Abstract
Background
Endometriosis is a common gynaecological condition affecting 10% to 15% of reproductive‐age women and may cause dyspareunia, dysmenorrhoea, and infertility. One treatment strategy is combining surgery and medical therapy to reduce the recurrence of endometriosis. Though the combination of surgery and medical therapy appears to be beneficial, there is a lack of clarity about the appropriate timing of when medical therapy should be used in relation with surgery, that is, before, after, or both before and after surgery, to maximize treatment response.
Objectives
To determine the effectiveness of medical therapies for hormonal suppression before, after, or both before and after surgery for endometriosis for improving painful symptoms, reducing disease recurrence, and increasing pregnancy rates.
Search methods
We searched the Cochrane Gynaecology and Fertility (CGF) Group trials register, CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL, and two trials registers in November 2019 together with reference checking and contact with study authors and experts in the field to identify additional studies.
Selection criteria
We included randomized controlled trials (RCTs) which compared medical therapies for hormonal suppression before, after, or before and after, therapeutic surgery for endometriosis.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias. Where possible, we combined data using risk ratio (RR), standardized mean difference or mean difference (MD) and 95% confidence intervals (CI). Primary outcomes were: painful symptoms of endometriosis as measured by a visual analogue scale (VAS) of pain, other validated scales or dichotomous outcomes; and recurrence of disease as evidenced by EEC (Endoscopic Endometriosis Classification), rAFS (revised American Fertility Society), or rASRM (revised American Society for Reproductive Medicine) scores at second‐look laparoscopy.
Main results
We included 25 trials with 3378 women with endometriosis. We used the term "surgery alone" to refer to placebo or no medical therapy.
Presurgical medical therapy compared with placebo or no medical therapy
Compared to surgery alone, we are uncertain if presurgical medical hormonal suppression reduces pain recurrence at 12 months or less (dichotomous) (RR 1.10, 95% CI 0.72 to 1.66; 1 RCT, n = 262; very low‐quality evidence) or whether it reduces disease recurrence at 12 months – total (AFS score) (MD –9.6, 95% CI –11.42 to –7.78; 1 RCT, n = 80; very low‐quality evidence).
We are uncertain if presurgical medical hormonal suppression decreases disease recurrence at 12 months or less (EEC stage) compared to surgery alone (RR 1.11, 95% CI 0.86 to 1.43; 1 RCT, n = 262; very low‐quality evidence). We are uncertain if presurgical medical hormonal suppression improves pregnancy rates compared to surgery alone (RR 1.18, 95% CI 0.97 to 1.45; 1 RCT, n = 262; very low‐quality evidence). No trials reported pelvic pain at 12 months or less (continuous) or disease recurrence at 12 months or less.
Postsurgical medical therapy compared with placebo or no medical therapy
We are uncertain about the improvement observed in pelvic pain at 12 months or less (continuous) between postsurgical medical hormonal suppression and surgery alone (SMD ‐0.79, 95% CI ‐1.02 to ‐0.56; 3 RCTs, n = 340; I2 = 91%; very low‐quality evidence).
Compared to surgery alone, postsurgical medical therapy may decrease pain recurrence at 12 months or less (dichotomous) (RR 0.70, 95% CI 0.52 to 0.94; 5 RCTs, n = 657; I2 = 0%; low‐quality evidence).
We are uncertain if postsurgical medical hormonal suppression improves disease recurrence at 12 months – total (AFS score) compared to surgery alone (MD –2.29, 95% CI –4.01 to –0.57; 1 RCT, n = 51; very low‐quality evidence).
Disease recurrence at 12 months or less may be reduced with postsurgical medical hormonal suppression compared to surgery alone (RR 0.30, 95% CI 0.17 to 0.54; 4 RCTs, n = 433; I2 = 58%; low‐quality evidence).
We are uncertain if postsurgical medical hormonal suppression improves disease recurrence at 12 months or less (EEC stage) (RR 0.88, 95% CI 0.67 to 1.15; 1 RCT, n = 285; very low‐quality evidence).
Pregnancy rate is probably increased with postsurgical medical hormonal suppression compared to surgery alone (RR 1.19, 95% CI 1.02 to 1.38; 11 RCTs, n = 955; I2 = 27%; moderate‐quality evidence).
Pre‐ and postsurgical medical therapy compared with surgery alone or surgery and placebo
There were no trials identified in the search for this comparison.
Presurgical medical therapy compared with postsurgical medical therapy
We are uncertain about the difference in pain recurrence at 12 months or less (dichotomous) between postsurgical and presurgical medical hormonal suppression therapy (RR 1.40, 95% CI 0.95 to 2.07; 2 RCTs, n = 326; I2 = 2%; low‐quality evidence).
We are uncertain about the difference in disease recurrence at 12 months or less (EEC stage) between postsurgical and presurgical medical hormonal suppression therapy (RR 1.26, 95% CI 0.97 to 1.65; 1 RCT, n = 273; very low‐quality evidence).
We are uncertain about the difference in pregnancy rate between postsurgical and presurgical medical hormonal suppression therapy (RR 1.08, 95% CI 0.90 to 1.30; 1 RCT, n = 273; very low‐quality evidence).
No trials reported pelvic pain at 12 months or less (continuous), disease recurrence at 12 months – total (AFS score) or disease recurrence at 12 months or less (dichotomous).
Postsurgical medical therapy compared with pre‐ and postsurgical medical therapy
There were no trials identified in the search for this comparison.
Serious adverse effects for medical therapies reviewed
There was insufficient evidence to reach a conclusion regarding serious adverse effects, as no studies reported data suitable for analysis.
Authors' conclusions
Our results indicate that the data about the efficacy of medical therapy for endometriosis are inconclusive, related to the timing of hormonal suppression therapy relative to surgery for endometriosis. In our various comparisons of the timing of hormonal suppression therapy, women who receive postsurgical medical therapy compared with no medical therapy or placebo may experience benefit in terms of pain recurrence, disease recurrence, and pregnancy. There is insufficient evidence regarding hormonal suppression therapy at other time points in relation to surgery for women with endometriosis.
Plain language summary
Pre‐ and postsurgical medical therapy for endometriosis surgery
Review question
What are the effects of medical hormonal suppression therapies administered before or after (or both) surgical treatment of endometriosis compared to surgery alone or medical therapy before or after (or both) surgery?
Background
In endometriosis, tissue like the lining of the womb starts to grow in other places, such as the ovaries and fallopian tubes. It affects 10% to 15% of reproductive‐age women, and may cause pain in the lower tummy (pelvic pain) or back (which usually worsen during a woman's periods), painful sexual intercourse, and difficulty becoming pregnant.
Treatment to lower the levels of reproductive hormones (called medical hormonal suppression therapy) is common to reduce the size of endometrial tissue along with surgery to cut it away. Medical therapy can reduce pain or its reappearance, reduce disease recurrence (the chance of it coming back), and improve pregnancy rate. Potential benefits of medication may depend on whether it is given before or after surgery for endometriosis, but evidence is not clear.
Study characteristics
We found 25 randomized controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) with 3378 women who underwent surgery with or without medical therapy. We used the term "surgery alone" to refer to placebo or no medical therapy. The evidence is current to November 2019.
Key results
Medical therapy showed variable effects on pain, reappearance of pain or disease, and pregnancy rate when used before or after surgery for endometriosis. However, for outcomes disease recurrence and pregnancy, it may be most effective after surgery versus surgery alone compared to other comparisons reviewed.
Medical therapy before surgery compared with placebo or no medical therapy
Very weak evidence suggests that if pelvic pain recurrence at 12 months or less is 24% among women having surgery alone, the chance with medical therapy before surgery would be between 17% and 40%.
Very weak evidence suggests that if disease recurrence at 12 months or less is 45% among women having surgery alone, the chance with medical therapy before surgery would be between 39% and 65%.
Very weak evidence suggests that if pregnancy rate is 58% among women having surgery alone, the chance with medical therapy before surgery would be between 53% and 79%.
Medical therapy after surgery compared with placebo or no medical therapy
Weak evidence suggests that if pain recurrence at 12 months or less is 26% among women having surgery alone, the chance with medical therapy after surgery would be between 13% and 24%.
Weak evidence suggests that if disease recurrence at 12 months or less is 17% among women having surgery alone, the chance with medical therapy after surgery would be between 3% and 9%.
Very weak evidence suggests that if disease recurrence at 12 months or less (different classification used) is 45% among women having surgery alone, the chance with medical therapy after surgery would be between 30% and 52%.
Moderate‐quality evidence suggests that if pregnancy rate is 34% among women having surgery alone, the chance with medical therapy after surgery would be between 35% and 48%.
Medical therapy before surgery compared with medical therapy after surgery
Weak evidence suggests that if pelvic pain recurrence at 12 months or less is 20% among women having medical therapy after surgery, the chance with medical therapy before surgery would be between 19% and 41%.
Very weak evidence suggests that if disease recurrence at 12 months or less is 40% among women having medical therapy after surgery, the chance with medical therapy before surgery would be between 39% and 66%.
Very weak evidence suggests that if pregnancy rate is 60% among women having medical therapy after surgery, the chance with medical therapy before surgery would be between 54% and 78%.
Quality of the evidence
The evidence was of very low to moderate quality.
Summary of findings
Summary of findings 1. Presurgical medical therapy compared with placebo or no medical therapy.
| Presurgical medical therapy compared with placebo or no medical therapy for endometriosis | ||||||
|
Patient or population: women having endometriosis surgery Intervention: presurgical medical therapy Comparison: placebo or no medical therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no medical therapy | Presurgical medical therapies | |||||
|
Pain (continuous) Pelvic pain ≤ 12 months |
No trials reported on this outcome | |||||
|
Pain recurrence (dichotomous) Pain recurrence ≤ 12 months |
241 per 1000 | 265 per 1000 (174 to 400) | RR 1.10 (0.72 to 1.66) | 262 (1 study) | ⊕⊝⊝⊝ Verylowa,b | — |
| Disease recurrence at 3 months – total (AFS score) | The mean recurrence – AFS score – total AFS in the control groups was 44.1 | The mean recurrence – AFS score – total AFS in the intervention groups was 9.6 lower (11.42 to 7.78 lower) | — | 80 (1 study) | ⊕⊝⊝⊝ Verylowa,b | — |
| Disease recurrence ≤ 12 months (dichotomous) | No trials reported this outcome. | |||||
| Disease recurrence ≤ 12 months (EEC stage) | 453 per 1000 |
502 per 1000 (389 to 647) |
RR 1.11 (0.86 to 1.43) | 262 (1 study) | ⊕⊝⊝⊝ Verylowa,b | — |
| Pregnancy rate (dichotomous) | 547 per 1000 | 646 per 1000 (531 to 794) | RR 1.18 (0.97 to 1.45) | 262 (1 study) |
⊕⊝⊝⊝ Verylowa,b | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AFS: American Fertility Society; CI: confidence interval; EEC: Endoscopic Endometriosis Classification; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded once for serious risk of bias – no blinding and trial lacked details on allocation concealment. bDowngraded twice for very serious imprecision – evidence based on a single trial, wide confidence interval, small number of events.
Summary of findings 2. Postsurgical medical therapy compared with placebo or no medical therapy.
| Postsurgical medical therapy compared with placebo or no medical therapy for endometriosis | ||||||
|
Patient or population: women having endometriosis surgery Intervention: postsurgical medical therapy Comparison: placebo or no medical therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no medical therapy | Postsurgical medical therapies | |||||
|
Pain (continuous) Pelvic pain ≤ 12 months |
The mean pelvic pain score ≤ 12 months in the ranged across control groups from 0.35 to 7 | The mean pelvic pain score ≤ 12 months in the intervention groups was 0.79 lower (1.02 to 0.56 lower) | — | 340 (3 studies) |
⊕⊝⊝⊝ Very lowa,b | — |
|
Pain recurrence (dichotomous) Pain recurrence ≤ 12 months |
255 per 1000 |
178 per 1000 (132 to 239) |
RR 0.70 (0.52 to 0.94) | 657 (5 studies) | ⊕⊕⊝⊝ Lowa,c | |
| Disease recurrence at 12 months – total (AFS score) | The mean recurrence – AFS score – total AFS in the control groups was 3.1 | The mean recurrence – AFS score – total AFS in the intervention groups was 2.29 lower (4.01 to 0.57 lower) | — | 53 (1 study) | ⊕⊝⊝⊝ Very lowd,e | — |
| Disease recurrence ≤ 12 months (dichotomous) | 171 per 1000 |
51 per 1000 (29 to 92) |
RR 0.30 (0.17 to 0.54) | 433 (4 studies) |
⊕⊕⊝⊝ Lowa,f | — |
|
Disease recurrence ≤ 12 months (EEC stage) |
453 per 1000 |
398 per 1000 (303 to 520) |
RR 0.88 (0.67 to 1.15) | 285 (1 study) |
⊕⊝⊝⊝ Verylowa,d | — |
| Pregnancy rate (dichotomous) | 344 per 1000 |
409 per 1000 (351 to 475) |
RR 1.19 (1.02 to 1.38) | 955 (11 studies) |
⊕⊕⊕⊝ Moderatea | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AFS: American Fertility Society; CI: confidence interval; EEC: Endoscopic Endometriosis Classification; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded once for risk of bias – there are inadequate details on blinding and attrition. bDowngraded twice for very serious inconsistency – considerable heterogeneity. cDowngraded once for imprecision – small number of events
dDowngraded twice for very serious imprecision – small number of events/evidence is based on a single study. eDowngraded once for risk of bias – the trial lacked details on allocation concealment and randomization.. fDowngraded once for inconsistency – considerable heterogeneity.
Summary of findings 3. Presurgical medical therapy compared with postsurgical medical therapy.
| Presurgical medical therapy compared with postsurgical medical therapy for endometriosis | ||||||
|
Patient or population: women having endometriosis surgery Intervention: presurgical medical therapy Comparison: postsurgical medical therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Postsurgical medical therapy | Presurgical medical therapy | |||||
|
Pain (continuous) Pelvic pain ≤ 12 months |
No trials reported on this outcome | |||||
|
Pain recurrence (dichotomous) Pain recurrence ≤ 12 months |
199 per 1000 |
279 per 1000 (189 to 412) |
RR 1.40 (0.95 to 2.07) | 326 (2 studies) | ⊕⊕⊝⊝ Lowa,b | — |
| Disease recurrence at 12 months –total (AFS score) | No trials reported on this outcome | |||||
| Disease recurrence ≤ 12 months (dichotomous) | No trials reported on this outcome | |||||
| Disease recurrence ≤ 12 months (EEC stage) | 399 per 1000 |
502 per 1000 (387 to 658) |
RR 1.26 (0.97 to 1.65) | 273 (1 study) |
⊕⊝⊝⊝ Very lowa,b | — |
| Pregnancy rate (dichotomous) | 601 per 1000 |
649 per 1000 (541 to 782) |
RR 1.08 (0.90 to 1.30) | 273 (1 study) |
⊕⊝⊝⊝ Very lowa,b | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AFS: American Fertility Society; CI: confidence interval; EEC: Endoscopic Endometriosis Classification; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded once for serious risk of bias – no blinding and trial lacked details on allocation concealment. bDowngraded twice for very serious imprecision – evidence based on a single trial, wide confidence interval, and small number of events.
Background
Description of the condition
Endometriosis is a chronic inflammatory condition characterized by the presence of endometrial glands and stroma outside of the uterine cavity and diagnosed by surgery (Leyland 2010; Acien 2013). It affects 10% to 15% of reproductive‐age women (Macer 2012; Missmer 2003), and may cause dyspareunia, dysmenorrhoea, and infertility. As 30% to 50% of women with endometriosis may have difficulty conceiving (Macer 2012), more women with endometriosis are achieving pregnancy through assisted reproductive technology (ART) (Stephansson 2009).
Pathogenesis of endometriosis remain poorly understood and has been attributed to retrograde menstruation implantation, Mullerian remnant abnormalities, coelomic metaplasia, angiogenetic/lymphogenetic spread, metaplasia theory, and genetic/epigenetic theory (Koninckx 2019; Vercellini 2014). Clinical examination has low sensitivity and specificity for diagnosis of endometriosis, and laparoscopy remain the gold standard for diagnosis; however, recent studies looks promising for new sonographic and magnetic resonance imaging (MRI) techniques (Bazot 2017).
A number of classification systems for endometriosis have been developed, with the revised American Society for Reproductive Medicine (r‐ASRM) classification being the best‐known, originally developed in 1985 and revised in 1997 (ASRM 1997; Johnson 2017). However, classification systems have been criticized by women and care providers due to their poor correlation with disease symptoms and predictive prognosis (Johnson 2017).
Description of the intervention
As the presentation for endometriosis encompass two diverse spectra (i.e. pain and infertility), the choice of management strategies, whether medical or surgical, are made accordingly. For the treatment of pain, the choice between two alternatives is influenced by presence or absence of large endometriomas, ureteral/bowel stenosis, and desire for spontaneous pregnancy (Vercellini 2014).
Effect of surgery on pain is usually satisfactory but mostly temporary (Vercellini 2014), and has been reserved for women in whom medical therapy has failed or women with ovarian endometriomas greater than 3 cm in diameter (or both) (Leyland 2010). Laparoscopic treatment for minimal or mild endometriosis improves pregnancy; however, it's controversial for deeply infiltrating endometriosis.
Over the time, there has been increasing interest in combining medical and surgical therapy to reduce recurrence of endometriosis. The presurgical use of progestin, GnRHas, or danazol (an androgen receptor agonist) may decrease the extent of endometriosis and the size of endometriomas (ovarian endometriosis) making complete removal of endometriosis easier during laparoscopic surgery and increasing subsequent pregnancy rates (Bedaiwy 2017; Donnez 1987; Donnez 2004; Hemmings 1998). However, possible disadvantages of presurgical medical therapy, especially with danazol or GnRHas, are the adverse effects associated with these medications (e.g. hot flushes and vaginal dryness), which may influence women's willingness to use the therapy, and result only in a delay of surgery. However, benefits include inducing suppression of lesions that cannot be surgically removed, and reducing the risk of recurrence of endometriosis as a result of surgery (Kettel 1989; Thomas 1992). Similarly, postsurgical medical therapy to prevent recurrence of endometriomas has been recommended and is gaining popularity (Vercellini 2013). But there is hardly any information about comparison between presurgical and postsurgical medical therapy for the treatment of endometriosis.
How the intervention might work
For medical management of pain associated with endometriosis, combined hormonal contraceptive or progestins (e.g. dienogest) are recommended as first‐line therapy, while gonadotropin‐releasing hormone agonists (GnRHa) (with hormone therapy (HT) add back to control adverse events) or levonorgestrel‐releasing intrauterine system (LNG‐IUS) are recommended as second‐line therapeutic options (Bedaiwy 2017; Leyland 2010). Regarding mechanism of action suppression of endogenous oestrogen production is important for the successful treatment of endometriosis‐associated pain. Suppression of ovulation by hormonal contraceptives will in turn induce amenorrhoea, thereby creating a relatively hypo‐oestrogenic environment that will inhibit ectopic endometrial growth and prevent disease progression. Studies have also shown that progestins have both an anovulatory and an antiproliferative effect, while inhibiting the secretion of cytokines in the stroma of endometrial cells. Thus, inhibiting the growth of endometriotic tissue by inducing decidualization followed by atrophy of the endometriotic implants. Prolonged treatment with GnRHa leads to downregulation of the pituitary gonadotropin‐releasing hormone (GnRH) receptor with a subsequent decrease in pituitary secretion of luteinizing hormone (LH) and follicle‐stimulating hormone (FSH). This will in turn suppress ovarian follicular growth and ovulation, resulting in very low levels of circulating oestradiol and progesterone. Within one month of GnRH use, the circulating oestradiol concentrations will be in the menopausal range. Like dienogest, GnRHa may have direct effects on the endometrium and endometriotic implants (Bedaiwy 2017). GnRHa suppression with HT add‐back before in vitro fertilization (IVF) is also associated with improved pregnancy rate (Leyland 2010). However, we consider that LNG‐IUSs do not meet the inclusion requirement for systemic hormonal suppression, and, therefore, excluded them.
While presurgical medical therapy is generally used to treat existing endometriosis lesions, postsurgical medical therapy is generally used to prevent recurrence of endometriosis after surgical removal. Whether the hormonal medical therapy is used before, after or both before and after the surgery it is expected to improve the outcome, as compared to surgery alone.
Why it is important to do this review
A large number of studies and reviews compared various medical therapies for the treatment of endometriosis, with or without surgery and have clarified advantages and disadvantages in terms of efficacy, adverse events, and cost (Brown 2014; Vercellini 2014). Though the combination of surgery and medical therapy appears to be beneficial, the evidence is not conclusive (Somigliana 2017). Furthermore, there is a lack of clarity regarding when the medical therapy should be used in relation to surgery (i.e. before, after, or both before and after the surgery), in order to maximize response to therapy. The evidence about combined use of surgery and medication needs critical review, with a special focus on timing of initiating use of medical therapy in relation to surgery. It is necessary to evaluate the benefits and consider the harms prior to recommending any specific combination strategy. This review aimed to evaluate the use of medical therapy before, after, or both before and after surgery for endometriosis.
Objectives
To determine the effectiveness of medical therapies for hormonal suppression before, after, or both before and after surgery for endometriosis for improving painful symptoms, reducing disease recurrence, and increasing pregnancy rates.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials (RCT) where medical therapy for hormonal suppression of endometriosis was used before or after (or both) conservative surgery for endometriosis. We excluded quasi‐randomized trials.
Types of participants
The study population included women of reproductive age (no age restriction included in this review) who underwent therapeutic surgery for endometriosis. We excluded studies that did not clarify whether a therapeutic procedure was performed or not during laparoscopy/laparotomy.
All surgical procedures for the treatment of endometriosis that conserved the pelvic organs (such as ovarian cystectomy, drainage of endometriosis, excision, or ablation of endometriosis). We excluded women undergoing hysterectomy.
The diagnosis of endometriosis could have been made provisionally by clinical examination or ultrasound (or both) and confirmed during the therapeutic surgery, or could have been surgically confirmed endometriosis from prior surgery.
Women in at least one arm of trial would have medical therapy either before or after surgery.
Types of interventions
-
All systemic medical therapies for the hormonal suppression of endometriosis including GnRHas, danazol, progestogens, gestrinone, or the oral contraceptive pill (OCP) (or combinations of these) administered before or after (or both) surgery for endometriosis for the following comparisons:
presurgical medical therapy compared with placebo or no medical therapy;
postsurgical medical therapy compared with placebo or no medical therapy;
pre‐ and postsurgical medical therapy compared with placebo or no medical therapy;
presurgical medical therapy compared with postsurgical medical therapy;
postsurgical medical therapy compared with pre‐ and postsurgical medical therapy.
We used the term "surgery alone" to refer to placebo or no medical therapy.
The use of medical therapy was considered at any dosage and for a period of at least three months before or after surgery.
Only agents used with the aim of hormonal suppression were included (except for add‐back HTs to minimise adverse effects of primary hormonal agents).
We excluded LNG‐IUD as it is non‐systemic.
We excluded medical therapy with analgesics, anti‐inflammatory drugs, or antibiotics.
We excluded alternative, dietary, or complementary therapeutic strategies.
Types of outcome measures
We compared the effectiveness of the use and timing of medical therapy as an adjunct to surgery for endometriosis to surgery alone (placebo or no medical therapy).
Primary outcomes
Painful symptoms of endometriosis (including pelvic pain, dyspareunia, dysmenorrhea, pain recurrence) as measured by a visual analogue scale (VAS) of pain, other validated scales, or dichotomous outcomes.
Recurrence of disease as evidenced by EEC (Endoscopic Endometriosis Classification), rAFS (revised American Fertility Society), or rASRM scores at second‐look laparoscopy.
Secondary outcomes
Pregnancy rate per woman.
Ease of surgery, duration of surgery, postsurgical complications.
Levels of satisfaction of women.
Adverse effects (proportion of women with one or more reported adverse effects associated with medical therapy).
Search methods for identification of studies
Reports that described or might have described RCTs of hormonal suppression in the treatment of endometriosis before or after surgery were obtained from the following databases in consultation with Cochrane Gynaecology and Fertility (CGF) group information specialist.
Electronic searches
We searched the following electronic bibliographic databases, trial registers, and websites:
The CGF Group Specialized Register of Controlled Trials; ProCite platform (searched 20 November 2019; Appendix 1);
Cochrane Central Register of Controlled Trials; Ovid (CENTRAL; 2019, Issue 10) (Appendix 2);
MEDLINE – Epub ahead of print, In‐process & Other non‐indexed citations; Ovid platform (searched from 1946 to 20 November 2019; Appendix 3);
Embase; Ovid platform (searched from 1980 to 20 November 2019; Appendix 4);
PsycINFO; Ovid platform (searched from 1806 to 20 November 2019; Appendix 5);
CINAHL (Cumulative Index to Nursing and Allied Health Literature); Ebsco platform; (searched from 1961 to 20 November 2019; Appendix 6).
We combined the MEDLINE with the Cochrane highly sensitive search strategy for identifying RCTs, from Chapter 6 of the Cochrane Handbook of Systematic Reviews of Interventions (Lefebvre 2011). We combined the Embase searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (www.sign.ac.uk/what-we-do/methodology/search-filters/).
Searching other resources
Other electronic sources of trials included the following.
Trial registers for ongoing and registered trials: ClinicalTrials.gov (clinicaltrials.gov) and the World Health Organization International Trials Registry Platform search portal (apps.who.int/trialsearch/).
PubMed and Google Scholar, for recent trials not yet indexed in the major databases.
Reference lists and bibliographies of all relevant articles to identify additional trials for inclusion in this review.
We sent letters to experts within the field, pharmaceutical companies producing the products being reviewed, and authors of unpublished abstracts to identify unpublished trials of medical therapy before or after surgery for endometriosis.
We applied no language or date restrictions to the searches. We included a PRISMA flow chart to present the results of the search and the process of screening and selecting studies for inclusion in the review.
Data collection and analysis
Selection of studies
Two review authors (AC and VV) independently selected trials for inclusion using Covidence. We screened the titles and abstracts and discarded studies that were clearly ineligible, with an aim to be overly inclusive rather than risk losing relevant studies. We obtained full‐text articles. Both review authors independently assessed whether the studies met the inclusion criteria. We resolved disagreements by discussion between the review authors and lead author (IC). We sought further information from the authors where papers contained insufficient information to make a decision regarding eligibility. The selection process has been documented with a PRISMA flow chart (Figure 1).
1.
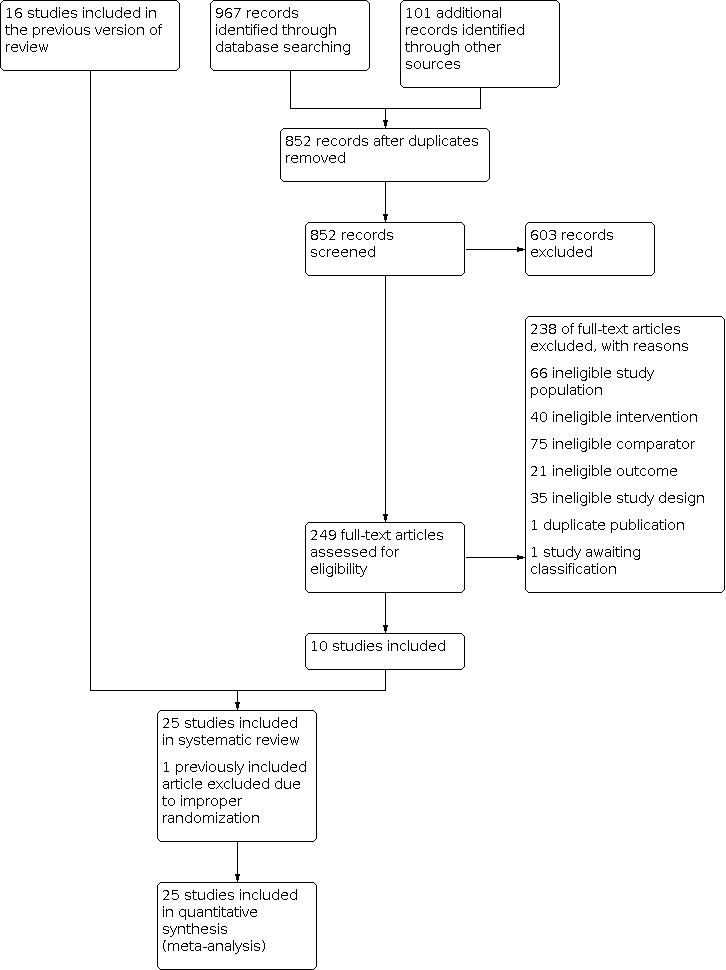
PRISMA study flow diagram.
Data extraction and management
Four review authors (AC, VV, AM and AZ) worked in pairs to extract data from eligible studies using a data extraction form designed and pilot‐tested by the authors. Two review authors extracted data for each study and resolved all disagreements by discussion. Data extracted included study characteristics and outcome data (see data extraction table for details in Appendix 7). We corresponded with study authors for further information on methods and results, as required.
Assessment of risk of bias in included studies
Four review authors (AC, VV, AM and AZ) working in pairs assessed risk of bias using the Cochrane 'Risk of bias' tool (Higgins 2011). Seven domains for each included study evaluated random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, risk of selective outcome reporting, and risk of other potential sources of bias (Appendix 8). Two review authors assessed each study and assigned judgements as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Section 8.5; Higgins 2011). We resolved disagreements by discussion among review authors and lead author (IC). All judgements are presented in the 'Risk of bias' table within the Characteristics of included studies table, which was later incorporated into the interpretation of review findings by means of sensitivity analysis.
We sought additional information on trial methodology or original trial data from the principal authors of trials that appeared to meet the eligibility criteria but were unclear in aspects of methodology or outcomes, or where the data were in a form unsuitable for meta‐analysis. In the process, our team made attempt to contact twelve authors to obtain additional information about their study methods to clarify some of the aspects affecting assessment of risk of bias.
Measures of treatment effect
For dichotomous data (e.g. pain recurrence), we used the numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel risk ratios (RRs) with 95% confidence intervals (CI). For continuous data (e.g. pain by VAS), if all studies reported the same outcomes using the same scales, we calculated mean differences (MDs) between treatment groups and 95% CI. If studies had used different scale, we planned to use standardized mean differences (SMDs) with 95% CIs.
We reversed the direction of effect of individual studies, if required, to ensure consistency across trials. We treated ordinal data (e.g. distribution of EEC stage) as dichotomous data.
We assessed whether the estimates calculated in the review for individual studies were compatible in each case with the estimates reported in the study publications.
Unit of analysis issues
The primary analysis was per woman randomized to treatment. We did not include reported data that were based on a different unit of analysis (e.g. per endometrioma cyst) in the meta‐analyses, but summarized them in an additional table.
Dealing with missing data
We analyzed data on an intention‐to‐treat basis where possible, and attempted to contact authors to obtain missing data. Where studies reported data by type of medical therapy, we combined these treatment groups and compared them to placebo or no medical therapy using MD and the standard deviation for continuous outcomes. Where the mean and standard deviation for the combined groups was not reported. we estimated it using the formulae described in Table 7.7a in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity using the I2 statistic. An I2 statistic greater than 50% indicated substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data.
Data synthesis
We performed statistical analysis in accordance with the guidelines for statistical analysis developed by the Cochrane using Review Manager 5 (Review Manager 2014).
If the studies were sufficiently similar, we used a fixed‐effect analysis. Where possible, we pooled the outcomes statistically. For dichotomous data (e.g. proportion of women with pain recurrence at 12 months), we expressed results for each study as RR with 95% CI and combined for meta‐analysis with Review Manager 5 software using the Mantel‐Haenszel method (Review Manager 2014).
For continuous outcomes (e.g. multidimensional pain scores), we combined means and standard deviations for each group in the meta‐analysis as MD or SMD and 95% CIs.
Comparison was organized for following study hypotheses:
presurgical medical therapy compared with placebo or no medical therapy;
postsurgical medical therapy compared with placebo or no medical therapy;
pre‐ and postsurgical medical therapy compared with placebo or no medical therapy;
presurgical medical therapy compared with postsurgical medical therapy;
postsurgical medical therapy compared with pre and postsurgical medical therapy.
Within each hypothesis, comparison were ordered for pain (VAS, 36‐item Short Form (SF‐36) pain score, pain recurrence, disease recurrence (continuous, dichotomous, EEC stage), and pregnancy rate (where data were available).
Subgroup analysis and investigation of heterogeneity
A priori, it was planned to look at the possible contribution of differences in trial design, medical therapy used, timing of therapy, dosage, mode of administration, and duration of therapy to any heterogeneity identified.
Sensitivity analysis
We conducted sensitivity analyses where there were sufficient trials included, in order to determine whether the conclusions were robust (i.e. whether conclusions would have differed if the inclusion of trials was restricted to those with low risk of bias). We performed this sensitivity analysis for the primary outcomes only. We also performed a sensitivity analysis comparing outcomes based on random‐effects model.
Overall quality of the body of evidence: 'Summary of findings' tables
We prepared 'Summary of findings' tables using GRADEpro and Cochrane methods (GRADEpro GDT; Higgins 2011). These tables evaluated the overall quality of the body of evidence for the main review outcomes for the main review comparisons. The main review outcomes were Pelvic pain at 12 months or less (continuous), Pain recurrence at 12 months or less (dichotomous), disease recurrence at 3 months ‐ Total (AFS score ‐ continuous), disease recurrence at 12 months or less (dichotomous), disease recurrence at 12 months or less (EEC stage‐ dichotomous), and pregnancy rate (dichotomous). The main review comparisons were:
presurgical medical therapy compared with placebo or no medical therapy;
postsurgical medical therapy compared with placebo or no medical therapy;
pre‐ and postsurgical medical therapy compared with placebo or no medical therapy;
presurgical medical therapy compared with postsurgical medical therapy;
postsurgical medical therapy compared with pre‐ and postsurgical medical therapy.
We assessed the quality of the evidence using GRADE working group criteria: risk of bias, consistency of effect, imprecision, indirectness, and publication bias. Two review authors independently made judgements about evidence quality (high, moderate, low, or very low), with disagreements resolved by discussion. Judgements were justified, documented, and incorporated into reporting of results for each outcome. We first extracted study data, formatted our comparisons in data tables, and prepared 'Summary of findings' tables before writing the results and conclusions of our review.
Results
Description of studies
Results of the search
The search retrieved 1084 articles, which included 16 articles included in previous version of the review (Furness 2004). We excluded one of the previously included articles because of the improper randomization procedure used in allocation of intervention (Batioglu 1997). Among the remaining newly retrieved 1068 articles, after removing the duplicates, 852 were left for analysis. A total of 265 articles were potentially eligible and were retrieved in full text. Upon closer examination, 238 articles did not meet the inclusion criteria due to ineligible study design, ineligible intervention, ineligible comparator, ineligible outcome, ineligible study population, or duplicate publication (Figure 1). Twenty‐five studies finally met our inclusion criteria, for both systematic review and meta‐analysis, including 3378 women (Alkatout 2013; Angioni 2015; Audebert 1998; Bianchi 1999; Busacca 2001; Cucinella 2013; Donnez 1994; Hornstein 1997; Huang 2018; Loverro 2001; Loverro 2008; Muzii 2000; Parazzini 1994; Rickes 2002; Seracchioli 2010a; Seracchioli 2010b; Sesti 2007; Sesti 2009; Shaw 2001; Tanmahasamut 2017; Telimaa 1987; Tsai 2004; Vercellini 1999; Yang 2006; Yang 2018). Fifteen were already included in the previous version of this review (Furness 2004). See Characteristics of included studies and Characteristics of excluded studies tables. We attempted to contact four authors to obtain additional information about the data presented in published paper; however only one responded with minimal additional information, which we included in the review. One study is awaiting classification (Roghaei 2010). We identified no ongoing studies.
Included studies
Study design and settings
We included 25 RCTs.
The original search identified 11 trials that met the inclusion criteria (Audebert 1998; Batioglu 1997; Bianchi 1999; Busacca 2001; Donnez 1994; Hornstein 1997; Loverro 2001; Muzii 2000; Parazzini 1994; Telimaa 1987; Vercellini 1999). The first updated search (in September 2010) identified a further six trials which met the inclusion criteria (Loverro 2008; Sesti 2007; Shaw 2001; Shawki 2002; Tsai 2004; Yang 2006). One trial was published only as an abstract, with insufficient information available to include it in this review (Shawki 2002). We attempted to contact the author to obtain additional information without success. This study was then listed under studies awaiting classification, in previous versions and has now been excluded from this review. One study was included in the previous review, but excluded in this updated version of the review, due to quasi‐randomization of this study (using odd and even numbers) (Batioglu 1997). This updated review identified 10 trials that met the inclusion criteria (Alkatout 2013; Angioni 2015; Cucinella 2013; Huang 2018; Rickes 2002; Seracchioli 2010a; Seracchioli 2010b; Sesti 2009; Tanmahasamut 2017; Yang 2018). Shawki 2002 was still not published, and, therefore, excluded from the recent updated review.
Of the 25 trials now included in this review, 13 were conducted in Italy (Angioni 2015; Bianchi 1999; Busacca 2001; Cucinella 2013; Loverro 2001; Loverro 2008; Muzii 2000; Parazzini 1994; Seracchioli 2010a; Seracchioli 2010b; Sesti 2007; Sesti 2009; Vercellini 1999); three in China (Huang 2018; Yang 2006; Yang 2018); two in Germany (Alkatout 2013; Rickes 2002); and one each in Belgium (Donnez 1994), Finland (Telimaa 1987), France (Audebert 1998), Taiwan (Tsai 2004), Thailand (Tanmahasamut 2017), UK/Republic of Ireland (Shaw 2001), and USA (Hornstein 1997).
Five reports declared pharmaceutical support for their studies (Audebert 1998; Hornstein 1997; Parazzini 1994; Shaw 2001; Vercellini 1999), while two had independent funding respectively from Fonds de la Recherche Scientifique (Donnez 1994) and Research and Science Foundation Farmos Ltd, Turku (Telimaa 1987). Yang 2006 received funding from the Natural Science Foundation of Heilongjiang Province. The remainder did not describe any form of funding or support.
Participants
The trials included 3378 women who underwent conservative therapeutic surgery for endometriosis, and were randomly allocated to pre‐ or postsurgical (or both) medical therapy or placebo or surgery alone, depending on the study's protocol. The ages of included women ranged from 18 to 50 years.
Interventions
Medical therapy included GnRHas (goserelin, leuprorelin, nafarelin, triptorelin), danazol, letrozole, progestogen (gestrinone, medroxyprogesterone acetate), and the combined OCP. Of the 25 studies included in this review, six mentioned the effects of the combined OCP (Cucinella 2013; Muzii 2000; Seracchioli 2010a; Seracchioli 2010b; Sesti 2007; Sesti 2009), two of which also mentioned the effect of triptorelin (Sesti 2007; Sesti 2009). Other participants were randomly allocated to medical therapy which included GnRHas (goserelin, leuprorelin, nafarelin, triptorelin) (Alkatout 2013; Angioni 2015; Audebert 1998; Busacca 2001; Donnez 1994; Hornstein 1997; Huang 2018; Loverro 2001; Loverro 2008; Parazzini 1994; Rickes 2002; Shaw 2001; Tsai 2004; Vercellini 1999; Yang 2018), danazol (Bianchi 1999; Telimaa 1987; Tsai 2004), or progestogen (gestrinone, desogestrel, medroxyprogesterone acetate) (Tanmahasamut 2017; Telimaa 1987; Yang 2006).
Presurgical medical therapy compared with placebo or no medical therapy
Three trials compared presurgical medical therapy for endometriosis to surgery alone (no medical therapy) (Alkatout 2013; Donnez 1994; Shaw 2001).
Alkatout 2013 compared three groups, with different timing of medical or surgical treatment. The study comprised 450 participants, aged 18 to 44 years, with symptomatic endometriosis. The authors compared two consecutive laparoscopic surgeries. Each group consisted of 150 participants, randomly allocated. For the analysis of presurgical medical therapy compared to surgery alone, we used only two groups in this review, namely medical therapy with subcutaneous leuprorelin acetate depot injected monthly for three months before surgery (group 1) and surgical treatment without hormonal postsurgical therapy (group 2). The authors performed a second‐look laparoscopy one to two months after conclusion of the HT in group 1 and five months after the first laparoscopy in group 2. They staged endometriosis according to the EEC, and obtained data regarding pregnancy rate and recurrence of pain symptoms.
Donnez 1994 included 80 women with infertility who were aged less than 35 years with laparoscopically confirmed ovarian endometriotic cysts, which were drained and flushed out laparoscopically. They randomized participants to receive a subcutaneous goserelin implant four‐weekly for 12 weeks or no treatment. Twelve weeks after the first‐look laparoscopy, they performed another laparoscopy during which a biopsy was done, and endometriosis and cyst wall vaporized. The same two observers used AFS scoring.
Shaw 2001 randomized 48 women aged 18 to 50 years who had been referred for management of symptoms or infertility due to endometrioma. After the cysts were aspirated, women received either goserelin four‐weekly for three months or no medical therapy. Following an ultrasound measurement of the residual cysts, women underwent definitive excision and were then followed for a further six months. Outcomes included size of endometrioma presurgery, proportion of participants who had complete excision of cysts, AFS scores, and recurrence of cysts measured by ultrasound at six months.
Postsurgical medical therapy compared with placebo or no medical therapy
Twenty‐two studies assessed postsurgical medical therapy for endometriosis. Seven compared postsurgical medical therapy to placebo (Hornstein 1997; Loverro 2008; Parazzini 1994; Sesti 2007; Sesti 2009; Tanmahasamut 2017; Telimaa 1987). Different forms of hormonal medication were compared to placebo, namely GnRHas, danazol, letrozole, OCP, and progestogen. The remaining 15 trials received the control group surgery alone with no medical therapy (Alkatout 2013; Angioni 2015; Bianchi 1999; Busacca 2001; Cucinella 2013; Huang 2018; Loverro 2001; Muzii 2000; Rickes 2002; Seracchioli 2010a; Shaw 2001; Tsai 2004; Vercellini 1999; Yang 2006; Yang 2018).
Two studies compared intranasal nafarelin (400 μg/day) with placebo over six months (Hornstein 1997) and three months (Parazzini 1994). Hornstein 1997 randomly allocated 49 to nafarelin and 44 to placebo; Parazzini 1994 randomly allocated 36 to nafarelin and 39 to placebo. three studies randomized women for postsurgical triptorelin depot or placebo (Loverro 2001; Loverro 2008; Sesti 2007; Sesti 2009).
Loverro 2001 analyzed 62 women and Loverro 2008 analysed 54 women with symptomatic endometriosis and compared triptorelin depot to placebo, evaluating pain recurrence, endometrioma relapse, and pregnancy rate. Sesti 2007 randomly allocated 234 women and Sesti 2009 randomly allocated 259 women with endometriosis to postsurgical medical therapy with GnRHas (either triptorelin or leuprorelin), continuous estroprogestin (OCP), dietary therapy (vitamins, minerals, lactic ferments, and fish oil), or placebo and evaluated pain (dysmenorrhoea, non‐menstrual pelvic pain, and dyspareunia) and quality of life. We combined data from the two hormonal suppression arms and compared them to placebo in the meta‐analysis. Data from the dietary therapy were not used in the meta‐analysis.
Telimaa 1987 compared danazol therapy with placebo. Telimaa 1987 had three groups, medroxyprogesterone acetate (MPA) 100 mg/day taken orally for six months (n = 17) and danazol 600 mg/day (200 mg three time daily) for six months (n = 18), compared to placebo (n = 16). Outcome measurements included pain recurrence, pregnancy rate, and disease recurrence determined by second‐look laparoscopy. Telimaa 1987 reported data separately for each group (see Table 4). In the meta‐analysis, we combined data from the medical therapy groups. The last placebo‐controlled trial of postsurgical medical therapy compared 20 women receiving desogestrel 0.075 mg with 20 women receiving placebo (Tanmahasamut 2017). The outcome measurement was pain recurrence, subdivided as overall pain, dysmenorrhoea, and noncyclic pelvic pain.
1. Descriptive data for trials not included in the meta‐analyses.
| Study ID | Comparison | Outcome | n | Conclusion |
| Angioni 2015 | Postsurgical vs no treatment, 2 groups (A = complete excision, B = incomplete excision, 1 = no therapy, 2 = GnRHas). 1A vs 2A | Differences in participant's quality of life by SF‐36 | 34/36 | Significant difference (P < 0.001) for general health, physical function, and vitality after 12 months of complete excision vs complete excision + GnRHas. |
| Postsurgical vs no therapy, 2 groups (A = complete excision, B = incomplete excision, 1 = no therapy, 2 = GnRHas). 1A 12 months' follow‐up vs baseline | 34 | Significant difference (P < 0.001) for general health, physical function, and vitality 12 months' follow‐up vs baseline for complete excision. | ||
| Postsurgical vs no therapy, 2 groups (A = complete excision, B = incomplete excision, 1 = no therapy, 2 = GnRHas). 2A 12 months' follow‐up vs baseline | 36 | Significant difference (P < 0.001) for general health, physical function, and vitality 12 months' follow‐up vs baseline for complete excision + GnRHas. | ||
| Audebert 1998 | Presurgical vs postsurgical GnRHa (nafarelin) | AFS scores – total | 25/28 | Total AFS score after 6 months was 0 in presurgery group and 6 in postsurgery group (P = 0.007); no SD or SE given and not calculable. |
| AFS scores – adhesion | 25/28 | Adhesion AFS score after 6 months was 0 in presurgery group and 2 in postsurgery group (P = 0.007), no SD or SE given and not calculable. | ||
| AFS scores – implant | 25/28 | Implant AFS score after 6 months was 0 in presurgery group and 4 in postsurgery group (P = 0.05), no SD or SE given and not calculable. | ||
| Ease of surgery | 25/28 | Surgery was easy in 56% of participants with GnRHa therapy presurgery compared to 35.7% in the postsurgery group. | ||
| Donnez 1994 | Presurgical GnRHa (goserelin) vs no medical therapy | Mean endometrioma size | 40/40 | Favouring goserelin: mean difference –1.81 cm (95% confidence interval –2.05 to 1.57). |
| Huang 2018 | Postsurgical vs no therapy, GnRHas | Total effective rate | 50/50 | Significant higher total effective rate compared to control group in favour of GnRHas group. |
| Seracchioli 2010a | Postsurgical vs no medical therapy (cyclic and continuous OC) | Cumulative pain‐free interval | 87/187 | Significant higher in continuous users vs cyclic (P < 0.0005) and in cyclic vs non‐users (P = 0.01) 18 months postsurgical. |
| Shaw 2001 | Presurgical GnRHa (goserelin) vs no medical therapy | Change in endometrioma size | 21/27 | Favouring goserelin: adjusted mean difference –1.25 cm (95% confidence interval –2.42 to –0.08). |
| Complete excision of cyst | 21/27 | No difference. 13/21 (72%) in GnRHa group and 16/27 (73%) in no medical therapy group had cysts completely excised at surgery. | ||
| Recurrence of residual cysts at 6 months | 21/27 | Favours goserelin. 2/21 (10%) in GnRHa and 4/27 (15%) in no medical treatment had recurrence of residual cysts. | ||
| Mean rAFS scores | 21/27 | No difference. 41.7 in GnRHa group and 42.5 in no medical treatment group (no SD given). | ||
| Telimaa 1987 | MPA vs placebo | Pain | 17/8 | Pain scores after 12 months assessed with 4‐point scales; 1.8 in MPA group and 4.4 in placebo group; "significant difference." |
| Danazol vs placebo | 18/8 | Pain scores after 12 months assessed with 4‐point scales; 2.5 in danazol group and 4.4 in placebo group; "significant difference." | ||
| MPA vs placebo | Participant satisfaction | 17/8 | Participant satisfaction achieved in 84% in MPA group and 24% in placebo group. | |
| Danazol vs placebo | 18/8 | Participant satisfaction achieved in 84% in danazol group and 24% in placebo group. | ||
| Tsai 2004 | Postsurgical leuprolide/danazol vs no therapy | Cumulative pregnancy rate at 12 months after clomiphene stimulation in both groups | 15/30 | No difference; 56.7% in leuprolide/danazol group and 54.5% in no therapy group |
| Yang 2006 | Postsurgical gestrinone vs no medical therapy | Disease recurrence at 6–30 months | 19/13 | Favoured medical therapy; 1/19 in gestrinone group and 4/13 in no medical therapy group (P < 0.05). |
| Yang 2018 | Postsurgical triptorelin acetate vs no medical therapy | Total effective rate | 65/65 | Significant higher total effective rate in triptorelin group vs control group (P = 0.009). |
| Levels of E2, LH, and FSH | 65/65 | Levels were significantly lower in the triptorelin acetate group than in control group (P < 0.001). |
AFS: American Fertility Society; FSH: follicle‐stimulating hormone; GnRHa: gonadotropin‐releasing hormone agonist; LH: luteinizing hormone; MPA: medroxyprogesterone acetate; n: number of participants; SD: standard deviation; SE: standard error; SF‐36: 36‐item Short Form.
Fifteen trials compared postsurgical medical therapy with GnRHas, danazol, progestogen, or OCPs with no postsurgical medical therapy (Alkatout 2013; Angioni 2015; Bianchi 1999; Busacca 2001; Cucinella 2013; Huang 2018; Loverro 2001; Muzii 2000; Rickes 2002; Seracchioli 2010a; Shaw 2001; Tsai 2004; Vercellini 1999; Yang 2006; Yang 2018). Bianchi 1999 compared postsurgical danazol 600 mg/day for three months with surgery alone in 53 women. Ten studies compared postsurgical GnRHas administered subcutaneously every four weeks with surgery alone (Alkatout 2013; Angioni 2015; Busacca 2001; Huang 2018; Loverro 2001; Rickes 2002; Tsai 2004; Vercellini 1999; Yang 2006; Yang 2018). Alkatout 2013 compared medical therapy with subcutaneous leuprorelin acetate 3.75 mg depot injected monthly for three months after surgery (group 3) compared with no hormonal therapy after surgery (group 2). In both groups, 150 participants were randomized. Busacca 2001 randomized 44 participants to receive leuprolide acetate depot 3.75 mg every four weeks for eight weeks (three injections) compared to 45 participants without postsurgical therapy. Outcome measurements were pain recurrence, disease recurrence, and pregnancy rate at 18 months. Tsai 2004 randomly allocated 15 women to postsurgical therapy with either GnRHas (leuprolide, n = 8) or danazol (n = 7), and the remaining 30 to no postsurgical medical therapy prior to controlled ovarian hyperstimulation with clomiphene followed by intrauterine insemination (IUI) or IVF.
Two studies compared triptorelin acetate depot 3.75 mg with no postsurgical therapy in 79 women (Angioni 2015) and 65 women (Yang 2018). Both treated women with endometriosis for six months with triptorelin acetate depot. Huang 2018 compared GnRHas with no medical therapy after surgery for four to six months, but there is no mention of which GnRHas was used. Rickes 2002 and Vercellini 1999 both compared goserelin with no hormonal therapy. Rickes 2002 enrolled 110 women with stage II to IV endometriosis and randomized them to two groups, 55 women received goserelin after surgery, and 55 women received surgery alone. After randomizations and therapy with GnRHas or no medical therapy, women were divided in two groups for ART, namely IUI or IVF/intracytoplasmic sperm injection (ICSI). Vercellini 1999 compared 133 women in the goserelin group versus 134 in the control group for six months after surgery. Loverro 2001 compared postsurgical triptorelin, administered subcutaneously every four weeks for 12 weeks, with surgery alone in groups of 62 women with endometriosis. In China, Yang 2006 compared postsurgical therapy with traditional Chinese medicine, gestrinone, or no therapy in 52 women and reported the pregnancy rate and recurrence of endometriosis with a nine‐ and 30‐month follow‐up.
Four studies investigated the effectiveness of OCPs (Cucinella 2013; Muzii 2000; Seracchioli 2010a; Seracchioli 2010b). Cucinella 2013 randomized 130 women into three groups with two monophasic and one multiphasic OCP. Non‐users did not received HT after surgery and were the control group. Muzii 2000 compared surgery plus six months of therapy with low‐dose cyclical OCP to surgery alone. Seracchioli 2010a and Seracchioli 2010b compared cyclic and continuous OCP use to non‐users. Group A (non‐users) included 69 (Seracchioli 2010a) and 87 (Seracchioli 2010b) women. Group B (cyclic OC users) included after randomizations 75 (Seracchioli 2010a) and 92 (Seracchioli 2010b) women, compared to group C (continuous OC users) with 73 (Seracchioli 2010a) and 95 (Seracchioli 2010b) women. Therapy with OCP was continued for at least 24 months.
Pre‐ and postsurgical medical therapy compared with surgery alone or surgery and placebo
We found no studies comparing pre‐ and postsurgical medical therapy with surgery alone or surgery and placebo.
Presurgical medical therapy compared with postsurgical medical therapy
Two studies compared presurgical medical therapy with postsurgical medical therapy (Alkatout 2013; Audebert 1998). Audebert 1998 compared medical therapy with intranasal nafarelin administered daily for six months before surgery with intranasal nafarelin administered daily for six months after surgery. Outcomes were pain, AFS scores, and ease of surgery. Alkatout 2013 compared three groups, with different timings of medical or surgical treatment. For the analysis of presurgical medical therapy compared to postsurgical medical therapy, we used only two groups, namely medical therapy with subcutaneous leuprorelin acetate depot injected monthly for three months before surgery (group 1) compared to postsurgical hormonal therapy with the same medication as group 1 (group 3). The study performed a second‐look laparoscopy at one to two months after conclusion of therapy. Authors staged endometriosis according to the EEC, and reported data for pregnancy rate and recurrence of pain symptoms.
Postsurgical medical therapy compared with pre‐ and postsurgical medical therapy
We found no studies comparing postsurgical medical therapy with pre‐ and postsurgical medical therapy.
Outcomes
Twenty‐two studies reported one or more of our primary outcomes (Alkatout 2013; Angioni 2015; Audebert 1998; Bianchi 1999; Busacca 2001; Cucinella 2013; Donnez 1994; Hornstein 1997; Huang 2018; Loverro 2001; Loverro 2008; Muzii 2000; Parazzini 1994; Seracchioli 2010a; Seracchioli 2010b; Sesti 2007; Sesti 2009; Tanmahasamut 2017; Telimaa 1987; Tsai 2004; Vercellini 1999; Yang 2018). The other three studies only mentioned secondary outcomes (Rickes 2002; Shaw 2001; Yang 2006).
Excluded studies
We excluded 238 studies from the review due to their non‐conformity with review objectives and methods for following reasons:
66 had ineligible study population;
40 had ineligible intervention;
75 had ineligible comparator;
21 had ineligible outcome;
35 had ineligible study design;
one was a duplicate publication of another study already included in review (Alkatout 2013).
Five key excluded studies are listed below and are described in further detail in the Characteristics of excluded studies table.
Morgante 1999: all participants received triptorelin for six months postsurgery before randomization to danazol or no therapy.
Schindler 1998: a prospective multicentre phase three study published in German. Preliminary translation suggested treatment was not randomly assigned.
Shawki 2002: data were not available at the time of writing this review.
Vercellini 2003: a pilot study using the LNG‐IUS for treatment of endometriosis postsurgery. This is a locally effective hormonal suppressive therapy and has low systemic effects.
Ylanen 2003: a dose finding study with no comparison of treatment modality with placebo or no medical therapy.
In addition, we excluded one previously included trial (Batioglu 1997), which was a quasi‐RCT, where randomization took place by even and odd numbers.
Risk of bias in included studies
Refer to the 'Risk of bias' tables and Figure 2 and Figure 3. Of the 25 trials included in this review, only four could be considered at low risk of bias overall (Parazzini 1994; Sesti 2007; Sesti 2009; Tanmahasamut 2017) (see Figure 3). We attempted to contact 12 authors to obtain additional information about their study methods to clarify some of the aspects affecting assessment of risk of bias; however, only two authors responded and the risk of bias assessment for their studies was updated accordingly.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Sixteen studies used computer‐generated randomization (Angioni 2015; Bianchi 1999; Busacca 2001; Cucinella 2013; Loverro 2008; Muzii 2000; Parazzini 1994; Rickes 2002; Seracchioli 2010a; Seracchioli 2010b; Sesti 2007; Sesti 2009; Shaw 2001; Tanmahasamut 2017; Tsai 2004; Vercellini 1999) and two used randomization tables (Donnez 1994; Huang 2018). These studies were at low risk of bias for this domain. The remainder of studies did not state their method of randomization and were, therefore, at unclear risk for this domain (Alkatout 2013; Audebert 1998; Hornstein 1997; Loverro 2001; Telimaa 1987; Yang 2006; Yang 2018).
Allocation concealment
Two studies reported adequate allocation concealment using telephone allocation (Parazzini 1994; Vercellini 1999). Six studies allocated participants using serially numbered opaque, sealed envelopes (Cucinella 2013; Seracchioli 2010a; Seracchioli 2010b; Sesti 2007; Sesti 2009; Tanmahasamut 2017), while Tsai 2004 allocated participants according to list "unknown to physicians." These nine studies were at low risk of bias. The remainder of the included studies did not describe their allocation methods and were at unclear risk of bias.
Blinding
Performance bias
Eight studies were double blinded and at low risk of performance bias (Audebert 1998; Cucinella 2013; Hornstein 1997; Parazzini 1994; Sesti 2007; Sesti 2009; Tanmahasamut 2017;Telimaa 1987). Loverro 2008 blinded participants to treatment allocation and used placebo injections, so were at low risk of performance bias.
One study was open label and at high risk of bias for performance bias (Vercellini 1999). There was insufficient information to assign the remaining studies, so they were at unclear risk of performance bias.
Detection bias
Eight studies were double blinded and at low risk of detection bias (Audebert 1998; Cucinella 2013; Hornstein 1997; Parazzini 1994; Sesti 2007; Sesti 2009; Tanmahasamut 2017; Telimaa 1987). For detection bias, if objective outcome measures were used, such as pregnancy rate and recurrence of endometriosis, detection bias was considered unlikely and assessed at low risk of bias. In case of subjective outcome measure used (pain), there was a high risk of bias. If both objective and subjective outcomes were included, there was an unclear risk of bias.
Seven studies used objective outcome measures, such as pregnancy rate and recurrence of endometriosis (Donnez 1994; Rickes 2002; Seracchioli 2010a; Shaw 2001; Tsai 2004; Yang 2006; Yang 2018). As a result, detection was unlikely, and detection bias was low risk. Three studies were at high risk of bias, due to use of a subjective outcome measure (pain) (Angioni 2015; Huang 2018; Seracchioli 2010b). One study was open label and at high risk of detection bias (Vercellini 1999). If both objective and subjective outcome measures were represented in the study, where there was no blinding, detection bias was assigned as unclear risk. Loverro 2008 was at unclear risk of bias because there was no information available to determine detection bias. There was insufficient information to assign the remaining studies, so they were at unclear risk of detection bias.
In all the studies included in this review, the adverse effects of the medication may have alerted the investigators and participants to the type of medical intervention.
Incomplete outcome data
Sixteen studies were at low risk of bias for this domain, six had no postrandomization losses (Bianchi 1999; Busacca 2001; Donnez 1994; Huang 2018; Parazzini 1994; Yang 2018), and 10 trials had few postrandomization losses (Angioni 2015: 11% evenly divided, Audebert 1998: 3%; Hornstein 1997: 15% evenly divided, Muzii 2000: 4%; Rickes 2002: 9.1%, Seracchioli 2010a: 9.2%; Sesti 2007: 5.1%; Sesti 2009: 7.3%; Tanmahasamut 2017: 5%; Telimaa 1987: 2%).
Outcome data was incomplete in six studies and at high risk of attrition bias (Alkatout 2013; Cucinella 2013; Loverro 2008; Seracchioli 2010b; Shaw 2001; Tsai 2004). Alkatout 2013 had a lost to follow‐up of 40/450 participants, which were unevenly divided between groups. Cucinella 2013 noted a lost to follow‐up of 8/38 (21%) participants in the non‐users group compared to 29/130 in the three treated groups combined. Loverro 2008 had a lost to follow‐up of 1/30 participants in the triptorelin group compared to 5/30 participants in the placebo group. Seracchioli 2010b had a lost to follow‐up of 37/239 participants, most of them in the non‐users group. Shaw 2001 noted a lost to follow‐up in the goserelin group of 7/21 (33%) participants and no therapy group of 11/27 (41%) participants. Tsai 2004 had a lost to follow‐up of four participants, all in the non‐users group (4/15). A further three studies were at unclear risk of bias for this domain (Loverro 2001; Vercellini 1999; Yang 2006).
Eleven studies reported pregnancy rates (Alkatout 2013; Bianchi 1999; Busacca 2001; Loverro 2001; Loverro 2008; Parazzini 1994; Rickes 2002; Telimaa 1987; Vercellini 1999; Yang 2006; Yang 2018).
Parazzini 1994 and Telimaa 1987 reported pregnancy rates 12 months after treatment commenced as an outcome for all participants in the trials; losses to follow‐up were small (Parazzini 1994: 9%; Telimaa 1987: 2%). Bianchi 1999 had also a follow‐up rate for pregnancy of 12 months, but the lost to follow‐up was not mentioned clearly. Busacca 2001 and Loverro 2001 reported pregnancy rates after 18 months' follow‐up in a subgroup of participants (Busacca 2001: 30%; Loverro 2001: 40% of participants). Busacca 2001 reported no losses to follow‐up in the group desiring pregnancy and Loverro 2001 did not state whether there were any losses to follow‐up. Alkatout 2013 and Vercellini 1999 reported 12 months' follow‐up. Alkatout 2013 compared three groups, namely HT (n = 125), surgery alone (n = 137), and HT plus surgery (n = 148). Pregnancy rate was assessed in 125 women in the HT group, 137 in the surgery alone group and 148 in HT plus surgery grooup. Vercellini 1999 reported pregnancy outcomes in a subgroup of 152 women desiring fertility (56% of participants) after two years of follow‐up; losses to follow‐up in these groups were very small. Loverro 2008 had a follow‐up period of five years, with an unclear lost to follow‐up risk of bias. Rickes 2002 reported the results of assisted reproduction, in five or six cycles of medication or five to six months without medication. The follow‐up from Yang 2018 was six to 30 months after treatment, with almost no lost to follow‐up.
Selective reporting
Twenty‐one studies reported our main review outcomes. Twenty were at low risk of selective reporting (Alkatout 2013; Angioni 2015; Audebert 1998; Bianchi 1999; Busacca 2001; Cucinella 2013; Hornstein 1997; Huang 2018; Loverro 2001; Loverro 2008; Muzii 2000; Parazzini 1994; Seracchioli 2010a; Sesti 2007; Sesti 2009; Tanmahasamut 2017; Telimaa 1987; Tsai 2004; Vercellini 1999; Yang 2018). We rated one study at unclear risk of selective reporting, as it reported insufficient data for review authors to make a judgement (Donnez 1994).
Other potential sources of bias
Eight studies were at unclear risk of other bias. One trial, which was described as an RCT, reported that participants were "randomly selected to receive" postsurgical medical therapy prior to ovarian stimulation over 13 years (1988 to 2001) (Tsai 2004). This might have given bias on pregnancy rate data. During this time there have been significant advancements in endoscopic technology. It is unclear whether this resulted in any bias in the results of the study. In addition, there were statistical differences between time from surgery to start of ovarian stimulation and number of oocytes and embryos per cycle in this study (Tsai 2004). One study reported differences between the groups at baseline with regard to disease severity, which may have introduced a bias into the results from this study (Shaw 2001). Sesti 2009 reported differences between the four groups, the rate of participants reporting dysmenorrhoea in the GnRHas group was significant lower compared to the other groups. Cucinella 2013, Loverro 2001, Muzii 2000, and Yang 2018 did not report the characteristics of each group at baseline.
Effects of interventions
See: Table 1; Table 2; Table 3
1. Presurgical medical therapy compared with placebo or no medical therapy
(Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Table 4)
Three studies compared presurgical medical therapy with placebo or no medical therapy (Alkatout 2013; Donnez 1994; Shaw 2001). There were too few studies to conduct any planned sensitivity analyses.
1.1 Pain (continuous)
No studies reported pain.
1.2 Pain recurrence (dichotomous)
One trial included pain as dichotomous outcome measure (Alkatout 2013).
We are uncertain if there is a difference in pelvic pain recurrence at 12 months or less (dichotomous) between presurgical medical hormonal suppression and surgery alone (RR 1.10, 95% CI 0.72 to 1.66; 1 RCT, n = 262; very low‐quality evidence). The evidence suggests that if the pelvic pain recurrence at 12 months or less (dichotomous) is assumed to be 24% among women with surgery alone, the chance following presurgical medical hormonal suppression would be between 17% and 40% (Analysis 1.1).
We are uncertain if there is a difference in dysmenorrhoea recurrence at 12 months or less (dichotomous) between presurgical medical hormonal suppression and surgery alone (RR 1.42, 95% CI 0.92 to 2.21; 1 RCT, n = 262; very low‐quality evidence; Analysis 1.1).
We are uncertain if there is a difference in dyspareunia recurrence at 12 months or less (dichotomous) between presurgical medical hormonal suppression and surgery alone (RR 1.46, 95% CI 0.88 to 2.44; 1 RCT, n = 262; very low‐quality evidence; Analysis 1.1).
1.3 Disease recurrence (continuous)
Two trials used AFS scores as the outcome measure in comparing medical therapy presurgery with surgery alone (Donnez 1994; Shaw 2001). There was insufficient evidence to determine whether there was a difference in endometrioma cyst size (Table 4), total AFS scores, and implant AFS scores comparing presurgical goserelin treatment with no treatment (Analysis 1.2).
We are uncertain about the improvement in disease recurrence at three months – total (AFS score) between presurgical medical hormonal suppression and surgery alone (mean recurrence score was 9.6 lower, 95% CI 11.42 to 7.78 lower; 1 RCT, n = 80; very low‐quality evidence; Analysis 1.2).
We are uncertain about the improvement in disease recurrence at three months – implant (AFS score) between presurgical medical hormonal suppression and surgery alone (mean recurrence score was 8.70 lower, 95% CI 10.67 to 6.73 lower; 1 RCT, n = 80; very low‐quality evidence; Analysis 1.2).
We are uncertain if there is a difference in disease recurrence at three months – adhesions (AFS score) between presurgical medical hormonal suppression and surgery alone (mean recurrence score was 0.90 lower, 95% CI 3.42 lower to 1.62 higher; 1 RCT, n = 80; very low‐quality evidence; Analysis 1.2).
1.4 Disease recurrence (dichotomous)
The distribution of EEC stage, mentioned in Alkatout 2013, showed insufficient evidence to determine a difference after presurgical therapy with leuprorelin compared to no presurgical therapy (Analysis 1.3).
We are uncertain if there is a difference observed in disease recurrence at 12 months or less (EEC stage) between presurgical medical hormonal suppression and surgery alone (RR 1.11, 95% CI 0.86 to 1.43; 1 RCT, n = 262; very low‐quality evidence; Analysis 1.3). The evidence suggests that if disease recurrence at 12 months or less by EEC stage is assumed to be 45% among women with surgery alone, the chance following presurgical medical hormonal suppression would be between 39% and 65%.
1.5 Pregnancy rate (dichotomous)
Alkatout 2013 compared pregnancy rate (pregnancies, abortions, and extrauterine pregnancies) for presurgical GnRHas therapy for two years after start of the study (Analysis 1.4). We are uncertain if presurgical medical hormonal suppression improves pregnancy rate compared to surgery alone (RR 1.18, 95% CI 0.97 to 1.45; 1 RCT, n = 262; very low‐quality evidence; Analysis 1.4). The evidence suggests that if pregnancy rate is assumed to be 58% among women with surgery alone, the pregnancy rate following presurgical medical hormonal suppression would be between 53% and 79%.
1.6 Ease of surgery, duration of surgery, postsurgical complications
No studies reported ease of surgery, duration of surgery, or postsurgical complications.
1.7 Levels of satisfaction of women
No studies reported levels of satisfaction of women participants.
1.8 Adverse events
No studies reported serious adverse events. Adverse events are reported in Table 5.
2. Adverse events.
| Trial ID | ADEs | Withdrawals due to ADEs |
| Alkatout 2013 | Not described. | None. |
| Angioni 2015 | Not described. | None. |
| Audebert 1998 | Adverse events were reported with equal frequency in both groups and were consistent with those published by other investigators. | 2 withdrawals after randomization from hot flushes and headaches. |
| Bianchi 1999 | Hyperandrogenism 16.7%, weight gain ≥ 3 kg 8.3%. | None. |
| Busacca 2001 | Most experienced menopausal symptoms, all became amenorrhoeic. | 1 withdrawal from unacceptable adverse events. |
| Cucinella 2013 | Comparable in 3 groups: headache in 10 participants, decreased libido in 9, spotting in 6, water retention in 4, vaginal dryness in 2, depression in 1, acne in 1, insomnia in 1. | 11 withdrawals from adverse events attributable to OC’s |
| Donnez 1994 | Not described. | None. |
| Hornstein 1997 | Not described. | Not due to ADEs. |
| Huang 2018 | Incidences of uterine bleeding, acne, and weight gain were significant lower in therapy group than control group while incidence of vaginal dryness was significant higher in control group. No differences regarding the incidence of hot flushes, sleep disorder, and headache between 2 groups. | None. |
| Loverro 2001 | Not described. | None. |
| Loverro 2008 | Not described. | None. |
| Muzii 2000 | Not described. | Not due to ADEs. |
| Parazzini 1994 | Amenorrhoea in all actively treated, 0 in placebo group. | None. |
| Rickes 2002 | Not described. | None. |
| Seracchioli 2010a | Not described. | Cyclic OC group: 4 did not complete study due to adverse events. Continuous OC group: 4 did not complete study due to adverse events. |
| Seracchioli 2010b | Not described. | Cyclic OC group: 8 did not complete study due to adverse events. Continuous OC group: 6 did not complete study due to adverse events. |
| Sesti 2007 | Menopausal symptoms, spotting, bloating, weight gain, and headache reported, but "well tolerated." | 4 withdrew from hormonal suppression group due to adverse events. |
| Sesti 2009 | GnRHas: 7 women experienced hot flushes, vaginal dryness, and reduced libido caused by hypo‐oestrogenism. OC: 4 women experienced breakthrough bleeding, headache, breast tension, nausea, and weight gain. |
11 in hormonal suppression therapy (GnRHas and OC). |
| Shaw 2001 | Hot flushes (62%), headaches (29%), and dysmenorrhoea (14%) in goserelin group and dysmenorrhoea (33%) in no therapy group. | 4 withdrew from goserelin group due to serious adverse events (only 1 related to therapy) |
| Tanmahasamut 2017 | Menstruation alterations (Amenorrhea, spotting, light bleeding), acne, breast pain, headache, nausea/vomiting, hair loss, mood change, rash. | Not due to ADE. |
| Telimaa 1987 | Weight increase MPA 1.9 kg (SD 1.3), danazol 3.4 kg (SD 2.3), placebo 0.4 kg (SD 2.6); breakthrough bleeding at 6 months: MPA 65%, danazol 56%, placebo 6%; acne at 6 months: danazol 56%, placebo 6%. | Not due to ADE. |
| Tsai 2004 | Not described. | Reasons for withdrawals not given. |
| Vercellini 1999 | Not described. | None. |
| Yang 2006 | Not described. | None. |
| Yang 2018 | 2 nausea and vomiting, pain at injection site, vertigo. | None. |
ADE: adverse drug effects; GnRHa: gonadotropin‐releasing hormone agonist; MPA: medroxyprogesterone acetate; OC: oral contraception; SD: standard deviation.
2. Postsurgical medical therapy compared with placebo or no medical therapy
(Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5; Table 4)
Twenty studies compared postsurgical medical therapy with placebo or no medical therapy (Alkatout 2013; Angioni 2015; Bianchi 1999; Busacca 2001; Cucinella 2013; Hornstein 1997; Huang 2018; Loverro 2001; Loverro 2008; Muzii 2000; Parazzini 1994; Seracchioli 2010a; Sesti 2007; Sesti 2009; Tanmahasamut 2017; Telimaa 1987; Tsai 2004; Vercellini 1999; Yang 2006; Yang 2018).
2.1 Pain (continuous)
Six trials reported he continuous outcome of pain measured by VAS (Angioni 2015; Hornstein 1997; Huang 2018; Parazzini 1994; Sesti 2007; Tanmahasamut 2017). Meta‐analysis was possible for the continuous outcome, mean VAS score of pelvic pain for 12 months' follow‐up or less, for three studies (Huang 2018; Parazzini 1994; Sesti 2007). We are uncertain whether postsurgical medical therapy improves pelvic pain at 12 months compared to placebo (SMD ‐0.79, 95% CI ‐1.02 to ‐0.56; 3 RCTs, n = 340; I2 = 91%; very low‐quality evidence; Analysis 2.1). A fourth study reported pain after 12 months using a 4‐point scale and presented mean scores without estimates of precision (Telimaa 1987). It was not possible to include these data in the meta‐analysis but the estimates are recorded in Table 4.
Angioni 2015 mentioned pain recurrence according to the SF‐36 subscale. There was no evidence of a difference (SMD 0.11, 95% CI ‐0.21 to 0.42; 1 RCT, n = 159; very low‐quality evidence).
In the meta‐analysis, two trials reported for the continuous outcome, mean VAS score for dysmenorrhoea and dyspareunia (Huang 2018; Sesti 2007). We are uncertain whether postsurgical medical therapy showed a reduction in dysmenorrhoea and dyspareunia at 12 months compared to placebo. These studies show inconsistent effects and considerable statistical heterogeneity (dysmenorrhoea: SMD ‐0.38, 95% CI ‐0.62 to ‐0.14; 2 RCTs, n = 287; I2 = 78%; very low‐quality evidence; dyspareunia: (SMD ‐0.44, 95% CI ‐0.67 to ‐0.20; 2 RCTs, n = 287; I2 = 37%; very low‐quality evidence; Analysis 2.1).
Two trials presented the change in pain score from the baseline to 12 months after surgery (Hornstein 1997; Tanmahasamut 2017). We are uncertain whether postsurgical medical therapy decreased pain scores after 12 months postsurgical, compared to no medical therapy (SMD –0.26, 95% CI –0.60 to 0.09; 2 RCTs, n = 129; very low‐quality evidence; Analysis 2.1). We are uncertain if postsurgical medical therapy leads to a change in dysmenorrhoea (SMD –0.28, 95% CI –0.90 to 0.34; 1 RCT, n = 40; very low‐quality evidence), dyspareunia (SMD –0.13, 95% CI –0.75 to 0.49; 1 RCT, n = 40; very low‐quality evidence), and overall pain (SMD –0.31, 95% CI –0.94 to 0.31; 1 RCT, n = 40; very low‐quality evidence) from baseline to 12 months after surgery, compared to no medical therapy (Analysis 2.1).
2.2 Pain recurrence (dichotomous)
Five trials measured pelvic pain recurrence during the first year after surgical treatment (Alkatout 2013; Bianchi 1999; Loverro 2001; Tanmahasamut 2017; Vercellini 1999). Compared to surgery alone, postsurgical medical therapy may decrease pain recurrence at 12 months or less (RR 0.70, 95% CI 0.52 to 0.94; 5 RCTs, n = 657; I2 = 0%; low‐quality evidence).
The evidence suggests that if pelvic pain recurrence at 12 months or less (dichotomous) is assumed to be 26% among women with surgery alone, the chance following presurgical medical hormonal suppression would be between 13% to 24% (Analysis 2.2).
Three trials reported pain recurrence during the second year after surgery (Busacca 2001; Muzii 2000; Vercellini 2003), but we are uncertain about the effect of postsurgical medical therapy on pain recurrence compared to surgery alone (RR 0.70, 95% CI 0.47 to 1.03; I2 = 0%; 3 RCTs, n = 312; very low‐quality evidence) (Analysis 2.2).
One study reported data on pain persistence or recurrence five years after therapy (Loverro 2008). Due to the wide 95% CIs, we are uncertain if there is a positive effect of postsurgical medical therapy on pain reduction compared to no therapy (RR 0.93, 95% CI 0.53 to 1.66; 1 RCT, n = 54; very low‐quality evidence; Analysis 2.2). Only one trial presented dysmenorrhoea recurrence (RR 0.82, 95% CI 0.50 to 1.35; 1 RCT, n= 285)
and dyspareunia recurrence (RR 0.53, 95% CI 0.27 to 1.03; 1 RCT, n = 285) (Alkatout 2013).
2.3 Disease recurrence (continuous)
One study reported AFS scores for disease recurrence (Telimaa 1987). After 12 months, a second‐look laparoscopy showed a reduction in AFS scores from baseline in all three groups: MPA, danazol, and placebo. There was a mean difference favouring MPA and danazol, when individually compared with placebo. There was no significant difference between the MPA and danazol groups in this respect. The results were inconclusive when mean AFS score were combined (MPA + danazol) and postsurgical medical therapies were compared with placebo (MD –2.29, 95% CI –4.01 to –0.57, 1 RCT, n = 51; very low‐quality evidence; Analysis 2.3).
2.4 Disease recurrence (dichotomous)
Disease recurrence, evaluated by gynaecological examination or ultrasonography, was measured at two time points: one year and two years after surgery. Four studies published the results of disease recurrence for 12 months' follow‐up or less (Bianchi 1999; Busacca 2001; Cucinella 2013; Yang 2018). There may be an decrease of disease recurrence in favour of postsurgical medical therapy, compared to no therapy (RR 0.30, 95% CI 0.17 to 0.54; I2 = 58%; 4 RCTs, n = 433; low‐quality evidence; Analysis 2.4). This suggest that if the change of disease recurrence following no medical therapy is 17%, the chance following postsurgical medical therapy would be between 3% and 9%.
Four studies reported disease recurrence two years after surgery (Cucinella 2013; Seracchioli 2010a; Sesti 2009; Tsai 2004). There may be a reduction of disease recurrence in favour of postsurgical hormonal therapy, compared to no postsurgical medical therapy (RR 0.40, 95% CI 0.27 to 0.58; I2 = 57%; 4 RCTs, n = 571; low‐quality evidence; Analysis 2.4).
Alkatout 2013 described distribution of EEC stage, comparing baseline and five months after starting hormonal therapy or five months after primary surgery. There was a reduction of EEC stages in the group with hormonal therapy, but due to this being a single study with wide 95% CIs, the results are inconclusive (RR 0.88, 95% CI 0.67 to 1.15; 1 RCT, n = 285; very low‐quality evidence; Analysis 2.4).
2.5 Pregnancy rate (dichotomous)
Eleven studies reported pregnancy (Alkatout 2013; Bianchi 1999; Busacca 2001; Loverro 2001; Loverro 2008; Parazzini 1994; Rickes 2002; Telimaa 1987; Vercellini 1999; Yang 2006; Yang 2018). Surgery plus medical therapy probably increases pregnancy rate compared to either surgery plus placebo or no medical therapy (RR 1.19, 95% CI 1.02 to 1.38; 11 RCTs, n = 955; I2 = 27%; moderate‐quality evidence; Analysis 2.5). This suggests that if the chance of pregnancy following no medical therapy is 34%, the chance following postsurgical medical therapy would be between 35% and 48%.
2.6 Ease of surgery, duration of surgery, postsurgical complications
No studies reported ease of surgery, duration of surgery, or postsurgical complications.
2.7 Levels of satisfaction of women
One study reported participant satisfaction (Telimaa 1987). There was an increase in participant satisfaction in both active treatment groups, which was statistically significantly greater compared to the placebo group, but there was no difference between the danazol and MPA groups (Table 4).
2.8 Adverse events
No studies reported serious adverse events. Adverse events are reported in Table 5.
Sensitivity analysis
Nine studies were at low risk of bias of selection bias (Cucinella 2013; Parazzini 1994; Seracchioli 2010a; Seracchioli 2010b; Sesti 2007; Sesti 2009; Tanmahasamut 2017; Tsai 2004; Vercellini 1999).
A sensitivity analysis was only performed for the primary outcomes included in the comparison of postsurgical medical therapy versus no medical therapy. Planned sensitivity analysis was not undertaken for the other comparisons because none of the trials were at low risk of bias.
Pain (continuous)
For this sensitivity analysis, three trials reported the continuous outcome of pain, measured by VAS (Parazzini 1994; Sesti 2007; Tanmahasamut 2017). Meta‐analysis was possible for the continuous outcome, mean VAS score of pelvic pain for 12 months' follow‐up or less, for two studies (Parazzini 1994; Sesti 2007). The pooled estimate showed a reduction in pelvic pain at 12 months favouring medical therapy compared to placebo (MD –1.18, 95% CI –1.45 to –0.91; I2 = 14%; 2 RCTs, n = 240; low‐quality evidence).
Sesti 2007 reported the continuous outcome, mean VAS score for dysmenorrhoea and dyspareunia. Postsurgical medical therapy decreases complaints of dysmenorrhoea or dyspareunia, compared to placebo or no medical therapy (dysmenorrhoea: MD –0.70, 95% CI –1.04 to –0.36; 1 RCT, n = 187; very low‐quality evidence; dyspareunia: MD –0.40, 95% CI –0.76 to –0.04; 1 RCT, n = 187; very low‐quality evidence).
Tanmahasamut 2017 reported the change in pain score from the baseline to 12 months after surgery. It found no difference between postsurgical medical therapy and placebo. Tanmahasamut 2017 also presented change in dysmenorrhoea, dyspareunia, and overall pain from baseline to 12 months after surgery, and found no evidence of a difference between medical therapy and placebo.
Pain (dichotomous)
Two studies reported pain recurrence during the first year after surgical treatment (Tanmahasamut 2017; Vercellini 1999). The pooled estimate from these trials showed a difference between medical therapy and surgery alone, in favour of postsurgical medical therapy (RR 0.53, 95% CI 0.29 to 0.96; I2 = 27%; 2 RCTs, n = 250; low‐quality evidence).
One trial reported pain recurrence during the second year after surgery (Vercellini 2003). There was no evidence of a difference between medical therapy after surgery and surgery alone (RR 0.64, 95% CI 0.39 to 1.05; 1 RCT, n = 155; very low‐quality evidence).
Disease recurrence (continuous)
Disease recurrence, evaluated by gynaecological examination or ultrasonography, was measured at two time points: one year and two years after surgery. One study published the results of disease recurrence for 12 months' follow‐up or less (Cucinella 2013). There was a difference in favour of hormonal therapy (RR 0.16, 95% CI 0.07 to 0.38; 1 RCT, n = 137; very low‐quality evidence).
A sensitivity analysis was also performed to compare outcomes based on random effects model. A difference in result was only observed in comparison postsurgical medical therapy compared with placebo or no medical therapy for the following outcomes: pelvic pain (MD ‐0.34, 95% CI ‐1.14 to 0.46; I2 = 94%; 4 RCTs, n= 419, dysmenorrhoea (MD ‐0.14, 95% CI ‐0.96 to 0.69; I2 = 80%; 3 RCTs, n = 366), and deep dyspareunia (MD ‐0.13, 95% CI ‐0.75 to 0.49; I2 = 89%; 3 RCTs; n = 366). Nevertheless, we did not consider this difference meaningful due to the very low quality evidence and very high heterogeneity. No difference in result was observed in the other analyses.
3. Pre‐ and postsurgical medical therapy compared with placebo or no medical therapy
We found no studies comparing pre‐ and postsurgical medical therapy with placebo or no medical therapy.
4. Presurgical medical therapy compared with postsurgical medical therapy
(Analysis 3.1; Analysis 3.2; Analysis 3.3; Table 4; Table 5)
Two studies compared presurgical medical therapy with postsurgical medical therapy (Alkatout 2013; Audebert 1998). There were too few studies to conduct any planned sensitivity analyses.
4.1 Pain (continuous)
No studies reported pain.
4.2 Pain recurrence (dichotomous)
Two studies reported pain recurrence (Alkatout 2013; Audebert 1998). We are uncertain whether there was a reduction in pelvic pain with presurgical medical therapy after 12 months' follow‐up, compared to postsurgical medical therapy. Alkatout 2013 showed a difference in favour of postsurgical hormonal therapy (group 3) compared to presurgical hormonal therapy (group 1), for dysmenorrhoea and dyspareunia. Audebert 1998 also compared these two groups for dysmenorrhoea and dyspareunia, but found no differences (Analysis 3.1).
We are uncertain if there is a difference in pelvic pain recurrence at 12 months or less (dichotomous) between postsurgical and presurgical medical hormonal suppression therapy (RR 1.40, 95% CI 0.95 to 2.07; I2 = 2%; 2 RCTs, n = 326; low‐quality evidence). The evidence suggests that if the pelvic pain recurrence at 12 months or less (dichotomous) is assumed to be 20% among women with postsurgical medical hormonal suppression alone, the chance following presurgical medical hormonal suppression would be between 19% and 41% (Analysis 3.1).
We are uncertain if there is a difference in dysmenorrhoea recurrence at 12 months or less (dichotomous) between postsurgical and presurgical medical hormonal suppression therapy (RR 1.73, 95% CI 1.09 to 2.74; I2 = not applicable; 2 RCTs; n = 326; very low‐quality evidence; Analysis 3.1).
We are uncertain whether there is a difference in dyspareunia recurrence at 12 months or less (dichotomous) between postsurgical and presurgical medical hormonal suppression therapy (RR 3.08, 95% CI 1.68 to 5.62; I2 = 0%; 2 RCTs, n = 326; very low‐quality evidence; Analysis 3.1).
One study compared postsurgical and presurgical hormonal suppression therapy for pelvic tenderness and pelvic induration at 12 months or less (Audebert 1998). We are uncertain if there is a difference in pelvic tenderness at 12 months or less (dichotomous) between postsurgical and presurgical medical hormonal suppression therapy (RR 0.95, 95% CI 0.52 to 1.72; 1 RCTs, n = 53; very low‐quality evidence; Analysis 3.1). We are uncertain if there is a difference observed in pelvic induration at 12 months or less (dichotomous) between postsurgical and presurgical medical hormonal suppression therapy (RR 1.63, 95% CI 0.94 to 2.81; 1 RCTs, n = 53; very low‐quality evidence; Analysis 3.1).
4.3 Disease recurrence (continuous)
Audebert 1998 reported that the presurgical nafarelin group had lower global AFS scores, adhesion scores and 'endometriosis scores' compared to the postsurgical nafarelin group, but data were presented in a form that was not suitable for inclusion in a forest plot so the data are recorded in Table 4.
4.4 Disease recurrence (dichotomous)
One study compared postsurgical and presurgical hormonal suppression therapy for disease recurrence using EEC staging (Alkatout 2013).
We are uncertain if there is a difference in disease recurrence at 12 months or less (EEC stage) between postsurgical and presurgical medical hormonal suppression therapy (RR 1.26, 95% CI 0.97 to 1.65; 1 RCT, n = 273; very low‐quality evidence). The evidence suggests that if the disease recurrence at 12 months or less (EEC stage) is assumed to be 40% among women with postsurgical medical hormonal suppression alone, the chance following presurgical medical hormonal suppression would be between 39% and 66% (Analysis 3.2).
4.5 Pregnancy rate (dichotomous)
One study reported pregnancy rate (Alkatout 2013). We are uncertain if pregnancy rate is improved with presurgical medical hormonal suppression therapy compared to postsurgical medical hormonal suppression therapy (RR 1.08, 95% CI 0.90 to 1.30; 1 RCT, n = 273; very low‐quality evidence).
The evidence suggests that if the pregnancy rate is assumed to be 60% among women with postsurgical medical hormonal suppression alone, the chance following presurgical medical hormonal suppression would be between 54% and 78%.
(Analysis 3.3).
4.6 Ease of surgery, duration of surgery, postsurgical complications
No studies reported ease of surgery, duration of surgery, postsurgical complications.
4.7 Levels of satisfaction of women
No studies reported levels of satisfaction of women participants.
4.8 Adverse events
No reported serious adverse events. Adverse events are reported in Table 5.
5. Postsurgical medical therapy compared with pre‐ and postsurgical medical therapy
We found no studies comparing postsurgical medical therapy with pre‐ and postsurgical medical therapy.
Discussion
Summary of main results
There is insufficient evidence to support the view that medical therapy for hormonal suppression of endometriosis prior to surgery is more effective than surgery alone. Two studies compared presurgical medical therapy with surgery alone. We are uncertain if AFS scores were improved in the medical therapy group, because of the very low‐quality evidence. There was uncertainty that disease recurrence at 12 months or less, measured by EEC stage, was improved after presurgical hormonal therapy (very low‐quality evidence). Results were inconclusive regarding pregnancy rate, with very low‐quality evidence. A sensitivity analysis of the data from these studies was not possible, as only one study reported each outcome.
There was uncertainty that postsurgical hormonal suppression of endometriosis compared to surgery alone (either no medical therapy or placebo) showed a reduction in pain after 12 months (pelvic pain, dysmenorrhoea, and dyspareunia) measured by VAS score (very low‐quality evidence). Pain recurrence at 12 months or less (dichotomous) showed inconclusive data about the effect of postsurgical medical therapy compared to no medical therapy or placebo (very low‐quality evidence). There may be a benefit for disease recurrence (AFS scores), when postsurgical medical therapy is compared to no medical therapy (low‐quality evidence). The meta‐analysis showed that postsurgical medical therapy group probably improves pregnancy rate, compared to no medical therapy, with moderate‐quality evidence. A sensitivity analysis was conducted for the primary outcomes. Nine studies were at low risk of bias of selection bias. For this sensitivity analysis, for the continuous outcome of pain, measured by VAS, the pooled estimate may have reduced pelvic pain at 12 months favouring medical therapy compared to placebo (low‐quality evidence). There was inconclusive evidence regarding the effects of postsurgical medical therapy compared to no medical therapy, for dysmenorrhoea and dyspareunia (very low‐quality evidence). Disease recurrence may show an improvement in favour of postsurgical hormonal therapy, compared to no medical therapy (low‐quality evidence). The sensitivity analysis did not change the conclusion of the results.
There were no trials that compared hormonal suppression of endometriosis before and after surgery with surgery alone.
There may be some improvement in favour of postsurgery hormonal suppression for dysmenorrhoea and dyspareunia compared to presurgery hormonal suppression (low‐quality evidence). We are uncertain about the effect of postsurgical medical therapy compared to presurgical medical therapy on pelvic pain recurrence. Results were inconclusive regarding pregnancy rate when presurgical hormonal therapy was compared to postsurgical hormonal therapy. All outcomes, except pelvic pain recurrence at 12 months or less, were of very low‐quality evidence, related to a single study included in the analysis. Pelvic pain recurrence at 12 months or less was of low‐quality evidence.
There were no trials that compared hormonal suppression of endometriosis after surgery with before and after surgery
In summary, data included were inconsistent about the use of medical therapy after surgery for endometriosis, regarding reduction and recurrence of pain. Recurrence of endometriosis may also decrease with postsurgical hormonal therapy, but again, data were inconsistent and of low‐ to moderate‐quality evidence. When used prior to surgery, medical therapy was inconclusive about the effect on cyst size and the effect on AFS scores was conflicting. There is inconclusive evidence that medical therapy pre‐ or postsurgery improves pregnancy rates. No conclusions can be drawn with respect to the outcomes of facilitating surgery, duration of surgery, postsurgical complications, or levels of satisfaction of participants from the trials included in this review. Adverse effects were described but not quantified, so no direct comparisons between the included trials were possible.
Overall completeness and applicability of evidence
Given the thorough search strategy and clear inclusion and exclusion criteria, this review provides a comprehensive overview of the current scientific evidence pertaining to the timing of medical hormonal suppression therapy for women undergoing surgery for endometriosis. All 25 studies included in systematic review were also included in meta‐analyses. All women of reproductive age, as defined by authors of included studies, were included in review without any additional age limitation. The review presented five different comparisons based on variable potential timing of administration of medical therapy. Most of the eligible studies compared medical therapy after surgery with placebo or no medical therapy and the evidence answers the review question adequately and is generally in line with current practice. However, there were very few eligible studies that compared presurgical medical therapy with placebo or no medical therapy or postsurgical medical therapy; and none compared pre‐ and postsurgical medical therapy with placebo or no medical therapy or postsurgical medical therapy resulting in inadequate answers for the review questions.
Quality of the evidence
We prepared 'Summary of findings' tables using GRADEpro and Cochrane methods.
We judged the evidence for the comparisons 'presurgical medical therapy compared with placebo or no medical therapy' and 'presurgical medical therapy compared with postsurgical medical therapy' as very low quality. The comparison 'postsurgical medical therapy compared with placebo or no medical therapy' was moderate‐ to very low‐quality evidence, depending on the outcome measurement.
A strength of this review is that all the included studies involved women with laparoscopically diagnosed endometriosis and laparoscopic assessment of the extent of the endometriosis. Weaknesses of this review are that the included studies were small and many were at risk of bias. Several additional studies were identified on this review, and even though there was a spread of different times at which a range of different outcomes were measured, the additional studies allowed for some studies to be combined in meta‐analysis for some outcomes.
The quality of evidence for the pregnancy rate or disease recurrence at 12 months or less among the studies comparing postsurgical medical therapy with surgery alone was moderate. Magnitude of risk reduction of disease recurrence was large with moderate variability among studies (I2 = 58%), but in case of pregnancy rate the magnitude of effect size was modest with relatively large variability between studies (I2 = 24%). Though these results can support the practice of postsurgical medical therapy, additional methodologically robust studies measuring these outcomes can help in improving the quality of evidence.
The quality of evidence for most of the other outcomes reviewed was either very low or low, primarily due to few studies with small sizes reporting each outcome or poorly reported methods. And as result this evidence may not be considered sufficient to recommend practice change.
Potential biases in the review process
Potential biases in the review process were minimized by searching published and unpublished literature from a variety of sources with no restrictions on date of publication or language. At least two review authors independently extracted data and conducted the risk of bias assessment.
The main problem remains is that the small number of trials per outcome across multiple comparisons makes meta‐analysis difficult. The publications included spanned over 31 years (from 1987 to 2018) during which time the methods and data reporting practices have changed significantly. Attempts to contact authors for older publications were often unsuccessful, resulting in poor scoring in risk of bias assessment due to inadequate information. Inaccessibility to useful information available with these authors, but not included in papers, might have also limited the meta‐analysis.
Agreements and disagreements with other studies or reviews
Several systematic reviews have been conducted to investigate individual or grouped hormonal suppression therapies in management of endometriosis. As a result, postsurgical therapy with either OCP or GnRHas have become standard care in women with endometriosis, as recommended by the guideline of the European Society of Human Reproduction and Embryology (ESHRE) (Dunselman 2014). presurgical hormonal therapy is less‐common practice, and has not been recommended by the ESHRE guideline.
However, in our review of the literature, we identified no other review pertaining to the timing of medical management in women undergoing surgery for endometriosis. We believe this updated review will contribute to the better management of endometriosis by practicing gynaecologists regarding best time to use these medicines.
Authors' conclusions
Implications for practice.
Our results indicate that the efficacy of medical therapy for endometriosis related to the timing of therapy relative to surgery for endometriosis is inconclusive. In our various comparisons of the timing for therapy, we have found some evidence to indicate that postsurgical medical therapy compared with no medical therapy after surgery may be beneficial with respect to pain recurrence, disease recurrence and pregnancy. The evidence on pain outcomes is inconclusive. There is insufficient evidence regarding medical therapy at other time points in relation to surgery for women with endometriosis.
Implications for research.
Our review is unable to provide conclusive evidence for the appropriate time of use of medical therapy relative to surgery, primarily due to few studies for each comparison and small sample size for most of included studies. As this is not a heavily researched area, a number of factors need special attention for any future research on this topic.
Research conducted to assess the effects of medical therapy pre or postsurgery for endometriosis is associated with many difficulties. Not many women are likely to consent to undergo second‐look laparoscopy, just to assess the results of previous treatment modalities. Hence, recruiting large numbers of participants for randomized trials is difficult. Women with subfertility due to endometriosis may also not accept treatment that may improve pain and other symptoms but reduces or delays their chance of conceiving. Despite these difficulties, it would be valuable to have adequately powered trials to determine if there is a significant benefit in adjunctive medical therapy before or after surgery for endometriosis.
Due to nature of hormonal suppression medical therapy and its associated therapeutic and adverse effects, which would be obvious to both the participant and outcome assessor, maintaining blinding is difficult. But still blinded outcome assessment is possible and is highly desirable.
A key missing limitation is that although multiple studies were included in each comparison, they did not report the same outcome. Even if they reported the same outcome (e.g. pain), they used different presentations (pelvic pain, dyspareunia, dysmenorrhea), scales (continuous or dichotomous), or measures of occurrence (presence, continuation, recurrence), which makes trial interpretation and evidence synthesis difficult. Due to heterogeneity of the outcome measures, a meta‐analysis was possible only with a small number of studies, with a small number of participants for each outcome measure. A core outcome set for endometriosis‐associated outcome measures has been developed (Duffy 2020) and new trials should consider using it to provide more comparable data for future research.
Despite these difficulties, it would be valuable to have well‐designed, adequately powered, and well‐conducted trials to determine if there is a significant benefit in adjunctive medical therapy before or after surgery for endometriosis. Data to quantify the number and degree of adverse events experienced as a result of medical therapy would enable better assessment of the comparative benefits and harms of medical therapy.
What's new
| Date | Event | Description |
|---|---|---|
| 18 December 2020 | Amended | Forest plot numbering updated |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 3, 2004
| Date | Event | Description |
|---|---|---|
| 25 November 2020 | Amended | Study Roghaei 2010 placed in awaiting assessment; correction of results |
| 13 May 2020 | New search has been performed | We updated the review. We included 10 new studies (Alkatout 2013; Angioni 2015; Cucinella 2013; Huang 2018; Rickes 2002; Seracchioli 2010a; Seracchioli 2010b; Sesti 2009; Tanmahasamut 2017; Yang 2018). |
| 13 May 2020 | New citation required and conclusions have changed | The addition of new studies has led to a change in the conclusion about postsurgical medical therapy. Our results indicate that the efficacy of medical therapy for endometriosis may be related to the timing of therapy relative to surgery for endometriosis. In our various comparisons of the timing of therapy, we have found that postsurgical therapy compared with no medical therapy may be beneficial with respect to pelvic pain, disease recurrence, and pregnancy. There is insufficient evidence to recommend medical therapy at other time points in relation to surgery for women with endometriosis. |
| 2 May 2011 | Amended | Summary of findings tables added |
| 20 September 2010 | New search has been performed | Substantive update September 2010 ‐ 5 new trials included. Risk of bias assessment on all included studies. Minor changes to the objectives ‐ hypotheses deleted |
| 7 November 2008 | Amended | Converted to new review format. |
| 26 May 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank the Cochrane Gynaecology and Fertility Group, for their help, advice, and support during the preparation of the review. For the current update of the review, we would like to thank Marian Showell (Information Specialist) who updated the searches.
We would like to thank Sue Furness, Christine Yap, Ying Cheong, Cindy Farquhar, and James Duffy for their contribution to previous versions of the review.
We would like to thank Virginia Minogue (consumer), Katie Stocking, Jane Thomas, and Rui Wang for their valuable peer review comments.
Appendices
Appendix 1. The Cochrane Gynaecology and Fertility specialised register search strategy
Procite Platform
Searched 20 November 2019
Keywords CONTAINS "endometrioma" or "endometriosis" or "The Endometriosis Health Profile" or "endometriosis‐outcome" or "endometriosis scores" or "Endometriosis‐Symptoms" or "endometriotic cysts" or "adenomyosis" or "dyschezia" or Title CONTAINS "endometrioma" or "endometriosis" or "The Endometriosis Health Profile" or "endometriosis‐outcome" or "endometriosis scores" or "Endometriosis‐Symptoms" or "endometriotic cysts" or "adenomyosis" or "dyschezia" AND Keywords CONTAINS "oral contraceptive" or "Oral Contraceptive Agent" or "oral contraceptive pill" or "oral contraceptives" or "oral dydrogesterone" or "OCP pretreatment" or "Danazol" or "GnRH a" or "GnRH agonist" or "GnRH agonists" or "GnRHa" or "Gonadorelin" or "Gonadotrophin releasing agonist" or "gonadotrophin‐releasing hormone (GnRH)" or "Gonadotrophin releasing hormones" or "gonadotropin releasing hormone agonist" or "gonadotropin‐releasing hormone" or "LHRH" or "gestrinone" or "*Gestagen" or "Pretreatment" or "Medical" or "Progesterone" or "progestagen" or "progestin" or "progestin implant" or "progestins" or "Medroxyprogesterone" or "levonorgestrel intrauterine system" or "levonorgestrel‐releasing intrauterine device" or "Levonorgestrel‐Therapeutic‐Use" or "LNG‐IUS" or "Letrozole" or "nafarelin" or "Zoladex" or "triptorelin" or "Goserelin" or "goserelin pretreatment" or "Goserelin Acetate" or "leuprolide" or "leuprorelin" or "mifepristone"
(633 records)
Appendix 2. CENTRAL search strategy
Ovid Platform
Searched 20 November 2019 (Issue 10)
1 exp Endometriosis/ (796) 2 Endometrio*.tw. (2600) 3 dyschezia.tw. (41) 4 adenomyosis.tw. (210) 5 or/1‐4 (2811) 6 exp Contraceptives, Oral/ (4684) 7 (oral contraceptive* or contraceptive* pill*).tw. (4124) 8 OCP*.tw. (381) 9 exp progestins/ or 20‐alpha‐dihydroprogesterone/ or algestone/ or algestone acetophenide/ or allylestrenol/ or desogestrel/ or dydrogesterone/ or flurogestone acetate/ or gestrinone/ or progesterone/ (2893) 10 (progestogen* or gestrinone or gestagen).tw. (1308) 11 (progestin* or progesterone*).tw. (7401) 12 (desogestrel or dydrogesterone).tw. (911) 13 hormon* treatment*.tw. (1709) 14 hormon* therap*.tw. (4557) 15 exp Medroxyprogesterone Acetate/ (1147) 16 medroxyprogesterone.tw. (1992) 17 provera.tw. (152) 18 exp Intrauterine Devices, Medicated/ or exp Levonorgestrel/ (1289) 19 (levonorgestrel adj2 intrauterine).tw. (479) 20 IUD.tw. (1419) 21 (LNG* or mirena*).tw. (876) 22 medical treatment*.tw. (6164) 23 medical therap*.tw. (4862) 24 hormon* supress*.tw. (0) 25 exp Aromatase Inhibitors/ (760) 26 letrozole.tw. (1865) 27 exp gonadotropin‐releasing hormone/ or buserelin/ or goserelin/ or leuprolide/ or nafarelin/ or triptorelin pamoate/ (2723) 28 (GnRH or lhrh or gn‐rh or lfrh or lh‐rh or lhfshrh).tw. (4459) 29 Gonadotrop?in‐Releasing Hormone*.tw. (2024) 30 (gonadorelin or luliberin or luteinizing hormone‐releasing hormone or cystorelin).tw. (464) 31 (dirigestran or factrel or gonadoliberin).tw. (6) 32 (buserelin or goserelin or leuprolide or nafarelin or triptorelin).tw. (2241) 33 (deslorelin or leuprorelin).tw. (259) 34 dienogest.tw. (237) 35 GnRHa*.tw. (526) 36 zoladex.tw. (333) 37 exp Danazol/ (234) 38 danazol.tw. (430) 39 exp Mifepristone/ (542) 40 mifepristone.tw. (1029) 41 magnesium.tw. (6383) 42 zinc.tw. (5156) 43 or/6‐42 (50763) 44 exp Gynecologic Surgical Procedures/ (4640) 45 surg$.tw. (196423) 46 Laparoscopy/ (4383) 47 Laparoscop*.tw. (18877) 48 celioscop*.tw. (13) 49 peritoneoscop*.tw. (26) 50 exp Minimally Invasive Surgical Procedures/ (27606) 51 exp Surgical Procedures, Operative/ (121457) 52 nerve ablation*.tw. (70) 53 UAE.tw. (463) 54 preoperat*.tw. (34247) 55 postoperati*.tw. (91865) 56 LUNA.tw. (56) 57 perioperati*.tw. (16662) 58 (operation or operative).tw. (62303) 59 presacral neurectomy.tw. (17) 60 (pre treatment or pretreatment).tw. (21850) 61 adenomyomectom*.tw. (10) 62 or/44‐61 (319471) 63 5 and 43 and 62 (656)
Appendix 3. MEDLINE search strategy
Ovid Platform
Searched from 1946 to 20 November 2019
1 exp Endometriosis/ (19780) 2 Endometrio*.tw. (26536) 3 dyschezia.tw. (248) 4 adenomyosis.tw. (2310) 5 or/1‐4 (31207) 6 exp Contraceptives, Oral/ (44313) 7 (oral contraceptive* or contraceptive* pill*).tw. (24546) 8 OCP*.tw. (3740) 9 exp progestins/ or 20‐alpha‐dihydroprogesterone/ or algestone/ or algestone acetophenide/ or allylestrenol/ or desogestrel/ or dydrogesterone/ or flurogestone acetate/ or gestrinone/ or progesterone/ (65699) 10 (progestogen* or gestrinone or gestagen).tw. (6275) 11 (progestin* or progesterone*).tw. (84971) 12 (desogestrel or dydrogesterone).tw. (1495) 13 hormon* treatment*.tw. (10732) 14 hormon* therap*.tw. (21268) 15 exp Medroxyprogesterone Acetate/ (4689) 16 medroxyprogesterone.tw. (5877) 17 provera.tw. (931) 18 exp Intrauterine Devices, Medicated/ or exp Levonorgestrel/ (6029) 19 (levonorgestrel adj2 intrauterine).tw. (1213) 20 IUD.tw. (6669) 21 (LNG* or mirena*).tw. (2033) 22 medical treatment*.tw. (44641) 23 medical therap*.tw. (27272) 24 hormon* supress*.tw. (5) 25 exp Aromatase Inhibitors/ (6941) 26 letrozole.tw. (2399) 27 exp gonadotropin‐releasing hormone/ or buserelin/ or goserelin/ or leuprolide/ or nafarelin/ or triptorelin pamoate/ (30878) 28 (GnRH or lhrh or gn‐rh or lfrh or lh‐rh or lhfshrh).tw. (29546) 29 Gonadotrop?in‐Releasing Hormone*.tw. (15707) 30 (gonadorelin or luliberin or luteinizing hormone‐releasing hormone or cystorelin).tw. (5724) 31 (dirigestran or factrel or gonadoliberin).tw. (158) 32 (buserelin or goserelin or leuprolide or nafarelin or triptorelin).tw. (4628) 33 (deslorelin or leuprorelin).tw. (676) 34 dienogest.tw. (393) 35 GnRHa*.tw. (1426) 36 zoladex.tw. (383) 37 exp Danazol/ (2274) 38 danazol.tw. (2384) 39 exp Mifepristone/ (5702) 40 mifepristone.tw. (3210) 41 magnesium.tw. (51895) 42 zinc.tw. (102119) 43 or/6‐42 (447101) 44 exp Gynecologic Surgical Procedures/ (76582) 45 surg$.tw. (1679888) 46 Laparoscopy/ (75633) 47 Laparoscop*.tw. (110200) 48 celioscop*.tw. (555) 49 peritoneoscop*.tw. (746) 50 exp Minimally Invasive Surgical Procedures/ (455594) 51 exp Surgical Procedures, Operative/ (2847799) 52 nerve ablation*.tw. (267) 53 UAE.tw. (3068) 54 preoperat*.tw. (250462) 55 postoperati*.tw. (462096) 56 LUNA.tw. (798) 57 perioperati*.tw. (78826) 58 (operation or operative).tw. (498368) 59 presacral neurectomy.tw. (105) 60 (pre treatment or pretreatment).tw. (188874) 61 adenomyomectom*.tw. (39) 62 or/44‐61 (4108447) 63 5 and 43 and 62 (2944) 64 randomized controlled trial.pt. (458772) 65 controlled clinical trial.pt. (92329) 66 randomized.ab. (408806) 67 randomised.ab. (81650) 68 placebo.tw. (193271) 69 clinical trials as topic.sh. (183298) 70 randomly.ab. (288659) 71 trial.ti. (180948) 72 (crossover or cross‐over or cross over).tw. (76033) 73 or/64‐72 (1201786) 74 exp animals/ not humans.sh. (4446637) 75 73 not 74 (1107059) 76 63 and 75 (425)
Appendix 4. Embase search strategy
Ovid Platform
Searched from 1980 to 20 November 2019
1 exp endometriosis/ (34629) 2 Endometrio*.tw. (41965) 3 dyschezia.tw. (570) 4 adenomyosis.tw. (3843) 5 or/1‐4 (50452) 6 exp oral contraceptive agent/ (53674) 7 (oral contraceptive* or contraceptive* pill*).tw. (25819) 8 OCP*.tw. (5589) 9 exp gestagen/ (144615) 10 exp desogestrel/ (3098) 11 exp progesterone/ (76118) 12 (progestogen* or gestrinone).tw. (5821) 13 (progestin* or progesterone*).tw. (97383) 14 (desogestrel or dydrogesterone).tw. (1899) 15 gestagen*.tw. (1264) 16 hormon*?treatment*.tw. (13909) 17 hormon* therap*.tw. (33490) 18 exp medroxyprogesterone acetate/ (16160) 19 medroxyprogesterone.tw. (6828) 20 provera.tw. (3024) 21 exp intrauterine contraceptive device/ (15580) 22 exp levonorgestrel/ or exp levonorgestrel releasing intrauterine system/ (12047) 23 (levonorgestrel adj2 intrauterine).tw. (1990) 24 IUD.tw. (6438) 25 (LNG* or mirena*).tw. (4325) 26 medical treatment*.tw. (68061) 27 medical therap*.tw. (45446) 28 hormon* supress*.tw. (4) 29 exp aromatase inhibitor/ (30429) 30 letrozole.tw. (5080) 31 exp gonadorelin/ (32415) 32 exp buserelin acetate/ (1013) 33 exp goserelin/ (6981) 34 exp leuprorelin/ (11027) 35 exp nafarelin/ (985) 36 exp triptorelin/ (5247) 37 (GnRH or lhrh or gn‐rh or lfrh or lh‐rh or lhfshrh).tw. (36716) 38 Gonadotrop?in‐Releasing Hormone*.tw. (18461) 39 (gonadorelin or luliberin or luteinizing hormone‐releasing hormone or cystorelin).tw. (5453) 40 (dirigestran or factrel or gonadoliberin).tw. (278) 41 (buserelin or goserelin or leuprolide or nafarelin or triptorelin).tw. (6890) 42 (deslorelin or leuprorelin).tw. (1016) 43 dienogest.tw. (815) 44 GnRHa*.tw. (2381) 45 zoladex.tw. (2117) 46 exp danazol/ (8284) 47 danazol.tw. (3163) 48 exp mifepristone/ (12518) 49 mifepristone.tw. (4531) 50 magnesium.tw. (60310) 51 zinc.tw. (122416) 52 or/6‐51 (623725) 53 exp gynecologic surgery/ (140228) 54 surg$.tw. (2374757) 55 exp laparoscopy/ (151459) 56 Laparoscop*.tw. (193324) 57 celioscop*.tw. (309) 58 peritoneoscop*.tw. (670) 59 exp minimally invasive surgery/ (39825) 60 surgery/ (507176) 61 nerve ablation*.tw. (519) 62 UAE.tw. (5606) 63 preoperat*.tw. (370655) 64 postoperati*.tw. (656692) 65 LUNA.tw. (1474) 66 perioperati*.tw. (131286) 67 (operation or operative).tw. (710854) 68 presacral neurectomy.tw. (108) 69 (pre treatment or pretreatment).tw. (250913) 70 adenomyomectom*.tw. (103) 71 or/53‐70 (3351639) 72 5 and 52 and 71 (6181) 73 Clinical Trial/ (949846) 74 Randomized Controlled Trial/ (576640) 75 exp randomization/ (84997) 76 Single Blind Procedure/ (37214) 77 Double Blind Procedure/ (164636) 78 Crossover Procedure/ (61263) 79 Placebo/ (329802) 80 Randomi?ed controlled trial$.tw. (216279) 81 Rct.tw. (34845) 82 random allocation.tw. (1952) 83 randomly.tw. (423005) 84 randomly allocated.tw. (33698) 85 allocated randomly.tw. (2476) 86 (allocated adj2 random).tw. (810) 87 Single blind$.tw. (23712) 88 Double blind$.tw. (197195) 89 ((treble or triple) adj blind$).tw. (1049) 90 placebo$.tw. (294010) 91 prospective study/ (565049) 92 or/73‐91 (2340314) 93 case study/ (65394) 94 case report.tw. (385251) 95 abstract report/ or letter/ (1070443) 96 or/93‐95 (1511200) 97 92 not 96 (2287942) 98 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5834925) 99 97 not 98 (2129230) 100 72 and 99 (1199)
Appendix 5. PsycINFO search strategy
Ovid Platform
Searched from 1806 to 20 November 2019
1 exp gynecological disorders/ (1767) 2 endometrio$.tw. (273) 3 adenomyosis.tw. (9) 4 or/1‐3 (1961) 5 exp oral contraceptives/ (932) 6 oral contraceptive$.tw. (1459) 7 contraceptive$ pill$.tw. (314) 8 OCP.tw. (125) 9 exp gonadotropic hormones/ (4178) 10 gonadotropic hormone$.tw. (65) 11 (GnRH or lhrh or gn‐rh or lfrh or lh‐rh or lhfshrh).tw. (1196) 12 Gonadotropin‐Releasing Hormone.tw. (758) 13 (gonadorelin or luliberin or luteinizing hormone‐releasing hormone or cystorelin).tw. (233) 14 (dirigestran or factrel or gonadoliberin).tw. (2) 15 drug therapy/ or exp hormone therapy/ (135651) 16 danazol.tw. (16) 17 exp progestational hormones/ (2357) 18 (progestin$ or progestogen$ or gestrinon$ or progesterone).tw. (4703) 19 progestagen$.tw. (36) 20 or/5‐19 (145075) 21 exp Surgery/ (69152) 22 exp gynecology/ (792) 23 surg$.tw. (47921) 24 Laparoscop$.tw. (489) 25 or/21‐24 (98994) 26 4 and 20 and 25 (31) 27 random.tw. (56626) 28 control.tw. (433547) 29 double‐blind.tw. (22457) 30 clinical trials/ (11485) 31 placebo/ (5406) 32 exp Treatment/ (1020157) 33 or/27‐32 (1407757) 34 26 and 33 (24)
Appendix 6. CINAHL search strategy
Ebsco Platform
Searched from 1961 to 20 November 2019
S69 S56 AND S68 120 S68 S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 OR S66 OR S67 1,358,983 S67 TX allocat* random* 11,111 S66 (MH "Quantitative Studies") 23,702 S65 (MH "Placebos") 11,481 S64 TX placebo* 59,846 S63 TX random* allocat* 11,111 S62 (MH "Random Assignment") 56,174 S61 TX randomi* control* trial* 178,064 S60 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) 1,035,594 S59 TX clinic* n1 trial* 253,599 S58 PT Clinical trial 86,295 S57 (MH "Clinical Trials+") 269,268 S56 S42 AND S55 553 S55 S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 936,428 S54 TX adenomyomectom* 33 S53 TX (pre treatment or pretreatment) 19,876 S52 TX presacral neurectomy 14 S51 TX(operation or operative) 119,100 S50 TX LUNA 2,059 S49 TX (preoperat* or postoperati* or perioperat*) 299,304 S48 TX UAE 1,711 S47 TX nerve ablation* 312 S46 TX Laparoscop* 32,595 S45 (MM "Surgery, Laparoscopic+") 4,646 S44 TX surg* 804,638 S43 (MM "Minimally Invasive Procedures") OR (MM "Surgery, Urogenital+") OR (MM "Ultrasonic Surgical Procedures") OR (MM "Bloodless Medical and Surgical Procedures") 32,337 S42 S3 AND S41 1,081 S41 S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 103,166 S40 TX (magnesium or zinc) 14,915 S39 TX Mifepristone 1,166 S38 (MM "Mifepristone") 534 S37 TX danazol 244 S36 (MM "Danazol") 58 S35 TX zoladex 26 S34 TX GnRHa* 148 S33 TX dienogest 164 S32 TX (deslorelin or leuprorelin) 60 S31 TX (buserelin or goserelin or leuprolide or nafarelin or triptorelin) 826 S30 TX(dirigestran or factrel or gonadoliberin) 1 S29 TX (gonadorelin or luliberin or luteinizing hormone‐releasing hormone or cystorelin) 1,657 S28 TX Gonadotrop?in‐Releasing Hormone* 133 S27 TX (GnRH or lhrh or gn‐rh or lfrh or lh‐rh or lhfshrh) 1,196 S26 (MM "Nafarelin") 7 S25 (MM "Leuprolide") 180 S24 (MM "Goserelin") 120 S23 (MM "Gonadorelin+") 1,069 S22 TX letrozole 805 S21 TX medical therap* 19,875 S20 TX medical treatment* 22,400 S19 TX (LNG* or mirena*) 618 S18 TX(levonorgestrel N2 intrauterine) 611 S17 (MM "Levonorgestrel") 867 S16 (MM "Intrauterine Devices") 1,709 S15 TX provera 187 S14 TX Medroxyprogesterone 1,667 S13 TX hormon* therap* 28,031 S12 TX hormon* treatment* 3,337 S11 TX(desogestrel or dydrogesterone) 173 S10 TX (progestin* or progesterone*) 8,461 S9 TX (progestogen* or gestrinone or gestagen) 754 S8 TX (progestin* or progesterone*) 8,461 S7 (MM "Progestational Hormones+") OR (MM "Hormone Replacement Therapy") OR (MM "Medroxyprogesterone Acetate") 7,921 S6 TX OCP* 466 S5 TX(oral contraceptive* or contraceptive* pill*) 8,097 S4 (MM "Contraceptives, Oral") 2,747 S3 S1 OR S2 6,661 S2 TX Endometrio* 6,661 S1 (MM "Endometriosis") 3,338
Appendix 7. Data extraction table
| Study Background/risk of bias extracted by | |
| Study identification | |
| Covidence study number | |
| Covidence study name, year | |
| Citation (Authors, title, journal, year, volume, issue, page) | |
| PMID | |
| First author | |
| Funding/Sponsor | |
| Study Location/Country(ies) | |
| No. of centers | |
| Study design | |
| Hypothesis tested (based on hypothesis tag) (H1 to H5) H1: Medical therapy for hormonal suppression of endometriosis prior to surgery is more effective than surgery alone (no medical therapy). H2: Medical therapy for hormonal suppression of endometriosis after surgery is more effective than surgery alone (placebo or no medical therapy). H3: Medical therapy for hormonal suppression of endometriosis before and after surgery is more effective than surgery alone. H4: Medical therapy for hormonal suppression of endometriosis before surgery is more effective than medical therapy for hormonal suppression of endometriosis after surgery. H5: Medical therapy for hormonal suppression of endometriosis pre and post surgery is more effective than medical therapy post surgery. | |
| Study Design (RCT, Parallel/Cross‐over) | |
| Blinding (Not mentioned/No/Single/Double/Triple) | |
| Duration of intervention (medical therapy) | |
| Recruitment period (From ‐ To) | |
| 2nd look laparoscopy (Yes/No, If yes‐ timing) | |
| Follow‐up period | |
| Inclusion criteria | |
| Age (years) | |
| Endometriosis ‐ method of diagnosis (laparotomy, Laparoscopy, ultrasound, histology, Not clear) | |
| Endometriosis ‐ staging classification used | |
| Endometriosis ‐ stage (list stages, unclear) | |
| other (including general comments) | |
| Exclusion criteria | |
| Age | |
| Endometriosis ‐ stage (list stages, unclear) | |
| Prior hormonal therapy (medicines, duration | |
| Medical illnesses | |
| Surgery | |
| Other | |
| Pain evaluation scale(s) used | |
| Power calculation done | |
| Premature stoppage of study | |
| Primary outcomes (list) | |
| Secondary outcomes (list) | |
| outcome assessment method, frequency | |
| Risk of Bias | |
| Randomization /Random sequence generation (Unclear/Low/High risk) | |
| Allocation concealment (Unclear/Low/High risk) | |
| Blinding of participants and personnel (Unclear/Low/High risk) | |
| Blinding of outcome assessment (Unclear/Low/High risk) | |
| Incomplete outcome data (attrition bias) (Unclear/Low/High risk) | |
| Selective reporting (Unclear/Low/High risk) | |
| Other bias (Unclear/Low/High risk) | |
| Comments on Risk of Bias | |
| Study Participants | |
| Number assessed | |
| Number invited (Total) | |
| Number randomized | |
| Number NOT completed study | |
| Difference between groups at baseline | |
| Stratification | |
| Group 1 ‐ Treatment/Intervention (medicine, dose, route, timing) | |
| Group 2 ‐ Control ‐ (Placebo or no medical therapy) | |
| Group 3 ‐ Another Treatment/Intervention (medicine, dose, route, timimg | |
| Group 4 ‐ Another control group | |
| Name of data extractor | |
| Statistical Data ‐ Group label in study | |
| No. of participants started intervention | |
| No. of participants completed intervention | |
| No. of participants analysed | |
| Outcomes | |
| 1 Presurgical medical therapy compared to no medical therapy for endometriosis surgery | |
| 1.1 Recurrence ‐ AFS Score | |
| 1.1.1 Total AFS | |
| 1.1.1 Mean | |
| 1.1.1 SD | |
| 1.1.1 Total (n) | |
| 1.1.2 Implant AFS | |
| 1.1.2 Mean | |
| 1.1.2 SD | |
| 1.1.2 Total (n) | |
| 1.1.3 Adhesion AFS | |
| 1.1.3 Mean | |
| 1.1.3 SD | |
| 1.1.3 Total (n) | |
| Any Data with comments for H1 that does not fit in above cells (Please be descriptive in recording such data) | |
| 2 Postsurgical medical therapy versus placebo or no medical therapy | |
| Type and time of last outcome assessment (if less than 12 months) | |
| Any special comment/problem with outcome data extraction for this type of studies | |
| 2.1 Pain (VAS) | |
| 2.1.1 Pelvic pain at 12 months | |
| 2.1.1 Mean | |
| 2.1.1 SD | |
| 2.1.1 Total (n) | |
| 2.1.2 Dysmenorrhoea at 12 months | |
| 2.1.2 Mean | |
| 2.1.2 SD | |
| 2.1.2 Total (n) | |
| 2.1.3 Deep Dyspareunia at 12 months | |
| 2.1.3 Mean | |
| 2.1.3 SD | |
| 2.1.3 Total (n) | |
| 2.1.4 Change in pelvic pain score at 12 months | |
| 2.1.4 Mean | |
| 2.1.4 SD | |
| 2.1.4 Total (n) | |
| 2.2 Pain (dichotomous) | |
| 2.2.1 Pain recurrence </= 12 months | |
| 2.2.1 Events | |
| 2.2.1 Total (n) | |
| 2.2.2 Pain recurrence 13‐24 months | |
| 2.2.2 Events | |
| 2.2.2 Total (n) | |
| 2.2.3 pain persistence/recurrence 5 years | |
| 2.2.3 Events | |
| 2.2.3 Total (n) | |
| 2.3 Recurrence ‐ AFS Score | |
| 2.3.1 Total AFS score (12 months) | |
| 2.3.1 Mean | |
| 2.3.1 SD | |
| 2.3.1 Total (n) | |
| 2.4 Disease/symptom recurrence | |
| 2.4.1 Disease/symptoms recurrence at 12 months | |
| 2.4.1 Events | |
| 2.4.1 Total (n) | |
| 2.4.2 Disease/symptoms recurrence at 24 months | |
| 2.4.2 Events | |
| 2.4.2 Total (n) | |
| 2.5 Pregnancy | |
| 2.5 Events | |
| 2.5 Total (n) | |
| 2.6 Recurrence EEC stage Not sure | |
| 2.6.1 EEC stage 0 events | |
| 2.6.2 EEC stage I events | |
| 2.6.3 EEC stage II events | |
| 2.6.4 EEC stage III events | |
| 2.6.5 EEC stage Total (n) | |
| Any Data for H2 that does not fit in above cells (Please be descriptive in recording such data) | |
| 3 Before and after medical therapy versus surgery alone | |
| Any Data with comments for H3 that does not fit in above cells (Please be descriptive in recording such data) | |
| 4 Presurgical versus postsurgical medical therapy | |
| 4.1 Pain (Dichotomous) | |
| 4.1.1 Dysmenorrhoea | |
| 4.1.1 Events | |
| 4.1.1 Total (n) | |
| 4.1.2 Dyspareunia | |
| 4.1.2 Events | |
| 4.1.2 Total (n) | |
| 4.1.3 Pelvic pain | |
| 4.1.3 Events | |
| 4.1.3 Total (n) | |
| 4.1.4 Pelvic tenderness | |
| 4.1.4 Events | |
| 4.1.4 Total (n) | |
| 4.1.5 Pelvic induration | |
| 4.1.5 Events | |
| 4.1.5 Total (n) | |
| Any Data with comments for H4 that does not fit in above cells (Please be descriptive in recording such data) | |
| 5 Postsurgical medical therapy versus pre‐ and postsurgical medical therapy with GnRHa | |
| 5.1 Recurrence ‐ AFS Score | |
| 5.1.1 Total AFS score | |
| 5.1.1 Mean | |
| 5.1.1 SD | |
| 5.1.1 Total (n) | |
| 5.1.2 Implant AFS score | |
| 5.1.2 Mean | |
| 5.1.2 SD | |
| 5.1.2 Total (n) | |
| 5.1.3 Adhesion AFS score | |
| 5.1.3 Mean | |
| 5.1.3 SD | |
| 5.1.3 Total (n) | |
| 5.2 Pregnancy Rate | |
| 5.2 Events | |
| 5.2 Total (n) | |
| Any Data with comments for H5 that does not fit in above cells (Please be descriptive in recording such data) |
Appendix 8. Criteria for judging risk of bias in the 'Risk of bias' assessment tool
Adequate sequence generation: use of a random number table, use of a computerized system, central randomization by statistical co‐ordinating centre, randomization by an independent service using minimization technique, permuted block allocation or Zelan technique were considered adequate. If the paper merely stated 'randomized' or 'randomly allocated' with no further information this was assessed as being unclear.
Allocation concealment: centralized allocation including access by telephone call or fax, or pharmacy‐controlled randomization, using sequentially numbered, sealed opaque envelopes were considered adequate. Where there was no mention of allocation concealment methods, this domain was assessed as unclear.
Blinding of participants and personnel: lack of blinding of participants, carers, or people delivering the interventions may cause bias in the estimated effects of both assignment to intervention and of adhering to intervention. Unless the trial was specifically described as double blind, or there was a statement about blinding in the methods section of the paper, it was assumed that blinding of participants and clinical staff did not occur.
Blinding of outcome assessment: blinding of outcome assessors to avoid bias in measuring the outcome was considered separately. Unless the trial specifically described the blinding of outcome assessors, it was assumed that blinding of outcome assessors did not occur.
Outcome data: outcome data were considered complete if all participants randomized were included in the analysis of the outcome(s).
Selective outcome reporting: a trial was assessed as being at low risk of bias due to selective outcome reporting if the outcomes of interest described in the methods section were systematically reported in the results section. Where reported outcomes did not include those outcomes specified or expected in trials of treatments for endometriosis, this domain was assessed as unclear.
Other bias: imbalance in potentially important prognostic factors between the treatment groups at baseline, or the use of a co‐intervention in only one group (e.g. analgesics) were examples of potential sources of bias that were noted.
Data and analyses
Comparison 1. Presurgical medical therapy compared with placebo or no medical therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain recurrence (dichotomous) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Pain recurrence ≤ 12 months | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.72, 1.66] |
| 1.1.2 Dysmenorrhoea recurrence ≤ 12 months | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.92, 2.21] |
| 1.1.3 Dyspareunia recurrence ≤ 12 months | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.88, 2.44] |
| 1.2 Disease recurrence (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Disease recurrence at 3 months – total (AFS score) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐9.60 [‐11.42, ‐7.78] |
| 1.2.2 Disease recurrence at 3 months – implant (AFS score) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐8.70 [‐10.67, ‐6.73] |
| 1.2.3 Disease recurrence at 3 months – adhesion (AFS score) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐3.42, 1.62] |
| 1.3 Disease recurrence (dichotomous) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Disease recurrence ≤ 12 months (EEC stage) | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.86, 1.43] |
| 1.4 Pregnancy rate (dichotomous) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
1.1. Analysis.

Comparison 1: Presurgical medical therapy compared with placebo or no medical therapy, Outcome 1: Pain recurrence (dichotomous)
1.2. Analysis.

Comparison 1: Presurgical medical therapy compared with placebo or no medical therapy, Outcome 2: Disease recurrence (continuous)
1.3. Analysis.

Comparison 1: Presurgical medical therapy compared with placebo or no medical therapy, Outcome 3: Disease recurrence (dichotomous)
1.4. Analysis.

Comparison 1: Presurgical medical therapy compared with placebo or no medical therapy, Outcome 4: Pregnancy rate (dichotomous)
Comparison 2. Postsurgical medical therapy compared with placebo or no medical therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Pain (continuous) | 6 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 Pelvic pain ≤ 12 months (visual analogue scale (VAS)) | 3 | 340 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.79 [‐1.02, ‐0.56] |
| 2.1.2 Dysmenorrhoea ≤ 12 months (VAS) | 2 | 287 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.62, ‐0.14] |
| 2.1.3 Dyspareunia ≤ 12 months (VAS) | 2 | 287 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.67, ‐0.20] |
| 2.1.4 Pain ≤ 12 months (36‐item Short Form (SF‐36) pain score) | 1 | 159 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.21, 0.42] |
| 2.1.5 Change in pelvic pain score ≤ 12 months (VAS) | 2 | 129 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.60, 0.09] |
| 2.1.6 Change in dysmenorrhea score ≤ 12 months (VAS) | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.90, 0.34] |
| 2.1.7 Change in dyspareunia score ≤ 12 months (VAS) | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.75, 0.49] |
| 2.1.8 Change in overall pain score ≤ 12 months (VAS) | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.94, 0.31] |
| 2.2 Pain recurrence (dichotomous) | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.2.1 Pain recurrence ≤ 12 months | 5 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.52, 0.94] |
| 2.2.2 Pain recurrence 13–24 months | 3 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.47, 1.03] |
| 2.2.3 Pain persistence/recurrence at 5 years | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.53, 1.66] |
| 2.2.4 Dysmenorrhoea recurrence ≤ 12 months | 1 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.50, 1.35] |
| 2.2.5 Dyspareunia recurrence ≤ 12 months | 1 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.27, 1.03] |
| 2.3 Disease recurrence (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 Disease recurrence at 12 months – total (American Fertility Society (AFS) score) | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐2.29 [‐4.01, ‐0.57] |
| 2.4 Disease recurrence (dichotomous) | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.4.1 Disease recurrence ≤ 12 months | 4 | 433 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.17, 0.54] |
| 2.4.2 Disease recurrence 13–24 months | 4 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.27, 0.58] |
| 2.4.3 Disease recurrence ≤ 12 months (Endoscopic Endometriosis Classification (EEC) stage) | 1 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.67, 1.15] |
| 2.5 Pregnancy rate (dichotomous) | 11 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.02, 1.38] |
2.1. Analysis.

Comparison 2: Postsurgical medical therapy compared with placebo or no medical therapy, Outcome 1: Pain (continuous)
2.2. Analysis.

Comparison 2: Postsurgical medical therapy compared with placebo or no medical therapy, Outcome 2: Pain recurrence (dichotomous)
2.3. Analysis.

Comparison 2: Postsurgical medical therapy compared with placebo or no medical therapy, Outcome 3: Disease recurrence (continuous)
2.4. Analysis.

Comparison 2: Postsurgical medical therapy compared with placebo or no medical therapy, Outcome 4: Disease recurrence (dichotomous)
2.5. Analysis.

Comparison 2: Postsurgical medical therapy compared with placebo or no medical therapy, Outcome 5: Pregnancy rate (dichotomous)
Comparison 3. Presurgical medical therapy compared with postsurgical medical therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Pain recurrence (dichotomous) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1.1 Dysmenorrhoea recurrence ≤ 12 months | 2 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.09, 2.74] |
| 3.1.2 Dyspareunia recurrence ≤ 12 months | 2 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [1.68, 5.62] |
| 3.1.3 Pelvic pain recurrence ≤ 12 months | 2 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.95, 2.07] |
| 3.1.4 Pelvic tenderness ≤ 12 months | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.52, 1.72] |
| 3.1.5 Pelvic induration ≤ 12 months | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.94, 2.81] |
| 3.2 Disease recurrence (dichotomous) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.2.1 Disease recurrence ≤ 12 months (Endoscopic Endometriosis Classification (EEC) stage) | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.97, 1.65] |
| 3.3 Pregnancy rate (dichotomous) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
3.1. Analysis.

Comparison 3: Presurgical medical therapy compared with postsurgical medical therapy, Outcome 1: Pain recurrence (dichotomous)
3.2. Analysis.

Comparison 3: Presurgical medical therapy compared with postsurgical medical therapy, Outcome 2: Disease recurrence (dichotomous)
3.3. Analysis.

Comparison 3: Presurgical medical therapy compared with postsurgical medical therapy, Outcome 3: Pregnancy rate (dichotomous)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alkatout 2013.
| Study characteristics | ||
| Methods | No. of centres: 1 Location: Department of Obstetrics & Gynaecology, Kiel University, Kiel, Germany Recruitment period: NR |
|
| Participants | Inclusion criteria: aged 18–44 years with symptomatic endometriosis in whom 2 consecutive laparoscopic interventions were to be assessed. Undergoing diagnostic hysteroscopy and laparoscopy for treatment of endometriosis. Endometriosis diagnosed or confirmed at laparoscopy, rated according to the EEC introduced by Kurt Semm and Liselotte Mettler Exclusion criteria: deep endometriosis with bladder or rectum excision; previous hormone therapy for endometrioses; previous surgery for endometriosis No. randomized: 450 No. analyzed: 410 |
|
| Interventions | Postsurgical medical therapy Group 1: (n = 150) medical therapy, leuprorelin acetate 3.75 mg depot SC monthly for 3 months Group 2: (n = 150) surgical therapy Group 3: (n = 150) combined (medical + surgical) treatment, surgery + leuprorelin acetate 3.75 mg depot SC monthly for 3 months |
|
| Outcomes | Response rate to EEC stages 0 and 1 of ≥ 75% Recurrence rate of pain (dysmenorrhoea, dyspareunia, abdominal pain) Pregnancy rate |
|
| Notes | Power calculation: NR Funding: none declared Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | All participants were allocated exactly according to the random principle. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not mentioned, not placebo controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not blinded, no blinding of outcome assessment. For pregnancy rate and EEC stage, detection bias was low risk. For pain as high risk of bias, as this represents a subjective outcome measure. Therefore, overall detection bias as unclear. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 410/450 participants returned for second‐look pelviscopy and assessment of finding. Unevenly divided over 3 arms. |
| Selective reporting (reporting bias) | Low risk | All main outcomes reported. |
| Other bias | Unclear risk | insufficient information. |
Angioni 2015.
| Study characteristics | ||
| Methods | No. of centres: 1 Location: Department of Obstetrics and Gynecology, University of Cagliari, Italy Recruitment period: January 2006 to December 2011 |
|
| Participants | Inclusion criteria: reproductive age, ≤ 40 years. Underwent a complete biochemical, ultrasonographic, and MRI evaluation. presurgical diagnostic hysteroscopy performed Laparoscopic diagnosis of deep endometriosis with complete or incomplete surgical treatment. Participants symptom score before surgery was required to have a total score of ≥ 6 (of a possible 15), including a total of ≥ 2 in the symptoms of dysmenorrhoea, dyspareunia, and pelvic pain. Exclusion criteria: previous medical or surgical therapy for endometriosis, infiltrating in the rectum > 3 cm or rectal stenosis (Enzian score E4c), or both; presence of other disease that might cause pelvic pain and diagnosis of liver, endocrine, or neoplastic disease No. randomized: 159 No. analyzed: 142 |
|
| Interventions | Postsurgical medical therapy Group 1: (n = 80) participants in which a complete excision of all endometriotic implant was achieved during surgery Group 2: (n = 79) participants in whom surgery did not allowed a complete removal of all infiltrating implants for lack of consent to a radical excision |
|
| Outcomes | Modified Biberoglu and Behrman symptom scale; 0 (no discomfort) to 3 (severe symptoms) in each of 5 categories namely, dysmenorrhoea, dyspareunia, pelvic pain, pelvic tenderness, and induration. Score range 6–15, based on participant diary and monthly pelvic examination SF‐36 fulfilled by participant before surgery and at 1‐year follow‐up |
|
| Notes | Power calculation: yes Funding: none declared Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomizations sequence. |
| Allocation concealment (selection bias) | Unclear risk | NR. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not mentioned, not placebo controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, no blinding of outcome assessment. Pain represents a subjective outcome measure; therefore, detection bias is assigned as high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17/159 participants withdrew. 10 in group 1 (complete excision) and 7 in group 2 (incomplete excision). Group 1 (1A + 1B = without GnRHas) 12 withdrew. Group 2 (2A + 2B = with GnRHas) 5 withdrew. All because they got pregnant or need hormonal therapy or repeated surgery for important recurrence of pain. |
| Selective reporting (reporting bias) | Low risk | All main outcomes were reported. |
| Other bias | Low risk | The groups were similar with respect to their demographic and clinical characteristics. |
Audebert 1998.
| Study characteristics | ||
| Methods | Location: France No. of centres: multicentre Recruitment period: December 1990 to March 1993 |
|
| Participants | Inclusion criteria: aged < 40 years, stage III–IV endometriosis, pelvic pain, dysmenorrhoea, or dyspareunia Exclusion criteria: aged > 40 years, hormonal therapy for endometriosis within 3 months (including OCP, progestins); significant medical illness, e.g. liver, heart, renal disease; abnormal PAP smear; pregnancy; surgery for endometriosis within 6 months No. randomized: 55 No. analyzed: 53 |
|
| Interventions | Group 1: (n = 28) presurgery medical therapy with nafarelin nasal 400 μg daily for 6 months Group 2: (n = 25) postsurgery medical therapy with nafarelin nasal 400 μg daily for 6 months |
|
| Outcomes | Pain: dysmenorrhoea, dyspareunia, pelvic pain, pelvic tenderness, pelvic induration AFS scores: global, adhesions, endometriosis Ease of surgery |
|
| Notes | Power calculation: NR Funding: Syntex Pharmaceuticals International for supply of Nafarelin, grant for trial Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised;" no details of method of sequence generation provided. |
| Allocation concealment (selection bias) | Unclear risk | NR. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind, probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants included in analysis. |
| Selective reporting (reporting bias) | Low risk | Important outcomes – AFS scores, recurrence, and surgical difficulty reported. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
Bianchi 1999.
| Study characteristics | ||
| Methods | No. of centres: 1 Location: University of Milan, Italy Recruitment period: July 1994 to October 1996 |
|
| Participants | Inclusion criteria: aged < 40 years Exclusion criteria: medical or surgical treatment for endometriosis, concurrent disease that might affect fertility or cause pelvic pain, no pain symptoms, not seeking pregnancy, liver or endocrine disease No. randomized: 77 No. analyzed: 77 |
|
| Interventions | Postsurgical medical therapy Group 1: (n = 36) danazol oral 600 mg daily for 3 months Group 2: (n = 41) no therapy |
|
| Outcomes | Pain recurrence AFS scores Pregnancy rates Adverse events of medication |
|
| Notes | Power calculation: NR Funding: NR Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done according to a computer generated list." |
| Allocation concealment (selection bias) | Unclear risk | NR. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NR, no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not blinded, no blinding of outcome assessment. For pregnancy rate and AFC score assigned as low risk. For pain assigned as high risk of bias, as this represents a subjective outcome measure. Therefore, overall detection bias as unclear risk assigned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants included in analyses. |
| Selective reporting (reporting bias) | Low risk | Important outcomes – pregnancy rate, recurrence of endometriosis pain, and adverse effects reported. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
Busacca 2001.
| Study characteristics | ||
| Methods | No. of centres: 1 Location: University of Milan, Italy Recruitment period: July 1997 to December 1999 |
|
| Participants | Inclusion criteria: aged < 40 years, laparoscopic diagnosis of endometriosis stage III–IV Exclusion criteria: previous medical or surgical therapy for endometriosis; other diseases that might affect fertility or cause pelvic pain; liver, endocrine, or neoplastic disease No. randomized: 89 No. analyzed: 89 |
|
| Interventions | Postsurgical medical therapy Group 1: (n = 44) leuprolide acetate SC 3.5 mg 4 weekly × 3 doses Group 2: (n = 45) no therapy |
|
| Outcomes | Pain: pelvic pain recurrence at 18 months AFS scores: objective disease recurrence Cumulative pregnancy rates at 18 months |
|
| Notes | Power calculation: yes Funding: NR Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed according to a computer‐generated list unknown to the physicians. |
| Allocation concealment (selection bias) | Unclear risk | NR. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NR, no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR, no placebo. For pregnancy rate and AFC score assigned as low risk. For pain assigned as high risk of bias, as this represents a subjective outcome measure. Therefore, overall detection bias as unclear risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Important outcomes of pregnancy and recurrence of endometriosis and pain reported. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
Cucinella 2013.
| Study characteristics | ||
| Methods | No. of centres: 3 Location: Departments of Obstetric and Gynaecology at the universities of Naples, Messinna, and Palermo, Italy Recruitment period: September 2009 to August 2010 |
|
| Participants | Inclusion criteria: aged 18–40 years, not attempting to conceive, either at time of study entry or for ≥ 2 years after surgery; conservative surgery for ovarian endometrioma (stripping technique), staging by the ASRM. Exclusion criteria: previously undergone surgical treatment for endometriosis or taken recent medical therapy for this pathology (stopped ≥ 6 months before surgery); contraindications for OCPs; did not wish to postpone pregnancy for ≥ 2 years after surgery No. randomized: 130 (+38 non‐users) No. analyzed: 130 |
|
| Interventions | Postsurgical medical therapy Group 1: (n = 38) non‐users Group 2: (n = 43) monophasic pill with ethinyl estradiol 20 μg and desogestrel 0.15 mg daily Group 3: (n = 44) monophasic pill with ethinyl estradiol 20 μg and gestodene 0.075 mg daily Group 4: (n = 43) multiphasic pill with oestradiol valerate 2 mg for 22 days, with dienogest 2 mg for first 5 days and 3 mg on the remaining 17 days, the other 4: pill with only oestradiol valerate and 2: placebo pill |
|
| Outcomes | Endometrioma recurrence Timing of recurrence endometrioma Cumulative recurrence Endometrioma diameter Menstrual bleeding profile |
|
| Notes | Power calculation: NR Funding: none declared Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomizations sequence. |
| Allocation concealment (selection bias) | Low risk | Numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial participants, key personnel who performed trial, and outcome assessors did not know to which group the trial participants had been assigned and they did not know the randomizations schedule, which was kept by an external person until the code break after the study completion. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Scans were performed by experienced operators, blinded to study allocation. Data analysis was performed by different operators than those who performed trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not completed study: A = 21% (n = 8, 2 pregnancy, 2 started OCPs because of referred dysmenorrhoeal, 3 unrelated, 1 excluded for cyst persistence at 1‐month follow‐up). B/C/D = 22% (n = 29, 14 unrelated, 11 adverse events (8,5%), 4 cyst persistence at 1‐month follow‐up). |
| Selective reporting (reporting bias) | Low risk | All main outcomes are reported. |
| Other bias | Unclear risk | No other source of bias identified. |
Donnez 1994.
| Study characteristics | ||
| Methods | No. of centres: 1 Location: Catholic University of Louvain, Belgium Recruitment period: January 1990 to December 1990 |
|
| Participants | Inclusion criteria: aged < 35 years, infertility, laparoscopic confirmed ovarian endometriotic cysts (AFS moderate n = 41, AFS severe n = 39) Exclusion criteria: none No. randomized: 80 No. analyzed: 80 |
|
| Interventions | Medical therapy presurgery Group 1: (n = 40) goserelin SC 4 weekly × 4 Group 2: (n = 40) no therapy |
|
| Outcomes | AFS scores: total, implants, adhesions, moderate, severe scores Ovarian cyst diameter Degree of active endometriosis as determined histologically from ovarian cyst wall biopsy |
|
| Notes | Power calculation: NR Funding: Fonds de la Recherche Scientifique Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised according to official randomisation tables." |
| Allocation concealment (selection bias) | Unclear risk | NR. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NR, no placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | NR, no placebo used. Outcome measures were objective measurements, therefore, assigned low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants included in outcome assessment. |
| Selective reporting (reporting bias) | Unclear risk | AFS scores at second‐look laparoscopy – no pregnancy data reported. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
Hornstein 1997.
| Study characteristics | ||
| Methods | No. of centres: 13 Location: North America Recruitment period: NR |
|
| Participants | Inclusion criteria: aged 18–47 years, normal menstrual cycles of 24–36 days, clinical pelvic pain, dysmenorrhoea, dyspareunia Exclusion criteria: received medical therapy for endometriosis within 3 months, abnormal bone density, significant medical illness, laboratory abnormalities, pregnancy, and lactation No. randomized: 109 No. analyzed: 93 7 in nafarelin group and 8 in placebo group withdrew after 90 days of therapy 1 in placebo group excluded because missed 5 days of medication |
|
| Interventions | Postsurgery medical therapy Group 1: (n = 49) nafarelin nasal 400 μg daily × 6 months Group 2: (n = 44) placebo |
|
| Outcomes | Pain Physician scores for tenderness and induration on physical examination at end of medical therapy and 6 months after medical therapy |
|
| Notes | Power calculation: NR Funding: Syntex laboratory, CA Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "multicenter, prospective, randomised, double‐blind study." Comment: method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | NR. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double blind" but authors acknowledged difficulty of maintaining blinding with this treatment. Placebo controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double blind" but authors acknowledged difficulty of maintaining blinding with this treatment. Placebo controlled. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants in medical therapy group and 8 participants in placebo group were excluded from analyses because they withdrew before completing 90 days of therapy. Remaining 93 participants included in analysis even though 39 in medical therapy group and 43 in placebo group terminated the study early. |
| Selective reporting (reporting bias) | Low risk | Primary outcome was time to requiring alternative treatment; pain and recurrence also reported. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
Huang 2018.
| Study characteristics | ||
| Methods | No. of centres: 1 Location: Department of Gynaecology of Wuhan Children's Hospital, Tongji Medical College, Huazhong University of Science & Technology, China Recruitment period: January 2011 to December 2014 |
|
| Participants | Inclusion criteria: women with endometriosis confirmed by histology; not planning to conceive immediately; no contraindications against laparoscopy and GnRHas Exclusion criteria: hormone therapy 3 months prior to surgery; endocrine, immune, metabolic diseases, or malignant tumours; laparoscopy or GnRHas previously; contraindications against either laparoscopy or GnRHas No. randomized: 100 No. analyzed: 100 |
|
| Interventions | Postsurgery medical therapy Group 1: (n = 50) GnRHas Group 2: (n = 50) surgery alone |
|
| Outcomes | Pain scores Complications Gynaecological examination results, adjuvant examination results Clinical efficacy (including recurrence rate) |
|
| Notes | Power calculation: NR Funding: none Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used. |
| Allocation concealment (selection bias) | Unclear risk | NR. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding, not placebo controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, no blinding of outcome assessment. Pain represents a subjective outcome measure, therefore detection bias assigned as high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All main outcomes were reported. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
Loverro 2001.
| Study characteristics | ||
| Methods | No. of centres: 1 Location: Bari, Italy Recruitment period: January 1996 to January 1997 |
|
| Participants | Inclusion criteria: AFS score III–IV Exclusion criteria: NR No. randomized: 62 No. analyzed: 62? |
|
| Interventions | Postsurgery medical therapy Group 1: (n = 33) triptorelin 3.75 mg SC every 4 weeks × 3 months Group 2: (n = 29) no therapy |
|
| Outcomes | Pain Pregnancy rates |
|
| Notes | Power calculation: NR Funding: NR Clinical trial registration number: NR Pregnancy outcomes and pain recurrence were only expressed as percentages in each group so numbers calculated were rounded up to the nearest whole number. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Prospective and randomised" – no details of method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | NR. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NR, information missing to determine the risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR, information missing to determine the risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated how many women are included in the outcomes – only percentages reported. |
| Selective reporting (reporting bias) | Low risk | Important outcomes – time to relapse (pelvic pain) and pregnancy reported. |
| Other bias | Unclear risk | No information on comparability of groups at baseline given. |
Loverro 2008.
| Study characteristics | ||
| Methods | No. of centres: 1 Location: Italy Recruitment period: January 1998 to January 1999 |
|
| Participants | Inclusion criteria: women of reproductive age with stage III–IV endometriosis, associated with chronic pelvic pain, adnexal mass, or infertility, who had undergone complete laparoscopic excision, had rAFS score > 15 and no previous hormonal therapy Exclusion criteria: NR No. randomized: 60 No. analyzed: 54 |
|
| Interventions | Postsurgical triptorelin vs placebo Group 1: (n = 29) triptorelin 3.75 mg depot monthly on day 20 of cycle for 3 months Group 2: (n = 25) placebo monthly on day 20 of cycle for 3 months |
|
| Outcomes | Pain persistence Pregnancy |
|
| Notes | Power calculation: NR Funding: NR Clinical trial registration number: NR Email sent to contact author February 2010 requesting further information – no reply received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a computer generated randomizations table." |
| Allocation concealment (selection bias) | Unclear risk | NR. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded to treatment allocation. Placebo injections used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Nothing mentioned about detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 participant in triptorelin group and 5 participants in placebo group lost to follow‐up. Possibility of bias. |
| Selective reporting (reporting bias) | Low risk | Pain, relapse, and pregnancy reported (for those who desired pregnancy). |
| Other bias | Low risk | Groups appeared similar at baseline. |
Muzii 2000.
| Study characteristics | ||
| Methods | No. of centres: 2 Location: university departments, Rome, Italy Recruitment period: January 1994 to June 1997 |
|
| Participants | Inclusion criteria: aged 20–35 years, moderate to severe dysmenorrhoea or chronic pelvic pain (or both), not desiring fertility Exclusion criteria: treatment for endometriosis in previous 6 months No. randomized: 70 No. analyzed: 68 |
|
| Interventions | Postsurgical medical therapy Group 1: (n = 35) cyclic monophasic OCP (ethinyl estradiol 0.03 mg, gestodene 0.075 mg) for 21 days with 7 pill‐free days for 6 months Group 2: (n = 35) no therapy |
|
| Outcomes | Recurrence of pain and time to recurrence Recurrence of cysts |
|
| Notes | Power calculation: yes Funding: NR. Drugs supplied by Population Council Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly allocated to one of two management arms on the basis of a computer generated sequence." |
| Allocation concealment (selection bias) | Unclear risk | NR. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NR, no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NR, no placebo. For recurrence, detection bias assigned as low risk. For pain assigned as high risk of bias, as this represents a subjective outcome measure. Therefore, overall detection bias as unclear risk assigned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 postrandomization withdrawals. Unlikely to have introduced bias. |
| Selective reporting (reporting bias) | Low risk | Important outcomes reported – recurrence of endometriosis, pain, AFS scores. Participants not desiring pregnancy. |
| Other bias | Unclear risk | No information of the baseline characteristics of the groups reported. |
Parazzini 1994.
| Study characteristics | ||
| Methods | No. of centres: 6 Location: university centres in Italy Recruitment period: January 1990 to July 1991 |
|
| Participants | Inclusion criteria: aged < 38 years, normal medical examination, unexplained infertility for ≥ 1 year, with/without chronic pelvic pain, endometriosis stage III–IV, partners with normal sperm analysis and postcoital tests Exclusion criteria: previous laparoscopic/clinical diagnosis of endometriosis, other diseases that might cause infertility or pelvic pain, previous treatment for endometriosis or infertility No. randomized: 75 No. analyzed: 75 (pregnancy rates), 68 (pain scores) |
|
| Interventions | Postsurgical medical therapy Group 1: (n = 36) nafarelin nasal 400 μg daily for 3 months Group 2: (n = 39) placebo |
|
| Outcomes | Pain (multidimensional and 10‐point linear scale) score at 12 months Pregnancy rates Adverse drug outcome (amenorrhoea) |
|
| Notes | Power calculation: yes (post‐hoc?) Funding: Recordati Milan provided nafarelin Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated randomizations list." |
| Allocation concealment (selection bias) | Low risk | Assigned by telephone call 7 days from surgery. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind but authors acknowledged that adverse effects of treatment make maintaining blinding difficult. Placebo controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded regarding medication treatment. But, authors acknowledged that adverse effects of treatment make maintaining blinding difficult. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up, all randomized participants included in analyses. |
| Selective reporting (reporting bias) | Low risk | Pregnancy rate and pelvic pain reported. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
Rickes 2002.
| Study characteristics | ||
| Methods | No. of centres: 1 Location: Clinic for Reproductive Medicine and Gynecologic Endocrinology, Faculty of Medicine, Otto‐von‐Guericke University, Magdeburg, Germany Recruitment period: May 1999 to May 2001 |
|
| Participants | Inclusion criteria: endometriosis diagnosed by videolaparoscopy, with biopsy for histological confirmation (in cases of doubt). Stage 2–4 on ASRM guidelines. Exclusion criteria: lack of desire to conceive, aged > 40 years, dependence on testicular sperm in ART No. randomized: 110 No. analyzed: 100 |
|
| Interventions | Postsurgical medical therapy Group 1: (n = 55) therapy with GnRHa (goserelin 3.6 mg, received first SC dose on day 3 after surgery, monthly over 5 or 6 cycles) Group 2: (n = 55) therapy without GnRHa |
|
| Outcomes | Pregnancy screened by β‐hCG assay Valid ultrasonographic evidence of amniotic sac. Measurement of endometrial depth |
|
| Notes | Power calculation: NR Funding: NR Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocks of 6, randomized by computer. |
| Allocation concealment (selection bias) | Unclear risk | NR; most probably not done. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding mentioned, not placebo controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding mentioned. For pregnancy, detection bias assigned as low risk, as this represents an objective outcome measure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/55 participants in GnRHa group not completed study and 7/55 assigned to surgery chose to leave the study. |
| Selective reporting (reporting bias) | Low risk | All main outcomes were reported. |
| Other bias | Low risk | Only included Stage II–IV. The demographic characteristics of the 2 randomized groups did not differ statistically in terms of mean age, duration of infertility, ratio of primary to secondary infertility, mean stage of endometriosis, and mean endometriosis score. |
Seracchioli 2010a.
| Study characteristics | ||
| Methods | No. of centres: 1 Location: Minimally Invasive Gynaecological Surgery Unit of S. Orsola University Hospital, Bologna, Italy Recruitment period: June 2002 to May 2006 |
|
| Participants | Inclusion criteria: aged 20–40 years, 2 transvaginal ultrasonographic examination of ovarian endometrioma of which the diameter was ≥ 4 cm, at 6–8 weeks before surgery and on the day before surgery. Not attempting to conceive either at time of study entry or for ≥ 2 years after surgery Exclusion criteria: OCP for < 6 months before surgery; contraindications for OC; unwilling to tolerate the absence of menstruation; lack of the desire to postpone pregnancy for ≥ 2 years after surgery. No. randomized: 239 No. analyzed: 217 |
|
| Interventions | Postsurgical medical therapy Group 1: non‐users Group 2: cyclic OC users – daily for 21 days, followed by 7‐day interval (low‐dose monophasic combined OC (ethinyl E2 0.020 mg and gestodene 0.075 mg daily), which started on the day of discharge after surgery and lasted for 24 months) Group 3: continuous OC users – continuous therapy no pill‐free interval (low‐dose monophasic combined OC (ethinyl E2 0.020 mg and gestodene 0.075 mg daily), which started on the day of discharge after surgery and lasted for 24 months) |
|
| Outcomes | Recurrence rate Recurrence‐free survival Size and rate of growth of recurrent cysts Time of recurrence |
|
| Notes | Power calculation: NR Funding: none declared Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomizations. |
| Allocation concealment (selection bias) | Low risk | Numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No placebo used. Blinding not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All scans were performed by experienced operators, blinded to the study allocation. All objective outcomes included in this study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Group 1: 10 participants did not complete the study (4 spontaneous pregnancy, 6 started OC) = 12.6%. Group 2: cyclic users: 6 did not complete study (2 unrelated, 4 adverse events) = 7.6%. Group 3: continuous users: 6 did not complete study (2 unrelated, 4 adverse events) = 7.6%. |
| Selective reporting (reporting bias) | Low risk | All main outcomes are reported. |
| Other bias | Low risk | None detected. The 3 groups were homogeneous with regard to mean age, mean body mass index, endometriosis stage, mean endometrioma diameter, and the proportion of participants with bilateral cysts. |
Seracchioli 2010b.
| Study characteristics | ||
| Methods | No. of centres: 1 Locations: Minimally Invasive Gynaecological Surgery Unit of S. Orsola University Hospital, Bologna, Italy Recruitment period: June 2002 to May 2006 |
|
| Participants | Inclusion criteria: aged 20–40 years, 2 ultrasounds controls, laparoscopic excision of ovarian endometrioma; classification by AFS system; nulliparous; not attempting to conceive either at time of study entry or for ≥ 2 years after surgery; related symptoms (dysmenorrhoea, dyspareunia, chronic pelvic pain); pain evaluation: moderate or severe pain in ≥ 1 type of pain (VAS 4–10) Exclusion criteria: hormonal therapy for ≥ 6 months before surgery; gastrointestinal or urological diseases or diagnosis of current pelvic inflammatory disease; previous surgical treatment for endometriosis; contraindications for OC; deep endometriosis during surgery; unacceptable for women to have absence of menstruation for ≥ 2 years induced by OC No. randomized: 311 No. analyzed: 274 Withdrew: group non‐users: 17; group cyclic users: 8; group non‐cyclic users: 6 Missing: group non‐users: 0; group cyclic users: 3; group non‐cyclic users: 3 |
|
| Interventions | Postsurgical medical therapy Group 1: (n = 103) cyclic OC use (low‐dose monophasic combined OC: ethinyl E2 0.02 mg and gestodene 0.075 mg daily) Group 2: (n = 104) non‐users, no medical therapy Group 3: (n = 104) continuous OC use (low‐dose monophasic combined OC: ethinyl E2 0.020 mg and gestodene 0.075 mg daily) |
|
| Outcomes | Pain recurrence (cumulative pain‐free survival VAS scores, differences in VAS score from 6–24 months during study period in 3 study groups) | |
| Notes | Power calculation: NR Funding: nothing to declare Clinical trial registration number: NR Email send to contact author Oct 2019 requesting further information – no reply received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomizations sequence. |
| Allocation concealment (selection bias) | Low risk | Numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding mentioned, no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding mentioned, no placebo. For pain assigned as high risk of bias, as this represents a subjective outcome measure. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Group non‐users: 17/104 (16.3%) (7 pregnancy, 10 adverse events ‐‐> start OC); group cyclic: 11/103 (10.6%) (3 unrelated, 8 adverse events OC), group non‐cyclic: 9/104 (8.6%) (3 unrelated, 6 adverse events). |
| Selective reporting (reporting bias) | Low risk | Pain recurrence, cumulative pain‐free survival, VAS scores, differences in VAS score mentioned. |
| Other bias | Low risk | Groups similar at baseline for mean age, mean body mass index, and endometriosis stage according to the revised AFS classification. |
Sesti 2007.
| Study characteristics | ||
| Methods | No. of centres: 1 Location: Rome, Italy Recruitment period: January 1999 to May 2005 |
|
| Participants | Inclusion criteria: women of reproductive age < 40 years with endometriosis‐related symptoms (dysmenorrhoea, pelvic pain, deep dyspareunia); laparoscopic diagnosis of St III –IV endometriosis; desiring pregnancy; nulliparous Exclusion criteria: concurrent disease, such as cancer or pelvic inflammatory disease; previous surgery for endometriosis; contraindications to oestrogens/progestins No. randomized: 234 No. analyzed: 222 |
|
| Interventions | Group 1: (n = 115) placebo for 6 months Group 2: (n = 119) postsurgical medical or dietary therapy Participants in group 2 received:
|
|
| Outcomes | Pelvic pain | |
| Notes | Power calculation: yes Funding: NR Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised according to a computer generated randomizations sequence." |
| Allocation concealment (selection bias) | Low risk | Allocated by serially numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Because of blinding, low risk of detection bias. Quote: "neither the surgeons not the patients were aware of the regimen prescribed during the study period." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants in placebo group and 3 participants in GnRHa groups were lost to follow‐up and reasons given. 2 lost to follow‐up from each of OCP and diet groups but reasons not given. 222 participants evaluated. |
| Selective reporting (reporting bias) | Low risk | Pain and health‐related quality of life reported. No pregnancy outcome in a group of women desiring pregnancy. No result of the original main variables was excluded from published reports. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
Sesti 2009.
| Study characteristics | ||
| Methods | No. of centres: 1 Location: Endometriosis Center, Section of Gynecology, Tor Vergata University Hospital, Rome, Italy Recruitment period: January 2004 to August 2006 |
|
| Participants | Inclusion criteria: aged 20–40 years at time of surgery; ultrasonographic evidence of endometrioma; moderate‐to‐severe endometriosis‐related painful symptoms (VAS ≥ 4 on a 10‐point scale); first surgery for endometriosis; conservative treatment with retention of uterus and ovaries; complete excision of all evident ovarian and peritoneal disease Exclusion criteria: 6 months of oestrogen‐suppressing drugs before first surgery; previous surgical treatment for endometriosis; surgical findings of concomitant deeply infiltrating endometriosis; contraindications to oestrogens and progestins; attempting to conceive at study entry No. randomized: 259 No. analyzed: 240 |
|
| Interventions | Postsurgical medical therapy Group 1: (n = 65) surgery + GnRH surgery and tryptorelin or leuprorelin, 3.75 mg every 28 days Group 2: (n = 65) surgery + placebo (n = 65) Group 3: (n = 64) surgery + continuous low‐dose monophasic OC (ethinyl estradiol 0.03 mg + gestoden 0.75 mg) Group 4: (n = 65) surgery + dietary, consisting of nutritional intake in addition to vitamins (B6, A, C, and E), mineral salts (calcium, magnesium, selenium, zinc, iron), lactic ferments VSL3 (Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus bulgaricus, Streptococcus thermophilus), omega‐3 and omega‐6 fatty acids (fish oil), which secured nutritional rate at 1600–2000 calories. |
|
| Outcomes | Recurrence rate endometrioma | |
| Notes | Power calculation: yes Funding: NR Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed according to a computer‐generated randomizations sequence using serially numbered, opaque, sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Randomization was performed according to a computer‐generated randomizations sequence using serially numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of surgeons and participant. Placebo controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of ultrasonography operator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar attrition between arms. |
| Selective reporting (reporting bias) | Low risk | All main outcomes were reported. |
| Other bias | Unclear risk | Data at baseline were not statistically different among the groups except for the rate of participants reporting dysmenorrhoea in the GnRHa group than participants in the other groups. |
Shaw 2001.
| Study characteristics | ||
| Methods | No. of centres: 7 Location: UK and Republic of Ireland Recruitment period: NR |
|
| Participants | Inclusion criteria: women aged 18–50 years referred for symptom management or infertility Exclusion criteria: cervical intraepithelial neoplasia No. randomized: 48 No. analyzed: 40 |
|
| Interventions | presurgical goserelin vs no therapy Group 1: (n = 21) goserelin 3.6 mg SC monthly for 3 months presurgically Group 2: (n = 27) no medical therapy |
|
| Outcomes | Change in endometrioma size Recurrence Pregnancy |
|
| Notes | Power calculation: yes Funding: Astra Zeneca, Macclesfield, UK Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were stratified by endometrioma size. Quote: "randomly allocated using computer generated randomizations lists." |
| Allocation concealment (selection bias) | Unclear risk | NR. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding mentioned. For endometrioma size, recurrence, and pregnancy rate, detection bias assigned as low risk, as this represents an objective outcome measure. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 33% (7) of participants in group 1 and 41% (11) of participants in group 2 withdrew from trial before surgery. Reasons given but were different in each group. 4 withdrawals from goserelin group presurgery due to serious adverse events (migraine/headache/hot flushes, groin pain, muscle cramps, pain iliac fossa/sciatica). However, all participants were included in outcome evaluation provided there was > 1 postbaseline measurement of endometrioma, but these numbers were not stated. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome was change in size of endometrioma; range of other outcomes including ease of surgery and pregnancy reported. |
| Other bias | Unclear risk | Some differences between the groups at baseline in mean endometrioma size. Difficulty in recruiting participants made trial underpowered, with different numbers in each group. |
Tanmahasamut 2017.
| Study characteristics | ||
| Methods | No. of centres: 1 Location: Department of Obstetrics and Gynaecology, Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand Recruitment period: April 2012 to October 2014 |
|
| Participants | Inclusion criteria: endometriosis confirmed by laparoscopic surgery; moderate‐to‐severe dysmenorrhoea or pelvic pain (or both) > 6 months Exclusion criteria: no endometriosis by laparoscopy; current treatment for endometriosis other than analgesic medications No. randomized: 40 No. analyzed: 38 |
|
| Interventions | Postsurgical medical therapy Group 1: (n = 20) desogestrel 0.075 mg per tablet/day Group 2: (n = 20) placebo |
|
| Outcomes | Endometriosis‐related pain Participant satisfaction Adverse events |
|
| Notes | Power calculation: yes Funding: none declared Clinical trial registration number: NCT01559480 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomizations. List of medication serial numbers and treatment codes were stored confidentially. |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomizations sequence using serially numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo controlled. Double blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nurse who was unaware of treatment group, assessed participants for subjective outcomes and reported numbers of leftover trial or rescue (or both) medication. Data collected separate from gynaecologist, in separate medical record to maintain double‐blind status. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Desogestrel group: 1 lost to follow‐up; placebo group: 1 participant received depot medroxyprogesterone acetate (severe dysmenorrhoea at month 3). |
| Selective reporting (reporting bias) | Low risk | All important outcomes reported. |
| Other bias | Low risk | No difference at baseline. |
Telimaa 1987.
| Study characteristics | ||
| Methods | No. of centres: 1 Location: University of Oulu, Finland Recruitment period: NR |
|
| Participants | Inclusion criteria: advanced endometriosis Exclusion criteria: NR No. randomized: 60 No. analyzed: 51 (pain), 59 (pregnancy) |
|
| Interventions | Postsurgical medical therapy Group 1: (n = 20) danazol 600 mg orally daily × 180 days Group 2: (n = 20) medroxyprogesterone acetate 100 mg daily × 180 days Group 3: (n = 20) placebo |
|
| Outcomes | Pain scores AFS scores Pregnancy rates Participant satisfaction Adverse drug reactions: weight gain, breakthrough bleeding, acne |
|
| Notes | Power calculation: NR Funding: Research and Science Foundation Farmos Ltd, Turku; Cultural Foundation of Keski‐Pohjanmaa, Finland; Farmos Group, Turku, Finland supplied drugs Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised;" no information on method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | NR. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, placebo controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind, placebo controlled. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for postrandomization exclusions similar in each group. Exclusion due to pregnancy: group 1: 3; group 2: 2; group 3: 3. 1 adverse event in placebo group. |
| Selective reporting (reporting bias) | Low risk | Important outcomes of pregnancy recurrence and pain reported. |
| Other bias | Low risk | Groups appeared comparable at baseline and authors stated that "no other medication was used during the trial." |
Tsai 2004.
| Study characteristics | ||
| Methods | No. of centres: 1 Location: Taiwan Recruitment period: June 1988 to December 2001 |
|
| Participants | Inclusion criteria: women of reproductive age with infertility and stage III or IV endometriosis planning to undergo controlled ovarian hyperstimulation and intrauterine insemination or in vitro fertilization and embryo transfer. All had surgery for endometriosis – either laparotomy or laparoscopy for cystectomy, adhesiolysis, ablation of endometriosis Exclusion criteria: NR No. randomized: 45 No. analyzed: 41 |
|
| Interventions | Postsurgical medical therapy (either danazol or GnRHa) Group 1: (n = 15) either 3 months of danazol 400 mg orally, twice daily for 3 months or leuprolide acetate 3.75 mg depot SC every 28 days for 3 months Group 2: (n = 30) no postsurgical medical therapy |
|
| Outcomes | Pregnancy rate Recurrence |
|
| Notes | Power calculation: NR Funding: NR Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "simple randomisation with a computer generated list unknown to physicians." |
| Allocation concealment (selection bias) | Low risk | List "unknown to physicians." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NR, no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | NR, no placebo. For pregnancy rate and recurrence, detection bias is assigned as low risk, as this represents an objective outcome measure. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 lost to follow‐up from group 1 (27%)#. |
| Selective reporting (reporting bias) | Low risk | Pregnancy and recurrence reported. |
| Other bias | Unclear risk | 13 years of recruitment –? associated changes in surgical techniques over this time. |
Vercellini 1999.
| Study characteristics | ||
| Methods | No. of centres: 19 Location: Italy Recruitment period: February 1992 to June 1994 |
|
| Participants | Inclusion criteria: premenopausal, endometriosis score ≥ 4 points, chronic pelvic pain Exclusion criteria: NR No. randomized: 269 No. analyzed: 210 |
|
| Interventions | Postsurgical medical therapy Group 1: (n = 133) goserelin SC 3.6 mg every 4 weeks × 6 months Group 2: (n = 134) no therapy |
|
| Outcomes | Pain recurrence Pregnancy rates |
|
| Notes | Power calculation: yes Funding: Zeneca Pharmaceuticals provided drugs and financial support Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised in a proportion of 1:1 … in accordance with a computer‐generated randomisation sequence." |
| Allocation concealment (selection bias) | Low risk | Centralized randomization, allocation obtained by telephone call. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study, no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label study, no placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 269 participants randomized, 2 excluded because case record forms not completed, 26 participants withdrew from treatment group and 31 participants (22%) withdrew from control group, for reasons other than symptom recurrence or were excluded due to major protocol violations. Attrition appears to be high but was balanced across the intervention groups. So it is unclear if it caused attrition bias, so it was assigned as unclear risk of bias. |
| Selective reporting (reporting bias) | Low risk | Important outcomes of recurrence, dysmenorrhoea and pregnancy reported. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
Yang 2006.
| Study characteristics | ||
| Methods | No. of centres: 1 Location: Harbin, China Recruitment period: March 2002 to March 2004 |
|
| Participants | Inclusion criteria: women with stage 3 endometriosis who had undergone conservative or semi‐conservative surgery, with normal renal function and blood count, aged 23–42 years Exclusion criteria: history of hypertension, heart disease, diabetes mellitis, had undergone hormone therapy in 6 months prior to surgery No. randomized: 52 No. analyzed: unclear |
|
| Interventions | Postsurgical Group 1: (n = 20) yiweining 200 mL orally twice daily, for 3 months, starting on 7th postsurgical day Group 2: (n = 19) gestrinone 2.5 mg twice weekly, SC? for 3–6 months, starting on 7th postsurgical day Group 3: (n = 13) control – no therapy |
|
| Outcomes | Pregnancy Recurrence of endometriosis Adverse effects |
|
| Notes | Power calculation: NR Funding: Fund of National Science of Heilongjiang Province Clinical trial registration number: NR Email sent to corresponding author requesting additional information April 2010 – mailbox not found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly divided" – no details of method of sequence generation given. |
| Allocation concealment (selection bias) | Unclear risk | NR. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NR, no placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | NR, no placebo used. For pregnancy rate and recurrence, detection bias is assigned as low risk, as this represents an objective outcome measure. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear how many of the randomized participants are included in the outcomes (% only given). |
| Selective reporting (reporting bias) | Unclear risk | Pregnancy and recurrence reported – no details as criteria for measuring these. |
| Other bias | Low risk | Groups are similar in age and type of surgery at baseline. |
Yang 2018.
| Study characteristics | ||
| Methods | No. of centres: 1 Location: Department of Urology, Zhongda Hospital, Southeast University, Jiang Su, China Recruitment period: January 2005 to March 2016 |
|
| Participants | Inclusion criteria: diagnosis confirmed by laparoscopy or B‐ultrasound examination; postsurgical biopsy; normal sexual life; without contraception, but no pregnancy for > 1 year and having indications for laparoscopic surgery Exclusion criteria: recent use of hormones; abnormal menstruation and other symptoms; other diseases such as pelvic floor dysfunction, severe cardiovascular, hepatic, or renal dysfunction; other causes of dysmenorrhoea; allergy to medicines used in study; person's spouse had sexual dysfunction or seminal abnormality No. randomized: 130 No. analyzed: 130 |
|
| Interventions | Postsurgical medical therapy Group 1: (n = 65) surgery + triptorelin acetate 3.75 mg SC every 4 weeks for 6 months Group 2: (n = 65) surgery alone |
|
| Outcomes | Clinical effect Levels of E2, LH, and FSH Pregnancy rate Recurrence rate Adverse reaction |
|
| Notes | Power calculation: NR Funding: NR Clinical trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly divided, not mentioned how. |
| Allocation concealment (selection bias) | Unclear risk | NR. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding mentioned, no placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding mentioned. For pregnancy rate and recurrence, detection bias is assigned as low risk, as this represents an objective outcome measure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | Low risk | All main outcomes reported. |
| Other bias | Unclear risk | No other bias identified. |
AFS: American Fertility Society; ART: assisted reproductive technology; ASRM: American Society for Reproductive Medicine; β‐hCG: β‐human chorionic gonadotropin; EEC: Endoscopic Endometriosis Classification; FSH: follicle‐stimulating hormone; GnRHa: gonadotropin‐releasing hormone agonist; LH: luteinizing hormone; MRI: magnetic resonance imaging; n: number of participants; NR: not reported; OC: oral contraceptive; OCP: oral contraceptive pill; PAP: Papanicolaou; rAFS: revised American Fertility Society; SC: subcutaneous; SF‐36: 36‐item Short Form; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Batioglu 1997 | Quasi‐randomized trial, by even and odd numbers. |
| Morgante 1999 | All participants received triptorelin for 6 months postsurgery before randomization to danazol or no therapy. |
| Schindler 1998 | Prospective multicentre phase 3 study published in German. Preliminary translation suggests that treatment was not randomly assigned. |
| Shawki 2002 | Data were not available at the time of writing this review. |
| Vercellini 2003 | Pilot study using the levonorgestrel‐releasing intrauterine system for treatment of endometriosis postsurgery. This is a locally effective hormonal suppressive therapy and has low systemic effects. |
| Ylanen 2003 | Dose‐finding study with no comparison of treatment modality with placebo or no medical therapy. |
Characteristics of studies awaiting classification [ordered by study ID]
Roghaei 2010.
| Methods | No. of centres: NR Location: teaching hospitals in Isfahan, Iran Recruitment period: September 2008 to July 2009 Due to data discrepancy in the published paper, we have contacted the authors for more information. |
| Participants | Inclusion criteria: aged 18–45 years; endometriosis laparoscopic staged 1 month before surgery; reproductive age; regular menstrual cycles (18–45 days); chronic pelvic pain and dysmenorrhoea for ≥ 2 weeks each month during past 3 months Exclusion criteria: hormone therapy during past 3 months; osteopenia; history of convulsions or pulmonary, cardiac, hepatic, renal or cerebrovascular diseases; abnormal vaginal bleeding of unknown cause; ovarian cyst > 2 cm; smoking history; hypersensitivity to danazol and letrozole; pregnancy No. randomized: 106 No. analyzed: 79 |
| Interventions | Postsurgical medical therapy Group 1: (n = 38) letrozole tablets 2.5 mg/day, calcium 1000 mg/day, and vitamin D 800 IU/day Group 2: (n = 37) danazol tablets 600 mg/day, calcium 1000 mg/day, and vitamin D 800 IU/day Group 3: (n = 31) placebo: 2 calcium tablets 500 mg each/day, and vitamin D 800 IU/day |
| Outcomes | Symptom improvement (dyspareunia, dysmenorrhoea, chronic pelvic pain) |
| Notes | Power calculation: NR Funding: NR Clinical trial registration number: NR |
Differences between protocol and review
Clarifications to the original protocol
We consider that levonorgestrel‐releasing intrauterine devices do not meet the inclusion requirement for systemic hormonal suppression and, therefore, we excluded the trial by Vercellini 2003.
We updated the quality assessment of included studies 'Assessment of risk of bias of included studies' in line with the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). We used additional headings available in Review Manager 5 to make the structure of the Methods, Results, and Discussion sections of the review clearer.
It was planned to undertake sensitivity analysis in this update to investigate whether the conclusions would differ if analysis was restricted to trials with low risk of bias.
Pentoxifylline is a medical therapy for endometriosis which is evaluated in a separate systematic review (Lv 2009).
We made minor changes to the format of the objectives of this review – the hypotheses were deleted from the updated review.
For the 2020 update, we moved pregnancy rate per woman to secondary outcomes and changed the title to "Pre‐ and postsurgical medical therapy for endometriosis surgery".
Contributions of authors
IC: took the lead in developing the review protocol and search strategy; screening the articles, risk of bias assessment, data extraction, data analysis, grading and interpretation; writing and revising all versions of this review update.
VV: contributed to the background, search strategy, data extraction, risk of bias, analysis, results, and discussion of the review and this update.
AC: contributed to the background, search strategy, data extraction, risk of bias, analysis, results, and discussion of the review and this update.
AM: feedback on methodology of design, data extraction, risk of bias, interpretation, and manuscript review.
AZ: feedback on methodology of design, data extraction, risk of bias, interpretation, and manuscript review.
AB: feedback on methodology and design, interpretation, and manuscript review.
CA: assistance with search strategy, screen, retrieval of articles, and manuscript review.
JM: feedback on methodology and design, interpretation, and manuscript review.
Sources of support
Internal sources
Singhealth Research, Singapore General Hospital, Singapore
External sources
No sources of support supplied
Declarations of interest
IC: none.
VV: none.
AC: none.
AM: participated in speaker bureau and/or advisory boards for Allergan, Abbvie, Bayer, Hologic, Medtronic, Pfizer, Baxter
AZ: received honoraria for educational presentations outside the submitted work.
AB: Dr. Black has received a honorarium for participating as a consultant on advisory boards, presenting at continuing medical education events, and developing patient and health care provider educational tools from the following companies: Pfizer, Merck, Bayer.
CA: none.
JM: none.
Edited (no change to conclusions)
References
References to studies included in this review
Alkatout 2013 {published data only}
- Alkatout I, Mettler L, Beteta C, Hedderich J, Jonat W, Schollmeyer T, et al. Combined surgical and hormone therapy for endometriosis is the most effective treatment: prospective, randomized, controlled trial. Journal of Minimally Invasive Gynaecology 2013;20(4):473-81. [DOI] [PubMed] [Google Scholar]
Angioni 2015 {published data only}
- Angioni S, Pontis A, Dessole M, Surico D, De Cicco Nardone C, Melis I. Pain control and quality of life after laparoscopic en-block resection of deep infiltrating endometriosis (DIE) vs. incomplete surgical treatment with or without GnRHa administration after surgery. Archives of Gynecology and Obstetrics 2015;291:363-70. [DOI] [PubMed] [Google Scholar]
Audebert 1998 {published data only}
- Audebert A, Descamps P, Marret H, Ory-Lavollee L, Bailleul F, Hamamah S. Pre or post-operative medical treatment with nafarelin in stage III–IV endometriosis: a French multicenter study. Obstetrics and Gynecology 1998;79:145-8. [DOI] [PubMed] [Google Scholar]
Bianchi 1999 {published data only}
- Bianchi S, Busacca M, Agnoli B, Candiani M, Calia C, Vignali M. Effects of 3 month therapy with danazol after laparoscopic surgery for stage III/IV endometriosis: a randomized study. Human Reproduction 1999;14(5):1335-7. [DOI] [PubMed] [Google Scholar]
Busacca 2001 {published data only}
- Busacca M, Somigliana E, Bianchi S, Marinis SD, Calia C, Candiani M, et al. Post-operative GnRH analogue treatment after conservative surgery for symptomatic endometriosis stage III–IV: a randomized controlled trial. Human Reproduction 2001;16(11):2399-402. [DOI] [PubMed] [Google Scholar]
Cucinella 2013 {published data only}
- Cucinella G, Granese R, Calagna G, Svelato A, Saitta S, Tonni G, et al. Oral contraceptives in the prevention of endometrioma recurrence: does the different progestins used make a difference? Archives of Gynecology and Obstetrics 2013;288:821-7. [DOI] [PubMed] [Google Scholar]
Donnez 1994 {published data only}
- Donnez J, Anaf V, Nisolle M, Clerckx-Braun F, Gillerot S, Casanas-Roux F. Ovarian endometrial cysts: the role of gonadotropin-releasing hormone agonist and/or drainage. Fertility and Sterility 1994;62(1):63-6. [DOI] [PubMed] [Google Scholar]
Hornstein 1997 {published data only}
- Hornstein MD, Hemmings R, Yuzpe AA, Heinrichs WL. Use of nafarelin versus placebo after reductive laparoscopic surgery for endometriosis. Fertility and Sterility 1997;68(5):860-4. [DOI] [PubMed] [Google Scholar]
Huang 2018 {published data only}
- Huang C, Wu M, Liu Z, Shi H, Han Y, Song X. Clinical efficacy and safety of gonadotropin-releasing hormone agonist combined with laparoscopic surgery in the treatment of endometriosis. International Journal of Clinical and Experimental Medicine 2018;11:4132-7. [Google Scholar]
Loverro 2001 {published data only}
- Loverro G, Santillo V, Pansini MV, Lorusso F, Depalo R, Selvaggi L. Are GnRH agonists helpful in the therapy of endometriosis after surgical treatment? Human Reproduction 2001;16 Suppl(1):96. [Google Scholar]
Loverro 2008 {published data only}
- Loverro G, Carriero C, Rossi AC, Putignano G, Nicolardi V, Selvaggi L. A randomized study comparing triptorelin or expectant management following conservative laparoscopic surgery for symptomatic stage III–IV endometriosis. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2008;136(2):194-8. [DOI] [PubMed]
Muzii 2000 {published data only}
- Muzii L, Marana R, Caruana P, Catalano GF, Margutti F, Panici PB. Postoperative administration of monophasic combined oral contraceptives after laparoscopic treatment of ovarian endometriomas: a prospective, randomized trial. American Journal of Obstetrics and Gynecology 2000;183(3):588-92. [DOI] [PubMed] [Google Scholar]
Parazzini 1994 {published data only}
- Parazzini F, Fedele L, Busacca M, Falsetti L, Pellegrini S, Venturini PL, et al. Postsurgical medical treatment of advanced endometriosis: results of a randomized clinical trial. American Journal of Obstetrics and Gynecology 1994;171:1205-7. [DOI] [PubMed] [Google Scholar]
Rickes 2002 {published data only}
- Rickes D, Nickel I, Kropf S, Kleinstein J. Increased pregnancy rates after ultralong postoperative therapy with gonadotropin-releasing hormone analogs in patients with endometriosis. Fertility and Sterility 2002;78:757-62. [DOI] [PubMed] [Google Scholar]
Seracchioli 2010a {published data only}
- Seracchioli R, Mabrouk M, Frascà C, Manuzzi L, Montanari G, Keramyda A, et al. Long-term cyclic and continuous oral contraceptive therapy and endometrioma recurrence: a randomized controlled trial. Fertility and Sterility 2010;93:52-6. [DOI] [PubMed] [Google Scholar]
Seracchioli 2010b {published data only}
- Seracchioli R, Mabrouk M, Frascà C, Manuzzi L, Savelli L, Venturoli S. Long-term oral contraceptive pills and postoperative pain management after laparoscopic excision of ovarian endometrioma: a randomized controlled trial. Fertility and Sterility 2010;94:464-71. [DOI] [PubMed] [Google Scholar]
Sesti 2007 {published data only}
- Sesti F, Pietropolli A, Capozzolo T, Broccoli P, Pierangeli S, Bollea MR, et al. Hormonal suppression treatment or dietary therapy versus placebo in the control of painful symptoms after conservative surgery for endometriosis stage III-IV. A randomized comparative trial. Fertility and Sterility 2007;88(6):1541-7. [DOI] [PubMed]
Sesti 2009 {published data only}
- Sesti F, Capozzolo T, Pietropolli A, Marziali M, Bollea MR, Piccione E. Recurrence rate of endometrioma after laparoscopic cystectomy: a comparative randomized trial between post-operative hormonal suppression treatment or dietary therapy vs. placebo. European Journal of Obstetrics and Gynaecology and Reproductive Biology 2009;147:72-7. [DOI] [PubMed] [Google Scholar]
Shaw 2001 {published data only}
- Shaw R, Garry R, McMillan L, Sutton C, Wood S, Harrison R, et al. A prospective randomized open study comparing goserelin (Zoladex) plus surgery and surgery alone in the management of ovarian endometriomas. Gynaecological Endoscopy 2001;10:151-7. [Google Scholar]
Tanmahasamut 2017 {published data only}
- Tanmahasamut P, Saejong R, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Sanga-areekul N. Postoperative desogestrel for pelvic endometriosis-related pain: a randomized controlled trial. Gynecological Endocrinology 2017;33:534-9. [DOI] [PubMed] [Google Scholar]
Telimaa 1987 {published data only}
- Telimaa S, Ronnberg L, Kauppila A. Placebo-controlled comparison of danazol and high-dose medroxyprogesterone acetate in the treatment of endometriosis after conservative surgery. Gynecological Endocrinology 1987;1(4):363-71. [DOI] [PubMed] [Google Scholar]
Tsai 2004 {published data only}
- Tsai Y-L, Hwang J-L, Loo T-C, Cheng W-C, Chuang J, Seow K-M. Short-term postoperative GnRH analogue or danazol treatment after conservative surgery for stage III or IV endometriosis before ovarian stimulation: a prospective, randomized study. Journal of Reproductive Medicine 2004;49(12):955-9. [PubMed]
Vercellini 1999 {published data only}
- Vercellini P, Crosignani PG, Fadini R, Radici E, Belloni C, Sismondi P. A gonadotrophin-releasing hormone agonist compared with expectant management after conservative surgery for symptomatic endometriosis. British Journal of Obstetrics and Gynaecology 1999;106:672-7. [DOI] [PubMed] [Google Scholar]
Yang 2006 {published data only (unpublished sought but not used)}
- Yang D, Ma W, Qu F, MA B. Comparative study on the efficiency of yiweining and gestrinone for post-operational treatment of stage III endometriosis. Chinese Journal of Integrative Medicine 2006;12(3):218-20. [DOI] [PubMed] [Google Scholar]
Yang 2018 {published data only}
- Yang Y, Zhu W, Chen S, Zhang G, Chen M, Zhuang Y. Laparoscopic surgery combined with GnRH agonist in endometriosis. Journal of the College of Physicians and Surgeons Pakistan 2018;29:313-6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Batioglu 1997 {published data only}
- Batioglu S, Haberai A, Celikkanat H. Comparison of GnRH agonist administration before and after laparoscopic drainage of endometriomas. Journal of Gynecologic Surgery 1997;13(1):17-21. [Google Scholar]
Morgante 1999 {published data only}
- Morgante G, Ditto A, Marca AL, Leo VD. Low-dose danazol after combined surgical and medical therapy reduces the incidence of pelvic pain in women with moderate and severe endometriosis. Human Reproduction 1999;14(9):2371-4. [DOI] [PubMed] [Google Scholar]
Schindler 1998 {published data only}
- Schindler AE, Buhler K, Lubben G, Kienle E. Managment of endometriosis through a combined medical-surgical approach [Was leistet die kombinierte chirurgisch-hormonell Therapie zum Management der Endometriose]. Zentralblatt fur Gynakologie 1998;120:183-90. [PubMed] [Google Scholar]
Shawki 2002 {published data only}
- Shawki O, Hamza H, Sattar M. Mild endometriosis, to treat or not treat: randomized controlled trial comparing diagnostic laparoscopy with no further treatment versus post operative Zoladex in cases with infertility associated with stage I, II endometriosis. Fertility and Sterility 2002;77 Suppl 1:13. [Google Scholar]
Vercellini 2003 {published data only}
- Vercellini P, Frontino G, Giorgi OD, Aimi G, Zaina B, Crosignani PG. Comparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertility and Sterility 2003;80(2):305-9. [DOI] [PubMed] [Google Scholar]
Ylanen 2003 {published data only}
- Ylanen K, Laatikainen T, Lahteenmaki P, Moo-Young AJ. Subdermal progestin implant (Nestorone) in the treatment of endometriosis: clinical response to various doses. Acta Obstetricia et Gynecologica Scandinavica 2003;82:167-72. [PubMed] [Google Scholar]
References to studies awaiting assessment
Roghaei 2010 {published data only}
- Roghaei MA, Tehrany HG, Taherian A, Koleini N. Effects of letrozole compared with danazol on patients with confirmed endometriosis: a randomized clinical trial. International Journal of Fertility and Sterility 2010;4:67-72. [Google Scholar]
Additional references
Acien 2013
- Acien Pedro, Velasco Irene. Endometriosis: a disease that remains enigmatic. ISRN Obstetrics and Gynecology 2013;2013:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
ASRM 1997
- American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertility and Sterility 1997;67(5):817-21. [DOI] [PubMed] [Google Scholar]
Bazot 2017
- Bazot M, Darai E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertility and Sterility 2017;108(6):886-94. [DOI] [PubMed] [Google Scholar]
Bedaiwy 2017
- Bedaiwy MA, Allaire C, Alfaraj S. Long-term medical management of endometriosis with dienogest and with a gonadotropin-releasing hormone agonist and add-back hormone therapy. Fertility and Sterility 2017;107(3):537-48. [DOI] [PubMed] [Google Scholar]
Brown 2014
- Brown J, Farquhar C. Endometriosis: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD009590. [DOI: 10.1002/14651858.CD009590.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Donnez 1987
- Donnez J, Lemaire-Rubbers M, Karaman Y, Nisolle-Pochet M, Casanas-Roux F. Combined (hormonal and microsurgical) therapy in infertile women with endometriosis. Fertility and Sterility 1987;48(2):239-42. [DOI] [PubMed] [Google Scholar]
Donnez 2004
- Donnez J, Pirard C, Smets M, Jadoul P, Squifflet J. Surgical management of endometriosis. Best Practice & Research: Clinical Obstetrics & Gynaecology 2004;18(2):329-48. [DOI] [PubMed] [Google Scholar]
Duffy 2020
- Duffy JMN, Hirsch M, Vercoe M, Abbott J, Barker C, Collura B, et al. A core outcome set for future endometriosis research: an international consensus development study. BJOG: An International Journal of Obstetrics & Gynaecology 2020;127:967– 974. [DOI] [PubMed] [Google Scholar]
Dunselman 2014
- Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D'Hooghe T, De Bie B, et al. ESHRE guideline: management of women with endometriosis. Human Reproduction 2014;3:400-12. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Hemmings 1998
- Hemmings R. Combined treatment of endometriosis, GnRH agonists and laparoscopic surgery. Journal of Reproductive Medicine 1998;43 Suppl(2):316-20. [PubMed] [Google Scholar]
Higgins 2009
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Intervention. Chichester (UK): John Wiley & Sons, 2009. [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Johnson 2017
- Johnson NP, Hummelshoj L, Adamson GD, Keckstein J, Taylor HS, Abrao Mauricio S, et al. World endometriosis society consensus on the classification of endometriosis. Human Reproduction 2017;32(2):315-24. [DOI] [PubMed] [Google Scholar]
Kettel 1989
- Kettel LM, Murphy AA. Combination medical and surgical therapy for infertile patients with endometriosis. Obstetrics and Gynecology Clinics of North America 1989;16(1):167-77. [PubMed] [Google Scholar]
Koninckx 2019
- Koninckx PR, Ussia A Adamyan L, Wattiez A, Gomel V, Martin DC. Pathogenesis of endometriosis: the genetic/epigenetic theory. Fertility and Sterility 2019;111(2):327-40. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Leyland 2010
- Leyland N, Casper R, Laberge P, Singh SS, Allen L, Arendas K, et al. Endometriosis: diagnosis and management. Journal of Obstetrics and Gynaecology Canada 2010;32(7):S1-3. [PubMed] [Google Scholar]
Lv 2009
- Lv D, Song H, Li Y, Clarke J, Shi G. Pentoxifylline versus medical therapies for subfertile women with endometriosis. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD007677. [DOI: 10.1002/14651858.CD007677] [DOI] [PubMed] [Google Scholar]
Macer 2012
- Macer ML, Taylor HS. Endometriosis and infertility. A review of the pathogenesis and treatment of endometriosis-associated infertility. Obstetrics and Gynecology Clinics of North America 2012;39(4):535-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Missmer 2003
- Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstetrics and Gynecology Clinics of North America 2003;30(1):1-19, vii. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Somigliana 2017
- Somigliana E, Busnelli A, Benaglia L, Vigana P, Leonardi M, Paffoni A, et al. Postoperative hormonal therapy after surgical excision of deep endometriosis. European Journal of Obstetrics and Gynecology and Reproductive Biology 2017;209:77-80. [DOI] [PubMed] [Google Scholar]
Stephansson 2009
- Stephansson O, Kieler H, Granath F, Falconer H. Endometriosis, assisted reproduction technology, and risk of adverse pregnancy outcome. Human Reproduction 2009;24(9):2341-7. [DOI] [PubMed] [Google Scholar]
Thomas 1992
- Thomas EJ. Combining medical and surgical treatment for endometriosis: the best of both worlds? British Journal of Obstetrics and Gynaecology 1992;99 Suppl 7:5-8. [DOI] [PubMed] [Google Scholar]
Vercellini 2013
- Vercellini P, Matteis SD, Somigliana E, Buggio L, Frattaruolo MP, Fedele L. Long-term adjuvant therapy for the prevention of postoperative endometrioma recurrence: a systematic review and meta-analysis. Acta Obstetricia et Gynecologica Scandinavica 2013;92(1):8-16. [DOI] [PubMed] [Google Scholar]
Vercellini 2014
- Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. National Review of Endocrinology 2014;10:261-75. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Furness 2004
- Furness S, Yap C, Farquhar C, Cheong YC. Pre and post-operative medical therapy for endometriosis surgery. Cochrane Database of Systematic Reviews 2004, Issue 3. Art. No: CD003678. [DOI: 10.1002/14651858.CD003678.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


