Abstract
The diagnostic potential of D‐dimer and fibrinogen to detect periprosthetic joint infection (PJI) of the hip and knee is not well‐understood. The aim of this study was to determine whether D‐Dimer and fibrinogen can be used as effective biomarkers to screen PJI. A systematic review of the literature indexed in Web of Science, PubMed, Cochrane Library, Embase, and Google Scholar databases was performed. All studies using D‐dimer levels in serum or plasma, or fibrinogen levels in plasma, for the diagnosis of PJI were included. Meta‐analysis estimates, including sensitivity, specificity, diagnostic odds ratios (DOR), and the area under the summary receiver operating characteristic curve (AUSROC), were calculated using a random‐effects model, and used to assess the diagnostic accuracy of these biomarkers. A total of nine studies were analyzed, and their quality was considered to be acceptable. D‐dimer gave a limited diagnostic value if serum and plasma combined: sensitivity (0.77, 95% confidence interval [CI] [0.63 to 0.87]), specificity (0.67, 95% CI [0.54 to 0.78]), DOR (6.81, 95% CI [2.67 to 17.37]), and AUSROC (0.78, 95% CI [0.74 to 0.82]). Plasma D‐dimer levels were associated with less satisfactory sensitivity (0.65, 95% CI 0.57 to 0.71), specificity (0.58, 95% CI 0.50 to 0.66), DOR (2.52, 95% CI 1.64 to 3.90), and AUSROC (0.65, 95% CI 0.61 to 0.69). Serum D‐dimer levels showed higher corresponding values of 0.89 (95% CI 0.79 to 0.94), 0.76 (95% CI 0.55 to 0.89), 24.24 (95% CI 10.07 to 58.32), and 0.91 (95% CI 0.88 to 0.93). Plasma fibrinogen showed acceptable corresponding values of 0.79 (95% CI 0.70 to 0.85), 0.73 (95% CI 0.57 to 0.85), 10.14 (95% CI 6.16 to 16.70), and 0.83 (95% CI 0.79 to 0.86). Serum D‐dimer may be an effective marker for the diagnosis of PJI in hip and knee arthroplasty patients, and it may show higher diagnostic potential than plasma fibrinogen. Plasma D‐dimer may have limited diagnostic potential.
Keywords: D‐dimer, Fibrinogen, Meta‐analysis, Periprosthetic joint infection, Plasma, Serum
Introduction
Total joint arthroplasty (TJA) is a successful surgical treatment for advanced hip and knee diseases 1 , 2 . However, periprosthetic joint infection (PJI) that occurs after TJA is a catastrophic complication, leading to prolonged treatment, increased hospital expenses, and even higher morbidity and mortality rates 3 , 4 . The incidence rate of PJI is estimated to range between 0.7% and 2.4% 5 , 6 , and is expected to increase rapidly with an increase in prevalence of primary TJA. The number of revision knee arthroplasties is expected to grow at a high rate of nearly 90% each year and is expected to reach 47,313 cases by 2050, which is mainly due to the increase in PJI 7 . An early and accurate diagnosis of PJI is important for patients and surgeons. This helps plan and execute an optimal therapy scheme, manage patients’ emotions and expectations, and ensure retention of the implanted prosthesis as well as joint function 8 . PJI screening is of prime importance, especially for those patients with chronic infection (which occurred after 3 months of the index procedure) caused by low‐virulence pathogens. Markers in blood, serum, or plasma are first‐line screening tools and play a critical role in PJI screens, since they are convenient, fast, and inexpensive to assay. C‐reactive protein (CRP) and the erythrocyte sedimentation rate (ESR) have been used to identify PJI in the clinic, as recommended by the Musculoskeletal Infection Society (MSIS) workgroup 9 . However, these indices have been associated with a high rate of false negatives, since they can fall within the normal range when the patient has an infection with a weakly virulent organism 10 , such as Cutibacterium acnes 11 . Therefore, it is very important to find and evaluate new indicators to diagnose PJI to improve the diagnostic accuracy and avoid missed diagnosis of PJI.
Recent studies report that D‐dimer and fibrinogen could be useful for PJI screening, and serum D‐dimer has been adopted as a PJI marker by the 2018 criteria of the International Consensus Meeting (ICM) 12 . However, the sample sizes of these studies are limited and there are inconsistencies in the diagnostic accuracy of D‐dimer 13 , 14 ; more importantly, the D‐dimer reported in some studies was tested from serum 13 , 14 , while others were tested from plasma 15 , 16 . In addition, plasma fibrinogen needs further discussion before it can be applied for diagnosing PJI in clinic. Hence, this systematic review and meta‐analysis were conducted to: (i) synthesize the available information on the use and diagnostic accuracy of D‐dimer levels in serum and plasma as well as fibrinogen levels in plasma for PJI screening; and (ii) more importantly, compare the diagnostic accuracy of D‐dimer between serum and plasma for PJI screening.
Methods
Study Design
This systematic review and meta‐analysis was performed to the aim to evaluate the diagnostic values of D‐dimer and fibrinogen, following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) Statement. It has also been registered at the International Prospective Register of Systematic Reviews (CRD42020170438). The serum D‐dimer means the values were tested in post‐coagulation serum, while the values of plasma D‐dimer and fibrinogen were tested in plasma.
Search Strategy
We searched the related literature using electronic databases, including PubMed, Web of Science, the Cochrane Library, Embase, and Google Scholar. Relevant literature was examined from database inception to August 2020 with no language restrictions. The vocabulary and syntax of search strings were adjusted for each database as necessary. The following search strategy were used for searches: (((((infection[MeSH Terms]) OR infection) OR infections)) AND (((fibrinogen[MeSH Terms]) OR fibrinogen) OR D‐dimer)) AND ((((((arthroplasty[MeSH Terms]) OR arthroplasty) OR arthroplasties) OR replacement[MeSH Terms]) OR replacement) OR replacements). The search strategy in Pubmed was shown in Supplementary Table S1. All references cited in these studies and relevant review articles were analyzed manually. Furthermore, unpublished and gray literature found in established orthopaedic journals (e.g., The Journal of Bone and Joint Surgery, Clinical Orthopaedics and Related Research, The Journal of Arthroplasty, and The Bone & Joint Journal) between January 2016 and August 2020 was also evaluated.
Inclusion and Exclusion Criteria
Two reviewers (XH and XJW) independently screened the literature, and all disagreements were resolved after discussion with a third reviewer (YJL). The studies that evaluated the diagnostic values of D‐dimer and fibrinogen for identifying PJI in patients who underwent revision knee or hip arthroplasty in comparison with the diagnostic results of reference standard were included. Inclusion criteria: (i) Participants: patients who had undergone revision knee or hip arthroplasty due to PJI or aseptic mechanical failure; (ii) Interventions: not applicable; (iii) Comparisons: not applicable; (iv) Outcomes: diagnostic values of plasma fibrinogen and D‐dimer tested in serum or plasma for identifying PJI. Exclusion criteria: (i) Studies that tested unrelated biomarkers and studies reporting insufficient information to calculate sensitivity and specificity were excluded; (ii) Case reports, commentaries, expert opinions, and reviews were also excluded. A PRISMA flow diagram of the literature screening process used in this study was constructed (Fig. 1).
Fig. 1.
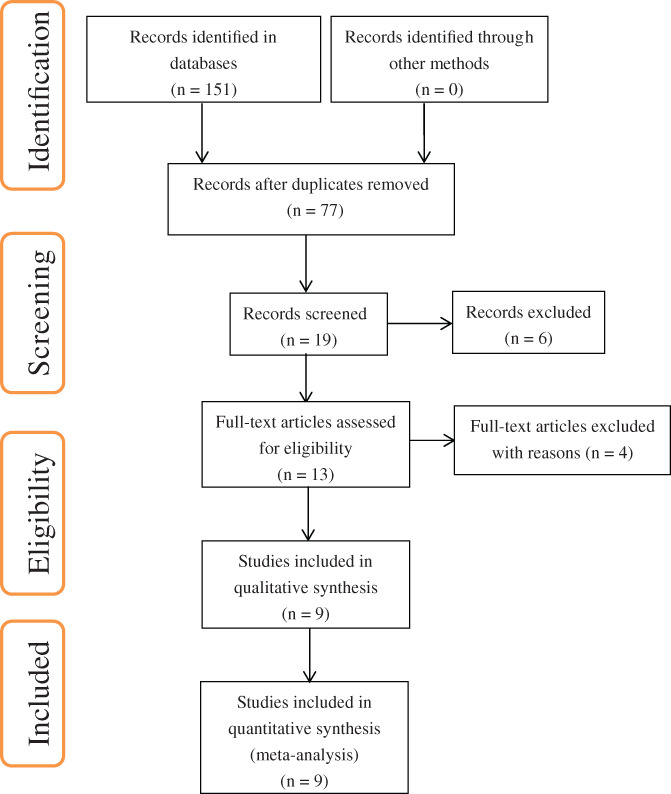
PRISMA flow diagram of the literature screening process.
Data Extraction and Quality Assessment
Two reviewers (XH and XJW) independently extracted relevant data from the included studies using a standardized form. Data included sensitivity, specificity, positive and negative predictive values; numbers, age range, sex ratio, and inclusion criteria of patients; cutoff values for the markers being tested and their origin, whether derived from the Youden index or predetermined by the authors; reference standards; study design; and location and name of study site.
The quality assessment of the included studies was conducted using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) tool 17 . This tool uses 14 questions based on the following four key domains to assess the risk of bias: patient selection, index test, reference standard, as well as flow and timing. On the one hand, the information is recorded based on the signaling questions, which are answered as “yes,” “no,” or “unclear” and phrased such that “yes” indicates low risk of bias, and used to determine the risk of bias for each domain, which is judged as “low,” “high,” or “unclear” according to the answers to all signaling questions. On the other hand, review authors record the information on which the judgment of applicability concerns is determined and then rate their concern that the study does not match the review question. The applicability concerns are rated as “low,” “high,” or “unclear,” focusing on the first three domains.
Statistical Analyses
The bivariate model retains the two‐dimensional property of the original data and considers the negative correlation between sensitivity and specificity, which is based on a random effects model and takes heterogeneity among included studies into consideration. The comprehensive evaluation value of sensitivity and specificity and the negative correlation value between them can be obtained by fitting the model. Therefore, the bivariate model is used to synthesize the entire pooled dataset to ensure the reliable estimation of diagnostic accuracy 18 , 19 .
Pooled values for sensitivity, specificity, positive and negative likelihood ratios (LRs), and diagnostic odds ratios (DORs) were calculated along with 95% confidence intervals (CIs). What's more, the summarized receiver operating characteristic (SROC) curves were constructed, and the area under the SROC curves (AUSROCs) was used to evaluate the diagnostic potential of the markers. Spearman's correlation coefficients between sensitivity and specificity were used to determine threshold effects, and a P value <0.05 was defined to indicate a significant threshold effect. A visual analysis of the SROC curve of sensitivity and specificity was used to assess the threshold effect across studies: a “shoulder‐arm” pattern suggests the existence of a diagnostic threshold bias 20 . To assess the diagnostic accuracy of D‐dimer, data for serum or plasma D‐dimer were analyzed first together, then separately. All statistical analyses were performed using MIDAS commands in Stata 11 (StataCorp., College Station, Texas).
Results
Of a total of 151 records identified from various databases, nine unique studies were found to satisfy the inclusion criteria and were therefore included in the synthesis and meta‐analysis (Fig. 1). Five studies 13 , 14 , 21 , 22 , 23 evaluated the diagnostic potential of serum D‐dimer against a reference standard in 609 patients, including 218 with PJI and 391 without PJI. Two studies 15 , 16 assessed the diagnostic accuracy of plasma D‐dimer in 789 patients, including 177 with PJI and 612 without PJI. One of these studies 16 evaluated the diagnostic accuracy of both plasma D‐dimer and fibrinogen, and we analyzed the data for each marker separately. The diagnostic potential of plasma fibrinogen was also evaluated by three studies 16 , 24 , 25 , in 1088 patients, including 346 with PJI and 742 without PJI. The details of all studies included are summarized in Table 1.
TABLE 1.
Characteristics of studies included in meta‐analysis
| Study | Location | Study design | Reference standard | Inclusion criteria | Origin and values of cutoffs | Patients: All (PJI/Non‐PJI) | Age (years), and sex (Male/Female) |
|---|---|---|---|---|---|---|---|
| Serum D‐dimer | |||||||
| Shahi et al. 13 (2017) | Thomas Jefferson Univ., Philadelphia, USA | P | MSIS (2013) | Patients undergoing primary and revision hip or knee arthroplasty | Youden index; 850 ng/mL | 195 (57/138) | PJI: 59.7 (49–76)*, (24/33); Non‐PJI: NR (44–81)*, (77/61) |
| Xiong et al. 18 (2019) | The First Affiliated Hospital of Nanchang Univ., Nanchang, China | P | MSIS (2011) | Patients with suspected infections after TJA, and those prepared for revision arthroplasty | Youden index; 756 ng/mL | 80 (26/54) |
PJI: (65.42 ± 10.8) # (7/19); Non‐PJI: (59.76 + 12.53) # (25/29) |
| Huang et al. 19 (2019) | People's Hospital of Zhengzhou Univ., Zhengzhou, China | R | MSIS (NR) | Patients with primary OA or secondary to hip congenital, PJI, and aseptic loosening | Reference to previous studies; 850 ng/mL | 101 (31/70) | NR |
| Qin et al. 20 (2020) | The First Affiliated Hospital of Chongqing Medical Univ., Chongqing, China | P | MSIS (2013) | Patients presenting with pain after TJA for surgical revision | Youden index; 1170 ng/mL | 122 (55/67) |
PJI: (65.89 ± 10.72) #, (28/27); Non‐PJI: (64.66 ± 10.39) # , (25/42) |
| Pannu et al. 12 (2020) | Cleveland Clinic Florida, Weston, USA | R | MSIS (2013) | Patients with revision TJA | Youden index; 850 ng/mL | 111 (49/62) |
PJI: (70 ± 10) #, (22/27) Non‐PJI: (68 ± 10) #, (27/35) |
| Plasma D‐dimer | |||||||
| Li et al. 21 (2019) | Chinese PLA General Hospital and Beijing Jishuitan Hospital, Beijing, China | R | MSIS (2013) | Patients managed with revision hip or knee arthroplasty |
Youden index; 1.25 mg/mL |
565 (95/470) |
PJI: 63.7 (18–89) *, (43/52); Non‐PJI: 61.3(23–86) *, (205/265) |
|
Pei et al. 22 2019 |
West China Hospital, Sichuan Univ., Chengdu, China | R | MSIS (2013) | Patients who have undergone revision hip or knee arthroplasty | Youden index; 1.02 mg/L | 224 (82/142) | NR |
| Plasma fibrinogen | |||||||
| Klim et al. 23 (2018) | Medical Univ. of Graz, Graz, Austria. | P | MSIS (2011) | Patients scheduled to have revision hip or knee arthroplasty |
NR; 519 mg/dL (equal to 5.19 g/L |
84 (55/29) |
PJI: (65.7 ± 15.8) # , NR; Non‐PJI: (65.1 ± 14.6) #, NR |
| Li et al. 21 (2019) | Chinese PLA General Hospital and Beijing Jishuitan Hospital, Beijing, China | R | MSIS (2013) | Patients managed with revision hip or knee arthroplasty | Youden index; 4.01 g/L | 565 (95/470) |
PJI: 63.7 (18–89) *, (43/52); Non‐PJI: 61.3 (23–86) *, (205/265) |
|
Xu et al. 24 (2019) |
West China Hospital, Sichuan Univ., Chengdu, China | R | MSIS (2013) | Patients who have undergone revision hip or knee arthroplasty | Youden index; 3.57 g/L | 439 (196/243) | NR |
MSIS, Musculoskeletal Infection Society; NR, not reported; OA, osteoarthritis; P, Prospective; PJI, Periprosthetic joint infection defined by their reference standard; R, Retrospective; TJA, Total joint arthroplasty.
Age is presented as mean (range);
Age is presented as mean ± standard deviation.
Among the included studies, the study of Li et al. 16 classified patients into two groups based on the presence of comorbidities found while evaluating the diagnostic value of plasma D‐dimer and fibrinogen; moreover, comorbidities were further divided into three categories, which included malignancy, autoimmune disease, and cardiovascular and cerebrovascular disease. Similarly, the study of Xu et al. 25 also divided their patients into two groups based on whether they found comorbidities while evaluating the diagnostic accuracy of plasma fibrinogen. All patients with comorbidities in both studies were included and analyzed. Almost all studies included (n = 7) used the Youden index to derive cutoff values. One exception was a study in which Huang et al. 22 determined the cutoff based on previous studies 14 , 26 , and the another study in which Klim et al. 24 used two cutoff values, of which we chose to use the value closest to the largest value based on the Youden index during our analyses.
Quality Assessment
The quality of the included studies was assessed using the QUADAS‐2 tool. Risk of bias and applicability concerns were shown in Table 2. In general, the quality of the included studies was considered to be acceptable. Regarding patient selection, reference standard domain, and flow and timing domain, all included studies had low risk of bias. Regarding the index test domain, the included studies had high risk of bias due to seven studies determining the cutoffs based on the Youden index instead of pre‐specified cutoffs. In addition, there was a low risk for concern applicability for all included studies.
TABLE 2.
Quality assessment of studies included in meta‐analysis
| Study | Risk of bias | Applicability concerns | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | ||||||||||||
| Q1 | Q2 | Q3 | F | Q4 | Q5 | F | Q6 | Q7 | F | Q8 | Q9 | Q10 | Q11 | F | Q12 | Q13 | Q14 | |
| Serum D‐dimer | ||||||||||||||||||
| Shahi et al. 13 (2017) | Y | Y | Y | L | Y | N | H | Y | Y | L | Y | Y | Y | Y | L | L | L | L |
| Xiong et al. 18 (2019) | Y | Y | Y | L | Y | N | H | Y | Y | L | Y | Y | Y | Y | L | L | L | L |
| Huang et al. 19 (2019) | Y | Y | Y | L | U | Y | U | Y | Y | L | Y | Y | Y | Y | L | L | L | L |
| Qin et al. 20 (2020) | Y | Y | Y | L | Y | N | H | Y | Y | L | Y | Y | Y | Y | L | L | L | L |
| Pannu et al. 12 (2020) | Y | Y | Y | L | Y | N | H | Y | Y | L | Y | Y | Y | Y | L | L | L | L |
| Plasma D‐dimer | ||||||||||||||||||
| Li et al. 21 (2019) | Y | Y | Y | L | U | N | H | Y | Y | L | Y | Y | Y | Y | L | L | L | L |
| Pei et al. 22 (2019) | Y | Y | Y | L | Y | N | H | Y | Y | L | Y | Y | Y | Y | L | L | L | L |
| Plasma fibrinogen | ||||||||||||||||||
| Klim et al. 23 (2018) | Y | Y | Y | L | Y | U | U | Y | Y | L | Y | Y | Y | Y | L | L | L | L |
| Li et al. 21 (2019) | Y | Y | Y | L | U | N | H | Y | Y | L | Y | Y | Y | Y | L | L | L | L |
| Xu et al. 24 (2019) | Y | Y | Y | L | Y | N | H | Y | Y | L | Y | Y | Y | Y | L | L | L | L |
Y indicates “yes”, N indicates “No” and U indicates “unclear” for the signaling questions of each domain. F, final judgment of risk of bias or clinical applicability of each domain. L indicates “low risk of bias”, H indicates “high risk of bias” and U indicates “unclear” for the risk of bias of each domain or clinical applicability of the first three domains. Q1, Was a consecutive or random sample of patients enrolled? Q2, Was a case–control design avoided? Q3, Did the study avoid inappropriate exclusions? Q4, Were the index test results interpreted without knowledge of the results of the reference standard? Q5, If a threshold was used, was it pre‐specified? Q6, Is the reference standard likely to correctly classify the target condition? Q7, Were the reference standard results interpreted without knowledge of the results of the index test? Q8, Was there an appropriate interval between index test(s) and reference standard? Q9, Did all patients receive a reference standard? Q10, Did all patients receive the same reference standard? Q11, Were all patients included in the analysis? Q12, Is there concern that the included patients do not match the review question? Q13, Are there concerns that the index test, its conduct, or interpretation differ from the review question? Q14, Are there concerns that the target condition as defined by the reference standard does not match the review question?
Diagnostic Potential of D‐Dimer for PJI Screening
Our first step was to assess the diagnostic accuracy of D‐dimer used for PJI screening by combining the data available on serum and plasma D‐dimer. Pooling results for serum and plasma D‐dimer gave a sensitivity of 0.77 (95% CI 0.63–0.87) and specificity of 0.67 (95% CI 0.54–0.78) (Fig. 2A). The pooled positive and negative LRs were 2.34 (95% CI 1.53–3.57) and 0.34 (95% CI 0.19–0.61), respectively (Table 3). The DOR and AUSROC values were 6.81 (95% CI 2.67–17.37) and 0.78 (95% CI 0.74–0.82), respectively (Table 3, Fig. 3A).
Fig. 2.
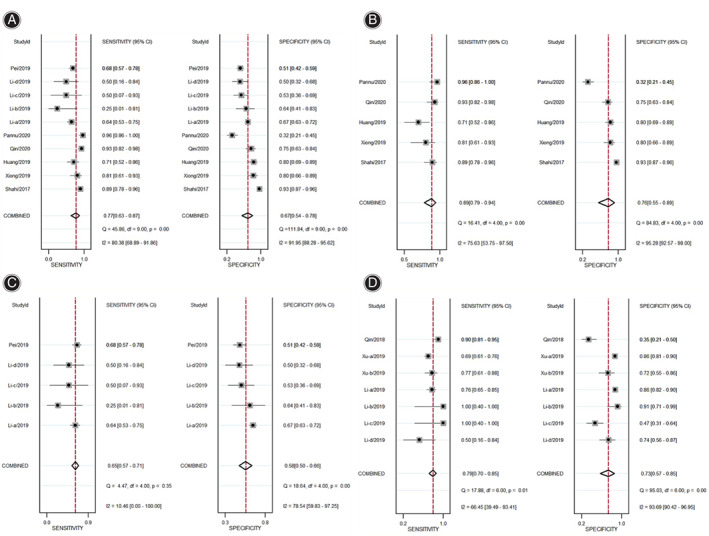
Sensitivity and specificity of markers used for PJI screening: (A) D‐dimer (serum and plasma combined); (B) serum D‐dimer; (C) plasma D‐dimer; (D) plasma fibrinogen.
TABLE 3.
Summary results of key diagnostic characteristics for potential markers
| Potential marker | AUSROC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Positive LR(95% CI) | Negative LR(95% CI) | DOR(95% CI) |
|---|---|---|---|---|---|---|
| D‐dimer (serum and plasma combined) | 0.78 (0.74 to 0.82) | 0.77 (0.63 to 0.87) | 0.67 (0.54 to 0.78) | 2.34 (1.53 to 3.57) | 0.34 (0.19 to 0.61) | 6.81 (2.67 to 17.37) |
| Serum D‐dimer | 0.91 (0.88 to 0.93) | 0.89 (0.79 to 0.94) | 0.76 (0.55 to 0.89) | 3.56 (1.85 to 7.24) | 0.15 (0.08 to 0.27) | 24.24 (10.07 to 58.32) |
| Plasma D‐dimer | 0.65 (0.61 to 0.69) | 0.65 (0.57 to 0.71) | 0.58 (0.50 to 0.66) | 1.54 (1.24 to 1.91) | 0.61 (0.48 to 0.77) | 2.52 (1.64 to 3.90) |
| Plasma fibrinogen | 0.83 (0.79 to 0.86) | 0.79 (0.70 to 0.85) | 0.73 (0.57 to 0.85) | 2.95 (1.85 to 4.72) | 0.29 (0.23 to 0.37) | 10.14 (6.16 to 16.70) |
AUSROC, area under the summary receiver operating characteristic curve; DOR, diagnostic odds ratio; LR, likelihood ratio.
Fig. 3.
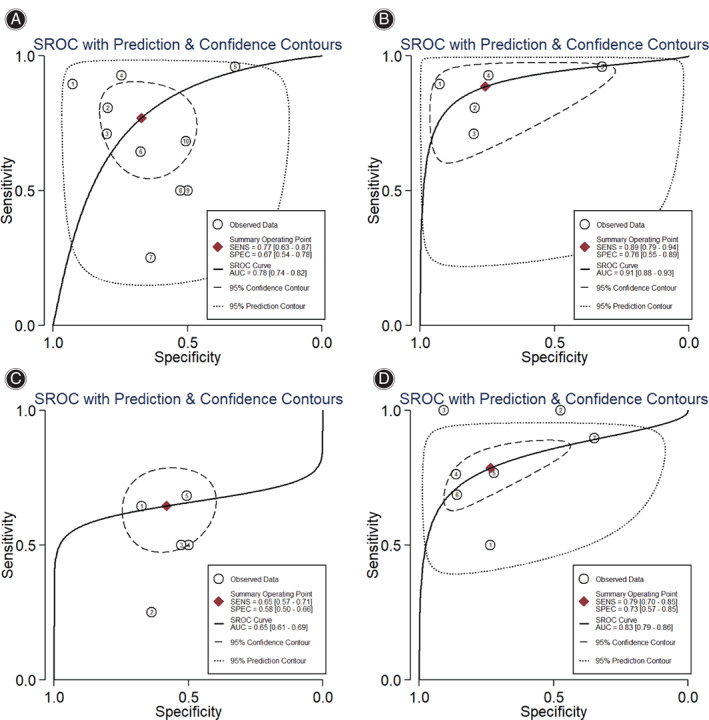
Summary receiver operating characteristic (SROC) curves with prediction and confidence contours for sensitivity (SENS) and specificity (SPEC) of markers: (A) D‐dimer (serum and plasma combined); (B) serum D‐dimer; (C) plasma D‐dimer; (D) plasma fibrinogen.
Next the diagnostic potential of D‐dimer level was assessed separately for serum or plasma. Serum D‐dimer gave a pooled sensitivity of 0.89 (95% CI 0.79–0.94) and specificity of 0.76 (95% CI 0.55–0.89) (Fig. 2B) for serum D‐dimer. Plasma D‐dimer showed lower pooled sensitivity (0.65, 95% CI = 0.57–0.71) and specificity (0.58, 95% CI = 0.50–0.66) than serum D‐dimer (Fig. 2C). Serum D‐dimer showed higher pooled positive LR (3.56, 95% CI 1.85–7.24) and lower negative LR (0.15, 95% CI 0.08–0.27) than plasma D‐dimer, which gave the corresponding values of 1.54 (95% CI 1.24–1.91) and 0.61 (95% CI 0.48–0.77; Table 3). Serum D‐dimer had a higher DOR (24.24, 95% CI 10.07–58.32) and AUSROC (0.91, 95% CI 0.88–0.93) than plasma D‐dimer, which gave the corresponding values of 2.52 (95% CI 1.64–3.9) and 0.65 (95% CI 0.61–0.69) (Table 3, Fig. 3B and C).
Diagnostic Potential of Plasma Fibrinogen for PJI Screening
We found that the diagnostic potential of plasma fibrinogen used for PJI screening was lower than that of serum D‐dimer, but higher than plasma D‐dimer. The pooled values for diagnostic sensitivity and specificity of plasma fibrinogen were 0.79 (95% CI 0.70–0.85) and 0.73 (95% CI 0.57–0.85), respectively, (Fig. 2D). Pooled positive and negative LRs were 2.95 (95% CI 1.85–4.72) and 0.29 (95% CI 0.23–0.37) (Table 3), DOR was 10.14 (95% CI 6.16–16.70), and AUSROC was 0.83 (95% CI 0.79–0.86) (Table 3, Fig. 3D).
Threshold Effect of Tested Markers and Optimal Cutoff Value for Serum D‐Dimer
No significant correlation was observed between sensitivity and specificity for any of these markers: Spearman's correlation coefficient was 0.237 (P = 0.510) for serum and plasma D‐dimer combined, −0.7 (P = 0.188) for serum D‐dimer, −0.103 (P = 0.870) for plasma D‐dimer, and 0.126 (P = 0.788) for plasma fibrinogen, and all P values >0.05. Furthermore, no “shoulder‐arm” pattern between sensitivity and specificity was observed in the SROC curves of these markers, confirming that there was no threshold effect. In addition, a cutoff value of 850 μg/mL of serum D‐dimer was used in three of five studies; the cutoff values for two studies 16 , 20 were calculated based on the Youden index, while the cutoff value in the third study 18 was based on previous studies (Table 4). However, no additional estimates were calculated due to the lack of sufficient studies.
TABLE 4.
Diagnostic potential of serum D‐dimer (cutoff value = 850 ng/mL)
| Study | Patients: All (PJI/Non‐PJI) | AUC | Sensitivity | Specificity | PPV | NPV | Positive LR | Negative LR | Accuracy |
|---|---|---|---|---|---|---|---|---|---|
| Shahi et al. 13 (2017) | 195 (57/138) | NR | 89.47% | 92.75% | 83.61% | 95.52% | 12.34 | 0.11 | 91.79% |
| Huang et al. 19 (2019) | 101 (31/70) | NR | 70.97% | 80.00% | 61.12% | 86.17% | 3.55 | 0.36 | 77.23% |
| Pannu et al. 12 (2020) | 111 (49/62) | 0.742 | 95.92% | 32.26% | 52.81% | 90.91% | 1.42 | 0.13 | 60.36% |
AUC, area under the curve; LR, likelihood ratio; NPV, negative predictive value; NR, not reported; PPV, positive predictive value.
Discussion
This appears to be the first systematic review and meta‐analysis evaluating the diagnostic accuracy of D‐dimer and fibrinogen to identify PJI in revision hip or knee arthroplasty patients. Firstly, the evidences of plasma fibrinogen, serum or plasma D‐dimer for screening PJI were synthesized, and then the diagnostic accuracy of D‐dimer between serum and plasma were assessed separately to compare their diagnostic values. Our results suggest that serum D‐dimer has a higher diagnostic accuracy for identifying PJI before revision hip or knee arthroplasty, and the diagnostic value of plasma fibrinogen is inferior to serum D‐dimer, while plasma D‐dimer has limited diagnostic potential.
The timely, accurate diagnosis of PJI remains a significant challenge for clinical surgeons 27 . Although the MSIS workgroup has provided a definition and criteria for identifying PJI, clear‐cut clinical diagnoses remain problematic when it comes to clinical practice, especially for patients with occult infections 28 . Compared with synovial markers, blood/serum/plasma markers are more important for screening infection because they are the earliest referenced indicators that clinicians are exposed to when PJI is clinically suspected. Therefore, an accurate biomarker in blood, serum, or plasma can efficiently reduce the chances of a missed diagnosis and inaccurate management of infection. Additionally, for patients with autoimmune diseases 29 or infections caused by weakly virulent organisms 10 , the use of CRP and ESR may be ineffective. Fibrinolytic system is strongly associated with inflammatory system 30 , and fibrinolysis and coagulation indicators, such as D‐dimer 31 , fibrinogen degradation products 32 , fibrinogen 33 , and even platelets 34 , play an important role in inflammatory processes. Scholars have begun to pay attention to the values of these markers for identifying PJI. Moreover, the ICM (2018) Criteria had recommended the serum D‐dimer as a marker for identifying PJI 12 .
D‐dimer, the product of fibrin(ogen) degradation, is familiar to clinicians because it is also used to exclude venous thromboembolism 35 and monitor the status of postoperative fibrinolytic response 36 . However, there is increasing evidence supporting the association between D‐dimer and infections 31 , 37 . One of the first studies to evaluate the use of serum D‐dimer in PJI screening showed that its diagnostic accuracy was higher than that of ESR and serum CRP 14 . Then, several researchers also evaluated the diagnostic values of serum/plasma D‐dimer for PJI with their own data. We synthesized these studies and the results showed the serum D‐dimer is an excellent marker for screening infection, while plasma D‐dimer may be limited. However, the discrepancy is still uncertain. Fibrinogen is first converted into fibrin monomers by the action of thrombin, and the fibrin monomers are polymerized into soluble fibrin dimers, trimers, oligomers, and multimers 38 , 39 . These fibrin polymers are bound by non‐covalent bonds and are soluble in urea or chloroacetic acid, also known as soluble fibrin monomer complex (SFMC), which may aggregate into insoluble fibrin as the serum sample coagulates completely in vitro 40 , 41 . Hence, these substances containing D‐dimer structure are consumed in serum samples due to re‐coagulation, leading to soluble D‐dimer levels that are lower than the corresponding levels in plasma samples. Conversely, a fibrinolytic response secondary to coagulation in vitro may lead to a false increase in the concentration of D‐dimer in serum samples 42 . Future studies comparing the diagnostic accuracy of serum and plasma D‐dimer should simultaneously collect serum and plasma samples from the same patients undergoing revision knee or hip arthroplasty.
Plasma fibrinogen is a large, hexameric, homodimeric glycoprotein of 340 kDa 43 , and it is used as an index of coagulation function during routine preoperative screening. The glycoprotein secreted from the liver is converted into fibrin to stop excessive bleeding, stabilize blood clots, and promote hemostasis after tissue and vascular injury 44 . Plasma fibrinogen is also associated with the activation and mediation of inflammatory processes 45 , and its secretion can be upregulated by inflammatory events 43 . Our results indicate that plasma fibrinogen may also be a useful biomarker for PJI screening. Furthermore, both the studies of Li et al. 16 and Xu et al. 25 revealed that fibrinogen may be preferable for detecting PJI in patients with coagulation‐related comorbidities and inflammatory arthritis, while traditional diagnostic indices, CRP and ESR, may be confusing in such conditions 29 .
Larger studies should verify and extend our finding that serum D‐dimer may be an effective biomarker for periprosthetic joint infection screening, especially since the present study had important limitations. First, the limited number of included studies meant that we were unable to investigate sources of heterogeneity among the studies or assess the presence of publication bias. Second, our study suggested that serum D‐dimer and plasma fibrinogen may be useful for screening PJI; however, there is no primary study comparing the diagnostic values between serum D‐dimer and plasma fibrinogen. Therefore, future prospective studies are needed to compare the serum D‐dimer and plasma fibrinogen in the same subject. Additionally, although the 2018 criteria of the ICM suggest a cutoff value of 860 μg/L of serum D‐dimer for diagnosing chronic PJI 12 , we could not determine an optimal threshold for serum D‐dimer or plasma fibrinogen because of the lack of relevant data. Despite these issues, our study substantiates the clinical applicability of D‐dimer and plasma fibrinogen for PJI screening and lays a solid foundation for future research on the optimization of diagnostic criteria for PJI.
Conclusions
The serum D‐dimer may be a promising biomarker for screening PJI, and the diagnostic accuracy of plasma fibrinogen was inferior to it; however, plasma D‐dimer may be limited for identifying PJI. More large‐sample studies on these markers are needed due to the limited number of included studies.
Author Contributions
HX and JWX: screened the literature and extracted relevant data, drafted the work, and revised it critically for important intellectual content. JLY: analyzed and interpreted data for the work. ZYH: participated in final approval of the version of this paper to be published. FXP contributed to the conception and design of the work, and revised the manuscript. All authors read and approved the final manuscript.
Availability of Data and Materials
Please contact author for data requests.
Supporting information
Supplementary Table 1 Search strategy in Pubmed
Acknowledgments
We thank all authors of included studies. We also thank A. Chapin Rodríguez, PhD, from Creaducate Enterprises Ltd. for editing the English text of a draft of this manuscript.
Grant Sources: The research was supported by Post‐Doctor Research Project, West China Hospital, Sichuan University (2018HXBH073); National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z2018B11).
Disclosure: No conflict of interest exits in the submission of this manuscript. The manuscript is approved by all authors for publication.
References
- 1. Pivec R, Johnson AJ, Mears SC, Mont MA. Hip arthroplasty. Lancet, 2012, 380: 1768–1777. [DOI] [PubMed] [Google Scholar]
- 2. Alipour M, Tabari M, Keramati M, Zarmehri AM, Makhmalbaf H. Effectiveness of oral tranexamic acid administration on blood loss after knee artroplasty: a randomized clinical trial. Transfus Apher Sci, 2013, 49: 574–577. [DOI] [PubMed] [Google Scholar]
- 3. Parvizi J, Shohat N, Gehrke T. Prevention of periprosthetic joint infection: new guidelines. Bone Joint J, 2017, 99: 3–10. [DOI] [PubMed] [Google Scholar]
- 4. Cozzi Lepri A, del Prete A, Soderi S, et al. The identification of pathogens associated with periprosthetic joint infection in two‐stage revision. Eur Rev Med Pharmacol Sci, 2019, 23: 101–116. [DOI] [PubMed] [Google Scholar]
- 5. Saleh A, Ramanathan D, Siqueira MBP, Klika AK, Barsoum WK, Rueda CAH. The diagnostic utility of synovial fluid markers in periprosthetic joint infection: a systematic review and meta‐analysis. J Am Acad Orthop Surg, 2017, 25: 763–772. [DOI] [PubMed] [Google Scholar]
- 6. Kurtz SM, Lau E, Watson H, et al. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty, 2012, 27: 61–65. [DOI] [PubMed] [Google Scholar]
- 7. Klug A, Gramlich Y, Rudert M, et al. The projected volume of primary and revision total knee arthroplasty will place an immense burden on future health care systems over the next 30years. Knee Surg Sports Traumatol Arthrosc, 2020. https://10.1007/s00167-020-06154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint Arthroplasty for Periprosthetic infection. J Arthroplasty, 2015, 30: 1492–1497. [DOI] [PubMed] [Google Scholar]
- 9. Marson BA, Deshmukh SR, Grindlay DJC, et al. Alpha‐defensin and the synovasure lateral flow device for the diagnosis of prosthetic joint infection: a systematic review and meta‐analysis. Bone Joint J, 2018, 100: 703–711. [DOI] [PubMed] [Google Scholar]
- 10. Kheir MM, Tan TL, Shohat N, Foltz C, Parvizi J. Routine diagnostic tests for periprosthetic joint infection demonstrate a high false‐negative rate and are influenced by the infecting organism. J Bone Joint Surg Am, 2018, 100: 2057–2065. [DOI] [PubMed] [Google Scholar]
- 11. Kanafani ZA, Sexton DJ, Pien BC, Varkey J, Basmania C, Kaye KS. Postoperative joint infections due to Propionibacterium species: a case‐control study. Clin Infect Dis, 2009, 49: 1083–1085. [DOI] [PubMed] [Google Scholar]
- 12. Shohat N, Bauer T, Buttaro M, et al. Hip and knee section, what is the definition of a periprosthetic joint infection (PJI) of the knee and the hip? Can the same criteria be used for both joints?: proceedings of international consensus on orthopedic infections. J Arthroplasty, 2019, 34: S325–S327. [DOI] [PubMed] [Google Scholar]
- 13. Pannu TS, Villa JM, Patel PD, Riesgo AM, Barsoum WK, Higuera CA. The utility of serum d‐dimer for the diagnosis of periprosthetic joint infection in revision total hip and knee arthroplasty. J Arthroplasty, 2020, 35: 1692–1695. [DOI] [PubMed] [Google Scholar]
- 14. Shahi A, Kheir MM, Tarabichi M, Hosseinzadeh HRS, Tan TL, Parvizi J. Serum D‐dimer test is promising for the diagnosis of periprosthetic joint infection and timing of reimplantation. J Bone Joint Surg Am, 2017, 99: 1419–1427. [DOI] [PubMed] [Google Scholar]
- 15. Xu H, Xie J, Huang Q, Lei Y, Zhang S, Pei F. Plasma fibrin degradation product and D‐dimer are of limited value for diagnosing periprosthetic joint infection. J Arthroplasty, 2019, 34: 2454–2460. [DOI] [PubMed] [Google Scholar]
- 16. Li R, Shao HY, Hao LB, et al. Plasma fibrinogen exhibits better performance than plasma D‐dimer in the diagnosis of periprosthetic joint infection: a multicenter retrospective study. J Bone Joint Surg Am, 2019, 101: 613–619. [DOI] [PubMed] [Google Scholar]
- 17. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Internal Med, 2011, 155: 529–536. [DOI] [PubMed] [Google Scholar]
- 18. Reitsma JB, Glas AS, Rutjes AW, et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol, 2005, 58: 982–990. [DOI] [PubMed] [Google Scholar]
- 19. Harbord RM, Whiting P, Sterne JA, et al. An empirical comparison of methods for meta‐analysis of diagnostic accuracy showed hierarchical models are necessary. J Clin Epidemiol, 2008, 61: 1095–1103. [DOI] [PubMed] [Google Scholar]
- 20. Pei Y, Yang Y, Feng Y, et al. Diagnostic accuracy of artery peak velocity variation measured by bedside real‐time ultrasound for prediction of fluid responsiveness: a meta‐analysis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue, 2020, 32: 99–105. [DOI] [PubMed] [Google Scholar]
- 21. Xiong L, Li S, Dai M. Comparison of D‐dimer with CRP and ESR for diagnosis of periprosthetic joint infection. J Orthop Surg Res, 2019, 14: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang J, Zhang Y, Wang Z, et al. The serum level of D‐dimer is not suitable for distinguishing between prosthetic joint infection and aseptic loosening. J Orthop Surg Res, 2019, 14: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qin L, Li F, Gong X, Wang J, Huang W, Hu N. Combined measurement of D‐dimer and C‐reactive protein levels: highly accurate for diagnosing chronic periprosthetic joint infection. J Arthroplasty, 2020, 35: 229–234. [DOI] [PubMed] [Google Scholar]
- 24. Klim SM, Amerstorfer F, Gruber G, et al. Fibrinogen ‐ a practical and cost efficient biomarker for detecting periprosthetic joint infection. Sci Rep, 2018, 8: 8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu H, Xie J, Yang J, et al. Plasma fibrinogen and platelet count are referable tools for diagnosing periprosthetic joint infection: a single‐center retrospective cohort study. J Arthroplasty, 2019, 8: 8802 [DOI] [PubMed] [Google Scholar]
- 26. Lee YS, Lee YK, Han SB, Nam CH, Parvizi J, Koo KH. Natural progress of D‐dimer following total joint arthroplasty: a baseline for the diagnosis of the early postoperative infection. J Orthop Surg Res, 2018, 13: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bingham JS, Hassebrock JD, Christensen AL, et al. Screening for Periprosthetic joint infections with ESR and CRP: the ideal cutoffs. J Arthroplasty, 2019, 35: 1351–1354. [DOI] [PubMed] [Google Scholar]
- 28. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid alpha‐Defensin and C‐reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am, 2014, 96: 1439–1445. [DOI] [PubMed] [Google Scholar]
- 29. Shohat N, Goswami K, Fillingham Y, et al. Diagnosing Periprosthetic joint infection in inflammatory arthritis: assumption is the enemy of true understanding. J Arthroplasty, 2018, 33: 3561–3566. [DOI] [PubMed] [Google Scholar]
- 30. Sitter T, Toet K, Fricke H, Schiffl H, Held E, Kooistra T. Modulation of procoagulant and fibrinolytic system components of mesothelial cells by inflammatory mediators. Am J Physiol, 1996, 271: R1256–R1263. [DOI] [PubMed] [Google Scholar]
- 31. Rodelo JR, de la Rosa G, Valencia ML, et al. D‐dimer is a significant prognostic factor in patients with suspected infection and sepsis. Am J Emerg Med, 2012, 30: 1991–1999. [DOI] [PubMed] [Google Scholar]
- 32. Fujimoto T, Kaneko T, Sunakawa T, Ikegami H, Musha Y. Elevation of fibrin degradation product (FDP) values prevents the negative conversion of serum CRP values after total knee arthroplasty. J Orthop, 2018, 15: 940–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood, 2019, 133: 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lood C, Tyden H, Gullstrand B, et al. Decreased platelet size is associated with platelet activation and anti‐phospholipid syndrome in systemic lupus erythematosus. Rheumatology, 2017, 56: 408–416. [DOI] [PubMed] [Google Scholar]
- 35. Hu W, Wang Y, Li J, et al. The predictive value of d‐dimer test for venous thromboembolism during puerperium: a prospective cohort study. Clin Appl Thromb Hemost, 2020, 26: 1076029620901786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pong RP, Leveque JA, Edwards A, et al. Effect of tranexamic acid on blood loss, D‐dimer, and fibrinogen kinetics in adult spinal deformity surgery. J Bone Joint Surg Am, 2018, 100: 758–764. [DOI] [PubMed] [Google Scholar]
- 37. Sharma A, Sikka M, Gomber S, et al. Plasma fibrinogen and D‐dimer in children with sepsis: a single‐center experience. Iran J Pathol, 2018, 13: 272–275. [PMC free article] [PubMed] [Google Scholar]
- 38. Mega JL, Morrow DA, de Lemos JA, Mohanavelu S, Cannon CP, Sabatine MS. Thrombus precursor protein and clinical outcomes in patients with acute coronary syndromes. J Am Coll Cardiol, 2008, 51: 2422–2429. [DOI] [PubMed] [Google Scholar]
- 39. LaCapra S, Arkel YS, Ku DH, Gibson D, Lake C, Lam X. The use of thrombus precursor protein, D‐dimer, prothrombin fragment 1.2, and thrombin antithrombin in the exclusion of proximal deep vein thrombosis and pulmonary embolism. Blood Coagul Fibrinolysis, 2000, 11: 371–377. [DOI] [PubMed] [Google Scholar]
- 40. Kochi M, Shimomura M, Hinoi T, et al. Possible role of soluble fibrin monomer complex after gastroenterological surgery. World J Gastroenterol, 2017, 23: 2209–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rivera‐Caravaca JM, Roldan V, Romera M, et al. Soluble fibrin monomer complex and prediction of cardiovascular events in atrial fibrillation: the observational Murcia atrial fibrillation project. J Gen Intern Med, 2018, 33: 847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Korte W, Riesen W. Latex‐enhanced immunoturbidimetry allows D‐dimer determination in plasma and serum samples. Clin Chem, 2000, 46: 871–872. [PubMed] [Google Scholar]
- 43. Pieters M, Wolberg AS. Fibrinogen and fibrin: an illustrated review. Res Pract Thromb Haemost, 2019, 3: 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Undas A, Ariens RA. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler Thromb Vasc Biol, 2011, 31: e88–e99. [DOI] [PubMed] [Google Scholar]
- 45. Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol, 2012, 34: 43–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Search strategy in Pubmed
Data Availability Statement
Please contact author for data requests.


