Abstract
The impact of SARS-CoV-2 and COVID-19 disease susceptibility varies depending on the age and health status of an individual. Currently, there are more than 140 COVID-19 vaccines under development. However, the challenge will be to induce an effective immune response in the elderly population. Analysis of B cell epitopes indicates the minor role of the stalk domain of spike protein in viral neutralization due to low surface accessibility. Nevertheless, the accumulation of mutations in the receptor-binding domain (RBD) might reduce the vaccine efficacy in all age groups. We also propose the concept of chimeric vaccines based on the co-expression of SARS-CoV-2 spike and influenza hemagglutinin (HA) and matrix protein 1 (M1) proteins to generate chimeric virus-like particles (VLP). This review discusses the possible approaches by which influenza-specific memory repertoire developed during the lifetime of the elderly populations can converge to mount an effective immune response against the SARS-CoV-2 spike protein with the possibilities of designing single vaccines for COVID-19 and influenza.
Highlights
Immunosenescence aggravates COVID-19 symptoms in elderly individuals.
Low immunogenicity of SARS-CoV-2 vaccines in elderly population.
Tapping the memory T and B cell repertoire in elderly can enhance vaccine efficiency.
Chimeric vaccines can mount effective immune response against COVID-19 in elderly.
Chimeric vaccines co-express SARS-CoV-2 spike and influenza HA and M1 proteins.
Keywords: Immunosenescence, COVID-19, SARS-CoV-2, Influenza, vaccine, virus-like particle, memory cells, hemagglutinin
Introduction
The appearance of novel coronavirus known as SARS-CoV-2 is accountable for the disease called COVID-19 which caused more than 2 million global deaths. Coronaviruses are widespread family of viruses existing in numerous species of animals, including camels, cattle, cats, bats, and birds. Zoonotic transmission of coronaviruses seldom transpires in the human population. The previous human to human transmission of coronaviruses occurred in 2003 and 2014 with SARS-CoV and the middle-eastern and the respiratory syndrome coronavirus (MERS-CoV), respectively. Current literature describes two types of the coronaviruses alphacoronaviruses (229E and NL63) and betacoronaviruses (OC43 and HKU1) that circulates in human causing common flu [1]. The pathogenic strains that infect human are SARS-CoV-1, MERS-CoV and SARS-CoV-2, all of which belong to betacoronaviruses [2]. SARS-CoV-2 has 29.9 kb single stranded positive sense RNA genome that encodes 29 viral genes. Around 16 of these elements codes for nonstructural proteins that are copied as pair of large polyproteins (Orf1a and Orf1b) that are further processed into different polypeptides via viral proteases. Other elements codes for several viral proteins that forms variety of accessory and structural components including the spike (S), envelope (E), membrane (M) and nucelocapsid (N) proteins. The interaction between the SARS-CoV-2 spike protein and the angiotensin-converting enzyme 2 (ACE2) receptor on host cell membrane helps the virus to penetrate the cell [3]. The trimeric S protein comprises of two subunits, S1 and S2, which mediates receptor binding and membrane fusion [4,5]. Hence the spike protein has been emphasized and used as a prime candidate for vaccine development globally [6,7].
Development of vaccine is time consuming and challenging especially for the mutating RNA viruses. However, the mutation rate of SARS-CoV-2 is lower than other single stranded RNA viruses like influenza or HIV [8]. RNA viruses have the tendency to lose infectivity after acquiring mutation but few quasi-species retain infectivity and others become highly infective. These highly infective species bypass the existing immunity to establish themselves in the circulation as a new variant. The repeated viral mutation is well observed during flu season caused by influenza viruses. Every year new flu vaccine is developed based on the previous year’s epidemiological data. SARS-CoV-2 rapid multiplication and infectivity allows the virus to acquire mutations such as D164G, A222V, L18F, P681H and N501Y mutations in the Spike (S) proteins, P323L in the NSP12 protein and R203K, G204R and A220V in the nucleocapsid proteins [9]. Current epidemiological studies have identified new variants in UK (B.1.1.7), South Africa (B.1.351) and Brazil (P.1) and all three have now been detected in the USA [10]. Current vaccines under use can neutralize emerging strains but to what extent they can protect elderly population remains a major concern.
Earlier reports by CDC indicated that older adults (≥ 65 years) comprising 9% of the global population accounted for more than 80% of COVID-19 related mortality [11]. This emphasizes the severity and vulnerability of the elderly individuals to SARS-CoV-2 infection. The SARS-CoV-2 infection can overwhelmingly excite the immune system in the elderly patients thereby leading to acute respiratory distress syndrome (ARDS) which is a natural outcome of immunopathological episodes and remains a critical reason for mortality [12]. The weakened immune system of older adults is characterized by steady decline of innate and adaptive immune responses poses a major challenge for developing a vaccine that will effectively work for people of all ages [13–15]. The bottleneck to developing such vaccines is the limited knowledge about the correlates of vaccine-induced protection in older adults. Previous studies have reported various physiological and immunological changes in the human body with aging [16,17]. Therefore, vaccination does not always ensure protection to an older individual due to their inherent loss of immune functions and heightened susceptibility to infectious diseases. Thus, we must consider additional safeguards while designing a vaccine for these groups. In this review, we emphasize on the elderly population (≥ 65 years age) and their immune correlation against vaccination based on previous and current studies. We further discuss the innovative approaches of hybrid vaccines containing influenza and SARS-CoV-2 motifs to overcome the suboptimal immune response during vaccination in elderly population.
Immunosenescence and aging aggravates COVID-19 disease
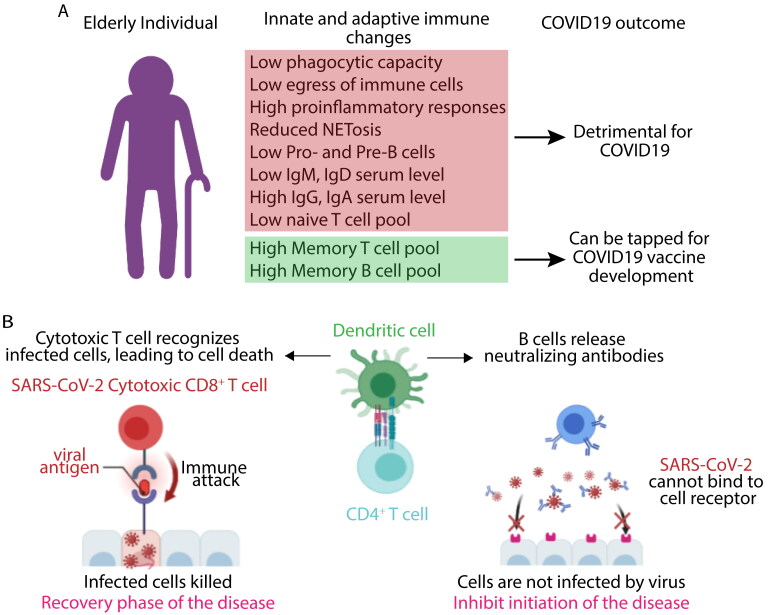
Multifaceted alterations in immune system are the hallmark of the aging process. Immunosenescence is defined as gradual decrease in immune function with age leading to augmented susceptibility to infectious diseases [18]. Studies have reported a systemic inflammatory state with aging, known as ‘inflammaging’ leading to a chronic low-grade inflammation in the lung of elderly people. The respiratory system undergoes a set of functional and structural changes with age. The progressive decline in lung functions including anatomical changes of the thoracic cage leading reduction in chest wall compliance, decreased strength of respiratory muscle affecting airway clearance are observed with aging [19,20]. Uncontrolled inflammation may impair lung, heart and kidney functions due to impeded resolution and presence of frailty in the older individuals [21]. Older individual has dysregulated innate immune cells lacking efficient phagocytosis and egress mechanism from the site of infection which further increases proinflammatory response by engaging with pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) resulting in further tissue damage [22,23]. Innate immune response is primarily responsible for initial inflammations leading to the accumulation of phagocytes (neutrophils and macrophages) at the site of infection. The phagocytes clear-up the virus infected cells and dead or apoptotic cells damaged due to viral multiplication [24,25]. Alveolar macrophages (Aφs) provide the first line defense against respiratory pathogen and plays a pivotal role maintaining tissue homeostasis [26]. In a resting state, Aφs binds to the alveolar type II epithelium and expresses regulatory ligands (CD200 and TGFβ) and anti-inflammatory cytokines, such as IL10 and TGF β [27,28]. Upon sensing any danger signals or insult, Aφs undergo molecular reprogramming to regulate a complex interaction between suppressive and activating signals [29,30]. Neutrophils from aged animals also show a similar reduced ability to mount an innate immune response like formation of neutrophil extracellular traps (NETs) [31,32]. Lower expression of Atg5 in aged mice leads to reduced autophagy mediated NET formation [33]. Innate immune responses promote adaptive immunity by stimulating the antigen presenting cells (APCs) to capture and present antigen and by producing cytokines, chemokines and co stimulatory molecules that is required for optimum T helper cell response at the secondary lymphoid organs [34]. Antibodies and CD4+ T cells are essential to neutralize virus particles by opsonization and killing of virus infected cells by cytotoxic T cells [35,36]. Aging significantly influences thymic involution with a gradual declination of the structural integrity of the thymus in aged people resulting in a steady decrease in the T cell production [37]. Older individuals have reduced number of naïve T helper cell populations and distorted lymph node architecture critical to retain naïve cells which promote germinal center reaction after activation by APCs to produce affinity matured and class switched antibodies (Figure 1A) [38,39].
Figure 1.
Factors influencing COVID-19 outcomes in the elderly population. A. Immunosenescence mediated changes in innate and adaptive immune response might affect the COVID19 disease outcome. The immunosenescence is characterized by thymic involution, modified T and B cell responses due to alteration in the naïve/memory lymphocyte population, and heightened serum levels of IgG and IgA with a lower level of IgM and IgD, and a weak response to newly encountered pathogens/antigens such as SARS-CoV2 or influenza or after vaccination which may lead to severe disease outcome specially within the elderly population. B. Both the humoral (mediated by SARS-CoV-2 specific neutralizing antibodies) and cellular immune response (mediated by CD8 + T cells that kills virus infected cells) are indispensable for effective COVID-19 immune response. Diverse memory T and B cells in elderly individual might act as a reservoir that can be tapped to raise a strong adaptive immune response against SARS-CoV-2 by tweaking novel vaccine design.
Outcome of any infectious disease depends on both the arm of the immune responses. Aging leads to gradual changes in every aspect of host immunity affecting both the innate and adaptive immune arm resulting in heightened inflammatory response or cytokines storms during robust viral replication and cell damage that defines the disease pathophysiology of COVID-19 [40,41]. Recent clinical studies have reported significant upregulation in proinflammatory cytokines (TNF-α, IL-1β, IL-6, IL-8, G-CSF and GM-CSF) and chemokines such as MCP1, IP10 and MIP1α in severely affected COVID-19 individuals [42–44]. Cytokines storms are characteristics for many viral diseases like SARS, MERS and H5N1 infection including the current COVID-19, providing them an inflammatory disease signature [45,46]. High SARS-CoV-2 replication in the type 2 pneumocytes causes it to differentiate from a quiescent state to a highly proliferating inflammatory state resulting in an impairment of suppressing signals [47].
Severely affected SARS-CoV-2 patients of all ages have very low number of T regulatory (Tregs) cells [48]. Patients severely suffering from COVID-19 were reported to have an uncontrolled inflammation associated with pneumonia, myocarditis and microvascular thrombosis, and cytokine storms which can be effectively dealt with increased Tregs [49]. Several clinical studies with SARS-CoV-2 patients have reported the presence of proinflammatory cytokines including IL6, IL8 and IL12, IFN γ and IL-2 [50,51]. ACE2, the key receptor of SARS-CoV-2, is reported to be downregulated after SARS-CoV-2 infection, thereby leading to ARDS and cardiac injury due to increase accumulation of angiotensin II [52,53].
COVID-19 vaccination and immune response within the elderly population (≤65 years)
All the COVID-19 vaccines are designed to induce an effective antiviral response and the efficacy is analyzed by measuring the neutralizing antibody titer against the spike protein or receptors binding domain of spike [54]. The total number of CD4+ and CD8+ T cells were dramatically reduced in COVID-19 patients, especially among elderly and ICU patients. Moreover, T cells in these individuals shows high expression of PD-1 and Tim-3 expression compared to healthy controls, an indicative of T cell exhaustion [55]. A recent study compared T cell memory response in COVID-19 recovered patients (28 with mild disease and 14 with severe disease) and indicated that mild cases have higher proportions of SARS-CoV-2 specific CD8+ T cells whereas individual with severe COVID-19 have more SARS-CoV-2 specific antibody titer compared to mild case [48]. These finding suggest that humoral immune responses alone were unable to protect an individual from severity of the disease. Earlier study by Wang et al, have found that H7N9 infected influenza patients discharged within 2–3 weeks have early prominent H7N9-specific CD8+ T cell responses, while individuals with prolonged hospital stays have late recruitment of CD8+ or CD4+ T cells and antibodies simultaneously [56]. These studies suggest that recovery phases of acute viral infection are dependent on effective CD8+ T cell response. All these observations suggest an optimum T cell response is indispensable for the resolution of COVID-19 disease which may be lacking in elderly population and provide rationalization for vulnerability toward severe SARS-CoV-2 infection (Figure 1B). The use of spike as a vaccine candidate can be effective to induce effective CD8+ T cell responses, as new findings also suggest that during natural infection a significant number of CD8+ T cell responses were observed with spike derived peptide pool during ex vivo challenge [48,56,57].
Inovio pharmaceuticals had developed INO-4800, a novel DNA based vaccine candidate which is administered directly into the skin of the recipient through Cellectra 2000 [58,59]. As soon as the genetic sequence of the novel corona virus was published [60], Inovio constructed a DNA based vaccine without further delay utilizing their proprietary DNA medicine platform technology. It successfully prompted antigen specific robust T cell mediated immune response and neutralizing antibody production that effectively blocked the binding affinity of the spike protein to ACE2 receptor. Phase 1 clinical trial with INO-4800 exhibited excellent safety and tolerability and remained immunogenic in 100% (38/38) of the vaccinated individuals by evoking either or both humoral or cellular immune responses [59]. Currently a study with populations 51 years and older is underway (NCT04336410).
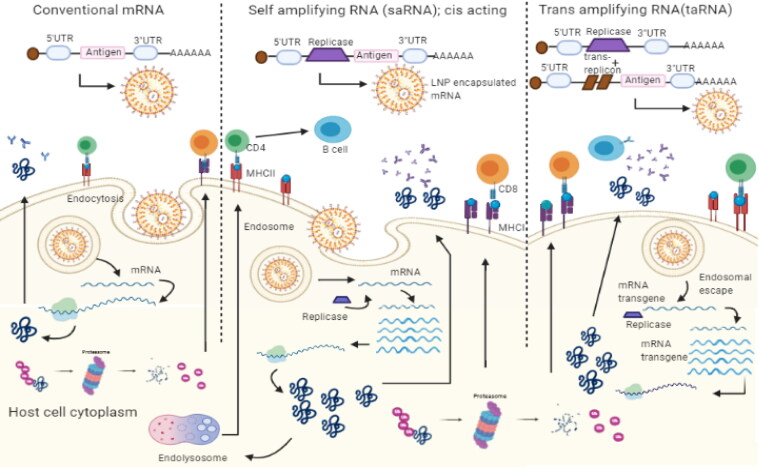
The mRNA vaccines are similar to DNA vaccines off late gaining much required attention due to their robust immunogenicity, easy production scale up, and the development of lipid nanoparticles for efficient delivery [61]. With this new technology viral antigen can be delivered as mRNA sequence within lipid nanoparticles (LNP) instead of viral protein molecules [62–64]. The antigen is then expressed in the recipient cells of the vaccinated individual to mount humoral and cell mediated immune responses (Figure 2). There are several mRNA vaccines that are under development or in trials for COVID-19 (Table 1).
Figure 2.
Overview of mRNA vaccines mediated immune responses. The mRNA incorporated in the lipid nanoparticles are delivered to host cells by intramuscular injection. Inside the cells, mRNA is translated into antigenic proteins which is subsequently processed into peptides by the proteasomal degradation or endosomal lysis and represented by MHC molecules on the plasma membrane to mount an immune response. There are some variations within mRNA vaccine architecture based on mRNA molecules either consisting of the only the target antigen (conventional mRNA vaccines) or target antigen along with replication machinery (self-amplifying or trans-amplifying mRNA vaccines) to amplify the mRNA molecules further after entering the host cells for a longer and higher amount antigen expression.
Table 1.
Major COVID-19 vaccines available or under development.
| Company/Institute | Vaccine name | Backbone | Target antigen |
Age | Clinical status (Trial No.) |
|---|---|---|---|---|---|
| Inovio Pharmaceuticals | INO-4800 | DNA-based | SARS-CoV-2 spike protein | ≥19 to ≤64 | Phase 2/3 (NCT04447781) |
| Codagenix/Serum Institute of India | CDX-005 | Deoptimized live attenuated virus | Whole virus particle | No information | Preclinical/Phase 1 trial |
| Moderna/NIAID | mRNA-1273 | mRNA | Stabilized spike protein | ≥18 | Phase1/2 /3 completed and under emergency use (NCT04470427) |
| BioNTech and Pfizer | BNT162 | mRNA | Spike protein | ≥18 to ≤55; 56 to ≤85 |
Phase1/2 /3 completed and under emergency use (NCT04380701) |
| Novavax | NVX-CoV2373 | Protein Subunit | Full length S trimers/ nanoparticle +Matrix M | ≥ 18 to 59 ≥ 18 to 84 |
Phase 2 (NCT04533399, NCT04368988). Phase 3 under trial |
| AstraZeneca | ChAdOx1 nCoV-19(AZD1222 ) |
Non-replicating chimpanzee adenovirus | Spike protein | ≥18 to ≤55 ≥18 |
In use (NCT04324606, NCT04516746) |
| Bharat Biotech | Covaxin (BBV152B) | Inactivated SARS-CoV-2 virus with 6 µg-Algel-IMDG | A whole virion | ≥18 | Phase III completed (NCT0464181) |
Moderna in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) have developed a LNP encapsulated mRNA vaccine named mRNA-1273 that encodes full-length perfusion stabilized spike protein [65]. The perfusion form encompasses a transmembrane anchor and an unaltered S1-S2 cleavage site. The substitution mutation of two proline residues in the S2 region stabilizes the newly designed protein [66]. The genetic similarity of the MERS-CoV and SARS-CoV-2 lead to the development of a single synthetic viral RNA molecule as a vaccine candidate making it safer for human use. The phase 1 and phase 2 clinical trials were carried out to evaluate the safety, reactogenicity, and immunogenicity of the vaccine in human. Antigen-specific neutralizing antibody developed in a Rhesus macaque model also enhanced protection in the lung without any symptoms of pathological changes [67]. The participants in this trial were between the age group of 18–70 years. Vaccine trials have found mild adverse events of fatigue, chills, headache, and pain at the site of the injection area were observed. This study also reported the presence of neutralizing antibodies in the serum with strong CD4+ mediated T cell responses. The phase III trial had 25.3% population with 65 years and older age. The vaccine evoked elevated local and systemic adverse reactions compared to those in the placebo group during phase III trial, usually enduring a few days. The reactogenicity were commonly mild to moderate and generally less frequent in older adults (≥65 years) as compared to younger participants [68].
In USA, the BNT162 mRNA vaccine developed by BioNTech and Pfizer, encodes an optimized SARS-CoV-2 receptor-binding domain (RBD) antigen which is currently used for vaccination [69]. A prime-boost regimen strategy was explored with this vaccine and reported to be well tolerated during the early-stage human trial and elicited dose-dependent immunogenicity [70]. During December 2020, monitoring by the Vaccine Adverse Event Reporting System identified 21 cases of anaphylaxis (which is a severe, life-threatening allergic reaction that occurs rarely after vaccination) following administration of a reported 1,893,360 primary doses of the Pfizer-BioNTech COVID-19 vaccine (11.1 cases per million doses); 71% of these appeared within 15 minutes of vaccination. Recent reports of 23 elderly patients death in Norway after having regular doses of Pfizer/BioNTech mRNA vaccines created panic in certain parts of the world about their safety and tolerability [71]. The higher dose of conventional mRNA vaccine can induce an adverse side effect in the vulnerable frail group. Health authorities are investigating the reason behind such deaths in the elderly population [71]. Both Moderna and Pfizer/BioNTech vaccines require two doses and the reactogenicity is typically higher after the booster dose.
Novavax investigated their proposed vaccine, NVX-CoV2373, full length S subunit protein, with or without Matrix-MTM as an adjuvant. The anti-Spike IgG antibody was found to be present in the all-vaccine recipient right after the single dose and the presence of the adjuvant further augmented the polyfunctional CD4 + T cell responses [72]. In this study participants were between 18 to 59 years of age were divided in to multiple groups to carry out the immunogenicity of the vaccine. The phase 3 trial aimed in enrolling 30,000 people in the United States and Mexico with two cohorts: individuals 18 through 64 years old and those ≥ 65 years older, with a goal of enrolling at least 25% of all volunteers who are.
Live attenuated vaccines are produced by creating a genetically weakened variant of the virus that replicates to a constrained extent, causing no or mild disease but induces immune responses comparable to that induced by natural infection [73]. Codagenix, Inc. in collaboration with Serum Institute of India had developed CDX-005, an engineered live attenuated virus with the help of codon deoptimization software platform [74]. Although the amino acid sequence of the constructed mutated whole virus particle is structurally identical to the wild type SARS-CoV2, it is unable to replicate in the host. The newly designed nonpathogenic attenuated virus reported to generate strong humoral and T cell mediated immune responses in the preclinical studies with mice and guinea pigs and with non-human primates.
The ChAdOx1 nCoV-19 vaccine developed by the University of Oxford in collaboration with AstraZeneca and the Serum Institute of India, expresses a full-length, wild-type version of the Spike protein. The ChAdOx1 nCoV-19 vaccines are typically based on a modified adenovirus that has been engineered to express the Spike protein and has been disabled from replication in vivo by the deletion of parts of its genome. During safety and immunogenicity study with ChAdOx1 nCoV-19 the local and systemic reactions including pain, feeling feverish, colds, muscle ache, headache, and malaise were more frequent in the ChAdOx1 nCoV-19 groups which were lessened by the use of prophylactic paracetamol [75]. A recent study was conducted from four ongoing blinded, randomized, controlled trials done across the UK, Brazil, and South Africa. In this trial, in Britain, the registered population was predominantly white and, in younger age groups, incorporated more female participants due to the focus on recruitment of health-care workers whereas in Brazil, there was a larger proportion of nonwhite ethnicities. Study participants aged 18 years and older were randomly assigned to ChAdOx1 nCoV-19 vaccine or control. Overall vaccine efficacy across both groups was around 70·4% [76]. The phase 1/2 trial proposes two-dose regime where the booster dose induces a strong T cell response and anti-spike neutralizing antibody production and the vaccine is currently under phase 3 trial [77]. Recently many European countries halted the use of AstraZeneca’s vaccine in March 2021 following the report of symptoms that led to at least 15 deaths. However, vaccination was resumed on European Medicines Agency (EMA) recommendation stating the benefits of vaccine outweighs its risks [78].
All these vaccines use various advanced technologies and are reported to mount a protective immune response by generating antigen specific neutralizing antibodies and an optimum T cell response. Most effective COVID-19 vaccine must induce an effective CD8+ T cell response with neutralizing antibody titer. However, current determinant of vaccine efficacy doesn’t provide enough importance to CD8+ T cell response. Also, it is not known how long vaccine induced neutralizing antibody titer will provide protection from infection or reinfection. Current COVID-19 vaccine development must include the capability to efficiently induce CD8+ T cell memory response that will provide an additional layer of protection to avoid severe consequences during future reinfection. Vaccines developed using the whole virus not only induced CD8+ T cell response against Spike but also for other viral proteins like M, Nucleoprotein ORF3a, ORF6 and ORF7a [57,79–81]. Therefore, spike-based vaccines lack diversified CD8+ T cell responses, limiting the breadth of immune response which may play important role during resolution phase of the infection. Thus, all the next gen mRNA-based vaccines have an inherent drawback of lower capacity to induce diverse immune response particularly within elderly individuals with low antigens specific naïve CD4+ or CD8+ T cells [82]. Although the current phase 3 trial results of mRNA-1273 vaccine suggests that it is safe and effective at preventing symptomatic COVID-19 in adults but its efficacy within elderly population (≥ 65 years) remains matter of concern. Recently, BioNTech and Pfizer have also expanded this study to determine vaccine efficacy within the elderly group but the data is yet to be public (NCT04368728) [70]. Gradual mutations in spike protein will make the current vaccines no longer effective similar to flu vaccines. However live attenuated SARS-CoV-2 strains can overcome such challenges by mounting an effective CD8+ T cell response to various relatively conserved proteins.
Lessons learned from earlier vaccination strategies for elderly population
New vaccines are being developed by modifying the antigen, the route of administration, higher dose administration and implementing adjuvants. Administering higher dose via parenteral route activates follicular DCs and subsequently antigen presentation and B cell activation in the older population [83]. Majority of the commercialized licensed vaccines imparts reduced immunogenicity and lesser protective efficacy among elderly population due to evident decrease in naïve T cell population, distorted lymphoid architecture whereas polyfunctional CD8+ T cell remain unaffected with aging [84]. The commercially available widely used vaccines for elderly people especially in the developing countries have recommended guidelines for vaccination in elderly people against infections like influenza, pneumonia, herpes zoster, and tetanus (Table 2). Waning immune system generates a weaker immune response toward vaccination in elderly population and requires repeated boosters.
Table 2.
List of non-COVID-19 vaccines available for elderly population.
| Vaccine name | Formulation | Immune response | Schedule | Refs |
|---|---|---|---|---|
| Pneumococcal polysaccharide vaccine (Pneumovac23) | T cell independent antigen response. | It generates IgM dominated antibody response but lacks immunological memory. | Suboptimal response of Pneumovac23 in the elder individual requires repeated booster. | [103] |
| Pneumococcal conjugate vaccine (Prevnar13) | Carrier protein conjugated with capsular polysaccharide. | T cell dependent response, opsanophagocytic antibody production. | A prime boost strategy by priming with Prevnar13 and boosting with pneumovac23 is recommended in elderly. | [104] |
| Shingles vaccine (Zostavax) |
Comprising of at least 2000 PFU of highly potent live attenuated varicella zoster virus (oka strain) | Induces T cell and antibody response with moderate efficacy. | Cellular immunity increases with booster dose which was given more than 10 years after the first dose. | [130,131] |
| Shingles Subunit zoster vaccine (Shingrix) |
Recombinant vaccine contains viral glycoprotein E along with ASO1 based liposomal adjuvant. | Generates T cell and humoral response and induces robust memory response. | Cellular immunity increases with booster dose which was given more than 10 years after the first dose. | [85,91] |
| Influenza trivalent inactivated influenza vaccine (TIV) |
TIV contains antigens from two influenza A (H1N1 and H3N2) and one influenza B (Victoria/Yamagata) strains and MF59 as adjuvant. | It efficaciously prevented hospitalizations due to flu and generates robust T cell response in elderly (≥ 65 years). | Due to antigenic drift, annual vaccination schedule was adapted. | [132,133] |
| Influenza (H1N1/pdm2009) | Contains H1N1 antigen along with ASO3 as adjuvant. | Provides higher antigen-sparing capacity. It generates higher HAI titer values compared to whole-virion vaccine. | Two dose strategies were considered for older individuals. | [134] |
| Influenza vaccine (Inflexal-V) | Virosomal influenza vaccine containing surface antigen HA and neuraminidase (NA). | Mimics natural infection and reported to be efficacious especially in immune-compromised individuals. | Licensed for people of all age groups and adapted annually. | [135] |
| Hepatitis vaccine (Twinrix) | Contains combination of inactivated hepatitis A and B virus surface antigen. | It induced higher seropositive rates in people above 40 years compared to other monovalent vaccines. | Three doses over a span of 6 months. | [136] |
| Recombinant hepatitis B vaccine (Engerix-B) | DNA vaccine containing HBsAg surface antigen of hepatitis B. | Confers protective efficacy for up to 10 years and effectively immunogenic for diabetes mellitus patients. | Three doses over a span of 6 months. | [137] |
| Respiratory Syncytial Virus vaccine (RSV F) | Nanoparticles based vaccine contains recombinant F protein with aluminum phosphate and Matrix-M1 as adjuvant. | Induces strong neutralizing antibody titers in elderly between 60 and 80 years old compared to non-adjuvant vaccines. | Completed Phase-I and started Phase-II trial (NCT03026348). Single and two dose regimen were compared. | [138] |
| Multivalent vector-based RSV vaccine (MVA-BN-RSV) | Contains non-replicating modified vaccinia Ankara viral vector F and G antigens from both A and B subtypes and two internal proteins N and M2. | Induces Th1 type cell mediated immune responses in adults aged ≥55 years that can persist up to 6 months. | Completed Phase-II trial (NCT02873286); boosted annually. | [102] |
| Lipid based vaccine platform DepoVax (DPX-RSV[A]) vaccine | Contains the ectodomain of the small hydrophobic glycoprotein (SHe) of RSV subgroup A. | Highly immunogenic in healthy people between ages 50 and 64 years; IgG responses persisted over a year. | Completed Phase-I trial (NCT02472548) | [139] |
Adjuvants are recognized to enhance the potency and endurance of specific immune responses generated to the administered antigen. However, live attenuated bacteria or viruses do not always require any adjuvants due to their structural components or genetic material that can induce the host’s innate immune system. One such licensed attenuated vaccine, Zostavax, was reported to be 33% protective but can effectively reduce hospitalization suffering from herpes zoster and postherpetic neuralgia among the elderly [85], whereas TIV (trivalent inactivated influenza vaccine) contains two antigens from influenza A (H1N1, H3N2 strain) and one from influenza B strain (Victoria or Yamagata) along with MF59, is an oil in water emulsion based adjuvant composed of squalene, tween 20 and span 85. A cumulative effect of all of the components of MF59 was reported to impart enhanced immunogenicity by activating the genes supporting trans-endothelial cell migration, favoring antigen uptake and its transportation to the lymph node [86,87]. Matrix-MTM and ASO3 are also reported to enhance the antigen uptake and favor the augmentation of Th1 and Th2 responses which will be beneficial for elderly group as they have dysregulated T cell machinery [88]. ASO3 is an immuno-enhancing oil in water emulsion-based adjuvant, comprising of squalene, polysorbate 80 and α-tocopherol, used in the Influenza (H1N1/pdm2009) vaccine formulation [89]. It was reported to generate microneutralizing antibodies which can persist up to more than a year after immunization with a much lower dose of antigen whereas other non-adjuvanted vaccine required higher dose of antigen but showed a declination in antibody responses over this time [90]. Another liposome-based adjuvant, ASO1, comprises MPL (Lipid molecule named 3-O-desacyl-40-monophosphoryl lipid A obtained from Salmonella minnesota) and QS-21 (a lytic saponin fraction of QuilA) was used to enhance vaccine efficacy of a subunit zoster vaccine named Shingrix [78]. A significant increase in humoral as well as cellular immunity including cytotoxic T cell responses with a vaccine efficacy of more than 90% was observed compared to non-adjuvanted vaccine [91]. Influenza virosomes have also been used as an adjuvant and carrier system owing to its excellent antigen delivery mechanism to the target sites [92]. Structurally the modified virus is composed of unilamellar phospoholipid membrane incorporating surface hemagglutinin (HA) and neuraminidase (NA) antigen but lacks the viral inner core and genetic information making it replication deficient. Mimicking the natural infection, virosome adjuvanted influenza vaccine effectively induced humoral antibody and cytotoxic T cell activity in elderly people [93,94]. Microparticles of β-d-[2 ->1] poly(fructo-furanosyl)α-d-glucose (delta inulin) proved to be safe and nontoxic adjuvant for human use. It skewed immune responses to the Th1 or Th2 depending upon the type of antigen it is administered with, functioning as an amplifier. It is effectively used in Hepatitis B, influenza, and HIV [95–97]. The most extensively used adjuvant is Matrix-M, comprises cholesterol, phospholipids and saponin, augments humoral and cellular immune responses. Although, the mechanism underlying the potency of this adjuvant is not yet resolved but it creates a milieu of activated T and B lymphocytes, NK cells, neutrophils and monocytes at local lymph nodes [98,99]. Researchers had employed vector-based vaccines with influenza nucleoprotein and matrix protein and reported to mount a robust immune response that compensates for annual influenza vaccination [100]. Non replicating viral vectors are also used to deliver potential vaccine candidate. Modified vaccinia Ankara viral vector containing five respiratory syncytical virus (RSV) proteins were being tested in a clinical trial and reported to induce Th1 type cell mediated immune responses among older adults [101,102].
A 23 valent polysaccharide based pneumococcal vaccine, containing a T cell independent antigen, elicites exclusively humoral immunity but fails to generate immunologic memory owing to its lack of direct recognition by T cells [103]. Nonetheless, chemical conjugation of polysaccharide to a carrier protein makes 13 valent conjugate pneumococcal vaccine into a T cell dependent antigen which eventually generates high affinity matured antibody and immunological memory [104]. A prime-boost regimen with Prevnar13 followed by Pneumovac23 further augmented the immune responses among elderly. This prime-boost strategy resulted in improved serotype specific IgG responses [105]. Much deeper perceptive is required to develop potential vaccine candidate for the elderly people to boost their immune system.
In addition to the appropriate selection of adjuvants and suitable modification of antigens, vaccine efficacy largely depends on several host factors. Considering the weaken immune system of the elderly people and several other co-morbid clinical conditions, effective vaccination must overcome these hurdles successfully. An aged immune system harbors multitude of changes especially a steady decline in naïve CD4+ T cell number due to inability of lymphocyte generation in the primary lymphoid organ. Parallelly, alternation of secondary lymphoid structure and function results in altered T cell trafficking and a restricted T cell repertoire [16,106]. Depletion of naïve CD4+ T cells during vaccination in elderly people significantly reduces the vaccine efficacy. The influenza specific memory T cells are directed toward conserved protein sequences across numerous influenza strains available. Therefore, these memory T cells can successfully confer cross protection against numerous strains of influenza and strategically bestows an alternative approach to treat elderly people with an added advantage. Older individuals have encountered several influenza strains throughout their life span and unknowingly carries diverse influenza specific memory CD4+ T cell populations [107].
SARS-CoV-2 mutations and vaccine effectiveness
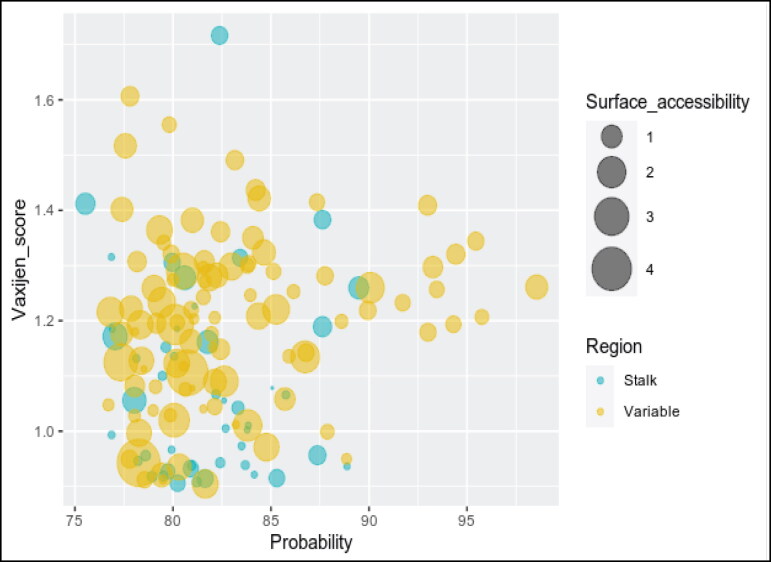
Researchers around the world are toiling to develop targeted vaccines against COVID-19 in record time. The spike glycoprotein present on the surface of the coronavirus that helps in receptor binding and membrane fusion was proposed to be a potential vaccine candidate by various research groups [108]. The viral S protein consists of two subunits: S1 and S2. The S1 domain contains the receptor-binding domain (RBD) crucial for the initial attachment of the virus to the host cell, and S2 prompted viral fusion with the host cells to initiate the infection [109,110]. Numerous studies have identified the probable B cell epitopes from the spike protein which often acquires point mutations [111–114]. Further studies also indicate that the variable region is more prone to point mutations than the stalk region [115,116]. So, the efficacy of the spike-based vaccines can be compromised by acquired mutations over time. Numerous naturally occurring spike variants like Q414E, N439K, G446V, K458N, I472V, A475V, T478I, V483I, F490L, H519P, D614G and Q321L have been reported to decrease the sensitivity to neutralizing antibody and convalescent sera [117]. Most of these naturally occurring mutations found in RBD domain of variable region of spike protein. Our analysis of B cell epitopes and surface accessibility of spike protein, S1 region (S1: aa13 to aa685 and RBD: aa319 to aa514) and stalk domain (S2: aa686 to aa1273) shows that stalk directed B cells epitopes are less accessible (Figure 3). But accumulation of RBD mutations may influence vaccine effectiveness both in general as well as in the elderly populations. Consistent with our findings the RBD of spike is a target for 90% of the neutralizing SARS-CoV-2 immune sera indicating their immunodominance and the less accessible stalk B cell epitopes probably plays minor role in viral neutralization [113]. The E484K a receptor-binding-domain mutation, and 501V2 mutation N501Y was stated to be linked with escape mutant from neutralizing antibodies [118,119]. The current BNT162b2 vaccine elicited sera can neutralize N501Y variants [120]. However, convalescent sera from COVID-19 individual were unable to neutralize a novel bat coronavirus (W1V1–CoV) that is highly homologous to SARS-CoV-2 and uses ACE2 receptor for host cell entry indicating their pandemic potential in the future [121,122]. Immune selection of antigenic variants seldom occurs within immune-compromised or elderly populations due to their inadequate immune response or sustained pathogen presence [123]. Altogether these findings indicate that humoral response against spike-based vaccines may reduce their efficacy with the emergence of new antigenic variants inadequate immune response within the elderly population may facilitate such antigenic variant selection.
Figure 3.
Bubble plot analysis of the SARS-CoV-2 spike protein. The plot shows the probable B cell epitopes from the variable (S1 domain: aa13 to aa685) and stalk region (S2 domain: aa686 to aa1273) of the Spike protein. The corresponding Vaxijen score and the percentage of candidate vaccine probability shown in y and x axis, respectively. The size of each bubble signifies the respective surface accessibility. The B cell epitopes from the stalk S2 domain have less surface accessibility making it a poor vaccine candidate compared to the highly accessible B cell epitopes in the variable S1 domain and RBD.
Strategies to induce SARS-CoV-2 specific immune response within elderly population
Researchers discovered constant regions of influenza virus trimeric HA especially in the stalk domain and utilized it to generate neutralizing antibodies which will impart universal protection against several influenza strains [124–126]. Analyzing and identifying the conserved region of the spike protein of SARS-CoV-2 can lead to generation of a hybrid or chimeric vaccine candidate harboring both the influenza and SARS-CoV-2 antigens part is possible. Our Vaxijen based prediction shows the presence of some B cell epitopes in the stalk region but due to their low surface accessibility stalk region, unlike influenza HA stalk may not be an appropriate vaccine candidate (Figure 3). A hybrid/chimeric protein can be generated by combining the HA and spike protein as the formation of the trimeric assembly largely depends on the residues present in the stalk region. The residues from the stalk region form numerous H-bond interaction to form the trimeric assembly of the Spike protein and the residues from the variable region also gets stabilized by interacting with the stalk region (Figure 4A) [109,127]. The baculovirus insect cell expression system can be used to produce hybrid and/or chimeric VLPs comprising the SARS-COV-2 spike protein, stalk region of HA and the influenza M1 protein (Figure 4B) [128,129]. Chimeric protein with intact stalk region for the HA protein and the Spike variable or RBD domain will allow utilization of influenza specific CD4+ T cell populations to mount an effective antibody response in germinal center reactions. Each VLP will have both Spike, HA and M1 capable of utilizing corresponding CD4+ T cells help during germinal center reaction with spike specific B cells subsequently promoting affinity maturation and class switching (Figure 4C). This influenza specific CD4+ T cells can interact with spike specific B cells when the same B cell clone portrays MHC II containing influenza specific peptides for T cell help during germinal center reaction in the secondary lymph node. This may overcome the lack of spike antigen specific naïve or matured CD4+ T cells population which lack in aged individual due to age related defects. This strategy was explored widely against polysaccharide vaccines, where polysaccharide is attached directly to a protein molecule to mount strong T cell dependent immune responses to produce high affinity and class switched antibodies. The repetitive antigenic structure present on the surface of the VLPs makes it easier to be recognized and prompted a strong induction of humoral and cell mediated immune responses.
Figure 4.
Structure and design of hybrid or chimeric VLP for SARS-CoV-2 vaccination. (A) The trimeric assembly of SARS-COV-2 Spike protein and Influenza HA. Residues from stalk region represented in a cartoon format, differnt colors signifies three different chains. The residues from the variable region those are making H-bond with the stalk are labeled (representative from a single chain) whereas all the other residues shown as a ribbon diagramme. Upper figure showing the H-bond interaction profile for a single chain stalk region (pink) within the trimeric assembly. (B) Influnza Hemagglutinin (HA) and SARS-CoV-2 Spike contain head and stalk domain. Matrix protein 1 (M1), either with chimeric Spike (stalk from HA with head from Spike). (C) Schematic diagram showing cooperation between influenza induced memory T cells and development of spike specific immune response.
This novel approach of designing of chimeric or hybrid vaccine will give additional protection against influenza viruses responsible for thousands of deaths every year during seasonal flu. Recently phase 1 clinical trials were conducted with chimeric HA (consisting of constant stalk region with variable head domain of different strain of influenza viruses) inducing broadly protective HA stalk directed antibodies in vaccinated individuals [125]. Natural head domain of HA replaced with spike head can induce spike specific antibodies as well as antibodies directed against the HA stalk. This will further boost influenza specific immunity within vaccinated individual.
Conclusions
The terrific brunt of SARS-CoV-2 infection and the scarcity of available effective treatments specially to cure elderly individuals require more rational approach. Multifactorial changes in developing an appropriate cell mediated immune responses in elderly individual is of paramount need for designing an efficacious vaccine. The vaccines from Moderna, Pfizer and AstraZeneca, which are under emergency use in the USA and Europe will protect an individual from COVID-19 but the durability within normal or elderly population is unknown. The disease manifestation of COVID-19 has similarities with seasonal flu caused by influenza viruses. The ultimate goal is to design a universal vaccine for influenza, SARS-CoV-2 or other coronaviruses. In this article we highlighted the design of vaccine molecule having multiple antigens from different viruses can complement each other to mount an effective immune response especially in the elderly population with low naïve immune cell population. Stalk region of HA is relatively conserved and stalk directed antibodies can give protection to wide range of influenza strains. Therefore, chimeric vaccines for both influenza and SARS-CoV-2 will be a significant boost to deal global challenges against seasonal or pandemic flu viruses. Researchers should move forward with the idea of making a single chimeric molecule as vaccine candidates for both influenza and SARS-CoV-2. The idea of making chimeric molecules can be incorporated with mRNA, DNA, and adenovirus-based vaccine delivery system. This will have a significant impact on worldwide vaccination drive and their economic impact in the future to save human lives.
Funding sources
This research did not receive any specific grants from funding agencies in public, commercial, or not-for-profit sectors.
Disclosure statement
The authors declare no financial interest to disclose.
References
- 1.Cui J, Li F, Shi ZL.. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He G, Sun Z, Zhao Y, et al. Zhou Q: β-coronavirus infectious diseases: recommended strategies for the prevention and control of transmission. Int J Clin Exp Pathol. 2020;13:1060–1065. [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Astuti IY. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metab Syndr. 2020;14:407–412. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim D, Lee JY, Yang JS, et al. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181:914–921, e910. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong Y, Dai T, Wei Y, et al. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct Target Ther. 2020;5:237. doi: 10.1038/s41392-020-00352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funk CD, Laferrière C, Ardakani A.. A snapshot of the global race for vaccines targeting SARS-CoV-2 and the COVID-19 pandemic. Front Pharmacol. 2020;11(937):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callaway E. The coronavirus is mutating – does it matter? Nature. 2020;585:174–177. doi: 10.1038/d41586-020-02544-6. [DOI] [PubMed] [Google Scholar]
- 9.Vilar S, Isom DG.. One year of SARS-CoV-2: how much has the virus changed? [DOI] [PMC free article] [PubMed]
- 10.Galloway SE, Paul P, MacCannell DR, et al. Emergence of SARS-CoV-2 B.1.1.7 Lineage - United States, December 29, 2020–January 12, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:95–99. doi: 10.15585/mmwr.mm7003e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Driscoll M, Ribeiro Dos SG, Wang L, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2020 ;590(7844):140–145. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Klein SL, Garibaldi BT, et al. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65:101205. doi: 10.1016/j.arr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Giudice G, Goronzy JJ, Grubeck-Loebenstein B, et al. Fighting against a protean enemy: immunosenescence, vaccines, and healthy aging. NPJ Aging Mech Dis. 2017;4:1. doi: 10.1038/s41514-017-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson KL, Wu YC, Barnett Y, et al. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8:18–25. doi: 10.1111/j.1474-9726.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haq K, McElhaney JE.. Immunosenescence: Influenza vaccination and the elderly. Curr Opin Immunol. 2014;29:38–42. doi: 10.1016/j.coi.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikolich-Žugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol. 2018;19(1):10–19. doi: 10.1038/s41590-018-0205-0. [DOI] [PubMed] [Google Scholar]
- 18.Cambier J. Immunosenescence: a problem of lymphopoiesis, homeostasis, microenvironment, and signaling. Immunol Rev. 2005;205:5–6. doi: 10.1111/j.0105-2896.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- 19.Campisi J. Cellular senescence and lung function during aging, Yin and Yang. Ann Am Thorac Soc. 2016;13 Suppl 5:S402–S406. doi: 10.1513/AnnalsATS.201609-703AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray MA, Chotirmall SH.. The impact of immunosenescence on pulmonary disease. Mediators Inflamm. 2015;2015:692546. doi: 10.1155/2015/692546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rea IM, Gibson DS, McGilligan V, et al. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol. 2018;9(586):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong CK, Smith CA, Sakamoto K, et al. Aging impairs alveolar macrophage phagocytosis and increases influenza-induced mortality in mice. J Immunol. 2017;199:1060–1068. doi: 10.4049/jimmunol.1700397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Beek AA, Van den Bossche J, Mastroberardino PG, et al. Metabolic alterations in aging macrophages: ingredients for inflammaging? Trends Immunol. 2019;40:113–127. doi: 10.1016/j.it.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Nainu F, Shiratsuchi A, Nakanishi Y. Induction of apoptosis and subsequent phagocytosis of virus-infected cells as an antiviral mechanism. Front Immunol. 2017;8(1220):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujimoto I, Pan J, Takizawa T, et al. Virus clearance through apoptosis-dependent phagocytosis of influenza A virus-infected cells by macrophages. J Virol. 2000;74:3399–3403. doi: 10.1128/jvi.74.7.3399-3403.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puttur F, Gregory LG, Lloyd CM.. Airway macrophages as the guardians of tissue repair in the lung. Immunol Cell Biol. 2019;97:246–257. doi: 10.1111/imcb.12235. [DOI] [PubMed] [Google Scholar]
- 27.Snelgrove RJ, Goulding J, Didierlaurent AM, et al. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 28.Yu X, Buttgereit A, Lelios I, et al. The cytokine TGF-β promotes the development and homeostasis of alveolar macrophages. Immunity. 2017;47:903–912.e904. doi: 10.1016/j.immuni.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Kulikauskaite J, Wack A.. Teaching old dogs new tricks? The plasticity of lung alveolar macrophage subsets. Trends Immunol. 2020;41:864–877. doi: 10.1016/j.it.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussell T, Bell TJ.. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 31.Wenisch C, Patruta S, Daxböck F, et al. Effect of age on human neutrophil function. J Leukoc Biol. 2000;67(1):40–45. doi: 10.1002/jlb.67.1.40. [DOI] [PubMed] [Google Scholar]
- 32.Ortmann W, Kolaczkowska E.. Age is the work of art? Impact of neutrophil and organism age on neutrophil extracellular trap formation. Cell Tissue Res. 2018;371:473–488. doi: 10.1007/s00441-017-2751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu F, Zhang C, Zou Z, et al. Aging-related Atg5 defect impairs neutrophil extracellular traps formation. Immunology. 2017;151:417–432. doi: 10.1111/imm.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruddle NH, Akirav EM.. Secondary lymphoid organs: responding to genetic and environmental cues in ontogeny and the immune response. J Immunol. 2009;183:2205–2212. doi: 10.4049/jimmunol.0804324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goronzy JJ, Fang F, Cavanagh MM, et al. Naive T cell maintenance and function in human aging. J Immunol. 2015;194:4073–4080. doi: 10.4049/jimmunol.1500046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swain SL, McKinstry KK, Strutt TM.. Expanding roles for CD4+ T cells in immunity to viruses. Nat Rev Immunol. 2012;12:136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer S, Albergante L, Blackburn CC, et al. Thymic involution and rising disease incidence with age. Proc Natl Acad Sci USA. 2018;115:1883–1888. doi: 10.1073/pnas.1714478115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner VM, Mabbott NA.. Influence of ageing on the microarchitecture of the spleen and lymph nodes. Biogerontology. 2017;18:723–738. doi: 10.1007/s10522-017-9707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunn-Walters DK. The ageing human B cell repertoire: a failure of selection? Clin Exp Immunol. 2016;183:50–56. doi: 10.1111/cei.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 41.Vellas C, Delobel P, de Souto Barreto P, et al. COVID-19, virology and geroscience: a perspective. J Nutr Health Aging. 2020;24:685–691. doi: 10.1007/s12603-020-1416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laing AG, Lorenc A, Del Molino Del Barrio I, et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med. 2020;26:1623–1635. doi: 10.1038/s41591-020-1038-6. [DOI] [PubMed] [Google Scholar]
- 44.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biswas A, Bhattacharjee U, Chakrabarti AK, et al. Emergence of novel coronavirus and COVID-19: whether to stay or die out? Crit Rev Microbiol. 2020;46:182–193. doi: 10.1080/1040841X.2020.1739001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang YY, Li BR, Ning BT.. The comparative immunological characteristics of SARS-CoV, MERS-CoV, and SARS-CoV-2 coronavirus infections. Front Immunol. 2020;11:2033. doi: 10.3389/fimmu.2020.02033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li S, Jiang L, Li X, et al. Clinical and pathological investigation of patients with severe COVID-19. JCI Insight. 2020;5(12):5. doi: 10.1172/jci.insight.138070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng Y, Mentzer AJ, Liu G, et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnstone J, Parsons R, Botelho F, et al. Immune biomarkers predictive of respiratory viral infection in elderly nursing home residents. PLoS One. 2014;9:e108481. doi: 10.1371/journal.pone.0108481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conti P, Ronconi G, Caraffa A, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:327–331. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 51.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ciulla MM. SARS-CoV-2 downregulation of ACE2 and pleiotropic effects of ACEIs/ARBs. Hypertens Res. 2020;43:985–986. doi: 10.1038/s41440-020-0488-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng H, Wang Y, Wang GQ.. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020;92:726–730. doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Premkumar L, Segovia-Chumbez B, Jadi R, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 2020:5(48):eabc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with Coronavirus Disease 2019 (COVID-19). Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Z, Wan Y, Qiu C, et al. Recovery from severe H7N9 disease is associated with diverse response mechanisms dominated by CD8+ T cells. Nat Commun. 2015;6:6833. doi: 10.1038/ncomms7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501. e1415. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel A, Walters J, Reuschel EL, et al. Intradermal-delivered DNA vaccine provides anamnestic protection in a rhesus macaque SARS-CoV-2 challenge model. bioRxiv. 2020. bioRxiv. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tebas P, Yang S, Boyer JD, et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial. EClin Med. 2021;31:100689. doi: 10.1016/j.eclinm.2020.100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu A, Peng Y, Huang B, et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang C, Maruggi G, Shan H, et al. Advances in mRNA vaccines for infectious diseases. Front Immunol. 2019;10(594);1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kowalski PS, Rudra A, Miao L, et al. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol Ther. 2019;27:710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reichmuth AM, Oberli MA, Jaklenec A, et al. mRNA vaccine delivery using lipid nanoparticles. Ther Deliv. 2016;7:319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bloom K, van den Berg F, Arbuthnot P.. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2020;28(3-4):117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corbett KS, Edwards DK, Leist SR, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. 2020;383(16):1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walsh EE, Frenck RW Jr., Falsey AR, et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Torjesen I. Covid-19: Norway investigates 23 deaths in frail elderly patients after vaccination. BMJ. 2021;372:n149. doi: 10.1136/bmj.n149. [DOI] [PubMed] [Google Scholar]
- 72.Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Todorov G, Uversky VN.. A possible path towards rapid development of live-attenuated SARS-CoV-2 vaccines: plunging into the natural pool. Biomolecules. 2020;10(10):1438. doi: 10.3390/biom10101438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.https://www.pharmaceutical-technology.com/news/codagenix-sii-dosing-vaccine/.
- 75.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barrett JR, Belij-Rammerstorfer S, Dold C, et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;27(2):279–288. doi: 10.1038/s41591-020-01179-4. [DOI] [PubMed] [Google Scholar]
- 78.Graham F. Daily briefing: Oxford–AstraZeneca COVID vaccine is back on track. Nature. 2021. [DOI] [PubMed] [Google Scholar]
- 79.Sattler A, Angermair S, Stockmann H, et al. SARS-CoV-2-specific T cell responses and correlations with COVID-19 patient predisposition. J Clin Invest. 2020;130:6477–6489. doi: 10.1172/JCI140965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Candia P, Prattichizzo F, Garavelli S, et al. T cells: warriors of SARS-CoV-2 infection. Trends Immunol. 2021;42:18–30. doi: 10.1016/j.it.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183(1):158–168. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saule P, Trauet J, Dutriez V, et al. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. 2006;127:274–281. doi: 10.1016/j.mad.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 83.El Shikh ME, El Sayed RM, Sukumar S, et al. Activation of B cells by antigens on follicular dendritic cells. Trends Immunol. 2010;31:205–211. doi: 10.1016/j.it.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lelic A, Verschoor CP, Ventresca M, et al. The polyfunctionality of human memory CD8+ T cells elicited by acute and chronic virus infections is not influenced by age. PLoS Pathog. 2012;8(12):e1003076. doi: 10.1371/journal.ppat.1003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Izurieta HS, Wernecke M, Kelman J, et al. Effectiveness and duration of protection provided by the live-attenuated Herpes Zoster Vaccine in the Medicare population ages 65 years and older. Clin Infect Dis. 2017;64:785–793. doi: 10.1093/cid/ciw854. [DOI] [PubMed] [Google Scholar]
- 86.Calabro S, Tritto E, Pezzotti A, et al. The adjuvant effect of MF59 is due to the oil-in-water emulsion formulation, none of the individual components induce a comparable adjuvant effect. Vaccine. 2013;31:3363–3369. doi: 10.1016/j.vaccine.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 87.Del Giudice G, Rappuoli R.. Inactivated and adjuvanted influenza vaccines. Curr Top Microbiol Immunol. 2015;386:151–180. doi: 10.1007/82_2014_406. [DOI] [PubMed] [Google Scholar]
- 88.Magnusson SE, Reimer JM, Karlsson KH, et al. Immune enhancing properties of the novel Matrix-M™ adjuvant leads to potentiated immune responses to an influenza vaccine in mice. Vaccine. 2013;31:1725–1733. doi: 10.1016/j.vaccine.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 89.De Serres G, Gariépy MC, et al. Short and long-term safety of the 2009 AS03-adjuvanted pandemic vaccine. PLoS One. 2012;7(7):e38563. doi: 10.1371/journal.pone.0038563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen WH, Jackson LA, Edwards KM, et al. Persistence of antibody to Influenza A/H5N1 vaccine virus: impact of AS03 adjuvant. Clin Vaccine Immunol. 2016;23:73–77. doi: 10.1128/CVI.00475-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–2096. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 92.Moser C, Amacker M, Zurbriggen R.. Influenza virosomes as a vaccine adjuvant and carrier system. Expert Rev Vaccines. 2011;10:437–446. doi: 10.1586/erv.11.15. [DOI] [PubMed] [Google Scholar]
- 93.Conne P, Gauthey L, Vernet P, et al. Immunogenicity of trivalent subunit versus virosome-formulated influenza vaccines in geriatric patients. Vaccine. 1997;15(15):1675–1679. doi: 10.1016/S0264-410X(97)00087-X. [DOI] [PubMed] [Google Scholar]
- 94.Glück R, Mischler R, Finkel B, et al. Immunogenicity of new virosome influenza vaccine in elderly people. Lancet. 1994;344:160–163. doi: 10.1016/S0140-6736(94)92758-8. [DOI] [PubMed] [Google Scholar]
- 95.Saade F, Honda-Okubo Y, Trec S, et al. A novel hepatitis B vaccine containing Advax™, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine. 2013;31:1999–2007. doi: 10.1016/j.vaccine.2012.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Honda-Okubo Y, Saade F, Petrovsky N.. Advax™, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine. 2012;30:5373–5381. doi: 10.1016/j.vaccine.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dolter KE, Evans CF, Ellefsen B, et al. Immunogenicity, safety, biodistribution and persistence of ADVAX, a prophylactic DNA vaccine for HIV-1, delivered by in vivo electroporation. Vaccine. 2011;29:795–803. doi: 10.1016/j.vaccine.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 98.Reimer JM, Karlsson KH, Lövgren-Bengtsson K, et al. Matrix-M™ adjuvant induces local recruitment, activation and maturation of central immune cells in absence of antigen. PLoS One. 2012;7:e41451. doi: 10.1371/journal.pone.0041451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bengtsson KL, Karlsson KH, Magnusson SE, et al. Matrix-M adjuvant: enhancing immune responses by ‘setting the stage’ for the antigen. Expert Rev Vaccines. 2013;12:821–823. doi: 10.1586/14760584.2013.814822. [DOI] [PubMed] [Google Scholar]
- 100.Antrobus RD, Lillie PJ, Berthoud TK, et al. A T cell-inducing influenza vaccine for the elderly: safety and immunogenicity of MVA-NP + M1 in adults aged over 50 years. PLoS One. 2012;7:e48322. doi: 10.1371/journal.pone.0048322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Altenburg AF, Kreijtz JH, de Vries RD, et al. Modified vaccinia virus Ankara (MVA) as production platform for vaccines against influenza and other viral respiratory diseases. Viruses. 2014;6:2735–2761. doi: 10.3390/v6072735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jordan E, Lawrence SJ, Meyer TPH, et al. Broad antibody and cellular immune response from a phase 2 clinical trial with a novel multivalent poxvirus-based respiratory syncytial virus vaccine. J Infect Dis. 2021;223:1062–1072. doi: 10.1093/infdis/jiaa460. [DOI] [PubMed] [Google Scholar]
- 103.Suzuki M, Dhoubhadel BG, Ishifuji T, et al. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infect Dis. 2017;17:313–321. doi: 10.1016/S1473-3099(17)30049-X. [DOI] [PubMed] [Google Scholar]
- 104.van Deursen AMM, van Houten MA, Webber C, et al. Immunogenicity of the 13-valent pneumococcal conjugate vaccine in older adults with and without comorbidities in the community-acquired pneumonia immunization trial in adults (CAPiTA). Clin Infect Dis. 2017;65:787–795. doi: 10.1093/cid/cix419. [DOI] [PubMed] [Google Scholar]
- 105.Nived P, Jönsson G, Settergren B, et al. Prime-boost vaccination strategy enhances immunogenicity compared to single pneumococcal conjugate vaccination in patients receiving conventional DMARDs, to some extent in abatacept but not in rituximab-treated patients. Arthritis Res Ther. 2020;22(1):36. doi: 10.1186/s13075-020-02256-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weyand CM, Goronzy JJ.. Aging of the immune system. Mechanisms and therapeutic targets. Ann Am Thorac Soc. 2016;13 Suppl 5:S422–S428. doi: 10.1513/AnnalsATS.201602-095AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crooke SN, Ovsyannikova IG, Poland GA, et al. Immunosenescence and human vaccine immune responses. Immun Ageing. 2019;16:25. doi: 10.1186/s12979-019-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dai L, Gao GF.. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21:73–82. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cai Y, Zhang J, Xiao T, et al. Distinct conformational states of SARS-CoV-2 spike protein. Science. 2020;369:1586–1592. doi: 10.1126/science.abd4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huang Y, Yang C, Xu XF, et al. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang D, Mai J, Zhou W, et al. Immunoinformatic analysis of T- and B-cell epitopes for SARS-CoV-2 vaccine design. Vaccines (Basel). 2020;8(3):355. doi: 10.3390/vaccines8030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shi R, Shan C, Duan X, et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 2020;584:120–124. doi: 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- 113.Piccoli L, Park YJ, Tortorici MA, et al. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell. 2020;183(4):1024–1042. doi: 10.1016/j.cell.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dearlove B, Lewitus E, Bai H, et al. A SARS-CoV-2 vaccine candidate would likely match all currently circulating variants. Proc Natl Acad Sci USA. 2020;117:23652–23662. doi: 10.1073/pnas.2008281117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Laha S, Chakraborty J, Das S, et al. Characterizations of SARS-CoV-2 mutational profile, spike protein stability and viral transmission. Infect Genet Evol. 2020;85:104445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pokhrel S, Kraemer BR, Mochly-Rosen D. Natural variants in SARS-CoV-2 S protein pinpoint structural and functional hotspots; implications for prophylaxis strategies. 2021. [DOI] [PMC free article] [PubMed]
- 117.Li Q, Wu J, Nie J, et al. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell. 2020;182:1284–1294,e1289. doi: 10.1016/j.cell.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weisblum Y, Schmidt F, Zhang F, et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife. 2020;9:9. doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fratev F. The N501Y and K417N mutations in the spike protein of SARS-CoV-2 alter the interactions with both hACE2 and human derived antibody: a free energy of perturbation study. 2020. bioRxiv. doi:. [DOI] [PubMed]
- 120.Xie X, Zou J, Fontes-Garfias CR, et al. Neutralization of SARS-CoV-2 spike69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nature Medicine 2021;27:620–621. [DOI] [PubMed]
- 121.Krammer F, Pica N, Hai R, et al. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol. 2013;87:6542–6550. doi: 10.1128/JVI.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Garcia-Beltran WF, Lam EC, Astudillo MG, et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184:476–488,e411. doi: 10.1016/j.cell.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Georgieva M, Buckee CO, Lipsitch M.. Models of immune selection for multi-locus antigenic diversity of pathogens. Nat Rev Immunol. 2019;19:55–62. doi: 10.1038/s41577-018-0092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Krammer F, Pica N, Hai R, Tan GS, et al. Hemagglutinin stalk-reactive antibodies are boosted following sequential infection with seasonal and pandemic H1N1 Influenza Virus in mice. J Virol. 2012;86:10302–10307. doi: 10.1128/JVI.01336-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nachbagauer R, Feser J, Naficy A, et al. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat Med. 2021;27:106–114. doi: 10.1038/s41591-020-1118-7. [DOI] [PubMed] [Google Scholar]
- 126.Biswas A, Chakrabarti AK, Dutta S.. Current challenges: from the path of "original antigenic sin" towards the development of universal flu vaccines. Int Rev Immunol. 2020;39:21–36. doi: 10.1080/08830185.2019.1685990. [DOI] [PubMed] [Google Scholar]
- 127.Xiong X, Qu K, Ciazynska KA, et al. A thermostable, closed SARS-CoV-2 spike protein trimer. Nat Struct Mol Biol. 2020;27:934–941. doi: 10.1038/s41594-020-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mortola E, Roy P.. Efficient assembly and release of SARS coronavirus-like particles by a heterologous expression system. FEBS Lett. 2004;576:174–178. doi: 10.1016/j.febslet.2004.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu YV, Massare MJ, Barnard DL, et al. Chimeric severe acute respiratory syndrome coronavirus (SARS-CoV) S glycoprotein and influenza matrix 1 efficiently form virus-like particles (VLPs) that protect mice against challenge with SARS-CoV. Vaccine. 2011;29(38):6606–6613. doi: 10.1016/j.vaccine.2011.06.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Levin MJ, Oxman MN, Zhang JH, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825–835. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Levin MJ, Schmader KE, Pang L, et al. Cellular and humoral responses to a second dose of herpes zoster vaccine administered 10 years after the first dose among older adults. J Infect Dis. 2016;213:14–22. doi: 10.1093/infdis/jiv480. [DOI] [PubMed] [Google Scholar]
- 132.Jefferson T, Rivetti D, Rivetti A, et al. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366:1165–1174. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- 133.Ciabattini A, Nardini C, Santoro F, et al. Vaccination in the elderly: the challenge of immune changes with aging. Semin Immunol. 2018;40:83–94. doi: 10.1016/j.smim.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 134.Nicholson KG, Abrams KR, Batham S, et al. Immunogenicity and safety of a two-dose schedule of whole-virion and AS03A-adjuvanted 2009 influenza A (H1N1) vaccines: a randomised, multicentre, age-stratified, head-to-head trial. Lancet Infect Dis. 2011;11:91–101. doi: 10.1016/S1473-3099(10)70296-6. [DOI] [PubMed] [Google Scholar]
- 135.Herzog C, Hartmann K, Künzi V, et al. Eleven years of Inflexal V-a virosomal adjuvanted influenza vaccine. Vaccine. 2009;27:4381–4387. doi: 10.1016/j.vaccine.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 136.Joines RW, Blatter M, Abraham B, et al. A prospective, randomized, comparative US trial of a combination hepatitis A and B vaccine (Twinrix) with corresponding monovalent vaccines (Havrix and Engerix-B) in adults. Vaccine. 2001;19(32):4710–4719. doi: 10.1016/S0264-410X(01)00240-7. [DOI] [PubMed] [Google Scholar]
- 137.Keating GM, Noble S.. Recombinant hepatitis B vaccine (Engerix-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs. 2003;63:1021–1051. doi: 10.2165/00003495-200363100-00006. [DOI] [PubMed] [Google Scholar]
- 138.https://clinicaltrials.gov/ct2/show/NCT03026348.
- 139.Langley JM, MacDonald LD, Weir GM, et al. A respiratory syncytial virus vaccine based on the small hydrophobic protein ectodomain presented with a novel lipid-based formulation is highly immunogenic and safe in adults: a first-in-humans study. J Infect Dis. 2018;218:378–387. doi: 10.1093/infdis/jiy177. [DOI] [PMC free article] [PubMed] [Google Scholar]