Abstract
Background
Neonatal sepsis is a major cause of morbidity and mortality. It is the third leading cause of neonatal mortality globally constituting 13% of overall neonatal mortality. Despite the high burden of neonatal sepsis, high‐quality evidence in diagnosis and treatment is scarce. Due to the diagnostic challenges of sepsis and the relative immunosuppression of the newborn, many neonates receive antibiotics for suspected sepsis. Antibiotics have become the most used therapeutics in neonatal intensive care units, and observational studies in high‐income countries suggest that 83% to 94% of newborns treated with antibiotics for suspected sepsis have negative blood cultures. The last Cochrane Review was updated in 2005. There is a need for an updated systematic review assessing the effects of different antibiotic regimens for late‐onset neonatal sepsis.
Objectives
To assess the beneficial and harmful effects of different antibiotic regimens for late‐onset neonatal sepsis.
Search methods
We searched the following electronic databases: CENTRAL (2021, Issue 3); Ovid MEDLINE; Embase Ovid; CINAHL; LILACS; Science Citation Index EXPANDED and Conference Proceedings Citation Index – Science on 12 March 2021. We also searched clinical trials databases and the reference lists of retrieved articles for randomised controlled trials (RCTs) and quasi‐RCTs.
Selection criteria
We included RCTs comparing different antibiotic regimens for late‐onset neonatal sepsis. We included participants older than 72 hours of life at randomisation, suspected or diagnosed with neonatal sepsis, meningitis, osteomyelitis, endocarditis, or necrotising enterocolitis. We excluded trials that assessed treatment of fungal infections.
Data collection and analysis
Three review authors independently assessed studies for inclusion, extracted data, and assessed risk of bias. We used the GRADE approach to assess the certainty of evidence. Our primary outcome was all‐cause mortality, and our secondary outcomes were: serious adverse events, respiratory support, circulatory support, nephrotoxicity, neurological developmental impairment, necrotising enterocolitis, and ototoxicity. Our primary time point of interest was at maximum follow‐up.
Main results
We included five RCTs (580 participants). All trials were at high risk of bias, and had very low‐certainty evidence.
The five included trials assessed five different comparisons of antibiotics.
We did not conduct a meta‐analysis due to lack of relevant data.
Of the five included trials one trial compared cefazolin plus amikacin with vancomycin plus amikacin; one trial compared ticarcillin plus clavulanic acid with flucloxacillin plus gentamicin; one trial compared cloxacillin plus amikacin with cefotaxime plus gentamicin; one trial compared meropenem with standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin); and one trial compared vancomycin plus gentamicin with vancomycin plus aztreonam.
None of the five comparisons found any evidence of a difference when assessing all‐cause mortality, serious adverse events, circulatory support, nephrotoxicity, neurological developmental impairment, or necrotising enterocolitis; however, none of the trials were near an information size that could contribute significantly to the evidence of the comparative benefits and risks of any particular antibiotic regimen.
None of the trials assessed respiratory support or ototoxicity.
The benefits and harms of different antibiotic regimens remain unclear due to the lack of well‐powered trials and the high risk of systematic errors.
Authors' conclusions
Current evidence is insufficient to support any antibiotic regimen being superior to another. RCTs assessing different antibiotic regimens in late‐onset neonatal sepsis with low risks of bias are warranted.
Plain language summary
Antibiotic regimens for late‐onset neonatal sepsis
Review question
We reviewed the available evidence on different antibiotic regimens for newborns (from 72 hours of life to one month of life) with late‐onset sepsis.
Background
Sepsis in newborns is a severe and potential lethal condition, caused by the body's response to an infection. Neonatal sepsis is the third leading cause of neonatal death globally. Despite this high burden of sepsis in newborns, high‐quality evidence in diagnosis and treatment is scarce. This Cochrane Review was originally published in 2005. To identify the most appropriate antibiotic policies for neonatal sepsis, there is a need to base these policies on an updated well‐conducted review. Therefore, there is a need for such a review assessing the effects of different antibiotic regimens for late‐onset neonatal sepsis.
Study characteristics
The evidence is current to March 2021. We included five trials randomising 580 participants. The five trials compared five different antibiotic regimens.
Key results
We included five trials: one trial compared cefazolin plus amikacin with vancomycin plus amikacin; one trial compared ticarcillin plus clavulanic acid with flucloxacillin plus gentamicin; one trial compared cloxacillin plus amikacin with cefotaxime plus gentamicin; one trial compared meropenem with standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin); and one trial compared vancomycin plus gentamicin with vancomycin plus aztreonam.
None of the five antibiotic comparisons showed that the choice of antibiotics influenced the effects on death from all‐causes, serious adverse events (i.e. major complications), circulatory support, nephrotoxicity (toxicity in the kidneys), neurological developmental impairment (disabilities in the functioning of the brain that affect a child's behaviour, memory, or ability to learn), or necrotising enterocolitis (tissues in the gut become inflamed and start to die). Current evidence cannot confirm or reject, one antibiotic regimen being superior to another due to scarce data.
Quality of the evidence
Our conclusions are based on very low‐quality evidence. The five trials were at high risk of bias (i.e. the trials were conducted in a way that may have skewed results to the positive side). In addition, the five trials included few participants, making the results of this review imprecise.
Summary of findings
Background
Description of the condition
Definition
Sepsis is defined as a life‐threatening organ dysfunction caused by a dysregulated host response to infection (Singer 2016). There is currently no international consensus on specific criteria for neonatal sepsis (Wynn 2014; Wynn 2016). The most used neonatal sepsis criteria in clinical trials are based on a combination of clinical and laboratory parameters (see Table 6) (Morris 2016; Wynn 2014).
1. Commonly used clinical and laboratory criteria of sepsis.
| Clinical criteria | Laboratory criteria |
|
|
WBC: white blood cell.
Sepsis that occurs before 28 days after birth is termed neonatal sepsis (Bakhuizen 2014; Camacho‐Gonzalez 2013). Depending on the time of onset, neonatal sepsis is termed either early‐onset sepsis or late‐onset sepsis. The most accepted distinction between these two subgroups is cases occurring before 72 hours after birth and after 72 hours after birth, but other definitions exist (e.g. 48 hours and seven days after birth (Bakhuizen 2014; Bizzarro 2008; Camacho‐Gonzalez 2013; Manan 2016; Metsvaht 2010; Shah 2014; Shane 2013; Shane 2014; Tripathi 2012; Zaidi 2009; Zea‐Vera 2015). This distinction is based on the different aetiologies and pathophysiology of pathogens typically seen before and after 72 hours of age (Camacho‐Gonzalez 2013; Metsvaht 2010; Shah 2014; Shane 2013).
Late‐onset sepsis frequently presents with clinical deterioration including apnoea, tachypnoea, increased ventilatory requirement, hypotension, abnormal heart rate, hyperglycaemia, abnormal temperature (hypothermia or hyperthermia), cyanosis, acidosis, feeding intolerance, abdominal distension, lethargy, and skin mottling (Craft 2000; Tsai 2014). As some of these clinical manifestations can be non‐specific, it can be difficult to clinically distinguish between sepsis and deep‐seated infections, such as meningitis, osteomyelitis, and necrotising enterocolitis (NEC) (Camacho‐Gonzalez 2013; Zea‐Vera 2015).
Epidemiology
Since there is neither consensus on criteria for neonatal sepsis nor agreement on the cut‐off between early‐onset and late‐onset sepsis (48 hours, 72 hours, or seven days) (see 'Definition' section above), it is difficult to estimate the exact incidence of neonatal sepsis (Bakhuizen 2014). Studies from the USA and Australia suggest that late‐onset sepsis constitutes 3 per 1000 to 6 per 1000 live births, while early‐onset sepsis ranges from 0.9 per 1000 to 3.5 per 1000 live births (Isaacs 1999; Schuchat 2000; Vergnano 2005; Vergnano 2011).
Late‐onset sepsis is believed to be more common in preterm (less than 37 weeks' gestation) and low birthweight (less than 2500 g) neonates (Stoll 2011; Tsai 2014). Large non‐randomised studies suggest that late‐onset sepsis has decreased significantly in recent decades in high‐income countries (Horbar 2017; Stoll 2015).
Neonatal sepsis is a major cause of morbidity and mortality. It is the third leading cause of neonatal mortality globally, constituting 13% of overall neonatal mortality (Lawn 2005; Liu 2012). In high‐income countries, the mortality rate due to neonatal sepsis ranges from 5% to 20%, and neonatal sepsis results in major disability or death in 39% of all cases despite initiation of conventional treatment. Mortality rates higher than 70% can be observed in some low‐ and middle‐income countries (LMICs) (Bakhuizen 2014; Kabwe 2016; Weston 2011; Wynn 2014).
Sepsis during the neonatal period can result in several complications, such as multiple organ failure, cerebral haemorrhage, periventricular leukomalacia, meningitis, and respiratory distress syndrome (Sharma 2007; Stoll 2010). In survivors, sepsis is associated with serious long‐term morbidity, such as cerebral palsy, cognitive and psychomotor delay, auditory and visual impairment, and bronchopulmonary dysplasia (Bakhuizen 2014; Benjamin 2006; Klinger 2010; Schlapbach 2011). Most of these associations are based on observational cohort studies and therefore do not distinguish between causality and association. It remains uncertain whether it is possible to prevent these subsequent sequela by treating neonatal sepsis with an appropriate empirical antibiotic regimen (Bakhuizen 2014).
Aetiology
The pathogens that cause late‐onset sepsis include Gram‐positive and Gram‐negative bacteria, as well as fungal infections (Boghossian 2013). The mortality and the distribution pattern of pathogens that cause late‐onset infection differ between LMICs and high‐income countries. Important variations may be observed within and between individual neonatal intensive care units (NICUs) in each country. The predominant organisms responsible for neonatal sepsis within regions have also changed over time (Dong 2015; Stoll 1996).
The most common aetiological pathogen responsible for late‐onset sepsis is coagulase‐negative staphylococci, constituting 53% to 78% of all cases of late‐onset sepsis in high‐income countries (Bizzarro 2005; Bizzarro 2008; Dong 2015; Isaacs 1996; Rubin 2002; Stoll 2011; Weston 2011). However, since coagulase‐negative staphylococci are skin commensals, these organisms are also common blood culture contaminants and there is a lack of consensus regarding how to interpret blood cultures that are positive for coagulase‐negative staphylococci (Rubin 2002; Weinstein 2003). Other bacteria prevalent in late‐onset sepsis are Escherichia coli, group B Streptococcus, Klebsiella pneumoniae, Enterococcus, Candida, and Pseudomonas (Isaacs 1996; Rubin 2002; Stoll 2011; Vergnano 2011).
In LMICs, coagulase‐negative staphylococci are still very common, constituting 36% to 47% of all cases of late‐onset sepsis (Dong 2015; Hammoud 2012). The second most common Gram‐positive pathogen is Staphylococcus aureus (Dong 2015; Zaidi 2005). Gram‐negative pathogens are relatively more common in LMICs (Dong 2015; Zaidi 2005). The most frequent Gram‐negative pathogens are Klebsiella species, E coli, Pseudomonas, and Salmonella species (Breurec 2016; Hammoud 2012; Vergnano 2005; WHO 1999; Zaidi 2005). The pathogen with the highest case fatality ratio is considered to be Pseudomonas aeruginosa (Hammoud 2012; Tsai 2014).
Late‐onset sepsis has several risk factors. Major risk factors are immaturity, mechanical ventilation, intravascular catheterisation, failure of early enteral feeding with breast milk, prolonged duration of parenteral nutrition, surgery, underlying respiratory and cardiovascular diseases, and hospitalisation (Boghossian 2013; Leal 2012; Stoll 2002; Tröger 2014; Tsai 2014). Furthermore, neonates are theoretically immunocompromised as several components of the immune system are not fully developed at birth (Camacho‐Gonzalez 2013; Kumar 2016). Preterm neonates are especially immunocompromised due to even more immature innate and adaptive immune systems (Kan 2016; Rogosch 2012; Walker 2011; Ygberg 2012; Zemlin 2007).
Description of the intervention
Antibiotics are antimicrobial drugs that treat and prevent bacterial infections by either killing the bacteria or inhibiting their growth (Waksman 1947). Early initiation of antibiotic therapy on neonates with suspected sepsis reduces both mortality and morbidity (Bakhuizen 2014). The choice of antibiotic used is often empirical and based on several factors, such as age at onset, likely pathogens, and antibiotic susceptibility patterns (Dong 2015; Manan 2016; Rubin 2002).
The most used first‐line treatment is a beta‐lactam antibiotic (most commonly ampicillin, flucloxacillin, or penicillin) combined with an aminoglycoside (most commonly gentamicin) (Dong 2015; Vergnano 2011). However, there has been an increased use of alternatives, such as vancomycin and cephalosporins, due to increased drug resistance among the most common pathogen (e.g. coagulase‐negative staphylococci) (Dong 2015; Rubin 2002).
Most guidelines recommend a penicillin plus an aminoglycoside for all cases of neonatal sepsis (Cortese 2016; Manan 2016; Muller‐Pebody 2011; Vergnano 2005; Vergnano 2011; WHO 2013). However, other protocols exist where a cephalosporin or a glycopeptide is used as a first‐line option to treat late‐onset sepsis (Fernando 2008; Marchant 2013; Stockmann 2014). Guidelines may differ due to local antibiotic resistance of the most common pathogens or whether the empirical regimen is supposed to cover the common but low virulence coagulase‐negative staphylococci (Bizzarro 2015; Marchant 2013). Vancomycin is to be considered if staphylococcal infection is suspected (Stockmann 2014).
Antibiotic susceptibility
Antibiotic resistance is a growing problem that increases the morbidity, mortality, and costs associated with infections globally (Cohen 1992; Foster 2006; Huynh 2016; Vergnano 2005). Studies indicate that bacterial resistance to antibiotics results primarily from the selective pressure exerted by the use and overuse of antibiotics (Foster 2006; Kunin 1990; McGowan 1994; Murray 1994; Sáez‐Llorens 2000). The spread of drug‐resistant organisms in hospitals is a recognised problem, although neonates admitted from the community may also carry drug‐resistant pathogens (Bhutta 1996). Studies that compare antibiotic susceptibility over time in the same unit show increased resistance to the most used antibiotics (Vergnano 2005).
The pathogens that cause neonatal infections and their antibiotic susceptibility patterns change over time and may differ between countries (Breurec 2016; Isaacs 2003; May 2005; Stoll 2003; Stoll 2005; Vergnano 2011). Furthermore, the definition and epidemiology of neonatal sepsis differs between countries (Vergnano 2005). This makes the comparison of antibiotic susceptibility between countries difficult. When comparing the epidemiology of neonatal sepsis in LMICs with high‐income countries, some important differences emerge in the pattern of aetiological pathogens and their antibiotic resistance (Khatua 1986; Tallur 2000; Tessin 1990; Vesikari 1985).
In high‐income countries, most pathogens that cause late‐onset sepsis (84%) were susceptible to the commonly used empiric antibiotics (penicillin/gentamicin and flucloxacillin/gentamicin) (Vergnano 2011).
In LMICs, estimations suggest that up to 70% of pathogens isolated from neonatal sepsis may not be covered by the recommended empirical regimen of ampicillin and gentamicin (Zaidi 2005). Some studies in LMICs have shown almost universal resistance (92% to 100% resistance) among some of the most common pathogens to first‐ and second‐line antibiotics (Dagnew 2013; Kabwe 2016; Zaidi 2005).
In addition to antibiotic coverage, supportive care aiming to reverse the life‐threatening organ dysfunction caused by a dysregulated host response to infection is also part of the care for neonates with sepsis. This includes respiratory support, maintenance of peripheral perfusion (intravenous fluids and inotropics), phototherapy, temperature, and glucose regulation (Seale 2015; WHO 2013).
Adverse events
Use of ampicillin has been associated in some studies with adverse events, such as rashes, diarrhoea, nausea, and nephrotoxicity (Golan 2011; Katzung 2009; Mrvos 2013). Contrary to these findings, one systematic review of randomised controlled trials (RCTs) showed that ampicillin only increased the incidence of candidiasis with no significant increase in rashes, diarrhoea, nausea, or nephrotoxicity (Gillies 2015).
Aminoglycosides (e.g. gentamicin) have been shown to be toxic (nephrotoxicity and ototoxicity) in adults. However, their toxicity in neonates remains unclear (Huth 2011; Jackson 1971; Mattie 1989; McGlone 2008; Mingeot‐Leclercq 1999; Musiime 2015; Schultze 1971; Selimoglu 2007; Wargo 2014).
The most common adverse effects caused by vancomycin are fever, phlebitis, and, in rare cases, nephrotoxicity and ototoxicity (Rybak 2009). However, in addition to the development of resistance towards vancomycin, one must also consider that observational studies suggest a three‐ to four‐fold increase in nephrotoxicity when aminoglycosides are combined with vancomycin (Farber 1983; Hailemeskel 1999; Rybak 2009; Sorrell 1985).
Cefotaxime, which is considered an alternative first‐line antibiotic, might have a broad spectrum of activity. However, cefotaxime is also associated with increased risk of death and invasive candidiasis in non‐randomised studies (Clark 2006a; Cotten 2006; Stockmann 2014).
In addition to the specific adverse effects of each antibiotic, extended use of antibiotics is also associated with higher risk of neonatal candidaemia (Filioti 2007; Spiliopoulou 2012).
How the intervention might work
Antibiotics are antimicrobial drugs that treat and prevent bacterial infections by either killing or inhibiting the growth of the bacteria (Waksman 1947). They can be classified based on:
their mechanism of action (bactericidal or bacteriostatic);
bacterial spectrum (broad or narrow); and
chemical structure (e.g. penicillins, macrolides, quinolones, tetracyclines, or aminoglycosides) (Bérdy 2005).
A combination of different antibiotics might have several advantages. First, it is thought to provide an enhanced effect beyond the additive effects of the individual therapies (Allan 1985). Second, it can be used to broaden the spectrum of antibiotic coverage when used empirically to increase the chances of covering the alleged causative bacteria. Third, a combination therapy is thought to suppress the development of subpopulations of micro‐organisms resistant to antibiotics (Allan 1985; Milatovic 1987; Tamma 2012).
Why it is important to do this review
Despite the high burden of neonatal sepsis, high‐quality evidence in diagnosis and treatment is scarce (Zea‐Vera 2015). Yet, in adults, appropriate empirical antibiotic treatment halves the fatality associated with sepsis (Ibrahim 2000; Leibovici 1998; Paul 2010). Due to the diagnostic challenges of sepsis, and the relative immunosuppression of the newborn, many neonates receive antibiotics for suspected sepsis. In fact, antibiotics have become the most used therapeutics in NICUs (Clark 2006b). Studies suggest that up to 95% of newborns treated with antibiotics for suspected sepsis prove to have no evidence of infection (Bedford Russell 2015; Cantey 2015; Luck 2003). This presumed inappropriate use of antibiotics seems to contribute to the development and spread of resistant pathogens in NICUs, and seems to be associated with adverse events (e.g. invasive candidiasis and increased antimicrobial resistance) (Clark 2006a; Cordero 2003; Cotten 2006; Cotten 2009; Foster 2006; Kuppala 2011).
The Cochrane Review published in 2005 concluded that there was inadequate evidence from RCTs in favour of any particular antibiotic regimen for the treatment of suspected late‐onset neonatal sepsis (Gordon 2005). No other systematic review has been conducted to date to assess the effects of different antibiotic regimens for suspected late‐onset sepsis. Therefore, there is a need for an updated systematic review that assesses the effects of different antibiotic regimens for late‐onset sepsis.
Objectives
To assess the beneficial and harmful effects of different antibiotic regimens for late‐onset neonatal sepsis.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs, quasi‐RCTs, and cluster‐RCTs. We included trials regardless of publication type (e.g. full‐text or abstract), publication status (e.g. preprint or published), publication date, and language. We excluded crossover trials.
Types of participants
We included participants suspected of or diagnosed with late‐onset sepsis (as defined by trial authors).
We included participants if described as newborns or 72 hours of life or more (at randomisation), suspected or diagnosed with neonatal sepsis, meningitis, osteomyelitis, endocarditis, or NEC.
We excluded trials that assessed treatment of fungal infections.
Types of interventions
We accepted any type of antibiotic or combination of antibiotics, such as the following.
Broad‐spectrum beta‐lactam antibiotics, defined as broad‐spectrum penicillins (e.g. ampicillin, amoxicillin, piperacillin, ticarcillin, carbenicillin, and mezlocillin), cephalosporins (e.g. cefazolin, cephalexin, cefuroxime, cefotetan, cefoxitin, ceftriaxone, cefotaxime, ceftazidime, cefepime, cefazolin, ceftobiprole, ceftolozane, and cefoperazone), carbapenems (e.g. imipenem, meropenem, doripenem, and ertapenem), and monobactams (e.g. aztreonam). Narrow‐spectrum antibiotics included narrow‐spectrum penicillins (e.g. oxacillin, cloxacillin, dicloxacillin, nafcillin, methicillin, and penicillin G).
Beta‐lactam antibiotics with beta‐lactamase inhibitors such as avibactam, clavulanic acid, sulbactam, and tazobactam.
Combination of beta‐lactam with aminoglycoside (e.g. gentamicin).
Combination of beta‐lactam with glycopeptide (e.g. vancomycin and teicoplanin).
Combination of glycopeptide with aminoglycoside.
We planned to assess the following comparisons.
Aminoglycoside added to any type of antibiotic versus any type of antibiotic (same antibiotic as in the experimental group).
Broad‐spectrum beta‐lactam antibiotic and aminoglycoside versus narrow‐spectrum beta‐lactam antibiotic (as defined in the above) and aminoglycoside (same aminoglycoside as in the experimental group).
Beta‐lactam antibiotic (as defined in the above) and aminoglycoside versus beta‐lactam antibiotic and glycopeptide.
Any other used antibiotic regimen (not included in the above‐mentioned comparisons) versus any other used antibiotic regimen (not included in the above‐mentioned comparisons).
Types of outcome measures
Primary outcomes
All‐cause mortality.
Secondary outcomes
Proportion of participants with one or more serious adverse events. We defined a serious adverse event as any untoward medical occurrence that resulted in death, was life‐threatening, jeopardised the participant, was persistent, led to significant disability, hospitalisation, or prolonged hospitalisation (ICH‐GCP 2015). As we expected the reporting of serious adverse events in many trials to be very heterogeneous and not strictly according to the recommendations regarding good clinical practice from the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH‐GCP) (ICH‐GCP 2015), we included the event as a serious adverse event if the trial authors either: used the term 'serious adverse event' but not refer to ICH‐GCP, or reported the proportion of participants with an event we consider fulfilled the ICH‐GCP definition (e.g. death or developed shock). If several such events were reported, we chose the highest proportion reported in each trial to avoid double‐counting.
Respiratory support, defined as the need for respiratory support, such as non‐invasive ventilation (e.g. continuous positive airway pressure (CPAP)) or invasive ventilation (e.g. respirator).
Circulatory support, defined as the need for circulatory support such as fluid bolus or vasoactive medication (e.g. inotropes or vasopressors).
Nephrotoxicity measured as decreased urine output, decreased estimated creatine clearance, or increase in S‐creatinine according to guidelines (such as "Paediatric Risk, Injury, Failure, Loss, End‐Stage Kidney Disease (pRIFLE) system", "Acute Kidney Injury Network (AKIN) guideline", and "Kidney Disease: Improving Global Outcomes (KDIGO) guideline") (McWilliam 2017) or as defined by the trial author.
Presence of moderate‐to‐severe neurological developmental and sensory impairment (defined as a functional abnormality in the function of the brain, spinal cord, muscles, nerves, eyes, or ears, or as any significant lag in a child's physical or motor, cognitive, behavioural, emotional or social development, in comparison with other children of the same age and sex within similar environments. If formal evaluation tools were used to assess neurodevelopmental impairment, we planned to use a threshold of –2 standard deviations (SDs) of the normal. Furthermore, severe brain injury per se is included, such as intraventricular haemorrhage grade 3 and 4 (Papile 1978; Volpe 2008), and periventricular leukomalacia.
NEC during or after treatment, defined by Bell's criteria 2 (Bell 1978) (participants with NEC at baseline were not included in the analysis of this outcome).
Ototoxicity as defined by the trial authors.
We assessed all dichotomised outcomes as proportions.
We used the trial results reported at maximum follow‐up (our primary time point of interest).
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for Specialized Register; neonatal.cochrane.org/resources-review-authors). We searched for errata or retractions from included studies published in full‐text on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Electronic searches
We conducted a comprehensive literature search including: the Cochrane Central Register of Controlled Trials (CENTRAL 2021, Issue 3) in the Cochrane Library; Ovid MEDLINE (1946 to 12 March 2021); Embase via Ovid (1974 to 12 March 2021); CINAHL (EBSCOhost; 12 March 2021); LILACS (Bireme; 1982 to 12 March 2021); and Science Citation Index EXPANDED and Conference Proceedings Citation Index – Science (1990 to 12 March 2021). We have included the search strategies for each database in Appendix 1.
We searched ZETOC for abstracts of scientific conferences or symposia (zetoc.jisc.ac.uk/).
We searched clinical trial registries for ongoing or recently completed trials. We searched the World Health Organization's International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en/), and the U.S. National Library of Medicine's ClinicalTrials.gov (clinicaltrials.gov), via Cochrane CENTRAL. Additionally, we searched the ISRCTN Registry for any unique trials not identified through the Cochrane CENTRAL search (www.isrctn.com/).
We applied no language restrictions. If we identified any papers in a language not known by the review author group, we sought translation assistance and acknowledged the translators in the Acknowledgements section of the review.
Searching other resources
We checked the reference lists of all relevant primary trials and reviews for additional references.
To identify unpublished trials we also searched clinical trial registers of Europe and the USA, websites of pharmaceutical companies, and websites of the US Food and Drug Administration (FDA) and the European Medicines Agency.
Data collection and analysis
Selection of studies
Three review authors (SKK, CN, and SS) independently screened titles and abstracts. We retrieved all relevant full‐text study reports/publication and three review authors (SKK, CN, and SS) independently screened the full texts and identified trials for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion or, if required, by consulting another review author (JCJ). We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), and a Characteristics of excluded studies table.
Where studies had multiple publications, we collated the reports of the same study so that each study, rather than each report, was the unit of interest for the review, and such studies had a single identifier with multiple references.
Data extraction and management
We used data collection forms for trial characteristics and outcome data that we piloted on at least one trial included in the review. Three review authors (SKK, CN, and SS) extracted trial characteristics from included trials. We extracted the following trials characteristics.
Methods: trial design, total duration of the trial, number of trial centres and location, trial setting, withdrawals, and date of the trial.
Participants: number of participants in each intervention group, mean age, age range, gender, diagnostic criteria, inclusion criteria, and exclusion criteria.
Interventions: intervention and comparison.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Three review authors (SKK, CN and SS) independently extracted outcome data from included trials. We noted in the Characteristics of included studies table if the trial authors did not report outcome data in a usable way. We resolved disagreements by consensus or by involving another review author (JCJ). One review author (SKK) transferred data into the Review Manager 5 (Review Manager 2020). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (SS) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (SKK and SS) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane 'Risk of bias' tool for the following domains (Higgins 2011).
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved any disagreements by discussion or by consulting a third review author (JCJ). See Appendix 2 for a more detailed description of risk of bias for each domain.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Korang 2021), and reported any deviations from it in the Differences between protocol and review section.
Measures of treatment effect
Dichotomous outcomes
We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials and the neonatal unit (or subunit) for cluster‐RCTs. For cluster‐RCTs, we planned to undertake analyses at the level of the individual while accounting for the clustering in the data using the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Dealing with missing data
We did not impute missing values for any outcomes in our primary analysis. In two of our sensitivity analyses, we planned to impute data (see Sensitivity analysis).
We contacted investigators and trial sponsors to verify key trial characteristics and obtain missing numerical outcome data when possible (e.g. when we identified a study as an abstract only).
Assessment of heterogeneity
We planned to visually inspect forest plots to assess signs of heterogeneity, and we planned to explore possible heterogeneity in our prespecified subgroup analyses. We inspected trial characteristics across trials to identify clinical heterogeneity. We assessed the presence of statistical heterogeneity using the Chi² test (threshold P < 0.10) and measured the quantities of heterogeneity using the I² statistic (Higgins 2002; Higgins 2003). If we had detected moderate or high heterogeneity (I² statistic of 50% or greater), we planned to explore the possible causes (e.g. differences in study design, participants, interventions, or completeness of outcome assessments).
Assessment of reporting biases
We planned to use a funnel plot to assess publication bias if 10 or more trials met the inclusion criteria. We also planned to visually inspect funnel plots to assess the risk of bias. As we planned to report results when we analysed dichotomous outcomes using RRs, we did not use any tests to assess funnel plot asymmetry when analysing dichotomous outcomes (Higgins 2019).
Data synthesis
Meta‐analysis
We planned to undertake this meta‐analysis according to the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We planned to use Review Manager 5 to analyse data (Review Manager 2020).
We planned to assess our intervention effects using fixed‐effect meta‐analyses (Demets 1987), in accordance with the policies of Cochrane Neonatal. We used one primary outcome and, therefore, we considered a P value of 0.05 or less as the threshold for statistical significance (Jakobsen 2014). We used the eight‐step procedure to assess if the thresholds for significance were crossed (Jakobsen 2014). Where data were only available from one trial, we planned to use Fisher's exact test for dichotomous data (Fisher 1922).
Where a trial reported multiple trial arms, we planned to include only the relevant trial arms. If two comparisons were combined in the same meta‐analysis, we would halve the control group to avoid double‐counting.
Trial sequential analysis
Traditional meta‐analysis runs the risk of random errors due to sparse data and repetitive testing of accumulating data when updating reviews. Therefore, we planned to perform trial sequential analysis (TSA) on the outcomes, to calculate the required information size and the cumulative Z‐curve's breach of relevant trial sequential monitoring boundaries (Brok 2008; Brok 2009; Thorlund 2009; Thorlund 2011; TSA 2011; Wetterslev 2008; Wetterslev 2009; Wetterslev 2017). We wished to control the risks of type I errors and type II errors. A more detailed description of TSA can be found at www.ctu.dk/tsa/. We planned to assess our TSA intervention effects with both a random‐effects model (DerSimonian 1986), and a fixed‐effect model (Demets 1987).
For dichotomous outcomes, we planned to estimate the required information size based on the observed, unweighted proportion of participants with an outcome in the control group (the cumulative proportion of participants with an event in the control groups relative to all participants in the control groups), a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and diversity as suggested by the trials in the meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses for our primary outcome.
High risk of bias trials compared with low risk of bias trials.
Gestational age: term (37 weeks or greater) compared with preterm.
Trials from high‐income countries compared with trials from LMICs, as defined by the World Bank (World Bank 2017).
Late‐onset sepsis defined by: onset after 48 hours, after 72 hours, after one week, or as defined by the trial authors.
Clinically suspected sepsis compared with culture‐supported suspicion of severe bacterial infection.
Trials including participants with coagulase‐negative staphylococci versus trials excluding participants with coagulase‐negative staphylococci.
We planned to use the formal test for subgroup interactions in Review Manager 5 (Review Manager 2020). We did not perform any of the above subgroups due to a lack of data.
Sensitivity analysis
To assess the potential impact of the missing data, we planned to perform the two following sensitivity analyses on the primary outcome and the secondary outcome serious adverse events.
'Best‐worst‐case' scenario: we planned to assume that all participants lost to follow‐up in the experimental group had survived and had no serious adverse event; and all those participants with missing outcomes in the control group had not survived and had a serious adverse event.
'Worst‐best‐case' scenario: we planned to assume that all participants lost to follow‐up in the experimental group had not survived and had a serious adverse event; and that all those participants lost to follow‐up in the control group had survived and had no serious adverse event.
We planned to present results of both scenarios in our review, but we did not perform any of the sensitivity analyses due to a lack of data.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence of the following (clinically relevant) outcomes: all‐cause mortality (primary outcome), and five secondary outcomes (serious adverse events, circulatory failure, nephrotoxicity, neurological developmental impairment, and NEC) at maximum follow‐up.
Three review authors (SKK, SS, and CN) independently assessed the certainty of the evidence for each of the outcomes above. We considered evidence from RCTs as high certainty but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used GRADEpro GDT to create five 'Summary of findings' tables to report the certainty of the evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: we are very uncertain about the estimate.
Results
Description of studies
We assessed all studies according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019), and the protocol for this review (Korang 2021). Characteristics of each study can be found in the Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
Our initial search identified 3356 references. We deemed 55 studies relevant and obtained full texts for further evaluation (see Figure 1). Of these, we included five trials (Ceriani 2014; Lutsar 2020; Miall‐Allen 1988; Millar 1992; Ramasamy 2014). We identified no ongoing trials relevant to the review.
1.
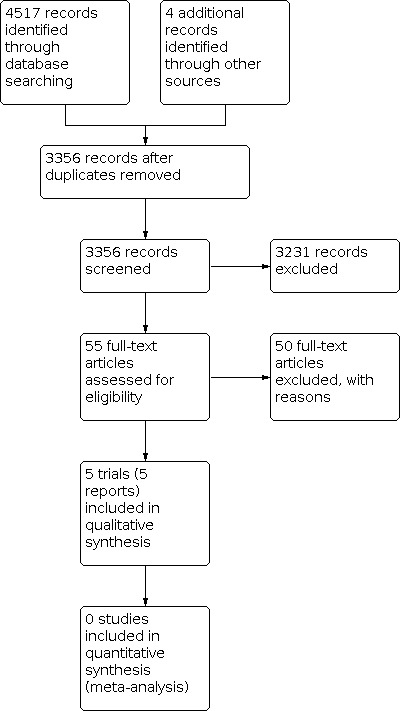
Study flow diagram.
Included studies
Five trials met our inclusion criteria (Ceriani 2014; Lutsar 2020; Miall‐Allen 1988; Millar 1992; Ramasamy 2014). For detailed descriptions, see the Characteristics of included studies table. Four of the trials were single centre, and one was multicentre.
Participants
The five trials included 580 participants. The mean proportion of girls was 46% among the trials that reported gender.
Interventions
The trials reported five different antibiotic regimens.
One trial assessed cefazolin plus amikacin compared with vancomycin plus amikacin (Ceriani 2014).
One trial assessed ticarcillin plus clavulanic acid compared with flucloxacillin plus gentamicin (Miall‐Allen 1988).
One trial assessed cloxacillin plus amikacin compared with cefotaxime plus gentamicin (Ramasamy 2014).
One trial assessed meropenem compared with standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin) (Lutsar 2020).
One trial assessed vancomycin plus gentamicin compared with vancomycin plus aztreonam (Millar 1992).
Co‐interventions
Participants in all five trials received standard care in addition to the allocated antibiotic regimen.
Outcomes
All five included trials reported all‐cause mortality and serious adverse events. None of the trials reported serious adverse events according to the ICH‐GCP, neither did they report serious adverse events as a composite outcome. Therefore, we reported the proportion of participants with an event we considered fulfilled the ICH‐GCP definition (e.g. shock or death). As there were several such events, we chose the highest proportion reported in each trial to avoid double‐counting. One trial reported circulatory support (Ramasamy 2014), neurological developmental impairment (Lutsar 2020), and nephrotoxicity (Ramasamy 2014). Two trials reported NEC (Lutsar 2020; Millar 1992). None of the trials reported respiratory support or ototoxicity.
Antibiotic resistance in included trials
One trial (from different countries in Europe) reported 31 cases of resistance (towards meropenem) out of the 63 participants with positive cultures in the meropenem group, compared with 45 cases of resistance (towards one of the allocated antibiotics) out of the 75 participants with positive cultures in the standard care group (Lutsar 2020).
One trial (from England) reported one case of resistance (towards gentamicin) out of the four participants with positive cultures in the flucloxacillin plus gentamicin group, but there was no resistance among the five participants with positive cultures in the ticarcillin plus clavulanic acid group (Miall‐Allen 1988).
The remaining three trials (from Argentina, England, and India) did not report resistance among the included participants towards the allocated antibiotics (Ceriani 2014; Millar 1992; Ramasamy 2014).
Excluded studies
We assessed 50 trials as relevant upon review of the abstract, but later excluded them upon review of the full publication.
We excluded 24 trials because they were a mix of early‐ and late‐onset neonatal sepsis (Adelman 1987a; Adelman 1987b; Baqui 2013; Begue 1998; De Louvois 1992; Faix 1988; Fogel 1983; Gokalp 1991; Haffejee 1984; Hall 1988; Lee 2005; Marks 1978; Mir 2017; Molyneux 2017; Odio 1987; Odio 1995; Taheri 2011; Tessin 1988; Tessin 1989; Tshefu 2015a; Tshefu 2015b; Umaña 1990; Wiese 1988; Zaidi 2013).
In eight trials both groups received the same antibiotics (Auriti 2005; Chowdhary 2006; Gathwala 2010; Hansen 1980; Langhendries 1993; McCracken 1976; Mulubwa 2020; Rohatgi 2017).
Four trials included only early‐onset neonatal sepsis (Hammerberg 1989; Metsvaht 2010; Snelling 1983; Tewari 2014).
One trial included adults (Bassetti 1991).
Two trials were not randomised (Ebrahim 1969; Oral 1998).
Eleven trials did not include neonates with late‐onset sepsis (Alinejad 2018; Aronoff 1984; Chartrand 1984; Collins 1998; Deville 2003; Feigin 1976; Jantausch 2003; Kaplan 2003; Lönnerholm 1982; Viganò 1995; Wells 1984).
When the participant age was unclear or separate data were not available for late‐onset sepsis, we contacted the study authors. However, we obtained no additional information on these trials.
Risk of bias in included studies
We assessed all the included studies at overall high risk of bias (Figure 2). We contacted the authors for clarification, as some data were missing and several bias domains were unclear.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three trials used a computer program to generate the sequence resulting in assessment of low risk (Ceriani 2014; Lutsar 2020; Ramasamy 2014). Two trials did not describe how allocation sequence generation was performed resulting in assessment of unclear (Miall‐Allen 1988; Millar 1992).
Three trials allocated with concealment using serially numbered opaque sealed envelopes or had the randomisation list kept confidential and were at low risk of bias (Ceriani 2014; Lutsar 2020; Ramasamy 2014). Two trials did not describe allocation concealment and were at unclear risk of bias (Miall‐Allen 1988; Millar 1992).
Blinding
Two trials did not blind participants or treatment providers resulting in assessment of high risk of performance bias (Ceriani 2014; Lutsar 2020). Two trials did not describe methods of blinding (Miall‐Allen 1988; Millar 1992), and one trial was only described as single‐blinded (without specifications) (Ramasamy 2014), resulting in unclear risk of performance bias.
Two trials did not blind outcome assessors resulting in assessment of high risk of detection bias (Ceriani 2014; Lutsar 2020). Three trials did not describe whether outcome assessors were blinded resulting in unclear risk of detection bias (Miall‐Allen 1988; Millar 1992; Ramasamy 2014).
Incomplete outcome data
All trials used either intention‐to‐treat analysis or had no or few dropouts resulting in assessment of low risk of attrition bias.
Selective reporting
All trials reported mortality resulting in assessment of low risk of reporting bias.
Other potential sources of bias
Review authors observed no other biases.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings 1. Cefazolin plus amikacin compared with vancomycin plus amikacin for late‐onset neonatal sepsis.
| Cefazolin + amikacin compared with vancomycin + amikacin for late‐onset neonatal sepsis | ||||||
|
Patient or population: newborns with late‐onset sepsis Settings: neonatal intensive care unit in Argentina Intervention: cefazolin + amikacin Comparison: vancomycin + amikacin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Vancomycin + amikacin | Cefazolin + amikacin | |||||
|
All‐cause mortality maximum follow‐up |
135 per 1000 | 94 per 1000 (39 to 223) |
RR 0.70 (0.29 to 1.66) |
109 (1) | ⊕⊝⊝⊝a Very low | OIS: 3022 (RR 0.80, α 0.05, β 0.20) |
|
Serious adverse events maximum follow‐up |
135 per 1000 | 94 per 1000 (39 to 223) |
RR 0.70 (0.29 to 1.66) |
109 (1) | ⊕⊝⊝⊝a Very low | OIS: 3022 (RR 0.80, α 0.05, β 0.20) Serious adverse events were deaths. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OIS: optimal information size; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded three levels because of very serious risk of bias, very serious imprecision of results, and serious risk of indirectness.
Summary of findings 2. Ticarcillin plus clavulanic acid compared with flucloxacillin and gentamicin for late‐onset neonatal sepsis.
| Ticarcillin + clavulanic acid compared with flucloxacillin + gentamicin for late‐onset neonatal sepsis | ||||||
|
Patient or population: newborns with late‐onset sepsis Settings: neonatal intensive care unit in England Intervention: ticarcillin + clavulanic acid Comparison: flucloxacillin + gentamicin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Flucloxacillin + gentamicin | Ticarcillin + clavulanic acid | |||||
|
All‐cause mortality maximum follow‐up |
143 per 1000 | 28 per 1000 (1 to 546) |
RR 0.20 (0.01 to 3.82) |
28 (1) | ⊕⊝⊝⊝a Very low | OIS: 4306 (RR 0.80, α 0.05, β 0.20) |
|
Serious adverse events maximum follow‐up |
143 per 1000 | 28 per 1000 (1 to 546) |
RR 0.20 (0.01 to 3.82) |
28 (1) | ⊕⊝⊝⊝a Very low | OIS: 4306 (RR 0.80, α 0.05, β 0.20) Serious adverse events were deaths. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OIS: optimal information size; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded three levels because of very serious risk of bias, very serious imprecision of results, and serious risk of indirectness.
Summary of findings 3. Cloxacillin plus amikacin compared with cefotaxime plus gentamicin for neonatal late‐onset sepsis.
| Cloxacillin + amikacin compared with cefotaxime + gentamicin for neonatal late‐onset sepsis | ||||||
|
Patient or population: newborns with late‐onset sepsis Settings: neonatal intensive care unit in India Intervention: cloxacillin + amikacin Comparison: cefotaxime + gentamicin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Cefotaxime + gentamicin | Cloxacillin + amikacin | |||||
|
All‐cause mortality maximum follow‐up |
200 per 1000 | 76 per 1000 (22 to 254) |
RR 0.38 (0.11 to 1.27) |
90 (1) | ⊕⊝⊝⊝a Very low | OIS: 2894 (RR 0.80, α 0.05, β 0.20) |
|
Serious adverse events maximum follow‐up |
200 per 1000 | 100 per 1000 (34 to 296) |
RR 0.50 (0.17 to 1.48) |
90 (1) | ⊕⊝⊝⊝a Very low | OIS: 2894 (RR 0.80, α 0.05, β 0.20) Serious adverse events were participants who developed shock. |
|
Circulatory support maximum follow‐up |
200 per 1000 | 100 per 1000 (34 to 296) |
RR 0.50 (0.17 to 1.48) |
90 (1) | ⊕⊝⊝⊝a Very low | OIS: 2894 (RR 0.80, α 0.05, β 0.20) |
|
Nephrotoxicity maximum follow‐up |
100 per 1000 | 25 per 1000 (3 to 205) |
RR 0.25 (0.03 to 2.05) |
90 (1) | ⊕⊝⊝⊝a Very low | OIS: 6428 (RR 0.80, α 0.05, β 0.20) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OIS: optimal information size; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded three levels because of very serious risk of bias, very serious imprecision of results, and serious risk of indirectness.
Summary of findings 4. Meropenem compared with standard care for neonatal late‐onset sepsis.
| Meropenem compared with standard care for neonatal late‐onset sepsis | ||||||
|
Patient or population: newborns with late‐onset sepsis Settings: neonatal intensive care units in Europe Intervention: meropenem Comparison: standard care (ampicillin + gentamicin or cefotaxime + gentamicin) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Meropenem | Standard care | |||||
|
All‐cause mortality maximum follow‐up |
52 per 1000 | 74 per 1000 (29 to 188) |
RR 1.42 (0.56 to 3.62) |
271 (1) | ⊕⊝⊝⊝a Very low |
OIS: 12976 (RR 0.80, α 0.05, β 0.20) |
|
Serious adverse events maximum follow‐up |
133 per 1000 | 205 per 1000 (120 to 355) |
RR 1.54 (0.90 to 2.66) |
271 (1) | ⊕⊝⊝⊝a Very low | OIS: 4662 (RR 0.80, α 0.05, β 0.20) |
|
Neurological developmental impairment maximum follow‐up |
178 per 1000 | 155 per 1000 (91 to 263) |
RR 0.87 (0.51 to 1.48) |
271 (1) | ⊕⊝⊝⊝a Very low | OIS: 3336 (RR 0.80, α 0.05, β 0.20) This outcome was number of participants with intracranial bleeding. |
|
Necrotising enterocolitis maximum follow‐up |
119 per 1000 | 81 per 1000 (39 to 168) |
RR 0.68 (0.33 to 1.42) |
271 (1) | ⊕⊝⊝⊝a Very low | OIS: 5324 (RR 0.80, α 0.05, β 0.20) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CIS: optimal information size; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded three levels because of very serious risk of bias, very serious imprecision of results, and serious risk of indirectness.
Summary of findings 5. Vancomycin plus gentamicin compared with vancomycin plus aztreonam for late‐onset neonatal sepsis.
| Vancomycin + gentamicin compared with vancomycin + aztreonam for late‐onset neonatal sepsis | ||||||
|
Patient or population: newborns with late‐onset sepsis Settings: neonatal intensive care units in England Intervention: vancomycin + gentamicin Comparison: vancomycin + aztreonam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Vancomycin + aztreonam | Vancomycin + gentamicin | |||||
|
All‐cause mortality maximum follow‐up |
150 per 1000 | 98 per 1000 (30 to 320) |
RR 0.65 (0.20 to 2.13) |
81 (1) | ⊕⊝⊝⊝a Very low | OIS: 4072 (RR 0.80, α 0.05, β 0.20) |
|
Serious adverse events maximum follow‐up |
150 per 1000 | 98 per 1000 (30 to 320) |
RR 0.65 (0.20 to 2.13) |
81 (1) | ⊕⊝⊝⊝a Very low | OIS: 4072 (RR 0.80, α 0.05, β 0.20) |
|
Necrotising enterocolitis maximum follow‐up |
NA | NA |
RR 12.69 (0.74 to 218.09) |
81 (1) | ⊕⊝⊝⊝a Very low | OIS: NA |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NA: not available; OIS: optimal information size; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded three levels because of very serious risk of bias, very serious imprecision of results, and serious risk of indirectness.
We included five trials (580 participants) that met all the inclusion criteria (Ceriani 2014; Lutsar 2020; Miall‐Allen 1988; Millar 1992; Ramasamy 2014). We were able to assess in part all‐cause mortality, serious adverse events, circulatory support, nephrotoxicity, neurological developmental impairment, and NEC as primary and secondary outcomes. However, the five trials assessed comparisons with different antibiotic regimens. Hence, we performed no meta‐analyses, no TSA, and no subgroup analysis on any outcomes. We estimated the optimal information size for all of the outcomes and the optimal information size was not reached for any of the comparisons (Table 1; Table 2; Table 3; Table 4; Table 5).
Cefazolin plus amikacin compared with vancomycin plus amikacin
We found one trial comparing cefazolin plus amikacin with vancomycin plus amikacin (Table 1).
Primary outcome
All‐cause mortality
One trial randomising 109 participants comparing cefazolin plus amikacin with vancomycin plus amikacin showed no evidence of a difference in all‐cause mortality (RR 0.70, 95% CI 0.29 to 1.66; very low‐certainty evidence; Analysis 1.1) (Ceriani 2014).
1.1. Analysis.

Comparison 1: Cefazolin plus amikacin versus vancomycin plus amikacin, Outcome 1: All‐cause mortality
Secondary outcomes
Serious adverse events
One trial randomising 109 participants comparing cefazolin plus amikacin with vancomycin plus amikacin showed no evidence of a difference in serious adverse events (RR 0.70, 95% CI 0.29 to 1.66; very low‐certainty evidence; Analysis 1.2) (Ceriani 2014).
1.2. Analysis.

Comparison 1: Cefazolin plus amikacin versus vancomycin plus amikacin, Outcome 2: Serious adverse events
Respiratory support
The trial did not report respiratory support.
Circulatory support
The trial did not report circulatory support.
Nephrotoxicity
The trial did not report nephrotoxicity.
Neurological developmental impairment
The trial did not report neurological developmental impairment.
Necrotising enterocolitis
The trial did not report NEC.
Ototoxicity
The trial did not report ototoxicity.
Ticarcillin plus clavulanic acid compared with flucloxacillin plus gentamicin
We found one trial comparing ticarcillin plus clavulanic acid with flucloxacillin plus gentamicin (Table 2).
Primary outcome
All‐cause mortality
One trial randomising 28 participants comparing ticarcillin plus clavulanic acid with flucloxacillin plus gentamicin showed no evidence of a difference in all‐cause mortality (RR 0.20, 95% CI 0.01 to 3.82; very low‐certainty evidence; Analysis 2.1) (Miall‐Allen 1988).
2.1. Analysis.

Comparison 2: Ticarcillin plus clavulanic acid versus flucloxacillin plus gentamicin, Outcome 1: All‐cause mortality
Secondary outcomes
Serious adverse events
One trial randomising 28 participants comparing ticarcillin plus clavulanic acid with flucloxacillin plus gentamicin showed no evidence of a difference in serious adverse events (RR 0.20, 95% CI 0.01 to 3.82; Analysis 2.2; very low‐certainty evidence) (Miall‐Allen 1988).
2.2. Analysis.

Comparison 2: Ticarcillin plus clavulanic acid versus flucloxacillin plus gentamicin, Outcome 2: Serious adverse events
Respiratory support
The trial did not report respiratory support.
Circulatory support
The trial did not report circulatory support.
Nephrotoxicity
The trial did not report nephrotoxicity.
Neurological developmental impairment
The trial did not report neurological developmental impairment.
Necrotising enterocolitis
The trial did not report NEC.
Ototoxicity
The trial did not report ototoxicity.
Cloxacillin plus amikacin compared with cefotaxime plus gentamicin
We found one study comparing cloxacillin plus amikacin with cefotaxime plus gentamicin (Table 3).
Primary outcome
All‐cause mortality
One trial randomising 90 participants comparing cloxacillin plus amikacin with cefotaxime plus gentamicin showed no evidence of a difference in all‐cause mortality (RR 0.38, 95% CI 0.11 to 1.27; very low‐certainty evidence; Analysis 3.1) (Ramasamy 2014).
3.1. Analysis.

Comparison 3: Cloxacillin plus amikacin versus cefotaxime plus gentamicin, Outcome 1: All‐cause mortality
Secondary outcomes
Serious adverse events
One trial randomising 90 participants comparing cloxacillin plus amikacin with cefotaxime plus gentamicin showed no evidence of a difference in serious adverse events (RR 0.50, 95% CI 0.17 to 1.48; very low‐certainty evidence; Analysis 3.2) (Ramasamy 2014).
3.2. Analysis.

Comparison 3: Cloxacillin plus amikacin versus cefotaxime plus gentamicin, Outcome 2: Serious adverse events
Respiratory support
The trial did not report respiratory support.
Circulatory support
One trial randomising 90 participants comparing cloxacillin plus amikacin with cefotaxime plus gentamicin showed no evidence of a difference in circulatory support (RR 0.50, 95% CI 0.17 to 1.48; very low‐certainty evidence; Analysis 3.3) (Ramasamy 2014).
3.3. Analysis.

Comparison 3: Cloxacillin plus amikacin versus cefotaxime plus gentamicin, Outcome 3: Circulatory support
Nephrotoxicity
One trial randomising 90 participants comparing cloxacillin plus amikacin with cefotaxime plus gentamicin showed no evidence of a difference in nephrotoxicity (RR 0.25, 95% CI 0.03 to 2.05; very low‐certainty evidence; Analysis 3.4) (Ramasamy 2014).
3.4. Analysis.

Comparison 3: Cloxacillin plus amikacin versus cefotaxime plus gentamicin, Outcome 4: Nephrotoxicity
Neurological developmental impairment
The trial did not report neurological developmental impairment.
Necrotising enterocolitis
The trial did not report NEC.
Ototoxicity
The trial did not report ototoxicity.
Meropenem compared with standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin)
We found one trial comparing meropenem compared with standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin) (Table 4).
Primary outcome
All‐cause mortality
One trial randomising 271 participants comparing meropenem with standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin) showed no evidence of a difference in all‐cause mortality (RR 1.42, 95% CI 0.56 to 3.62; very low‐certainty evidence; Analysis 4.1) (Lutsar 2020).
4.1. Analysis.

Comparison 4: Meropenem versus standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin), Outcome 1: All‐cause mortality
Secondary outcomes
Serious adverse events
One trial randomising 271 participants comparing meropenem with standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin) showed no evidence of a difference in serious adverse events (RR 1.54, 95% CI 0.90 to 2.66; very low‐certainty evidence; Analysis 4.2) (Lutsar 2020).
4.2. Analysis.

Comparison 4: Meropenem versus standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin), Outcome 2: Serious adverse events
Respiratory support
The trial did not report respiratory support.
Circulatory support
The trial did not report circulatory support.
Nephrotoxicity
The trial did not report nephrotoxicity.
Neurological developmental impairment
One trial randomising 271 participants comparing meropenem with standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin) showed no evidence of a difference in neurological developmental impairment (RR 0.87, 95% CI 0.51 to 1.48; very low‐certainty evidence; Analysis 4.3) (Lutsar 2020).
4.3. Analysis.

Comparison 4: Meropenem versus standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin), Outcome 3: Neurological developmental impairment
Necrotising enterocolitis
One trial randomising 271 participants comparing meropenem with standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin) showed no evidence of a difference in NEC (RR 0.68, 95% CI 0.33 to 1.42; very low‐certainty evidence; Analysis 4.4) (Lutsar 2020).
4.4. Analysis.

Comparison 4: Meropenem versus standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin), Outcome 4: Necrotising enterocolitis
Ototoxicity
The trial did not report ototoxicity.
Vancomycin plus gentamicin compared with vancomycin plus aztreonam
We found one trial comparing vancomycin plus gentamicin compared with vancomycin plus aztreonam (Table 5).
Primary outcome
All‐cause mortality
One trial randomising 81 participants comparing vancomycin plus gentamicin with vancomycin plus aztreonam showed no evidence of a difference in all‐cause mortality (RR 0.65, 95% CI 0.20 to 2.13; very low‐certainty evidence; Analysis 5.1) (Millar 1992).
5.1. Analysis.

Comparison 5: Vancomycin plus gentamicin versus vancomycin plus aztreonam, Outcome 1: All‐cause mortality
Secondary outcomes
Serious adverse events
One trial randomising 81 participants comparing vancomycin plus gentamicin with vancomycin plus aztreonam showed no evidence of a difference in serious adverse events (RR 0.65, 95% CI 0.20 to 2.13; very low‐certainty evidence; Analysis 5.2) (Millar 1992).
5.2. Analysis.

Comparison 5: Vancomycin plus gentamicin versus vancomycin plus aztreonam, Outcome 2: Serious adverse events
Respiratory support
The trial did not report respiratory support.
Circulatory support
The trial did not report circulatory support.
Nephrotoxicity
The trial did not report nephrotoxicity.
Neurological developmental impairment
The trial did not report neurological developmental impairment.
Necrotising enterocolitis
One trial randomising 81 participants comparing vancomycin plus gentamicin with vancomycin plus aztreonam showed no evidence of a difference in NEC (RR 12.69, 95% CI 0.74 to 218.09; very low‐certainty evidence; Analysis 5.3) (Millar 1992).
5.3. Analysis.

Comparison 5: Vancomycin plus gentamicin versus vancomycin plus aztreonam, Outcome 3: Necrotising enterocolitis
Ototoxicity
The trial did not report ototoxicity.
Discussion
Summary of main results
Evidence from five RCTs including 580 participants contributed data to our predefined outcomes. There is insufficient information to assess the relative effects of any of the antibiotics compared. Furthermore, these trials had high risk of bias. In summary, the certainty of the evidence was very low.
We conducted no meta‐analyses due to a lack of relevant data. The optimal information size was not reached for any of the comparisons (Table 1; Table 2; Table 3; Table 4; Table 5).
When assessing all‐cause mortality: one trial randomising 109 participants showed no evidence of a difference when comparing cefazolin plus amikacin with vancomycin plus amikacin (RR 0.70, 95% CI 0.29 to 1.66; very low‐certainty evidence) (Ceriani 2014); one trial randomising 28 participants showed no evidence of a difference when comparing ticarcillin plus clavulanic acid with flucloxacillin plus gentamicin (RR 0.20, 95% CI 0.01 to 3.82; very low‐certainty evidence) (Miall‐Allen 1988); one trial randomising 90 participants showed no evidence of a difference when comparing cloxacillin plus amikacin with cefotaxime plus gentamicin (RR 0.38, 95% CI 0.11 to 1.27; very low‐certainty evidence) (Ramasamy 2014); one trial randomising 71 participants showed no evidence of a difference when comparing meropenem with standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin) (RR 1.42, 95% CI 0.56 to 3.62; very low‐certainty evidence) (Lutsar 2020); one trial randomising 81 participants showed no evidence of a difference when comparing vancomycin plus gentamicin with vancomycin plus aztreonam (RR 0.65, 95% CI 0.20 to 2.13; very low‐certainty evidence) (Millar 1992).
When assessing serious adverse events: one trial randomising 109 participants showed no evidence of a difference when comparing cefazolin plus amikacin with vancomycin plus amikacin (RR 0.70, 95% CI 0.29 to 1.66; very low‐certainty evidence) (Ceriani 2014); one trial randomising 28 participants showed no evidence of a difference when comparing ticarcillin plus clavulanic acid with flucloxacillin plus gentamicin (RR 0.20, 95% CI 0.01 to 3.82; very low‐certainty evidence) (Miall‐Allen 1988); one trial randomising 90 participants showed no evidence of a difference when comparing cloxacillin plus amikacin with cefotaxime plus gentamicin (RR 0.50, 95% CI 0.17 to 1.48; very low‐certainty evidence) (Ramasamy 2014); one trial randomising 271 participants showed no evidence of a difference when comparing meropenem with standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin) (RR 1.54, 95% CI 0.90 to 2.66; very low‐certainty evidence) (Lutsar 2020); one trial randomising 81 participants showed no evidence of a difference when comparing vancomycin plus gentamicin with vancomycin plus aztreonam (RR 0.65, 95% CI 0.20 to 2.13; very low‐certainty evidence) (Millar 1992).
None of the trials reported respiratory support.
When assessing circulatory support, one trial randomising 90 participants showed no evidence of a difference when comparing cloxacillin plus amikacin with cefotaxime plus gentamicin (RR 0.50, 95% CI 0.17 to 1.48; very low‐certainty evidence) (Ramasamy 2014).
When assessing nephrotoxicity, one trial randomising 90 participants showed no evidence of a difference when comparing cloxacillin plus amikacin with cefotaxime plus gentamicin (RR 0.25, 95% CI 0.03 to 2.05; very low‐certainty evidence) (Ramasamy 2014).
When assessing neurological developmental impairment, one trial randomising 271 participants showed no evidence of a difference when comparing meropenem with standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin) (RR 0.87, 95% CI 0.51 to 1.48; very low‐certainty evidence) (Lutsar 2020).
When assessing NEC: one trial randomising 271 participants showed no evidence of a difference when comparing meropenem with standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin) (RR 0.68, 95% CI 0.33 to 1.42; very low‐certainty evidence) (Lutsar 2020); one trial randomising 81 participants showed no evidence of a difference when comparing vancomycin plus gentamicin with vancomycin plus aztreonam (RR 12.69, 95% CI 0.74 to 218.09; very low‐certainty evidence) (Millar 1992).
None of the trials reported ototoxicity.
The benefits and harms of different antibiotic regimens remain unclear owing to the lack of well‐powered trials, and the high risk of systematic errors.
Overall completeness and applicability of evidence
We were unable to perform any meta‐analyses due to lack of relevant data and the identified trials were underpowered. Therefore, it was not possible to conclude whether one antibiotic regimen was superior to another in neonates with late‐onset sepsis. More and larger RCTs with low risk of bias are needed.
Quality of the evidence
Heterogeneity
As no meta‐analysis was performed, we did not assess heterogeneity.
Risk of systematic error ('bias')
We found no trials and no outcome results at low risk of bias.
It was not possible to assess publication bias, as we were only able to include five studies.
Risk of random error ('play of chance')
It was not possible to perform TSA, as we performed no meta‐analyses.
GRADE
We assessed the certainty of the evidence for each outcome by using the GRADE approach (Table 1; Table 2; Table 3; Table 4; Table 5). The GRADE assessment generally showed that the evidence was of very‐low certainty.
Potential biases in the review process
The main limitation of this review was the low number of randomised participants and hence paucity of evidence for the use of different antibiotic regimens. Another limitation was that some trials did not distinguish between early‐onset and late‐onset neonatal sepsis, which resulted in a large number of potentially relevant trials that were excluded. We used the broadest possible definition of late‐onset sepsis and the broadest choice of possible antibiotic regimens. This could potentially have caused the inclusion of trials with very a heterogeneous population and many different interventions. Despite this broad approach, we only found five trials.
If that had been the case, we would have considered whether meta‐analysis could be justified.
As indicated in our Background section, there might be substantial differences between the pathogens across countries. The optimal antibiotic regimen might, therefore, vary according to country and local risks of antibiotic resistance. We did not include enough trials to confirm that this was the case.
Despite the anticipated differences between antibiotic resistance at different sites, there could still be important differences between antibiotic regimens on clinical outcomes that would lead to generalised recommendations (Paul 2010).
For future updates, we will systematically assess the clinical heterogeneity (Barbateskovic 2021).
Agreements and disagreements with other studies or reviews
The additional trials included in this version did not change the overall conclusions and recommendations of the former review (Gordon 2005). The largest included trial only randomised 271 participants and compared meropenem with standard care (Lutsar 2020). The authors found no evidence to suggest that meropenem was superior to standard care. Although the largest of our included trials, this sample size is presumably underpowered to detect any evidence of a difference between two antibiotic regimens on clinically important outcomes such as mortality and serious adverse events.
Authors' conclusions
Implications for practice.
Current evidence does not allow confirmation, or rejection, of one antibiotic regimen being superior to another.
Implications for research.
The primary focus should be to develop an international consensus definition of neonatal sepsis (McGovern 2020; Wynn 2014; Wynn 2016).
Then high‐quality randomised controlled trials are needed to assess the effects of different antibiotic regimens for sepsis in newborn infants. Such trials should:
randomise a sufficient number of participants to demonstrate a reliable result;
assess all‐cause mortality and serious adverse events;
be conducted with low risk of bias;
adhere to consensus definitions of suspected and diagnosed late‐onset neonatal when such emerge;
measure antibiotic resistance among the culture‐positive participants;
Assess differences between sites, countries, and regions included.
What's new
| Date | Event | Description |
|---|---|---|
| 12 March 2021 | Amended | The authors have revised the protocol prior to conducting the updated review (Korang 2021). This protocol and the subsequent review will replace the review of "Antibiotic regimens for suspected late onset sepsis in newborn infants" (Gordon 2005). |
History
Protocol first published: Issue 12, 2020 Review first published: Issue 4, 2021
| Date | Event | Description |
|---|---|---|
| 7 July 2016 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank Cochrane Neonatal: Colleen Ovelman, Managing Editor; Jane Cracknell, Assistant Managing Editor; Roger Soll, Co‐coordinating editor; and Bill McGuire, Co‐coordinating Editor, who provided editorial and administrative support.
Carol Friesen, Cochrane Neonatal Information Specialist and Sarah Klingenberg Cochrane Hepato‐Biliary Information Specialist designed the literature searches.
As Cochrane Neonatal Associate Editors, Jeffrey Horbar and Vibhuti Shah peer reviewed and offered feedback for this review.
The Methods section of this review was based on a standard template used by Cochrane Neonatal.
We thank the Danish/Spanish nurse Cindy Bustamante for her help in translating Ceriani 2014 .
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 3) in the Cochrane Library
#1 MeSH descriptor: [Infant] explode all trees
#2 (infan* or newborn or neonat* or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
#3 #1 or #2
#4 MeSH descriptor: [Neonatal Sepsis] explode all trees
#5 (sepsis NEAR/3 (neonat* or neo nat*))
#6 (sepsis NEAR/3 (newborn* or new born* or newly born*))
#7 (septic* NEAR/3 (neonat* or neo nat*))
#8 (septic* NEAR/3 (newborn* or new born* or newly born*))
#9 (infect* NEAR/3 (neonat* or neo nat*))
#10 (infect* NEAR/3 (newborn* or new born* or newly born*))
#11 (bacter* NEAR/3 (neonat* or neo nat*))
#12 (bacter* NEAR/3 (newborn* or new born* or newly born*))
#13 (gram NEAR/2 negative)
#14 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13
#15 MeSH descriptor: [Anti‐Bacterial Agents] explode all trees
#16 (antibiot* OR antimicrob* OR lactam* OR aminoglycoside* OR glycoprotein OR penicillin OR oxacillin OR cloxacillin OR dicloxacillin OR nafcillin OR methicillin OR ampicillin OR amoxicillin OR piperacillin OR ticarcillin OR carbenicillin OR mezlocillin OR cephalosporins OR cefazolin OR cephalexin OR cefuroxime OR cefotetan OR cefoxitin OR ceftriaxone OR cefotaxime OR ceftazidime OR cefepime OR cefazolin OR ceftobiprole OR cefoperazone OR carbapenems OR imipenem OR meropenem OR doripenem OR ertapenem OR monobactams OR aztreonam)
#17 #15 OR #16
#18 #3 and #14 and #17
MEDLINE Ovid (1946 to March 2021)
1. exp Infant/
2. (infan* or newborn or neonat* or premature or preterm or very low birth weight or low birth weight or VLBW or LBW).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
3. 1 or 2
4. exp Neonatal Sepsis/
5. (sepsis adj3 (neonat$ or neo nat$)).ti,ab.
6. (sepsis adj3 (newborn$ or new born$ or newly born$)).ti,ab.
7. (septic$ adj3 (neonat$ or neo nat$)).ti,ab.
8. (septic$ adj3 (newborn$ or new born$ or newly born$)).ti,ab.
9. (infect$ adj3 (neonat$ or neo nat$)).ti,ab.
10. (infect$ adj3 (newborn$ or new born$ or newly born$)).ti,ab.
11. (bacter$ adj3 (neonat$ or neo nat$)).ti,ab.
12. (bacter$ adj3 (newborn$ or new born$ or newly born$)).ti,ab.
13. (gram adj2 negative).ti,ab.
14. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13
15. exp Anti‐Bacterial Agents/
16. (antibiot* or antimicrob* or lactam* or aminoglycoside* or glycoprotein or penicillin or oxacillin or cloxacillin or dicloxacillin or nafcillin or methicillin or ampicillin or amoxicillin or piperacillin or ticarcillin or carbenicillin or mezlocillin or cephalosporins or cefazolin or cephalexin or cefuroxime or cefotetan or cefoxitin or ceftriaxone or cefotaxime or ceftazidime or cefepime or cefazolin or ceftobiprole or cefoperazone or carbapenems or imipenem or meropenem or doripenem or ertapenem or monobactams or aztreonam).ti,ab.
17. 15 or 16
18. 3 and 14 and 17
19. (randomized controlled trial or controlled clinical trial).pt. or clinical trials as topic.sh. or trial.ti.
20. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
21. 18 and (19 or 20)
Embase Ovid (1974 to March 2021)
1. exp infant/
2. (infan* or newborn or neonat* or premature or preterm or very low birth weight or low birth weight or VLBW or LBW).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
3. 1 or 2
4. exp newborn sepsis/
5. (sepsis adj3 (neonat$ or neo nat$)).ti,ab.
6. (sepsis adj3 (newborn$ or new born$ or newly born$)).ti,ab.
7. (septic$ adj3 (neonat$ or neo nat$)).ti,ab.
8. (septic$ adj3 (newborn$ or new born$ or newly born$)).ti,ab.
9. (infect$ adj3 (neonat$ or neo nat$)).ti,ab.
10. (infect$ adj3 (newborn$ or new born$ or newly born$)).ti,ab.
11. (bacter$ adj3 (neonat$ or neo nat$)).ti,ab.
12. (bacter$ adj3 (newborn$ or new born$ or newly born$)).ti,ab.
13. (gram adj2 negative).ti,ab.
14. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13
15. exp antiinfective agent/
16. (antibiot* or antimicrob* or lactam* or aminoglycoside* or glycoprotein or penicillin or oxacillin or cloxacillin or dicloxacillin or nafcillin or methicillin or ampicillin or amoxicillin or piperacillin or ticarcillin or carbenicillin or mezlocillin or cephalosporins or cefazolin or cephalexin or cefuroxime or cefotetan or cefoxitin or ceftriaxone or cefotaxime or ceftazidime or cefepime or cefazolin or ceftobiprole or cefoperazone or carbapenems or imipenem or meropenem or doripenem or ertapenem or monobactams or aztreonam).ti,ab.
17. 15 or 16
18. 3 and 14 and 17
19. Randomized controlled trial/ or Controlled clinical study/ or trial.ti.
20. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
21. 18 and (19 or 20)
CINAHL (EBSCOhost; March 2021)
S14 S10 AND S13
S13 S11 OR S12
S12 TX ( random* or blind* or placebo* or meta‐analys* ) OR TI trial
S11 PT randomized controlled trial OR PT controlled clinical trial
S10 S3 AND S6 AND S9
S9 S7 OR S8
S8 TI ( (antibiot* or antimicrob* or lactam* or aminoglycoside* or glycoprotein or penicillin or oxacillin or cloxacillin or dicloxacillin or nafcillin or methicillin or ampicillin or amoxicillin or piperacillin or ticarcillin or carbenicillin or mezlocillin or cephalosporins or cefazolin or cephalexin or cefuroxime or cefotetan or cefoxitin or ceftriaxone or cefotaxime or ceftazidime or cefepime or cefazolin or ceftobiprole or cefoperazone or carbapenems or imipenem or meropenem or doripenem or ertapenem or monobactams or aztreonam)
S7 MH antibiotics
S6 S4 OR S5
S5 TI ( (((sepsis or septic* or infect* or bacter*) N3 (neonat* or neo nat* or newborn* or new born* or newly born*)) or (gram N2 negative)) ) OR AB ( (((sepsis or septic* or infect* or bacter*) N3 (neonat* or neo nat* or newborn* or new born* or newly born*)) or (gram N2 negative)) )
S4 MH Neonatal Sepsis
S3 S1 OR S2
S2 TX (infan* or newborn or neonat* or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
S1 MH infant
LILACS (Bireme; 1982 to March 2021)
(infan$ or newborn or neonat$ or premature or preterm or very low birth weight or low birth weight or VLBW or LBW) and (((sepsis or septic$ or infect$ or bacter$) and (neonat$ or neo nat$ or newborn$ or new born$ or newly born$)) or (gram near negative)) and (antibiot$ OR antimicrob$ OR lactam$ OR aminoglycoside$ OR glycoprotein OR penicillin OR oxacillin OR cloxacillin OR dicloxacillin OR nafcillin OR methicillin OR ampicillin OR amoxicillin OR piperacillin OR ticarcillin OR carbenicillin OR mezlocillin OR cephalosporins OR cefazolin OR cephalexin OR cefuroxime OR cefotetan OR cefoxitin OR ceftriaxone OR cefotaxime OR ceftazidime OR cefepime OR cefazolin OR ceftobiprole OR cefoperazone OR carbapenems OR imipenem OR meropenem OR doripenem OR ertapenem OR monobactams OR aztreonam) [Words] and (random$ or blind$ or placebo$ or meta‐analys$) [Words]
Science Citation Index EXPANDED (1900 to March 2021) and Conference Proceedings Citation Index – Science (1990 to March 2021) (Web of Science)
#5 #4 AND #3 AND #2 AND #1
#4 TI=(random* or blind* or placebo* or meta‐analys* or trial*) OR TS=(random* or blind* or placebo* or meta‐analys*)
#3 TS=(antibiot* OR antimicrob* OR lactam* OR aminoglycoside* OR glycoprotein OR penicillin OR oxacillin OR cloxacillin OR dicloxacillin OR nafcillin OR methicillin OR ampicillin OR amoxicillin OR piperacillin OR ticarcillin OR carbenicillin OR mezlocillin OR cephalosporins OR cefazolin OR cephalexin OR cefuroxime OR cefotetan OR cefoxitin OR ceftriaxone OR cefotaxime OR ceftazidime OR cefepime OR cefazolin OR ceftobiprole OR cefoperazone OR carbapenems OR imipenem OR meropenem OR doripenem OR ertapenem OR monobactams OR aztreonam)
#2 TS=(((sepsis or septic* or infect* or bacter*) and (neonat* or neo nat* or newborn* or new born* or newly born*)) or (gram near negative))
#1 TS=(infan* or newborn or neonat* or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. 'Risk of bias' tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality of the included trials. For each included trial, we sought information regarding the method of randomisation, blinding, and reporting of all outcomes of all infants enrolled in the trial. We assessed each criterion as being at low, high, or unclear risk of bias. Three review authors independently assessed each study. We resolved any disagreement by discussion. We added this information to the 'Characteristics of included studies' table. We evaluated the following issues and entered the findings into the 'Risk of bias' table.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk, or unclear risk for participants; and
low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we reincluded missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Were reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk (where not all the study's prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified outcomes of interest and were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could have put it at risk of bias as:
low risk;
high risk;
unclear risk.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Cefazolin plus amikacin versus vancomycin plus amikacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.29, 1.66] |
| 1.2 Serious adverse events | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.29, 1.66] |
Comparison 2. Ticarcillin plus clavulanic acid versus flucloxacillin plus gentamicin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 All‐cause mortality | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 3.82] |
| 2.2 Serious adverse events | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 3.82] |
Comparison 3. Cloxacillin plus amikacin versus cefotaxime plus gentamicin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 All‐cause mortality | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.11, 1.27] |
| 3.2 Serious adverse events | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.17, 1.48] |
| 3.3 Circulatory support | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.17, 1.48] |
| 3.4 Nephrotoxicity | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.05] |
Comparison 4. Meropenem versus standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 All‐cause mortality | 1 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.56, 3.62] |
| 4.2 Serious adverse events | 1 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.90, 2.66] |
| 4.3 Neurological developmental impairment | 1 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.51, 1.48] |
| 4.4 Necrotising enterocolitis | 1 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.33, 1.42] |
Comparison 5. Vancomycin plus gentamicin versus vancomycin plus aztreonam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 All‐cause mortality | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.20, 2.13] |
| 5.2 Serious adverse events | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.20, 2.13] |
| 5.3 Necrotising enterocolitis | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.69 [0.74, 218.09] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ceriani 2014.
| Study characteristics | ||
| Methods | Design: randomised controlled trial, single centre Duration: 7 days; however, if pretreatment cultures were positive or there was a lack of clinical improvement, treatment continued to 10 days Date: March 2006 to August 2010 Location: La División de Neonatologia del Hospital Italiano de Buenos Aires, Argentina |
|
| Participants | 109 neonates aged > 3 days of life with suspected or confirmed neonatal sepsis Gender (boys/girls): not specified Age: not specified Diagnostic criteria: confirmed sepsis defined when, before a clinical picture compatible with sepsis, the blood culture or CSF were positive. Sepsis was most likely described when cultures were negative and the newborn presented clinical signs of sepsis and ≥ 2 of the following test results diagnostics: < 5000 white blood cells/mm³, < 1500 neutrophils/mm³, NI/NT ≥ 0.2, C‐reactive protein > 10 mg/L, and number of platelets < 100,000/mm³. Nosocomial or late‐onset sepsis was considered when the clinical signs of bacterial infection showed up after the 3rd day of life and even before discharge. Assessed by physicians to have neonatal sepsis and indicated to get vancomycin. Exclusion criteria: received vancomycin or other antibiotics prior to arrival or randomisation. |
|
| Interventions |
Intervention 1: cefazolin + amikacin Intervention 2: vancomycin + amikacin Antibiotics were administered intravenously at doses and intervals according to gestational and postnatal age (not specified). |
|
| Outcomes |
Primary outcomes
Follow‐up: 7‐10 days after end of treatment |
|
| Notes | In Spanish, translated with help from Spanish nurse Cindy Bustamante and Translated with www.DeepL.com/Translator. Funded by Carlos A Gia. Email: jose.ceriani@hospitalitaliano.org.ar |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computer program to perform sequence generation. |
| Allocation concealment (selection bias) | Low risk | Allocation details stored in opaque envelopes with sequential numbering. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not performed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Low risk | Reported all‐cause mortality. |
| Other bias | Low risk | Appeared free of other components that could have put it at risk of bias. |
Lutsar 2020.
| Study characteristics | ||
| Methods | Design: randomised clinical trial, multicentre Duration: 8–14 days to allocated treatment Date: September 2012 to November 2014 Location: 18 NICUs in Estonia, Greece, Italy, Lithuania, Spain, and Turkey |
|
| Participants | 272 neonates aged > 3 days of life with clinical or culture confirmed late‐onset neonatal sepsis Gender (boys/girls): 53% boys in both groups Age (median in days): 16 in both groups Diagnostic criteria: postnatal age between 72 hours and 90 days and clinical or culture confirmed late‐onset sepsis. Culture‐confirmed late‐onset sepsis defined as the presence of ≥ 1 positive culture from a normally sterile site and ≥ 1 abnormal clinical or laboratory parameter within the 24 hours prior to randomisation. If postmenstrual age > 44 weeks, the International Paediatric Sepsis Consensus Conference criteria had to be met (Goldstein 2005). For neonates with postmenstrual age < 44 weeks, the criteria defined by the European Medicines Agency Expert Meeting on Neonatal and Paediatric Sepsis were used (Oeser 2013) and the presence of ≥ 2 clinical and 2 laboratory parameters within the 24 hours prior to randomisation. Exclusion criteria: administration of any systemic antibiotics for > 24 hours within the 7 days prior to randomisation unless the change was driven by lack of efficacy, late‐onset sepsis caused by micro‐organisms suspected or known to be resistant to study antibiotics, severe congenital malformations if the baby was not expected to survive > 3 months, presence of renal failure or requirement of haemofiltration or peritoneal dialysis (or a combination) and known intolerance of study medication. |
|
| Interventions |
Intervention 1: meropenem 20 mg/kg 8 hourly with the exception of those with gestational age (GA) < 32 weeks or postnatal age < 2 weeks who received the same dose 12 hourly. Intervention 2: standard care (ampicillin + gentamicin or cefotaxime + gentamicin depending on site) |
|
| Outcomes |
Composite primary endpoint
Secondary outcomes
Resolution or improvement of clinical and laboratory parameters was evaluated by the study statistician using predefined algorithms. Clinical relapses were defined as recurrence of late‐onset sepsis together with initiation of a new course of antibiotic treatment, and microbiological relapse as an isolation of a phenotypically similar organism from a normally sterile site in a neonate with signs of infection. An adverse event was defined as any untoward medical occurrence or deviation of laboratory parameters of any causality in a neonate receiving study treatment. Follow‐up: 28 days after start of treatment |
|
| Notes | Email: Irja.lutsar@ut.ee | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation using a computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Randomisation list was kept confidential to trial team and sites received automated treatment assignment centrally via the e‐CRF. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not performed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 dropout in the standard care group. |
| Selective reporting (reporting bias) | Low risk | Followed the prepublished protocol, and reported mortality. |
| Other bias | Low risk | Appeared free of other components that could have put it at risk of bias. |
Miall‐Allen 1988.
| Study characteristics | ||
| Methods | Design: randomised controlled study Duration: maximum 10 days, but until 48 hours after participant were asymptomatic and afebrile Date: not specified Location: Hammersmith Hospital, London, UK |
|
| Participants | 28 neonates with suspected infection after 48 hours of age Gender (boys/girls): 13/15 Age (mean in days): 17.5 (intervention 1), 18.2 (intervention 2) Inclusion criteria: > 48 hours after birth with confirmed sepsis, signs highly suggestive of sepsis or who were at particular high risk of developing sepsis Exclusion criteria: administration of any systemic antibiotics in the 24 hours preceding entry to the trial |
|
| Interventions |
Intervention 1: ticarcillin + clavulanic acid 80 mg/kg 12 hourly or 8 hourly if neonate weighed > 2 kg Intervention 2: flucloxacillin 25 mg/kg 12 hourly + gentamicin 2.5 mg/kg 12 hourly |
|
| Outcomes |
Follow‐up: 4–6 weeks after end of treatment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 dropout in the ticarcillin + clavulanic acid group. The reason was clearly stated. |
| Selective reporting (reporting bias) | Low risk | Trial reported all‐cause mortality. |
| Other bias | Low risk | No other biases identified |
Millar 1992.
| Study characteristics | ||
| Methods | Design: randomised controlled study Duration: median duration of antibiotic treatment in both groups was 5 days Date: February 1989 and April 1990 Location: Peter Congdon Regional Neonatal Unit in Leeds, UK |
|
| Participants | 81 neonates with suspected sepsis after the first week of life Gender: not reported Age: not reported Inclusion criteria: neonates < 33 weeks' gestational age with episodes of suspected bacterial infection occurring after the 1st week of life. Diagnosis of suspected infection was made clinically and the clinical signs included apnoea, bradycardia, metabolic acidosis, hypotension, unstable temperature, and poor peripheral perfusion. Exclusion criteria: not reported |
|
| Interventions |
Intervention 1: intravenous vancomycin 22 mg/kg every 12 hours with gentamicin 3 mg/kg every 12 hours (41 neonates) Intervention 2: intravenous vancomycin 22 mg/kg every 12 hours with aztreonam 15 mg/kg every 12 hours (40 neonates) |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Low risk | Reported mortality. |
| Other bias | Low risk | No other biases identified. |
Ramasamy 2014.
| Study characteristics | ||
| Methods | Design: randomised controlled trial Duration: all neonates were given antibiotics for ≥ 10 days. Duration of antibiotics was extended if required depending on clinical response and repeat blood culture report Date: not described Location: extramural nursery of the Paediatrics Department, JIPMER, Pondicherry, a tertiary care teaching hospital in India |
|
| Participants | 90 neonates with suspected late‐onset neonatal sepsis Gender (boys/girls): 56/34 Age: not reported Inclusion criteria: neonates aged 3–28 days with the evidence of late‐onset sepsis by both clinical and laboratory parameters. The clinical parameters used were poor feeding, poor activity, seizures, apnoea, respiratory distress, umbilical discharge and abdominal distension, whereas the septic screen tests included microerythrocyte sedimentation rate, C‐reactive protein, absolute neutrophil count, and band cell count. Neonates with ≥ 1 of the above clinical parameters and 2 positive septic screen tests were included in the study. Exclusion criteria: babies with major congenital anomalies, extreme prematurity (< 28 weeks), very low birthweight (< 1500 g), congenital heart disease, severe asphyxia (5‐minute Apgar < 5), and who had received antibiotics before admission |
|
| Interventions |
Intervention 1: cefotaxime + gentamicin (50 neonates) Intervention 2: cloxacillin + amikacin (40 neonates) Antibiotics were administered intravenously but doses and intervals were not specified. |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Follow‐up: 2 weeks and 1 month after discharge |
|
| Notes | Email: drnbiswal@yahoo.com | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Allocations kept in sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as single‐blinded but it was unclear in what way. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as single‐blinded but it was unclear in what way. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Low risk | Reported all‐cause mortality. |
| Other bias | Low risk | No other biases identified. |
CSF: cerebrospinal fluid; DIC: disseminated intravascular coagulation; e‐CRF: electronic case report form; NICU: neonatal intensive care unit; NI/NT: neutrophil ratio immature on total neutrophils.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adelman 1987a | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Adelman 1987b | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Alinejad 2018 | Participants did not have late‐onset neonatal sepsis. |
| Aronoff 1984 | Did not included neonates with sepsis. |
| Auriti 2005 | Both groups received amoxicillin. |
| Baqui 2013 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Bassetti 1991 | Participants were adults. |
| Begue 1998 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Chartrand 1984 | Did not included neonates with sepsis. |
| Chowdhary 2006 | Both groups received the same antibiotics. |
| Collins 1998 | Participants did not have late‐onset neonatal sepsis. |
| De Louvois 1992 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Deville 2003 | Did not have late‐onset sepsis. |
| Ebrahim 1969 | Not a randomised controlled trial. |
| Faix 1988 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Feigin 1976 | Participants did not have late‐onset neonatal sepsis. |
| Fogel 1983 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Gathwala 2010 | Both groups received the same antibiotics. |
| Gokalp 1991 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Haffejee 1984 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Hall 1988 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Hammerberg 1989 | Only included participants with early‐onset neonatal sepsis. |
| Hansen 1980 | Both groups received ampicillin + gentamycin. |
| Jantausch 2003 | Did not have late‐onset sepsis. |
| Kaplan 2003 | Did not have late‐onset sepsis. |
| Langhendries 1993 | Both groups received the same antibiotic. |
| Lee 2005 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Lönnerholm 1982 | Participants were not suspected for sepsis, a severe infection or deep‐seated infection. |
| Marks 1978 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| McCracken 1976 | Both groups received the same antibiotic. |
| Metsvaht 2010 | Included only participants with early‐onset sepsis. |
| Mir 2017 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Molyneux 2017 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Mulubwa 2020 | Both groups received the same antibiotic. |
| Odio 1987 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Odio 1995 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Oral 1998 | Not a randomised controlled trial. |
| Rohatgi 2017 | Both groups received the same antibiotics. |
| Snelling 1983 | Included only participants with early‐onset sepsis. |
| Taheri 2011 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Tessin 1988 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Tessin 1989 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Tewari 2014 | Included only participants with early‐onset sepsis. |
| Tshefu 2015a | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Tshefu 2015b | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Umaña 1990 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Viganò 1995 | Did not have late‐onset sepsis. |
| Wells 1984 | Did not have late‐onset sepsis. |
| Wiese 1988 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
| Zaidi 2013 | Included a mix of early‐ and late‐onset neonatal sepsis. Unable to provide separate data for late‐onset. |
Differences between protocol and review
We decided to describe the antibiotic resistance occurring within the included trials towards the allocated antibiotic regimens narratively. We did this to further strengthen the review as recommended by Leibovici and colleagues (Leibovici 2016).
We decided to include two subgroups assessing the different inclusion criteria for sepsis (primarily for future updates).
Contributions of authors
SKK: conceived, designed, and drafted the review. He extracted, analysed and interpreted the data.
SS: extracted data, commented on, and revised the review.
CN: extracted data, commented on, and revised the review.
MG: provided general advice and revised the review.
GG: provided general advice and revised the review.
ULT: provided general advice and revised the review.
JCJ: conceived, designed, provided general advice, and revised the review. He analysed and interpreted the data.
All authors agreed on the final review version.
Sources of support
Internal sources
The Royal Prince Alfred Hospital Newborn Care, RPA Hospital, NSW, Australia
External sources
-
National Institute for Health Research (NIHR), UK
Editorial support for Cochrane Neonatal has been funded with funds from a UK NIHR Cochrane Programme Grant (16/114/03). The views expressed in this publication are those of the review authors and not necessarily those of the National Health Service, the NIHR, or the UK Department of Health.
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
The project received no funding.
SKK: none.
SS: none.
CN: none.
MG: none.
GG: none.
ULT: none.
JCJ: none.
New
References
References to studies included in this review
Ceriani 2014 {published data only}
- Ceriani Cernadas JM, Fernandez Jonusas S, Marquez M, Garsd A, Mariani G. Clinical outcome of neonates with nosocomial suspected sepsis treated with cefazolin or vancomycin: a non-inferiority, randomized, controlled trial. Archivos Argentinos de Pediatría 2014;112(4):308-14. [DOI: 10.5546/aap.2014.308] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lutsar 2020 {published data only}
- Lutsar I, Chazallon C, Trafojer U, Vincent MC, Cinzia A, Chiara B, et al, NeoMero Consortium. Meropenem vs standard of care for treatment of neonatal late onset sepsis (NeoMero1): a randomised controlled trial. Plos One 2020;15(3):e0229380. [DOI: 10.1371/journal.pone.0229380] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miall‐Allen 1988 {published data only}
- Miall-Allen VM, Whitelaw AG, Darrell JH. Ticarcillin plus clavulanic acid (Timentin) compared with standard antibiotic regimes in the treatment of early and late neonatal infections. British Journal of Clinical Practice 1988;42(7):273-9. [PMID: ] [PubMed] [Google Scholar]
Millar 1992 {published data only}
- Millar MR, MacKay P, Levene M, Langdale V, Martin C. Enterobacteriaceae and neonatal necrotising enterocolitis. Archives of Disease in Childhood 1992;67(1 Spec No):53-6. [DOI: 10.1136/adc.67.1_spec_no.53] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramasamy 2014 {published data only}
- Ramasamy S, Biswal N, Bethou A, Mathai B. Comparison of two empiric antibiotic regimen in late onset neonatal sepsis – a randomized controlled trial. Journal of Tropical Pediatrics 2014;60(1):83-6. [DOI: 10.1093/tropej/fmt080] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adelman 1987a {published data only}
- Adelman RD, Wirth F, Rubio T. A controlled study of the nephrotoxicity of mezlocillin and gentamicin plus ampicillin in the neonate. Journal of Pediatrics 1987;111(6 Pt 1):888-93. [DOI: 10.1016/s0022-3476(87)80212-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Adelman 1987b {published data only}
- Adelman RD, Wirth F, Rubio T. A controlled study of the nephrotoxicity of mezlocillin and amikacin in the neonate. American Journal of Diseases of Children 1987;141(11):1175-8. [DOI: 10.1001/archpedi.1987.04460110045019] [PMID: ] [DOI] [PubMed] [Google Scholar]
Alinejad 2018 {published data only}
- Alinejad S, Yousefichaijan P, Rezagholizamenjany M, Rafie Y, Kahbazi M, Arjmand A. Nephrotoxic effect of gentamicin and amikacin in neonates with Infection. Nephro-Urology Monthly 2018;10(2):e58580. [DOI: 10.5812/numonthly.58580] [DOI] [Google Scholar]
Aronoff 1984 {published data only}
- Aronoff SC, Reed MD, O'Brien CA, Blumer JL. Comparison of the efficacy and safety of ceftriaxone to ampicillin/chloramphenicol in the treatment of childhood meningitis. Journal of Antimicrobial Chemotherapy 1984;13(2):143-51. [DOI: 10.1093/jac/13.2.143] [PMID: ] [DOI] [PubMed] [Google Scholar]
Auriti 2005 {published data only}
- Auriti C, Rava L, Di Ciommo V, Ronchetti MP, Orzalesi M. Short antibiotic prophylaxis for bacterial infections in a neonatal intensive care unit: a randomized controlled trial. Journal of Hospital Infection 2005;59(4):292-8. [DOI: 10.1016/j.jhin.2004.09.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Baqui 2013 {published data only}
- Baqui AH, Saha SK, Ahmed A, Shahidullah M, Quasem I, Roth DE, et al. Safety and efficacy of simplified antibiotic regimens for outpatient treatment of serious infection in neonates and young infants 0-59 days of age in Bangladesh: design of a randomized controlled trial. Pediatric Infectious Disease Journal 2013;32(Suppl 1):S12-8. [DOI: 10.1097/INF.0b013e31829ff790] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bassetti 1991 {published data only}
- Bassetti D, Cruciani M, Solbiati M, Rubini F, Gandola L, Valenti G, et al. Comparative efficacy of ceftriaxone versus ceftazidime in the treatment of nosocomial lower respiratory tract infections. Chemotherapy 1991;37(5):371-5. [DOI: 10.1159/000238881] [PMID: ] [DOI] [PubMed] [Google Scholar]
Begue 1998 {published data only}
- Begue P, Astruc J, Francois P, Floret D. Comparison of ceftriaxone and cefotaxime in severe pediatric bacterial infections: a multicentrique study [Evaluation de la ceftriaxone et du cefotaxime dans l'infection bacterienne severe en pediatrie: etude multicentrique]. Medecine et Maladies Infectieuses 1998;28(4):300-6. [DOI: 10.1016/S0399-077X(98)80054-1] [DOI] [Google Scholar]
Chartrand 1984 {published data only}
- Chartrand SA, Marks MI, Scribner RK, Johnston JT, Frederick DF. Moxalactam therapy of haemophilus influenzae type B meningitis in children. Journal of Pediatrics 1984;104(3):454-9. [DOI: 10.1016/s0022-3476(84)81116-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chowdhary 2006 {published data only}
- Chowdhary G, Dutta S, Narang A. Randomized controlled trial of 7-day vs. 14-day antibiotics for neonatal sepsis. Journal of Tropical Pediatrics 2006;52(6):427-32. [DOI: 10.1093/tropej/fml054] [PMID: ] [DOI] [PubMed] [Google Scholar]
Collins 1998 {published data only}
- Collins MD, Dajani AS, Kim KS, King DR, Kaplan SL, Azimi PH, et al. Comparison of ampicillin/sulbactam plus aminoglycoside vs. ampicillin plus clindamycin plus aminoglycoside in the treatment of intraabdominal infections in children. The Multicenter Group. Pediatric Infectious Disease Journal 1998;17(3 Suppl):S15-8; discussion S20-1. [DOI: 10.1097/00006454-199803001-00005] [PMID: ] [DOI] [PubMed] [Google Scholar]
De Louvois 1992 {published data only}
- De Louvois J, Dagan R, Tessin I. A comparison of ceftazidime and aminoglycoside based regimens as empirical treatment in 1316 cases of suspected sepsis in the newborn. European Society for Paediatric Infectious Diseases – Neonatal Sepsis Study Group. European Journal of Pediatrics 1992;151(12):876-84. [DOI: 10.1007/BF01954122] [PMID: ] [DOI] [PubMed] [Google Scholar]
Deville 2003 {published data only}
- Deville JG, Adler S, Azimi PH, Jantausch BA, Morfin MR, Beltran S, et al. Linezolid versus vancomycin in the treatment of known or suspected resistant Gram-positive infections in neonates. Pediatric Infectious Disease Journal 2003;22(9 Suppl):S158-63. [DOI: 10.1097/01.inf.0000086955.93702.c7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ebrahim 1969 {published data only}
- Ebrahim GJ. Sepsis of the new-born (a therapeutic trial with gentamicin). East African Medical Journal 1969;46(1):30-3. [PMID: ] [PubMed] [Google Scholar]
Faix 1988 {published data only}
- Faix RG, Polley TZ, Grasela TH. A randomized, controlled trial of parenteral clindamycin in neonatal necrotizing enterocolitis. Journal of Pediatrics 1988;112(2):271-7. [DOI: 10.1016/s0022-3476(88)80069-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Feigin 1976 {published data only}
- Feigin RD, Stechenberg BW, Chang MJ, Dunkle LM, Wong ML, Palkes H, et al. Prospective evaluation of treatment of Hemophilus influenzae meningitis. Journal of Pediatrics 1976;88(4 Pt 1):542-8. [DOI] [PubMed] [Google Scholar]
Fogel 1983 {published data only}
- Fogel D, Farfel L, Miskin A, Mogilner BM. Comparison between the combination of azlocillin-gentamicin and ampicillin-gentamicin in the treatment of a nursery population. Israel Journal of Medical Sciences 1983;19(11):1009-15. [PMID: ] [PubMed] [Google Scholar]
Gathwala 2010 {published data only}
- Gathwala G, Sindwani A, Singh J, Choudhry O, Chaudhary U. Ten days vs. 14 days antibiotic therapy in culture-proven neonatal sepsis. Journal of Tropical Pediatrics 2010;56(6):433-5. [DOI: 10.1093/tropej/fmq012] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gokalp 1991 {published data only}
- Gokalp AS, Oguz A, Gultekin A, Icagasioglu D. Neonatal sepsis in Turkey: the comparison between penicillin plus aminoglycoside and ampicillin plus third-generation cephalosporin chemotherapies. Materia Medica Polona. Polish Journal of Medicine and Pharmacy 1991;23(3):226-28. [DOI: 10.1093/tropej/36.4.200] [PMID: ] [DOI] [PubMed] [Google Scholar]
Haffejee 1984 {published data only}
- Haffejee IE. A therapeutic trial of cefotaxime versus penicillin-gentamicin for severe infections in children. Journal of Antimicrobial Chemotherapy 1984;14(Suppl B):147-52. [DOI: 10.1093/jac/14.suppl_b.147] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hall 1988 {published data only}
- Hall MA, Ducker DA, Lowes JA, McMichael J, Clarke P, Rowe D, et al. A randomised prospective comparison of cefotaxime versus netilmicin/penicillin for treatment of suspected neonatal sepsis. Drugs 1988;35(Suppl 2):169-77. [DOI] [PubMed] [Google Scholar]
Hammerberg 1989 {published data only}
- Hammerberg O, Kurnitzki C, Watts J, Rosenbloom D. Randomized trial using piperacillin versus ampicillin and amikacin for treatment of premature neonates with risk factors for sepsis. European Journal of Clinical Microbiology & Infectious Diseases 1989;8(3):241-4. [DOI: 10.1007/BF01965268] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hansen 1980 {published data only}
- Hansen TN, Ritter DA, Speer ME, Kenny JD, Rudolph AJ. A randomized, controlled study of oral gentamicin in the treatment of neonatal necrotizing enterocolitis. Journal of Pediatrics 1980;97(5):836-39. [DOI: 10.1016/s0022-3476(80)80283-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jantausch 2003 {published data only}
- Jantausch BA, Deville J, Adler S, Morfin MR, Lopez P, Edge-Padbury B, et al. Linezolid for the treatment of children with bacteremia or nosocomial pneumonia caused by resistant Gram-positive bacterial pathogens. Pediatric Infectious Disease Journal 2003;22(9 Suppl):S164-71. [DOI: 10.1097/01.inf.0000086956.45566.55] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kaplan 2003 {published data only}
- Kaplan SL, Deville JG, Yogev R, Morfin MR, Wu E, Adler S, et al, Linezolid Pediatric Study Group. Linezolid versus vancomycin for treatment of resistant Gram-positive infections in children. Pediatric Infectious Disease Journal 2003;22(8):677-86. [DOI: 10.1097/01.inf.0000078160.29072.42] [PMID: ] [DOI] [PubMed] [Google Scholar]
Langhendries 1993 {published data only}
- Langhendries JP, Battisti O, Bertrand JM, François A, Darimont J, Tulkens PM, et al. Once daily administration of amikacin. Adaptation to neonatology for infants less than 3 days of postnatal age [Administration en dose unique journalière de l'amikacine. Adaptation à la néonatologie pour des enfants traités avant le 3ème jour d'âge postnatal]. Médecine et Maladies Infectieuses 1993;23(7):44-54. [DOI: 10.1016/S0399-077X(05)80984-9] [DOI] [Google Scholar]
Lee 2005 {published data only}
- Lee SJ, Park EA. Efficacy and safety of amoxicillin-sulbactam and ampicillin-sulbactam in full term neonates. Journal of the Korean Society of Neonatology 2005;12(1):17-24. [Google Scholar]
Lönnerholm 1982 {published data only}
- Lönnerholm G, Bengtsson S, Ewald U. Oral pivampicillin and amoxycillin in newborn infants. Scandinavian Journal of Infectious Diseases 1982;14(2):127-30. [DOI: 10.3109/inf.1982.14.issue-2.10] [PMID: ] [DOI] [PubMed] [Google Scholar]
Marks 1978 {published data only}
- Marks S, Marks MI, Dupont C, Hammerberg S. Evaluation of three antibiotic programs in newborn infants. Canadian Medical Association Journal 1978;118(6):659-62. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
McCracken 1976 {published data only}
- McCracken GH, Mize SG. A controlled study of intrathecal antibiotic therapy in Gram-negative enteric meningitis of infancy. Report of the Neonatal Meningitis Cooperative Study Group. Journal of Pediatrics 1976;89(1):66-72. [DOI: 10.1016/s0022-3476(76)80929-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Metsvaht 2010 {published data only}
- Metsvaht T, Ilmoja ML, Parm U, Maipuu L, Merila M, Lutsar I. Comparison of ampicillin plus gentamicin vs penicillin plus gentamicin in empiric treatment of neonates at risk of early onset sepsis. Acta Paediatrica, International Journal of Paediatrics 2010;99(5):665-72. [DOI: 10.1111/j.1651-2227.2010.01687.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Metsvaht T, Ilmoja ML, Parm U, Merila M, Maipuu L, Muursepp P, et al. Ampicillin versus penicillin in the empiric therapy of extremely low-birthweight neonates at risk of early onset sepsis. Pediatrics International 2011;53(6):873-80. [DOI: 10.1111/j.1442-200X.2011.03468.x] [PMID: 21895866] [DOI] [PubMed] [Google Scholar]
- Parm U, Metsvaht T, Sepp E, Ilmoja ML, Pisarev H, Pauskar M, et al. Impact of empiric antibiotic regimen on bowel colonization in neonates with suspected early onset sepsis. European Journal of Clinical Microbiology & Infectious Diseases 2010;29(7):807-16. [DOI: 10.1007/s10096-010-0931-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mir 2017 {published data only}
- Mir F, Nisar I, Tikmani SS, Baloch B, Shakoor S, Jehan F, et al. Simplified antibiotic regimens for treatment of clinical severe infection in the outpatient setting when referral is not possible for young infants in Pakistan (Simplified Antibiotic Therapy Trial [SATT]): a randomised, open-label, equivalence trial. Lancet. Global Health 2017;5(2):e177-85. [DOI: 10.1016/S2214-109X(16)30335-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Molyneux 2017 {published data only}
- Molyneux EM, Dube Q, Banda FM, Chiume M, Singini I, Mallewa M, et al. The treatment of possible severe infection in infants: an open randomized safety trial of parenteral benzylpenicillin and gentamicin versus ceftriaxone in Infants <60 days of age in Malawi. Pediatric Infectious Disease Journal 2017;36(12):e328-33. [DOI: 10.1097/INF.0000000000001576] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulubwa 2020 {published data only}
- Mulubwa M, Griesel HA, Mugabo P, Dippenaar R, Wyk L. Assessment of vancomycin pharmacokinetics and dose regimen optimisation in preterm neonates. Drugs in Research & Development 2020;20(2):105-13. [DOI: 10.1007/s40268-020-00302-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Odio 1987 {published data only}
- Odio CM, Umana MA, Saenz A, Salas JL, McCracken GH Jr. Comparative efficacy of ceftazidime vs carbenicillin and amikacin for treatment of neonatal septicemia. Pediatric Infectious Disease Journal 1987;6(4):371-7. [DOI: 10.1097/00006454-198704000-00006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Odio 1995 {published data only}
- Odio CM. Cefotaxime for treatment of neonatal sepsis and meningitis. Diagnostic Microbiology and Infectious Disease 1995;22(1-2):111-7. [DOI: 10.1016/0732-8893(95)00093-p] [PMID: ] [DOI] [PubMed] [Google Scholar]
Oral 1998 {published data only}
- Oral R, Akisü, M, Kültürsay N, Vardar F, Tansuğ N. Neonatal Klebsiella pneumonia sepsis and imipenem/cilastatin. Indian Journal of Pediatrics 1998;65(1):121-9. [DOI: 10.1007/BF02849703] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rohatgi 2017 {published data only}
- Rohatgi S, Dewan P, Faridi MM, Kumar A, Malhotra RK, Batra P. Seven versus 10 days antibiotic therapy for culture-proven neonatal sepsis: a randomised controlled trial. Journal of Paediatrics and Child Health 2017;53(6):556-62. [DOI: 10.1111/jpc.13518] [PMID: ] [DOI] [PubMed] [Google Scholar]
Snelling 1983 {published data only}
- Snelling S, Hart CA, Cooke RW. Ceftazidime or gentamicin plus benzylpenicillin in neonates less than forty-eight hours old. Journal of Antimicrobial Chemotherapy 1983;12(Suppl A):353-6. [DOI: 10.1093/jac/12.suppl_a.353] [PMID: ] [DOI] [PubMed] [Google Scholar]
Taheri 2011 {published data only}
- Taheri PA, Eslamieh H, Salamati P. Is ceftizoxime an appropriate surrogate for amikacin in neonatal sepsis treatment? A randomized clinical trial. Acta Medica Iranica 2011;49(8):499-503. [PMID: ] [PubMed] [Google Scholar]
Tessin 1988 {published data only}
- Tessin I, Thiringer K, Trollfors B, Brorson JE. Comparison of serum concentrations of ceftazidime and tobramycin in newborn infants. European Journal of Pediatrics 1988;147(4):405-7. [DOI: 10.1007/BF00496420] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tessin 1989 {published data only}
- Tessin I, Trollfors B, Thiringer K, Thörn Z, Larsson P. Concentrations of ceftazidime, tobramycin and ampicillin in the cerebrospinal fluid of newborn infants. European Journal of Pediatrics 1989;148(7):679-81. [DOI: 10.1007/BF00441533] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tewari 2014 {published data only}
- Tewari VV, Jain N. Monotherapy with amikacin or piperacillin-tazobactum empirically in neonates at risk for early-onset sepsis: a randomized controlled trial. Journal of Tropical Pediatrics 2014;60(4):297-302. [DOI: 10.1093/tropej/fmu017] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tshefu 2015a {published data only}
- African Neonatal Sepsis Trial (AFRINEST) group, Tshefu A, Lokangaka A, Ngaima S, Engmann C, Esamai F, Gisore P, et al. Simplified antibiotic regimens compared with injectable procaine benzylpenicillin plus gentamicin for treatment of neonates and young infants with clinical signs of possible serious bacterial infection when referral is not possible: a randomised, open-label, equivalence trial. Lancet 2015;385(9979):1767-76. [DOI: 10.1016/S0140-6736(14)62284-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tshefu 2015b {published data only}
- African Neonatal Sepsis Trial (AFRINEST) group, Tshefu A, Lokangaka A, Ngaima S, Engmann C, Esamai F, Gisore P, et al. Oral amoxicillin compared with injectable procaine benzylpenicillin plus gentamicin for treatment of neonates and young infants with fast breathing when referral is not possible: a randomised, open-label, equivalence trial. Lancet 2015;385(9979):1758-66. [DOI: 10.1016/S0140-6736(14)62285-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Umaña 1990 {published data only}
- Umaña MA, Odio CM, Castro E, Salas JL, McCracken GH Jr. Evaluation of aztreonam and ampicillin vs amikacin and ampicillin for treatment of neonatal bacterial infections. Pediatrics Infectious Disease Journal 1990;9(3):175-80. [DOI: 10.1097/00006454-199003000-00006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Viganò 1995 {published data only}
- Viganò A, Principi N. A randomised comparison of isepamicin and amikacin in the treatment of bacterial infections in paediatric patients. Journal of Chemotherapy 1995;7(Suppl 2):95-101. [PMID: ] [PubMed] [Google Scholar]
Wells 1984 {published data only}
- Wells TG, Trang JM, Brown AL, Marmer BC, Jacobs RF. Cefotaxime therapy of bacterial meningitis in children. Journal of Antimicrobial Chemotherapy 1984;14(Suppl B):181-9. [DOI: 10.1093/jac/14.suppl_b.181] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wiese 1988 {published data only}
- Wiese G. Treatment of neonatal sepsis with ceftriaxone/gentamicin and with azlocillin/gentamicin: a clinical comparison of efficacy and tolerability. Chemotherapy 1988;34(2):158-63. [DOI: 10.1159/000238564] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zaidi 2013 {published data only}
- Zaidi AK, Tikmani SS, Sultana S, Baloch B, Kazi M, Rehman H, et al. Simplified antibiotic regimens for the management of clinically diagnosed severe infections in newborns and young infants in first-level facilities in Karachi, Pakistan: study design for an outpatient randomized controlled equivalence trial. Pediatric Infectious Disease Journal 2013;32(Suppl 1):S19-25. [DOI: 10.1097/INF.0b013e31829ff7aa] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Allan 1985
- Allan JD, Moellering RC Jr. Management of infections caused by Gram-negative bacilli: the role of antimicrobial combinations. Review of Infectious Diseases 1985;7(Suppl 4):S559-71. [DOI: 10.1093/clinids/7.supplement_4.s559] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bakhuizen 2014
- Bakhuizen SE, Haan TR, Teune MJ, Wassenaer-Leemhuis AG, Heyden JL, Ham DP, et al. Meta-analysis shows that infants who have suffered neonatal sepsis face an increased risk of mortality and severe complications. Acta Paediatrica 2014;103(12):1211-8. [DOI: 10.1111/apa.12764] [PMID: ] [DOI] [PubMed] [Google Scholar]
Barbateskovic 2021
Bedford Russell 2015
- Bedford Russell AR, Kumar R. Early onset neonatal sepsis: diagnostic dilemmas and practical management. Archives of Disease in Childhood. Fetal and Neonatal Edition 2015;100(4):F350-4. [DOI: 10.1136/archdischild-2014-306193] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1-7. [DOI: 10.1097/00000658-197801000-00001] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benjamin 2006
- Benjamin DK Jr, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, et al, National Institute of Child Health and Human Development Neonatal Research Network. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 2006;117(1):84-92. [DOI: 10.1542/peds.2004-2292] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bérdy 2005
- Bérdy J. Bioactive microbial metabolites. Journal of Antibiotics 2005;58(1):1-26. [DOI: 10.1038/ja.2005.1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bhutta 1996
- Bhutta ZA. Enterobacter sepsis in the newborn – a growing problem in Karachi. Journal of Hospital Infection 1996;34(3):211-6. [DOI: 10.1016/s0195-6701(96)90068-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bizzarro 2005
- Bizzarro MJ, Raskind C, Baltimore RS, Gallagher PG. Seventy-five years of neonatal sepsis at Yale: 1928-2003. Pediatrics 2005;116(3):595-602. [DOI: 10.1542/peds.2005-0552] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bizzarro 2008
- Bizzarro MJ, Dembry LM, Baltimore RS, Gallagher PG. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics 2008;121(4):689-96. [DOI: 10.1542/peds.2007-2171] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bizzarro 2015
- Bizzarro MJ, Shabanova V, Baltimore RS, Dembry LM, Ehrenkranz RA, Gallagher PG. Neonatal sepsis 2004-2013: the rise and fall of coagulase-negative Staphylococci. Journal of Pediatrics 2015;166(5):1193-9. [DOI: 10.1016/j.jpeds.2015.02.009] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boghossian 2013
- Boghossian NS, Page GP, Bell EF, Stoll BJ, Murray JC, Cotten CM, et al, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Late-onset sepsis in very low birth weight infants from singleton and multiple-gestation births. Journal of Pediatrics 2013;162(6):1120-4, 1124.e1. [DOI: 10.1016/j.jpeds.2012.11.089] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Breurec 2016
- Breurec S, Bouchiat C, Sire JM, Moquet O, Bercion R, Cisse MF, et al. High third-generation cephalosporin resistant Enterobacteriaceae prevalence rate among neonatal infections in Dakar, Senegal. BMC Infectious Diseases 2016;16(1):587. [DOI: 10.1186/s12879-016-1935-y] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. Journal of Clinical Epidemiology 2008;61(8):763-9. [DOI: 10.1016/j.jclinepi.2007.10.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive – trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. International Journal of Epidemiology 2009;38(1):287-98. [DOI: 10.1093/ije/dyn188] [PMID: ] [DOI] [PubMed] [Google Scholar]
Camacho‐Gonzalez 2013
- Camacho-Gonzalez A, Spearman PW, Stoll BJ. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatric Clinics of North America 2013;60(2):367-89. [DOI: 10.1016/j.pcl.2012.12.003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cantey 2015
- Cantey J, Wozniak PS, Sánchez PJ. Prospective surveillance of antibiotic use in the neonatal intensive care unit: results from the SCOUT study. Pediatric Infectious Disease Journal 2015;34(3):267-72. [DOI: 10.1097/INF.0000000000000542] [PMID: ] [DOI] [PubMed] [Google Scholar]
Clark 2006a
- Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics 2006;117(1):67-74. [DOI: 10.1542/peds.2005-0179] [PMID: ] [DOI] [PubMed] [Google Scholar]
Clark 2006b
- Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics 2006;117(6):1979-87. [DOI: 10.1542/peds.2005-1707] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cohen 1992
- Cohen ML. Epidemiology of drug resistance: implications for a post-antimicrobial era. Science 1992;257(5073):1050-5. [DOI: 10.1126/science.257.5073.1050] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cordero 2003
- Cordero L, Ayers LW. Duration of empiric antibiotics for suspected early-onset sepsis in extremely low birth weight infants. Infection Control and Hospital Epidemiology 2003;24(9):662-6. [DOI: 10.1086/502270] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cortese 2016
- Cortese F, Scicchitano P, Gesualdo M, Filaninno A, De Giorgi E, Schettini F, et al. Early and late infections in newborns: where do we stand? A review. Pediatrics and Neonatology 2016;57(4):265-73. [DOI: 10.1016/j.pedneo.2015.09.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cotten 2006
- Cotten CM, McDonald S, Stoll B, Goldberg RN, Poole K, Benjamin DK Jr, National Institute for Child Health and Human Development Neonatal Research Network. The association of third-generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics 2006;118(2):717-22. [DOI: 10.1542/peds.2005-2677] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cotten 2009
- Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sánchez PJ, et al, NICHD Neonatal Research Network. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 2009;123(1):58-66. [DOI: 10.1542/peds.2007-3423] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Craft 2000
- Craft AP, Finer NN, Barrington KJ. Vancomycin for prophylaxis against sepsis in preterm neonates. Cochrane Database of Systematic Reviews 2000, Issue 1. Art. No: CD001971. [DOI: 10.1002/14651858.CD001971] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dagnew 2013
- Dagnew M, Yismaw G, Gizachew M, Gadisa A, Abebe T, Tadesse T, et al. Bacterial profile and antimicrobial susceptibility pattern in septicemia suspected patients attending Gondar University Hospital, Northwest Ethiopia. BMC Research Notes 2013;6:283. [DOI: 10.1186/1756-0500-6-283] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Demets 1987
- Demets DL. Methods of combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341-50. [DOI: 10.1002/sim.4780060325] [PMID: ] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI: 10.1016/0197-2456(86)90046-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dong 2015
- Dong Y, Speer CP. Late-onset neonatal sepsis: recent developments. Archives of Disease in Childhood. Fetal and Neonatal Edition 2015;100(3):F257-63. [DOI: 10.1136/archdischild-2014-306213] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Farber 1983
- Farber BF, Moellering RC Jr. Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrobial Agents and Chemotherapy 1983;23(1):138-41. [DOI: 10.1128/aac.23.1.138] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernando 2008
- Fernando AM, Heath PT, Menson EN. Antimicrobial policies in the neonatal units of the United Kingdom and Republic of Ireland. Journal of Antimicrobial Chemotherapy 2008;61(3):743-5. [DOI: 10.1093/jac/dkm543] [PMID: ] [DOI] [PubMed] [Google Scholar]
Filioti 2007
- Filioti J, Spiroglou K, Roilides E. Invasive candidiasis in pediatric intensive care patients: epidemiology, risk factors, management, and outcome. Intensive Care Medicine 2007;33(7):1272-83. [DOI: 10.1007/s00134-007-0672-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fisher 1922
- Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society 1922;85(1):87-94. [DOI: 10.2307/2340521] [DOI] [Google Scholar]
Foster 2006
- Foster KR, Grundmann H. Do we need to put society first? The potential for tragedy in antimicrobial resistance. PLoS Medicine 2006;3(2):e29. [DOI: 10.1371/journal.pmed.0030029] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillies 2015
- Gillies M, Ranakusuma A, Hoffmann T, Thorning S, McGuire T, Glasziou P, et al. Common harms from amoxicillin: a systematic review and meta-analysis of randomized placebo-controlled trials for any indication. Canadian Medical Association Journal 2015;187(1):E21-31. [DOI: 10.1503/cmaj.140848] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Golan 2011
- Golan DE, Tashlian AH, Armstrong EJ, Armstrong AW. Principles of Pharmacology. 3rd edition. Philadelphia (PA): Lippincott Williams & Wilkins, 2011. [Google Scholar]
Goldstein 2005
- Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatric Critical Care Medicine 2005;6(1):2-8. [DOI: 10.1097/01.PCC.0000149131.72248.E6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gordon 2005
- Gordon A, Jeffery HE. Antibiotic regimens for suspected late onset sepsis in newborn infants. Cochrane Database of Systematic Reviews 2005, Issue 3. Art. No: CD004501. [DOI: 10.1002/14651858.CD004501.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 13 December 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Hailemeskel 1999
- Hailemeskel B, Namanny M, Wutoh A. Frequency of nephrotoxicity with vancomycin and aminoglycoside antibiotic therapy. Hospital Pharmacy 1999;34(12):1417-20. [DOI: 10.1177/194512539903401209] [DOI] [Google Scholar]
Hammoud 2012
- Hammoud MS, Al-Taiar A, Thalib L, Al-Sweih N, Pathan S, Isaacs D. Incidence, aetiology and resistance of late-onset neonatal sepsis: a five-year prospective study. Journal of Paediatrics and Child Health 2012;48(7):604-9. [DOI: 10.1111/j.1440-1754.2012.02432.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI: 10.1002/sim.1186] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI: 10.1136/bmj.327.7414.557] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA: on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6. [DOI] [PMC free article] [PubMed]
Horbar 2017
- Horbar JD, Edwards EM, Greenberg LT, Morrow KA, Soll RF, Buus-Frank ME, et al. Variation in performance of neonatal intensive care units in the United States. JAMA Pediatrics 2017;171(3):e164396. [DOI] [PubMed] [Google Scholar]
Huth 2011
- Huth ME, Ricci AJ, Cheng AG. Mechanisms of aminoglycoside ototoxicity and targets of hair cell protection. International Journal of Otolaryngology 2011;2011:937861. [DOI: 10.1155/2011/937861] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huynh 2016
- Huynh BT, Padget M, Garin B, Delarocque-Astagneau E, Guillemot D, BIRDY study group. Bacterial neonatal sepsis and antibiotic resistance in low-income countries. Lancet 2016;387(10018):533-4. [DOI: 10.1016/S0140-6736(16)00220-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ibrahim 2000
- Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000;118(1):146-55. [DOI: 10.1378/chest.118.1.146] [PMID: ] [DOI] [PubMed] [Google Scholar]
ICH‐GCP 2015
- International Conference on Harmonisation-Good Clinical Practice. ICH harmonised guideline: integrated addendum to ICH E6(R1): Guideline for Good Clinical Practice E6(R2) ICH Consensus Guideline (ICH-GCP), 2015. ichgcp.net (accessed prior to 19 April 2021).
Isaacs 1996
- Isaacs D, Barfield C, Clothier T, Darlow B, Diplock R, Ehrlich J, et al. Late-onset infections of infants in neonatal units. Journal of Paediatrics and Child Health 1996;32(2):158-61. [ 10.1111/j.1440-1754.1996.tb00914.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Isaacs 1999
- Isaacs D, Royle JA. Intrapartum antibiotics and early onset neonatal sepsis caused by group B Streptococcus and by other organisms in Australia. Australasian Study Group for Neonatal Infections. Pediatric Infectious Disease Journal 1999;18(6):524-8. [DOI: 10.1097/00006454-199906000-00009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Isaacs 2003
- Isaacs D, Australasian Study Group for Neonatal Infections. A ten year, multicentre study of coagulase negative staphylococcal infections in Australasian neonatal units. Archives of Disease in Childhood. Fetal and Neonatal Edition 2003;88(2):F89-93. [DOI: 10.1136/fn.88.2.f89] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jackson 1971
- Jackson GG, Arcieri G. Ototoxicity of gentamicin in man: a survey and controlled analysis of clinical experience in the United States. Journal of Infectious Diseases 1971;124(Suppl):S130-7. [DOI: 10.1093/infdis/124.supplement_1.s130] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Medical Research Methodology 2014;14:120. [DOI: 10.1186/1471-2288-14-120] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kabwe 2016
- Kabwe M, Tembo J, Chilukutu L, Chilufya M, Ngulube F, Lukwesa C, et al. Etiology, antibiotic resistance and risk factors for neonatal sepsis in a large referral center in Zambia. Pediatric Infectious Disease Journal 2016;35(7):e191-8. [DOI: 10.1097/INF.0000000000001154] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kan 2016
- Kan B, Razzaghian HR, Lavoie PM. An immunological perspective on neonatal sepsis. Trends in Molecular Medicine 2016;22(4):290-302. [DOI: 10.1016/j.molmed.2016.02.001] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Katzung 2009
- Katzung BG, Masters SB, Trevor AJ. Basic and Clinical Pharmacology. 11th edition. New York (NY): McGraw-Hill Medical Publishing Division, 2009. [Google Scholar]
Khatua 1986
- Khatua SP, Das AK, Chatterjee BD, Khatua S, Ghose B, Saha A. Neonatal septicemia. Indian Journal of Pediatrics 1986;53(4):509-14. [DOI: 10.1007/BF02749537] [PMID: ] [DOI] [PubMed] [Google Scholar]
Klinger 2010
- Klinger G, Levy I, Sirota L, Boyko V, Lerner-Geva L, Reichman B, Israel Neonatal Network. Outcome of early-onset sepsis in a national cohort of very low birth weight infants. Pediatrics 2010;125(4):e736-40. [DOI: 10.1542/peds.2009-2017] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kumar 2016
- Kumar SK, Bhat BV. Distinct mechanisms of the newborn innate immunity. Immunology Letters 2016;173:42-54. [DOI: 10.1016/j.imlet.2016.03.009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kunin 1990
- Kunin CM, Johansen KS, Worning AM, Daschner FD. Report of a symposium on use and abuse of antibiotics worldwide. Reviews of Infectious Diseases 1990;12(1):12-9. [DOI: 10.1093/clinids/12.1.12] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kuppala 2011
- Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. Journal of Pediatrics 2011;159(5):720-5. [DOI: 10.1016/j.jpeds.2011.05.033] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawn 2005
- Lawn JE, Cousens S, Zupan J, Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? Where? Why? Lancet 2005;365(9462):891-900. [DOI: 10.1016/S0140-6736(05)71048-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Leal 2012
- Leal YA, Álvarez-Nemegyei J, Velázquez JR, Rosado-Quiab U, Diego-Rodríguez N, Paz-Baeza E, et al. Risk factors and prognosis for neonatal sepsis in southeastern Mexico: analysis of a four-year historic cohort follow-up. BMC Pregnancy and Childbirth 2012;12:48. [DOI: 10.1186/1471-2393-12-48] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leibovici 1998
- Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik SD. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. Journal of Internal Medicine 1998;244(5):379-86. [DOI: 10.1046/j.1365-2796.1998.00379.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Leibovici 2016
- Leibovici L, Paul M, Garner P, Sinclair DJ, Afshari A, Pace NL, et al. Addressing resistance to antibiotics in systematic reviews of antibiotic interventions. Journal of Antimicrobial Chemotherapy 2016;71(9):2367-9. [DOI: 10.1093/jac/dkw135] [PMID: ] [DOI] [PubMed] [Google Scholar]
Liu 2012
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al, Child Health Epidemiology Reference Group of WHO and UNICEF . Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012;379(9832):2151-61; Erratum in: Lancet 2012;380(9850):1308. [DOI: 10.1016/S0140-6736(12)60560-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Luck 2003
- Luck S, Torny M, d'Agapeyeff K, Pitt A, Heath P, Breathnach A, et al. Estimated early-onset group B streptococcal neonatal disease. Lancet 2003;361(9373):1953-4. [DOI: 10.1016/S0140-6736(03)13553-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Manan 2016
- Manan MM, Ibrahim NA, Aziz NA, Zulkifly HH, Al-Worafi YM, Long CM. Empirical use of antibiotic therapy in the prevention of early onset sepsis in neonates: a pilot study. Archives of Medical Science 2016;12(3):603-13. [DOI: 10.5114/aoms.2015.51208] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marchant 2013
- Marchant EA, Boyce GK, Sadarangani M, Lavoie PM. Neonatal sepsis due to coagulase-negative Staphylococci. Clinical & Developmental Immunology 2013;2013:586076. [DOI: 10.1155/2013/586076] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattie 1989
- Mattie H, Craig WA, Pechère JC. Determinants of efficacy and toxicity of aminoglycosides. Journal of Antimicrobial Chemotherapy 1989;24(3):281-93. [DOI: 10.1093/jac/24.3.281] [PMID: ] [DOI] [PubMed] [Google Scholar]
May 2005
- May M, Daley AJ, Donath S, Isaacs D, Australasian Study Group for Neonatal Infections. Early onset neonatal meningitis in Australia and New Zealand, 1992-2002. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90(4):F324-7. [DOI: 10.1136/adc.2004.066134] [PMID: 15878934 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGlone 2008
- McGlone A, Cranswick N. Evidence behind the WHO guidelines: hospital care for children: what is the evidence of safety of gentamicin use in children? Journal of Tropical Pediatrics 2008;54(5):291-3. [DOI: 10.1093/tropej/fmn059] [PMID: ] [DOI] [PubMed] [Google Scholar]
McGovern 2020
- McGovern M, Giannoni E, Kuester H, Turner MA, den Hoogen A, Bliss JM, et al. Challenges in developing a consensus definition of neonatal sepsis. Pediatric Research 2020;88(1):14-26. [DOI] [PubMed] [Google Scholar]
McGowan 1994
- McGowan JE Jr. Do intensive hospital antibiotic control programs prevent the spread of antibiotic resistance? Infection Control and Hospital Epidemiology 1994;15(7):478-83. [DOI: 10.1086/646954] [PMID: ] [DOI] [PubMed] [Google Scholar]
McWilliam 2017
- McWilliam SJ, Antoine DJ, Smyth RL, Pirmohamed M. Aminoglycoside-induced nephrotoxicity in children. Pediatric Nephrology 2017;32(11):2015-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milatovic 1987
- Milatovic D, Braveny I. Development of resistance during antibiotic therapy. European Journal of Clinical Microbiology 1987;6(3):234-44. [DOI: 10.1007/BF02017607] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mingeot‐Leclercq 1999
- Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: nephrotoxicity. Antimicrobial Agents and Chemotherapy 1999;43(5):1003-12. [DOI: 10.1128/AAC.43.5.1003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI: 10.1016/j.jclinepi.2009.06.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morris 2016
- Morris JM, Roberts CL, Bowen JR, Patterson JA, Bond DM, Algert CS, et al, PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet 2016;387(10017):444-52. [DOI: 10.1016/S0140-6736(15)00724-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mrvos 2013
- Mrvos R, Pummer TL, Krenzelok EP. Amoxicillin renal toxicity: how often does it occur? Pediatric Emergency Care 2013;29(5):641-3. [DOI: 10.1097/PEC.0b013e31828e9e78] [PMID: ] [DOI] [PubMed] [Google Scholar]
Muller‐Pebody 2011
- Muller-Pebody B, Johnson AP, Heath PT, Gilbert RE, Henderson KL, Sharland M, iCAP Group. Empirical treatment of neonatal sepsis: are the current guidelines adequate? Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(1):F4-8. [DOI: 10.1136/adc.2009.178483] [PMID: ] [DOI] [PubMed] [Google Scholar]
Murray 1994
- Murray BE. Can antibiotic resistance be controlled? New England Journal of Medicine 1994;330(17):1229-30. [DOI: 10.1056/NEJM199404283301710] [PMID: ] [DOI] [PubMed] [Google Scholar]
Musiime 2015
- Musiime GM, Seale AC, Moxon SG, Lawn JE. Risk of gentamicin toxicity in neonates treated for possible severe bacterial infection in low- and middle-income countries: systematic review. Tropical Medicine & International Health 2015;20(12):1593-606. [DOI: 10.1111/tmi.12608] [PMID: ] [DOI] [PubMed] [Google Scholar]
Oeser 2013
- Oeser C, Lutsar I, Metsvaht T, Turner MA, Heath PT, Sharland M. Clinical trials in neonatal sepsis. Journal of Antimicrobial Chemotherapy 2013;68(12):2733-45. [DOI: 10.1093/jac/dkt297] [PMID: ] [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529-34. [DOI: 10.1016/s0022-3476(78)80282-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Paul 2010
- Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrobial Agents and Chemotherapy 2010;54(11):4851-63. [DOI: 10.1128/AAC.00627-10] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Rogosch 2012
- Rogosch T, Kerzel S, Hoss K, Hoersch G, Zemlin C, Heckmann M, et al. IgA response in preterm neonates shows little evidence of antigen-driven selection. Journal of Immunology 2012;189(11):5449-56. [DOI: 10.4049/jimmunol.1103347] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubin 2002
- Rubin LG, Sánchez PJ, Siegel J, Levine G, Saiman L, Jarvis WR, Pediatric Prevention Network. Evaluation and treatment of neonates with suspected late onset sepsis: a survey of neonatologists' practices. Pediatrics 2002;110(4):e42. [DOI: 10.1542/peds.110.4.e42] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rybak 2009
- Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC Jr, Craig WA, Billeter M, et al. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2009;29(11):1275-9. [DOI: 10.1592/phco.29.11.1275] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sáez‐Llorens 2000
- Sáez-Llorens X, Castrejón de Wong MM, Castaño E, De Suman O, De Morös D, De Atencio I. Impact of an antibiotic restriction policy on hospital expenditures and bacterial susceptibilities: a lesson from a pediatric institution in a developing country. Pediatric Infectious Disease Journal 2000;19(3):200-6. [DOI: 10.1097/00006454-200003000-00005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schlapbach 2011
- Schlapbach LJ, Aebischer M, Adams M, Natalucci G, Bonhoeffer J, Latzin P, et al, Swiss Neonatal Network and Follow-Up Group. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics 2011;128(2):e348-57. [DOI: 10.1542/peds.2010-3338] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schuchat 2000
- Schuchat A, Zywicki SS, Dinsmoor MJ, Mercer B, Romaguera J, O'Sullivan MJ, et al. Risk factors and opportunities for prevention of early-onset neonatal sepsis: a multicenter case-control study. Pediatrics 2000;105(1 Pt 1):21-6. [DOI: 10.1542/peds.105.1.21] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schultze 1971
- Schultze RG, Winters RE, Kauffman H. Possible nephrotoxicity of gentamicin. Journal of Infectious Diseases 1971;124(Suppl):S145-7. [DOI: 10.1093/infdis/124.supplement_1.s145] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Seale 2015
- Seale AC, Obiero CW, Berkley JA. Rational development of guidelines for management of neonatal sepsis in developing countries. Current Opinion in Infectious Diseases 2015;28(3):225-30. [DOI: 10.1097/QCO.0000000000000163] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Selimoglu 2007
- Selimoglu E. Aminoglycoside-induced ototoxicity. Current Pharmaceutical Design 2007;13(1):119-26. [DOI: 10.2174/138161207779313731] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shah 2014
- Shah BA, Padbury JF. Neonatal sepsis: an old problem with new insights. Virulence 2014;5(1):170-8. [DOI: 10.4161/viru.26906] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shane 2013
- Shane AL, Stoll BJ. Recent developments and current issues in the epidemiology, diagnosis, and management of bacterial and fungal neonatal sepsis. American Journal of Perinatology 2013;30(2):131-41. [DOI: 10.1055/s-0032-1333413] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shane 2014
- Shane AL, Stoll BJ. Neonatal sepsis: progress towards improved outcomes. Journal of Infection 2014;68(Suppl 1):S24-32. [DOI: 10.1016/j.jinf.2013.09.011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sharma 2007
- Sharma R, Tepas JJ 3rd, Hudak ML, Mollitt DL, Wludyka PS, Teng RJ, et al. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. Journal of Pediatric Surgery 2007;42(3):454-61. [DOI: 10.1016/j.jpedsurg.2006.10.038] [PMID: ] [DOI] [PubMed] [Google Scholar]
Singer 2016
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315(8):801-10. [DOI: 10.1001/jama.2016.0287] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sorrell 1985
- Sorrell TC, Collignon PJ. A prospective study of adverse reactions associated with vancomycin therapy. Journal of Antimicrobial Chemotherapy 1985;16(2):235-41. [DOI: 10.1093/jac/16.2.235] [PMID: ] [DOI] [PubMed] [Google Scholar]
Spiliopoulou 2012
- Spiliopoulou A, Dimitriou G, Jelastopulu E, Giannakopoulos I, Anastassiou ED, Christofidou M. Neonatal intensive care unit candidemia: epidemiology, risk factors, outcome, and critical review of published case series. Mycopathologia 2012;173(4):219-28. [DOI: 10.1007/s11046-011-9498-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stockmann 2014
- Stockmann C, Spigarelli MG, Campbell SC, Constance JE, Courter JD, Thorell EA, et al. Considerations in the pharmacologic treatment and prevention of neonatal sepsis. Paediatric Drugs 2014;16(1):67-81. [DOI: 10.1007/s40272-013-0057-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stoll 1996
- Stoll BJ, Gordon T, Korones SB, Shankaran S, Tyson JE, Bauer CR, et al. Late-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. Journal of Pediatrics 1996;129(1):63-71. [DOI: 10.1016/s0022-3476(96)70191-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stoll 2002
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110(2 Pt 1):285-91. [DOI: 10.1542/peds.110.2.285] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stoll 2003
- Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Seminars in Perinatology 2003;27(4):293-301. [DOI: 10.1016/s0146-0005(03)00046-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stoll 2005
- Stoll BJ, Hansen NI, Higgins RD, Fanaroff AA, Duara S, Goldberg R, et al, National Institute of Child Health and Human Development. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of Gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002-2003. Pediatric Infectious Disease Journal 2005;24(7):635-9. [DOI: 10.1097/01.inf.0000168749.82105.64] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stoll 2010
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126(3):443-56. [DOI: 10.1542/peds.2009-2959] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoll 2011
- Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Meurs KP, et al, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics 2011;127(5):817-26. [DOI: 10.1542/peds.2010-2217] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoll 2015
- Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 2015;314(10):1039-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tallur 2000
- Tallur SS, Kasturi AV, Nadgir SD, Krishna BV. Clinico-bacteriological study of neonatal septicemia in Hubli. Indian Journal of Pediatrics 2000;67(3):169-74. [DOI: 10.1007/BF02723654] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tamma 2012
- Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with Gram-negative bacteria. Clinical Microbiology Reviews 2012;25(3):450-70. [DOI: 10.1128/CMR.05041-11] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tessin 1990
- Tessin I, Trollfors B, Thiringer K. Incidence and etiology of neonatal septicaemia and meningitis in western Sweden 1975-1986. Acta Paediatrica Scandinavica 1990;79(11):1023-30. [DOI: 10.1111/j.1651-2227.1990.tb11378.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? International Journal of Epidemiology 2009;38(1):276-86. [DOI: 10.1093/ije/dyn179] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA), 2011. ctu.dk/tsa/files/tsa_manual.pdf (accessed 7 January 2015).
Tripathi 2012
- Tripathi N, Cotten CM, Smith PB. Antibiotic use and misuse in the neonatal intensive care unit. Clinics in Perinatology 2012;39(1):61-8. [DOI: 10.1016/j.clp.2011.12.003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tröger 2014
- Tröger B, Göpel W, Faust K, Müller T, Jorch G, Felderhoff-Müser U, et al, German Neonatal Network. Risk for late-onset blood-culture proven sepsis in very-low-birth weight infants born small for gestational age: a large multicenter study from the German Neonatal Network. Pediatric Infectious Disease Journal 2014;33(3):238-43. [DOI: 10.1097/INF.0000000000000031] [PMID: ] [DOI] [PubMed] [Google Scholar]
TSA 2011
- Copenhagen Trial Unit. TSA – Trial Sequential Analysis, 2011. ctu.dk/tsa/ (accessed 7 January 2015).
Tsai 2014
- Tsai MH, Hsu JF, Chu SM, Lien R, Huang HR, Chiang MC, et al. Incidence, clinical characteristics and risk factors for adverse outcome in neonates with late-onset sepsis. Pediatric Infectious Disease Journal 2014;33(1):e7-13. [DOI: 10.1097/INF.0b013e3182a72ee0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vergnano 2005
- Vergnano S, Sharland M, Kazembe P, Mwansambo C, Heath PT. Neonatal sepsis: an international perspective. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90(3):F220-4. [DOI: 10.1136/adc.2002.022863] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vergnano 2011
- Vergnano S, Menson E, Kennea N, Embleton N, Russell AB, Watts T, et al. Neonatal infections in England: the NeonIN surveillance network. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(1):F9-14. [DOI: 10.1136/adc.2009.178798] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vesikari 1985
- Vesikari T, Janas M, Grönroos P, Tuppurainen N, Renlund M, Kero P, et al. Neonatal septicaemia. Archives of Disease in Childhood 1985;60(6):542-6. [DOI: 10.1136/adc.60.6.542] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Volpe 2008
- Volpe JJ. Intracranial hemorrhage: germinal matrix intraventricular hemorrhage. In: Neurology of the Newborn. 5th edition. Philadelphia (PA): Saunders Elsevier, 2008:517-88. [Google Scholar]
Waksman 1947
- Waksman SA. What is an antibiotic or an antibiotic substance? Mycologia 1947;39(5):565-9. [DOI: 10.1056/NEJM194712042372302.] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walker 2011
- Walker JC, Smolders MA, Gemen EF, Antonius TA, Leuvenink J, Vries E. Development of lymphocyte subpopulations in preterm infants. Scandinavian Journal of Immunology 2011;73(1):53-8. [DOI: 10.1111/j.1365-3083.2010.02473.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wargo 2014
- Wargo KA, Edwards JD. Aminoglycoside-induced nephrotoxicity. Journal of Pharmacy Practice 2014;27(6):573-7. [DOI: 10.1177/0897190014546836] [PMID: ] [DOI] [PubMed] [Google Scholar]
Weinstein 2003
- Weinstein MP. Blood culture contamination: persisting problems and partial progress. Journal of Clinical Microbiology 2003;41(6):2275-8. [DOI: 10.1128/jcm.41.6.2275-2278.2003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weston 2011
- Weston EJ, Pondo T, Lewis MM, Martell-Cleary P, Morin C, Jewell B, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005-2008. Pediatric Infectious Disease Journal 2011;30(11):937-41. [DOI: 10.1097/INF.0b013e318223bad2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. Journal of Clinical Epidemiology 2008;61(1):64-75. [DOI: 10.1016/j.jclinepi.2007.03.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Medical Research Methodology 2009;9:86. [DOI: 10.1186/1471-2288-9-86] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Medical Research Methodology 2017;17(1):39. [DOI: 10.1186/s12874-017-0315-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1999
- World Health Organization Young Infants Study Group. Bacterial etiology of serious infections in young infants in developing countries: results of a multicenter study. Pediatric Infectious Disease Journal 1999;18(10 Suppl):S17-22. [DOI: 10.1097/00006454-199910001-00004] [PMID: ] [DOI] [PubMed] [Google Scholar]
WHO 2013
- World Health Organization. Pocket book of hospital care for children: second edition. Guidelines for the management of common childhood illnesses. apps.who.int/iris/bitstream/10665/81170/1/9789241548373_eng.pdf?ua=1 (accessed 16 February 2018).
World Bank 2017
- World Bank. World Bank country and lending groups. datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed 9 December 2019).
Wynn 2014
- Wynn JL, Wong HR, Shanley TP, Bizzarro MJ, Saiman L, Polin RA. Time for a neonatal-specific consensus definition for sepsis. Pediatric Critical Care Medicine 2014;15(6):523-8. [DOI: 10.1097/PCC.0000000000000157] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wynn 2016
- Wynn JL. Defining neonatal sepsis. Current Opinion in Pediatrics 2016;28(2):135-40. [DOI: 10.1097/MOP.0000000000000315] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ygberg 2012
- Ygberg S, Nilsson A. The developing immune system – from foetus to toddler. Acta Paediatrica 2012;101(2):120-7. [DOI: 10.1111/j.1651-2227.2011.02494.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zaidi 2005
- Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. Hospital-acquired neonatal infections in developing countries. Lancet 2005;365(9465):1175-88. [DOI: 10.1016/S0140-6736(05)71881-X] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zaidi 2009
- Zaidi AK, Thaver D, Ali SA, Khan TA. Pathogens associated with sepsis in newborns and young infants in developing countries. Pediatric Infectious Disease Journal 2009;28(1 Suppl):S10-8. [DOI: 10.1097/INF.0b013e3181958769] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zea‐Vera 2015
- Zea-Vera A, Ochoa TJ. Challenges in the diagnosis and management of neonatal sepsis. Journal of Tropical Pediatrics 2015;61(1):1-13. [DOI: 10.1093/tropej/fmu079] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zemlin 2007
- Zemlin M, Hoersch G, Zemlin C, Pohl-Schickinger A, Hummel M, Berek C, et al. The postnatal maturation of the immunoglobulin heavy chain IgG repertoire in human preterm neonates is slower than in term neonates. Journal of Immunology 2007;178(2):1180-8. [DOI: 10.4049/jimmunol.178.2.1180] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Korang 2021
- Korang SK, Safi S, Gupta M, Greisen G, Lausten-Thomsen U, Jakobsen JC. Antibiotic regimens for late-onset neonatal sepsis. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013836. [DOI: 10.1002/14651858.CD013836] [DOI] [PMC free article] [PubMed] [Google Scholar]


