Abstract
Objective
To determine factors associated with diagnostic delays and outcomes in juvenile dermatomyositis (JDM) in the Childhood Arthritis and Rheumatology Research Alliance Legacy Registry (CLR).
Methods
This was a cross‐sectional study of subjects aged 0 to 17 years with JDM enrolled to the CLR from 2010 to 2015. Access to care was measured by calculating the distance from the subject zip code of residence to the treating pediatric rheumatology center and determining the state density of pediatric rheumatologists based on the 2015 American College of Rheumatology Workforce Study. Delay was categorized as early (<30 days), typical (1‐3 months), moderate (3‐12 months), and severe (>12 months). Ordered generalized additive models were used to determine the association between these measures and diagnostic delays.
Results
The median time to diagnosis was 3.1 months; 37.2% of patients experienced moderate delays, and 14.6% experienced severe delays. In a univariate analysis, younger age of disease onset and male sex were associated with delays. Using a generalized additive model accounting for age, sex, race, and ethnicity, increasing distance from treating pediatric rheumatologist and younger age at disease onset were associated with diagnostic delay. There was no association between the state density of rheumatologists and diagnostic delays in this model.
Conclusion
In the CLR, we found moderate to severe diagnostic delays in the majority of subjects with JDM. Our data suggest that access to care, measured as the distance traveled to treating rheumatologist, is an important factor associated with delays in care but also highlight age as a contributing factor, suggesting that JDM may be less recognizable in young children.
Significance & Innovations.
The majority of subjects with juvenile dermatomyositis enrolled to the Childhood Arthritis and Rheumatology Research Alliance Legacy Registry between 2010 and 2015 experienced diagnostic delays of more than 3 months, with 14% having delays of more than 1 year.
Delays were associated with increasing distance traveled to treating pediatric rheumatologist and younger age at disease onset, highlighting factors that impede timely diagnosis.
This study emphasizes the need to expand access to pediatric rheumatology care through expanding both the size and reach of the pediatric rheumatology workforce as well as the need to enhance education of primary care clinicians regarding the diverse presentations of juvenile dermatomyositis.
INTRODUCTION
Juvenile dermatomyositis (JDM) is a rare pediatric autoimmune disorder characterized by muscle inflammation that is distinguished from other juvenile idiopathic inflammatory myopathies by distinctive rashes in stereotypical distributions. Untreated, JDM can cause long‐term damage and functional decline (1, 2). Past studies have shown that delayed diagnosis, race, and socioeconomic factors are associated with disease morbidity, including the development of calcinosis (3, 4), and it has been shown that early, aggressive treatment leads to better outcomes (5). A recent study in childhood‐onset systemic lupus erythematosus (SLE) identified significant delays to first pediatric rheumatology visit and found that severe delays were associated with younger age of onset and low income (6), but this has not been explored in patients with JDM.
Currently there are fewer than 400 board‐certified pediatric rheumatologists (PRs) practicing in the United States. Analyses of data from the most recent 2017 American Board of Pediatrics Workforce Data Book found that nine states have no PRs (7). Limited access to a PR and rare disease manifestations are associated with diagnostic delays in childhood rheumatic diseases (8). A regional survey of primary care pediatricians in Minnesota, North Dakota, and South Dakota regarding referral practices to PRs suggested that the distance to nearest PR in addition to perceived wait time influenced their referral practices and may lead to late referral/diagnosis (9).
In this study, we investigated whether distance from the treating rheumatology center and state density of PRs are associated with diagnostic delays in subjects with JDM enrolled to the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Legacy Registry (CLR). Our goal was to learn which factors might be associated with diagnostic delays in JDM to inform future research, raise awareness, and motivate initiatives to address these factors in order to improve diagnosis and outcomes in children with JDM.
PATIENTS AND METHODS
Patients
This was a cross‐sectional study of patients meeting definite or probable diagnostic criteria for JDM (n = 668) enrolled in the CLR—the first iteration of a registry of children and young adults with rheumatic disease designed to allow larger‐scale research on rare pediatric conditions—from pediatric rheumatology centers across the United States between 2010 and 2015. This was a convenience cohort, and subjects could be enrolled at any point in the disease course. Overall, these subjects trended toward milder disease (10), with the majority of patients having no weakness, and they had a median disease duration of 3.8 years (interquartile range [IQR] 1.6‐6.7 years) (10). Those with incomplete data for the variables “date of symptom onset,” “date of diagnosis,” and “zip code” were excluded, resulting in a data set of 522 individuals.
Methods
The driving distance in miles from the subject zip code centroid to the treating rheumatology center zip code centroid as defined by Google Maps was calculated using the R package “gmapsdistance”. To identify outliers, we first imposed a cutoff of less than 250 miles driving distance on the basis of prior work analyzing the effect of distance in other subspecialties (11, 12). Knowing that some patients may truly travel more than 403.2 kilometers (i.e., 250 miles) to pediatric rheumatology centers, we then individually examined these entries for inclusion using the following logic: patients traveling more than 250 miles were included if they were traveling to an institution within their own state, to a bordering state, or to a known encatchment area (ie, Idaho is an encatchment area for both Seattle and Colorado centers). The remaining observations not meeting this criteria (n = 20) were deemed to be outliers and were removed from the analysis on the assumption that these were patients traveling by air for second opinions or data entry errors. The density of PRs per state was obtained from the 2015 American College of Rheumatology (ACR) Workforce Study (13) and were calculated by dividing the state population of children by the number of practicing PRs within that state. Additional covariates were obtained from the CLR and included age, sex, race, and household income. Race was defined as white, Black, and other minority race because of a low percentage of nonwhite subjects in the registry. Subjects classified as other minority race identified as Asian, American Indian or Alaskan Native, Native Hawaiian or Pacific Islander, or multiracial/other. Hispanic ethnicity was assessed as a separate variable. Our primary outcome was diagnostic delay, which was defined as early (<30 days), typical (1‐3 months), moderate delay (3‐12 months), and severe delay (>12 months). These categories were chosen on the basis of existing literature evaluating delays in pediatric lupus (6). The first day of the month was used to calculate the time to diagnosis for patients who reported only the month and year of symptom onset.
Statistical analysis
Subjects were stratified by delay category and comparisons were made using χ2 tests for categorical variables and Kruskal‐Wallis tests for continuous variables, which all demonstrated a non‐normal distribution. To model the relationship between the primary predictors (distance to a PR and density of PRs) and diagnostic delay, we used an ordered generalized additive model (GAM). GAMs allow for the addition of smoothed predictors that may not be linearly related to the outcome and are useful for evaluating data with a geospatial element. In addition to the P value, we also assessed predictor significance using the Akaike information criterion (AIC), an estimator of the quality of the model that also penalizes the inclusion of additional terms, with and without each predictor. Predictors that improved (decreased) the AIC by two or more points were considered to be important in the model. To test for residual spatial autocorrelation, Moran’s I test was run on the residuals from the model. We additionally evaluated for correlation between distance traveled and density of PRs using the Pearson correlation coefficient.
RESULTS
Patient characteristics
A total of 494 patients were included after applying exclusion criteria for missing variables and removing 18 outliers. Overall patient characteristics are displayed in Supplementary Table 1, and the geographic locations of these observations are displayed on a US map in Supplementary Figure 1. Median age at disease onset was 5 years (IQR 3‐9), and 72.0% of subjects were female. The majority (78.6%) of subjects identified as white, whereas 11.8% identified as Black, and 9.6% identified as another minority. Additionally, 14.6% reported Hispanic ethnicity. The median disease duration was 1.9 years (IQR 0.5‐4.4 years). The median time to diagnosis was 3.1 months, with the highest percentage of patients, 37.2%, presenting with moderate diagnostic delays of 3 to 12 months and 14.6% of patients presenting with severe delays of more than 1 year. Early diagnosis was seen in 16.4% of subjects, and typical diagnosis was made in 31.8% of subjects. The median distance subjects traveled to the treating pediatric rheumatology center was 42 miles (IQR 18‐113). The median density of children per PR was approximately 250 000 (IQR 147 000‐399 000).
Factors associated with diagnostic delay
Stratified by delay, factors associated with diagnostic delay in a univariate analysis included younger age at disease onset and male sex (see Table 1). In a GAM (reduced to a total number of 480 subjects because of incomplete data for 14 observations), which included a smoothed effect of distance to treating PR, smoothed density of PRs, age, sex, race, and Hispanic ethnicity, distance to treating PR (P = 0.02) and younger age at disease onset (P = 0.01) were significantly associated with diagnostic delays (see Table 2). The odds ratios in this model represent the odds of being in a higher delay category (ie, increasing by one or more delay category) for every unit increase in the covariate. The relationship between distance to treating PR and delay is graphically displayed in Figure 1, which demonstrates increasing log odds of increasing one delay category with increasing distance traveled. However, the confidence of this estimate greatly decreases for observations of more than 400 miles, as there are very few observations in this range. This model performs well at predicting subjects in the typical diagnosis and moderate delay categories but performs less well at predicting those in the early diagnosis and severe delay categories, indicating some unmeasured variables may be more important in predicting time to diagnosis for these two categories.
Table 1.
Patient characteristics stratified by time to diagnosis
| Early (<30 d) | Typical (1‐3 mo) | Moderate Delay (3‐12 mo) | Severe Delay (>12 mo) | P Value | |
|---|---|---|---|---|---|
| Subjects, n | 81 | 157 | 184 | 72 | |
| Age, median (IQR), y | 6.0 (4.0‐10.0) | 5.0 (4.0‐10.0) | 5.0 (3.0‐9.0) | 4.0 (3.0‐8.0) | 0.04 |
| Female sex, n (%) | 51 (64.6) | 118 (75.6) | 136 (76.8) | 43 (60.6) | 0.02 |
| Race, n (%) | 0.30 | ||||
| White | 59 (72.8) | 124 (79.0) | 150 (82.0) | 53 (75.7) | |
| Black | 9 (11.1) | 20 (12.7) | 21 (11.5) | 8 (11.4) | |
| Other minority | 13 (16.0) | 13 (8.3) | 12 (6.6) | 9 (12.9) | |
| Hispanic ethnicity (%) | 14 (17.3) | 17 (10.8) | 25 (13.6) | 16 (22.2) | 0.12 |
| Distance from pediatric rheumatology center, median (IQR), mi | 42 (17‐81) | 42 (20‐119) | 46 (18‐124) | 41 (18‐113) | 0.48 |
| kilometers | 67.6 (27.4‐130.4) | 67.6 (32.2‐209.9) | 74.0 (29.0‐228.5) | 66.0 (29.0‐182.0) | |
| Population of children per pediatric rheumatologist, median (IQR) | 214 994 (142 609‐298 318) | 249 921 (146 572‐399 046) | 249 921 (146 572‐369 729) | 249 921 (161 577‐484 672) | 0.16 |
IQR, interquartile range.
Bold value indicated significance, p < 0.05.
Early = Early Time to Diagnosis
Typical = Typical Time to Diagnosis
Table 2.
Predictors of delay in an ordered GAM
| Predictor | OR | 95% CI | P Value |
|---|---|---|---|
| Distance (smoothed) | See Figure 1 | 0.02 | |
| Density of rheumatologists (smoothed) | See Figure 1 | 0.35 | |
| Age of JDM onset | 0.94 | 0.91‐0.98 | 0.01 |
| Female sex | 0.91 | 0.63‐1.33 | 0.63 |
| Black race | 1.05 | 0.63‐1.75 | 0.84 |
| Other minority race | 0.65 | 0.36‐1.20 | 0.17 |
| Hispanic ethnicity | 1.36 | 0.83‐2.24 | 0.22 |
CI, confidence interval; GAM, generalized additive model; JDM, juvenile dermatomyositis; OR, odds ratio.
The OR represents the odds of increasing in one delay category. For the category of race, white race is the baseline category and ORs for Black and other minority races are reported relative to this category.
Bold value indicated significance, p < 0.05
Figure 1.
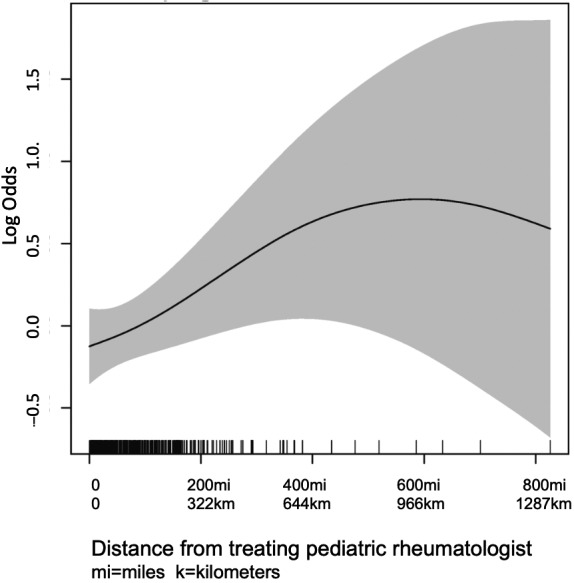
Log odds of increasing by one or more delay categories as distance from treating pediatric rheumatologist increases with surrounding confidence intervals (shaded). There are few observations traveling more than 400 miles.
Younger age at disease onset was associated with diagnostic delay with a reduction in the odds of being in a higher delay category with each year increase in age (odds ratio 0.94 per year increase; 95% confidence interval 0.91‐0.98; adjusted P = 0.01). There was no association between number of children per PR and diagnostic delay (adjusted P = 0.35; Figure 1). There was also no association between sex and diagnostic delays in a multivariate model accounting for these other factors considering a P value cutoff of less than 0.05. However, age, sex, race, and distance traveled all enhanced model fit using the criteria of decreasing the model AIC by more than two points, suggesting that sex and race might also be associated with diagnostic delay. Density of PRs and Hispanic ethnicity did not enhance model fit by this criteria. There was no evidence of residual spatial autocorrelation in this model by Moran’s I test (P = 0.08), so a spatial term including smoothed latitude and longitude was not included in the model.
The Pearson correlation coefficient between distance traveled and state density of PRs was 0.13, suggesting a positive but weak correlation between these variables (Supplementary Figure 2). Further investigation of this relationship revealed that many of the states that have few PRs are also sparsely populated, so although the distance traveled is high, the density of PRs is low because of a lower number of children for that state (eg, North Dakota, South Dakota, and Idaho).
DISCUSSION
In this study, we have shown that the majority of subjects with JDM enrolled in the CLR between 2010 and 2015 experienced significant diagnostic delays of greater than 3 months. Factors associated with diagnostic delays included younger age at disease onset and greater distance from a treating pediatric rheumatology center. In a univariate analysis, male sex was also associated with delayed diagnosis, although this association did not hold up when controlling for other factors. The sex and age results suggest that JDM may be considered less often in young children and male children, which may be due to the epidemiology of JDM relative to other pediatric rheumatic diseases. These findings are in accordance with other studies that have described diagnostic delays in pediatric rheumatic diseases, including pediatric lupus and juvenile idiopathic arthritis (6, 8), but they emphasize that JDM may be associated with even greater delays.
Our findings show that in JDM, distance traveled to pediatric rheumatologist is associated with time to diagnosis. This is a finding of key importance, as delays in diagnosis are known to be associated with increased morbidity in patients with JDM (4). Prior work has shown poorer outcomes in other chronic pediatric conditions, such as type 1 diabetes mellitus and depression, for individuals who live further distances from their specialists (14, 15). Living greater distances from specialist care likely makes frequent‐interval follow‐up visits challenging, and close monitoring is necessary in chronic conditions such as JDM to assess and detect disease activity in order to consider modifications in therapy. In addition to increasing the pediatric rheumatology workforce, strategies such as partnering with local adult rheumatologists and primary care providers and performing telemedicine, which has become significantly more prevalent in the era of coronavirus disease 2019, may be possible approaches to narrow this disparity.
Interestingly, living in a state with fewer PRs was not found to be associated with delay to diagnosis, as was the case in pediatric lupus (6). We had initially theorized that patients living in states with a lower density of PRs might be the same patients driving longer distances to a treating center; however, this was not supported by our analysis. Based on our analysis, we found that patients who drove longer distances lived in states with less population density, so the relative density of PRs was not as sparse as one might think.
Younger age at onset was associated with significantly longer time to diagnosis in our study population. Similar findings were reported in patients with SLE (6) and are likely due to the low incidence of JDM and limited recognition of these rare conditions by primary care providers. Increased education and outreach to community providers around the presenting symptoms of JDM, particularly the bimodal age distribution of children affected, rash morphologies, and patterns of weakness, is needed to help remedy this issue.
We recognize that there are limitations to this study. Because we only have information regarding patients enrolled to the CLR, our findings may not be generalizable to the US population as a whole. Definite or probable JDM were not separated at the time of enrollment, so they could not be subanalyzed. Because this was a voluntary registry, there may be selection bias in the patients enrolled, with patients who were English speaking likely being asked to participate at higher rates in addition to other potential factors biasing enrollment. Additionally, almost all patients enrolled to the CLR had insurance, and this analysis may not be generalizable to patients who do not have health insurance. There was also less participation in the CLR from underserved states. Notably, we did not have subjects in our study from 14 states (Alaska, Arizona, Arkansas, Florida, Hawaii, Louisiana, Mississippi, Montana, Nebraska, Nevada, New Mexico, North Dakota, South Dakota, and Wyoming), which have less access to pediatric rheumatology care. Ten of these states had only one or two PRs, and two states had no PRs according to the 2015 ACR Workforce Study (13). Many of these states make up the Midwest region of the country, from which we had few observations in which patients may have to travel great distances to reach care (Supplementary Figure 1). We expect that the findings in this study may underestimate the diagnostic delays in these states that are not represented in the CLR.
Additional limitations included missing data, which reduced our total data set to about half the original size; measuring distance by zip code centroid rather than the exact address, which adds some noise to the measurement; and our inability to account for unmeasured confounders (for example, mode of transportation; traffic; access to pediatric primary care; socioeconomic status, such as income and parental education level; primary language; and immigration status) that may have impacted access to care, all of which could introduce possible bias. We also had limited information regarding disease features at JDM presentation. Thus, we could not include details of initial disease presentation in our analysis (eg, severity of disease, presenting rashes, systemic features, arthritis, muscle enzymes, etc). It may be that patients that were sicker presented to care earlier, whereas patients with milder disease experienced more delays regardless of age or distance to referral center. Lastly, these findings may be unique to the US population, limiting the generalizability to the international community.
Despite these limitations, a significant strength of this study is our use of the large national cohort of patients with JDM enrolled in the CLR, which included the typical age and racial diversity reported from other JDM cohorts (16, 17, 18), suggesting that the data are representative of patients with JDM in the United States. This study highlights how large disease registries can be integrated with other publicly available data sets, such as geographical data and workforce data sets, to answer important epidemiological questions. Another strength of this work is the multimodal approach to looking at access pediatric rheumatology care using both geospatial data and number of PRs available to a community as measured by density. By using two different variables to address this concept, we hopefully decreased the impact of the unmeasured confounders mentioned above in the overall answering of our initial research question.
Most importantly, our study highlights the significant diagnostic delays experienced by children with JDM associated with several demographic and socioeconomic factors, including access to pediatric rheumatology care. These barriers will require creative solutions from many angles to improve outcomes for children with JDM. First, it is key to increase the pediatric rheumatology workforce throughout the United States. In addition, increasing exposure to pediatric rheumatology in medical schools and residency programs, as well as providing continuing education to primary care providers, will be essential in improving early recognition of JDM, especially in the youngest patients. Using technology such as telemedicine, which providers are becoming increasingly more adept at, to decrease the burden of travel for patients and partnering with local clinicians may help to improve the frequency of assessments and adjustment of therapy and ultimately decrease functional disability.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Susan Kim had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Neely, Shalen, Sturrock, Kim.
Acquisition of data
Neely, Shalen, Kim.
Analysis and interpretation of data
Neely, Shalen, Sturrock, Kim.
Supporting information
Supplementary Material
Acknowledgments
The authors thank all participants and hospital sites that recruited patients for the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. The authors thank the following CARRA Registry site principal investigators and research coordinators: L. Abramson, E. Anderson, M. Andrew, N. Battle, M. Becker, H. Benham, T. Beukelman, J. Birmingham, P. Blier, A. Brown, H. Brunner, A. Cabrera, D. Canter, D. Carlton, B. Caruso, L. Ceracchio, E. Chalom, J. Chang, P. Charpentier, K. Clark, J. Dean, F. Dedeoglu, B. Feldman, P. Ferguson, M. Fox, K. Francis, M. Gervasini, D. Goldsmith, G. Gorton, B. Gottlieb, T. Graham, T. Griffin, H. Grosbein, S. Guppy, H. Haftel, D. Helfrich, G. Higgins, A. Hillard, J.R. Hollister, J. Hsu, A. Hudgins, C. Hung, A. Huttenlocher, N. Ilowite, A. Imlay, L. Imundo, C.J. Inman, J. Jaquith, R. Jerath, L. Jung, P. Kahn, A. Kapedani, D. Kingsbury, K. Klein, M. Klein‐Gitelman, A. Kunkel, S. Lapidus, S. Layburn, T. Lehman, C. Lindsley, M. Macgregor‐Hannah, M. Malloy, C. Mawhorter, D. McCurdy, K. Mims, N. Moorthy, D. Morus, E. Muscal, M. Natter, J. Olson, K. O’Neil, K. Onel, M. Orlando, J. Palmquist, M. Phillips, L. Ponder, S. Prahalad, M. Punaro, D. Puplava, S. Quinn, A. Quintero, C. Rabinovich, A. Reed, C. Reed, S. Ringold, M. Riordan, S. Roberson, A. Robinson, J. Rosette, D. Rothman, D. Russo, N. Ruth, K. Schikler, A. Sestak, B. Shaham, Y. Sherman, M. Simmons, N. Singer, S. Spalding, H. Stapp, R. Syed, E. Thomas, K. Torok, D. Trejo, J. Tress, W. Upton, R. Vehe, E. von Scheven, L. Walters, J. Weiss, P. Weiss, N. Welnick, A. White, J. Woo, J. Wootton, A. Yalcindag, C. Zapp, L. Zemel, and A. Zhu.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Neely was supported by a Cure JM Fellowship Grant during the period of time this work was conducted. The Childhood Arthritis and Rheumatology Research Alliance (CARRA) Legacy Registry was supported by a grant from National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institute of Health under award number RC2AR058934. The CARRA Legacy Registry was also supported by CARRA, Friends of CARRA, the Arthritis Foundation, and the Duke Clinical Research Institute.
Drs. Neely and Shalen contributed equally to this work.
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1. Gowdie PJ, Allen RC, Kornberg AJ, Akikusa JD. Clinical features and disease course of patients with juvenile dermatomyositis. Int J Rheum Dis 2013;16:561–7. [DOI] [PubMed] [Google Scholar]
- 2. Ravelli A, Trail L, Ferrari C, Ruperto N, Pistorio A, Pilkington C, et al. Long‐term outcome and prognostic factors of juvenile dermatomyositis: a multinational, multicenter study of 490 patients. Arthritis Care Res 2010;62:63–72. [DOI] [PubMed] [Google Scholar]
- 3. Phillippi K, Hoeltzel M, Byun Robinson A, Kim S. Childhood Arthritis and Rheumatology Research Alliance Legacy Registry Investigators. Race, income, and disease outcomes in juvenile dermatomyositis. J Pediatr 2017;184:38–44.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saini I, Kalaivani M, Kabra SK. Calcinosis in juvenile dermatomyositis: frequency, risk factors and outcome. Rheumatol Int 2016;36:961–5. [DOI] [PubMed] [Google Scholar]
- 5. Kim S, El‐Hallak M, Dedeoglu F, Zurakowski D, Fuhlbrigge RC, Sundel RP. Complete and sustained remission of juvenile dermatomyositis resulting from aggressive treatment. Arthritis Rheum 2009;60:1825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rubinstein TB, Mowrey WB, Ilowite NT, et al. Delays to care in pediatric lupus patients: data from the Childhood Arthritis and Rheumatology Research Alliance Legacy Registry. Arthritis Care Res 2018;70:420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. The American Board of Pediatrics . Pediatric physicians workforce data book, 2017‐2018. 2017. URL: https://www.abp.org/sites/abp/files/pdf/pediatricphysiciansworkforcedatabook2017‐2018.pdf.
- 8. Foster HE, Eltringham MS, Kay LJ, Friswell M, Abinun M, Myers A. Delay in access to appropriate care for children presenting with musculoskeletal symptoms and ultimately diagnosed with juvenile idiopathic arthritis. Arthritis Care Res 2007;57:921–7. [DOI] [PubMed] [Google Scholar]
- 9. Correll CK, Spector LG, Zhang L, Binstadt BA, Vehe RK. Barriers and alternatives to pediatric rheumatology referrals: survey of general pediatricians in the United States. Pediatr Rheumatol Online J 2015;13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Robinson AB, Hoeltzel MF, Wahezi DM, Becker ML, Kessler EA, Schmeling H, et al. Clinical characteristics of children with juvenile dermatomyositis: the Childhood Arthritis and Rheumatology Research Alliance Registry. Arthritis Care Res 2014;66:404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adler JT, Bababekov YJ, Markmann JF, Chang DC, Yeh H. Distance is associated with mortality on the waitlist in pediatric liver transplantation. Pediatr Transplant 2017;21. [DOI] [PubMed] [Google Scholar]
- 12. Hamilton EC, Nguyen HT, Chang Y‐C, Eberth JM, Cormier J, Elting LS, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr 2016;175:182–7. [DOI] [PubMed] [Google Scholar]
- 13. Battafarano DF, Ditmyer M, Bolster MB, Fitzgerald JD, Deal C, Bass AR, et al. 2015 American College of Rheumatology workforce study: supply and demand projections of adult rheumatology workforce, 2015–2030. Arthritis Care Res 2018;70:617–26. [DOI] [PubMed] [Google Scholar]
- 14. Fox DA, Islam N, Amed S. Type 1 diabetes outcomes: does distance to clinic matter? [Original Article]. Pediatr Diabetes 2018;19:1331–6. [DOI] [PubMed] [Google Scholar]
- 15. Upadhyay N, Aparasu R, Rowan PJ, Fleming ML, Balkrishnan R, Chen H. The association between geographic access to providers and the treatment quality of pediatric depression. J Affect Disord 2019;253:162–70. [DOI] [PubMed] [Google Scholar]
- 16. Shah M, Mamyrova G, Targoff IN, Huber AM, Malley JD, Rice MM, et al. The clinical phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine 2013;92:25–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khojah A, Liu V, Savani SI, Morgan G, Shore R, Bellm J, et al. Studies of 96 children with juvenile dermatomyositis: P155/140, is associated with loss of nailfold capillaries, but not generalized lipodystrophy. Arthritis Care Res 2020. E‐pub ahead of print.5 [DOI] [PubMed] [Google Scholar]
- 18. Mendez EP, Lipton R, Ramsey‐Goldman R, Roettcher P, Bowyer S, Dyer A, et al. US incidence of juvenile dermatomyositis, 1995–1998: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Registry. Arthritis Care Res 2003;49:300–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


