Summary
The transcription factor SOX10 mediates the differentiation of neural crest–derived cells, and SOX10 by immunohistochemistry (IHC) is used primarily for the diagnosis of melanoma. SOX10 expression has been previously documented in benign breast myoepithelial cells. However there is limited literature on its expression in triple-negative breast carcinoma (TNBC). The aim was to study the clinical, pathologic and molecular profiles of SOX10+ tumors in TNBC. Tissue microarrays of TNBC were evaluated for SOX10 expression in 48 cases. SOX10 expression was correlated with clinical and pathologic features such as age, grade, and stage. Gene expression was analyzed on RNA extracted from formalin-fixed paraffin-embedded (FFPE) specimens with Affymetrix 2.0 HTA. Co-expression of SOX10 with androgen receptor (AR), WT1, gross cystic disease fluid protein-15 (GCDFP-15), mammaglobin, epidermal growth factor receptor (EGFR), CK5/6 and GATA transcription factor 3 (GATA3) were also assessed. The mean age was 59.38 (range, 28–90 years). Overall, 37.5% cases (18/48) were SOX10+. There was no association between SOX10 expression and age, grade or stage of patients; 6 of 10 (60%) cases of basal-like 1 (BL1), and 5 of 8 cases of unstable (UNS) molecular subtype were SOX10+. One of 5 basal-like-2 (BL2), 1 of 6 immunomodulatory (IM), 1 of 4 mesenchymal (M), 1 of 5 luminal androgen receptor (LAR) and 2 of 8 mesenchymal stem cell (MSL) showed lower frequencies of SOX10 expression. There was negative correlation between SOX10 and AR+ subtypes (P < .002). SOX10 was positively correlated with WT1 (P = .05). SOX10 did not show significant correlation with mammaglobin, GCDFP15, EGFR, CK5/6 and GATA3. SOX10 expression in the basal-like and unstable molecular subtypes supports the concept that these neoplasms show myoepithelial differentiation.
Keywords: Triple-negative breast cancer, Basal-like, SOX10, Androgen receptor, WT1, Molecular subtypes
1. Introduction
The transcription factor SOX10 belongs to the SOX [sex-determining region Y (SRY)-related high mobility group (HMG)-box] gene family, which regulates Wnt/beta catenin signaling in diverse developmental processes [1]. It mediates differentiation of neural crest cells into melanocytes, oligodendrocyte and glia [2–8] and contributes to stem/progenitor activity and induces a mesenchymal transition in mammary cells [9].
SOX10 is expressed in many different cells and tissues—melanocytes, Schwann cells and corresponding tumors [10]. In addition, it has also been found in some solid tumors—breast cancers [11], salivary tumors [12–14], hepatocellular carcinomas [15], ovarian tumors [16], nasopharyngeal carcinomas [17], prostate cancers [18], and digestive cancers [19]. In addition, SOX10 expression by immunohistochemistry (IHC) has previously been documented in myoepithelial cells of the breast, salivary glands, and bronchial glands [11,20].
In the breast, SOX10 plays a role in Notch4 and PBP (peroxisome-proliferative–activated receptor-binding protein)–mediated mammary epithelial cell growth in vitro [21]. There is very limited literature on expression of SOX10 in breast carcinomas, and SOX10 expression in the molecular subtype of the triple-negative carcinoma has not been well examined. Here, we report the first systematic study of the expression of SOX10 in triple-negative breast carcinoma subdivided by molecular subtype, including basal-like 1 (BL1), basal-like 2 (BL2), mesenchymal (M), mesenchymal stem cell (MSL), immunomodulatory (IM), luminal androgen receptor (LAR), Unstable (UNS) and the correlations with immunohistochemical markers androgen receptor (AR), WT-1, GATA3, mammaglobin and GCDFP-15 with clinicopathological parameters.
2. Materials and methods
2.1. Tissue microarray construction and case selection
The use of archival tissue for this study was approved by the Institutional Review Board at University Hospitals Cleveland Medical Center (protocol 01-13-43C). Tissue microarrays (TMAs) were constructed from archival FFPE blocks using 1.5 mm cores in duplicate pairs after macro-dissection by an expert breast pathologist. Tissue microarrays of TNBC were evaluated for SOX10 and AR expression by IHC in 48 cases. SOX10 expression was correlated with clinical and pathologic features such as age, grade, and stage. Co-expression of SOX10 with other hormone-related proteins including AR, WT1, gross cystic disease fluid protein-15 (GCDFP-15), mammaglobin and GATA transcription factor 3 (GATA3) were also assessed (Table 3).
Table 3.
SOX10, AR, WT1, GATA3, mammaglobin, EGFR, CK5/6 and GCDFP15 expression in TNBC patients
| Immunohistochemical markers | SOX10 negative | SOX10 positive | Total | P |
|---|---|---|---|---|
| Androgen receptor | ||||
| <10% | 15 (50%) | 17 (94.4%) | 32 (66.7%) | .002 |
| >10% | 15 (50%) | 1 (5.6%) | 16 (33.3%) | |
| Total | 30 | 18 | 48 | |
| WT1 | ||||
| Negative | 25 (86.2%) | 11 (61.1%) | 36 (76.6%) | .05 |
| Positive | 4 (13.8) | 7 (38.9) | 11 (23.4%) | |
| Total | 29 | 18 | 47 | |
| GATA3 | ||||
| Negative | 9 (30%) | 7 (38.9%) | 16 (33.3%) | .545 |
| Positive | 21 (70%) | 11 (61.1%) | 32 (66.7%) | |
| Total | 30 | 18 | 48 | |
| GCDFP-15 | ||||
| Negative | 20 (69%) | 15 (88.2%) | 35 (76.1%) | .172 |
| Positive | 9 (31%) | 2 (11.8%) | 11 (23.9%) | |
| Total | 29 | 17 | 46 | |
| Mammaglobin | ||||
| Negative | 19 (63.3%) | 11 (64.7%) | 30 (63.6%) | .591 |
| Positive | 11 (36.7%) | 6 (35.3%) | 17 (36.2%) | |
| Total | 30 | 17 | 47 | |
| EGFR | ||||
| Negative | 11 (40.7%) | 7 (41.2%) | 18 (40.9%) | .611 |
| Positive | 16 (59.3%) | 10 (58.8%) | 26 (59.1%) | |
| Total | 27 | 17 | 44 | |
| CK5/6 | ||||
| Negative | 3 (10.7%) | 0 (0%) | 3 (6.7%) | .597 |
| Positive | 25 (89.3%) | 17 (100%) | 42 (93.3%) | |
| Total | 28 | 17 | 45 | |
2.2. Immunohistochemical staining
The antibodies used for immunohistochemistry in this study are shown in Table 1. All immunostains were performed T1 using formalin-fixed, paraffin-embedded tissue sections. The staining was interpreted by two pathologists on a multiview microscope. Immunohistochemical staining was performed on 5 μm TMA sections using the Ventana Benchmark Ultra platform (Ventana Medical Systems, Tucson, AZ.). Briefly, unstained sections of tissue were prepared from paraffin blocks and baked for 30 minutes at 60°C in a Boekel Lab oven. The slides were deparaffinized, and antigen was retrieved with standard Cell Conditioning 1 (Ventana Medical Systems, AZ, USA), then incubated at 37°C with the primary antibody. Primary antibodies for estrogen receptor, progesterone receptor, HER2, androgen receptor, WT-1, GATA-3, Mammaglobin, EGFR, CK5/6 and GCDFP-15 were used. All IHC markers were assessed by light microscopy. Scoring of immunostained slides was done according to the percentage of tumor cells exhibiting nuclear (ER, PR, WT-1, GATA-3, and SOX10) and membrane (HER2, EGFR and GCDFP15) staining and cytoplasmic CK5/6, mammaglobin. ER/PR staining was performed to assess and/or confirm clinical status. Tumors were considered positive for ER or PR if there were >1% staining in tumor nuclei. HER2 was evaluated using current 2013 ASCO/CAP guidelines. AR positivity was defined as greater than or equal to 10% staining in tumor nuclei [22]. The IHC staining ER, PR, WT1, SOX10, EGFR, CK5/6, and GATA-3 immunohistochemistry was considered positive when more than 1% tumor cells were stained (Fig. 1).
Table 1.
Antibodies used for Immunohistochemistry
| Antibody | Clone | Dilution | Source |
|---|---|---|---|
| ER | SPI | Predilute | Ventana |
| PR | IE2 | Predilute | Ventana |
| HER2 | 4B5 | Predilute | Ventana |
| GATA3 | L50-023 | Predilute | Cell Marque |
| SOX10 | Monoclonal | Predilute | Biocare |
| WT1 | WT49 | Predilute | Leica |
| GCDFP15 | EP1582Y | Predilute | Cell Marque |
| Mammaglobin | 31A5 | Predilute | Cell Marque |
| AR | SP107 | Predilute | Cell Marque |
| CK5/6 | D5/16B4 | Predilute | Ventana |
| EGFR | - | - | Outside hospital |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor, EGFR epidermal growth factor receptor.
Fig. 1.
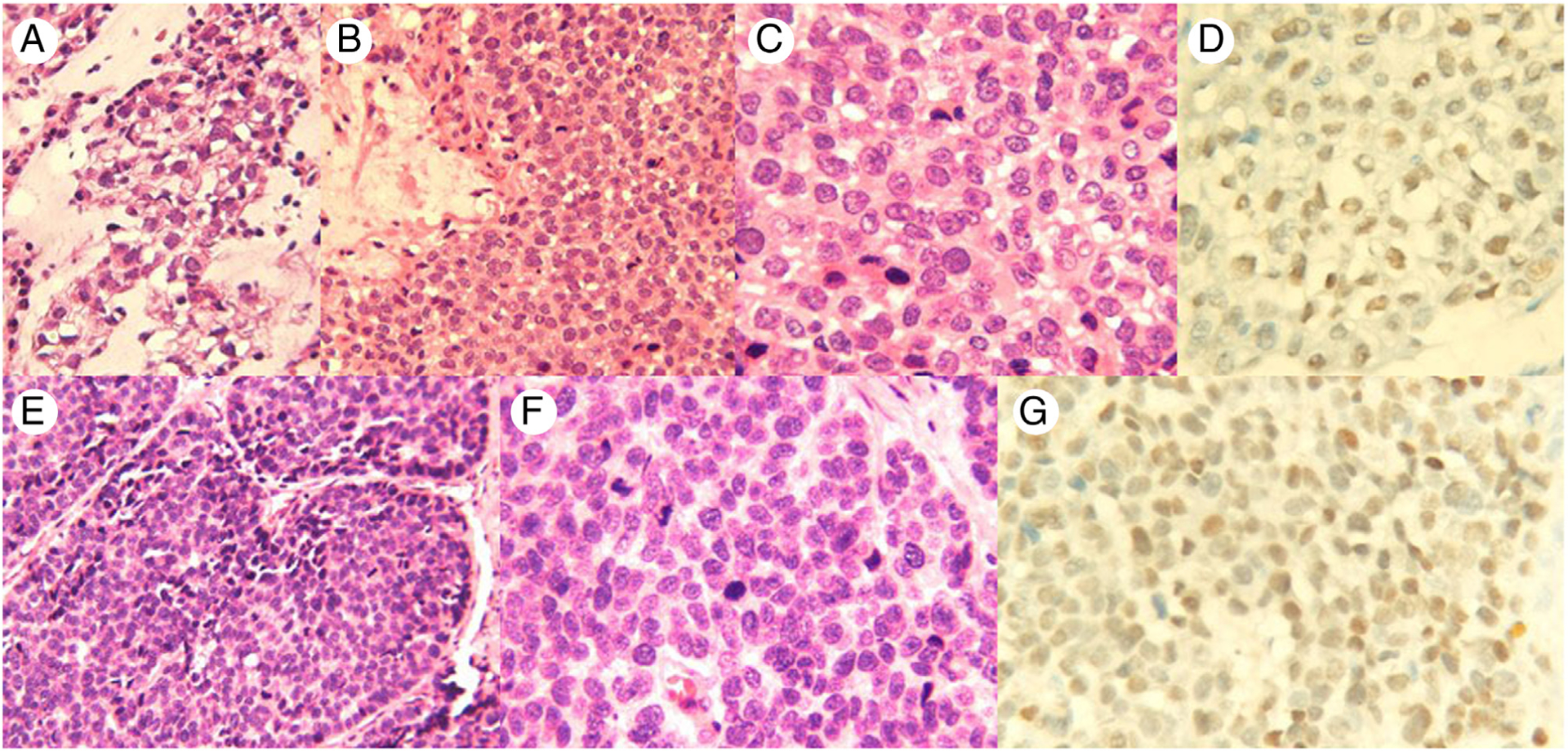
Case 1, A-D, The tumor cells show focal clear cell change and chondromyxoid background (H&E, A and B 200×; C, 400×); SOX10-positive triple-negative breast cancer (D, SOX10 IHC, 400x,). Case 2, E-G, The tumor cells are poorly differentiated and are arranged in solid nests (H&E, E, 200×; F, 400×); SOX10-positive triple-negative breast cancer (G, SOX10 IHC, 400×).
2.3. Molecular analysis
DNA/RNA is extracted from tumor tissue adjacent to the punches taken for TMA construction from the same FFPE block for molecular analysis. Samples from the patients had tumor DNA/RNA extracted from 2 mm cores of macrodissected FFPE tissue blocks using the Qiagen AllPrep DNA/RNA FFPE Kit (cat# 80234). DNA and RNA concentration and quality were assessed using a NanoDrop spectrophotometer. Gene expression was analyzed on RNA extracted from FFPE specimens with Affymetrix 2.0 HTA. Pietenpol TNBC molecular subtypes were calculated using the online TNBC type tool [23].
2.4. Statistical analysis
The software SPSS version 22 for Windows (SPSS Inc, IL., USA) was used for statistical analysis. Associations between SOX10 expression and clinicopathological factors were evaluated using χ2 test and Fisher’s exact test. Disease-free survival (DFS) was measured from the date of diagnosis to the date of death, recurrence or metastasis, and censored at the date of last follow-up for survivors without recurrence. Survivor distributions (DFS) were estimated using Kaplan-Meier methods, and differences of survivals between groups were examined by log-rank test. P ≤ .05 was considered statistically significant.
3. Results
3.1. Clinicopathologic characteristics of patients
Table 2 summarizes clinicopathological characteristics of T2 48 cases of TNBC. All patients were women with a mean age of 59.38 (range, 28–90 years). Overall, 37.5% of cases 18 of 48 were SOX10+ by IHC; 13 of 45 (28.9%) cases showed axillary lymph node metastasis. Tumor recurrence was noted in 10 of 48 cases. When the study group was classified according to the gene profiling, there were 21.7% BL1, 17.4% MSL and UNS each, 13% IM, 10.9% LAR and BL2 each and 8.75% were M subtype. Clinicopathologic characteristics between SOX10 negative and positive were compared in Table 2. Tumor size of SOX10-positive cases was greater than SOX10-negative cases but was not statistically significant (P = .07). When comparing the tumors by molecular subtype, 6 of 10 (60%) cases of BL1 were SOX10 positive, and 5 of 8 cases were of the UNS molecular subtype. SOX10 positivity was seen less frequently in other subtypes: BL2 (1/5), IM (1/6), M (1/4), LAR (1/5) and MSL (2/8). This demonstrated that BL1 and UNS subtype showed higher proportion to be SOX10 positive. There was no statistical significance in age, lymph node metastasis and tumor recurrence between SOX10-positive and -negative cases.
Table 2.
Clinicopathological characteristics of TNBC
| Clinicopathological parameters | SOX10 negative | SOX10 positive | Total | P |
|---|---|---|---|---|
| Age (y, mean ± SD) | 58.17 ± 14.56 | 58.83 ± 13.626 | − | .63 |
| Size (cm, mean ± SD) | 2.7 ± 1.9 | 3.1 ± 2.7 | − | .076 |
| TNBC subtypes | ||||
| BL1 | 4 (40%) | 6 (60%) | 10 (21.7%) | .273 |
| MSL | 6 (75%) | 2 (25%) | 8 (17.4%) | |
| LAR | 4 (80%) | 1 (20%) | 5 (10.9%) | |
| IM | 5 (83.3%) | 1 (16.7%) | 6 (13%) | |
| BL2 | 4 (80%) | 1 (20%) | 5 (10.9%) | |
| M | 3 (75%) | 1 (25%) | 4 (8.7%) | |
| UNS | 3 (37.5%) | 5 (62.5%) | 8 (17.4%) | |
| Total | 29 | 17 | 46 | |
| T stage | ||||
| T1 | 13 (44.85) | 9 (50%) | 22 (46.8%) | .376 |
| T2 | 13 (44.8%) | 5 (27.8%) | 18 (38.3%) | |
| T3 | 3 (10.3%) | 4 (22.2%) | 7 (14.9%) | |
| Total | 29 | 18 | 47 | |
| N stage | ||||
| N0 | 19 (67.9%) | 13 (76.5%) | 32 (71.1%) | .258 |
| N1 | 5 (17.9%) | 4 (23.5%) | 9 (20%) | |
| N3 | 4 (14.3%) | 0 (0%) | 4 (8.9%) | |
| Total | 28 | 17 | 45 | |
| Recurrence | ||||
| Absent | 23 (76.7%) | 15 (83.3%) | 38 (79.2%) | .722 |
| Present | 7 (23.3%) | 3 (30.0%) | 10 (20.8%) | |
| Total | 30 | 18 | 48 |
3.2. IHC staining results
Table 3 demonstrates IHC characteristics of SOX10-positive (Fig. 1) and -negative cases in TNBC. WT-1 was expressed with higher incidence in SOX10-positive TNBC (38.3%) than those of SOX10-negative TNBC (13.8%) (P = .05). There was significantly lower expression of AR in SOX10-positive TNBC (0.5%) than those of SOX10-negative TNBC (50%) with P value of .002. GATA3 expression was present in 66.6% (32/48) of all cases. GCDFP-15 expression was present in 23.9% (11/46) of TNBC while mammaglobin was expressed in 36.1% (17/47) of TNBC cases. EGFR was positive in 26 of 44 (59.1%) cases and CK5/6 positive in 42 of 45 (93.3%) cases. GATA-3 was positive in 11 of 18 (61.1%) SOX10+ cases, GCDFP-15 was positive in 2 of 17 (11.8%) SOX10+ cases, mammaglobin was positive in 6 of 17 (35.3%) SOX10+ cases. EGFR was positive in 10 of 17 (58.8%) SOX10+ cases. CK5/6 was positive in all SOX10-positive cases. SOX10 and GATA-3, GCDFP-15 and mammaglobin expression was not 100% concordant between cases. There was no significant association of SOX10 expression with mammaglobin, GCDFP-15, EGFR, CK5/6 and GATA-3 expression.
3.3. Follow-up and survival
The median follow up was 184.9 months with range 1 to 286 months. The 6-year disease-free survival (DFS) was 63.9%. There was no significant difference in DFS between SOX10-positive and -negative cases (P = .214, log rank test, Fig. 2).
Fig. 2.
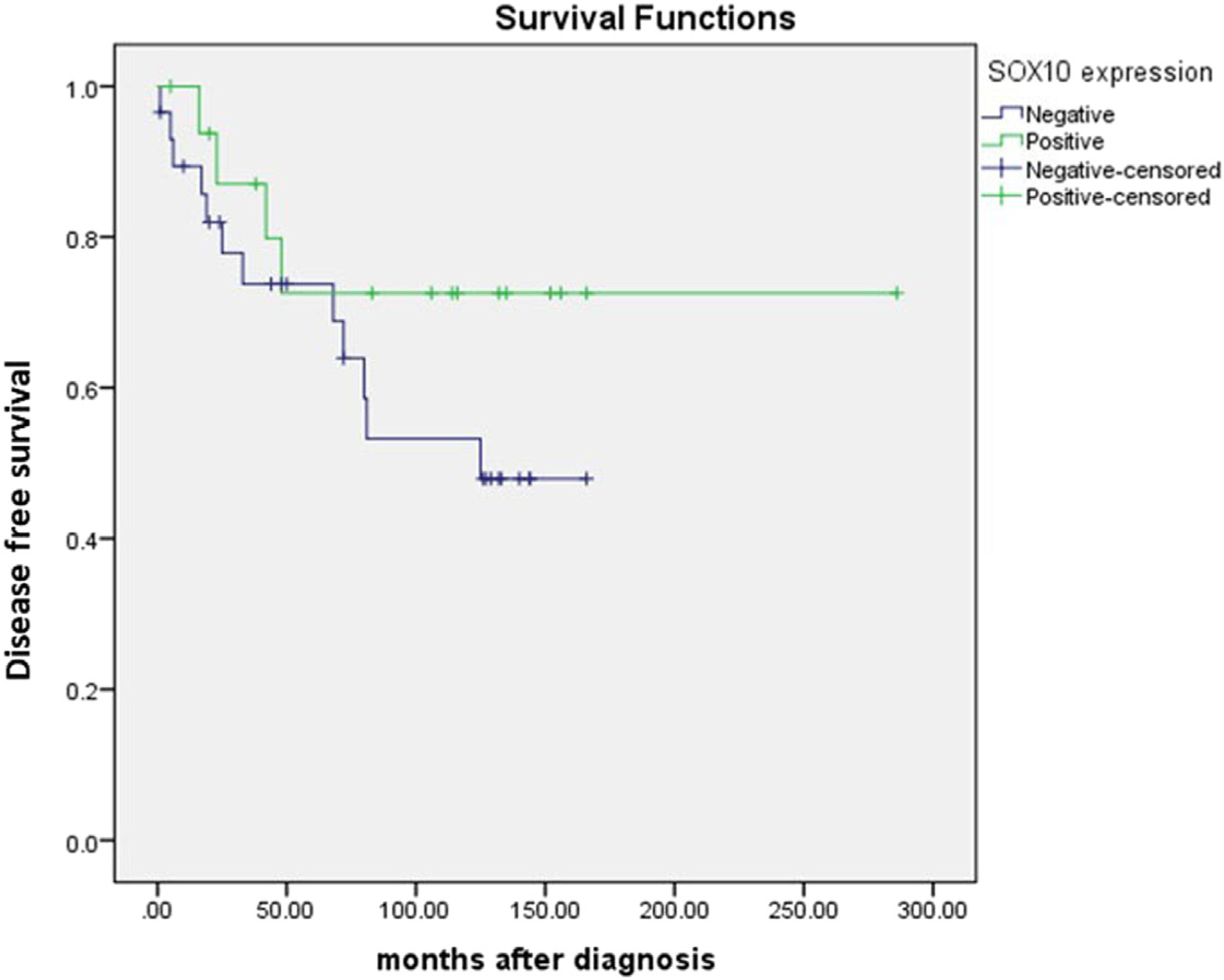
Disease-free survival in SOX10-positive and -negative cases.
4. Discussion
Breast cancer is a heterogeneous disease, which can be classified into biologically, morphologically and clinically meaningful entities. Approximately 12 to 17% are triple-negative breast cancers lacking expression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) [24]. These lesions are also characterized by a high incidence of TP53 mutations, as well as low levels of RB and high levels of p16 proteins [25] Triple-negative breast cancers are more prevalent in patients below the age of 50 years [26,27]. In particular, triple-negative breast cancers are clinically problematic as there is no approved targeted systemic therapy for these lesions; Currently, chemotherapy is the only systemic therapy available for triple-negative breast cancers and is curative in a subset of patients with chemotherapy-sensitive disease [28]. TNBC itself is a heterogeneous group and further divided into basal type and non-basal type based on five biomarkers with BLBC being ER, PR, HER2 negative and positive for basal cytokeratin and EGFR. However, basal-like carcinomas themselves are heterogeneous [25]. Notably, metaplastic carcinomas have also been shown to have a basal-like phenotype by IHC [29–31] and genomic profiling [32].
Recently SOX10 has been described as an additional marker in triple-negative breast cancer, especially basal-like and metaplastic subtype by immunohistochemistry. Cimino-Mathews et al described SOX10 labeling in 40% of primary invasive ductal carcinomas, with labeling seen in 66% (38/58) of the basal-like, unclassified triple-negative, and metaplastic carcinomas as compared with 5% (2/42) of the luminal A, luminal B, and Her-2 carcinomas [11]. Authors also suggest that since SOX10 labels the benign myoepithelial cells of the breast, as well as myoepithelial cell–derived neoplasms of the salivary glands, the SOX10 labeling in a subset of triple-negative breast carcinomas may be attributable to a basal-like, or myoepithelial cell–like phenotype of some of these neoplasms [11]. In our study, we found 37.5% of TNBC cases positive for SOX10 which was less than previous study [11]. This difference may be due heterogeneity within TNBC subgroups. Also the previous study divided TNBC based on immunohistochemistry while in this study molecular studies based sub classification was used [11]. However 60% of basal-like subtype and 62% of UNS subtype was positive for SOX10 similar to previous studies [11]. It suggests that the unclassified triple-negative carcinomas that demonstrate SOX10 labeling also show basal-like or myoepithelial differentiation that is not usually detected by IHC for EGFR or CK5/6. Also, immunoreactivity for SOX10 may be useful in supporting breast origin in a metastasis from a triple-negative, basal-like, or UNS subtype, although it has its limitations as the full spectrum of SOX10 immunoreactivity in all the neoplasms has not yet completely studied.
This study also showed that the incidence of AR expression is lower in the tumor cells of SOX10-positive TNBC than in those of SOX10-negative TNBC (P = .002). This further supports that SOX10 expression is due to myoepithelial differentiation of TNBC.
GATA3 expression was present in 66.6% (32/48) of all cases similar to SOX10 frequency in basal-like and UNS subtype. SOX10 immunohistochemistry can be used to support a breast origin in a metastatic triple-negative carcinoma in patients with a history of triple-negative breast carcinoma, as well as to suggest breast as a site of origin in a metastatic carcinoma of unknown primary. SOX10 and GATA3 were equally positive in our study, although GATA3 expression is less specific for breast carcinoma as GATA3 labels many other types of carcinomas [33] than does SOX10 [34]. Also the SOX10 and GATA3 expression was not 100% concordant between cases and thus both can be used in the evaluation of a carcinoma of unknown primary.
GCDFP-15 expression was present in 23.9% (11/46) of TNBC while mammaglobin was expressed in 36.1% (17/47) of TNBC cases. The SOX10, GCDFP-15 and mammaglobin expression were not 100% concordant between cases. These findings also suggest that immunohistochemistry for SOX10 in addition with GATA3 [35], could have clinical utility in supporting classification of an ER− carcinoma of unknown origin as a primary breast carcinoma, particularly since triple-negative breast carcinomas typically show lower expression for other mammary-specific markers such as GCDFP and mammaglobin [36].
WT1 is a transcription factor which regulates the epithelial-mesenchymal balance during embryonic development and can lead to the formation of Wilms’ tumor in case of mutation. Its expression has also been reported in several solid tumor types, including breast cancer, and shown to have poor outcome. However, published data is inconsistent and the role of WT1 in this malignancy remains unclear [37]. In a study by Artibani et al, author found that WT1 plays a role in regulating the epithelial-mesenchymal balance of breast cancer cells and that WT1-expressing tumors are mainly associated with a mesenchymal phenotype and the known association between WT1 expression in breast cancer and poor prognosis is potentially due to cancer-related epithelial-to-mesenchymal transition (EMT) and poor chemotherapy response [37]. They also showed that WT1 expression in breast cancer occurs at low frequency (10%−30%), and it was lower than in the healthy mammary gland [38]. Also, WT1 expression was significantly higher in ER-positive (luminal) than in ER-negative (basal) tumors [37]. In our study, we found that WT-1 was positive in 23.4% cases similar to previous study. Also, significant correlation of WT-1 and SOX10, further supports the mesenchymal differentiation in TNBC cases. Further studies are needed to fully understand the role of Sox10 in TNBC and its role in myoepithelial differentiation and survival of tumor cells.
In summary, strong nuclear SOX10 labeling is seen in a subset of triple-negative breast carcinomas, most notably the basal-like and unstable subtypes. SOX10 expression in these tumor types in addition to inverse correlation with AR supports myoepithelial differentiation. SOX10 expression is not only found in melanoma, but also in triple-negative breast carcinoma and can be used as additional diagnostic marker with GATA3 in metastasis of unknown origin.
Acknowledgments
We thank Amad Awadallah for his expert technical assistance with immunohistochemistry.
Acknowledgment of sources of support, conflicts of interest and disclaimer—none.
Footnotes
The authors declare no conflict of interest and no funding support to disclose.
References
- [1].Gubbay J, Collignon J, Koopman P, et al. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 1990;346:245–50. [DOI] [PubMed] [Google Scholar]
- [2].Haldin CE, LaBonne C. SoxE factors as multifunctional neural crest regulatory factors. Int J Biochem Cell Biol 2010;42:441–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Harris ML, Baxter LL, Loftus SK, et al. Sox proteins in melanocyte development and melanoma. Pigment Cell Melanoma Res 2010;23: 496–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hong CS, Saint-Jeannet JP. Sox proteins and neural crest development. Semin Cell Dev Biol 2005;16:694–703. [DOI] [PubMed] [Google Scholar]
- [5].Kelsh RN. Sorting out Sox10 functions in neural crest development. BioEssays 2006;28:788–98. [DOI] [PubMed] [Google Scholar]
- [6].Kim J, Lo L, Dormand E, et al. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron 2003; 38:17–31. [DOI] [PubMed] [Google Scholar]
- [7].Mollaaghababa R, Pavan WJ. The importance of having your SOX on: role of SOX10 in the development of neural crest-derived melanocytes and glia. Oncogene 2003;22:3024–34. [DOI] [PubMed] [Google Scholar]
- [8].Stolt CC, Wegner M. SoxE function in vertebrate nervous system development. Int J Biochem Cell Biol 2010;42:437–40. [DOI] [PubMed] [Google Scholar]
- [9].Dravis C, Spike BT, Harrell JC, et al. Sox10 regulates stem/progenitor and mesenchymal cell states in mammary epithelial cells. Cell Rep 2015;12:2035–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].de la Rocha AM, Sampron N, Alonso MM, et al. Role of SOX family of transcription factors in central nervous system tumors. Am J Cancer Res 2014;4:312–24. [PMC free article] [PubMed] [Google Scholar]
- [11].Cimino-Mathews A, Subhawong AP, Elwood H, et al. Neural crest transcription factor Sox10 is preferentially expressed in triple-negative and metaplastic breast carcinomas. HUM PATHOL 2013;44:959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ivanov SV, Panaccione A, Nonaka D, et al. Diagnostic SOX10 gene signatures in salivary adenoid cystic and breast basal-like carcinomas. Br J Cancer 2013;109:444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hsieh MS, Lee YH, Chang YL. SOX10-positive salivary gland tumors: a growing list, including mammary analogue secretory carcinoma of the salivary gland, sialoblastoma, low-grade salivary duct carcinoma, basal cell adenoma/adenocarcinoma, and a subgroup of mucoepidermoid carcinoma. HUM PATHOL 2016;56:134–42. [DOI] [PubMed] [Google Scholar]
- [14].Ohtomo R, Mori T, Shibata S, et al. SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: a clue to the histogenesis for tumor diagnosis. Mod Pathol 2013;26:1041–50. [DOI] [PubMed] [Google Scholar]
- [15].Zhou D, Bai F, Zhang X, et al. SOX10 is a novel oncogene in hepatocellular carcinoma through Wnt/beta-catenin/TCF4 cascade. Tumour Biol 2014;35:9935–40. [DOI] [PubMed] [Google Scholar]
- [16].Kwon AY, Heo I, Lee HJ, et al. Sox10 expression in ovarian epithelial tumors is associated with poor overall survival. Virchows Arch 2016; 468:597–605. [DOI] [PubMed] [Google Scholar]
- [17].Zhao Y, Liu ZG, Tang J, et al. High expression of Sox10 correlates with tumor aggressiveness and poor prognosis in human nasopharyngeal carcinoma. OncoTargets Ther 2016;9:1671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhong WD, Qin GQ, Dai QS, et al. SOXs in human prostate cancer: implication as progression and prognosis factors. BMC Cancer 2012;12: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tong X, Li L, Li X, et al. SOX10, a novel HMG-box-containing tumor suppressor, inhibits growth and metastasis of digestive cancers by suppressing the Wnt/beta-catenin pathway. Oncotarget 2014;5:10571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol 2008;32:1291–8. [DOI] [PubMed] [Google Scholar]
- [21].Zhu YT, Jia Y, Hu L, et al. Peroxisome-proliferator-activated receptor-binding protein (PBP) is essential for the growth of active Notch4-immortalized mammary epithelial cells by activating SOX10 expression. Biochem J 2009;425:435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gucalp A, Tolaney S, Isakoff SJ, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast Cancer. Clin Cancer Res 2013;19:5505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen X, Li J, Gray WH, et al. TNBCtype: a subtyping tool for triple-negative breast Cancer. Cancer Inform 2012;11:147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010;363:1938–48. [DOI] [PubMed] [Google Scholar]
- [25].Rakha E, Reis-Filho JS. Basal-like breast carcinoma: from expression profiling to routine practice. Arch Pathol Lab Med 2009;133:860–8. [DOI] [PubMed] [Google Scholar]
- [26].Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007;13:2329–34. [DOI] [PubMed] [Google Scholar]
- [27].Subhawong AP, Subhawong T, Nassar H, et al. Most basal-like breast carcinomas demonstrate the same Rb−/p16+ immunophenotype as the HPV-related poorly differentiated squamous cell carcinomas which they resemble morphologically. Am J Surg Pathol 2009;33:163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13: 4429–34. [DOI] [PubMed] [Google Scholar]
- [29].Reis-Filho JS, Milanezi F, Steele D, et al. Metaplastic breast carcinomas are basal-like tumours. Histopathology 2006;49:10–21. [DOI] [PubMed] [Google Scholar]
- [30].Gobbi H, Simpson JF, Jensen RA, et al. Metaplastic spindle cell breast tumors arising within papillomas, complex sclerosing lesions, and nipple adenomas. Modern Pathol 2003;16:893–901. [DOI] [PubMed] [Google Scholar]
- [31].Koker MM, Kleer CG. p63 expression in breast cancer: a highly sensitive and specific marker of metaplastic carcinoma. Am J Surg Pathol 2004; 28:1506–12. [DOI] [PubMed] [Google Scholar]
- [32].Weigelt B, Kreike B, Reis-Filho JS. Metaplastic breast carcinomas are basal-like breast cancers: a genomic profiling analysis. Breast Cancer Res Treat 2009;117:273–80. [DOI] [PubMed] [Google Scholar]
- [33].Asch-Kendrick R, Cimino-Mathews A. The role of GATA3 in breast carcinomas: a review. HUM PATHOL 2016;48:37–47. [DOI] [PubMed] [Google Scholar]
- [34].Miettinen M, McCue PA, Sarlomo-Rikala M, et al. Sox10—a marker for not only schwannian and melanocytic neoplasms but also myoepithelial cell tumors of soft tissue: a systematic analysis of 5134 tumors. Am J Surg Pathol 2015;39:826–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cimino-Mathews A, Subhawong AP, Illei PB, et al. GATA3 expression in breast carcinoma: utility in triple-negative, sarcomatoid, and metastatic carcinomas. HUM PATHOL 2013;44:1341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lewis GH, Subhawong AP, Nassar H, et al. Relationship between molecular subtype of invasive breast carcinoma and expression of gross cystic disease fluid protein 15 and mammaglobin. Am J Clin Pathol 2011;135: 587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Artibani M, Sims AH, Slight J, et al. WT1 expression in breast cancer disrupts the epithelial/mesenchymal balance of tumour cells and correlates with the metabolic response to docetaxel. Sci Rep 2017; 7:45255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Silberstein GB, Van Horn K, Strickland P, et al. Altered expression of the WT1 wilms tumor suppressor gene in human breast cancer. Proc Natl Acad Sci U S A 1997;94:8132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


