Abstract
The preimplantation embryo has a remarkable ability to execute its developmental program using regulatory information inherent within itself. Nonetheless, the uterine environment is rich in cell signaling molecules termed embryokines that act on the embryo during the morula-to-blastocyst transition, promoting blastocyst formation and programming the embryo for subsequent developmental events. Programming can not only affect developmental processes important for continuance of development in utero but also affect characteristics of the offspring during postnatal life. Given the importance of embryokines for regulation of embryonic development, it is likely that some causes of infertility involve aberrant secretion of embryokines by the uterus. Embryokines found to regulate development of the bovine embryo include insulin-like growth factor 1, colony stimulating factor 2 (CSF2), and dickkopf WNT signaling pathway inhibitor 1. Embryo responses to CSF2 exhibit sexual dimorphism, suggesting that sex-specific programming of postnatal function is caused by maternal signals acting on the embryo during the preimplantation period that regulate male embryos differently than female embryos.
Keywords: embryokine, blastocyst, preimplantation embryo, uterus, IGF1, CSF2, DKK1
The uterine environment is rich in cell signaling molecules that act on the embryo during the morula-to-blastocyst transition (termed embryokines) to facilitate blastocyst formation and program the embryo for subsequent developmental events.
Introduction
The totipotent and unicellular zygote possesses all of the information required to organize itself into a multicellular and differentiated blastocyst. In the cow, the model species for this paper, embryos produced by in vitro fertilization can proceed to the blastocyst stage when cultured on a plastic substratum in a simple defined medium without protein [1, 2]. The developmental program of the embryo leading to blastocyst formation is initially executed under the control of mRNA inherited from the oocyte and then from embryonic genes that become activated in a sequential manner over the course of development [3].
Ordinarily, of course, the embryo develops not in a culture dish but rather in the oviduct and uterus. The milieu in which it executes its developmental program includes molecules secreted by oviductal and endometrial cells, filtrate from blood [4] and membrane-associated molecules of the oviductal and endometrial epithelium. Several cell signaling molecules secreted by the maternal reproductive tract, called embryokines [5, 6], can regulate developmental processes of the conceptus.
The uterine environment is often inadequate to support development of the embryo. Using results from experiments attempting to establish twin pregnancies in embryo transfer recipients, McMillan [7] has estimated that only about 50–70% of bovine females are capable of supporting the development of a conceptus to term. The uterine environment during the preimplantation period is also important for programming the developing conceptus for postnatal life. A variety of changes in maternal physiology during the preimplantation period can change postnatal phenotype of the resulting offspring, with male offspring often being affected differently than females [8, 9].
Actions of embryokines released into the uterine lumen modify the developmental program of the morula to facilitate blastocyst formation and program the conceptus for subsequent success in executing other developmental events. Later developmental events include those important for continuance of development in utero, but also those that affect characteristics of the offspring at birth and duringpostnatal life. This insight not only highlights the importance of the maternal environment during the preimplantation period for birth of a healthy offspring but also reinforces the notion that pregnancy success can be enhanced in human medicine and animal production by manipulation of maternal signaling to the embryo.
In this paper, we will summarize evidence from experiments using the cow that the uterine environment exerts regulatory effects on the embryo at a key time in its development—when it transitions from being a group of totipotent blastomeres at the morula stage to a blastocyst that has begun the first of a multitude of differentiation events. Unless otherwise stated, the evidence presented here regarding embryo function derives from experiments in the cow.
Embryonic development in the uterus following exit from the oviduct to formation of the blastocyst
The bovine embryo first moves into the uterine lumen between days 4 and 5 after insemination [10]. At day 5, embryos range in development from the 16-cell stage to the compact morula stage [11, 12]. Compaction, observed by the loss of clearly visualized cell borders and involving formation of tight junctions at the basolateral membranes of outer cells of the embryo [13], occurs at about the 32-cell stage and continues until the 64-cell stage when the blastocyst first begins to form [12]. Blastocyst formation occurs at day 7, or less frequently at day 6 [11, 12]. At this time, the embryo resides in the anterior third of the uterine horn ipsilateral to the side of ovulation [14].
Initially, the blastocyst is composed of two cell types, the trophectoderm (TE), destined to give rise to the placenta, and the inner cell mass (ICM). By days 9–10 of development, the hypoblast forms as a unicellular layer at the base of the ICM [15] and is destined to give rise to the yolk sac. The remainder of the ICM, which will form the fetus, is now referred to as the epiblast.
Formation of the blastocyst is dependent upon transport of water across the outer cells of the embryo largely through actions of Na+/K+ ATPase [16, 17]. Activity of this enzyme leads to an increase in energetic requirements for the embryo—it has been estimated that 36% of ATP produced by the blastocyst is consumed by Na+/K+ ATPase [18]. ATP production, oxygen consumption, mitochondrial activity, and uptake of pyruvate and glucose increase with compaction [19, 20].
The transcription factor caudal type homeobox 2 (CDX2) is critical for proper development of the TE. Knockdown of CDX2 expression does not prevent blastocyst formation but function of the TE is disrupted [21–23]. Development of cells positive for CDX2 is dependent upon expression of yes associated protein 1 (YAP1) [22]. Moreover, CDX2 expression is regulated by TEA domain transcription factor 4 (TEAD4) in a process dependent on cellular communication network factor 2 (CCN2) [23].
The mechanism for control of CDX2 by YAP1 and TEAD4 are likely to differ between species. In the mouse, polarization of outer cells of the compact morulae is required to sequester angiomotin (AMOT) to the apical part of the cell and prevent its phosphorylation and inactivation of YAP1 [24]. In cattle, polarization of the morula is much less extensive than in mice [27] and knockdown of AMOT expression decreases the number of cells positive for CDX2 in the blastocyst [24]. Similarly, Rho-associated coil containing protein kinase (ROCK), which contributes to TE formation in mice by promoting polarity [28, 29], appears to play a different role in cattle, as inhibition of the enzyme increases nuclei positive for YAP1 and CDX2 in the bovine blastocyst [30]. Other transcription factor genes that are preferentially expressed in the TE include CCN2, GATA binding protein 2 (GATA2), GATA3, inhibitor of DNA binding 2, TEAD4, and transcription factor AP-2α (TFAP2A), and transcription factor AP-2γ (TFAP2C) [25, 31, 32].
The epiblast of the cow overexpresses several transcription factor genes associated with pluripotency including Kruppel like factor 4, POU class 5 homeobox 1 (POU5F1), SRY-box 2, Nanog homeobox (NANOG), and spalt like transcription factor 4 [31, 32]. In the cow and human, but not the mouse, POU5F1 (previously OCT4) is expressed in both ICM and TE, although in lower amounts in the TE [21, 31]. Studies using embryos produced by transfer of nuclei from POU5F1 knockout fibroblasts revealed that POU5F1 is required for formation of the epiblast [33].
Formation of both epiblast and hypoblast is dependent upon activation of Janus kinase (JAK)/STAT signaling because inhibition of JAK1/2 leads to a reduction in markers for both cell types [34]. Hypoblast cells overexpress GATA6 as compared to epiblast cells [31, 32]. Fibroblast growth factor 4 (FGF4), the growth factor produced by the epiblast that controls hypoblast formation in mice, is overexpressed in the epiblast of the cow [31]. In addition, FGF4 [35] or FGF2 [36] can promote differentiation of GATA6+ cells in the blastocyst. The physiological relevance of these actions of FGFs is unresolved. Inhibition of mitogen-activated protein kinase (MAPK) signaling either did not reduce hypoblast cell number in the blastocyst [24] or caused only a slight reduction [35]. Similarly, inhibition of FGF receptors was without effect on the number of hypoblast cells [35]. Correct placement of cells destined to be hypoblast may depend on chemokine signaling because inhibitors of the C-C motif chemokine receptor 3 or knockdown of C-C motif chemokine ligand 24 caused a reduction in cells positive for GATA6 on the outside of the ICM [37].
Characteristics of the uterine milieu during development of the blastocyst
Embryo development in vitro can change many characteristics of the blastocyst including its transcriptome [38, 39] and competence to establish pregnancy [40]. Experiments in which embryos grow in the reproductive tract or culture dish for specific periods of development revealed that the maternal environment can affect blastocyst gene expression and DNA methylation either before or after embryonic genome activation [41–43].
In part, the mother shapes the intrinsic developmental program of the embryo through the secretion of embryokines. The endometrium transcribes a plethora of transcripts encoding for cell signaling proteins during the period of development corresponding to the morula to blastocyst transition (days 5–7 of development). Among the genes expressed in the endometrium during this time are those encoding for growth factors, cytokines, chemokines, and WNT-regulatory molecules (Figure 1). From a set of 93 cell signaling genes evaluated [44], the most abundant transcripts at day 5 after ovulation were for WNT family member 5A (WNT5A), teratocarcinoma-derived growth factor 1 (TDGF1), chemokine (C-X-C motif) ligand 3, CCN2 (formerly termed CTGF), and secreted frizzled related protein 1 (SFRP1). At day 7, the most abundant transcripts were TDGF1, WNT5A, CCN2, vascular endothelial growth factor B and IK cytokine. Several cell signaling proteins have also been identified in uterine fluid at days 7 or 8 including colony-stimulating factor 2 (CSF2) [44, 45], dickkopf WNT signaling pathway inhibitor 1 (DKK1) [44] and CCN2, heparin binding EGF like growth factor, KIT ligand, and stanniocalcin 1 [46].
Figure 1.
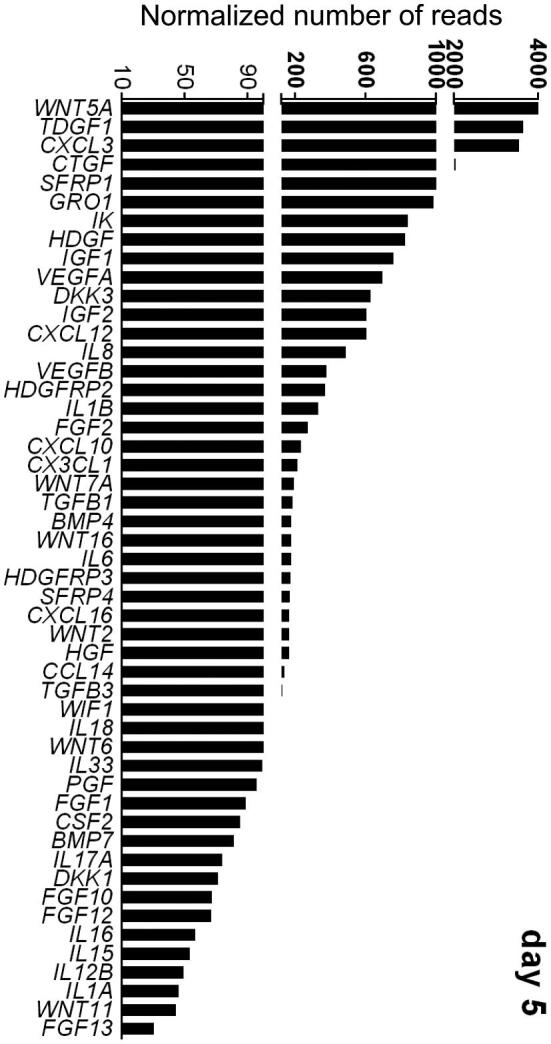
Transcript abundance of selected genes encoding for cell signaling proteins in the endometrium of the cow at day 5 after ovulation. Shown are the top 50 expressed genes from a larger set of 93 genes. Data are least-squares means. The figure is reproduced from ref. 44.
A variety of small molecules implicated in cell signaling also exist in uterine fluid. Tríbulo et al. [47] identified 31 metabolites in uterine flushings collected during the first 7 days after ovulation that have been reported to regulate cellular function, including amino acids and their metabolites and intermediates of oxidative respiration. A total of 14 metabolites identified by Tríbulo et al. [47] have been reported to affect preimplantation embryo development.
Transcription of cell signaling genes is probably under hormonal control; transcripts for 34 of 93 genes evaluated changed in abundance from days 0 to 7 after ovulation [44]. The embryo itself modifies the endometrial transcriptome as indicated by experiments in which endometrial explants were cultured with embryos or embryo conditioned culture medium [48, 49] as well as experiments evaluating changes in endometrial gene expression at day 7 of pregnancy in regions near the developing embryo [14].
Embryokines act on uterine-stage embryos to increase competence to develop to the blastocyst stage
A large number of regulatory molecules produced by the reproductive tract can modulate development of the embryo to the blastocyst stage. In the cow, however, only a few have been shown to act at the 16-cell stage or later, i.e. after the embryo is present in the uterus.
Insulin-like growth factor 1
Insulin-like growth factor 1 (IGF1) is both a hormone, being released by the liver to mediate actions of somatotropin, and a paracrine growth factor. Transcripts for IGF1 are present in the endometrium [44, 50] and immunoreactive IGF1 can be detected in uterine flushings [50]. Accordingly, changes in maternal physiology, systemically or locally, could modify the nature of regulation of the embryo by this growth factor.
The embryo expresses insulin like growth factor 1 receptor: transcript abundance increases as the embryo reaches the morula and blastocyst stages [51, 52]. Culture of in vitro produced embryos with IGF1 increased the proportion that became blastocysts [53–55]. Similarly, treatment of superovulated cows with somatotropin, which increases IGF1 concentrations in uterine flushing [50], increased the percent of transferrable quality embryos at day 7 of development [56]. IGF1 exerts its effects on competence of the embryo to develop to the blastocyst after the embryo has entered the uterus. This is so because addition of IGF1 to cultured embryos at day 4 of development increased the percent of embryos that reached the blastocyst stage while addition of IGF1 from days 0 to 4 was without effect [57].
IGF1 can affect cellular function through MAPK-mediated pathways that regulate proliferation and phosphatidylinositol 3-kinase (PI3K)/AKT-mediated pathways that block apoptosis responses [58]. The mechanism by which IGF1 improves developmental competence probably involves regulation of cell proliferation. Culture of in vitro produced embryos with IGF1 increased cell number in the morula [59] and blastocyst [53, 54]. Moreover, effects of IGF1 on cell number [59] and development to the blastocyst stage [57] are blocked by an inhibitor of MAPK.
IGF1 can also reduce effects of heat shock on development to the blastocyst stage [54, 59]. It may be that IGF1 plays two roles in the function of the embryo in the uterus—promotion of proliferation and protection from stresses such as heat shock that can compromise embryonic survival. In addition, IGF1 can block induction of apoptosis by heat shock in the morula and this action of IGF1 can be blocked by PI3K or AKT inhibitors [59, 60]. Paradoxically, however, the protective effects of IGF1 against heat shock are not due to its antiapoptotic actions [59].
Colony-stimulating factor 2
Also called granulocyte-macrophage colony stimulating factor, CSF2 is expressed by the endometrium during the period encompassing development to the blastocyst stage [44]. The protein has been immunolocalized throughout the endometrium with most intense labeling in the luminal and glandular epithelium [44, 45, 61] and the protein can be identified in uterine flushings [44, 45].
CSF2 was first shown to be embryotrophic in a study by de Moraes et al. [62] in which addition of CSF2 to culture medium either after fertilization or at day 5 of development increased the percent of embryos becoming blastocysts. The overall percent of embryos becoming blastocysts in this experiment was low however. Subsequently, it was shown that effectiveness of CSF2 for increasing development to the blastocyst stage depends on the performance of the of in vitro production system [63]. When the percent of embryos becoming blastocysts was low, CSF2 increased the number of embryos developing to the blastocyst stage. When development was high however, CSF2 reduced blastocyst yield. There was no effect of CSF2 when development to the blastocyst stage was intermediate. It may be, therefore, that one function of CSF2 is to protect embryos from stress and that this action is prominent when embryo culture conditions are suboptimal. Consistent with this idea is the finding that CSF2 blocked induction of apoptosis in the blastocyst caused by heat shock [64], reduced expression of stress-response genes in the mouse blastocyst [65] and increased blastocoel re-expansion of frozen mouse embryos after thawing [66].
Addition of CSF2 at day 5 of development increased the percent of embryos becoming blastocysts for female embryos while having no effect on males [67]. Moreover, expression of several genes in the blastocyst was affected by CSF2 in a sex-dependent manner including NANOG and POU5F1. As will be discussed further, there is additional evidence for sexual dimorphism in embryonic responses to CSF2. It is possible that signal transduction for CSF2 is affected by sex or that sex differences in epigenetic programming [68, 69] or microRNA accumulation [70] result in differential effects of CSF2 on gene expression. Also, there are differences between male and female embryos in response to various stresses [71, 72] and it may be that CSF2 interacts with sex and stress of culture to affect development of female embryos differently than male embryos.
Prototypically, CSF2 signals through a receptor composed of two subunits—the α-subunit (CSF2RA) which is specific to CSF2, and the β-subunit (CSF2RB), which also functions as part of the interleukin (IL)-3 and IL5 receptor and which increases receptor complex affinity [73]. The receptor-ligand complex exists as a heterohexameric protein with two molecules of ligand and each receptor subunit [73]. Despite the effectiveness of CSF2 for modifying function of the embryo, there is an absence of expression of CSFRB at all stages of development through the blastocyst stage [63], suggesting signaling by CSF2 in the embryo is distinct from the prototypical mechanism.
Other embryokines acting on the morula or blastocyst
The gene for the beta A subunit of activin A is expressed in the bovine endometrium [44]. Addition of activin A at day 5 increased the percent of embryos becoming blastocysts [55, 74–76]. Activin A treatment also increased expression of selected genes in the blastocyst [74].
Addition of leukemia inhibitory factor (LIF) to culture medium at day 4 increased the percent of embryos becoming blastocysts [77]. When added at day 5 [78] or day 6 [79], however, LIF decreased the percent of morulae developing to advanced blastocyst stages (expanded or hatched). Hepatoma-derived growth factor mRNA and protein are present in endometrium [44, 48, 80], can decrease competence of embryos to develop to the blastocyst stage when added at day 5 (early morula) but increase development when added at day 6 to more advanced morulae or early blastocysts [48, 80]. These experiments, as well as those with LIF, point out that there can be stage-specific effects of embryokines on the embryo that are not always captured in a static culture system.
Programming actions of CSF2 on embryonic survival, fetal development, and postnatal phenotype
Actions of embryokines on the morula-to-blastocyst stage embryo can sometimes program embryonic phenotype to affect competence for survival to term and to alter the physiological and morphological characteristics of the resultant fetus and neonate. In the cow, one such molecule is CSF2. Addition of CSF2 to culture medium at day 5 of development for embryos produced in vitro using X-sorted semen (i.e. predominately female embryos) increased pregnancies and calves per transfer when embryos were transferred into lactating recipients [81, 82] (Figure 2).
Figure 2.
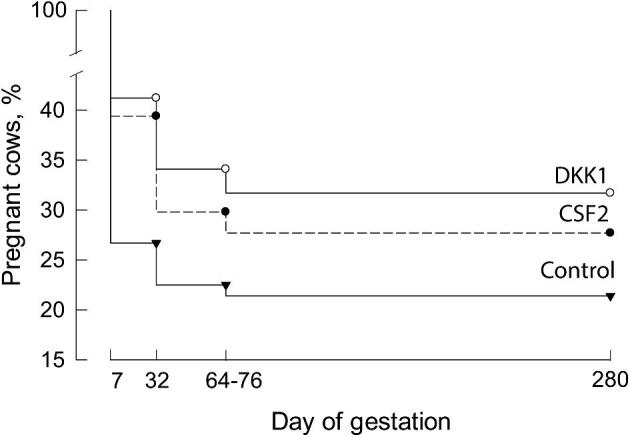
Proportion of cows pregnant at various days of gestation as affected by type of embryo transferred into the uterus. Cows received either a control embryo (inverted triangle), an embryo cultured with CSF2 (closed circle) or an embryo cultured with DKK1 (open circle). Data at day 280 represent percent of cows that calved. The figure is reproduced with slight modification from ref. 81.
The mechanisms by which CSF2 increase embryonic competence for development to term are not known but it is likely that changes in the transcriptome are involved. Treatment with CSF2 from days 6 to 8 of development changed expression of 945 genes in the ICM and 886 genes in the TE of the blastocyst, using P < 0.05 and 1.5-fold changes in expression as cutoffs [83]. CSF2 may also improve survival of the ICM. Treatment of embryos with CSF2 from days 5 to 7 increased the competence of isolated ICM from the resulting blastocysts to survive passage on mitomycin C-treated fibroblasts [63]. This could be an important function of CSF2 because loss of the embryonic disk derived from the ICM by days 14–15 of pregnancy is commonly observed for in vitro produced embryos [84, 85].
The studies in the preceding paragraph were performed without reference to embryo sex. Given sexual dimorphism of preimplantation embryos in response to CSF2 [67, 86], there is a need to evaluate CSF2-induced changes in the blastocyst separately for male and female embryos. In the one study, CSF2 regulated 5 of 90 genes examined at the blastocyst stage in a sex-specific manner [67]. In particular, CSF2 decreased expression of DEAD-box helicase 3 Y-linked and two pluripotency genes, NANOG and POU5F1, in males but not females and decreased myogeneic factor 6 (MYF6) and receptor interacting serine/threonine kinase 3 (RIPK3) in females but not males. MYF6 is a member of a family of transcription factors involved in skeletal muscle formation [87] and RIPK3 is a receptor interacting protein kinase that promotes death by necroptosis [88].
Evidence that actions of CSF2 on the morula or blastocyst can program development later in gestation can be seen as early as day 15 of pregnancy. This is an important time in development of the bovine embryo because it corresponds to the process of trophoblast elongation. The embryo undergoes rapid increases in length from about 2–5 mm on days 13 to 14 to 60–150 mm on day 16 [89, 90]. Elongation occurs coincident with upregulation of the antiluteolytic hormone interferon-tau (IFNT) that blocks prostaglandin release from the uterus and allows the corpus luteum to persist [91]. In an experiment by Dobbs et al. [86], in vitro produced embryos were treated with vehicle or CSF2 from days 5 to 7 after fertilization, transferred to recipient females and then flushed from the reproductive tract at day 15 of gestation. Key results are shown in Figure 3. For vehicle-treated embryos, female embryos were larger at day 15 than male embryos and there was more accumulation of IFNT in the uterine lumen. The increased size of female embryos probably reflects earlier onset of elongation because there were no differences in size due to sex at day 14 [92] and male embryos were longer at day 16 [93]. Treatment with CSF2 affected male embryos in the opposite manner than female embryos. In particular, CSF2 treatment increased embryo length and IFNT accumulation in male embryos but decreased length and IFNT accumulation in female embryos. Moreover, there were differences in gene expression and DNA methylation in the trophoblast. Almost exclusively, genes and methylated regions regulated by CSF2 in the male embryos were different than those regulated in the female embryos. Moreover, a large proportion of differentially-regulated genes and differentially-methylated CpG probes were part of a set of genes and methylation sites that were regulated by sex (Figure 3).
Figure 3.
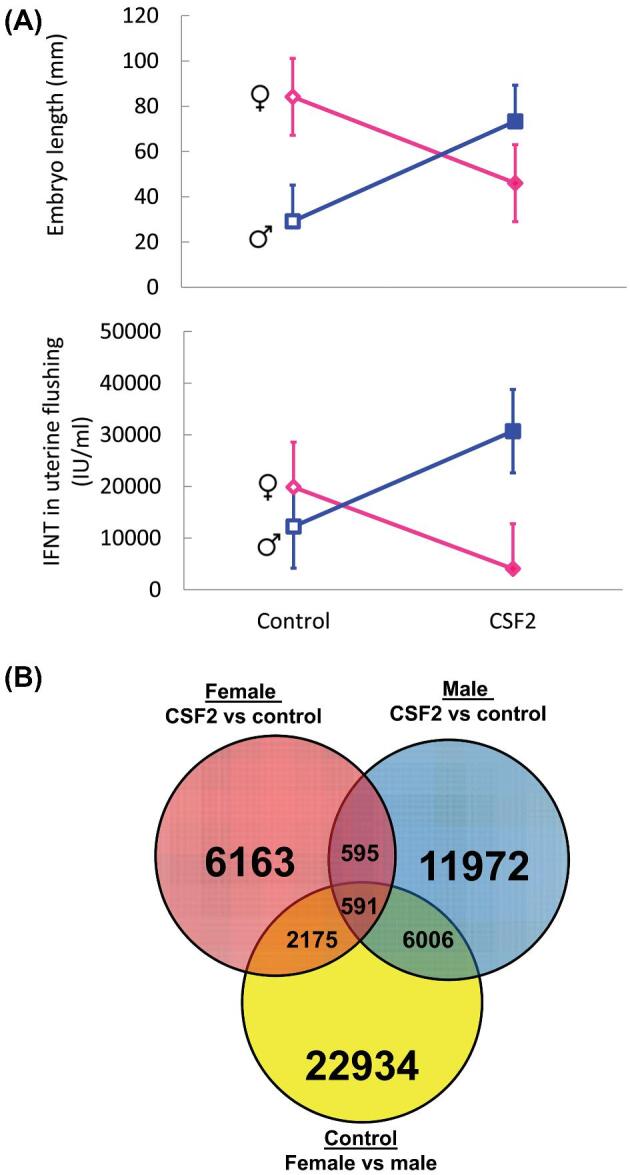
Sex-dependent developmental programming of trophoblast elongation at day 15 of pregnancy by actions of CSF2 from day 5 to 7 of development. Panel A: Embryo length and accumulation of IFNT in uterine flushing. Panel B: Venn diagram illustrating relationship between differentially methylated probes of DNA isolated from trophoblast. Note that a large proportion of probes regulated by CSF2 in either females or males are also differentially methylated between female and male embryos. The figure is reproduced from ref. 86.
At least in the female embryo (experiments have been limited to that sex), actions of CSF2 during the morula and blastocyst stages continue to program development into the fetal and postnatal periods. Siqueira et al. [94] evaluated characteristics of the fetus and placenta at day 86 of gestation for female conceptuses derived from embryos produced by artificial insemination, transfer of an in vitro produced embryo, or transfer of an in vitro produced embryo exposed to CSF2 from days 5 to 7 of development. Fetuses were larger for the two groups produced by in vitro fertilization than for fetuses produced by artificial insemination, but CSF2 did not alleviate this effect. Expression of 92 genes in liver, placenta, and muscle was determined. For liver, there was aberrant expression of 7 genes for fetuses derived from the IVF group compared to the artificial insemination group; CSF2 treatment alleviated this effect for 6 genes. Similarly, for placenta, there were 8 genes affected by IVF and CSF2 alleviated this effect for each gene. Gene expression in muscle was most disrupted by in vitro production (30 genes showed differences between fetuses produced by artificial insemination vs embryo transfer) but gene expression for CSF2 fetuses was in most cases similar to that of fetuses derived from embryos cultured without CSF2.
More detailed and well-powered studies are needed, but there is evidence that programming effects of CSF2 can persist into the postnatal period. Specifically, growth rates of heifer calves derived from embryos produced in vitro in culture medium containing CSF2 from days 5 to 7 of development were heavier in weight from 5 to 13 months of age than calves derived from embryos produced in vitro without CSF2 or calves derived using artificial insemination [95]. There were no treatment effects on birth weight or weights at 3 or 4 months of age. This effect of CSF2 in cattle is consistent with observations in mice where addition of CSF2 to cultured mouse embryos reduced some of the negative effects of culture on postnatal growth and reproductive function [96].
WNT signaling in the morula and blastocyst
WNT proteins are a group of conserved cell signaling proteins encoded by multiple genes (19 in the bovine) that regulate proliferation and differentiation of stem and progenitor cells in embryonic development and the adult [97]. WNT proteins can regulate cell function by binding with one or more seven transmembrane Frizzled (FZD) receptors (10 FZD genes have been identified in the cow), the orphan receptor RAR related orphan receptor A (ROR), or with the non-catalytic tyrosine kinase receptor or co-receptor receptor-like tyrosine kinase (Ryk) [98]. Canonical WNT signaling involves binding of WNT to FZD, recruitment of the co-receptor LDL receptor related protein (LRP) 5 or LRP6, and downstream inactivation of a protein complex that targets β-catenin (CTNNB) for destruction in the proteosome. As a result, CTNNB accumulates in the cytoplasm, translocates to the nucleus and interacts with TCF and LEF family transcription factors to regulate gene expression [99]. Binding of WNT to FZD can also lead to Ca++ mediated signaling (with downstream activation of calcineurin, calcium/calmodulin dependent protein kinase II, and protein kinase C) as well as activation of the planar cell polarity (PCP) pathway that leads to ROCK and JNK-mediated rearrangement of the cytoskeleton [99, 100]. Activation of Ryk can also lead to CTNNB-dependent signaling while activation of ROR causes Ca++ dependent signaling [98].
A variety of soluble WNT antagonists modify WNT signaling, including soluble FZD receptors and WNT antagonists [98] as well as DKK1, which interferes with association of FZD to LRP5/6 [101]. Whether or not a specific WNT activates canonical WNT signaling or other signaling pathways depends on the particular WNT, as well as the availability of receptors, co-receptors, and regulatory molecules. Some, like WNT2, WNT3, WNT3A, and WNT8A are considered more likely to activate canonical signaling, whereas others such as WNT4, WNT5A, WNT5B, WNT6, WNT7A, and WNT11 are considered to be more likely to activate other, non-canonical pathways [102]. The actual pathways activated depend upon complex interactions between various molecules involved in WNT signaling [103]. Non-canonical signaling pathways can be antagonistic to canonical signaling. For example, activation of ROR2 or PCP signaling can lead to inhibition of CTNNB/TCF regulation of gene expression [103, 104].
The morula and blastocyst possesses transcripts for WNT2, WNT2B, WNT6, WNT8A, WNT10A, WNT11 and WNT16 as well as for several FZD receptor genes, LRP6 and RYK [105]. Transcripts for all 19 WNT genes are expressed in the endometrium during days 5– 7 [44]. The most abundant transcripts at day 5 were for WNT5A, WNT7A, WNT16, WNT2 and WNT6 and the most abundant at day 7 were WNT5A, WNT7A, WNT11, WNT6, and WNT16. Transcripts for inhibitors of WNT signaling are also abundant in the endometrium at this time. At day 5, transcripts in descending abundance that encoded for WNT inhibitory proteins were SFRP1, DKK3, SFRP4, WIF1, and DKK1 [44].
There are several lines of evidence that inhibition of canonical WNT signaling is important for development of the embryo to the blastocyst stage of development. Activation of canonical WNT signaling, either by addition of a WNT agonist [106, 107] or by inhibition of the CTNNB1 destruction pathway [107, 108] reduced the percent of embryos becoming blastocysts. In contrast, administration of an inhibitor of WNT acylation to block secretion of embryonic WNT proteins did not affect development of cultured embryos to the blastocyst stage [107]. Moreover, there was no evidence for accumulation of CTNNB1 in the nucleus of the embryo [105], suggesting that some aspects of canonical signaling are inoperative in the embryo. Inhibition of WNT signaling may also be important for maintaining the pluripotency of the ICM. A recent report describing development of primed embryonic stem cells from the ICM of the bovine blastocyst utilized a culture system that incorporated IWR-1 [109], which inhibits CTNNB1-mediated WNT signaling [110].
One of the WNT genes expressed in the bovine endometrium is WNT7A [44]. This WNT can activate canonical signaling [111] and also affect cell function, independent of CTNNB1-mediated pathways through actions involving FZD-mediated activation of AKT and ROR signaling [112] and inactivation of S-phase kinase-associated protein 2 [113]. Addition of WNT7A to cultured bovine embryos from days 5– 7 of development increased the proportion of embryos becoming a blastocyst [55, 107]. Effects of WNT7A occurred without affecting abundance of CTNNB1 and reduced pJNK [107]. Effects of WNT7A on development to the blastocyst stage occurred for both male and female embryos [55].
Programming of embryonic survival and fetal development by the WNT antagonist DKK1
Addition of DKK1 to in vitro produced embryos between days 5–7 after fertilization increases the proportion of the resultant blastocysts that established and maintain pregnancy after transfer into recipients [81] (Figure 2). There are additional, indirect lines of evidence for DKK1 being important for embryonic survival. Transcript abundance for DKK1 in the endometrium was reduced in lactating cows [114], which is a condition associated with reduced fertility [115, 116]. Moreover, persistentlyinfertile heifers had lower expression of DKK1 in the endometrium than heifers that repeatedly became pregnant [117].
DKK1 functions as an inhibitor of canonical WNT signaling through binding and internalization of LRP5/6 [97, 99], but it can also inhibit the WNT/Ca++ pathway [118] and either inhibit [118, 119] or activate [120–122] the PCP pathway. In the bovine embryo, DKK1 treatment reduced accumulation of CTNNB1 in the blastocyst but did not affect amounts of pJNK (a downstream target of the PCP pathway) in the morula [107].
The changes in the blastocyst caused by DKK1 that increase competence for subsequent development are not known. Treatment with DKK1 does not increase the percent of embryos that develop to the blastocyst stage in culture. Depending on the experiment, the percent of embryos becoming blastocyst was either unaffected by DKK1 or slightly lower [81, 106, 107]. In one study [81], DKK1 reduced the number of cells in the blastocyst, while increasing the percent of cells that were TE as compared to ICM. In the same study, the percent of cells in the ICM of the day 8 blastocyst that were NANOG+ (i.e. epiblast) were decreased, while the percent that were GATA6+ (i.e. hypoblast) was increased. In a subsequent study however, DKK1 had no effect on the number or cells in the blastocyst, whether considered as total cells, TE cells or ICM cells [107]. In the latter study, DKK1 had no effect on labeling of morula or blastocysts for the TE marker CDX2 but DKK1 reduced intensity of YAP1 in YAP1+ cells. Furthermore, treatment of embryos with DKK1 at day 5 of development reduced expression of AMOT in the day 6 morula [123]. Based on the role of YAP1 and AMOT in formation of TE in cattle [24], the observed changes should reduce differentiation of TE cells.
The lack of repeatable changes in properties of the blastocyst in vitro makes it difficult to determine whether female embryos respond differently to DKK1 than male embryos. Denicol et al. [123] observed 50 genes in the morula that were regulated by DKK1 in a sex-dependent manner as determined by microarray analysis, but none of a subset of 5 of these 50 genes was affected by the interaction between DKK1 and sex when measured by reverse transcription-polymerase chain reaction with a separate set of embryos. In another experiment [107], there were no interactions between DKK1 and sex on development of embryos to the blastocyst stage or blastocyst cell numbers.
There are indications that DKK1 actions on the morula or blastocyst can program fetal development because calves derived from in vitro produced embryos treated with DKK1 from days 5 to 7 of development had lower birth weight than calves derived from embryos not treated with DKK1 [124]. In this experiment, serum was present in the medium used to produce embryos and it is not known whether DKK1 interacted with regulatory molecules in serum.
As mentioned above, DKK1 is not the only soluble inhibitor of WNT signaling whose gene is expressed in the bovine endometrium. Indeed, transcript abundance is even higher for the related gene, DKK3 [44]. Little is known about DKK3, but analysis of its amino acid sequence as compared to other DKK family members has revealed that it is evolutionarily-distinct from DKK1, DKK2, and DKK4 and may not regulate WNT signaling [125].
Conclusions, caveats, and future directions
The preimplantation embryo has a remarkable ability to execute its developmental program using regulatory information inherent within itself. Nonetheless, as described in this review, developmental processes in the embryo are shaped by maternal regulatory signals. Inappropriate amounts of maternal cell signaling molecules in the uterine lumen may compromise the capability of the embryo for sustained development to term. Opportunities for alterations in the uterine environment are many. Among the physiological conditions shown to affect expression of cell signaling genes in the endometrium of the cow are lactation [114], negative energy balance [126], subclinical endometritis [127], and exposure to sperm [128] and seminal plasma [129]. In the case of assisted reproduction, in which the embryo develops in an artificial environment much different from that of the reproductive tract, disruption in development is more extreme [130] and embryo competence for development to term can be compromised [40].
The molecular and cellular biology of the preimplantation embryo is important not only for survivability of the embryo but also for shaping the phenotype of the resultant postnatal organism. Several changes in maternal physiology during the preimplantation period have been demonstrated to modify the phenotype of the resultant offspring, often in a sexually dimorphic manner [6, 7].
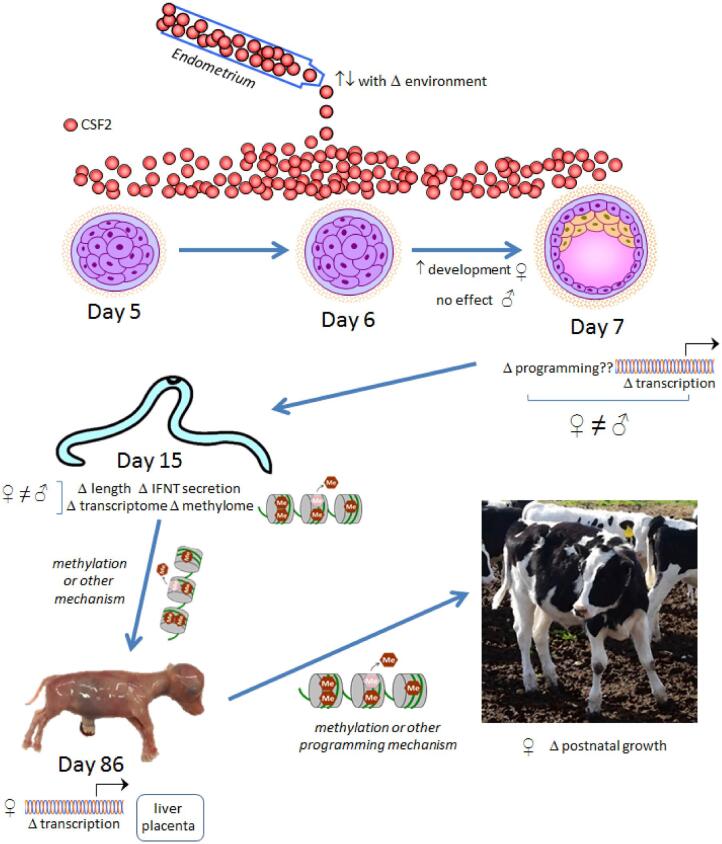
An example of how changes in embryokine secretion by the endometrium could affect postnatal phenotype is shown in Figure 4 using the example of CSF2. When the embryo enters the uterus at day 4 or 5 of development, it is exposed to CSF2 secreted by the maternal endometrium. Colony stimulating factor 2 acts in a sex-dependent manner to affect competence of the embryo to develop to the blastocyst stage and to initiate the process of trophoblast elongation necessary for pregnancy maintenance. Also, CSF2 acts at the morula or blastocyst stage to modify the developmental program of the embryo. Caused at least in part by changes in DNA methylation (as observed at day 15 of gestation), exposure to CSF2 during the morula to blastocyst period changes gene expression in the female fetus and placenta at day 86 of gestation and leads to changes in postnatal function characterized by increased body weight in the juvenile period. It is not well understood, but expression of CSF2 can be modified by physiological alternations in maternal function including increased expression after exposure to seminal plasma [129] and, at least in the oviduct, by decreased expression associated with obesity [131]. Changing the abundance of CSF2 in the uterus in response to changes in maternal physiology could conceivably change how the embryokine alters the developmental program of the embryo to modify postnatal function.
Figure 4.
Diagram using CSF2 as an example of how changes in embryokine secretion by the endometrium could affect postnatal phenotype. When the embryo enters the uterus at day 4 or 5 of development it is exposed to CSF2 secreted by the endometrium. CSF2 acts in a sex-dependent manner to affect competence of the embryo to develop to the blastocyst stage and to initiate the process of trophoblast elongation necessary for pregnancy maintenance. CSF2 also acts at the morula or blastocyst stage to modify the developmental program of the embryo. Exposure to CSF2 during the morula to blastocyst period changes gene expression in the female fetus and placenta at day 86 of gestation, caused at least in part by changes in DNA methylation. (as observed at day 15 of gestation), and leads to changes in postnatal function characterized by increased body weight in the juvenile period.
There are caveats to consider when attempting to understand experiments on the role of specific embryokines in development of the preimplantation embryo. The model of the in vitro produced embryo is a useful one for understanding maternal regulation of embryonic function because of the ease of manipulation of the environment of the embryo and ready accessibility of the embryo for examination. However, the alterations in embryonic function caused by culture are extensive [38–43] and it is possible that some cell signaling molecules alleviate actions of culture stress on the embryo that would not be relevant to the situation in vivo. Accordingly, there is a need to confirm the importance of putative embryokines identified in vitro for function of the embryo developing in vivo. In addition, it is important to understand the degree to which embryokines act in concert to regulate the embryo, similar to the actions of cytokines on immune cells [132].
There are practical implications for understanding mechanisms by which embryokines regulate the survivability and developmental program of the preimplantation embryo. The obvious one is to provide cultured embryos with embryokines that optimize pregnancy outcomes and minimize changes in fetal development or adult life associated with assisted reproduction technologies [133, 134]. It might also be possible to provide embryokines to females to promote embryonic survival or shape the postnatal phenotype of the offspring. There are few successful examples of this approach in cattle although injection of somatotropin, which, among other actions, increases circulating concentrations of IGF1, has been reported to increase embryonic survival under some situations [53, 135]. It may also be possible to change the endocrine regulation of the endometrium to improve the uterine capacity to support embryonic development. In cattle, efforts to increase pregnancy rate by increasing progesterone support of the uterus have yielded mixed results [136], but regulating the growth of the preovulatory follicle, which could affect the oocyte and the reproductive tract, has increased subsequent embryonic survival [137].
Acknowledgements
Authors thank all the colleagues who contributed to the research leading up to the ideas expressed here. Thanks are also expressed to John J. Bromfield, for providing important editorial suggestions for improvement of the text.
Author Biographical
 Peter J. Hansen is a Distinguished Professor and L.E. ``Red'' Larson Professor of Animal Sciences at the University of Florida. His research focuses on the biology of pregnancy and embryonic survival and development of methods to improve fertility and assisted reproductive technologies in livestock (particularly dairy cattle). Particular emphasis is placed on elucidating effects of elevated temperature on pregnancy, characterizing the nature of maternal control of early embryonic development and identifying genes controlling embryonic survival and fertility. In addition, work is underway to develop methods to improve dairy cow fertility during heat stress and to increase profitable uses of embryo transfer. Hansen received the B.S. in Agricultural Sciences from the University of Illinois in 1978 and the M.S. and Ph.D. degrees from the University of Wisconsin in 1980 and 1983. He did a postdoctoral fellowship at the University of Florida from 1983-1984 before joining the faculty at Florida as an assistant professor in 1984. He has published over 290 peer-reviewed research papers and mentored 50 graduate students, 15 postdoctoral fellows and 49 visiting scientists. Among his awards are the Trainee Mentoring Award (2010) and Research Award (2014) from the Society for the Study of Reproduction.
Peter J. Hansen is a Distinguished Professor and L.E. ``Red'' Larson Professor of Animal Sciences at the University of Florida. His research focuses on the biology of pregnancy and embryonic survival and development of methods to improve fertility and assisted reproductive technologies in livestock (particularly dairy cattle). Particular emphasis is placed on elucidating effects of elevated temperature on pregnancy, characterizing the nature of maternal control of early embryonic development and identifying genes controlling embryonic survival and fertility. In addition, work is underway to develop methods to improve dairy cow fertility during heat stress and to increase profitable uses of embryo transfer. Hansen received the B.S. in Agricultural Sciences from the University of Illinois in 1978 and the M.S. and Ph.D. degrees from the University of Wisconsin in 1980 and 1983. He did a postdoctoral fellowship at the University of Florida from 1983-1984 before joining the faculty at Florida as an assistant professor in 1984. He has published over 290 peer-reviewed research papers and mentored 50 graduate students, 15 postdoctoral fellows and 49 visiting scientists. Among his awards are the Trainee Mentoring Award (2010) and Research Award (2014) from the Society for the Study of Reproduction.
References
- 1. Pinyopummintr T, Bavister BD. In vitro-matured/in vitro-fertilized bovine oocytes can develop into morulae/blastocysts in chemically defined, protein-free culture media. Biol Reprod 1991; 45(5):736–742. [DOI] [PubMed] [Google Scholar]
- 2. Block J, Bonilla L, Hansen PJ. Effect of addition of hyaluronan to embryo culture medium on survival of bovine embryos in vitro following vitrification and establishment of pregnancy after transfer to recipients. Theriogenology 2009; 71(7):1063–1071. [DOI] [PubMed] [Google Scholar]
- 3. Graf A, Krebs S, Zakhartchenko V, Schwalb B, Blum H, Wolf E. Fine mapping of genome activation in bovine embryos by RNA sequencing. Proc Natl Acad Sci 2014; 111(11):4139–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fahning ML, Schultz RH, Graham EF. The free amino acid content of uterine fluids and blood serum in the cow. Reproduction 1967; 13(2):229–236. [DOI] [PubMed] [Google Scholar]
- 5. Hansen PJ, Dobbs KD, Denicol AC. Programming of the preimplantation embryo by the embryokine colony stimulating factor 2. Anim Reprod Sci 2014; 149(1-2): 59–66. [DOI] [PubMed] [Google Scholar]
- 6. Hansen PJ, Denicol AC, Dobbs KB. Maternal embryokines that regulate development of the bovine preimplantation embryo. Turk J Vet Anim Sci 2014; 38:589–598. [Google Scholar]
- 7. McMillan WH. Statistical models predicting embryo survival to term in cattle after embryo transfer. Theriogenology 1998; 50(7):1053–1070. [DOI] [PubMed] [Google Scholar]
- 8. Hansen PJ, Dobbs KB, Denicol AC, Siqueira LG. Sex and the preimplantation embryo: implications of sexual dimorphism in the preimplantation period for maternal programming of embryonic development. Cell Tissue Res 2016; 363(1):237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fleming TP, Eckert JJ, Denisenko O. The role of maternal nutrition during the periconceptional period and its effect on offspring phenotype. Adv Exp Med Biol 2017; 1014:87–105. [DOI] [PubMed] [Google Scholar]
- 10. Hackett AJ, Durnford R, Mapletoft R, Marcus G. Location and status of embryos in the genital tract of superovulated cows 4 to 6 days after insemination. Theriogenology 1993; 40(6):1147–1153. [Google Scholar]
- 11. Betteridge KJ, Fleéhon J-E. The anatomy and physiology of pre-attachment bovine embryos. Theriogenology 1988; 29(1):155–187. [Google Scholar]
- 12. Van Soom A, Boerjan ML, Bols PE, Vanroose G, Lein A, Coryn M, de Kruif A. Timing of compaction and inner cell allocation in bovine embryos produced in vivo after superovulation. Biol Reprod 1997; 57(5):1041–1049. [DOI] [PubMed] [Google Scholar]
- 13. Barcroft LC, Hay-Schmidt A, Caveney A, Gilfoyle E, Overstrom EW, Hyttel P, Watson AJ. Trophectoderm differentiation in the bovine embryo: characterization of a polarized epithelium. Reproduction 1998; 114(2):327–339. [DOI] [PubMed] [Google Scholar]
- 14. Sponchiado M, Gomes NS, Fontes PK, Martins T, Del Collado M, Pastore AA, Pugliesi G, Nogueira MFG, Binelli M. Pre-hatching embryo-dependent and -independent programming of endometrial function in cattle. PLoS One 2017; 12(4):e0175954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maddox-Hyttel P, Alexopoulos NI, Vajta G, Lewis I, Rogers P, Cann L, Callesen H, Tveden-Nyborg P, Trounson A. Immunohistochemical and ultrastructural characterization of the initial post-hatching development of bovine embryos. Reproduction 2003; 125:607–623. [PubMed] [Google Scholar]
- 16. Betts DH, MacPhee DJ, Kidder GM, Watson AJ. Ouabain sensitivity and expression of Na/K-ATPase α- and β-subunit isoform genes during bovine early development. Mol Reprod Dev 1997; 46(2):114–126. [DOI] [PubMed] [Google Scholar]
- 17. Donnay I, Leese HJ. Embryo metabolism during the expansion of the bovine blastocyst. Mol Reprod Dev 1999; 53(2):171–178. [DOI] [PubMed] [Google Scholar]
- 18. Houghton FD, Humpherson PG, Hawkhead JA, Hall CJ, Leese HJ. Na+, K+, ATPase activity in the human and bovine preimplantation embryo. Dev Biol 2003; 263(2):360–366. [DOI] [PubMed] [Google Scholar]
- 19. Thompson JG, Partridge RJ, Houghton FD, Cox CI, Leese HJ. Oxygen uptake and carbohydrate metabolism by in vitro derived bovine embryos. Reproduction 1996; 106(2):299–306. [DOI] [PubMed] [Google Scholar]
- 20. Tarazona AM, Rodríguez JI, Restrepo LF, Olivera-Angel M. Mitochondrial activity, distribution and segregation in bovine oocytes and in embryos produced in vitro. Reprod Domest Anim 2006; 41(1):5–11. [DOI] [PubMed] [Google Scholar]
- 21. Berg DK, Smith CS, Pearton DJ, Wells DN, Broadhurst R, Donnison M, Pfeffer PL. Trophectoderm lineage determination in cattle. Dev Cell 2011; 20(2):244–255. [DOI] [PubMed] [Google Scholar]
- 22. Goissis MD, Cibelli JB. Functional characterization of CDX2 during bovine preimplantation development in vitro. Mol Reprod Dev 2014; 81(10):962–970. [DOI] [PubMed] [Google Scholar]
- 23. Sakurai N, Takahashi K, Fujii T, Hirayama H, Kageyama S, Hashizume T, Sawai K. The necessity of OCT-4 and CDX2 for early development and gene expression involved in differentiation of inner cell mass and trophectoderm lineages in bovine embryos. Cell Reprogram 2016; 18(5):309–318. [DOI] [PubMed] [Google Scholar]
- 24. Negrón-Pérez VM, Hansen PJ. Role of yes-associated protein 1, angiomotin, and mitogen-activated kinase kinase 1/2 in development of the bovine blastocyst. Biol Reprod 2018; 98(2):170–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Akizawa H, Kobayashi K, Bai H, Takahashi M, Kagawa S, Nagatomo H, Kawahara M. Reciprocal regulation of TEAD4 and CCN2 for the trophectoderm development of the bovine blastocyst. Reproduction 2018; 155(6):563–571. [DOI] [PubMed] [Google Scholar]
- 26. Lorthongpanich C, Doris TPY, Limviphuvadh V, Knowles BB, Solter D. Developmental fate and lineage commitment of singled mouse blastomeres. Development 2012; 139(20):3722–3731. [DOI] [PubMed] [Google Scholar]
- 27. Koyama H, Suzuki H, Yang X, Jiang S, Foote RH. Analysis of polarity of bovine and rabbit embryos by scanning electron microscopy. Biol Reprod 1994; 50(1):163–170. [DOI] [PubMed] [Google Scholar]
- 28. Kono K, Tamashiro DA, Alarcon VB. Inhibition of RHO‐ROCK signaling enhances ICM and suppresses TE characteristics through activation of Hippo signaling in the mouse blastocyst. Dev Biol 2014; 394(1): 142–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mihajlović AI, Bruce AW. Rho-associated protein kinase regulates subcellular localisation of Angiomotin and Hippo-signalling during preimplantation mouse embryo development. Reprod Biomed Online 2016; 33(3):381–390. [DOI] [PubMed] [Google Scholar]
- 30. Negrón-Pérez VM, Rodrigues LT, Mingoti GZ, Hansen PJ. Role of ROCK signaling in formation of the trophectoderm of the bovine preimplantation embryo. Mol Reprod Dev 2018; 85(5):374–375. [DOI] [PubMed] [Google Scholar]
- 31. Negrón-Pérez VM, Zhang Y, Hansen PJ. Single-cell gene expression of the bovine blastocyst. Reproduction 2017; 154(5):627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wei Q, Zhong L, Zhang S, Mu H, Xiang J, Yue L, Dai Y, Han J. Bovine lineage specification revealed by single-cell gene expression analysis from zygote to blastocyst. Biol Reprod 2017; 97(1):5–17. [DOI] [PubMed] [Google Scholar]
- 33. Simmet K, Zakhartchenko V, Philippou-Massier J, Blum H, Klymiuk N, Wolf E. OCT4/POU5F1 is required for NANOG expression in bovine blastocysts. Proc Natl Acad Sci USA 2018; 115(11):2770–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meng F, Forrester-Gauntlett B, Turner P, Henderson H, Oback B. Signal inhibition reveals JAK/STAT3 pathway as critical for bovine inner cell mass development. Biol Reprod 2015; 93(6):132. [DOI] [PubMed] [Google Scholar]
- 35. Kuijk EW, van Tol LTA, Van de Velde H, Wubbolts R, Welling M, Geijsen N, Roelen BA. The roles of FGF and MAP kinase signaling in the segregation of the epiblast and hypoblast cell lineages in bovine and human embryos. Development 2012; 139(5):871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang QE, Fields SD, Zhang K, Ozawa M, Johnson SE, Ealy AD. Fibroblast growth factor 2 promotes primitive endoderm development in bovine blastocyst outgrowths. Biol Reprod 2011; 85(5):946–953. [DOI] [PubMed] [Google Scholar]
- 37. Negrón-Pérez VM, Vargas-Franco D, Hansen PJ. Role of chemokine (C-C motif) ligand 24 in spatial arrangement of the inner cell mass of the bovine embryo. Biol Reprod 2017; 96(5):948–959. [DOI] [PubMed] [Google Scholar]
- 38. Driver AM, Peñagaricano F, Huang W, Ahmad KR, Hackbart KS, Wiltbank MC, Khatib H. RNA-Seq analysis uncovers transcriptomic variations between morphologically similar in vivo- and in vitro-derived bovine blastocysts. BMC Genomics 2012; 13(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heras S, De Coninck DI, Van Poucke M, Goossens K, Bogado Pascottini O, Van Nieuwerburgh F, Deforce D, De Sutter P, Leroy JL, Gutierrez-Adan A, Peelman L, Van Soom A. Suboptimal culture conditions induce more deviations in gene expression in male than female bovine blastocysts. BMC Genomics 2016; 17(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ferraz PA, Burnley C, Karanja J, Viera-Neto A, Santos JE, Chebel RC, Galvão KN. Factors affecting the success of a large embryo transfer program in Holstein cattle in a commercial herd in the southeast region of the United States. Theriogenology 2016; 86(7):1834–1841. [DOI] [PubMed] [Google Scholar]
- 41. Gad A, Hoelker M, Besenfelder U, Havlicek V, Cinar U, Rings F, Held E, Dufort I, Sirard MA, Schellander K, Tesfaye D. Molecular mechanisms and pathways involved in bovine embryonic genome activation and their regulation by alternative in vivo and in vitro culture conditions. Biol Reprod 2012; 87(4):100. [DOI] [PubMed] [Google Scholar]
- 42. Salilew-Wondim D, Saeed-Zidane M, Hoelker M, Gebremedhn S, Poirier M, Pandey HO, Tholen E, Neuhoff C, Held E, Besenfelder U, Havlicek V, Rings Fet al. Genome-wide DNA methylation patterns of bovine blastocysts derived from in vivo embryos subjected to in vitro culture before, during or after embryonic genome activation. BMC Genomics 2018; 19(1):424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Salilew-Wondim D, Fournier E, Hoelker M, Saeed-Zidane M, Tholen E, Looft C, Neuhoff C, Besenfelder U, Havlicek V, Rings F, Gagné D, Sirard MAet al. Genome-wide DNA methylation patterns of bovine blastocysts developed in vivo from embryos completed different stages of development in vitro. PLoS One 2015; 10(11):e0140467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tríbulo P, Siqueira LGB, Oliveira LJ, Scheffler T, Hansen PJ. Identification of potential embryokines in the bovine reproductive tract. J Dairy Sci 2018; 101(1):690–704. [DOI] [PubMed] [Google Scholar]
- 45. de Moraes AA, Paula-Lopes FF, Chegini N, Hansen PJ. Localization of granulocyte-macrophage colony-stimulating factor in the bovine reproductive tract. J Reprod Immunol 1999; 42(2):135–145. [DOI] [PubMed] [Google Scholar]
- 46. Muñoz M, Martin D, Carrocera S, Alonso-Guervos M, Mora MI, Corrales FJ, Peynot N, Giraud-Delville C, Duranthon V, Sandra O, Gómez E. Localisation of stem cell factor, stanniocalcin-1, connective tissue growth factor and heparin-binding epidermal growth factor in the bovine uterus at the time of blastocyst formation. Reprod Fertil Dev 2017; 29(11):2127–2139. [DOI] [PubMed] [Google Scholar]
- 47. Tríbulo P, Balzano-Nogueira L, Conesa A, Siqueira LG, Hansen PJ. Changes in the uterine metabolome of the cow during the first 7 days after estrus. Mol Reprod Dev 2019; 86(1):75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gómez E, Carrocera S, Martin D, Sánchez-Calabuig MJ, Gutiérrez-Adán A, Murillo A, Muñoz M. Hepatoma-derived growth factor: Protein quantification in uterine fluid, gene expression in endometrial-cell culture and effects on in vitro embryo development, pregnancy and birth. Theriogenology 2017; 96:118–125. [DOI] [PubMed] [Google Scholar]
- 49. Passaro C, Tutt D, Mathew DJ, Sanchez JM, Browne JA, Boe-Hansen GB, Fair T, Lonergan P. Blastocyst-induced changes in the bovine endometrial transcriptome. Reproduction 2018; 156(3):219–229. [DOI] [PubMed] [Google Scholar]
- 50. Bilby TR, Guzeloglu A, Kamimura S, Pancarci SM, Michel F, Head HH, Thatcher WW. Pregnancy and bovine somatotropin in nonlactating dairy cows: I. Ovarian, conceptus, and insulin-like growth factor system responses. J Dairy Sci 2004; 87(10):3256–3267. [DOI] [PubMed] [Google Scholar]
- 51. Lonergan P, Rizos D, Gutierrez-Adán A, Moreira PM, Pintado B, de la Fuente J, Boland MP. Temporal divergence in the pattern of messenger RNA expression in bovine embryos cultured from the zygote to blastocyst stage in vitro or in vivo. Biol Reprod 2003; 69(4):1424–1431. [DOI] [PubMed] [Google Scholar]
- 52. Jiang Z, Sun J, Dong H, Luo O, Zheng X, Obergfell C, Tang Y, Bi J, O’Neill R, Ruan Y, Chen J, Tian XC. Transcriptional profiles of bovine in vivo pre-implantation development. BMC Genomics 2014; 15(1):756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moreira F, Paula-Lopes FF, Hansen PJ, Badinga L, Thatcher WW. Effects of growth hormone and insulin-like growth factor-I on development of in vitro derived bovine embryos. Theriogenology 2002; 57(2):895–907. [DOI] [PubMed] [Google Scholar]
- 54. Jousan FD, Hansen PJ. Insulin-like growth factor-I as a survival factor for the bovine preimplantation embryo exposed to heat shock. Biol Reprod 2004; 71(5):1665–1670. [DOI] [PubMed] [Google Scholar]
- 55. Tríbulo P, Jumatayeva G, Lehloenya K, Moss JI, Negrón-Pérez VM, Hansen PJ. Effects of sex on response of the bovine preimplantation embryo to insulin-like growth factor 1, activin A, and WNT7A. BMC Dev Biol 2018; 18(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moreira F, Badinga L, Burnley C, Thatcher WW. Bovine somatotropin increases embryonic development in superovulated cows and improves post-transfer pregnancy rates when given to lactating recipient cows. Theriogenology 2002; 57(4):1371–1387. [DOI] [PubMed] [Google Scholar]
- 57. Bonilla AQ, Ozawa M, Hansen PJ. Timing and dependence upon mitogen-activated protein kinase signaling for pro-developmental actions of insulin-like growth factor 1 on the preimplantation bovine embryo. Growth Horm IGF Res 2011; 21(2):107–111. [DOI] [PubMed] [Google Scholar]
- 58. Murillo-Cuesta S, Rodríguez-de la Rosa L, Cediel R, Lassaletta L, Varela-Nieto I. The role of insulin-like growth factor-I in the physiopathology of hearing. Front Mol Neurosci 2011; 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jousan FD, Hansen PJ. Insulin-like growth factor-I promotes resistance of bovine preimplantation embryos to heat shock through actions independent of its anti-apoptotic actions requiring PI3K signaling. Mol Reprod Dev 2007; 74(2):189–196. [DOI] [PubMed] [Google Scholar]
- 60. Jousan FD, Oliveira LJ, Hansen PJ. Short-Term culture of in vitro produced bovine preimplantation embryos with insulin-like growth factor-i prevents heat shock-induced apoptosis through activation of the phosphatidylinositol 3-kinase/Akt pathway. Mol Reprod Dev 2008; 75(4):681–688. [DOI] [PubMed] [Google Scholar]
- 61. Emond V, MacLaren LA, Kimmins S, Arosh JA, Fortier MA, Lambert RD. Expression of cyclooxygenase-2 and granulocyte-macrophage colony-stimulating factor in the endometrial epithelium of the cow is up-regulated during early pregnancy and in response to intrauterine infusions of interferon-tau. Biol Reprod 2004; 70(1):54–64. [DOI] [PubMed] [Google Scholar]
- 62. de Moraes AA, Hansen PJ. Granulocyte-macrophage colony-stimulating factor promotes development of in vitro produced bovine embryos. Biol Reprod 1997; 57(5):1060–1065. [DOI] [PubMed] [Google Scholar]
- 63. Dobbs KB, Khan FA, Sakatani M, Moss JI, Ozawa M, Ealy AD, Hansen PJ. Regulation of pluripotency of inner cell mass and growth and differentiation of trophectoderm of the bovine embryo by colony stimulating factor 2. Biol Reprod 2013; 89(6):141. [DOI] [PubMed] [Google Scholar]
- 64. Loureiro B, Oliveira LJ, Favoreto MG, Hansen PJ. Colony-stimulating factor 2 inhibits induction of apoptosis in the bovine preimplantation embryo. Am J Reprod Immunol 2011; 65(6):578–588. [DOI] [PubMed] [Google Scholar]
- 65. Chin PY, Macpherson AM, Thompson JG, Lane M, Robertson SA. Stress response genes are suppressed in mouse preimplantation embryos by granulocyte-macrophage colony-stimulating factor (GM-CSF). Hum Reprod 2009; 24(12):2997–3009. [DOI] [PubMed] [Google Scholar]
- 66. Papayannis M, Eyheremendy V, Sanjurjo C, Blaquier J, Raffo FG. Effect of granulocyte-macrophage colony stimulating factor on growth, resistance to freezing and thawing and re-expansion of murine blastocysts. Reprod Biomed Online 2007; 14(1):96–101. [DOI] [PubMed] [Google Scholar]
- 67. Siqueira LG, Hansen PJ. Sex differences in response of the bovine embryo to colony-stimulating factor 2. Reproduction 2016; 152(6):645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gebert C, Wrenzycki C, Herrmann D, Gröger D, Thiel J, Reinhardt R, Lehrach H, Hajkova P, Lucas-Hahn A, Carnwath JW, Niemann H. DNA methylation in the IGF2 intragenic DMR is re-established in a sex-specific manner in bovine blastocysts after somatic cloning. Genomics 2009; 94(1):63–69. [DOI] [PubMed] [Google Scholar]
- 69. Dobbs KB, Rodriguez M, Sudano MJ, Ortega MS, Hansen PJ. Dynamics of DNA methylation during early development of the preimplantation bovine embryo. PLoS One 2013; 8(6):e66230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gross N, Kropp J, Khatib H. Sexual dimorphism of miRNAs secreted by bovine In vitro-produced embryos. Front Genet 2017; 8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Green MP, Harvey AJ, Spate LD, Kimura K, Thompson JG, Roberts RM. The effects of 2,4-dinitrophenol and D-glucose concentration on the development, sex ratio, and interferon-tau (IFNT) production of bovine blastocysts. Mol Reprod Dev? 2016; 83(1):50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dallemagne M, Ghys E, De Schrevel C, Mwema A, De Troy D, Rasse C, Donnay I. Oxidative stress differentially impacts male and female bovine embryos depending on the culture medium and the stress condition. Theriogenology 2018; 117:49–56. [DOI] [PubMed] [Google Scholar]
- 73. Broughton SE, Dhagat U, Hercus TR, Nero TL, Grimbaldeston MA, Bonder CS, Lopez AF, Parker MW. The GM-CSF/IL-3/IL-5 cytokine receptor family: from ligand recognition to initiation of signaling. Immunol Rev 2012; 250(1):277–302. [DOI] [PubMed] [Google Scholar]
- 74. Park JE, Oh HJ, Hong SG, Jang G, Kim MK, Lee BC. Effects of activin A on the in vitro development and mRNA expression of bovine embryos cultured in chemically-defined two-step culture medium. Reprod Domest Anim 2010; 45:585–593. [DOI] [PubMed] [Google Scholar]
- 75. Trigal B, Gómez E, Díez C, Caamaño JN, Martín D, Carrocera S, Muñoz M. In vitro development of bovine embryos cultured with activin A. Theriogenology 2011; 75(3):584–588. [DOI] [PubMed] [Google Scholar]
- 76. Kannampuzha-Francis J, Tribulo P, Hansen PJ. Actions of activin A, connective tissue growth factor, hepatocyte growth factor and teratocarcinoma-derived growth factor 1 on the development of the bovine preimplantation embryo. Reprod Fertil Dev 2017; 29(7):1329–1339. [DOI] [PubMed] [Google Scholar]
- 77. Kocyigit A, Cevik M. Effects of leukemia inhibitory factor and insulin-like growth factor-I on the cell allocation and cryotolerance of bovine blastocysts. Cryobiology 2015; 71(1):64–69. [DOI] [PubMed] [Google Scholar]
- 78. Vejlsted M, Avery B, Gjorret JO, Maddox-Hyttel P. Effect of leukemia inhibitory factor (LIF) on in vitro produced bovine embryos and their outgrowth colonies. Mol Reprod Dev 2005; 70(4):445–454. [DOI] [PubMed] [Google Scholar]
- 79. Rodríguez A, De Frutos C, Díez C, Caamaño JN, Facal N, Duque P, García-Ochoa C, Gómez E. Effects of human versus mouse leukemia inhibitory factor on the in vitro development of bovine embryos. Theriogenology 2007; 67(5):1092–1095. [DOI] [PubMed] [Google Scholar]
- 80. Gómez E, Correia-Álvarez E, Caamaño JN, Díez C, Carrocera S, Peynot N, Martín D, Giraud-Delville C, Duranthon V, Sandra O, Muñoz M. Hepatoma-derived growth factor: from the bovine uterus to the in vitro embryo culture. Reproduction 2014; 148(4):353–365. [DOI] [PubMed] [Google Scholar]
- 81. Denicol AC, Block J, Kelley DE, Pohler KG, Dobbs KB, Mortensen CJ, Ortega MS, Hansen PJ. The WNT signaling antagonist Dickkopf-1 directs lineage commitment and promotes survival of the preimplantation embryo. FASEB J 2014; 28(9):3975–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Loureiro B, Bonilla L, Block J, Fear JM, Bonilla AQ, Hansen PJ. Colony-stimulating factor 2 (CSF-2) improves development and posttransfer survival of bovine embryos produced in vitro. Endocrinology 2009; 150(11):5046–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ozawa M, Sakatani M, Dobbs KB, Kannampuzha-Francis J, Hansen PJ. Regulation of gene expression in the bovine blastocyst by colony stimulating factor 2. BMC Res Notes 2016; 9(1):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fischer-Brown AE, Lindsey BR, Ireland FA, Northey DL, Monson RL, Clark SG, Wheeler MB, Kesler DJ, Lane SJ, Weigel KA, Rutledge JJ. Embryonic disc development and subsequent viability of cattle embryos following culture in two media under two oxygen concentrations. Reprod Fertil Dev 2004; 16(8):787–793. [DOI] [PubMed] [Google Scholar]
- 85. Loureiro B, Block J, Favoreto MG, Carambula S, Pennington KA, Ealy AD, Hansen PJ. Consequences of conceptus exposure to colony-stimulating factor 2 on survival, elongation, interferon-τ secretion, and gene expression. Reproduction 2011; 141(5):617–624. [DOI] [PubMed] [Google Scholar]
- 86. Dobbs KB, Gagné D, Fournier E, Dufort I, Robert C, Block J, Sirard MA, Bonilla L, Ealy AD, Loureiro B, Hansen PJ. Sexual dimorphism in developmental programming of the bovine preimplantation embryo caused by colony-stimulating factor 2. Biol Reprod 2014; 91(3):80. [DOI] [PubMed] [Google Scholar]
- 87. Zammit PS. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin Cell Dev Biol 2017; 72:19–32. [DOI] [PubMed] [Google Scholar]
- 88. Orozco S, Oberst A. RIPK3 in cell death and inflammation: the good, the bad, and the ugly. Immunol Rev 2017; 277(1):102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Betteridge KJ, Eaglesome MD, Randall GC, Mitchell D. Collection, description and transfer of embryos from cattle 10-16 days after oestrus. Reproduction 1980; 59(1):205–216. [DOI] [PubMed] [Google Scholar]
- 90. Berg DK, van Leeuwen J, Beaumont S, Berg M, Pfeffer PL. Embryo loss in cattle between Days 7 and 16 of pregnancy. Theriogenology 2010; 73(2):250–260. [DOI] [PubMed] [Google Scholar]
- 91. Spencer TE, Hansen TR. Implantation and establishment of pregnancy in ruminants. Advances in Anat Embryol Cell Biol 2015; 216:105–135. [DOI] [PubMed] [Google Scholar]
- 92. Kimura K, Spate LD, Green MP, Murphy CN, Seidel GE Jr, Roberts RM. Sexual dimorphism in interferon-tau production by in vivo-derived bovine embryos. Mol Reprod Dev 2004; 67(2):193–199. [DOI] [PubMed] [Google Scholar]
- 93. Bertolini M, Beam SW, Shim H, Bertolini LR, Moyer AL, Famula TR, Anderson GB. Growth, development, and gene expression by in vivo- and in vitro-produced day 7 and 16 bovine embryos. Mol Reprod Dev 2002; 63(3):318–328. [DOI] [PubMed] [Google Scholar]
- 94. Siqueira LG, Tribulo P, Chen Z, Denicol AC, Ortega MS, Negrón-Pérez VM, Kannampuzha-Francis J, Pohler KG, Rivera RM, Hansen PJ. Colony-stimulating factor 2 acts from days 5 to 7 of development to modify programming of the bovine conceptus at day 86 of gestation. Biol Reprod 2017; 96(4):743–757. [DOI] [PubMed] [Google Scholar]
- 95. Kannampuzha-Francis J, Denicol AC, Loureiro B, Kaniyamattam K, Ortega MS, Hansen PJ. Exposure to colony stimulating factor 2 during preimplantation development increases postnatal growth in cattle. Mol Reprod Dev 2015; 82(11):892–897. [DOI] [PubMed] [Google Scholar]
- 96. Sjöblom C, Roberts CT, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor alleviates adverse consequences of embryo culture on fetal growth trajectory and placental morphogenesis. Endocrinology 2005; 146(5):2142–2153. [DOI] [PubMed] [Google Scholar]
- 97. Steinhart Z, Angers S. Wnt signaling in development and tissue homeostasis. Development 2018; 145(11):dev146589. [DOI] [PubMed] [Google Scholar]
- 98. Hosseini V, Dani C, Geranmayeh MH, Mohammadzadeh F, Nazari Soltan Ahmad S, Darabi M. Wnt lipidation: Roles in trafficking, modulation, and function. J Cell Physiol 2018; 234:8040–8054.. [DOI] [PubMed] [Google Scholar]
- 99. Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat Rev 2018; 62:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. De A. Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin 2011; 43(10):745–756. [DOI] [PubMed] [Google Scholar]
- 101. Feng Q, Gao N. Keeping Wnt signalosome in check by vesicular traffic. J Cell Physiol 2015; 230(6):1170–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chae WJ, Bothwell ALM. Canonical and non-canonical Wnt signaling in immune cells. Trends Immunol 2018; 39(10):830–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol 2009; 129(7):1614–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development 2009; 136(19):3205–3214. [DOI] [PubMed] [Google Scholar]
- 105. Tribulo P, Moss JI, Ozawa M, Jiang Z, Tian XC, Hansen PJ. WNT regulation of embryonic development likely involves pathways independent of nuclear CTNNB1. Reproduction 2017; 153(4):405–419. [DOI] [PubMed] [Google Scholar]
- 106. Denicol AC, Dobbs KB, McLean KM, Carambula SF, Loureiro B, Hansen PJ. Canonical WNT signaling regulates development of bovine embryos to the blastocyst stage. Sci Rep 2013; 3(1):1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tribulo P, Leão BCDS, Lehloenya KC, Mingoti GZ, Hansen PJ. Consequences of endogenous and exogenous WNT signaling for development of the preimplantation bovine embryo. Biol Reprod 2017; 96(6):1129–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Aparicio IM, Garcia-Herreros M, Fair T, Lonergan P. Identification and regulation of glycogen synthase kinase-3 during bovine embryo development. Reproduction 2010; 140(1):83–92. [DOI] [PubMed] [Google Scholar]
- 109. Bogliotti YS, Wu J, Vilarino M, Okamura D, Soto DA, Zhong C, Sakurai M, Sampaio RV, Suzuki K, Izpisua Belmonte JC, Ross PJ. Efficient derivation of stable primed pluripotent embryonic stem cells from bovine blastocysts. Proc Natl Acad Sci USA 2018; 115(9):2090–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lu J, Ma Z, Hsieh JC, Fan CW, Chen B, Longgood JC, Williams NS, Amatruda JF, Lum L, Chen C. Structure-activity relationship studies of small-molecule inhibitors of Wnt response. Bioorg Med Chem Lett 2009; 19(14):3825–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Huang X, Zhu H, Gao Z, Li J, Zhuang J, Dong Y, Shen B, Li M, Zhou H, Guo H, Huang R, Yan J. Wnt7a activates canonical Wnt signaling, promotes bladder cancer cell invasion, and is suppressed by miR-370-3p. J Biol Chem 2018; 293(18):6693–6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. von Maltzahn J, Bentzinger CF, Rudnicki MA. Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic growth pathway in skeletal muscle. Nat Cell Biol 2012; 14(2):186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bikkavilli RK, Avasarala S, Van Scoyk M, Arcaroli J, Brzezinski C, Zhang W, Edwards MG, Rathinam MK, Zhou T, Tauler J, Borowicz S, Lussier YAet al. Wnt7a is a novel inducer of β-catenin-independent tumorsuppressive cellular senescence in lung cancer. Oncogene 2015; 34(42):5317–5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cerri RL, Thompson IM, Kim IH, Ealy AD, Hansen PJ, Staples CR, Li JL, Santos JE, Thatcher WW. Effects of lactation and pregnancy on gene expression of endometrium of Holstein cows at day 17 of the estrous cycle or pregnancy. J Dairy Sci 2012; 95(10):5657–5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sartori R, Bastos MR, Wiltbank MC. Factors affecting fertilisation and early embryo quality in single- and superovulated dairy cattle. Reprod Fertil Dev 2010; 22(1):151–158. [DOI] [PubMed] [Google Scholar]
- 116. Hansen PJ. Challenges to fertility in dairy cattle: From ovulation to the fetal stage of pregnancy. Rev Brasil Reprod Anim 2011; 35:229–238. [Google Scholar]
- 117. Minten MA, Bilby TR, Bruno RG, Allen CC, Madsen CA, Wang Z, Tibary A, Neibergs HL, Geary TW, Bauersachs S. Effects of fertility on gene expression and function of the bovine endometrium. PLoS One 2013; 8(8):e69444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhuang X, Zhang H, Li X, Li X, Cong M, Peng F, Yu J, Zhang X, Yang Q, Hu G. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat Cell Biol 2017; 19(10):1274–1285. [DOI] [PubMed] [Google Scholar]
- 119. Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S, Trümper L, Binder C. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci 2006; 103(14):5454–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Caneparo L, Huang YL, Staudt N, Tada M, Ahrendt R, Kazanskaya O, Niehrs C, Houart C. Dickkopf-1 regulates gastrulation movements by coordinated modulation of Wnt/betacatenin and Wnt/PCP activities, through interaction with the Dally-like homolog Knypek. Genes Dev 2007; 21(4):465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Killick R, Ribe EM, Al-Shawi R, Malik B, Hooper C, Fernandes C, Dobson R, Nolan PM, Lourdusamy A, Furney S, Lin K, Breen Get al. Clusterin regulates β-amyloid toxicity via Dickkopf-1-driven induction of the wnt-PCP-JNK pathway. Mol Psychiatry 2014; 19(1):88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Krause U, Ryan DM, Clough BH, Gregory CA. An unexpected role for a Wnt-inhibitor: Dickkopf-1 triggers a novel cancer survival mechanism through modulation of aldehyde-dehydrogenase-1 activity. Cell Death Dis 2014; 5(2):e1093–e1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Denicol AC, Leão BC, Dobbs KB, Mingoti GZ, Hansen PJ. Influence of sex on basal and dickkopf-1 regulated gene expression in the bovine morula. PLoS One 2015; 10(7):e0133587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tríbulo P, Bernal Ballesteros BH, Ruiz A, Tríbulo A, Tríbulo RJ, Tríbulo HE, Bo GA, Hansen PJ. Consequences of exposure of embryos produced in vitro in a serum-containing medium to dickkopf-related protein 1 and colony stimulating factor 2 on blastocyst yield, pregnancy rate, and birth weight1. J Anim Sci 2017; 95(10):4407–4412. [DOI] [PubMed] [Google Scholar]
- 125. Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 2006; 25(57):7469–7481. [DOI] [PubMed] [Google Scholar]
- 126. Wathes DC, Cheng Z, Chowdhury W, Fenwick MA, Fitzpatrick R, Morris DG, Patton J, Murphy JJ. Negative energy balance alters global gene expression and immune responses in the uterus of postpartum dairy cows. Physiol Genomics 2009; 39(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Salilew-Wondim D, Ibrahim S, Gebremedhn S, Tesfaye D, Heppelmann M, Bollwein H, Pfarrer C, Tholen E, Neuhoff C, Schellander K, Hoelker M. Clinical and subclinical endometritis induced alterations in bovine endometrial transcriptome and miRNome profile. BMC Genomics 2016; 17(1):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Elweza AE, Ezz MA, Acosta TJ, Talukder AK, Shimizu T, Hayakawa H, Shimada M, Imakawa K, Zaghloul AH, Miyamoto A. A proinflammatory response of bovine endometrial epithelial cells to active sperm in vitro. Mol Reprod Dev 2018; 85(3):215–226. [DOI] [PubMed] [Google Scholar]
- 129. Ibrahim LA, Rizo JA, Fontes PLP, Lamb GC, Bromfield JJ. Seminal plasma modulates expression of endometrial inflammatory mediators in the bovine. Biol Reprod 2018; (in press). Published online ahead of print 17 October 2018; DOI : 10.1093/biolre/ioy226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hansen PJ, Block J, Loureiro B, Bonilla L, Hendricks KE. Effects of gamete source and culture conditions on the competence of in vitro-produced embryos for post-transfer survival in cattle. Reprod Fertil Dev 2010; 22(1):59–66. [DOI] [PubMed] [Google Scholar]
- 131. Nahar A, Maki S, Kadokawa H. Suppressed expression of granulocyte macrophage colony-stimulating factor in oviduct ampullae of obese cows. Anim Reprod Sci 2013; 139(1-4):1–8. [DOI] [PubMed] [Google Scholar]
- 132. Mittrücker HW, Visekruna A, Huber M. Heterogeneity in the differentiation and function of CD8+ T cells. Arch Immunol Ther Exp 2014; 62(6):449–458. [DOI] [PubMed] [Google Scholar]
- 133. Farin CE, Farmer WT, Farin PW. Pregnancy recognition and abnormal offspring syndrome in cattle. Reprod Fertil Dev 2010; 22(1):75–87. [DOI] [PubMed] [Google Scholar]
- 134. Siqueira LGB, Dikmen S, Ortega MS, Hansen PJ. Postnatal phenotype of dairy cows is altered by in vitro embryo production using reverse X-sorted semen. J Dairy Sci 2017; 100(7):5899–5908. [DOI] [PubMed] [Google Scholar]
- 135. Ribeiro ES, Bruno RG, Farias AM, Hernández-Rivera JA, Gomes GC, Surjus R, Becker LF, Birt A, Ott TL, Branen JR, Sasser RG, Keisler DHet al. Low doses of bovine somatotropin enhance conceptus development and fertility in lactating dairy cows. Biol Reprod 2014; 90(1):10. [DOI] [PubMed] [Google Scholar]
- 136. Wiltbank MC, Souza AH, Carvalho PD, Cunha AP, Giordano JO, Fricke PM, Baez GM, Diskin MG. Physiological and practical effects of progesterone on reproduction in dairy cattle. Animal 2014; 8(s1):70–81. [DOI] [PubMed] [Google Scholar]
- 137. Geary TW, Smith MF, MacNeil MD, Day ML, Bridges GA, Perry GA, Abreu FM, Atkins JA, Pohler KG, Jinks EM, Madsen CA. Triennial Reproduction Symposium: influence of follicular characteristics at ovulation on early embryonic survival. J Anim Sci 2013; 91:3014–3021. [DOI] [PubMed] [Google Scholar]