Key Points
Question
In statin-treated patients at high cardiovascular risk with elevated triglyceride levels and low levels of high-density lipoprotein cholesterol treated with ω-3 fatty acids, are achieved levels of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) associated with cardiovascular outcomes?
Findings
In a secondary analysis of a randomized clinical trial studying a carboxylic acid formulation of ω-3 fatty acids, plasma levels of EPA and DHA were measured 12 months after randomization in 10 382 patients. There was no association between achieved or change in level of either ω-3 fatty acid and major adverse cardiovascular events.
Meaning
These findings do not support the concept that achieving higher EPA plasma levels through pharmacological means reduces adverse cardiovascular outcomes, nor were higher DHA levels associated with harm.
Abstract
Importance
In patients treated with ω-3 fatty acids, it remains uncertain whether achieved levels of eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA) are associated with cardiovascular outcomes.
Objective
To determine the association between plasma levels of EPA and DHA and cardiovascular outcomes in a trial of ω-3 fatty acids compared with corn oil placebo.
Design, Setting, and Participants
A double-blind, multicenter trial enrolled patients at high cardiovascular risk with elevated triglyceride levels and low levels of high-density lipoprotein cholesterol at 675 centers (enrollment from October 30, 2014, to June 14, 2017; study termination January 8, 2020; last visit May 14, 2020).
Interventions
Participants were randomized to receive 4 g daily of ω-3 carboxylic acid (CA) or an inert comparator, corn oil.
Main Outcomes and Measures
The primary prespecified end point was a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina requiring hospitalization. The primary outcome measure was the hazard ratio, adjusted for baseline characteristics, for patients treated with the ω-3 CA compared with corn oil for the top tertile of achieved EPA and DHA plasma levels 12 months after randomization.
Results
Of the 13 078 total participants, 6539 (50%) were randomized to receive ω-3 CA and 6539 (50%) randomized to corn oil. ω-3 Fatty acid levels were available at both baseline and 12 months after randomization in 10 382 participants (5175 ω-3 CA patients [49.8%] and 5207 corn oil–treated patients [50.2%]; mean [SD] age, 62.5 [8.9] years, 3588 [34.6%] were women, 9025 [86.9%] were White, and 7285 [70.2%] had type 2 diabetes). The median plasma levels at 12 months in ω-3 CA patients were 89 μg/mL (interquartile range [IQR], 46-131 μg/mL) for EPA and 91 μg/mL (IQR, 71-114 μg/mL) for DHA with top tertile levels of 151 μg/mL (IQR, 132-181 μg/mL) and 118 μg/mL (IQR, 102-143 μg/mL), respectively. Compared with corn oil, the adjusted hazard ratios for the highest tertile of achieved plasma levels were 0.98 (95% CI, 0.83-1.16; P = .81) for EPA, and 1.02 (95% CI, 0.86-1.20; P = .85 for DHA. Sensitivity analyses based on changes in plasma and red blood cell levels of EPA and DHA and primary and secondary prevention subgroups showed similar results.
Conclusions and Relevance
Among patients treated with ω-3 CA, the highest achieved tertiles of EPA and DHA were associated with neither benefit nor harm in patients at high cardiovascular risk.
Trial Registration
ClinicalTrials.gov Identifier: NCT02104817
This secondary analysis of a randomized clinical trial investigates the association between plasma levels of eicosapentaenoic acid and docosahexaenoic acid and cardiovascular outcomes in a trial of ω-3 fatty acids compared with corn oil placebo.
Introduction
Two large prospective, randomized clinical trials have reported divergent results for treatment of patients at elevated cardiovascular risk with high doses of ω-3 fatty acids, resulting in scientific controversy. The Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT)1 studied a purified formulation of eicosapentaenoic acid (EPA) compared with a mineral oil placebo, reporting a large reduction in major adverse cardiovascular events, with a primary end point hazard ratio (HR) of 0.75 (95% CI, 0.68-0.83). The Long-Term Outcomes Study to Assess Statin Residual Risk with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia (STRENGTH) trial2 studied a mixture of EPA and docosahexaenoic acid (DHA) compared with a corn oil placebo, and reported a neutral result (HR, 0.99; 95% CI, 0.90-1.09).
Several hypotheses have been proposed to explain the different results for these 2 trials. One hypothesis relates to the potential adverse effects of mineral oil as the placebo in the REDUCE-IT trial, which may have elevated risk in the placebo treatment group, resulting in a false-positive result.2,3,4 Two additional hypotheses may explain the disparate results for the 2 trials. These include the possibility that the moderately higher plasma levels of EPA achieved in REDUCE-IT were responsible for the observed benefits or that harmful effects from the administration of DHA in the STRENGTH trial counteracted potential beneficial effects of EPA. To address these latter 2 possibilities, this post hoc analysis of the STRENGTH trial was conducted to assess the association between cardiovascular outcomes and achieved levels of EPA, DHA, or changes in levels of these fatty acids.
Methods
Study Organization and Oversight
The trial was coordinated by the Cleveland Clinic Coordinating Center for Clinical Research. The trial organization is fully described in a design article and the primary publication reporting the results.2,5 Oversight of the trial was provided by an academic executive committee, and analyses were conducted independently by statisticians at Cleveland Clinic Coordinating Center for Clinical Research. The study design was approved by institutional review boards at each site prior to enrollment. An independent data monitoring committee monitored the trial.
Study Population and Procedures
Details of the study design have been published previously.2,5 All patients provided written informed consent. Both primary-prevention patients at high risk for future cardiovascular events and secondary-prevention patients were eligible to participate using predefined criteria. At least 50% of randomized patients were required to satisfy criteria for secondary cardiovascular prevention. Other key entry criteria included the requirement for treatment with a statin, a low-density lipoprotein (LDL) cholesterol level of less than 100 mg/dL (to convert to millimoles per liter, multiply by 0.0259) unless taking maximally tolerated statin dose for at least 4 weeks, triglyceride levels between 180 and 500 mg/dL (to convert to millimoles per liter, multiply by 0.0113), and a high-density lipoprotein cholesterol level of less than 42 mg/dL for men or less than 47 mg/dL for women. Patients were excluded if they had experienced an ischemic cardiovascular event within the preceding 30 days, consumed more than 1 g per day of an ω-3 dietary supplement, or used fibrates or weight loss drugs.
Patients were randomized in a 1:1 ratio to treatment with ω-3 carboxylic acid (CA), 4 g, daily or a matching corn oil comparator for a maximum duration of 5 years. Corn oil was selected because it was considered an inert comparator without effects on biochemical parameters associated with cardiovascular risk.6 Plasma and red blood cell concentrations of EPA and DHA were determined by OmegaQuant at baseline and 12 months after randomization. Patient race and ethnicity were reported by participants using an open-ended question to account for ethnic variability in baseline systemic ω-3 fatty acid concentrations or response to treatment.
Study End Points
The primary efficacy end point was a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, and hospitalization for unstable angina. Achieved levels and changes in plasma EPA and DHA levels were described in the protocol as prespecified efficacy parameters. For the current analysis, event rates for patients by tertiles of achieved plasma EPA and DHA in the ω-3 CA treatment group were compared with event rates for patients randomized to the corn oil placebo. As sensitivity analyses, event rates for tertiles of change in plasma levels and both achieved and change in red blood cell EPA and DHA levels were compared with event rates for patients randomized to the corn oil group. Additional analyses examined outcomes for the primary and secondary prevention subgroups.
Statistical Analysis
The statistical analysis plan and detailed statistical methods were reported in the original STRENGTH trial publication.2 For the current analyses, multivariable models were developed to adjust for potential imbalances in baseline characteristics. Stepwise selection was used to identify candidate variables and bootstrap resampling used to select a parsimonious model. All demographic characteristics, medical history, baseline laboratory markers, and medications were considered for model inclusion. Estimates of adjusted HRs and 95% CIs for each tertile of fatty acid in the ω-3 CA group compared with corn oil were calculated using Cox proportional hazards models with covariates identified during model selection. Each tertile of fatty acid was compared with placebo using the Wald χ2 statistic. Univariable biochemical parameters are presented as median with first (Q1) and third (Q3) quartiles. Significance testing was performed using 2-sided tests (α = .05). All analyses were conducted using SAS, version 9.4 (SAS Institute Inc).
Early Trial Termination
As described previously, on January 8, 2020, when 1384 primary end points had been positively adjudicated, the independent data monitoring committee recommended termination of the trial owing to a low probability of demonstrating a clinical benefit of ω-3 CA compared with corn oil. At the completion of the study, 1580 patients had experienced a positively adjudicated first primary end point event.
Results
Study Population
A total of 13 078 patients were enrolled at 675 sites in 22 countries between October 30, 2014, and June 14, 2017. Of these, EPA and DHA levels were available in 10 382 patients at both baseline and 12 months after randomization. In patients with available EPA and DHA levels, median (IQR) follow-up was 43 (38.4-49.2) months. Complete follow-up for assessment of the primary end point was available in 10 153 patients (97.8%). The baseline characteristics of participants with available ω-3 fatty acid levels are shown in Table 1 for those randomized to corn oil, ω-3 CA, and tertiles of achieved plasma EPA. For all patients with measured ω-3 fatty acid levels, mean (SD) age was 62.4 (8.9) years, 3588 (34.6%) were women, and established cardiovascular disease was present in 5618 (54.1%). The baseline characteristics were balanced among patients receiving corn oil, ω-3 CA, and tertiles of achieved EPA levels (Table 1). The characteristics of the patients in whom ω-3 fatty acid levels were not available are shown in eTable 1 in the Supplement.
Table 1. Baseline Patient Characteristics by Plasma EPA Level.
| Characteristic | No. (%) | |||||
|---|---|---|---|---|---|---|
| All patients (N = 10 382) | Corn oil (n = 5207) | All EPA (n = 5175) | Plasma EPA | |||
| Tertile 1 (n = 1733) | Tertile 2 (n = 1727) | Tertile 3 (n = 1715) | ||||
| Age, mean (SD), y | 62.5 (8.9) | 62.5 (8.9) | 62.5 (8.9) | 61.2 (9.0) | 62.1 (8.7) | 64.2 (8.6) |
| Female | 3588 (34.6) | 1817 (34.9) | 1771 (34.2) | 632 (36.5) | 512 (29.6) | 627 (36.6) |
| Male | 6794 (65.4) | 3390 (65.1) | 3404 (65.8) | 1101 (63.5) | 1215 (70.4) | 1088 (63.4) |
| BMI, mean (SD) | 32.5 (5.6) | 32.5 (5.5) | 32.5 (5.6) | 33.0 (5.8) | 33.5 (5.7) | 31.1 (5.1) |
| Race/ethnicity | ||||||
| White | 9025 (86.9) | 4544 (87.3) | 4481 (86.6) | 1548 (89.3) | 1531 (88.7) | 1402 (81.7) |
| Black | 277 (2.7) | 136 (2.6) | 141 (2.7) | 55 (3.2) | 48 (2.8) | 38 (2.2) |
| Asian | 564 (5.4) | 264 (5.1) | 300 (5.8) | 42 (2.4) | 60 (3.5) | 198 (11.5) |
| Othera | 516 (5.0) | 263 (5.1) | 253 (4.9) | 88 (5.1) | 88 (5.1) | 77 (4.5) |
| Established CVD at baseline | 5618 (54.1) | 2840 (54.5) | 2778 (53.7) | 912 (52.6) | 936 (54.2) | 930 (54.2) |
| Coronary disease | 4629 (44.6) | 2328 (44.7) | 2301 (44.5) | 699 (40.3) | 803 (46.5) | 799 (46.6) |
| Cerebrovascular disease | 780 (7.5) | 393 (7.6) | 387 (7.5) | 169 (9.8) | 116 (6.7) | 102 (6.0) |
| Peripheral vascular disease | 385 (3.7) | 205 (3.9) | 180 (3.5) | 56 (3.2) | 55 (3.2) | 69 (4.0) |
| Aortic disease | 380 (3.7) | 206 (4.0) | 174 (3.4) | 65 (3.8) | 49 (2.8) | 60 (3.5) |
| Diabetes at baseline | 7285 (70.2) | 3663 (70.4) | 3622 (70.0) | 1205 (69.5) | 1268 (73.4) | 1149 (67.0) |
| History of hypertension | 9141 (88.1) | 4564 (87.7) | 4577 (88.4) | 1559 (90.0) | 1548 (89.6) | 1470 (85.7) |
| eGFR, mean (SD), mL/min/1.73 m2 | 77.2 (19.3) | 77.2 (19.5) | 77.1 (19.7) | 78.7 (19.5) | 77.3 (19.7) | 75.3 (19.9) |
| Medication use | ||||||
| High-intensity statin | 5485 (52.8) | 2750 (52.8) | 2735 (52.9) | 1112 (64.2) | 909 (52.6) | 714 (41.6) |
| Other statin | 4897 (47.2) | 2457 (47.2) | 2422 (47.1) | 621 (35.8) | 818 (47.4) | 1001 (58.4) |
| Antiplatelet agents | 7343 (70.7) | 3694 (70.9) | 3649 (70.5) | 1231 (71.0) | 1245 (72.1) | 1173 (68.4) |
| RAAS blockers | 8540 (82.3) | 4279 (82.2) | 4261 (82.3) | 1426 (82.3) | 1453 (84.1) | 1382 (80.6) |
| β-Blockers | 6897 (66.4) | 3473 (66.7) | 3424 (66.2) | 1117 (64.5) | 1165 (67.5) | 1142 (66.6) |
| Laboratory data, median (IQR) | ||||||
| Cholesterol, mg/dL | ||||||
| Total | 162 (139-190) | 162 (139-190) | 162 (140-190) | 166 (141-200) | 155 (134-180) | 166 (146-191) |
| LDL | 76 (57-101) | 76 (57-101) | 76 (57-101) | 81 (59-111) | 71 (53-93) | 78 (61-99) |
| HDL | 36 (31-40) | 36 (31-40) | 36 (31-40) | 35 (31-40) | 35 (31-39) | 36 (32-41) |
| Triglyceride, mg/dL | 240 (192-309) | 241 (191-310) | 239 (193-308) | 235 (188-305) | 235 (189-293) | 250 (202-328) |
| Non-HDL cholesterol, mg/dL | 126 (105-154) | 126 (104-154) | 126 (105-153) | 129 (106-164) | 119 (99-144) | 129 (110-153) |
| Apolipoprotein B, mg/dL | 57 (44-73) | 57 (44-73) | 57 (44-73) | 59 (46-77) | 55 (43-70) | 57 (44-73) |
| Apolipoprotein C-III, mg/dL | 17 (14-21) | 17 (14-21) | 17 (14-21) | 16 (13-20) | 17 (14-20) | 18 (15-22) |
| hs-CRP, mg/dLb | 0.21 (0.11-0.42) | 0.21 (0.11-0.41) | 0.22 (0.11-0.43) | 0.25 (0.12-0.47) | 0.22 (0.11-0.42) | 0.19 (0.09-0.39) |
| Plasma EPA, ug/mL | 21 (13-34) | 22 (13-34) | 21 (13-34) | 17 (11-28) | 19 (12-31) | 28 (18-45) |
| RBC EPA, % of total | 0.6 (0.4-1.0) | 0.6 (0.4-1.0) | 0.6 (0.4-1.0) | 0.5 (0.3-0.8) | 0.6 (0.4-0.9) | 0.8 (0.5-1.2) |
| Plasma DHA, ug/mL | 63 (47-85) | 63 (47-85) | 62 (47-85) | 60 (44-82) | 60 (46-78) | 70 (52-96) |
| RBC DHA, % of total | 5 (4.0-6.2) | 5 (4.0-6.2) | 5 (4.0-6.2) | 5 (3.7-6.0) | 5 (3.9-6.0) | 5 (4.2-6.6) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CVD, cardiovascular disease; DHA, docosahexaenoic acid; eGFR, estimated glomerular filtration rate; EPA, eicosapentaenoic acid; HDL, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; IQR, interquartile range; LDL, low-density lipoprotein cholesterol; RAAS, renin-angiotensin-aldosterone system; RBC, red blood cell.
SI conversion factor: To convert apolipoprotein to grams per liter, multiply by 0.01; cholesterol levels to millimoles per liter, multiply by 0.0259; CRP to milligrams per liter, multiply by 10; triglycerides to millimoles per liter, multiply by 0.0113.
The other race/ethnicity category includes American Indian or Alaska native, Native Hawaiian or Pacific Islander, multiple (mixed) race, and other.
All follow-up measures were at 12 months, except for hs-CRP, which was measured at 60 months (n = 1390 in corn oil placebo and n=1372 in ω-3 carboxylic acid).
Biochemical Parameters
At baseline, in all patients with available ω-3 fatty acid levels at 12 months, median (IQR) levels of LDL cholesterol were 76 mg/dL (57-101 mg/dL), high-density lipoprotein cholesterol levels were 36 mg/dL (31-40 mg/dL), triglyceride levels were 240 mg/dL (192-309 mg/dL), and high-sensitivity C-reactive protein was 0.21 mg/dL (0.11-0.42 mg/dL) (to convert to milligrams per liter, multiply by 10) (Table 1). These values were similar for patients treated with corn oil placebo, ω-3 CA, and each of the 3 tertiles of achieved plasma EPA levels (Table 1).
Table 2 shows biochemical measures for patients 12 months after randomization. The median (IQR) for plasma EPA was 89 μg/mL (46-131 μg/mL) and the median (IQR) DHA level was 91 (71-114). The top tertile of plasma EPA level achieved a median (IQR) level of 151 μg/mL (132-181 μg/mL) and the top tertile of DHA achieved a median (IQR) level of 118 μg/mL (102-143 μg/mL). At 12 months after randomization, the median (IQR) level of LDL cholesterol was 76 mg/dL (56-101 mg/dL), high-density lipoprotein cholesterol level was 37 mg/dL (32-43), and triglyceride level was 212 mg/dL (IQR, 159-288 mg/dL). The median (IQR) high-sensitivity C-reactive protein determined at the end of treatment was 0.18 mg/dL (0.09-0.38 mg/dL) (Table 2). eTable 2 in the Supplement shows the changes in these biochemical markers from baseline to 12 months. eFigure 1 in the Supplement shows the association between achieved EPA and DHA levels as a scatterplot and eFigure 2 in the Supplement shows this association for change in EPA and DHA levels with coefficient of determination values (r2) of 0.55 and 0.49, respectively.
Table 2. Biochemical Measurements 12 Months After Randomization by Tertile of Plasma EPA Level.
| Variable | Median (IQR) | |||||
|---|---|---|---|---|---|---|
| All patients (n = 10 382) | Corn oil (n = 5207) | All EPA (n = 5175) | Plasma EPA | |||
| Tertile 1 (n = 1733) | Tertile 2 (n = 1727) | Tertile 3 (n = 1715) | ||||
| Laboratory data | ||||||
| Cholesterol, mg/dL | ||||||
| Total | 158 (134-189) | 162 (138-192) | 154 (131-185) | 158 (131-197) | 144 (125-170) | 161 (141-192) |
| LDL | 76 (56-101) | 75 (55-100) | 76 (57-103) | 76 (56-110) | 71 (52-91) | 82 (63-107) |
| HDL | 37 (32-43) | 37 (32-42) | 37 (32-43) | 37 (32-43) | 37 (32-42) | 38 (33-43) |
| Triglyceride, mg/dL | 212 (159-288) | 235 (178-315) | 191 (145-255) | 198 (146-270) | 177 (139-228) | 198 (153-271) |
| Non-HDL cholesterol, mg/dL | 120 (97-150) | 124 (101-152) | 116 (94-146) | 119 (94-156) | 107 (88-132) | 123 (102-153) |
| Apolipoprotein B, mg/dL | 56 (44-70) | 56 (45-70) | 55 (44-70) | 56 (44-71) | 52 (42-66) | 57 (47-74) |
| Apolipoprotein C-III, mg/dL | 17 (13-21) | 18 (14-23) | 16 (13-20) | 16 (12-20) | 15 (12-19) | 17 (13-21) |
| hs-CRP, mg/dLa | 0.18 (0.09-0.38) | 0.18 (0.09-0.40) | 0.17 (0.08-0.37) | 0.18 (0.09-0.40) | 0.18 (0.09-0.38) | 0.15 (0.08-0.32) |
| Plasma EPA, ug/mL | 35 (16-92) | 19 (12-31) | 89 (46-131) | 30 (17-47) | 90 (76-103) | 151 (132-181) |
| RBC EPA, % of total | 1.0 (0.5-2.8) | 0.6 (0.4-0.9) | 2.8 (1.5-4.0) | 1.0 (0.6-1.7) | 2.9 (2.4-3.6) | 4.3 (3.5-5.1) |
| Plasma DHA, ug/mL | 75 (53-101) | 58 (44-80) | 91 (71-114) | 67 (53-84) | 88 (76-103) | 118 (102-143) |
| RBC DHA, % of total | 5.8 (4.5-6.8) | 4.8 (3.8-6.0) | 6.6 (5.7-7.3) | 5.7 (4.7-6.5) | 6.6 (6.1-7.3) | 7.1 (6.4-7.8) |
Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HDL, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; IQR, interquartile range; LDL, low-density lipoprotein cholesterol; RBC, red blood cell.
SI conversion factor: To convert apolipoprotein to grams per liter, multiply by 0.01; cholesterol levels to millimoles per liter, multiply by 0.0259; hs-CRP to milligrams per liter, multiply by 10; triglycerides to millimoles per liter, multiply by 0.0113.
All follow-up measures were at 12 months, except for hs-CRP, which was measured at 60 months (n = 1390 in corn oil placebo and n = 1372 in ω-3 CA).
Clinical End Points
In patients with available ω-3 fatty acid levels at 12 months, the primary end point of cardiovascular death, myocardial infarction, stroke, coronary revascularization, or unstable angina requiring hospitalization occurred in 574 of 5207 patients (11.0%) treated with corn oil and 575 of 5175 patients (11.1%) treated with ω-3 CA. Event rates and HRs for tertiles of achieved plasma EPA and DHA levels compared with corn oil placebo are shown in Table 3. In ω-3 CA–treated patients, for the top tertile of achieved EPA levels, 194 of 1715 (11.3%) experienced a primary end point compared with 574 of 5207 (11.0%) for corn oil–treated patients (adjusted HR, 0.98; 95% CI, 0.83-1.16; P = .81). For the top tertile of achieved level of DHA, the event rate was 11.4% (adjusted HR, 1.02; 95% CI, 0.86-1.20; P = .85) compared with placebo.
Table 3. Association Between Tertiles of Achieved Plasma EPA and DHA Levels and Cardiovascular Eventsa.
| Variable | Plasma, μg/mL | No. with events/total No. (%) | Adjusted HR (95% CI)b | P value |
|---|---|---|---|---|
| Achieved plasma EPA | ||||
| Corn oil | NA | 574/5207 (11.0) | 1 [Reference] | NA |
| Achieved plasma EPA tertiles | <62.4 | 173/1733 (10.0) | 0.99 (0.84-1.18) | .95 |
| 62.5-116.3 | 208/1727 (12.0) | 1.10 (0.93-1.28) | .27 | |
| >116.4 | 194/1715 (11.3) | 0.98 (0.83-1.16) | .81 | |
| Achieved plasma DHA | ||||
| Corn oil | NA | 574/5207 (11.0) | 1 [Reference] | NA |
| Achieved plasma DHA tertiles | <78.2 | 188/1735 (10.8) | 1.03 (0.87-1.22) | .74 |
| 78.3-105.1 | 191/1722 (11.1) | 1.03 (0.87-1.21) | .74 | |
| >105.2 | 196/1718 (11.4) | 1.02 (0.86-1.20) | .85 | |
Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HR, hazard ratio; NA, not applicable.
Cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, and revascularization.
Adjusted for baseline fatty acid levels, region, cardiovascular disease, age, sex, diabetes, creatinine, non–high-density lipoprotein cholesterol, high-sensitivity C-reactive protein, antiplatelets agents, β-blockers, and renin angiotensin inhibitors.
Table 4 shows the event rates and HR for changes in EPA and DHA levels compared with corn oil. In patients with the largest increase in EPA levels (top tertile), the event rate was 11.7% (202 of 1724) compared with 11.0% (574 of 5207) for the corn oil group (HR, 1.03; 95% CI, 0.88-1.21; P = .69). In patients in the top tertile for increase in DHA levels, the event rate was 12.1% (208 of 1725) (HR, 1.12; 95% CI, 0.96-1.32; P = .16). eTables 3 and 4 in the Supplement show similar analyses of event rates for tertiles of achieved and changes in red blood cell levels of EPA and DHA, showing no significant association with cardiovascular outcome. eTables 5 and 6 in the Supplement show results for patients in the primary and secondary prevention populations, respectively, and also show no significant association with cardiovascular outcome.
Table 4. Association Between Tertiles of Change in Plasma EPA and DHA Levels and Cardiovascular Eventsa.
| Variable | Plasma, μg/mL | No. with events/total No. (%) | Adjusted HR (95% CI)b | P value |
|---|---|---|---|---|
| Change in plasma EPA | ||||
| Corn oil | NA | 574/5207 (11.0) | 1 [Reference] | NA |
| Tertiles of change in plasma EPA | <38.0 | 173/1727 (10.0) | 0.98 (0.82-1.16) | 0.77 |
| 38.1-88.5 | 200/1724 (11.6) | 1.06 (0.90-1.24) | 0.49 | |
| >88.6 | 202/1724 (11.7) | 1.03 (0.88-1.21) | 0.69 | |
| Change in plasma DHA | ||||
| Corn oil | NA | 574/5207 (11.0) | 1 [Reference] | NA |
| Tertiles of change in plasma DHA | <11.2 | 191/1724 (11.1) | 1.02 (0.86-1.2) | 0.87 |
| 11.2-38.4 | 176/1726 (10.2) | 0.94 (0.79-1.11) | 0.45 | |
| >38.5 | 208/1725 (12.1) | 1.12 (0.96-1.32) | 0.16 | |
Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HR, hazard ratio; NA, not applicable.
Cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, and revascularization.
Adjusted for baseline fatty acid levels, region, cardiovascular disease, age, gender, diabetes, creatinine, non–high-density lipoprotein cholesterol, high-sensitivity C-reactive protein, antiplatelets agents, β-blockers, and renin angiotensin inhibitors.
Figure, A shows Kaplan-Meier curves for time to event for the top tertile of achieved EPA levels compared with corn oil placebo, and Figure, B shows time-to-event curves for the top tertile of plasma DHA. eFigures 3 and 4 in the Supplement show time-to-event curves for the top tertile for changes in EPA and DHA levels, respectively.
Figure. Time to Event of First Incidence of Any Component of the Primary Composite End Point .
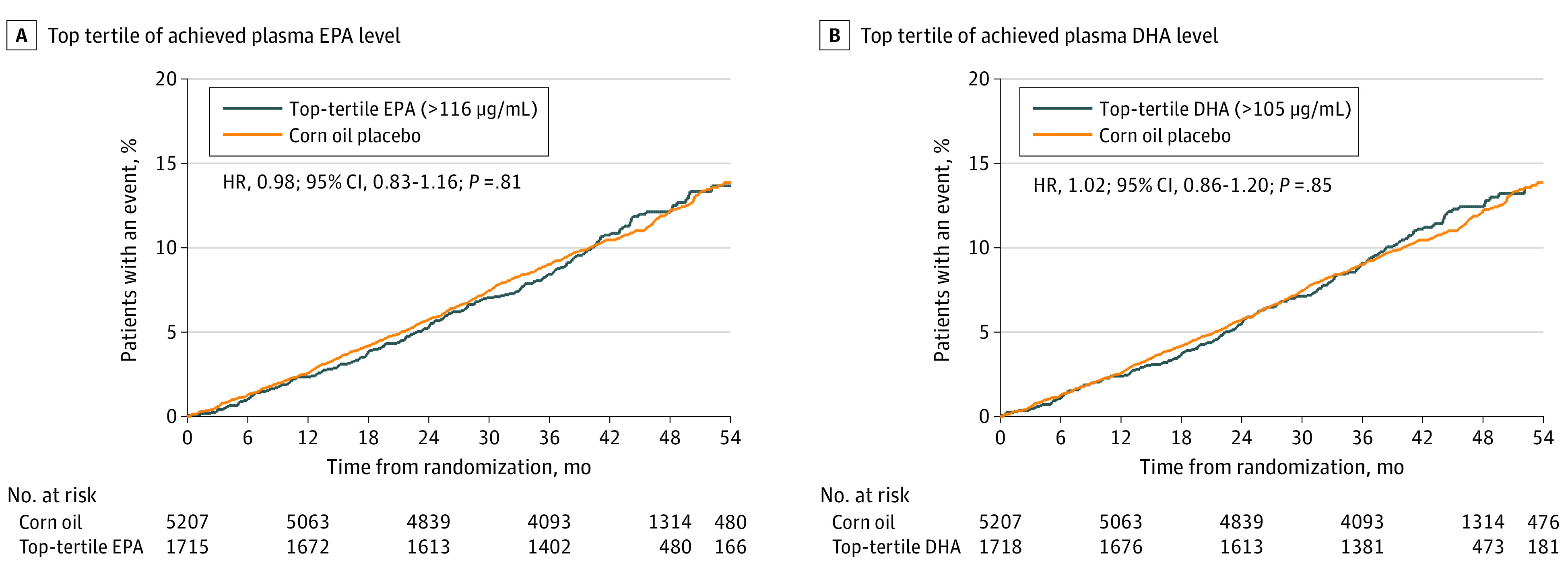
A, Time to event of first incidence of any component of the primary composite end point for patients in the top tertile of achieved plasma eicosapentaenoic acid (EPA) level. B, Time to event of first incidence of any component of the primary composite end point for patients in the top tertile of achieved plasma docosahexaenoic acid (DHA) level.
Discussion
This post hoc analysis was undertaken to address whether achieving high levels of EPA after treatment with ω-3 CA is associated with cardiovascular benefit and secondarily whether achieving high levels of plasma DHA is associated with harm. The relevance of these findings pertains to the divergent results from the REDUCE-IT and STRENGTH trials, which represents a puzzling and not easily explained conundrum. The REDUCE-IT Trial administered 4 g of purified EPA (icosapent ethyl) to achieve high blood levels of this fatty acid. The STRENGTH trial administered a mixture of EPA and DHA in a formulation with enhanced bioavailability, achieving blood levels of EPA that were markedly increased but modestly lower than those achieved in REDUCE-IT. To assess whether the higher EPA levels achieved in REDUCE-IT might explain the difference in results for the 2 trials, this analysis assessed outcomes for patients in the STRENGTH trial who achieved the highest levels of EPA. Conversely, administration of DHA in STRENGTH could have caused harm, thereby undermining the benefits of EPA. Accordingly, this study also looked at outcomes in patients who achieved high levels of DHA.
In the STRENGTH trial, the top tertile of achieved median EPA level was 151 μg/mL, which compares favorably with the median level (144 μg/mL) reported in the icosapent ethyl treatment group in the REDUCE-IT trial. For the primary end point, patients in the highest tertile of achieved EPA levels in STRENGTH showed a neutral HR of 0.98 (95% CI, 0.83-1.16; P = .81) (Table 3). Patients in the highest tertile of DHA achieved a median level of 118 μg/mL and also showed a neutral HR of 1.02 (95% CI, 0.86-1.20, P = .85) (Table 3). Sensitivity analyses based on the highest tertile of change in EPA or DHA levels showed similarly neutral results (Table 4). Because plasma levels may not reflect tissue levels of EPA or DHA, additional analyses assessing red blood cell EPA and DHA levels were undertaken. Neither absolute nor change in red blood cell levels of EPA or DHA showed evidence of benefit or harm (eTables 3 and 4 in the Supplement). Results were also neutral in the primary and secondary prevention subgroups (eTables 5 and 6 in the Supplement). Together, these analyses provide no evidence that achieving higher levels of EPA or DHA are associated with either cardiovascular benefit or harm.
Controversy over the cardiovascular effects of ω-3 fatty acid administration has persisted for decades. Many trials have examined the effects of ω-3 fatty acids on cardiovascular outcomes, generating inconsistent results. More than 20 years ago, the GISSI-Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico) reported that supplementation with just 1 g of a mixture of EPA and DHA in patients after myocardial infarction decreased major cardiovascular events.7 However, this trial was open label, LDL-C levels averaged nearly 140 mg/dL, only a minority of patients were treated with a statin, and the study was never successfully replicated. A decade later, the Japan EPA Lipid Intervention Study (JELIS) study reported a reduction in cardiovascular events after administration of 1.8 g of purified EPA, but this study was also open label, mean baseline LDL-C levels were 180 mg/dL, and patients were treated with a mean dose of only 10 mg of pravastatin or 5 mg of simvastatin.8 Because neither study met contemporary standards for high-quality evidence, guidelines did not adopt administration of ω-3 fatty acids as class 1 evidence-based therapy.
The Effect of Vascepa on Improving Coronary Atherosclerosis in People With High Triglycerides Taking Statin Therapy (EVAPORATE) Trial also purported to show a benefit of icosapent ethyl using computed tomographic angiography with measurement of the volume of low attenuation plaque as the primary end point.9 However, this study was small (68 patients), had twice as much plaque at baseline in the EPA treatment group as the placebo group, and reported a 17% reduction in low attenuation plaque in the treatment group but a 109% increase in low attenuation plaque in the mineral oil placebo group after 18 months. Given the large baseline differences and identical plaque volume measurements in both treatment groups at follow-up, the reported differences seem most likely a result of regression to the mean.
Contemporary studies were conducted with higher standards and consistently failed to demonstrate significant benefits. The ORIGIN (Outcome Reduction with an Initial Glargine Intervention) Trial randomized 12 536 patients with diabetes to 1 g of ω-3 fatty acids or olive oil placebo. After a median follow-up of 6.2 years, there was no reduction in cardiovascular events (HR, 0.98; 95% CI, 0.87-1.10; P = .72).10 The ASCEND Trial (A Study of Cardiovascular Events in Diabetes) also administered 1 g of ω-3 fatty acids to patients with diabetes. Compared with olive oil placebo, after a median follow-up of 7.4 years, there was no effect on serious vascular events (HR, 0.97; 95% CI, 0.87-1.08; P = .55).11 In 2019, the Vitamin D and Omega-3 Trial (VITAL) Trial randomized 25 871 patients to 1 g of ω-3 fatty acids or matching placebo.12 During a median follow-up of 5.3 years, there was no cardiovascular benefit (HR, 0.92; 95% CI, 0.80-1.06; P = .24). These very large, long-term trials cast significant doubt on the cardiovascular benefits of ω-3 fatty acid supplementation.
In this secondary analysis of the STRENGTH trial, the absence of an association between ω-3 fatty acid levels and outcomes requires consideration of other explanations for the different results from the REDUCE-IT trial. Considerable discussion has centered on the choice of comparators in the 2 trials.2,3,4 The STRENGTH trial used corn oil as a placebo comparator because it was considered neutral without significant effects on biochemical parameters associated with cardiovascular risk, whereas the REDUCE-IT trial used mineral oil as the comparator. The US Food and Drug Administration had raised concerns about the use of mineral oil as a comparator during the initial submission for approval of the purified EPA product used in REDUCE-IT.3 Similar concerns emerged during the Food and Drug Administration review of the REDUCE-IT Trial.3 In both cases, the Food and Drug Administration concluded that there was insufficient evidence to conclude that toxicity from the use of mineral oil as a placebo substantially altered outcomes. However, these decisions were made before completion of the STRENGTH trial, which provided additional insight into the effects of the placebo comparators.
There are alternative explanations for the differences observed between the STRENGTH and REDUCE-IT trials. The STRENGTH trial used a carboxylic formulation of both EPA and DHA, which is different from the REDUCE-IT trial, which used a formulation of EPA ethyl ester. Although both therapies raised EPA levels, differences in the pharmacology of the 2 products cannot be excluded as a potential factor explaining the divergent results. It is also conceivable that harmful effects by DHA or competitive binding to receptors not yet characterized might have counterbalanced beneficial effects of EPA resulting in the neutral outcomes observed in the STRENGTH trial.
In this analysis, the absence of a benefit from achieving high levels of EPA or harm from achieving high levels of DHA strengthens the concerns that the choice of comparator may have influenced the divergent results observed in the 2 trials. Given the importance of balancing benefits and risks, these results of both trials must be considered in the context of the safety of the administered drugs. In both the STRENGTH and REDUCE-IT trials, the incidence of atrial fibrillation was significantly increased. In REDUCE-IT, bleeding was also increased while in STRENGTH it was not.1,2
Limitations
This study has important limitations. First, although ω-3 fatty acid levels were prespecified as efficacy end points, this study represents a postrandomization analysis and lacks the rigor of a randomized comparison. Accordingly, these results should be considered as exploratory, hypothesis-generating analyses rather than a definitive finding. Second, analysis of results by tertiles reduces statistical power since each subgroup represents only one-third of the treatment population. However, the relatively narrow confidence intervals for the primary outcome reduce any concern about this source of statistical error. Third, imbalances in baseline characteristics for tertiles can influence this type of analysis. Although a multivariable analysis was used, residual confounding is always a possibility. Fourth, the trial evaluated only patients at high cardiovascular risk. The association between ω-3 fatty acid blood levels and outcomes in lower risk populations may be different. Fifth, there was a moderate association between achieved or change in EPA and DHA levels (r2 = 0.55 and 0.49, respectively), which precludes completely independent assessment of the associations between these fatty acids and outcome.
Conclusions
The top tertiles of achieved EPA and DHA levels in the STRENGTH trial showed neither benefit nor harm. These findings suggest that supplementation of ω-3 fatty acids in high-risk cardiovascular patients is neutral even at the highest achieved levels. Accordingly, the choice of placebo comparator may play an important role in determining outcome for trials of ω-3 products. Additional research is needed with trials specifically designed to compare corn oil with mineral oil and compare purified EPA with other formulations of ω-3 fatty acids.
eTable 1. Baseline characteristics for patients with and without omega-3 fatty acid levels available
eTable 2. Absolute changes biochemical measurements from baseline to 12 months by tertile of on-treatment plasma EPA level
eTable 3. Relationship Between Tertiles of Achieved Red Blood Cell EPA and DHA Levels and Cardiovascular Events
eTable 4. Relationship Between Tertiles of Change in Red Blood Cell EPA and DHA Levels and Cardiovascular Events
eTable 5. Relationship Between Tertiles of Change in Plasma EPA and DHA Levels and Cardiovascular Events in the Primary Prevention Population
eTable 6. Relationship Between Tertiles of Achieved Plasma EPA and DHA Levels and Cardiovascular Events in the Secondary Prevention Population
eFigure 1. Relationship between Plasma EPA and DHA Levels 12 Months after Randomization
eFigure 2. Relationship between Change in Plasma EPA and DHA Levels 12 Months after Randomization
eFigure 3. Time to Event First Incidence of Any Component of the Primary Composite Endpoint for Patients in the Top Tertile of Change in EPA Level
eFigure 4. Time to Event First Incidence of Any Component of the Primary Composite Endpoint for Patients in the Top Tertile of Change in DHA Level
References
- 1.Bhatt DL, Steg PG, Miller M, et al. ; REDUCE-IT Investigators . Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11-22. doi: 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 2.Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324(22):2268-2280. doi: 10.1001/jama.2020.22258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration Briefing Document. Endocrinologic and Metabolic Drugs Advisory Committee Meeting November 14, 2019. Accessed April 9, 2021. https://www.fda.gov/media/132477/download
- 4.Curfman G. Do omega-3 fatty acids benefit health? JAMA. 2020;324(22):2280-2281. doi: 10.1001/jama.2020.22898 [DOI] [PubMed] [Google Scholar]
- 5.Nicholls SJ, Lincoff AM, Bash D, et al. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: Rationale and design of the STRENGTH trial. Clin Cardiol. 2018;41(10):1281-1288. doi: 10.1002/clc.23055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degirolamo C, Rudel LL. Dietary monounsaturated fatty acids appear not to provide cardioprotection. Curr Atheroscler Rep. 2010;12(6):391-396. doi: 10.1007/s11883-010-0133-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico . Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354(9177):447-455. doi: 10.1016/S0140-6736(99)07072-5 [DOI] [PubMed] [Google Scholar]
- 8.Budoff MJ, Bhatt DL, Kinninger A, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J. 2020;41(40):3925-3932. doi: 10.1093/eurheartj/ehaa652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saito Y, Yokoyama M, Origasa H, et al. ; JELIS Investigators, Japan . Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis. 2008;200(1):135-140. doi: 10.1016/j.atherosclerosis.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 10.Bosch J, Gerstein HC, Dagenais GR, et al. ; ORIGIN Trial Investigators . n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367(4):309-318. doi: 10.1056/NEJMoa1203859 [DOI] [PubMed] [Google Scholar]
- 11.Bowman L, Mafham M, Wallendszus K, et al. ; ASCEND Study Collaborative Group . Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N Engl J Med. 2018;379(16):1540-1550. doi: 10.1056/NEJMoa1804989 [DOI] [PubMed] [Google Scholar]
- 12.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group . Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380(1):23-32. doi: 10.1056/NEJMoa1811403 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline characteristics for patients with and without omega-3 fatty acid levels available
eTable 2. Absolute changes biochemical measurements from baseline to 12 months by tertile of on-treatment plasma EPA level
eTable 3. Relationship Between Tertiles of Achieved Red Blood Cell EPA and DHA Levels and Cardiovascular Events
eTable 4. Relationship Between Tertiles of Change in Red Blood Cell EPA and DHA Levels and Cardiovascular Events
eTable 5. Relationship Between Tertiles of Change in Plasma EPA and DHA Levels and Cardiovascular Events in the Primary Prevention Population
eTable 6. Relationship Between Tertiles of Achieved Plasma EPA and DHA Levels and Cardiovascular Events in the Secondary Prevention Population
eFigure 1. Relationship between Plasma EPA and DHA Levels 12 Months after Randomization
eFigure 2. Relationship between Change in Plasma EPA and DHA Levels 12 Months after Randomization
eFigure 3. Time to Event First Incidence of Any Component of the Primary Composite Endpoint for Patients in the Top Tertile of Change in EPA Level
eFigure 4. Time to Event First Incidence of Any Component of the Primary Composite Endpoint for Patients in the Top Tertile of Change in DHA Level


