Abstract
Although the structure and composition of collagen have been studied by polarized light microscopy since the early 19th century, many studies and reviews have paid little or no attention to the morphological problems of histopathological diagnosis. The morphology of collagen fibers is critical in guiding mechanical and biological properties in both normal and pathological tendons. Highlighting the organization and spatial distribution of tendon‐containing collagen fibers can be very useful for visualizing a tendon's ultrastructure, biochemical and indirect mechanical properties, which benefits other researchers and clinicians. Picrosirius red (PSR) staining, relying on the birefringence of collagen fibers, is one of the best understood histochemical methods that can highly and specifically underline fibers better than other common staining techniques when combined with polarized light microscopy (PLM). Polarized light microscopy provides complementary information about collagen fibers, such as orientation, type and spatial distribution, which is important for a comprehensive assessment of collagen alteration in a tendon. Here, this brief review serves as a simplistic and important primer to research developments in which differential staining of collagen types by the Picrosirius‐polarization method is increasing in diverse studies of the medical field, mainly in the assessment of the morphology, spatial distribution, and content of collagen in tendons.
Keywords: Birefringence, Collagen, Morphology, Picrosirius red staining, Polarized light microscopy
Introduction
A tendon is most often thought of as a bright white connective tissue, which primarily contains dense longitudinal fibrils (10–350 nm in diameter) that braid spatially into rope‐like collagen fibers (1–20 μm in diameter), transferring tensile loads from muscle to bone to facilitate joint motions 1 , 2 . The distinct arrangement of collagen fibers, together with their interactions with the noncollagenous matrix rich in hydrophilic proteoglycans, endows a normal tendon with unique mechanical functions, such as resistance to tension and the transmission of forces 3 , 4 . Moreover, the degradation and loss of collagen fibers are generally associated with functional decline 5 , 6 . Accordingly, understanding the architecture and biomechanics of collagen fibers is fundamental to exploring how compositional differences initiate altered biological and mechanical behaviors of a tendon 7 .
The distribution and quantitative estimation of collagen fibers can be obtained by many microscopic techniques and morphological methods applied to sections with different degrees of satisfaction. Among these methods, light microscopy, which is commonly used in histology, does not provide sufficient imaging resolution to reveal the detailed architecture of tendons at the ultrastructural level but shows the general morphology of tenocytes and the tendon texture 8 . Transmission and scanning electron microscopy can provide high‐resolution images of well‐defined individual collagen fibrils but these are relatively expensive, involve complicated and time‐consuming specimen preparation, and provide nanolevel details that are redundant for many studies 9 . Alternative optical techniques are thus needed to better visualize collagen fibers in both physiological and pathological tissues.
Furthermore, among the various histological methods, immunohistochemistry allows a detailed phenotypic analysis of specific collagen fibers and their spatial distribution patterns 10 . However, it is affected by several limitations and pitfalls, such as complex and time‐consuming protocols and expensive reagents, in contrast to histochemistry. Moreover, collagen staining patterns exhibit marked variability in assays for different commercially available antibodies 11 . Compared to them, histochemistry represents a simple and quick method to detect the total collagen fiber content in tendon sections. In this regard, the majority of stains that are currently used are traditional trichrome stains (e.g., the Masson, Mallory, and van Gieson methods). Further studies, however, have found that these methods underestimate the collagen fiber content in certain circumstances 7 , 12 . One explanation for this funding is that these techniques lack selective precision, failing to detect the very thin collagen fibers observed in electron microscopy. Except for these confounding issues, the tendency of a stain to fade prompted Puchtler et al. 12 to seek a better assessment method for collagen alternation, that is, Picrosirius red staining.
Picrosirius red staining (also called PSR or “Sirius red” staining) is a selective histochemical method suitable for both morphological and semiquantitative detection in paraffin‐embedded sections of both normal and pathological tendons. In sections that are analyzed with this procedure under polarized light microscopy (PLM), a complex mixture of different colors, representing various collagen types, is simultaneously presented, which is useful for identifying and subsequently analyzing the differential distribution patterns of the structurally distinct collagen types in a tendon 13 , 14 . Furthermore, PLM can be performed with a light microscope by adding two filters, a polarizer and an analyzer, and is a powerful and robust tool for the assessment of fiber properties in tissues containing collagen fibers 15 . Jungueira et al. 16 first described a method for the detecting and subsequent analysis of collagen fibers stained using a combination of Sirius red staining and polarized light microscopy. Currently, with image analysis technologies, this method exploits elaborate data processing and polarized light imaging and continues to be widely used for the identification and analysis of the fiber anisotropy, orientation 17 , 18 , and crimp pattern 19 in animals 20 and humans. In this mini‐review, we discuss the basic concepts of tendon morphology related to polarization microscopy; readers who are interested in a more in‐depth discussion of this topic can consult many excellent studies and reviews 1 , 5 , 7 , 21 , 22 .
The Picrosirius‐polarization method for collagen: fundamental knowledge.
Anisotropic objects can exhibit a variety of phenomena, including birefringence, which is mainly emphasized in previous studies. In the phenomenon of birefringence, as light enters anisotropic compounds or structures, the light beam is split into two beams with different velocities whose vibration planes are perpendicular to each other 23 . Birefringence is a measure of the difference in the refractive indices of two separate components (the fast ray and slow ray) of the incident ray passing through a sample 24 . Examples of tissues with birefringent constructs include tendons, dermis, muscle, cartilage, and so on.
The presence of anisotropy is attributed to pressure, stretching or directional movement, which usually triggers a remarkably regular pattern of collagen molecules and, consequently, birefringence. In contrast, factors causing a random alignment of molecules, such as healing, usually induce an absence of anisotropy 21 . Interestingly, only the birefringence of longitudinally cut collagen fibers is evaluated, because the longitudinally cut fibers are anisotropic while cross‐sectioned fibers are isotropic 12 . This phenomenon was also discussed in research by Rieppo et al. 25 . Furthermore, polarized light imaging (linear, elliptical, and circular) was utilized to visualize the birefringence of collagen fibers stained by Sirius red revealing changes in the hue, alignment, and distribution patterns in sections. In addition, this technique can measure the relative phase change between two polarized lights, which is termed retardance, to quantify the birefringent property of a tendon. When rotating the sections, destructive interference between the ordinary and extraordinary rays emitted from a biological sample can be observed through the ocular lens of PLM, which is characterized by a dark background, and the angle of the microscope is recorded. Subsequently, the optical retardation of the sample is obtained by the calculation formulas mentioned in previous literature 26 . The relative magnitude of the retardance is positively correlated with the density, alignment, and thickness of biological samples 27 .
Picrosirius red dyes are elongated birefringent molecules, and when bound within the upper groove of well‐oriented collagen molecules that are eosinophilic, the dyes orient parallel to the long axis of each collagen fiber, thereby greatly enhancing the normal birefringence stemming from a uniform fiber distribution 21 , 28 . Undoubtedly, collagen fibers stained with Sirius red can be detected with polarized light, where type I collagen fibers appear bright and red‐yellow, in sharp contrast to type III collagen which appears green 16 , 24 . Therefore, this method is widely employed for quantitative estimations of tissues, because it enables morphology identification and a better quantitative assessment than the van Gieson method 7 . In addition, morphometric image analysis has been widely applied to assess the properties of collagen fibers due to its precision, objectivity, and reproducibility 29 , 30 . Therefore, PSR staining can be employed with less fading than the van Gieson or other methods and subsequently visualized with much better results with the aid of PLM in combination with morphometric image analysis due to the varying birefringent properties of cationic collagen fibers combined with the elongated, anionic Sirius red dye 12 , 31 .
Assessment of the Collagen Fiber Morphology
Photographs and descriptions of the patterns of various tendon tissues have been presented previously. Most of the literature has focused on directly observing and analyzing the differences in the hue, alignment, and distribution patterns of collagen fibers in tendon sections. For instance, according to the description by Jiang et al. 20 , when using polarized light microscopy large amounts of orientationally ordered type I collagen were clearly visualized using the special stain (PSR) in healthy, asymptomatic Achilles tendons, which appear as thick, brilliant (strongly birefringent) red‐yellow fibers resulting from the interaction of the molecules of type I collagen with the Sirius red dye; in contrast, type III collagen is weakly birefringent and characterized by thin greenish fibers. Cury et al. 32 reported similar colored patterns when studying the changes in collagen fiber types of adult and elderly rat calcaneal tendons. Moreover, in 2019, Lee et al. 33 utilized a variety of microscopes, such as polarized light microscope (Fig. 1), electron microscope and second harmonic microscope, to characterize the structure of collagen in rat tendons.
Fig 1.
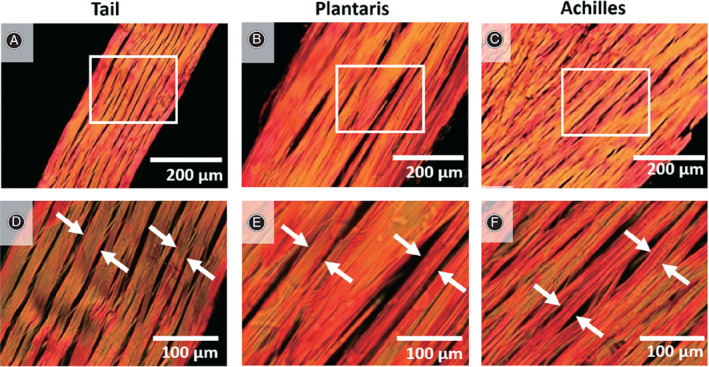
Assessment of collagen fiber structure in longitudinal direction. (A‐C) Rat tail, plantaris, and Achilles tendons were cut in longitudinal direction, stained with picrosirius red, and showed at low magnification. White boxes represent areas observed at (D‐F) high magnifications, and collagen fibers are highlighted (white arrows). Adapted with permission from Lee et al. 33 .
Moreover, various research groups have found that a few weakly birefringent collagen fibers observed with PLM are explained by immature type III collagen fibers that arranged in a disorderly manner, forming a network structure and causing a decrease in their anisotropy 12 . Supporting these notions, Brown et al. 34 found that the Picrosirius‐polarization method allows one to highlight type I collagen, which appears as a well‐defined, red or yellow fibrillary element that stands out from the green‐stained type III collagen fibers scattered around type I collagen in a sparse network. In 2019, Hutson et al. 35 investigated and subsequently compared the microstructure of collagen fibers in rat tails that had been fixed in formalin, flash frozen, or preserved in an ammonium sulfate solution. The authors used Picrosirius red staining to observe different colors and the distribution pattern; more importantly, they quantified and analyzed the area percentage of total tissue regions that exhibit birefringence and found that the three preservation methods had little impact on the collagen fiber measurements. However, under pathological conditions in which collagen degradation, consequent molecular disorder and irregular arrangement, and content change occur, the birefringence will exist in a different pattern than that in normal tissue 36 . The results of these studies have deepened scholars' understanding of the possibilities and limitations of the Picrosirius‐polarization method in analyzing collagen fibers.
Furthermore, many scholars have also proposed and used other microscopic techniques to investigate the impact of different treatments or pathological conditions on collagen fibers. For example, Yildirim et al. 37 utilized second‐harmonic microscopy to distinguish normal and scarred tissues by determining the orientation and density of collagen fibers in the image. Oliveira et al. 38 revealed the morphology of the collagen fibers in the Achilles tendons of diabetic rats using atomic force microscopy. The authors found that the tendons of diabetic rats showed pathological changes, such as thickening of the tendon, disordered arrangement of the fibers, and diminished biomechanical properties, compared with healthy tendons. Additionally, Zabrzyński et al. 39 presented electron microscopy images of collagen fibers, revealing that the collagen fibrils in pathological tendons are more loosely and disorderly arranged than normal tendons, with fewer coarse collagen fibers and more fine collagen molecules.
Notably, in numerous studies, scholars did not use the microscopy alone to observe the collagen fibers in the tendon but combined the microscopy with other technologies to better observe and analyze the collagen. For instance, Wegner et al. 40 extended the versatility of PSR by using fluorescent imaging to detect the Sirius red signal and applying morphometric image analysis. The authors compared this method with polarization microscopy for collagen imaging and noted that more stained collagen molecules were detected by fluorescent microscopy than by polarized light imaging. Although the collagen fibers appeared as a single color under fluorescent light, there was no apparent shift in the collagen fiber color or density as the sample was rotated. Additionally, to better visualize and describe the complicated three‐dimensional collagen fiber architectural features of the eye, in 2018 Yang et al. 18 proposed and validated 3dPLM as an imaging technique based on PLM that allows for the visualization and further quantification of the collagen fiber orientation in three‐dimensional ocular tissues; in turn, this enables an observation of the tendon's architecture and enriched knowledge of the role of collagen fiber biomechanics in both normal and pathological tissues.
Another consideration is the crimping phenomenon (length and angle of the crimp peaks). The crimp period, showing alternating dark and light transverse bands, is determined by calculating the length of a line (parallel to the longitudinal axis of the tendon) connecting the apex of adjacent peaks 4 , 41 . Using polarized light microscopy alone, Mazon et al. 42 noted that the calcaneal tendon of rats has a characteristic crimp pattern with a uniform periodic visible pattern, which is similar to the previous findings by Novak et al. 43 (Fig. 2). Moreover, the presence of a crimp period in paraffin‐embedded tissue sections was also confirmed by using hematoxylin/eosin staining, analyzing the tendon sections by light microscopy 6 , 44 , and using van Gieson staining 45 . Furthermore, Franchi et al. 46 described the structural modifications of collagen fibers in crimps of both relaxed and stretched rat Achilles tendons using polarized light microscopy. The authors found that when a rat Achilles tendon is physiologically relaxed in vivo, the tendon crimps increase in number (39.6 in relaxed tendons vs 74.4 in stretched tendons, P < 0.01) and show more undulation with a decrease in the crimp top angle (148° in relaxed tendons vs 165° in stretched tendons, P < 0.005). According to the current interpretation, the crimp can indicate the actual functional condition and act as a shock absorber during physiological stretching of the tendon 47 .
Fig 2.
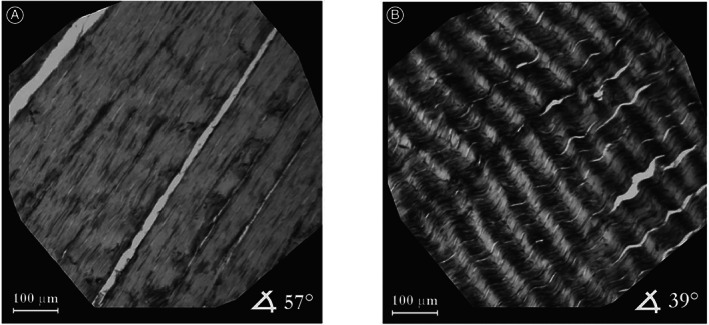
Typical spatial distribution of local orientations of collagen fibers in Achilles tendons, resolution 1 × 1 pixel. Image (A) shows the area where nearly all collagen fiber bundles are aligned at 57°. In contrast, image (B) depicts a specimen of Achilles tendon containing undulated collagen fibers. Adapted with permission from Novak et al. 43 .
Evaluation of the Collagen Fiber Content and Birefringence
In addition to evaluating the hue of fibers and the spatial distribution of the various colors, combined with image‐analysis software, the Picrosirius‐polarization method has a key advantage over traditional techniques in both identifying fibers and subsequently quantifying the collagen content and birefringence, such as the proportions of different colored fibers and retardance 7 . However, there is very little literature reporting on the measurement of the collagen fiber content of the tendon using polarized light microscopy. Terena et al. 48 described the effect of low‐level laser therapy on the collagen fiber content in tendons during compensatory overload of the plantar muscle in rats. After color separation of the images using the Image J program, the authors found that the irradiated hypertrophy group had a greater proportion of type I collagen in the tendons in comparison to the hypertrophy group after 7 and 14 days. Besides, Zerbinati et al. 49 observed the effect of subcutaneous injection of calcium hydroxylapatite (CaHA) on the collagen fibers of the skin. Under polarization, the authors found that the skin of the experimental group exhibited decreased type I collagen content (40.59% in the control group vs 15.54% in CaHA injection group, P < 0.01) and an elevated immature type III collagen level (1.76% in the control group vs 34.42% in CaHA injection group, P < 0.01).
Undoubtedly, the spatial distribution pattern of different collagen fibers stained by Sirius red, from weakly anisotropic to strongly anisotropic staining, can be clearly visualized using a polarizing microscope with image analysis technologies 48 . While the thicker type I collagen fibers appear red/yellow in color, the thinner mature type III collagen fibers have a green color. This differential staining permits a quantitative assessment of types I and III collagen by calculating the ratio of different color areas. In previous research by Alves et al. 50 , the collagen proportional area was also utilized in transmission electron microscopy to observe the changes in the microarchitecture of collagen fibers in sections of a reconstructed anterior cruciate ligament with a hamstring autograft in vivo. Additionally, to better visualize and analyze the different structural appearances of collagen fibers in horse tendons, in 2012, Södersten et al. 51 employed an immunohistochemistry method and found that more intense immunoreactivity for type III collagen was observed in injured tissues and the disorganized regions than in normal tissue. Recently, in 2019, Woessner et al. 52 utilized quantitative polarized light imaging (QPLI) to investigate and analyze the differences in the collagen fiber content and alignment of mouse skin wounds. QPLI‐derived maps of the collagen fiber organization illustrated the alignment of the fibers, average light retardation (thickness of the collagen fiber layer), and changes in the collagen‐positive pixel density (collagen content) over time. The authors found that the collagen fiber content and layer thickness increased with time in the wound bed. Moreover, a significant increase in the fiber directional variance from day 5 to 10 (P = 0.003) indicated that more locally formed fibers were randomly distributed.
In principle, immature collagen fibers are scattered and disordered under the microscope, and highly oriented collagen fibers develop gradually during the maturation process 12 . The above findings aligned with the research of Kim et al. 53 , and the authors noted that type I collagen fibers are closely related to mechanical strength, with a relatively larger average fiber diameter, while type III collagen fibers are involved in tendon injury repair. Additionally, the data of Provenzano et al. 54 indicated that the production of type III collagen fibers slowly decreases during development and then increases by a considerable amount after injury in the healing process.
In addition to the collagen proportional area analyzed by morphology in morphometric image analysis, in recent years, some scholars have also proposed and adopted complicated formulas to quantify and analyze the birefringence of biological samples using retardation, which is not commonly discussed in previous literature 4 , 26 , 55 . Notably, the measurement of birefringence using crossed light in biological samples requires the tendon to be unstained. Several years ago, Vidal et al. 56 demonstrated that the optical retardation of collagen after Picrosirius staining increased by five to six times. This result can explain why most scholars have tended to stain collagen fibers when characterizing collagen fibers with a polarizing microscope. In fact, the relative magnitude of the retardation is correlated with the density, orderly alignment, and thickness of the samples. In 2018, Spiesz et al. 4 compared the birefringence of the two main components of equine tendons and found that fascicles have a higher retardation than the interfascicular matrix. Therefore, the authors demonstrated that fascicles in the tendon contain a higher density of collagen fibers than the interfascicular matrix. Furthermore, in research by Silva et al. 26 using rat models, the experimental group was treated with a low‐power red laser parallel to the long axis of the tendon. As indicated by their calculations and analysis of the birefringence of nonirradiated and irradiated collagen in unstained paraffin sections, the authors found that the irradiated samples presented significantly higher retardation values than the control samples, regardless of whether the samples were immersed in water or glycerin. Therefore, the authors demonstrated that a low‐power red laser parallel to the long axis of the tendon promotes further arrangement of the collagen fibers in a healthy tendon. This conclusion is consistent with the conclusion described by Terena et al. 48 .
Summary
In conclusion, PLM in combination with Sirius red staining is an inexpensive, simple, and reliable assessment method that can be used to reveal the presence of the collagen fibers, describe their distribution patterns, and perform quantitative detection of the fibers. Indeed, this method allows one to clearly visualize well‐defined type I collagen, which stands out from green‐stained type III collagen, and to obtain limited quantitative results by image analysis in paraffin‐embedded sections of the tendon, under both normal and pathological conditions. However, there are still many confounding problems that needed to be elucidated. On one hand, most experimental studies are limited to smaller animals (e.g., rats and rabbits), and there are few corresponding clinical studies. On the other hand, these technologies, which focus only on two‐dimensional measurements, have a low measurement accuracy and provide limited information about the complex three‐dimensional orientation of collagen fibers. Nevertheless, with the emergence of staining, microscopy, and image analysis technologies and scientific concepts in multiple disciplines and fields, the architecture and composition of tendons will be further studied.
Acknowledgements
The authors are grateful for the support from Library of Tianjin Medical University. We would also like to thank the friends who gave us help in the creation and revision of this article.
Grant Sources: This work was supported by National Key R&D Program of China (No.2018YFC1106700).
Disclosure: All authors listed meet the authorship criteria according to the latest guidelines of the International Committee of Medical Journal Editors, and all authors are in agreement with the manuscript with no competing interests.
Contributor Information
Yong‐cheng Hu, Email: yongchenghu@126.com.
Bin Liu, Email: liubin@cmde.org.cn.
References
- 1. Thorpe CT, Screen HR. Tendon structure and composition. Adv Exp Med Biol, 2016, 920: 3–10. [DOI] [PubMed] [Google Scholar]
- 2. Tresoldi I, Oliva F, Benvenuto M, et al. Tendon's ultrastructure. Muscles Ligaments Tendons J, 2013, 3: 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Franchi M, Quaranta M, De Pasquale V, et al. Tendon crimps and peritendinous tissues responding to tensional forces. Eur J Histochem, 2007, 51: S9–S14. [PubMed] [Google Scholar]
- 4. Spiesz EM, Thorpe CT, Thurner PJ, Screen HRC. Structure and collagen crimp patterns of functionally distinct equine tendons, revealed by quantitative polarised light microscopy (qPLM). Acta Biomater, 2018, 70: 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lattouf R, Younes R, Lutomski D, et al. Picrosirius red staining: a useful tool to appraise collagen networks in normal and pathological tissues. J Histochem Cytochem, 2014, 62: 751–758. [DOI] [PubMed] [Google Scholar]
- 6. Chen CH, Liu X, Yeh ML, et al. Pathological changes of human ligament after complete mechanical unloading. Am J Phys Med Rehabil, 2007, 86: 282–289. [DOI] [PubMed] [Google Scholar]
- 7. Rich L, Whittaker P. Collagen and Picrosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. J Morphol Sci, 2005, 22: 97–104. [Google Scholar]
- 8. Pang X, Wu JP, Allison GT, et al. Three dimensional microstructural network of elastin, collagen, and cells in Achilles tendons. J Orthop Res, 2017, 35: 1203–1214. [DOI] [PubMed] [Google Scholar]
- 9. Starborg T, Kalson NS, Lu Y, et al. Using transmission electron microscopy and 3View to determine collagen fibril size and three‐dimensional organization. Nat Protoc, 2013, 8: 1433–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Segnani C, Ippolito C, Antonioli L, et al. Histochemical detection of collagen fibers by Sirius red/fast green is more sensitive than van Gieson or Sirius red alone in Normal and inflamed rat colon. PLoS One, 2015, 10: e0144630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garewal H, Ramsey L, Fass R, et al. Perils of immunohistochemistry: variability in staining specificity of commercially available COX‐2 antibodies on human colon tissue. Dig Dis Sci, 2003, 48: 197–202. [DOI] [PubMed] [Google Scholar]
- 12. Puchtler H, Waldrop FS, Valentine LS. Polarization microscopic studies of connective tissue stained with Picro‐Sirius red FBA. Beitr Pathol, 1973, 150: 174–187. [DOI] [PubMed] [Google Scholar]
- 13. Junqueira LC, Cossermelli W, Brentani R. Differential staining of collagens type I, II and III by Sirius red and polarization microscopy. Arch Histol Jpn, 1978, 41: 267–274. [DOI] [PubMed] [Google Scholar]
- 14. Montes GS. Structural biology of the fibres of the collagenous and elastic systems. Cell Biol Int, 1996, 20: 15–27. [DOI] [PubMed] [Google Scholar]
- 15. Changoor A, Tran‐Khanh N, Méthot S, et al. A polarized light microscopy method for accurate and reliable grading of collagen organization in cartilage repair. Osteoarthr Cartil, 2011, 19: 126–135. [DOI] [PubMed] [Google Scholar]
- 16. Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J, 1979, 11: 447–455. [DOI] [PubMed] [Google Scholar]
- 17. Jan NJ, Grimm JL, Tran H, et al. Polarization microscopy for characterizing fiber orientation of ocular tissues. Biomed Opt Express, 2015, 6: 4705–4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang B, Jan NJ, Brazile B, Voorhees A, Lathrop KL, Sigal IA. Polarized light microscopy for 3‐dimensional mapping of collagen fiber architecture in ocular tissues. J Biophotonics, 2018, 11: e201700356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kalson NS, Malone PS, Bradley RS, Withers PJ, Lees VC. Fibre bundles in the human extensor carpi ulnaris tendon are arranged in a spiral. J Hand Surg Eur Vol, 2012, 37: 550–554. [DOI] [PubMed] [Google Scholar]
- 20. Jiang Y, Zhang Y, Yang J, Luo J, Qin T. [Histomorphological and biomechanical characteristics of decellularized bovine tendons]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi, 2013, 27: 565–570. [PubMed] [Google Scholar]
- 21. Wolman M. Polarized light microscopy as a tool of diagnostic pathology. J Histochem Cytochem, 1975, 23: 21–50. [DOI] [PubMed] [Google Scholar]
- 22. Wolman M, Kasten FH. Polarized light microscopy in the study of the molecular structure of collagen and reticulin. Histochemistry, 1986, 85: 41–49. [DOI] [PubMed] [Google Scholar]
- 23. Caamaño JN, Muñoz M, Diez C, Gómez E. Polarized light microscopy in mammalian oocytes. Reprod Domest Anim, 2010, 45: S49–S56. [DOI] [PubMed] [Google Scholar]
- 24. Rittié L. Method for Picrosirius red‐polarization detection of collagen fibers in tissue sections. Methods Mol Biol, 2017, 1627: 395–407. [DOI] [PubMed] [Google Scholar]
- 25. Rieppo J, Hallikainen J, Jurvelin JS, Kiviranta l HHJ, Hyttinen MM. Practical considerations in the use of polarized light microscopy in the analysis of the collagen network in articular cartilage. Microsc Res Tech, 2008, 71: 279–287. [DOI] [PubMed] [Google Scholar]
- 26. Silva DF, Gomes AS, de Campos Vidal B, Ribeiro MS. Birefringence and second harmonic generation on tendon collagen following red linearly polarized laser irradiation. Ann Biomed Eng, 2013, 41: 752–762. [DOI] [PubMed] [Google Scholar]
- 27. Shen Y, Betzendahl I, Tinneberg HR, Eichenlaub‐Ritter U. Enhanced polarizing microscopy as a new tool in aneuploidy research in oocytes. Mutat Res, 2008, 651: 131–140. [DOI] [PubMed] [Google Scholar]
- 28. Devendra A, Niranjan KC, Swetha A, Kaveri H. Histochemical analysis of collagen reorganization at the invasive front of oral squamous cell carcinoma tumors. J Investig Clin Dent, 2017, 9: e12283. [DOI] [PubMed] [Google Scholar]
- 29. Oberholzer M, Ostreicher M, Christen H, Brühlmann M. Methods in quantitative image analysis. Histochem Cell Biol, 1996, 105: 333–355. [DOI] [PubMed] [Google Scholar]
- 30. Junquiera LC, Bignolas G, Brentani RR. A simple and sensitive method for the quantitative estimation of collagen. Anal Biochem, 1979, 94: 96–99. [DOI] [PubMed] [Google Scholar]
- 31. Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol, 1994, 89: 397–410. [DOI] [PubMed] [Google Scholar]
- 32. Cury DP, Dias FJ, Miglino MA, Watanabe IS. Structural and ultrastructural characteristics of bone‐tendon junction of the calcaneal tendon of adult and elderly Wistar rats. PLoS One, 2016, 11: e0153568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee AH, Elliott DM. Comparative multi‐scale hierarchical structure of the tail, plantaris, and Achilles tendons in the rat. J Anat, 2019, 234: 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brown SR, Cleveland EM, Deeken CR, Huitron SS, Aluka KJ, Davis KG. Type I/type III collagen ratio associated with diverticulitis of the colon in young patients. J Surg Res, 2017, 207: 229–234. [DOI] [PubMed] [Google Scholar]
- 35. Hutson HN, Kujawa C, Eliceiri K, Campagnola P, Masters KS. Impact of tissue preservation on collagen fiber architecture. Biotech Histochem, 2019, 94: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Borges LF, Taboga SR, Gutierrez PS. Simultaneous observation of collagen and elastin in normal and pathological tissues: analysis of Sirius‐red‐stained sections by fluorescence microscopy. Cell Tissue Res, 2005, 320: 551–552. [DOI] [PubMed] [Google Scholar]
- 37. Yildirim M, Quinn KP, Kobler JB, Zeitels SM, Georgakoudi I, Ben‐Yakar A. Quantitative differentiation of normal and scarred tissues using second‐harmonic generation microscopy. Scanning, 2016, 38: 684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oliveira RR, Medina de Mattos R, Magalhães Rebelo L, et al. Experimental diabetes alters the morphology and nano‐structure of the Achilles tendon. PLoS One, 2017, 12: e0169513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zabrzyński J, Gagat M, Paczesny Ł, Łapaj Ł, Grzanka D. Electron microscope study of the advanced tendinopathy process of the long head of the biceps brachii tendon treated arthroscopically. Folia Morphol, 2018, 77: 371–377. [DOI] [PubMed] [Google Scholar]
- 40. Wegner KA, Keikhosravi A, Eliceiri KW, Vezina CM. Fluorescence of Picrosirius red multiplexed with immunohistochemistry for the quantitative assessment of collagen in tissue sections. J Histochem Cytochem, 2017, 65: 479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Franchi M, Quaranta M, Macciocca M, et al. Collagen fibre arrangement and functional crimping pattern of the medial collateral ligament in the rat knee. Knee Surg Sports Traumatol Arthrosc, 2010, 18: 1671–1678. [DOI] [PubMed] [Google Scholar]
- 42. Mazon J, de Aro AA, Simões PW, Pimentel ER. Effect of different resistance‐training protocols on the extracellular matrix of the calcaneal tendon of rats. Ann Anat, 2018, 216: 75–81. [DOI] [PubMed] [Google Scholar]
- 43. Novak K, Polzer S, Tichy M, Bursa J. Automatic evaluation of collagen fiber directions from polarized light microscopy images. Microsc Microanal, 2015, 21: 863–875. [DOI] [PubMed] [Google Scholar]
- 44. Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford), 2003, 43: 131–142. [DOI] [PubMed] [Google Scholar]
- 45. Negrín R, Duboy J, Olavarría F, et al. Biomechanical and histological comparison between the cryopreserved and the lyophilized Gracilis tendon allograft for MPFL reconstruction, a cadaveric experimental study. J Exp Orthop, 2016, 3: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Franchi M, Fini M, Quaranta M, et al. Crimp morphology in relaxed and stretched rat Achilles tendon. J Anat, 2007, 210: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Steplewski A, Fertala J, Tomlinson R, et al. The impact of cholesterol deposits on the fibrillar architecture of the Achilles tendon in a rabbit model of hypercholesterolemia. J Orthop Surg Res, 2019, 14: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Terena SML, Fernandes KPS, Bussadori SK, et al. Infrared laser improves collagen organization in muscle and tendon tissue during the process of compensatory overload. Photomed Laser Surg, 2018, 36: 130–136. [DOI] [PubMed] [Google Scholar]
- 49. Zerbinati N, Calligaro A. Calcium hydroxylapatite treatment of human skin: evidence of collagen turnover through picrosirius red staining and circularly polarized microscopy. Clin Cosmet Investig Dermatol, 2018, 11: 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alves A, Gritsch K, Sirieix C, et al. Computerized histomorphometric study of the splenic collagen polymorphism: a control‐tissue for polarization microscopy. Microsc Res Tech, 2015, 78: 900–907. [DOI] [PubMed] [Google Scholar]
- 51. Södersten F, Hultenby K, Heinegård D, Johnston C, Ekman S. Immunolocalization of collagens (I and III) and cartilage oligomeric matrix protein in the normal and injured equine superficial digital flexor tendon. Connect Tissue Res, 2013, 54: 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Woessner AE, McGee JD, Jones JD, Quinn KP. Characterizing differences in the collagen fiber organization of skin wounds using quantitative polarized light imaging. Wound Repair Regen, 2019, 27: 711–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim SG, Akaike T, Sasagaw T, Atomi Y, Kurosawa H. Gene expression of type I and type III collagen by mechanical stretch in anterior cruciate ligament cells. Cell Struct Funct, 2002, 27: 139–144. [DOI] [PubMed] [Google Scholar]
- 54. Provenzano PP, Vanderby R Jr. Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biol, 2006, 25: 71–84. [DOI] [PubMed] [Google Scholar]
- 55. Yakovlev DD, Shvachkina ME, Sherman MM, Spivak AV, Pravdin AB, Yakovlev DA. Quantitative mapping of collagen fiber alignment in thick tissue samples using transmission polarized‐light microscopy. J Biomed Opt, 2016, 21: 71111. [DOI] [PubMed] [Google Scholar]
- 56. Vidal BC, Mello ML, Pimentel ER. Polarization microscopy and microspectrophotometry of Sirius red, Picrosirius and Chlorantine fast red aggregates and of their complexes with collagen. Histochem J, 1982, 14: 857–878. [DOI] [PubMed] [Google Scholar]


