ABSTRACT
Background: COVID-19 has caused calamitous health, economic and societal consequences globally. Currently, there is no effective treatment for the infection. Areas covered: We have recently described the NZACE2-Pātari project, which seeks to administer modified Angiotensin Converting Enzyme 2 (ACE2) molecules early in the infection to intercept and block SARS-CoV-2 binding to the pulmonary epithelium. Expert opinion: Since the nasopharyngeal mucosa is infected in the first asymptomatic phase of the infection, treatment of the nose is likely to be safe and potentially effective. The intercepted virus will be swallowed and destroyed in the stomach. There is however a limited window of opportunity to alter the trajectory of the infection in an individual patient, which requires access to rapid testing for SARS-CoV-2. The proposed strategy is analogous to passive immunization of viral infections such as measles and may be of particular benefit to immunodeficient and unvaccinated individuals.
KEYWORDS: COVID-19, nasal therapy, nzace2-pātari, sars-cov-2
1. Introduction
SARS-CoV-2, the zoonotic agent responsible for COVID-19 originated in the Hubei province of China [1]. COVID-19 has caused catastrophic health, economic and societal consequences globally. There is currently no universally effective treatment for the infection. Over the last fifteen months, an unprecedented international scientific effort has been launched to understand the infection and to develop new treatments and vaccines. References relevant to the current article have been identified from the published literature to 11 March 2021. Only peer reviewed articles have been included. The reader is advised to seek up-to-date references as this field is changing each day.
2. Immunosenescence and co-morbidities
COVID-19 causes a steep age-related mortality gradient. Death rates approaching 30% were seen in those over 80 years of age in China and elsewhere [2]. In addition to older age, comorbidities including obesity, diabetes, hypertension, renal impairment, malignancy, pulmonary and cardiac disease were associated with poor prognosis. Precisely how these comorbidities influence adverse outcomes is incompletely understood.
Black and South Asian individuals are also vulnerable to severe disease [3]. Part of this ethnic/racial predisposition could be confounded by a higher prevalence of comorbidities and poor access to healthcare [4,5].
3. The phases of the infection
COVID-19 evolves in three overlapping clinical phases (Figure 1) [6]. In the incubation period, the nasopharyngeal mucosa is infected [7]. The virus gains access to the respiratory epithelium by binding angiotensin converting enzyme 2 (ACE2) on the cell surface [8]. Host proteases facilitate viral entry by cleaving the S1 subunit of the viral spike (S) glycoprotein, allowing the S2 subunit to fuse with the host cell [9].
Figure 1.
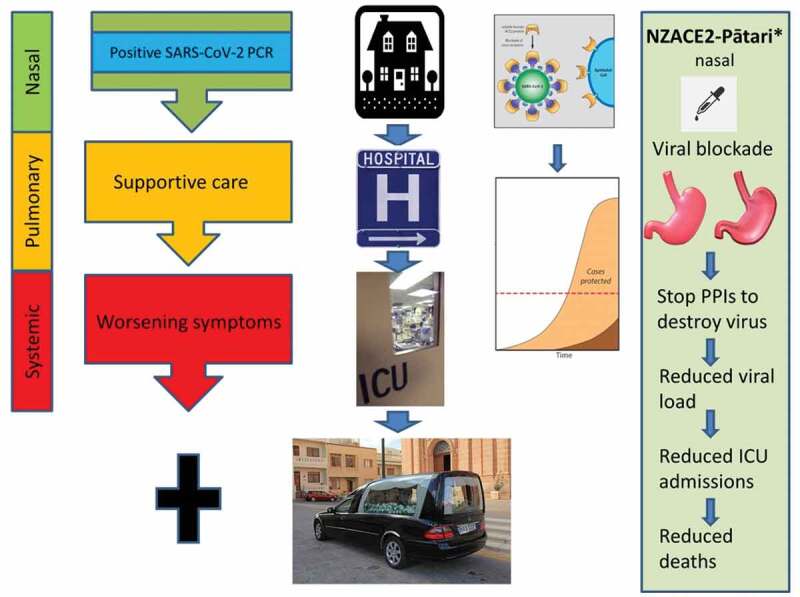
The three overlapping clinical phases of COVID-19 [6]. The asymptomatic (incubation) nasal phase is followed by the pulmonary and systemic phases leading to multi-organ failure in some patients. Administration of NZACE2-Pātari by a nasal dropper may intercept of SARS-CoV-2 leading to a reduced viral load and milder disease [35,36]. Stopping proton pump inhibitors (PPIs) may allow the stomach to function as an effective antiviral organ [10]. Because the drug will be administered several times a day, there will be no requirement to fast. * NZACE2-Pātari has been created but has not entered clinical trials at the time of writing. The simplest trial design would be to randomize unvaccinated households with an infected individual to receive either placebo or active drug to determine infection rates. This diagram was constructed from free images on the Internet
Following fusion with the epithelial cell membrane, viral RNA is released, which hijacks cellular organelles for production of progeny virions. Release of daughter virus causes destruction of host pneumocytes.
In older individuals and those with comorbidities, there is often progression to lung involvement. In this second pulmonary phase, typically around day 5, patients experience increasing breathlessness, fever, fatigue and myalgia. Laboratory correlates of worsening disease include neutrophilia, lymphopenia, raised inflammatory markers including C – reactive protein (CRP) and ESR [11].
Around day 10, those destined to suffer the systemic phase will progress to acute respiratory distress syndrome (ARDS) and experience dysfunction of other target organs including the heart, brain, kidneys and activation of the coagulation cascade [12]. The systemic phase is associated with viral sepsis leading to a cytokine storm, with release of multiple cytokines including IL6. Persistent elevation of troponin may signify myocarditis leading to heart failure. Patients entering the systemic phase have a high mortality rate from multi-organ failure in spite of invasive ventilation and extra-corporeal membrane oxygenation (ECMO).
Survivors of COVID-19 are often left with disabilities including strokes, myocarditis, renal impairment, pulmonary dysfunction and a condition similar to chronic fatigue syndrome [13,14]. ‘Long COVID’ is being increasingly recognized in COVID-19 survivors [15]. Their long-term prognosis is unknown. A pandemic psychiatric morbidity is likely to be a major consequence of COVID-19 [16].
In contrast to the severe outcomes noted above, in many cases the disease is mild and some patients are asymptomatic. The explanation for asymptomatic infection, even in older persons is poorly understood [17]. These asymptomatic individuals can however transmit the virus and are a logistical and societal challenge to countries attempting to eradicate the infection [18].
4. The immune response to SARS-CoV-2
The correlates of protective immunity to SARS-CoV-2 are not currently understood [19]. The initial nasal phase is largely asymptomatic. The virus engages a series of ploys to avoid detection and activation of the innate immune system [20]. Cytoplasmic pattern recognition molecules such as Toll-like receptors, RIG-like receptors, MDA5 and protein kinase R (PKR) are normally triggered by the presence of viral RNA or its variants including double-stranded RNA during viral replication [20]. Viral endonucleases cloak the viral RNA preventing activation of pathogen pattern recognition molecules [20]. In addition anti-interferon antibodies [21] impede the action of type 1 interferons [22]. During the initial five days, there is thus unopposed viral replication in the nasal mucosa. Other arms of the innate immune system such as NK cells are also rendered ineffective [23]. The complement cascade may have a role in aggravating the infection [24]. Activation of the inflammasome may lead to pyroptosis and could contribute to the cytokine storm in patients experiencing multi-organ failure [20].
The adaptive immune system is also subverted by the virus with the loss of CD8 cells [25]. The S protein is highly glycosylated rendering it less immunogenic [26]. Humoral immunity to SARS-CoV-2 may not be protective and there is the possibility of antibody disease enhancement (ADE) [27]. Some patients dying from the infection had both high viral loads and antibody titers, indicating these antibodies were unable to neutralize the virus. The in vitro correlates of ADE are only partially understood [28].
5. Treatment strategies for COVID-19
There is currently no universally effective curative treatment for COVID-19. Repurposing existing drugs has largely been disappointing with multiple trials showing the lack of efficacy of treatments such as hydroxychloroquine [29]. Dexamethasone has shown modest benefits for severely ill patients [30]. Although a large number of vaccines have entered production and in some cases emergency approval for use, they face many logistical challenges including global distribution, long-term efficacy and the risk of adverse effects including vaccine-induced ADE or thrombotic events. Selection of escape mutants by vaccines remains a concern. Very recently, the Astra-Zeneca vaccine was shown to be less effective against the South African variant (B.1.351) and the planned roll-out for HCWs has been suspended in South Africa. There are also increasing fears monoclonal antibodies such as bamlanivimab, casirivimab and indevimab may be rendered ineffective by viral evolution, particularly those bearing the E484K substitution [31].
6. The NZACE2-Pātari project to treat COVID-19
We have recently described the NZACE2-Pātari project which aims to intercept SARS-CoV-2 and block infection of respiratory epithelial cells [32]. We have constructed modified ACE2 molecules, which will be administered by an inhaler during the early phases of the infection [33,34]. We expect our drugs may mitigate the viral pneumonia and consequently reduce the risk patients will progress to the systemic phase of the infection, which carries high morbidity and mortality.
As part of this project, we also plan to administer the ACE2-derived drugs (NZACE2-Pātari) by the nasal route (Figure 1). In this article we explore the risks and benefits of nasal administration of these drugs. We expect NZACE2-Pātari will result in a reduction in the number of virions that are able to infect the nasal mucosa. Consequently, there will be fewer virions that can reach the lungs by microaspiration. A lower viral burden at each stage of the infection may reduce the numbers of patients entering the pulmonary and systemic phases of the disease. Current data indicates a lower viral burden is associated with milder disease [35,36]. Using this strategy, we expect disease severity will be mitigated.
7. Doses of modified ACE2 (NZACE2-Pātari) required to treat nasal infection
We have calculated the doses of ACE2 molecules needed to neutralize binding of SARS-CoV-2 to the nose as follows [36,37].
Viral particles/ml nasal fluid [36,37] = 1.4 x 10(6)/ml (some samples have higher values)
Spikes per virion = 57
Nasal extracellular fluid = 5 ml (volume may be increased later in infection)
Total number of ACE2 molecules needed to bind every spike on every virus in the nose
= 57 x 5 x 1.4 x10(6)
ACE2 mw = 92 463kDa (with CHO) kg/mol
Avogadro’s number = 6.02 x 10(23)/mol
Amount of ACE2 to bind every spike = 1.5 x 10(7) x 57 × 5 x 9.246 x10(4)/6.02 x 10(23)
= 7.9 x 10(−9) kg
= 7.9 µg ACE2
If spike is a trimer x3 = 23.7 µg ACE2
8. Administration of NZACE2-Pātari to the nose
Nasal NZACE2-Pātari can be easily administered by a dropper (Figure 1). A dropper is a low-pressure device and is unlikely to denature the molecules by shear stress. NZACE2-Pātari will be administered with patients leaning back over the bed and then rotating their head laterally to ensure coverage of the nasal mucosa. Patients would receive 4 mg of NZACE2-Pātari to the nose over 2 days. From the calculations presented here, we would expect SARS-CoV-2 to be overwhelmed by NZACE2-Pātari, which stoichiometrically far exceeds the number of virions (by approximately 170x). Some studies have suggested higher nasal viral loads (1.5x 10 (7)) but most virus will likely be bound to NZACE2-Pātari [38].
Nasal secretions may increase toward day 5 of infection, but the proposed dose should compensate for higher nasal mucous production. Furthermore, viral titers decrease toward the end of the nasal phase [38]. Repeated administration of the drugs over 2 days will substantially reduce the viral load and may alter the trajectory of the infection (Figure 1). The SARSCoV-2/NZACE2-Pātari complexes will reach the pharynx by naso-ciliary transport and be swallowed leading to hydrolytic destruction in the stomach as discussed below. It is acknowledged some viral particles may escape binding to NZACE2-Pātari, particularly if there is a delay in diagnosis but we expect the overall viral burden will be reduced, mitigating disease severity [35].
9. Advantage of the nasal route
The NZACE2-Pātari project exploits a critical vulnerability of the virus, which is the obligate requirement for the receptor-binding domain (RBD) of the S glycoprotein to bind cellular ACE2 to infect cells. The RBD is therefore a stable target for antiviral strategies and unlikely to mutate, without loss of pathogenicity [39].
The nasal route for therapeutic administration is very attractive for several reasons. Delivery of drugs to the site of infection allows smaller doses to maximize therapeutic effect and minimize adverse reactions. The administration of nasal drops is simple, it does not require any special skill and self–medication is possible once dispensed by a health-care worker (HCW). Therefore, nasal delivery increases the likelihood of patient compliance and reduces treatment cost.
The solution used to reconstitute these products is simple. The formulation will include a protein such as human serum albumin to stabilize NZACE2-Pātari. It will also contain mucoadhesives to increase the duration of the drug in the nasal cavity. The drugs could be lyophilized and reconstituted when needed. This would allow the easier global deployment of the drug as transporting lyophilized proteins carries low risk of inactivation in hostile environmental conditions around the world.
Overdosage is unlikely to be a problem. These drugs are likely to have a high therapeutic index. Any excess drug will be swallowed and hydrolyzed in the stomach. We expect excess drug also to be transported to the pharynx, which may provide anti-viral activity for the SARS-CoV-2 throat infection. A further advantage of the nasal route is that a portion of drugs could reach the brain from the nasal cavity [40]. The brain is a primary target for SARS-CoV-2 and if NZACE2-Pātari enters the brain it may provide a direct neuroprotective effect against the virus [41,42]. We acknowledge NZACE2-Pātari will have no efficacy in patients who develop viral sepsis leading to neurological damage.
In the initial nasal incubation phase of COVID-19 there is minimal inflammation hence the patient is asymptomatic. This offers NZACE2-Pātari the advantage of reduced degradation by cellular proteases, which could otherwise shorten the drugs half-life. Furthermore, in the absence of severe inflammation, an intact muco-ciliary clearance system allows transit of SARS-CoV-2/NZACE2-Pātari complexes to the pharynx. Once swallowed, this will lead to hydrolytic destruction of the virus in the stomach, as detailed below.
10. Safety, potential risks and adverse reactions to nasal NZACE2-Pātari
The nose appears to be very safe compared to most other routes of drug delivery. Administration of drugs to the nose such as erythropoietin or glucagon did not cause serious adverse reactions [43,44]. Similarly, nasal ketorolac challenges in highly sensitized Samter’s triad patients rarely caused bronchospasm [45].
The angiotensin converting catalytic site of NZACE2-Pātari has been rendered inactive by a single amino acid substitution (R273A) while the affinity for the virus has been improved by removing an N-glycosylation site (N90D). ACE2 catalyses the production of angiotensin 1–7 and 1–9, which have a role in blood pressure control [46]. We would not expect adverse cardiovascular events with supraphysiological doses of inactivated ACE2 administered to the nose, even if some of the drug enters the circulation. A biosimilar drug, APN01 (containing the active ACE2 catalytic site) was well tolerated in high doses, when administered intravenously for ARDS [47]. Since the correlates of protective immunity for COVID-19 are not understood, we are not administering modified ACE2 conjugated to an Fc receptor. The consequences of early activation of macrophages are unknown at this time. There is a risk this could trigger ARDS. Such conjugates could be considered in the future, with better understanding of the immunological conundrum posed by SARS-CoV-2.
Nasal adverse reactions are typically allergies and given the short duration of treatment, we would not expect patients to become sensitized to the modified ACE2 drugs. Any such local reaction can be treated with a decongestant or an antimuscarinic agent such as Ipratropium. A short course of treatment is unlikely to trigger an autoimmune neurological disorder. These risks must be balanced against a lethal infection with a propensity to cause severe long-term disability, for which there is no widely available curative treatment.
11. The role of the stomach in mitigating COVID-19
The stomach is likely to play an important role in the defense against SARS-CoV-2 (Figure 1) [48]. The virus is inactivated by pH ranges (1.5–3) found in the stomach [49]. It is likely much of the virus from the nasal infection is swallowed and destroyed in the stomach [48].
There are now multiple studies showing that the use of proton pump inhibitors (PPIs) is associated with worse outcomes in COVID-19 [50,51]. There is more than one interpretation of this observation. First, it is possible the active viral burden is increased by a high stomach pH. This would allow intact virus to travel to the small intestine and gain entry through gut epithelial cells [52]. Diarrhea and abdominal discomfort can be symptoms of COVID-19 [53].
Secondly, the use of PPIs may be more common in those with obesity and type 2 diabetes. These individuals are at increased risk of gastroesophageal reflux (GER). It is possible the use of PPIs allows the stomach to act as a reservoir for active virus, which leads to greater microaspiration into the lungs from GER.
Recent data from China suggests that young HCWs exposed to greater inocula of the virus before the use of personal protective equipment (PPE), had more severe outcomes [54]. The result of increased GER with active virus might have the same consequence as those exposed to a greater pulmonary inoculum of the virus before the use of PPE. Lower rates of GER in children may be an important age-related protective factor against COVID-19 and may be one explanation for severe outcomes for those with obesity and type 2 diabetes.
In the absence of effective antiviral drugs, the stomach will play an important role in destroying swallowed SARS-CoV-2 complexed to NZACE2-Pātari (Figure 1). A critical part of our strategy is to stop PPIs temporarily. The stomach would no longer serve as a reservoir of active viral particles to aggravate the pneumonitis or to gain entry through the gut. Alternatives to PPIs may need to be considered including metoclopramide for symptomatic GER. Elevating the bed at night and avoiding dinner just before bedtime are other simple measures to reduce GER.
12. Clinical utility
NZACE2-Pātari is best suited to treating unvaccinated patients in the earliest phases of the disease [32]. They could be deployed at the onset of a disease outbreak in a nursing home, when unvaccinated patients first test positive. They could be used in prisons and refugee centers, where physical and social distancing is difficult.
NZACE2-Pātari may have a role at the NZ border to treat infected travelers and their contacts. These drugs could be used for infected staff at the border including military and hotel personnel. If effective, they will assist front-line hospital nursing and medical staff, who are at high risk of infection [55].
NZACE2-Pātari may mitigate community outbreaks, where unvaccinated contacts can be rapidly traced, tested and offered treatment if they are infected. This will reduce the R0 of the virus. This will have major economic benefits as it may reduce the need for stringent quarantines.
This strategy may be particularly effective for unvaccinated patients with comorbidities including obesity and diabetes, who are at risk of severe outcomes. It may also be beneficial for infected Māori and Pacifika patients who have an increased burden of comorbidities and would be expected have severe outcomes [3]. Intervention rates in Māori and Pacifika patients have been shown in multiple studies to lag behind patients of other ethnicities, which could contribute to severe outcomes [56]. COVID-19 is likely to exacerbate preexisting economic, health, and social disparities in vulnerable groups [4,57].
Because multiple new SARS-CoV-2 variants, the B.1.1.7 (UK), B.1.1.248 (Brazil), B 1.617 (India),B.1.427/B.1.429 (California) and B.1.351 (South Africa) require ACE2 to gain entry, we would expect NZACE2-Pātari to remain effective. NZACE2-Pātari will also serve as insurance against future coronavirus infections utilizing ACE2 to gain entry into cells. It will also assist in the unfortunate event where COVID-19 vaccines cause ADE in some patients [58]. This could cause serious reputational damage to vaccines in general, leading to resurgence of vaccine-preventable diseases [59–61]. In the event SARS-CoV-2 vaccines cause ADE, NZACE2-Pātari could mitigate the situation by outcompeting such vaccine-induced antibodies.
They could be of benefit to immunodeficient patients and the elderly, who respond poorly to many vaccines [62–65]. Many immunodeficient patients are also disadvantaged as they have pulmonary disease from the infective and inflammatory sequelae of immune system failure. If proven to be safe and effective, these drugs will help a broad array of unvaccinated, infected individuals early in the disease.
13. Caveats
Our strategy is analogous to administering normal immune globulin as postexposure prophylaxis for measles. We plan to treat newly diagnosed unvaccinated patients in the presymptomatic nasal phase (Figure 1). These patients will have a positive SARS-CoV-2 test, ideally by PCR or by a rapid antigen test. Testing is a challenge in many parts of the world and turnaround times vary greatly for PCR tests. The PCR result or antigen detection test should be available on the same day. If proven safe and effective, the global deployment of NZACE2-Pātari will depend on access to rapid testing for SARS-CoV-2.
In the event a patient receives NZACE2-Pātari treatment in the absence of disease, it is however very unlikely this will cause adverse effects. Ideally, patients should only need treatment once, to minimize the small risk of sensitization to NZACE2-Pātari, which has two amino acid differences from wild-type ACE2. Again, an adverse reaction is likely to result in temporary nasal obstruction.
The nose is a very attractive route to safely treat COVID-19, given the limited understanding of the correlates of protective immunity. There is however a small window of opportunity to influence disease severity. As noted above, nasal NZACE2-Pātari is less likely to be effective once patients enter the pulmonary and systemic phases. The key to success is early identification and treatment of COVID-19 patients in the presymptomatic nasal phase.
4. Expert opinion
Since SARS-CoV-2 binds cell surface ACE2 to infect cells, this represents an attractive target to intercept the virus. Mutation of the receptor-binding domain of the viral spike glycoprotein is unlikely to be tolerated without loss of pathogenicity. The receptor-binding domain is thus a stable target for antiviral strategies.
The viral infection evolves in three overlapping clinical stages; the nasal, pulmonary and systemic phases. SARS-CoV-2 deploys strategies to avoid provoking an inflammatory response during the initial nasal incubation period. As a result, the virus is able to exponentially multiply, unchallenged. Patients destined to have more severe disease then progress to the pulmonary and systemic phases with dysfunction of multiple organs including the lungs, brain, heart and kidneys as well as the coagulation cascade. Targeting the nose during the incubation period is thus an attractive strategy before the second pulmonary or third systemic phases, for which there is no universally effective treatment.
In the absence of widely available effective antiviral drugs, we have identified the stomach as a key antiviral organ. The virus is destroyed by low pH levels typically found in the stomach. Current data indicates patients being treated with proton pump inhibitors have a high mortality. Our interpretation of this data is that the swallowed virus remains intact in patients on PPIs and can infect the lungs by gastroesophageal reflux or gain access to other organs through cells of the small intestine.
The nose and the stomach are, therefore, the key organs allowing interception and destruction of the virus before the pulmonary and systemic phases of infection. Treating the nose is very attractive as there is a very low risk of adverse effects and furthermore, treating the site of infection requires minimal doses of the drug. Our calculations based on published data show that the virus can be overwhelmed with a total dose of 4 mg, particularly if the modified ACE2 molecules (NZACE2-Pātari) have a higher affinity for the virus compared to wild-type ACE2 molecules expressed on the cell surface. Treating the nose with frequent dosing will intercept each wave of daughter virus released from the nasal mucosa.
NZACE2-Pātari can be synthesized in molecular biology laboratories and lyophilized for easy transport globally. The drug can be easily reconstituted and self-administered by a nasal pipette. The risk of nasal adverse effects is very low and can be treated with a decongestant. If the treatment is coupled with a point of care rapid antigen test, treatment could be commenced within 24–48 hours of infection. Early treatment is the key to mitigating disease severity and reducing viral transmission.
NZACE2-Pātari could be deployed in many clinical settings including outbreaks of the virus in nursing homes, prisons, refugee camps and other areas where social and physical distancing is difficult. If safe and effective, this strategy may mitigate disease severity in individual unvaccinated patients and may rapidly bring the infection under control, if there is a new cluster of infection.
Repurposing existing drugs for COVID-19 has been disappointing. The recent successes of the Pfizer, Moderna, Johnson & Johnson and Astra-Zeneca vaccines are encouraging but not all patients will be protected by vaccines. Immunodeficient patients may not respond to vaccines. Similarly, those who are not immunized will have no protection against the virus and its variants. Escape mutants as noted above with the Astra-Zeneca vaccine, remain a serious concern. The recent description of rare cases of vaccine associated/induced thrombosis and thrombocytopenia could cause reputational damage to adenovirus based vaccines. Furthermore, the stringent storage and transport requirements for mRNA-based vaccines will create logistical challenges. If proven to be safe and effective, NZACE2-Pātari may mitigate the health crises in countries such as Brazil and India until new drugs and vaccines are developed and widely deployed.
Acknowledgments
The NZACE2-Pātari project has not reached clinical trials. Prototypes of these drugs have been produced and were partially tested in vitro. This project has been suspended because of a lack of funding and institutional support. We are gifting our ideas so colleagues with funding and facilities can bring these treatments to fruition to save lives. Science will ultimately prevail against SARS-CoV-2.
Funding Statement
This paper was not funded
Article highlights
There is currently no effective treatment for COVID-19
Safe and effective vaccines face financial and logistical challenges, which will hinder global deployment
The virus initially infects the nasopharyngeal mucosa by binding cell-surface ACE2. By evading the innate immune system the virus is able to multiply exponentially and in some cases infect the lungs and other organs.
Here we describe the potential use of recombinant modified ACE2 molecules to target the virus in the nasal phase
Treating the virus in the asymptomatic incubation phase may alter the prognostic trajectory of the infection
If deployed globally with rapid testing, this treatment may help mitigate the ongoing pandemic
Rapid viral evolution may render some vaccines and monoclonal antibodies ineffective
In contrast, modified ACE2 molecules are likely to be effective even with rapid viral evolution, as the virus requires ACE2 to gain entry into cells
Author contributions
RA conceived the idea of intercepting the virus with ACE2 molecules and wrote the first draft of the manuscript. All other authors were part of the writing team and modified and edited the manuscript.
Declaration of interest
The project team may patent future modified ACE2 molecules to facilitate global use. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Zhou P, Yang XL, Wang XG, et al., A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798): 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Early description of COVID-19.
- 2.Zhou F, Yu T, Du R, et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229): 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Risk factors for severe outcomes from COVID-19.
- 3.Kirby T. Evidence mounts on the disproportionate effect of COVID-19 on ethnic minorities. Lancet Respir Med. 2020;8(6):547–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abedi V, Olulana O, Avula V, et al. Racial, Economic, and Health Inequality and COVID-19 Infection in the United States. J Racial Ethn Health Disparities. 2020. DOI: 10.1007/s40615-020-00833-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price-Haywood EG, Burton J, Fort D, et al. Mortality among black patients and white patients with covid-19. Epub 2012020 May 2011627 N Engl J Med. 2020;382(26):2534–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223): 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Clinical description of risk factors for severe outcomes in COVID-19.
- 7.Hou YJ, Okuda K, Edwards CE, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182(2):429–446.e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tai W, He L, Zhang X, et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17(6):613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;4(20):30229–30224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almario CV, Chey WD, Spiegel BMR. Increased risk of COVID-19 among users of proton pump inhibitors. Am J Gastroenterol. 2020;115(10):1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019. (COVID-19): A Review. JAMA. 2020. Aug 25;324(8):782–793. DOI: 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 12.Baek WK, Sohn SY, Mahgoub A, et al. Review of severe acute respiratory syndrome coronavirus 2. Cureus. 2020;12(5):e7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almqvist J, Granberg T, Tzortzakakis A, et al. Neurological manifestations of coronavirus infections - a systematic review. Ann Clin Transl Neurol. 2020;7(10):2057–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo YL. Covid-19, fatigue and dysautonomia. J Med Virol. 2020;93(3). DOI: 10.1002/jmv.26552 [DOI] [PubMed] [Google Scholar]
- 15.Editorial. Facing up to long COVID. Lancet. 2020; 396(10266):1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazza MG, De Lorenzo R, Conte C, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blain H, Rolland Y, Tuaillon E, et al. Efficacy of a test-retest strategy in residents and health care personnel of a nursing home facing a COVID-19 outbreak. J Am Med Dir Assoc. 2020;21(7):933–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker M, Kvalsvig A, Verrall AJ, et al. elimination strategy for the COVID-19 pandemic and what is required to make it work. NZ Med J. 2020;133(1512):10–14. [PubMed] [Google Scholar]
- 19.Manners C, Larios Bautista E, Sidoti H, et al. Protective adaptive immunity against severe acute respiratory syndrome coronaviruses 2 (SARS-CoV-2) and implications for vaccines. Cureus. 2020;12(6):e8399. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Detailed description of the immunology of COVID-19.
- 20.Bouayad A. Innate immune evasion by SARS-CoV-2: comparison with SARS-CoV. Rev Med Virol. 2020;30(6):e2135. [DOI] [PubMed] [Google Scholar]
- 21.Bastard P, Rosen LB, Zhang Q, et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585. DOI: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Important observation on the role of interferons in protecting against COVID-19.
- 22.Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045.e1039. Epub 2020 May 1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Potential JA. Effect of Novel Coronavirus SARS-CoV-2 on NK cells; A perspective on potential therapeutic interventions. Front Immunol. 2020;11:1692. eCollection 02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laurence J, Mulvey JJ, Seshadri M, et al. Anti-complement C5 therapy with eculizumab in three cases of critical COVID-19. Clin Immunol. 2020;219:108555. Epub 102020 Aug 108556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, John Wherry E. T cell responses in patients with COVID-19. Nat Rev Immunol. 2020;20(9):529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Showing the critical role of T cells in protection against COVID-19.
- 26.Watanabe Y, Allen JD, Wrapp D, et al. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020;369(330–333):330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Negro F. Is antibody-dependent enhancement playing a role in COVID-19 pathogenesis? Swiss Med Wkly. 2020;150:w20249. eCollection 22020 Apr 20246. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Liu Z, Li S, et al. Enhancement versus neutralization by SARS-CoV-2 antibodies from a convalescent donor associates with distinct epitopes on the RBD. Cell Rep. 2021;34(5):108699. Epub 102021 Jan 108612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for covid-19. N Engl J Med. 2020;383(6):517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.RECOVERY Collaborative Group: Dexamethasone in Hospitalized Patients with Covid-19 . Preliminary report. N Engl J Med. 2021;384(8):693-704. DOI: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z, VanBlargan LA, Bloyet LM, et al. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe. 2021;27(21):00044–00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ameratunga R, Lehnert K, Leung E, et al. Inhaled modified angiotensin converting enzyme 2 (ACE2) as a decoy to mitigate SARS-CoV-2 infection. N Z Med J. 2020;133(1515):112–118. [PubMed] [Google Scholar]
- 33.Chan KK, Dorosky D, Sharma P, et al. Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science. 2020;369(6508):1261–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monteil V, Kwon H, Prado P, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–913.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burgess S, Smith D, Kenyon JC, et al. Lightening the viral load to lessen covid-19 severity. BMJ. 2020;371:m4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsukagoshi H, Shinoda D, Saito M, et al. Relationships between viral load and the clinical course of COVID-19. Viruses 2021;13((2), 304):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. Jama. 2020;323(18):1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lei C, Qian K, Li T, et al. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nat Commun.. 2020. Apr 24;11(1):2070 doi: 10.1038/s41467-020-16048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Xiong G, Tsang WC, et al. Nose-to-brain delivery. J Pharmacol Exp Ther. 2019;370(3):593–601. [DOI] [PubMed] [Google Scholar]
- 41.Gänger S, Schindowski K. Tailoring formulations for intranasal nose-to-brain delivery: a review on architecture, physico-chemical characteristics and mucociliary clearance of the nasal olfactory mucosa. Pharmaceutics 2018;10(3):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song E, Zhang C, Israelow B, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med.. 2021. Mar 1;218(3):e20202135. DOI: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santos-Morales O, Díaz-Machado A, Jiménez-Rodríguez D, et al. Nasal administration of the neuroprotective candidate NeuroEPO to healthy volunteers: a randomized, parallel, open-label safety study. BMC Neurol. 2017;17(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sherr JL, Ruedy KJ, Foster NC, et al. Glucagon nasal powder: a promising alternative to intramuscular glucagon in youth with type 1 diabetes. Diabetes Care. 2016;39(4):555–562. Epub 2016 Feb 2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quiralte-Castillo J, Ávila-Castellano MR, Cimbollek S, et al. Nasal ketorolac challenge using acoustic rhinometry in patients with aspirin-exacerbated respiratory disease. J Investig Allergol Clin Immunol. 2017;27(3):169–174. Epub 12016 Oct 18119. [DOI] [PubMed] [Google Scholar]
- 46.Chamsi-Pasha MA, Shao Z, Tang WH. Angiotensin-converting enzyme 2 as a therapeutic target for heart failure. Curr Heart Fail Rep. 2014;11(1):58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan A, Benthin C, Zeno B, et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyake S, Ashikari K, Kato S, et al. SARS-CoV-2 prevalence in saliva and gastric and intestinal fluid in patients undergoing gastrointestinal endoscopy in COVID-19 endemic areas: prospective cross-sectional study in Japan. Dig Endosc. 2021;6(10):13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan KH, Sridhar S, Zhang RR, et al. Factors affecting stability and infectivity of SARS-CoV-2. J Hosp Infect. 2020;106(2):226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Almario CV, Chey WD, Spiegel BMR. Increased risk of COVID-19 among users of proton pump inhibitors. Am J Gastroenterol. 2020;115(10):1707-1715. DOI: 10.14309/ajg.0000000000000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee SW, Ha EK, Yeniova A, et al. Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching. Gut.2020;gutjnl-2020-323672. doi: 10.1136/gutjnl-2020-323672 [DOI] [PubMed] [Google Scholar]
- 52.Pamplona J, Solano R, Soler C, et al. Epidemiological approximation of the enteric manifestation and possible fecal-oral transmission in COVID-19: a preliminary systematic review. Eur J Gastroenterol Hepatol. 2020;17(10):0000000000001934. [DOI] [PubMed] [Google Scholar]
- 53.Lin L, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020;69(6):997–1001. Epub 322020 Apr 321012. [DOI] [PubMed] [Google Scholar]
- 54.Zhao Y, Liang W, Luo Y, et al. Personal protective equipment protecting healthcare workers in the Chinese epicentre of COVID-19. Clin Microbiol Infect. 2020;23(20):30437-30437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heinzerling A, Stuckey MJ, Scheuer T, et al. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient - solano county, california, february 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graham R, Masters-Awatere B. Experiences of māori of Aotearoa New Zealand’s public health system: a systematic review of two decades of published qualitative research. Aust N Z J Public Health. 2020;44(3):193–200. [DOI] [PubMed] [Google Scholar]
- 57.Steyn N, Binny RN, Hannah K, et al. Estimated inequities in COVID-19 infection fatality rates by ethnicity for Aotearoa New Zealand. NZ Med J. 2020;133(1520):28–39. [PubMed] [Google Scholar]
- 58.Lambert PH, Ambrosino DM, Andersen SR, et al. Consensus summary report for CEPI/BC March 12- 13, 2020 meeting: assessment of risk of disease enhancement with COVID-19 vaccines. Vaccine. 2020;38(31):4783–4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ameratunga R, Gillis D, Gold M, et al. Evidence refuting the existence of autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA). J Allergy Clin Immunol Pract. 2017;5(6):1551–1555.e1551. [DOI] [PubMed] [Google Scholar]
- 60.Ameratunga R, Langguth D, Perspective: HD. Scientific and ethical concerns pertaining to animal models of autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA). Autoimmun Rev. 2018;17(5):435–439. [DOI] [PubMed] [Google Scholar]
- 61.Elwood JM, Ameratunga R. Autoimmune diseases after hepatitis B immunization in adults: literature review and meta-analysis, with reference to ‘autoimmune/autoinflammatory syndrome induced by adjuvants’ (ASIA). Vaccine. 2018;36(38):5796–5802. [DOI] [PubMed] [Google Scholar]
- 62.Ameratunga R, Ahn Y, Steele R, et al. The natural history of untreated primary hypogammaglobulinemia in adults: implications for the diagnosis and treatment of common variable immunodeficiency disorders (CVID). Front Immunol. 2019;10(1541). DOI: 10.3389/fimmu.2019.01541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ameratunga R, Allan C, Woon ST. Defining common variable immunodeficiency disorders in 2020. Immunol Allergy Clin North Am. 2020;40(3):403–420. [DOI] [PubMed] [Google Scholar]
- 64.Ameratunga R, Brewerton M, Slade C, et al. Comparison of diagnostic criteria for common variable immunodeficiency disorder. Front Immunol. 2014;5(415). DOI: 10.3389/fimmu.2014.00415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ameratunga R, Lederman HM, Sullivan KE, et al. Defective antigen-induced lymphocyte proliferation in the X-linked hyper-IgM syndrome. J Pediatr. 1997;131(1 Pt 1):147–150. [DOI] [PubMed] [Google Scholar]


