Abstract
Zona pellucida (ZP), which enwraps the oocyte during folliculogenesis, initially forms in the primary follicle and plays an important role in female fertility. Here, we investigated a mouse strain (“mutant mice” for short) carrying two types of ZP defects in folliculogenesis, i.e., ZP thinned (but intact) and ZP cracked, caused by targeted mutation in the Zp1 gene. Using this mutant mouse strain and wild-type mouse as control, we studied the effects of the ZP defects on the development of oocytes and granulosa cells during folliculogenesis. For each ZP defect, we examined the morphology of transzonal projections and apoptosis of granulosa cells in the corresponding growing follicles, as well as the morphology of corresponding ovulated eggs and their abilities to develop into viable individuals. Our results suggested that ZP integrity rather than thickness or porosity is crucial for preventing the ectopia of granulosa cells, maintaining adequate routine bilateral signaling between oocyte and surrounding granulosa cells, and thus for ensuring the survival of granulosa cells and the establishment of the full developmental competence of oocytes. This is the first study to elucidate the effects of different degrees of ZP defects caused by the same gene mutation, on the apoptosis of granulosa cells and developmental competence of oocytes, and to explore the potential mechanisms underlying these effects.
Keywords: infertility, zona pellucida, granulosa cells, apoptosis, oocyte development, oocyte-follicle interactions, embryo culture, blastocyst
Zona pellucida integrity rather than thickness or porosity is important for maintaining transzonal projections, ensuring the survival of granulosa cells and the establishment of the full developmental competence of oocytes during folliculogenesis.
Introduction
Zona pellucida (ZP) is the eggshell-like extracellular matrix encapsulating mammalian oocytes, fertilized eggs, and preimplantation embryos. It plays an important role in folliculogenesis, fertilization, and development of preimplantation embryos [1–3]. Zona pellucida is composed of zona pellucida glycoproteins (ZPGs), and human ZP contains four ZPGs, hZP1-4, which are synthesized and secreted by the oocyte [4]. Defects in ZP are thought to be closely related to poor outcomes in in vitro fertilization (IVF) treatment: defective ZP reduces implantation rate and live birth rate, making it an important cause of infertility [5]. In 2014, we reported a familial infertility case characterized by complete absence of eggs’ ZP and failure of IVF and identified the pathogenic mutation of the human ZP1 gene [6]. Thereafter, several more cases of human ZP defects and infertility caused by ZP mutations have been revealed. For example, in 2017 Chen et al. [7] found that ZP3 mutation led to absence of ZP and degeneration of oocytes. Liu et al. [8, 9] reported that coexistence of ZP2 and ZP3 heterozygous mutations in a female resulted in eggs with very thin or even completely absent ZP and those eggs showed polyspermy or degradation following IVF. In 2018, Dai et al. [10] demonstrated that ZP2 mutations caused thinned ZP and IVF failure. In mice, knockout of Zp1 resulted in thinned and loosened ZP and loss of embryos due to precocious hatching [11]. Knockout of mouse Zp2 or Zp3 led to absence of ZP in mature eggs, and blastocysts originating from these eggs lost the ability to develop into viable individuals when transferred into uterus [12, 13]. The above studies illustrated that ZP defects caused by mutations of ZP genes are deleterious to the oocyte's full developmental competence, i.e., the ability to undergo meiotic maturation, be fertilized, and give rise to a healthy embryo and viable new-born individual.
It is well known that there is a complex bilateral signaling between the oocyte and surrounding granulosa cells to coordinate the synchronous development of both cells: promoting the proliferation and differentiation of granulosa cells, inhibiting their apoptosis, and boosting the growth and development of the oocyte to ensure that the ultimate mature egg has full developmental competence [14, 15]. Therefore, to some extent, excessive apoptosis of granulosa cells may be a sign of impaired developmental competence of oocytes. For instance, Host et al. [16] reported that a higher degree of apoptosis was detected in cumulus cells from cumulus oocyte complexes (COCs) with immature oocytes, when comparing with mature oocytes in humans. Furthermore, apoptosis in cumulus cells impaired the fertilization rate of metaphase II oocytes after intracytoplasmic sperm injection.
Transzonal projections (TZPs) are filamentary structures composed of cytoplasmic tips that extend from the oocyte and compassing granulosa cells, spanning the ZP to form gap junctions [17]. TZPs are maintained and stabilized by ZP [18]. In mice, gap junctions of TZPs are mainly composed of connexin 37, allowing small molecules (<1 kDa) to pass through [19]. Simon et al. [19] found that gap junctions between the oocyte and surrounding granulosa cells disappeared in mice lacking connexin 37. Follicles of this mouse strain could not develop beyond the preantral stage, resulting in the oocytes’ inability to complete meiosis [19]. Recently, Komatsu et al. [20] found that in addition to gap junctions, TZPs can also be formed by direct fusion of cell membranes, which can mediate the transportation of big molecules (>10 kDa), such as growth and differentiation factor-9 (GDF-9), one of the key molecules involved in bilateral signaling. In mice lacking the Gdf-9 gene, folliculogenesis was blocked at the primary stage [21].
Based on all the above studies, we hypothesize that ZP defects attenuate TZPs and bilateral signaling in folliculogenesis, and as a result contribute to the apoptosis of granulosa cells and the loss of the oocytes’ developmental competence. After alignment with human DNA sequences, we used an applicable CRISPR/Cas9 system to edit the mouse DNA in order to gain a mouse strain (“mutant mice” for short) carrying a mutation in the Zp1 gene similar to that we previously reported in humans [6]. Unlike humans, the eggs of the mutant mice mainly presented two morphologic types: one with thinned but intact ZP and the other without ZP, originating from growing follicles with thinned but intact ZP and cracked ZP, respectively. In this article, for each ZP defect in folliculogenesis, we examined the morphology of TZPs and apoptosis of granulosa cells in the growing follicles, as well as the potential of corresponding eggs to develop into viable individuals in order to test our hypothesis.
Materials and Methods
Animals
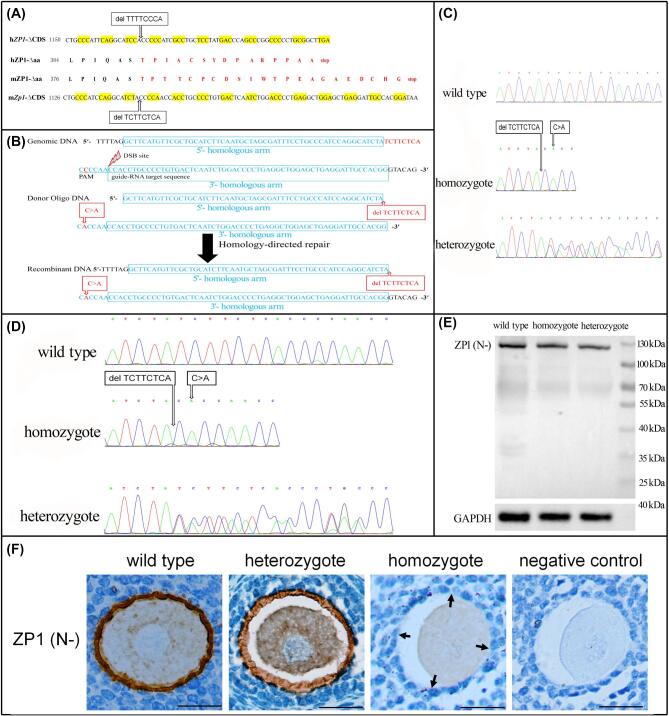
We established a mutation in C57BL/6J mouse Zp1 similar to that identified in human ZP1 [6] (an 8bp-deletion at nucleotides 1169-1176 (TTTTCCCA) of human ZP1 CDS, i.e., CCDS31572.1). According to the BLAST search result, we determined that an 8 bp-deletion at nucleotides 1145-1152 (TCTTCTCA) of mouse Zp1 CDS (CCDS37918.1) would result in a premature stop codon, encoding a truncated mouse ZP1 protein of 403 aa (Figure 1A). According to the designed CRISPR/Cas9 system (Figure 1B), we performed zygote microinjections and blastocyst transfers. Mating of F0 generation mutant mice and wild type was arranged in order to generate heterozygous F1. Sanger sequencing confirmed the expected various DNA sequences of F1 and F2 mice with different genotypes (Figure 1C). These were also confirmed at the cDNA level (Figure 1D). Finally, we performed western blot (Figure 1E) and immunohistochemistry (Figure 1F) to ascertain the changes of mutant ZP1 protein. Using the ZP1 (N-) antibody (sc-49582, Santa-Cruz, raised against a peptide mapping near the N-terminus of ZP1), mutant ZP1 was successfully detected only in the cytoplasm of the oocyte and was undetectable in the ZP in homozygous mice (Figure 1F), but unexpectedly, the molecular weight seemed unchanged (Figure 1E).
Figure 1.
Targeted mutation in mouse Zp1 gene. (A) Nucleotide and protein alignments of Zp1 mutant mouse and ZP1 mutant human. hZP1-ΔCDS: coding sequence of mutant human ZP1; hZP1-Δaa: amino acid sequence of mutant human ZP1; mZP1-Δaa: amino acid sequence of mutant mouse ZP1; mZp1-ΔCDS: coding sequence of mutant mouse Zp1. (B) Scheme of CRISPR/Cas9-mediated genomic editing. c.1154C > A is a synonymous mutation for the 8bp-deleted Zp1 and was deliberately introduced in order to avoid potential improper cut for the second time of mutant DNA; DSB: double-strand break; PAM: protospacer adjacent motif. (C, D) Sanger sequencing of F1 and F2 mice at the genomic DNA (C) and cDNA (D) levels. (E) Western blot of moue ZP1 protein. (F) Detection of mouse ZP1 protein in growing follicles. Arrows indicated absent signal of mutant ZP1 in ZP, scale bar 20 μm.
In this study, wild-type and homozygous mutant mice of F2 and F3 generations were used. Only the ones aged 10–13 weeks and weighing 25–30 g were studied. All mice were housed in an environmentally controlled room maintained at 23 ± 1°C with a 12 h light/dark cycle. Animal care and the experiments using them were conducted in accordance with the Guidelines for Animal Experimentation, Central South University, Changsha, Hunan, China, and were approved by The Animal Care and Use Committee of the university.
Ovarian histology
The mice were sacrificed by cervical vertebra dislocation and their ovaries were isolated, weighed, and fixed in 4% paraformaldehyde solution for 16–20 h. After dehydration, ovaries were embedded in paraffin. Twelve ovaries (6 wild type and 6 mutant) were assembled into a wax block to make tissue microarrays. To highlight the ZP, periodic acid Schiff's (PAS) staining was performed. Paraffin sections of ovarian tissue chips were generated at a thickness of 4 μm and an interval of 100 μm, mounted sections were stained with PAS reagent and hematoxylin, and scanned with a slice scanner (pannoramic MIDI, 3DHISTECH Corporation). The area of each tissue section was measured by Image-pro plus 6.0 (Media Cybernetics Corporation), and the growing follicles were classified and counted. Apoptosis of the follicles was detected by a terminal deoxynucleotidyl transferase 2′-deoxyuridine, 5′-triphosphate nick end labeling (TUNEL) system (Roche11684817910), following the manufacturer's instructions and dyeing the tissue microarrays with DAB chromogenic agent (DAKO, K5007) and hematoxylin. To reveal the morphology of TZPs, phalloidin staining was performed. After fixation and dehydration, ovaries were made into frozen sections with 10 μm thickness. The sections were washed with PBS and incubated with 1:500 diluted phalloidin-iFluor 555 reagent (Abcam 176756) at room temperature for 90 min. After washing with PBS again, the sections were stained with DAPI for 3 min, then mounted and observed under a fluorescence microscope (Olympus BX53).
Isolation of ovulated eggs
Female mice were intraperitoneally injected with 10 IU pregnant mare serum gonadotropin (PMSG) between 5:00 p.m. and 6:00 p.m. and 10 IU human chorionic gonadotropin (hCG) 48 h later. After 15 h, mice were sacrificed, and the fallopian tubes were isolated. Then, each fallopian tube was placed into an oil-covered G-MOPS plus (vitrolife corporation) droplet of 30 μl, which had been previously equilibrated in a CO2-free environment at 37°C for 1 h at least. Under the microscope, the enlarged ampulla of the fallopian tube was torn out, the COCs were dragged into the droplet and then transferred into a 50 μl droplet of M2 medium with hyaluronidase (1 mg/ml), which was preheated at 37°C. Then, the COCs were gently blown with a pipette carrying a fine tip until the granulosa cells dispersed. The detached eggs were washed three times in G-MOPS plus droplets and photographed with a relief contrast (RC) microscope (IX73, Olympus corporation).
Transmission electron microscopy of COCs
Isolated COCs were washed in 0.1M PBS and fixed in 2.5% glutaraldehyde for 6 h. After washing in PBS 3 times and embedding in 3% agarose, the agar blocks were placed in 1% osmic acid for 1.5 h, dehydrated in a series of graded acetone solutions, and immersed in a mixture of acetone and embedding medium (epon-812, dodecyl succinic anhydride, methyl nonyl acetaldehyde, and dimethyl phthalate-30) at 1:1 for 6 h. Then the agar blocks were embedded in the embedding medium overnight. Ultrathin sections were made, counterstained with uranyl acetate and lead citrate, and examined with a Tecnai G2 Spirit TWIN transmission electron microscope (FEI Company).
Collection and culture of zygotes and preimplantation embryos
After injection of PMSG and hCG, the mice were mated with fertile males. Vaginal plug was checked the next morning (0.5 day post coitus, 0.5 DPC). Approximately, 22 h after injection of hCG, the females were killed and COCs were isolated. Then COCs were washed 3 times with pre-balanced (in the environment of 37°C and 5% CO2 for 8–12 h) G-1 plus oil-covered droplets and were transferred into G-1 plus droplets (50 μl for COCs from each fallopian tube) for culture. After 5 h of culture, the granulosa cells were dispersed, the detached zygotes were washed with preheated G-MOPS plus droplets, and the pronuclei were observed with RC microscope. The unfertilized or polyspermic eggs were abandoned and the normal zygotes (with two pronuclei) were further separately cultured in G-1 plus droplets of 10 μl for each zygote. The embryos were photographed 40, 64, 88, and 110 h after injection of hCG. For better control of mutant ZP free eggs, wild-type eggs were collected and the ZP was removed by acidic tyrode's solution (Sigma-Aldrich Corporation, T1788) 13 h after injection of hCG. These eggs were co-incubated for 1 h with capacitated sperms at a final concentration of 5000/ml in pre-balanced G-IVF (Vitrolife Corporation) droplets. After careful washing, all eggs were cultured separately, washed again 12 h later, and photographed. After in vitro culture, blastocysts of 4.5 DPC were transferred to 3.5 DPC pseudopregnant female mice. For each pseudopregnant mouse, 8–12 blastocysts were transplanted and evenly distributed in both the uteri.
Statistics
Statistical analysis was performed using SPSS 19. Data were represented as mean ± standard deviation for measurement data or by rate ± standard error for counting data. The measurement data were tested by t-test, and the counting data were tested by χ2 test. P < 0.05 was considered to be statistically significant.
Results
The number and composition of growing follicles in mutant mice were unaffected
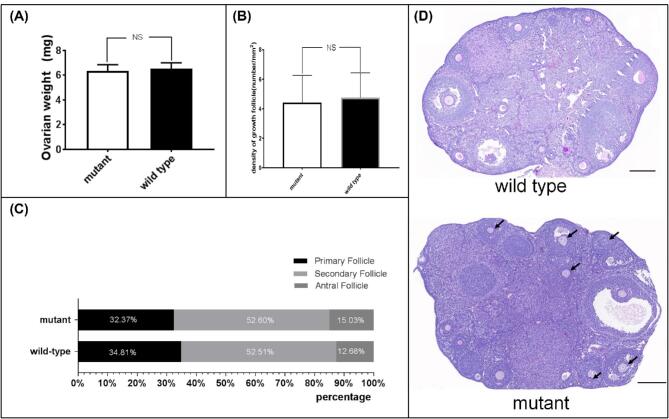
From a total of 20 mice (10 mutant and 10 wild type), 40 ovaries were obtained and weighed. The mean weight of paired ovaries (left plus right) was 6.53 mg in wild-type and 6.34 mg in mutant mice. No significant statistical difference was observed between two groups (P = 0.400) (Figure 2A). In order to evaluate the density and composition of growing follicles, 72 discontinuous ovarian tissue sections, derived from 6 wild-type and 6 mutant ovaries, were stained by the PAS method. The number of wild-type sections equaled that of mutant ones. The average density of the growing follicles in wild-type and mutant mice was 4.76/mm2 and 4.42/mm2, respectively, and no statistically significant difference was observed (P = 0.412) (Figure 2B). Referring to Gook et al. [22], the growing follicles were classified into primary follicle, with only one layer of cubic or columnar granular cells encompassing the oocyte; secondary follicle, in which the oocyte was surrounded by at least two layers of cubic granulosa cells and no obvious antrum could be observed; and antral follicle, in which an obvious antrum could be observed. According to statistics, 339 wild-type follicles were found, with constituent ratios of primary follicle 34.81%, secondary follicle 52.51%, and antral follicle 12.68%, respectively. A total of 346 mutant follicles were found with constituent ratios of primary follicle 32.37%, secondary follicle 52.60%, and antral follicle 15.03%, respectively (Figure 2C). The difference of constituent ratios between mutant and wild type growing follicles was not statistically significant (P = 0.612). These results strongly suggested that the total number and composition of growing follicles in mutant mice seemed to be unaffected.
Figure 2.
The weight of ovaries and the density and composition of growing follicles. (A) weights of paired ovaries (left plus right) were showed (mean ± SD); (B) density of growing follicles in each tissue section was calculated and exhibited (mean ± SD); (C) constituent ratios of growing follicles; (D) general morphology of ovaries (PAS staining). NS: not significant; arrows indicate cracked ZP; scale bar 200 μm.
Sparse TZPs and increased apoptosis of granulosa cells were observed in growing follicles with cracked ZP
The ZP in follicles was conspicuously red in PAS (Figure 3A–F). The primary follicles of mutant mice seemed morphologically normal with intact ZP, while the secondary and antral follicles presented two types: ZP intact and ZP cracked, and the latter accounted for the majority (51% in secondary follicle and 73% in antral follicle). The ZP-intact follicles (Figure 3C and D) were morphologically almost the same as the wild type (Figure 3A and B), but the ZP of mature oocyte (Figure 3D) was significantly thinner than that of the wild type (Figure 3B). In ZP-cracked follicles, granulosa cells were ectopic in the perivitelline space, and at the ectopic zone, part of the ZP was peeled from the oocyte (Figure 3E and F). The ectopic granulosa cells closest to the oocyte directly contacted the oocyte instead of via the ZP.
Figure 3.
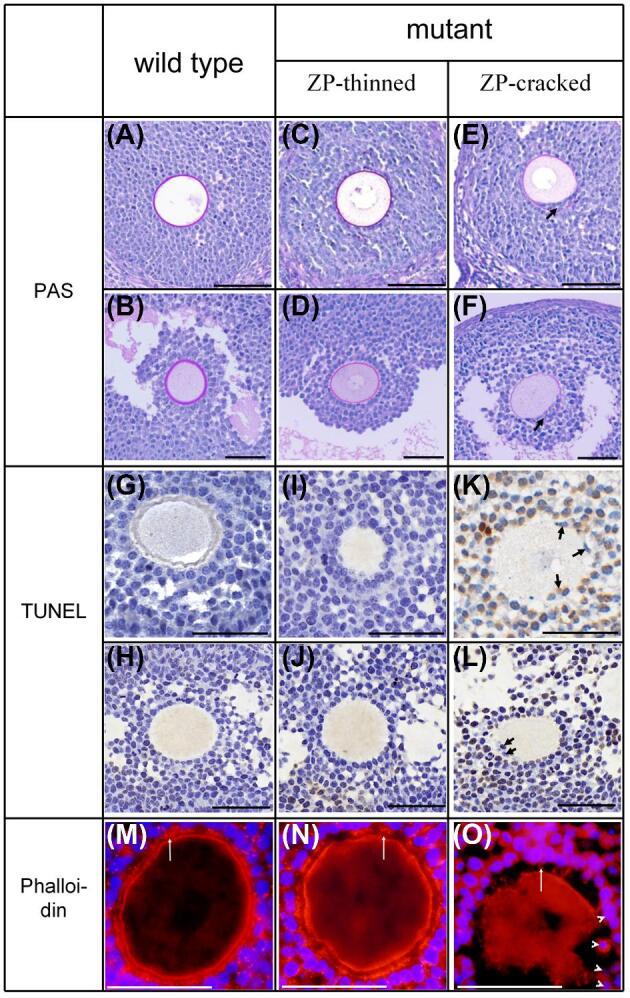
Histology assay of growing follicles. PAS (A–F), TUNEL (G–L) assay, and phalloidin (M–O) staining are presented. Follicles are divided into 3 groups: wild-type follicles, including secondary (A, G, M) and antral (B, H) follicles; mutant follicles with intact ZP, including secondary (C, I, N) and antral (D, J) follicles; mutant follicles with cracked ZP and ectopic granulosa cells, including secondary (E, K, O) and antral (F, L) follicles. Black arrows and white arrow heads indicate ectopic granulosa cells, white arrows indicate TZPs. Scale bar 50 μm.
For the TUNEL analysis, 6 tissue sections from 3 mice were stained and considered for statistical analysis for each genotype. The growing follicles of the total 12 sections were classified into 3 groups: wild type, ZP-thinned, and ZP-cracked, the latter two were both from the mutant mice. Since the overwhelming majority of early stage secondary follicles in mutant mice was ZP-intact, only the secondary follicles with more than 3 layers of granulosa cells were considered. According to this screen criterion, a total of 29 secondary follicles (including 16 wild type, 6 ZP-thinned, and 7 ZP-cracked) and 19 antral follicles (including 9 wild type, 4 ZP-thinned, and 6 ZP-cracked) were statistically analyzed. As the overwhelming majority of apopotic granulosa cells of antral follicles were cumulus cells, mural granulosa cells were not included into counting. A total of 8959 granulosa cells of secondary follicles (including 4669 wild type, 2028 ZP-thinned, and 2262 ZP-cracked) and 2482 granulosa cells of antral follicles (including 1215 wild type, 504 ZP-thinned, and 763 ZP-cracked) were counted. The results of statistical analysis are presented in Figure 4. These results showed that apoptosis of granulosa cells encompassing the oocyte was very slight in the ZP-intact secondary (Figure 3I) and antral follicles (Figure 3J) of mutant mice, consistent with those of wild type (Figure 3G and H). However, apoptosis of granulosa cells was significantly increased in ZP-cracked follicles with multiple granulosa cell ectopia (Figure 3K and L).
Figure 4.
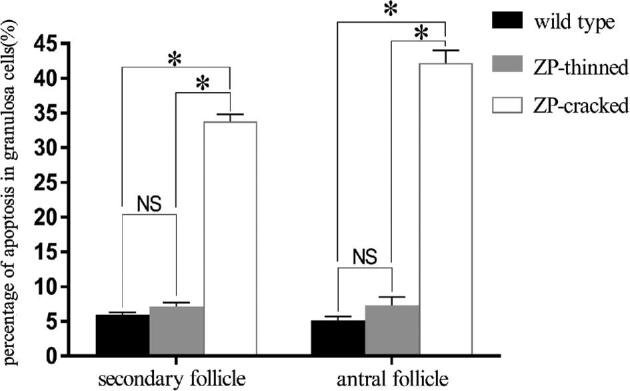
Apoptosis of granulosa cells in secondary and antral follicles. *P < 0.05, NS: not significant.
Phalloidin staining showed that the TZPs in ZP-intact growing follicles of mutant mice (Figure 3N), as well as in the ZP-cracked growing follicles with very few ectopic granulosa cells, were rather abundant, consistent with that in the wild type (Figure 3M). However, in the ZP-cracked growing follicles with multiple ectopic granulosa cells (Figure 3O, the white arrow heads), the density of TZPs was significantly decreased not only in the ectopic zone but also in the normal zone (Figure 3O).
Two types of eggs, i.e., ZP thinned (intact but loosened) and ZP free, were observed in the mutant mice
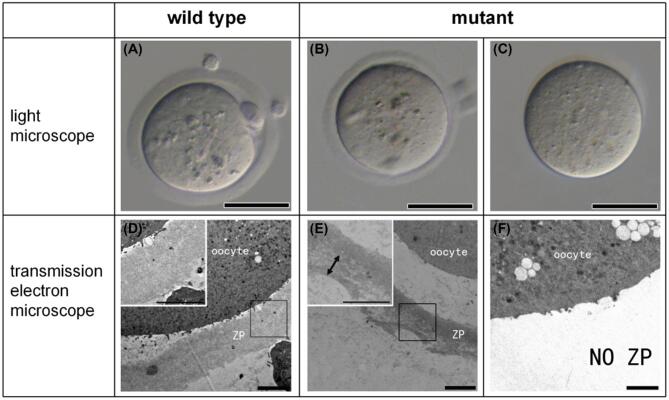
Under light microscope, the eggs of mutant mice mainly presented two types of morphologies: one with thinned but intact ZP (Figure 5B), accounting for about 50%; the other without ZP (Figure 5C), accounting for about 40%. The former originated from the ZP-intact follicles (Figure 3D) and the latter from the ZP-cracked ones (Figure 3F). Compared with the wild type (Figure 5D), the ZP of the mutant mice (Figure 5E) was loosened, particularly the outermost layer (Figure 5E, the double arrow).
Figure 5.
Zona pellucida morphology of ovulated eggs under light microscope (A–C) and transmission electron microscope (D, E). (A, D) Eggs of wild-type mice; (B, E) ZP-intact eggs of mutant mice; (C, F) ZP-free eggs of mutant mice. The inserts in the top left corner are enlarged views of the corresponding boxes, double arrow heads indicate the outermost layer of ZP, scale bar: A–C, 50 μm; D–F, 4 μm.
The developmental potential of ZP-thinned eggs was normal, but that of ZP-free eggs was reduced
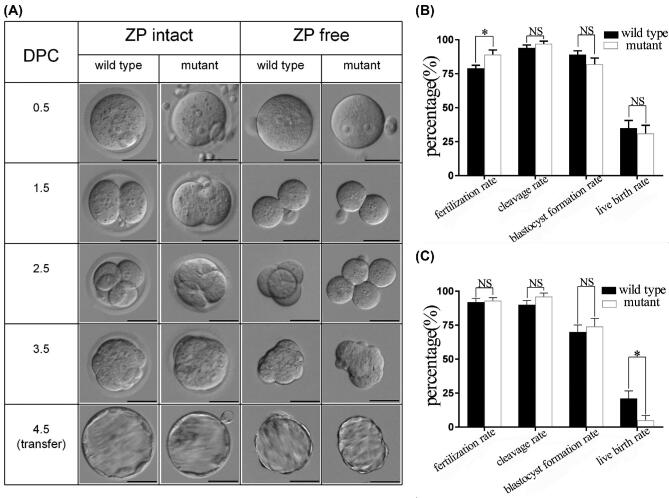
The eggs of mutant mice were divided into 2 groups: ZP intact (but thinned and loosened) and ZP free, correspondingly the wild-type eggs with intact ZP or removed ZP was set as control. Progression of fertilization and embryonic development was recorded by photos (Figure 6A) and a table (Table 1), and the statistical results of the data were presented in the column diagrams (Figure 6B and C), no dead birth was observed after transfer of blastocysts. The fertilization rate of ZP-intact eggs in mutant was higher than that in wild type (P = 0.042), but there was no statistically significant difference in cleavage rate, blastocyst formation rate, and live birth rate (Figure 6B). Only 5% of the blastocysts issuing from ZP-free eggs in mutant mice developed into viable individuals after transplantation, while in wild type the live birth rate of blastocysts issuing from ZP-removed eggs was 21%, the difference was statistically significant (P = 0.034). However, there was no significant difference in preimplantation developmental potential (fertilization rate, cleavage rate, and blastocyst formation rate) between mutant ZP-free eggs and wild-type ones (Figure 6C). Altogether, although ZP was thinned and loosened, ZP-intact eggs of the mutant mice had normal developmental competence, but ZP-free eggs of the mutant mice had significantly impaired developmental competence, showing a severely reduced live birth rate.
Figure 6.
Progression of embryogenesis. (A) Image of embryos in different days post coitus; (B) column graph of embryogenesis issuing from ZP-intact eggs; (C) column graph of embryogenesis issuing from ZP-free eggs. Scale bar: 50 μm, DPC: day post coitus, *P < 0.05, NS: not significant.
Table 1.
Development of zygotes originates from ZP-intact and ZP-free eggs.
| ZP-intact | ZP-free | |||
|---|---|---|---|---|
| wild type | mutant | wild type | mutant | |
| Eggs | 159 | 83 | 97 | 59 |
| Zygotes# | 125 (79%) | 74 (89%) | 89 (92%) | 55 (93%) |
| Two-cell embryos** | 117 (94%) | 72 (97%) | 80 (90%) | 53 (96%) |
| Blastocysts* | 104 (89%) | 59 (82%) | 56 (70%) | 39 (74%) |
| Transferred‡ | 72 | 59 | 52 | 38 |
| Live births§ | 25 (35%) | 18 (31%) | 11 (21%) | 2 (5%) |
#Number of zygotes (fertilization rate).
**Number of embryos (cleavage rate).
*Number of embryos (blastocyst formation rate).
‡Number of cultured blastocysts transferred to pseudopregnant females.
§Number of live births (live birth rate).
Discussion
Using CRISPR/Cas9 system, we successfully obtained Zp1 c.1145_1152delTCTTCTCA homozygous mutant mice. In folliculogenesis, ZP in the secondary and antral follicles of the mutant mice showed varying degrees of defects: thinned (but intact) and cracked ZP. In the ZP-thinned follicles, the density of TZPs and apoptosis of granulosa cells were not affected, whereas in the ZP-cracked follicles with multiple ectopic granulosa cells lodging in the perivitelline space, density of TZPs was significantly reduced and the apoptosis of granulosa cells was significantly increased. Zona pellucida of ovulated eggs in mutant mice was thinned (loosened but still intact) or absent, originating from ZP-thinned or ZP-cracked follicles, respectively. The ZP-thinned eggs showed comparable developmental competence when compared with the ZP-intact wild-type eggs, but the ZP-free eggs showed significantly impaired competence characterized by dramatically reduced live birth rate when compared with ZP-removed wild-type eggs. These findings supported that ZP integrity rather than thickness or porosity in folliculogenesis is crucial for the survival of granulosa cells and developmental potential of the oocyte. This study is expected to advance the assessment of the eggs’ developmental potential in patients with ZP morphological abnormalities, but major differences in species and pathogenic genes must be carefully considered. Similar mutation of human ZP1 led to absence of ZP in all the retrieved eggs, which could not be fertilized in vitro [6]. The developmental potential of these human ZP-free eggs is significantly weaker than that of mouse ZP-free eggs. We hypothesize that since the number and transport mechanism of human ZPGs are different from those of mouse, it results in the failure of ZP formation throughout folliculogenesis and therefore a worse developmental potential of eggs in humans. In 2018, Dai et al. [10] reported a case of thinned ZP caused by human ZP2 mutation, and unlike the ZP-thinned eggs of mutant mice described in this article, these human ZP-thinned eggs could not be fertilized in vitro. This difference may be attributed to the different function of ZP1 and ZP2: ZP2 which is absent in the human thinned ZP plays a role of recognizing the sperm during fertilization while ZP1 does not.
In the ZP-cracked follicles of mutant mice, the ectopic granulosa cells closest to the oocyte directly contacted the oocyte instead of via the ZP, thus disturbing the normal bilateral signaling between the oocyte and surrounding granulosa cells mediated by TZPs. At the beginning, when very few granulosa cells were ectopic, only the TZPs at the ectopic zone disappeared and the apoptosis of granulosa cells did not increase significantly. However, when multiple granulosa cells were ectopic in the perivitelline space, TZPs at the normal zone (no ectopic granulosa cell) were also dramatically reduced, and the reason for this needs to be further explored. Previous studies have shown that the resumption of meiosis of oocytes induced by leuteinizing hormone is accompanied by the disappearance of TZPs, and the microfilament disruptor cytochalasin E can accelerate the disappearance of TZPs and prevent cumulus expansion [23]. Intriguingly, in mice lacking GDF-9, a growth factor produced by the oocyte, TZPs were reduced and frequently lay parallel rather than perpendicular to the oocyte surface [21]. These findings suggest that changes in the metabolism of granulosa cells or oocytes can affect the density and distribution of TZPs. In addition, in a growing follicle, there are gap junctions and even fusion of cell membranes not only between granulosa cells and the oocyte, but also between granulosa cells themselves [20, 24, 25]. In view of these facts, we hypothesize that multiple ectopic granulosa cells disturb the normal bilateral signaling and result in considerable changes in metabolism of themselves and the oocyte. Metabolism of the granulosa cells without ectopia may also be affected because of the disturbance of extensive communication not only between granulosa cells and the oocyte, but also between granulosa cells themselves. These putative metabolic changes might lead to the ubiquitous diminishment of TZPs. Ubiquitous reduction of TZPs further affects the routine and proper bilateral signaling between the granulosa cells and the oocyte, thus promoting the apoptosis of granulosa cells and hindering the establishment of the oocyte's full developmental competence. Conversely, the density and distribution of TZPs in the ZP-intact follicles of mutant mice are normal, and the development of oocytes and granulosa cells is not impaired, suggesting that the integrity of ZP is crucial to the development of both cells.
In the case report of 2014, we suggested that mutations in a single ZP protein might hinder the secretion of other ZP proteins by shifting the intracellular interaction of ZPs [6]. Subsequent reports on ZP2/ZP3 mutations further supported this idea by using cell lines transfected with recombinant plasmids [7, 10]. Compared with Zp1 null mice, the phenotype of our mouse strain seems to be more serious: the ZP-cracked growing follicles and ZP-free eggs are in a higher proportion and the litter size is smaller (2.3 VS 3.6). One plausible explanation is that mutant ZP1 protein hinders the transport of wild-type ZP2/ZP3, but this explanation has at least two obvious problems: first, the differences in animal feeding and experimental operations between different laboratories restrict the significance of phenotypic comparison; second, whether intracellular interaction of ZPs is related to the transport and secretion of ZPs is still controversial. Dai et al. [10] suggested that wild-type ZPs did not interact intracellularly, while Zhou et al. [26] recorded results to the contrary. In view of these problems, we cannot rule out that there is no substantial difference between our mouse strain and Zp1 null mouse.
In the Zp2-null mice, ZP could not be stably maintained in folliculogenesis and the antral follicles possessed no ZP [12]. In Zp3-null mice, ZP was absent throughout folliculogenesis [13]. The blastocysts derived from ZP-free eggs of Zp2-null mice and Zp3-null mice lost their competence to develop into individuals [12]. In this study, the developmental potential of blastocysts originating from mutant ZP-free eggs is significantly reduced, which is consistent with the above two Zp-null mouse strains. In addition, ZP was thinned in both Zp1-null mouse strain and the strain carrying a single Zp3 allele [11, 27], and the blastocysts of these two strains possessed unimpaired developmental potential [12]. In this article, the developmental potential of blastocysts derived from ZP-thinned eggs does not decrease, consistent with the above two strains. These facts further support our conclusion that ZP integrity in folliculogenesis is important for maintaining adequate routine bilateral signaling between oocyte and surrounding granulosa cells, thus ensuring the establishment of the fully developmentally competent oocytes. The challenge in the future is to further confirm the molecular metabolic changes in the oocyte and surrounding granulosa cells caused by the crack of ZP, and the effects of these changes on the development of both cells. These endeavors will improve our understanding of ZP functions in the establishment of the eggs’ developmental competence.
Notes
Edited by Dr. Jodi Flaws, PhD, University of Illinois
Footnotes
Grant Support: This study was supported by National Key R&D Program of China (2016YFC1201805 and 2017YFC1001100), National Natural Science Foundation of China (81471453 and 81501248), Natural Science Foundation of Hunan Province of China (2015JJ2166 and 2017JJ3425), and construction project of the center of reproductive health, Central South University (164990007).
Author contributions
Hong-Mei Xiao conceived, initiated, and designed the research. Yan Wang, Chao Lv, Hua-Lin Huang, and Ming-Hua Zeng performed the experiments. Yan Wang analyzed the data. Da-Jing Yi, Hang-Jing Tan, Tian-Liu Peng, Wen-Xian Yu, and Hong-Wen Deng contributed to discussion. Hong-Wen Deng contributed to the revision and finalization of the manuscript. Yan Wang and Hong-Mei Xiao wrote the paper.
References
- 1. Wassarman PM, Litscher ES. The mouse egg's zona pellucida. Curr Top Dev Biol 2018; 130:331–356. [DOI] [PubMed] [Google Scholar]
- 2. Gupta SK. The human egg's zona pellucida. Curr Top Dev Biol 2018; 130:379–411. [DOI] [PubMed] [Google Scholar]
- 3. Wassarman PM, Litscher ES. Influence of the zona pellucida of the mouse egg on folliculogenesis and fertility. Int J Dev Biol 2012; 56(10-12):833–839. [DOI] [PubMed] [Google Scholar]
- 4. Lefievre L, Conner SJ, Salpekar A, Olufowobi O, Ashton P, Pavlovic B, Lenton W, Afnan M, Brewis IA, Monk M, Hughes DC, Barratt CL. Four zona pellucida glycoproteins are expressed in the human. Hum Reprod 2004; 19(7):1580–1586. [DOI] [PubMed] [Google Scholar]
- 5. Sauerbrun-Cutler MT, Vega M, Breborowicz A, Gonzales E, Stein D, Lederman M, Keltz M. Oocyte zona pellucida dysmorphology is associated with diminished in-vitro fertilization success. J Ovarian Res 2015; 8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang HL, Lv C, Zhao YC, Li W, He XM, Li P, Sha AG, Tian X, Papasian CJ, Deng HW, Lu GX, Xiao HM. Mutant ZP1 in familial infertility. N Engl J Med 2014; 370(13):1220–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen T, Bian Y, Liu X, Zhao S, Wu K, Yan L, Li M, Yang Z, Liu H, Zhao H, Chen ZJ. A recurrent missense mutation in ZP3 causes empty follicle syndrome and female infertility. Am J Hum Genet 2017; 101(3):459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu W, Li K, Bai D, Yin J, Tang Y, Chi F, Zhang L, Wang Y, Pan J, Liang S, Guo Y, Ruan Jet al. Dosage effects of ZP2 and ZP3 heterozygous mutations cause human infertility. Hum Genet 2017; 136(8):975–985. [DOI] [PubMed] [Google Scholar]
- 9. Liu W, Bai D, Chen J, Gao S. Additive-effect pattern of both ZP2 and ZP3 in human and mouse. Hum Genet 2017; 136(11-12):1493–1495. [DOI] [PubMed] [Google Scholar]
- 10. Dai C, Hu L, Gong F, Tan Y, Cai S, Zhang S, Dai J, Lu C, Chen J, Chen Y, Lu G, Du Jet al. ZP2 pathogenic variants cause in vitro fertilization failure and female infertility. Genet Med 2019; 21(2):431–440. [DOI] [PubMed] [Google Scholar]
- 11. Rankin T, Talbot P, Lee E, Dean J. Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development 1999; 126(17):3847–3855. [DOI] [PubMed] [Google Scholar]
- 12. Rankin TL, O’Brien M, Lee E, Wigglesworth K, Eppig J, Dean J. Defective zonae pellucidae in Zp2-null mice disrupt folliculogenesis, fertility and development. Development 2001; 128(7):1119–1126. [DOI] [PubMed] [Google Scholar]
- 13. Liu C, Litscher ES, Mortillo S, Sakai Y, Kinloch RA, Stewart CL, Wassarman PM. Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proc Natl Acad Sci USA 1996; 93(11):5431–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kidder GM, Vanderhyden BC. Bidirectional communication between oocytes and follicle cells: ensuring oocyte developmental competence. Can J Physiol Pharmacol 2010; 88(4):399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hussein TS, Froiland DA, Amato F, Thompson JG, Gilchrist RB. Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J Cell Sci 2005; 118(22):5257–5268. [DOI] [PubMed] [Google Scholar]
- 16. Host E, Gabrielsen A, Lindenberg S, Smidt-Jensen S. Apoptosis in human cumulus cells in relation to zona pellucida thickness variation, maturation stage, and cleavage of the corresponding oocyte after intracytoplasmic sperm injection. Fertil Steril 2002; 77(3):511–515. [DOI] [PubMed] [Google Scholar]
- 17. Kidder GM, Mhawi AA. Gap junctions and ovarian folliculogenesis. Reproduction 2002; 123(5):613–620. [DOI] [PubMed] [Google Scholar]
- 18. Clarke HJ. Regulation of germ cell development by intercellular signaling in the mammalian ovarian follicle. Wiley Interdiscip Rev Dev Biol 2018; 7(1). doi: 10.1002/wdev.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature 1997; 385(6616):525–529. [DOI] [PubMed] [Google Scholar]
- 20. Komatsu K, Masubuchi S. Mouse oocytes connect with granulosa cells by fusing with cell membranes and form a large complex during follicle development. Biol Reprod 2018; 99(3):527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carabatsos MJ, Elvin J, Matzuk MM, Albertini DF. Characterization of oocyte and follicle development in growth differentiation factor-9-deficient mice. Dev Biol 1998; 204(2):373–384. [DOI] [PubMed] [Google Scholar]
- 22. Gook DA, Edgar DH, Borg J, Martic M. Detection of zona pellucida proteins during human folliculogenesis. Hum Reprod 2008; 23(2):394–402. [DOI] [PubMed] [Google Scholar]
- 23. Yi YJ, Nagyova E, Manandhar G, Procházka R, Sutovsky M, Park CS, Sutovsky P. Proteolytic activity of the 26S proteasome is required for the meiotic resumption, germinal vesicle breakdown, and cumulus expansion of porcine cumulus-oocyte complexes matured in vitro. Biol Reprod 2008; 78(1):115–126. [DOI] [PubMed] [Google Scholar]
- 24. Sommersberg B, Bulling A, Salzer U, Fröhlich U, Garfield RE, Amsterdam A, Mayerhofer A. Gap junction communication and connexin 43 gene expression in a rat granulosa cell line: regulation by follicle-stimulating hormone. Biol Reprod 2000; 63(6):1661–1668. [DOI] [PubMed] [Google Scholar]
- 25. Ackert CL, Gittens JE, O’Brien MJ, Eppig JJ, Kidder GM. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol 2001; 233(2):258–270. [DOI] [PubMed] [Google Scholar]
- 26. Zhou Z, Ni C, Wu L, Chen B, Xu Y, Zhang Z, Mu J, Li B, Yan Z, Fu J, Wang W, Zhao Let al. Novel mutations in ZP1, ZP2, and ZP3 cause female infertility due to abnormal zona pellucida formation. Hum Genet 2019; 138(4):327–337. [DOI] [PubMed] [Google Scholar]
- 27. Wassarman PM, Qi H, Litscher ES. Mutant female mice carrying a single mZP3 allele produce eggs with a thin zona pellucida, but reproduce normally. Proc Biol Sci 1997; 264(1380):323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]