Abstract
Epilepsy is a common neurological disease that affects more than 50 million people worldwide. Neuroinflammation plays an important role in epilepsy. Activation of the immune system and an excessive inflammatory response can increase the frequency of seizures and increase the susceptibility to epilepsy. Therefore, anti-inflammatory therapies may have antiepileptic effects. Connexin 43 (Cx43) is a major component of astroglial hemichannels and gap junctions. Gap junctions are important for the direct exchange of substances and information between cells, as well as regulating the neuroinflammatory response, changing neuronal excitability, neuronal apoptosis, and synaptic remodeling. Cx43-mediated gap junction pathway can be crucial in epilepsy-induced neuroinflammatory cascades. Further, pro-inflammatory cytokines may in turn directly affect the expression of the Cx43 protein in astrocytes. Therefore, examining the association between neuroinflammation and epilepsy can be instrumental in uncovering the pathogenesis of epilepsy, which can lead to the development of novel and more effective antiepileptic drugs.
Keywords: epilepsy, neuroinflammation, Cx43
1. Introduction
Epilepsy is a common neurological disease that affects >50 million people worldwide. Approximately 30% of epileptic patients are resistant to various antiepileptic drugs and eventually develop refractory epilepsy (1,2). Although novel treatment strategies have been developed over the past few years, certain cases of refractory epilepsy cannot be controlled yet (3). In addition, most available treatments are focused on decreasing seizures by inhibiting the excitability of the nervous system (4). Therefore, the development of new antiepileptogenic therapies that can resolve seizures is imminent (5). Recent studies reported that neuroinflammation plays an important role in the development of epilepsy and abnormal neural circuits (6–11). Further, anti-inflammatory therapies were reported to have possible antiepileptogenic potential (12,13). Therefore, uncovering the role of inflammatory reactions in epilepsy can lead to the development of more effective and novel antiepileptic drugs which can decrease the disability rate and improve the quality of life in epileptic patients.
2. Inflammatory reaction in the central nervous system
The central nervous system can be termed as an ‘immunity exemption zone’ because the blood-brain barrier restricts the entry of various substances in the blood circulation (14–17). However, accumulating evidence indicates that the immune exemption of the central nervous system is relative (18,19). Engelhardt et al (20) reported that although immature T cells cannot pass through the blood-brain barrier, activated T cells can directly attach to the surface of the cerebral vascular endothelium and pass through the blood-brain barrier in the direction of blood flow. After crossing the blood-brain barrier, T cells can mediate inflammation in the central nervous system (20).
The central nervous system has a special ‘immune defense line’. Cells involved in the brain's inflammatory reaction include the microglia, astrocytes and endothelial cells. Microglia are the immune sentinels of the central nervous system; they can be activated by injury stimuli and induce the corresponding inflammatory reaction, which maintains homeostasis in the central nervous system (21). The number of microglia is relatively small, accounting for ~5% of the glial population (22–24). Following infection or damage to the central nervous system, the resting microglia acquire antigenicity, their shape becomes extended, and they are activated into macrophages to participate in the inflammatory reaction together with T cells inoculated within the blood circulation (25). However, hyperactivated microglia are important sources of pro-inflammatory factors as well as oxidative stress, and can cause neurotoxicity.
Astrocytes are also important contributors to the inflammatory reaction in the nervous system (26,27). Astrocytes can play a role in neuronal migration movement, maintain the potassium concentration in the central nervous system, regulate neuronal excitability, and present antigens to autoreactive T cells.
Microglia are activated leading to the production of inflammatory factors following trauma or other injuries. Activated microglia can release cytotoxic substances and cytokines (28). Further, tissue injury could lead to the infiltration of circulating immune cells (29). Additionally, brain tissues are also exposed to systemic inflammatory response, which further aggravates the immune response and leads to secondary neuronal damage (30).
3. Role of the inflammatory reaction in the development of epilepsy
Activation of the immune system and an excessive inflammatory response play crucial roles in the development of chronic seizures (31–33). Moreover, neuronal inflammation associated with inflammatory cytokines signaling pathways may trigger epileptogenesis (34). Of note, patients diagnosed with relapsing remitting multiple sclerosis with epilepsy showed more extensive cortical inflammation compared with patients diagnosed with relapsing remitting multiple sclerosis without epilepsy (35). Therefore, examining the association between neuroinflammation and epilepsy can help uncover the pathogenesis. Clinical and animal studies suggested that the immune response is triggered during the pathophysiology of epilepsy and that the inflammatory reaction within the brain may be involved in the development of epilepsy (36,37). Further, the dysregulation of immunoinflammatory reactions during the pathological course of epilepsy was associated with seizure-induced plasticity (10). Rana and Musto (37) reported that neuronal inflammation generated by neural death and astrocytes proliferation was associated with the microglial activation in damaged areas such as the amygdala, piriform and hippocampus in a rat model of lithium-pilocarpine-induced epilepsy (38). This dysfunction can facilitate the occurrence of epileptic seizures or epilepsy-induced neuronal damage (39). Indeed, inflammatory reactions were found to increase the propensity for seizures, change neuronal excitability, damage the blood-brain barrier, and mediate neuronal apoptosis and synaptic remodeling by activating intracellular signaling pathways (40). Seizures can activate microglia and neurons in the brain, and produce a series of inflammatory reactions without additional exogenous stimuli (32,33). Consequently, microglia and neurons secrete large amounts of pro-inflammatory factors and prostaglandins, and activate the complement system, which ultimately promotes neuronal death and synapse regeneration, leading to chronic spontaneous seizures (10,39).
Noteworthy, adenosine triphosphate (ATP) was shown to activate the sterile inflammatory process through interactions with purinergic receptors (40). The ATP-gated ionotropic P2X7 receptor (P2X7R) can mediate the regulation of neuroinflammation and immune reactions in the central nervous system (41). Neuronal injury was reported to activate microglia and increase the expression of P2X7R (42,43). Monif et al (44) reported that the overexpression of P2X7R is sufficient to drive microglial activation. Moreover, the Fas ligand derived from microglia exacerbates P2X7-mediated microglial activation and triggers a vicious cycle of neuronal death (45,46). Low concentrations of ATP act as chemotactic agents for microglia recognition and migration to guide them to the site of injury (47). ATP-stimulated and P2X4R-mediated microglial activation might have an initial protective effect. Activated microglia can remove potentially necrotic cell debris and promote tissue repair, thereby contributing to neuroprotection. Further, activated microglia release neurotrophic factors through activated P2X4Rs and contribute to neuronal survival (48). P2X7R-activated microglia in neuron-microglia co-culture protect neurons from glutamate toxicity primarily by releasing tumor necrosis factor (TNF)-α. The depletion of microglia can lead to an increase in the levels of cytokines and chemokines such as interleukin (IL)-1β, TNF-α, cytokine-induced neutrophil chemoattractant 1 and monocyte chemoattractant protein-1 in the brain, which aggravates brain damage (49). In later stages of brain injury, ATP can stimulate the overexpression of microglia P2X7R, leading to microglia activation and proliferation, as well as cell death (50). Over-activated microglia upregulate the expression of surface immunomodulatory proteins and release of neurotoxic proinflammatory factors such as IL-1β, IL-6, and TNF-α, which can promote further activation of microglia. Long-term inflammatory reactions result in neuronal death that affects both healthy and damaged cells (51,52).
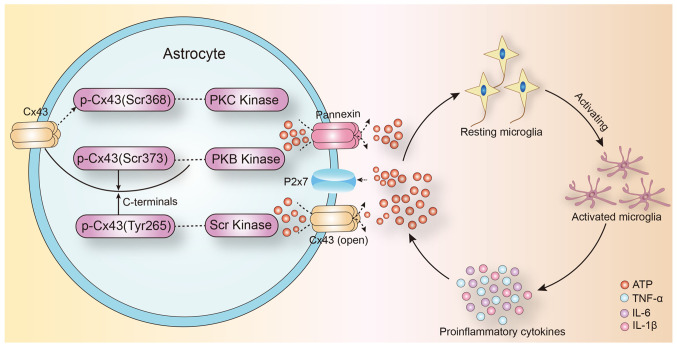
The expression of connexin 43 (Cx43) in astrocytes is affected by inflammatory cytokines (53). Treatment with IL-1β, for 24 h, inhibited the gap junction communication between the human embryonic astrocytes and decreased Cx43 mRNA and protein expression (54). IL-1β had a transient inhibitory effect on gap junction communication between primary astrocytes, and this inhibitory effect was produced through p38-dependent signaling pathway (55). Therefore, Cx43-mediated gap junction communication in astrocytes is closely correlated to the inflammatory response in the central nervous system. They regulate inflammation in the brain and selectively regulate the opening of gap junction communication by intracranial inflammatory factors (Fig. 1).
Figure 1.
Proposed role of Cx43 pathway and ATP in inflammatory activation of microglia in epilepsy. Under physiological conditions, Cx43 is phosphorylated and the channel is closed. Cx43 is dephosphorylated by a variety of protein kinases or protein phosphorylases in epilepsy. The change in Cx43 conformation leads to abnormal opening of the channels. The release of the intracellular ATP mediates the activation and differentiation of microglia into proinflammatory phenotypes, and mediates the process of neuroinflammatory response in epilepsy. Cx43, connexin 43; TNF, tumor necrosis factor; IL, interleukin; ATP, adenosine triphosphate.
4. Regulation of astrocyte glial junction in epilepsy
Cx43 is a major component of astroglial hemichannels and gap junctions (56). Gap junctions play an important role in neuroinflammatory reactions (52). However, the impact of astrocyte Cx43, its hemichannel, and gap junctions on regulating the neuroinflammatory response in epilepsy is still unclear.
Astrocytes are the largest glial cell population in the central nervous system. Cx43 is a gap junction protein that is mainly expressed in astrocytes and mediates over 95% of the gap junction communication in the brain (57). Under physiological conditions, gap junctions allow the exchange of small molecules (<1.5 kDa) between cells. ATP mediates the migration of activated microglia to the injured area, especially in the initial phase of inflammation (41). Further, extracellular ATP induces the release of endogenous ATP from microglia and attracts distant microglia to the injury site, which leads to the promotion of the inflammatory cascade. ATP released by astrocyte hemichannels establishes an ATP gradient in the extracellular environment that can trigger microglial activation (58,59). Increased extracellular ATP concentration in the injury site mediates the activation of microglia around the lesion (60). Of note, the local injection of ATP mimicked the traumatic brain injury-induced microglial activation and the administration of the gap junction channel blocker, carbenic acid, significantly inhibited the microglial activation (61,62). Following injury, extracellular ATP is released from the open hemichannels and mediates a rapid reaction to microglial damage (63). Jesus et al (64) demonstrated that targeted knockout of astrocyte Cx43 expression decreased proinflammatory cytokine levels in the brain following lipopolysaccharide injection. In addition, hemichannel modulators like Cx43 mimetic peptide and Cx43 antisense oligonucleotides could inhibit the inflammatory response mediated by microglial activation following spinal cord injury (65). Taken together, it is plausible that astrocyte gap junction channel can act as a ‘switch’ in the inflammatory signaling cascade by promoting the release of ATP into the extracellular space. The inflammatory response can affect neuronal excitability, neuronal apoptosis and synaptic remodeling. These factors can lead to the development of abnormal neural excitability, which contributes to the pathogenesis of epilepsy. At the same time, pro-inflammatory cytokines can directly affect the expression of Cx43 protein in astrocytes (Fig. 1).
5. Conclusion
The occurrence, development and maintenance of epileptic seizures progress through a complicated process. The neuroinflammatory reaction can aggravate epilepsy and maintain recurrent episodes by increasing neuronal excitability, mediating neuronal apoptosis and remodeling the synapses. Therefore, controlling the neuroinflammatory reaction can mitigate the downstream cascade. The gap junction pathway mediated by astrocyte Cx43 can play a crucial role in controlling the epilepsy-induced neuroinflammatory cascade. Therefore, Cx43 can be a potential target for managing epileptic inflammatory reactions. Studies that examine the correlation between neuroinflammation and gap junctions will lead to a better understanding of epilepsy pathogenesis and can uncover new treatment targets.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- Cx43
connexin 43
- ATP
adenosine triphosphate
- IL
interleukin
- TNF
tumor necrosis factor
Funding Statement
This study was funded by the National Natural Science Foundation of China (grant nos. 81801284, 81771396 and 31371125) and Jilin Provincial Ring-fenced Funding for Industrial Innovation Project (grant no. 2017C029-1).
Funding
This study was funded by the National Natural Science Foundation of China (grant nos. 81801284, 81771396 and 31371125) and Jilin Provincial Ring-fenced Funding for Industrial Innovation Project (grant no. 2017C029-1).
Availability of data and materials
Not applicable.
Authors' contributions
CX and JX searched for literature; GW and JW wrote this review; JL and XW revised this review. All authors read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Beghi E, Giussani G. Treatment of epilepsy in light of the most recent advances. Lancet Neurol. 2019;18:7–8. doi: 10.1016/S1474-4422(18)30412-5. [DOI] [PubMed] [Google Scholar]
- 2.Brewer MK, Grossman TR, McKnight TR, Goldberg YP, Landy H, Gentry MS. The 4th International Lafora Epilepsy Workshop: Shifting paradigms, paths to treatment, and hope for patients. Epilepsy Behav. 2019;90:284–286. doi: 10.1016/j.yebeh.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conway CR, Udaiyar A, Schachter SC. Neurostimulation for depression in epilepsy. Epilepsy Behav. 2018;88S:25–32. doi: 10.1016/j.yebeh.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Johnson EL. Seizures and epilepsy. Med Clin North Am. 2019;103:309–324. doi: 10.1016/j.mcna.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Klein P, Dingledine R, Aronica E, Bernard C, Blümcke I, Boison D, Brodie MJ, Brooks-Kayal AR, Engel J, Jr, Forcelli PA, et al. Commonalities in epileptogenic processes from different acute brain insults: Do they translate? Epilepsia. 2018;59:37–66. doi: 10.1111/epi.13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vezzani A, Balosso S, Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol. 2019;15:459–472. doi: 10.1038/s41582-019-0217-x. [DOI] [PubMed] [Google Scholar]
- 7.Hodges SL, Lugo JN. Therapeutic role of targeting mTOR signaling and neuroinflammation in epilepsy. Epilepsy Res. 2020;161:106282. doi: 10.1016/j.eplepsyres.2020.106282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eastman CL, D'Ambrosio R, Ganesh T. Modulating neuroinflammation and oxidative stress to prevent epilepsy and improve outcomes after traumatic brain injury. Neuropharmacology. 2020;172:107907. doi: 10.1016/j.neuropharm.2019.107907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terrone G, Balosso S, Pauletti A, Ravizza T, Vezzani A. Inflammation and reactive oxygen species as disease modifiers in epilepsy. Neuropharmacology. 2020;167:107742. doi: 10.1016/j.neuropharm.2019.107742. [DOI] [PubMed] [Google Scholar]
- 10.Sanz P, Garcia-Gimeno MA. Reactive glia inflammatory signaling pathways and epilepsy. Int J Mol Sci. 2020;21:4096. doi: 10.3390/ijms21114096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukherjee S, Arisi GM, Mims K, Hollingsworth G, O'Neil K, Shapiro LA. Neuroinflammatory mechanisms of post-traumatic epilepsy. J Neuroinflammation. 2020;17:193. doi: 10.1186/s12974-020-01854-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korff CM, Dale RC. The immune system in pediatric seizures and epilepsies. Pediatrics. 2017;140:e20163534. doi: 10.1542/peds.2016-3534. [DOI] [PubMed] [Google Scholar]
- 13.Bauer J, Becker AJ, Elyaman W, Peltola J, Rüegg S, Titulaer MJ, Varley JA, Beghi E. Innate and adaptive immunity in human epilepsies. Epilepsia. 2017;58(Suppl 3):57–68. doi: 10.1111/epi.13784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura R, Yoshida K, Toda M. Current understanding of lymphatic vessels in the central nervous system. Neurosurg Rev. 2020;43:1055–1064. doi: 10.1007/s10143-019-01133-0. [DOI] [PubMed] [Google Scholar]
- 15.Kipnis J. Multifaceted interactions between adaptive immunity and the central nervous system. Science. 2016;353:766–771. doi: 10.1126/science.aag2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein RS, Garber C, Howard N. Infectious immunity in the central nervous system and brain function. Nat Immunol. 2017;18:132–141. doi: 10.1038/ni.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wesselingh R, Butzkueven H, Buzzard K, Tarlinton D, O'Brien TJ, Monif M. Innate immunity in the central nervous system: A missing piece of the autoimmune encephalitis puzzle? Front Immunol. 2019;10:2066. doi: 10.3389/fimmu.2019.02066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peralta Ramos JM, Iribarren P, Bousset L, Melki R, Baekelandt V, Van der Perren A. Peripheral inflammation regulates CNS immune surveillance through the recruitment of inflammatory monocytes upon systemic α-synuclein administration. Front Immunol. 2019;10:80. doi: 10.3389/fimmu.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci. 2017;20:136–144. doi: 10.1038/nn.4475. [DOI] [PubMed] [Google Scholar]
- 20.Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18:123–131. doi: 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- 21.Greenhalgh AD, Zarruk JG, Healy LM, Baskar Jesudasan SJ, Jhelum P, Salmon CK, Formanek A, Russo MV, Antel JP, McGavern DB, et al. Peripherally derived macrophages modulate microglial function to reduce inflammation after CNS injury. PLoS Biol. 2018;16:e2005264. doi: 10.1371/journal.pbio.2005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohlen CJ, Friedman BA, Dejanovic B, Sheng M. Microglia in brain development, homeostasis, and neurodegeneration. Annu Rev Genet. 2019;53:263–288. doi: 10.1146/annurev-genet-112618-043515. [DOI] [PubMed] [Google Scholar]
- 23.Tan YL, Yuan Y, Tian L. Microglial regional heterogeneity and its role in the brain. Mol Psychiatry. 2020;25:351–367. doi: 10.1038/s41380-019-0609-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Illes P, Rubini P, Ulrich H, Zhao Y, Tang Y. Regulation of microglial functions by purinergic mechanisms in the healthy and diseased CNS. Cells. 2020;9:1108. doi: 10.3390/cells9051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu M, Rayasam A, Kijak JA, Choi YH, Harding JS, Marcus SA, Karpus WJ, Sandor M, Fabry Z. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat Commun. 2019;10:229. doi: 10.1038/s41467-018-08163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wheeler MA, Jaronen M, Covacu R, Zandee SEJ, Scalisi G, Rothhammer V, Tjon EC, Chao CC, Kenison JE, Blain M, et al. Environmental control of astrocyte pathogenic activities in CNS inflammation. Cell. 2019;176:581–596.e18. doi: 10.1016/j.cell.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothhammer V, Quintana FJ. Control of autoimmune CNS inflammation by astrocytes. Semin Immunopathol. 2015;37:625–638. doi: 10.1007/s00281-015-0515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson WD, Greenhalgh AD, Takwale A, David S, Vadigepalli R. Novel influences of IL-10 on CNS inflammation revealed by integrated analyses of cytokine networks and microglial morphology. Front Cell Neurosci. 2017;11:233. doi: 10.3389/fncel.2017.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiang N, Serhan CN. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Aspects Med. 2017;58:114–129. doi: 10.1016/j.mam.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dupuis N, Auvin S. Inflammation and epilepsy in the developing brain: Clinical and experimental evidence. CNS Neurosci Ther. 2015;21:141–151. doi: 10.1111/cns.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukhtar I. Inflammatory and immune mechanisms underlying epileptogenesis and epilepsy: From pathogenesis to treatment target. Seizure. 2020;82:65–79. doi: 10.1016/j.seizure.2020.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Wyatt-Johnson SK, Brewster AL. Emerging Roles for Microglial Phagocytic Signaling in Epilepsy. Epilepsy Curr. 2020;20:33–38. doi: 10.1177/1535759719890336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiragi T, Ikegaya Y, Koyama R. Microglia after seizures and in epilepsy. Cells. 2018;7:26. doi: 10.3390/cells7040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paudel YN, Shaikh MF, Shah S, Kumari Y, Othman I. Role of inflammation in epilepsy and neurobehavioral comorbidities: Implication for therapy. Eur J Pharmacol. 2018;837:145–155. doi: 10.1016/j.ejphar.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Calabrese M, De Stefano N, Atzori M, Bernardi V, Mattisi I, Barachino L, Rinaldi L, Morra A, McAuliffe MM, Perini P, et al. Extensive cortical inflammation is associated with epilepsy in multiple sclerosis. J Neurol. 2008;255:581–586. doi: 10.1007/s00415-008-0752-7. [DOI] [PubMed] [Google Scholar]
- 36.Drion CM, van Scheppingen J, Arena A, Geijtenbeek KW, Kooijman L, van Vliet EA, Aronica E, Gorter JA. Effects of rapamycin and curcumin on inflammation and oxidative stress in vitro and in vivo - in search of potential anti-epileptogenic strategies for temporal lobe epilepsy. J Neuroinflammation. 2018;15:212. doi: 10.1186/s12974-018-1247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rana A, Musto AE. The role of inflammation in the development of epilepsy. J Neuroinflammation. 2018;15:144. doi: 10.1186/s12974-018-1192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung KH, Chu K, Lee ST, Kim JH, Kang KM, Song EC, Kim SJ, Park HK, Kim M, Lee SK, et al. Region-specific plasticity in the epileptic rat brain: A hippocampal and extrahippocampal analysis. Epilepsia. 2009;50:537–549. doi: 10.1111/j.1528-1167.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- 39.Vezzani A, Rüegg S. Introduction to the 2nd Meeting on Immunity and Inflammation in Epilepsy (IIE2016) Epilepsia. 2017;58(Suppl 3):7–10. doi: 10.1111/epi.13780. [DOI] [PubMed] [Google Scholar]
- 40.Mazarati AM, Lewis ML, Pittman QJ. Neurobehavioral comorbidities of epilepsy: Role of inflammation. Epilepsia. 2017;58(Suppl 3):48–56. doi: 10.1111/epi.13786. [DOI] [PubMed] [Google Scholar]
- 41.Allsopp RC, Dayl S, Schmid R, Evans RJ. Unique residues in the ATP gated human P2X7 receptor define a novel allosteric binding pocket for the selective antagonist AZ10606120. Sci Rep. 2017;7:725. doi: 10.1038/s41598-017-00732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nuka E, Ohnishi K, Terao J, Kawai Y. ATP/P2X7 receptor signaling as a potential anti-inflammatory target of natural polyphenols. PLoS One. 2018;13:e0204229. doi: 10.1371/journal.pone.0204229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zumerle S, Calì B, Munari F, Angioni R, Di Virgilio F, Molon B, Viola A. Intercellular calcium signaling induced by ATP potentiates macrophage phagocytosis. Cell Rep. 2019;27:1–10.e4. doi: 10.1016/j.celrep.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monif M, Burnstock G, Williams DA. Microglia: Proliferation and activation driven by the P2X7 receptor. Int J Biochem Cell Biol. 2010;42:1753–1756. doi: 10.1016/j.biocel.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 45.Li R, Shang Y, Hu X, Yu Y, Zhou T, Xiong W, Zou X. ATP/P2X7r axis mediates the pathological process of allergic asthma by inducing M2 polarization of alveolar macrophages. Exp Cell Res. 2020;386:111708. doi: 10.1016/j.yexcr.2019.111708. [DOI] [PubMed] [Google Scholar]
- 46.Ryoden Y, Fujii T, Segawa K, Nagata S. Functional expression of the P2X7 ATP receptor requires eros. J Immunol. 2020;204:559–568. doi: 10.4049/jimmunol.1900448. [DOI] [PubMed] [Google Scholar]
- 47.Burnstock G, Knight GE. The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal. 2018;14:1–18. doi: 10.1007/s11302-017-9593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amadio S, Parisi C, Piras E, Fabbrizio P, Apolloni S, Montilli C, Luchetti S, Ruggieri S, Gasperini C, Laghi-Pasini F, et al. Modulation of P2X7 receptor during inflammation in multiple sclerosis. Front Immunol. 2017;8:1529. doi: 10.3389/fimmu.2017.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei L, Syed Mortadza SA, Yan J, Zhang L, Wang L, Yin Y, Li C, Chalon S, Emond P, Belzung C, et al. ATP-activated P2X7 receptor in the pathophysiology of mood disorders and as an emerging target for the development of novel antidepressant therapeutics. Neurosci Biobehav Rev. 2018;87:192–205. doi: 10.1016/j.neubiorev.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Beamer E, Fischer W, Engel T. The ATP-gated P2X7 receptor as a target for the treatment of drug-resistant epilepsy. Front Neurosci. 2017;11:21. doi: 10.3389/fnins.2017.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaczmarek-Hajek K, Zhang J, Kopp R, Grosche A, Rissiek B, Saul A, Bruzzone S, Engel T, Jooss T, Krautloher A, et al. Re-evaluation of neuronal P2X7 expression using novel mouse models and a P2X7-specific nanobody. Elife. 2018;7:e36217. doi: 10.7554/eLife.36217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim Y, Davidson JO, Gunn KC, Phillips AR, Green CR, Gunn AJ. Role of hemichannels in CNS inflammation and the inflammasome pathway. Adv Protein Chem Struct Biol. 2016;104:1–37. doi: 10.1016/bs.apcsb.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Das A, Wallace GC IV, Holmes C, McDowell ML, Smith JA, Marshall JD, Bonilha L, Edwards JC, Glazier SS, Ray SK, et al. Hippocampal tissue of patients with refractory temporal lobe epilepsy is associated with astrocyte activation, inflammation, and altered expression of channels and receptors. Neuroscience. 2012;220:237–246. doi: 10.1016/j.neuroscience.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.John GR, Scemes E, Suadicani SO, Liu JS, Charles PC, Lee SC, Spray DC, Brosnan CF. IL-1beta differentially regulates calcium wave propagation between primary human fetal astrocytes via pathways involving P2 receptors and gap junction channels. Proc Natl Acad Sci USA. 1999;96:11613–11618. doi: 10.1073/pnas.96.20.11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zvalova D, Cordier J, Mesnil M, Junier MP, Chneiweiss H. p38/SAPK2 controls gap junction closure in astrocytes. Glia. 2004;46:323–333. doi: 10.1002/glia.10334. [DOI] [PubMed] [Google Scholar]
- 56.De Bock M, Wang N, Decrock E, Bultynck G, Leybaert L. Intracellular cleavage of the Cx43 C-terminal domain by matrix-metalloproteases: A novel contributor to inflammation? Mediators Inflamm. 2015;2015:257471. doi: 10.1155/2015/257471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jourdeuil K, Taneyhill LA. The gap junction protein connexin 43 controls multiple aspects of cranial neural crest cell development. J Cell Sci. 2020;133:jcs235440. doi: 10.1242/jcs.235440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nikolic L, Shen W, Nobili P, Virenque A, Ulmann L, Audinat E. Blocking TNFα-driven astrocyte purinergic signaling restores normal synaptic activity during epileptogenesis. Glia. 2018;66:2673–2683. doi: 10.1002/glia.23519. [DOI] [PubMed] [Google Scholar]
- 59.Ghaemi A, Alizadeh L, Babaei S, Jafarian M, Khaleghi Ghadiri M, Meuth SG, Kovac S, Gorji A. Astrocyte-mediated inflammation in cortical spreading depression. Cephalalgia. 2018;38:626–638. doi: 10.1177/0333102417702132. [DOI] [PubMed] [Google Scholar]
- 60.Webster KM, Sun M, Crack P, O'Brien TJ, Shultz SR, Semple BD. Inflammation in epileptogenesis after traumatic brain injury. J Neuroinflammation. 2017;14:10. doi: 10.1186/s12974-016-0786-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Illes P, Burnstock G, Tang Y. Astroglia-derived ATP modulates CNS neuronal circuits. Trends Neurosci. 2019;42:885–898. doi: 10.1016/j.tins.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Nakamura Y, Park JH, Hayakawa K. Therapeutic use of extracellular mitochondria in CNS injury and disease. Exp Neurol. 2020;324:113114. doi: 10.1016/j.expneurol.2019.113114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li W, Li J, Sama AE, Wang H. Carbenoxolone blocks endotoxin-induced protein kinase R (PKR) activation and high mobility group box 1 (HMGB1) release. Mol Med. 2013;19:203–211. doi: 10.2119/molmed.2013.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jesus LB, Santos AB, Jesus EEV, Santos RGD, Grangeiro MS, Bispo-da-Silva A, Arruda MR, Argolo DS, Pinheiro AM, El-Bachá RS, et al. IDO, COX and iNOS have an important role in the proliferation of Neospora caninum in neuron/glia co-cultures. Vet Parasitol. 2019;266:96–102. doi: 10.1016/j.vetpar.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Kwan T, Floyd CL, Kim S, King PH. RNA binding protein human antigen R is translocated in astrocytes following spinal cord injury and promotes the inflammatory response. J Neurotrauma. 2017;34:1249–1259. doi: 10.1089/neu.2016.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.