Abstract
Background
We summarized the protocol-specified corticosteroid tapering regimens in clinical trials of moderate–severe ulcerative colitis (UC) and Crohn’s disease (CD) and calculated differences in rates of clinical remission vs corticosteroid-free clinical remission (CSF-CR).
Methods
Through a systematic literature review through February 28, 2019, we identified 16 randomized controlled trials (RCTs) of biologics or small molecules in patients with moderate–severe UC or CD who reported CSF-CR as an outcome. We estimated the relative risk and 95% confidence interval of achieving CSF-CR vs overall clinical remission in patients treated with active intervention or placebo through random-effects meta-analysis.
Results
Across trials of UC (11 trials) and CD (5 trials), a median of 53% and 49% of participants were on corticosteroids at the time of trial entry, respectively. Participants were allowed to enter trials at a median corticosteroid dose (range) of 35 (20–40) mg/d. Doses were kept stable for a median (range) of 8 (5–10) weeks during induction therapy, after which a mandatory and structured taper was implemented, albeit with the investigators’ discretion depending on clinical status. Pooled rates of CSF-CR in patients with UC and CD treated with placebo were 9.7% and 19.1%, respectively. In UC and CD trials, the rate of CSF-CR was 24% and 18% lower than the rate of overall clinical remission, respectively.
Conclusions
Protocol-specified corticosteroid tapering regimens vary across trials. These findings will help to inform the design and interpretation of future clinical trials and highlight the need for standardization.
Keywords: end points, clinical trials, ulcerative colitis, Crohn’s disease, maintenance therapy
Protocol-specified corticosteroid tapering regimens vary across trials. Most trials keep the dose of corticosteroids stable during induction therapy, after which a mandatory and structured taper is implemented, albeit with the investigators’ discretion depending on clinical status. Rates of corticosteroid-free clinical remission are 18%–24% lower than overall rates clinical remission.
INTRODUCTION
Corticosteroids are potent, nonselective systemic anti-inflammatory drugs frequently used for acute management of symptoms in patients with ulcerative colitis (UC) and Crohn’s disease (CD). Although effective for induction of clinical remission, they are not as effective as maintenance agents and are inherently associated with systemic toxicities including mood changes, insomnia, polyphagia and weight gain, and increased risk of infection.1 Long-term use of corticosteroids is associated with additional side effects including bone loss and risk of osteoporosis, glaucoma, increased risk of diabetes and cardiovascular diseases, thromboembolism, poor wound healing, and increased risk of mortality. Hence, avoidance of corticosteroids is an important patient- and physician-preferred treatment goal for managing UC and CD in clinical practice.
Clinical trials of UC and CD typically enroll patients with moderately to severely active disease, of whom a substantial proportion will require corticosteroids to manage their disease at the time of trial entry. Management of corticosteroids during a clinical trial is important for two main reasons. First, corticosteroids can mask clinical symptoms of active disease and can thus influence the end point of clinical remission. Second, corticosteroid-free clinical remission (CSF-CR) is sometimes used as a primary or secondary end point in clinical trials. Thus, the corticosteroid tapering regimens should be transparently documented within clinical trials and ideally be consistent across trials to enable fair comparisons across studies. In a prior synthesis evaluating outcomes reported in clinical trials of CD, complete tapering off corticosteroids was required for the definition of clinical response or remission in 13 induction (16.0%) and 20 maintenance (45.5%) trials.2 In this study, we aimed to synthesize how corticosteroids are handled in clinical trials of biologics, and we targeted small molecules in patients with moderate to severe UC and CD. In addition, we calculated differences between CSF-CR and overall clinical remission, along with pooled rates of CSF-CR in patients treated with placebo. This information would be helpful in the design and interpretation of ongoing and future clinical trials in the field and in the development of a core outcome set.
METHODS
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) standards and followed an a priori protocol.3
Selection Criteria
Studies included in this meta-analysis were placebo-controlled phase II or III RCTs in adults (age >17 years) with moderate to severe UC or CD treated with biologic agents (anti-TNF agents, anti-integrin agents, anti-interleukin-12 and/or -23) or targeted small molecules (janus kinase inhibitors, sphingosine-1 phosphate receptor agonist) that also reported rates of both CSF-CR and overall clinical remission.
We excluded the following studies: (1) active comparator trials without a placebo arm; (2) trials that did not report how corticosteroids were tapered and did not report CSF-CR or clinical remission as an outcome; (3) trials of probiotics, antibiotics, 5-aminosalicylates or sulfasalazine, thiopurines, methotrexate, tacrolimus, cyclosporine, complementary therapy, and trials of hospitalized patients with severe or fulminant IBD; and (4) pediatric studies.
Search Strategy
We originally searched MEDLINE (1948–2017), EMBASE (1947–2017), and the Cochrane Central Register of Controlled Trials (1994–2017), without language restrictions, from inception to March 1, 2017. The original search was subsequently updated to February 28, 2019. The key search terms were derived to identify clinical trials (including concepts of blinding, randomization, and placebo control) in patients with IBD of any disease extent. Key search terms were then combined using Boolean operators. The detailed search strategy is outlined in Supplementary Tables 1 and 2. Two study investigators (S.S. and J.G.) independently reviewed the title and abstract of studies identified in the search to exclude studies that did not address the research question of interest on the basis of prespecified inclusion and exclusion criteria. The full text of the remaining articles was examined to determine whether it contained relevant information. Conflicts in study selection at this stage were resolved by consensus, referring back to the original article, in consultation with a senior investigator. Second, we searched the bibliographies of these selected articles, systematic reviews, and clinical trial registries (www.clinicaltrials.gov) to identify any additional studies. Third, we conducted a manual search of abstracts from major gastroenterology conferences (Digestive Disease Week, American College of Gastroenterology annual meeting, Advances in Inflammatory Bowel Diseases meeting organized by the Crohn’s and Colitis Foundation of America, European Crohn’s and Colitis Organization annual meeting, and United European Gastroenterology Week) from 2012 to 2018 to identify additional abstracts on the topic. Finally, we contacted experts in the field to identify other unpublished studies.
Data Abstraction and Quality Assessment
Data on study-, participant-, disease-, and treatment-related characteristics were abstracted onto a standardized form by 2 authors (S.S. and J.G.) independently, and discrepancies were resolved by consensus, referring to the original article, in consultation with a third reviewer. We abstracted data on how corticosteroids were handled in clinical trials (proportion of patients on corticosteroids at trial entry, maximum dose allowed, corticosteroid tapering regime) and rates of CSF-CR vs overall clinical remission in patients with UC and CD receiving active intervention and placebo. Two study investigators (C.M., T.N.) independently rated the quality of included studies using the Cochrane Risk of Bias Tool.
Outcomes Assessed
We synthesized data on how corticosteroids were tapered across clinical trials, qualitatively and semiquantitatively, assessing what proportion of patients were on corticosteroids at trial entry, the maximum dose permitted, and corticosteroid tapering protocols (for how long the corticosteroid dose was kept stable, taper initiation and ending time points, taper protocol). We quantitatively assessed the pooled placebo rate of CSF-CR and the rate of CSF-CR vs overall clinical remission in patients with UC and CD who received active intervention or placebo. Clinical remission across trials was defined as Mayo Clinic Score [MCS] ≤2 with no individual subscore of >1 (for patients with UC) and Crohn’s Disease Activity Index (CDAI) <150 (for patients with CD). All analyses were stratified by UC and CD.
Statistical Analysis
We used the random-effects model described by DerSimonian and Laird to calculate the relative risk (RR) and 95% confidence interval (CI) of achieving CSF-CR vs overall clinical remission in patients receiving active intervention or placebo.4 We assessed heterogeneity between study-specific estimates using the inconsistency index (I2) and used cutoffs of <30%, 30%–59%, 60%–75%, and >75% to suggest low, moderate, substantial, and considerable heterogeneity, respectively.5 Due to the small number of studies, a reliable assessment of publication bias could not be estimated. All analysis was performed using Comprehensive Meta-Analysis (CMA), version 2 (Biostat, Englewood, NJ, USA).
RESULTS
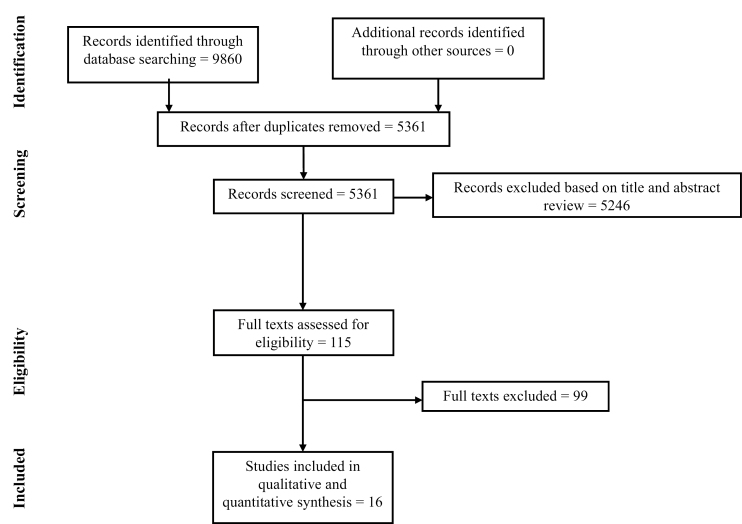
In total, 9860 records were identified through the database search through 2017; 5361 citations were screened after removing duplicate entries (Fig. 1). After updating the literature search and screening titles and abstracts, 115 trials were included in full-text review. Finally, 16 trials (11 RCTs of UC,6–15 5 RCTs of CD16–20) were included in the quantitative synthesis. Only trials of maintenance therapy reported the end point of CSF-CR (only for a subset of patients who were on corticosteroids at trial entry); none of the trials of induction therapy reported CSF-CR. CSF-CR was the primary end point for only 3 clinical trials of biologic agents, which did not report overall CR and were excluded from quantitative synthesis.21–23 The overall risk of bias was low in the included trials.
FIGURE 1.
Study selection flowsheet.
Corticosteroid Tapering in Clinical Trials
In RCTs of UC (11 trials) and CD (5 trials), a median (range) of 53% (28%–81%) and 49% (42%–56%) of participants were receiving corticosteroids at the time of trial entry, respectively. Participants could enter trials at median corticosteroid dose (range) of 35 (20–40) mg/d. After trial entry, corticosteroid dose was kept stable for a median (range) of 8 (5–10) weeks, generally throughout the duration of induction therapy. After that, a structured taper was initiated, which if enforced fully would have allowed corticosteroids to be fully tapered off by a median (range) of 17 (14–20) weeks. However, during corticosteroid taper, if patients had clinical worsening of disease, in most trials investigators were allowed to increase the corticosteroid dose back up to the dose used at trial entry (but not higher), and then advised to resume the taper within 2–4 weeks. However, in clinical trials of tofacitinib, corticosteroid dose escalation was not permitted, and patients were deemed treatment failures if they required corticosteroid rescue.15 Once patients had then tapered off corticosteroids, resumption of corticosteroids was not allowed. There are limited data on what proportion of patients across trials were tapered off corticosteroids by when, and what proportion were corticosteroid-dependent throughout the trial (but still classified as having achieved clinical remission). No data were available on cumulative steroid exposure throughout the trial. Tables 1 and 2 details how corticosteroids were tapered across trials, including the maximum allowed dose at trial entry and corticosteroid taper schedule and regimen.
TABLE 1.
Characteristics of Trials in Patients With Moderate–Severe Ulcerative Colitis Reporting Overall and Corticosteroid-Free Clinical Remission, and How Corticosteroids Were Handled During the Trial
| Trial and Intervention Characteristics | Definition and Timing of Outcome (CRem) | Maximum Prednisone Dose Equivalent at Trial Entry | Taper Initiation | Anticipated Taper Completeda | Taper Schedule | Concomitant Medications at Time of Trial Entry in Placebo and Intervention Arms | ||
|---|---|---|---|---|---|---|---|---|
| Immunomodulators, % | Corticosteroids, % | |||||||
| ACT 16 | 62 sites; P: 121; I: IFX, 121 |
MCS≤2; W54 | 40 mg/d | Week 8 | Week 20 | Decrease by 5 mg per week until 20 mg/d, then 2.5 mg per week | P: 44; I: 55 |
P: 65; I: 58 |
| ACT 26 | 55 sites; P: 123; I: IFX, 121 |
MCS≤2; W30 | 40 mg/d | Week 8 | Week 20 | Decrease by 5 mg per week until 20 mg/d, then 2.5 mg per week | P: 44; I: 43 |
P: 49; I: 50 |
| Jiang et al.7 | 1 site (China); P: 41; I: IFX, 41 |
MCS≤2; W30 | 40 mg/d | Week 8 | Week 20 | Decrease by 5 mg per week until 20 mg/d, then 2.5 mg per week | P: 32; I: 29 |
P: 51; I: 54 |
| NCT015512908 | 12 sites (China); P: 49; I: IFX, 50 |
MCS≤2; W26 | 30 mg/d | Week 8 | Week 14 | Decrease by 5 mg per week until 20 mg/d, then 2.5 mg per week | NR | P: 80; I: 60 |
| ULTRA 29 | 103 sites; P: 246; I: ADA, 248 |
MCS≤2; W52 | 20 mg/d | Week 8 | Week 14 | Decrease by 5 mg per week until 10 mg/d, then 2.5 mg per week | P: 51; I: 58 |
P: 75; I: 81 |
| Suzuki et al.10 | 65 sites; P: 96; I: ADA, 90 |
MCS≤2; W52 | 20 mg/d | Week 8 | Week 14 | Decrease by 5 mg per week until 10 mg/d, then 2.5 mg per week | P: 54; I: 46 |
P: 60; I: 63 |
| PURSUIT-M11 | 251 sites; P: 156; I: GLM, 154 |
MCS≤2; W52 | 40 mg/d | Week 6 | Week 18 | Decrease by 5 mg per week until 20 mg/d, then 2.5 mg per week | P: 33; I: 31 |
P: 53; I: 51 |
| PURSUIT-J12 | 49 sites (Japan); P: 31; I: GLM, 32 |
MCS≤2; W54 | 40 mg/d | Week 6 | Week 18 | Decrease by 5 mg per week until 20 mg/d, then 2.5 mg per week | P: 42; I: 50 |
P: 29; I: 28 |
| GEMINI I13 | 211 sites; P: 149; I: VDZ, 257 |
MCS≤2; W52 | 30 mg/d | Week 6 | Week 14 | Decrease by 5 mg per week until 10 mg/d, then 2.5 mg per week | P: 30; I: 35 |
P: 56; I: 53 |
| Motoya 201914 | 100 sites (Japan); P: 42; I: VDZ, 41 |
MCS≤2; W60 | 40 mg/d | Week 10 | Week 20 | Decrease by 2.5–5 mg per week until 10 mg/d, then 2.5–5 mg per week | P: 52; I: 49 |
P: 31; I: 32 |
| OCTAVE-Sustain15 | 178 sites; P: 198; I: tofacitinib, 395 |
MCS≤2, with rectal bleeding score 0; W52 | 25 mg/d | Week 5 | Week 14 | Decrease by 5 mg per week until 20 mg/d, then 2.5 mg per week | P: 0; I: 0 |
P: 51; I: 51 |
aUniform corticosteroid taper was not forced during corticosteroid taper. If patients had clinical worsening of disease, investigators were allowed to increase the dose back up to corticosteroid dose at trial entry (but not higher), and then advised to resume taper within 2–4 weeks.
Abbreviations: ADA, adalimumab; GLM, golimumab; I, intervention; IFX, infliximab; MCS, Mayo Clinic Score; P, placebo; VDZ, vedolizumab.
TABLE 2.
Characteristics of Trials in Patients With Moderate–Severe Crohn’s Disease Reporting Overall and Corticosteroid-Free Clinical Remission, and How Corticosteroids Were Handled During the Trial
| Trial and Intervention Characteristics | Definition and Timing of Outcome (CRem) | Maximum Prednisone Dose Equivalent at Trial Entry | Taper Initiation | Anticipated Taper Completeda | Taper Schedule | Concomitant Medications at Time of Trial Entry in Placebo and Intervention Arms | ||
|---|---|---|---|---|---|---|---|---|
| Immunomodulators, % | Corticosteroids, % | |||||||
| CLASSIC-II (maintenance phase)16 | 62 sites; P: 18; I: ADA, 37 |
CDAI<150; W56 | 30 mg/d | Week 8 | Week 16 | Decrease by 5 mg per week until 10 mg/d, then 2.5 mg per week | P: 6; I: 24 |
P: 56; I: 46 |
| CHARM17 | 92 sites; P: 170; I: ADA, 329 |
CDAI<150; W56 | 30 mg/d | Week 8 | Week 16 | Decrease by 5 mg per week until 10 mg/d, then 2.5 mg per week | P+I: 41 | P+I: 42 |
| GEMINI II18 | 285 sites; P: 153; I: VDZ, 308 |
CDAI<150; W52 | 30 mg/d | Week 6 | Week 14 | Decrease by 5 mg per week until 10 mg/d, then 2.5 mg per week | P: 32; I: 33 |
P: 54; I: 53 |
| CERTIFI19 | 153 sites; P: 72; I: UST, 73 |
CDAI<150; W22 | 40 mg/d | Week 8 | Week 18 | Decrease by 5 mg per week until 10 mg/d, then 2.5 mg per week | P: 23; I: 24 |
P: 55; I: 48 |
| IM-UNITI20 | 260 sites; P: 131; I: UST, 257 |
CDAI<150; W44 | 40 mg/d | Week 8 | Week 18 | Decrease by 5 mg per week until 10 mg/d, then 2.5 mg per week | P: 35; I: 33 |
P: 44; I: 49 |
aUniform corticosteroid taper was not forced during corticosteroid taper. If patients had clinical worsening of disease, investigators were allowed to increase the dose back up to corticosteroid dose at trial entry (but not higher), and then advised to resume taper within 2–4 weeks.
Abbreviations: ADA, adalimumab; CDAI, Crohn’s Disease Activity Index; I, intervention; P, placebo; UST, ustekinumab; VDZ, vedolizumab.
Placebo Rate for Corticosteroid-Free Clinical Remission
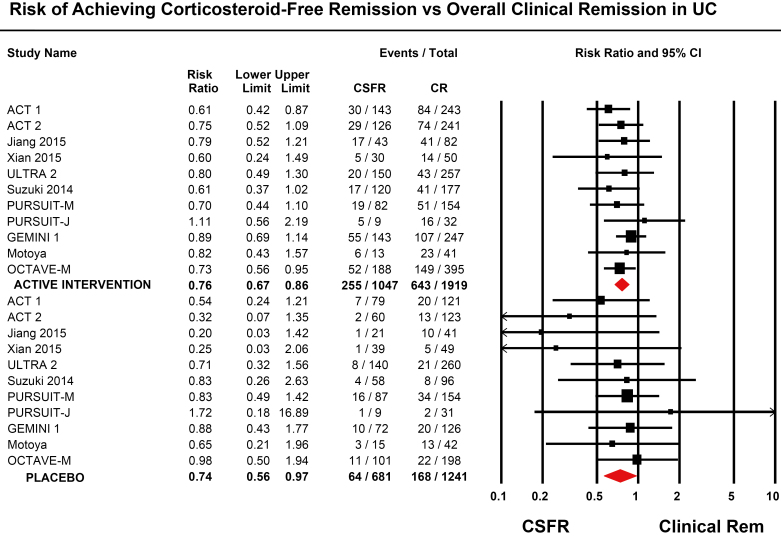
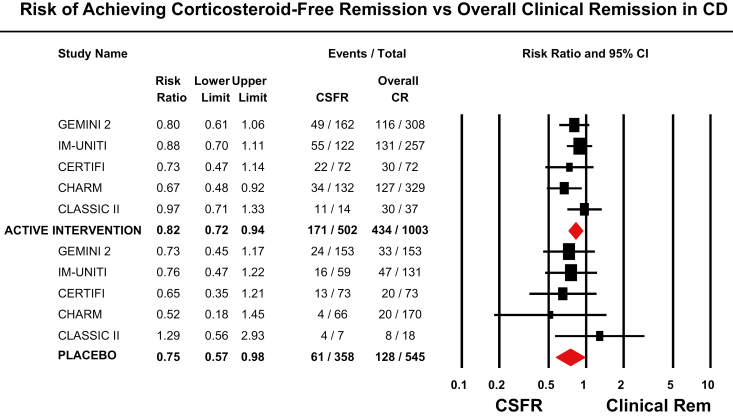
In trials of UC, pooled rates of CSF-CR in patients treated with placebo was 9.7% (95% CI, 6.8%–13.6%), as compared with an overall rate of clinical remission of 14.2% (95% CI, 10.6%–18.7%). In trials of CD, the pooled rate of CSF-CR in patients treated with placebo was 19.1% (95% CI, 11.3%–30.2%), as compared with an overall clinical remission rate of 25.7% (95% CI, 16.4%–37.7%). As compared with rate of overall clinical remission in placebo-treated patients, the rate of CSF-CR in placebo-treated patients with UC was 26% lower (RR, 0.74; 95% CI, 0.56–0.97), with minimal heterogeneity (I2 = 0%) and no significant difference by type of intervention (P = 0.33) (Fig. 2). Similarly, as compared with the rate of overall clinical remission in placebo-treated patients, the rate of CSF-CR in placebo-treated patients with CD was 25% lower (RR, 0.75; 95% CI, 0.57–0.98), with minimal heterogeneity (I2 = 0%) and no significant difference by type of intervention (P = 0.96) (Fig. 3). Due to the small number of studies available, metaregression to identify factors associated with CSF-CR in placebo-treated patients was not reported.
FIGURE 2.
Risk of achieving corticosteroid-free remission vs overall clinical remission in patients with moderate to severe ulcerative colitis.
FIGURE 3.
Risk of achieving corticosteroid-free remission vs overall clinical remission in patients with moderate to severe Crohn’s disease.
Overall vs Corticosteroid-Free Clinical Remission With Active Intervention
In patients with UC (11 trials), the rate of CSF-CR was 24% lower than the rate of overall clinical remission (RR, 0.76; 95% CI, 0.67–0.86), with minimal heterogeneity (I2 = 0%) and no significant difference by type of intervention (P = 0.82) (Fig. 2). In patients with CD (5 trials), the rate of CSF-CR was 18% lower than the rate of overall clinical remission (RR, 0.82; 95% CI, 0.72–0.94), with minimal heterogeneity (I2 = 0%) and no significant difference by type of intervention (P = 0.65) (Fig. 3).
Publication Bias
Due to small number of studies of UC and CD, formal assessment of funnel plot asymmetry was not performed.
DISCUSSION
Corticosteroids are effective for short-term symptom management but cause considerable short- and long-term side effects. Avoidance of corticosteroids is an important patient- and physician-preferred treatment outcome when managing UC and CD in clinical practice. In clinical trials, CSF-CR has been reported as both a primary and secondary efficacy end point. Logically, it would be important to ensure that there is standardization of steroid-tapering regimes across trials, especially in registration or head–head trials, as this can inherently influence symptoms that constitute the primary trial end point. To our knowledge, there has been limited discussion on CSF-CR as an end point when evaluating the efficacy of biologic agents and targeted small-molecule inhibitors. In this systematic review, we have synthesized how corticosteroids are tapered in clinical trials of moderate to severe CD and UC and the association between overall and CSF-CR. We observed that ~50% of participants are on corticosteroids at trial entry, with a maximum permitted dose ranging from 20 to 40 mg/d prednisone (it should be noted that these trials are thus almost uniformly underpowered for the subgroup analysis of CSF-CR). Across trials, the dose of corticosteroids remained stable during induction therapy for a median duration of 8 weeks, and a tapering schedule was implemented during maintenance, although it was not strictly enforced in the majority of trials (if strictly enforced, then patients across trials would have been uniformly off corticosteroids by week 20). If during the corticosteroid taper process patients experienced worsening symptoms, investigators were generally allowed to increase the corticosteroid dose back up to baseline dose at trial entry, and then encouraged to resume tapering in 2–4 weeks. However, there are limited data on what proportion of patients required resumption of corticosteroids, over what period of time, the dosing strategy used, and how many were still corticosteroid-dependent despite being classified as having achieved clinical remission. Furthermore, no data were available on the cumulative dose of corticosteroid exposure used throughout the duration of the trial, which could enable a fairer comparison between treatment arms within a trial and enable comparisons across trials. Across trials, and in both the placebo and active intervention arms, rates of CSF-CR were approximately 20%–25% lower than overall clinical remission, indicating that this is a more stringent end point to achieve. In general, the CSF-CR end point was less likely to achieve statistical significance, likely due to both the stringency of the end point and inadequate statistical power. Taken together, these data highlight that handling of steroids within pivotal clinical trial programs requires greater attention. With the increasing number of biologics and targeted small-molecule inhibitors in various stages of development and the large proportion of UC and CD patients in practice who remain steroid dependent, findings from our analysis may help inform the design and interpretation of ongoing and future clinical trials and provide insight into how clinical trial findings regarding corticosteroid tapering may be applied to clinical practice. Some specific trial design aspects that need to be considered include recommending a standard fixed maximum permissible dose of corticosteroids at trial entry, defining standard timing and protocols for corticosteroid taper during or immediately after induction, and developing consistent policies around re-escalation or re-initiation of corticosteroids after tapering during the maintenance phase of trials and implications in defining treatment failures.
How corticosteroids are handled in clinical trials can potentially influence the efficacy of medications and rates of adverse events and infections. In the SONIC study comparing infliximab + azathioprine vs monotherapy with either agent in immunosuppressive-naïve patients with CD, combination therapy was consistently demonstrated to be more effective for achieving and maintaining CSF-CR, the primary trial end point.22 In contrast, in the COMMIT trial in a similar patient population, infliximab + methotrexate was not shown to be more effective than infliximab monotherapy for achieving and maintaining CSF-CR.24 One proposed explanation was the use of prednisone induction therapy in the latter clinical trial, with a forced taper off by week 14, which may have augmented the efficacy of infliximab, potentially diluting the additive effect of methotrexate. Similarly, in the recently presented abstract of the VARSITY trial comparing vedolizumab vs adalimumab in patients with moderate–severe UC, vedolizumab was significantly more effective than adalimumab across multiple end points, including clinical, endoscopic, and histological remission.25 In patients who were taking steroids at trial entry, the rate of CSF-CR was numerically but not statistically higher in adalimumab-treated patients vs vedolizumab-treated patients; however, cumulative steroid exposure is yet to be reported.
Concomitant use of corticosteroids has been consistently shown to increase the risk of serious infections across biologic agents and may be associated with increased mortality in patients with CD.26, 27 In clinical practice, the true measure of effectiveness of a medication is being able to achieve and maintain remission off corticosteroids. In clinical trials, we observed that the rate of CSF-CR was 20%–25% lower than the rate of clinical remission, indicating that it is a more stringent end point to achieve. This information may be helpful in informing shared decision-making regarding efficacy of drug intervention. Likewise, there is limited guidance and considerable variability in how providers taper corticosteroids when starting a new biologic in practice, possibly due to the near-complete absence of randomized controlled trial data on this topic. Anecdotally, most providers generally attempt tapering off corticosteroids within 6–10 weeks of starting a new therapy. In contrast, in clinical trials, we observed that across studies the dose of corticosteroids is kept stable for ~8 weeks after enrollment, typically to coincide with the end of induction, followed by a subsequent taper that would have resulted in complete discontinuation by 20 weeks; however, a uniform policy of forced corticosteroid taper is typically not implemented. This difference may also potentially impact observed variability in remission rates, along with safety signals and adverse events. We do note that in the recent maintenance trial of tofacitinib for UC strict steroid tapering was required, and the rates of CSF-CR and CR were nearly identical for that reason. This successful trial demonstrates that a strict steroid-tapering protocol is achievable in a clinical trial with an effective therapy.
This is the first systematic synthesis of how corticosteroids are tapered across clinical trials in UC and CD. However, there are some limitations that should be acknowledged. First, we focused only on patients with moderate–severe UC and CD treated with biologic agents or targeted small molecules and did not address conventional therapies like thiopurines and methotrexate. This review was intended to reflect modern management and evolving trials in UC and CD. Second, we limited our analysis to placebo-controlled trials and excluded active comparator trials. This was meant to ensure consistency in estimating the association between CSF-CR and overall clinical remission and to ascertain placebo rates for achieving CSF-CR. Third, due to the limited number of trials, we were unable to ascertain factors influencing placebo rates of achieving CSF-CR through study-level metaregression or to perform subgroup analysis.
In conclusion, there is considerable variability in how corticosteroids are tapered across clinical trials, including differences in proportions of participants and maximum doses of corticosteroids on trial entry, timing and protocol for prednisone taper vis-à-vis assessment of primary outcomes, and lack of uniformly enforced corticosteroid taper. CSF-CR may be a patient-preferred outcome, and its rate is consistently 20%–25% lower than overall clinical remission. Hence, as core outcome sets for clinical trials in patients with UC and CD are being developed, CSF-CR should be an integral part of these and requires greater attention from those involved in designing and approving clinical trials for novel therapeutic agents to ensure consistency and enhance comparability of findings.
Supplementary Material
Supported by: This study did not receive any direct funding.
Conflicts of interest: John George has no conflicts to declare. Siddharth Singh is supported by the NIDDK K23DK117058, the American College of Gastroenterology Junior Faculty Development Award, and the Crohn’s and Colitis Foundation Career Development Award (#404614); has received research grant support from Pfizer and AbbVie; has received consulting fees from AbbVie, Takeda, and AMAG Pharmaceuticals; and has received honoraria from Pfizer for grant review. Parambir S. Dulai has received research grant support from Takeda, Pfizer, Janssen, Prometheus, Polymedco, and ALPCO; has served as a consultant for Takeda, Janssen, Prometheus Labs, and Abbvie. Christopher Ma has served as a consultant for Janssen, AbbVie, Pfizer, and Robarts Clinical Trials, Inc. Tran Nguyen is an employee of Robarts Clinical Trials Inc. Brian Feagan has received grant/research support from Millennium Pharmaceuticals, Merck, Tillotts Pharma, AbbVie, Novartis Pharmaceuticals, Centocor, Elan/Biogen, UCB Pharma, Bristol-Myers Squibb, Genentech, ActoGenix, and Wyeth Pharmaceuticals; has received consulting fees from Millennium Pharmaceuticals, Merck, Centocor, Elan/Biogen, Janssen-Ortho, Teva Pharmaceuticals, Bristol-Myers Squibb, Celgene, UCB Pharma, AbbVie, AstraZeneca, Serono, Genentech, Tillotts Pharma, Unity Pharmaceuticals, Albireo Pharma, Given Imaging, Salix Pharmaceuticals, Novonordisk, GSK, ActoGenix, Prometheus Therapeutics and Diagnostics, Athersys, Axcan, Gilead, Pfizer, Shire, Wyeth, Zealand Pharma, Zyngenia, GiCare Pharma, and Sigmoid Pharma; and has received speaker’s bureau fees from UCB, AbbVie, and J&J/Janssen. William J. Sandborn has received research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, Abbvie, Janssen, Takeda, Lilly, Celgene/Receptos; has received consulting fees from Abbvie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Prizer, Precision IBD, Progenity, Prometheus Laboratories, Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust, HART), Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, Tigenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, and Vivelix Pharmaceuticals; and has received stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Precision IBD, Progenity, Ritter Pharmaceuticals, Ventyx Biosciences, Vimalan Biosciences (spouse: Opthotech – consultant, stock options; Progenity – consultant, stock; Oppilan Pharma – employee, stock options; Escalier Biosciences – employee, stock options; Precision IBD – employee, stock options; Ventyx Biosciences – employee, stock options; Vimalan Biosciences – employee, stock options). Vipul Jairath receives salary support from the John and Susan McDonald Endowed IBD Chair at Western University, London, Ontario, Canada; has received consulting fees from AbbVie, Eli Lilly, GlaxoSmithKline, Arena Pharmaceuticals, Genentech, Pendopharm, Sandoz, Merck, Takeda, Janssen, Robarts Clinical Trials, Topivert, and Celltrion; and has received speaker fees from Takeda, Janssen, Shire, Ferring, AbbVie, and Pfizer.
Author contributions: Study concept and design: S.S. Acquisition of data: J.G., C.M., T.N., S.S. Analysis and interpretation of data: J.G., S.S., P.S.D., B.G.F., W.J.S., V.J. Drafting of the manuscript: J.G., S.S. Critical revision of the manuscript for important intellectual content: P.S.D., C.M., T.N., B.G.F., W.J.S., V.J. Approval of the final manuscript: J.G., S.S., P.S.D., C.M., T.N., B.G.F., W.J.S., V.J. Guarantor of the article: S.S.
REFERENCES
- 1. Huscher D, Thiele K, Gromnica-Ihle E, et al. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis. 2009;68:1119–1124. [DOI] [PubMed] [Google Scholar]
- 2. Ma C, Hussein IM, Al-Abbar YJ, et al. Heterogeneity in definitions of efficacy and safety endpoints for clinical trials of Crohn’s disease: a systematic review. Clin Gastroenterol Hepatol. 2018;16:1407–1419.e22. [DOI] [PubMed] [Google Scholar]
- 3. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- 4. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 5. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. [DOI] [PubMed] [Google Scholar]
- 7. Jiang XL, Cui HF, Gao J, Fan H. Low-dose infliximab for induction and maintenance treatment in Chinese patients with moderate to severe active ulcerative colitis. J Clin Gastroenterol. 2015;49:582–588. [DOI] [PubMed] [Google Scholar]
- 8. Xian-Janssen Pharmaceuticals Ltd. NCT01551290: a study to evaluate the effectiveness and safety of infliximab in Chinese patients with active ulcerative colitis. Available at: https://clinicaltrials.gov/ct2/show/NCT01551290. Accessed March 12, 2012.
- 9. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–265.e1. [DOI] [PubMed] [Google Scholar]
- 10. Suzuki Y, Motoya S, Hanai H, et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol. 2014;49:283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sandborn WJ, Feagan BG, Marano C, et al. ; PURSUIT-SC Study Group . Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85–95; quiz e14. [DOI] [PubMed] [Google Scholar]
- 12. Hibi T, Imai Y, Senoo A, et al. Efficacy and safety of golimumab 52-week maintenance therapy in Japanese patients with moderate to severely active ulcerative colitis: a phase 3, double-blind, randomized, placebo-controlled study (PURSUIT-J study). J Gastroenterol. 2017;03:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group . Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 14. Motoya S, Watanabe K, Ogata H, et al. Vedolizumab in Japanese patients with ulcerative colitis: a phase 3, randomized, double-blind, placebo-controlled study. PLoS One. 2019;14:e0212989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sandborn WJ, Su C, Sands BE, et al. ; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators . Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–1736. [DOI] [PubMed] [Google Scholar]
- 16. Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65. [DOI] [PubMed] [Google Scholar]
- 18. Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group . Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721. [DOI] [PubMed] [Google Scholar]
- 19. Sandborn WJ, Gasink C, Gao LL, et al. ; CERTIFI Study Group . Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–1528. [DOI] [PubMed] [Google Scholar]
- 20. Feagan BG, Sandborn WJ, Gasink C, et al. ; UNITI–IM-UNITI Study Group . Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960. [DOI] [PubMed] [Google Scholar]
- 21. Lémann M, Mary JY, Duclos B, et al. ; Groupe d’Etude Therapeutique des Affections Inflammatoires du Tube Digestif (GETAID) . Infliximab plus azathioprine for steroid-dependent Crohn’s disease patients: a randomized placebo-controlled trial. Gastroenterology. 2006;130:1054–1061. [DOI] [PubMed] [Google Scholar]
- 22. Colombel JF, Sandborn WJ, Reinisch W, et al. ; SONIC Study Group . Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. [DOI] [PubMed] [Google Scholar]
- 23. Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392–400.e3. [DOI] [PubMed] [Google Scholar]
- 24. Feagan BG, McDonald JW, Panaccione R, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology. 2014;146:681–688.e1. [DOI] [PubMed] [Google Scholar]
- 25. Schreiber S, Peyrin-Biroulet L, Loftus EV Jr, et al. VARSITY: a double-blind, double-dummy, randomised, controlled trial of vedolizumab versus adalimumab in patients with active ulcerative colitis. Paper presented at: European Crohn’s and Colitis Organization Annual Meeting, Copenhagen, Denmark; 2019. [Google Scholar]
- 26. Singh S, Facciorusso A, Dulai PS, et al. Comparative risk of serious infections with biologic and/or immunosuppressive therapy in patients with inflammatory bowel diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019; Mar 12: 1542–3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewis JD, Scott FI, Brensinger CM, et al. Increased mortality rates with prolonged corticosteroid therapy when compared with antitumor necrosis factor-α-directed therapy for inflammatory bowel disease. Am J Gastroenterol. 2018;113:405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.