Abstract
About 10% of women of reproductive age are unable to conceive or carry a pregnancy to term. Female factors alone account for at least 35% of all infertility cases and comprise a wide range of causes affecting ovarian development, maturation of oocytes, and fertilization competence, as well as the potential of a fertilized egg for preimplantation development, implantation, and fetal growth. Genetic abnormalities leading to infertility in females comprise large chromosome abnormalities, submicroscopic chromosome deletion and duplications, and DNA sequence variations in the genes that control numerous biological processes implicated in oogenesis, maintenance of ovarian reserve, hormonal signaling, and anatomical and functional development of female reproductive organs. Despite the great number of genes implicated in reproductive physiology by the study of animal models, only a subset of these genes is associated with human infertility. In this review, we mainly focus on genetic alterations identified in humans and summarize recent knowledge on the molecular pathways of oocyte development and maturation, the crucial role of maternal-effect factors during embryogenesis, and genetic conditions associated with ovarian dysgenesis, primary ovarian insufficiency, early embryonic lethality, and infertility.
Keywords: female infertility, follicular development, genetics, oocyte development, preimplantation embryo, premature ovarian failure, X chromosome
Overview
Infertility is a disease of the reproductive system, encompasses a wide spectrum of conditions that affect the capacity of an individual to reproduce. A diagnosis of female infertility, defined as the inability to establish a clinical pregnancy after 12 months of regular unprotected sexual intercourse with a healthy partner, can also lead to anxiety and depression. Genetic, endocrine, physiological, anatomical, and immunological abnormalities of the reproductive system can affect a woman's likelihood of becoming pregnant and delivering a living child.
The high prevalence of reproductive disorders and infertility, affecting ∼10–15% of couples worldwide, is perhaps not surprising as successful reproduction requires the precise regulation of complex processes essential for development of functional gonads and other reproductive organs, sex determination, gametogenesis, neuroendocrine competency, and ability to carry a pregnancy. Oogenesis is a process by which the mammalian egg becomes competent for fertilization and involves complex interaction between the oocyte and somatic cells that surround it, including the interplay of multiple transcriptional regulators. The disruption of these transcriptional regulators leads to ovarian dysgenesis or disorders of sex development. Newborn girls’ ovaries have, on average, 1–2 million of primordial oocytes, and by the time of puberty, only about 400,000 remain. Individual oocytes are enveloped by somatic cells to form follicles and stay arrested at the diplotene stage of meiosis I (MI) until puberty. Completion of MI and ovulation are triggered by pituitary gonadotropins (Figure 1). The initial endowment of follicles (ovarian reserve) determines a woman's reproductive potential and, subsequently, her reproductive lifespan and age of menopause onset (between the ages of 40 and 58). Diminished ovarian reserve, either due to a low number of follicles at birth or puberty or to a rapid decline in the ovarian follicle pool after puberty, is associated with irregular menstruation, follicle exhaustion, and premature menopause (before age 40).
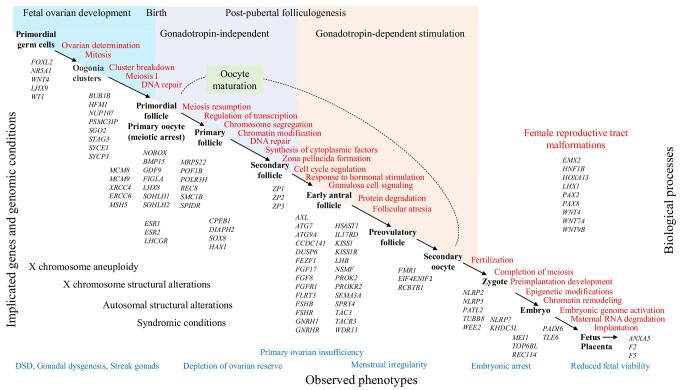
Figure 1.
Biological processes and genetic causes implicated in female infertility. Large X chromosome alterations are the frequent cause of ovarian dysgenesis. A less severe phenotype manifesting as primary or secondary amenorrhea as well as reduced fetal viability can be seen in patients with balanced rearrangements, submicroscopic alterations, and single gene defects. Reproductive potential in patients with genomic alterations and syndromic conditions greatly depends on diagnosis, genes affected, and concomitant manifestations. Causative genes are displayed beneath the key biological processes, however can be involved in multiple pathways, affecting several stages of oocyte development.
Multiple genes have been implicated in the production and maturation of germ cells, defects in which can cause accelerated cell apoptosis and follicle atresia, resulting in primary ovarian insufficiency (POI). Follicular atresia is one of the major mechanisms for eliminating germ cells from the ovary. About 1–2% of women are affected by POI, a phenotypically and etiologically heterogeneous condition characterized by primary or secondary amenorrhea, infertility, decreased estrogen production, elevated gonadotropins (follicle stimulating hormone and luteinizing hormone), and increased risk for osteoporosis and cardiovascular disease. POI is a term that is increasingly used and has been adopted to encompass diagnostically similar conditions, including hypergonadotropic hypogonadism, premature ovarian failure, and ovarian dysgenesis. POI is etiologically due to intrinsic problems within the ovary itself, which may include iatrogenic causes, such as surgery or radiation, or disruption of genes that primarily affect ovarian function. In a more severe presentation, POI presents with streak ovaries due to the depletion of germ cells and surrounding somatic, granulosa cells. Other causes of POI include abnormal chromosome segregation, deficiency in DNA repair, insufficient hormonal synthesis or signaling, defects in pathways that affect folliculogenesis, oocyte maturation and/or ovulation.
Functional disruption at the level of the hypothalamus or pituitary glands leads to hypogonadotropic hypogonadism, small ovaries, amenorrhea, and infertility. Infertility can also result from fertilization failure, either due to oocyte maturation arrest and the inability to produce a haploid zygote or to a defective zona pellucida.
Embryo development is initially regulated by maternal transcripts that are synthetized and stored in the cytoplasm during oocyte maturation. The euploid maternal genome and the cytoplasmic components are essential for normal embryonic development. Other pathologies, such as disruption of reproductive tract development, endometriosis, uterine fibroids, polycystic ovary syndrome, or autoimmune factors, may have a negative impact on implantation and pregnancy, leading to infertility or recurrent pregnancy loss.
Numerous genetic studies in animal models have identified thousands of genes that are essential for mammalian reproduction. Only a small proportion (less than 200) of these genes shows strong association with human infertility. Recent applications of whole exome and genome sequencing to families with infertility, have led to unbiased approaches and discovered new genes and novel variants involved in human infertility. These family-based approaches and recent genome-wide association studies have unearthed a small number of human infertility genes, and more discoveries are expected in the near future.
X Chromosome and female infertility
In the normal ovary, the primordial germ cells carry two X chromosomes, one of which is initially inactivated similarly to any other somatic cell. Importantly, the second X chromosome is reactivated prior to meiosis as the presence of two transcriptionally active X chromosomes is essential for oogenesis [1, 2]. In females, both X chromosomes must be active during meiosis to pair efficiently like autosome homologues [3]. Moreover, ∼20% of X-linked genes escape inactivation and continue to be expressed from the inactive X chromosome, maintaining the dosage of female-specific transcripts in surrounding somatic cells [1–3].
In humans, chromosomal abnormalities such as monosomy X (Turner syndrome), cytogenetically visible deletions and duplications, and balanced and unbalanced X-autosome rearrangements are associated with an accelerated loss of primordial oocytes during female fetal development, resulting in streak gonads at birth [4, 5]. They account for close to 10% of cases of POI [6]. The mechanism of such massive germ cell loss is not well understood; however, some studies suggest the presence of a surveillance mechanism that may detect and eliminate cells with unpaired or unsynapsed chromatin, such as cells with monosomy X [7, 8]. It is also possible that accelerated germ cell depletion is caused by inadequate signaling from surrounding somatic granulosa cells that also carry a single X chromosome [9]. Failure in either dosage of X-linked genes or the X chromosome structural integrity can lead to POI [10]. Despite great importance of X chromosome in ovarian biology, only few X-linked genes have been implicated in ovarian function. BMP15, FMR1, and PGRMC1 genes are located on the human X chromosome and have been linked with ovarian development and POI. Structural X chromosome abnormalities, rather than gene-specific abnormalities, may be the major reason for germ cell loss.
Beside POI, X chromosome abnormalities such as deletions, duplications, inversions, complex rearrangements, X-autosome translocations, and single-gene sequence variants, carried by at least 5–6% of women, can lead to recurrent fetal losses [11, 12]. In females, X chromosome abnormalities may result in early embryonic or fetal lethality, particularly of male conceptuses. To date, ∼30% of X-linked genes are associated with a specific phenotype in humans, while pathogenic variants in a significant number of X-linked genes, such as BCOR, EBP, FLNA, HCCS, IKBKG, MECP2, OFD1, OTC, and REP1, are presumed to be male-lethal [12–14]. In addition, germline mosaicism is another explanation of infertility and recurrent miscarriages of affected fetuses with X-linked or autosomal dominant lethal alterations. Maternal germline mosaicism for pathogenic variants has been reported for male-lethal genes, including IKBKG [15], FLNA [16], and MECP2 [17], and is likely an under-recognized condition. Female fetuses can also be affected by male-lethal X-linked disorders in cases of skewed X chromosome inactivation.
Genetic causes of gonadal dysgenesis due to defects in sex determination
Sexual development includes two processes: “sex determination,” in which the undifferentiated gonad develops into a testis or ovary based on XY or XX chromosomal complement; and “sex differentiation,” which leads to sex-specific hormone production governing anatomical and psychological differences between males and females.
XY gonadal dysgenesis
Phenotypic females with defects in sex determination, also known as Swyer syndrome, commonly present as XY individuals with female-appearing external genitalia and Müllerian structures (uterus and fallopian tubes), but dysgenic and non-functional gonads, lack of spontaneous pubertal development, absent breast development, primary amenorrhea, and infertility [18–20]. Approximately 10–15% of individuals with XY gonadal dysgenesis have deletions or pathogenic sequence variants affecting the SRY gene on the Y chromosome. Other causes comprise Y chromosome structural alterations, mosaicism for a cell line with monosomy X, heterozygous deletions, and sequence variants in the NR5A1 gene, duplications in the Xp21 region involving NR0B1, WNT4 gene duplications, and deletions and loss-of-function pathogenic variants in the AR, MAP3K1, GATA4, DMRT1, DMRT2, ZNRF3, and DHH genes [21–27]. Disorders of sexual development are also often seen as a manifestation of other syndromic conditions.
XX gonadal dysgenesis
While many genes have been identified as key regulators of the testis developmental pathways, less is known about the mechanisms triggering ovarian development. Most of the current knowledge of key regulators involves genes predominantly expressed in the somatic compartment of the gonad. In XX individuals, the absence of SRY is not a default mechanism for ovarian differentiation and requires the activation of genes associated with female pathway development and suppression of male development factors [28–30]. Our knowledge of the genetic regulators of ovarian developments is based on studying animal models, as well as human families afflicted with ovarian dysgenesis. Animal, mainly mouse studies, imply numerous biological processes that are essential for ovarian determination, differentiation, and maintenance. Ovarian development is orchestrated by several transcription factors, including SOHLH1, SOHLH2, NOBOX, LHX8, FIGLA, GATA4, WNT4, WT1, LHX9, FOXL2, and NR5A1 [31–37], and comprises mitotic proliferation of XX primordial germ cells, their progression into meiosis, and the formation of primordial follicles (Figure 1). Mouse studies show that Sohlh1 and Sohlh2 affect differentiation of both male and female gametes, while Nobox, Lhx8, and Figla are gamete-specific genes known to regulate oogenesis, without affecting male germ cell differentiation. In humans, the gonadal development in females and males is identical within first four weeks after conception. In fact, orthologues of above mouse genes are present in the human genome. Heterozygous and biallelic pathogenic variants in SOHLH1, SOHLH2, NOBOX, FIGLA, WNT4, WT1, FOXL2, and NR5A1 genes were found in women with a wide spectrum of reproductive abnormalities, including isolated as well as syndromic conditions associated with complete gonadal dysgenesis and less severe forms of POI [37–40]. Phenotypic expression and severity of impaired gonadogenesis and oogenesis may depend on the type of alteration (loss of function vs. gain of function) and the effects on specific protein domains.
Defects in early oogenesis and chromosome segregation as cause of infertility
Once primordial germ cells cease proliferation, they become oogonia, and enter meiosis circa 12 weeks of human gestation. During the initial stages of meiosis, germ cells undergo a set of highly organized processes that include homologous chromosome pairing, alignment, synapsis, and crossover [41–43]. The accurate segregation of chromosomes involves multiple processes to establish a physical connection of the homologous chromosomes via centromere interaction and recombination, to ensure correct positioning (chromosome alignment) at the spindle equator at metaphase I and to control a proper meiotic chromosome segregation [44, 45]. During MI prophase, homologous chromosomes pair and undergo recombination, that consists of programmed DNA double-strand breaks (DSB) governed by SPO11 protein, a crossover event resulting in an exchange of large chromosomal segments, and a repair of DNA breaks. In humans, a total number of DSB repair events outnumber the count of crossover events by more than 10-fold. Cell cycle checkpoint responds to incorrectly repaired/unrepaired DNA breaks and to a non-disjunction of chromosomes by inducing cell cycle arrest and activating apoptotic programs.
Programmed cell death is a naturally occurring mechanism to eliminate abnormal, damaged or excess cells at every stage of oogenesis. The fetal ovary contains ∼7 million germ cells, only ∼400 000 (5%) of which will remain at puberty, and ∼400 oocytes (0.005% from the original germ cell pool) will ovulate during a female reproductive lifespan [46–48]. Accelerated apoptosis and disruption in signaling can lead to depletion of the primordial follicle pool, incomplete development of follicles, or failure of normal sexual differentiation, resulting in dysgenic or streak gonads.
Premature loss of germ cells is a common cause of human gonadal dysgenesis, primary amenorrhea, and infertility in human females with defects in genes implicated in germ cell mitosis and meiosis (Table 1) and genes important in chromosome segregation, such as BUB1B, STAG3, SYCE1, SYCP3, HFM1, PSMC3IP, and SGO2, as well as genes involved in DNA damage repair, such as MCM8, MCM9, XRCC4, ERCC6, and BRCA2 [49–60].
Table 1.
Genes implicated in non-syndromic POI, gonadal dysgenesis, and female infertility.
| Gene | Phenotype MIM/[ref] | Phenotype, reproductive outcomes | Genetic defects | Mode of inheritance |
|---|---|---|---|---|
| ANXA5 | #614391 | Susceptibility to recurrent pregnancy loss, RPRGL3 | Sequence variants in the promoter | AD |
| ATG7, ATG9A | [199] | Primary ovarian insufficiency | Sequence variants | AD |
| AXL | [200] | Hypogonadotropic hypogonadism with or without anosmia | Sequence variants | AD |
| BMP15 | #300510 | Ovarian dysgenesis, primary amenorrhea, POF4 | Sequence variants, deletions | X-linked |
| BUB1B | #176430 | Premature chromatid separation, recurrent pregnancy loss | Sequence variants | AD |
| CCDC141 | [116] | Kallmann syndrome | Sequence variants | AR |
| CPEB1 | [201, 202] | Primary ovarian insufficiency | Deletions | AD |
| DIAPH2 | #300511 | Primary or secondary amenorrhea, POF2A | Sequence variants, deletions | X-linked |
| DUSP6 | #615269 | Hypogonadotropic hypogonadism HH19 with or without anosmia | Sequence variants | AD |
| EIF4ENIF1 | [115] | Primary ovarian insufficiency | Sequence variants | AD |
| ERCC6 | #616946 | Primary ovarian insufficiency, POF11 | Sequence variants | AD |
| ESR1 | #615363 | Estrogen resistance, primary amenorrhea | Sequence variants | AR |
| ESR2 | #618187 | Ovarian dysgenesis, ODG8, primary amenorrhea | Sequence variants | AD |
| F2 | #614390 | Susceptibility to recurrent pregnancy loss, RPRGL2 | Sequence variants | AD |
| F5 | #614389 | Susceptibility to recurrent pregnancy loss, RPRGL1 | Sequence variants | AD |
| FEZF1 | #616030 | Hypogonadotropic hypogonadism HH22 with or without anosmia | Sequence variants | AR |
| FGF17 | #615270 | Hypogonadotropic hypogonadism HH20 with or without anosmia | Sequence variants | AD |
| FGF8 | #612702 | Hypogonadotropic hypogonadism HH6 with or without anosmia | Sequence variants | AD |
| FGFR1 | #147950 | Hypogonadotropic hypogonadism HH2 with or without anosmia | Sequence variants | AD |
| FIGLA | #612310 | Ovarian insufficiency, POF6 | Sequence variants | AD, AR |
| FLRT3 | #615271 | Hypogonadotropic hypogonadism HH21 with or without anosmia | Sequence variants | AD |
| FMR1 | #311360 #300869 |
Primary ovarian insufficiency, POF1 | Expansion of trinucleotide (CCG)n repeats, duplication | X-linked |
| FSHB | #229070 | Ovarian insufficiency, hypogonadotropic hypogonadism HH24 | Sequence variants | AR/AD |
| FSHR | #233300 | Ovarian dysgenesis, primary amenorrhea, ODG1 | Sequence variants | AR |
| GDF9 | #618014 | Primary ovarian insufficiency, POF14 | Sequence variants, duplication | AR, digenic |
| GNRH1 | #614837 | Hypogonadotropic hypogonadism HH12 with or without anosmia | Sequence variants | AR |
| GNRHR | #146110 | Hypogonadotropic hypogonadism HH7, primary amenorrhea | Sequence variants | AR |
| HAX1 | [203] | Primary ovarian insufficiency, short stature | Sequence variants | AD |
| HFM1 | #615724 | Ovarian dysgenesis, ovarian insufficiency, POF9 | Sequence variants | AR |
| HS6ST1 | #614880 | Hypogonadotropic hypogonadism HH15 with or without anosmia | Sequence variants | AD |
| IL17RD | #615267 | Hypogonadotropic hypogonadism HH18 with or without anosmia | Sequence variants | AD, AR |
| KHDC3L | [166] | Preimplantation embryonic lethality | Sequence variants | AR |
| KISS1 | #614842 | Hypogonadotropic hypogonadism HH13 with or without anosmia | Sequence variants | AR |
| KISS1R | #614837 | Hypogonadotropic hypogonadism, HH8, primary amenorrhea | Sequence variants | AR |
| LHB | #228300 | Hypogonadotropic hypogonadism, HH23 | Sequence variants | AR |
| LHCGR | #238320 | Luteinizing hormone resistance, amenorrhea, and infertility | Sequence variants | AR |
| LHX8 | [99] | Ovarian dysgenesis, ovarian insufficiency | Sequence variants | AD |
| MCM8 | #236700 | Ovarian dysgenesis, primary amenorrhea, POF10 | Sequence variants | AR |
| MCM9 | #616185 | Ovarian dysgenesis, primary amenorrhea, ODG4 | Sequence variants | AR |
| MRPS22 | #618117 | Ovarian dysgenesis, ODG7, primary amenorrhea | Sequence variants | AR |
| MSH5 | #617442 | Primary ovarian insufficiency, POF13 | Sequence variants | AR |
| NLRP2, NLRP5 | [160] | Primary infertility, Oocyte maturation defect, | Sequence variants | AR |
| NOBOX | #611548 | Ovarian dysgenesis, primary ovarian insufficiency, POF5 | Sequence variants | AD, AR |
| NR5A1 | #612964 | Gonadal dysgenesis, ovotesticular DSD, secondary ovarian insufficiency, POF7 | Del/dup, Sequence variants | AD |
| NSMF | #614838 | Hypogonadotropic hypogonadism HH9 with or without anosmia | Sequence variants | AD |
| NUP107 | #618078 | Ovarian dysgenesis, ODG6, primary amenorrhea | Sequence variants | AR |
| PADI6 | #617234 | Preimplantation embryonic lethality, PREMBL12 | Sequence variants | AR |
| PATL2 | #617743 | Primary infertility, oocyte maturation defect, OOMD4, early embryonic arrest | Sequence variants | AR |
| POF1B | #300604 | Primary ovarian insufficiency, POF2B | Sequence variants | X-linked |
| POLR3H | [204] | Primary ovarian insufficiency | Sequence variants | AR |
| PROK2 | #610628 | Hypogonadotropic hypogonadism HH4 with or without anosmia | Sequence variants | AD |
| PROKR2 | #244200 | Hypogonadotropic hypogonadism HH3 with or without anosmia | Sequence variants | AD |
| PRLR | #615555 | Hyperprolactinemia, amenorrhea | Sequence variants | AR |
| PSMC3IP | #614324 | Ovarian dysgenesis, ODG3, primary amenorrhea | Sequence variants | AR |
| REC8 | [99] | Ovarian dysgenesis, ovarian insufficiency | Sequence variants | unknown |
| SALL4 | [205] | Primary ovarian insufficiency | Sequence variants | AD |
| SEMA3A | #614897 | Hypogonadotropic hypogonadism HH16 with or without anosmia | Sequence variants | AD |
| SGO2 | [58] | Primary ovarian insufficiency | Sequence variants | AR |
| SLC29A3 | #602782 | Histiocytosis-lymphadenopathy plus syndrome | Sequence variants | AR |
| SMC1B | [99] | Ovarian dysgenesis, ovarian insufficiency | Sequence variants | unknown |
| SOHLH1 | #617690 | Ovarian dysgenesis, ODG5, primary ovarian insufficiency | Sequence variants | AR |
| SOHLH2 | #616066 | Primary ovarian insufficiency | Sequence variants | AD |
| SOX8 | [192] | Ovarian dysgenesis, primary or secondary amenorrhea | Sequence variants | AD |
| SPIDR | [206] | Ovarian dysgenesis | Sequence variants | AR |
| SPRY4 | #615266 | Hypogonadotropic hypogonadism HH17 with or without anosmia | Sequence variants | AD |
| STAG3 | #615723 | Ovarian dysgenesis, ovarian insufficiency, POF8 | Sequence variants | AR |
| SYCE1 | #616947 | Ovarian dysgenesis, ovarian insufficiency POF12 | Sequence variants | AR |
| SYCP3 | #270960 | Susceptibility to recurrent pregnancy loss, RPRGL4 | Sequence variants, deletions | AD |
| TAC3 | #614839 | Hypogonadotropic hypogonadism HH10 with or without anosmia | Sequence variants | AR |
| TACR3 | #614840 | Hypogonadotropic hypogonadism HH11 with or without anosmia | Sequence variants | AR |
| TLE6 | #616814 | Preimplantation embryonic lethality, PREMBL1 | Sequence variants | AR |
| TUBB8 | #616780 | Primary infertility, oocyte maturation defect, OOMD2 | Sequence variants (de novo, paternal transmission) | AD, AR |
| WDR11 | #614858 | Hypogonadotropic hypogonadism HH14 with or without anosmia | Sequence variants | AD |
| WEE2 | #617996 | Primary infertility, oocyte maturation defect, OOMD5 | Sequence variants | AR |
| WNT4 | #158330 | Müllerian aplasia, hyperandrogenism, primary amenorrhea | Sequence variants | AD |
| ZP1 | #615774 | Primary infertility, oocyte maturation defect, OOMD1 | Sequence variants | AR |
| ZP2 | [150] | Primary infertility | Sequence variants | AR, digenic |
| ZP3 | #617712 | Primary infertility, oocyte maturation defect, OOMD3 | Sequence variants (de novo, paternal transmission) | AD |
There is compelling evidence that infertile women have a higher incidence of aneuploid oocytes [61, 62]. Rare deleterious alterations in genes required for chromosome synapsis and segregation during meiosis, such as SYCP3 [49], STAG3 [50], HFM1 [51], SYCE1 [52, 53], NUP107 [54, 55], PSMC3IP [56, 57], SGO2 [58], REC8 [59], SMC1B [59], and BUB1B [60], are the cause of meiotic disturbances, aneuploidy, streak gonads, primary ovarian insufficiency, embryonic arrest, recurrent miscarriage, risk of affected offspring with chromosomal abnormalities, and infertility (Table 1). Meiosis-specific cohesin complexes consist of two structural proteins (SMC1α/SMC1β and SMC3), encoded by SMC1A and SMC1B, respectively; an α-kleisin protein (RAD21, RAD21L, or REC8); and a stromal antigen protein (STAG1, STAG2, or STAG3) [63]. Pathogenic variants in REC8, SMC1B, and STAG3 are implicated in human infertility [50, 58]. Defects in assembly of the meiotic cohesion and/or synaptonemal complex lead to multi-chromosome aneuploidy during the second meiotic division, resulting in follicle atresia. Such genetic defects have been observed in patients with idiopathic infertility and recurrent miscarriages.
Female meiosis is highly prone to chromosome segregation errors, particularly in older women, resulting in a high proportion of aneuploid zygotes and at least 10% of aneuploid human fetuses [64–66]. The gradual loss of cohesins or cohesin-related proteins such as SGO2, has been implicated in meiotic non-disjunction and age-related aneuploidy and infertility [67]. The BUB1B gene encodes a mitotic checkpoint serine/threonine kinase B, a protein that plays an important role in regulation of the spindle-assembly checkpoint. Heterozygous and biallelic alterations in BUB1B are rare findings among fetuses and liveborn individuals affected by mosaic variegated aneuploidy [68, 69]. Similarly, chromosome mis-segregations are observed in patients with homozygous pathogenic variants in the CEP57 gene [70, 71]. Impaired spindle checkpoint function results in aneuploidies involving multiple different chromosomes and is likely to cause oocyte maturation arrest, early embryonic lethality, or miscarriage [60, 72]. The presumed inheritance of these genes is listed in Tables 1 and 2.
Table 2.
Syndromic conditions accompanied by ovarian insufficiency and female infertility.
| Gene | Phenotype MIM/[ref] | Phenotype, reproductive outcomes | Genetic defects | Mode of inheritance |
|---|---|---|---|---|
| AARS2 | #615889 | Leukoencephalopathy, progressive, with ovarian failure | Sequence variants | AR |
| AIRE | #340300 | Autoimmune polyendocrinopathy syndrome | Sequence variants | AD, AR |
| ANOS1 | #308700 | Kallmann syndrome | Sequence variants, deletions | X-linked |
| AR | #300068 | Androgen insensitivity, ovarian dysgenesis, XY females | Sequence variants, deletions | X-linked |
| ATM | #208900 | Ataxia-telangiectasia | Sequence variants, deletions | AR |
| BRCA2 | [73] | Ovarian dysgenesis, microcephaly, cancer predisposition | Sequence variants | AR |
| CCDC39 | #613807 | Ciliary dyskinesia, recurrent respiratory infections, infertility | Sequence variants | AR, digenic |
| CHD7 | #214800 | CHARGE syndrome, hypogonadotropic hypogonadism, ovarian dysgenesis | Sequence variants, deletions | AD |
| CLPP | #614129 | Perrault syndrome 3, progressive hearing loss, primary ovarian insufficiency | Sequence variants | AR |
| CYP17A1 | #202110 | Congenital adrenal hyperplasia due to 17-hydroxylase deficiency | Sequence variants, deletions | AR |
| CYP21A2 | #201910 | Congenital adrenal hyperplasia due to 21-hydroxylase deficiency | Sequence variants, deletions | AR |
| DCAF17 | #241080 | Hypogonadism, ovarian dysgenesis, partial alopecia, Woodhouse–Sakati syndrome | Sequence variants | AR |
| DDX11 | #613398 | Warsaw breakage syndrome | Sequence variants | AR |
|
EIF2B1/B2/
B3/B4/B5 |
#603896 | Leukoencephalopathy with vanishing white matter, gonadal dysgenesis, primary or secondary amenorrhea | Sequence variants | AR |
| ERAL1 | #617565 | Perrault syndrome 6, progressive hearing loss, primary ovarian insufficiency | Sequence variants | AR |
| ERCC2 | #601675 | Trichothiodystrophy 1, brittle, sulfur-deficient hair, ichthyosis, developmental disabilities, decreased fertility, short stature, infections | Sequence variants | AR |
| ERCC3 | #616390 | Trichothiodystrophy 2 | Sequence variants | AR |
| FANCA, multiple | #227650 | Fanconi anemia | Sequence variants, deletions | AR |
| FOXL2 | #110100 | Blepharophimosis with ovarian dysgenesis, POF3 | Sequence variants, deletions, duplications, translocations | AD |
| GALT | #230400 | Galactosemia, hypoglycemia, renal tubular dysfunction, muscle hypotonia, hypogonadism | Sequence variants, deletions | AR |
| H6PD | #604931 | Cortisone reductase deficiency 1, hirsutism, oligomenorrhea, infertility | Sequence variants | AR |
| HARS2 | #614926 | Perrault syndrome 2, sensorineural deafness, primary amenorrhea, streak gonads | Sequence variants | AR |
| HSD11B1 | #614662 | Cortisone reductase deficiency 2, oligomenorrhea, infertility | Sequence variants | AD |
| HSD17B4 | #233400 | Perrault syndrome 1, progressive hearing loss, primary ovarian insufficiency | Sequence variants | AR |
| KHDC3L | #614293 | Hydatidiform mole, recurrent pregnancy loss, gestational trophoblastic neoplasia | Sequence variants | AR |
| LARS2 | #615300 | Perrault syndrome 4, progressive hearing loss, primary ovarian insufficiency | Sequence variants | AR |
| LMNA | #151660 | Lipodystrophy, familial partial, type 2, abnormal distribution of subcutaneous fat, polycystic ovary syndrome, infertility, spontaneous abortions, fetal death | Sequence variants | AD |
| LMNA | #212112 | Malouf syndrome, cardiomyopathy, skeletal anomalies, amenorrhea | Sequence variants | AD |
| MEI1 | [172] | Hydatidiform mole, recurrent pregnancy loss, gestational trophoblastic neoplasia | ||
| MKKS | #236700 | McKusick-Kaufman syndrome, hypogenitalism, hydrometrocolpos | Sequence variants | AR |
| NBN | #251260 | Nijmegen breakage syndrome, primary amenorrhea, microcephaly and immunodeficiency | Sequence variants | AR |
| NLRP7 | #231090 | Hydatidiform mole, recurrent pregnancy loss, gestational trophoblastic neoplasia | Sequence variants, deletions | AR |
| NR0B1 | #300018 | 46,XY sex reversal 2, dosage-sensitive | Duplications | X-linked |
| NR3C1 | #615962 | Glucocorticoid resistance, infertility, male-pattern baldness, hirsutism, and menstrual irregularities | Sequence variants | AD |
| PGM1 | #614921 | Congenital disorder of glycosylation, cleft lip and bifid uvula, hepatopathy, hypoglycemia, short stature, and exercise intolerance, rhabdomyolysis, dilated cardiomyopathy, and hypogonadotropic hypogonadism | Sequence variants | AR |
| PMM2 | #212065 | Ovarian dysgenesis, cerebellar atrophy, mental impairment, pigmentary retinopathy | Sequence variants | AR |
| PNPLA6 | #215470 | Boucher-Neuhauser syndrome, pinocerebellar ataxia, hypogonadotropic hypogonadism, visual impairment | Sequence variants | AR |
| POLG | #157640 | Progressive external ophthalmoplegia, amenorrhea | Sequence variants | AD |
| PPARG | #151660 | Lipodystrophy, familial partial, type 3, insulin-resistant diabetes mellitus, infertility | Sequence variants | AD |
| PROP1 | #262600 | Combined pituitary hormone deficiency, growth failure | Sequence variants, deletions | AR |
| RCBTB1 | [119] | Retinitis pigmentosa, goiter, primary ovarian insufficiency, intellectual disability | Sequence variants | AR |
| REC114 | [172] | Hydatidiform mole, recurrent pregnancy loss, gestational trophoblastic neoplasia | Sequence variants | AR |
| RECQL2 | #210900 | Werner syndrome | Sequence variants | AR |
| RECQL3 | #277700 | Bloom syndrome | Sequence variants | AR |
| RNF216 | #212840 | Cerebellar ataxia and hypogonadotropic hypogonadism | Sequence variants, digenic | AR |
| SLC29A3 | #602782 | Histiocytosis-lymphadenopathy plus syndrome, joint deformities, sensorineural hearing loss, and subsequent development of generalized lymphadenopathy and swellings in the eyelids, hypogonadotropic hypogonadism, amenorrhea | Sequence variants | AR |
| SOX10 | [117] | Kallmann syndrome, deafness | Sequence variants | AD |
| TOP6BL | [172] | Hydatidiform mole, recurrent pregnancy loss, gestational trophoblastic neoplasia | Sequence variants | AR |
| TP63 | #603543 | Limb-mammary syndrome, hand/foot anomalies and hypoplasia/aplasia of the mammary gland, amenorrhea, absence of the uterus and ovaries | Sequence variants | AD |
| TRIM37 | #253250 | Mulibrey nanism, gonadal dysgenesis | Sequence variants | AR |
| TWNK | #616138 | Perrault syndrome 5, progressive hearing loss, primary ovarian insufficiency | Sequence variants | AR |
| WT1 | #256370 | Nephrotic syndrome, gonadal dysgenesis | Sequence variants | AD |
| XPA, XPC, ERCC2, ERCC3, ERCC4 | #278700 | Xeroderma pigmentosum | Sequence variants | AR |
| XRCC4 | #616541 | Short stature, microcephaly, and endocrine dysfunction, ataxia | Sequence variants | AR |
DNA repair genes and their role in POI and human female infertility
Many studies implicate DNA repair genes in follicle maturation and quality, reproductive aging, and subsequent age of menopause onset [73–84]. Beside their role in meiosis, many DNA damage response genes also play essential roles in cell cycle control during mitosis. Diversity of DNA repair mechanisms, differential expression in various tissues, existence of the alternative tissue-specific DNA repair pathways, incomplete penetrance, environmental exposures, and other risk factors are the explanations for phenotypic variability in patients with defects in DNA repair genes. Thus, affected individuals may present with a spectrum phenotype manifesting as a syndromic condition or isolated tissue-specific phenotypes. Biallelic defects in DNA repair genes are the cause of syndromic conditions such as Fanconi anemia (FANCA, FANCB, FANCC, BRCA2, FANCD2, FANCE, FANCF, XRCC9, FANCI, BRIP1, PHF9, PALB2, RAD51C, SLX4, ERCC4, RAD51, BRCA1, UBE2T, XRCC2, MAD2L2, RFWD3), Werner syndrome (RECQL2), Warsaw breakage syndrome (DDX11), LIG4 syndrome (LIG4), Bloom syndrome (BLM), Nijmegen breakage syndrome (NBN), ataxia-telangiectasia (ATM), trichothiodystrophy 1 (ERCC2, ERCC3), and xeroderma pigmentosum (XPA, XPC, ERCC2, ERCC3, ERCC4). These syndromes are rare genetic conditions characterized by susceptibility to chromosome breakage and genome instability, triggering permanent cell cycle arrest, and apoptosis of the affected cells. As a result, patients with pathogenic variants present with growth retardation, skin lesions, bone marrow and immune deficiencies, neurodevelopmental problems, endocrine and early gonadal dysfunction, and predisposition to cancers.
There are also a number of patients with biallelic variants in DNA repair genes such as MCM8, MCM9, XRCC4, and MSH5 who present with a non-syndromic primary ovarian insufficiency [78–83]. Homozygous pathogenic variants in the MCM8 or MCM9 genes were discovered in women with hypergonadotropic primary amenorrhea, hypothyroidism, growth retardation, and absent or very small ovaries. None of the affected women had cancer at the time of investigation; however, excessive chromosomal breakage was observed in their somatic cells [78, 79]. A single report indicated an affected family with MCM9 pathogenic variants in association with primary hypergonadotropic hypogonadism with early-onset colorectal cancer [84]. The heterozygous mutation carriers appeared healthy and fertile, although they also had an increased number of chromosome breakages in comparison to wildtype MCM8 and MCM9 individuals. Interestingly, population-based genome-wide studies found the strongest association with a non-synonymous single nucleotide polymorphism (rs16991615) in coding exon 9 of the MCM8 gene and the age of menopause in Caucasian, European, African American [85], and Hispanic women. These results indicate that MCM8, and probably other DNA repair genes, regulate the reproductive span from gonadal formation to menopause, and that heterozygous variants may confer risk loci for primary ovarian insufficiency, diminished ovarian reserves, and infertility [85]. Variants in MCM8 also correlate with antral follicle count [86]. The mechanisms of MCM8 and MCM9 action are currently under intensive study. Both MCM8 and MCM9 are members of a complex that is rapidly recruited at the DNA damage sites to resolve double-strand breaks [87]. During meiosis, programmed double-strand breaks are generated to attain accurate segregation and crossover of homologous chromosomes. To maintain germ cells’ genomic integrity and prevent DNA from possible damage, breaks must be repaired via a homologous recombination-mediated mechanism. Inability to efficiently repair chromosomal ends activates cell apoptosis, leading to depletion of germ cells and dramatic loss of ovarian reserves [75, 87]. It is possible that deficiency in MCM8/MCM9 leads to oocyte loss due to unrepaired double-strand DNA breaks.
Abnormal folliculogenesis and female infertility
The number of primordial follicles at birth, their quality, and the rate of germ cell depletion are the factors that define reproductive lifespan in women [88–91]. In some women, oocyte depletion occurs during the fetal stages of oogenesis, endowing them with a relatively small pool by the time of puberty. In others, there is accelerated loss of oocytes after the establishment of the primordial follicle pool, and can be due to environmental exposures such as cigarette smoking. Elimination of oocytes is accelerated with maternal age and is related to deterioration of oocyte quality. In fact, about 40–60% of oocytes from women of 40 years are aneuploid [92], while the oocyte quality of younger women with diminished ovarian reserve are unknown.
Diminished ovarian reserve (DOR) is another term used for women with a relatively low antral follicle count on ultrasound examinations (<8) and low AMH levels and affects 10% of women undergoing infertility treatment [93]. DOR is more prevalent than POI but, in biological reality, may very well have similar genetic underpinnings, except that less penetrant mutations may be more frequent in DOR individuals.
Folliculogenesis involves proliferation and differentiation of granulosa cells and maturation of the oocyte. This process includes a series of signaling events between the cells within the follicle. Although the granulosa and theca cells have long been implicated in ovarian function, animal studies have revealed a synergistic effect of GDF9 and BMP15 on granulosa cell differentiation, folliculogenesis, and ovulation [94–97]. BMP15 and GDF9, members of the transforming growth factor-beta (TGFβ) superfamily, are expressed in oocytes through folliculogenesis and are involved in a cascade of regulatory processes directing granulosa cell proliferation, steroidogenesis, cumulus expansion, and apoptosis. GDF9 has been implicated in the transition from the primary to the secondary follicle stage [96]. BMP15 stimulates follicle maturation during the gonadotropin-independent phase and regulates granulosa cell sensitivity to follicle-stimulating hormone (FSH). It has been observed that a high level of GDF9 and BMP15 in the follicle is associated with good embryo morphology. Human GDF9 and BMP15 gene variants, mostly heterozygous, have been detected in up to 10% of women with hypergonadotropic ovarian failure, POI, primary and secondary amenorrhea, and polycystic ovary syndrome [94, 98, 99]. However, more convincing functional studies are needed to determine the true role of these variants in female infertility.
Multiple oocyte-specific transcription factors, including FIGLA, NOBOX, LHX8, SOHLH1, and SOHLH2, also control follicular development and play a role in cytoplasmic maturation by regulating the expression of maternal-effect factors essential for future embryonic activation [28, 30, 100]. Heterozygous and homozygous sequence variants in these transcription factors were found to be associated with ovarian dysgenesis and POI [37, 101–109]. In mice, these genes cause phenotypes in autosomal recessive fashion, and this is likely to be true in humans, but further studies are necessary to determine whether heterozygous variants in these genes can cause pathology.
Pleotropic and syndromic conditions resulting in POI and female infertility
Expansion of CGG repeats in the 5’UTR of the FMR1 gene is another genetic condition strongly associated with diminished ovarian reserve and primary ovarian insufficiency, as the FMR1 protein is expressed in neurons and granulosa cells. FMR1 is an mRNA-binding protein that also regulates translation. Fragile X intellectual disability syndrome is caused by inactivation of the FMR1 gene, located on the X chromosome, mainly due to DNA hypermethylation of the promoter and the CGG repeat region when the repeat size exceeds 200. Full mutation alleles arise from premutation alleles with 55–200 CGG repeats that demonstrate a repeat length instability and a tendency to expand from one generation to the next. The prevalence of premutation alleles in the general population is estimated to be 1 per 150–300 females, with the highest incidence, 1 per 100 females, in Colombia and Israel [110, 111].
Interestingly, women with 55–200 CGG repeats (premutation alleles) have elevated mRNA expression and decreased protein level posing a significant risk for premature ovarian aging and infertility, while females with over 200 CGG repeats, full-mutation carriers, do not have any form of ovarian dysfunction. FMR1 premutation alleles confer risk for adult neurodegenerative disorder in males as well as POI in females. A phenomenon in which a pathogenic variant is the cause of multiple apparently unrelated conditions has been defined as pleiotropy. Such effects can be explained by the failure of a single molecular function involved in multiple biological processes, variably affecting cellular physiology. Premutation carriers were noted to have significantly elevated FSH correlated with increasing CGG repeats and decreased inhibin B/A, progesterone, and antimülerian hormone levels.
The exact mechanism of oocyte toxicity in women with FMR1 premutation alleles is unknown, although mRNA overexpression may result in RNA/protein gain-of-function disorder affecting follicular maturation, as well as increasing the degree of follicular apoptosis [112–114]. Another gene, eukaryotic translation initiation factor 4E nuclear import factor 1 (EIF4ENIF1) has been recently implicated in ovarian germ cell development and primary ovarian insufficiency [115]. Defects in eIF4ENIF1 may result in decreased mRNA degradation and increased mRNA stability, similar to the mechanism of fragile X primary ovarian insufficiency.
A significant number of syndromic conditions, such Perrault syndrome (HSD17B4, HARS2, CLPP, LARS2, C10ORF2), blepharophimosis with ptosis, and epicanthus inversus syndrome (FOXL2); leukoencephalopathy with vanishing white matter due to pathogenic variants in the EIF2B1, EIF2B2, EIF2B4, and EIF2B5 genes; galactosemia and carbohydrate-deficient glycoprotein syndromes with pathogenic variants in GALT and PMM2 genes, and many others (Table 2) have ovarian dysgenesis and/or primary ovarian insufficiency among their clinical manifestations [116–122].
Mitochondria and female infertility
Defects in the POLG gene, which encodes polymerase gamma, responsible for mitochondrial DNA synthesis, are the cause of progressive external ophthalmoplegia and are also reported in multiple women with POI [123–124]. The association of nuclear and mitochondrial genes responsible for mitochondria function with female oogenesis and fertility is not surprising as the number of mitochondria increases at least 1000-fold in human oocytes [125–126]. Mitochondria are essential for multiple processes during oogenesis, such as ATP production, apoptosis, and calcium homeostasis [127]. Mitochondrial dysfunction can lead to defects in oocyte maturation, impaired spindle assembly and chromosome missegregation, poor pre-implantation development, and implantation failure [124]. Mitochondrial inheritance is exclusively maternal and is considered to be a key determinant of female reproductive aging and infertility [128]. In addition to POLG, a number of nuclear genes regulate mitochondrial function, such as the genes responsible for Perrault syndrome (HARS2, HSD17B4, LARS2). The clinical significance of other genes involved in mitochondrial DNA maintenance, repair, and response to oxidative stress, such as RAD51, RAD51C, and XRCC3, remains to be established.
Deficiencies in hormonal signaling and response in female infertility
The gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), play a pivotal role in regulation of folliculogenesis, oocyte maturation, ovulation, implantation, and estradiol synthesis [129–130]. Recruitment of primordial follicles is a gonadotropin-independent process, while subsequent follicle growth is gonadotropin-sensitive. Upon binding to the receptors, gonadotropins stimulate oocyte development from early antral stage to maturation and convert androgen to estrogen in granulosa cells. FSH is a pituitary glycoprotein hormone composed of an alpha chain (CGA) that is similar to other pituitary glycoproteins such as LH, chorionic gonadotropin, and thyroid-stimulating hormone, and a beta chain encoded by FSHB. Patients with pathogenic variants in the FSHB (FSH beta subunit) and the FSHR (FSH receptor) genes develop follicles up to preantral stages, but further maturation is blocked. Defects in FSHB lead to a low production of FSH, resulting in hypogonadotropic hypogonadism [131, 132], whereas changes in FSHR cause hypergonadotropic hypogonadism [133–135]. The severity of the phenotype in patients with variations in the FSHR gene correlates with the effect of the genetic change in the FSHR function. Pathogenic variants in FSHR, specifically the c.566C > T change, are more frequent in a Finnish population [133], but are rare in other ethnic groups [133, 134].
Hypogonadotropic hypogonadism is a rare disorder characterized by the deficiency of gonadotropin-releasing hormone (GnRH) due to its impaired production, secretion, or function. It often manifests as incomplete or absent puberty and infertility. GnRH acts via the GnRH receptor, which affects both synthesis and release of LH and FSH, which in turn, control gonadal maturation, which then feeds back via Inhibin and Activin to complete the hypothalamic–pituitary–gonadal axis [136]. A considerable proportion of patients affected by hypogonadotropic hypogonadism have genetic changes leading to an isolated or a syndromic condition with X-linked, autosomal recessive, and autosomal dominant inheritance. Hypogonadotropic hypogonadism [137] is a genetically heterogeneous condition with both sporadic and familial cases and more than 25 causative genes identified to date (Table 2). The most commonly affected genes are ANOS1 (KAL1), SOX10, IL17RD, FGFR1, CHD7, PROKR2, GNRHR, and TACR3, while pathogenic variants in AXL, DMXL2, FEZF1, FGF17, FGF8, GNRH1, HESX1, HS6ST1, KISS1, KISS1R, LEP, LEPR, NR0B1, NSMF, OTUD4, PCSK1, PNPLA6, PROK2, RNF216, SEMA3A, SEMA7A, TAC3, and WDR11 are less frequent. Kallmann syndrome is characterized by a defective sense of smell in approximately 50% of patients with hypogonadotropic hypogonadism. The presence of anosmia should alert clinicians for a possible congenital hypogonadotropic hypogonadism and facilitate the search for a definitive molecular diagnosis in affected patients.
Genetics of polycystic ovaries
Development of preovulatory follicles depends greatly on regulation by a number of factors, such as FSH, oocyte-secreted proteins, and insulin-like growth factors [130]. Mature oocytes are surrounded by granulosa and thecal cells, which produce an androgen substrate, a prerequisite for ovarian estrogen biosynthesis. Importantly, hormonal imbalances related to ovarian factors or to systemic hormonal dysregulation is a frequent cause of POI as well as polycystic ovary syndrome (PCOS).
PCOS is the most common form of female infertility, affecting about 10% of women of reproductive age, and is associated with poor conception rates and high (30–50%) pregnancy loss rates. Multiple studies have established a strong correlation between enlarged polycystic ovaries and obesity, hirsutism, and amenorrhea. Hyperplasia of theca and stromal cells, elevated testosterone and LH, and low estradiol and FSH levels have been observed in women with hirsutism and oligomenorrhea [137–140]. Genomic changes in the FSHR, BMP15, and LHCGR genes as well as noncoding RNAs have been proposed to disturb a fine balance between LH and FSH hormonal stimulation of the thecal and granulosa cell compartments, leading to excessive androstenedione and low estradiol concentration in follicles.
LHCGR is a receptor for both LH and chorionic gonadotropin. LHCGR variants have been described in patients with non-syndromic infertility, cystic ovaries, empty follicles, and primary and secondary amenorrhea [141, 142]. The LHCGR c.935G > A polymorphism, promoter hypomethylation, and reduced expression of LHCGR were observed in women with PCOS; however, the molecular causes of PCOS remain to be elucidated.
Genome wide association studies (GWAS) studies have been very helpful in identifying novel loci that play important roles in human reproduction. A GWAS among Han Chinese women with PCOS identified eight highly significant loci. Among these are three novel loci that contain LH–choriogonadotropin receptor (LHCGR), thyroid adenoma-associated gene (THADA), and differentially expressed in normal and neoplastic cells (DENN)–MAPK activating death domain (MADD) containing 1A (DENND1A). THADA and DENND1A loci associations have been replicated in some of the European cohorts [139]. Eight additional loci were identified in a follow up study [143]. Further functional studies are necessary to fully understand the role of these genes in human reproductive pathology.
Other endocrine disturbances
Endocrine disorders such as diabetes, hypothyroidism, conditions affecting the adrenal glands, or hypothalamus are frequently accompanied by delay or lack of development of secondary sexual characteristics, irregular menstrual cycles, or amenorrhea. Females affected by congenital endocrinopathies, such hyperprolactinemia (PRLR), combined pituitary hormone deficiency (PROP1); autoimmune polyendocrinopathy syndrome (AIRE) frequently present with primary ovarian insufficiency and infertility (Table 2). It is also important to point out that pathogenic variants in DNA damage repair genes can affect endocrine function, and sometimes endocrine dysfunction can be the first presenting sign of a more serious condition such as Fanconi anemia [144].
Genetic causes of fertilization failure
Ovulation in mammals is regulated by a cyclic secretion of LH. Eggs surrounded by the zona pellucida and somatic cumulus cells may be fertilized by a capacitated sperm. Sperm capacitation is a complex process, comprising structural and molecular changes to the acrosome, that happen to the spermatozoa residing within the female reproductive tract [145]. Female-specific factors that affect sperm capacitation and the sperm's ability to recognize and penetrate through the cumulus cell layer and zona pellucida are unknown, but genetic defects in genes responsible for this process are likely to cause fertilization failure and infertility. These patients present with primary infertility despite normal ovarian reserve, regular menstrual cycles, and normal sex hormone levels.
The zona pellucida, an extracellular matrix surrounding the developing oocyte, contains four types of glycoprotein, ZP1, ZP2, ZP3, and ZP4. During oocyte development, the zona pellucida can be first observed in primary follicles as an external coat that physically separates the oocyte from the surrounding granulosa cells. The zona pellucida plays an important role in protection of the growing oocyte, oocyte-follicle cell interactions, and sperm recognition and binding [146]. Right after fertilization, the zona pellucida undergoes changes which prevent polyspermy, support blastocyst development, and preclude premature implantation.
Nucleotide sequence variations in the human zona pellucida genes may cause defects in the formation of zona pellucida and subsequent infertility. An autosomal recessive inheritance has been documented in women with loss-of-function ZP1 variants and absence of the zona pellucida around their oocytes [147] and in women with pathogenic sequence variants in ZP2 and dysfunctional zona pellucida [148]. Empty follicle syndrome has been reported to cause infertility in women with heterozygous pathogenic variants in the ZP3 gene [149]. Oocyte retrieval after an IVF treatment cycle showed a highly disorganized follicle content with absent or degenerated oocytes lacking zonae pellucidae. ZP3 expression is limited to oocytes; thus, the infertile phenotype is limited to females who have a de novo alterations or inherit pathogenic heterozygous variants from their fathers [148]. Digenic inheritance of two inherited variants in the ZP2 and ZP3 genes can also result in oocyte maturation defects and infertility [150].
In humans, ZP2, ZP3, and ZP4 glycoproteins form long filaments connected by ZP1, providing an interactive oocyte-granulosa cell environment. In the absence of a functional zona pellucida, intercellular interaction between the developing oocyte and granulosa cells is interrupted, resulting in oocyte degeneration prior to ovulation. During fertilization, the zona pellucida glycoproteins serve as sperm receptors, providing highly species-specific cell recognition and binding. To date, female-specific alterations leading to inability of sperm binding are unknown, but males with defects in zona pellucida binding proteins may produce dysmorphic spermatozoa with incompetence or reduced ability to penetrate the zona pellucida. Successful interaction between a single sperm and the zona pellucida as well as the activation of a blocking mechanism that prevents the fusion of additional sperm with the egg is critical for fertilization. Polyspermic fertilization, which leads to the formation of non-viable polyploid embryos, is prevented by the release of zinc ions and an enzyme ovastacin (MIM* 608860) stored in egg cortical granules to immobilize egg-surrounding spermatozoa immediately after fertilization and to cleave the sperm-binding region within ZP2 [151]. Defects in genes implicated in the polyspermy blockage have yet to be discovered in humans.
Maternal effect genes and post-fertilization preimplantation embryonic failure
Identification of human genes that result in early embryonic lethality, before implantation, is challenging. Recent evidence from assisted reproductive technologies and embryo research suggests that idiopathic infertility may be caused by very early forms of embryonic lethality. It is estimated that up to 70% of human embryos produced in IVF are viable, while the remaining embryos arrest during cleavage-stage embryogenesis [152]. After fertilization, the zygotic genome is transcriptionally silent, and the first few cell divisions of the embryo depend on maternally derived cytoplasmic factors accumulated during oocyte maturation. These maternal components include RNA, proteins, subcellular organelles and macromolecules that are encoded by maternal-effect genes [153–155].
Maternal factors play three major roles in preimplantation development: remodeling of condensed maternal and paternal genomes to form the diploid cell upon fertilization; gradual degradation of maternal RNA and proteins; and transcriptional activation of the embryonic genome. Failure in any of these biological processes leads to female infertility. Based on animal models, many genes are involved in early, post-fertilization cleavage events, including chromatin remodeling, epigenetic reprogramming, embryo genome activation, and cell specification.
A few of these genes have been identified in humans very recently, in part due to application of whole exome sequencing to phenotypically well-characterized clinical cases. Women with recurrent unsuccessful attempts of in vitro fertilization combined with intracytoplasmic sperm injection are diagnosed with primary female infertility as the majority of their embryos fail to develop. Biallelic alterations in PADI6 [156], PATL2 [157, 158], TLE6 [159], NLRP2 [160], NLRP5 [160], and WEE2 [161], as well as heterozygous and homozygous pathogenic variants in TUBB8 [162, 163], were found to be responsible for a spectrum of early embryonic lethality phenotypes, including oocyte maturation arrest, failure of fertilization, early embryonic arrest at the blastomere stage, and implantation failure [164]. The subcortical maternal complex, uniquely expressed in mammalian oocytes is composed of at least four proteins: OOEP, NLRP5, TLE6, and KHDC3L [165]. Maternal nucleotide variants in the TLE6 or NLRP5 gene have been linked to arrested embryo development on Day 3 and failure to form blastocysts [159, 160]. Pathogenic variants in KHDC3L are associated with recurrent hydatidiform mole and have also been found in patients with embryos arrested at the morula stage [166].
Loss-of-function variants of PATL2 were reported in infertile females affected by oocyte germinal vesicle arrest and primary infertility [157, 158]. PATL2 encodes an RNA-binding protein that acts as a translational repressor and is highly expressed in human germinal vesicle and polar body MI oocytes. The exact role of PATL2 and mechanism leading to oocyte maturation arrest is unknown, although PATL2 deficiency may impact canonical translational repression and further hinder protein synthesis. Defects in WEE2 result in female infertility due to inability of the oocyte to exit metaphase II [161]. Oocytes from women with loss-of-function pathogenic variants in WEE2 fail to complete MII exit and form pronuclei, resulting in fertilization failure. The TUBB8 gene encodes a beta-tubulin subunit that is essential for oocyte meiotic spindle assembly. Oocytes of patients with pathogenic variants in TUBB8 show no spindle formation or disorganized spindle and commonly fail to extrude a polar body. In some instances, the first polar body can be extruded in the absence of a properly assembled spindle; however, those embryos still fail to progress beyond the 4- or 8-cell stage [167].
Post-implantation pathologies: hydatidiform moles
Triploid human pregnancy, a conception that contains an additional set of chromosomes resulting in a total of 69 chromosomes in each cell, is an aberrant condition characterized by early embryonic arrest or first trimester miscarriage. Triploid embryos resulting from fertilization by two spermatozoa are detected in ∼1–2% of conceptions [168] and are thought to be caused by inefficient polyspermy blockage by the zona pellucida. Triploidy may also occur from the fertilization of a diploid egg by a haploid sperm (digynic triploidy) or fertilization of a haploid egg by a diploid sperm (diandric triploidy or partial hydatidiform mole). Although rare, recurrent familial cases of triploidy due to a failure to extrude the polar body in meiosis suggest a genetic predisposition [169–171]. Such oocyte maturation defects may lead to an early miscarriage of an unrecognized pregnancy and a diagnosis of primary infertility. Complete hydatidiform mole, a pregnancy formed by the fusion of two sperm or by duplication of a paternal haploid genome in the absence of a maternal counterpart, can be caused by biallelic pathogenic variants in the MEI1, REC114, or TOP6BL genes [172]. These genes are involved in double-strand DNA break formation, and defects can lead to extrusion of chromosomes from oocytes into the first polar body, creating an “empty” egg. Women with such a meiotic abnormality may suffer infertility, recurrent miscarriage, complete hydatidiform mole with excessive trophoblastic proliferation, and failure of embryonic development.
Another cause of a highly recurrent hydatidiform mole with a diploid (normal) set of chromosomes is autosomal recessive inheritance of pathogenic variants in the NLRP7 and KHDC3L genes [173–175]. Interestingly, hydatidiform moles in this condition are not androgenetic but have biparental genomic complements. In such condition, a normal haploid oocyte has been fertilized by a haploid spermatozoon; however, the maternal genome failed to establish a maternally-specific gene expression profile, resulting in trophoblastic proliferation and embryonic failure attributed to abnormal imprinting similar to a phenotype seen in androgenetic molar conceptuses. To date, NLRP7 biallelic sequence variants, genomic deletions, and complex rearrangements have been observed in affected females [173]. In addition, a heterozygous carrier of a pathogenic variant in the NLRP7 gene with a history of recurrent miscarriages and abnormal imprinting in two deceased children has been reported [176]. Although women who carry biallelic variants in these genes are healthy, they have a risk of reproductive failure and a possible impact on the fetal imprinting profile and viability. Other genes involved in establishing a female-specific DNA methylation pattern in mature oocytes remain to be elucidated.
Female infertility due to recurrent miscarriages and fetal lethality
Recurrent miscarriages, defined as two or more consecutive pregnancy losses, may be attributed to maternal, placental or fetal causes of infertility. It has been estimated that up to 60% of all conceptions will abort spontaneously within the first 12 weeks of gestation [66]. Beside fetal aneuploidy, which is responsible for 50–60% of overall fetal losses, many risk factors have been recognized to cause recurrent miscarriages, including anatomical uterine anomalies, polycystic ovary syndrome, antiphospholipid syndrome (up to 15%), endocrinological abnormalities (∼10%), unbalanced products of parental balanced translocations (2–4%), X chromosome aberrations, thrombophilic conditions such as heterozygous variants in the coagulation factor II gene (F2) and coagulation factor V gene (F5), placental insufficiency due to defects in the placental anticoagulant protein annexin A5 (ANXA5), as well as other genetic conditions affecting fetal viability [177–180]. Numerous studies have investigated the role of polymorphisms in the methylenetetrahydrofolate reductase gene (MTHFR) in the risk of recurrent miscarriages [181]; however, the associations are inconsistent, and MTHFR genotyping has no clinical utility at the present. Whole exome and genome sequencing approaches may help in defining the monogenic and complex genetic causes of diminished fetal viability in couples with recurrent miscarriages and stillborn children.
Infertility due to developmental defects in female reproductive organs
Genetic factors play a crucial role in organogenesis of the reproductive system. Beside gonadal dysgenesis, malformation of fallopian tubes and uterus, uterine leiomyoma, or endometriosis, can lead to infertility, miscarriage, premature labor, or fetal death [123, 182]. Abnormalities in the female reproductive tract occur in up to 3.0% of live births in humans and include agenesis, atresia, and abnormal septation of the oviducts (fallopian tubes), uterus, cervix, or vagina, which embryologically arise from the Müllerian duct. Heterozygous sequence variants, deletions, and expansion in the polyadenosine tail of the HOXA13 gene are well-known causes of hand-foot-genital syndrome characterized by fusion of Müllerian structures and uterine malformations [183, 184]. Rare variants in other Homeobox A family genes (HOXA10 and HOXA11) as well as aberrant expression of HOXA11-AS1 antisense RNA (a long noncoding RNA) have been identified in individuals with sporadic uterine malformations and infertile women with endometriosis [185, 186]. Heterozygous pathogenic variants in HNF1B, LHX1, WNT4, WNT7A, and WNT9B have been reported in individuals with syndromic and variable uterine malformations, including Mayer-Rokitansky-Küster-Hauser syndrome [187]. Less solid evidence links PAX2, PAX8, and EMX2 genes with female reproductive tract malformations [188].
Concluding remarks and future studies
Mammalian reproduction is regulated by a large number of genes that are essential for differentiation of highly specialized cells, complex intra- and intercellular signaling, and dynamic tissue-specific and organ-specific interactions among hypothalamus, pituitary, and the reproductive tract. Studies conducted on animal models have identified thousands of candidate genes and micro RNAs that might be involved in fertility [189]. Pathogenic alterations in a subset of these genes have been found to cause ovarian dysfunction and infertility in humans. With the advances of exome and genome sequencing and careful phenotyping, substantial progress has been made in the discovery of genes causing isolated and syndromic forms of primary ovarian insufficiency, preimplantation development [153, 190], and germline-limited alterations associated with lethal X-linked and autosomal phenotypes. It is evident that ovarian function depends on the level and timing of transcription of many genes and is controlled by non-coding regulatory DNA sequences [191–193], microRNAs [194–196], and small non-coding RNA molecules [197] that will be the focus of future studies.
For many genes, ovarian dysgenesis and primary ovarian insufficiency are the result of homozygous or biallelic pathogenic variants; however, little is known about the consequences of heterozygous pathogenic variants in these genes. Digenic and oligogenic inheritance in several affected patients points toward a possible polygenic cause of POI and infertility resulting from the cumulative effects of less harmful alterations among genes involved in a highly complex functional interplay. Heterozygous changes in key genes may confer susceptibility loci, while genomic changes in other loci push the effects of any damage above a “threshold.” Polygenic inheritance may explain developmental failure in embryos resulting from poor-quality oocytes related to female factors of infertility or incompetence of an embryo or fetus to develop postfertilization due to a biparental polygenic burden. Additional studies, including whole genome sequencing and high-throughput functional assays will continue to enlarge our understanding of the interplay between genomic and reproductive health. Furthermore, a possibility of in vitro gametogenesis from stem cells [198], may offer opportunities to edit pathogenic variants prior to fertilization, and restore fertility in affected individuals.
Author Biographical
 Aleksandar Rajkovic is Chief Genomics Officer, Stuart Lindsay Distinguished Professor in Experimental Pathology I and the Director of the Genomic Medicine Initiative at the University of California, San Francisco. He investigates the genetic underpinnings of the formation and differentiation of gametes and reproductive tract, their role of these genes in human disease, embryo lethality and origin of heritable human disorders. His laboratory discovered numerous transcriptional regulators such as Sohlh1, Sohlh2, Lhx8, and Nobox that regulate gamete development and reproductive tract development. These transcription factors are necessary to drive oocyte growth, and synthesis of maternal effect genes that are essential for early embryogenesis and are likely involved in setting of epigenetic marks. Mutations in these oocyte-specific transcriptional regulators associate with human condition of premature ovarian insufficiency and infertility, emphasizing the importance of these pathways to women's health. His laboratory also discovered that DNA damage repair genes such as MCM8 and MCM9 are mutated in women with infertility and the lab is exploring the link between DNA damage repair genes with infertility phenotypes and accelerated overall aging, as well as the effect of these genes on the overall health of offspring and genesis of structural birth defects. These and other studies indicate that many of the reproductive disorders are developmental in origin.
Aleksandar Rajkovic is Chief Genomics Officer, Stuart Lindsay Distinguished Professor in Experimental Pathology I and the Director of the Genomic Medicine Initiative at the University of California, San Francisco. He investigates the genetic underpinnings of the formation and differentiation of gametes and reproductive tract, their role of these genes in human disease, embryo lethality and origin of heritable human disorders. His laboratory discovered numerous transcriptional regulators such as Sohlh1, Sohlh2, Lhx8, and Nobox that regulate gamete development and reproductive tract development. These transcription factors are necessary to drive oocyte growth, and synthesis of maternal effect genes that are essential for early embryogenesis and are likely involved in setting of epigenetic marks. Mutations in these oocyte-specific transcriptional regulators associate with human condition of premature ovarian insufficiency and infertility, emphasizing the importance of these pathways to women's health. His laboratory also discovered that DNA damage repair genes such as MCM8 and MCM9 are mutated in women with infertility and the lab is exploring the link between DNA damage repair genes with infertility phenotypes and accelerated overall aging, as well as the effect of these genes on the overall health of offspring and genesis of structural birth defects. These and other studies indicate that many of the reproductive disorders are developmental in origin.
 Svetlana Yatsenko is the Associated Professor at the Departments of Pathology, Obstetrics, Gynecology & Reproductive Sciences, and Magee Womens Research Institute, Director of Cytogenetics and Genomics Laboratories, at the University of Pittsburgh Medical Center, Pittsburgh. Her main research interests are centered on study of molecular bases and mechanisms of genomic alterations and complex chromosomal rearrangements, as well as gene dosage effect that lead to congenital disorders, birth defects, preimplantation development and prenatal phenotypes, fetal viability, malformations of reproductive system, gonadal dysgenesis and dysfunction, and disorders of sexual development. Her laboratory is also focused on study of alterations involving the X chromosome and their contribution to premature ovarian insufficiency, fetal lethality, sexual dimorphism and femalepredominant diseases.
Svetlana Yatsenko is the Associated Professor at the Departments of Pathology, Obstetrics, Gynecology & Reproductive Sciences, and Magee Womens Research Institute, Director of Cytogenetics and Genomics Laboratories, at the University of Pittsburgh Medical Center, Pittsburgh. Her main research interests are centered on study of molecular bases and mechanisms of genomic alterations and complex chromosomal rearrangements, as well as gene dosage effect that lead to congenital disorders, birth defects, preimplantation development and prenatal phenotypes, fetal viability, malformations of reproductive system, gonadal dysgenesis and dysfunction, and disorders of sexual development. Her laboratory is also focused on study of alterations involving the X chromosome and their contribution to premature ovarian insufficiency, fetal lethality, sexual dimorphism and femalepredominant diseases.
Footnotes
Grant Support: R01HD070647, R21HD074278
References
- 1. Arnold AP, Reue K, Eghbali M, Vilain E, Chen X, Ghahramani N, Itoh Y, Li J, Link JC, Ngun T, Williams-Burris SM. The importance of having two X chromosomes. Phil Trans R Soc B 2016; 371:20150113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Payer B, Lee JT. X chromosome dosage compensation: how mammals keep the balance. Annu Rev Genet 2008; 42:733–772. [DOI] [PubMed] [Google Scholar]
- 3. Heard E, Turner J. Function of the sex chromosomes in mammalian fertility. Cold Spring Harb Perspect Biol 2011; 3:a002675–a002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tuke MA, Ruth KS, Wood AR, Beaumont RN, Tyrrell J, Jones SE, Yaghootkar H, Turner CLS, Donohoe ME, Brooke AM, Collinson MN, Freathy RMet al. Mosaic Turner syndrome shows reduced penetrance in an adult population study. Genet Med 2019; 21:877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abir R, Fisch B, Nahum R, Orvieto R, Nitke S, Ben Rafael Z. Turner's syndrome and fertility: current status and possible putative prospects. Hum Reprod Update 2001; 7:603–610. [DOI] [PubMed] [Google Scholar]
- 6. Lakhal B, Braham R, Berguigua R, Bouali N, Zaouali M, Chaieb M, Veitia RA, Saad A, Elghezal H. Cytogenetic analyses of premature ovarian failure using karyotyping and interphase fluorescence in situ hybridization (FISH) in a group of 1000 patients. Clin Genet 2010; 78:181–185. [DOI] [PubMed] [Google Scholar]
- 7. Demain LA, Conway GS, Modi DN, Sane S, Bhartiya D. Accelerated germ cell apoptosis in sex chromosome aneuploid fetal human gonads. Mol Hum Reprod 2003; 9:219–225. [DOI] [PubMed] [Google Scholar]
- 8. Cloutier JM, Mahadevaiah SK, ElInati E, Nussenzweig A, Tóth A, Turner JM. Histone H2AFX links meiotic chromosome asynapsis to prophase i oocyte loss in mammals. PLoS Genet 2015; 11:e1005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Persani L, Rossetti R, Di Pasquale E, Cacciatore C, Fabre S. The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders. Hum Reprod Update 2014; 20:869–883. [DOI] [PubMed] [Google Scholar]
- 10. Yatsenko SA, Wood-Trageser M, Chu T, Jiang H, Rajkovic A. A high-resolution X chromosome copy-number variation map in fertile females and women with primary ovarian insufficiency. Genet Med 2019; (in press). Published online ahead of print 5 April 2019; DOI 10.1038/s41436-019-0505-2. [DOI] [PubMed] [Google Scholar]
- 11. Lanasa MC, Hogge WA, Hoffman EP. The X chromosome and recurrent spontaneous abortion: the significance of transmanifesting carriers. Am J Hum Genet 1999; 64:934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martin J, Asan, Yi Y, Alberola T, Rodríguez-Iglesias B, Jiménez-Almazán J, Li Q, Du H, Alama P, Ruiz A, Bosch E, Garrido Net al. Comprehensive carrier genetic test using next-generation deoxyribonucleic acid sequencing in infertile couples wishing to conceive through assisted reproductive technology. Fertil Steril 2015; 104:1286–1293. [DOI] [PubMed] [Google Scholar]
- 13. Morleo M, Franco B. Dosage compensation of the mammalian X chromosome influences the phenotypic variability of X-linked dominant male-lethal disorders. J Med Genet 2008; 45:401–408. [DOI] [PubMed] [Google Scholar]
- 14. Franco B, Ballabio A. X-inactivation and human disease: X-linked dominant male-lethal disorders. Curr Opin Genet Develop 2006; 16:254–259. [DOI] [PubMed] [Google Scholar]
- 15. Aarabi M, Sniezek O, Jiang H, Saller DN, Bellissimo D, Yatsenko SA, Rajkovic A. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet 2018; 137:175–181. [DOI] [PubMed] [Google Scholar]
- 16. Robertson SP, Thompson S, Morgan T, Holder-Espinasse M, Martinot-Duquenoy V, Wilkie AO, Manouvrier-Hanu S. Postzygotic mutation and germline mosaicism in the otopalatodigital syndrome spectrum disorders. Eur J Hum Genet 2006; 14:549–554. [DOI] [PubMed] [Google Scholar]
- 17. Archer HL, Whatley SD, Evans JC, Ravine D, Huppke P, Kerr A, Bunyan D, Kerr B, Sweeney E, Davies SJ, Reardon W, Horn Jet al. Gross rearrangements of the MECP2 gene are found in both classical and atypical Rett syndrome patients. J Med Genet 2006; 43:451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eggers S, Ohnesorg T, Sinclair A. Genetic regulation of mammalian gonad development. Nat Rev Endocrinol 2014; 11:673–683. [DOI] [PubMed] [Google Scholar]
- 19. Ono M, Harley VR. Disorders of sex development: new genes, new concepts. Nat Rev Endocrinol 2013; 2:79–91. [DOI] [PubMed] [Google Scholar]
- 20. King TF, Conway GS. Swyer syndrome. Curr Opin Endocrinol Diabetes Obes 2014; 6:504–510. [DOI] [PubMed] [Google Scholar]
- 21. Yatsenko SA, Rajkovic A. Chromosomal causes of infertility: the story continues. In: Sermon K, Viville S (eds.), Textbook of Human Reproductive Genetics. United Kingdom: Cambridge University Press; 2014:97–112. [Google Scholar]
- 22. Croft B, Ohnesorg T, Hewitt J, Bowles J, Quinn A, Tan J, Corbin V, Pelosi E, van den Bergen J, Sreenivasan R, Knarston I, Robevska Get al. Human sex reversal is caused by duplication or deletion of core enhancers upstream of SOX9. Nat Commun 2018; 9:5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harris A, Siggers P, Corrochano S, Warr N, Sagar D, Grimes DT, Suzuki M, Burdine RD, Cong F, Koo BK, Clevers H, Stévant Iet al. ZNRF3 functions in mammalian sex determination by inhibiting canonical WNT signaling. Proc Natl Acad Sci USA 2018; 115:5474–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ounap K, Uibo O, Zordania R, Kiho L, Ilus T, Oiglane-Shlik E, Bartsch O. Three patients with 9p deletions including DMRT1 and DMRT2: a girl with XY complement, bilateral ovotestes, and extreme growth retardation, and two XX females with normal pubertal development. Am J Med Genet 2004; 130A:415–423. [DOI] [PubMed] [Google Scholar]
- 25. Chassot AA, Gillot I, Chaboissier MC. R-spondin1, WNT4, and the CTNNB1 signaling pathway: strict control over ovarian differentiation. Reproduction 2014; 148: R97–R110. [DOI] [PubMed] [Google Scholar]
- 26. Hughes IA, Deeb A. Androgen resistance. Best Pract Res Clin Endocrinol Metab 2006; 20:577–598. [DOI] [PubMed] [Google Scholar]
- 27. Umehara F, Tate G, Itoh K, Yamaguchi N, Douchi T, Mitsuya T, Osame M. A novel mutation of desert hedgehog in a patient with 46, XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Am J Hum Genet 2000; 67:1302–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pangas SA, Rajkovic A. Transcriptional regulation of early oogenesis: in search of masters. Hum Reprod Update 2006; 12:65–76. [DOI] [PubMed] [Google Scholar]
- 29. Ballow DJ, Xin Y, Choi Y, Pangas SA, Rajkovic A. Sohlh2 is a germ cell-specific bHLH transcription factor. Gene Expr Patterns 2006; 6:1014–1018. [DOI] [PubMed] [Google Scholar]
- 30. Choi Y, Rajkovic A. Characterization of NOBOX DNA binding specificity and its regulation of Gdf9 and Pou5f1 promoters. J Biol Chem 2006; 281:35747–35756. [DOI] [PubMed] [Google Scholar]
- 31. Miyamoto Y, Taniguchi H, Hamel F, Silversides DW, Viger RS. A GATA4/WT1 cooperation regulates transcription of genes required for mammalian sex determination and differentiation. BMC Mol Biol 2008; 9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klattig J, Sierig R, Kruspe D, Makki MS, Englert C. WT1-mediated gene regulation in early urogenital ridge development. Sex Dev 2007; 1:238–254. [DOI] [PubMed] [Google Scholar]
- 33. Elzaiat M, Todeschini AL, Caburet S, Veitia RA. The genetic make-up of ovarian development and function: the focus on the transcription factor FOXL2. Clin Genet 2017; 91:173–182. [DOI] [PubMed] [Google Scholar]
- 34. Jameson JL, Achermann JC, Ozisik G, Meeks JJ. Battle of the sexes: new insights into genetic pathways of gonadal development. Trans Am Clin Climatol Assoc 2003; 114:51–65. [PMC free article] [PubMed] [Google Scholar]
- 35. Wang H, Zhang L, Wang N, Zhu H, Han B, Sun F, Yao H, Zhang Q, Zhu W, Cheng T, Cheng K, Liu Yet al. Next-generation sequencing reveals genetic landscape in 46, XY disorders of sexual development patients with variable phenotypes. Hum Genet 2018; 137:265–277. [DOI] [PubMed] [Google Scholar]
- 36. El-Khairi R, Achermann JC. Steroidogenic factor-1 and human disease. Semin Reprod Med 2012; 30:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shin YH, Ren Y, Suzuki H, Golnoski KJ, Ahn HW, Mico V, Rajkovic A. Transcription factors SOHLH1 and SOHLH2 coordinate oocyte differentiation without affecting meiosis I. J Clin Invest 2017; 127:2106–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schumacher V, Schärer K, Wühl E, Altrogge H, Bonzel KE, Guschmann M, Neuhaus TJ, Pollastro RM, Kuwertz-Bröking E, Bulla M, Tondera AM, Mundel Pet al. Spectrum of early onset nephrotic syndrome associated with WT1 missense mutations. Kidney Int 1998; 53:1594–1600. [DOI] [PubMed] [Google Scholar]
- 39. Hoefele J, Kemper MJ, Schoenermarck U, Mueller S, Klein HG, Lemke A. Truncating Wilms tumor suppressor gene 1 mutation in an XX female with adult-onset focal segmental Glomerulosclerosis and streak ovaries: a case report. Nephron 2017; 135:72–76. [DOI] [PubMed] [Google Scholar]
- 40. Bashamboo A, Donohoue PA, Vilain E, Rojo S, Calvel P, Seneviratne SN, Buonocore F, Barseghyan H, Bingham N, Rosenfeld JA, Mulukutla SN, Jain Met al. A recurrent p.Arg92Trp variant in steroidogenic factor-1 (NR5A1) can act as a molecular switch in human sex development. Hum Mol Genet 2016; 25:3446–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gray S, Cohen PE. Control of meiotic crossovers: from double-strand break formation to designation. Annu Rev Genet 2016; 50:175–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. MacLennan M, Crichton JH, Playfoot CJ, Adams IR. Oocyte development, meiosis and aneuploidy. Semin Cell Dev Biol 2015; 45:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zickler D, Kleckner N. Recombination, Pairing, and Synapsis of Homologs during Meiosis. Cold Spring Harb Perspect Biol 2015; 7:pii: a016626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baudat F, Imai Y, de Massy B. Meiotic recombination in mammals: localization and regulation. Nat Rev Genet 2013; 14:794–806. [DOI] [PubMed] [Google Scholar]
- 45. Handel MA, Schimenti JC. Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet 2010; 11:124–136. [DOI] [PubMed] [Google Scholar]
- 46. Guigon CJ, Magre S. Contribution of germ cells to the differentiation and maturation of the ovary: insights from models of germ cell depletion. Biol Reprod 2006; 74:450–458. [DOI] [PubMed] [Google Scholar]
- 47. De Felici M, Klinger FG, Farini D, Scaldaferri ML, Iona S, Lobascio M. Establishment of oocyte population in the fetal ovary: primordial germ cell proliferation and oocyte programmed cell death. Reprod Biomed Online 2005; 10:182–191. [DOI] [PubMed] [Google Scholar]
- 48. Wood MA, Rajkovic A. Genomic markers of ovarian reserve. Semin Reprod Med 2013; 31:399–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sazegari A, Kalantar SM, Pashaiefar H, Mohtaram S, Honarvar N, Feizollahi Z, Ghasemi N. The T657C polymorphism on the SYCP3 gene is associated with recurrent pregnancy loss. J Assist Reprod Genet 2014; 31:1377–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Caburet S, Arboleda VA, Llano E, Overbeek PA, Barbero JL, Oka K, Harrison W, Vaiman D, Ben-Neriah Z, García-Tuñón I, Fellous M, Pendás AMet al. Mutant cohesin in premature ovarian failure. N Engl J Med 2014; 370:943–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang J, Zhang W, Jiang H, Wu BL, Primary Ovarian Insufficiency Collaboration. Mutations in HFM1 in recessive primary ovarian insufficiency. N Engl J Med 2014; 370:972–974. [DOI] [PubMed] [Google Scholar]
- 52. de Vries L, Behar DM, Smirin-Yosef P, Lagovsky I, Tzur S, Basel-Vanagaite L. Exome sequencing reveals SYCE1 mutation associated with autosomal recessive primary ovarian insufficiency. J Clin Endocrinol Metab 2014; 99:E2129–E2132. [DOI] [PubMed] [Google Scholar]
- 53. Tšuiko O, Nõukas M, Žilina O, Hensen K, Tapanainen JS, Mägi R, Kals M, Kivistik PA, Haller-Kikkatalo K, Salumets A, Kurg A. Copy number variation analysis detects novel candidate genes involved in follicular growth and oocyte maturation in a cohort of premature ovarian failure cases. Hum Reprod 2016; 31:1913–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weinberg-Shukron A, Renbaum P, Kalifa R, Zeligson S, Ben-Neriah Z, Dreifuss A, Abu-Rayyan A, Maatuk N, Fardian N, Rekler D, Kanaan M, Samson AOet al. A mutation in the nucleoporin-107 gene causes XX gonadal dysgenesis. J Clin Invest 2015; 125:4295–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ren Y, Diao F, Katari S, Yatsenko S, Jiang H, Wood-Trageser MA, Rajkovic A. Functional study of a novel missense single-nucleotide variant of NUP107 in two daughters of Mexican origin with premature ovarian insufficiency. Mol Genet Genomic Med 2018; 6:276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zangen D, Kaufman Y, Zeligson S, Perlberg S, Fridman H, Kanaan M, Abdulhadi-Atwan M, Abu Libdeh A, Gussow A, Kisslov I, Carmel L, Renbaum Pet al. XX ovarian dysgenesis is caused by a PSMC3IP/HOP2 mutation that abolishes coactivation of estrogen-driven transcription. Am J Hum Genet 2011; 89:572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Al-Agha AE, Ahmed IA, Nuebel E, Moriwaki M, Moore B, Peacock KA, Mosbruger T, Neklason DW, Jorde LB, Yandell M, Welt CK. Primary ovarian insufficiency and azoospermia in carriers of a homozygous PSMC3IP stop gain mutation. J Clin Endocrinol Metab 2018; 103:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Faridi R, Rehman AU, Morell RJ, Friedman PL, Demain L, Zahra S, Khan AA, Tohlob D, Assir MZ, Beaman G, Khan SN, Newman WGet al. Mutations of SGO2 and CLDN14 collectively cause coincidental Perrault syndrome. Clin Genet 2017; 91:328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Garcia-Cruz R, Brieño MA, Roig I, Grossmann M, Velilla E, Pujol A, Cabero L, Pessarrodona A, Barbero JL, Garcia Caldés M. Dynamics of cohesin proteins REC8, STAG3, SMC1 beta and SMC3 are consistent with a role in sister chromatid cohesion during meiosis in human oocytes. Hum Reprod 2010; 25:2316–2327. [DOI] [PubMed] [Google Scholar]
- 60. Suo L, Zhou YX, Jia LL, Wu HB, Zheng J, Lyu QF, Sun LH, Sun H, Kuang YP. Transcriptome profiling of human oocytes experiencing recurrent total fertilization failure. Sci Rep 2018; 8:17890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kort JD, McCoy RC, Demko Z, Lathi RB. Are blastocyst aneuploidy rates different between fertile and infertile populations? J Assist Reprod Genet 2018; 35:403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shahine LK, Marshall L, Lamb JD, Hickok LR. Higher rates of aneuploidy in blastocysts and higher risk of no embryo transfer in recurrent pregnancy loss patients with diminished ovarian reserve undergoing in vitro fertilization. Fertil Steril 2016; 106:1124–1128. [DOI] [PubMed] [Google Scholar]
- 63. Ishiguro KI. The cohesin complex in mammalian meiosis. Genes Cells 2019; 24:6–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Babariya D, Fragouli E, Alfarawati S, Spath K, Wells D. The incidence and origin of segmental aneuploidy in human oocytes and preimplantation embryos. Hum Reprod 2017; 32:2549–2560. [DOI] [PubMed] [Google Scholar]
- 65. Pylyp LY, Spynenko LO, Verhoglyad NV, Mishenko AO, Mykytenko DO, Zukin VD. Chromosomal abnormalities in products of conception of first-trimester miscarriages detected by conventional cytogenetic analysis: a review of 1000 cases. J Assist Reprod Genet 2018; 35:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Macklon NS, Geraedts JP, Fauser BC. Conception to ongoing pregnancy: the 'black box' of early pregnancy loss. Hum Reprod Update 2002; 8:333–343. [DOI] [PubMed] [Google Scholar]
- 67. Tsutsumi M, Fujiwara R, Nishizawa H, Ito M, Kogo H, Inagaki H, Ohye T, Kato T, Fujii T, Kurahashi H. Age-related decrease of meiotic cohesins in human oocytes. PLoS One 2014; 9:e96710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rio Frio T, Lavoie J, Hamel N, Geyer FC, Kushner YB, Novak DJ, Wark L, Capelli C, Reis-Filho JS, Mai S, Pastinen T, Tischkowitz MDet al. Homozygous BUB1B mutation and susceptibility to gastrointestinal neoplasia. N Engl J Med 2010; 363:2628–2637. [DOI] [PubMed] [Google Scholar]
- 69. Yamaguchi T, Yamaguchi M, Akeno K, Fujisaki M, Sumiyoshi K, Ohashi M, Sameshima H, Ozaki M, Kato M, Kato T, Hosoba E, Kurahashi H. Prenatal diagnosis of premature chromatid separation/mosaic variegated aneuploidy (PCS/MVA) syndrome. J Obstet Gynaecol Res 2018; 44:1313–1317. [DOI] [PubMed] [Google Scholar]
- 70. Brightman DS, Ejaz S, Dauber A. Mosaic variegated aneuploidy syndrome caused by a CEP57 mutation diagnosed by whole exome sequencing. Clin Case Rep 2018; 6:1531–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. De la Torre-García O, Mar-Aldama R, Salgado-Sangri R, Diaz-Gomez N, Bonilla-Arcaute L, Diaz-Ponce-Medrano J, Guevara-Yañez R, Córdova EJ, Monge-Cazares T, Orozco L, Martínez-Hernández A. A homozygous CEP57 c.915_925dupCAATGTTCAGC mutation in a patient with mosaic variegated aneuploidy syndrome with rhizomelic shortening in the upper and lower limbs and a narrow thorax. Eur J Med Genet. 2019; 62:195–197. [DOI] [PubMed] [Google Scholar]
- 72. Watanabe K, Takao D, Ito KK, Takahashi M, Kitagawa D. The Cep57-pericentrin module organizes PCM expansion and centriole engagement. Nat Commun 2019; 10:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Weinberg-Shukron A, Rachmiel M, Renbaum P, Gulsuner S, Walsh T, Lobel O, Dreifuss A, Ben-Moshe A, Zeligson S, Segel R, Shore T, Kalifa Ret al. Essential role of BRCA2 in ovarian development and function. N Engl J Med 2018; 379:1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Katari S, Aarabi M, Kintigh A, Mann S, Yatsenko SA, Sanfilippo JS, Zeleznik AJ, Rajkovic A. Chromosomal instability in women with primary ovarian insufficiency. Hum Reprod 2018; 33:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yatsenko SA, Rajkovic A. Reproductive aging and MCM8/9. Oncotarget 2015; 6:15750–15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shin YH, McGuire MM, Rajkovic A. Mouse HORMAD1 is a meiosis i checkpoint protein that modulates DNA double- strand break repair during female meiosis. Biol Reprod 2013; 89:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shin YH, Choi Y, Erdin SU, Yatsenko SA, Kloc M, Yang F, Wang PJ, Meistrich ML, Rajkovic A. Hormad1 mutation disrupts synaptonemal complex formation, recombination, and chromosome segregation in mammalian meiosis. PLoS Genet 2010; 6:e1001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wood-Trageser MA, Gurbuz F, Yatsenko SA, Jeffries EP, Kotan LD, Surti U, Ketterer DM, Matic J, Chipkin J, Jiang H, Trakselis MA, Topaloglu AKet al. MCM9 mutations are associated with ovarian failure, short stature, and chromosomal instability. Am J Hum Genet 2014; 95:754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. AlAsiri S, Basit S, Wood-Trageser MA, Yatsenko SA, Jeffries EP, Surti U, Ketterer DM, Afzal S, Ramzan K, Faiyaz-Ul Haque M, Jiang H, Trakselis MAet al. Exome sequencing reveals MCM8 mutation underlies ovarian failure and chromosomal instability. J Clin Invest 2015; 125:258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tenenbaum-Rakover Y, Weinberg-Shukron A, Renbaum P, Lobel O, Eideh H, Gulsuner S, Dahary D, Abu-Rayyan A, Kanaan M, Levy-Lahad E, Bercovich D, Zangen D. Minichromosome maintenance complex component 8 (MCM8) gene mutations result in primary gonadal failure. J Med Genet 2015; 52:391–399. [DOI] [PubMed] [Google Scholar]
- 81. Desai S, Wood-Trageser M, Matic J, Chipkin J, Jiang H, Bachelot A, Dulon J, Sala C, Barbieri C, Cocca M, Toniolo D, Touraine Pet al. MCM8 and MCM9 nucleotide variants in women with primary ovarian insufficiency. J Clin Endocrinol Metab 2017; 102:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mandon-Pépin B, Touraine P, Kuttenn F, Derbois C, Rouxel A, Matsuda F, Nicolas A, Cotinot C, Fellous M. Genetic investigation of four meiotic genes in women with premature ovarian failure. Eur J Endocrinol 2008; 158:107–115. [DOI] [PubMed] [Google Scholar]
- 83. Guo T, Zhao S, Zhao S, Chen M, Li G, Jiao X, Wang Z, Zhao Y, Qin Y, Gao F, Chen ZJ. Mutations in MSH5 in primary ovarian insufficiency. Hum Mol Genet 2017; 26:1452–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Goldberg Y, Halpern N, Hubert A, Adler SN, Cohen S, Plesser-Duvdevani M, Pappo O, Shaag A, Meiner V. Mutated MCM9 is associated with predisposition to hereditary mixed polyposis and colorectal cancer in addition to primary ovarian failure. Cancer Genet 2015; 208:621–624. [DOI] [PubMed] [Google Scholar]
- 85. Chen CT, Liu CT, Chen GK, Andrews JS, Arnold AM, Dreyfus J, Franceschini N, Garcia ME, Kerr KF, Li G, Lohman KK, Musani SKet al. Meta-analysis of loci associated with age at natural menopause in African-American women. Hum Mol Genet 2014; 23:3327–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schuh-Huerta SM, Johnson NA, Rosen MP, Sternfeld B, Cedars MI, Reijo Pera RA. Genetic markers of ovarian follicle number and menopause in women of multiple ethnicities. Hum Genet 2012; 131:1709–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lee KY, Im JS, Shibata E, Park J, Handa N, Kowalczykowski SC, Dutta A. MCM8-9 complex promotes resection of double-strand break ends by MRE11-RAD50-NBS1 complex. Nat Commun 2015; 6:7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Desai S, Rajkovic A. Genetics of reproductive aging from gonadal dysgenesis through menopause. Semin Reprod Med 2017; 35:147–159. [DOI] [PubMed] [Google Scholar]
- 89. Rajkovic A, Pangas S. Ovary as a biomarker of health and longevity: insights from genetics. Semin Reprod Med 2017; 35:231–240. [DOI] [PubMed] [Google Scholar]
- 90. Spencer KL, Malinowski J, Carty CL, Franceschini N, Fernández-Rhodes L, Young A, Cheng I, Ritchie MD, Haiman CA, Wilkens L, Chunyuanwu, Matise TCet al. Genetic variation and reproductive timing: African American women from the population architecture using genomics and epidemiology (PAGE) study. PLoS One 2013; 8:e55258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Grive KJ, Freiman RN. The developmental origins of the mammalian ovarian reserve. Development 2015; 142:2554–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Treff NR. Genome-wide analysis of human preimplantation aneuploidy. Semin Reprod Med 2012; 30:283–288. [DOI] [PubMed] [Google Scholar]
- 93. Levi AJ, Raynault MF, Bergh PA, Drews MR, Miller BT, Scott RT. Reproductive outcome in patients with diminished ovarian reserve. Fertil Steril 2001; 76:666–669. [DOI] [PubMed] [Google Scholar]
- 94. Kumar R, Alwani M, Kosta S, Kaur R, Agarwal S. BMP15 and GDF9 gene mutations in premature ovarian failure. J Reprod Infertil 2017; 18:185–189. [PMC free article] [PubMed] [Google Scholar]
- 95. Patiño LC, Walton KL, Mueller TD, Johnson KE, Stocker W, Richani D, Agapiou D, Gilchrist RB, Laissue P, Harrison CA. BMP15 mutations associated with primary ovarian insufficiency reduce expression, activity, or synergy with GDF9. J Clin Endocrinol Metab 2017; 102:1009–1019. [DOI] [PubMed] [Google Scholar]
- 96. Sanfins A, Rodrigues P, Albertini DF. GDF-9 and BMP-15 direct the follicle symphony. J Assist Reprod Genet 2018; 35:1741–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Richards JS, Ren YA, Candelaria N, Adams JE, Rajkovic A. Ovarian follicular theca cell recruitment, differentiation, and impact on fertility: 2017 update. Endocr Rev 2018; 39:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhao H, Qin Y, Kovanci E, Simpson JL, Chen ZJ, Rajkovic A. Analyses of GDF9 mutation in 100 Chinese women with premature ovarian failure. Fertil Steril 2007; 88:1474–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bouilly J, Beau I, Barraud S, Bernard V, Azibi K, Fagart J, Fèvre A, Todeschini AL, Veitia RA, Beldjord C, Delemer B, Dodé Cet al. Identification of multiple gene mutations accounts for a new genetic architecture of primary ovarian insufficiency. J Clin Endocrinol Metab 2016; 101:4541–4550. [DOI] [PubMed] [Google Scholar]
- 100. Conti M, Franciosi F. Acquisition of oocyte competence to develop as an embryo: integrated nuclear and cytoplasmic events. Hum Reprod Update 2018; 24:245–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gallardo TD, John GB, Bradshaw K, Welt C, Reijo-Pera R, Vogt PH, Touraine P, Bione S, Toniolo D, Nelson LM, Zinn AR, Castrillon DH. Sequence variation at the human FOXO3 locus: a study of premature ovarian failure and primary amenorrhea. Hum Reprod 2008; 23:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Choi Y, Yuan D, Rajkovic A. Germ cell-specific transcriptional regulator sohlh2 is essential for early mouse folliculogenesis and oocyte-specific gene expression. Biol Reprod 2008; 79:1176–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Choi Y, Ballow DJ, Xin Y, Rajkovic A. Lim homeobox gene, lhx8, is essential for mouse oocyte differentiation and survival. Biol Reprod 2008; 79:442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pangas SA, Choi Y, Ballow DJ, Zhao Y, Westphal H, Matzuk MM, Rajkovic A. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc Natl Acad Sci 2006; 103:8090–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Qin Y, Zhao H, Kovanci E, Simpson JL, Chen ZJ, Rajkovic A. Analysis of LHX8 mutation in premature ovarian failure. Fertil Steril 2008; 89:1012–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Qin Y, Choi Y, Zhao H, Simpson JL, Chen ZJ, Rajkovic A. NOBOX homeobox mutation causes premature ovarian failure. Am J Hum Genet 2007; 81:576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 2004; 305:1157–1159. [DOI] [PubMed] [Google Scholar]
- 108. Qin Y, Zhao H, Kovanci E, Simpson JL, Chen ZJ, Rajkovic A. Mutation analysis of NANOS3 in 80 Chinese and 88 Caucasian women with premature ovarian failure. Fertil Steril 2007; 88:1465–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhao H, Chen ZJ, Qin Y, Shi Y, Wang S, Choi Y, Simpson JL, Rajkovic A. Transcription factor FIGLA is mutated in patients with premature ovarian failure. Am J Hum Genet 2008; 82:1342–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Seltzer MM, Baker MW, Hong J, Maenner M, Greenberg J, Mandel D. Prevalence of CGG expansions of the FMR1 gene in a US population-based sample. Am J Med Genet 2012; 159B:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hunter J, Rivero-Arias O, Angelov A, Kim E, Fotheringham I, Leal J. Epidemiology of fragile X syndrome: a systematic review and meta-analysis. Am J Med Genet 2014; 164A:1648–1658. [DOI] [PubMed] [Google Scholar]
- 112. Rehnitz J, Alcoba DD, Brum IS, Dietrich JE, Youness B, Hinderhofer K, Messmer B, Freis A, Strowitzki T, Germeyer A. FMR1 expression in human granulosa cells increases with exon 1 CGG repeat length depending on ovarian reserve. Reprod Biol Endocrinol 2018; 16:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lekovich J, Man L, Xu K, Canon C, Lilienthal D, Stewart JD, Pereira N, Rosenwaks Z, Gerhardt J. CGG repeat length and AGG interruptions as indicators of fragile X-associated diminished ovarian reserve. Genet Med 2018; 20:957–964. [DOI] [PubMed] [Google Scholar]
- 114. Eslami H, Eslami A, Favaedi R, Asadpour U, Zari Moradi S, Eftekhari-Yazdi P, Madani T, Shahhoseini M, Mohseni Meybodi A. Epigenetic aberration of FMR1 gene in infertile women with diminished ovarian reserve. Cell J 2018; 20:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kasippillai T, MacArthur DG, Kirby A, Thomas B, Lambalk CB, Daly MJ, Welt CK. Mutations in eIF4ENIF1 are associated with primary ovarian insufficiency. J Clin Endocr Metab 2013; 98:E1534–E1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hutchins BI, Kotan LD, Taylor-Burds C, Ozkan Y, Cheng PJ, Gurbuz F, Tiong JD, Mengen E, Yuksel B, Topaloglu AK, Wray S. CCDC141 mutation identified in anosmic hypogonadotropic hypogonadism (Kallmann Syndrome) alters GnRH neuronal migration. Endocrinology 2016; 157:1956–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Pingault V, Bodereau V, Baral V, Marcos S, Watanabe Y, Chaoui A, Fouveaut C, Leroy C, Vérier-Mine O, Francannet C, Dupin-Deguine D, Archambeaud Fet al. Loss-of-function mutations in SOX10 cause Kallmann syndrome with deafness. Am J Hum Genet 2013; 92:707–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yang X, Touraine P, Desai S, Humphreys G, Jiang H, Yatsenko A, Rajkovic A. Gene variants identified by whole-exome sequencing in 33 French women with premature ovarian insufficiency. J Assist Reprod Genet 2019; 36:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Coppieters F, Ascari G, Dannhausen K, Nikopoulos K, Peelman F, Karlstetter M, Xu M, Brachet C, Meunier I, Tsilimbaris MK, Tsika C, Blazaki SVet al. Isolated and syndromic retinal dystrophy caused by biallelic mutations in RCBTB1, a gene implicated in ubiquitination. Am J Hum Genet 2016; 99:470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gurbuz F, Desai S, Diao F, Turkkahraman D, Wranitz F, Wood-Trageser M, Shin YH, Kotan LD, Jiang H, Witchel S, Gurtunca N, Yatsenko Set al. Novel inactivating mutations of the DCAF17 gene in American and Turkish families cause male infertility and female subfertility in the mouse model. Clin Genet 2018; 93:853–859. [DOI] [PubMed] [Google Scholar]
- 121. Ghaddhab C, Morin C, Brunel-Guitton C, Mitchell GA, Van Vliet G, Huot C. Premature ovarian failure in French Canadian Leigh syndrome. J Pediatr 2017; 184:227–229.e1. [DOI] [PubMed] [Google Scholar]
- 122. Molho-Pessach V, Lerer I, Abeliovich D, Agha Z, Abu Libdeh A, Broshtilova V, Elpeleg O, Zlotogorski A. The H syndrome is caused by mutations in the nucleoside transporter hENT3. Am J Hum Genet 2008; 83:529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tong ZB, Sullivan SD, Lawless LM, Vanderhoof V, Zachman K, Nelson LM. Five mutations of mitochondrial DNA polymerase-gamma (POLG) are not a prevalent etiology for spontaneous 46,XX primary ovarian insufficiency. Fertil Steril 2010; 94:2932–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Newman WG. Genetics of mitochondrial dysfunction and infertility. Clin Genet 2017; 91:199–207. [DOI] [PubMed] [Google Scholar]
- 125. Cao L, Shitara H, Horii T, Nagao Y, Imai H, Abe K, Hara T, Hayashi J, Yonekawa H. The mitochondrial bottleneck occurs without reduction of mtDNA content in female mouse germ cells. Nat Genet 2007; 39:386–390. [DOI] [PubMed] [Google Scholar]
- 126. Wai T, Teoli D, Shoubridge EA. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat Genet 2008; 40:1484–1488. [DOI] [PubMed] [Google Scholar]
- 127. Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA. The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod 2010; 83:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. May-Panloup P, Boucret L, Chao de la Barca JM, Desquiret-Dumas V, Ferré-L’Hotellier V, Morinière C, Descamps P, Procaccio V, Reynier P. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum Reprod Update 2016; 22:725–743. [DOI] [PubMed] [Google Scholar]
- 129. Messinis IE, Messini CI, Dafopoulos K. The role of gonadotropins in the follicular phase. Ann NY Acad Sci 2010; 1205:5–11. [DOI] [PubMed] [Google Scholar]
- 130. Hsueh AJ, Kawamura K, Cheng Y, Fauser BC. Intraovarian control of early folliculogenesis. Endocr Rev 2015; 36:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Siegel ET, Kim HG, Nishimoto HK, Layman LC. The molecular basis of impaired Follicle-Stimulating hormone action. Reprod Sci 2013; 20:211–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tian Y, Zhao H, Chen H, Peng Y, Cui L, Du Y, Wang Z, Xu J, Chen ZJ. Variants in FSHB are associated with polycystic ovary syndrome and luteinizing hormone level in Han chinese women. J Clin Endocrinol Metab 2016; 101:2178–2184. [DOI] [PubMed] [Google Scholar]
- 133. Jiang M, Aittomäki K, Nilsson C, Pakarinen P, Iitiä A, Torresani T, Simonsen H, Goh V, Pettersson K, de la Chapelle A, Huhtaniemi I. The frequency of an inactivating point mutation (566C→T) of the human follicle-stimulating hormone receptor gene in four populations using allele-specific hybridization and time-resolved fluorometry. J Clin Endocrinol Metab 1998; 83:4338–4343. [DOI] [PubMed] [Google Scholar]
- 134. Katari S, Wood-Trageser MA, Jiang H, Kalynchuk E, Muzumdar R, Yatsenko SA, Rajkovic A. Novel inactivating mutation of the FSH receptor in two siblings of indian origin with premature ovarian failure. J Clin Endocrinol Metab 2015; 100:2154–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Gromoll J, Simoni M. Genetic complexity of FSH receptor function. Trend Endocrinol Metab 2005; 16:368–373. [DOI] [PubMed] [Google Scholar]
- 136. Brown JL, Roberson M. Novel insights into Gonadotropin-releasing hormone action in the pituitary gonadotrope. Semin Reprod Med. 2017; 35:130–138. [DOI] [PubMed] [Google Scholar]
- 137. Trofimova T, Lizneva D, Suturina L, Walker W, Chen YH, Azziz R, Layman LC. Genetic basis of eugonadal and hypogonadal female reproductive disorders. Best Pract Res Clin Obstet Gynaecol 2017; 44:3–14. [DOI] [PubMed] [Google Scholar]
- 138. Jones MR, Goodarzi MO. Genetic determinants of polycystic ovary syndrome: progress and future directions. Fertil Steril 2016; 106:25–32. [DOI] [PubMed] [Google Scholar]
- 139. McAllister JM, Legro RS, Modi BP, Strauss JF 3rd. Functional genomics of PCOS: from GWAS to molecular mechanisms. Trend Endocrinol Metab 2015; 26:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Liu YD, Li Y, Feng SX, Ye DS, Chen X, Zhou XY, Chen SL. Long noncoding RNAs: potential regulators involved in the pathogenesis of polycystic ovary syndrome. Endocrinology 2017; 158:3890–3899. [DOI] [PubMed] [Google Scholar]
- 141. Richards JS, Ascoli M. Endocrine, endocrine, paracrine, and autocrine signaling pathways that regulate ovulation. Trend Endocrinol Metab 2018; 29:313–325. [DOI] [PubMed] [Google Scholar]
- 142. Zielen AC, Khan MJ, Pollock N, Jiang H, Ahmed J, Nazli R, Jabeen M, Yatsenko A, Rajkovic A. A novel homozygous frame-shift variant in the LHCGR gene is associated with primary ovarian insufficiency in a Pakistani family. Clin Genet 2018; 94:396–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Shi Y, Zhao H, Shi Y, Cao Y, Yang D, Li Z, Zhang B, Liang X, Li T, Chen J, Shen J, Zhao Jet al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet 2012; 44:1020–1025. [DOI] [PubMed] [Google Scholar]
- 144. Giri N, Batista DL, Alter BP, Stratakis CA. Endocrine abnormalities in patients with Fanconi anemia. J Clin Endocrinol Metab 2007; 92:2624–2631. [DOI] [PubMed] [Google Scholar]
- 145. Puga Molina LC, Luque GM, Balestrini PA, Marín-Briggiler CI, Romarowski A, Buffone MG. Molecular basis of human sperm capacitation. Front Cell Dev Biol 2018; 6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Gupta SK. The human egg's zona pellucida. Curr Top Dev Biol 2018;130:379–411. [DOI] [PubMed] [Google Scholar]
- 147. Huang HL, Lv C, Zhao YC, Li W, He XM, Li P, Sha AG, Tian X, Papasian CJ, Deng HW, Lu GX, Xiao HM. Mutant ZP1 in familial infertility. N Engl J Med 2014; 370:1220–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Dai C, Hu L, Gong F, Tan Y, Cai S, Zhang S, Dai J, Lu C, Chen J, Chen Y, Lu G, Du Jet al. ZP2 pathogenic variants cause in vitro fertilization failure and female infertility. Genet Med 2019; 21;431–440. [DOI] [PubMed] [Google Scholar]
- 149. Chen T, Bian Y, Liu X, Zhao S, Wu K, Yan L, Li M, Yang Z, Liu H, Zhao H, Chen ZJ. A recurrent missense mutation in ZP3 causes empty follicle syndrome and female infertility. Am J Hum Genet 2017; 101:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Liu W, Bai D, Chen J, Gao S. Additive-effect pattern of both ZP2 and ZP3 in human and mouse. Hum Genet 2017; 136:1493–1495. [DOI] [PubMed] [Google Scholar]
- 151. Tokuhiro K, Dean J. Glycan-Independent gamete recognition triggers egg zinc sparks and ZP2 cleavage to prevent polyspermy. Dev Cell 2018; 46:627–640.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update 1997; 3:367–382. [PubMed] [Google Scholar]
- 153. Yanez LZ, Han J, Behr BB, Reijo Pera RA, Camarillo DB. Human oocyte developmental potential is predicted by mechanical properties within hours after fertilization. Nat Commun 2016; 7:10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Liu C, Ma Y, Shang Y, Huo R, Li W. Post-translational regulation of the maternal-to-zygotic transition. Cell Mol Life Sci 2018; 75:1707–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lu X, Gao Z, Qin D, Li L. A maternal functional module in the mammalian oocyte-to-embryo transition. Trend Mol Med 2017; 23:1014–1023. [DOI] [PubMed] [Google Scholar]
- 156. Xu Y, Shi Y, Fu J, Yu M, Feng R, Sang Q, Liang B, Chen B, Qu R, Li B, Yan Z, Mao Xet al. Mutations in PADI6 cause female infertility characterized by early embryonic arrest. Am J Hum Genet 2016; 99:744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Huang L, Tong X, Wang F, Luo L, Jin R, Fu Y, Zhou G, Li D, Song G, Liu Y, Zhu F. Novel mutations in PATL2 cause female infertility with oocyte germinal vesicle arrest. Hum Reprod 2018; 33:1183–1190. [DOI] [PubMed] [Google Scholar]
- 158. Chen B, Zhang Z, Sun X, Kuang Y, Mao X, Wang X, Yan Z, Li B, Xu Y, Yu M, Fu J, Mu Jet al. Biallelic mutations in PATL2 cause female infertility characterized by oocyte maturation arrest. Am J Hum Genet 2017; 101:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Alazami AM, Awad SM, Coskun S, Al-Hassan S, Hijazi H, Abdulwahab FM, Poizat C, Alkuraya FS. TLE6 mutation causes the earliest known human embryonic lethality. Genome Biol 2015; 16:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Mu J, Wang W, Chen B, Wu L, Li B, Mao X, Zhang Z, Fu J, Kuang Y, Sun X, Li Q, Jin Let al. Mutations in NLRP2 and NLRP5 cause female infertility characterised by early embryonic arrest. J Med Genet 2019; DOI: 10.1136/jmedgenet-2018-105936. [DOI] [PubMed] [Google Scholar]
- 161. Sang Q, Li B, Kuang Y, Wang X, Zhang Z, Chen B, Wu L, Lyu Q, Fu Y, Yan Z, Mao X, Xu Yet al. Homozygous mutations in WEE2 cause fertilization failure and female infertility. Am J Hum Genet 2018; 102:649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Feng R, Sang Q, Kuang Y, Sun X, Yan Z, Zhang S, Shi J, Tian G, Luchniak A, Fukuda Y, Li B, Yu Met al. Mutations in TUBB8 and human oocyte meiotic arrest. N Engl J Med 2016; 374:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Chen B, Li B, Li D, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L, Kuang Y, Sang Q, Wang L. Novel mutations and structural deletions in TUBB8: expanding mutational and phenotypic spectrum of patients with arrest in oocyte maturation, fertilization or early embryonic development. Hum Reprod 2017; 32:457–464. [DOI] [PubMed] [Google Scholar]
- 164. Qian J, Nguyen NMP, Rezaei M, Huang B, Tao Y, Zhang X, Cheng Q, Yang H, Asangla A, Majewski J, Slim R. Biallelic PADI6 variants linking infertility, miscarriages, and hydatidiform moles. Eur J Hum Genet 2018; 26:1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Zhu K, Yan L, Zhang X, Lu X, Wang T, Yan J, Liu X, Qiao J, Li L. Identification of a human subcortical maternal complex. Mol Hum Reprod 2015; 21:320–329. [DOI] [PubMed] [Google Scholar]
- 166. Wang X, Song D, Mykytenko D, Kuang Y, Lv Q, Li B, Chen B, Mao X, Xu Y, Zukin V, Mazur P, Mu Jet al. Novel mutations in genes encoding subcortical maternal complex proteins may cause human embryonic developmental arrest. Reprod Biomed Online 2018; 36:698–704. [DOI] [PubMed] [Google Scholar]
- 167. Feng R, Yan Z, Li B, Yu M, Sang Q, Tian G, Xu Y, Chen B, Qu R, Sun Z, Sun X, Jin Let al. Mutations in TUBB8 cause a multiplicity of phenotypes in human oocytes and early embryos. J Med Genet 2016; 53:662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Gardner AJ, Evans JP. Mammalian membrane block to polyspermy: new insights into how mammalian eggs prevent fertilisation by multiple sperm. Reprod Fertil Dev 2006; 18:53–61. [DOI] [PubMed] [Google Scholar]
- 169. Check JH, Katsoff B, Summers-Chase D, Breitbart J. A case report supporting the concept that some women have a predisposition for maternal meiosis errors resulting in digyny. Clin Exp Obstet Gynecol 2009; 36:133–134. [PubMed] [Google Scholar]
- 170. Filges I, Manokhina I, Peñaherrera MS, McFadden DE, Louie K, Nosova E, Friedman JM, Robinson WP. Recurrent triploidy due to a failure to complete maternal meiosis II: whole-exome sequencing reveals candidate variants. Mol Hum Reprod 2015; 21:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Khawajkie Y, Buckett W, Nguyen NMP, Mechtouf N, Ao A, Arseneau J, Slim R. Recurrent triploid digynic conceptions and mature ovarian teratomas: are they different manifestations of the same genetic defect? Genes Chromosomes Cancer 2017; 56:832–840. [DOI] [PubMed] [Google Scholar]
- 172. Nguyen NMP, Ge ZJ, Reddy R, Fahiminiya S, Sauthier P, Bagga R, Sahin FI, Mahadevan S, Osmond M, Breguet M, Rahimi K, Lapensee Let al. Causative mutations and mechanism of androgenetic hydatidiform moles. Am J Hum Genet 2018; 103:740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Nguyen NM, Slim R. Genetics and epigenetics of recurrent hydatidiform moles: basic science and genetic counselling. Curr Obstet Gynecol Rep 2014; 3:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Nguyen NMP, Khawajkie Y, Mechtouf N, Rezaei M, Breguet M, Kurvinen E, Jagadeesh S, Solmaz AE, Aguinaga M, Hemida R, Harma MI, Rittore Cet al. The genetics of recurrent hydatidiform moles: new insights and lessons from a comprehensive analysis of 113 patients. Mod Pathol 2018; 31:1116–1130. [DOI] [PubMed] [Google Scholar]
- 175. Parry DA, Logan CV, Hayward BE, Shires M, Landolsi H, Diggle C, Carr I, Rittore C, Touitou I, Philibert L, Fisher RA, Fallahian Met al. Mutations causing familial biparental hydatidiform mole implicate C6orf221 as a possible regulator of genomic imprinting in the human oocyte. Am J Hum Genet 2011; 89: 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Soellner L, Begemann M, Degenhardt F, Geipel A, Eggermann T, Mangold E. Maternal heterozygous NLRP7 variant results in recurrent reproductive failure and imprinting disturbances in the offspring. Eur J Hum Genet 2017; 25:924–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Shamseldin HE, Kurdi W, Almusafri F, Alnemer M, Alkaff A, Babay Z, Alhashem A, Tulbah M, Alsahan N, Khan R, Sallout B, Al Mardawi Eet al. Molecular autopsy in maternal-fetal medicine. Genet Med 2018; 20:420–427. [DOI] [PubMed] [Google Scholar]
- 178. Vaiman D. Genetic regulation of recurrent spontaneous abortion in humans. Biomed J 2015; 38:11–24. [DOI] [PubMed] [Google Scholar]
- 179. Arias-Sosa LA, Acosta ID, Lucena-Quevedo E, Moreno-Ortiz H, Esteban-Pérez C, Forero-Castro M. Genetic and epigenetic variations associated with idiopathic recurrent pregnancy loss. J Assist Reprod Genet 2018; 35:355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Pereza N, Ostojić S, Kapović M, Peterlin B. Systematic review and meta-analysis of genetic association studies in idiopathic recurrent spontaneous abortion. Fertil Steril 2017; 107:150–159.e2. [DOI] [PubMed] [Google Scholar]
- 181. Shi X, Xie X, Jia Y, Li S. Maternal genetic polymorphisms and unexplained recurrent miscarriage: a systematic review and meta-analysis. Clin Genet 2017; 91:265–284. [DOI] [PubMed] [Google Scholar]
- 182. Wang X, Mittal P, Castro CA, Rajkovic G, Rajkovic A. Med12 regulates ovarian steroidogenesis, uterine development and maternal effects in the mammalian egg. Biol Reprod 2017; 97:822–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Utsch B, Becker K, Brock D, Lentze MJ, Bidlingmaier F, Ludwig M. A novel stable polyalanine [poly(A)] expansion in the HOXA13 gene associated with hand-foot-genital syndrome: proper function of poly(A)-harbouring transcription factors depends on a critical repeat length? Hum Genet 2002; 110:488–494. [DOI] [PubMed] [Google Scholar]
- 184. Tas E, Sebastian J, Madan-Khetarpal S, Sweet P, Yatsenko AN, Pollock N, Rajkovic A, Schneck FX, Yatsenko SA, Witchel SF. Familial deletion of the HOXA gene cluster associated with Hand-Foot-Genital syndrome and phenotypic variability. Am J Med Genet 2017; 173:221–224. [DOI] [PubMed] [Google Scholar]
- 185. Wang M, Hao C, Huang X, Bao H, Qu Q, Liu Z, Dai H, He S, Yan W. Aberrant expression of lncRNA (HOXA11-AS1) and homeobox A (HOXA9, HOXA10, HOXA11, and HOXA13) genes in infertile women with endometriosis. Reprod Sci 2018; 25:654–661. [DOI] [PubMed] [Google Scholar]
- 186. Du H, Taylor HS. The role of hox genes in female reproductive tract development, adult function, and fertility. Cold Spring Harb Perspect Med 2016; 6:a023002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Jacquinet A, Millar D, Lehman A. Etiologies of uterine malformations. Am J Med Genet 2016; 170:2141–2172. [DOI] [PubMed] [Google Scholar]
- 188. Mullen RD, Behringer RR. Molecular genetics of Müllerian duct formation, regression and differentiation. Sex Dev 2014; 8:281–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Jagarlamudi K, Rajkovic A. Oogenesis: transcriptional regulators and mouse models. Mol Cell Endocrinol 2012; 356:31–39. [DOI] [PubMed] [Google Scholar]
- 190. Amargant F, Barragan M, Vassena R, Vernos I. Insights of the tubulin code in gametes and embryos: from basic research to potential clinical applications in humans. Biol Reprod 2019; 100:575–589. [DOI] [PubMed] [Google Scholar]
- 191. Dangle P, Touzon MS, Reyes-Múgica M, Witchel SF, Rajkovic A, Schneck FX, Yatsenko SA. Female-to-male sex reversal associated with unique Xp21.2 deletion disrupting genomic regulatory architecture of the dosage-sensitive sex reversal region. J Med Genet 2017; 54:705–709. [DOI] [PubMed] [Google Scholar]
- 192. Portnoi MF, Dumargne MC, Rojo S, Witchel SF, Duncan AJ, Eozenou C, Bignon-Topalovic J, Yatsenko SA, Rajkovic A, Reyes-Mugica M, Almstrup K, Fusee Let al. Mutations involving the SRY-related gene SOX8 are associated with a spectrum of human reproductive anomalies. Hum Mol Genet 2018; 27:1228–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Verdin H, D’haene B, Beysen D, Novikova Y, Menten B, Sante T, Lapunzina P, Nevado J, Carvalho CM, Lupski JR, De Baere E. Microhomology-mediated mechanisms underlie non-recurrent disease-causing microdeletions of the FOXL2 gene or its regulatory domain. PLoS Genet 2013; 9:e1003358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Zhao H, Rajkovic A. MicroRNAs and mammalian ovarian development. Semin Reprod Med 2008; 26:461–468. [DOI] [PubMed] [Google Scholar]
- 195. Ahn HW, Morin RD, Zhao H, Harris RA, Coarfa C, Chen ZJ, Milosavljevic A, Marra MA, Rajkovic A. MicroRNA transcriptome in the newborn mouse ovaries determined by massive parallel sequencing. Mol Hum Reprod 2010; 16:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Sirotkin AV, Lauková M, Ovcharenko D, Brenaut P, Mlyncek M. Identification of microRNAs controlling human ovarian cell proliferation and apoptosis. J Cell Physiol 2010; 223:49–56. [DOI] [PubMed] [Google Scholar]
- 197. Sirotkin AV. RNA interference and ovarian functions. J Cell Physiol 2010; 225:354–363. [DOI] [PubMed] [Google Scholar]
- 198. Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, Shimamoto S, Imamura T, Nakashima K, Saitou M, Hayashi K. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 2016; 539:299–303. [DOI] [PubMed] [Google Scholar]
- 199. Delcour C, Amazit L, Patino LC, Magnin F, Fagart J, Delemer B, Young J, Laissue P, Binart N, Beau I. ATG7 and ATG9A loss-of-function variants trigger autophagy impairment and ovarian failure. Genet Med 2019; 21:930–938. [DOI] [PubMed] [Google Scholar]
- 200. Salian-Mehta S, Xu M, Knox AJ, Plummer L, Slavov D, Taylor M, Bevers S, Hodges RS, Crowley WF Jr, Wierman ME. Functional consequences of AXL sequence variants in hypogonadotropic hypogonadism. J Clin Endocrinol Metab 2014; 99:1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. McGuire MM, Bowden W, Engel NJ, Ahn HW, Kovanci E, Rajkovic A. Genomic analysis using high-resolution single-nucleotide polymorphism arrays reveals novel microdeletions associated with premature ovarian failure. Fertil Steril 2011; 95:1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Hyon C, Mansour-Hendili L, Chantot-Bastaraud S, Donadille B, Kerlan V, Dodé C, Jonard S, Delemer B, Gompel A, Reznik Y, Touraine P, Siffroi JPet al. Deletion of CPEB1 gene: a rare but recurrent cause of premature ovarian insufficiency. J Clin Endocrinol Metab 2016; 101:2099–2104. [DOI] [PubMed] [Google Scholar]
- 203. Cekic S, Saglam H, Gorukmez O, Yakut T, Tarim O, Kilic SS. Delayed puberty and gonadal failure in patients with HAX1 mutation. J Clin Immunol 2017; 37:524–528. [DOI] [PubMed] [Google Scholar]
- 204. Franca MM, Han X, Funari MFA, Lerario AM, Nishi MY, Fontenele EGP, Domenice S, Jorge AAL, Garcia-Galiano D, Elias CF, Mendonca BB. Exome sequencing reveals POLR3H gene as a novel cause of primary ovarian insufficiency. J Clin Endocrinol Metab 2019; (in press). Published online ahead of print 4 March 2019; DOI 10.1210/jc.2018-02485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Wang Q, Li D, Cai B, Chen Q, Li C, Wu Y, Jin L, Wang X, Zhang X, Zhang F. Whole-exome sequencing reveals SALL4 variants in premature ovarian insufficiency: an update on genotype-phenotype correlations. Hum Genet 2019; 138:83–92. [DOI] [PubMed] [Google Scholar]
- 206. Smirin-Yosef P, Zuckerman-Levin N, Tzur S, Granot Y, Cohen L, Sachsenweger J, Borck G, Lagovsky I, Salmon-Divon M, Wiesmüller L, Basel-Vanagaite L. A biallelic mutation in the homologous recombination repair gene SPIDR is associated with human gonadal dysgenesis. J Clin Endocrinol Metab 2017; 102:681–688. [DOI] [PubMed] [Google Scholar]