In this review, the authors focus on ferroptosis in innate and adaptive immunity. They also discuss and highlight the impact of ferroptotic death in infection, inflammation, and immune diseases.
Abstract
Ferroptosis is a type of regulated necrosis that is triggered by a combination of iron toxicity, lipid peroxidation, and plasma membrane damage. The upstream inducers of ferroptosis can be divided into two categories (biological versus chemical) and activate two major pathways (the extrinsic/transporter versus the intrinsic/enzymatic pathways). Excessive or deficient ferroptotic cell death is implicated in a growing list of physiological and pathophysiological processes, coupled to a dysregulated immune response. This review focuses on new discoveries related to how ferroptotic cells and their spilled contents shape innate and adaptive immunity in health and disease. Understanding the immunological characteristics and activity of ferroptotic death not only illuminates an intersection between cell death and immunity but may also lead to the development of novel treatment approaches for immunopathological diseases.
Introduction
In healthy tissues, cell death is an evolutionarily conserved process that maintains a stable cell population by removing superfluous, damaged, or aged cells. The direct consequence of this process is that >150 billion dead cells are routinely produced every day in the human body (Bianconi et al., 2013). Clearing these dead cell corpses without destroying healthy neighboring cells through phagocytosis (within tissues) or shedding (from epithelial surfaces) plays a vital role in sustaining cell homeostasis (Boada-Romero et al., 2020; Doran et al., 2020; Martinez et al., 2011; Qu et al., 2007). A dysregulated cell death machinery as well as abnormal host response are hallmarks of multiple pathologies, including cancer and inflammation-related diseases, such as coronavirus disease 2019 (Barnes et al., 2020; Tang et al., 2020). For this reason, deconvoluting the risk factors and outcomes of cell death may be important for the development of new therapeutic strategies.
A large number of regulated cell death (RCD) modalities have been identified. Such RCD subroutines involve different signal transductions, molecular mechanisms, and immunological outcomes (Tang et al., 2019). Although apoptosis may occur, at least in some cases, in a caspase-independent manner, RCD is generally divided into apoptotic and nonapoptotic subgroups, based on the response to pharmacological or genetic inhibition of caspases (which constitute a cysteine protease family; Galluzzi et al., 2018). Hence, cell death that is not slowed down in its kinetics by caspase inhibition generally is considered to be “nonapoptotic.” Among these nonapoptotic RCD modalities, ferroptosis was originally discovered as an iron-dependent cell death in cancer cells (Dixon et al., 2012), but recently has attracted great attention due to its extensive implications in health and disease (Jiang et al., 2021a; Stockwell et al., 2020; Tang et al., 2021), coupled to significant effects on metabolism and immunity. Genetic, biochemical, and imaging methods have suggested that ferroptosis is mechanistically different from the other three most studied forms of RCD, namely apoptosis, necroptosis, and pyroptosis (Tang and Kroemer, 2020). Accordingly, an emerging area of basic and applied research explores the heterogeneous mechanisms that trigger ferroptosis and its interaction with the immune system.
In this review, we focus on the impact of ferroptosis on innate and adaptive immunity; discuss its implication in infection, inflammation, and immune diseases; and highlight some suggestions to guide this research field.
Signaling and process of ferroptosis
In the past 5 yr, combining genetic technology and pharmacological approaches, our understanding of the molecular machinery of ferroptosis has rapidly improved and been fine-tuned at the epigenetic, transcriptional, and posttranslational levels (Chen et al., 2020a). In general, ferroptosis is a type of oxidative cell death driven by lipid peroxidation. Various oxidation and antioxidant pathways couple autophagy and membrane repair mechanisms to shape the process of lipid peroxidation and plasma membrane damage during ferroptosis (Fig. 1). Complementing previous reviews (Jiang et al., 2021a; Stockwell et al., 2020; Tang et al., 2021), here we summarize the key ferroptotic signaling pathways that can be initiated in a heterogeneous fashion in response to a variety of different triggers but then converge on a common machinery of cell destruction.
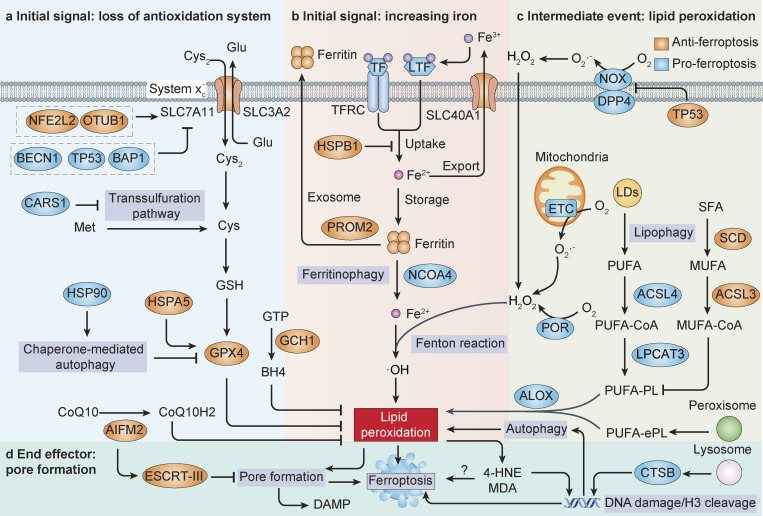
Figure 1.
Core mechanisms of ferroptosis. (a and b) The initiation of ferroptosis requires two key signals, namely the inhibiting antioxidant SLC7A11-GSH-GPX4 system and the accumulation of free iron. This process is further regulated by epigenetic, transcriptional, and posttranslational mechanisms. For example, the expression and activity of SLC7A11 is regulated by protein–protein interaction (e.g., by BECN1), gene expression (e.g., by NFE2L2, TP53, and BAP1), and protein stabilization (e.g., by OTUB1), whereas autophagy promotes ferroptosis partly by degrading GPX4 or ferritin. Alternatively, CoQ10 or tetrahydrobiopterin (BH4) inhibits ferroptosis independently of GSH. (c) The generation of polyunsaturated phospholipid (by ACSL4 and LPCAT3) or PUFA-ePL (by peroxisomal enzymes) and subsequent activation of ALOX have a main role in promoting lipid peroxidation. This process requires hydrogen peroxide (H2O2) production from an iron-mediated Fenton reaction or the activation of POR, NOX, or mitochondria electron transport chain pathways. (d) At the final step of ferroptosis, lipid peroxidation or its secondary products (e.g., 4-HNE and MDA) directly or indirectly induce pore formation in plasma or organelle membrane, which eventually triggers cell death. The lysosomal enzyme CTSB mediates ferroptosis by transporting to the nucleus and inducing DNA damage or histone H3 cleavage. In contrast, endosomal sorting complex required for transport-III (ESCRT-III)–mediated membrane repair prevents ferroptotic cell death and/or DAMP release.
Initial signals
Our initial understanding of the molecular mechanisms controlling ferroptosis came from studies using small-molecule compounds that selectively kill cancer cells with oncogenic RAS mutations (Dolma et al., 2003). Therefore, for a long time, the RAS-RAF–mitogen-activated protein kinase kinase–extracellular-regulated kinase (ERK signaling cascade) was considered a unique ignitor of ferroptotic cell death. However, subsequent studies have shown that ferroptosis can occur in a way that does not depend on RAS, especially in the context of tissue damage caused by drugs or environmental stresses (e.g., ischemia reperfusion injury and circadian clock disturbance). Currently, two key initial signals have been proposed to trigger ferroptosis: (a) excessive iron accumulation and (b) the inhibition of glutathione peroxidase 4 (GPX4).
Iron is an essential element, but iron overload can cause various cell deaths, including ferroptosis (Dixon et al., 2012). In theory, increasing the concentration of free intracellular iron by intervening at any level of iron metabolism (including its absorption, storage, utilization, and outflow) can induce ferroptosis (Chen et al., 2020b). For example, degradation of the iron storage protein ferritin by ferritinophagy promotes ferroptosis (Gao et al., 2016; Hou et al., 2016). The production of iron-mediated ROS mediated by the Fenton reaction or the oxidation of iron-binding enzymes eventually triggers lipid peroxidation during ferroptosis. An unresolved question is why other metal ions fail to induce ROS-dependent ferroptosis. It also remains to be determined how different ROS generators, such as dysfunctional mitochondria (Gao et al., 2019) and dipeptidyl peptidase 4 (DPP4)–mediated nicotinamide adenine dinucleotide phosphate oxidase (NOX) activation (Dixon et al., 2012; Xie et al., 2017; Yang et al., 2019), may synergize with iron toxicity to cause fatal lipid peroxidation.
GPX4 is a glutathione (GSH) and selenium-dependent glutathione peroxidase that can detoxify lipid hydroperoxides formed during oxidative stress. The production of GSH, a cysteine-containing tripeptide and important intracellular antioxidant, mainly depends on amino acid antiporter system xc−-mediated cystine uptake and the attendant reduction of cystine to cysteine (Dixon et al., 2012) or, alternatively, on the generation of cysteine through the transsulfuration pathway regulated by cysteinyl–transfer RNA synthetase 1 (CARS1; Hayano et al., 2016). The pharmacological inhibition of system xc− (e.g., using erastin, sorafenib, and sulfasalazine) or GPX4 (e.g., using RSL3, FIN56, and ML210) is a so-called classical chemical method that induces ferroptosis. The inhibition of GSH synthesis by cystine starvation not only triggers GSH depletion, but also induces the accumulation of glutamate to promote ferroptotic cell death (Kang et al., 2021). Moreover, system xc−, in particular its key subunit solute carrier family 7 member 11 (SLC7A11/xCT), is regulated by multiple mechanisms for controlling its activity, such as protein–protein interactions (e.g., by beclin 1 [BECN1]; Song et al., 2018), gene expression (e.g., by nuclear factor erythroid 2-like 2 [NFE2L2/NRF2]; Chen et al., 2017; tumor protein p53 [TP53]; Jiang et al., 2015; and BRCA1-associated protein 1 [BAP1]; Zhang et al., 2018) and protein stabilization (e.g., by ovarian tumor deubiquitinase, ubiquitin aldehyde binding 1 [OTUB1]; Liu et al., 2019). In addition to inhibiting GPX4 activity, several ferroptosis activators also cause GPX4 degradation in an autophagy- or ubiquitin–proteasome system-dependent manner (Liu et al., 2021; Wu et al., 2019b; Yang et al., 2020; Zhu et al., 2017).
Other internally synthesized antioxidants (e.g., resulting from apoptosis-inducing factor mitochondria-associated 2 [AIFM2/FSP1]–mediated coenzyme Q production; Bersuker et al., 2019; Doll et al., 2019; and GTP cyclohydrolase 1–regulated tetrahydrobiopterin synthesis; Kraft et al., 2020) play an alternative role in reducing ferroptotic cell death. An increased cell density, adhesion, and connection can limit the initiation of ferroptosis in cancer cells partly through Hippo pathway–mediated cadherin 1 expression or the activation of the integrin pathway (Brown et al., 2017; Wu et al., 2019a; Yang et al., 2019). After initiation, ferroptosis can spread from cell to cell in a wave-like manner (Katikaneni et al., 2020; Kim et al., 2016; Linkermann et al., 2014; Riegman et al., 2020), which likely causes widespread tissue damage. Ferroptosis may be further doubly regulated by an amino acid sensor: mechanistic target of rapamycin kinase (Baba et al., 2018; Conlon et al., 2021; Liu et al., 2021; Yi et al., 2020; Zhang et al., 2021) or an energy sensor: adenosine monophosphate–activated protein kinase (AMPK; Lee et al., 2020; Li et al., 2020a; Song et al., 2021; Song et al., 2018; Zhao et al., 2020). These findings underscore the complexity and plasticity of ferroptosis regulation, which is strongly affected by the cellular context.
Intermediate events
During ferroptosis, lipid oxidation occurs as an “intermediate event.” Lipids are hydrocarbon-containing biomolecules that form the basis of the structure and function of cellular membranes. Although the sources, types, and functions of lipids vary, in general terms, polyunsaturated fatty acids (PUFAs) and monounsaturated fatty acids (MUFAs) play completely opposite roles in ferroptosis due to their differential susceptibility to oxidation (Zheng and Conrad, 2020). During ferroptosis, PUFAs, especially arachidonic acids (AAs), are highly susceptible to peroxidation, thus causing the destruction of lipid bilayers and disrupting membrane function. The incorporation of PUFAs into cellular phospholipids (especially phosphatidylethanolamine) requires the contribution of specific enzymes involved in fatty acid synthesis (acyl–coenzyme A [CoA] synthetase long-chain family member 4 [ACSL4]) and lipid remodeling (lysophosphatidylcholine acyltransferase 3 [LPCAT3]). ACSL4 esterifies AA to generate AA-CoA (Doll et al., 2017; Kagan et al., 2017; Yuan et al., 2016), which is subsequently incorporated in phospholipid membranes by LPCAT3 (Dixon et al., 2015; Doll et al., 2017). Conversely, MUFAs generated by stearoyl-CoA desaturase (Tesfay et al., 2019) or activated by ACSL3 (Magtanong et al., 2019) inhibit ferroptosis by displacing PUFAs from phosphatidylethanolamine, thus reducing available substrates for lipid peroxidation. In addition to free PUFAs, i.e., the polyunsaturated ether phospholipids (PUFA-ePLs) synthesized in peroxisome (Zou et al., 2020a), lysosomal degradation of lipid droplets by lipophagy (Bai et al., 2019) or glutamine-derived anaplerotic flux that provides α-ketoglutarate to the tricarboxylic acid cycle in mitochondria (Gao et al., 2015) may also generate sources for lipid peroxidation in ferroptosis. Finally, several lipid-peroxidizing enzymes (e.g., lipoxygenases [ALOXs]; Chu et al., 2019; Kuang et al., 2021; Li et al., 2021; Wenzel et al., 2017; Yang et al., 2016; and cytochrome P450 oxidoreductase [POR]; Yan et al., 2021; Zou et al., 2020b) catalyze the oxidation of PUFA to drive ferroptosis. Additional studies will be needed to precisely determine the dynamic relationship of lipid peroxidation reactions affecting organellar membranes within the cell and the plasma membrane at its surface.
End effectors
Although the literature on ferroptosis frequently alludes to “effector” or “executioner” or “mediator” molecules, the last step of the ferroptosis signal is still enigmatic. A ruptured and intact plasma membrane is generally considered as a sign of necrotic and apoptotic cell death, respectively. Caspase-3 is an effector of apoptosis due to its ability to cleave proteins. Mixed-lineage kinase domain-like pseudokinase and gasdermin D are effectors of necroptosis and pyroptosis, respectively, because of their capacity to form pores as they insert into the plasma membrane. Although a cell-free system suggested that POR-mediated lipid peroxidation has potential pore-forming ability (Yan et al., 2021), it seems that POR only partially mediates ferroptosis. Cathepsin B (CTSB) belongs to the family of lysosomal cysteine proteases and has recently been regarded as one of the mediators of ferroptosis through its ability to cause nuclear damage (Kuang et al., 2020; Nagakannan et al., 2021). However, the rupture of the lysosomal membrane that leads to CTSB activation is observed in various lethal subroutines, suggesting that CTSB cannot be a specific executor of ferroptosis. Another hypothesis postulates that the effector of ferroptosis is not a protein but rather a toxic lipid, such as 4-hydroxynonenal (4-HNE) or malondialdehyde (MDA). However, so far there is no direct evidence that these lipotoxic products attain concentrations high enough to induce ferroptosis. Nevertheless, in the context of oxidative reactions, yet-to-be-identified proteins may cause membrane rupture, which can be repaired by the endosomal sorting complex required for transport-III–dependent membrane scission machinery (Dai et al., 2020c; Pedrera et al., 2020).
Ferroptosis in immune cells
The immune system is usually divided into two arms: innate immunity and adaptive immunity. Ferroptosis impacts immune cells in two fundamentally different ways. On one hand, ferroptosis affects the number and function of the immune cells themselves. On the other hand, ferroptotic cells can be recognized by immune cells and then trigger a range of inflammatory or specific responses. In this section, we focus on the effects of ferroptotic cell death on innate immune cells (macrophages and neutrophils; Fig. 2) as well as on adaptive immune cells (T and B lymphocytes; Fig. 3).
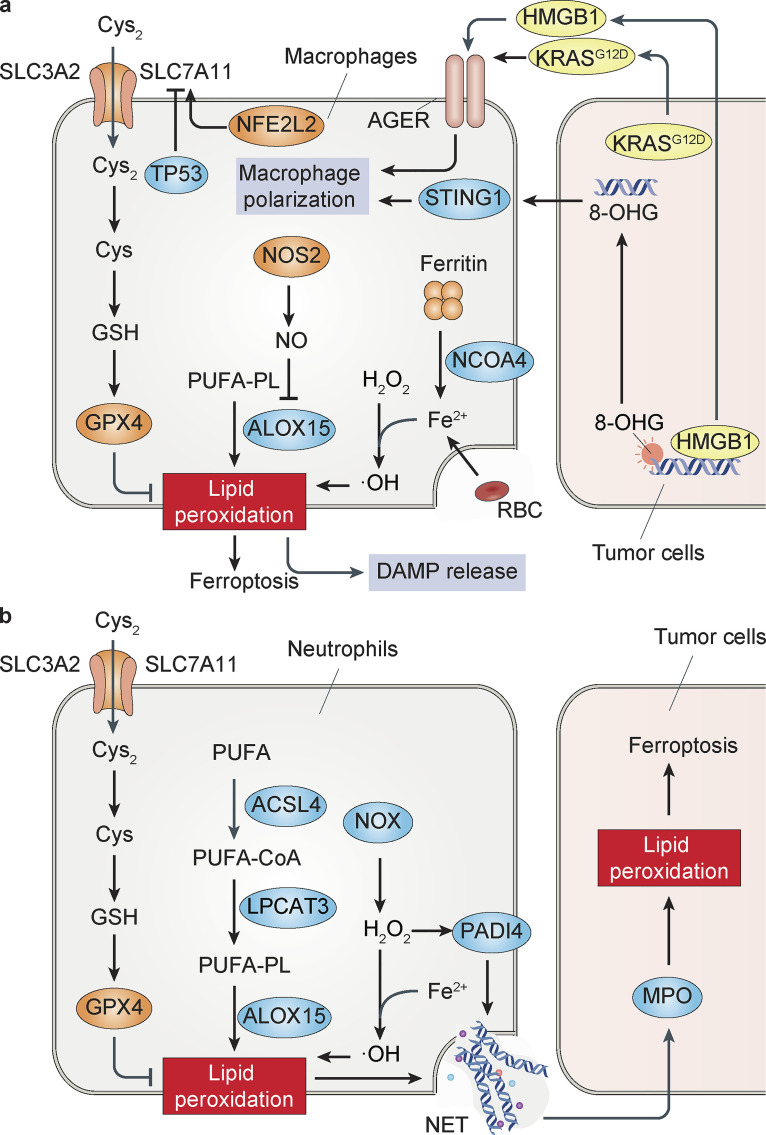
Figure 2.
Models of ferroptosis in innate immune cells. (a) Ferroptosis in macrophages. The activation of the SLC7A110-GSH-GPX4 axis or an increase in iron storage protein ferritin by nuclear receptor coactivator 4–mediated ferritinophagy prevents lipid peroxidation in macrophages. In contrast, erythrophagocytosis or exogenous ferric ions can trigger iron-dependent ferroptosis in macrophages. Compared with M2 microglia/macrophages, M1 cells are resistant to ferroptosis due to the loss of ALOX15 activity by NOS2-mediated NO production. The released DAMPs (e.g., HMGB1, KRASG12D, and 8-OHG) by ferroptotic cancer cells cause inflammation-related immunosuppression through AGER- or STING1-mediated macrophage polarization. (b) Ferroptosis in neutrophils. The activation of NOX and PADI4 is essential for NET formation in neutrophils. The release of myeloperoxidase (MPO) by neutrophils through NET induces lipid peroxidation and subsequent ferroptosis in glioblastoma cells.
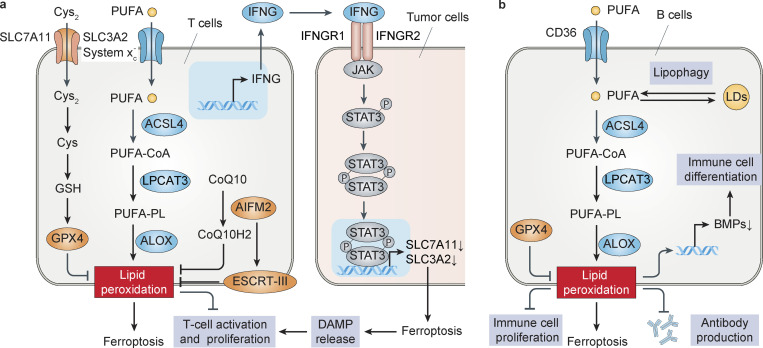
Figure 3.
Models of ferroptosis in adaptive immune cells. (a) Ferroptosis in T cells. SLC7A11, GPX4, or AIFM2 inhibits, whereas ACSL4 and CD36 promotes, ferroptosis in CD8+ T cells. IFNG released by CD8+ T cells induces ferroptotic tumor cell death by activating STAT3-dependent down-regulation of system xc− subunit (SLC3A2 and SLC7A11) expression. In addition, ferroptotic cancer cells activate CD8+ T cell–mediated immunogenic cell death by releasing DAMPs (e.g., HMGB1 and ATP). (b) Ferroptosis in B cells. The depletion of GPX4 induces ferroptotic cell death and impairs IgM antibody responses in B1 and MZB cells. CD36 increases the absorption of fatty acids and the sensitivity to ferroptosis of B1 and MZB cells, and the degradation of lipid droplets (LDs) caused by lipophagy may promote ferroptosis by increasing the concentration of free fatty acids within cells. Lipid peroxidation promotes human peripheral blood mononuclear cell proliferation and differentiation into B cells and natural killer cells by inhibiting BMPs.
Macrophages
For the maintenance of homeostasis, macrophages constantly remove unwanted cells, including cells that undergo cell death. Human monocyte–derived macrophages are less effective in phagocytosing ferroptotic Jurkat T cells as compared with their apoptotic counterparts, likely because the latter express more “eat me” signals, for example, in the form of phosphatidylserine that is exposed on the outer leaflet of the plasma membrane (Klöditz and Fadeel, 2019). This finding reinforces the notion that nonapoptotic cell death, including ferroptosis, is more likely to cause inflammation than apoptosis. In contrast, ferroptotic cells can produce oxygenated phosphatidylethanolamines on the outer plasma membrane that can be recognized by TLR2 on macrophages, leading to the clearance of ferroptotic cells (Luo et al., 2021).
Macrophages also participate in iron homeostasis by scavenging aged erythrocytes and recovering their iron. This may trigger the ferroptosis of macrophages, thereby limiting their immune activity. For example, the transfusion of refrigerator storage–damaged red blood cells leads to increased erythrophagocytosis, which is reduced by ferroptosis inhibitor ferrostatin-1 in splenic red pulp macrophages (Youssef et al., 2018). Similarly, exogenous ferric citrate–induced cell death can be inhibited by ferrostatin-1 in bone marrow–derived macrophages (BMDMs; Wang et al., 2017), documenting a direct role for iron in triggering ferroptosis in macrophages. As a stress protection mechanism, iron induces the up-regulation of Slc7a11 mRNA by activating the ROS-NFE2L2 axis, which reduces ferroptotic cell death in BMDMs (Wang et al., 2017). In contrast, Gpx4−/− BMDMs are more sensitive to cell death induced by bacterial infection or cytosolic LPS by pyroptosis, but not by ferroptosis (Kang et al., 2018). These data indicate that SLC7A11 and GPX4 profoundly affect the regulation of several cell death modalities in macrophages.
Macrophages can polarize their functions in a continuum between two extremes (M1 versus M2) as they receive and integrate environmental signals (Murray, 2017). M1 macrophages express CD16, CD32, CD80, CD86, CD64, and nitric oxide synthase 2 (NOS2/iNOS) as phenotypic markers, while CD163 and CD206 are the major markers of M2 macrophages. LPS, IFN-γ, and TNF polarize macrophages toward the M1 phenotype, thereby inducing the secretion of cytokines, such as IL-1, IL-12, IL-18, and TNF (Murray, 2017). In contrast, M2 macrophage activation can be induced by CSF1, IL-4, IL-13, IL-10, and TGFB1 (Murray, 2017). Functionally, M1 macrophages are proinflammatory, while the M2 state is considered to be anti-inflammatory. An imbalance of macrophage M1-M2 polarization contributes to various diseases or inflammatory conditions (Murray, 2017). Mounting evidence suggests that macrophage polarization and ferroptosis can influence each other at the cell-autonomous level or by communication with other cells in a context-dependent manner. On one hand, compared with M2 microglia (brain macrophages), M1 cells are resistant to ferroptosis due to the loss of arachidonate 15-lipoxygenase (ALOX15) activity secondary to NOS2-mediated nitric oxide (NO) production, which finally promotes inflammatory responses (Kapralov et al., 2020). On the other hand, ferroptotic pancreatic cancer cells release damage-associated molecular patterns (DAMPs; e.g., mutant KRASG12D and 8-hydroxyguanine [8-OHG]) and cause inflammation-related immunosuppression through macrophage polarization in xenograft or transgenic mouse models (Dai et al., 2020a; Dai et al., 2020b). Hypoxia-induced down-regulation of nuclear receptor coactivator 4 (a cargo receptor of ferritinophagy that promotes ferroptosis; Gao et al., 2016; Hou et al., 2016) and TP53 polymorphisms (e.g., P47S) further reduce the sensitivity of macrophages to ferroptosis (Singh et al., 2020). Since different populations of macrophages in distinct tissues exhibit highly heterogeneous transcription and epigenetic programs, it will be important to carefully evaluate transcriptional profiles with respect to their impact on ferroptosis sensitivity of macrophages.
Neutrophils
Neutrophil granulocytes are the first immune cells recruited to sites of inflammation, where they fight pathogens through phagocytosis, degranulation, and the release of neutrophil extracellular traps (NETs) coupled to the death of the cells. The activation of NOX (Bianchi et al., 2009) and peptidyl arginine deiminase 4 (PADI4, best known as PAD4; Li et al., 2010) is essential for NET formation. As a downstream mechanism of PADI4, the production of ROS by sulfasalazine promotes the nonenzymatic formation of ether-linked oxidized phospholipids (oxPLs), leading to NET formation in neutrophils in vitro and in vivo (Yotsumoto et al., 2017). In contrast, the effect of sulfasalazine on NET formation by lipid peroxidation does not seem to be related to sulfasalazine-induced SLC7A11 inhibition, ALOX activation, and subsequent ferroptosis (Yotsumoto et al., 2017). Although the mechanism and trigger for the membrane rupture step remain unclear, these findings support the notion that lipid peroxidation is an upstream signal shared by various types of RCDs, in this case NET-induced death and ferroptosis.
The accumulation of neutrophils triggers and regulates the initial inflammatory response after trauma, which is not only beneficial to the early repair process, but potentially also leads to subsequent tissue damage. Neutrophils induce lipid peroxidation within tumor cells, for instance by transferring myeloperoxidase into glioblastoma cells (Yee et al., 2020). Ferroptotic stimulation promotes the recruitment of neutrophils to the myocardium after heart transplantation, leading to heart damage in mouse models (Li et al., 2019a). Since this ferroptosis-related sterile inflammation of the heart is inhibited by ferrostatin-1 (Li et al., 2019a), further investigation is urgently awaited to clarify whether the depletion of ALOX15 (a direct target of ferrostatin-1; Anthonymuthu et al., 2021) also protects against myocardial ischemia–reperfusion-induced cardiomyocyte loss. Irrespective of this uncertainty, altogether the current evidence suggests that neutrophils participate in sustaining inflammation caused by ferroptotic tissue damage.
T cells
Several in vitro and in vivo observations suggest that the activity and function of cytotoxic T cells (CD8+) and helper T cells (CD4+) are regulated by lipid peroxidation and ferroptosis. First, human naive CD4+ T cells almost lack SLC7A11, which, however, is strongly up-regulated during T cell activation (Garg et al., 2011; Levring et al., 2012). Second, T cell activation and proliferation require a reducing extracellular microenvironment to maintain intracellular GSH levels, as this can be achieved in vitro by adding β-mercaptoethanol to the culture medium (Pruett et al., 1989). Third, the genetic depletion of Gpx4 or treatment with GPX4 inhibitors (e.g., RSL3, ML162, and ML210) induces lipid peroxidation and concomitantly ferroptotic cell death in T cells in vitro (Drijvers et al., 2021; Matsushita et al., 2015). In contrast, the overexpression of Gpx4 and Aifm2 or knockout of Acsl4 protects CD8+ T cells from ferroptosis (Drijvers et al., 2021). Fourth, the T cell–specific deletion of Gpx4 does not affect thymopoiesis in mice, but compromises CD8+ T cell homeostasis in the periphery (Matsushita et al., 2015). Fifth, Gpx4-deficient CD4+ or CD8+ T cells fail to expand in the context of acute infections, and this defect is rescued by supplementation of vitamin E, a potent lipid-soluble antioxidant (Matsushita et al., 2015). Although in the memory phase of viral infection the depletion of the Gpx4 gene does not affect T cell recall responses upon secondary infection (Matsushita et al., 2015), future studies must delineate the role of ferroptosis in distinct T cell populations, including naive, effector, memory, and regulatory T cells.
The activation of CD8+ T cells also has the ability to enhance the sensitivity of surrounding non–T cells to ferroptosis, especially in cancer therapy (Wang et al., 2019a). Mechanistically, IFNγ secreted by CD8+ T cells down-regulates the expression of system xc− subunits (solute carrier family 3 member 2 [SLC3A2] and SLC7A11) and hence impairs the uptake of cystine by tumor cells, thus facilitating ferroptosis induction secondary to GSH depletion (Wang et al., 2019a). As mentioned earlier, ferroptotic cancer cells can be perceived as immunogenic and hence activate CD8+ T cell–mediated antitumor immune responses (Efimova et al., 2020). These findings establish a potential positive feedback mechanism in which CD8+ T cells trigger immunogenic cell death through the induction of ferroptosis. Potentially, such a feedforward mechanism might amplify anticancer immune responses and facilitate antigen spreading.
B cells
After maturation, B lymphocytes either recirculate through secondary lymphoid organs as part of a long-lived pool (as follicular or B2 B cells) or join more static compartments enriched in the marginal zone of the spleen (marginal zone B [MZB] cells), peritoneal, and pleural cavities (B1 B cells). GPX4 is required for the development, maintenance, and antibody responses of B1 and MZB cells, but not for that of follicular B cells (Muri et al., 2019). The conditional deletion of Gpx4 in B1 and MZB cells induces ferroptotic cell death and impairs IgM antibody responses to Streptococcus pneumoniae (Muri et al., 2019) in mice. Compared with follicular B cells, both B1 and MZB cells express higher levels of the fatty acid transporter protein CD36, causing increased fatty acid uptake and ferroptosis sensitivity (Muri et al., 2019). These findings extend our understanding of the redox regulation mechanisms of B cell homeostasis.
Two other examples illustrate the importance of ferroptosis in the B cell compartment. First, compared with follicular B2 cells, the cell survival and self-renewal of B1a B cells is more dependent on the uptake of exogenous fatty acids and their mobilization by autophagy (Clarke et al., 2018). Fatty acids are mainly stored in the form of lipid droplets, which inhibits the oxidation of PUFAs (Bailey et al., 2015). The degradation of lipid droplets by autophagy (“lipophagy”) promotes ferroptotic cell death by increasing intracellular free fatty acids (Singh et al., 2009). Therefore, lipophagy may regulate the function of B cells through ferroptosis, a conjecture that requires further experimental exploration. Second, lipid peroxidation caused by the ferroptosis activator erastin promotes the proliferation and differentiation of human peripheral blood mononuclear cells into B cells and natural killer cells through the down-regulation of bone morphogenetic protein (BMP) family members (Wang et al., 2018). BMPs belong to the TGFB superfamily, which is involved in regulating cell proliferation, differentiation, and activity of B, natural killer, and myeloid cells (Wang et al., 2014). These findings uncover alternative mechanisms through which lipid peroxidation may govern B cell differentiation and activity.
DAMPs and pattern recognition receptors in ferroptosis
DAMPs are endogenous molecules that are released from, or exposed on, injured or stressed cells (Tang et al., 2012; Zindel and Kubes, 2020). Like pathogen-associated molecular patterns derived from infectious microbes, DAMPs bind to pattern recognition receptors (PRRs), which signal for the activation of downstream transcription factors (especially NF-κB and interferon regulatory factor [IRF]) and the production of immune factors, including a variety of cytokines and chemokines. The DAMP-PRR axis plays a core role in bridging cell death and immune response in the context of tissue damage and tumor immunity (Tang et al., 2012; Zindel and Kubes, 2020). Several PRRs, such as membrane bound TLR4, advanced glycosylation end-product–specific receptor (AGER, also known as RAGE), and cytoplasmic DNA sensor cyclic guanosine monophosphate–adenosine monophosphate synthase (CGAS), may integrate signals from DAMPs emitted by ferroptotic cells (Fig. 4).
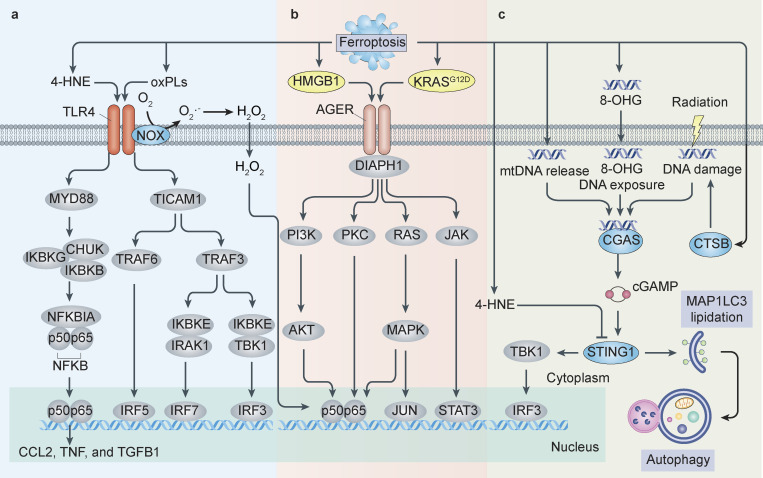
Figure 4.
Models of DAMPs and PRRs in ferroptosis. (a) TLR4 pathway in ferroptosis. TLR4 plays a central role in innate immunity by signaling to adaptor MYD88 or TICAM1/TRIF to induce proinflammatory cytokines. The activation of TLR4 induces type I IFN signaling in vascular endothelial cells, triggering neutrophil recruitment and subsequent ferroptosis-mediated cardiac damage. TLR4-dependent NF-κB activation promotes ferroptosis-related inflammation through the production of proinflammatory cytokines (cytokines C-C motif chemokine ligand 2 [CCL2], TNF, and TGFB1) during rhabdomyolysis-associated kidney damage. Moreover, lipid oxidation products (e.g., 4-HNE and oxPLs) can trigger inflammation partly through activating TLR4 signaling. The interaction of TLR4 and NOX4 may enhance ferroptotic cell death–mediated inflammation. (b) AGER signaling in ferroptosis. AGER recognizes various DAMPs, leading to the activation of signaling pathways, including the protein kinase C [PKC], JAK-STAT, phophatidylinositol 3-kinase [PI3K]–protein kinase B, and MAPK–NF-κB pathways. In addition, AGER is responsible for HMGB1- or KRASG12D-mediated macrophage activation and polarization in response to ferroptotic cancer cells. (c) STING1 pathway in ferroptosis. In this pathway, 4-HNE inhibits STING1 activity by the carbonylation of STING1, whereas the release of 8-OHG from ferroptotic cancer cells activates the STING1-dependent inflammatory pathway in macrophages by the DNA sensor CGAS. STING1 directly promotes ferroptosis by increasing MAP1LC3 lipidation, thereby activating autophagy-dependent cell death caused by zalcitabine-induced mitochondrial DNA (mtDNA) damage and CTSB-mediated genomic DNA damage.
TLR4 pathway
TLR4, which is one of the PRRs activated by HMGB1, plays a central role in triggering innate immune responses by the activation of two main signal transduction pathways. One pathway is activated by the adaptor MYD88 to induce NF-κB–dependent proinflammatory cytokines, and another pathway is activated by the adaptor TCR adaptor molecule 1 (TICAM1/TRIF) to trigger type I IFN production. After heart transplantation, the activation of TLR4-TICAM1 type I IFN signaling in vascular endothelial cells triggers neutrophil recruitment and subsequent ferroptosis-mediated cardiac damage (Li et al., 2019a). Conversely, TLR4-dependent NF-κB activation and subsequent production of proinflammatory cytokines (cytokines C-C motif chemokine ligand 2, TNF, and TGFB1) favors ferroptosis-related inflammation during rhabdomyolysis-associated kidney damage (Guerrero-Hue et al., 2019). Moreover, lipid oxidation products (e.g., 4-HNE and oxPLs) trigger inflammation in vitro or in vivo partly through activating TLR4 signaling (Imai et al., 2008; Wang et al., 2019b). These findings establish a potential role for TLR4 in activating different adaptor proteins to mediate ferroptosis-related inflammatory responses through lipid DAMPs. The production and release of prostaglandin E2 during lipid peroxidation may play a protective role in limiting sterile inflammation in certain settings (Loynes et al., 2018).
In addition to its immunomodulatory roles, TLR4 may also directly promote cell death (including apoptosis and ferroptosis) by binding to NOX4 or its isoenzymes (Park et al., 2004). For example, during heart failure, TLR4 and NOX4 are up-regulated while ferroptosis occurs, and the inhibition of either TLR4 or NOX4 reduces cardiomyocyte death (Chen et al., 2019). Of note, the role of NOX proteins in heart failure may depend on the isoform, activation level, and cellular distribution (Zhang et al., 2013), and it will be important to understand how these factors influence the propensity of heart muscle cells to undergo ferroptosis.
AGER pathway
AGER is a member of the immunoglobulin superfamily, which recognizes various DAMPs (e.g., HMGB1 and S100) to activate a range of signaling pathways, including but not limited to the activation of the protein kinase C pathway, tyrosine phosphorylation of the JAK signal transducer and activator of transcription (STAT) pathway, and activation of the MAPK–NF-κB signaling pathway (Sparvero et al., 2009). AGER signaling has been involved in acute or chronic inflammatory diseases, such as sepsis, pancreatitis, and diabetes (Deane et al., 2012; Hudson and Lippman, 2018; Kang et al., 2016; Sirois et al., 2013). Consistently, there are multiple mechanisms that contain AGER-mediated inflammation.
Two studies using AGER neutralizing antibodies or short hairpin RNAs document the critical role of AGER in mediating ferroptotic cell–initiated inflammation and immunity. The earlier study reported that AGER, not TLR4, is responsible for HMGB1-mediated TNF production in macrophages responding to RSL3-induced ferroptotic cells (Wen et al., 2019). The later study showed that AGER expression in macrophages facilitates the uptake of mutant KRASG12D protein contained in exosomes, which are released by ferroptotic pancreatic cancer cells in an autophagy-dependent manner (Dai et al., 2020a). This may result in AGER-dependent M2 macrophage polarization and subsequently stimulate tumor growth. In other scenarios, AGER-mediated extracellular HMGB1/LPS uptake causes activation of the caspase-11–dependent inflammasome, leading to pyroptosis of macrophages (Deng et al., 2018). It would be interesting to further identify the structural basis for the recognition and uptake of different DAMPs by AGER in the context of distinct RCD modalities.
STING1 pathway
CGAS is a cytoplasmic sensor of pathogen or host-derived DNA that catalyzes the production of a second messenger, cyclic guanosine monophosphate–adenosine monophosphate (cGAMP; Li and Chen, 2018). The cGAMP accumulating in the cytosol then activates stimulator of interferon response cGAMP interactor 1 (STING1/TMEM173), an ER-associated membrane protein. STING1 stimulates a type I IFN response through activating TANK binding kinase 1 (TBK1), leading to the activation of the transcription factor IRF3 (Li and Chen, 2018). In addition, STING1 induces the activation of autophagy (Gui et al., 2019) or immunocoagulation (Zhang et al., 2020) through a mechanism independent of TBK1-IRF3 signaling. Accordingly, STING1 is a multifunctional regulator of various types of RCD, including apoptosis, necroptosis, pyroptosis, and ferroptosis, highlighting its broad role in regulating cell death–mediated immune responses.
Ferroptotic DAMPs have peculiar effects on STING1 activation. The increase in lipid peroxidation caused by GPX4 depletion limits the STING1-mediated type I IFN antiviral immune response during herpes simplex virus 1 infection in mice (Jia et al., 2020). Mechanistically, 4-HNE inhibits STING1 activation by its direct carbonylation in mouse primary peritoneal macrophages (Jia et al., 2020). In contrast, the release of oxidized nucleobases (e.g., 8-OHG) from ferroptotic cells activates the STING1-dependent inflammatory pathway in surrounding macrophages (Dai et al., 2020b), illustrating yet another example how distinct DAMPs shape the immune response.
STING1 directly promotes ferroptosis by overactivating autophagy in the context of DNA damage. Although autophagy has a preponderantly cytoprotective role, autophagy plays a prodeath role in the context of ferroptosis. Autophagy promotes ferroptosis through eliminating antiferroptotic proteins such as ferritin, GPX4, and aryl hydrocarbon receptor nuclear translocator-like (ARNTL, best known as BMAL1) or by digesting lipid droplets (Liu et al., 2020a). STING1 stimulates the formation of autophagosomes (Gui et al., 2019) and thus promotes ferroptotic cell death under zalcitabine-induced mitochondrial DNA damage or nuclear CTSB-related genomic DNA damage (Hämälistö et al., 2020; Li et al., 2021). It will be important to evaluate the likely complex relationship between STING1 activation, autophagy status, and ferroptotic effects during radiotherapy (Lang et al., 2019; Liu et al., 2020a; Yamazaki et al., 2020).
Ferroptosis in infectious, inflammatory, and immune diseases
Accumulating preclinical data suggest the involvement of ferroptosis-relevant regulators and pathways in human disorders (Jiang et al., 2021a; Stockwell et al., 2020; Tang et al., 2021). Therefore, targeting ferroptosis by pharmacological modulators (activators or inhibitors) may represent a possible avenue for treating multiple pathologies. Ferroptotic damage has been implicated in several common immune diseases, such as nonalcoholic steatohepatitis, diabetes, neuroinflammation, asthma, and rheumatoid arthritis, but the exact contribution of ferroptosis to these pathologies is not well understood (Jiang et al., 2021a; Stockwell et al., 2020; Tang et al., 2021). Here, we will discuss the role of ferroptosis in infectious, inflammatory, and immune diseases through several well-studied examples.
Bacterial infection
The activation of ferroptosis is implicated in bacterial infection–induced injury of host tissues. For example, the Gram-negative bacterium Pseudomonas aeruginosa is a major pathogen in cystic fibrosis, a chronic inflammatory disease in which airway tissues contain elevated levels of oxidized AA-phospholipids (Bragonzi et al., 2009; Dar et al., 2018). P. aeruginosa expresses pLoxA (its mammalian orthologue is ALOX15), leading to lipid peroxidation and subsequent ferroptosis in human bronchial epithelial cells (Dar et al., 2018). This process can be blocked by baicalein (an ALOX inhibitor) and ferrostatin-1 (a ferroptosis inhibitor; Dar et al., 2018), suggesting that such inhibitors might be developed as a new therapy for cystic fibrosis infection, perhaps in the form of inhalable aerosols.
Mycobacterium tuberculosis is another pathogen that may kill host cells via ferroptosis (Amaral et al., 2019), contradicting previous reports suggesting that necroptosis would play a major role in tuberculosis (Pajuelo et al., 2018). However, most conclusions regarding the pathological role of ferroptosis in M. tuberculosis–induced lung damage are based on pharmacological studies using ferrostatin-1 or N-acetyl cysteine (Amaral et al., 2016; Amaral et al., 2019; Venketaraman et al., 2008), which may have off-target effects on other cell death subroutines. Thus, genetic animal studies must clarify the potential contribution of ferroptotic host damage to the pathogenesis of tuberculosis.
Of note, in a mouse model of polymicrobial sepsis induced by cecal ligation and puncture, the conditional depletion of Gpx4 in myeloid cells accelerates the systemic inflammatory response and multiorgan failure that reportedly rely on pyroptosis rather than ferroptosis (Kang et al., 2018). Nonetheless, ferroptosis-inhibitory drugs (dexrazoxane, ferrostatin-1, and irisin) can protect against CLP- or LPS-induced tissue damage (Li et al., 2020b; Wei et al., 2020). Further elucidation of the role of GPX4 in different types of RCD, including pyroptosis, is important for understanding its contribution to infectious diseases.
Inflammatory bowel disease (IBD)
IBD is a common term for two distinct diseases (Crohn’s disease and ulcerative colitis), which are characterized by chronic inflammation of the gastrointestinal tract. Epidemiological, human, and animal studies suggest that ferroptosis is involved in Crohn’s disease, as shown by four lines of evidence. First, the increase in the incidence of IBD in the population is related to a Western diet rich in PUFAs (Mayr et al., 2020). Second, small intestinal epithelial cells from Crohn’s disease patients show impaired GPX4 activity and increased lipid peroxidation (Mayr et al., 2020). Third, PUFAs (e.g., AA), but not MUFAs (e.g., palmitoleic acid and oleic acid), trigger ACSL4-mediated lipid peroxidation and the GPX4-repressible production of cytokines (e.g., IL-6 and C-X-C motif chemokine ligand 1) by intestinal epithelial cells (Mayr et al., 2020). Fourth, in mice that were fed a Western-style diet rich in PUFAs, the heterozygous deletion of Gpx4 in intestinal epithelial cells accelerated the local infiltration of neutrophils and monocytes as signs of an inflammatory response (Mayr et al., 2020). These preclinical studies identify that dietary PUFAs cause Crohn’s disease through promoting ferroptosis.
The analysis of gene expression profiles and lipid peroxidation products (e.g., MDA) related to ferroptosis in patients with colitis ulcerosas suggest that ferroptotic damage may also occur in secondary IBD (Xu et al., 2020). Indeed, in mice, several ferroptosis inhibitors (ferrostatin-1, GSK2606414, and curculigoside) alleviate experimental colitis induced with dextran sulfate sodium by inhibiting ER stress or via the up-regulation of GPX4 expression (Wang et al., 2020; Xu et al., 2020). It is worth noting that sulfasalazine, a US Food and Drug Administration–approved IBD drug, is an inhibitor of system xc− and hence a potential ferroptosis inducer. Whether this contributes to the mode of action of sulfasalazine (or explains the fact that this drug fails in most patients) remains a matter of speculation.
Acute pancreatitis
Pancreatitis, a severe condition and a risk factor for pancreatic cancer, is caused by acinar cell death–mediated DAMP release and consequent sterile inflammation (Kang et al., 2014). Cerulein (an analogue of cholecystokinin) and the amino acid L-arginine are the two most commonly used agents for triggering experimental pancreatitis in mice. The conditional depletion of Arntl, a central component of the circadian clock, in the pancreas increases L-arginine–induced acute pancreatitis in mice partly through ferroptotic damage–induced HMGB1 release (Liu et al., 2020b). Thus, the administration of liproxstatin-1 or an anti-HMGB1 neutralizing antibody decreases Arntl deficiency–induced acute pancreatitis in mice. Mechanistically, pancreatic aryl hydrocarbon receptor nuclear translocator-like acts as a key transcriptional factor to up-regulate the expression of ferroptosis-inhibitory genes (e.g., Slc7a11 and Gpx4) to suppress oxidative tissue injury (Liu et al., 2020b). Moreover, high-iron diets or the conditional depletion of Gpx4 in the pancreas exacerbates cerulein-induced pancreatitis in mice by activating DNA damage-induced systemic inflammation (Dai et al., 2020b). These findings uncover a pathological link between ferroptotic damage and pancreatitis. Since L-arginine can be converted into NO, the role of NO in ferroptosis-related pancreatitis should be clarified in the future.
Ischemia reperfusion injury (IRI)
IRI arises from the restriction of blood supply to an organ, followed by the reestablishment of blood supply and reoxygenation. IRI is an inflammatory condition that drives innate and adaptive immune responses involved in multiple pathways. The depletion of Tlr2, Tlr4, and Tlr9 in mice confers significant protection from IRI in various experiment models. Ferroptosis-mediated sterile inflammation is also involved in the progression of IRI in various tissues, such as heart (Gao et al., 2015), liver (Friedmann Angeli et al., 2014), kidney (Linkermann et al., 2014), brain (Tuo et al., 2017), and gut (Li et al., 2019b). Consistent with this, ferrostatin-1 or an ACSL4 inhibitor protects against IRI in mouse models. However, other RCD subroutines, such as necroptosis, also contribute to the development of IRI in kidney (Linkermann et al., 2013) and heart (Zhang et al., 2016). This pathway is thought to provide an additional mechanism for triggering sterile inflammation. Thus, it is difficult to determine the specific involvement of ferroptotic damage in IRI, calling for additional investigation, preferentially in genetic models of ferroptosis and necroptosis inhibition.
Tumorigenesis and therapy
Inflammation is a double-edged sword in tumor immunity (Greten and Grivennikov, 2019). On one hand, chronic inflammation provides a supporting environment for tumor transformation, proliferation, and metastasis. On the other hand, acute inflammation caused by anticancer treatments can trigger protective antitumor immunity. This pattern is also seen in the effect of ferroptotic damage on tumors. As illustrated in a previous section of this article, “Ferroptosis in immune cells,” ferroptosis induced by chemotherapy, radiotherapy, and immunotherapy may trigger the release of DAMPs, leading to dendritic cell recruitment, dendritic cell–mediated antigen capture and presentation, and finally, the induction of cytotoxic T cell responses. In contrast, IL-4 induction 1, a secreted amino acid oxidase produced by antigen-presenting cells, can protect cancer cells from ferroptosis (Zeitler et al., 2021). In addition, a combination of cyst(e)inase (a cystine- and cysteine-degrading enzyme that should induce ferroptosis) and immune checkpoint inhibitors can elicit a synergistic tumor suppressive effect in mouse models (Wang et al., 2019a), but the rationale for this effect is still elusive. One possibility is that immune checkpoint inhibitors may enhance the expression of components of system xc−, NFE2L2 target genes, or TYRO3 protein tyrosine kinase, thereby providing negative feedback to limit the anticancer activity of immune checkpoint inhibitors (Jiang et al., 2021b). Indeed, the expression of SLC3A2 negatively correlates with the clinical benefit of cancer immunotherapy with the checkpoint inhibitor nivolumab (Wang et al., 2019a). Although cancer cells in an early stage of ferroptosis may induce a tumor vaccine–like effect and hence elicit adaptive antitumor immunity (Efimova et al., 2020), it is still unclear whether ferroptotic tumor cells induce a long-lasting immunological memory.
In the past decade, a variety of genetically engineered mouse models have been developed to improve the understanding of immune responses during tumorigenesis. For example, two basic genetically engineered mouse models, namely Pdx1-Cre;KrasG12D/+ mice (termed KC) and Pdx1-Cre;KrasG12D/+;Tp53R172H/+ mice (termed KPC), are used to study the initiation and advancement of pancreatic cancer. In KC mice with additional pancreatic Gpx4 depletion or a high-iron diet, the administration of the ferroptosis inhibitor lipoxstatin-1 protects against pancreatic tumorigenesis (Dai et al., 2020b). Several mechanisms putatively explain this observation. First, the depletion of Gpx4 in pancreatic adenocarcinomas promotes oxidative DNA damage and the release of 8-OHG, which leads to macrophage M2 polarization by activating the STING1-dependent DNA sensor pathway (Dai et al., 2020b). Second, KRASG12D released by ferroptotic cells causes AGER-dependent macrophage M2 polarization by the activation of STAT3-mediated fatty acid oxidation (Dai et al., 2020a). Consistently, Gpx4 deletion in myeloid cells (macrophages and neutrophils) is sufficient to initiate tumorigenesis in mice (Canli et al., 2017). Gpx4-deficient macrophages secrete elevated levels of hydrogen peroxide, which causes DNA mutations and intestinal tumorigenesis (Canli et al., 2017). Tumor necrosis induced by neutrophils is mediated by the induction of ferroptosis, which promotes glioblastoma growth in vivo (Yee et al., 2020). Furthermore, a high expression of ferroptosis-related genes is associated with poor survival of glioblastoma patients (Yee et al., 2020). These observations suggest that ferroptosis has a protumorigenic potential and are contrary to the observation that the deletion of pancreatic Slc7a11 in KPC mice (in contrast to KC mice with a Tp53 mutation) suppresses rather than exacerbates pancreatic tumor growth (Badgley et al., 2020). Hence, the contribution of ferroptosis to tumorigenesis might be highly context dependent.
The lymphatic system is a part of the circulatory system and the immune system. The increased level of MUFAs (e.g., oleic acid) in melanoma tumor cells caused by the lymph fluid (which contains more oleic acid than plasma) promotes ferroptosis resistance in an acyl-CoA synthetase long-chain family member 3–dependent fashion, thus favoring tumor metastasis (Ubellacker et al., 2020). However, CD36-mediated fatty acid uptake promotes ferroptosis in tumor-infiltrating CD8+ T cells, which limits antitumor immunity (Ma et al., 2021). These data further increase the complexity of the local and systemic immune mechanisms that regulate the sensitivity to ferroptosis. The ambiguous role of ferroptosis in tumor immunity also means that manipulation of the ferroptotic pathway for tumor treatment is still challenging and requires careful evaluation (Chen et al., 2021b).
Conclusions and perspectives
The balance between cell survival and cell death is critical to many aspects of homeostasis in multicellular organisms. In recent years, significant progress has been made in delineating the mechanisms that modulate ferroptosis susceptibility in tissues challenged by a variety of metabolic, infectious, or oncogenic stressors. Recent work has begun to clarify the complex roles of such ferroptosis-relevant pathways in different aspects of immune function. On one hand, the ferroptotic demise of immune cells may compromise immune responses. On the other hand, ferroptosis in nonimmune cells causes the release of DAMPs, which alert immune cells. Regarding the immunomodulatory effects of ferroptosis, there are still several unresolved questions, such as how ferroptosis affects the prognosis of human diseases, and how different immune cell subpopulations sense ferroptosis and then amplify or attenuate the subsequent response to assure a whole spectrum of graduated responses to ferroptotic perturbations. Further research on the context-dependent effects of important ferroptotic modulators (e.g., GPX4, SLC7A11, and ACSL4) in various immune cells may enable us to disentangle the likely complex immunomodulatory effects of ferroptosis that may have important repercussions in infection and cancer. In several models, including solid organs, cell cultures, and zebrafish, ferroptosis spreads between neighboring cells in a non–cell-autonomous manner (Katikaneni et al., 2020; Kim et al., 2016; Linkermann et al., 2014; Riegman et al., 2020). However, the role of cell–cell propagation of ferroptosis in regulating the activity of tissue-resident immune cells remains to be determined (Riegman et al., 2019). Viral infection often triggers host cell death with iron dyshomeostasis (Drakesmith and Prentice, 2008), and it remains to be investigated whether ferroptosis is involved in this process. Another big unknown is whether there are specific pathogen–associated molecular patterns or DAMPs that might trigger ferroptosis in tissue-resident immune cells, thereby affecting recognition, specificity, sensitivity, and memory.
The major challenge is how to apply the theoretical framework of ferroptosis to the diagnosis and therapy of human diseases. Although the accumulation of lipid peroxidation is a sign of ferroptosis, this change may also be part of other RCD modalities, such as apoptosis, necroptosis, pyroptosis, and NET. To distinguish the relative pathogenic contribution of ferroptosis and other cell death modalities, we must develop a panoply of exquisitely specific pharmacological inhibitors, organoid models, and other technologies applicable to primary patient–derived samples. To bridge ferroptosis with clinically relevant metrics of disease, scientists may need to focus on exploring specific biomarkers of ferroptosis that may be collected through novel technologies (e.g., single-cell transcriptomics, proteomics, and metabolomics; Chen et al., 2021a). As the activity of ferroptosis inhibitors (such as ferrostatin-1) in vivo is still controversial, more genetic evidence is needed to prove the link between ferroptosis and disease.
Acknowledgments
We thank Dave Primm (Department of Surgery, University of Texas Southwestern Medical Center) for his critical reading of the manuscript. We thank our numerous colleagues in the field of ferroptosis. We also apologize to the researchers who were not referenced due to space limitations.
G. Kroemer is supported by the Agence Nationale de la Recherche–Projets blancs; Agence Nationale de la Recherche under the frame of the ERA-Net for Research on Rare Diseases (E-Rare-2); Association pour la Recherche sur le Cancer; Cancéropôle Ile de France; Chancellerie des Universités de Paris (Legs Poix); a donation by Elior; European Research Area Network on Cardiovascular Diseases (MINOTAUR); Fondation Carrefour; Fondation pour la Recherche Médicale; Gustave Roussy Odyssea; the European Union Horizon 2020 Framework Programme Project Oncobiome; High-end Foreign Expert Program in China (GDW20171100085 and GDW20181100051); Institut National de la Santé et de la Recherche Médicale (Heterogeneity of Tumors & Ecosystem); Institut National du Cancer; Institut Universitaire de France; LabEx Immuno-Oncology; LeDucq Foundation; Ligue Contre le Cancer (équipe labellisée); Recherche Hospitalo-Universitaire Torino Lumière; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination; and SIRIC Cancer Research and Personalized Medicine.
Author contributions: X. Chen, R. Kang, G. Kroemer, and D. Tang wrote the manuscript. G. Kroemer and D. Tang edited, reviewed, and approved the manuscript before submission.
References
- Amaral, E.P., Conceição E.L., Costa D.L., Rocha M.S., Marinho J.M., Cordeiro-Santos M., D’Império-Lima M.R., Barbosa T., Sher A., and Andrade B.B.. 2016. N-acetyl-cysteine exhibits potent anti-mycobacterial activity in addition to its known anti-oxidative functions. BMC Microbiol. 16:251. 10.1186/s12866-016-0872-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral, E.P., Costa D.L., Namasivayam S., Riteau N., Kamenyeva O., Mittereder L., Mayer-Barber K.D., Andrade B.B., and Sher A.. 2019. A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis. J. Exp. Med. 216:556–570. 10.1084/jem.20181776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthonymuthu, T.S., Tyurina Y.Y., Sun W.Y., Mikulska-Ruminska K., Shrivastava I.H., Tyurin V.A., Cinemre F.B., Dar H.H., VanDemark A.P., Holman T.R., et al. 2021. Resolving the paradox of ferroptotic cell death: Ferrostatin-1 binds to 15LOX/PEBP1 complex, suppresses generation of peroxidized ETE-PE, and protects against ferroptosis. Redox Biol. 38:101744. 10.1016/j.redox.2020.101744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba, Y., Higa J.K., Shimada B.K., Horiuchi K.M., Suhara T., Kobayashi M., Woo J.D., Aoyagi H., Marh K.S., Kitaoka H., and Matsui T.. 2018. Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 314:H659–H668. 10.1152/ajpheart.00452.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgley, M.A., Kremer D.M., Maurer H.C., DelGiorno K.E., Lee H.J., Purohit V., Sagalovskiy I.R., Ma A., Kapilian J., Firl C.E.M., et al. 2020. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 368:85–89. 10.1126/science.aaw9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, Y., Meng L., Han L., Jia Y., Zhao Y., Gao H., Kang R., Wang X., Tang D., and Dai E.. 2019. Lipid storage and lipophagy regulates ferroptosis. Biochem. Biophys. Res. Commun. 508:997–1003. 10.1016/j.bbrc.2018.12.039 [DOI] [PubMed] [Google Scholar]
- Bailey, A.P., Koster G., Guillermier C., Hirst E.M., MacRae J.I., Lechene C.P., Postle A.D., and Gould A.P.. 2015. Antioxidant Role for Lipid Droplets in a Stem Cell Niche of Drosophila. Cell. 163:340–353. 10.1016/j.cell.2015.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S., et al. 2020. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 217:e20200652. 10.1084/jem.20200652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersuker, K., Hendricks J.M., Li Z., Magtanong L., Ford B., Tang P.H., Roberts M.A., Tong B., Maimone T.J., Zoncu R., et al. 2019. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 575:688–692. 10.1038/s41586-019-1705-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi, M., Hakkim A., Brinkmann V., Siler U., Seger R.A., Zychlinsky A., and Reichenbach J.. 2009. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood. 114:2619–2622. 10.1182/blood-2009-05-221606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianconi, E., Piovesan A., Facchin F., Beraudi A., Casadei R., Frabetti F., Vitale L., Pelleri M.C., Tassani S., Piva F., et al. 2013. An estimation of the number of cells in the human body. Ann. Hum. Biol. 40:463–471. 10.3109/03014460.2013.807878 [DOI] [PubMed] [Google Scholar]
- Boada-Romero, E., Martinez J., Heckmann B.L., and Green D.R.. 2020. The clearance of dead cells by efferocytosis. Nat. Rev. Mol. Cell Biol. 21:398–414. 10.1038/s41580-020-0232-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragonzi, A., Paroni M., Nonis A., Cramer N., Montanari S., Rejman J., Di Serio C., Döring G., and Tümmler B.. 2009. Pseudomonas aeruginosa microevolution during cystic fibrosis lung infection establishes clones with adapted virulence. Am. J. Respir. Crit. Care Med. 180:138–145. 10.1164/rccm.200812-1943OC [DOI] [PubMed] [Google Scholar]
- Brown, C.W., Amante J.J., Goel H.L., and Mercurio A.M.. 2017. The α6β4 integrin promotes resistance to ferroptosis. J. Cell Biol. 216:4287–4297. 10.1083/jcb.201701136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli, Ö., Nicolas A.M., Gupta J., Finkelmeier F., Goncharova O., Pesic M., Neumann T., Horst D., Löwer M., Sahin U., and Greten F.R.. 2017. Myeloid Cell-Derived Reactive Oxygen Species Induce Epithelial Mutagenesis. Cancer Cell. 32:869–883.e5. 10.1016/j.ccell.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Chen, D., Tavana O., Chu B., Erber L., Chen Y., Baer R., and Gu W.. 2017. NRF2 Is a Major Target of ARF in p53-Independent Tumor Suppression. Mol. Cell. 68:224–232.e4. 10.1016/j.molcel.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X., Comish P.B., Tang D., and Kang R.. 2021a. Characteristics and Biomarkers of Ferroptosis. Front. Cell Dev. Biol. 9:637162. 10.3389/fcell.2021.637162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X., Kang R., Kroemer G., and Tang D.. 2021b. Broadening horizons: the role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 18:280–296. 10.1038/s41571-020-00462-0 [DOI] [PubMed] [Google Scholar]
- Chen, X., Li J., Kang R., Klionsky D.J., and Tang D.. 2020a. Ferroptosis: machinery and regulation. Autophagy.:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X., Xu S., Zhao C., and Liu B.. 2019. Role of TLR4/NADPH oxidase 4 pathway in promoting cell death through autophagy and ferroptosis during heart failure. Biochem. Biophys. Res. Commun. 516:37–43. 10.1016/j.bbrc.2019.06.015 [DOI] [PubMed] [Google Scholar]
- Chen, X., Yu C., Kang R., and Tang D.. 2020b. Iron Metabolism in Ferroptosis. Front. Cell Dev. Biol. 8:590226. 10.3389/fcell.2020.590226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, B., Kon N., Chen D., Li T., Liu T., Jiang L., Song S., Tavana O., and Gu W.. 2019. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat. Cell Biol. 21:579–591. 10.1038/s41556-019-0305-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, A.J., Riffelmacher T., Braas D., Cornall R.J., and Simon A.K.. 2018. B1a B cells require autophagy for metabolic homeostasis and self-renewal. J. Exp. Med. 215:399–413. 10.1084/jem.20170771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon, M., Poltorack C.D., Forcina G.C., Armenta D.A., Mallais M., Perez M.A., Wells A., Kahanu A., Magtanong L., Watts J.L., et al. 2021. A compendium of kinetic modulatory profiles identifies ferroptosis regulators. Nat. Chem. Biol. 10.1038/s41589-021-00751-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, E., Han L., Liu J., Xie Y., Kroemer G., Klionsky D.J., Zeh H.J., Kang R., Wang J., and Tang D.. 2020a. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 16:2069–2083. 10.1080/15548627.2020.1714209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, E., Han L., Liu J., Xie Y., Zeh H.J., Kang R., Bai L., and Tang D.. 2020b. Ferroptotic damage promotes pancreatic tumorigenesis through a TMEM173/STING-dependent DNA sensor pathway. Nat. Commun. 11:6339. 10.1038/s41467-020-20154-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, E., Meng L., Kang R., Wang X., and Tang D.. 2020c. ESCRT-III-dependent membrane repair blocks ferroptosis. Biochem. Biophys. Res. Commun. 522:415–421. 10.1016/j.bbrc.2019.11.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar, H.H., Tyurina Y.Y., Mikulska-Ruminska K., Shrivastava I., Ting H.C., Tyurin V.A., Krieger J., St Croix C.M., Watkins S., Bayir E., et al. 2018. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. J. Clin. Invest. 128:4639–4653. 10.1172/JCI99490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane, R., Singh I., Sagare A.P., Bell R.D., Ross N.T., LaRue B., Love R., Perry S., Paquette N., Deane R.J., et al. 2012. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Invest. 122:1377–1392. 10.1172/JCI58642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, M., Tang Y., Li W., Wang X., Zhang R., Zhang X., Zhao X., Liu J., Tang C., Liu Z., et al. 2018. The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis. Immunity. 49:740–753.e7. 10.1016/j.immuni.2018.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., et al. 2012. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 149:1060–1072. 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, S.J., Winter G.E., Musavi L.S., Lee E.D., Snijder B., Rebsamen M., Superti-Furga G., and Stockwell B.R.. 2015. Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem. Biol. 10:1604–1609. 10.1021/acschembio.5b00245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll, S., Freitas F.P., Shah R., Aldrovandi M., da Silva M.C., Ingold I., Goya Grocin A., Xavier da Silva T.N., Panzilius E., Scheel C.H., et al. 2019. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 575:693–698. 10.1038/s41586-019-1707-0 [DOI] [PubMed] [Google Scholar]
- Doll, S., Proneth B., Tyurina Y.Y., Panzilius E., Kobayashi S., Ingold I., Irmler M., Beckers J., Aichler M., Walch A., et al. 2017. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13:91–98. 10.1038/nchembio.2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolma, S., Lessnick S.L., Hahn W.C., and Stockwell B.R.. 2003. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 3:285–296. 10.1016/S1535-6108(03)00050-3 [DOI] [PubMed] [Google Scholar]
- Doran, A.C., Yurdagul A. Jr., and Tabas I.. 2020. Efferocytosis in health and disease. Nat. Rev. Immunol. 20:254–267. 10.1038/s41577-019-0240-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakesmith, H., and Prentice A.. 2008. Viral infection and iron metabolism. Nat. Rev. Microbiol. 6:541–552. 10.1038/nrmicro1930 [DOI] [PubMed] [Google Scholar]
- Drijvers, J.M., Gillis J.E., Muijlwijk T., Nguyen T.H., Gaudiano E.F., Harris I.S., LaFleur M.W., Ringel A.E., Yao C.H., Kurmi K., et al. 2021. Pharmacologic Screening Identifies Metabolic Vulnerabilities of CD8+ T Cells. Cancer Immunol. Res. 9:184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova, I., Catanzaro E., Van der Meeren L., Turubanova V.D., Hammad H., Mishchenko T.A., Vedunova M.V., Fimognari C., Bachert C., Coppieters F., et al. 2020. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J. Immunother. Cancer. 8:e001369. 10.1136/jitc-2020-001369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann Angeli, J.P., Schneider M., Proneth B., Tyurina Y.Y., Tyurin V.A., Hammond V.J., Herbach N., Aichler M., Walch A., Eggenhofer E., et al. 2014. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16:1180–1191. 10.1038/ncb3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi, L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W., et al. 2018. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25:486–541. 10.1038/s41418-017-0012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, M., Monian P., Quadri N., Ramasamy R., and Jiang X.. 2015. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell. 59:298–308. 10.1016/j.molcel.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, M., Monian P., Pan Q., Zhang W., Xiang J., and Jiang X.. 2016. Ferroptosis is an autophagic cell death process. Cell Res. 26:1021–1032. 10.1038/cr.2016.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, M., Yi J., Zhu J., Minikes A.M., Monian P., Thompson C.B., and Jiang X.. 2019. Role of Mitochondria in Ferroptosis. Mol. Cell. 73:354–363.e3. 10.1016/j.molcel.2018.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg, S.K., Yan Z., Vitvitsky V., and Banerjee R.. 2011. Differential dependence on cysteine from transsulfuration versus transport during T cell activation. Antioxid. Redox Signal. 15:39–47. 10.1089/ars.2010.3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten, F.R., and Grivennikov S.I.. 2019. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. 51:27–41. 10.1016/j.immuni.2019.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Hue, M., García-Caballero C., Palomino-Antolín A., Rubio-Navarro A., Vázquez-Carballo C., Herencia C., Martín-Sanchez D., Farré-Alins V., Egea J., Cannata P., et al. 2019. Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death. FASEB J. 33:8961–8975. 10.1096/fj.201900077R [DOI] [PubMed] [Google Scholar]
- Gui, X., Yang H., Li T., Tan X., Shi P., Li M., Du F., and Chen Z.J.. 2019. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature. 567:262–266. 10.1038/s41586-019-1006-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämälistö, S., Stahl J.L., Favaro E., Yang Q., Liu B., Christoffersen L., Loos B., Guasch Boldú C., Joyce J.A., Reinheckel T., et al. 2020. Spatially and temporally defined lysosomal leakage facilitates mitotic chromosome segregation. Nat. Commun. 11:229. 10.1038/s41467-019-14009-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano, M., Yang W.S., Corn C.K., Pagano N.C., and Stockwell B.R.. 2016. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 23:270–278. 10.1038/cdd.2015.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, W., Xie Y., Song X., Sun X., Lotze M.T., Zeh H.J. III, Kang R., and Tang D.. 2016. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 12:1425–1428. 10.1080/15548627.2016.1187366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, B.I., and Lippman M.E.. 2018. Targeting RAGE Signaling in Inflammatory Disease. Annu. Rev. Med. 69:349–364. 10.1146/annurev-med-041316-085215 [DOI] [PubMed] [Google Scholar]
- Imai, Y., Kuba K., Neely G.G., Yaghubian-Malhami R., Perkmann T., van Loo G., Ermolaeva M., Veldhuizen R., Leung Y.H., Wang H., et al. 2008. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 133:235–249. 10.1016/j.cell.2008.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, M., Qin D., Zhao C., Chai L., Yu Z., Wang W., Tong L., Lv L., Wang Y., Rehwinkel J., et al. 2020. Redox homeostasis maintained by GPX4 facilitates STING activation. Nat. Immunol. 21:727–735. 10.1038/s41590-020-0699-0 [DOI] [PubMed] [Google Scholar]
- Jiang, L., Kon N., Li T., Wang S.J., Su T., Hibshoosh H., Baer R., and Gu W.. 2015. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 520:57–62. 10.1038/nature14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X., Stockwell B.R., and Conrad M.. 2021a. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22:266–282. 10.1038/s41580-020-00324-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Z., Lim S.O., Yan M., Hsu J.L., Yao J., Wei Y., Chang S.S., Yamaguchi H., Lee H.H., Ke B., et al. 2021b. TYRO3 induces anti-PD-1/PD-L1 therapy resistance by limiting innate immunity and tumoral ferroptosis. J. Clin. Invest. 131:e139434. 10.1172/JCI139434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan, V.E., Mao G., Qu F., Angeli J.P., Doll S., Croix C.S., Dar H.H., Liu B., Tyurin V.A., Ritov V.B., et al. 2017. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13:81–90. 10.1038/nchembio.2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, R., Chen R., Xie M., Cao L., Lotze M.T., Tang D., and Zeh H.J. III. 2016. The Receptor for Advanced Glycation End Products Activates the AIM2 Inflammasome in Acute Pancreatitis. J. Immunol. 196:4331–4337. 10.4049/jimmunol.1502340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, R., Lotze M.T., Zeh H.J., Billiar T.R., and Tang D.. 2014. Cell death and DAMPs in acute pancreatitis. Mol. Med. 20:466–477. 10.2119/molmed.2014.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, R., Zeng L., Zhu S., Xie Y., Liu J., Wen Q., Cao L., Xie M., Ran Q., Kroemer G., et al. 2018. Lipid Peroxidation Drives Gasdermin D-Mediated Pyroptosis in Lethal Polymicrobial Sepsis. Cell Host Microbe. 24:97–108.e4. 10.1016/j.chom.2018.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, Y.P., Mockabee-Macias A., Jiang C., Falzone A., Prieto-Farigua N., Stone E., Harris I.S., and DeNicola G.M.. 2021. Non-canonical Glutamate-Cysteine Ligase Activity Protects against Ferroptosis. Cell Metab. 33:174–189.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapralov, A.A., Yang Q., Dar H.H., Tyurina Y.Y., Anthonymuthu T.S., Kim R., St Croix C.M., Mikulska-Ruminska K., Liu B., Shrivastava I.H., et al. 2020. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat. Chem. Biol. 16:278–290. 10.1038/s41589-019-0462-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katikaneni, A., Jelcic M., Gerlach G.F., Ma Y., Overholtzer M., and Niethammer P.. 2020. Lipid peroxidation regulates long-range wound detection through 5-lipoxygenase in zebrafish. Nat. Cell Biol. 22:1049–1055. 10.1038/s41556-020-0564-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.E., Zhang L., Ma K., Riegman M., Chen F., Ingold I., Conrad M., Turker M.Z., Gao M., Jiang X., et al. 2016. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat. Nanotechnol. 11:977–985. 10.1038/nnano.2016.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöditz, K., and Fadeel B.. 2019. Three cell deaths and a funeral: macrophage clearance of cells undergoing distinct modes of cell death. Cell Death Discov. 5:65. 10.1038/s41420-019-0146-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft, V.A.N., Bezjian C.T., Pfeiffer S., Ringelstetter L., Müller C., Zandkarimi F., Merl-Pham J., Bao X., Anastasov N., Kössl J., et al. 2020. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent. Sci. 6:41–53. 10.1021/acscentsci.9b01063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang, F., Liu J., Li C., Kang R., and Tang D.. 2020. Cathepsin B is a mediator of organelle-specific initiation of ferroptosis. Biochem. Biophys. Res. Commun. 533:1464–1469. 10.1016/j.bbrc.2020.10.035 [DOI] [PubMed] [Google Scholar]
- Kuang, F., Liu J., Xie Y., Tang D., and Kang R.. 2021. MGST1 is a redox-sensitive repressor of ferroptosis in pancreatic cancer cells. Cell Chem. Biol.:S2451-9456(21)00006-4. org/ 10.1016/j.chembiol.2021.01.006 [DOI] [PubMed] [Google Scholar]
- Lang, X., Green M.D., Wang W., Yu J., Choi J.E., Jiang L., Liao P., Zhou J., Zhang Q., Dow A., et al. 2019. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov. 9:1673–1685. 10.1158/2159-8290.CD-19-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H., Zandkarimi F., Zhang Y., Meena J.K., Kim J., Zhuang L., Tyagi S., Ma L., Westbrook T.F., Steinberg G.R., et al. 2020. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 22:225–234. 10.1038/s41556-020-0461-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levring, T.B., Hansen A.K., Nielsen B.L., Kongsbak M., von Essen M.R., Woetmann A., Odum N., Bonefeld C.M., and Geisler C.. 2012. Activated human CD4+ T cells express transporters for both cysteine and cystine. Sci. Rep. 2:266. 10.1038/srep00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C., Dong X., Du W., Shi X., Chen K., Zhang W., and Gao M.. 2020a. LKB1-AMPK axis negatively regulates ferroptosis by inhibiting fatty acid synthesis. Signal Transduct. Target. Ther. 5:187. 10.1038/s41392-020-00297-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C., Zhang Y., Liu J., Kang R., Klionsky D.J., and Tang D.. 2021. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy. 17:948–960. 10.1080/15548627.2020.1739447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N., Wang W., Zhou H., Wu Q., Duan M., Liu C., Wu H., Deng W., Shen D., and Tang Q.. 2020b. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic. Biol. Med. 160:303–318. 10.1016/j.freeradbiomed.2020.08.009 [DOI] [PubMed] [Google Scholar]
- Li, P., Li M., Lindberg M.R., Kennett M.J., Xiong N., and Wang Y.. 2010. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 207:1853–1862. 10.1084/jem.20100239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T., and Chen Z.J.. 2018. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 215:1287–1299. 10.1084/jem.20180139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., Feng G., Gauthier J.M., Lokshina I., Higashikubo R., Evans S., Liu X., Hassan A., Tanaka S., Cicka M., et al. 2019a. Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J. Clin. Invest. 129:2293–2304. 10.1172/JCI126428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Feng D., Wang Z., Zhao Y., Sun R., Tian D., Liu D., Zhang F., Ning S., Yao J., and Tian X.. 2019b. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 26:2284–2299. 10.1038/s41418-019-0299-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkermann, A., Bräsen J.H., Darding M., Jin M.K., Sanz A.B., Heller J.O., De Zen F., Weinlich R., Ortiz A., Walczak H., et al. 2013. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA. 110:12024–12029. 10.1073/pnas.1305538110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkermann, A., Skouta R., Himmerkus N., Mulay S.R., Dewitz C., De Zen F., Prokai A., Zuchtriegel G., Krombach F., Welz P.S., et al. 2014. Synchronized renal tubular cell death involves ferroptosis. Proc. Natl. Acad. Sci. USA. 111:16836–16841. 10.1073/pnas.1415518111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Kuang F., Kroemer G., Klionsky D.J., Kang R., and Tang D.. 2020a. Autophagy-Dependent Ferroptosis: Machinery and Regulation. Cell Chem. Biol. 27:420–435. 10.1016/j.chembiol.2020.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, T., Jiang L., Tavana O., and Gu W.. 2019. The Deubiquitylase OTUB1 Mediates Ferroptosis via Stabilization of SLC7A11. Cancer Res. 79:1913–1924. 10.1158/0008-5472.CAN-18-3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., Wang Y., Liu J., Kang R., and Tang D.. 2020b. The circadian clock protects against ferroptosis-induced sterile inflammation. Biochem. Biophys. Res. Commun. 525:620–625. 10.1016/j.bbrc.2020.02.142 [DOI] [PubMed] [Google Scholar]
- Liu, Y., Wang Y., Liu J., Kang R., and Tang D.. 2021. Interplay between MTOR and GPX4 signaling modulates autophagy-dependent ferroptotic cancer cell death. Cancer Gene Ther. 28:55–63. 10.1038/s41417-020-0182-y [DOI] [PubMed] [Google Scholar]
- Loynes, C.A., Lee J.A., Robertson A.L., Steel M.J., Ellett F., Feng Y., Levy B.D., Whyte M.K.B., and Renshaw S.A.. 2018. PGE2 production at sites of tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of inflammation resolution in vivo. Sci. Adv. 4:eaar8320. 10.1126/sciadv.aar8320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, X., Gong H.-B., Gao H.-Y., Wu Y.-P., Sun W.-Y., Li Z.-Q., Wang G., Liu B., Liang L., Kurihara H., et al. 2021. Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ. 10.1038/s41418-020-00719-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X., Xiao L., Liu L., Ye L., Su P., Bi E., Wang Q., Yang M., Qian J., and Yi Q.. 2021. CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metab.:S1550-4131(21)00071-1. 10.1016/j.cmet.2021.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magtanong, L., Ko P.J., To M., Cao J.Y., Forcina G.C., Tarangelo A., Ward C.C., Cho K., Patti G.J., Nomura D.K., et al. 2019. Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem. Biol. 26:420–432.e9. 10.1016/j.chembiol.2018.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez, J., Almendinger J., Oberst A., Ness R., Dillon C.P., Fitzgerald P., Hengartner M.O., and Green D.R.. 2011. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc. Natl. Acad. Sci. USA. 108:17396–17401. 10.1073/pnas.1113421108 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Matsushita, M., Freigang S., Schneider C., Conrad M., Bornkamm G.W., and Kopf M.. 2015. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 212:555–568. 10.1084/jem.20140857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr, L., Grabherr F., Schwärzler J., Reitmeier I., Sommer F., Gehmacher T., Niederreiter L., He G.W., Ruder B., Kunz K.T.R., et al. 2020. Dietary lipids fuel GPX4-restricted enteritis resembling Crohn’s disease. Nat. Commun. 11:1775. 10.1038/s41467-020-15646-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muri, J., Thut H., Bornkamm G.W., and Kopf M.. 2019. B1 and Marginal Zone B Cells but Not Follicular B2 Cells Require Gpx4 to Prevent Lipid Peroxidation and Ferroptosis. Cell Rep. 29:2731–2744.e4. 10.1016/j.celrep.2019.10.070 [DOI] [PubMed] [Google Scholar]
- Murray, P.J. 2017. Macrophage Polarization. Annu. Rev. Physiol. 79:541–566. 10.1146/annurev-physiol-022516-034339 [DOI] [PubMed] [Google Scholar]
- Nagakannan, P., Islam M.I., Conrad M., and Eftekharpour E.. 2021. Cathepsin B is an executioner of ferroptosis. Biochim. Biophys. Acta Mol. Cell Res. 1868:118928. 10.1016/j.bbamcr.2020.118928 [DOI] [PubMed] [Google Scholar]
- Pajuelo, D., Gonzalez-Juarbe N., Tak U., Sun J., Orihuela C.J., and Niederweis M.. 2018. NAD+ Depletion Triggers Macrophage Necroptosis, a Cell Death Pathway Exploited by Mycobacterium tuberculosis. Cell Rep. 24:429–440. 10.1016/j.celrep.2018.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H.S., Jung H.Y., Park E.Y., Kim J., Lee W.J., and Bae Y.S.. 2004. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J. Immunol. 173:3589–3593. 10.4049/jimmunol.173.6.3589 [DOI] [PubMed] [Google Scholar]
- Pedrera, L., Espiritu R.A., Ros U., Weber J., Schmitt A., Stroh J., Hailfinger S., von Karstedt S., and García-Sáez A.J.. 2020. Ferroptotic pores induce Ca2+ fluxes and ESCRT-III activation to modulate cell death kinetics. Cell Death Differ. 10.1038/s41418-020-00691-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett, S.B., Obiri N., and Kiel J.L.. 1989. Involvement and relative importance of at least two distinct mechanisms in the effects of 2-mercaptoethanol on murine lymphocytes in culture. J. Cell. Physiol. 141:40–45. 10.1002/jcp.1041410107 [DOI] [PubMed] [Google Scholar]
- Qu, X., Zou Z., Sun Q., Luby-Phelps K., Cheng P., Hogan R.N., Gilpin C., and Levine B.. 2007. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 128:931–946. 10.1016/j.cell.2006.12.044 [DOI] [PubMed] [Google Scholar]
- Riegman, M., Bradbury M.S., and Overholtzer M.. 2019. Population Dynamics in Cell Death: Mechanisms of Propagation. Trends Cancer. 5:558–568. 10.1016/j.trecan.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegman, M., Sagie L., Galed C., Levin T., Steinberg N., Dixon S.J., Wiesner U., Bradbury M.S., Niethammer P., Zaritsky A., and Overholtzer M.. 2020. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat. Cell Biol. 22:1042–1048. 10.1038/s41556-020-0565-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, K.S., Leu J.I., Barnoud T., Vonteddu P., Gnanapradeepan K., Lin C., Liu Q., Barton J.C., Kossenkov A.V., George D.L., et al. 2020. African-centric TP53 variant increases iron accumulation and bacterial pathogenesis but improves response to malaria toxin. Nat. Commun. 11:473. 10.1038/s41467-019-14151-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M., Tanaka K., Cuervo A.M., and Czaja M.J.. 2009. Autophagy regulates lipid metabolism. Nature. 458:1131–1135. 10.1038/nature07976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois, C.M., Jin T., Miller A.L., Bertheloot D., Nakamura H., Horvath G.L., Mian A., Jiang J., Schrum J., Bossaller L., et al. 2013. RAGE is a nucleic acid receptor that promotes inflammatory responses to DNA. J. Exp. Med. 210:2447–2463. 10.1084/jem.20120201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, X., Liu J., Kuang F., Chen X., Zeh H.J. III, Kang R., Kroemer G., Xie Y., and Tang D.. 2021. PDK4 dictates metabolic resistance to ferroptosis by suppressing pyruvate oxidation and fatty acid synthesis. Cell Rep. 34:108767. 10.1016/j.celrep.2021.108767 [DOI] [PubMed] [Google Scholar]
- Song, X., Zhu S., Chen P., Hou W., Wen Q., Liu J., Xie Y., Liu J., Klionsky D.J., Kroemer G., et al. 2018. AMPK-Mediated BECN1 Phosphorylation Promotes Ferroptosis by Directly Blocking System Xc- Activity. Curr. Biol. 28:2388–2399.e5. 10.1016/j.cub.2018.05.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparvero, L.J., Asafu-Adjei D., Kang R., Tang D., Amin N., Im J., Rutledge R., Lin B., Amoscato A.A., Zeh H.J., and Lotze M.T.. 2009. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J. Transl. Med. 7:17. 10.1186/1479-5876-7-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell, B.R., Jiang X., and Gu W.. 2020. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol. 30:478–490. 10.1016/j.tcb.2020.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, D., and Kroemer G.. 2020. Ferroptosis. Curr. Biol. 30:R1292–R1297. 10.1016/j.cub.2020.09.068 [DOI] [PubMed] [Google Scholar]
- Tang, D., Chen X., Kang R., and Kroemer G.. 2021. Ferroptosis: molecular mechanisms and health implications. Cell Res. 31:107–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, D., Comish P., and Kang R.. 2020. The hallmarks of COVID-19 disease. PLoS Pathog. 16:e1008536. 10.1371/journal.ppat.1008536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, D., Kang R., Coyne C.B., Zeh H.J., and Lotze M.T.. 2012. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol. Rev. 249:158–175. 10.1111/j.1600-065X.2012.01146.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, D., Kang R., Berghe T.V., Vandenabeele P., and Kroemer G.. 2019. The molecular machinery of regulated cell death. Cell Res. 29:347–364. 10.1038/s41422-019-0164-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfay, L., Paul B.T., Konstorum A., Deng Z., Cox A.O., Lee J., Furdui C.M., Hegde P., Torti F.M., and Torti S.V.. 2019. Stearoyl-CoA Desaturase 1 Protects Ovarian Cancer Cells from Ferroptotic Cell Death. Cancer Res. 79:5355–5366. 10.1158/0008-5472.CAN-19-0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo, Q.Z., Lei P., Jackman K.A., Li X.L., Xiong H., Li X.L., Liuyang Z.Y., Roisman L., Zhang S.T., Ayton S., et al. 2017. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol. Psychiatry. 22:1520–1530. 10.1038/mp.2017.171 [DOI] [PubMed] [Google Scholar]
- Ubellacker, J.M., Tasdogan A., Ramesh V., Shen B., Mitchell E.C., Martin-Sandoval M.S., Gu Z., McCormick M.L., Durham A.B., Spitz D.R., et al. 2020. Lymph protects metastasizing melanoma cells from ferroptosis. Nature. 585:113–118. 10.1038/s41586-020-2623-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venketaraman, V., Millman A., Salman M., Swaminathan S., Goetz M., Lardizabal A., David Hom, and Connell N.D.. 2008. Glutathione levels and immune responses in tuberculosis patients. Microb. Pathog. 44:255–261. 10.1016/j.micpath.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Wang, D., Xie N., Gao W., Kang R., and Tang D.. 2018. The ferroptosis inducer erastin promotes proliferation and differentiation in human peripheral blood mononuclear cells. Biochem. Biophys. Res. Commun. 503:1689–1695. 10.1016/j.bbrc.2018.07.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., An P., Xie E., Wu Q., Fang X., Gao H., Zhang Z., Li Y., Wang X., Zhang J., et al. 2017. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology. 66:449–465. 10.1002/hep.29117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R.N., Green J., Wang Z., Deng Y., Qiao M., Peabody M., Zhang Q., Ye J., Yan Z., Denduluri S., et al. 2014. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 1:87–105. 10.1016/j.gendis.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S., Liu W., Wang J., and Bai X.. 2020. Curculigoside inhibits ferroptosis in ulcerative colitis through the induction of GPX4. Life Sci. 259:118356. 10.1016/j.lfs.2020.118356 [DOI] [PubMed] [Google Scholar]
- Wang, W., Green M., Choi J.E., Gijón M., Kennedy P.D., Johnson J.K., Liao P., Lang X., Kryczek I., Sell A., et al. 2019a. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 569:270–274. 10.1038/s41586-019-1170-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Wang W., Yang H., Shao D., Zhao X., and Zhang G.. 2019b. Intraperitoneal injection of 4-hydroxynonenal (4-HNE), a lipid peroxidation product, exacerbates colonic inflammation through activation of Toll-like receptor 4 signaling. Free Radic. Biol. Med. 131:237–242. 10.1016/j.freeradbiomed.2018.11.037 [DOI] [PubMed] [Google Scholar]
- Wei, S., Bi J., Yang L., Zhang J., Wan Y., Chen X., Wang Y., Wu Z., Lv Y., and Wu R.. 2020. Serum irisin levels are decreased in patients with sepsis, and exogenous irisin suppresses ferroptosis in the liver of septic mice. Clin. Transl. Med. 10:e173. 10.1002/ctm2.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, Q., Liu J., Kang R., Zhou B., and Tang D.. 2019. The release and activity of HMGB1 in ferroptosis. Biochem. Biophys. Res. Commun. 510:278–283. 10.1016/j.bbrc.2019.01.090 [DOI] [PubMed] [Google Scholar]
- Wenzel, S.E., Tyurina Y.Y., Zhao J., St Croix C.M., Dar H.H., Mao G., Tyurin V.A., Anthonymuthu T.S., Kapralov A.A., Amoscato A.A., et al. 2017. PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals. Cell. 171:628–641.e26. 10.1016/j.cell.2017.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J., Minikes A.M., Gao M., Bian H., Li Y., Stockwell B.R., Chen Z.N., and Jiang X.. 2019a. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 572:402–406. 10.1038/s41586-019-1426-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z., Geng Y., Lu X., Shi Y., Wu G., Zhang M., Shan B., Pan H., and Yuan J.. 2019b. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc. Natl. Acad. Sci. USA. 116:2996–3005. 10.1073/pnas.1819728116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Y., Zhu S., Song X., Sun X., Fan Y., Liu J., Zhong M., Yuan H., Zhang L., Billiar T.R., et al. 2017. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 20:1692–1704. 10.1016/j.celrep.2017.07.055 [DOI] [PubMed] [Google Scholar]
- Xu, M., Tao J., Yang Y., Tan S., Liu H., Jiang J., Zheng F., and Wu B.. 2020. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis. 11:86. 10.1038/s41419-020-2299-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki, T., Kirchmair A., Sato A., Buqué A., Rybstein M., Petroni G., Bloy N., Finotello F., Stafford L., Navarro Manzano E., et al. 2020. Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat. Immunol. 21:1160–1171. 10.1038/s41590-020-0751-0 [DOI] [PubMed] [Google Scholar]
- Yan, B., Ai Y., Sun Q., Ma Y., Cao Y., Wang J., Zhang Z., and Wang X.. 2021. Membrane Damage during Ferroptosis Is Caused by Oxidation of Phospholipids Catalyzed by the Oxidoreductases POR and CYB5R1. Mol. Cell. 81:355–369.e10. [DOI] [PubMed] [Google Scholar]
- Yang, L., Chen X., Yang Q., Chen J., Huang Q., Yao L., Yan D., Wu J., Zhang P., Tang D., et al. 2020. Broad Spectrum Deubiquitinase Inhibition Induces Both Apoptosis and Ferroptosis in Cancer Cells. Front. Oncol. 10:949. 10.3389/fonc.2020.00949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, W.H., Ding C.C., Sun T., Rupprecht G., Lin C.C., Hsu D., and Chi J.T.. 2019. The Hippo Pathway Effector TAZ Regulates Ferroptosis in Renal Cell Carcinoma. Cell Rep. 28:2501–2508.e4. 10.1016/j.celrep.2019.07.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, W.S., Kim K.J., Gaschler M.M., Patel M., Shchepinov M.S., and Stockwell B.R.. 2016. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA. 113:E4966–E4975. 10.1073/pnas.1603244113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee, P.P., Wei Y., Kim S.Y., Lu T., Chih S.Y., Lawson C., Tang M., Liu Z., Anderson B., Thamburaj K., et al. 2020. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat. Commun. 11:5424. 10.1038/s41467-020-19193-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, J., Zhu J., Wu J., Thompson C.B., and Jiang X.. 2020. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc. Natl. Acad. Sci. USA. 117:31189–31197. 10.1073/pnas.2017152117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsumoto, S., Muroi Y., Chiba T., Ohmura R., Yoneyama M., Magarisawa M., Dodo K., Terayama N., Sodeoka M., Aoyagi R., et al. 2017. Hyperoxidation of ether-linked phospholipids accelerates neutrophil extracellular trap formation. Sci. Rep. 7:16026. 10.1038/s41598-017-15668-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef, L.A., Rebbaa A., Pampou S., Weisberg S.P., Stockwell B.R., Hod E.A., and Spitalnik S.L.. 2018. Increased erythrophagocytosis induces ferroptosis in red pulp macrophages in a mouse model of transfusion. Blood. 131:2581–2593. 10.1182/blood-2017-12-822619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, H., Li X., Zhang X., Kang R., and Tang D.. 2016. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem. Biophys. Res. Commun. 478:1338–1343. 10.1016/j.bbrc.2016.08.124 [DOI] [PubMed] [Google Scholar]
- Zeitler, L., Fiore A., Meyer C., Russier M., Zanella G., Suppmann S., Gargaro M., Sidhu S.S., Seshagiri S., Ohnmacht C., et al. 2021. Anti-ferroptotic mechanism of IL4i1-mediated amino acid metabolism. eLife. 10:e64806. 10.7554/eLife.64806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Zeng L., Xie M., Liu J., Zhou B., Wu R., Cao L., Kroemer G., Wang H., Billiar T.R., et al. 2020. TMEM173 Drives Lethal Coagulation in Sepsis. Cell Host Microbe. 27:556–570.e6. 10.1016/j.chom.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M., Perino A., Ghigo A., Hirsch E., and Shah A.M.. 2013. NADPH oxidases in heart failure: poachers or gamekeepers? Antioxid. Redox Signal. 18:1024–1041. 10.1089/ars.2012.4550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T., Zhang Y., Cui M., Jin L., Wang Y., Lv F., Liu Y., Zheng W., Shang H., Zhang J., et al. 2016. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat. Med. 22:175–182. 10.1038/nm.4017 [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Shi J., Liu X., Feng L., Gong Z., Koppula P., Sirohi K., Li X., Wei Y., Lee H., et al. 2018. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol. 20:1181–1192. 10.1038/s41556-018-0178-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Swanda R.V., Nie L., Liu X., Wang C., Lee H., Lei G., Mao C., Koppula P., Cheng W., et al. 2021. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat. Commun. 12:1589. 10.1038/s41467-021-21841-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y., Li M., Yao X., Fei Y., Lin Z., Li Z., Cai K., Zhao Y., and Luo Z.. 2020. HCAR1/MCT1 Regulates Tumor Ferroptosis through the Lactate-Mediated AMPK-SCD1 Activity and Its Therapeutic Implications. Cell Rep. 33:108487. 10.1016/j.celrep.2020.108487 [DOI] [PubMed] [Google Scholar]
- Zheng, J., and Conrad M.. 2020. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 32:920–937. 10.1016/j.cmet.2020.10.011 [DOI] [PubMed] [Google Scholar]
- Zhu, S., Zhang Q., Sun X., Zeh H.J. III, Lotze M.T., Kang R., and Tang D.. 2017. HSPA5 Regulates Ferroptotic Cell Death in Cancer Cells. Cancer Res. 77:2064–2077. 10.1158/0008-5472.CAN-16-1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindel, J., and Kubes P.. 2020. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. 15:493–518. 10.1146/annurev-pathmechdis-012419-032847 [DOI] [PubMed] [Google Scholar]
- Zou, Y., Henry W.S., Ricq E.L., Graham E.T., Phadnis V.V., Maretich P., Paradkar S., Boehnke N., Deik A.A., Reinhardt F., et al. 2020a. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature. 585:603–608. 10.1038/s41586-020-2732-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, Y., Li H., Graham E.T., Deik A.A., Eaton J.K., Wang W., Sandoval-Gomez G., Clish C.B., Doench J.G., and Schreiber S.L.. 2020b. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat. Chem. Biol. 16:302–309. 10.1038/s41589-020-0472-6 [DOI] [PMC free article] [PubMed] [Google Scholar]