Chitosan/Gelatin/PVA Hydrogel Incorporating Honey for Wound Healing Applications:An In Vitro, In Vivo Study. Int J Pharm. 2021;592:120068. doi: 10.1016/j.ijpharm.2020.120068. https://doi.org/10.1016/j.ijpharm.2020.120068. [DOI] [PubMed] [Google Scholar]
- 52.Yeo M, Kim G. Three-Dimensional Microfibrous Bundle Structure Fabricated Using an Electric Field-Assisted/Cell Printing Process for Muscle Tissue Regeneration. ACS Biomater Sci Eng. 2018;4:728–38. doi: 10.1021/acsbiomaterials.7b00983. https://doi.org/10.1021/acsbiomaterials.7b00983. [DOI] [PubMed] [Google Scholar]
- 53.Merceron TK, Burt M, Seol YJ, et al. A 3D Bioprinted Complex Structure for Engineering the Muscle-tendon Unit. Biofabrication. 2015;7:35003. doi: 10.1088/1758-5090/7/3/035003. https://doi.org/10.1088/1758-5090/7/3/035003. [DOI] [PubMed] [Google Scholar]
- 54.Venus M, Waterman J, McNab I. Basic Physiology of the Skin. Surgery. 2010;28:469–72. https://doi.org/10.1016/j.mpsur.2010.07.011. [Google Scholar]
- 55.García-Lizarribar A, Fernández-Garibay X, Velasco-Mallorquí F, et al. Composite Biomaterials as Long-Lasting Scaffolds for 3D Bioprinting of Highly Aligned Muscle Tissue. Macromol Biosci. 2018;18:1800167. doi: 10.1002/mabi.201800167. http://doi.org/10.1002/mabi.201800167. [DOI] [PubMed] [Google Scholar]
- 56.Kim BS, Kwon YW, Kong JS, et al. 3D Cell Printing of In Vitro Stabilized Skin Model and In Vivo Pre-vascularized Skin Patch Using Tissue-specific Extracellular Matrix Bioink:A Step towards Advanced Skin Tissue Engineering. Biomaterials. 2018;168:38–53. doi: 10.1016/j.biomaterials.2018.03.040. https://doi.org/10.1016/j.biomaterials.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 57.Skardal A, Murphy SV, Crowell K, et al. A Tunable Hydrogel System for Long-term Release of Cell-secreted Cytokines and Bioprinted In Situ Wound Cell Delivery. J Biomed Mater Res Part B Appl Biomater. 2017;105:1986–2000. doi: 10.1002/jbm.b.33736. https://doi.org/10.1002/jbm.b.33736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jorgensen AM, Varkey M, Gorkun A, et al. Bioprinted Skin Recapitulates Normal Collagen Remodeling in Full-Thickness Wounds. Tissue Eng Part A. 2020;26:512–26. doi: 10.1089/ten.tea.2019.0319. https://doi.org/10.1089/ten.tea.2019.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen S, Nakamoto T, Kawazoe N, et al. Engineering Multi-layered Skeletal Muscle Tissue by Using 3D Microgrooved Collagen Scaffolds. Biomaterials. 2015;73:23–31. doi: 10.1016/j.biomaterials.2015.09.010. https://doi.org/10.1016/j.biomaterials.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 60.Mozetic P, Giannitelli SM, Gori M, et al. Engineering Muscle Cell Alignment through 3D Bioprinting. J Biomed Mater Res Part A. 2017;105:2582–8. doi: 10.1002/jbm.a.36117. https://doi.org/10.1002/jbm.a.36117. [DOI] [PubMed] [Google Scholar]
- 61.Kim JH, Kim I, Seol YJ, et al. Neural Cell Integration into 3D Bioprinted Skeletal Muscle Constructs Accelerates Restoration of Muscle Function. Nat Commun. 2020;11:1–12. doi: 10.1038/s41467-020-14930-9. https://doi.org/10.1038/s41467-020-14930-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baltazar T, Merola J, Catarino C, et al. Three Dimensional Bioprinting of a Vascularized and Perfusable Skin Graft Using Human Keratinocytes, Fibroblasts, Pericytes, and Endothelial Cells. Tissue Eng Part A. 2020;26:227–38. doi: 10.1089/ten.tea.2019.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ng WL, Qi JTZ, Yeong WY, et al. Proof-of-concept:3D Bioprinting of Pigmented Human Skin Constructs. Biofabrication. 2018;10:25005. doi: 10.1088/1758-5090/aa9e1e. https://doi.org/10.1088/1758-5090/aa9e1e. [DOI] [PubMed] [Google Scholar]
- 64.Kageyama T, Yan L, Shimizu A, et al. Preparation of Hair Beads and Hair Follicle Germs for Regenerative Medicine. Biomaterials. 2019;212:55–63. doi: 10.1016/j.biomaterials.2019.05.003. https://doi.org/10.1016/j.biomaterials.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 65.Frías-Sánchez A, Quevedo-Moreno D, Samandari M, et al. Biofabrication of Muscle Fibers Enhanced with Plant Viral Nanoparticles Using Surface Chaotic Flows. Biofabrication. 2021;2021:abd9d7. doi: 10.1088/1758-5090/abd9d7. https://doi.org/10.1088/1758-5090/abd9d7. [DOI] [PubMed] [Google Scholar]
- 66.Ceballos-González CF, Bolívar-Monsalve EJ, Quevedo-Moreno DA, et al. Micro-biogeography Greatly Matters for Competition:Continuous Chaotic Bioprinting of Spatially-controlled Bacterial Microcosms. BioRxiv. 2020;2020:199307. https://doi.org/10.1101/2020.07.12.199307. [Google Scholar]
- 67.Kim WJ, Lee H, Lee JU, et al. Efficient Myotube Formation in 3D Bioprinted Tissue Construct by Biochemical and Topographical Cues. Biomaterials. 2020;230:119632. doi: 10.1016/j.biomaterials.2019.119632. https://doi.org/10.1016/j.biomaterials.2019.119632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Organizacion Nacional de Transplantes. [Last accessed on 2020 Oct 01];El Registro Mundial de Trasplantes cifra en 139.024 los Trasplantes Realizados en el Mundo en el Último año, con un Aumento del 2,3%, viewed October 1, 2020. 2019 Available from: http://www.ont.es/prensa/NotasDePrensa/28%2008%202019%20%20REGISTRO%20MUNDIAL%20DE%20TRASPLANTES.pdf .
- 69.Organ Procurement and Transplantation Network. National Data, viewed October 5, 2020. 2020 Available from: https://www.optn.transplant.hrsa.gov/data/view-data-reports/national-data/# .
- 70.Murphy SV, De Coppi P, Atala A. Opportunities and Challenges of Translational 3D Bioprinting. Nat Biomed Eng. 2020;4:370–80. doi: 10.1038/s41551-019-0471-7. https://doi.org/10.1038/s41551-019-0471-7. [DOI] [PubMed] [Google Scholar]
- 71.Mirdamadi E, Tashman JW, Shiwarski DJ, et al. FRESH 3D Bioprinting a Full-Size Model of the Human Heart. ACS Biomater Sci Eng. 2020;6:6453–9. doi: 10.1021/acsbiomaterials.0c01133. https://doi.org/10.1021/acsbiomaterials.0c01133. [DOI] [PubMed] [Google Scholar]
- 72.Turunen S, Kaisto S, Skovorodkin I, et al. 3D Bioprinting of the Kidney Hype or Hope? AIMS Cell Tissue Eng. 2018;2:119–62. https://doi.org/10.3934/celltissue.2018.3.119. [Google Scholar]
- 73.Ali M, Kumar A Pr, Yoo JJ, et al. A Photo-Crosslinkable Kidney ECM-Derived Bioink Accelerates Renal Tissue Formation. Adv Healthc Mater. 2019;8:1800992. doi: 10.1002/adhm.201800992. http://doi.org/10.1002/adhm.201800992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Irvine SA, Agrawal A, Lee BH, et al. Printing Cell-laden Gelatin Constructs by Free-form Fabrication and Enzymatic Protein Crosslinking. Biomed Microdevices. 2015;17:16. doi: 10.1007/s10544-014-9915-8. https://doi.org/10.1007/s10544-014-9915-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ozbolat IT, Hospodiuk M. Current Advances and Future Perspectives in Extrusion-based Bioprinting. Biomaterials. 2016;76:321–43. doi: 10.1016/j.biomaterials.2015.10.076. https://doi.org/10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 76.Gudapati H, Dey M, Ozbolat I. A Comprehensive Review on Droplet-based Bioprinting:Past, Present and Future. Biomaterials. 2016;102:20–42. doi: 10.1016/j.biomaterials.2016.06.012. https://doi.org/10.1016/j.biomaterials.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 77.Ng WL, Lee JM, Zhou M, et al. Vat Polymerization-based Bioprinting-Process, Materials, Applications and Regulatory Challenges. Biofabrication. 2020;12:22001. doi: 10.1088/1758-5090/ab6034. https://doi.org/10.1088/1758-5090/ab6034. [DOI] [PubMed] [Google Scholar]
- 78.Liu W, Zhang YS, Heinrich MA, et al. Rapid Continuous Multimaterial Extrusion Bioprinting. Adv Mater. 2017;29:1–8. doi: 10.1002/adma.201604630. https://doi.org/10.1002/adma.201604630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu W, Zhong Z, Hu N, et al. Coaxial Extrusion Bioprinting of 3D Microfibrous Constructs with Cell-favorable Gelatin Methacryloyl Microenvironments. Biofabrication. 2018;10:024102. doi: 10.1088/1758-5090/aa9d44. https://doi.org/10.1088/1758-5090/aa9d44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yeo M, Ha J, Lee H, et al. Fabrication of hASCs-Laden Structures Using Extrusion-based Cell Printing Supplemented with an Electric Field. Acta Biomater. 2016;38:33–43. doi: 10.1016/j.actbio.2016.04.017. http://doi.org/10.1016/j.actbio.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 81.Campos DFD, Philip MA, Gürzing S, et al. Synchronized Dual Bioprinting of Bioinks and Biomaterial Inks as a Translational Strategy for Cartilage Tissue Engineering. 3D Print Addit Manuf. 2019;6:63–71. https://doi.org/10.1089/3dp.2018.0123. [Google Scholar]
- 82.Betsch M, Cristian C, Lin YY, et al. Incorporating 4D into Bioprinting:Real-Time Magnetically Directed Collagen Fiber Alignment for Generating Complex Multilayered Tissues. Adv Healthc Mater. 2018;7:1800894. doi: 10.1002/adhm.201800894. https://doi.org/10.1002/adhm.201800894. [DOI] [PubMed] [Google Scholar]
- 83.Köpf M, Campos DFD, Blaeser A, et al. A Tailored Three-dimensionally Printable Agarose-collagen Blend Allows Encapsulation, Spreading, and Attachment of Human Umbilical Artery Smooth Muscle Cells. Biofabrication. 2016;8:25011. doi: 10.1088/1758-5090/8/2/025011. https://doi.org/10.1088/1758-5090/8/2/025011. [DOI] [PubMed] [Google Scholar]
- 84.Tetsuka H, Shin SR. Materials and Technical Innovations in 3D Printing in Biomedical Applications. J Mater Chem B. 2020;8:2930–50. doi: 10.1039/d0tb00034e. https://doi.org/10.1039/d0tb00034e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hong J, Shin Y, Kim S, et al. Complex Tuning of Physical Properties of Hyperbranched Polyglycerol-Based Bioink for Microfabrication of Cell-Laden Hydrogels. Adv Funct Mater. 2019;29:1808750. https://doi.org/10.1002/adfm.20180∮. [Google Scholar]
- 86.Lam T, Dehne T, Krüger JP, et al. Photopolymerizable Gelatin and Hyaluronic Acid for Stereolithographic 3D Bioprinting of Tissue-engineered Cartilage. J Biomed Mater Res Part B Appl Biomater. 2019;107:2649–57. doi: 10.1002/jbm.b.34354. https://doi.org/10.1002/jbm.b.34354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jung H, Min K, Jeon H, et al. 2016,Physically Transient Distributed Feedback Laser Using Optically Activated Silk Bio-Ink. Adv Opt. 4:1738–43. https://doi.org/10.1002/adom.201600369. [Google Scholar]
- 88.Heinrich MA, Liu W, Jimenez A, et al. 3D Bioprinting:From Benches to Translational Applications. Small. 2019;15:1–47. doi: 10.1002/smll.201805510. https://doi.org/10.1002/smll.201805510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gao G, Huang Y, Schilling AF, et al. Organ Bioprinting:Are We There Yet? Adv Healthc Mater. 2018;7:1–8. doi: 10.1002/adhm.201701018. https://doi.org/10.1002/adhm.201701018. [DOI] [PubMed] [Google Scholar]
- 90.Badwaik R. 3D Printed Organs:The Future of Regenerative Medicine. J Clin Diagnostic Res. 2019;13:13256. https://doi.org/10.7860/jcdr/2019/42546.13256. [Google Scholar]
- 91.Paxton N, Smolan W, Böck T, et al. Proposal to Assess Printability of Bioinks for Extrusion-based Bioprinting and Evaluation of Rheological Properties Governing Bioprintability. Biofabrication. 2017;9:44107. doi: 10.1088/1758-5090/aa8dd8. https://doi.org/10.1088/1758-5090/aa8dd8. [DOI] [PubMed] [Google Scholar]
- 92.Ding H, Chang RC. Printability Study of Bioprinted Tubular Structures Using Liquid Hydrogel Precursors in a Support Bath. Appl Sci. 2018;8:403. https://doi.org/10.3390/app8030403. [Google Scholar]
- 93.Diamantides N, Wang L, Pruiksma T, et al. Correlating Rheological Properties and Printability of Collagen Bioinks:The Effects of Riboflavin Photocrosslinking and pH. Biofabrication. 2017;9:34102. doi: 10.1088/1758-5090/aa780f. https://doi.org/10.1088/1758-5090/aa780f. [DOI] [PubMed] [Google Scholar]
- 94.Bulanova EA, Koudan EV, Degosserie J, et al. Bioprinting of a Functional Vascularized Mouse Thyroid Gland Construct. Biofabrication. 2017;9:34105. doi: 10.1088/1758-5090/aa7fdd. https://doi.org/10.1088/1758-5090/aa7fdd. [DOI] [PubMed] [Google Scholar]
- 95.Kiyotake EA, Douglas AW, Thomas EE, et al. Development and Quantitative Characterization of the Precursor Rheology of Hyaluronic Acid Hydrogels for Bioprinting. Acta Biomater. 2019;95:176–87. doi: 10.1016/j.actbio.2019.01.041. https://doi.org/10.1016/j.actbio.2019.01.041. [DOI] [PubMed] [Google Scholar]
- 96.Zhang B, Luo Y, Ma L, et al. 3D Bioprinting:An Emerging Technology Full of Opportunities and Challenges. Biodesign Manuf. 2018;1:2–13. https://doi.org/10.1007/s42242-018-0004-3. [Google Scholar]
- 97.Hölzl K, Lin S, Tytgat L, et al. Bioink Properties Before, during and after 3D Bioprinting. Biofabrication. 2016;8:32002. doi: 10.1088/1758-5090/8/3/032002. https://doi.org/10.1088/1758-5090/8/3/032002. [DOI] [PubMed] [Google Scholar]
- 98.Donderwinkel I, Van Hest JCM, Cameron NR. Bio-inks for 3D bioprinting:Recent advances and future prospects. Polym. 2017;8(31):4451–71. https://doi.org/10.1039/c7py00826k. [Google Scholar]
- 99.Li J, Chen M, Fan X, et al. Recent Advances in Bioprinting Techniques:Approaches, Applications and Future Prospects. J Transl Med. 2016;14:1–15. doi: 10.1186/s12967-016-1028-0. https://doi.org/10.1186/s12967-016-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rocca M, Fragasso A, Liu W, et al. Embedded Multimaterial Extrusion Bioprinting. SLAS Technol. 2018;23:154–63. doi: 10.1177/2472630317742071. https://doi.org/10.1177/2472630317742071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trujillo-De Santiago G, Alvarez MM, Samandari M, et al. Chaotic Printing:Using Chaos to Fabricate Densely Packed Micro-and Nanostructures at High Resolution and Speed. Mater Horizons. 2018;5:813–22. doi: 10.1039/C8MH00344K. https://doi.org/10.1039/c8mh00344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chávez-Madero C, Díaz de León-Derby M, Samandari M, et al. Using Chaotic Advection for Facile High-throughput Fabrication of Ordered Multilayer Micro and Nanostructures:Continuous Chaotic Printing. Biofabrication. 2020;12:35023. doi: 10.1088/1758-5090/ab84cc. https://doi.org/10.1088/1758-5090/ab84cc. [DOI] [PubMed] [Google Scholar]
- 103.Gungor-Ozkerim PS, Inci I, Zhang YS, et al. Bioinks for 3D Bioprinting:an Overview. Biomater Sci. 2018;6:915–46. doi: 10.1039/c7bm00765e. https://doi.org/10.1039/c7bm00765e.Bioinks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Campos DFD, Zhang S, Kreimendahl F, et al. Hand-held Bioprinting for De Novo Vascular Formation Applicable to Dental Pulp Regeneration. Connect Tissue Res. 2020;61:205–15. doi: 10.1080/03008207.2019.1640217. https://doi.org/10.1080/03008207.2019.1640217. [DOI] [PubMed] [Google Scholar]
- 105.Di Bella C, Duchi S, O'Connell CD, et al. In Situ Handheld Three-dimensional Bioprinting for Cartilage Regeneration. J Tissue Eng Regen Med. 2018;12:611–21. doi: 10.1002/term.2476. https://doi.org/10.1002/term.2476. [DOI] [PubMed] [Google Scholar]
- 106.Singh S, Choudhury D, Yu F, et al. In Situ Bioprinting Bioprinting from Benchside to Bedside? Acta Biomater. 2020;101:14–25. doi: 10.1016/j.actbio.2019.08.045. https://doi.org/10.1016/j.actbio.2019.08.045. [DOI] [PubMed] [Google Scholar]
- 107.Albanna M, Binder KW, Murphy SV, et al. In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds. Sci Rep. 2019;9:1–15. doi: 10.1038/s41598-018-38366-w. https://doi.org/10.1038/s41598-018-38366-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Russell CS, Mostafavi A, Quint JP, et al. In Situ Printing of Adhesive Hydrogel Scaffolds for the Treatment of Skeletal Muscle Injuries. ACS Appl Bio Mater. 2020;3:1568–79. doi: 10.1021/acsabm.9b01176. https://doi.org/10.1021/acsabm.9b01176. [DOI] [PubMed] [Google Scholar]
- 109.Bernal PN, Delrot P, Loterie D, et al. Volumetric Bioprinting of Complex Living-Tissue Constructs within Seconds. Adv Mater. 2019;31:1904209. doi: 10.1002/adma.201904209. https://doi.org/10.1002/adma.201904209. [DOI] [PubMed] [Google Scholar]
- 110.Navarro J, Calderon GA, Miller JS, et al., editors. Academic Press; London: 2019. Bioinks for Three-Dimensional Printing in Regenerative Medicine; pp. 805–830. [Google Scholar]
- 111.Choi YJ, Jun YJ, Kim DY, et al. A 3D Cell Printed Muscle Construct with Tissue-derived Bioink for the Treatment of Volumetric Muscle Loss. Biomaterials. 2019;206:160–9. doi: 10.1016/j.biomaterials.2019.03.036. https://doi.org/10.1016/j.biomaterials.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 112.Chu DT, Nguyen TPT, Nguyen LBT, et al. Adipose Tissue Stem Cells for Therapy:An Update on the Progress of Isolation, Culture, Storage, and Clinical Application. J Clin Med. 2019;8:917. doi: 10.3390/jcm8070917. https://doi.org/10.3390/jcm8070917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu W, Zhang XX, Yang P, et al. Alginate-honey Bioinks with Improved Cell Responses for Applications as Bioprinted Tissue Engineered Constructs. ACS Appl Mater Interfaces. 2019;6:1–10. https://doi.org/10.1007/s40898-017-0003-8. [Google Scholar]
- 114.Zhou F, Hong Y, Liang R, et al. Rapid Printing of Bio-inspired 3D Tissue Constructs for Skin Regeneration. Biomaterials. 2020;258:120287. doi: 10.1016/j.biomaterials.2020.120287. https://doi.org/10.1016/j.biomaterials.2020.120287. [DOI] [PubMed] [Google Scholar]
- 115.Kim H, Park MN, Kim J, et al. Characterization of Cornea-specific Bioink:High Transparency, Improved In Vivo Safety. J Tissue Eng. 2019;10:2041731418823382. doi: 10.1177/2041731418823382. https://doi.org/10.1177/2041731418823382 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ouyang L, Yao R, Zhao Y, et al. Effect of Bioink Properties on Printability and Cell Viability for 3D Bioplotting of Embryonic Stem Cells. Biofabrication. 2016;8:1–12. doi: 10.1088/1758-5090/8/3/035020. https://doi.org/10.1088/1758-5090/8/3/035020. [DOI] [PubMed] [Google Scholar]
- 117.Campos DFD, Blaeser A, Korsten A, et al. The Stiffness and Structure of Three-dimensional Printed Hydrogels Direct the Differentiation of Mesenchymal Stromal Cells toward Adipogenic and Osteogenic Lineages. Tissue Eng Part A. 2015;21:740–56. doi: 10.1089/ten.TEA.2014.0231. https://doi.org/10.1089/ten.tea.2014.0231. [DOI] [PubMed] [Google Scholar]
- 118.Ma H, Zhou Q, Chang J, et al. Grape Seed-Inspired Smart Hydrogel Scaffolds for Melanoma Therapy and Wound Healing. ACS Nano. 2019;13:4302–11. doi: 10.1021/acsnano.8b09496. https://doi.org/10.1021/acsnano.8b09496. [DOI] [PubMed] [Google Scholar]
- 119.Park J, Lee SJ, Chung S, et al. Cell-laden 3D bioprinting hydrogel matrix depending on different compositions for soft tissue engineering:Characterization and evaluation. Mater Sci Eng C. 2017;71:678–84. doi: 10.1016/j.msec.2016.10.069. https://doi.org/10.1016/j.msec.2016.10.069. [DOI] [PubMed] [Google Scholar]
- 120.Zhu W, Cui H, Boualam B, et al. 3D Bioprinting Mesenchymal Stem Cell-laden Construct with Core-shell Nanospheres for Cartilage Tissue Engineering. Nanotechnology. 2018;29:185101. doi: 10.1088/1361-6528/aaafa1. https://doi.org/10.1088/1361-6528/aaafa1. [DOI] [PubMed] [Google Scholar]
- 121.Huang S, Yao B, Xie J, et al. 3D Bioprinted Extracellular Matrix Mimics Facilitate Directed Differentiation of Epithelial Progenitors for Sweat Gland Regeneration. Acta Biomater. 2016;32:170–7. doi: 10.1016/j.actbio.2015.12.039. https://doi.org/10.1016/j.actbio.2015.12.039. [DOI] [PubMed] [Google Scholar]
- 122.Gu Q, Tomaskovic-Crook E, Wallace GG, et al. 3D Bioprinting Human Induced Pluripotent Stem Cell Constructs for In Situ Cell Proliferation and Successive Multilineage Differentiation. Adv Healthc Mater. 2017;6:1700175. doi: 10.1002/adhm.201700175. https://doi.org/10.1002/adhm.201700175. [DOI] [PubMed] [Google Scholar]
- 123.Li Y, Jiang X, Li L, et al. 3D Printing Human Induced Pluripotent Stem Cells with Novel Hydroxypropyl Chitin Bioink:Scalable Expansion and Uniform Aggregation. Biofabrication. 2018;10:44101. doi: 10.1088/1758-5090/aacfc3. https://doi.org/10.1088/1758-5090/aacfc3. [DOI] [PubMed] [Google Scholar]
- 124.Choe G, Oh S, Seok JM, et al. Graphene Oxide/alginate Composites as Novel Bioinks for Three-dimensional Mesenchymal Stem Cell Printing and Bone Regeneration Applications. Nanoscale. 2019;11:23275–85. doi: 10.1039/c9nr07643c. https://doi.org/10.1039/c9nr07643c. [DOI] [PubMed] [Google Scholar]
- 125.Gonzalez-Fernandez T, Rathan S, Hobbs C, et al. Pore-forming bioinks to Enable Spatio-temporally Defined Gene Delivery in Bioprinted Tissues. J Control Release. 2019;301:13–27. doi: 10.1016/j.jconrel.2019.03.006. https://doi.org/10.1016/j.jconrel.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 126.Sodupe-Ortega E, Sanz-Garcia A, Pernia-Espinoza A, et al. Accurate Calibration in Multi-material 3D Bioprinting for Tissue Engineering. Materials (Basel) 2018;11:1–19. doi: 10.3390/ma11081402. https://doi.org/10.3390/ma11081402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu J, Li L, Suo H, et al. 3D Printing of Biomimetic Multi-layered GelMA/nHA Scaffold for Osteochondral Defect Repair. Mater Des. 2019;171:107708. https://doi.org/https://doi.org/10.1016/j.matdes.2019.107708. [Google Scholar]
- 128.Admane P, Gupta AC, Jois P, et al. 2019,Direct 3D Bioprinted Full-thickness Skin Constructs Recapitulate Regulatory Signaling Pathways and Physiology of Human Skin. Bioprinting. 15:e00051. https://doi.org/10.1016/j.bprint.2019.e00051. [Google Scholar]
- 129.Kwak H, Shin S, Lee H, et al. Formation of a Keratin Layer with Silk Fibroin-polyethylene Glycol Composite Hydrogel Fabricated by Digital Light Processing 3D Printing. J Ind Eng Chem. 2019;72:232–40. https://doi.org/10.1016/j.jiec.2018.12.023. [Google Scholar]
- 130.Pereira RF, Sousa A, Barrias CC, et al. A Single-component Hydrogel Bioink for Bioprinting of Bioengineered 3D Constructs for Dermal Tissue Engineering. Mater Horizons. 2018;5:1100–11. https://doi.org/10.1039/c8mh00525g. [Google Scholar]
- 131.Gholami P, Ahmadi-Pajouh MA, Abolftahi N, et al. Segmentation and Measurement of Chronic Wounds for Bioprinting. IEEE J Biomed Health. 2018;22:1269–77. doi: 10.1109/JBHI.2017.2743526. https://doi.org/10.1109/JBHI.2017.2743526. [DOI] [PubMed] [Google Scholar]
- 132.Chen Y, Wang Y, Yang Q, et al. A Novel Thixotropic Magnesium Phosphate-based Bioink with Excellent Printability for Application in 3D Printing. J Mater Chem B. 2018;6:4502–13. doi: 10.1039/c8tb01196f. https://doi.org/10.1039/C8TB01196F. [DOI] [PubMed] [Google Scholar]
- 133.Zhu K, Chen N, Liu X, et al. A General Strategy for Extrusion Bioprinting of Bio-Macromolecular Bioinks through Alginate-Templated Dual-Stage Crosslinking. Macromol Biosci. 2019;18:1800127. doi: 10.1002/mabi.201800127. https://doi.org/10.1002/mabi.201800127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cao X, Ashfaq R, Cheng F, et al. A Tumor-on-a-Chip System with Bioprinted Blood and Lymphatic Vessel Pair. Adv Funct Mater. 2019;29:1807173. doi: 10.1002/adfm.201807173. https://doi.org/10.1002/adfm.201807173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sánchez-Salazar MG, Álvarez MM, Trujillo-de Santiago G. Advances in 3D Bioprinting for the Biofabrication of Tumor Models. Bioprinting. 2021;21:e00120. https://doi.org/10.1016/j.bprint.2020.e00120. [Google Scholar]
- 136.Lucey BP, Nelson-Rees WA, Hutchins GM, et al. Historical Perspective Henrietta Lacks, HeLa Cells, and Cell Culture Contamination. Arch Pathol Lab Med. 2009;133:1463–7. doi: 10.5858/133.9.1463. https://doi.org/10.1043/1543-2165-133.9.1463. [DOI] [PubMed] [Google Scholar]
- 137.Saygili E, Dogan-Gurbuz AA, Yesil-Celiktas O, et al. 3D Bioprinting:A Powerful Tool to Leverage Tissue Engineering and Microbial Systems. Bioprinting. 2020;18:e00071. https://doi.org/10.1016/j.bprint.2019.e00071. [Google Scholar]
- 138.Schmieden DT, Vázquez SJB, Sangüesa H, et al. Printing of Patterned, Engineered E. coli Biofilms with a Low-Cost 3D Printer. ACS Synth Biol. 2018;7:1328–37. doi: 10.1021/acssynbio.7b00424. https://doi.org/10.1021/acssynbio.7b00424 . [DOI] [PubMed] [Google Scholar]
- 139.Ning E, Turnbull G, Clarke J, et al. 3D Bioprinting of Mature Bacterial Biofilms for Antimicrobial Resistance Drug Testing. Biofabrication. 2019;11:045018. doi: 10.1088/1758-5090/ab37a0. https://doi.org/10.1088/1758-5090/ab37a0. [DOI] [PubMed] [Google Scholar]
- 140.Huang Y, Xia A, Yang G, et al. Bioprinting Living Biofilms through Optogenetic Manipulation. ACS Synth Biol. 2018;7:1195–200. doi: 10.1021/acssynbio.8b00003. https://doi.org/10.1021/acssynbio.8b00003. [DOI] [PubMed] [Google Scholar]
- 141.Balasubramanian S, Aubin-Tam ME, Meyer AS. 3D Printing for the Fabrication of Biofilm-Based Functional Living Materials. ACS Synth Biol. 2019;8:1564–7. doi: 10.1021/acssynbio.9b00192. https://doi.org/10.1021/acssynbio.9b00192. [DOI] [PubMed] [Google Scholar]
- 142.Hynes WF, Chacón J, Segrè D, et al. Bioprinting Microbial Communities to Examine Interspecies Interactions in Time and Space. Biomed Phys Eng Express. 2018;4:055010. https://doi.org/10.1088/2057-1976/aad544. [Google Scholar]
- 143.Lode A, Krujatz F, Brüggemeier S, et al. Green Bioprinting:Fabrication of Photosynthetic Algae-laden Hydrogel Scaffolds for Biotechnological and Medical Applications. Eng Life Sci. 2015;15:177–83. https://doi.org/10.1002/elsc.201400205. [Google Scholar]
- 144.Maharjan S, Alva J, Cámara C, et al. Symbiotic Photosynthetic Oxygenation within 3D-Bioprinted Vascularized Tissues. Matter. 2021;4:217–40. doi: 10.1016/j.matt.2020.10.022. https://doi.org/10.1016/j.matt.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cui X, Dean D, Ruggeri ZM, et al. Cell Damage Evaluation of Thermal Inkjet Printed Chinese Hamster Ovary Cells. Biotechnol Bioeng. 2010;106:963–9. doi: 10.1002/bit.22762. https://doi.org/10.1002/bit.22762. [DOI] [PubMed] [Google Scholar]
- 146.Ozler SB, Bakirci E, Kucukgul C, et al. Three-dimensional Direct Cell Bioprinting for Tissue Engineering. J Biomed Mater Res Part B Appl Biomater. 2017;105:2530–44. doi: 10.1002/jbm.b.33768. https://doi.org/10.1002/jbm.b.33768. [DOI] [PubMed] [Google Scholar]
- 147.Hoffman AS. Hydrogels for Biomedical Applications. Adv Drug Deliv Rev. 2012;64:18–23. doi: 10.1016/s0169-409x(01)00239-3. https://doi.org/10.1016/j.addr.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 148.Chiew CSC, Poh PE, Pasbakhsh P, et al. Physicochemical Characterization of Halloysite/Alginate Bionanocomposite Hydrogel. Appl Clay Sci. 2014;101:444–54. https://doi.org/10.1016/j.clay.2014.09.007. [Google Scholar]
- 149.Xiong R, Zhang Z, Chai W, et al. Freeform Drop-on-demand Laser Printing of 3D Alginate and Cellular Constructs. Biofabrication. 2015;7:45011. doi: 10.1088/1758-5090/7/4/045011. https://doi.org/10.1088/1758-5090/7/4/045011. [DOI] [PubMed] [Google Scholar]
- 150.Freeman FE, Kelly DJ. Tuning Alginate Bioink Stiffness and Composition for Controlled Growth Factor Delivery and to Spatially Direct MSC Fate within Bioprinted Tissues. Sci Rep. 2017;7:17042. doi: 10.1038/s41598-017-17286-1. https://doi.org/10.1038/s41598-017-17286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jeon O, Lee YB, Hinton TJ, et al. Cryopreserved Cell-laden Alginate Microgel Bioink for 3D Bioprinting of Living Tissues. Mater Today Chem. 2019;12:61–70. doi: 10.1016/j.mtchem.2018.11.009. https://doi.org/10.1016/j.mtchem.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hiller T, Berg J, Elomaa L, et al. Generation of a 3D Liver Model Comprising Human Extracellular Matrix in an Alginate/Gelatin-Based Bioink by Extrusion Bioprinting for Infection and Transduction Studies. Int J Mol Sci. 2018;19:1–17. doi: 10.3390/ijms19103129. https://doi.org/10.3390/ijms19103129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.de Ruijter M, Ribeiro A, Dokter I, et al. Simultaneous Micropatterning of Fibrous Meshes and Bioinks for the Fabrication of Living Tissue Constructs. Adv Healthc Mater. 2019;8:e1800418. doi: 10.1002/adhm.201800418. https://doi.org/10.1002/adhm.201800418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yue K, Trujillo-de Santiago G, Alvarez MM, et al. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials. 2015;73:254–71. doi: 10.1016/j.biomaterials.2015.08.045. https://doi.org/10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee BH, Lum N, Seow LY, et al. Synthesis and Characterization of Types A and B Gelatin Methacryloyl for Bioink Applications. Materials (Basel) 2016;9:4–7. doi: 10.3390/ma9100797. https://doi.org/10.3390/ma9100797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen X, Du W, Cai Z, et al. Uniaxial Stretching of Cell-Laden Microfibers for Promoting C2C12 Myoblasts Alignment and Myofibers Formation. ACS Appl Mater Interfaces. 2020;12:2162–70. doi: 10.1021/acsami.9b22103. https://doi.org/10.1021/acsami.9b22103. [DOI] [PubMed] [Google Scholar]
- 157.Wang Z, Tian Z, Menard F, et al. Comparative Study of Gelatin Methacrylate Hydrogels from Different Sources for Biofabrication Applications. Biofabrication. 2017;9:44101. doi: 10.1088/1758-5090/aa83cf. https://doi.org/10.1088/1758-5090/aa83cf. [DOI] [PubMed] [Google Scholar]
- 158.Offengenden M, Chakrabarti S, Wu J. Chicken Collagen Hydrolysates Differentially Mediate Anti-inflammatory Activity and Type I Collagen Synthesis on Human Dermal Fibroblasts. Food Sci Hum Wellness. 2018;7:138–47. https://doi.org/10.1016/j.fshw.2018.02.002. [Google Scholar]
- 159.Lee J, Park CH, Kim CS. Microcylinder-laden Gelatin-based Bioink Engineered for 3D Bioprinting. Mater Lett. 2018;233:24–7. https://doi.org/10.1016/j.matlet.2018.08.138. [Google Scholar]
- 160.Ying GL, Jiang N, Maharjan S, et al. Aqueous Two-Phase Emulsion Bioink-Enabled 3D Bioprinting of Porous Hydrogels. Adv Mater. 2018;30:1805460. doi: 10.1002/adma.201805460. https://doi.org/10.1002/adma.201805460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yin J, Yan M, Wang Y, et al. 3D Bioprinting of Low-Concentration Cell-Laden Gelatin Methacrylate (GelMA) Bioinks with a Two-Step Cross-linking Strategy. ACS Appl Mater Interfaces. 2018;10:6849–57. doi: 10.1021/acsami.7b16059. https://doi.org/10.1021/acsami.7b16059. [DOI] [PubMed] [Google Scholar]
- 162.Na K, Shin S, Lee H, et al. Effect of Solution Viscosity on Retardation of Cell Sedimentation in DLP 3D Printing of Gelatin Methacrylate/Silk Fibroin Bioink. J Ind Eng Chem. 61:340–7. https://doi.org/10.1016/j.jiec.2017.12.032. [Google Scholar]
- 163.Wang Z, Abdulla R, Parker B, et al. A Simple and High-Resolution Stereolithography-based 3D Bioprinting System Using Visible Light Crosslinkable Bioinks. Biofabrication. 2015;7:45009. doi: 10.1088/1758-5090/7/4/045009. https://doi.org/10.1088/1758-5090/7/4/045009. [DOI] [PubMed] [Google Scholar]
- 164.Rutz AL, Hyland KE, Jakus AE, et al. A Multimaterial Bioink Method for 3D Printing Tunable, Cell-Compatible Hydrogels. Adv Mater. 2015;27:1607–14. doi: 10.1002/adma.201405076. https://doi.org/10.1002/adma.201405076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shin JH, Kang HW. The Development of Gelatin-Based Bio-Ink for Use in 3D Hybrid Bioprinting. Int J Precis Eng Manuf. 2018;19:767–71. https://doi.org/10.1007/s12541-018-0092-1. [Google Scholar]
- 166.Nichol JW, Koshy ST, Bae H, et al. Cell-laden Microengineered Gelatin Methacrylate Hydrogels. Biomaterials. 2010;31:5536–44. doi: 10.1016/j.biomaterials.2010.03.064. https://doi.org/10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Peak CW, Stein J, Gold KA, et al. Nanoengineered Colloidal Inks for 3D Bioprinting. Langmuir. 2018;34:917–25. doi: 10.1021/acs.langmuir.7b02540. https://doi.org/10.1021/acs.langmuir.7b02540. [DOI] [PubMed] [Google Scholar]
- 168.Nemir S, Hayenga HN, West JL. PEGDA Hydrogels with Patterned Elasticity:Novel Tools for the Study of Cell Response to Substrate Rigidity. Biotechnol Bioeng. 2010;105:636–44. doi: 10.1002/bit.22574. https://doi.org/10.1002/bit.22574. [DOI] [PubMed] [Google Scholar]
- 169.López-Marcial GR, Zeng AY, Osuna C, et al. Agarose-Based Hydrogels as Suitable Bioprinting Materials for Tissue Engineering. ACS Biomater Sci Eng. 2018;4:3610–6. doi: 10.1021/acsbiomaterials.8b00903. https://doi.org/10.1021/acsbiomaterials.8b00903. [DOI] [PubMed] [Google Scholar]
- 170.Abelseth E, Abelseth L, De la Vega L, et al. 3D Printing of Neural Tissues Derived from Human Induced Pluripotent Stem Cells Using a Fibrin-Based Bioink. ACS Biomater Sci Eng. 2019;5:234–3. doi: 10.1021/acsbiomaterials.8b01235. https://doi.org/10.1021/acsbiomaterials.8b01235. [DOI] [PubMed] [Google Scholar]
- 171.Cappelletti RM. Fibrinogen and Fibrin:Structure and Functional Aspects. J Thromb Haemost. 2012;3:1894–904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 172.de Melo BAG, Jodat YA, Cruz EM, et al. Strategies to Use Fibrinogen as Bioink for 3D Bioprinting Fibrin-based Soft and Hard Tissues. Acta Biomater. 2020;117:60–76. doi: 10.1016/j.actbio.2020.09.024. https://doi.org/10.1016/j.actbio.2020.09.024. [DOI] [PubMed] [Google Scholar]
- 173.Chameettachal S, Midha S, Ghosh S. Regulation of Chondrogenesis and Hypertrophy in Silk Fibroin-Gelatin-Based 3D Bioprinted Constructs. ACS Biomater Sci Eng. 2016;2:1450–63. doi: 10.1021/acsbiomaterials.6b00152. https://doi.org/10.1021/acsbiomaterials.6b00152. [DOI] [PubMed] [Google Scholar]
- 174.Rodriguez MJ, Dixon TA, Cohen E, et al. 3D Freeform Printing of Silk Fibroin. Acta Biomater. 2018;71:379–87. doi: 10.1016/j.actbio.2018.02.035. https://doi.org/10.1016/j.actbio.2018.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chawla S, Midha S, Sharma A, et al. Silk-Based Bioinks for 3D Bioprinting. Adv Healthc Mater. 2018;7:1–23. doi: 10.1002/adhm.201701204. https://doi.org/10.1002/adhm.201701204. [DOI] [PubMed] [Google Scholar]
- 176.Kuss MA, Harms R, Wu S, et al. Short-term Hypoxic Preconditioning Promotes Prevascularization in 3D Bioprinted Bone Constructs with Stromal Vascular Fraction Derived Cells. RSC Adv. 2017;7:29312–20. doi: 10.1039/c7ra04372d. https://doi.org/10.1039/c7ra04372d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mouser VHM, Abbadessa A, Levato R, et al. Development of a Thermosensitive HAMA-containing Bio-ink for the Fabrication of Composite Cartilage Repair Constructs. Biofabrication. 2017;9:15026. doi: 10.1088/1758-5090/aa6265. https://doi.org/10.1088/1758-5090/aa6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ma K, Zhao T, Yang L, et al. Application of Robotic-assisted In Situ 3D Printing in Cartilage Regeneration with HAMA Hydrogel:An In Vivo Study. J Adv Res. 2020;23:123–32. doi: 10.1016/j.jare.2020.01.010. https://doi.org/10.1016/j.jare.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhou M, Lee BH, Tan YJ, et al. Microbial Transglutaminase Induced Controlled Crosslinking of Gelatin Methacryloyl to Tailor Rheological Properties for 3D Printing. Biofabrication. 2019;11:25011. doi: 10.1088/1758-5090/ab063f. https://doi.org/10.1088/1758-5090/ab063f. [DOI] [PubMed] [Google Scholar]
- 180.Salinas-Fernández S, Santos M, Alonso M, et al. Genetically Engineered Elastin-like Recombinamers with Sequence-based Molecular Stabilization as Advanced Bioinks for 3D Bioprinting. Appl Mater Today. 2020;18:100500. https://doi.org/10.1016/j.apmt.2019.100500. [Google Scholar]
- 181.Rinoldi C, Costantini M, Kijeńska-Gawrońska E, et al. Tendon Tissue Engineering:Effects of Mechanical and Biochemical Stimulation on Stem Cell Alignment on Cell-Laden Hydrogel Yarns. Adv Healthc Mater. 2019;8(7):1801218. doi: 10.1002/adhm.201801218. https://doi.org/10.1002/adhm.201801218. [DOI] [PubMed] [Google Scholar]
- 182.Swaminathan S, Hamid Q, Sun W, et al. Bioprinting of 3D Breast Epithelial Spheroids for Human Cancer Models. Biofabrication. 2019;11:025003. doi: 10.1088/1758-5090/aafc49. https://doi.org/10.1088/1758-5090/aafc49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gu Q, Tomaskovic-Crook E, Lozano R, et al. Functional 3D Neural Mini-Tissues from Printed Gel-Based Bioink and Human Neural Stem Cells. Adv Healthc Mater. 2016;5:1429–38. doi: 10.1002/adhm.201600095. https://doi.org/https://doi.org/10.1002/adhm.201600095. [DOI] [PubMed] [Google Scholar]
- 184.Zhang K, Fu Q, Yoo J, et al. 3D Bioprinting of Urethra with PCL/PLCL Blend and Dual Autologous Cells in Fibrin Hydrogel:An In Vitro Evaluation of Biomimetic Mechanical Property and Cell Growth Environment. Acta Biomater. 2017;50:154–64. doi: 10.1016/j.actbio.2016.12.008. https://doi.org/10.1016/j.actbio.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 185.Wang Y, Shi W, Kuss M, et al. 3D Bioprinting of Breast Cancer Models for Drug Resistance Study. ACS Biomater Sci Eng. 2018;4:4401–11. doi: 10.1021/acsbiomaterials.8b01277. https://doi.org/10.1021/acsbiomaterials.8b01277. [DOI] [PubMed] [Google Scholar]
- 186.Kuss M, Kim J, Qi D, et al. Effects of Tunable, 3D-bioprinted Hydrogels on Human Brown Adipocyte Behavior and Metabolic Function. Acta Biomater. 2018;71:486–95. doi: 10.1016/j.actbio.2018.03.021. https://doi.org/10.1016/j.actbio.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ebrahimi M, Ostrovidov S, Salehi S, et al. Enhanced Skeletal Muscle Formation on Microfluidic Spun Gelatin Methacryloyl (GelMA) Fibres Using Surface Patterning and Agrin Treatment. J Tissue Eng Regen Med. 2018;12:2151–63. doi: 10.1002/term.2738. https://doi.org/10.1002/term.2738. [DOI] [PubMed] [Google Scholar]
- 188.Wang Y, Kankala RK, Zhu K, et al. Coaxial Extrusion of Tubular Tissue Constructs Using a Gelatin/GelMA Blend Bioink. ACS Biomater Sci Eng. 2019;5:5514–24. doi: 10.1021/acsbiomaterials.9b00926. https://doi.org/10.1021/acsbiomaterials.9b00926. [DOI] [PubMed] [Google Scholar]
- 189.Parthiban SP, Rana D, Jabbari E, et al. Covalently Immobilized VEGF-Mimicking Peptide with Gelatin Methacrylate Enhances Microvascularization of Endothelial Cells. Acta Biomater. 2017;51:330–40. doi: 10.1016/j.actbio.2017.01.046. https://doi.org/10.1016/j.actbio.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 190.Shao L, Gao Q, Zhao H, et al. Fiber-Based Mini Tissue with Morphology-Controllable GelMA Microfibers. Small. 2018;14:1–8. doi: 10.1002/smll.201802187. https://doi.org/10.1002/smll.201802187. [DOI] [PubMed] [Google Scholar]
- 191.Lee VK, Kim DY, Ngo H, et al. Creating Perfused Functional Vascular Channels Using 3D Bio-printing Technology. Biomaterials. 2014;35:8092–102. doi: 10.1016/j.biomaterials.2014.05.083. https://doi.org/10.1016/j.biomaterials.2014.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jia W, Gungor-Ozkerim PS, Zhang YS, et al. Direct 3D Bioprinting of Perfusable Vascular Constructs Using a Blend Bioink. Biomaterials. 2016;106:58–68. doi: 10.1016/j.biomaterials.2016.07.038. https://doi.org/10.1016/j.biomaterials.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shao L, Gao Q, Xie C, et al. Sacrificial Microgel-laden Bioink-enabled 3D Bioprinting of Mesoscale Pore Networks. Biodesign Manuf. 2020;3:30–9. https://doi.org/10.1007/s42242-020-00062-y. [Google Scholar]
- 194.Haring AP, Thompson EG, Tong Y, et al. Process-and Bio-inspired Hydrogels for 3D Bioprinting of Soft Free-standing Neural and Glial Tissues. Biofabrication. 2019;11:25009. doi: 10.1088/1758-5090/ab02c9. https://doi.org/10.1088/1758-5090/ab02c9. [DOI] [PubMed] [Google Scholar]
- 195.Saadati A, Hassanpour S, Hasanzadeh M, et al. Immunosensing of Breast Cancer Tumor Protein CA 15-3 (Carbohydrate Antigen 15.3) Using a Novel Nano-bioink:A New Platform for Screening of Proteins in Human Biofluids by Pen-on-paper Technology. Int J Biol Macromol. 2019;132:748–58. doi: 10.1016/j.ijbiomac.2019.03.170. https://doi.org/10.1016/j.ijbiomac.2019.03.170. [DOI] [PubMed] [Google Scholar]
- 196.Mokhtari H, Kharaziha M, Karimzadeh F, et al. An Injectable Mechanically Robust Hydrogel of Kappa-carrageenan-dopamine Functionalized Graphene Oxide for Promoting Cell Growth. Carbohydr Polym. 2019;214:234–49. doi: 10.1016/j.carbpol.2019.03.030. https://doi.org/10.1016/j.carbpol.2019.03.030. [DOI] [PubMed] [Google Scholar]
- 197.Aldrich A, Kuss MA, Duan B, et al. 3D Bioprinted Scaffolds Containing Viable Macrophages and Antibiotics Promote Clearance of Staphylococcus aureus Craniotomy-Associated Biofilm Infection. ACS Appl Mater Interfaces. 2019;11:12298–307. doi: 10.1021/acsami.9b00264. https://doi.org/10.1021/acsami.9b00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Noh I, Kim N, Tran HN, et al. 3D Printable Hyaluronic Acid-based Hydrogel for its Potential Application as a Bioink in Tissue Engineering. Biomater Res. 2019;23:3. doi: 10.1186/s40824-018-0152-8. https://doi.org/10.1186/s40824-018-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Arya N, Forget A, Sarem M, et al. RGDSP Functionalized Carboxylated Agarose as Extrudable Carriers for Chondrocyte Delivery. Mater Sci Eng C. 2019;99:103–11. doi: 10.1016/j.msec.2019.01.080. https://doi.org/10.1016/j.msec.2019.01.080. [DOI] [PubMed] [Google Scholar]
- 200.Ooi HW, Mota C, ten Cate AT, et al. Thiol-Ene Alginate Hydrogels as Versatile Bioinks for Bioprinting. Biomacromolecules. 2018;19:3390–400. doi: 10.1021/acs.biomac.8b00696. https://doi.org/10.1021/acs.biomac.8b00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kérourédan O, Bourget JM, Rémy M, et al. Micropatterning of Endothelial Cells to Create a Capillary-like Network with Defined Architecture by Laser-assisted Bioprinting. J Mater Sci Mater Med. 2019;30:28. doi: 10.1007/s10856-019-6230-1. https://doi.org/10.1007/s10856-019-6230-1. [DOI] [PubMed] [Google Scholar]
- 202.Miri AK, Nieto D, Iglesias L, et al. Microfluidics-Enabled Multimaterial Maskless Stereolithographic Bioprinting. Adv Mater. 2018;30:1800242. doi: 10.1002/adma.201800242. https://doi.org/10.1002/adma.201800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jang J, Park HJ, Kim SW, et al. 3D Printed Complex Tissue Construct Using Stem Cell-laden Decellularized Extracellular Matrix Bioinks for Cardiac Repair. Biomaterials. 2017;112:264–74. doi: 10.1016/j.biomaterials.2016.10.026. https://doi.org/10.1016/j.biomaterials.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 204.Shin S, Kwak H, Hyun J. Melanin Nanoparticle-Incorporated Silk Fibroin Hydrogels for the Enhancement of Printing Resolution in 3D-Projection Stereolithography of Poly(ethylene glycol)-Tetraacrylate Bio-ink. ACS Appl Mater Interfaces. 2018;10:23573–82. doi: 10.1021/acsami.8b05963. https://doi.org/10.1021/acsami.8b05963. [DOI] [PubMed] [Google Scholar]
- 205.Laternser S, Keller H, Leupin O, et al. A Novel Microplate 3D Bioprinting Platform for the Engineering of Muscle and Tendon Tissues. SLAS Technol Transl Life Sci Innov. 2018;23:599–613. doi: 10.1177/2472630318776594. https://doi.org/10.1177/247263031≈594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhu K, Shin SR, van Kempen T, et al. Gold Nanocomposite Bioink for Printing 3D Cardiac Constructs. Adv Funct Mater. 2017;27:1605352. doi: 10.1002/adfm.201605352. https://doi.org/10.1002/adfm.201605352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhai X, Ruan C, Ma Y, et al. 3D-Bioprinted Osteoblast-Laden Nanocomposite Hydrogel Constructs with Induced Microenvironments Promote Cell Viability, Differentiation, and Osteogenesis both In Vitro and In Vivo. Adv Sci. 2018;5:1700550. doi: 10.1002/advs.201700550. https://doi.org/10.1002/advs.201700550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lee D, Lee D, Won Y, et al. Insertion of Vertically Aligned Nanowires into Living Cells by Inkjet Printing of Cells. Small. 2016;12:1446–57. doi: 10.1002/smll.201502510. https://doi.org/10.1002/smll.201502510. [DOI] [PubMed] [Google Scholar]
- 209.Hashmi MU, Khan F, Khalid N, et al. Hydrogels Incorporated with Silver Nanocolloids Prepared from Antioxidant Rich Aerva javanica as Disruptive Agents against Burn Wound Infections. Colloids Surfaces A Physicochem Eng Asp. 2017;529:475–86. https://doi.org/10.1016/j.colsurfa.2017.06.036 . [Google Scholar]
- 210.Maharjan B, Kumar D, Awasthi GP, et al. Synthesis and Characterization of Gold/Silica Hybrid Nanoparticles Incorporated Gelatin Methacrylate Conductive Hydrogels for H9C2 Cardiac Cell Compatibility Study. Compos Part B Eng. 2019;177:107415. https://doi.org/10.1016/j.compositesb.2019.107415. [Google Scholar]
- 211.Hasnidawani JN, Azlina HN, Norita H, et al. Synthesis of ZnO Nanostructures Using Sol-Gel Method. Procedia Chem. 2016;19:211–6. https://doi.org/10.1016/j.proche.2016.03.095. [Google Scholar]
- 212.Salavati-Niasari M, Soofivand F, Sobhani-Nasab A, et al. Facile Synthesis and Characterization of CdTiO3 Nanoparticles by Pechini Sol-gel Method. J Mater Sci Mater Electron. 2017;28:14965–73. https://doi.org/10.1007/s10854-017-7369-5. [Google Scholar]
- 213.Rahali K, Ben Messaoud G, Kahn CJF, et al. 2017,Synthesis and Characterization of Nanofunctionalized Gelatin Methacrylate Hydrogels. Int J Mol Sci. 18:2675. doi: 10.3390/ijms18122675. https://doi.org/10.3390/ijms18122675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kusior A, Kollbek K, Kowalski K, et al. Sn and Cu Oxide Nanoparticles Deposited on TiO2 Nanoflower 3D Substrates by Inert Gas Condensation Technique. Appl Surf Sci. 2016;380:193–202. https://doi.org/10.1016/j.apsusc.2016.01.204. [Google Scholar]
- 215.Iqbal J, Abbasi BA, Ahmad R, et al. Biogenic Synthesis of Green and Cost Effective Iron Nanoparticles and Evaluation of their Potential Biomedical Properties. J Mol Struct. 2020;1199:126979. https://doi.org/10.1016/j.molstruc.2019.126979. [Google Scholar]
- 216.Nunes R, Baião A, Monteiro D, et al. Zein Nanoparticles as Low-cost, Safe, and Effective Carriers to Improve the Oral Bioavailability of Resveratrol. Drug Deliv Transl Res. 2020;10:826–37. doi: 10.1007/s13346-020-00738-z. https://doi.org/10.1007/s13346-020-00738-z. [DOI] [PubMed] [Google Scholar]
- 217.Peak CW, Singh KA, Adlouni M, et al. Printing Therapeutic Proteins in 3D using Nanoengineered Bioink to Control and Direct Cell Migration. Adv Healthc Mater. 2019;8:1801553. doi: 10.1002/adhm.201801553. https://doi.org/10.1002/adhm.201801553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chimene D, Peak CW, Gentry JL, et al. Nanoengineered Ionic-Covalent Entanglement (NICE) Bioinks for 3D Bioprinting. ACS Appl Mater Interfaces. 2018;10:9957–68. doi: 10.1021/acsami.7b19808. https://doi.org/10.1021/acsami.7b19808. [DOI] [PubMed] [Google Scholar]
- 219.Wilson SA, Cross LM, Peak CW, et al. Shear-Thinning and Thermo-Reversible Nanoengineered Inks for 3D Bioprinting. ACS Appl Mater Interfaces. 2017;9:43449–58. doi: 10.1021/acsami.7b13602. https://doi.org/10.1021/acsami.7b13602. [DOI] [PubMed] [Google Scholar]
- 220.Ahlfeld T, Cidonio G, Kilian D, et al. Development of a Clay Based Bioink for 3D Cell Printing for Skeletal Application. Biofabrication. 2017;9:34103. doi: 10.1088/1758-5090/aa7e96. https://doi.org/10.1088/1758-5090/aa7e96. [DOI] [PubMed] [Google Scholar]
- 221.Olsen TR, Casco M, Herbst A, et al. Longitudinal Stretching for Maturation of Vascular Tissues Using Magnetic Forces. Bioengineering. 2016;3:29. doi: 10.3390/bioengineering3040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ojansivu M, Rashad A, Ahlinder A, et al. Wood-based Nanocellulose and Bioactive Glass Modified Gelatin-alginate Bioinks for 3D Bioprinting of Bone Cells. Biofabrication. 2019;11:35010. doi: 10.1088/1758-5090/ab0692. https://doi.org/10.1088/1758-5090/ab0692. [DOI] [PubMed] [Google Scholar]
- 223.Reakasame S, Trapani D, Detsch R, et al. 2018,Cell Laden Alginate-keratin Based Composite Microcapsules Containing Bioactive Glass for Tissue Engineering Applications. J Mater Sci Mater Med. 29:185. doi: 10.1007/s10856-018-6195-5. https://doi.org/10.1007/s10856-018-6195-5. [DOI] [PubMed] [Google Scholar]
- 224.Trampe E, Koren K, Akkineni AR, et al. Functionalized Bioink with Optical Sensor Nanoparticles for O2 Imaging in 3D-Bioprinted Constructs. Adv Funct Mater. 2018;28:1804411. https://doi.org/10.1002/adfm.201804411. [Google Scholar]
- 225.Allig S, Mayer M, Thielemann C. Workflow for Bioprinting of Cell-laden Bioink. Lek Tech. 48:46–51. [Google Scholar]
- 226.DeSimone E, Schacht K, Pellert A, et al. Recombinant Spider Silk-Based Bioinks. Biofabrication. 2017;9:44104. doi: 10.1088/1758-5090/aa90db. https://doi.org/10.1088/1758-5090/aa90db. [DOI] [PubMed] [Google Scholar]
- 227.Echalier C, Levato R, Mateos-Timoneda MA, et al. Modular Bioink for 3D Printing of Biocompatible Hydrogels:Sol-gel Polymerization of Hybrid Peptides and Polymers. RSC Adv. 2017;7:12231–35. https://doi.org/10.1039/C6RA2⅜F. [Google Scholar]
- 228.Gao G, Zhang XF, Hubbell K C, et al. NR2F2 Regulates Chondrogenesis of Human Mesenchymal Stem Cells in Bioprinted Cartilage. Biotechnol Bioeng. 2017;114:208–16. doi: 10.1002/bit.26042. https://doi.org/10.1002/bit.26042. [DOI] [PubMed] [Google Scholar]
- 229.Daly AC, Cunniffe GM, Sathy BN, et al. 3D Bioprinting of Developmentally Inspired Templates for Whole Bone Organ Engineering. Adv Healthc Mater. 2016;5:2353–62. doi: 10.1002/adhm.201600182. https://doi.org/10.1002/adhm.201600182. [DOI] [PubMed] [Google Scholar]
- 230.Lee DY, Lee H, Kim Y, et al. Phage as Versatile Nanoink for Printing 3-D Cell-laden Scaffolds. Acta Biomater. 2016;29:112–24. doi: 10.1016/j.actbio.2015.10.004. https://doi.org/10.1016/j.actbio.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 231.Lozano R, Stevens L, Thompson BC, et al. 3D Printing of Layered Brain-like Structures Using Peptide Modified Gellan Gum Substrates. Biomaterials. 2015;67:264–73. doi: 10.1016/j.biomaterials.2015.07.022. https://doi.org/10.1016/j.biomaterials.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 232.Schacht K, Jüngst T, Schweinlin M, et al. 2015,Biofabrication of Cell-Loaded 3D Spider Silk Constructs. Angew Chemie Int Ed. 54:2816–20. doi: 10.1002/anie.201409846. https://doi.org/10.1002/anie.201409846. [DOI] [PubMed] [Google Scholar]
- 233.Jia J, Richards DJ, Pollard S, et al. Engineering Alginate as Bioink for Bioprinting. Acta Biomater. 2014;10:4323–31. doi: 10.1016/j.actbio.2014.06.034. https://doi.org/10.1016/j.actbio.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chung JHY, Naficy S, Yue Z, et al. Bio-ink Properties and Printability for Extrusion Printing Living Cells. Biomater Sci. 2013;1:763–73. doi: 10.1039/c3bm00012e. https://doi.org/10.1039/C3BM00012E. [DOI] [PubMed] [Google Scholar]
- 235.Poon YF, Cao Y, Liu Y, et al. Hydrogels Based on Dual Curable Chitosan-graft-Polyethylene Glycol-graft-Methacrylate:Application to Layer-by-Layer Cell Encapsulation. ACS Appl Mater Interfaces. 2010;2:2012–25. doi: 10.1021/am1002876. https://doi.org/10.1021/am1002876. [DOI] [PubMed] [Google Scholar]
- 236.Ji S, Almeida E, Guvendiren M. 3D Bioprinting of Complex Channels within Cell-laden Hydrogels. Acta Biomater. 2019;95:214–24. doi: 10.1016/j.actbio.2019.02.038. https://doi.org/10.1016/j.actbio.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 237.Park J, Lee SJ, Lee H, et al. Three Dimensional Cell Printing with Sulfated Alginate for Improved Bone Morphogenetic Protein-2 Delivery and Osteogenesis in Bone Tissue Engineering. Carbohydr Polym. 2018;196:217–24. doi: 10.1016/j.carbpol.2018.05.048. https://doi.org/10.1016/j.carbpol.2018.05.048. [DOI] [PubMed] [Google Scholar]
- 238.Luo Y, Luo G, Gelinsky M, et al. 3D Bioprinting Scaffold Using Alginate/Polyvinyl Alcohol Bioinks. Mater Lett. 2017;189:295–8. https://doi.org/10.1016/j.matlet.2016.12.009. [Google Scholar]
- 239.Poldervaart MT, Wang H, van der Stok J, et al. Sustained Release of BMP-2 in Bioprinted Alginate for Osteogenicity in Mice and Rats. PLoS One. 2013;8:e72610. doi: 10.1371/journal.pone.0072610. https://doi.org/10.1371/journal.pone.0072610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Cunniffe GM, Gonzalez-Fernandez T, Daly A, et al. Three-Dimensional Bioprinting of Polycaprolactone Reinforced Gene Activated Bioinks for Bone Tissue Engineering. Tissue Eng Part A. 2017;23:891–900. doi: 10.1089/ten.tea.2016.0498. https://doi.org/10.1089/ten.tea.2016.0498. [DOI] [PubMed] [Google Scholar]
- 241.Rathan S, Dejob L, Schipani R, Haffner B, Möbius ME, Kelly DJ. Fiber Reinforced Cartilage ECM Functionalized Bioinks for Functional Cartilage Tissue Engineering. Adv Healthc Mater. 8:1801501. doi: 10.1002/adhm.201801501. [DOI] [PubMed] [Google Scholar]
- 242.Kesti M, Fisch P, Pensalfini M, et al. 2016,Guidelines for Standardization of Bioprinting:A Systematic Study of Process Parameters and their Effect on Bioprinted Structures. BioNanoMaterials. 17:193–204. https://doi.org/10.1515/bnm-2016-0004. [Google Scholar]
- 243.Acosta-Vélez GF, Zhu TZ, Linsley CS, et al. Photocurable Poly(Ethylene Glycol) as a Bioink for the Inkjet 3D Pharming of Hydrophobic Drugs. Int J Pharm. 2018;546:145–53. doi: 10.1016/j.ijpharm.2018.04.056. https://doi.org/10.1016/j.ijpharm.2018.04.056. [DOI] [PubMed] [Google Scholar]
- 244.Gao G, Lee JH, Jang J, et al. Tissue Engineered Bio-Blood-Vessels Constructed Using a Tissue-Specific Bioink and 3D Coaxial Cell Printing Technique:A Novel Therapy for Ischemic Disease. Adv Funct Mater. 2017;27:1700798. https://doi.org/10.1002/adfm.201700798. [Google Scholar]
- 245.Acosta-Vélez GF, Linsley CS, Craig MC, et al. Photocurable Bioink for the Inkjet 3D Pharming of Hydrophilic Drugs. Bioengineering. 2017;4:11. doi: 10.3390/bioengineering4010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Erdem A, Darabi MA, Nasiri R, et al. 3D Bioprinting of Oxygenated Cell-Laden Gelatin Methacryloyl Constructs. Adv Healthc Mater. 2020;9:1–12. doi: 10.1002/adhm.201901794. https://doi.org/10.1002/adhm.201901794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


