Abstract
Although the majority of patients with acute myeloid leukemia (AML) treated with intensive chemotherapy achieve a complete remission (CR), many are destined to relapse if treated with intensive chemotherapy alone. Allogeneic stem cell transplant (allo-SCT) represents a pivotally important treatment strategy in fit adults with AML because of its augmented anti-leukemic activity consequent upon dose intensification and the genesis of a potent graft-versus-leukemia effect. Increased donor availability coupled with the advent of reduced intensity conditioning (RIC) regimens has dramatically increased transplant access and consequently allo-SCT is now a key component of the treatment algorithm in both patients with AML in first CR (CR1) and advanced disease. Although transplant related mortality has fallen steadily over recent decades there has been no real progress in reducing the risk of disease relapse which remains the major cause of transplant failure and represents a major area of unmet need. A number of therapeutic approaches with the potential to reduce disease relapse, including advances in induction chemotherapy, the development of novel conditioning regimens and the emergence of the concept of post-transplant maintenance, are currently under development. Furthermore, the use of genetics and measurable residual disease technology in disease assessment has improved the identification of patients who are likely to benefit from an allo-SCT which now represents an increasingly personalized therapy. Future progress in optimizing transplant outcome will be dependent on the successful delivery by the international transplant community of randomized prospective clinical trials which permit examination of current and future transplant therapies with the same degree of rigor as is routinely adopted for non-transplant therapies.
Keywords: acute myeloid leukemia, allogeneic stem cell transplantation, graft-versus-leukemia, chemotherapy, minimal residual disease, measurable residual disease (MRD)
Introduction
It is more than sixty years since allogeneic stem cell transplantation (allo-SCT) was pioneered as a novel and potentially curative therapeutic modality in patients with chemotherapy-resistant acute myeloid leukemia (AML) (1, 2). Subsequent analyses have confirmed the role of allo-SCT as the optimal treatment strategy in adults with AML in first complete remission (CR1) consequent upon its ability to reduce the risk of disease relapse by more than 60% compared with intensive chemotherapy alone. Remarkably the magnitude of the augmented anti-leukemic activity of allo-SCT, result from both dose intensification and the genesis of a potent graft-versus-leukemia (GVL) effect, is similar in all biological subtypes of AML (3).
The survival benefit of the augmented anti-leukemic activity of allo-SCT is blunted by its attendant transplant related mortality (TRM). It is therefore essential to a) identify patients whose outcome with intensive chemotherapy (IC) is such that the enhanced anti-leukemic activity of allo-SCT is otiose b) identify patients whose outcome with IC is such that deployment of the enhanced anti-leukemic activity of an allograft should be considered and c) define as precisely as possible the patient population in which allo-SCT can be delivered with an acceptable morbidity and mortality. Thus the identification of patients likely to benefit from allo-SCT requires a dynamic assessment which incorporates both the predicted risk of disease relapse if the patient were to receive IC alone coupled with a prediction of the TRM were the patient to proceed to transplant (4). Accurate prediction of these parameters has been refined by progress in both risk stratification utilizing clinical, cytogenetic and molecular genetic data as well as advances in prediction of the risk of allo-SCT (5–9). Increasingly, randomized controlled trials are informing critical questions concerning relapse risk in patients treated with IC alone (10) and informing the personalization of transplant strategies (11–14). Cooperative transplant trials networks such as the US BMT CTN and the UK transplant cooperative IMPACT will play an increasingly important role in optimizing outcomes after allo-SCT in AML (15).
Who and When Should Patients With AML Be Transplanted?
The focus of therapeutic endeavor in newly diagnosed AML in recent years has primarily been on improving induction chemotherapy (16, 17). However, the increasing availability of allo-SCT coupled with the recognition that a substantial proportion of patients treated with IC alone are destined to relapse has prioritized the development of algorithms designed to identify patients likely to benefit from allo-SCT in CR1. The advent of more accurate risk stratification, utilizing genetic and measurable residual disease (MRD) analysis, coupled with increased sophistication in predicting and reducing TRM has improved decision making concerning the delivery of optimal consolidation therapy in adult AML (18).
The importance of correctly identifying patients in first CR1 who are likely to relapse is predicated by the poor, incomplete rates of remission salvage, such that a significant proportion of patient who relapse do not reach a second CR (CR2) (19). Furthermore, the use of additional intensive chemotherapy and concomitant infections often result in patients with impaired fitness prior to an allo-SCT in CR2. Studies recurrently show that patients with active disease have poorer outcomes as compared to those patients transplanted in CR, thus this should be a critical goal prior to proceeding to transplant (20, 21). Whilst patients transplanted with CR with incomplete count recovery (CRi) have inferior outcomes to patients with AML in CR, this is as a result of increased non-relapse mortality (NRM) but not necessarily relapse risk (22). Other studies have shown the number of courses of consolidation chemotherapy delivered prior to transplant do not improve patient outcome (23).
Who Should Be Transplanted With Refractory or Relapsed Disease?
The aim of therapy in fit adults with relapsed with AML is to proceed to allo-SCT once a 2nd CR has been achieved (24). This is based on studies demonstrating very poor outcomes in patients who are not allografted in CR2 (19, 25, 26). However, there may be a subset of patients with core-binding factor translocated AML who may achieve long term remission with autologous transplantation, or in a minority, salvage chemotherapy (19, 27). A number of prognostic systems exist for patients with relapsed/refractory AML (28, 29) which may help to identify subgroups of patients with AML who are likely to have long-term survival following an allo-SCT. Important factors identified in these prognostic systems include, duration of CR1, age at relapse and cytogenetic risk at diagnosis.
Retrospective analyses of allo-SCT for AML in CR2 have demonstrated overall survival (OS) of 30-60%, with acceptable rates of TRM despite intensive pre-treatment in this cohort of patients (30–32). Results have also been encouraging in the use of alternative donors in transplantation at CR2 (32). A formal comparison of myeloablative (MAC) versus RIC regimens in this setting is not possible, but registry studies show no significant differences in OS between patients treated with the differing conditioning intensities (32). Despite this, in fit younger patients who might tolerate a MAC regimen, this is probably the preferred treatment strategy to reduce further disease relapse which remains the major risk facing this patient cohort.
A particularly challenging group of patients with AML are those with primary refractory disease, defined as failure to achieve remission following two cycles of induction chemotherapy (33). Numerous studies have shown that patients transplanted with active disease have poorer outcomes than those in remission (20, 31, 34). However, studies have demonstrated approximately 20-30% of patients with primary refractory disease may have long term survival after an allo-SCT (35) and recent work has identified risk factors that may identify patients who are likely to have primary refractory disease at an earlier stage (36). In the evolving landscape of genetic stratification, these scoring systems are likely to be refined, and the long term impact of novel salvage options from targeted therapies remains to be seen (37, 38). One recent study underlined the particularly poor outcome of patients with TP53 mutant AML, when they were transplanted with active disease (39). A challenge in assessments of such genetic risk factors will be the clonal evolution which occurs in patients with AML following treatment (40).
Finally, for patients who relapse following an allo-SCT, the outcome is very poor (41). However, for some patients, especially ones with a durable remission since transplant, and with disease control at the time of second allo-SCT, this procedure may provide an OS at 2 years of 25% (42). In patients who received an unrelated donor transplant, no advantage for change in donor in this setting could be demonstrated.
Who Should Be Transplanted in First Complete Remission?
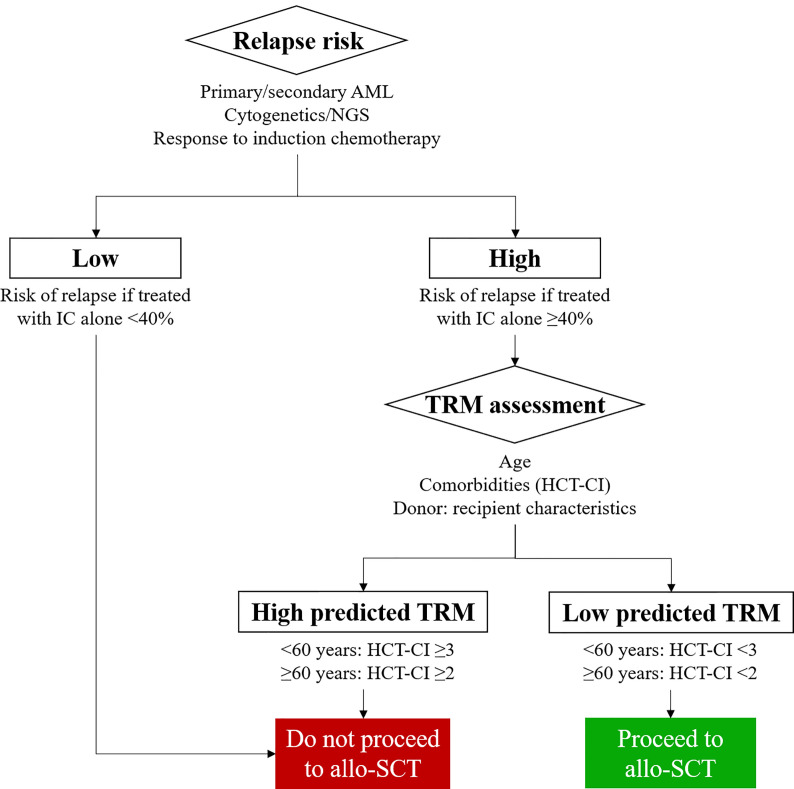
Donor versus no donor studies were the first to demonstrate the ability of allo-SCT to increase disease free survival (DFS) and OS in patients transplanted using a myeloablative HLA matched sibling allo-SCT (43). A selection strategy to identify patients who should be transplanted in CR1 was articulated by Cornelissen and colleagues with the European LeukaemiaNet (ELN) AML working party (4) and is based on the competing risks of relapse with chemotherapy alone versus risk of relapse after an allo-SCT and the concomitant TRM ( Figure 1 ). Underpinning this treatment algorithm is the observation that the risk of relapse following allo-SCT is more than halved as compared to that observed in patients treated with IC alone (3)- regardless of cytogenetic risk group. At the same time recent reductions in transplant toxicity permit delivery of an allo-SCT with an NRM of 15% or less in fit adults with a well matched sibling or volunteer unrelated donor. On this basis the ELN group recommend consideration of allo-SCT in fit adults with AML in CR1 who have a predicted relapse risk of 35-40% and a suitable donor (33). Thus adults with AML in CR1 who fulfill ELN criteria for good risk disease on the basis of cytogenetics or the presence of an NPM1 mutation without FLT3-ITD mutation, and demonstrate a good response to induction chemotherapy by MRD criteria are not routinely deemed eligible for an allo-SCT in CR1. Conversely, all other adults in CR1 in whom the predicted risk of relapse of >40% if they are treated with IC alone should, in principle, be considered transplant candidates providing a suitable stem cell donor is available (44).
Figure 1.
Identifying patients with Acute Myeloid Leukemia (AML) who are likely to benefit from allogeneic stem cell transplantation (allo-SCT). MRD, Measurable Residual Disease; TRM, Transplant related mortality; HCT-CI, Hematopoietic cell transplantation - specific comorbidity index; NGS, next generation sequencing.
Risk stratification in patients with AML in CR1 is based on clinical (5) factors, such as age and gender, as well as cytogenetic risk based on karyotyping results (6) ( Table 1 ). This has been refined in recent years by the discovery of further mutations of prognostic significance in genes such as FLT3 (45), NPM1 (46), ASXL1 (47), RUNX1 (48) and TP53 (49) as reflected in the 2017 ELN classification (33). Increasingly mutational information is available for patients as a result of next generation sequencing (NGS) technology assaying panels of commonly affected myeloid genes (33). This is of further importance as these genetic markers are now commonly used as both therapeutic targets (50, 51) and as prognostic markers of response to therapies (52). The results of these large scale sequencing efforts of AML samples at diagnosis, in combination with data relating to treatment use and clinical outcome will likely refine these risk categories. This will provide a “personalized” risk score for individuals patients based on a number of these clinical factors and allow for incorporation of combinations of genetic mutations, such as that seen recently in the study of myeloproliferative neoplasms (53, 54). It is increasingly becoming apparent that both clinical and mutational characteristics determine the kinetics of disease relapse. Importantly patients with a FLT3 mutation are amongst those likely to relapse early in whom the timing of transplant should not be delayed (55).
Table 1.
Factors determining disease risk in AML.
| Clinical Variables | Molecular variables | Dynamic variables |
|---|---|---|
| • Age | • Cytogenetic | • Response to course 1 by morphology |
| • Gender | • Next generation sequencing of genes e.g. FLT3, NPM1, RUNX1, ASXL1, TP53 | • Response to treatment by MRD |
| • Presenting white cell count | ||
| • Primary versus secondary disease | ||
| • Performance status |
Incorporation of MRD Risk Stratification
An important development in risk stratification has been the incorporation of MRD monitoring to routinely assess patients’ response to chemotherapy (56) ( Table 2 and Figure 2 ). The kinetics and depth of response has been identified as being critical in re-assessing the risk of relapse in patients with otherwise favorable and intermediate risk disease. The impact of MRD monitoring appears to be the most important, independent prognostic factor in many scenarios (57, 58). The selection of the optimal MRD monitoring modality depends on the presence of leukemia specific molecular, cytogenetic or immuno-phenotypic dependent on the AML subtype. Each MRD monitoring technique has its own advantages and disadvantages, and all require expertise in the delivery of reliable results ( Table 2 ).
Table 2.
Relative merits of different MRD monitoring methodologies.
| Method | Multi-parameter Flow Cytometry (MFC MRD) | Quantitative PCR (RQ-PCR) | Next generation sequencing (NGS) |
|---|---|---|---|
| Advantages | Rapid results Widely applicable to many patents |
Sensitive Easily compared with sequential results due to quantitative range Widely accepted standardisation |
Applicable to many patients Error correction increases sensitivity |
| Disadvantages | Reliant on expertise of reporting lab Phenotype of AML cells may change over time |
Restricted molecular targets (e.g. Core binding factor translocations, NPM1c mutant) | Ongoing development of technology Expense |
| Examples of use | Risk stratification in younger adults, post induction chemotherapy, with NPM1 negative AML. | Risk stratification post chemotherapy to determine relapse risk in NPM1 mutant AML. | Pre-transplant MRD monitoring. |
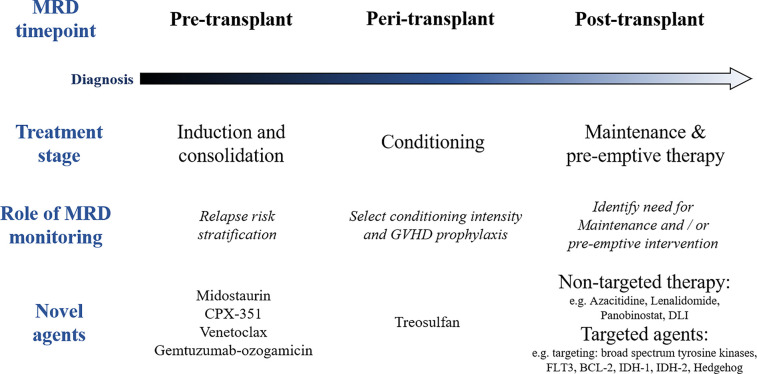
Figure 2.
Role of measurable residual disease (MRD) and novel agents at different stages of the treatment pathway in acute myeloid leukemia (AML). GVHD, graft versus host disease; DLI, donor lymphocyte infusion.
Examples of Different Uses of MRD Risk Stratification
Real-time quantitative polymerase chain reaction (RQ-PCR) monitoring of disease specific transcripts provides a sensitive and disease specific assay of MRD for patients with AML expressing a detectable fusion gene transcript (e.g. Core-binding factor (CBF) fusion gene, KMT2A fusion genes, mutant NPM1). In the case of AML with CBF translocation, although age can influence prognosis (59), the depth of response to course 1 and 2 of IC (57) are critical determinants of relapse risk. In cases with residual levels of CBF fusion transcripts at the end of treatment (60), relapse risk depends on level of transcripts, but low levels of CBF fusion gene transcripts may persist after end of treatment without affecting long-term survival. Failure to achieve a 3-log reduction in CBF fusion transcript after two cycles of chemotherapy is associated with an over 50% relapse risk in the monitoring studies of two large cooperative groups, suggesting possible benefit from an allo-SCT in these patients (57, 61).
In younger adults with NPM1 mutant AML, RQ-PCR positivity in the peripheral blood after two cycles of chemotherapy is an important predictor of relapse, identifying a population of patients who should be considered allo-SCT mandatory (58). This is supported by data which points to the beneficial effect of allo-SCT in patients with mutant NPM1 residual disease post induction chemotherapy (62). Recent studies have confirmed that, in younger adults at least, NPM1 is also a predictive biomarker. Patients with NPM1 mutant AML who have a less than 4-log reduction in peripheral blood NPM1 MRD levels demonstrated improved survival after allo-SCT compared with patients who received chemotherapy alone (62). The low relapse risk for patients who are negative for mutant NPM1 transcripts in the peripheral blood after two cycles of intensive chemotherapy outweighs other poor prognostic factors such as concomitant FLT3-ITD mutation or poor risk genotypes (7). The degree to which NPM1 mutations are a prognostic or predictive biomarker in older patients (over the age of 60 years) remains unclear (63). In part this may be due to the increased association of other poor risk cytogenetic features in more elderly patients with NPM1 mutant AML (64). Of note in patients with adverse risk cytogenetics, the presence of NPM1 mutation has no impact on survival outcomes.
A number of large prospective studies have confirmed the prognostic significance of multi-parametric flow cytometry (MFC) determined MRD in adults with newly diagnosed AML treated with IC. In younger adults, MFC MRD+ positive patients with standard risk, NPM1- mutated AML appeared to benefit from allo-SCT in CR1 (10) and data on this group of patients continues to be accrued, including the benefits of intensifying chemotherapy in patients with a suboptimal MRD response after first course of intensive chemotherapy. In older patients, a higher level of MRD after induction treatment is also prognostic of a worse outcome (65). However, in this age group, although MFC MRD negativity, offered improved overall survival, relapse rates remained high.
Early studies suggest a promise for NGS technology for MRD assessment (66), which has the advantage that it may be applicable for many forms of AML. Error correction methodology has become incorporated in this technology to enable higher levels of sensitivity (67), but is currently limited to research settings due to the costs. Furthermore, there has not yet been an upfront comparison of these different MRD technologies independently, or in combination, to compare technical specifications. A recent large study suggested there was an additive prognostic value of NGS MRD over MFC MRD, but interestingly the persistence of age related clonal hematopoiesis after treatment did not result in an increased relapsed rate (66)
Improving Assessment of Transplant Related Mortality in Patients With AML
A critical factor to understand whether a patient with AML is suitable for an allo-SCT is estimation of the TRM associated with the procedure and whether it is outweighed by the improvement in relapse risk delivered by the transplant process (68–70) ( Figure 1 ). Furthermore, these considerations are central to any discussion with patient and family as to whether the increased risk of an allograft is justifiable. The European Society for Blood and Marrow Transplantation (EBMT) risk score, originally developed in patients allografted for chronic myeloid leukemia (CML) (71), was subsequently shown to be applicable in other disease settings (72), and provided the first attempt to provide a quantifiable estimate of TRM and transplant outcome which could be routinely applied in clinic. However in patients allografted for AML more emphasis is now placed on the Hematopoietic cell transplantation-specific comorbidity index (HCT-CI) score which incorporates a weighted score based on the presence of pre-transplant comorbidities (8). This has been shown to be valid in patients undergoing an allo-SCT for myelodysplastic syndrome (MDS) or AML (9) and more recently combined with age (73), to demonstrate the varying effects of these comorbidities based on a patients’ age. Of note, this analysis showed that younger patients with comorbidities were at a significant disadvantage to older fit individuals with no other significant comorbidities.
Unfortunately no scoring system for TRM can include the importance of a clinical assessment of patients based on the “end of bed” assessment and knowledge of how patients have tolerated recent intensive treatment. Thus despite improvements in mathematical modeling techniques to predict treatment related risk on a personalized basis to account for the dynamic interactions between different variables (74, 75), there remains a considerable limitation in the ability of these scoring systems to predict TRM. Finally, the majority of these scoring systems were developed in the era of sibling or matched unrelated donor transplantation, thereby limiting their use for those with alternative donor sources, which are now of increasing use; such as for recipients of haploidentical donor or umbilical cord stem cells transplants.
What Is the Impact of Patients’ Age in Considering Transplant Eligibility?
It is commonly recognized that an important challenge in the management of patients with AML is the increased frequency of this disease with age. Furthermore, the older patient faces a combined challenge of increased frequency of comorbidities and higher risk genetic features (76). Nevertheless patients over the age of 70 years with AML are routinely transplanted with acceptable results (77) but careful assessment of transplant suitability is required. The widely used, updated HCT-CI score allows some adjustments due to age (73), and this analysis showed that younger patients with comorbidities were at a significant disadvantage to older fit individuals with no other significant comorbidities. Nonetheless the HCT-CI score is still of importance in this population, as it has been shown that in patients above 60 years of age a HCT-CI score of 2 or greater results in substantially higher TRM than otherwise expected (78). Future developments to improve assessment of transplant eligibility in this cohort should involve geriatric assessments that encompass an assessment of the functional status of the patient (79).
How Should Patients With AML in CR1 Be Transplanted?
The major causes of treatment failure in adults allografted for AML are transplant toxicity and disease relapse. Whilst significant progress has been made over recent decades in reducing TRM the risk of disease relapse remains stubbornly high. The key considerations in patients with allo-SCT-mandatory AML include identifying which patients should receive RIC as opposed to a MAC allo-SCT and, in patients lacking a well-matched sibling or unrelated donor, what is the preferential alternative donor stem cell source? The development of strategies with the ability to reduce the risk of disease relapse post-transplant also represents a major unmet need.
Strategies to Improve Outcomes Pre-Transplant
The design of novel treatment strategies with the potential to reduce the risk of disease relapse post allo-SCT remains a priority if we are to increase the number of patients with AML who benefit from transplant. A number of questions remain regarding the optimal management of patients’ pathway before, during and after an allo-SCT ( Figure 2 ). This debate has been reinvigorated in recent years by two key innovations: the widespread use of MRD technologies in patients with AML (80) and the increasing availability of novel pharmacological agents that may be applied at different treatment stages (81) ( Figure 2 ). The adverse impact of pre-transplant MRD on post-transplant outcomes has been increasingly widely recognized (14, 82) and this may inform pre-transplant treatment strategies. Furthermore, emerging data suggest that conditioning intensity and potentially graft-versus-host disease prophylaxis strategies may influence the poor prognostic impact of pre-transplant MRD (83). Finally, post-transplant monitoring of MRD may become important in identifying patients who should receive pre-emptive treatment (84) and is likely to be important in future maintenance strategies in patients post allo-SCT.
How Important Is Pre-Transplant MRD?
A number of retrospective studies have demonstrated the adverse prognostic significance of patients with MFC MRD positivity prior to transplant (82), with some likening the outcomes of these patients post allo-SCT to those with active disease (85). This draws comparison to the outcomes of younger adults with a partial response to the first cycle of induction chemotherapy who have a similar overall outcome as compared to patients who have a CR or CRi but have MFC MRD positivity (10). Two prospective studies have demonstrated the importance of pre-transplant MRD (14, 83) in patients with AML or high risk MDS. The FIGARO study investigated the impact of pre-transplant MFC MRD in 244 patients entered into a randomized comparison between FLAMSA-Bu-RIC regimen and a control RIC arm. This identified a poor prognostic impact of a 0.2% threshold of residual disease. However, even in the MRD positive arm, only approximately 50% of patients relapsed: not only suggesting further strategies to identify patients at risk of relapse are required (14), but contrary to previously held opinions, this sizeable proportion of patients with high risk AML may be salvageable with an allo-SCT.
The importance of pre-transplant MRD persists regardless of the technique used to monitor MRD. RT-PCR monitoring of CBF fusion transcripts prior to allo-SCT for patients in CR2, show that those with MRD negativity have a reduced risk of relapse as compared to those with MRD positive disease pre-transplant (86).
Can We Improve Transplant Outcomes in Patients With Evidence of Pre-Transplant MRD?
It remains unknown whether additional courses of chemotherapy or whether further alterations to transplant management in patients with pre-transplant MRD would be of benefit. However, in recent years a number of provocative results have provided impetus to design clinical trials to tackle the poor prognostic impact of pre-transplant MRD.
Pre-Transplant Strategies to Alter Impact of Pre-Transplant MRD?
Studies of novel agents in recent years such as midostaurin and the liposomal cytarabine-daunorubicin preparation CPX-351, suggest that the benefits of these drugs may extend to patients who receive an allo-SCT (16, 50) ( Figure 2 ). This provides interesting preliminary data that this may be through improving quality of remissions pre-transplant which may in future studies be measured as pre-transplant MRD. In the case of the FLT3 inhibitor midostaurin which was added to intensive induction and consolidation, the overall survival benefit of the addition of midostaurin appeared to persist in the majority of patients who were allografted in first remission. Notably midostaurin was not administered as post-transplant maintenance in this study. Likewise, CPX-351 demonstrated improved remission rates and OS in patients receiving this drug over standard remission induction therapy in patients with secondary AML. In patients who subsequently received an allo-SCT, those who had received CPX-351 had improved survival as compared to those in the control arm, but the numbers in the study were small, and a smaller proportion were in a remission at time of transplant in the control arm (16). Definitive studies including the incorporation of pre-transplant MRD will be important in validating or refuting the role of pre-transplant therapy in influencing pre-transplant MRD status.
In patients with comorbidities and a high chance of induction related death following intensive chemotherapy, in whom a curative pathway is still intended (87, 88), a less intensive approach may be valid prior to transplant. With the increasing availability of venetoclax based regimens, data will likely emerge as to the transplant outcomes of patients who have a remission following these lower intensity approaches as compared to conventional intensive induction regimens. At present, data on this cohort remains limited, as these regimens have been developed in cohorts of less fit individuals in which the overall transplant rates have been low (89). Certainly, it is well established that patients with AML who have non-proliferative disease, or transformed MDS can have durable remissions with azacitidine alone (90), and patients who proceed to transplant in remission may have long term outcomes which is comparable to those who have remissions from IC (91–93). Although, remission rates for patients receiving non-intensive treatment such as Azacitidine are likely to be inferior as compared to conventional induction chemotherapy alone (94–96), it is unclear whether for patients who do remit, pre-transplant MRD levels are affected by treatment intensity, and whether this has subsequent impact on post-transplant outcomes.
Can Changes in Conditioning and GVHD Prophylaxis Alter the Impact of Pre-Transplant MRD?
MRD as measured by error corrected NGS was performed in patients with AML who were enrolled onto the BMT CTN 0901 study which performed a randomized comparison of RIC versus MAC regimens (15). In a comparison of patients who were NGS MRD positive pre-transplant, patients who received a RIC regimen had an inferior outcome to those who were MRD negative at the same timepoint (83). In contrast, in patients transplanted with a MAC regimen, levels of MRD pre-transplant did not appear to affect outcomes post-transplant. This suggested that it was possible to alter transplant conditioning to improve outcomes of patients with MRD pre-transplant, but in practice would be limited to younger patients who would be eligible to receive a MAC regimen regardless (see below).
For those with NPM1 mutant transcripts pre-transplant, the risk of relapse post-transplant is increased. However, this is also dependent on the concomitant FLT3-ITD mutation status (97). The identification of T-cell depletion as an adverse risk factor in the whole cohort, and in those with positive NPM1 MRD pre-transplant, suggest a possible transplant strategy that may improve outcomes for this subset of patients.
Improving Conditioning Regimens for Patients With AML
Transplant conditioning regimens have evolved since the establishment of allo-SCT as a pivotal tool in reducing relapse risk in patients with AML. MAC regimens established the benefits of an allo-SCT in patients with AML (43, 98) but patients over the age of 40 experienced excess toxicity historically. In the last two decades the increased use of RIC regimens has allowed the routine delivery of an allo-SCT to patients over the age of 70 (77). In recent years the efforts of a number transplant cooperative groups have delivered important randomized controlled trials to optimize transplant conditioning regimens to further inform choice of conditioning regimens (12, 15, 99, 100).
What Is the Optimal Conditioning Intensity?
A MAC regimen by definition requires the infusion of donor stem cells to rescue recipients from permanent bone marrow aplasia. The original studies in allo-SCT used conditioning regimens based on radiotherapy (1). This established the basic principles required of any conditioning regimen in acute leukemia, which is to allow durable engraftment of donor hematopoiesis as well as the delivery of an anti-leukemic effect, which is in turn related to the intensity of conditioning (101).
Cyclophosphamide (Cy) based conditioning combined with total body irradiation (TBI) or busulphan are acceptable MAC regimens. The development of intravenous preparations of busulphan has improved the pharmacokinetics of this agent (102) and has practical advantages over TBI based regimens. Measuring busulphan pharmacokinetics may help predict optimal doses in conditioning (103). Cy/TBI regimens are still commonly used and may be better for patients with either central nervous system (CNS) disease or myeloid sarcoma. Nevertheless, a pivotal randomized controlled trial that demonstrated the superior tolerability of a Fludarabine/Busulphan (Flu/Bu4: 12.8 mg/kg over 4 days of IV busulfan) combination over a standard Cyclophosphamide/Busulphan combination, with acceptable tolerability in patients up to the age of 65 (12). This has resulted in the Flu/Bu4 regimen being accepted as a standard of care for fit patients where a MAC regimen is desired.
RIC regimens result in varying duration of cytopenias and are defined as containing less than ≤8 Gy Total Body Irradiation (TBI) or ≤8 mg/kg busulfan (104). The optimal RIC regimen has not been established. A number of RIC regimens have been developed over the last twenty years to enable a tolerable conditioning regimen to be delivered in patients due to either comorbidities or increased age, with varying levels of toxicity and anti-leukemic potency (e.g. Flu/Bu2: 6.4mg/kg, 2 days of IV busulphan) (105), and Flu/melphalan (140 mg/m2 of IV melphalan on 1 day) (106). The variability in the effectiveness of these regimens are exemplified by two randomized controlled trials (RCT) of RIC regimens. One study which compared the outcomes of a Flu/2Gy TBI regimen with a Flu/Bu2 regimen demonstrated increased TRM but notable decrease in relapse rates with the Flu/Bu2 regimen (107). In contrast, a recent Flu/Treosulfan study showed superior toxicity incidence to a Flu/Bu2 comparison, but is notable for a TRM in the Flu/Bu2 arm that is far in excess of historical expectations (108).
Given the improved tolerability of novel MAC regimens (12) alongside widespread experience with RIC regimens an important question arose as to whether a MAC or RIC regimen should be selected when either is available in high risk MDS and AML (109, 110). Despite this interest it was surprising that two RCTs comparing RIC and MAC regimens closed early to recruitment but did not demonstrate significant differences in relapse free or overall survival (99, 100, 111). In contrast, a Blood and Marrow Transplant Clinical Trials Network (BMT CTN) study (15) which studied a randomized comparison of RIC versus MAC regimens demonstrated a lower rate of TRM, but higher relapse risk resulting in an inferior relapse free survival (RFS) in patients receiving in the RIC arm as compared to those who received a MAC regimen. However, this study is notable for the higher than expected relapse risk in patients who received a RIC regimen.
The high relapse rates associated with RIC regimens, for patients with high risk AML resulted in the development of the FIGARO study, which compared the outcomes of a standard RIC arm with an augmented RIC schedule with sequential chemotherapy (FLAMSA-Bu) which had shown promising results in early studies in patients with primary refractory disease (112). However, this randomized controlled study demonstrated no improvement in relapse risk from the FLAMSA-Bu regimen as compared to a standard control arm (14).
GVHD Prophylaxis Strategies
The introduction of Ciclosporin was critical in establishing the deliverability of allo-SCT in patients with acute leukemia (113, 114) reducing the risk of graft-versus-host disease (GVHD). However, studies that demonstrated an inverse relationship between GVHD and relapse risk form the basis of the evidence underlying the GVL (115, 116). Commensurate with this observation, further studies demonstrated a relationship between ciclosporin exposure and risk of relapse, in the context of T-cell depleted allo-SCT (21, 117). Tacrolimus (FK506) has also been compared with Ciclosporin in a number of randomized trials with varying results (118–120), suggesting a reduction in acute GVHD with the use of Tacrolimus but no significant effect on OS or RFS. Other agents such as Sirolimus (121, 122) and Mycophenolate mofetil (123, 124) have also been used either as an addition or substitute for historical Ciclosporin/Methotrexate combination without a definitive improvement in overall outcomes.
In vivo T-cell depletion can be achieved by either Anti-thymocyte globulin (ATG) or Alemtuzumab. Studies demonstrate an improvement in risk of acute GVHD without significant changes in OS (125, 126). However a US retrospective study suggested that ATG compromised relapse risk in patients undergoing a RIC allo-SCT (127) which has led to a discrepancy in the uptake of ATG on the two continents (128). More recent data suggest that variations in vivo levels of ATG may result in differences in relapse risk as well as NRM (129). It is also important to note that there appear to be different immunosuppressive properties dependent on the source of ATG, which is critical when different studies are compared (130). The humanized anti-CD52 antibody, Alemtuzumab has also been used extensively as a method of in vivo T-cell depletion (131, 132), with control of GVHD particularly notable in the HLA-mismatch setting (133). In more recent years, the use of post-transplant Cyclophosphamide which was pioneered for use in the haploidentical donor allo-SCT setting (134) has been used in the volunteer unrelated donor setting (135) but formal assessment in the clinical trial setting is awaited.
The variation in relapse rate from study to study for these different GVHD prophylaxis studies suggest the need to perform adequately powered studies with suitable endpoints, in order to determine the optimal GVHD prophylaxis strategies in AML.
How to Improve Outcomes of Patients With AML Post-Transplant
Improving Monitoring of Disease Post-Transplant
Whilst the cornerstone of post-transplant care remains careful clinical assessment and review, post-transplant disease monitoring to identify patients at risk of relapse, and timely intervention is becoming more important. This is particularly important with the increased use of RIC allo-SCT which is associated with a higher risk of relapse (15). Furthermore, the use of pre-emptive treatment before fulminant hematological relapse may increase the efficacy of interventions such as donor lymphocyte infusion (DLI) or Azacitidine (136–139).
MRD Monitoring Post-Transplant
Prior to hematological relapse, the prognosis of which is usually very poor, early disease re-emergence can be detected by several techniques. The ELN guidelines formally recommend monitoring for MRD post-transplant (33). Similar to pre-transplant, the optimal method for monitoring MRD will be dependent on disease characteristics, and availability of technology, and expertise in the treating center. Post-transplant MRD monitoring has prognostic value. For example, the (8, 21) fusion transcript RUNX1/RUNX1T1 is suitable for MRD monitoring and has been investigated post-transplant (60, 140, 141). Similar to pre-transplant, detectable RUNX1/RUNX1T1 transcripts at 3 months after transplant was a more potent predictor of relapse than presence of c-KIT mutations (141). The most prognostic threshold of MRD may be different after transplant, as compared to that of the pre-transplant setting. For example, one study determined the prognostic impact of NPM1 MRD pre- and post-transplant and found that 1% increase in transcripts pre-transplant and a 10% increase post-transplant were predictive of outcome (142). A combination of multiple methods to detect MRD may be required to provide the most accurate prognostic information. For example combining NGS MRD for NPM1 with multicolor flow cytometry may improve relapse prediction over either modality alone (143).
Discrepancies between the most discriminatory MRD thresholds at different treatment stages illustrate how the pre- and post-transplant bone marrow environment is different; post-transplant, there is a complex immunological milieu of developing tolerance and GVL. As not all patients with MRD relapse, it is postulated that the GVL effect may eradicate residual disease without the need for further intervention. Although it is also logical that early intervention for patients with molecular MRD would be beneficial, there is limited evidence to support this strategy. In a sub-analysis of patients included in the UK AML17 trial, the provision of post-transplant MRD information to clinicians did not affect outcomes – although this was not a randomized comparison, and not a main aim of the study (97).
Chimerism
Post-transplant monitoring of host-donor hematopoietic chimerism is a widely used post-transplant monitoring strategy, particularly after RIC allo-SCT. Chimerism can be measured in the whole blood, or specifically in T cells (CD3+ selected) or myeloid cells (CD33+). It is known that patients with mixed chimerism post-RIC allo-SCT do have an increased risk of relapse (144), although it should be noted that chimerism and residual disease are conceptually different. Mixed chimerism does not necessarily mean the presence of residual disease, nor does complete chimerism confirm its absence. In haploidentical allo-SCT disease relapse can occur due to acquired uniparental disomy of chromosome 6p leading to loss of the mismatched HLA-haplotype on leukemia cells and subsequent immune escape (145, 146). In this context, chimerism measurement by disparate methodologies can yield different results: recipient non-HLA marker based chimerism shows an increase during relapse, whilst HLA marker based chimerism remains low in disease relapse driven by a loss of HLA (147). Nevertheless chimerism monitoring, post RIC allo-SCT is an important way of identifying patients at high risk of relapse in whom intervention with pre-emptive DLI may be beneficial. Patients who achieve full donor chimerism (FDC) with DLI have a comparable outcome to those who reach FDC spontaneously (148, 149).
There may be ways to improve the performance of chimerism monitoring, including earlier use post-transplant (150), in CD34+ cells (151–154), and, in combination with monitoring for MRD. Waterhouse et al. compared the utility of chimerism and molecular monitoring including WT1 over-expression. Of 15/70 patients in whom increasing mixed chimerism was detected, all had a positive MRD marker and/or increased WT1 expression. They found that in half, detectable MRD and mixed chimerism occurred at the same time but in the other half, mixed chimerism preceded MRD positivity (155). The FIGARO study demonstrated that the risk of relapse following pre-transplant MRD positivity, is reduced by the achievement of full donor chimerism (14), and is a key finding that should direct future treatment strategies to identify methods of increasing the rate of achieving full donor chimerism.
Post-Transplant Maintenance Strategies to Reduce Relapse
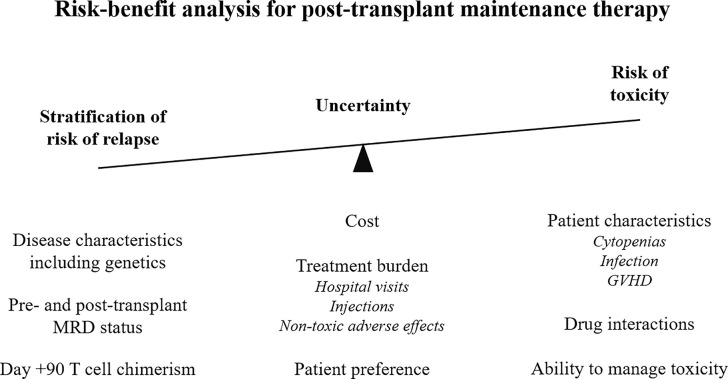
Post-transplant pharmacological interventions may have direct activity on malignant cells, and there is improving understanding that modulation of the complex immunological environment may provide additional benefit. There is improving interest in assessing the impact of routine, maintenance treatments, which do not significantly add to the burden of toxicity which includes infection, organ toxicity, and GVHD ( Table 3 and Figure 3 ).
Table 3.
Examples of post-transplant maintenance strategies.
| Mechanism | Examples of use | ||
|---|---|---|---|
| Non-targeted agents | Azacitidine | Epigenetic modulator | RICAZA (2016) Phase II trial, azacitidine single agent, n=37. Reduced GvHD (156). RELAZA (2012) Phase II trial, azacitadine single agent for mixed CD34+ chimerism, n=20. 80% responded (157). RELAZA2 (2018) Phase II trial, azacitadine single agent for MRD+ patients, n=55. Relapse free survival at 12 months 46% (84). Oran et al. (2020) Phase III trial, n=187. No difference in relapse free survival or overall survival (158). |
| Oral azacitidine | Epigenetic modulator | On-going phase III trial NCT04173533 (oral azacitidine versus placebo). |
|
| Panobinostat | Epigenetic modulator | On-going phase II trial NCT04326764 | |
| Lenalidomide | Immunomodulator | LENAMAINT (2012) Phase II trial, n=10. Stopped early due to high incidence of severe acute GVHD (159). |
|
| Targeted agents | Sorafenib | Broad-spectrum tyrosine kinase inhibitor | SORMAIN study (2020) Randomised phase II, n=83, FLT3-ITD. Improved relapse free survival at 2 years (85% versus 53%) (160). Xuan et al. (13) Randomised phase III, n=202, FLT3-ITD. Reduced relapse at 1 year (7% versus 24%) (13). |
| Midostaurin | Broad-spectrum tyrosine kinase inhibitor | RADIUS study (2020) Phase II, n=60 (161). |
|
| Gilteritinib | FLT-3 inhibitor | On-going phase III trial NCT02997202 (gilteritinib versus placebo). |
|
| Venetoclax | BCL-2 inhibitor | Kent et al. (2020) (abstract) Phase II, n=23. 6 month leukemia free survival: 87% (162). On-going trials Venetoclax + azacitidine. NCT04161885 (phase III) and NCT04128501 (phase II). |
|
| Glasdegib | Hedgehog inhibitor | Kent et al. (2020) Phase II, n=31, high risk patients. No apparent benefit (163). |
|
| Ivosidenib | IDH-1 inhibitor | On-going phase I trial NCT03728335 | |
| Enasidenib | IDH-2 inhibitor | On-going phase I trial NCT03564821 | |
| Cellular therapy | Prophylactic donor lymphocyte infusion (DLI) | Graft-versus-leukemia effect | Schmid et al. (2019) Retrospective matched-pair study of prophylactic DLI for high-risk disease. Overall survival benefit (69.8% vs. 40.2%) (164). On-going phase II trial NCT02856464 |
Figure 3.
Risk-benefit analysis for maintenance therapy post-allogeneic stem cell transplant. MRD, measurable residual disease; GVHD, graft versus host disease.
Non-Targeted Agents
Non-targeted agents which modulate the immune system and tumor microenvironment have the advantage that they are generalizable, are not dependent on specific mutations and may maintain efficacy across the patchwork of clonally heterogeneous disease which is rapidly changing in the post-transplant bone marrow (165, 166).
Azacitidine is an epigenetic modulator that has efficacy in AML both as sole therapy and in combination with other treatments. Post-transplant, in the RICAZA study, Azacitadine was shown to be well tolerated and may both reduce the risk of GVHD through regulatory T-cell expansion and augment the GVL through upregulation of cancer associated antigens on leukemia cells (139, 156, 167). Azacitadine has also been studied in the RELAZA (157) and RELAZA2 (84) studies whereby patients were with mixed CD34+ chimerism and MRD positivity respectively were offered single-agent Azacitadine. In RELAZA, 80% patients responded and Azacitadine delayed relapse. In RELAZA2, relapse free survival at 12 months was 46% in those who had MRD detected and received Azacitadine, suggesting a delaying of haematological relapse. Despite this, a phase 3 RCT of azacitadine versus observation did not show evidence of survival benefit when used as post-transplant maintenance for patients with high risk AML, although this study was limited by the short duration of time that patients remained on treatment (158). The oral formulation of Azacitidine (CC-486) and Panobinostat, another epigenetic modulator have also shown promise in early phase studies and are both the subject of on-going RCTs (NCT04173533 and NCT04326764 respectively) (168, 169). Lenalidomide, an immunomodulator, in combination with Azacitidine is also active in post-transplant relapse (170) but is associated with GVHD when used as monotherapy in the maintenance setting (159) thus indicating the importance of studying the effects of drugs in this specific treatment stage.
DLI can induce remission in patients with hematological relapse, eradicate MRD and promote reversion to full donor chimerism. Alternatively, prophylactic DLI can be delivered to patients at high risk of relapse regardless of detectable disease. A recent observational, matched-pair study found that prophylactic DLI in patients with high-risk AML increased OS at five years by 30% (164). The on-going prospective, 2-arm, phase II PRO-DLI randomized trial will add valuable further information in this area (171). There are also developing technology to manipulate DLI to improve efficacy and limit toxicity. These are reviewed elsewhere, and studies are on-going (172).
Targeted Agents and Future Areas of Development
Routine application of NGS for DNA mutations have allowed for the identification of dysregulated, druggable pathways in AML. Many are only applicable to a subset of patients, but may also offer the first rung on the ladder of personalized medicine. The major challenges include identification of suitable, druggable targets in the context of clonal heterogeneity (165), and proving clinical efficacy when patient subgroups are relatively small.
An ever-expanding list of targeted treatments directed against key pathways in AML have received Food and Drug Administration FDA approval in recent years. FLT3, as described above is a tyrosine kinase, mutations in which are known to be associated with poor outcomes. In patients with FLT3 mutations, the use of post-transplant sorafenib, a broad-spectrum tyrosine kinase inhibitor (including FLT3), was associated with improved survival compared with placebo (13), findings that were consistent with the phase II SORMAIN study (160). As discussed earlier, the use of another broad-spectrum FLT3 inhibitor, midostaurin along with induction chemotherapy improves outcomes in FLT3-mutated AML (50). In the post-transplant setting, evidence of benefit from midostaurin is limited to a randomized phase II study (RADIUS) which showed a reduction in relapse with midostaurin treatment post-transplant albeit compared with historical controls (173).
Despite some evidence of benefit, there remain concerns about the off-target toxicity and adverse events associated with the broad-spectrum tyrosine kinase inhibitors. The aforementioned SORMAIN study found that the patients most likely to benefit from sorafenib post-transplant were those in whom MRD was detectable (160). For treatments where there are concerns over toxicity, especially in patients with more comorbidities, it is clear that post-transplant disease monitoring can add vital information for assessment of the risk-benefit equation. Second generation drugs which are potent, more specific FLT3 inhibitors are now available and have efficacy as monotherapy in relapsed AML (37). Clinical evaluation of Gilteritinib for post-transplant maintenance is underway (174).
Other targets of small molecule inhibitors include the anti-apoptotic protein BCL2, the Hedgehog signaling pathway, and isocitrate dehydrogenase 1 and 2 (IDH1 & 2). Venetoclax is a selective BCL2 inhibitor which is currently licensed in combination with Azacitidine for the treatment of older patients who are not suitable for intensive treatment and was found to have a substantial survival benefit in this cohort when compared with Azacitidine monotherapy (89). In a small study in the post-transplant maintenance setting, Venetoclax was reported to be safe and well tolerated but further studies are required to demonstrate benefit (162). Venetoclax is also being assessed in combination with Azacitadine as maintenance therapy post-transplant (175, 176) but its application may be limited by concerns over myelosuppression.
Glasdegib is an inhibitor of the Hedgehog signaling pathway which has evidence of modest benefit in combination with low dose Cytarabine for patients unfit for intensive treatment (38). It has been recently evaluated in a small single arm study in unselected high-risk patients in the post-transplant maintenance setting. However, there was no clear evidence of benefit either measured by MRD elimination, change in chimerism status, or clinical outcomes. Additionally, treatment was complicated by adverse events requiring pausing or cessation of treatment (163). Further studies in patients who are most likely to benefit as identified by genetic pre-stratification are required.
IDH1 and 2 are proteins which mediate the conversion of isocitrate to alpha-ketoglutarate. Gain in function mutations result in DNA and histone hypermethylation and altered downstream gene expression contributing to oncogenesis. Ivosidenib and Enasidenib, IDH1 and IDH2 inhibitors respectively both have evidence of efficacy in single-arm studies in AML (177–179) and are currently being evaluated for post-transplant maintenance (180, 181).
In summary, there is emerging, encouraging evidence that post-transplant maintenance therapies can reduce the risk of relapse, modulate the risk of GVHD, and improve survival. However, their use must be balanced in order to weigh up the additional toxicity and financial burden against the magnitude of the clinical effect. Detailed molecular analysis of a patient’s disease and post-transplant disease monitoring will allow further stratification and potentially identify the patients who are most likely to benefit from treatment (summarized in Figure 3 ).
Conclusion
The establishment of large transplant trial networks has improved the scientific rationale behind transplant practice at every stage of the treatment pathway. This has improved the identification of which patients who are most likely to benefit from an allo-SCT, and also provides a rigorous assessment of novel agents that may benefit patients. Finally, by embedding correlative translational science in these studies, this further improves our knowledge and understanding of the scientific basis of clinical practice. This is of direct benefit to patients, and subsequently provides a vital starting place for future studies.
Author Contributions
All authors contributed to the writing of this review article. All authors contributed to the article and approved the submitted version.
Funding
Research support and clinical trials funding from CRUK, Bloodwise and Cure Leukaemia acknowledged. Core funding to the Birmingham ECMC Centre program is gratefully acknowledged.
Conflict of Interest
CC has received honoraria from Celgene, Daichi-Sankyo, Novartis and Pfizer as well as research funding from Celgene. JL has received travel funding from Novartis and Daichi-Sankyo, honoraria from Pfizer, Janssen and Amgen.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer CS has declared past co-authorships with one of the authors CC, to the handling editor, at the time of review.
References
- 1. Thomas ED, Lochte HL, Jr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med (1957) 257(11):491–6. 10.1056/NEJM195709122571102 [DOI] [PubMed] [Google Scholar]
- 2. Appelbaum FR. Hematopoietic-Cell Transplantation at 50. N Engl J Med (2007) 357(15):1472–5. 10.1056/NEJMp078166 [DOI] [PubMed] [Google Scholar]
- 3. Cornelissen JJ, Breems D, Putten WLJV, Gratwohl AA, Passweg JR, Pabst T, et al. Comparative Analysis of the Value of Allogeneic Hematopoietic Stem-Cell Transplantation in Acute Myeloid Leukemia With Monosomal Karyotype Versus Other Cytogenetic Risk Categories. J Clin Oncol (2012) 30(17):2140–6. 10.1200/JCO.2011.39.6499 [DOI] [PubMed] [Google Scholar]
- 4. Cornelissen J. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol (2012) 9:579–90. 10.1038/nrclinonc.2012.150 [DOI] [PubMed] [Google Scholar]
- 5. Wheatley K, Burnett AK, Goldstone AH, Gray RG, Hann IM, Harrison CJ, et al. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council’s Adult and Childhood Leukaemia Working Parties. Br J Haematol (1999) 107(1):69–79. 10.1046/j.1365-2141.1999.01684.x [DOI] [PubMed] [Google Scholar]
- 6. Grimwade D. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood (2010) 116:354–65. 10.1182/blood-2009-11-254441 [DOI] [PubMed] [Google Scholar]
- 7. Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N Engl J Med (2016) 374(23):2209–21. 10.1056/NEJMoa1516192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood (2005) 106(8):2912–9. 10.1182/blood-2005-05-2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sorror ML, Giralt S, Sandmaier BM, De Lima M, Shahjahan M, Maloney DG, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood (2007) 110(13):4606–13. 10.1182/blood-2007-06-096966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freeman SD, Hills RK, Virgo P, Khan N, Couzens S, Dillon R, et al. Measurable Residual Disease at Induction Redefines Partial Response in Acute Myeloid Leukemia and Stratifies Outcomes in Patients at Standard Risk Without NPM1 Mutations. J Clin Oncol (2018) 36(15):1486–97. 10.1200/JCO.2017.76.3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fuchs EJ, O’Donnell PV, Eapen M, Logan BR, Antin JH, Dawson P, et al. Double unrelated umbilical cord blood versus HLA-haploidentical bone marrow transplantation (BMT CTN 1101). Blood (2021) 137(3):420–8. 10.1182/blood.2020007535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rambaldi A, Grassi A, Masciulli A, Boschini C, Mico MC, Busca A, et al. Busulfan plus cyclophosphamide versus busulfan plus fludarabine as a preparative regimen for allogeneic haemopoietic stem-cell transplantation in patients with acute myeloid leukaemia: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol (2015) 16(15):1525–36. 10.1016/S1470-2045(15)00200-4 [DOI] [PubMed] [Google Scholar]
- 13. Xuan L, Wang Y, Huang F, Fan Z, Xu Y, Sun J, et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: an open-label, multicentre, randomised phase 3 trial. Lancet Oncol (2020) 21(9):1201–12. 10.1016/S1470-2045(20)30455-1 [DOI] [PubMed] [Google Scholar]
- 14. Craddock C, Jackson A, Loke J, Siddique S, Hodgkinson A, Mason J, et al. Augmented Reduced-Intensity Regimen Does Not Improve Postallogeneic Transplant Outcomes in Acute Myeloid Leukemia. J Clin Oncol (2020) 0(0):JCO.20.02308. 10.1200/JCO.20.02308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J Clin Oncol (2017) 35(11):1154–61. 10.1200/JCO.2016.70.7091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J Clin Oncol (2018) 36(26):2684–92. 10.1200/JCO.2017.77.6112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Löwenberg B, Pabst T, Vellenga E, van Putten W, Schouten HC, Graux C, et al. Cytarabine Dose for Acute Myeloid Leukemia. N Engl J Med (2011) 364(11):1027–36. 10.1056/NEJMoa1010222 [DOI] [PubMed] [Google Scholar]
- 18. Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced Mortality after Allogeneic Hematopoietic-Cell Transplantation. N Engl J Med (2010) 363(22):2091–101. 10.1056/NEJMoa1004383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burnett AK, Goldstone A, Hills RK, Milligan D, Prentice A, Yin J, et al. Curability of Patients With Acute Myeloid Leukemia Who Did Not Undergo Transplantation in First Remission. J Clin Oncol (2013) 31(10):1293–301. 10.1200/JCO.2011.40.5977 [DOI] [PubMed] [Google Scholar]
- 20. Michallet M, Bilger K, Garban F, Attal M, Huyn A, Blaise D, et al. Allogeneic Hematopoietic Stem-Cell Transplantation After Nonmyeloablative Preparative Regimens: Impact of Pretransplantation and Posttransplantation Factors on Outcome. J Clin Oncol (2001) 19(14):3340–9. 10.1200/JCO.2001.19.14.3340 [DOI] [PubMed] [Google Scholar]
- 21. Charles C, Sandeep N, Andrew P, Cassandra B, Laura B, Emmanouil N, et al. Factors predicting long-term survival after T-cell depleted reduced intensity allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica (2010) 95(6):989–95. 10.3324/haematol.2009.013920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Innes AJ, Woolley P, Szydlo RM, Lozano S, Fernando F, Bansal D, et al. Complete remission with incomplete count recovery (CRi) prior to allogeneic HCT for acute myeloid leukaemia is associated with a high non-relapse mortality. Leukemia (2020) 34(2):667–70. 10.1038/s41375-019-0572-z [DOI] [PubMed] [Google Scholar]
- 23. Tallman MS, Rowlings PA, Milone G, Zhang MJ, Perez WS, Weisdorf D, et al. Effect of postremission chemotherapy before human leukocyte antigen-identical sibling transplantation for acute myelogenous leukemia in first complete remission. Blood (2000) 96(4):1254–8. 10.1182/blood.V96.4.1254 [DOI] [PubMed] [Google Scholar]
- 24. DeWolf S, Tallman MS. How I treat relapsed or refractory AML. Blood (2020) 136(9):1023–32. 10.1182/blood.2019001982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gale RP, Horowitz MM, Rees JK, Gray RG, Oken MM, Estey EH, et al. Chemotherapy versus transplants for acute myelogenous leukemia in second remission. Leukemia (1996) 10(1):13–9. [PubMed] [Google Scholar]
- 26. Ganzel C, Sun Z, Cripe LD, Fernandez HF, Douer D, Rowe JM, et al. Very poor long-term survival in past and more recent studies for relapsed AML patients: The ECOG-ACRIN experience. Am J Hematol (2018) 93(8):1074–81. 10.1002/ajh.25162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gorin NC, Labopin M, Frassoni F, Milpied N, Attal M, Blaise D, et al. Identical outcome after autologous or allogeneic genoidentical hematopoietic stem-cell transplantation in first remission of acute myelocytic leukemia carrying inversion 16 or t(8;21): a retrospective study from the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol (2008) 26(19):3183–8. 10.1200/JCO.2007.15.3106 [DOI] [PubMed] [Google Scholar]
- 28. Breems DA, Van Putten WL, Huijgens PC, Ossenkoppele GJ, Verhoef GE, Verdonck LF, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol (2005) 23(9):1969–78. 10.1200/JCO.2005.06.027 [DOI] [PubMed] [Google Scholar]
- 29. Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol (2010) 28(23):3730–8. 10.1200/JCO.2010.28.8852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tauro S, Craddock C, Peggs K, Begum G, Mahendra P, Cook G, et al. Allogeneic stem-cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease-free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. J Clin Oncol (2005) 23(36):9387–93. 10.1200/JCO.2005.02.0057 [DOI] [PubMed] [Google Scholar]
- 31. Weisdorf DJ, Millard HR, Horowitz MM, Hyare PS, Champlin R, Ho V, et al. Allogeneic transplantation for advanced acute myeloid leukemia: The value of complete remission. Cancer (2017) 123(11):2025–34. 10.1002/cncr.30536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gilleece MH, Labopin M, Savani BN, Yakoub-Agha I, Socie G, Gedde-Dahl T, et al. Allogeneic haemopoietic transplantation for acute myeloid leukaemia in second complete remission: a registry report by the Acute Leukaemia Working Party of the EBMT. Leukemia (2019) 34(1):87–99. 10.1038/s41375-019-0527-4 [DOI] [PubMed] [Google Scholar]
- 33. Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood (2017) 129(4):424–47. 10.1182/blood-2016-08-733196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Craddock C, Nagra S, Peniket A, Brookes C, Buckley L, Nikolousis E, et al. Factors predicting long-term survival after T-cell depleted reduced intensity allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica (2010) 95(6):989–95. 10.3324/haematol.2009.013920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Craddock C, Labopin M, Pillai S, Finke J, Bunjes D, Greinix H, et al. Factors predicting outcome after unrelated donor stem cell transplantation in primary refractory acute myeloid leukaemia. Leukemia (2011) 25(5):808–13. 10.1038/leu.2011.13 [DOI] [PubMed] [Google Scholar]
- 36. Ferguson P, Hills RK, Grech A, Betteridge S, Kjeldsen L, Dennis M, et al. An operational definition of primary refractory acute myeloid leukaemia allowing early identification of patients who may benefit from allogeneic stem cell transplantation. Haematologica (2016) 101(11):1351–8. 10.3324/haematol.2016.148825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perl AE, Altman JK, Cortes J, Smith C, Litzow M, Baer MR, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1-2 study. Lancet Oncol (2017) 18(8):1061–75. 10.1016/S1470-2045(17)30416-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cortes JE, Khaled S, Martinelli G, Perl AE, Ganguly S, Russell N, et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol (2019) 20(7):984–97. 10.1016/S1470-2045(19)30150-0 [DOI] [PubMed] [Google Scholar]
- 39. Najima Y, Sadato D, Harada Y, Oboki K, Hirama C, Toya T, et al. Prognostic impact of TP53 mutation, monosomal karyotype, and prior myeloid disorder in nonremission acute myeloid leukemia at allo-HSCT. Bone Marrow Transplant (2021) 56(2):334–46. 10.1038/s41409-020-01016-9 [DOI] [PubMed] [Google Scholar]
- 40. Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature (2012) 481(7382):506–10. 10.1038/nature10738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood (2012) 119(6):1599–606. 10.1182/blood-2011-08-375840 [DOI] [PubMed] [Google Scholar]
- 42. Christopeit M, Kuss O, Finke J, Bacher U, Beelen DW, Bornhauser M, et al. Second allograft for hematologic relapse of acute leukemia after first allogeneic stem-cell transplantation from related and unrelated donors: the role of donor change. J Clin Oncol (2013) 31(26):3259–71. 10.1200/JCO.2012.44.7961 [DOI] [PubMed] [Google Scholar]
- 43. Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA (2009) 301(22):2349–61. 10.1001/jama.2009.813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med (2008) 358(18):1909–18. 10.1056/NEJMoa074306 [DOI] [PubMed] [Google Scholar]
- 45. Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood (2001) 98(6):1752–9. 10.1182/blood.V98.6.1752 [DOI] [PubMed] [Google Scholar]
- 46. Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med (2005) 352(3):254–66. 10.1056/NEJMoa041974 [DOI] [PubMed] [Google Scholar]
- 47. Metzeler KH, Becker H, Maharry K, Radmacher MD, Kohlschmidt J, Mrozek K, et al. ASXL1 mutations identify a high-risk subgroup of older patients with primary cytogenetically normal AML within the ELN Favorable genetic category. Blood (2011) 118(26):6920–9. 10.1182/blood-2011-08-368225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mendler JH, Maharry K, Radmacher MD, Mrozek K, Becker H, Metzeler KH, et al. RUNX1 mutations are associated with poor outcome in younger and older patients with cytogenetically normal acute myeloid leukemia and with distinct gene and MicroRNA expression signatures. J Clin Oncol (2012) 30(25):3109–18. 10.1200/JCO.2011.40.6652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ortmann CA, Kent DG, Nangalia J, Silber Y, Wedge DC, Grinfeld J, et al. Effect of Mutation Order on Myeloproliferative Neoplasms. N Engl J Med (2015) 372(7):601–12. 10.1056/NEJMoa1412098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med (2017) 377(5):454–64. 10.1056/NEJMoa1614359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Burd A, Levine RL, Ruppert AS, Mims AS, Borate U, Stein EM, et al. Precision medicine treatment in acute myeloid leukemia using prospective genomic profiling: feasibility and preliminary efficacy of the Beat AML Master Trial. Nat Med (2020) 26(12):1852–8. 10.1038/s41591-020-1089-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. DiNardo CD, Tiong IS, Quaglieri A, MacRaild S, Loghavi S, Brown FC, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood (2020) 135(11):791–803. 10.1182/blood.2019003988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grinfeld J, Nangalia J, Baxter EJ, Wedge DC, Angelopoulos N, Cantrill R, et al. Classification and Personalized Prognosis in Myeloproliferative Neoplasms. N Engl J Med (2018) 379(15):1416–30. 10.1056/NEJMoa1716614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fenwarth L, Thomas X, de Botton S, Duployez N, Bourhis JH, Lesieur A, et al. A personalized approach to guide allogeneic stem cell transplantation in younger adults with acute myeloid leukemia. Blood (2021) 137(4):524–32. 10.1182/blood.2020005524 [DOI] [PubMed] [Google Scholar]
- 55. Craddock C, Versluis J, Labopin M, Socie G, Huynh A, Deconinck E, et al. Distinct factors determine the kinetics of disease relapse in adults transplanted for acute myeloid leukaemia. J Internal Med (2018) 283(4):371–9. 10.1111/joim.12720 [DOI] [PubMed] [Google Scholar]
- 56. Schuurhuis GJ, Heuser M, Freeman S, Bene MC, Buccisano F, Cloos J, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood (2018) 131(12):1275–91. 10.1182/blood-2017-09-801498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jourdan E, Boissel N, Chevret S, Delabesse E, Renneville A, Cornillet P, et al. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood (2013) 121(12):2213–23. 10.1182/blood-2012-10-462879 [DOI] [PubMed] [Google Scholar]
- 58. Ivey A, Hills RK, Simpson MA, Jovanovic JV, Gilkes A, Grech A, et al. Assessment of Minimal Residual Disease in Standard-Risk AML. N Engl J Med (2016) 374(5):422–33. 10.1056/NEJMoa1507471 [DOI] [PubMed] [Google Scholar]
- 59. Prébet T, Boissel N, Reutenauer S, Thomas X, Delaunay J, Cahn J-Y, et al. Acute Myeloid Leukemia With Translocation (8;21) or Inversion (16) in Elderly Patients Treated With Conventional Chemotherapy: A Collaborative Study of the French CBF-AML Intergroup. J Clin Oncol (2009) 27(28):4747–53. 10.1200/JCO.2008.21.0674 [DOI] [PubMed] [Google Scholar]
- 60. Yin JA, O’Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood (2012) 120(14):2826–35. 10.1182/blood-2012-06-435669 [DOI] [PubMed] [Google Scholar]
- 61. Rücker FG, Agrawal M, Corbacioglu A, Weber D, Kapp-Schwoerer S, Gaidzik VI, et al. Measurable residual disease monitoring in acute myeloid leukemia with t(8;21)(q22;q22.1): results from the AML Study Group. Blood (2019) 134(19):1608–18. 10.1182/blood.2019001425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Balsat M, Renneville A, Thomas X, Botton SD, Caillot D, Marceau A, et al. Postinduction Minimal Residual Disease Predicts Outcome and Benefit From Allogeneic Stem Cell Transplantation in Acute Myeloid Leukemia With NPM1 Mutation: A Study by the Acute Leukemia French Association Group. J Clin Oncol (2017) 35(2):185–93. 10.1200/JCO.2016.67.1875 [DOI] [PubMed] [Google Scholar]
- 63. Ostronoff F, Othus M, Lazenby M, Estey E, Appelbaum FR, Evans A, et al. Prognostic significance of NPM1 mutations in the absence of FLT3-internal tandem duplication in older patients with acute myeloid leukemia: a SWOG and UK National Cancer Research Institute/Medical Research Council report. J Clin Oncol (2015) 33(10):1157–64. 10.1200/JCO.2014.58.0571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Angenendt L, Röllig C, Montesinos P, Martínez-Cuadrón D, Barragan E, García R, et al. Chromosomal Abnormalities and Prognosis in NPM1-Mutated Acute Myeloid Leukemia: A Pooled Analysis of Individual Patient Data From Nine International Cohorts. J Clin Oncol (2019) 37(29):2632–42. 10.1200/JCO.19.00416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Freeman SD, Virgo P, Couzens S, Grimwade D, Russell N, Hills RK, et al. Prognostic Relevance of Treatment Response Measured by Flow Cytometric Residual Disease Detection in Older Patients With Acute Myeloid Leukemia. J Clin Oncol (2013) 31(32):4123–31. 10.1200/JCO.2013.49.1753 [DOI] [PubMed] [Google Scholar]
- 66. Jongen-Lavrencic M, Grob T, Hanekamp D, Kavelaars FG, al Hinai A, Zeilemaker A, et al. Molecular Minimal Residual Disease in Acute Myeloid Leukemia. N Engl J Med (2018) 378(13):1189–99. 10.1056/NEJMoa1716863 [DOI] [PubMed] [Google Scholar]
- 67. Ghannam J, Dillon LW, Hourigan CS. Next-generation sequencing for measurable residual disease detection in acute myeloid leukaemia. Br J Haematol (2020) 188(1):77–85. 10.1111/bjh.16362 [DOI] [PubMed] [Google Scholar]
- 68. Loke J, Malladi R, Moss P, Craddock C. The role of allogeneic stem cell transplantation in the management of acute myeloid leukaemia: a triumph of hope and experience. Br J Haematol (2020) 188(1):129–46. 10.1111/bjh.16355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood (2016) 127(1):62–70. 10.1182/blood-2015-07-604546 [DOI] [PubMed] [Google Scholar]
- 70. Brunet S, Labopin M, Esteve J, Cornelissen J, Socié G, Iori AP, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol (2012) 30(7):735–41. 10.1200/JCO.2011.36.9868 [DOI] [PubMed] [Google Scholar]
- 71. Gratwohl A, Hermans J, Goldman JM, Arcese W, Carreras E, Devergie A, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet (1998) 352(9134):1087–92. 10.1016/S0140-6736(98)03030-X [DOI] [PubMed] [Google Scholar]
- 72. Gratwohl A, Stern M, Brand R, Apperley J, Baldomero H, de Witte T, et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Cancer (2009) 115(20):4715–26. 10.1002/cncr.24531 [DOI] [PubMed] [Google Scholar]
- 73. Sorror ML, Storb RF, Sandmaier BM, Maziarz RT, Pulsipher MA, Maris MB, et al. Comorbidity-Age Index: A Clinical Measure of Biologic Age Before Allogeneic Hematopoietic Cell Transplantation. J Clin Oncol (2014) 32: (29):3249–56. 10.1200/JCO.2013.53.8157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gerstung M, Papaemmanuil E, Martincorena I, Bullinger L, Gaidzik VI, Paschka P, et al. Precision oncology for acute myeloid leukemia using a knowledge bank approach. Nat Genet (2017) 49(3):332–40. 10.1038/ng.3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shouval R, Labopin M, Bondi O, Mishan-Shamay H, Shimoni A, Ciceri F, et al. Prediction of Allogeneic Hematopoietic Stem-Cell Transplantation Mortality 100 Days After Transplantation Using a Machine Learning Algorithm: A European Group for Blood and Marrow Transplantation Acute Leukemia Working Party Retrospective Data Mining Study. J Clin Oncol (2015) 33(28):3144–51. 10.1200/JCO.2014.59.1339 [DOI] [PubMed] [Google Scholar]
- 76. Schiffer C, Lee E, Tomiyasu T, Wiernik P, Testa J. Prognostic impact of cytogenetic abnormalities in patients with de novo acute nonlymphocytic leukemia. Blood (1989) 73: (1):263–70. 10.1182/blood.V73.1.263.bloodjournal731263 [DOI] [PubMed] [Google Scholar]
- 77. Muffly L, Pasquini MC, Martens M, Brazauskas R, Zhu X, Adekola K, et al. Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood (2017) 130(9):1156–64. 10.1182/blood-2017-03-772368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nikolousis E, Nagra S, Pearce R, Perry J, Kirkland K, Byrne J, et al. Impact of pre-transplant co-morbidities on outcome after alemtuzumab-based reduced intensity conditioning allo-SCT in elderly patients: a British Society of Blood and Marrow Transplantation study. Bone Marrow Transplant (2015) 50(1):82–6. 10.1038/bmt.2014.215 [DOI] [PubMed] [Google Scholar]
- 79. Muffly LS, Boulukos M, Swanson K, Kocherginsky M, Cerro PD, Schroeder L, et al. Pilot Study of Comprehensive Geriatric Assessment (CGA) in Allogeneic Transplant: CGA Captures a High Prevalence of Vulnerabilities in Older Transplant Recipients. Biol Blood Marrow Transplant (2013) 19(3):429–34. 10.1016/j.bbmt.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 80. Freeman SD, Hourigan CS. MRD evaluation of AML in clinical practice: are we there yet? Hematol Am Soc Hematol Educ Program (2019) 2019(1):557–69. 10.1182/hematology.2019000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Richard-Carpentier G, DiNardo CD. Single-agent and combination biologics in acute myeloid leukemia. Hematol Am Soc Hematol Educ Program (2019) 2019(1):548–56. 10.1182/hematology.2019000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Buckley SA, Wood BL, Othus M, Hourigan CS, Ustun C, Linden MA, et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: a meta-analysis. Haematologica (2017) 102(5):865–73. 10.3324/haematol.2016.159343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hourigan CS, Dillon LW, Gui G, Logan BR, Fei M, Ghannam J, et al. Impact of Conditioning Intensity of Allogeneic Transplantation for Acute Myeloid Leukemia With Genomic Evidence of Residual Disease. J Clin Oncol (2020) 38(12):1273–83. 10.1200/JCO.19.03011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Platzbecker U, Middeke JM, Sockel K, Herbst R, Wolf D, Baldus CD, et al. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): an open-label, multicentre, phase 2 trial. Lancet Oncol (2018) 19(12):1668–79. 10.1016/S1470-2045(18)30580-1 [DOI] [PubMed] [Google Scholar]
- 85. Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y, et al. Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia: Time to Move Toward a Minimal Residual Disease-Based Definition of Complete Remission? J Clin Oncol (2016) 34(4):329–36. 10.1200/JCO.2015.63.3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Halaburda K, Labopin M, Mailhol A, Socié G, Craddock C, Aljurf M, et al. Allogeneic stem cell transplantation in second complete remission for core binding factor acute myeloid leukemia: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica (2020) 105(6):1723–30. 10.3324/haematol.2019.222810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Krug U, Röllig C, Koschmieder A, Heinecke A, Sauerland MC, Schaich M, et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: a web-based application for prediction of outcomes. Lancet (2010) 376(9757):2000–8. 10.1016/S0140-6736(10)62105-8 [DOI] [PubMed] [Google Scholar]
- 88. Walter RB, Othus M, Borthakur G, Ravandi F, Cortes JE, Pierce SA, et al. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: a novel paradigm for treatment assignment. J Clin Oncol (2011) 29(33):4417–23. 10.1200/JCO.2011.35.7525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med (2020) 383(7):617–29. 10.1056/NEJMoa2012971 [DOI] [PubMed] [Google Scholar]
- 90. Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol (2009) 10(3):223–32. 10.1016/S1470-2045(09)70003-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Damaj G, Duhamel A, Robin M, Beguin Y, Michallet M, Mohty M, et al. Impact of azacitidine before allogeneic stem-cell transplantation for myelodysplastic syndromes: a study by the Societe Francaise de Greffe de Moelle et de Therapie-Cellulaire and the Groupe-Francophone des Myelodysplasies. J Clin Oncol (2012) 30(36):4533–40. 10.1200/JCO.2012.44.3499 [DOI] [PubMed] [Google Scholar]
- 92. Gerds AT, Gooley TA, Estey EH, Appelbaum FR, Deeg HJ, Scott BL. Pretransplantation therapy with azacitidine vs induction chemotherapy and posttransplantation outcome in patients with MDS. Biol Blood Marrow Transplant (2012) 18(8):1211–8. 10.1016/j.bbmt.2012.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Voso MT, Leone G, Piciocchi A, Fianchi L, Santarone S, Candoni A, et al. Feasibility of allogeneic stem-cell transplantation after azacitidine bridge in higher-risk myelodysplastic syndromes and low blast count acute myeloid leukemia: results of the BMT-AZA prospective study. Ann Oncol (2017) 28(7):1547–53. 10.1093/annonc/mdx154 [DOI] [PubMed] [Google Scholar]
- 94. Alessandrino EP, Porta MGD, Pascutto C, Bacigalupo A, Rambaldi A. Should Cytoreductive Treatment Be Performed Before Transplantation in Patients With High-Risk Myelodysplastic Syndrome? J Clin Oncol (2013) 31(21):2761–2. 10.1200/JCO.2012.48.0525 [DOI] [PubMed] [Google Scholar]
- 95. Sébert M, Komrokji RS, Sekeres MA, Prebet T, Cluzeau T, Santini V, et al. Impact of baseline cytogenetic findings and cytogenetic response on outcome of high-risk myelodysplastic syndromes and low blast count AML treated with azacitidine. Leuk Res (2017) 63:72–7. 10.1016/j.leukres.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 96. Burnett AK, Russell NH, Hills RK, Kell J, Freeman S, Kjeldsen L, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol (2012) 30(32):3924–31. 10.1200/JCO.2012.42.2964 [DOI] [PubMed] [Google Scholar]
- 97. Dillon R, Hills R, Freeman S, Potter N, Jovanovic J, Ivey A, et al. Molecular MRD status and outcome after transplantation in NPM1-mutated AML. Blood (2020) 135(9):680–8. 10.1182/blood.2019002959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cornelissen JJ, van Putten WL, Verdonck LF, Theobald M, Jacky E, Daenen SM, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood (2007) 109(9):3658–66. 10.1182/blood-2006-06-025627 [DOI] [PubMed] [Google Scholar]
- 99. Bornhäuser M, Kienast J, Trenschel R, Burchert A, Hegenbart U, Stadler M, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol (2012) 13(10):1035–44. 10.1016/S1470-2045(12)70349-2 [DOI] [PubMed] [Google Scholar]
- 100. Kroger N, Iacobelli S, Franke GN, Platzbecker U, Uddin R, Hubel K, et al. Dose-Reduced Versus Standard Conditioning Followed by Allogeneic Stem-Cell Transplantation for Patients With Myelodysplastic Syndrome: A Prospective Randomized Phase III Study of the EBMT (RICMAC Trial). J Clin Oncol (2017) 35(19):2157–64. 10.1200/JCO.2016.70.7349 [DOI] [PubMed] [Google Scholar]
- 101. Clift RA, Buckner CD, Appelbaum FR, Bearman SI, Petersen FB, Fisher LD, et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood (1990) 76(9):1867–71. 10.1182/blood.V76.9.1867.1867 [DOI] [PubMed] [Google Scholar]
- 102. Andersson BS, Kashyap A, Gian V, Wingard JR, Fernandez H, Cagnoni PJ, et al. Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: a phase II study. Biol Blood Marrow Transplant (2002) 8(3):145–54. 10.1053/bbmt.2002.v8.pm11939604 [DOI] [PubMed] [Google Scholar]
- 103. Esteves I, Santos FPS, Fernandes JF, Seber A, Oliveira JSR, Hamerschlak N, et al. Pharmacokinetics analysis results are similar for oral compared to intravenous busulfan in patients undergoing hematopoietic stem cell transplantation, except for the earlier onset of mucositis. A controlled clinical study. Bone Marrow Transplant (2019) 54(11):1799–804. 10.1038/s41409-019-0521-5 [DOI] [PubMed] [Google Scholar]
- 104. Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant (2009) 15(12):1628–33. 10.1016/j.bbmt.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood (1998) 91(3):756–63. 10.1182/blood.V91.3.756.756_756_763 [DOI] [PubMed] [Google Scholar]
- 106. Giralt S, Estey E, Albitar M, van Besien K, Rondon G, Anderlini P, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood (1997) 89(12):4531–6. 10.1182/blood.V89.12.4531 [DOI] [PubMed] [Google Scholar]
- 107. Blaise D, Tabrizi R, Boher JM, Le Corroller-Soriano AG, Bay JO, Fegueux N, et al. Randomized study of 2 reduced-intensity conditioning strategies for human leukocyte antigen-matched, related allogeneic peripheral blood stem cell transplantation: prospective clinical and socioeconomic evaluation. Cancer (2013) 119(3):602–11. 10.1002/cncr.27786 [DOI] [PubMed] [Google Scholar]
- 108. Beelen DW, Trenschel R, Stelljes M, Groth C, Masszi T, Reményi P, et al. Treosulfan or busulfan plus fludarabine as conditioning treatment before allogeneic haemopoietic stem cell transplantation for older patients with acute myeloid leukaemia or myelodysplastic syndrome (MC-FludT.14/L): a randomised, non-inferiority, phase 3 trial. Lancet Haematol (2020) 7(1):e28–39. 10.1016/S2352-3026(19)30157-7 [DOI] [PubMed] [Google Scholar]
- 109. Martino R, Iacobelli S, Brand R, Jansen T, van Biezen A, Finke J, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood (2006) 108(3):836–46. 10.1182/blood-2005-11-4503 [DOI] [PubMed] [Google Scholar]
- 110. Martino R, de Wreede L, Fiocco M, van Biezen A, von dem Borne PA, Hamladji RM, et al. Comparison of conditioning regimens of various intensities for allogeneic hematopoietic SCT using HLA-identical sibling donors in AML and MDS with <10% BM blasts: a report from EBMT. Bone Marrow Transplant (2013) 48(6):761–70. 10.1038/bmt.2012.236 [DOI] [PubMed] [Google Scholar]
- 111. Fasslrinner F, Schetelig J, Burchert A, Kramer M, Trenschel R, Hegenbart U, et al. Long-term efficacy of reduced-intensity versus myeloablative conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: retrospective follow-up of an open-label, randomised phase 3 trial. Lancet Haematol (2018) 5(4):e161–e9. 10.1016/S2352-3026(18)30022-X [DOI] [PubMed] [Google Scholar]
- 112. Schmid C, Schleuning M, Ledderose G, Tischer J, Kolb HJ. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol (2005) 23(24):5675–87. 10.1200/JCO.2005.07.061 [DOI] [PubMed] [Google Scholar]
- 113. Powles RL, Clink HM, Spence D, Morgenstern G, Watson JG, Selby PJ, et al. Cyclosporin a to prevent graft-versus-host disease in man after allogeneic bone-marrow transplantation. Lancet (1980) 315(8164):327–9. 10.1016/S0140-6736(80)90881-8 [DOI] [PubMed] [Google Scholar]
- 114. Storb R, Deeg HJ, Pepe M, Appelbaum F, Anasetti C, Beatty P, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood (1989) 73(6):1729–34. 10.1182/blood.V73.6.1729.1729 [DOI] [PubMed] [Google Scholar]
- 115. Weiden PL, Flournoy N, Thomas ED, Prentice R, Fefer A, Buckner CD, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med (1979) 300(19):1068–73. 10.1056/NEJM197905103001902 [DOI] [PubMed] [Google Scholar]
- 116. Inamoto Y, Flowers ME, Lee SJ, Carpenter PA, Warren EH, Deeg HJ, et al. Influence of immunosuppressive treatment on risk of recurrent malignancy after allogeneic hematopoietic cell transplantation. Blood (2011) 118(2):456–63. 10.1182/blood-2011-01-330217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bacigalupo A, Van Lint MT, Occhini D, Gualandi F, Lamparelli T, Sogno G, et al. Increased risk of leukemia relapse with high-dose cyclosporine A after allogeneic marrow transplantation for acute leukemia. Blood (1991) 77(7):1423–8. 10.1182/blood.V77.7.1423.bloodjournal7771423 [DOI] [PubMed] [Google Scholar]
- 118. Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood (1998) 92(7):2303–14. [PubMed] [Google Scholar]
- 119. Hiraoka A, Ohashi Y, Okamoto S, Moriyama Y, Nagao T, Kodera Y, et al. Phase III study comparing tacrolimus (FK506) with cyclosporine for graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation. Bone Marrow Transplant (2001) 28(2):181–5. 10.1038/sj.bmt.1703097 [DOI] [PubMed] [Google Scholar]
- 120. Ram R, Gafter-Gvili A, Yeshurun M, Paul M, Raanani P, Shpilberg O. Prophylaxis regimens for GVHD: systematic review and meta-analysis. Bone Marrow Transplant (2009) 43(8):643–53. 10.1038/bmt.2008.373 [DOI] [PubMed] [Google Scholar]
- 121. Pidala J, Kim J, Jim H, Kharfan-Dabaja MA, Nishihori T, Fernandez HF, et al. A randomized phase II study to evaluate tacrolimus in combination with sirolimus or methotrexate after allogeneic hematopoietic cell transplantation. Haematologica (2012) 97(12):1882–9. 10.3324/haematol.2012.067140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Furlong T, Kiem HP, Appelbaum FR, Carpenter PA, Deeg HJ, Doney K, et al. Sirolimus in combination with cyclosporine or tacrolimus plus methotrexate for prevention of graft-versus-host disease following hematopoietic cell transplantation from unrelated donors. Biol Blood Marrow Transplant (2008) 14(5):531–7. 10.1016/j.bbmt.2008.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bolwell B, Sobecks R, Pohlman B, Andresen S, Rybicki L, Kuczkowski E, et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant (2004) 34(7):621–5. 10.1038/sj.bmt.1704647 [DOI] [PubMed] [Google Scholar]
- 124. Perkins J, Field T, Kim J, Kharfan-Dabaja MA, Fernandez H, Ayala E, et al. A randomized phase II trial comparing tacrolimus and mycophenolate mofetil to tacrolimus and methotrexate for acute graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant (2010) 16(7):937–47. 10.1016/j.bbmt.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 125. Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol (2009) 10(9):855–64. 10.1016/S1470-2045(09)70225-6 [DOI] [PubMed] [Google Scholar]
- 126. Socié G, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood (2011) 117(23):6375–82. 10.1182/blood-2011-01-329821 [DOI] [PubMed] [Google Scholar]
- 127. Soiffer RJ, LeRademacher J, Ho V, Kan F, Artz A, Champlin RE, et al. Impact of immune modulation with anti–T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood (2011) 117(25):6963–70. 10.1182/blood-2011-01-332007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ruutu T, van Biezen A, Hertenstein B, Henseler A, Garderet L, Passweg J, et al. Prophylaxis and treatment of GVHD after allogeneic haematopoietic SCT: a survey of centre strategies by the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant (2012) 47(11):1459–64. 10.1038/bmt.2012.45 [DOI] [PubMed] [Google Scholar]
- 129. Admiraal R, Nierkens S, de Witte MA, Petersen EJ, Fleurke GJ, Verrest L, et al. Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: a multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol (2017) 4(4):e183–e91. 10.1016/S2352-3026(17)30029-7 [DOI] [PubMed] [Google Scholar]
- 130. Atta EH, de Sousa AM, Schirmer MR, Bouzas LF, Nucci M, Abdelhay E. Different outcomes between cyclophosphamide plus horse or rabbit antithymocyte globulin for HLA-identical sibling bone marrow transplant in severe aplastic anemia. Biol Blood Marrow Transplant (2012) 18(12):1876–82. 10.1016/j.bbmt.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 131. Chakraverty R, Peggs K, Chopra R, Milligan DW, Kottaridis PD, Verfuerth S, et al. Limiting transplantation-related mortality following unrelated donor stem cell transplantation by using a nonmyeloablative conditioning regimen. Blood (2002) 99(3):1071–8. 10.1182/blood.V99.3.1071 [DOI] [PubMed] [Google Scholar]
- 132. Perez-Simon JA, Kottaridis PD, Martino R, Craddock C, Caballero D, Chopra R, et al. Nonmyeloablative transplantation with or without alemtuzumab: comparison between 2 prospective studies in patients with lymphoproliferative disorders. Blood (2002) 100(9):3121–7. 10.1182/blood-2002-03-0701 [DOI] [PubMed] [Google Scholar]
- 133. Mead AJ, Thomson KJ, Morris EC, Mohamedbhai S, Denovan S, Orti G, et al. HLA-mismatched unrelated donors are a viable alternate graft source for allogeneic transplantation following alemtuzumab-based reduced-intensity conditioning. Blood (2010) 115(25):5147–53. 10.1182/blood-2010-01-265413 [DOI] [PubMed] [Google Scholar]
- 134. Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant (2008) 14(6):641–50. 10.1016/j.bbmt.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Battipaglia G, Labopin M, Kroger N, Vitek A, Afanasyev B, Hilgendorf I, et al. Posttransplant cyclophosphamide vs antithymocyte globulin in HLA-mismatched unrelated donor transplantation. Blood (2019) 134(11):892–9. 10.1182/blood.2019000487 [DOI] [PubMed] [Google Scholar]
- 136. Schmid C, Labopin M, Nagler A, Bornhauser M, Finke J, Fassas A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol (2007) 25(31):4938–45. 10.1200/JCO.2007.11.6053 [DOI] [PubMed] [Google Scholar]
- 137. Hofmann S, Götz M, Schneider V, Guillaume P, Bunjes D, Döhner H, et al. Donor Lymphocyte Infusion Induces Polyspecific CD8+ T-Cell Responses With Concurrent Molecular Remission in Acute Myeloid Leukemia With NPM1 Mutation. J Clin Oncol (2013) 31(3):e44–7. 10.1200/JCO.2011.41.1116 [DOI] [PubMed] [Google Scholar]
- 138. Wermke M, Thiede C, Kiani A, Ehninger G, Bornhäuser M, Platzbecker U. Successful treatment of molecular relapse in NPM1-positive AML using 5-azacytidine. Leukemia (2010) 24(1):236–7. 10.1038/leu.2009.204 [DOI] [PubMed] [Google Scholar]
- 139. Craddock C, Labopin M, Robin M, Finke J, Chevallier P, Yakoub-Agha I, et al. Clinical activity of azacitidine in patients who relapse after allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica (2016) 101(7):879–83. 10.3324/haematol.2015.140996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Qin YZ, Wang Y, Xu LP, Zhang XH, Chen H, Han W, et al. The dynamics of RUNX1-RUNX1T1 transcript levels after allogeneic hematopoietic stem cell transplantation predict relapse in patients with t(8;21) acute myeloid leukemia. J Hematol Oncol (2017) 10(1):44–. 10.1186/s13045-017-0414-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wang Y, Wu DP, Liu QF, Qin YZ, Wang JB, Xu LP, et al. In adults with t(8;21)AML, posttransplant RUNX1/RUNX1T1-based MRD monitoring, rather than c-KIT mutations, allows further risk stratification. Blood (2014) 124(12):1880–6. 10.1182/blood-2014-03-563403 [DOI] [PubMed] [Google Scholar]
- 142. Shayegi N, Kramer M, Bornhäuser M, Schaich M, Schetelig J, Platzbecker U, et al. The level of residual disease based on mutant NPM1 is an independent prognostic factor for relapse and survival in AML. Blood (2013) 122(1):83–92. 10.1182/blood-2012-10-461749 [DOI] [PubMed] [Google Scholar]
- 143. Zhou Y, Othus M, Walter RB, Estey EH, Wu D, Wood BL. Deep NPM1 Sequencing Following Allogeneic Hematopoietic Cell Transplantation Improves Risk Assessment in Adults with NPM1-Mutated AML. Biol Blood Marrow Transplant (2018) 24(8):1615–20. 10.1016/j.bbmt.2018.04.017 [DOI] [PubMed] [Google Scholar]
- 144. McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood (2001) 97(11):3390–400. 10.1182/blood.V97.11.3390 [DOI] [PubMed] [Google Scholar]
- 145. Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MTL, et al. Loss of Mismatched HLA in Leukemia after Stem-Cell Transplantation. N Engl J Med (2009) 361(5):478–88. 10.1056/NEJMoa0811036 [DOI] [PubMed] [Google Scholar]
- 146. Villalobos IB, Takahashi Y, Akatsuka Y, Muramatsu H, Nishio N, Hama A, et al. Relapse of leukemia with loss of mismatched HLA resulting from uniparental disomy after haploidentical hematopoietic stem cell transplantation. Blood (2010) 115(15):3158–61. 10.1182/blood-2009-11-254284 [DOI] [PubMed] [Google Scholar]
- 147. Ahci M, Toffalori C, Bouwmans E, Crivello P, Brambati C, Pultrone C, et al. A new tool for rapid and reliable diagnosis of HLA loss relapses after HSCT. Blood (2017) 130(10):1270–3. 10.1182/blood-2017-05-784306 [DOI] [PubMed] [Google Scholar]
- 148. Brierley CK, Jones FM, Hanlon K, Peniket AJ, Hatton C, Collins GP, et al. Impact of graft-versus-lymphoma effect on outcomes after reduced intensity conditioned-alemtuzumab allogeneic haematopoietic stem cell transplantation for patients with mature lymphoid malignancies. Br J Haematol (2019) 184(4):547–57. 10.1111/bjh.15685 [DOI] [PubMed] [Google Scholar]
- 149. Liesveld JL, Rothberg PG. Mixed chimerism in SCT: Conflict or peaceful coexistence? Bone Marrow Transplant (2008) 42:297–310. 10.1038/bmt.2008.212 [DOI] [PubMed] [Google Scholar]
- 150. Kinsella FAM, Inman CF, Gudger A, Chan YT, Murray DJ, Zuo J, et al. Very early lineage-specific chimerism after reduced intensity stem cell transplantation is highly predictive of clinical outcome for patients with myeloid disease. Leuk Res (2019) 83:106173–. 10.1016/j.leukres.2019.106173 [DOI] [PubMed] [Google Scholar]
- 151. Thiede C, Lutterbeck K, Oelschlägel U, Kiehl M, Steudel C, Platzbecker U, et al. Detection of relapse by sequential monitoring of chimerism in circulating CD34+ cells. Ann Hematol (2002) 81(Suppl 2):S27–8. [PubMed] [Google Scholar]
- 152. Bornhäuser M, Oelschlaegel U, Platzbecker U, Bug G, Lutterbeck K, Kiehl MG, et al. Monitoring of donor chimerism in sorted CD34+ peripheral blood cells allows the sensitive detection of imminent relapse after allogeneic stem cell transplantation. Haematologica (2009) 94(11):1613–7. 10.3324/haematol.2009.007765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Rosenow F, Berkemeier A, Krug U, Müller-Tidow C, Gerss J, Silling G, et al. CD34(+) lineage specific donor cell chimerism for the diagnosis and treatment of impending relapse of AML or myelodysplastic syndrome after allo-SCT. Bone Marrow Transplant (2013) 48(8):1070–6. 10.1038/bmt.2013.2 [DOI] [PubMed] [Google Scholar]
- 154. Hoffmann JC, Stabla K, Burchert A, Volkmann T, Bornhäuser M, Thiede C, et al. Monitoring of acute myeloid leukemia patients after allogeneic stem cell transplantation employing semi-automated CD34+ donor cell chimerism analysis. Ann Hematol (2014) 93(2):279–85. 10.1007/s00277-013-1961-4 [DOI] [PubMed] [Google Scholar]
- 155. Waterhouse M, Pfeifer D, Duque-Afonso J, Follo M, Duyster J, Depner M, et al. Droplet digital PCR for the simultaneous analysis of minimal residual disease and hematopoietic chimerism after allogeneic cell transplantation. Clin Chem Lab Med (2019) 57(5):641–7. 10.1515/cclm-2018-0827 [DOI] [PubMed] [Google Scholar]
- 156. Craddock C, Jilani N, Siddique S, Yap C, Khan J, Nagra S, et al. Tolerability and Clinical Activity of Post-Transplantation Azacitidine in Patients Allografted for Acute Myeloid Leukemia Treated on the RICAZA Trial. Biol Blood Marrow Transplant (2016) 22(2):385–90. 10.1016/j.bbmt.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Platzbecker U, Wermke M, Radke J, Oelschlaegel U, Seltmann F, Kiani A, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: Results of the RELAZA trial. Leukemia (2012) 26(3):381–9. 10.1038/leu.2011.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Oran B, de Lima M, Garcia-Manero G, Thall PF, Lin R, Popat U, et al. A phase 3 randomized study of 5-azacitidine maintenance vs observation after transplant in high-risk AML and MDS patients. Blood Adv (2020) 4(21):5580–8. 10.1182/bloodadvances.2020002544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Sockel K, Bornhaeuser M, Mischak-Weissinger E, Trenschel R, Wermke M, Unzicker C, et al. Lenalidomide maintenance after allogeneic HSCT seems to trigger acute graft-versus-host disease in patients with high-risk myelodysplastic syndromes or acute myeloid leukemia and del(5q): results of the LENAMAINT trial. Haematologica (2012) 97(9):e34–5. 10.3324/haematol.2012.067629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Burchert A, Bug G, Fritz LV, Finke J, Stelljes M, Röllig C, et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia With FLT3-Internal Tandem Duplication Mutation (SORMAIN). J Clin Oncol (2020) 38(26):2993–3002. 10.1200/JCO.19.03345 [DOI] [PubMed] [Google Scholar]
- 161. Maziarz RT, Levis M, Patnaik MM, Scott BL, Mohan SR, Deol A, et al. Midostaurin after allogeneic stem cell transplant in patients with FLT3-internal tandem duplication-positive acute myeloid leukemia. Bone Marrow Transplant (2020). 10.1038/s41409-020-01153-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kent A, Pollyea DA, Winters A, Jordan CT, Smith C, Gutman JA. Venetoclax Is Safe and Tolerable As Post-Transplant Maintenance Therapy for AML Patients at High Risk for Relapse. Blood (2020) 136(Supplement 1):11–2. 10.1182/blood-2020-138832 [DOI] [PubMed] [Google Scholar]
- 163. Kent A, Vasu S, Schatz D, Monson N, Devine S, Smith C, et al. Glasdegib as maintenance therapy for patients with AML and MDS patients at high risk for postallogeneic stem cell transplant relapse. Blood Adv (2020) 4(13):3102–8. 10.1182/bloodadvances.2020001991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Schmid C, Labopin M, Schaap N, Veelken H, Schleuning M, Stadler M, et al. Prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation in acute leukaemia - a matched pair analysis by the Acute Leukaemia Working Party of EBMT. Br J Haematol (2019) 184(5):782–7. 10.1111/bjh.15691 [DOI] [PubMed] [Google Scholar]
- 165. Quek L, Ferguson P, Metzner M, Ahmed I, Kennedy A, Garnett C, et al. Mutational analysis of disease relapse in patients allografted for acute myeloid leukemia. Blood Adv (2016) 1(3):193–204. 10.1182/bloodadvances.2016000760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Jan M, Leventhal MJ, Morgan EA, Wengrod JC, Nag A, Drinan SD, et al. Recurrent genetic HLA loss in AML relapsed after matched unrelated allogeneic hematopoietic cell transplantation. Blood Adv (2019) 3(14):2199–204. 10.1182/bloodadvances.2019000445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Goodyear O, Dennis M, Jilani N, Loke J, Siddique S, Ryan G, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood (2012) 119:3361–9. 10.1182/blood-2011-09-377044 [DOI] [PubMed] [Google Scholar]
- 168. ClinicalTrials.gov. National Library of Medicine (U.S.) Randomised Study of Oral Azacitidine vs Placebo Maintenance in AML or MDS Patients After Allo-SCT Identifier NCT04173533. Available at: https://clinicaltrials.gov/ct2/show/NCT04173533 (Retrieved April 1, 2021).
- 169. ClinicalTrials.gov. National Library of Medicine (U.S.) Panobinostat Maintenance After HSCT for High-risk AML and MDS. Identifier: NCT04326764. Available from: https://clinicaltrials.gov/ct2/show/NCT04326764 (Retrieved April 1, 2021).
- 170. Craddock C, Slade D, De Santo C, Wheat R, Ferguson P, Hodgkinson A, et al. Combination Lenalidomide and Azacitidine: A Novel Salvage Therapy in Patients Who Relapse After Allogeneic Stem-Cell Transplantation for Acute Myeloid Leukemia. J Clin Oncol (2019) 37(7):580–8. 10.1200/JCO.18.00889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. IMPACT Partnership (Internet) . PRO-DLI: A Phase II Prospective Trial of Prophylactic Donor Lymphocyte Infusions for the Prevention of Relapse post HSCT in patients with High Risk Myeloid Malignancy. Available from: https://www.impactpartnership.org.uk/the-trials/pro-dli/ (updated November 12 2020; cited April 1 2020).
- 172. Greiner J, Götz M, Bunjes D, Hofmann S, Wais V. Immunological and Clinical Impact of Manipulated and Unmanipulated DLI after Allogeneic Stem Cell Transplantation of AML Patients. J Clin Med (2019) 9(1). 10.3390/jcm9010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Schlenk RF, Weber D, Fiedler W, Salih HR, Wulf G, Salwender H, et al. Midostaurin added to chemotherapy and continued single-agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood (2019) 133(8):840–51. 10.1182/blood-2018-08-869453 [DOI] [PubMed] [Google Scholar]
- 174. ClinicalTrials.gov. National Library of Medicine (U.S.). A Trial of the FMS-like Tyrosine Kinase 3 (FLT3) Inhibitor Gilteritinib Administered as Maintenance Therapy Following Allogeneic Transplant for Patients With FLT3/Internal Tandem Duplication (ITD) Acute Myeloid Leukemia (AML). Identifier NCT02997202. Available from: https://clinicaltrials.gov/ct2/show/NCT02997202 (Retrieved April 1, 2021).
- 175. ClinicalTrials.gov. National Library of Medicine (U.S.). Venetoclax and Azacitidine for the Treatment of Acute Myeloid Leukemia in the Post-Transplant Setting. Identifier NCT04128501. Available from: https://clinicaltrials.gov/ct2/show/NCT04128501 (Retrieved April 1, 2021).
- 176. ClinicalTrials.gov. National Library of Medicine (U.S.). (2000, February 29 -). A Study Evaluating Safety and Efficacy of Venetoclax in Combination With Azacitidine Versus Standard of Care After Allogeneic Stem Cell Transplantation (SCT) in Participants With Acute Myeloid Leukemia (AML) (VIALE-T). Identifier NCT04161885. Retrieved April 1, 2021 from: https://clinicaltrials.gov/ct2/show/NCT04161885.
- 177. Roboz GJ, DiNardo CD, Stein EM, de Botton S, Mims AS, Prince GT, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood (2020) 135(7):463–71. 10.1182/blood.2019002140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N Engl J Med (2018) 378(25):2386–98. 10.1056/NEJMoa1716984 [DOI] [PubMed] [Google Scholar]
- 179. Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood (2017) 130(6):722–31. 10.1182/blood-2017-04-779405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. ClinicalTrials.gov. National Library of Medicine (U.S.). Enasidenib as Maintenance Therapy in Treating Patients With Acute Myeloid Leukemia With IDH2 Mutation After Donor Stem Cell Transplant. Identifier NCT03728335. Available from: https://clinicaltrials.gov/ct2/show/NCT03728335 (Retrieved April 1, 2021).
- 181. ClinicalTrials.gov. National Library of Medicine (U.S.). IDH1 Inhibition Using Ivosidenib as Maintenance Therapy for IDH1-mutant Myeloid Neoplasms Following Allogeneic Stem Cell Transplantation. Identifier NCT03564821. Available from: https://clinicaltrials.gov/ct2/show/NCT03564821 (Retrieved April 1 2021).