ABSTRACT
Aim: This study aims to determine the prognostic value of the Glasgow Prognostic Score (GPS) and fibrinogen to albumin ratio (FAR) in patients with COVID-19.
Methods: Electronic database records of 400 patients with COVID-19 were retrospectively analyzed and the initial levels of CRP, albumin, fibrinogen values were recorded. The ground-glass opacities (GGO) and consolidations were evaluated on thorax CT. Hospital mortality and the need for intensive care unit (ICU) transfer were determined as adverse outcomes.
Results: It was determined that 345 patients (86.25%) were discharged while 31 patients (7.75%) were transferred to ICU in addition to 24 patients who died (6%). The rates of deaths and transfers to ICU were significantly increased in GPS 2 group compared to both GPS 0 and 1 groups. Additionally, increased FAR was observed in patients who died and transferred to ICU compared to the discharged patients. The FAR was significantly increased in patients with diffuse GGO. Logistic regression analysis indicated that FAR ≥144.59 and the presence of GPS 2 were independent predictors of the adverse outcomes in COVID-19 patients.
Conclusion: Our results demonstrated that the GPS and FAR could possess a predictive value for adverse outcomes in patients with COVID-19.
KEYWORDS: COVID-19, GPS, FAR, mortality, need for intensive care unit
1. Introduction
In COVID-19, some cases progress rapidly and can be mortal while many cases are mild [1]. Activation of coagulation and consumption of clotting factors occur in severe COVID-19 cases [2]. It was determined that the activation of the coagulation system could be related to the sustained inflammatory response in COVID-19 [3,4]. It was recognized that a large and sustained systemic inflammatory response is the cause for the emergence of multiple organ failures in COVID-19 cases [5,6].
Glasgow Prognostic Score (GPS) is a scoring system based on inflammation, which is created by evaluating C-reactive protein (CRP) and albumin [7]. GPS is a reliable and practical scoring system in many types of diseases and cancers, such as esophagus cancer, lung cancer, and chronic obstructive pulmonary disease (COPD) [8–10]. In the recent literature, only one study demonstrated that perioperative GPS (poGPS) was independently associated with the 30-day mortality rate in COVID-19 patients [11]. Similarly, fibrinogen to albumin ratio (FAR) is utilized as an effective marker of inflammation, and it was demonstrated that elevated FARs were present in some conditions such as severe infections and malignant disorders [12]. After the outbreak of COVID-19, many studies based on clinical features, CT imaging features, and laboratory results have been published. The results of the two meta-analyzes that summarize laboratory findings in COVID-19 showed that the most common laboratory findings were increased CRP, decreased albumin, decreased eosinophil, increased IL-6, lymphopenia and increased LDH [13,14]. During the pandemic, a huge knowledge has been gained about laboratory abnormalities in COVID-19, but less clear information has been provided on laboratory parameters ratios. In addition, the changes of albumin, fibrinogen, and CRP levels may not be simultaneously observed in the COVID-19 patients. Therefore, we think that the use of GPS and FAR indexes which were calculated with routinely measured albumin, CRP and levels may be more effective for predicting the outcomes in patients with COVID-19. In this study, we aimed to determine the prognostic value of GPS and FAR for mortality and transfers to intensive care unit (ICU) (adverse outcomes) in patients with COVID-19. Furthermore, we aimed to evaluate the correlations among GPS, FAR, and thorax CT findings.
2. Methods
In the current study, before initiating analyses, ethical approval was obtained from the institutional review board in Firat University (Decision No: 25.12.2020–432,316).
2.1. Sample
For the sample of the study, 400 patients with COVID-19 infections who were hospitalized in the pandemic clinics of the Faculty of Medicine at Firat University Hospital were retrospectively included. The presence of COVID-19 infection was diagnosed according to the laboratory diagnostic tests, radiological imaging, and symptoms. Posteroanterior (PA) chest radiographs and/or thorax computed tomography (CT) and laboratory parameters were evaluated in patients with COVID-19 before the treatment. The treatments of the cases were carried out according to the ‘COVID-19 (2019-nCoV Disease) Guide’ prepared by the Ministry of Health for COVID-19 in Turkey. Treatment applied in hospitalized patients according to these guidelines was stated below:
a) Uncomplicated patients: Favipiravir (2 × 1600 mg loading dose, 2 × 600 mg maintenance dose, 5 day)
b) Mild-Moderate Pneumonia (Those Without Signs of Severe Pneumonia): (Favipiravir 2 × 1600 mg loading dose, 2 × 600 mg maintenance dose, 5–10 day).
c) Severe Pneumonia: (Favipiravir 2 × 1600 mg loading dose, 2 × 600 mg maintenance dose, 5–10 day).
In addition, oxygen therapy (nasal kanula, high-flow nasal oxygen), dexamethasone 6 mg/day or equivalent corticosteroid (patients who need oxygen in room air), ampiric antimicrobial therapy (if necessary according to clinical and laboratory findings), Tocilizumab or anakinra (In cases who develop macrophage activation syndrome and do not respond to glucocorticoid therapy for at least 24 hours) and low-molecular-weight heparin (LMWH) for thrombosis prophylaxis were applied.
2.2. Data collection
Demographic characteristics of the cases and the laboratory data, which included CRP (mg/L), fibrinogen (mg/dL), and albumin (g/dL) values, in the pre-treatment period were obtained from the digital archive system in the hospital. Thorax CT findings that were recorded upon the initial application of the cases were evaluated. Hospital mortality rates and the need for ICU transfers were also included in the analyses.
The endpoints of our study were the presence of at least one of the three outcomes, which included death from COVID-19 (I), need for ICU transfer (II), and discharge from hospital (III). Furthermore, the cases with both mortality and transfer to ICU were defined as adverse outcomes in the sample.
The Glasgow Prognostic scores were determined by considering the serum albumin and CRP values [15]. GPS analysis was conducted according to the normal ranges of our hospital laboratory parameters, which were presented below.
Score 0: Normal CRP (≤5 mg/L) and normal albumin level (≥3.5 g/dL)
Score 1: At least one abnormal parameter of elevated CRP (>5 mg/L) or hypoalbuminemia (<3.5 g/dL)
Score 2: Elevated CRP (>5 mg/L) and hypoalbuminemia (<3.5 g/dL)
2.3. Radiological analyses
High-resolution computed tomography (HRCT) of thorax CT scans were evaluated while screening for pulmonary lesions. All the images were obtained via a 256-slice CT device (Revolution TM CT; General Electric Healthcare Company, Chicago, Illinois, USA). The CT scans were recorded while patients were at the end of inspiration and in the supine position. The axial images were obtained craniocaudally, and they covered the body parts from the thoracic inlet to the diaphragm. No contrast media was used during the scans. In the scans, the technical parameters for HRCT and thorax CT included 120 kV, 250 mA, 0.625 slice thickness, and 512 × 512 matrix. The reconstructed images were also obtained and used in the current study. A chest radiology specialist (Aydin AM, who had 23 years of experience in the profession) reviewed the thorax CT images. The thorax CT images were evaluated with both mediastinal (width: 350 HU, level: 40 HU) and lung (width: 1400 HU, level: −500 HU) window level settings.
In the study, we especially evaluated the two most common imaging features that were defined in previous studies. These included ground-glass opacities (GGO) and consolidation [6,7]. The presence of a single lobe lesion was considered as a limited involvement while multiple lobe lesions were considered as diffuse involvement for both GGO and consolidation.
2.4. Laboratory analyses
Complete blood cell counts were analyzed via a high-volume hematology analyzer (ADVIA 2120i, Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA). The blood samples were collected in potassium-ethylenediaminetetraacetic acid tubes and analyzed within 1 hour after venipuncture. The CRP levels were determined via a nephelometric analyzer (BN II System, Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA) by the immunonephelometry method. Thus, the complete blood cell count and CRP findings of the patients were included in the evaluation.
2.5. Statistical analyses
In the current study, the IBM SPSS Statistics 21 (Statistical Product and Service Solutions version 21.0, authorization code: d91314f638c364094170; Armonk, NY, USA) Package software was used for the statistical analyses. The results were presented as mean ± standard deviation. The level of statistical significance was regarded as p < 0.05. The pairwise group variables were compared by using the Mann–Whitney U-test because the data in our study did not demonstrate a normal distribution. On the other hand, the categorical variables were compared by using the chi-square test. Receiver operating characteristic (ROC) curve analysis was conducted to identify the optimal cutoff values of FAR to predict adverse outcomes in patients with COVID-19. Binary logistic regression analyses were used for univariate and multivariate analysis to evaluate the potential risk factors for mortality rate and transfer to ICU and odds ratios (ORs) were calculated with a 95% confidence interval (CI).
3. Results
In the study, 400 patients with COVID-19 infections were retrospectively evaluated. Two hundred and thirty-five (58.75%) of the patients were males (mean age of 55.51 ± 18.88) and 165 (41.25%) of the patients were females (mean age of 55.79 ± 19.54). It was determined that 345 patients (86.25%) were discharged while 31 patients (7.75%) were transferred to ICU, in addition to 24 patients who died (6%). There was no significant difference in age between discharged patients and patients who died and between those transferred to ICU and dead patients, but the mean age was statistically higher in transferred to ICU patients than discharged patients (p < 0.001). In addition, there was no significant difference in gender between the discharged, died and transferred to ICU patients (X2: 1.538, p > 0.05). Demographic characteristics and laboratory parameters of COVID-19 patients who died, transferred to ICU and discharged was shown in (Table 1). Furthermore, it was determined that 309 patients (77.25%) had abnormal thorax CT findings, and 91 patients (22.75%) had normal thorax CT findings. As expected, increased mortality and transfer to ICU rates were observed in patients with abnormal thorax CT findings (Table 2).
Table 1.
Demographic characteristics and laboratory parameters in COVID-19 patients who died, transferred to ICU and discharged
| Discharged n = 345 |
Death n = 24 |
Transfer to ICU n = 31 |
Adverse Outcomes n = 55 |
|
|---|---|---|---|---|
| Sex (Male/female) | 200/145 | 17/7 | 18/13 | 35/20 |
| Age (Year) | 54.08 ± 18.66 c,f | 59.75 ± 21.43 | 69.7 ± 16.65 | 65.36 ± 19.35 |
| CRP (mg/L) | 49.93 ± 54.54a,c,e | 104.27 ± 63.87 | 85.11 ± 67.7 | 93.47 ± 66.15 |
| Ferritin (ng/mL) | 354.94 ± 376.43 | 473.05 ± 474.01 | 339.61 ± 314.18 | 397.84 ± 393.7 |
| Lymphocyte (%) | 20.27 ± 11.26 | 20.87 ± 11.5 | 19.25 ± 9.98 | 19.95 ± 10.6 |
| Neutrophil (%) | 69.24 ± 13.59 | 68.16 ± 14.17 | 70.4 ± 11.74 | 69.43 ± 12.78 |
| Platelet (103/μL) | 210.12 ± 80.03 | 226.66 ± 86.85 | 227.51 ± 81.7 | 227.14 ± 83.19 |
| WBC (103/mL) | 7.09 ± 3.58 | 6.96 ± 3.91 | 7.28 ± 3.75 | 7.14 ± 3.79 |
| D-Dimer (mg/L) | 0.95 ± 1.57b | 1.94 ± 2.69d | 1.26 ± 2.9 | 1.56 ± 2.81 |
| Procalcitonin (mg/L) | 0.56 ± 0.99a,e | 1.12 ± 1.01 | 0.75 ± 0.86 | 0.91 ± 0.94 |
|
a p < 0.001, b p < 0.05 compared with death patients, c p < 0.001, d p < 0.05 compared with transfer to ICU patients, e p < 0.001, f p < 0.01 compared with transfer to adverse outcomes | ||||
Table 2.
The ratios of patients according to the endpoints of the study and the results of limited or diffuse lesions in both GGOs and consolidations
| Discharged n = 345 |
Death n = 24 |
Transfer to ICU n = 31 |
Total n = 400 |
|
|---|---|---|---|---|
| Thorax CT | ||||
| Normal | 90 (98.9%) | 1 (1.1%) | 0 (0%) | 91 (100%) |
| Abnormal | 255 (82.5%) | 23 (7.4%) | 31 (10.1%) | 309 (100%) |
| X2: 16.031, p < 0.001 | ||||
| GGO | ||||
| Limited | 101 (74.8%) | 13 (9.6%) | 21 (15.6%) | 135 (100%) |
| Diffuse | 136 (88.9%) | 9 (5.9%) | 8 (5.2%) | 153 (100%) |
| Consolidation | ||||
| Limited | 43 (79.7%) | 4 (7.4%) | 7 (12.9%) | 54 (100%) |
| Diffuse | 68 (81.9%) | 7 (8.4%) | 8 (9.7%) | 83 (100%) |
| X2: 10.64, p < 0.01 for GGO, X2: 0.394, p > 0.05 for consolidation | ||||
Furthermore, increased FAR was observed in patients who died and who were transferred to ICU compared to the discharged patients (154.48 ± 68.22 for patients who died, 156.59 ± 66.31 for patients who were transferred to ICU, and 123.84 ± 45.75 for discharged patients) (Figure 1). Moreover, FAR was statistically increased in the adverse outcome group compared to the discharged patients (155.67 ± 66.53, 123.84 ± 45.75, respectively, p < 0.001) (Figure 1). Although fibrinogen levels were higher in patients who died and the patients who were transferred to ICU compared to those who were discharged, no statistically significant difference was observed in the analyses (Figure 2). Additionally, we discovered that albumin levels were significantly higher in patients who were discharged compared to patients who died and the patients who were transferred to ICU (Figure 2).
Figure 1.
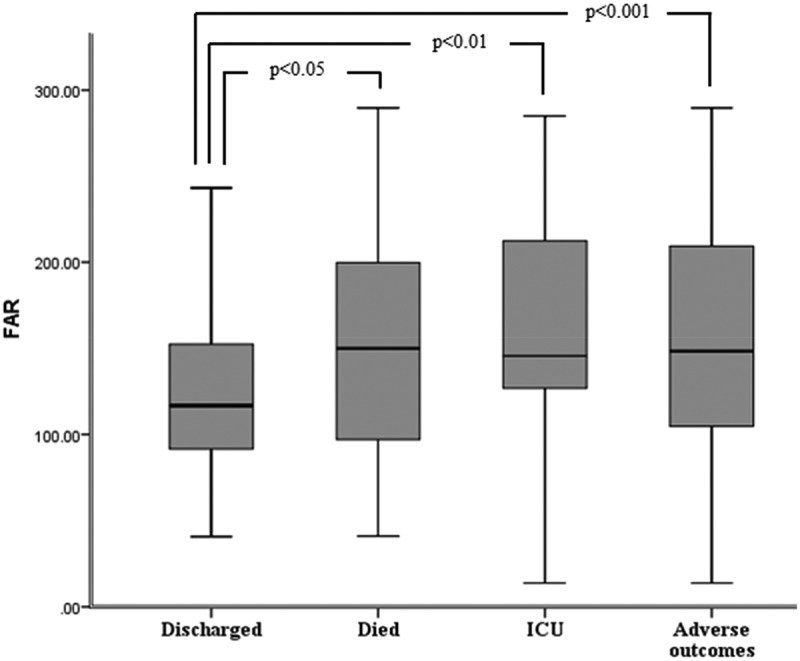
The FAR according to endpoints of the study
Figure 2.
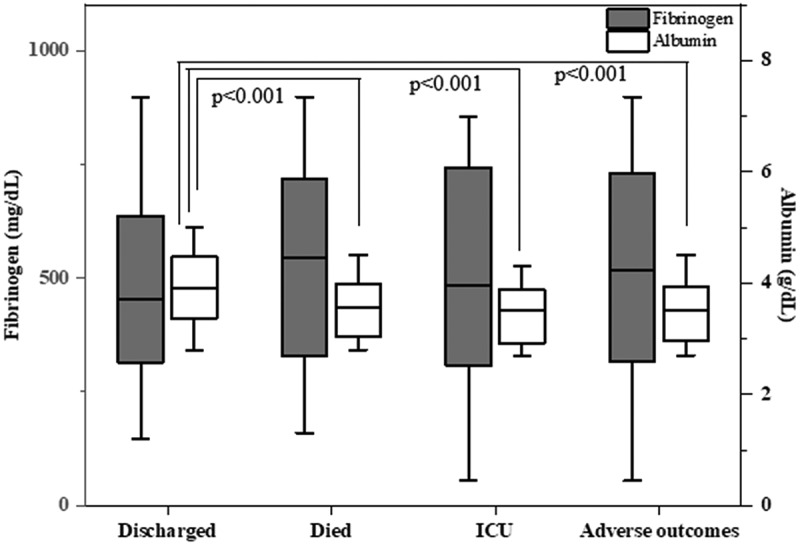
The fibrinogen and albumin levels according to endpoints of the study
GPS 0 was observed in 49 patients (12.25%). On the other hand, GPS 1 was observed in 287 patients (71.75%) while GPS 2 was observed in 64 patients (16%). The ratios of mortality were determined as 0% in GPS 0, 5.6% in GPS 1, and 12.5% in GPS 2. The ratios of transfer to ICU were found as 0% in GPS 0, 5.6% in GPS 1, and 23.4% in GPS 2. The ratios of death and transfers to ICU were significantly increased in GPS 2 group compared to both GPS 1 and GPS 0 groups. Additionally, these ratios were significantly increased in GPS 1 group compared to GPS 0 (X2: 38.357, p < 0.001) (Table 3). On the other hand, it was determined that FAR ratios were significantly increased with the higher the GPS score (91.79 ± 32.97 for GPS 0, 125.46 ± 43.4 for GPS 1, and 168.44 ± 62.34 for GPS 2; p < 0.001 for all the comparisons).
Table 3.
The GPS of the patients according to endpoints of the study
| GPS | Discharged n = 345 |
Death n = 24 |
Transfer to ICU n = 31 |
Total n = 400 |
|---|---|---|---|---|
| Score 0 | 49 (100%) | 0 | 0 | 49 |
| Score 1 | 255 (88.9%) | 16 (5.6%) | 16 (5.6%) | 287 (100%) |
| Score 2 | 41 (64.1%) | 8 (12.5%) | 15 (23.4%) | 64 (100%) |
| X2: 38.357, p < 0.001 | ||||
Within this framework, we discovered that limited or diffuse GGO affected the ratios of mortality and ICU transfers while the types of lesions did not affect the rate of mortality and ICU transfer. Moreover, increased ratios of mortality and ICU transfers were observed in patients with diffuse GGOs compared to those with limited GGOs (Table 2).
In the study, we evaluated GPS and FAR according to limited or diffuse GGO. Accordingly, it was observed that FAR was significantly increased in patients with diffuse GGO compared to patients with limited GGO (147.23 ± 52.87, 130.3 ± 42.41, respectively, p < 0.05). However, there was no significant difference between the patients with limited or diffuse GGO in terms of GPS (Table 4).
Table 4.
The GPS in patients with limited or diffuse GGO
| |
GGO |
Total |
|
|---|---|---|---|
| GPS | Diffuse n (%) |
Limited n (%) |
|
| Score 0 | 6 (28.6) | 15 (71.4) | 21 (100) |
| Score 1 | 104 (48.8) | 109 (51.2) | 213 (100) |
| Score 2 | 25 (46.3) | 29 (53.7) | 54 (100) |
| X2: 3.158, p > 0.05 | |||
When the cutoff value of FAR was taken as ≥144.59 by ROC analysis for the prediction of the adverse outcomes, FAR had an area under the curve (AUC) in the receiver operating characteristic (ROC) of 0.654 (0.566–0.742; 95% CI; p < 0.01). FAR level of 144.59 was taken as the cutoff value to predict the adverse outcomes in patients with COVID-19. Accordingly, FAR had a sensitivity of 72% and a specificity of 53% (ROC curve) (Figure 3).
Figure 3.
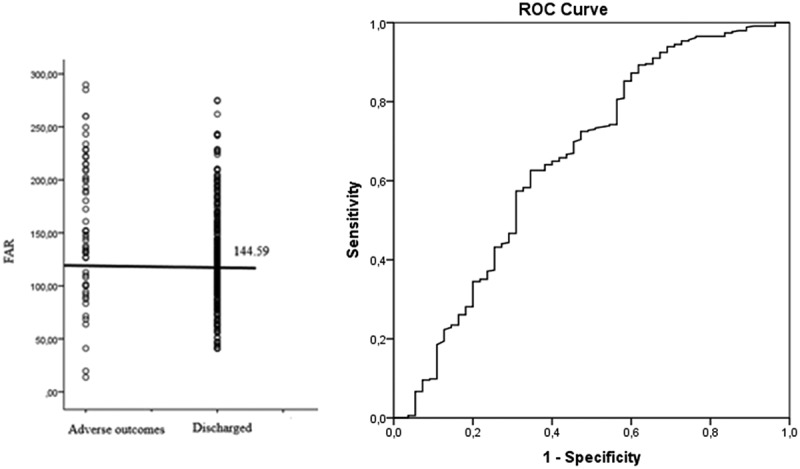
FAR with the cutoff value and the ROC curve for sensitivity and specificity
The results of the logistic regression analysis conducted for the potential predictors of an adverse outcome, death, and transfer to ICU were presented in (Table 5). The univariable logistic regression model showed the following parameters had statistical significance for death, including the presence of GPS 2, FAR ≥144.59 and FAR ≥144.59 and the presence of GPS 2 at the same time. The univariable logistic regression model showed the following parameters had statistical significance for transfer to ICU, including the presence of age≥65 year, the presence of GPS 2, FAR ≥144.59 and FAR ≥144.59 and the presence of GPS 2 at the same time. The multivariable logistic regression model indicated that age ≥65 year and having GPS 2 were likely to be independent predictors for transfer to ICU. The univariable logistic regression model indicated that age ≥65 year, FAR ≥144.59, the presence of GPS 2 and having GPS 2 and FAR ≥144.59 simultaneously were independent predictors of the adverse outcomes in COVID-19 patients. Also, age ≥65 year, having GPS 2 and FAR ≥144.59 were independent predictors for adverse outcomes in Covid-19 patients according to multivariable logistic regression model.
Table 5.
Results of logistic regression analysis of the potential predictors for outcomes of COVID-19 patients
| |
Age |
GPS 2 |
FAR≥144.59 |
FAR≥144.59+ GPS2 |
|---|---|---|---|---|
| Adverse Outcomes | ||||
| Univariable model | ||||
| p | <0.001 | <0.001 | <0.001 | <0.001 |
| OR (95%Cl) |
3.459 (1.919–6.235) |
5.329 (2.846–9.978) |
2.893 (1.621–5.164) |
5.744 (2.797–11.793) |
| Multivariable model | ||||
| p | <0.01 | <0.01 | <0.05 | |
| OR (95%Cl) |
2.829 (1.517–5.273) |
3.839 (1.410–10.745) |
2.344 (1.089–5.046) |
|
| Death | ||||
| Univariable model | ||||
| p | <0.01 | <0.01 | <0.001 | |
| OR (95%Cl) |
3.707 (1.494–9.203) |
3.065 (1.328–7.077) |
5.765 (2.171–15.307) |
|
| Multivariable model | ||||
| p | ||||
| OR (95%Cl) |
||||
| ICU | ||||
| Univariable model | ||||
| p | <0.001 | <0.001 | <0.01 | <0.001 |
| OR (95%Cl) |
5.270 (2.349–11.824) |
6.928 (3.188–15.057) |
2.756 (1.311–5.791) |
5.709 (2.360–13.812) |
| Multivariable model | ||||
| p | <0.01 | <0.01 | ||
| OR (95%Cl) |
4.037 (1.727–9.439) |
6.777 (2.093–21.949) |
||
4. Discussion
In our study, the results demonstrated that increased FAR and the presence of GPS2 were associated with adverse outcomes in patients with COVID-19. Accordingly, these variables can be considered as independent markers of poor prognosis in these patients. Furthermore, the ratios of mortality and transfer to ICU were increased in patients with diffuse GGO in addition to higher levels of FAR in patients with diffuse GGO.
The most important feature of severe COVID-19 infection is a marked systemic inflammatory response and aggressive inflammatory responses, which result in damages to the airway [13,16]. Disease severity in patients with COVID-19 depends on not only the viral infection but also the response of the host [17].
In the analyses of our study, GPS and FAR parameters were evaluated for an early prediction of disease progression. Meta-analysis results demonstrated that increased CRP level and decreased levels of albumin, which is a negative acute-phase protein, were among the most common laboratory findings [13,14]. In a previous study, it was determined that the systemic inflammatory response was independently correlated with circulating albumin concentrations [18]. In another study, it was reported that production and secretion of acute-phase proteins, the anti-proteases, and procoagulants, such as CRP and fibrinogen, were increased while the plasma concentrations of constitutive proteins, such as albumin and transferrin, were decreased [19]. The mechanism for hypoalbuminemia in COVID-19 has not been explained extensively, but it was reported as one of the possible mechanisms is intense systemic inflammation in severe COVID-19 [20]. The presence of hypoalbuminemia in COVID-19 cases was thought to be due to increased capillary permeability, which results in an escape of albumin into the interstitial area rather than a decreased albumin synthesis [21,22]. Decreased albumin levels may be caused by malfunctioning system organs such as vascular permeability, renal, and gastrointestinal in patients with severe COVID-19 [23]. Decreased albumin levels will possibly result in upregulation of ACE2 receptors that increase the COVID-19 infectivity because it has been found that albumin had the ability to downregulate ACE2 receptors [24]. Hypoalbuminemia was also reported as an independent predictive factor (OR, 6.394; 95% CI, 1.315–31.092) for mortality in patients with COVID-19 [25]. GPS, incorporating CRP and serum albumin levels, could indicate the presence of the systemic inflammatory response (CRP) [7]. GPS was used for evaluating systemic inflammatory response and survival in several conditions, such as cancer, COPD, and postoperative systemic inflammatory response [25,26]. Additionally, GPS was evaluated as a prognostic factor only in one study with COVID-19 cases, and it was independently associated with the 30-day mortality (OR 2.4, 95% CI; 1.1–5.1, p < 0.05) [11]. Similarly, in this study, we determined that both the rate of mortality and transfer to ICU were significantly higher with higher GPS. Furthermore, the presence of GPS 2 was an independent predictor of adverse outcomes in patients with COVID-19 according to multivariable logistic regression analysis. For these reasons, we think that GPS could be an indicator of adverse outcomes in patients with COVID-19.
Furthermore, coagulation-related abnormalities were observed in patients with COVID-19, especially those with severe diseases and frequent hypercoagulability [27,28]. Coagulation is an immune function and can play a defensive role against severe infections [29]. Additionally, inflammation is a well-recognized regulator of coagulation and fibrinolytic system activity [30]. In previous studies, the results indicated that the coagulation profile of patients with COVID-19, such as D-dimer, fibrin degradation products (FDP), and fibrinogen levels, was increased significantly [2,31]. Fibrinogen is considered an acute-phase reactant, and it plays a prominent role in regulating the inflammatory response [32]. The proinflammatory functions of fibrinogen and the derivative peptides of it result from their abilities to bind to and activate a wide range of immune cells [33]. FAR and platelet count were closely associated with the disease progression in COVID-19 cases. FAR levels were also significantly increased in patients with severe disease, and they returned to normal levels gradually while the patients were recovering from the disease [34]. In several studies, the researchers interpreted that increased FAR could result from cytokine storms induced by virus invasion [3,34]. Moreover, FAR was used as a marker of inflammation, and increased FAR levels were discovered in certain conditions, such as infection and malignant disorders [12]. As previously mentioned, we should also note that GPS and FAR indexes do not directly affect the outcomes in COVID-19 patients. In fact, the proteins (albumin, CRP, and fibrinogen), which determine the GPS and FAR, were determined to be responsible for the poor prognosis in patients with COVID-19 [12]. Already, fibrinogen, albumin and CRP are acute-phase responses to inflammation [6]. However, although it was reported that albumin, CRP, and fibrinogen abnormalities were prognostic markers in patients with COVID-19, changes in albumin, fibrinogen, and CRP levels were not observed simultaneously in the patients. For this reason, we think that the use of GPS and FAR indexes could present a better demonstration of these protein levels, and they can present great potential as prognostic factors in patients with COVID-19. Actually, when we evaluated the prognostic value of FAR for predicting the adverse outcomes in patients with COVID-19 by using ROC analysis, we determined that FAR had weak performance [AUC 0.654 (0.566–0.742; 95% CI; p < 0.01)]. When the optimal cut‐off value of FAR in terms of predicting the adverse outcomes in COVID‐19 patients was determined as 144.59, its sensitivity and its specificity were found to be 72% and 53%, respectively. To our knowledge, we saw that only one study evaluated FAR in patients with COVID-19. In this study, results showed that FAR (HR = 4.058, 95%CI = 1.246–13.222, p = 0.020) was independent factors for disease progression according to multivariate analysis. Area under the ROC curve (AUC) of FAR has been found as 0.730 (p = 0.001) in this study [34]. Also, we found that FAR was an independent predictor of the adverse outcomes in COVID-19 patients according to multivariable logistic regression analysis, although FAR has poor performance in ROC analysis. FAR can be easily calculated from routinely measured laboratory parameters in COVID-19 patients. For this reason, it may be a simple and useful index that can be used for predicting adverse outcomes in COVID-19 patients, has reasonable sensitivity and specificity.
In previous studies, it was reported that there were positive correlations between CRP and the diameters of the lung lesions. Accordingly, it was suggested that in the early stages of COVID-19, the CRP levels could indicate the lung lesions assessed by CT scan and disease severity [35,36]. Furthermore, it was stated that CRP could be a possibly promising marker in demonstrating the severity of the disease in these studies [35,36]. In our study, it was determined that GPS containing the CRP component was not significanty different according to radiologic evaluation. However, increased FARs were observed in patients with diffuse GGO. Therefore, we think that FAR could be a promising marker to indicate lung lesions and disease severity.
Our study has certain limitations. Firstly, our sample size was relatively small. Additionally, the study was designed retrospectively and conducted in a single health-care center. The relatively small sample size of the study may influence the interpretation of results. GPS and FAR indexes are easily measured and inexpensive. They should be prospectively studied in multicenter studies while covering higher numbers of patients to make use of the significant prognostic value of them even in a relatively small sample with COVID-19. Secondly, we could not evaluate all the nutritional parameters of the patients, such as BMI, and the comorbidities of patients because we were not able to reach all data. Since COVID-19 is an acute infection, it can be assumed that nutrition may not be effective in the development of hypoalbuminemia.
In conclusion, the initial evaluation of GPS and FAR indexes could be beneficial in the early identification of the adverse outcomes in COVID-19 patients. Because the underlying proteins vary depending on the patients, the determination of GPS and FAR can be a more appropriate approach in these patients.
Funding Statement
This paper was not funded.
Article highlights
Increased fibrinogen to albümin ratio (FAR) and Glasgow Prognostic Score (GPS) may be a predictor for advers outcomes in patients with COVID-19.
Increased FAR could indicate the diffuse lung lesions on thorax CT and disease severity.
Early identification of adverse outcomes in COVID-19 patients may be provide more appropriate treatment approach.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Lu R, Xiang X, Li J, et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This article described of the genomic structure of 2019-nCoV and it again highlights the hidden virus reservoir in wild animals and their potential to occasionally spill over into human populations.
- 2.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu XW, Wu XX, Jiang XG.. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective series. BMJ. 2020;368:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotamisligil GS. Foundations of immunometabolism and implications for metabolic health and disease. Immunity. 2017;47(3):406–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabay C, Kushner I, Epstein FH. Acute-phase proteins and other systemic responses to inflammation. New Engl J Med. 1999;340(6):448–454. [DOI] [PubMed] [Google Scholar]
- 7.McMillan DC. An inflammation-based prognostic score and its role in the nutrition based management of patients with cancer. Proc Nutr Soc. 2008;67(3):257–262. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi T, Teruya M, Kishiki T, et al. Inflammation-based prognostic score, prior to neoadjuvant chemoradiotherapy, predicts postoperative outcome in patients with esophageal squamous cell carcinoma. Surgery. 2008;144(5):729–735 [DOI] [PubMed] [Google Scholar]
- 9.Forrest LM, McMillan DC, McArdle CS, et al. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer. 2004;90(9):1704–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuluöztürk M, Deveci F. The Glasgow prognostic score can be a predictor of mortality in acute exacerbation of chronic obstructive pulmonary disease. Expert Rev Respir Med. 2020;14(5):521–525. [DOI] [PubMed] [Google Scholar]
- 11.Maguire D, Woods M, Richards C, et al. Prognostic factors in patients admitted to an urban teaching hospital with COVID-19 infection. J Transl Med. 2020;18(1):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun DW, Lin A, Lv G. Albumin-fibrinogen ratio and fibrinogen-pre-albumin ratio as promising prognostic markers for cancers: an updated meta-analysis. World J Surg Oncol. 2020;18(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang ZL, Hou YL, Li DT, et al. Laboratory findings of COVID-19: a systematic review and meta-analysis. Scand J Clin Lab Invest. 2020;80(6):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mc Millan D. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(7):223–226. [DOI] [PubMed] [Google Scholar]
- 16.Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136(1):95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tay MZ, Poh CM, Rénia L, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margarson MP, Soni N. Serum albumin: touchstone or totem? Anaesthesia. 1998;53(8):789–803. [DOI] [PubMed] [Google Scholar]
- 19.Myers MA Aspects of the acute phase response. PhD thesis, University of London, 1987. [Google Scholar]
- 20.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and Clinical Significance. JPEN J Parenter Enteral Nutr. 2019;43(2):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J, Cheng A, Kumar R, et al. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. 2020;92(10):2152–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hariyanto TI, Japar KV, Kwenandar F, et al. Inflammatory and hematologic markers as predictors of severe outcomes in COVID-19 infection: a systematic review and meta-analysis. Am J Emerg Med. 2021;41:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu BC, Gao J, Li Q, et al. Albumin caused the increasing production of angiotensin II due to the dysregulation of ACE/ACE2 expression in HK2 cells. Clin Chim Acta. 2009;403(1–2):23–30. [DOI] [PubMed] [Google Scholar]
- 25.Dupre A, Malik HS. Inflammation and cancer: what a surgical oncologist should know. Eur J Surg Oncol. 2018;44(5):566–570. [DOI] [PubMed] [Google Scholar]
- 26.Watt DG, McSorley ST, Park JH, et al. A Postoperative systemic inflammation score predicts short- and long-term outcomes in patients undergoing surgery for colorectal cancer. Ann Surg Oncol. 2017;24(4):1100–1109 [DOI] [PubMed] [Google Scholar]
- 27.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loof TG, Morgelin M, Johansson L, et al. Coagulation, an ancestral serine protease cascade, exerts a novel function in early immune defense. Blood. 2011;118(9):2589–2598 [DOI] [PubMed] [Google Scholar]
- 30.Esmon CT. Coagulation and inflammation. J Endotoxin Res. 2003;9(3):192–198. [DOI] [PubMed] [Google Scholar]
- 31.Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(7):1116–1120 [DOI] [PubMed] [Google Scholar]
- 32.Adams RA, Schachtrup C, Davalos D, et al. Fibrinogen signal transduction as a mediator and therapeutic target in inflammation: lessons from multiple sclerosis. Curr Med Chem. 2007;14(27):2925–2936 [DOI] [PubMed] [Google Scholar]
- 33.Adams RA, Passino M, Sachs BD, et al. Fibrin mechanisms and functions in nervous system pathology. Mol Interv. 2004;4(3):163–176 [DOI] [PubMed] [Google Scholar]
- 34.Bi X, Su Z, Yan H, et al. Prediction of severe illness due to COVID-19 based on an analysis of initial Fibrinogen to Albumin Ratio and Platelet count. Platelets. 2020;31(5):674–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumoto H, Kasai T, Sato A, et al. Association between C-reactive protein levels at hospital admission and long-term mortality in patients with acute decompensated heart failure. Heart Vessels. 2019;34(12):1961–1968 [DOI] [PubMed] [Google Scholar]
- 36.Wang L. C-reactive protein levels in the early stage of COVID19. Med Mal Infect. 2020;50(4):332–334. [DOI] [PMC free article] [PubMed] [Google Scholar]


