Significance
Studies of memory have established a distinction between ordinary recollection of the past (declarative memory), which depends on medial temporal lobe (MTL) structures and other (nondeclarative) forms of memory that are expressed through performance and depend on other brain systems. One phenomenon that has eluded classification is one-trial perceptual learning, whereby a degraded image of an object, which is difficult to identify, becomes recognizable after a single exposure to the original image. This effect was fully intact in memory-impaired patients with hippocampal or larger MTL lesions and persisted undiminished for more than 5 mo, despite impaired memory for the test format and the images themselves. Perceptual learning is MTL-independent (nondeclarative) and occurs without a requirement to consciously remember.
Keywords: hippocampus, perceptual learning, nondeclarative memory
Abstract
A degraded, black-and-white image of an object, which appears meaningless on first presentation, is easily identified after a single exposure to the original, intact image. This striking example of perceptual learning reflects a rapid (one-trial) change in performance, but the kind of learning that is involved is not known. We asked whether this learning depends on conscious (hippocampus-dependent) memory for the images that have been presented or on an unconscious (hippocampus-independent) change in the perception of images, independently of the ability to remember them. We tested five memory-impaired patients with hippocampal lesions or larger medial temporal lobe (MTL) lesions. In comparison to volunteers, the patients were fully intact at perceptual learning, and their improvement persisted without decrement from 1 d to more than 5 mo. Yet, the patients were impaired at remembering the test format and, even after 1 d, were impaired at remembering the images themselves. To compare perceptual learning and remembering directly, at 7 d after seeing degraded images and their solutions, patients and volunteers took either a naming test or a recognition memory test with these images. The patients improved as much as the volunteers at identifying the degraded images but were severely impaired at remembering them. Notably, the patient with the most severe memory impairment and the largest MTL lesions performed worse than the other patients on the memory tests but was the best at perceptual learning. The findings show that one-trial, long-lasting perceptual learning relies on hippocampus-independent (nondeclarative) memory, independent of any requirement to consciously remember.
A striking visual effect can be demonstrated by using a grayscale image of an object that has been degraded to a low-resolution, black-and-white image (1, 2). Such an image is difficult to identify (Fig. 1) but can be readily recognized after a single exposure to the original, intact image (Fig. 2) (3–6). Neuroimaging studies have found regions of the neocortex, including high-level visual areas and the medial parietal cortex, which exhibited a different pattern of activity when a degraded image was successfully identified (after seeing the intact image) than when the same degraded image was first presented and not identified (4, 5, 7). This phenomenon reflects a rapid change in performance based on experience, in this case one-trial learning, but the kind of learning that is involved is unclear.
Fig. 1.
A sample degraded image. Most people cannot identify what is depicted. See Fig. 2.
Fig. 2.
An intact version of the image in Fig. 1. When the intact version is presented just once directly after presentation of the degraded version, the ability to later identify the degraded image is greatly improved, even after many months. Reprinted from ref. (42), which is licensed under CC BY 4.0.
One possibility is that successful identification of degraded images reflects conscious memory of having recently seen degraded images followed by their intact counterparts. When individuals see degraded images after seeing their “solutions,” they may remember what is represented in the images, at least for a time. In one study, performance declined sharply from 15 min to 1 d after the solutions were presented and then declined more gradually to a lower level after 21 d (3). Alternatively, the phenomenon might reflect a more automatic change in perception not under conscious control (8). Once the intact image is presented, the object in the degraded image may be perceived directly, independently of whether it is remembered as having been presented. By this account, successful identification of degraded images is reminiscent of the phenomenon of priming, whereby perceptual identification of words and objects is facilitated by single encounters with the same or related stimuli (9–11). Some forms of priming persist for quite a long time (weeks or months) (12–14).
These two possibilities describe the distinction between declarative and nondeclarative memory (15, 16). Declarative memory affords the capacity for recollection of facts and events and depends on the integrity of the hippocampus and related medial temporal lobe structures (17, 18). Nondeclarative memory refers to a collection of unconscious memory abilities including skills, habits, and priming, which are expressed through performance rather than recollection and are supported by other brain systems (19–21). Does one-trial learning of degraded images reflect declarative or nondeclarative memory? How long does it last? In an early report that implies the operation of nondeclarative memory, two patients with traumatic amnesia improved the time needed to identify hidden images from 1 d to the next, but could not recognize which images they had seen (22). Yet, another amnesic patient reportedly failed such a task (23). The matter has not been studied in patients with medial temporal lobe (MTL) damage.
To determine whether declarative (hippocampus-dependent) or nondeclarative (hippocampus-independent) memory supports the one-trial learning of degraded images, we tested five patients with bilateral hippocampal lesions or larger MTL lesions who have severely impaired declarative memory. The patients were fully intact at perceptual learning, and performance persisted undiminished from 1 d to more than 5 mo. At the same time, the patients were severely impaired at remembering both the structure of the test and the images themselves.
Results
Naming.
Fig. 3 shows performance averaged across three iterations of the naming test using different materials. At study (i.e., the first exposure to degraded images), patients did as well as controls at identifying the 40 degraded images [22.8 ± 4.2% correct vs. 27.1 ± 3.3% correct; t(14) = 0.75, P = 0.47]. They were also as confident in their responses [rating on a 1 to 5 scale: 2.9 ± 0.4 for patients vs. 2.6 ± 0.3 for controls; t(14) = 0.70, P = 0.50]. Both groups easily identified with high confidence the intact images that followed immediately after the degraded images (patients: 96.8 ± 0.9% correct, confidence = 4.93 ± 0.02; controls: 97.6 ± 0.8% correct, confidence = 4.95 ± 0.02).
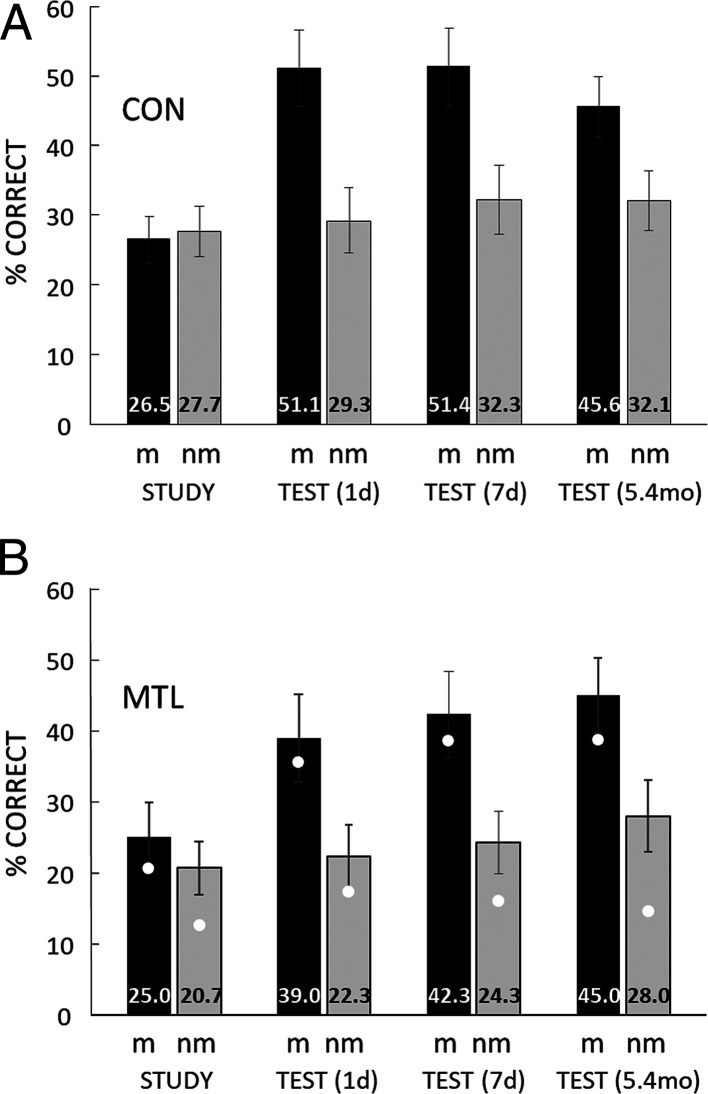
Fig. 3.
Naming. At study, participants tried to name what was depicted in each of 40 degraded, black-and-white images. Each image was followed by either the intact, matching (m) version of the same image or an intact image that did not match (nm). 1 d, 7 d, and again 5.4 mo later, participants saw the same 40 degraded images and tried to name what was depicted in each image. Percent correct naming scores are means from three iterations of this procedure using different materials. White circles show the scores for G.P., the patient with the most severe memory impairment and the largest MTL lesions. (A) CON = 11 controls. (B) MTL = 5 patients with MTL lesions. Error bars show SEM.
Fig. 3 also shows that presenting the matching, intact image at study directly after a degraded image facilitated the ability to later identify the degraded image. Thus, in both groups, and at each of three delays (1 d, 7 d, and 5.4 mo), participants were far better at naming degraded images that had earlier been paired with matching, intact images (m) than they were at naming degraded images that had earlier been paired with nonmatching, intact images (nm). For controls at each delay (Fig. 3A), ts(10) > 4.4, P < 0.001; for patients at each delay (Fig. 3B), ts(4) > 5.9, P < 0.004.
This beneficial effect on image identification was as strong and as long-lasting in the patients as in controls (CON). Three-factor ANOVAs carried out at each of the three delays for the data from Fig. 3 (CON/MTL, study/test, and match/nonmatch) yielded no effect of Group at any delay [Fs(1, 14) < 1.1, P > 0.31] and no interaction of Group with any other factor [Fs(1, 14) < 2.8, P > 0.11]. As Fig. 3 shows, there were large effects of study/test and match/nonmatch at each delay [Fs(1, 14) > 19.4, P < 0.001]. Importantly, the interaction of study/test × match/nonmatch at each delay [Fs(1, 14) > 55.9, P < 0.001] documents the key finding that the naming of degraded images robustly improved when degraded images had been followed at study by matching, intact images but not when they had been followed by nonmatching, intact images. Finally, the patients were not only as successful at naming degraded images as controls at each of the three delays, they also were as confident as controls in their responses to the degraded images (patients: 3.0 ± 0.4, 3.1 ± 0.4, 3.0 ± 0.4 at 1 d, 7 d, and 5.4 mo, respectively; controls: 2.9 ± 0.3, 3.1 ± 0.3, and 3.0 ± 0.2).
It is of interest that the ability to identify the degraded images that had been followed at study by nonmatching, intact images improved a little across test sessions (Fig. 3), presumably because the same degraded images were presented a total of four times: at study and at each of three delays [analysis of linear trend across the four tests; controls, F(1, 10) = 7.9, P = 0.02; patients, F(1, 4) = 14.4, P = 0.02]. This finding reflects a gradual, albeit modest, improvement in the ability to perceive degraded images simply as a result of repeated exposure to them. Note that this nonspecific effect was distinct from the robust, long-lasting facilitation of naming that was specific to, and dependent on, single exposures at study to matching, intact images that revealed what was depicted in the degraded images.
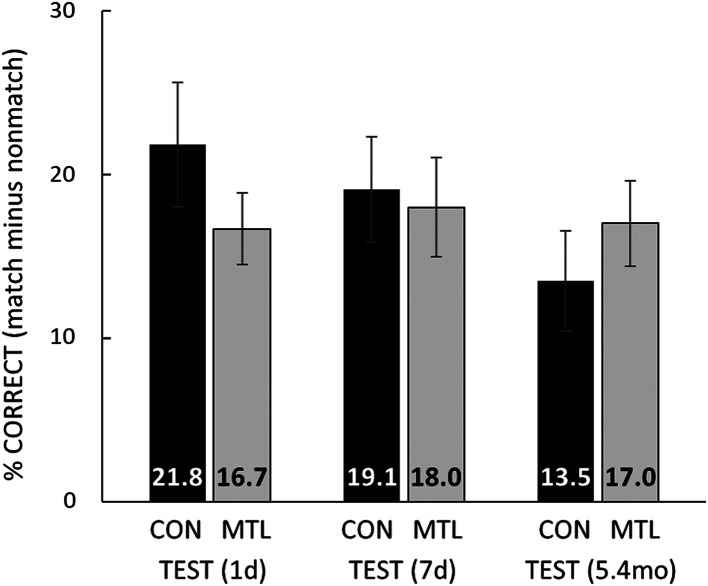
Fig. 4 shows directly the amount of facilitation of naming in each group at each delay. At each delay, the percent correct identification score for the 20 degraded images that had been followed at study by nonmatching, intact images was subtracted from the percent correct identification score for the 20 degraded images that had been followed at study by matching, intact images. The patients exhibited overall as strong a facilitation as controls, and the facilitation in each group persisted for at least 5.4 mo. These findings were documented by an ANOVA (Group and Delay), which yielded no effect of Group [F(1, 14) = 0.0, P = 0.90], no effect of Delay [F(2, 28) = 2.5, P = 0.10], and no interaction of Group × Delay [F(2, 28) = 2.6, P = 0.09]. The finding that the Delay effect and the Group × Delay interaction nevertheless approached significance likely reflects the fact that the facilitation exhibited by controls weakened across time [documented by an analysis of linear trend, F(1, 10) = 8.9, P = 0.01]. In contrast, the facilitation exhibited by the patients was sustained without decrement for as long as 5.4 mo [F(1, 4) = 0.0, P = 0.91]. The controls, but not the patients, may have drawn in part on declarative, conscious memory in order to name degraded images at the shorter delays. That is, shortly after study, controls not only perceived degraded images successfully but also explicitly remembered some of the solutions. This advantage became less available to controls as the delay increased but was not available to patients at any delay.
Fig. 4.
Naming. To illustrate how much the naming of degraded images benefited from earlier presentation of their intact, matching images, the percent correct naming score for the 20 degraded images that had earlier been followed by a nonmatching, intact image was subtracted from the percent correct naming score for the 20 degraded images that had earlier been followed by the matching, intact image. Scores at each delay are based on the data in Fig. 3. G.P.’s scores were 18.3%, 23.3%, and 25.0% at 1 d, 7 d, and 5.4 mo, respectively, the best of all the patients. CON = 11 controls; MTL = 5 patients with MTL lesions. Error bars show SEM.
Remembering.
Test 1.
Despite the fact that patients improved at identifying degraded images for as long as 5.4 mo after a single exposure to the corresponding, intact images, the patients had difficulty remembering intact images after only 1 d (Fig. 5, Left). Controls scored 98.0 ± 1.1% correct 1 d after studying 20 intact images, but patients scored only 79.5 ± 4.2% correct [t(14) = 5.86, P < 0.001; d′ = 3.7 ± 0.1 and 2.1 ± 0.2, respectively]. The controls were also more confident of their responses than patients [4.8 ± 0.1 vs. 4.2 ± 0.2; t(14) = 2.82, P = 0.01].
Fig. 5.
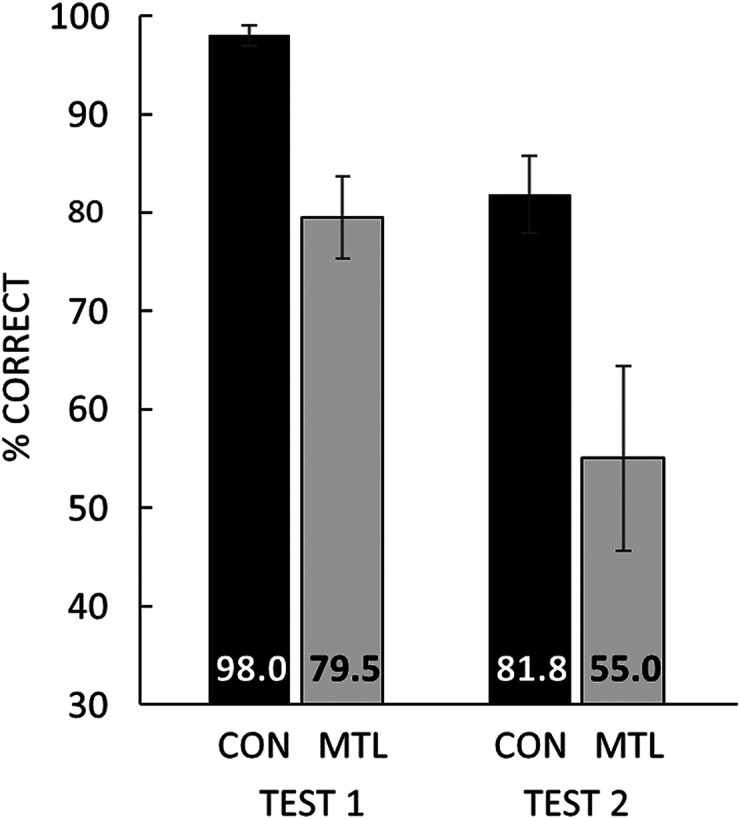
Remembering. Test 1. Participants saw 20 intact images and 1 d later took a yes/no recognition memory test for the 20 old images intermixed with 20 new images. Bars show percent correct scores for 11 controls (CON) and 5 patients with MTL lesions (MTL). d′ = 3.7 and 2.1 for CON and MTL groups, respectively. Test 2. Percent correct for eight multiple-choice questions about the format of the Naming test, which had last been encountered 6 d earlier. Chance = 33.3%. G.P. scored 65.0% correct on test 1 and 37.5% correct on test 2, the poorest of all the patients. Error bars show SEM.
Test 2.
The patients had difficulty remembering facts about the naming test, which they had last encountered 6 d earlier (Fig. 5, Right). Whereas the controls scored 81.8 ± 3.9% correct on multiple-choice questions about the naming test format (chance = 33.3%), the patients scored only 55.0 ± 9.4% correct [t(14) = 3.18, P = 0.007].
Remembering in Contrast to Naming.
To contrast remembering and naming, participants viewed 40 new degraded images and took a recognition memory test after 7 d at a point when their testing history was identical to what it was for the naming test (Fig. 3). That is, prior to the 7-d memory test, participants first tried to name 40 degraded images, 20 of which were followed by the intact, matching image, and they then took a naming test at 1 d. Not surprisingly, scores on these tests largely recapitulated the scores in Fig. 3. At study, controls correctly named 34.5 ± 4.2% of the 20 degraded images that were followed by intact, matching images and correctly named 28.6 ± 4.1% of the other 20 degraded images. For patients, the corresponding scores were 29.0 ± 7.0% and 25.0 ± 3.9% correct (compare to Fig. 3). At 1 d, the controls correctly named 59.1 ± 5.0% of the 20 images that had been matched at study and 31.8 ± 5.0% of the other 20 degraded images (patients, 47.0 ± 10.1% and 24.0 ± 6.6% correct) (compare to Fig. 3). The amount of facilitation at 1 d was robust and similar in the two groups (controls, 27.3 ± 5.2%; patients, 23.0 ± 4.9%) (compare to Fig. 4). Finally, the patients and controls expressed similar confidence in their responses throughout this stage of testing (patients at study, 2.9 ± 0.4; at 1 d, 3.0 ± 0.4; controls at study, 2.6 ± 0.3; at 1 d, 3.0 ± 0.2).
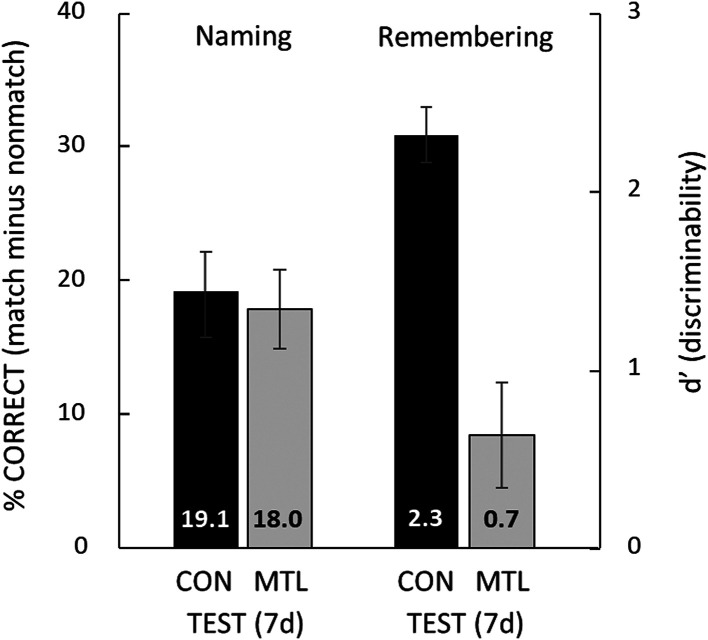
The finding of interest was obtained at 7 d after study when, instead of taking another naming test, participants now took a yes/no recognition memory test for the 40 degraded images intermixed with 40 new degraded images (chance = 50% correct). Fig. 6 compares naming at 7 d (reproduced from Fig. 4) with remembering at 7 d. Whereas naming scores were virtually identical in the two groups, recognition memory scores were markedly different. Controls remembered the degraded images quite well (d′ = 2.3 ± 0.2; 86.0 ± 1.6% correct), but the patients were severely impaired [d′ = 0.7 ± 0.3; 61.3 ± 5.3% correct; ts(14) > 5.42, P < 0.001]. Finally, the controls and the patients were similarly confident in their responses (3.7 ± 0.2 and 3.6 ± 0.3, respectively).
Fig. 6.
Remembering in contrast to naming. The percent correct naming scores are reproduced from the 7-d test in Fig. 4, which shows the amount of facilitation in naming (i.e., how much the naming of degraded images benefited from earlier presentation of their intact, matching images). G.P.’s score was 23.3%, the best of all the patients. For remembering (d′), the procedure at study and after 1 d was the same as for the naming test (Fig. 3). However, at 7 d after study, instead of taking another naming test, participants took a yes/no recognition memory test for the 40 old degraded images and 40 new degraded images. G.P. obtained a d′ score of 0.3 (55.0% correct), the poorest of all the patients. CON = 11 controls; MTL = 5 patients with MTL lesions. Error bars show SEM.
Discussion
The ability to identify degraded images substantially improved after single, brief exposures to the original, intact images. The improvement was evident at 1 d and persisted for more than 5 mo (Fig. 3). Memory-impaired patients with MTL lesions exhibited this effect at full strength and without decrement across the same time period (Fig. 4). Improved perceptual performance was unrelated to the ability to remember the images that had been presented. Thus, despite a robust and long-lasting improvement at identifying previously studied degraded images, the patients were severely impaired at remembering intact images even after 1 d (Fig. 5, Left), and in another session were impaired at remembering the format of the naming test (Fig. 5, Right). Notably, patient G.P., who has the most severe memory impairment and the largest MTL lesions, performed worse than the other four patients on the memory tests but was the best of the patients at perceptual learning (Fig. 4).
In a direct comparison of perceptual learning and remembering, at 7 d after learning patients and controls with identical testing histories took either a naming test with degraded images or a recognition memory test with degraded images. The patients improved as much as controls on the naming test but were severely impaired on the memory test (Fig. 6). Thus, patients could identify the degraded images, benefiting as much as controls from having previously seen the “solutions,” but the patients could not recognize the degraded images as familiar.
The successful performance of patients with MTL lesions suggests that the one-trial and long-lasting learning demonstrated here relies on nondeclarative (hippocampus-independent) memory. As a result of their experience, patients improve at perceiving degraded images but without remembering them. Note that at early intervals after learning controls may draw on declarative memory, thereby further improving their performance. In an earlier study (3), performance was much higher at 15 min after learning than after 1 d and continued to decline a little from 1 d to 21 d (figure 4 in ref. 3). In our study as well, performance of control participants measurably declined from 1 d to 5.4 mo (Fig. 4). In contrast, the patients exhibited consistent performance across the same time intervals, falling a little short of the controls at 1 d (albeit, not significantly), and even exceeding the controls by a little after 5.4 mo. Presumably, at shorter retention intervals, controls can remember some of the images and benefit by using declarative memory in the naming task.
Informal demonstrations using a single pair of images, as in Figs. 1 and 2, often begin with failure to identify the hidden object, followed some time later by confident and successful identification, as if the ability to identify degraded images might typically move from 0% correct initially to 100% correct on a later test. However, performance on tests involving multiple images does not behave this way. First, participants inevitably identify some degraded images spontaneously without benefit of seeing the solutions (see scores at study in Fig. 3) (3). Second, while presentation of the original, intact image directly after the degraded image often results in insight as to what the degraded image represents, sometimes participants do not see the connection between a degraded image and its original, so that there is no basis for identifying the degraded image when it is presented later. In our study, naming performance improved to 45.7% correct, averaged across both groups and three retention intervals (Fig. 3). The study by Ludmer et al. (3) reported a similar performance score across the interval 1 d to 21 d after learning.
An early hint of good performance by memory-impaired patients on perceptual learning came from a task in which drawings of common objects were first presented briefly in fragmented form and then in progressively more recognizable form until the object was identified (24, 25). When tested a second time, both patients and controls identified the objects at an earlier point in the series. Even the noted patient H.M. (26, 27) improved his performance when tested after 1 h, though he did not remember having taken the test before. However, the controls performed far better than the patients. As noted (25), the short retention interval and the small number of objects likely allowed a substantial contribution of declarative memory to task performance. That is, the controls were likely advantaged because they could remember some of the solutions or have available in memory many of the correct names, and thereby be aided in their guessing. Accordingly, at the time of this work it seemed possible that conscious remembering might be an important part of perceptual learning, and it was unclear if this should count as an example of learning that lies outside the province of the MTL, as had been demonstrated a few years earlier in the case of motor skill learning (28).
The present study demonstrates that when the possible contribution of declarative memory is limited by using a large number of images and long retention intervals, robust one-trial perceptual learning relies fully on nondeclarative memory. Participants are not asked to remember anything and are asked only to report what they see. Perceptual learning occurs without conscious control (8) and independent of any requirement to consciously remember. Brain activity elicited by successfully identified degraded images is sharpened in regions of the neocortex, including in the ventral visual stream (29). A similar idea involving sharpening has been suggested to underlie perceptual priming (30, 31). This pattern of activation is distinct from the activity associated with the same degraded images when they are not identified (5, 32, 33), and by 800 ms after image onset is similar to the activity associated with the corresponding, intact images (34). These cortical changes underlying one-trial perceptual learning occur independently of the MTL.
Methods
Participants.
Five memory-impaired patients participated, who have also been studied previously (35) (mean age = 66.0 ± 8.2 y; mean education = 13.1 ± 0.8 y). Four have bilateral lesions thought to be limited to the hippocampus (CA fields, dentate gyrus, and subicular complex), and one (G.P.) has larger MTL lesions (Table 1). For the five patients, the summed score for delayed recall (30 min) of two short prose passages (Weschler Memory Scale-Revised, WMS-R) averaged 1.2 segments (25 segments per passage). The average score for delayed reconstruction (10 to 15 min) of a complex diagram (36) was 5.8 (maximum score = 36). Paired-associate learning of 10 unrelated noun−noun pairs summed across each of three successive trials was 3.0 pairs (30 pairs total). Eleven healthy controls (four females) also participated (mean age = 72.8 ± 2.5 y; mean education = 14.4 ± 0.7 y). They scored 28.5 for the prose passages, 19.6 for the diagram, and 24.6 for paired-associate learning.
Table 1.
Characteristics of memory-impaired patients
| Patient | Age (y) | Education (y) | WAIS-III IQ | WMS-R | ||||
| Attention | Verbal | Visual | General | Delay | ||||
| D.A. | 37 | 12 | 95 | 104 | 90 | 91 | 90 | 56 |
| K.E. | 78 | 13.5 | 108 | 114 | 64 | 84 | 72 | 55 |
| L.J. | 82 | 12 | 101 | 105 | 83 | 60 | 69 | <50 |
| G.W. | 60 | 12 | 108 | 105 | 67 | 86 | 70 | <50 |
| G.P. | 73 | 16 | 98 | 102 | 79 | 62 | 66 | 50 |
WAIS-III is the Wechsler Adult Intelligence Scale-III and WMS-R is the Wechsler Memory Scale-Revised. The WMS-R does not provide numerical scores for individuals who score <50. The IQ score for D.A. is from the WAIS-IV.
Patients D.A. and G.W. became amnesic in 2011 and 2001, respectively, following a drug overdose and associated respiratory failure. K.E. became amnesic in 2004 after an episode of ischemia associated with kidney failure and toxic shock syndrome. L.J. (the only female) became amnesic during a 6-mo period in 1988 with no known precipitating event. Her memory impairment has been stable since that time. G.P. has severe memory impairment resulting from viral encephalitis in 1987.
Estimates of MTL damage were based on quantitative analysis of magnetic resonance images from the patients and from 19 age-matched, healthy males for K.E., G.W., and G.P., 11 age-matched, healthy females for patient L.J. (37), and 8 younger healthy males for D.A. Patients D.A., K.E., L.J., and G.W. have an average bilateral reduction in hippocampal volume of 35%, 49%, 46%, and 48%, respectively (all values at least 2.9 SDs from the control mean). On the basis of two patients (L.M. and W.H.) with similar bilateral volume loss in the hippocampus for whom detailed postmortem neurohistological information was obtained (38), the degree of volume loss in the four hippocampal patients may reflect nearly complete loss of hippocampal neurons. Significant volume loss in the parahippocampal gyrus (temporopolar, perirhinal, entorhinal, and parahippocampal cortices) was not detected (volumes were reduced by −5%, 11%, −17%, and 10%, respectively; all values within 2 SDs of the control mean). The negative values indicate volumes that were larger for a patient than for controls. These values are based on published guidelines for identifying the boundaries of the parahippocampal gyrus (39, 40). G.P. has an average bilateral reduction in hippocampal volume of 96%. The volume of the parahippocampal gyrus is reduced by 94%. G.P. also has a reduction of 24% (>3 SDs below control mean) in the left lateral temporal lobe and a reduction of 6% (<1 SD below control mean) in the right lateral temporal lobe. Eight coronal magnetic resonance images from each patient, together with detailed descriptions of the lesions, can be found elsewhere (41). All procedures were approved by the Institutional Review Board at the University of California San Diego, and participants gave written informed consent before participation.
Materials.
Images in grayscale and degraded images were constructed as previously described (8). Briefly, images were generated from photographs of single, real-world animate and inanimate objects selected from the Caltech database, the Pascal VOC database, and online search engines. Using MATLAB, images were first constructed in grayscale by resizing the original image to 9 × 9 cm and 500 × 500 pixels, and then applying a box filter (initially set at 10 × 10 pixels) for low-pass spatial filtering. Black-and-white degraded images were generated by thresholding the grayscale image to binarize it into black or white pixels. The threshold was set at the median intensity of each image. Images were then selected that were judged difficult to identify but that could be identified correctly when they were compared to the matching grayscale image. Next, groups of 40 to 60 degraded images were screened in pilot testing to construct sets of 40 where the mean probability of identification was 20 to 30% and where identification of each image improved after the matching, intact image was presented; 320 different images were used in the experimental conditions described below.
Procedure.
Naming.
The task began with nine practice trials in which a degraded image was presented on a computer screen, followed after 1 to 2 s of blank screen by its matching, intact image. Participants tried to name each image. The experimenter then presented the degraded image again, directing attention to the relationship between the two images. On a final (10th) practice trial, the degraded image was followed by a nonmatching, intact image, and the experimenter explained that sometimes the degraded image and the intact image would not match in this and all other tasks. The experimenter pressed a key to advance to the next item.
Participants were next told that they would see new images on the screen for 6 s each and should name each image, guessing if necessary. They were also asked to provide a confidence rating after each response (1 to 5 scale; 1 = pure guess, 5 = very confident). Participants then saw 40 degraded images: 20 were followed by the matching, intact image and 20 others, intermixed with the first 20, were followed by a nonmatching, intact image (6 s per image with a 1- to 2-s blank screen between images). Which 20 images were paired with their matching image and which 20 were paired with a nonmatching image was balanced across participants. The following day, again after 7 d, and again after 4.2 to 7.5 mo (mean = 5.4 mo), participants took four practice trials and then saw the same 40 degraded images for 6 s each with instructions to name them and provide a confidence rating. For each testing session (at study and at delays of 1 d, 7 d, and 5.4 mo), three different orders of the 40 degraded images were available, and these were assigned pseudorandomly across participants. No feedback (correct, incorrect) was given for any of the tests. The full task (study + three delays) was given a total of three times using three different sets of material across a period of 1.5 y.
Remembering.
Test 1.
Participants saw 20 novel grayscale images on a computer screen, each for 6 s followed by a blank screen for 1 to 2 s. They were instructed to name the images and to remember them for a later test. One day later, they took a yes/no recognition memory test for the 20 old images intermixed with 20 new images (6 s per image with a 1- to 2-s blank screen between images). After each response, participants provided a confidence rating from 1 to 5 (1 = pure guess, 5 = very confident).
Test 2.
Participants were presented with eight three-alternative, multiple-choice questions about the format of the naming test: for example, what color was the computer screen after the item disappeared (off-white, dark gray, or black)? At the time of this test, participants had previously encountered the naming test either 9 or 10 times, most recently 6 d earlier.
Remembering in contrast to naming.
To contrast remembering and naming directly, a remembering test was constructed using new materials. Up to 7 d after study, the experimental design and procedure were identical to the naming test described above. Thus, participants began by trying to name 40 degraded images and provide confidence ratings (1 to 5) for their responses. Twenty images were followed by the matching, intact image and 20 others, intermixed with the first 20, were followed by a nonmatching, intact image. Which 20 images were paired with their matching images and which were paired with a nonmatching image was balanced across subjects. The following day, again just as in the test of naming, participants saw the same 40 degraded images with instructions to name them and provide confidence ratings. Then, at 7 d after study, instead of taking another naming test, participants took a yes/no recognition memory test for the 40 old images intermixed with 40 new images. Which 40 images served as the 40 old images and which served as the 40 new images was balanced across participants. Memory performance at 7 d after study was compared to naming performance at 7 d after study (Fig. 4). Participants took this remembering test after all other testing was completed.
Acknowledgments
We thank John Wixted and Nicholas Spitzer for helpful comments and suggestions and Brenda Wong and Travis Ting for assistance. This work was supported by the Medical Research Service of the Department of Veterans Affairs (Program 5IK6CX001644) and National Institute of Mental Health Grant 24600. B.J.H. acknowledges support by NSF (BCS-1926780). The original image in Fig. 2 is courtesy of Caltech dataset, available for use under a Creative Commons Attribution 4.0 International License (42).
Footnotes
The authors declare no competing interest.
Data Availability
Data are available at the Open Science Framework repository at https://osf.io/4dmj5/.
References
- 1.Dallenbach K. M., A puzzle-picture with a new principle of concealment. Am. J. Psychol. 64, 431–433 (1951). [PubMed] [Google Scholar]
- 2.Porter P. B., Another puzzle-picture. Am. J. Psychol. 67, 550–551 (1954). [Google Scholar]
- 3.Ludmer R., Dudai Y., Rubin N., Uncovering camouflage: Amygdala activation predicts long-term memory of induced perceptual insight. Neuron 69, 1002–1014 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dolan R. J., et al., How the brain learns to see objects and faces in an impoverished context. Nature 389, 596–599 (1997). [DOI] [PubMed] [Google Scholar]
- 5.González-García C., Flounders M. W., Chang R., Baria A. T., He B. J., Content-specific activity in frontoparietal and default-mode networks during prior-guided visual perception. eLife 7, e36068 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albright T. D., On the perception of probable things: Neural substrates of associative memory, imagery, and perception. Neuron 74, 227–245 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorlin S., et al., Imaging prior information in the brain. Proc. Natl. Acad. Sci. U.S.A. 109, 7935–7940 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang R., Baria A. T., Flounders M. W., He B. J., Unconsciously elicited perceptual prior. Neurosci. Conscious. 2016, niw008 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graf P., Squire L. R., Mandler G., The information that amnesic patients do not forget. J. Exp. Psychol. Learn. Mem. Cogn. 10, 164–178 (1984). [DOI] [PubMed] [Google Scholar]
- 10.Tulving E., Schacter D. L., Priming and human memory systems. Science 247, 301–306 (1990). [DOI] [PubMed] [Google Scholar]
- 11.Schacter D. L., Cooper L. A., Tharan M., Rubens A. B., Preserved priming of novel objects in patients with memory disorders. J. Cogn. Neurosci. 3, 117–130 (1991). [DOI] [PubMed] [Google Scholar]
- 12.Cave C. B., Squire L. R., Intact and long-lasting repetition priming in amnesia. J. Exp. Psychol. Learn. Mem. Cogn. 18, 509–520 (1992). [DOI] [PubMed] [Google Scholar]
- 13.Cave C. B., Very long-lasting priming in picture naming. Psychol. Sci. 8, 322–325 (1997). [Google Scholar]
- 14.Wiggs C. L., Weisberg J., Martin A., Repetition priming across the adult lifespan—The long and short of it. Neuropsychol. Dev. Cogn. B. Aging Neuropsychol. Cogn. 13, 308–325 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Squire L. R., Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 99, 195–231 (1992). [DOI] [PubMed] [Google Scholar]
- 16.Squire L. R., Zola S. M., Structure and function of declarative and nondeclarative memory systems. Proc. Natl. Acad. Sci. U.S.A. 93, 13515–13522 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Squire L. R., Zola-Morgan S., The medial temporal lobe memory system. Science 253, 1380–1386 (1991). [DOI] [PubMed] [Google Scholar]
- 18.Eichenbaum H., Cohen N. J., From Conditioning to Conscious Recollection: Memory Systems of the Brain (Oxford University Press, New York, 2004). [Google Scholar]
- 19.Squire L. R., Zola-Morgan S., Memory: Brain systems and behavior. Trends Neurosci. 11, 170–175 (1988). [DOI] [PubMed] [Google Scholar]
- 20.Schacter D. L., Tulving E., “What are the memory systems of 1994?” in Memory Systems 1994, Schacter D. L., Tulving E., Eds. (The MIT Press, Cambridge, MA, 1994), pp. 1–38. [Google Scholar]
- 21.Squire L. R., Memory systems of the brain: A brief history and current perspective. Neurobiol. Learn. Mem. 82, 171–177 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Crovitz H. F., Harvey M. T., McClanahan S., Hidden memory: A rapid method for the study of amnesia using perceptual learning. Cortex 17, 273–278 (1981). [DOI] [PubMed] [Google Scholar]
- 23.Ramachandran V. S., “2-D or not 2-D—That is the question” in The Artful Eye, Gregory R. L., Harris J., Eds. (Oxford University Press, Oxford, 1994) pp. 249–267. [Google Scholar]
- 24.Warrington E. K., Weiskrantz L., New method of testing long-term retention with special reference to amnesic patients. Nature 217, 972–974 (1968). [DOI] [PubMed] [Google Scholar]
- 25.Milner B., Corkin S., Teuber H. L., Further analysis of the hippocampal amnesic syndrome: 14 year follow-up study of H.M. Neuropsychologia 6, 215–234 (1968). [Google Scholar]
- 26.Scoville W. B., Milner B., Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Squire L. R., The legacy of patient H.M. for neuroscience. Neuron 61, 6–9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milner B., “Les troubles de la memoire accompagnant des lesions hippocampiques bilaterales” in Physiologie de l’Hippocampe, Passouant B., Ed. (Centre National de la Recherche Scientifique, Paris, 1962), pp. 257–272. [Google Scholar]
- 29.González-García C., He B. J., A gradient of sharpening effects by perceptual prior across the human cortical hierarchy. J. Neurosci. 41, 167–178 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grill-Spector K., Henson R., Martin A., Repetition and the brain: Neural models of stimulus-specific effects. Trends Cogn. Sci. 10, 14–23 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Wiggs C. L., Martin A., Properties and mechanisms of perceptual priming. Curr. Opin. Neurobiol. 8, 227–233 (1998). [DOI] [PubMed] [Google Scholar]
- 32.van Loon A. M., et al., NMDA receptor antagonist ketamine distorts object recognition by reducing feedback to early visual cortex. Cereb. Cortex 26, 1986–1996 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Hsieh P. J., Vul E., Kanwisher N., Recognition alters the spatial pattern of FMRI activation in early retinotopic cortex. J. Neurophysiol. 103, 1501–1507 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flounders M. W., González-García C., Hardstone R., He B. J., Neural dynamics of visual ambiguity resolution by perceptual prior. eLife 8, e41861 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith C. N., Squire L. R., Awareness of what is learned as a characteristic of hippocampus-dependent memory. Proc. Natl. Acad. Sci. U.S.A. 115, 11947–11952 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osterrieth P. A., Le test de copie d’une figure complexe. Arch. Psychol. 30, 206–356 (1944). [Google Scholar]
- 37.Gold J. J., Squire L. R., Quantifying medial temporal lobe damage in memory-impaired patients. Hippocampus 15, 79–85 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rempel-Clower N. L., Zola S. M., Squire L. R., Amaral D. G., Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J. Neurosci. 16, 5233–5255 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frankó E., Insausti A. M., Artacho-Pérula E., Insausti R., Chavoix C., Identification of the human medial temporal lobe regions on magnetic resonance images. Hum. Brain Mapp. 35, 248–256 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Insausti R., et al., MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am. J. Neuroradiol. 19, 659–671 (1998). [PMC free article] [PubMed] [Google Scholar]
- 41.Urgolites Z. J., Smith C. N., Squire L. R., Eye movements support the link between conscious memory and medial temporal lobe function. Proc. Natl. Acad. Sci. U.S.A. 115, 7599–7604 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fei-Fei L., Fergus R., Perona P.. . Learning generative visual models from few training examples: an incremental Bayesian approach tested on 101 object categories. IEEE. CVPR 2004, Workshop on Generative-Model.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at the Open Science Framework repository at https://osf.io/4dmj5/.