Summary
The Wnt/β-catenin pathway is evolutionary conserved signaling system that regulates cell differentiation and organogenesis. We show that endothelial specific stabilization of Wnt/β-catenin signaling alters early vascular development in the embryo. The phenotype resembles that induced by upregulation of Notch signaling, including lack of vascular remodeling, altered elongation of the intersomitic vessels, defects in branching, and loss of venous identity. Both in vivo and in vitro data show that β-catenin upregulates Dll4 transcription and strongly increases Notch signaling in the endothelium, leading to functional and morphological alterations. The functional consequences of β-catenin signaling depend on the stage of vascular development and are lost when a gain-of-function mutation is induced at a late stage of development or postnatally. Our findings establish a link between Wnt and Notch signaling in vascular development. We propose that early and sustained β-catenin signaling prevents correct endothelial cell differentiation, altering vascular remodeling and arteriovenous specification.
Introduction
The Wnt canonical signaling pathway regulates fundamental aspects of development including cell-fate specification, proliferation, survival, and overall organogenesis (for review, see Angers and Moon, 2009; Clevers, 2006; Dickinson and McMahon, 1992; Junghans et al., 2005; Klaus and Birchmeier, 2008). Wnt proteins are a large family of secreted signaling molecules that signal through binding to a coreceptor complex formed by the seven-pass transmembrane proteins of the frizzled (Fzd) family and the lipoprotein receptor related 5/6 (Lrp5/6) proteins. This interaction leads to inactivation of a destruction complex that includes glycogen synthase kinase-3β (GSK-3β), the scaffolding protein axin, and adenomatosis polyposis coli (APC). The complex phosphorylates β-catenin and targets it for ubiquitin-proteasome-mediated degradation. In the presence of Wnt signaling, β-catenin is not phosphorylated and is therefore free to translocate to the nucleus and modulate cell transcription. β-catenin also links cadherins at cell-to-cell adherens junctions and stabilizes their interaction with the cytoskeleton.
It has been reported that canonical Wnt pathway may regulate cardiac and vascular development in the embryo (for review, see Goodwin and D’Amore [2002] and Parmalee and Kitajewski [2008]). Endothelial specific deletion of β-catenin alters the development of the embryonic vasculature and results in early lethality in utero at embryonic day (E)12.5 (Cattelino et al., 2003). The vessels show changes in vascular lumen, defects of vascular remodeling, and diffuse hemorrhages in different regions of the vascular tree. Furthermore, loss of β-catenin causes defective endocardial cushion and cardiac valve development due to altered endothelial mesenchymal transformation (Liebner et al., 2004). In absence of β-catenin, endothelial adherens junctions present a different molecular organization, which may contribute to vascular fragility. However, loss of Wnt signaling can also play a role in the observed phenotype. Consistently, ablation of Fzd5 (Ishikawa et al., 2001) and Wnt2 (Monkley et al., 1996) leads to a defective placenta vascularization and, in the case of Fzd5, also to defective remodeling of the yolk sac vasculature after E10.5. Furthermore, Wnt7b is responsible for hyaloid vessel regression in the retina (Lobov et al., 2005), and the binding of norrin to Fzd4 plays an important role in angiogenesis of the eye and the ear through stabilization of β-catenin (Xu et al., 2004; Ye et al., 2009). Finally, the differentiation of brain microvasculature to acquire blood brain barrier characteristics requires Wnt signaling and β-catenin transcriptional activity (Daneman et al., 2009; Liebner et al., 2008; Stenman et al., 2008).
Wnt cooperates frequently with the Notch signaling pathway in different cellular systems such as hemopoietic stem cells and heart development (Cohen et al., 2008; Grego-Bessa et al., 2007); however, little is known about the cooperation of Wnt and Notch in vascular development.
It has been reported that Notch signaling controls multiple aspects of endothelial cells (ECs) function such as growth, migration, lumen formation, and arteriovenous determination (Hellstrom et al., 2007; Hofmann and Iruela-Arispe, 2007; Lawson et al., 2001; Phng and Gerhardt, 2009; Roca and Adams, 2007; Suchting et al., 2007; Thurston et al., 2007). Recent studies in several experimental models show that this signaling pathway acts by limiting vascular sprouting. Suppression of Notch signaling by different tools markedly augments vascular density, branching, and hyperfusion of the capillary network.
In this work, we have studied endothelial-specific β-catenin gain-of-function (GOF) mutant mice to obtain a more comprehensive idea of the role of canonical Wnt signaling in ECs and to better define the mechanism of action of this pathway. We report that these β-catenin GOF mutant embryos die in utero with major alterations of vascular development. Vessels were unable to sprout and branch correctly, presented altered lumen, and showed a strongly affected arterial-venous specification. qRT-PCR analysis of ECs isolated from mutant embryos showed a significant increase in Dll4/Notch signaling. Isolated and cultured ECs expressing the same β-catenin GOF mutation also showed a strong increase in Dll4/Notch signaling accompanied by reduced sprouting activity. Additionally, chromatin immunoprecipitation and luciferase reporter assays showed β-catenin binding to the Dll4 promoter. These data determine a sequential and direct link between β-catenin and Notch signaling systems to tune ECs differentiation and vascular morphogenesis.
Results
Wnt/β-Catenin Signaling Is Detectable during Embryonic Vascular Development
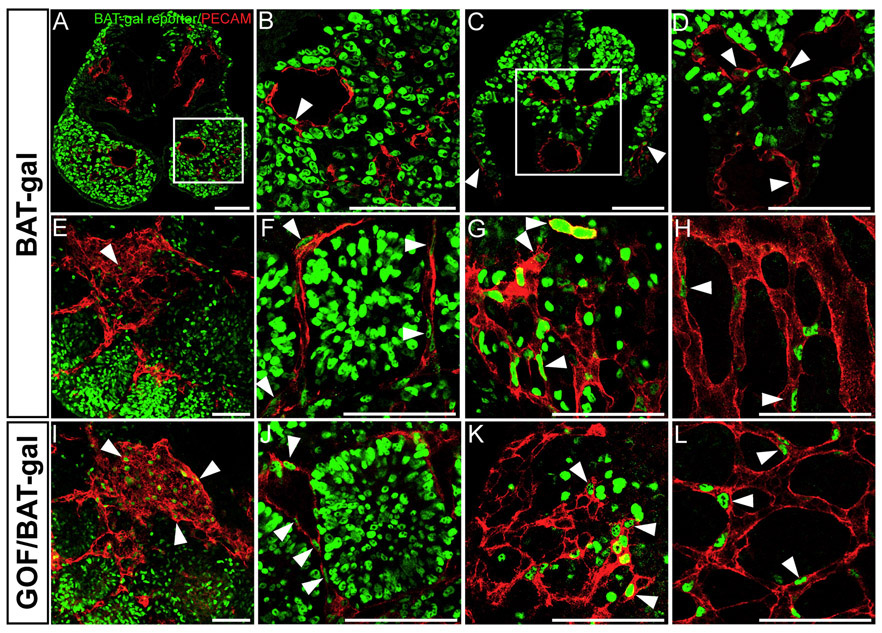
We first examined whether β-catenin signaling could be detected in newly forming vessels in the mouse embryo. Using BAT-gal reporter mice for Wnt/β-catenin signaling (Maretto et al., 2003), we observed b-catenin induced LacZ-reporter activity in ECs starting from E9.5 in several regions of the vascular tree. Confocal analysis of sectioned and whole-mounted BAT-gal embryos revealed ECs stained with β-galactosidase nuclei within the branchial arteries, (Figures 1A and 1B), dorsal aorta (DA), vitelline artery, umbilical vein (Figures 1C and 1D), caval vein, intersomitic vessels, tail microvasculature (Figures 1E–1G), and yolk sac microvasculature (Figure 1H). Additionally, β-galactosidase-positive ECs were also found within the endocardium and perineural vascular plexus, as reported previously (Daneman et al., 2009; Liebner et al., 2004, 2008; Stenman et al., 2008). Although reporter positive ECs were found throughout the vascular tree, they were four times more abundant within the vessels of the tail compared to the upper body.
Figure 1. Wnt/β-Catenin Signaling Is Active in Newly Forming Vessels in the Mouse Embryo.
(A–H) Confocal analysis of sectioned (A–D) and whole-mounted (E–L) E9.5 BAT-gal embryos double stained for β-galactosidase (green), to detect β-catenin induced expression of the Lac-Z reporter construct, and for PECAM (red) to detect the endothelium. In BAT-gal embryos, double positive cells (arrowheads) were found within the branchial arteries ([A and B], [B] represents the indicated area in [A]), the dorsal aorta and vitelline artery ([C and D], [D[represents the indicated area in [C]), (E) cardial vein, (F) ISV, (G) tail, and (H) yolk sac microvasculature. (I–L) In β-catenin GOF/BAT-gal embryos, an increased number of β-galactosidase and PECAM double-positive cells (I)–(L) were found throughout, the vascular tree compared to (E)–(H) control embryos. Representative single optical sections are shown. Control, n = 5; GOF, n = 4. Scale bars = 100 μm.
Endothelial β-CateninΔex3 Recombination Induces Embryonic Lethality
To understand the functional role of β-catenin signaling during vascular development, we selectively activated β-catenin transcriptional activity in ECs by crossing β-cateninlox(ex3)/lox(ex3) mice (Harada et al., 1999) with Tie2-Cre (Kisanuki et al., 2001) or VE-cadherin-Cre (Alva et al., 2006) transgenics. The recombined mutant β-cateninΔex3 lacks the exon 3 domain and cannot be phosphorylated and subsequently ubiquitinated and degraded in proteasomes, therefore it is free to translocate to the nucleus and activate cell transcription functioning as a GOF mutant. The ability of Tie2-Cre to recombine the floxed allele in the endothelium was tested by genomic PCR from embryo extracts. As reported in Figure S1 (available online) the β-cateninΔex3 allele was detected only in embryos expressing Tie2-Cre (Figure S1C) or VE-cadherin-Cre (data not shown). Further support that an efficient recombination of the β-cateninΔex3 allele was achieved and provided a robust β-catenin GOF phenotype, was given by a 4-fold increase in β-galactosidase reporter positive ECs throughout the vascular tree of β-catenin GOF embryos intercrossed with BAT-gal reporter mice compared to control embryos, 27.3 versus 6.8 reporter positive ECs per section, (compare Figures 1I-1L with Figures 1E-1H). Furthermore, cultured β-catenin GOF mutants ECs presented an effective recombination of the floxed gene (Figure S1C). No pup with endothelial β-cateninΔex3/wt/Tie2-Cre+, or VE-cadherin-Cre+ was born (Figure S1A). β-catenin GOF mutants were morphologically indistinguishable from control embryos (littermates that had not inherited the complete set of alleles) up to E8.5. However, 100% of the embryos died within E11.5 and E12.5 (Figure S1A). The intercross with either Tie2-Cre or VE-cadherin-Cre transgenics gave essentially the same lethality and embryonic phenotype, therefore, for the experiments described below, we focused on one mutant only i.e., β-cateninΔex3/wt/Tie2-Cre+ (referred to as β-catenin GOF mutant from here on).
Vascular Defects in Endothelial β-Catenin GOF Mutants
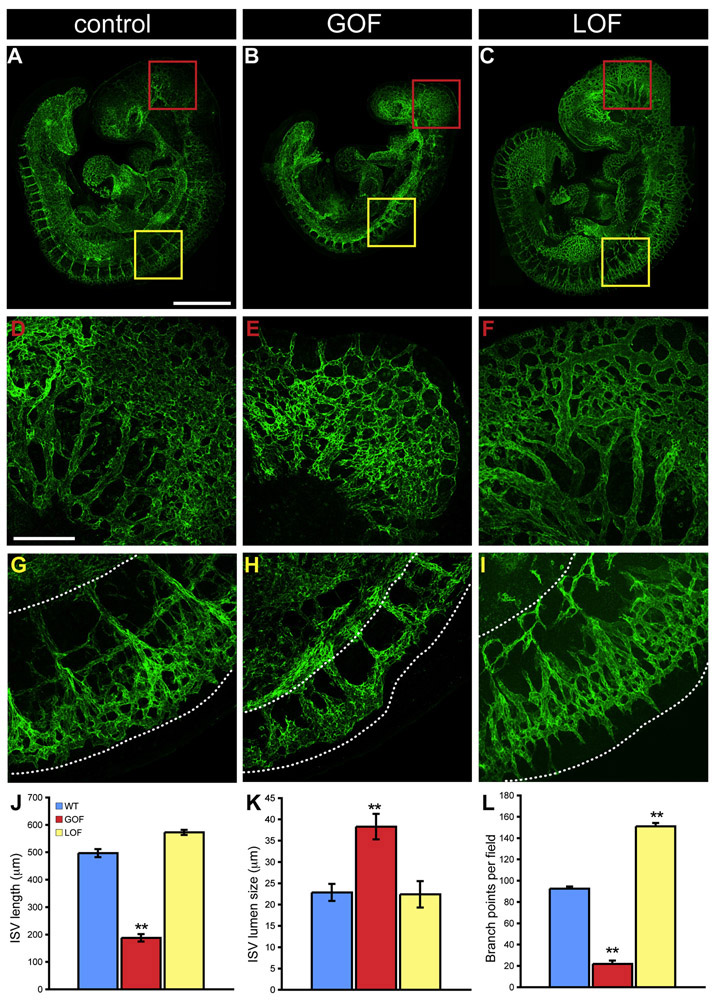
At E9.5, β-catenin GOF embryos were smaller and the vascular pattern was strongly defective in several regions of the vascular tree (Figure 2B). Intersomitic vessels (ISV) in both the occipital and trunk regions failed to elongate correctly and branching was severely altered. Irregular blind ending vessels and regions of vascular fusion and lacunae could be observed (compare Figures 2A and 2B, Figures 2D and 2E, and Figures 2G and 2H). A closer analysis (Figure S2) highlights strong reduction in the length of the ISV, and the absence of a perineural vascular plexus due to lack of correct branching and remodeling of small vessels in that region.
Figure 2. Endothelial β-Catenin GOF Mutation Strongly Affects Vascular Development.
(A–I) Whole-mount PECAM immunofluorescence staining of E9.5 control (somites n. 24-26) (A, D, and G), β-catenin GOF (B, E, and H), and LOF (C, F, and I) embryos. In β-catenin GOF embryos, the vascular pattern was strongly defective in several regions of the vascular tree. ISV in the trunk failed to elongate correctly and branching was severely altered. Irregular blind ending vessels and regions of vascular fusion and lacunae could be observed. The head vasculature did not undergo remodeling (D and E). LOF mutants showed a hyperbranched morphology at the perineural vascular plexus (compare [G] and [I]). (J–L) Quantitation of length, lumen size, and branch point of the ISV in the trunk of control, GOF, and LOF embryos. The dotted lines in (G)–(I) indicate the area of quantification. Error bars represent SD; p values are <0.05 (*) and <0.01 (**) by Student’s t test. Scale bars = 1000 μm in (A)–(C); 200 μm in (D)–(I).
For comparison, we studied loss-of-function (LOF) mutants of endothelial β-catenin described in detail in previous work (Cattelino et al., 2003). Analysis of these embryos, which at this early stage of development are still viable, revealed an opposite phenotype as compared to β-catenin GOF mutants with hyperbranching morphology at the perineural vascular plexus in some regions of the trunk (compare Figures 2G-2I). Quantification of the observed modifications showed a significant decrease in ISV length and branch points and an increase in ISV lumen size in GOF mutants (Figures 2J-2L) and increased ISV branching in LOF mutants (Figure 2L). Vascularization of the head (Figures 2D and 2E and Figures 4F and 4G) and yolk sac (Figures 4H and 4I) was also affected severely by β-catenin stabilization. Blood vessels in the perineural vascular plexus and the yolk sac did not undergo remodeling, and large arteries and veins were essentially missing. The vascular phenotype of β-catenin GOF mutants further progressed until E10.5, and the intersomitic, head, and tail vessels showed several areas of degeneration, regression, and disconnection (Figures S3A-S3H).
Figure 4. Endothelial β-Catenin GOF Mutation Upregulates Notch Signaling.
qRT-PCR analysis from (A) whole embryos (E9.5), and (B–D) Dynabeads isolated ECs. The RNA level obtained for controls was set to 1, and the ratio for β-catenin GOF versus controls or β-catenin LOF versus controls is shown for each gene. (A–C) Data show that several members of the Notch signaling pathway are upregulated by in vivo endothelial β-catenin GOF mutation and reduced in the absence of β-catenin. Error bars represent SD. Data are means of four experiments carried out in triplicate. p values are <0.05 (*) and <0.01 (**) by Student’s t test. (D) The γ-secretase inhibitor (DAPT) treatment of GOF embryos rescues the reduction of venous markers induced by the β-catenin GOF to control values. Error bars represent SD. Control n = 10, GOF untreated n = 8, GOF DAPT treated n = 8 embryos. p value is <0.01 (**) by Student’s t test. (E) DAPT treatment of GOF embryos improve vascular remodeling. The length of the major vitelline vessels was defined as continuous large arteries and veins with a diameter >20 μm; untreated embryos n = 8; DAPT treated embryos n = 4 means ± SEM; p value is < 0.01 (**) by Student’s t test. Confocal analysis of PECAM stained whole-mounted (F, G, J, and K) E9.5 embryos and (H, I, L, and M) yolk sacs, (F–I) untreated, and (J–M) treated with DAPT. The head vasculature of β-catenin GOF embryos lacked remodeling and formation of large veins (box in [G]) as compared to control embryos (box in [F]). Control, n = 10; GOF, n = 10 embryos. DAPT treatment, starting at E7.5, partially reversed the β-catenin GOF phenotype and promoted remodeling and formation of both large arteries and veins (box in K) compared to untreated β-catenin GOF embryos (box in [G]). DAPT treatment did not affect the development of the head vasculature in control embryos, compare (J) to (F). Yolk sacs of (H and I) untreated embryos and (L and M) DAPT treated embryos. β-catenin GOF embryos lacked remodeling of the yolk sac vasculature and formation of large vessels (compare [I] and [H]). A transient establishment of large vessels (red line in [I]) could be observed before they regressed. Control, n = 10; GOF, n = 10 embryos. DAPT treatment partially reversed the β-catenin GOF phenotype by promoting remodeling and formation of both larger arterial (red lines) and venous (green lines) vessels (M) compared to (I) untreated β-catenin GOF embryos. The DAPT treatment did not affect the development of the yolk sac vasculature in control embryos (L) compared to (H) (red and green lines correspond to arteries and veins, respectively). Control, n = 8; GOF, n = 4 embryos. Scale bars = 100 μm (F, G, J, and K); 500 μm (H, I, L, and M).
To investigate the degree of arterial-venous specification in β-catenin GOF mutants, we crossed β-cateninlox(ex3)/lox(ex3) mice with LacZ reporter strains to detect expression of EphB4, marking veins (Gerety et al., 1999), or ephrin-B2, marking arteries (Wang et al., 1998). Recombination of β-cateninΔex3 by crossing with Tie2-Cre transgenics revealed that the expression of EphB4 was strongly downregulated throughout the venous compartment of β-catenin GOF embryos as compared to controls (Figures 3A and 3C), indicating loss of endothelial venous identity. Ephrin-B2 was expressed at high levels all over the arterial compartment in β-catenin GOF embryos (Figure 3D), and no macroscopic differences could be observed when compared to control embryos (Figure 3B). However, detailed analysis of tissue sections showed that ECs expressing ephrin-B2 could be found both in arteries and veins of β-catenin GOF mutants as shown in Figures 3E-3H, where both ECs of the DA and anterior cardinal vein (ACV) expressed ephrin-B2, indicating the establishment of arterial cell identity in the venous compartment of the mutant embryo. This finding resembles the phenotype previously found in endothelial specific Notch-signaling GOF embryos (Kim et al., 2008; Trindade et al., 2008), where ephrin-B2 expressing cells, normally only found in the arteries, also could be found in the ACV. Furthermore, as reported in Figures 3I-3L, serial sections from the region where the ACV connects to the sinus venosus showed that the DA fused with the ACV β-catenin GOF embryos, leading to a bypass circulation from DA to the sinus venosus. The presence of this type of shunt is typical of conditions where arterial-venous identity is lost.
Figure 3. Endothelial β-Catenin GOF Mutation Prevents Venous Differentiation and Promotes Vascular Arterialization.
β-catenin GOF embryos were crossed with EphB4 and ephrin-B2 LacZ reporter mice to investigate venous and arterial specification, respectively. In β-catenin GOF embryos (C), the expression of EphB4 was markedly downregulated throughout the venous compartment compared to (A) control embryos, indicating reduced venous specification in the β-catenin GOF embryos. Macroscopic analysis of the ephrin-B2 expression showed an intense staining throughout the arterial compartment of both (B) control and (D) β-catenin GOF mutants embryos. Confocal analysis of cryosections showed that in addition to the DA and arterioles, ephrin-B2 expressing cells could also found within the ACV in β-catenin GOF mutants ([G] and magnified area in [H]) but not in control ([E] and magnified area in [F]) embryos. Control, n = 2; GOF, n = 2 embryos. Serial sections further revealed the presences of arteriovenous shunts (arrowhead) in the region where the ACV connects to the sinus venosus in seven of eight β-catenin GOF (K) and (L) but not in (I) and (J) control embryos. Control, n = 3; GOF, n = 8 embryos. Scale bars = 1 mm in (A)–(D); 50 μm in (E)–(L).
We counted the ratio of Ki67-positive ECs within the DA and ACV in tissue sections of β-catenin GOF and control embryos and no significant difference was found (0.18 ± 0.04, percentage increase in the β-catenin GOF over the control). The level of apoptosis detected within the embryo vasculature was not significantly modified (Figure S7).
Stabilization of β-Catenin Induces Notch Signaling
The vascular phenotype observed in β-catenin GOF mutant embryos was similar to that observed in Dll4/Notch GOF embryos (Trindade et al., 2008); we therefore tested whether β-catenin stabilization was coupled to increase in Notch signaling in these embryos. qRT-PCR analysis of embryo extracts showed that most EC markers were not modified significantly including pan-EC markers such as PECAM or VE-cadherin (Figure S4A). As expected, Axin2 (Figure 4A), which is a sensitive downstream target of β-catenin transcriptional activity, was strongly increased. We also found a consistent and marked augmentation of Dll4 and the dependent transcription factors Hey1 and 2, indicating a significant increase in Notch signaling in these embryos. Conversely, Notch1 and 4 and Jag1 remained essentially unchanged (Figure 4A). As these data were obtained from total embryo extracts, we could not discriminate whether the increase in Notch signaling was specific for ECs. Therefore, qRT-PCR analysis was also carried out on ECs freshly isolated from E9.5 embryo cell suspensions (Figure 4B). Consistent with the whole-embryo data, purified ECs from β-catenin GOF mutants presented significantly higher levels of Dll4, Hey1, and Hes. Hey2 was undetectable and did not increase in β-catenin GOF cells, whereas the downstream target of Notch signaling Nrarp was increased (Phng et al., 2009). Conversely, when β-catenin expression was abrogated as in LOF mutants, Hes1, Dll4, and Nrarp were significantly reduced whereas EphB4 was increased (Figure 4C).
To directly test whether β-catenin GOF mutation in vivo could increase Notch signaling we crossed TNR1 reporter mice (expressing a transgene composed of CBF-1 response element with four CBF-1 binding sites and a minimal SV40 promoter followed by an enhanced green fluorescent protein, GFP) (Duncan et al., 2005) with β-cateninΔex3/Tie2-Cre mutants. ECs were freshly isolated and qRT-PCR analysis of GFP expression was carried out. ECs from TNR1/β-catenin GOF mutants at E9.5 showed 40% increase in GFP as compared to control embryos, supporting the idea of increase in Notch signaling on β-catenin stabilization in the embryo.
To further prove the role of Notch activation in the observed phenotype, we treated pregnant mice with a γ-secretase inhibitor (DAPT) to inhibit Notch signaling in the embryos. Although we used relatively low doses of the drug to avoid embryo toxicity, we could see a partial but significant rescue of the phenotype in the β-catenin GOF mutant embryos. qRT-PCR analysis of ECs freshly isolated from DAPT treated embryos showed a normalization of the expression of the venous markers EphB4 and COUP-TFII, whereas no effect was detected on the level of ephrin-B2 expression (Figure 4D). Furthermore, analysis of both the head (Figures 4F, 4G, 4J, and 4K) and yolk sac vasculature (Figures 4H, 4I, 4L, and 4M) showed that the DAPT treatment improved the level of vascular remodeling and the formation of both large arteries and veins as indicated by a 4-fold increase in length of the major vitelline vessels (Figure 4E and highlighted in Figures 4H-4M).
We then intercrossed transgenic lines, allowing endothelial-specific and tamoxifen-inducible Cre-recombination, (Cdh5-CreERT2) (Sorensen et al., 2009) with β-cateninlox/lox, β-cateninlox(ex3)/lox(ex3) mutant mice. To investigate the effect of β-catenin stabilization or deletion on the developing retinal vasculature, we initiated tamoxifen treatments of mutant litters at postnatal day (p)1 or p3 and carried out analysis at p5–6 (Figures S5A and S5I). In both mutants, the radial expansion of the retinal vasculature was reduced as compared to controls, whereas the general morphology was similar (Figures S5B, S5C, S5J, and S5K). Quantification of the number of tip cells and filopodia per length of the vascular front did not reveal any difference in the two conditions (Figures S5B-S5P). Vascular density was reduced in LOF mutants (data not shown), in agreement with previous reports (Phng et al., 2009).
Furthermore, β-catenin GOF mutation was induced at late stages of embryonic development by treating pregnant mutant females with tamoxifen at E9.5 (Figure S5Q) and analyzing their vasculature up to E14.5 when recombination is expected to reach the highest level. We could not observe major differences in arteriovenous morphology and specification (data not shown). qRT-PCR analysis, carried out on freshly isolated ECs from β-catenin GOF and control embryos at E12.5 and E14.5 respectively, show only a small and not significant increase in Dll4 and downstream Notch effectors (Figure S5R) induced by β-catenin GOF mutation. Overall these observations indicate that endothelial cells are sensitive to β-catenin GOF mutation only at early stages of vascular development. At later stages, when arteriovenous identity is largely determined, the effect of sustained β-catenin signaling is lost.
β-Catenin GOF Mutation in Cultured ECs Affects Cell Sprouting
To further study the mechanism of action of β-catenin at a cellular and molecular level and to avoid indirect alterations due to defective organ perfusion, we isolated and cultured ECs from β-cateninlox(ex3)/wt/Cre− embryos. Recombination was induced in vitro by treating the cells with an adeno-Cre vector (Cattelino et al., 2003) (Figure S1C). qRT-PCR analysis of these cells showed a picture largely comparable to that obtained in the freshly isolated endothelium from β-catenin GOF embryos (Figure S4C). Axin2, Dll4, and Hey1 were strongly increased. Hey2, Hes, and Notch1 were not significantly changed whereas a small increase in Jag1 was detected.
Confluent β-catenin GOF ECs presented different morphology on recombination (compare Figures 5E and 5G): actin staining showed short stress fibers and irregular intercellular contacts whereas control cells were more elongated with longer and more organized stress fibers. Staining of β-catenin was concentrated at intercellular junctions in control cells but it was intense also in the nuclei in β-catenin GOF mutant cells indicating higher transcriptional signaling (compare Figures 5A and 5C). When a wound was induced in the cell monolayer, control and β-catenin GOF cells migrated into the empty space with different morphologies. Whereas control ECs formed several sprouting pseudopodia and filopodia (Figure 5F, arrowheads), β-catenin GOF mutant cells presented peripheral ruffles and lamellipodia at the front instead of filopodia (Figure 5H, arrows; Movies S1 and S2). In β-catenin GOF mutant cells and, to a much lower degree, in control cells, nuclear staining of β-catenin was increased in cells located at the migrating front compared to confluent cells (Figures 5A-5D).
Figure 5. β-Catenin GOF Mutation in Cultured ECs Affects Cell Sprouting and Pattern of Migration.
Cultured ECs β-cateninlox(ex3)/wt/Cre− were infected with adeno vector-expressing Cre-recombinase to obtained ECs line β-cateninΔex3/wt (GOF). Confluent cells were wounded to produce a cell-free zone. Immunofluorescence analysis of cells stained with (A–D) anti-β-catenin antibody and (E–H) phalloidin in confluent and wounded monolayer. β-catenin was concentrated in the nucleus in (C and D) β-catenin GOF mutant cells and in (B) control cells at the migrating front. Pseudopodia and filopodia are present in “migrating” control cells (arrowheads in [F]). In contrast, β-catenin GOF mutant cells present peripheral ruffles at the front (arrows in [H]) and no filopodia. (I–L) Nuclear NICD staining was strongly increased in (J) migrating control cells and more markedly in (L) β-catenin GOF cells. Dashed lines (J and L) indicate the migrating front. NICD nuclear staining was reduced in the presence of DAPT in both control and β-catenin GOF cells (M, N, O, and P). DAPT treatment induced more elongated filopodial protrusions ([R], arrowheads) in control cells and recovered a sprouting morphology and long actin fibers in β-catenin GOF cells (arrowheads in [T]). Scale bars = 20 μm.
To test whether, similarly to β-catenin GOF embryos, the increase in β-catenin signaling was accompanied by increase in Notch signaling, we stained the ECs with an antibody against the intracellular domain of Notch1 (NICD), which is released from the membrane and translocates to the nucleus on Notch activation (Artavanis-Tsakonas et al., 1999; Bray, 2006). As shown in Figure 5J, we found that Notch signaling was increased in control cells located at the migrating front as compared to confluent cells (Figure 5I). A similar pattern was also found in β-catenin GOF mutant cells but to a much stronger degree (Figure 5L). Inhibition of Notch signaling by DAPT treatment increased control cell sprouting into the wound (Figures 5Q and 5R). In addition, DAPT treatment of β-catenin GOF mutant cells partially recovered the mutant phenotype, cells showed filopodia (arrowheads) and higher sprouting activity (Figures 5S and 5T).
Finally, we infected the cells with a construct encoding the intracellular (IC) domain of Notch1 (N1IC) engineered into pHyTc retroviral vector. As reported in Figures S6E and S6F when we focused on the infected cells at the front by staining with an anti-HA-Tag antibody, constitutive Notch signaling induced peripheral ruffles and lamellipodia similarly to β-catenin GOF mutant cells. Furthermore neither β-catenin nor Notch GOF mutations did not change cell proliferation (Figure S6G).
β-Catenin Increases Notch Activity through Its Transcriptional Activity
To investigate whether the effect of β-catenin in inducing Notch activation and changes in cell morphology were mediated by its transcriptional activity, β-catenin GOF mutant cells were infected with a dominant-negative TCF4 mutant (dnTCF4) able to abrogate β-catenin transcriptional activity (Liebner et al., 2008). In these cells the transcription of both Axin2 and Dll4 were strongly reduced and Notch signaling was abrogated (Figures 6A and 6B). These cells express both Notch 1 and 4 as receptors and the effect of Dll4 reduction was apparent when they were in reciprocal contact as shown in the Figure 6B. To further prove that Notch signaling was increased by cell contact with β-catenin GOF mutant ECs, a coculture system with HeLa cells transfected with Notch 1 was used. As reported in Figure S6H β-catenin GOF endothelial cells increased NICD fluorescence intensity in the nucleus of HeLa transfected cells.
Figure 6. β-Catenin Increases Notch Signaling through Its Transcriptional Activity.
(A) qRT-PCR analysis of Axin2 and Dll4 mRNA levels in β-catenin GOF ECs shows a significant inhibition of Axin2 and Dll4 upregulation by cell infection with dnTCF4. Error bars represent the SD; data are means of three experiments carried out in triplicate. p value is <0.01 (**) by Student’s t test. (B) dnTCF4 abrogates the effect of β-catenin GOF mutation on nuclear NICD staining. Green nuclei show an anti-HA-Tag staining of dnTCF4 confirming infection of target cells. Scale bar = 20 μm. (C) ChIP analysis shows association of β-catenin to Dll4 promoter region. The positions of the putative Tcf-β-catenin binding site, T, are indicated. CDS, coding sequence. (D-G) Chromatin from control and β-catenin GOF cells was immunoprecipitated with β-catenin antibody and qRT-PCR on Region 1 and Region 2 was carried out. Quantitative and qualitative analysis on β-catenin-bound chromatin immunoprecipitated from confluent (D and E) and wounded (F and G) cells was carried out. In (D) and (F), the levels of DNA are normalized to input. Columns are means ± SD of triplicates from a representative experiment. (H) qRT-PCR analysis of Dll4 mRNA from control cells exposed for 10 hr to Wnt3a. After stimulation, Dll4 is upregulated to a level comparable to β-catenin GOF. Columns are mean ± SD of triplicates from a representative experiment. (I) Luciferase reporter assay. Luciferase activity was induced by control cells activation with Wnt3a (for 10 hr) and β-catenin GOF mutation. Point mutation of the TCF-β-catenin binding sites in the Region 2 of the Dll4 promoter significantly reduce the luciferase activity. Columns are mean ± SD of triplicates from a representative experiment.
In addition, infection of ECs with a transcriptionally active β-catenin mutant (LefΔN-βCTA) (Taddei et al., 2008) unable to bind cadherins, did reproduce the morphological changes induced by β-catenin GOF mutation, whereas a mutant able to link cadherins but unable to translocate to the nucleus (ΔC-Far) (Hagen et al., 2004) did not (Figures S6C and S6D).
To investigate whether β-catenin could interact directly with Dll4 promoter, we carried out chromatin immunoprecipitation assay (ChIP). Two consensus sequences for TCF-β-catenin binding sites were identified in the Dll4 promoter: Region 1, position −4137/−4121; Region 2, position −722/−706 (Figure 6C). β-catenin associates to Dll4 promoter in both confluent and wounded β-catenin GOF cells (Figures 6D-6G). Interestingly, in control cells, the binding of β-catenin to the Dll4 promoter could be detected in wounded monolayers only supporting the concept that Notch signaling is increased, through β-catenin transcriptional activity, when junctions are weaker (Figures 6F and 6G). To further test the specificity of β-catenin interaction with Dll4 promoter, we carried out a luciferase reporter assay by transfecting ECs with a construct where luciferase is under the control of Dll4 promoter (Seo et al., 2006) or the same promoter with a point mutation in the TCF-β-catenin binding site of the Region 2. As reported in Figures 6H and 6I both Wnt3a and β-catenin GOF mutation increased Dll4 expression and luciferase reporter activity only in the wild-type promoter sequence but not in the mutant.
Discussion
Wnt and Notch signaling pathways act in concert during embryo development in patterning processes and in cell fate decisions (Clevers, 2006; Phng and Gerhardt, 2009). Although the relevance of these two signaling systems is undoubted, the molecular mechanisms that mediate their reciprocal regulation are still not understood completely. We report that sustained β-catenin signaling in ECs leads to early embryonic death due to severe alterations in the development of the vascular system. The link between these alterations and high or uncontrolled Notch signaling is supported by several evidences: (1) in β-catenin GOF mutants, Dll4 and the downstream effectors are strongly increased in the embryo, in freshly isolated β-catenin GOF embryonic ECs and in β-catenin GOF cultured ECs—in contrast, freshly isolated ECs from LOF β-catenin embryos express decreased amounts of Dll4 and Notch effectors; (2) increased Notch nuclear signaling on β-catenin GOF mutation was detected in the mutant embryos and cultured cells; (3) the morphogenetic defects described in the β-catenin GOF mutants were comparable, at least in part, to those described when Notch signaling was increased by Dll4 upregulation in vivo, including lack of vascular remodeling in the yolk sac and in the head, impaired ECs migration, altered elongation of the intersomitic vessels, defects in branching, and loss of arterial-venous identity causing AV shunts leading to bypass circulation (Carlson et al., 2005; Trindade et al., 2008); and (4) DAPT treatment partially reverted the cellular and vascular phenotype.
To better understand the molecular basis of β-catenin and Dll4 interaction and to exclude indirect effects due to altered blood flow or organ perfusion, we focused on cultured β-catenin GOF mutant ECs. These cells showed increased Notch signaling and functional alterations similar to those reported for cells expressing a GOF mutant of Notch (Trindade et al., 2008). These include defects in actin organization and elongation, sprouting activity, upregulation of lamellipodia and abrogation of filopodia. DAPT treatment of β-catenin GOF mutant cells reverted significantly this phenotype.
Besides Notch activation, β-catenin stabilization may act through other pathways such as modification of junction organization and increase in cell-to-cell adhesion strength (Cattelino et al., 2003). Although we cannot fully rule out this possibility, the effect on cell migration and Dll4 expression are blocked by a dominant-negative mutant (dnTCF4) that inhibits only β-catenin transcriptional activity without modifying its recruitment and activity at junctions. Moreover, a β-catenin mutant (LefΔN-βCTA) with strong transcriptional activity but unable to bind cadherins, can reproduce the effect of inhibition of cell sprouting whereas a mutant that is able to link cadherins but unable to translocate to the nucleus (ΔC-Far) is inactive. Finally ChIP and luciferase reporter analysis showed β-catenin binding to Dll4 promoter in parallel with its upregulation.
β-catenin can also modulate the expression of other genes that may contribute to the observed embryo phenotype. This cannot be excluded and deserves further investigation; however, a significant part of the vascular defects detected in this study seems to be comparable to those observed in Dll4 sustained expression in vivo. This aspect and the extensive set of data discussed above make us rather confident that activation of the Notch system contributes at least to a good extent to the observed vascular phenotype in early embryo.
These data introduce what we believe to be a novel and important level of regulation of vascular angiogenesis and endothelial specification mediated by the cross talk between Wnt and Notch signaling pathways. In a previous work (Phng et al., 2009) reported that Notch can upregulate β-catenin signaling through induction of Nrarp. This factor acts as a feed back mechanism by limiting Notch on one side while upregulating Wnt signaling on the other. The final result of loss of Nrarp in vivo is vascular regression. Therefore, Wnt and Notch pathways can reciprocally modulate each other by induction of activators or repressors. Jag1, which is the second Notch ligand present in ECs, can antagonize Dll4 activity in postnatal retina (Benedito et al., 2009). Jag1 was found to be upregulated by β-catenin signaling in non-ECs (Estrach et al., 2006). However, in this study we found only very modest changes in the expression of this ligand in the embryo suggesting that, at early stages of vascular development, β-catenin signaling is switching the equilibrium in favor of Dll4.
In our experimental system, we amplified β-catenin signaling by maintaining high levels of the stabilized protein during vascular development. However, other data suggest that wildtype β-catenin signaling is important also in physiological vascular morphogenesis. In controls embryos, we were able to detect β-catenin signaling in several types of developing vessels. Cultured control ECs show increased nuclear β-catenin and signaling during migration. In the absence of β-catenin, quite a few vascular regions such as the perineural vascular plexus or retina vasculature present altered morphology. Furthermore, β-catenin signaling was shown to be required for brain angiogenesis and for the development of a stable vasculature (Cattelino et al., 2003; Daneman et al., 2009; Liebner et al., 2008; Lobov et al., 2005; Stenman et al., 2008).
The observations reported here also show that, surprisingly, activation of β-catenin signaling has different consequences depending on the stage of vascular development in the embryo. Activation of β-catenin does not modify arteriovenous specification in the late embryo or in the postnatal retina. In other cell systems, canonic Wnt signal controls progenitor cell expansion and lineage decision in early embryo in a temporally regulated way (see Clevers [2006] and Grigoryan et al. [2008]). Wnt signaling plays different and sometimes even opposite activities at different stages of embryo development (Ueno et al., 2007; Grigoryan et al., 2008). From E14.5 arteriovenous differentiation of endothelial cells is largely completed (for review, see Oliver and Srinivasan, 2010) and this could explain why late activation of β-catenin expression is no longer affecting vascular specification.
In cultured ECs, β-catenin translocation to the nucleus and Notch signaling is stronger in cells at the migrating front. This is detectable already in wild-type endothelium and is more evident in cells expressing β-catenin GOF mutation. This suggests that, because migrating cells have weaker and partially dismantled junctions, β-catenin interaction with cadherins is reduced in strength and the protein may be released in the cytoplasm and translocate to the nucleus. Taddei et al. (2008) showed that β-catenin signaling is increased in sparse cells as compared to confluent stable cell monolayers. Consistently, β-catenin binding to Dll4 promoter is higher in control wounded cell monolayers as compared to confluent cells and this parallels the increase in Notch signaling detected in these cells.
In conclusion, our study introduces canonical Wnt signaling as an important pathway in modulating endothelial differentiation and vascular development in the embryo. We propose that when ECs are activated by canonical Wnt signaling, β-catenin translocates to the nucleus and transcriptionally upregulates Dll4. Wnt and Notch systems are known in other cell systems to act in concert to determine cell fate and a complex system of activators and feedback inhibitors are needed to finely tune this interaction and promote correct vascular development and remodeling.
Experimental Procedures
Animals
The following transgenic mouse strains were used: β-cateninlox/lox (Brault et al., 2001), β-cateninlox(ex3)/lox(ex3) (Harada et al., 1999), BAT-gal (Maretto et al., 2003), ephrin-B2 (Wang et al., 1998), EphB4 (The Jackson Laboratory) (Gerety et al., 1999), and TNR1 (The Jackson Laboratory) (Duncan et al., 2005). β-catenin LOF embryos were generated as described (Cattelino et al., 2003). GOF embryos were generated by mating mice homozygous for the β-catenin exon 3 floxed allele (β-cateninlox(ex3)/lox(ex3)) with Tie2-Cre (Kisanuki et al., 2001) or VE-Cadherin-Cre (Alva et al., 2006) heterozygous mice, to produce β-cateninΔex3/wt/Cre+ and β-cateninlox(ex3)/wt/Cre− mice. All mouse strains were backcrossed into the C57Bl/6J background.
Notch signaling was inhibited by subcutaneously injection of 100 mg/kg DAPT (Alexis Bioscience) dissolved in 10% ethanol and 90% corn oil. DAPT solution was injected twice, at E7.5 and E8.5, in β-cateninlox(ex3)/lox(ex3)/Tie2-Cre pregnant animals and the embryos were dissected 24 hr later at E9.5. All experiments were carried out in accordance with the guidelines established in the Principles of Laboratory Animal Care (directive 86/609/EEC) and approved by the Italian Ministry of Health.
Immunohistochemistry and qRT-PCR
Embryos were dissected in PBS, fixed in 4% paraformaldehyde overnight, and used for whole-mount immunostaining as previously described (Cattelino et al., 2003; Liebner et al., 2004).
Individual control and mutant embryos were dissected in ice-cold PBS. Total mRNA was isolated with the RNeasy Mini Kit (QIAGEN) and 1 μg reverse transcribed with random hexamers (High-Capacity cDNA Archive Kit, Applied Biosystems) according to the manufacturer’s instructions. For the isolation of embryonic ECs, embryos were digested with collagenase type I (Roche) for 30 min at 37°C. The ECs were then separated using Dynabeads (Invitrogen) coated with PECAM antibody (BD Transduction) and RNA isolated by extraction with TRIzol (Invitrogen). cDNA synthesis and qPCR analysis were carried out as described in Taddei et al. (2008).
Cell Culture, Wound Healing Assay, and Immunofluorescence
ECs were isolated from E9.5 embryos β-cateninlox(ex3)/wt/Cre−, cultured and immortalized as described previously (Cattelino et al., 2003). The adeno-vector expressing Cre recombinase was used to obtained ECs line β-cateninΔex3/wt (GOF). As control ECs were infected with adeno-vector expressing GFP.
In some experiments cells were exposed to conditioned medium (CM) of L-cells, producing Wnt3a as described (Liebner et al., 2008). To define optimal conditions for cell activation, two CM dilution (1:2 and 1:10) have been used for different times of incubation (4, 10, 24, 48, and 72 hr). Dll4 was upregulated in a concentration and time-dependent way and the optimal condition of 1:2 dilution for 10 hr was selected for further experiments.
Wound assay and immunofluorescence experiments have been described previously (Cattelino et al., 2003).
ChIP Assay
ChIP assay was carried out as described previously (Taddei et al., 2008).
Luciferase Reporter Assay
A 3.7 kb fragment of the mouse Dll4 promoter (−3631/+76) subcloned in the pGL3 basic vector was kindly provide by T. Kume (Northwestern University School of Medicine, Chicago). The Dll4-mut was generate to mutate the TCF-β-catenin binding site TGGGGGACAAAGAGAGA to TGGGGGACCAAGA GAGA using QuickChange Site-Directed Mutagenesis Kit (Stratagene).
Control and GOF cells were transfected in 6-well plates using Lipofectamine 2000 (Invitrogen) and 48 hr posttransfection luciferase activity was measured. Each experiment was repeated three times with each reaction mixture in triplicate.
More details of the Experimental Procedures are reported in the indicated references and in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
This work was supported by the Fondation Leducq Transatlantic Network of Excellence, Associazione Italiana per la Ricerca sul Cancro, Association for International Cancer Research UK (07-0068), the European Community (Project Contracts: JUSTBRAIN; EUSTROKE contract 202213, OPTISTEM contract 223098, ANGIOSCAFF NMP3-LA-2008-214402, and ENDOSTEMCELLS Networks), Istituto Superiore di Sanita’, Italian Ministry of Health, and CARIPLO Foundation contract 2008.2463. D.N. was supported by fellowships from the Swedish Research Council and the Swedish Society for Medical Research.
Footnotes
Supplemental Information
Supplemental Information includes seven figures, two movies, Supplemental Experimental Procedures, and Supplemental References.
References
- Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, and Iruela-Arispe ML (2006). VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev. Dyn. 235, 759–767. [DOI] [PubMed] [Google Scholar]
- Angers S, and Moon RT (2009). Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol 10, 468–477. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, and Lake RJ (1999). Notch signaling: cell fate control and signal integration in development. Science 284, 770–776. [DOI] [PubMed] [Google Scholar]
- Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, and Adams RH (2009). The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137, 1124–1135. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, and Kemler R (2001). Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253–1264. [DOI] [PubMed] [Google Scholar]
- Bray SJ (2006). Notch signaling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol 7, 678–689. [DOI] [PubMed] [Google Scholar]
- Carlson TR, Yan Y, Wu X, Lam MT, Tang GL, Beverly LJ, Messina LM, Capobianco AJ, Werb Z, and Wang R (2005). Endothelial expression of constitutively active Notch4 elicits reversible arteriovenous malformations in adult mice. Proc. Natl. Acad. Sci. USA 102, 9884–9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, et al. (2003). The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J. Cell Biol. 162, 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H (2006). Wnt/beta-catenin signaling in development and disease. Cell 127, 469–480. [DOI] [PubMed] [Google Scholar]
- Cohen ED, Tian Y, and Morrisey EE (2008). Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development 135, 789–798. [DOI] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, and Barres BA (2009). Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. USA 106, 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson ME, and McMahon AP (1992). The role of Wnt genes in vertebrate development. Curr. Opin. Genet. Dev 2, 562–566. [DOI] [PubMed] [Google Scholar]
- Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, et al. (2005). Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat. Immunol 6, 314–322. [DOI] [PubMed] [Google Scholar]
- Estrach S, Ambler CA, Lo Celso C, Hozumi K, and Watt FM (2006). Jagged 1 is a beta-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development 133, 4427–4438. [DOI] [PubMed] [Google Scholar]
- Gerety SS, Wang HU, Chen ZF, and Anderson DJ (1999). Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol. Cell 4, 403–414. [DOI] [PubMed] [Google Scholar]
- Goodwin AM, and D’Amore PA (2002). Wnt signaling in the vasculature. Angiogenesis 5, 1–9. [DOI] [PubMed] [Google Scholar]
- Grego-Bessa J, Luna-Zurita L, del Monte G, Bolos V, Melgar P, Arandilla A, Garratt AN, Zang H, Mukouyama YS, Chen H, et al. (2007). Notch signaling is essential for ventricular chamber development. Dev. Cell 12, 415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan T, Wend P, Klaus A, and Birchmeier W (2008). Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 22, 2308–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen T, Sethi JK, Foxwell N, and Vidal-Puig A (2004). Signaling activity of beta-catenin targeted to different subcellular compartments. Biochem. J 379, 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, and Taketo MM (1999). Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 18, 5931–5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, et al. (2007). Dll4 signaling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445, 776–780. [DOI] [PubMed] [Google Scholar]
- Hofmann JJ, and Iruela-Arispe ML (2007). Notch signaling in blood vessels: who is talking to whom about what? Circ. Res. 100, 1556–1568. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Tamai Y, Zorn AM, Yoshida H, Seldin MF, Nishikawa S, and Taketo MM (2001). Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development 128, 25–33. [DOI] [PubMed] [Google Scholar]
- Junghans D, Haas IG, and Kemler R (2005). Mammalian cadherins and protocadherins: about cell death, synapses and processing. Curr. Opin. Cell Biol 17, 446–452. [DOI] [PubMed] [Google Scholar]
- Kim YH, Hu H, Guevara-Gallardo S, Lam MT, Fong SY, and Wang RA (2008). Artery and vein size is balanced by Notch and ephrin B2/EphB4 during angiogenesis. Development 135, 3755–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, and Yanagisawa M (2001). Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol 230, 230–242. [DOI] [PubMed] [Google Scholar]
- Klaus A, and Birchmeier W (2008). Wnt signaling and its impact on development and cancer. Nat. Rev. Cancer 8, 387–398. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, and Weinstein BM (2001). Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128, 3675–3683. [DOI] [PubMed] [Google Scholar]
- Liebner S, Cattelino A, Gallini R, Rudini N, Iurlaro M, Piccolo S, and Dejana E (2004). Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J. Cell Biol 166, 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, et al. (2008). Wnt/beta-catenin signaling controls development of the blood-brain barrier. J. Cell Biol 183, 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, et al. (2005). WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature 437, 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, and Piccolo S (2003). Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. USA 100, 3299–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, and Wainwright BJ (1996). Targeted disruption of the Wnt2 gene results in placentation defects. Development 122, 3343–3353. [DOI] [PubMed] [Google Scholar]
- Oliver G, and Srinivasan RS (2010). Endothelial cell plasticity: how to become and remain a lymphatic endothelial cell. Development 137, 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmalee NL, and Kitajewski J (2008). Wnt signaling in angiogenesis. Curr. Drug Targets 9, 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phng LK, and Gerhardt H (2009). Angiogenesis: a team effort coordinated by notch. Dev. Cell 16, 196–208. [DOI] [PubMed] [Google Scholar]
- Phng LK, Potente M, Leslie JD, Babbage J, Nyqvist D, Lobov I, Ondr JK, Rao S, Lang RA, Thurston G, et al. (2009). Nrarp coordinates endothelial Notch and Wnt signaling to control vessel density in angiogenesis. Dev. Cell 16, 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca C, and Adams RH (2007). Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 21, 2511–2524. [DOI] [PubMed] [Google Scholar]
- Seo S, Fujita H, Nakano A, Kang M, Duarte A, and Kume T (2006). The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev. Biol 294, 458–470. [DOI] [PubMed] [Google Scholar]
- Sorensen I, Adams RH, and Gossler A (2009). DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood 113, 5680–5688. [DOI] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, and McMahon AP (2008). Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322, 1247–1250. [DOI] [PubMed] [Google Scholar]
- Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, and Eichmann A (2007). The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc. Natl. Acad. Sci. USA 104, 3225–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, and Dejana E (2008). Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat. Cell Biol. 10, 923–934. [DOI] [PubMed] [Google Scholar]
- Thurston G, Noguera-Troise I, and Yancopoulos GD (2007). The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat. Rev. Cancer 7, 327–331. [DOI] [PubMed] [Google Scholar]
- Trindade A, Kumar SR, Scehnet JS, Lopes-da-Costa L, Becker J, Jiang W, Liu R, Gill PS, and Duarte A (2008). Overexpression of delta-like 4 induces arterialization and attenuates vessel formation in developing mouse embryos. Blood 112, 1720–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, and Murry CE (2007). Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl. Acad. Sci. USA 104, 9685–9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, and Anderson DJ (1998). Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93, 741–753. [DOI] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, et al. (2004). Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116, 883–895. [DOI] [PubMed] [Google Scholar]
- Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, and Nathans J (2009). Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell 139, 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.