Abstract
Background
One of the most feared symptoms associated with cancer is pain. Opioids remain the mainstay of pain treatment but corticosteroids are often used concurrently as co‐ or adjuvant analgesics. Due to their anti‐inflammatory mechanism of action, corticosteroids are said to provide effective analgesia for pain associated with inflammation and in the management of cancer‐related complications such as brain metastasis and spinal cord compression. However, corticosteroids have a wide range of adverse effects that are dose and time dependent.
Objectives
To evaluate the efficacy of corticosteroids in treating cancer‐related pain in adults.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 4), MEDLINE (OVID) (1966 to 29 September 2014), EMBASE (OVID) (1970 to 29 September 2014), CINAHL (1982 to 29 September 2014), Science Citation Index (Web of Science) (1899 to 29 September 2014) and Conference Proceedings Citation Index ‐ Science (Web of Science) (1990 to 29 September 2014).
Selection criteria
Any randomised or prospective controlled trial that included patients over 18 years with cancer‐related pain were eligible for the review. Corticosteroids were compared to placebo or usual treatment and/or supportive care.
Data collection and analysis
All review authors independently assessed trial quality and extracted data. We used arithmetic means and standard deviations for each outcome to report the mean difference (MD) with 95% confidence interval (CI).
Main results
Fifteen studies met the inclusion criteria, enrolling 1926 participants. The trial size varied from 20 to 598 patients. Most studies compared corticosteroids, particularly dexamethasone, to standard therapy. We included six studies with data at one week in the meta‐analysis for pain intensity; no data were available at that time point for the remaining studies. Corticosteroid therapy resulted in less pain (measured on a scale of 0 to 10 with a lower score indicating less pain) compared to control at one week (MD 0.84 lower pain, 95% CI 1.38 to 0.30 lower; low quality evidence). Adverse events were poorly documented. Factors limiting statistical analysis included the lack of standardised measurements of pain and the use of different agents, dosages, comparisons and routes of drug delivery. Subgroup analysis according to type of cancer was not possible. The quality of this evidence was limited by the risk of bias of the studies and small sample size. The results were also compromised by attrition, with data missing for the enrolled patients.
Authors' conclusions
The evidence for the efficacy of corticosteroids for pain control in cancer patients is weak. Significant pain relief was noted in some studies, albeit only for a short period of time. This could be important for patients with poor clinical status. Further trials, with increased numbers of participants, are needed to evaluate the safety and effectiveness of corticosteroids for the management cancer pain in adults, and to establish an ideal dose, duration of therapy and route of administration.
Plain language summary
Corticosteroids for the management of cancer pain in adults
Background: One of the most feared symptoms associated with cancer is pain. Opioids remain the mainstay of pain treatment but corticosteroids are often used at the same time, along with standard pain relievers. This review evaluates the clinical trial evidence up to 29 September 2014 to determine how effective corticosteroids are in treating cancer‐related pain in adults and how well tolerated this treatment is for these patients.
Study characteristics: We found 15 relevant studies with 1926 participants. The trial size varied from 20 to 598 patients and the duration of the included studies ranged from seven days to 42 weeks. Most studies compared corticosteroids, particularly dexamethasone, to standard therapy.
Key results and quality of the evidence: Overall, we found that the current evidence is based on studies that contain only a small number of patients. The following conclusions can be made from the available evidence: 1) the evidence for the efficacy of corticosteroids for pain control in cancer patients is weak (GRADE quality of evidence for pain outcome was low); 2) significant pain relief was noted in some studies, albeit only for a short period of time; this could be important for patients who have only a short time to live; 3) overall, more studies found corticosteroids not to be of benefit; 4) it was not possible to determine whether steroids are more effective for pain in specific cancers; and 5) the side effect profile of steroids, especially in the longer term, is not well described.
Summary of findings
Summary of findings 1. Corticosteroids for cancer‐related pain in adults.
| Corticosteroids for cancer‐related pain in adults | ||||||
| Patient or population: adult patients with cancer‐related pain Settings: in‐ and out‐patients Intervention: corticosteroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Corticosteroids | |||||
|
Pain at 1 week Scale from: 0 to 10 |
The mean pain at 1 week in the control group was 3.77 |
The mean pain at 1 week in the intervention groups was 0.84 lower (1.38 to 0.3 lower) | 315 (6 studies) | ⊕⊕⊝⊝ low1 | Pain (1 to 10) with lower score indicating less pain Quality of evidence low due to the small number of participants in each arm for the included studies |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1We downgraded the quality of evidence by two levels from high to low because of likely selection bias and the small number of patients in the included studies.
Background
Description of the condition
Cancer remains the leading cause of death worldwide. Over 12 million new cases are diagnosed each year (Foley 2011). The disease carries significant morbidity. Pain resulting directly or indirectly from the abnormal growth of malignant cells in normal tissue is the most common and most feared symptom associated with cancer (Van den Beuken‐van Everdingen 2007). It is estimated that one‐third of cancer patients on active therapy, and two‐thirds of those with advanced disease, experience pain that requires treatment with analgesic drugs (Foley 2011). Of concern, there is also considerable evidence that cancer pain is often under treated (Foley 2011). While opioids remain the mainstay of treatment for cancer pain, co‐analgesics or adjuvants are often used concurrently to optimise pain control. Corticosteroids (steroids) are commonly used in this context.
Description of the intervention
Steroids are essential for maintaining homeostasis and regulating a wide variety of physiological processes in the human body (Busillo 2013). Therapeutically, they are widely prescribed for the treatment of inflammation, auto‐immune disorders and malignancies (Busillo 2013). They are commonly used in the management of cancer pain.
How the intervention might work
Corticosteroids are used for relief of pain associated with space‐occupying lesions, not only in the brain, spinal cord and nerves, but also in the liver and soft tissues (eTG Complete 2014). They are used where there may be inflammation and oedema in confined spaces, including intracerebral, pelvic, retroperitoneal and spinal malignant disease, and are often used as an interim measure while awaiting more definitive therapies such as radiotherapy (eTG Complete 2014).
Corticosteroids have been proposed to have effects on all four stages of pain nociception including transduction, transmission, modulation and pain perception, although the exact mechanisms remain unclear (Leppert 2012). The anti‐inflammatory effect of corticosteroids may be due to (i) inhibition of the expression of collagenase, an enzyme involved in tissue degeneration during inflammatory mechanisms, (ii) inhibition of pro‐inflammatory cytokines, which have been implicated in a number of pain states, or (iii) by stimulating the synthesis of lipocortin, which in turn blocks the production of eicosanoids (Leppert 2012; Paulsen 2013).
It is proposed that the mechanism of pain relief for cancer‐related neuropathic pain is by the inhibition of prostaglandin production, reduction of inflammation thus decreasing capillary permeability and reducing oedema (Pharo 2005)
In summary, the mechanism of action of corticosteroids in the reduction of cancer pain remains unclear.
Why it is important to do this review
Corticosteroids are prescribed frequently in oncology practice to reduce swelling and pain caused by cancer and may also be used to control and prevent nausea and vomiting caused by chemotherapy. In addition, it is common in palliative care practice, especially for patients with advanced malignant disease, for a variety of symptom control indications including pain, nausea, mood elevation, anorexia and fatigue (Farr 1990; Hardy 2001; Riechelmann 2007). This is despite the fact that steroids are associated with significant side effects, especially following long‐term use (Hanks 2009). There is little objective evidence in the literature to support the use of corticosteroids for symptom control, and concerns have been raised about the 'uncontrolled' use of steroids in cancer patients (Gannon 2002; Twycross 1985). Patients who are started on steroids in the palliative care setting are often not closely monitored, allowing for the development of debilitating side effects, often in the context of limited clinical benefit. Some of these side effects include: proximal myopathy, oral candidiasis, symptomatic hyperglycaemia, psychological disturbances, gastrointestinal irritation, increased susceptibility to infections and the development of osteoporosis. For example, although steroids are frequently administered to assist with mood elevation, some studies have shown that corticosteroid therapy may result in more disturbing side effects such as insomnia, delirium, depression, anxiety and psychosis (Vyvey 2010). There is a relevant gap in the body of knowledge, in that most patients with cancer will be prescribed steroids at some stage during their disease course with very little evidence of effectiveness.
Objectives
To evaluate the efficacy of corticosteroids in treating cancer‐related pain in adults.
Methods
Criteria for considering studies for this review
Types of studies
Any randomised controlled or prospective controlled trial.
Types of participants
Participants with cancer‐related pain, aged 18 years and above.
Types of interventions
Types of interventions included any corticosteroid used to treat cancer‐related pain.
We considered all routes of drug administration.
Comparisons were:
placebo;
no intervention;
usual treatment or supportive care; or
non‐pharmacological treatment for pain.
Types of outcome measures
Primary outcomes
Patient‐reported pain intensity and pain relief using validated scales (visual analogue scale (VAS), verbal rating scale (VRS), numerical rating scale (NRS)).
Secondary outcomes
Adverse events
Quality of life
Patient satisfaction
Other relevant outcome measures, e.g. cost‐effectiveness data
Search methods for identification of studies
The search strategy attempted to identify as many trials as possible that met the inclusion criteria without limitation by language, publication type or status or by date.
Electronic searches
We searched the following electronic databases:
the Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 4);
MEDLINE (OVID) (1966 to 29 September 2014);
EMBASE (OVID) (1970 to 29 September 2014);
CINAHL (1982 to 29/ September 2014);
Science Citation Index (Web of Science) (1899 to 29 September 2014);
Conference Proceedings Citation Index ‐ Science (Web of Science) (1990 to 29 September 2014).
The MEDLINE search strategy was adapted by a Librarian (KR) from one originally devised by the Trials Search Co‐ordinator of the Cochrane Pain, Palliative and Supportive Care Review Group. The Cochrane Highly Sensitive Search Strategy (CHSS) filter for identifying randomised trials in MEDLINE via OVID was also modified and applied. This search was adapted and modified across the other databases. The search strategies are shown in Appendices 1 to 4 (Appendix 1; Appendix 2; Appendix 3; Appendix 4).
Searching other resources
We checked the bibliographic references of any relevant identified studies in order to find additional trials not identified by the electronic searches. We also searched www.ClinicalTrials.gov, the metaRegister of Controlled Trials (mRCT www.controlled-trials.com/mrct/), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) to identify any ongoing trials. In order to identify any unpublished or grey literature, we searched the Internet using the Google Scholar search engine (www.googlescholar.com), with selected terms from the above strategy. If only the abstract was published, we attempted to contact the authors for further details or for the unpublished paper. The searches were conducted by one of the review authors (KR) who is a Librarian. All searches were current as of 29 September 2014.
Data collection and analysis
Selection of studies
Four of the review authors (JH, PG, SJ‐M, KR) independently assessed the titles and abstracts of all the studies identified by the search for potential inclusion. Each of these authors independently selected all potentially relevant studies for inclusion by applying the selection criteria outlined in the 'Criteria for considering studies for this review' section. We then compared these four lists, discussed any differences and either included or excluded the papers based on a majority decision.
A PRISMA study flow diagram (Liberati 2009) is included in Figure 1 to document the screening process, as recommended in Part 2, Section 11.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
1.
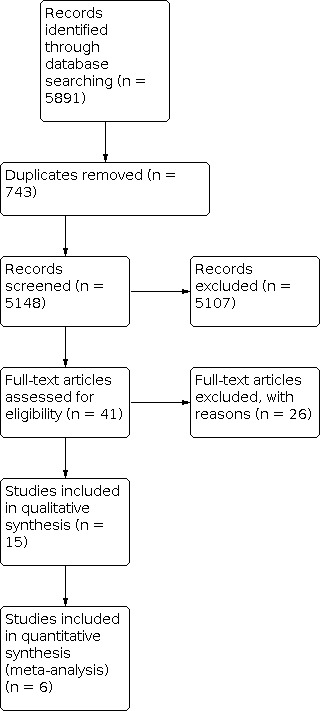
PRISMA Study flow diagram.
Data extraction and management
Four review authors (AL, AH, SK, SJ‐M) independently extracted data from the studies, using a piloted data extraction form. Data extracted included information about the year of study, study design, number of participants treated, participant demographic details, type of cancer, drug and dosing regimen, study design (placebo or active control) and methods, study duration and follow‐up, outcome measures (measurement of pain, pain scale), withdrawals and adverse events. We resolved potential disagreements by discussion.
Assessment of risk of bias in included studies
Six of the authors (AL, AH, JH, PG, SK, KR) independently assessed the risk of bias of each of the included studies by using the 'Risk of bias' assessment method outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved potential disagreements by discussion. For each study we assessed the risk of bias for the following domains.
Random sequence generation (checking for selection bias).
Allocation concealment (checking for selection bias).
Blinding of participants and personnel (checking for performance bias).
Blinding of outcome assessment (checking for detection bias).
Incomplete outcome data (checking for attrition bias).
Selective reporting (checking for reporting bias).
Size of study (checking for possible biases confounded by small size). We assessed studies as being at low risk of bias (200 or more participants per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); or high risk of bias (fewer than 50 participants per treatment arm).
We used the GRADE approach to assess the overall quality of the evidence for the primary outcome, with downgrading of the evidence from 'high quality' by one level for serious (or by two for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias (Langendam 2013). The outcome included in the 'Summary of findings' table was pain at week one (Table 1).
Measures of treatment effect
For continuous outcomes between groups, we measured arithmetic means and standard deviations (SD) and reported the mean difference (MD) with 95% CI. When an outcome was derived with different instruments measuring the same construct, we used the standardised mean difference (SMD) with 95% CIs.
Unit of analysis issues
We only included studies in which randomisation was by the individual patient; this included cross‐over or n = 1 studies.
Dealing with missing data
In cases where data were missing, we attempted to contact the authors to request the missing data. This strategy did not result in any additional data. We ascertained the method of assessing data processed from withdrawals where possible. It was not possible to assess the impact of missing data in sensitivity analyses due to the low study numbers. In all cases we aimed to perform intention‐to‐treat analyses.
Assessment of heterogeneity
There may be an effect of differences between patients, environment (inpatient versus outpatient) and outcome measures. We assessed heterogeneity by using the I2 statistic. We considered I2 values above 50% to represent substantial heterogeneity, in line with Higgins 2011, and assessed potential sources of heterogeneity through subgroup analyses.
Assessment of reporting biases
We interpreted the results of tests in the light of visual inspection of the funnel plot. If there was evidence of small study effects, we considered publication bias as only one of a number of possible explanations (Higgins 2011).
Data synthesis
We entered the data extracted from the included studies into Review Manager (RevMan 2014), which we used for data synthesis. Where appropriate, we pooled data for each dichotomous outcome and calculated RRs with 95% CIs using a random‐effects model.
Sensitivity analysis
When sufficient data were available, we examined the robustness of the meta‐analyses by conducting sensitivity analyses using different components of the 'Risk of bias' assessment, particularly those relating to whether allocation concealment and patient/assessor blinding were adequate. We conducted further sensitivity analyses to examine the impact of missing data on the results as a large proportion of the studies are at an 'unknown' or 'high' risk of attrition bias and, finally, sensitivity analyses to examine whether publication status and trial size influenced the results. Unfortunately, due to the low number of studies within each comparison, we were unable to perform any sensitivity analyses.
Results
Description of studies
See: 'Characteristics of included studies' and 'Characteristics of excluded studies' tables.
Results of the search
The PRISMA diagram (Figure 1) outlines the number of records identified in the search and the screening process for these papers. In the initial database search we identified 5891 records. Of these, 743 were duplicates and we rejected 5107 based on information given in the title and abstract.
We identified 41 publications for full‐text retrieval. We excluded 26 of these studies during screening. In three studies, only the abstract had been published and, as further detail was unavailable, we excluded them. Seven studies did not satisfy the inclusion criteria and we excluded 16 because pain was not an endpoint. The reasons for exclusion of each study are described in the 'Characteristics of excluded studies' table. A total of 15 studies met the inclusion criteria for this review. These included six placebo‐controlled studies, one placebo‐controlled cross‐over study, five studies with active controls, one open‐label and two low‐dose versus high‐dose studies (Table 2). We evaluated the results of six trials relative to pain intensity at one week (Basile 2012; Bruera 1985; Bruera 2004; Mercadante 2007; Paulsen 2014; Yennurajalingam 2013). The other trials could not be included in the meta‐analysis due to missing data at this time point.
1. Comparison of included studies.
| Comparison | Number of studies | References |
| Dexamethasone ‐ standard therapy | 5 | Basile 2012; Lauretti 2013; Lee 2008; Mercadante 2007; Teshima 1996 |
| (Methyl‐)prednisolone ‐ placebo | 5 | Bruera 1985; Della 1989; Paulsen 2014; Popiela 1989; Twycross 1985 |
| Dexamethasone ‐ placebo | 2 | Bruera 2004; Yennurajalingam 2013 |
| High‐dose versus low‐dose dexamethasone | 2 | Graham 2006; Vecht 1989 |
| Prednisone ‐ flutamide | 1 | Fossa 2001 |
Included studies
We identified 15 studies meeting the inclusion criteria (Basile 2012; Bruera 1985; Bruera 2004; Della 1989; Fossa 2001; Graham 2006; Lauretti 2013; Lee 2008; Mercadante 2007; Paulsen 2014; Popiela 1989; Teshima 1996; Twycross 1985; Vecht 1989; Yennurajalingam 2013). The 15 studies included 1926 enrolled participants. Trial size varied from 20 to 598 participants.
A detailed description of the included studies can be found in the 'Characteristics of included studies' table.
Primary disease sites
The primary disease sites addressed are tabulated in Table 3.
2. Primary sites of disease.
| Breast | Lung | Prostate | Ovary | Gastrointestinal | Genitourinary | Uterus | Other | Not specified | |
| Basile 2012 | x | x | |||||||
| Bruera 1985 | x | x | x | x | x | ||||
| Bruera 2004 | x | x | x | x | x | ||||
| Della 1989 | x | x | x | x | x | x | |||
| Fossa 2001 | x | ||||||||
| Graham 2006 | x | x | x | x | x | ||||
| Lauretti 2013 | x | x | x | x | x | ||||
| Lee 2008 | x | ||||||||
| Mercadante 2007 | x | ||||||||
| Paulsen 2014 | x | x | x | x | x | ||||
| Popiela 1989 | x | ||||||||
| Teshima 1996 | x | ||||||||
| Twycross 1985 | x | x | |||||||
| Vecht 1989 | x | ||||||||
| Yennurajalingam 2013 | x | x | x | x | x | x |
Most of the trials did not include patients with a specific cancer type, with the exception of Fossa 2001, Lee 2008 and Teshima 1996.
Types of studies
Studies were included in which steroids were used as part of a treatment regimen and pain was assessed as an outcome (Fossa 2001; Lee 2008). Two studies tested high‐dose versus low‐dose steroids (Graham 2006; Vecht 1989).
Pain requirement as an entry criteria
Eight of the 15 studies required that participants had pain at study entry (Basile 2012; Della 1989; Lauretti 2013; Mercadante 2007; Paulsen 2014; Popiela 1989; Teshima 1996; Yennurajalingam 2013).
Pain as primary endpoint
Of the 15 included studies, only nine trials were designed with pain relief as a primary outcome measure (Basile 2012; Bruera 1985; Graham 2006; Lauretti 2013; Mercadante 2007; Paulsen 2014; Teshima 1996; Twycross 1985; Vecht 1989). Of the remainder, six studies were designed to describe differences in chronic nausea, cancer‐related fatigue and quality of life (Bruera 2004; Della 1989; Fossa 2001; Lee 2008; Popiela 1989; Yennurajalingam 2013).
Types of corticosteroids studied
Dexamethasone was used in eight studies (Basile 2012; Bruera 2004; Graham 2006; Lauretti 2013; Lee 2008; Mercadante 2007; Vecht 1989; Yennurajalingam 2013), methylprednisolone in five studies (Bruera 1985; Della 1989; Paulsen 2014; Popiela 1989; Teshima 1996), and prednisone (Fossa 2001) and prednisolone (Twycross 1985) in one study each (Table 2).
Dexamethasone was administered orally in three studies (Bruera 2004; Mercadante 2007; Yennurajalingam 2013), and intravenously in five studies (Basile 2012; Graham 2006; Lauretti 2013; Lee 2008; Vecht 1989). Methylprednisolone was used in five trials, administered orally in two studies (Bruera 1985; Paulsen 2014), and intravenously in three studies (Della 1989; Popiela 1989; Teshima 1996). Prednisone (Fossa 2001) and prednisolone (Twycross 1985) were administered orally in both studies.
Additional details of dosage are provided in Table 4.
3. Target population, drug and dose of the 15 included studies.
| Study | Target population | Drug | Dose |
| Basile 2012 | Bone neoplasm | Dexamethasone | 4 mg/mL |
| Bruera 1985 | Advanced cancer | Methylprednisolone | 32 mg |
| Bruera 2004 | Advanced cancer | Dexamethasone | 20 mg |
| Della 1989 | Advanced cancer | Methylprednisolone | 125 mg |
| Fossa 2001 | Prostate cancer | Prednisone | 20 mg |
| Graham 2006 | Malignant spinal cord compression | Dexamethasone | 16 mg |
| Graham 2006 | Malignant spinal cord compression | Dexamethasone | 96 mg |
| Lauretti 2013 | Advanced cancer | Dexamethasone | 10 mg |
| Lee 2008 | Multiple myeloma | Dexamethasone | 40 mg |
| Mercadante 2007 | Adjuvant drug in advanced cancer patients | Dexamethasone | 8 mg |
| Paulsen 2014 | Advanced cancer | Methylprednisolone | 16 mg |
| Popiela 1989 | Advanced cancer | Methylprednisolone | 125 mg |
| Teshima 1996 | Bone metastases | Methylprednisolone | 500 mg |
| Twycross 1985 | Breast or bronchus cancer | Prednisolone | 15 mg |
| Vecht 1989 | Advanced cancer | Dexamethasone | 10 mg |
| Vecht 1989 | Advanced cancer | Dexamethasone | 100 mg |
| Yennurajalingam 2013 | Advanced cancer | Dexamethasone | 8 mg |
Pain and analgesic measurement tools
Different measurement tools were used to measure pain intensity.
Visual analogue scale 0 to 10 (four studies) (Basile 2012; Graham 2006; Lauretti 2013; Vecht 1989)
Visual analogue scale 0 to 100 (two studies) (Bruera 1985; Twycross 1985)
Numerical scale 0 to 10 (six studies) (Bruera 2004; Della 1989; Mercadante 2007; Paulsen 2014; Popiela 1989; Yennurajalingam 2013)
Quality of life questionnaire including pain (two studies) (Fossa 2001; Lee 2008)
Radiation Therapy Oncology Group (RTOG) pain scale (one study) (Teshima 1996)
Excluded studies
We excluded 26 studies (Campio 1983; Chanan‐Khan 2011; Coloma 2001; Datta 1997; Dutta 2012; Friedenberg 1991; Fuccio 2011; Gomez‐Hernandez 2010; Hird 2009; Laval 2000; North 2003; Richardson 2009; Richardson 2010; Richardson 2011; Richardson 2012; Rinehart 2010; Rizzo 2009; Sanguineti 2003; Schmuth 2002; Tantawy 2008; Tong 1982; Vecht 1994; Vij 2012; Yennurajalingam 2010; Yennurajalingam 2012; Yoshioka 2011). Reasons for exclusion are provided in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
We assessed each study using the Cochrane 'Risk of bias' tool. Overall findings are presented in the 'Risk of bias' graph (Figure 2), which reviews the authors' judgements about each risk of bias item presented as percentages across all included studies. Authors' judgements about each risk of bias for each included study is shown in 'Risk of bias' summary (Figure 3).
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies reported that they were randomised, but only six out of 15 properly described the method used to generate the random sequence. Four studies were randomised using a computer program (Della 1989; Lauretti 2013; Paulsen 2014; Popiela 1989). The participants from Graham 2006 were randomised using the Superdex website. Those from Teshima 1996 were randomly allocated according to Peto's balanced randomised list. We judged the six studies describing randomisation to have low risk and the other nine to have unclear risk of bias.
We judged seven studies to be low risk for allocation concealment; the trial medications were of identical appearance (Bruera 2004; Paulsen 2014), the packages were blinded (Della 1989; Lauretti 2013; Popiela 1989; Vecht 1989), or they used a password protected website to maintain blinding (Graham 2006). Two trials were at high risk, being open‐label or not placebo‐controlled (Lee 2008; Mercadante 2007). The remaining six trials were of unclear risk as not enough information was provided on the method of allocation concealment.
Blinding
Ten trials were at low risk, reporting blinding of patients and personnel (Basile 2012; Bruera 1985; Bruera 2004; Della 1989; Paulsen 2014; Popiela 1989; Twycross 1985; Vecht 1989; Yennurajalingam 2013). In five studies blinding was not possible due to different dosage intervals, because physicians were provided with patient's assigned treatment or because it was an open‐label trial, so we assessed them as having high risk (Fossa 2001; Graham 2006; Lee 2008; Mercadante 2007; Teshima 1996).
The outcome assessment was blind in eight trials, judged to have low risk (Bruera 1985; Bruera 2004; Della 1989; Paulsen 2014; Popiela 1989; Twycross 1985; Vecht 1989; Yennurajalingam 2013). In the other three trials it was unclear whether the outcome assessor was blinded or not (Basile 2012; Fossa 2001; Lauretti 2013). In four studies the outcome assessment was not blind because physicians were provided with the patient's assigned treatment, it was an open‐label study or there was no placebo given (Graham 2006; Lee 2008; Mercadante 2007; Teshima 1996). These studies are at high risk of bias.
Incomplete outcome data
It was not certain whether Lauretti 2013 reported all the outcomes that had been assessed, but in seven trials it appeared that additional pertinent outcomes should have been reported and their omission left a gap in the evidence (Bruera 1985; Fossa 2001; Graham 2006; Lee 2008; Mercadante 2007; Twycross 1985; Yennurajalingam 2013). We assessed these seven studies to have a high risk of bias. The remaining seven trials appear to have reported all relevant outcomes related to the subject matter (Basile 2012; Bruera 2004; Della 1989; Paulsen 2014; Popiela 1989; Teshima 1996; Vecht 1989).
Selective reporting
In two studies it was unclear if there was a reporting bias (Fossa 2001; Mercadante 2007). We found a high risk in Bruera 1985 and Teshima 1996, as some data evaluating pain and adverse events were not mentioned. In 11 trials, judged to be low risk, no reporting gaps were detected (Basile 2012; Bruera 2004; Della 1989; Graham 2006; Lauretti 2013; Lee 2008; Paulsen 2014; Popiela 1989; Twycross 1985; Vecht 1989; Yennurajalingam 2013).
Other potential sources of bias
Sample size was an issue. Small studies are thought to be at increased risk of bias as they are unlikely to be adequately powered. We considered only one of the studies large enough to give a low risk of bias (more than 200 patients per arm) (Lee 2008). We judged four studies to have an unclear risk of bias due to sample size (50 to 199 participants per arm) (Della 1989; Fossa 2001; Popiela 1989; Yennurajalingam 2013). We judged the remaining 10 trials to have a high risk of bias because of their small number of participants (Basile 2012; Bruera 1985; Bruera 2004; Graham 2006; Lauretti 2013; Mercadante 2007; Paulsen 2014; Teshima 1996; Twycross 1985; Vecht 1989).
Effects of interventions
See: Table 1
Primary outcome
Patient‐reported pain intensity and pain relief using validated scales
For the meta‐analysis, only those studies that provided mean pain intensity and the standard deviation at one week could be included. In this case, we evaluated six studies relative to pain intensity (Basile 2012; Bruera 1985; Bruera 2004; Mercadante 2007; Paulsen 2014; Yennurajalingam 2013). The other trials could not be included in the meta‐analysis due to missing data. Data were reported for baseline and after one week of intervention.
A total of 372 patients at baseline and 315 patients after one week of intervention were involved in these six studies. After one week of intervention, the intervention arm was favoured in all trials (Figure 4). The total mean difference is ‐0.84 with a 95% confidence interval of ‐1.38 to ‐0.30 (Analysis 1.1). While the study by Bruera 1985 was a cross‐over trial, inclusion in the meta‐analysis did not affect the overall review findings.
4.

Forest plot of pain at 1 week.
1.1. Analysis.

Comparison 1: Pain, Outcome 1: Pain at 1 week
Secondary outcomes
A meta‐analysis for secondary outcomes could not be undertaken as the data were heterogeneous with no consistency of measurement tools or outcome measures. Patient satisfaction and cost‐effectiveness data was not available.
Adverse events
Not all studies included information on adverse events and several reported no or only minimal adverse events compared to controls or placebo (Bruera 2004; Graham 2006; Lauretti 2013; Mercadante 2007; Yennurajalingam 2013). The most common adverse events attributed to steroids were restlessness and sleeplessness (Paulsen 2014), gastrointestinal and cardiovascular events (Popiela 1989), Cushingoid facies, anxiety, fluid retention (Bruera 1985), hypocalcaemia and hyperglycaemia (Della 1989). In the latter study, more patients randomised to steroids dropped out because of toxicity.
Quality of life/patient well‐being
Four studies reported on quality of life (Bruera 1985; Bruera 2004; Mercadante 2007; Yennurajalingam 2013). Quality of life or patient well‐being improved in three of four studies (Bruera 1985; Mercadante 2007; Yennurajalingam 2013).
Discussion
Summary of main results
The objective of this systematic review was to assess whether corticosteroids are effective in reducing cancer‐related pain. Fifteen randomised controlled trials with 1926 enrolled participants were included; six placebo‐controlled studies, one placebo‐controlled cross‐over study, five studies with active controls, one open‐label study and two low‐dose versus high‐dose studies. Included studies assessed either dexamethasone (at doses of 8 mg and 20 mg in an oral tablet or 4 mg/ml, 10 mg, 16 mg, 40 mg, 96 mg and 100 mg intravenously), methylprednisolone (16 mg and 32 mg orally or 125 mg and 500 mg intravenously) or prednisone 15 mg or prednisolone 20 mg orally.
For the meta‐analysis only six studies could be evaluated for pain intensity. Data were reported after one week of intervention, since this was the only time that could be standardised across all six trials. The following conclusion regarding the effectiveness of corticosteroids for pain relief in cancer patients should be interpreted with consideration of the small number of eligible studies. The quality of studies was generally poor with a high risk of bias identified.
There is some evidence to suggest that there is a benefit in favour of the use of corticosteroids (mean difference (MD) ‐0.84, 95% confidence interval (CI) ‐1.38 to ‐0.30) for cancer pain for up to one week of intervention. However, it is debatable if the reduction of a mean pain score of 0.8 with wide confidence intervals is clinically meaningful.
There were insufficient data to evaluate different subgroups such as drug type, route of administration, dosage and different primary disease types.
Further trials with increased numbers of participants are needed to evaluate the safety and effectiveness of corticosteroids for the management cancer pain in adults, and to establish an ideal dose, duration of therapy and route of administration.
Overall completeness and applicability of evidence
We identified 15 studies that met the inclusion criteria, but included only six studies in the meta‐analysis for pain intensity with insufficient data available for the remaining studies.
We did not attempt to classify specific pain syndromes. There were insufficient data for subgroup analyses.
Statistical analysis in relation to pain intensity in this review was limited by a number of factors. There is a lack of standardised measurement of pain. Several different tools have been used for pain measurement. In the meta‐analysis, visual analogue scale scores (0 to 10) and numerical scale scores (0 to 10) were compared.
The results are also influenced by differences in steroid type, dosage, comparators, routes of administration, primary disease type, aetiology of pain and heterogeneity of study populations. One of the studies had a single dose of intravenous dexamethasone as an intervention compared to multiple oral doses of dexamethasone or methylprednisolone. Comparators included in the meta‐analysis included both "standard treatment" (two studies) and placebo (four studies). Trials where dexamethasone was used primarily as an anticancer treatment rather than as a co‐analgesic were also included.
Reporting of data
Basile 2012 reported clear data for pain outcomes at baseline and after one week of intervention. Adverse events were not reported.
Bruera 1985 tabulated the intensity of pain (VAS), adverse events and quality of life in each group at baseline and after one week of intervention.
Bruera 2004 presented mean pain scores, intensity of nausea and fatigue, as well as quality of life scores for both patient groups.
Della 1989 presented graphical representation of mean change from baseline in Nurses' Observation Scale for Inpatient Evaluation (NOSIE) and linear analogue scale assessment (LASA) total score. No standard deviation was provided.
Fossa 2001 presented data in the form of graphs. No original data were presented and it was therefore not possible to include this trial in the meta‐analysis.
Graham 2006 published graphical representation of pain scores. Standard deviations were missing and therefore the trial could not be included in the meta‐analysis.
Lauretti 2013 was not included in the meta‐analysis as data on measured pain scores were not presented.
Lee 2008 had missing data on mean pain intensity and standard deviation. The study was not included in the meta‐analysis.
Mercadante 2007 tabulated the mean scores and 95% CI for pain intensity, intensity of nausea, fatigue, drowsiness and also quality of life scores.
Paulsen 2014 reported clear data for pain outcomes, fatigue and appetite at baseline and after one week of intervention.
Popiela 1989 published a graphical representation of adverse events and quality of life. No data were presented on pain intensity scores, therefore the study could not be included in the meta‐analysis.
Teshima 1996 presented pain scores and quality of life in a graphical field, but no standard deviation was provided. The trial was not included in the meta‐analysis.
Twycross 1985 tabulated pain intensity scores. These could not be included in the meta‐analysis as only the difference in outcome was reported at day eight with no baseline data available.
Vecht 1989 presented average pain scores and standard deviation assessed by a numerical rating scale in graphical form. No original data were presented and therefore it was not possible to include it in the meta‐analysis.
Yennurajalingam 2013 presented the mean and standard deviation of pain intensity, nausea, depression, fatigue, drowsiness and quality of life.
Quality of the evidence
The quality of the evidence was low. This is due to imprecision (likely selection bias and the small number of patients) in the included studies. Nine studies did not adequately describe sequence generation and one study did not provide information about allocation concealment. In five studies the blinding of participants and personnel was not provided and in four studies the outcome assessment was not blinded. In a number of studies it appeared that additional pertinent outcomes should have been reported and in two studies a risk of bias in selective reporting was identified. Sample size was of concern. Only one study had more than 200 participants in each arm. Ten studies had fewer than 50 participants in each arm.
Potential biases in the review process
Data extraction, including 'Risk of bias' assessment, was done independently by all authors to minimise bias. The conclusion that can be drawn is limited by the number and the quality of the included studies. Several trials were susceptible to bias and hampered by incomplete outcome data and small sample size.
Authors' conclusions
Implications for practice.
The evidence of the efficacy of corticosteroids for pain control in cancer patients is weak. Our meta‐analysis of studies with data at one week suggests that corticosteroids may relieve cancer pain, even if only for a short period of time. However, it is debatable if the reduction of a mean pain score of 0.8 with wide confidence intervals is clinically meaningful. In addition, any evidence of the efficacy of corticosteroids in the reduction of cancer pain must be weighed up against the associated significant side effects. Furthermore, we can make no recommendation regarding type of steroid, dose, route of delivery, side effect profile or treatment period.
In light of the above, we recommend that clinicians are cautious in their prescribing of steroids for pain management, that they assess benefit carefully, treat for the shortest possible time and discontinue early in the absence of symptom relief.
Implications for research.
Further trials with increased numbers of participants are needed to evaluate the safety and effectiveness of corticosteroids for the management cancer pain in adults, and to establish an ideal dose, duration of therapy and route of administration. Further adequately powered randomised controlled trials with pain as a primary outcome, measured with universally accepted standardised tools, using a single agent (dexamethasone), at a pre‐specified dose and route over a short time period are indicated. Longer‐term toxicity should be documented during a follow‐up period.
What's new
| Date | Event | Description |
|---|---|---|
| 21 January 2021 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 10, 2013 Review first published: Issue 4, 2015
| Date | Event | Description |
|---|---|---|
| 30 April 2015 | Review declared as stable | The authors state that it is unlikely that new evidence will become available in the short term for this topic. This review will be assessed for further updating in 2020. |
| 28 April 2015 | Amended | Corrections made to author affiliations. |
Notes
Assessed for updating in 2021
In January 2021 we did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be reassessed for updating in two years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
The authors acknowledge the support of Eve Pinkerton in the initial phase of this project and the staff of the University of Queensland Library.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. MEDLINE search strategy
1. exp Adrenal Cortex Hormones/
2. (corticoid* or corticosteroid* or glucocorticoid*).tw.
3. (adrenal adj2 hormone*).tw.
4. Betamethasone/
5. betamethasone.tw.
6. Fludrocortisone/
7. fludrocortisone.tw.
8. Cortisone/
9. (cortisone acetate or cortisone).tw.
10. deflazacort.tw.
11. Dexamethasone/
12. dexamethasone.tw.
13. Hydrocortisone/
14. hydrocortisone.tw.
15. Methylprednisolone/
16. methylprednisolone.tw.
17. Prednisolone/
18. prednisolone.tw.
19. Triamcinolone/
20. triamcinolone.tw.
21. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
22. exp Pain/
23. pain.tw.
24. Pain Measurement/
25. exp Analgesics/
26. exp Analgesia/
27. "analges*".tw.
28. (quality adj2 life).tw.
29. quality of life/
30. 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29
31. malignant.tw.
32. malignancy.tw.
33. "tumor*".tw.
34. "tumour*".tw.
35. "cancer*".tw.
36. "carcinoma*".tw.
37. exp Neoplasms/
38. 31 or 32 or 33 or 34 or 35 or 36 or 37
39. 21 and 30 and 38
40. randomized controlled trial.pt.
41. controlled clinical trial.pt.
42. randomized.ab.
43. randomised.ab.
44. placebo.ab.
45. drug therapy.fs.
46. randomly.ab.
47. trial.ab.
48. groups.ab.
49. 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47
50. exp animals/ not humans.sh.
51. 49 not 50
52. 39 and 51
Appendix 2. MEDLINE (in‐process & other non‐indexed citations)
1. (corticoid* or corticosteroid* or cortisone or betamethasone or deflazacort or dexamethasone or hydrocortisone or methylprednisolone or prednisolone or triamcinolone or fludrocortisone).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
2. (pain or analges* or "quality of life").mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
3. (malignan* or cancer* or carcinoma* or neoplas*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
4. 1 and 2 and 3
5. 1 and 3
6. ((pain or analges* or quality adj3 life).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
7. 5 and 6
Appendix 3. EMBASE search strategy
1. exp corticosteroid/
2. betamethasone.ti,ab.
3. cortisone.ti,ab.
4. deflazacort.ti,ab.
5. dexamethasone.ti,ab.
6. fludrocortisone.ti,ab.
7. hydrocortisone.ti,ab.
8. methylprednisolone.ti,ab.
9. prednisolone.ti,ab.
10. triamcinolone.ti,ab.
11. (corticoid* OR corticosteroid* OR glucocorticoid*).ti,ab.
12. 1 or 2 or 3 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11
13. exp pain/
14. pain assessment/
15. exp analgesic agent/
16. exp analgesia/
17. pain.ti,ab.
18. “analges*”.ti,ab.
19. (quality adj2 life).ti,ab.
20. 13 or 14 or 15 or 16 or 17 or 18 or 19
21. malignant.ti,ab.
22. malignancy.ti,ab.
23. “carcinoma$”.ti,ab.
24. “cancer$”.ti,ab.
25. “tumo*r$”.ti,ab.
26. exp neoplasm/
27. 21 or 22 or 23 or 24 or 25 or 26
28. 12 and 20 and 27
29. Clinical trial/
30. Randomized controlled trial/
31. Randomization/
32. Single blind procedure/
33. Double blind procedure/
34. Crossover procedure/
35. Placebo/
36. (randomised or randomized or placebo or randomly or trial or groups).ti,ab.
37. 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36
38. animal/
39. human/
40. 37 not (37 and 38)
41. 37 not 40
42. 28 not 41
Appendix 4. CENTRAL search strategy
#1 MeSH descriptor [Adrenal Cortex Hormones] explode all trees
#2 (corticoid* or corticosteroid* or glucocorticoid*):ti,ab,kw
#3 (betamethasone or fludrocortisone or cortisone or deflazacort or dexamethasone or hydrocortisone or methylprednisolone or prednisolone or triamcinolone):ti,ab,kw
#4 (#1 OR #2 OR #3)
#5 MeSH descriptor [ Pain] explode all trees
#6 MeSH descriptor [Pain Measurement] this term only
#7 MeSH descriptor [Analgeia] explode all trees
#8 MeSH descriptor [Analgesics] explode all trees
#9 MeSH descriptor [Quality of Life] this term only
#10 (pain or analges* or Quality near/3 Life):ti,ab,kw
#11 (#5 OR #6 OR #7 OR #8 OR #9 OR #10)
#12 (malignan* OR malignancy OR tumor* OR tumour* OR cancer* OR carcinoma*):ti,ab,kw
#13 MeSH descriptor [Neoplasms] explode all trees
#14 (#12 OR #1)
#15 (#4 AND #11 AND #14)
Data and analyses
Comparison 1. Pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain at 1 week | 6 | 315 | Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.38, ‐0.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Basile 2012.
| Study characteristics | ||
| Methods | Randomised controlled trial Study duration: 3 months |
|
| Participants | 20 consecutive patients (12 intervention, 8 control) with single‐level vertebral neoplasm or pathological fractures totally or partially refractory to analgesic treatment, with indication for vertebroplasty Inclusion criteria:
|
|
| Interventions | Intervention: intrasomatic injection of 4 mg/ml of dexamethasone phosphate through vertebroplasty needle followed by cement injection (group A) Control: standard vertebroplasty (group B) |
|
| Outcomes | Pain intensity using VAS at various time intervals: 6 hours to 3 months | |
| Notes | Baseline VAS 8/10 in both groups. Greater reduction in VAS in group A at early time points (including day 7), but no significant difference at last follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly divided into 2 groups Not specified how the random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was obtained by blind extraction of letters A or B in a closed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 3 interventional radiologists (all blinded to treatment allocation) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 patients died in group A, 3 patients died in group B during follow‐up |
| Selective reporting (reporting bias) | Low risk | No problem detected |
| Other bias | High risk | Sample size: 20 participants; < 50 participants per treatment arm |
Bruera 1985.
| Study characteristics | ||
| Methods | Prospective, randomised, double‐blind, cross‐over trial Study duration: 14 days |
|
| Participants | 40 terminally ill patients (in‐ and out‐patients) with malignant disease No specific anticancer treatment within 1 month Oral analgesia according to individual requirements |
|
| Interventions | 32 mg methylprednisolone (MP) daily (16 mg twice a day orally) or placebo for 5 days Days 5 to 7: treatment‐free Days 8 to 12: cross‐over to other arm then open label MP |
|
| Outcomes | Pain intensity using VAS (0 to 100) assessed at days 0, 5, 13 and 33 (response defined as > 30% improvement over placebo) Anxiety Daily oral analgesic consumption appetite Food consumption (% of each meal) Performance status Activity score |
|
| Notes | Pain one of several endpoints Intensity of pain and daily consumption of analgesics was significantly lower after MP compared with baseline or placebo Depression, appetite and food consumption also improved on MP |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly divided into 2 groups Not specified how the random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified, same investigator did all evaluations |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9 patients did not complete the trial and were not included in evaluation 3 patients reported no pain at baseline |
| Selective reporting (reporting bias) | High risk | Only 28 patients were evaluated for pain |
| Other bias | High risk | Sample size: 40 participants; < 50 participants per treatment arm |
Bruera 2004.
| Study characteristics | ||
| Methods | Double‐blind, parallel‐arm trial 5 international centres Study duration: 7 days |
|
| Participants | 51 participants (25 intervention, 26 control) with advanced cancer and chronic nausea (> 2 weeks) resulting from advanced cancer despite treatment with metoclopramide at a minimal daily dose of 40 to 60 mg for 2 days | |
| Interventions | Intervention: 20 mg/day dexamethasone orally in addition to metoclopramide (60 mg/day orally) Control: placebo in addition to metoclopramide (60 mg/day) |
|
| Outcomes | Pain, appetite, fatigue and nausea, measured on a 0 to 10 numerical rating scale (NRS) (0 = symptom absent, 10 = worst possible symptom) Quality of life: physical well‐being, social well‐being, functional well‐being, emotional well‐being Toxicity assessment: presence or absence of ankle oedema, insomnia, restlessness or other symptoms (patient‐rated) |
|
| Notes | Pain secondary outcome measure. Nausea as primary endpoint Pain intensity at baseline low in both arms. Authors therefore query meaningfulness of pain as outcome measure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not specified |
| Allocation concealment (selection bias) | Low risk | Capsules containing both drugs identical in appearance, randomisation in pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 of 25 patients receiving dexamethasone dropped out 5 of 26 patients receiving placebo dropped out |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | High risk | Sample size: 51 participants; < 50 participants per treatment arm |
Della 1989.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled, multi‐centre study Study duration: 8 weeks |
|
| Participants | 403 participants (207 intervention, 196 control) with "pre‐terminal" cancer Inclusion criteria:
|
|
| Interventions | Intervention: 125 mg/day IV methylprednisolone sodium succinate (MPSS) daily Control: placebo (88.8 mg mannitol) daily IV for maximum of 8 weeks |
|
| Outcomes | Linear Analogue Self‐Assessment scale (LASA), 10 questions on pain, appetite, well‐being, nausea, sleepiness, weakness (10‐point scale ranging from 'worst' to 'best') Nurses Observation Scale for Inpatient Evaluation (NOSIE), 21 questions, 5‐point scale ranging from 'never' to 'always' (total score= 50 + social competence + social interest ‐ irritability ‐ retardation ‐ depression) |
|
| Notes | Pain not primary outcome measure. Comparable LASA scores at baseline. MPSS produced significantly more improvement than placebo in LASA score for pain, appetite, vomiting and well‐being. Pain one of several endpoints | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme |
| Allocation concealment (selection bias) | Low risk | Double‐blind Study medication was provided in blinded packages that contained vials of either MPSS or placebo |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind Identity of the investigational therapy was not known by the investigator, his staff or the patients |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 30 MPSS‐treated and 33‐placebo treated patients dropped out, 83 MPSS‐treated and 59‐placebo treated patients died prior to completing 8 weeks of treatment |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Sample size: 403 participants; 50 to 199 participants per treatment arm |
Fossa 2001.
| Study characteristics | ||
| Methods | Randomised controlled trial Study duration: 24 weeks |
|
| Participants | 201 participants (101 prednisone, 100 flutamide) with castrate resistant prostate cancer (CRPC) and symptomatic metastatic disease Inclusion criteria:
|
|
| Interventions | Group F: 250 mg flutamide orally 3 times a day Group P: 5 mg prednisone orally 4 times a day Patients receiving LHRH analogues continued with this treatment |
|
| Outcomes | Quality of life (QoL): QLQ C‐30, a 30‐item questionnaire assessing a range of physical, emotional and social health issues
|
|
| Notes | Pain not primary endpoint Prednisone used as treatment of prostate cancer Statistically significant treatment effects following prednisone were noted for pain, nausea, vomiting and diarrhoea |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not described |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Flutamide 3 times a day, prednisone 4 times a day, therefore no blinding possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat Patients had to remain in the trial for at least 6 weeks to be assessable for response. They were otherwise included in the analysis as 'non‐assessable' |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Other bias | Unclear risk | Sample size: 201 participants; 50 to 199 participants per treatment arm |
Graham 2006.
| Study characteristics | ||
| Methods | Pilot randomised controlled trial 8 recruiting centres Study duration: 14 days |
|
| Participants | 20 participants with malignant spinal cord compression (MSCC) Inclusion criteria:
|
|
| Interventions | High‐dose: 96 mg dexamethasone intravenously day 0, continued to day 2 then weaned to 0 by day 15 or Low‐dose: 16 mg dexamethasone intravenously day 0, continued to day 2 then weaned to 0 by day 15 Radiotherapy in both arms |
|
| Outcomes | Visual analogue pain score (0 to 10) Toxicity (method not specified) Survival Ambulation and functional outcome (method not specified) |
|
| Notes | High‐dose versus low‐dose dexamethasone study terminated because of inadequate recruitment No significant difference in pain in the first week Analgesic use tended to be lower in high dose arm Pilot study, not powered for outcome, descriptive analysis Pain one of several endpoints |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomised via the Superdex website |
| Allocation concealment (selection bias) | Low risk | Website protected with password unique to each investigator |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Physicians were provided with patient's assigned treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Physicians were provided with patient's assigned treatment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 out of 20 patients not evaluable |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | High risk | Sample size: 20 participants (pilot study); < 50 participants per treatment arm |
Lauretti 2013.
| Study characteristics | ||
| Methods | Randomised, prospective, placebo‐controlled trial Study duration: 21 days |
|
| Participants | 72 participants (6 groups of n = 12) with moderate/severe chronic cancer pain Inclusion criteria:
|
|
| Interventions | Control group (CG): epidural 40 mg lidocaine diluted to 10 ml volume with N/saline Dexamethasone group (DG): 40 mg lidocaine plus 10 mg dexamethasone 2.5 Met group: 2.5 mg epidural methadone with 40 mg lidocaine 5 Met group: 5 mg epidural methadone plus 40 mg lidocaine 7.5 Met group: 7.5 mg epidural methadone plus 40 mg lidocaine 7.5 Met‐Dex group: 7.5 mg methadone with 40 mg lidocaine and 10 mg dexamethasone All delivered as sacral block with free access to oral morphine to maintain VAS < 4/10 |
|
| Outcomes | Analgesic use, pain score (VAS), adverse effects Assessed by patient daily diary |
|
| Notes | Very small numbers in each group Significantly less oral morphine consumption with dexamethasone |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using a randomised number generator in a computer program |
| Allocation concealment (selection bias) | Low risk | Drugs were diluted in a 10 ml covered syringe in order to maintain blindness |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Same anaesthetist, unaware of the study drug prepared by a second anaesthetist Patients blind to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 patients from GC, 3 from DG, 2 from 2.5 MetG, 2 from 5 MetG, 2 from 7.5 MetG and 1 from 7.5 MetDG were excluded due to incomplete data collection Minimum of 8 patients per group maintained for statistical purposes |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | High risk | Sample size: 72 participants; < 50 participants per treatment arm |
Lee 2008.
| Study characteristics | ||
| Methods | Prospective, randomised, open‐label study Multi‐site, international Study duration: 42 weeks |
|
| Participants | 598 participants (bortezomib 296, or dexamethasone 302) with relapsed multiple myeloma | |
| Interventions | Bortezomib (B): 1.3 mg/m2, days 1, 4, 8 and 11 for eight 3‐week cycles, then days 1, 8, 15 and 22 for three 5‐week cycles IV bolus Dexamethasone (D): 40 mg/day, days 1 to 4, 9 to 12 and 17 to 20 for four 5‐week cycles, then days 1 to 4 only for five 4‐week cycles, oral |
|
| Outcomes | Health‐related quality of life (HRQL) (EORTC QLQ‐C30), score range from 0 to 100; higher scores reflect better quality of life, for symptom scale, higher scores reflect worse symptoms Functional Assessment of Cancer Therapy Neurotoxicity questionnaire (FACT/GOG‐NTX), 11 individual items evaluating symptoms of neurotoxicity on a scale of 0 (not at all) to 4 (very much), items were reversed (reversed score = 4 ‐ row score) therefore total scores ranged from 0 to 44 with higher values indicating a lower burden of neurotoxicity Questionnaires administered at baseline and 6‐weekly to 42 weeks |
|
| Notes | Health related QoL and toxicity assessed in patients participating in an efficacy study of bortezomib versus dexamethasone in multiple myeloma Pain not primary outcome measure Benefit in pain scores in favour of B using available data but not when using imputed data sets Corticosteroids used as an anticancer treatment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised (1:1) Method not specified |
| Allocation concealment (selection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Amount of missing data increased over time due to attrition related to adverse events, disease progression, the premature termination of the dexamethasone arm of the study and death. Only 9% in the B and 5% in the D arm completed protocol‐specified treatment. Significant missing data with time |
| Selective reporting (reporting bias) | Low risk | None detected since pain not primary outcome |
| Other bias | Low risk | Sample size: 598 participants; ≥ 200 participants per treatment arm |
Mercadante 2007.
| Study characteristics | ||
| Methods | Prospective, randomised, controlled study of steroids as adjuvant drugs to opioids Study duration: 9 weeks |
|
| Participants | 76 patients with advanced cancer (31 opioid, 35 dexamethasone) with pain requiring strong opioids Other co‐analgesics allowed |
|
| Interventions | Group O: conventional opioid treatment Group OS: 8 mg dexamethasone orally along with conventional treatment |
|
| Outcomes | Average daily pain intensity measured using the patient's self report on NRS from 0 (absent) to 10 (maximum) Well‐being sensation, rated by means of a NRS from 0 to 10 Symptoms associated with opioid therapy or commonly present in advanced cancer patients (nausea and vomiting, weakness, drowsiness, constipation, confusion), scale from 0 to 3 (not at all, slight, a lot, awful) Opioid escalation index percentage (OEI%) and absolute dose (OEI mg) |
|
| Notes | No difference between groups in OEI Other co‐analgesics allowed, not standardised Difference in OEI between arms at baseline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients randomly divided into 2 groups Method not described |
| Allocation concealment (selection bias) | High risk | Method not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Half of the patients died before the third week making the interpretation of any long‐term corticosteroid benefit difficult |
| Selective reporting (reporting bias) | Unclear risk | Authors did not specify the adverse drug reactions where they claimed symptomatic improvement except for nausea, vomiting and constipation |
| Other bias | High risk | Sample size: 76 participants; < 50 participants per treatment arm |
Paulsen 2014.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled, double‐blind, parallel‐group, multi‐centre, phase III trial | |
| Participants | Patients with cancer experiencing pain > 4/10 receiving opioids | |
| Interventions | Methylprednisolone 16 mg or placebo 16 mg twice daily for 7 days | |
| Outcomes | Primary outcome: average pain intensity measured by BPI (0 to 10) Secondary outcome:
|
|
| Notes | 592 patients screened, 50 recruited over 3 to 4‐year time period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation conducted independently |
| Allocation concealment (selection bias) | Low risk | Methylprednisolone and placebo capsules were identical in appearance |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All parties blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomisation was blinded for all parties until the completion of data collection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 patient died in the placebo group and 1 patient withdrew from the intervention group as a result of malignant bowel obstruction; none lost to follow‐up ITT analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Sample size: 50 participants; < 50 participants per treatment arm |
Popiela 1989.
| Study characteristics | ||
| Methods | Randomised, prospective, double‐blind, placebo‐controlled, multi‐site clinical trial Study duration: 8 weeks |
|
| Participants | 173 female participants (85 intervention, 88 control) with advanced, terminal cancer and symptoms (pain, debility, nausea, cachexia etc) | |
| Interventions | Intervention: 125 mg infusion of methylprednisolone sodium succinate (MPSS) IV Control: matching placebo (P) Both for 56 consecutive days |
|
| Outcomes | Linear Analogue Self‐Assessment scale (LASA), patient ratings for pain, appetite, sense of well‐being completed weekly x 8 weeks Mortality Concomitant medications Adverse events |
|
| Notes | No significant changes with time for pain or sleep No significant changes in opioid use Better overall LASA score for patients on MPSS |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation specified by a computer‐generated randomisation scheme |
| Allocation concealment (selection bias) | Low risk | Double‐blind Study medication was provided in blinded packages containing vials of either placebo or MPSS |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, data from 2 sites removed ("significant investigator interaction") |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 38% MPSS and 30% P died before study completion ITT analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Sample size: 173 participants; 50 to 199 participants per treatment arm |
Teshima 1996.
| Study characteristics | ||
| Methods | Multi‐site, prospective randomised controlled trial of RT alone or RT and methylprednisolone for bone metastases Study duration: 14 days |
|
| Participants | 38 participants (20 intervention, 18 control) with bone metastases and clinical symptoms such as pain, loss of appetite, general fatigue, sleep disturbance, anxiety, depression and nausea and/or vomiting | |
| Interventions | Intervention: radiation combined with methylprednisolone 500 g IV daily x 3 days Control: radiation alone |
|
| Outcomes | RTOG pain score plus QoL (1 to 10), patient scored Performance status Urinary hydroxyproline/creatinine ratio Serum tartrate‐resistance acid phosphatase (tumour marker) |
|
| Notes | Pain not only endpoint No difference between groups with respect to pain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated to 1 of the 2 treatments according to Peto's balanced randomised list |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo, no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No placebo, no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts declared |
| Selective reporting (reporting bias) | High risk | Graphical presentation of pain scores and no mention of adverse events |
| Other bias | High risk | Sample size: 38 participants; < 50 participants per treatment arm |
Twycross 1985.
| Study characteristics | ||
| Methods | Double‐blind, randomised, placebo‐controlled trial Study duration: 7 days |
|
| Participants | 27 participants (16 intervention, 11 control) with cancer of the breast or lung | |
| Interventions | Intervention: 5 mg prednisolone by mouth 3 times a day Control: placebo of identical appearance |
|
| Outcomes | Visual analogue scales (VAS), 100 mm, relating to pain, nausea, mood, sleep, alertness and strength | |
| Notes | Poor quality RCT, no standard background dosing, changes in adjunct medication "kept to a minimum" Analgesics adjusted as required Difference in pain scales at baseline No significant difference at day 8 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised Method not specified |
| Allocation concealment (selection bias) | Unclear risk | 266 potentially eligible, only 56 randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 266 screened, 56 entered Double‐blind Placebo of identical appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 27/56 completed outcome assessments |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Sample size: 27 participants; < 50 participants per treatment arm |
Vecht 1989.
| Study characteristics | ||
| Methods | Randomised, multi‐centre controlled trial Study duration: 1 week |
|
| Participants | 37 participants (22 high‐dose, 15 low‐dose) with metastatic spinal cord compression (SCC) | |
| Interventions | High‐dose: 100 mg dexamethasone dissolved in glycerol and water Low‐dose: 10 mg dexamethasone dissolved in glycerol and water, both delivered immediately following diagnosis of SCC by myelography |
|
| Outcomes | NRS for pain (0 to 10) Ambulatory status (grade 1, walking independently – grade 5, no power in legs) Bladder function |
|
| Notes | High‐dose versus low‐dose dexamethasone plus radiotherapy for SCC No difference seen in pain, ambulation or bladder function |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised Not specified how the random sequence was generated 22 patients received high‐dose and 15 low‐dose (no explanation given) |
| Allocation concealment (selection bias) | Low risk | Coded ampoules blindly administered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Code broken by statistician at final analysis |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Code broken by statistician at final analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 40 patients randomised, 3 had insufficient data for analysis |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | High risk | Sample size: 37 participants; < 50 participants per treatment arm |
Yennurajalingam 2013.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled trial Study duration: 14 days |
|
| Participants | 120 participants (62 intervention, 58 control) with advanced cancer and ≥ 3 symptoms during the previous 24 hours (e.g. pain, fatigue, chronic nausea cluster) with average intensity of ≥ 4 on the Edmonton Symptom Assessment Scale (ESAS) | |
| Interventions | Intervention: 4 mg dexamethasone orally twice per day for 14 days Control: placebo orally twice per day for 14 days |
|
| Outcomes | ESAS to assess severity of common symptoms (e.g. pain, fatigue, nausea, depression, anxiety) rated on a NRS of 0 to 10 (0 = no symptoms, 10 = worst possible severity) Functional Assessment of Chronic Illness Therapy‐Fatigue (FACIT‐F), 27 questions, scale 0 to 4 (0 = not at all, 4 = very much) Functional Assessment of Cancer Therapy‐Anorexia‐Cachexia (FAACT), 12‐item symptom‐specific subscale, scale 0 to 4 Hospital Anxiety and Depression Scale (HADS), 14‐item questionnaire |
|
| Notes | Fatigue was primary outcome measure Pain as measured by ESAS significantly better on dexamethasone at day 8, but not day 15 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not described |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All members of the research team except the investigational pharmacist and statistician were blinded to treatment assignment throughout the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All members of the research team except the investigational pharmacist and statistician were blinded to treatment assignment throughout the study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 19 of 62 patients receiving dexamethasone were not evaluable 17 of 58 patients receiving placebo were not evaluable |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Unclear risk | Sample size: 120 participants; 50 to 199 participants per treatment arm |
BPI: Brief Pain Inventory
ECOG: Eastern Cooperative Oncology Group ESAS: Edmonton Symptom Assessment Scale ITT: intention‐to‐treat IV: intravenous LASA: Linear Analogue Self‐Assessment scale LHRH: Luteinizing Hormone Releasing Hormone
MP: methylprednisolone MPSS: methylprednisolone sodium succinate MSCC: malignant spinal cord compression NRS: numerical rating scale OEI: opioid escalation index P: placebo QoL: quality of life RCT: randomised controlled trial RT: radiation RTOG: Radiation Therapy Oncology Group SCC: spinal cord compression VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Campio 1983 | Abstract only; pain not an outcome measure |
| Chanan‐Khan 2011 | 2 arms not identical; measuring tumour flare reaction (TFR) of which pain is primary feature, more a measure of TFR |
| Coloma 2001 | Postoperative pain rather than cancer pain |
| Datta 1997 | Not all patients had pain at baseline |
| Dutta 2012 | Prophylactic steroids; primary endpoint was skin toxicity, not pain |
| Friedenberg 1991 | Not randomised; toxicity assessments included |
| Fuccio 2011 | Prophylactic steroids; pain not specifically measured |
| Gomez‐Hernandez 2010 | Prevention of postoperative pain |
| Hird 2009 | Non‐randomised; no toxicity assessment |
| Laval 2000 | Trial underpowered; focus on resolution of bowel obstruction and symptoms |
| North 2003 | Quality of life not pain as an outcome measure |
| Richardson 2009 | Pain not assessed |
| Richardson 2010 | Phase 1/2 trial; pain not an endpoint |
| Richardson 2011 | Pain not an endpoint |
| Richardson 2012 | Pain not assessed |
| Rinehart 2010 | Pain not an endpoint |
| Rizzo 2009 | No detailed results |
| Sanguineti 2003 | Assessed post‐radiation toxicity, not pain |
| Schmuth 2002 | Pain not assessed |
| Tantawy 2008 | Postoperative pain after oncologic pelvic surgery |
| Tong 1982 | Patients not treated with corticosteroids |
| Vecht 1994 | Pain not an endpoint |
| Vij 2012 | Pain not an endpoint |
| Yennurajalingam 2010 | Abstract only |
| Yennurajalingam 2012 | Abstract only |
| Yoshioka 2011 | Pain not an endpoint |
Differences between protocol and review
We updated the methods of the review to include the GRADE approach to assess the overall quality of the evidence for the primary outcome. There were insufficient data to evaluate different subgroups for this review, however we will perform subgroup analysis in any updated versions of this review. We will perform intention‐to‐treat analysis where possible in future updates.
Contributions of authors
All authors initiated and designed the study and drafted the protocol. All authors extracted the data and/or conducted the 'Risk of bias' assessment.
AH, SK, AL and PG supervised the statistical analysis. JH and PG commented on and revised the review, checked the data extraction and arbitrated in the event of disagreement between other authors.
Sources of support
Internal sources
Mater Research – The University of Queensland, School of Pharmacy and Griffith Health Institute Griffith University, The Mater Palliative Care Research Fund and St Vincent's Hospital Brisbane, Australia
External sources
No sources of support supplied
Declarations of interest
AH, PG, SK, AL, S‐JM, KR and JH all have no relevant conflicts of interest to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Basile 2012 {published and unpublished data}
- Basile A, Masala S, Banna G, Cotta E, Cavalli M, Fiumara P, et al. Intrasomatic injection of corticosteroid followed by vertebroplasty increases early pain relief rather than vertebroplasty alone in vertebral bone neoplasms: preliminary experience. Skeletal Radiology 2012;41(4):459-64. [DOI] [PubMed] [Google Scholar]
Bruera 1985 {published and unpublished data}
- Bruera E, Roca E, Cedaro L. Action of oral methylprednisolone in terminal cancer patients: a prospective randomized double-blind study. Cancer Treatment Reports 1985;69(7-8):751-4. [PubMed] [Google Scholar]
Bruera 2004 {published and unpublished data}
- Bruera E, Moyano JR, Sala R, Rico MA, Bosnjak S, Bertolino M, et al. Dexamethasone in addition to metoclopramide for chronic nausea in patients with advanced cancer: a randomized controlled trial. Journal of Pain and Symptom Management 2004;28(4):381-8. [DOI] [PubMed] [Google Scholar]
Della 1989 {published and unpublished data}
- Della Cuna GR, Pellegrini A, Piazzi M. Effect of methylprednisolone sodium succinate on quality of life in preterminal cancer patients: a placebo-controlled, multicenter study. The Methylprednisolone Preterminal Cancer Study Group. European Journal of Cancer & Clinical Oncology 1989;25(12):1817-21. [DOI] [PubMed] [Google Scholar]
Fossa 2001 {published and unpublished data}
- Fossa SD, Slee PH, Brausi M, Horenblas S, Hall RR, Hetherington JW, et al. Flutamide versus prednisone in patients with prostate cancer symptomatically progressing after androgen-ablative therapy: a phase III study of the European organization for research and treatment of cancer genitourinary group. Journal of Clinical Oncology 2001;19(1):62-71. [DOI] [PubMed] [Google Scholar]
Graham 2006 {published and unpublished data}
- Graham PH, Capp A, Dalaney G, Goozee G, Hickey B, Turner S, et al. A pilot randomised comparison of dexamethasone 96 mg vs 16 mg per day for malignant spinal-cord compression treated by radiotherapy: TROG 01.05 Superdex study. Clinical Oncology 2006;18(1):70-6. [DOI] [PubMed] [Google Scholar]
Lauretti 2013 {published and unpublished data}
- Lauretti GR, Rizzo CC, Mattos AL, Rodrigues SW. Epidural methadone results in dose-dependent analgesia in cancer pain, further enhanced by epidural dexamethasone. British Journal of Cancer 2013;108(2):259-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2008 {published and unpublished data}
- Lee SJ, Richardson PG, Sonneveld P, Schuster MW, Irwin D, San Miguel JF, et al. Bortezomib is associated with better health-related quality of life than high-dose dexamethasone in patients with relapsed multiple myeloma: results from the APEX study. British Journal of Haematology 2008;143(4):511-9. [DOI] [PubMed] [Google Scholar]
Mercadante 2007 {published and unpublished data}
- Mercadante SL, Berchovich M, Casuccio A, Fulfaro F, Mangione S. A prospective randomized study of corticosteroids as adjuvant drugs to opioids in advanced cancer patients. American Journal of Hospice and Palliative Medicine 2007;24(1):13-9. [DOI] [PubMed] [Google Scholar]
Paulsen 2014 {published data only}
- Paulsen Ø, Klepstad P, Rosland JH, et al. Efficacy of methylprednisolone on pain, fatigue, and appetite loss in patients with advanced cancer using opioids: a randomized, placebo-controlled, double-blind trial. Journal of Clinical Oncology 2014;32(29):3221-8. [DOI] [PubMed] [Google Scholar]
Popiela 1989 {published and unpublished data}
- Popiela T, Lucchi R, Giongo F. Methylprednisolone as palliative therapy for female terminal cancer patients. The Methylprednisolone Female Preterminal Cancer Study Group. European Journal of Cancer & Clinical Oncology 1989;25(12):1823-9. [DOI] [PubMed] [Google Scholar]
Teshima 1996 {published and unpublished data}
- Teshima T, Inoue T, Ikeda H, Murayama S, Yamazaki H, Ohtani M, et al. Symptomatic relief for patients with osseous metastasis treated with radiation and methylpredonisolone: a prospective randomized study. Radiation Medicine - Medical Imaging and Radiation Oncology 1996;14(4):185-8. [PubMed] [Google Scholar]
Twycross 1985 {published and unpublished data}
- Twycross RG, Guppy D. Prednisolone in terminal breast and bronchogenic cancer. Practitioner 1985;229(1399):57-9. [PubMed] [Google Scholar]
Vecht 1989 {published and unpublished data}
- Vecht CJ, Haaxma-Reiche H, Van Putten WLJ, Visser M, Vries EP, Twijnstra A. Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology 1989;39(9):1255-7. [DOI] [PubMed] [Google Scholar]
Yennurajalingam 2013 {published and unpublished data}
- Yennurajalingam S, Frisbee-Hume S, Palmer JL, Delgado-Guay MO, Bull J, Phan AT, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. Journal of Clinical Oncology 2013;31(25):3076-82. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Campio 1983 {published data only}
- Campio L, Hearron AE. Comparison of 6-methylprednisolone (6-MP) and placebo in improvement of the quality of life in pre-terminal cancer patients [abstract]. Journal of Steroid Biochemistry 1983;19 Suppl:92. [Google Scholar]
Chanan‐Khan 2011 {published data only}
- Chanan-Khan A, Miller KC, Lawrence D, Padmanabhan S, Miller A, Hernandez-Illatazurri F, et al. Tumor flare reaction associated with lenalidomide treatment in patients with chronic lymphocytic leukaemia predicts clinical response. Cancer 2011;117(10):2127-35. [DOI] [PubMed] [Google Scholar]
Coloma 2001 {published data only}
- Coloma M, Duffy LL, White PF, Kendall Tongier W, Huber PJ Jr. Dexamethasone facilitates discharge after outpatient anorectal surgery. Anesthesia and Analgesia 2001;92(1):85-8. [DOI] [PubMed] [Google Scholar]
Datta 1997 {published data only}
- Datta SN, Thomas K, Matthews PN. Is prednisolone as good as flutamide in hormone refractory metastatic carcinoma of the prostate? Journal of Urology 1997;158(1):175-7. [DOI] [PubMed] [Google Scholar]
Dutta 2012 {published data only}
- Dutta S, Sharma S, Gupta A, et al. Topical mometasone furoate (0.1%)-An effective prophylaxis for prevention of radiation dermatitis: A prospective randomized study. Journal of Cancer Research and Therapeutics 2012;8:175. [Google Scholar]
Friedenberg 1991 {published data only}
- Friedenberg WR, Kyle RA, Knospe WH, Bennett JM, Tsiatis AA, Oken MM. High-dose dexamethasone for refractory or relapsing multiple myeloma. American Journal of Hematology 1991;36(3):171-5. [DOI] [PubMed] [Google Scholar]
Fuccio 2011 {published data only}
- Fuccio L, Guido A, Laterza L, Eusebi LH, Busutti L, Bunkheila F, et al. Randomised clinical trial: preventive treatment with topical rectal beclomethasone dipropionate reduces post-radiation risk of bleeding in patients irradiated for prostate cancer. Alimentary Pharmacology & Therapeutics 2011;34(6):628-37. [DOI] [PubMed] [Google Scholar]
Gomez‐Hernandez 2010 {published data only}
- Gomez-Hernandez J, Orozco-Alatorre AL, Dominguez-Contreras M, Oceguera-Villanueva A, Gómez-Romo S, Alvarez Villaseñor AS, et al. Preoperative dexamethasone reduces postoperative pain, nausea and vomiting following mastectomy for breast cancer. BMC Cancer 2010;10:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hird 2009 {published data only}
- Hird A, Zhang L, Holt T, Fairchild A, DeAngelis C, Loblaw A, et al. Dexamethasone for the prophylaxis of radiation-induced pain flare after palliative radiotherapy for symptomatic bone metastases: a phase II study. Clinical Oncology (Royal College of Radiologists) 2009;21(4):329-35. [DOI] [PubMed] [Google Scholar]
Laval 2000 {published data only}
- Laval G, Girardier J, Lassaunire JM, Leduc B, Haond C, Schaerer R. The use of steroids in the management of inoperable intestinal obstruction in terminal cancer patients: do they remove the obstruction? Palliative Medicine 2000;14(1):3-10. [DOI] [PubMed] [Google Scholar]
North 2003 {published data only}
- North SA, Au H, Halls SB, Tkachuk L, Mackey JR. A randomized, phase III, double-blind, placebo-controlled trial of intrapleural instillation of methylprednisolone acetate in the management of malignant pleural effusion. Chest 2003;123(3):822-7. [DOI] [PubMed] [Google Scholar]
Richardson 2009 {published data only}
- Richardson PG, Weller E, Jagannath S, Avigan DE, Alsina M, Schlossman RL, et al. Multicenter, phase I, dose-escalation trial of lenalidomide plus bortezomib for relapsed and relapsed/refractory multiple myeloma. Journal of Clinical Oncology 2009;27(34):5713-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Richardson 2010 {published data only}
- Richardson PG, Siegel D, Baz R, et al. A Phase 1/2 multi-center, randomized, open label dose escalation study to determine the maximum tolerated dose, safety, and efficacy of pomalidomide alone or in combination with low-dose dexamethasone in patients with relapsed and refractory multiple myeloma who have received prior treatment that includes lenalidomide and bortezomib. Blood, ASH Annual Meeting Abstracts 2010;116:Abstract 864. [Google Scholar]
Richardson 2011 {published data only}
- Richardson PG, Siegel DS, Vij R, et al. Randomized, open label phase 1/2 study of pomalidomide (POM) alone or in combination with low-Dose Dexamethasone (LoDex) in patients (Pts) with relapsed and refractory multiple myeloma who have received prior treatment that includes lenalidomide (LEN) and bortezomib (BORT): Phase 2 results. Blood 2011;118:Abstract 634. [Google Scholar]
Richardson 2012 {published data only}
- Richardson PG, Jakubowiak A, Bahlis NJ, et al. Treatment outcomes with pomalidomide (POM) in combination with low-dose dexamethasone (LODEX) in patients with relapsed and refractory multiple myeloma (RRMM) and del(17p13) and/or t(4;14)(p16;q32) cytogenetic abnormalities who have received prior therapy with lenalidomide (LEN) and bortezomib (BORT). Blood 2012;120:Abstract 4053. [Google Scholar]
Rinehart 2010 {published data only}
- Rinehart JJ, Arnold SM, Kloecker GH, et al. Randomized phase II trial of carboplatin (C) and gemcitabine (G) with or without dexamethasone (Dex) in patients (pts) with stage IV non-small cell lung cancer (NSCLC). Journal of Clinical Oncology 2010;28(15):9150. [Google Scholar]
Rizzo 2009 {published data only}
- Rizzo CC, Lauretti GR, Mattos AL. The combination of epidural methadone and dexamethasone for cancer pain. European Journal of Pain 2009;13:258. [Google Scholar]
Sanguineti 2003 {published data only}
- Sanguineti G, Franzone P, Marcenaro M, Foppiano F, Vitale V. Sucralfate versus mesalazine versus hydrocortisone in the prevention of acute radiation proctitis during conformal radiotherapy for prostate carcinoma. A randomized study. Strahlentherapie und Onkologie 2003;179(7):464-70. [DOI] [PubMed] [Google Scholar]
Schmuth 2002 {published data only}
- Schmuth M, Wimmer MA, Hofer S, Sztankay A, Weinlich G, Linder DM, et al. Topical corticosteroid therapy for acute radiation dermatitis: a prospective, randomized, double-blind study. British Journal of Dermatology 2002;146(6):983-91. [DOI] [PubMed] [Google Scholar]
Tantawy 2008 {published data only}
- Tantawy AGM, El-Samahy KA, Omran AF. Effect of dexamethasone addition to epidural morphine on pain, and morphine-related side effects after pelvic oncologic surgery. Egyptian Journal of Anaesthesia 2008;24(1):75-80. [Google Scholar]
Tong 1982 {published data only}
- Tong D, Gillick L, Hendrickson FR. The palliation of symptomatic osseous metastases. Final results of the study by the Radiation Therapy Oncology Group. Cancer 1982;50(5):893-9. [DOI] [PubMed] [Google Scholar]
Vecht 1994 {published data only}
- Vecht CJ, Hovestadt A, Verbiest HBC, Vliet JJ, Putten WL. Dose-effect relationship of dexamethasone on Karnofsky performance in metastatic brain tumors: a randomized study of doses of 4, 8, and 16 mg per day. Neurology 1994;44(4):675-80. [DOI] [PubMed] [Google Scholar]
Vij 2012 {published data only}
- Vij R, Hofmeister CC, Richardson PG, et al. Pomalidomide (POM) with low-dose dexamethasone (LODEX) in patients with relapsed and refractory multiple myeloma (RRMM): Outcomes based on prior treatment exposure. Blood 2012;120:Abstract: 4070. [Google Scholar]
Yennurajalingam 2010 {published data only}
- Yennurajalingam S, Palmer JL, Reuben JM, et al. The effect of dexamethasone on symptom burden in patients with advanced cancer. Journal of Clinical Oncology 2010;28(15s):Abstract: TPS318. [Google Scholar]
Yennurajalingam 2012 {published data only}
- Yennurajalingam S, Susan FH, Marvin ODG, et al. Dexamethasone (DM) for cancer-related fatigue: A double-blinded, randomized, placebo-controlled trial. Journal of Clinical Oncology 2012;30(Suppl):Abstract 9002. [Google Scholar]
Yoshioka 2011 {published data only}
- Yoshioka H, Mio T, Nakatani K, et al. Randomized phase II trial of gemcitabine and carboplatin (G/C) with or without dexamethasone (DEX) pretreatment in chemotherapy-naive patients (pts) with advanced non-small cell lung cancer (NSCLC) - Results of kyoto thoracic oncology research group (KTORG) trial 0501. European Journal of Cancer 2011;47:625. [Google Scholar]
Additional references
Busillo 2013
- Busillo JM, Cidlowski JA. The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends in Endocrinology and Metabolism 2013;24(3):109-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
eTG Complete 2014
- Therapeutic Guidelines Ltd. Corticosteroids: use in pain management and palliative care. In: Therapeutic Guidelines: eTG Complete [electronic resource]. North Melbourne: Therapeutic Guidelines Limited, 2014. [Google Scholar]
Farr 1990
- Farr WC. The use of corticosteroids for symptom management in terminally ill patients. American Journal of Hospice and Palliative Care 1990;7:41. [DOI] [PubMed] [Google Scholar]
Foley 2011
- Foley MK. How well is cancer pain treated? Palliative Medicine 2011;25(5):398-401. [DOI] [PubMed] [Google Scholar]
Gannon 2002
- Gannon C, McNamara P. A retrospective observation of corticosteroid use at the end of life in a hospice. Journal of Pain and Symptom Management 2002;24(3):328-34. [DOI] [PubMed] [Google Scholar]
Hanks 2009
- Hanks G, Cherny N, Christakis N, Fallon M, Kaasa S, Portenoy R (editors). Oxford Textbook of Palliative Care. 4th edition. Oxford Press, 2009. [Google Scholar]
Hardy 2001
- Hardy J, Rees E, Ling J, Stone P, Burman R, Feuer D, et al. A prospective survey of the use of dexamethasone on a palliative care unit. Palliative Medicine 2001;15:3-8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;2:81-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leppert 2012
- Leppert W, Buss T. The role of corticosteroids in the treatment of pain in cancer patients. Current Pain and Headache Reports 2012;16:307-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of Internal Medicine 2009;151(4):W65-W94. [DOI: 10.7326/0003-4819-151-4-200908180-00136] [DOI] [PubMed] [Google Scholar]
Paulsen 2013
- Paulsen O, Aass N, Kaasa S, Dale O. Do corticosteroids provide analgesic effects in cancer patients? A systematic review. Journal of Pain and Symptom Management 2013;46:96-105. [DOI] [PubMed] [Google Scholar]
Pharo 2005
- Pharo GH, Zhou L. Pharmacologic management of cancer pain. Journal of the American Osteopathic Association 2005;105(11 Suppl 5):S21-8. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riechelmann 2007
- Riechelmann RP, Krzyanowska MK, O'Carroll A, Zimmermann C. Symptom and medication profiles among cancer patients attending a palliative care clinic. Supportive Care in Cancer 2007;15(12):1407-12. [DOI] [PubMed] [Google Scholar]
Van den Beuken‐van Everdingen 2007
- Van den Beuken-van Everdingen MHJ, De Rijke JM, Kessels AG, Schouten HC, Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Annals of Oncology 2007;18:1437-49. [DOI] [PubMed] [Google Scholar]
Vyvey 2010
- Vyvey M. Steroids as pain relief adjuvants. Canadian Family Physician 2010;56(12):1295-7. [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Hardy 2013
- Hardy JR, Jenkins-Marsh S, Pinkerton E, Rickett K, Good P. Corticosteroids for the management of cancer-related pain in adults. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD010756. [DOI: 10.1002/14651858.CD010756] [DOI] [PMC free article] [PubMed] [Google Scholar]


