Significance
Chronic inflammatory diseases are characterized by an imbalance between pathogenic T effector cells and regulatory T cells. In this report, we demonstrate that decitabine, a cytosine analog, has a rapid and sustained therapeutic effect in animal models of rheumatoid arthritis. Decitabine selectively targets T effector cells expressing ENT1, a nucleoside transporter, and generates robust regulatory T cells with the capacity to suppress immune-driven inflammation. This study identifies a path toward resetting tolerance in autoimmune disease using a repurposed drug.
Keywords: autoimmunity; DNA-methylation inhibitor; rheumatoid arthritis; indoleamine 2,3-dioxygenase
Abstract
Chronic inflammatory diseases like rheumatoid arthritis are characterized by a deficit in fully functional regulatory T cells. DNA-methylation inhibitors have previously been shown to promote regulatory T cell responses and, in the present study, we evaluated their potential to ameliorate chronic and acute animal models of rheumatoid arthritis. Of the drugs tested, decitabine was the most effective, producing a sustained therapeutic effect that was dependent on indoleamine 2,3-dioxygenase (IDO) and was associated with expansion of induced regulatory T cells, particularly at the site of disease activity. Treatment with decitabine also caused apoptosis of Th1 and Th17 cells in active arthritis in a highly selective manner. The molecular basis for this selectivity was shown to be ENT1, a nucleoside transporter, which facilitates intracellular entry of the drug and is up-regulated on effector T cells during active arthritis. It was further shown that short-term treatment with decitabine resulted in the generation of a population of regulatory T cells that were able to suppress arthritis upon adoptive transfer. In summary, a therapeutic approach using an approved drug is described that treats active inflammatory disease effectively and generates robust regulatory T cells with the IDO-dependent capacity to maintain remission.
Despite recent advances in the therapy of chronic inflammatory diseases such as rheumatoid arthritis (RA), the induction of drug-free disease remission remains an elusive goal for the majority of patients. One reason for this is the lack of available drugs with the capacity to target pathogenic T effector (Teff) cell subsets selectively, while sparing or increasing the activity of regulatory T (Treg) cells.
In health, Treg cells play a nonredundant role in maintaining self-tolerance by controlling the activity of Teff cells. However, chronic inflammation is associated with decreased Treg cell function, a phenomenon that has been documented in a number of autoimmune diseases, including RA, systemic lupus erythematosus, and type 1 diabetes (1–4). In the case of RA, we and others have previously shown that loss of Treg cell function is accompanied by reduced expression of CTLA-4 due to aberrant CpG methylation in the cis-regulatory regions of the gene (5). In addition, the importance of DNA demethylation at the FOXP3 locus in determining Treg cell function has been confirmed in a number of independent studies (6–12).
These findings led us to question whether DNA-demethylating agents could promote Treg cell responses in chronic inflammatory diseases. There are two broad classes of DNA-demethylating agents: nucleoside analogs and nonnucleoside analogs. Two nucleoside analogs, decitabine and azacytidine, are approved for use in cancer due to their ability to induce cell death at high dose and promote reexpression of silenced tumor suppressor genes at low dose (13). Serendipitously, it was observed that numbers of circulating Treg cells are increased in the peripheral blood of patients following treatment with azacytidine (14–16). In addition, nucleoside analog demethylating agents have been reported to promote the generation and suppressive function of induced Treg (iTreg) cells in vitro (14, 17, 18) and to protect against experimental autoimmune encephalomyelitis and allogeneic cardiac transplant rejection in vivo (19).
Against this background, we compared the ability of nucleoside- and nonnucleoside-based DNA-demethylating agents to promote induction of Treg cells in animal models of RA. We found that short-term treatment with the cytosine analog decitabine depleted pathogenic Teff cells and promoted Treg cell responses, leading to lasting disease remission.
Results
DNA-Demethylating Drugs Promote Generation of Treg Cells In Vitro.
We first assessed the ability of nucleoside- and nonnucleoside-based DNA-demethylating drugs to promote the generation of iTreg cells by stimulating naive CD4+ T cells with anti-CD3 antibody under Treg cell-inducing conditions (Table 1). Treatment with decitabine, psammaplin A, or zebularine resulted in a dose-dependent increase in the percentage and total number of iTreg cells in vitro, as well as increased FoxP3 and CD25 expression (SI Appendix, Fig. S1 A, B, and D). A fourth drug, procainamide, failed to increase numbers of iTreg cells (SI Appendix, Fig. S1C).
Table 1.
Potency of DNA-demethylating agents at optimal doses for induction of Treg cells in vitro
| %Treg cells | Total no. of Treg cells | MFI of Foxp3 | MFI of CD25 | |
| Decitabine (1 μM) | 30 ± 11 | 5,953 ± 2,795 | 2,897 ± 48 | 3,958 ± 535 |
| Psammaplin A (1 μM) | 10 ± 4 | 1,697 ± 311 | 1,369 ± 32 | 2,129 ± 394 |
| Procainamide (200 μM) | 1 ± 1 | 398 ± 303 | 505 ± 83 | 1,895 ± 118 |
| Zebularine (100 μM) | 23 ± 4 | 2,920 ± 851 | 1,939 ± 67 | 3,295 ± 198 |
Data are expressed as means ± SD.
Decitabine Induces Long-Term Remission of Collagen-Induced Arthritis.
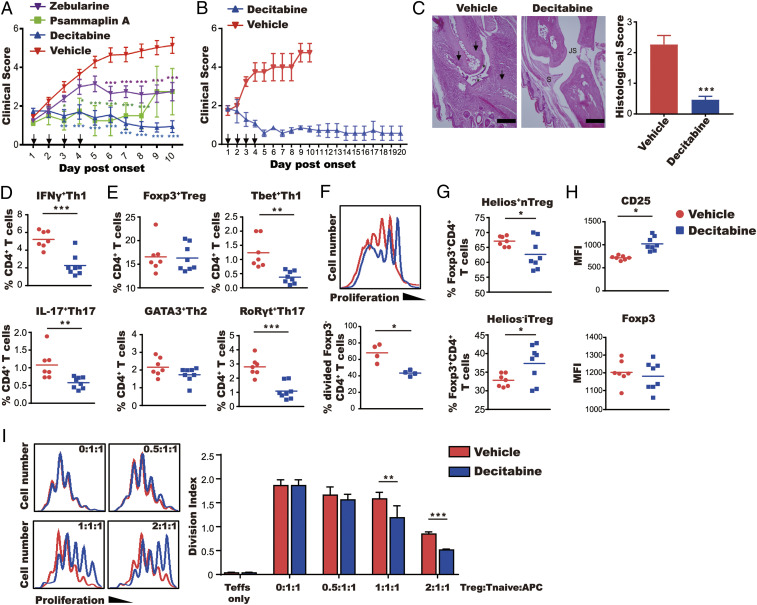
We next evaluated the efficacy of the DNA-demethylating drugs in collagen-induced arthritis (CIA) as a model for RA. DBA/1 mice were immunized with bovine type II collagen and treated with decitabine, zebularine, or psammaplin A at predetermined doses for 4 d only, starting after arthritis onset. There was a rapid reduction in clinical score (Fig. 1A) in mice treated with decitabine, and to a lesser extent zebularine, versus control mice that was sustained until the end of the experiment on day 10. Psammaplin A was also effective in reducing arthritis severity but the therapeutic effect was sustained only until day 8 (Fig. 1A). Thus, decitabine exhibited a superior therapeutic effect to zebularine and psammaplin A and was therefore used for all subsequent experiments. In a follow-up experiment, we demonstrated the ability of decitabine to maintain remission at least up to day 20, when the experiment was terminated (Fig. 1B). Histological assessment also confirmed the ability of decitabine to provide long-term protection against joint inflammation and erosion of cartilage and bone (Fig. 1C).
Fig. 1.
Decitabine has a sustained therapeutic effect on CIA. Mice with established arthritis were treated for 4 d with decitabine (1 mg⋅kg−1⋅d−1), psammaplin A (10 mg⋅kg−1⋅d−1), or zebularine (400 mg⋅kg−1⋅d−1). (A) Clinical scores (mean ± SEM; N = 10). (B) Clinical scores up to day 20 of mice treated with decitabine on days 1 to 4 (mean ± SEM; N = 7). (C) Representative images of proximal interphalangeal joint sections from mice treated with vehicle and decitabine are shown. Arrows indicate bone loss. Asterisks indicate damage of articular cartilage (JS, joint space; S, synovial membrane). (Scale bars, 100 μm.) The graph shows histological scores (mean ± SEM; N = 10). (D–H) Phenotypic characterization of Teff and Treg cells on day 10 of arthritis. Lymph node cells were stained with antibodies against cytokines (following stimulation with phorbol myristate acetate/ionomycin/brefeldin A) in D, lineage-specific transcription factors in E, or the nTreg cell marker Helios in G. Proliferation of FoxP3−CD4+ T cells was determined by CFSE labeling in F. Expression of FoxP3 and CD25 was quantified based on MFI in H. (I) Suppressive function of Treg cells was determined by culture with a fixed number of CFSE-labeled naive CD4+ T cells (CD25−CD4+) with mitomycin C-treated APCs from control mice under anti-CD3 antibody and IL-2 stimulation. Proliferation was determined by FACS (mean ± SEM; N = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
To establish its mechanism of action, type II collagen-immunized mice were treated with decitabine or vehicle for 4 d as in the previous experiment. Measurement of T cell subsets revealed a profound reduction in numbers of Th1 (IFNγ+CD4+ and Tbet+CD4+) and Th17 (IL-17+CD4+ and RoRγt+CD4+) cells in decitabine-treated mice (Fig. 1D and E). In addition, the number of proliferating FoxP3−CD4+ T cells from decitabine-treated mice ex vivo was markedly decreased compared with controls (Fig. 1F). Numbers of Treg (FoxP3+CD4+) cells were comparable in treated and control mice (Fig. 1E). However, the percentage of iTreg cells (as defined by Helios−FoxP3+CD4+ cells) within the total Treg cell population was increased in decitabine-treated versus control mice (Fig. 1G). Moreover, the suppressive activity of Treg cells from decitabine-treated mice was significantly increased compared with controls (Fig. 1I), which is in agreement with previous findings (18). Increased Treg cell function was consistent with the increased expression of CD25 on Treg cells from decitabine-treated mice (Fig. 1H). It was concluded that decitabine caused increased Treg cell activity as well as a reduction in pathogenic Th1/Th17 cells.
Indoleamine 2,3-Dioxygenase Is Required for Sustained Remission.
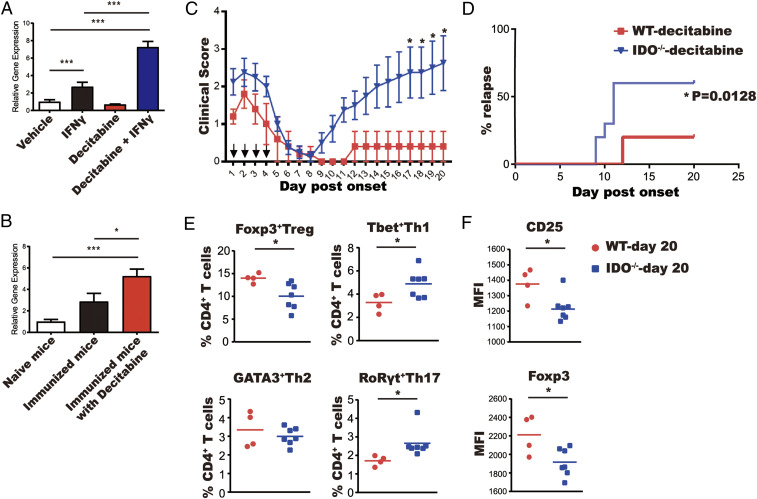
Indoleamine 2,3-dioxygenase 1 (IDO1) is important for maintaining robust Treg cell responses and its expression is also known to be suppressed by DNA methylation (20–22). This led us to investigate whether decitabine promotes IDO1 expression within the context of inflammation. Decitabine and IFNγ were found to act synergistically in the up-regulation of IDO1 gene expression in bone marrow-derived dendritic cells (BMDCs) in vitro, confirming previous findings (23) (Fig. 2A). In addition, increased IDO1 gene expression was observed in spleens and lymph nodes of type II collagen-immunized mice treated with decitabine (Fig. 2B). Thus, we further assessed whether the therapeutic effects of decitabine on CIA can be attributed to increased IDO1 expression. Arthritis was induced in Ido1−/− and wild-type mice, which were then treated with decitabine or vehicle for 4 d, as described above. Initially, decitabine had the same therapeutic effect in both groups but disease rapidly relapsed in Ido1−/− mice but not wild-type mice (Fig. 2 C and D). This was consistent with the fact that numbers of Treg cells and the expression of FoxP3 and CD25 in FoxP3+ Tregs were all significantly decreased in Ido1−/− mice compared with wild-type mice (Fig. 2 E and F).
Fig. 2.
IDO is induced by decitabine and is required for sustained disease remission. (A) IDO1 gene expression of BMDCs of C57BL/6 mice treated with IFNγ and/or decitabine was determined by qPCR. Values are the mean ± SEM (N = 3). (B) C57BL/6 mice were immunized with bovine type II collagen in CFA and treated with decitabine (1 mg⋅kg−1⋅d−1) or vehicle for 4 d. Spleens and lymph nodes were harvested on day 11 after immunization. IDO1 gene expression was determined by qPCR. Values are the mean ± SEM (N = 3). (C and D) Wild-type and Ido1−/− mice with CIA were treated for 4 d with decitabine (1 mg⋅kg−1⋅d−1) and monitored for 20 d. (C) Clinical scores. (D) % relapsed mice. (E and F) Wild-type and Ido1−/− mice treated with decitabine from C were culled on day 20. Lymph node cells were stained with lineage-specific transcription factors (E) and MFI of Treg immunoregulatory markers was determined by gating on Treg cells from E (F). *P < 0.05, **P < 0.01, ***P < 0.001.
Decitabine Selectively Targets ENT1+ T Cells.
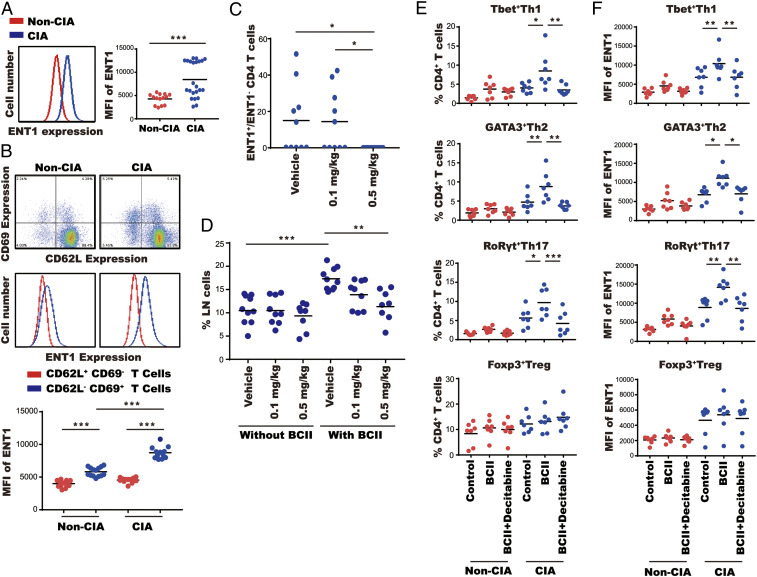
Decitabine is known to enter cells via the equilibrative nucleoside transporter 1 (ENT1), which is also known to be up-regulated on proliferating cells (13). We reasoned that this could explain the selective action of decitabine on Teff cells (Fig. 1). Indeed, ENT1 expression was higher in CD4+ T cells from type II collagen-immunized mice with active arthritis versus immunized mice without arthritis (Fig. 3A and SI Appendix, Fig. S2A). We also found a higher level of ENT1 expression in activated CD62L−CD69+CD4+ T cells compared with CD62+CD69−CD4+ nonactivated T cells (Fig. 3B). Moreover, ENT1 expression in activated CD62L−CD69+CD4+ T cells from arthritic mice was greater than activated CD62L−CD69+CD4+ T cells from nonarthritic mice (Fig. 3B). These results suggest that pathogenic Teff cells increase expression of ENT1 during active disease. We further showed that treatment with decitabine at 0.5 mg/kg led to complete elimination of ENT1+CD4+ cells from draining lymph nodes of arthritic mice (Fig. 3C). We ruled out the possibility that loss of ENT1+ cells was due to masking of ENT1 by decitabine, thereby preventing binding of the detection antibody (SI Appendix, Fig. S3). In addition, ex vivo stimulation with bovine type II collagen showed that decitabine treatment led to a dose-dependent reduction in antigen-stimulated proliferation of CD4+ T cells from arthritic mice, without affecting the basal rate of proliferation (Fig. 3D), thereby confirming the selective action of decitabine. Further analysis of CD4+ T cell subsets revealed that decitabine suppressed antigen-stimulated proliferation of Th1, Th2, and Th17 cells (but not Treg cells) from arthritic mice but had no effect on T cells from nonarthritic mice (Fig. 3 E and F). Taken together, these results demonstrate that ENT1 is up-regulated in pathogenic Teff cells during active disease and decitabine selectively depletes these cells.
Fig. 3.
Decitabine depletes ENT1+ cells. (A) ENT1 expression was determined in CD4+ T cells from lymph nodes of nonarthritic mice (red) versus arthritic mice (blue) by FACS. (B) ENT1 expression was determined in nonactivated CD4+ T cells (red; CD62L+CD69−) and activated CD4+ T cells (blue; CD62L−CD69+) from lymph nodes of nonarthritic mice versus arthritic mice by FACS. (C and D) Arthritic mice were treated for 4 d with decitabine (0.1 or 0.5 mg⋅kg−1⋅d−1). (C) The ratio of ENT1+:ENT1−CD4+ T cells was determined in lymph nodes by FACS. (D) lymph node cells were stimulated with or without bovine type II collagen (BCII) and IL-2; % CD4+ T cells in lymph nodes was determined by FACS. (E and F) lymph node cells from nonarthritic mice (red) or arthritic mice (blue) were stimulated with or without BCII, IL-2, and decitabine. % T cell subsets, Th1, Th2, Th17, and Treg cells (E) or ENT1 expression (F) in CD4+ T cells was determined by FACS. *P < 0.05, **P < 0.01, ***P < 0.001.
Depletion of Teff Cells by Decitabine Is Dependent on ENT1.
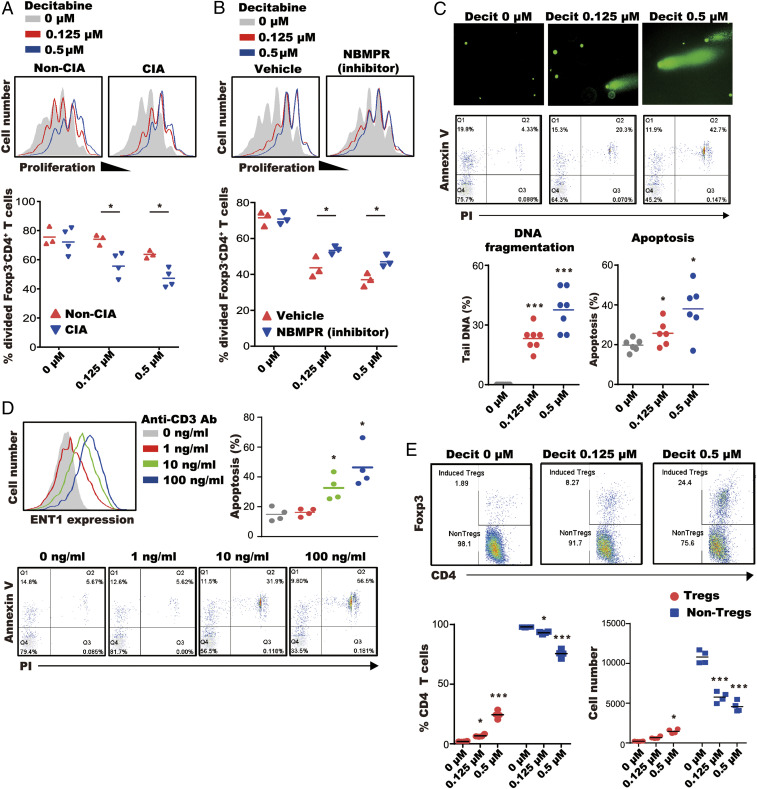
We next set out to address the mechanism by which decitabine depletes Teff cells. We first showed that proliferative responses of FoxP3−CD4+ T cells from arthritic mice were significantly more sensitive to the inhibitory effects of decitabine than those of nonarthritic mice (Fig. 4A). This was not due to an increased rate of proliferation in FoxP3−CD4+ T cells in arthritis because numbers of proliferating cells were similar between arthritic and nonarthritic mice without decitabine treatment (Fig. 4A). Rather, we attributed the greater sensitivity of FoxP3−CD4+ T cells from arthritic mice to increased expression of ENT1 (Fig. 4A). The involvement of ENT1 was subsequently confirmed by the fact that nitrobenzylthioinosine (NBMPR), a specific inhibitor of ENT1, significantly reduced the antiproliferative effect of decitabine but had no effect on proliferation in the absence of decitabine (Fig. 4B).
Fig. 4.
Decitabine inhibits proliferation of Teff cells and generates iTreg cells. (A) CFSE-stained spleen cells were treated with anti-CD3 antibody, IL-2, and decitabine. Proliferation of FoxP3−CD4+ T cells was determined by FACS. (B) CFSE-stained spleen cells from arthritic mice were stimulated with anti-CD3 antibody, IL-2, and decitabine ± ENT1 inhibitor (NBMPR; 100 μM). Proliferation of CFSE-labeled FoxP3−CD4+ T cells was determined by FACS. (C) Naive CD4+ T cells were treated with anti-CD3 antibody, IL-2, and decitabine. DNA fragmentation and apoptosis were determined by comet assay and annexin V/PI apoptosis kit. (D) Naive CD4+ T cells were treated with anti-CD3 antibody, IL-2, and decitabine (0.5 μM). ENT1 expression of naive CD4+ T cells was determined by FACS and apoptosis was determined by annexin V/PI apoptosis kit. (E) Naive CD4+ T cells treated with decitabine and anti-CD3 antibody/IL-2. Treg and non-Treg cells were quantified by FACS. *P < 0.05, **P < 0.01, ***P < 0.001.
To determine the mechanism by which decitabine reduces numbers of proliferating T cells, we looked for evidence of DNA fragmentation and apoptosis in the T cell population using the comet assay and annexin V/propidium iodide (PI) staining, respectively. Decitabine increased DNA fragmentation and annexin V staining of CD4+ T cells in a dose-dependent manner (Fig. 4C) and the percentage of apoptotic T cells correlated positively with ENT1 expression following anti-CD3 antibody stimulation (Fig. 4D). Furthermore, decitabine not only reduced the proliferation of FoxP3−CD4+ T cells but also enhanced the generation of iTreg cells under the conditions of anti-CD3 antibody stimulation (Fig. 4E). Taken together, the results are consistent with previous reports (13), and our own in vivo findings (Fig. 1), that decitabine depletes proliferating cells by DNA fragmentation and apoptosis while expanding iTreg cells, potentially by promoting the expression of regulatory genes previously silenced by DNA methylation.
Localization of Decitabine-Induced iTreg Cells at the Site of Disease Activity.
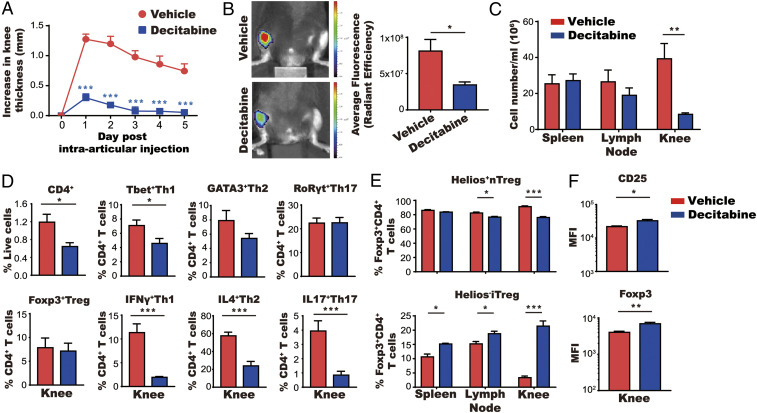
Administration of decitabine to mice with CIA led to an increase in the proportion of iTreg cells in draining lymph nodes (Fig. 1G). In order to assess the pathophysiological importance of this finding, we used the antigen-induced arthritis (AIA) model in which phenotypic analysis can be performed on cells extracted from inflamed knee joints ex vivo. C57BL/6 mice were immunized with methylated bovine serum albumin (mBSA), and then treated with decitabine for 5 d prior to intraarticular injection of mBSA. The severity of arthritis was profoundly reduced in decitabine-treated mice, as indicated by significant reductions in knee swelling, cathepsin activity, and inflammatory cell infiltration (Fig. 5 A–C). Phenotypic analysis of cells in the knee joints of decitabine-treated mice revealed reductions in CD4+ T cells and Th1, Th2, Th17, and Treg cells (Fig. 5D and SI Appendix, Fig. S4A). However, within the Treg cell population, there was a marked increase in the proportion of iTreg cells (as defined by Helios−FoxP3+CD4+ cells), particularly in the joints but also in the spleens and lymph nodes (Fig. 5E and SI Appendix, Fig. S4B). The expression of CD25 and Foxp3 was significantly increased in total Treg cells from decitabine-treated mice (Fig. 5F). Thus, decitabine treatment reduced inflammatory cell accumulation in the joint and increased the proportion of iTreg cells locally and systemically.
Fig. 5.
Accumulation of iTreg cells in the joint following treatment with decitabine. (A–C) Mice were immunized with mBSA and then treated for 5 d with decitabine (1 mg⋅kg−1⋅d−1) starting on day 10 after immunization (mean ± SEM; N = 5). Mice were given an intraarticular injection of mBSA 15 d after immunization. Knee swelling was monitored for 5 d (A). Cathepsin activity was measured using a fluorescent probe and detected using IVIS on day 2 of arthritis (B) and cell numbers in spleen, lymph node, and knee were counted on day 6 of arthritis (C). (D) Cells from arthritic joints were stained with antibodies against CD4+ T cell cytokines and lineage-specific transcription factors. Total numbers of cells are shown in SI Appendix, Fig. S4A. (E) Spleen, lymph node, and knee cells (following stimulation with mBSA) were stained with antibodies against the nTreg cell marker Helios. Total numbers of cells are shown in SI Appendix, Fig. S4B . (F) Expression of FoxP3 and CD25 of Treg cells gated from D was quantified based on MFI. *P < 0.05, **P < 0.01, ***P < 0.001.
Decitabine-Induced Treg Cells Possess Antiarthritic Activity.
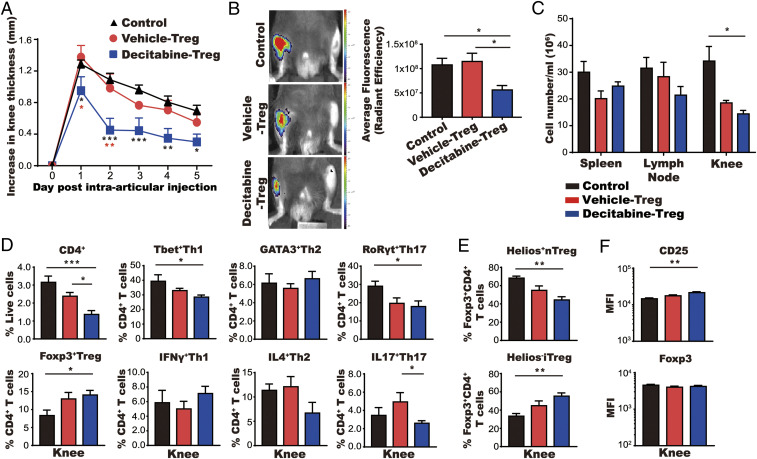
Finally, we confirmed the antiarthritic activity of decitabine-induced Treg cells by adoptive transfer from decitabine-treated donor mice to untreated, arthritic recipient mice. Treg cells were collected from spleens and lymph nodes of mBSA-immunized donor mice that had been treated previously with decitabine or vehicle alone. Treg cells (3 × 105 cells per mouse) were then injected intravenously into mBSA-immunized recipient mice prior to intraarticular injection of mBSA. Treg cells from decitabine-treated mice, but not control mice, significantly reduced inflammatory cell infiltration with significant reductions in CD4+ T cells and Th1, Th2, and Th17 cells (Fig. 6 A–D and SI Appendix, Fig. S5A). Moreover, numbers of Treg cells were increased in the arthritic joints of decitabine Treg-treated mice (Fig. 6D) and the mean fluorescence intensity (MFI) of CD25 was significantly increased on Treg cells from the knees of decitabine Treg-treated mice (Fig. 6F). The percentage and numbers of iTreg cells (Helios−FoxP3+CD4+) within the total Treg cell population were also significantly increased in the knees of mice that had received Treg cells from decitabine-treated mice versus control mice (Fig. 6E and SI Appendix, Fig. S5B). Hence, administration of decitabine-induced Treg cells had a profound and durable protective effect on development of arthritis.
Fig. 6.
Decitabine-induced Treg cells ameliorate arthritis. (A–C) Mice received Treg cells intravenously 10 d after immunization with mBSA (mean ± SEM; N = 6). Knee swelling was monitored for 5 d (A). Cathepsin activity was measured using a fluorescent probe and detected by IVIS on day 2 of arthritis (B) and cell numbers of spleen, lymph node, and knee were counted on day 6 of arthritis (C). (D and E) Cells from arthritic joints were stained with antibodies against CD4+ T cell cytokines and lineage-specific transcription factors in D and the nTreg cell marker Helios in E. Total numbers of cells are shown in SI Appendix, Fig. S5. (F) Expression of FoxP3 and CD25 of Treg cells gated from D was quantified based on MFI. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Chronic inflammation is associated with loss of Treg cell function due, at least in part, to DNA methylation at regulatory regions of functionally important genes, including FoxP3 and CTLA-4 (1–5). This led us to question whether DNA-methylation inhibitors would be effective in reestablishing tolerance in autoimmunity by augmenting Treg cell activity. We therefore evaluated the effect of short-term treatment of arthritis with three DNA-methylation inhibitors and found that one in particular, decitabine, was remarkably effective in suppressing disease activity and maintaining long-term remission. Amelioration of arthritis was accompanied by increased expression of Treg signature genes and CD25, increased Treg cell function, and reduced numbers of Th1 and Th17 cells. Importantly, decitabine-induced Treg cells were shown to be functionally active, as evidenced by their ability to suppress arthritis upon adoptive transfer. Phenotypic analysis of Treg cells in the joints and lymphoid organs of decitabine-treated mice revealed an increase in the proportion of iTreg cells (defined as Helios−FoxP3+CD4+), although total numbers of Treg cells (FoxP3+CD4+) were unchanged. On this basis it is proposed that treatment with decitabine increases the ratio of iTreg:nTregs (naive Tregs, defined as Helios+FoxP3+CD4+) but does not alter the size of the Treg cell pool.
It has been suggested that iTreg cells have a less stable phenotype than nTreg cells and can lose FoxP3 expression under inflammatory conditions (24). We found, however, that short-term treatment with decitabine induces Treg cells, expressing high levels of FoxP3 and CD25, even in inflammation. Furthermore, the increased expression of CD25 remained stable despite adoptive transfer to arthritic recipient mice. Thus, administration of decitabine to mBSA-immunized mice generated a robust and durable Treg cell population that was able to protect against disease upon transfer to mBSA-immunized mice injected intraarticularly with the same antigen. Several possible therapeutic strategies have been proposed to exploit the homeostatic potential of Treg cells, including administration of low-dose IL-2, expansion of autologous Treg cells in vitro, and generation of iTregs by T cell receptor (TCR) stimulation in the presence of IL-2 and TGF-β (25–29). However, functional instability of Treg cells is a major problem encountered with all of these approaches; therefore, the finding that decitabine treatment produces a phenotypically robust Treg cell population is noteworthy.
Given the importance of IDO in generation and amplification of Treg cell responses (20, 21), we hypothesized that deletion of IDO would reduce the efficacy of decitabine therapy. Indeed, this was confirmed by the fact that treatment of arthritic Ido1−/− mice failed to provide stable remission and was associated with reduced numbers of Treg cells and increased numbers of Th1 and Th17 cells. It is noteworthy that the induction of tolerogenic responses in macrophages by phagocytosis of apoptotic cells is dependent on IDO (30). Hence, it is possible that the tolerogenic uptake of apoptotic Teff cells following treatment with decitabine played a role in maintaining long-term remission of arthritis. Another consideration is that a related drug, 5-azacytidine, has recently been shown to mediate hematopoietic stem cell depletion (31), which could potentially allow for the subsequent reemergence of a more robust Treg cell population.
In addition to its effect on Treg cells, decitabine also caused rapid depletion of Teff cell populations. While this may have been caused partly by the increase in Treg cell activity, decitabine also had a direct effect on Teff cells, by inducing DNA damage and apoptotic cell death at submicromolar concentrations. This is consistent with the observations of Agrawal et al., who showed that high-dose decitabine causes cell death, an effect that masks any effects on DNA methylation (13). The intracellular entry point for decitabine is the nucleoside channel ENT1, and blockade of this channel impaired decitabine’s capacity to inhibit Teff cell proliferation. This provides an explanation for decitabine’s selectivity, as ENT1 expression was increased in Teff cells. Furthermore, ENT1 expression in Teff cells was elevated in arthritic mice compared with nonarthritic mice, despite the fact that T cell proliferation was comparable between the two groups. This suggests that there was an association between ENT1 expression and pathogenic T cells, although this remains to be characterized more fully.
A major obstacle to the therapeutic use of nucleoside analog-based drugs, like decitabine, in a nonfatal disease like RA is lack of specificity giving rise to off-target effects. Nevertheless, a degree of specificity is provided since decitabine incorporates into the DNA of cells in S phase (32), hence selectively targeting proliferating cells. More importantly, as discussed above, potentially pathogenic T cells from arthritic mice were more sensitive to the effects of decitabine than T cells from nonarthritic mice. Thus, the selective activity of decitabine is controlled at two levels: cellular proliferation and expression of ENT1. This potentially provides a therapeutic window which could allow immunomodulation with minimal side effects. It is worth emphasizing that no clinical signs of toxicity were observed in mice treated with decitabine at a dose of 1 mg/kg. Furthermore, a prospective, multicenter study of low‐dose decitabine in adults with refractory immune thrombocytopenia has recently been performed with no serious adverse events being recorded (33).
In summary, we have shown that a brief period of treatment with decitabine generates antiarthritic Treg cells, depletes Teff cells, and induces IDO-dependent disease remission in a validated model of RA. In view of its proven safety, and with further data on its effects on host immunity and gene expression, it should be possible to pursue this approach in human autoimmune disorders.
Materials and Methods
Study Design.
Studies were primarily designed to test the long-term therapeutic effects of short-term treatment of DNA-demethylating agents in immune-driven inflammation. All experiments were performed in a blinded fashion and key experiments were repeated to ensure reproducibility. For experiments involving genetically modified mice, age-matched littermates were housed together and randomly assigned to drug or vehicle treatment groups, thereby avoiding cage effects, body weight effects, and genetic drift. In general, animal numbers were determined on the basis of previous experience and from pilot studies. Clinical scores and paw or joint swelling were monitored daily after onset of arthritis. In accordance with our ethical approval, mice with CIA were killed 10 d after onset of arthritis. However, mice deemed to be in remission (defined as a clinical score of less than 2) were kept until day 20 or until they relapsed. For ex vivo studies, experiments were performed with at least three mice per study in addition to pilot optimization studies for dose and time point determination. All experimental procedures were approved by the Ethical Review Process Committee and the UK Home Office, in accordance with Animals (Scientific Procedures) Act 1986.
Reagents.
Full details are provided in SI Appendix.
Mice.
Full details are provided in SI Appendix.
CIA.
Immunization for CIA was carried out as previously described (34). In brief, a solution of bovine type II collagen (4 mg/mL) was emulsified with an equal volume of complete Freund’s adjuvant (CFA; BD Biosciences). DBA/1 mice (or wild-type and Ido1−/− mice on a C57BL/6N.Q, H-2q background) were immunized by subcutaneous injection of 100 μL emulsion.
The clinical severity of CIA was scored in each paw as follows: 0 = normal, 1 = slight swelling and/or erythema, 2 = marked swelling, 3 = ankylosis. Hindpaw thickness was measured using microcalipers (Kroeplin). For histological assessment, paws were fixed in 10% neutral-buffered formalin followed by decalcification with 5.5% ethylenediaminetetraacetate (EDTA) in buffered formalin. Fixed paws were embedded in paraffin, and sections were cut and stained with hematoxylin and eosin. Histopathologic changes in the joints were scored on a scale of 0 to 3, where 0 = normal, 1 = minimal synovitis without cartilage/bone erosion, 2 = synovitis with some marginal erosion but joint architecture maintained, and 3 = severe synovitis and erosion with loss of normal joint architecture.
Antigen-Induced Arthritis.
AIA was induced as previously described (35). Briefly, on day 0, 8- to 10-wk-old male C57BL/6 mice were anesthetized with 2% isoflurane and immunized subcutaneously with 100 μg of mBSA in CFA. Where indicated, mice were administered 1 mg/kg of decitabine or vehicle intraperitoneally from day 1 to day 5 for Treg purification or from day 10 to day 14 for normal AIA experiments. For adoptive transfer experiments, Treg cells from decitabine- or vehicle-treated mice were purified by FACS and then cultured overnight with 10 ng/mL IL-2 and 20 ng/mL IL-7. Recipient mice received an intravenous injection of 3 × 105 purified Treg cells on day 10 after immunization with mBSA. On day 15, mice were anesthetized with 2% isoflurane, both knees were shaved, and 125 μg of mBSA was administered intraarticularly in the right knee joint. Left knee joints received a vehicle control injection. Knee swelling was monitored daily using digital calipers for 5 d.
In Vivo Imaging System.
On day 1 of AIA, mice received an intravenous injection of 4 nmol ProSense 750 FAST imaging probe (PerkinElmer). Mice were imaged 20 h post intravenous injection using the In Vivo Imaging System (IVIS) Spectrum (PerkinElmer) with an excitation wavelength of 745 nm and an emission wavelength of 800 nm, automatic exposure, and medium binning. Images were analyzed using Living Image 4.7 software (PerkinElmer) to obtain the average fluorescence intensities of a circular region of interest encompassing the knee joint.
FACS.
Full details are provided in SI Appendix.
Proliferation/Viability Assay.
Spleen/lymph node cells (1 × 107) were labeled with the CellTrace CFSE Cell Proliferation Kit (5 μM; Invitrogen) and stimulated with anti-CD3 antibody (100 ng/mL or 1 μg/mL) and IL-2 (10 ng/mL) in the presence or absence of decitabine for 72 h. The cells were then stained with PerCP-Cy5.5–conjugated anti-CD4 antibody, PeCy7-conjugated anti-FoxP3 antibody, and Zombie Fixable Viability dye, and washed and analyzed by FACS to determine the proliferation of FoxP3−CD4+ T cells. For proliferation of naive CD4+ T cells purified by the Mojo Mouse CD4 Naïve T Cell Isolation Kit (BioLegend), 1 × 107 cells were labeled with the CellTrace CFSE Cell Proliferation Kit and stimulated with different doses of anti-CD3 antibody (1 to 100 ng/mL) and IL-2 (10 ng/mL) in the presence or absence of different doses of decitabine (0 to 0.5 μM) for 72 h. The cells were then stained with PerCP-Cy5.5–conjugated anti-CD4 antibody, AF-647–conjugated anti-ENT1 antibody, PeCy7-conjugated anti-FoxP3 antibody, and Zombie Fixable Viability dye, and washed and analyzed by FACS to determine the proliferation and numbers of CD4+ Teff cells and generation of FoxP3+CD4+ Treg cells.
Treg Suppression Assay.
Naive CD4+ T cells (CD25−CD4+) and CD25+CD4+ T (Treg) cells were isolated from a spleen/lymph node pooled cell suspension using the Regulatory T Cell Isolation Kit (Miltenyi Biotec) according to the manufacturer’s instructions. The remaining CD4− cells were treated with mitomycin C (20 μg/mL; Sigma-Aldrich) for 30 min and used as antigen-presenting cells (APCs). Naive CD4+ T cells were labeled with the CellTrace CFSE Cell Proliferation Kit (as described above) and cultured with mitomycin C-pretreated APCs and Treg cells in the presence of anti-CD3 antibody (1 μg/mL) and IL-2 (10 ng/mL) for 72 h. The ratio of Treg:naive CD4+ T:APC ranged from 2:1:1 to 0.0675:1:1. Proliferation of FoxP3−CD4+ T cells was analyzed and determined by FACS and FlowJo (v10).
Western Blotting.
Full details are provided in SI Appendix.
DNA Fragmentation and Apoptosis Assay.
Single-spleen cell suspensions were used to isolate naive CD4+ T cells by the Mojo Mouse CD4 Naïve T Cell Isolation Kit. Cells were then stimulated with anti-CD3 antibody (1, 10, and 100 ng/mL) and IL-2 (10 ng/mL) in the presence or absence of decitabine (0.125 and 0.5 μM) for 24, 48, or 72 h. A fraction of the cells were removed to perform the DNA fragmentation assay using the Comet Assay Kit (Abcam) according to the manufacturer’s instructions, and then analyzed using a Lecia microscope. The rest of the cells were stained by using the PB-Annexin V Apoptosis Detection Kit with PI (BioLegend) according to the manufacturer’s instructions, and then analyzed by FACS to determine the degree of apoptosis of CD4+ T cells.
iTreg Cell-Differentiation Assay.
A pooled single-cell suspension from spleens and lymph nodes was used to isolate Treg cells, naive CD4+ T cells, and APCs as described above. A 1:1 ratio of mitomycin C-pretreated APCs and naive CD4+ T cells was cultured in a 96-well U-bottom tissue-culture plate in the presence of anti-CD3 antibody (1 μg/mL) with TGF-β (5 ng/mL) and IL-2 (10 ng/mL). DNA-demethylating agents were added in serial concentrations. Numbers of iTreg cells were measured after 72 h by FACS.
Bone marrow-derived dendritic cells (BMDCs).
Bone marrow cells were harvested from femurs of mice and cultured in complete RPMI with 20 ng/ml GM-CSF and 10 ng/ml IL-4 for 3 d. Cells were then treated with 2 μM decitabine for 3 d followed by incubation for 7 d with 20 ng/ml GM-CSF and 10 ng/ml IL-4. BMDCs were then stimulated with 20 ng/ml IFNγ for 1 d, lysed and analyzed for gene expression by qPCR.
Reverse-Transcription qPCR.
Cell samples were lysed using RLT buffer (Qiagen) with 1-in-100 dilution of 2-mercaptoethanol (Sigma-Aldrich). RNA was purified using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions and stored at −80 °C until conversion to complementary DNA (cDNA). Reverse-transcription qPCR was carried out as previously described (36).
Statistical Analysis.
Clinical and histological scores were compared by unpaired t test or two-way ANOVA with Tukey’s or Sidak’s multiple comparison test, as appropriate. Numbers of relapsing mice were compared by survival curve comparison test. Phenotypic comparisons of T cell subsets were achieved using one-way ANOVA with Tukey’s multiple comparison or unpaired t test. Proliferative responses were compared by two-way ANOVA with Sidak’s multiple comparison test, one-way ANOVA with Tukey’s multiple comparison test, or unpaired t test, as appropriate. All calculations were performed using GraphPad Prism 7 software. A P value less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
This study was supported by research grants from Epsom Medical Research, Idogen AB, and the Ministry of Science and Technology, Taiwan (MOST-104-2911-I-182A-503).
Footnotes
Competing interest statement: A.S., L.G.S., and H.-O.S. are shareholders in Idogen.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2100939118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Scheinecker C., Bonelli M., Smolen J. S., Pathogenetic aspects of systemic lupus erythematosus with an emphasis on regulatory T cells. J. Autoimmun. 35, 269–275 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Lindley S., et al., Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes 54, 92–99 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Prakken B., Wehrens E., van Wijk F., Editorial: Quality or quantity? Unraveling the role of Treg cells in rheumatoid arthritis. Arthritis Rheum. 65, 552–554 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Flores-Borja F., Jury E. C., Mauri C., Ehrenstein M. R., Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc. Natl. Acad. Sci. U.S.A. 105, 19396–19401 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cribbs A. P., et al., Treg cell function in rheumatoid arthritis is compromised by ctla-4 promoter methylation resulting in a failure to activate the indoleamine 2,3-dioxygenase pathway. Arthritis Rheumatol. 66, 2344–2354 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Floess S., et al., Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 5, e38 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H. P., Leonard W. J., CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: A role for DNA methylation. J. Exp. Med. 204, 1543–1551 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polansky J. K., et al., DNA methylation controls Foxp3 gene expression. Eur. J. Immunol. 38, 1654–1663 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Ohkura N., et al., T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 37, 785–799 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Cribbs A. P., et al., Methotrexate restores regulatory T cell function through demethylation of the FoxP3 upstream enhancer in patients with rheumatoid arthritis. Arthritis Rheumatol. 67, 1182–1192 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Kennedy A., et al., A novel upstream enhancer of FOXP3, sensitive to methylation-induced silencing, exhibits dysregulated methylation in rheumatoid arthritis Treg cells. Eur. J. Immunol. 44, 2968–2978 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Ngalamika O., et al., Peripheral whole blood FOXP3 TSDR methylation: A potential marker in severity assessment of autoimmune diseases and chronic infections. Immunol. Invest. 44, 126–136 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Agrawal K., Das V., Vyas P., Hajdúch M., Nucleosidic DNA demethylating epigenetic drugs—A comprehensive review from discovery to clinic. Pharmacol. Ther. 188, 45–79 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Wijermans P., et al., Low-dose 5-aza-2′-deoxycytidine, a DNA hypomethylating agent, for the treatment of high-risk myelodysplastic syndrome: A multicenter phase II study in elderly patients. J. Clin. Oncol. 18, 956–962 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Goodyear O. C., et al., Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood 119, 3361–3369 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Stübig T., et al., 5-Azacytidine promotes an inhibitory T-cell phenotype and impairs immune mediated antileukemic activity. Mediators Inflamm. 2014, 418292 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu C. H., et al., DNA methyltransferase inhibitor promotes human CD4+CD25hFOXP3+ regulatory T lymphocyte induction under suboptimal TCR stimulation. Front. Immunol. 7, 488 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi J., et al., In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood 116, 129–139 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X., et al., Decitabine inhibits T cell proliferation via a novel TET2-dependent mechanism and exerts potent protective effect in mouse auto- and allo-immunity models. Oncotarget 8, 56802–56815 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu H., Gong J., Liu Y., Indoleamine 2, 3-dioxygenase regulation of immune response (review). Mol. Med. Rep. 17, 4867–4873 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Yang Y., Liu K., Chen Y., Gong Y., Liang Y., Indoleamine 2,3-dioxygenase (Ido) regulates Th17/Treg immunity in experimental IgA nephropathy. Folia Biol. (Praha) 65, 101–108 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Noonepalle S. K., et al., Promoter methylation modulates indoleamine 2,3-dioxygenase 1 induction by activated T cells in human breast cancers. Cancer Immunol. Res. 5, 330–344 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue Z. T., Sjögren H. O., Salford L. G., Widegren B., An epigenetic mechanism for high, synergistic expression of indoleamine 2,3-dioxygenase 1 (Ido1) by combined treatment with zebularine and IFN-γ: Potential therapeutic use in autoimmune diseases. Mol. Immunol. 51, 101–111 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Ohkura N., Kitagawa Y., Sakaguchi S., Development and maintenance of regulatory T cells. Immunity 38, 414–423 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann P., et al., Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood 108, 4260–4267 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Zheng S. G., Wang J., Wang P., Gray J. D., Horwitz D. A., IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J. Immunol. 178, 2018–2027 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Koreth J., et al., Interleukin-2 and regulatory T cells in graft-versus-host disease. N. Engl. J. Med. 365, 2055–2066 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saadoun D., et al., Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med. 365, 2067–2077 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Long S. A.et al.; Diabetes TrialNet and the Immune Tolerance Network , Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes 61, 2340–2348 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravishankar B., et al., Tolerance to apoptotic cells is regulated by indoleamine 2,3-dioxygenase. Proc. Natl. Acad. Sci. U.S.A. 109, 3909–3914 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shastri A., Will B., Steidl U., Verma A., Stem and progenitor cell alterations in myelodysplastic syndromes. Blood 129, 1586–1594 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Ali H. K., Jaekel N., Niederwieser D., The role of hypomethylating agents in the treatment of elderly patients with AML. J. Geriatr. Oncol. 5, 89–105 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Zhou H., et al., A prospective, multicenter study of low dose decitabine in adult patients with refractory immune thrombocytopenia. Am. J. Hematol. 94, 1374–1381 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Inglis J. J., Simelyte E., McCann F. E., Criado G., Williams R. O., Protocol for the induction of arthritis in C57BL/6 mice. Nat. Protoc. 3, 612–618 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Topping L. M., et al., Targeting extracellular vesicles to the arthritic joint using a damaged cartilage-specific antibody. Front. Immunol. 11, 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tseng W. Y., et al., TNF receptor 2 signaling prevents DNA methylation at the Foxp3 promoter and prevents pathogenic conversion of regulatory T cells. Proc. Natl. Acad. Sci. U.S.A. 116, 21666–21672 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.