In the most severe cases, coronavirus disease (COVID-19) leads to acute respiratory distress syndrome (ARDS) that is characterised by alveolar epithelial and endothelial injuries [1]. There are several ARDS biomarkers, which reflect alveolar tissue injuries [2]. The level of circulating soluble form of receptor for advanced glycation end-products (sRAGE) is correlated with type-1 alveolar epithelial injuries [3]. The elevation of angiopoietin-2 (ANG-2) indicates endothelial injury in patients with ARDS [4]. The increase in the circulating surfactant protein concentration indicates alveolar barrier disruption in ARDS cases [5]. Recently, Spadaro et al. reported that COVID-19 ARDS is characterised by increase in the circulating endothelial injury markers [6]. However, the detailed temporal characteristics of these markers remain unclear. In this preliminary study, we investigated the levels of circulating sRAGE, ANG-2, and surfactant protein D (SP-D) in serum samples of patients with COVID-19 with or without ARDS.
Patients who were diagnosed with COVID-19 by real-time polymerase chain reaction and admitted to Yokohama City University Hospital from January to August 2020 were included in this retrospective observational study (Ethics Reference Number: B200700100). Serum concentrations of sRAGE, ANG-2, and SP-D were measured using enzyme-linked immunosorbent assay kits (human RAGE: DY1145; human ANG-2: DY623; human SP-D: DY1920; R&D systems, Minneapolis, MN, USA). We compared the concentrations of these markers in patients with and without ARDS on hospital day 1 or 2. Moreover, we analysed temporal changes in these markers during the first 8 hospital days in those with ARDS. ARDS was diagnosed according to the Berlin Definition.
The data of those with and without ARDS were compared with the Mann–Whitney U test. Temporal changes in the markers were analysed using the Friedman and post-hoc Dunn’s tests. The peak day for each biomarker was observed using the Kruskal–Wallis and post-hoc Dunn’s tests. All statistical analyses were performed using Prism 9.0 software (Graphpad Software, San Diego CA, USA). The level of significance was set at P < 0.05.
Eleven and ten patients with and without ARDS, respectively, all with COVID-19, were included. Their characteristics are presented in Table 1. ARDS diagnosis was made on hospital day 1 or 2. The initial serum levels of sRAGE and SP-D were significantly higher in the ARDS than in the non-ARDS group; however, no significant difference was observed in the ANG-2 levels (Table 1).
Table 1.
Patient characteristics
| Non-ARDS (n = 10) | ARDS (n = 11) | P value | |
|---|---|---|---|
| Age, median (IQR), years | 57 (30–72) | 69 (64–76) | 0.2428 |
| Male/female, number | 8/2 | 10/1 | 0.5865 |
| APACHE2 score, median (IQR) | 7 (4.75–9.75) | 10 (9.00–14.00) | *0.0204 |
| P/F ratio at admission, median (IQR) | 395 (304–454) | 155 (108–203) | *< 0.0001 |
| Mechanical ventilation use, number | 0 | 11 | *< 0.0001 |
| Laboratory data on admission, median (IQR) | |||
| WBC count, cells/μL | 4600 (2525–7925) | 7000 (5800–9600) | 0.1567 |
| Neutrophil count, cells/μL | 2258 (1268–5416) | 5628 (4292–7350) | *0.0430 |
| Lymphocyte count, cells/μL | 1003 (674–1475) | 643 (342–788) | *0.0048 |
| Platelet count, ×103 cells/μL | 186 (86–317) | 220 (176–287) | 0.4262 |
| D-dimer, μg/mL | 0.67 (0.00–1.04) | 1.35 (0.65–2.52) | *0.0404 |
| CRP, mg/dL | 0.98 (0.28–2.06) | 14.62 (9.09–16.89) | *< 0.0001 |
| Creatinine, mg/dL | 0.75 (0.63–0.87) | 0.75 (0.59–0.89) | > 0.9999 |
| Total bilirubin, mg/dL | 0.55 (0.40–0.78) | 0.60 (0.50–1.00) | 0.5661 |
| Alveolar tissue injury marker levels on admission, median (IQR) | |||
| sRAGE, pg/mL | 896 (402–1718) | 2328 (1404–4982) | *0.0079 |
| ANG-2, pg/mL | 334 (65–781) | 699 (405–2201) | 0.1321 |
| SP-D, pg/mL | 2407 (772–3833) | 16,596 (6733–21,397) | *0.0062 |
ARDS acute respiratory distress syndrome, ANG-2 angiopoietin-2, SP-D surfactant protein D, sRAGE soluble form of receptor for advanced glycation end-products, WBC while blood cell, CRP c-reactive protein, IQR interquartile range
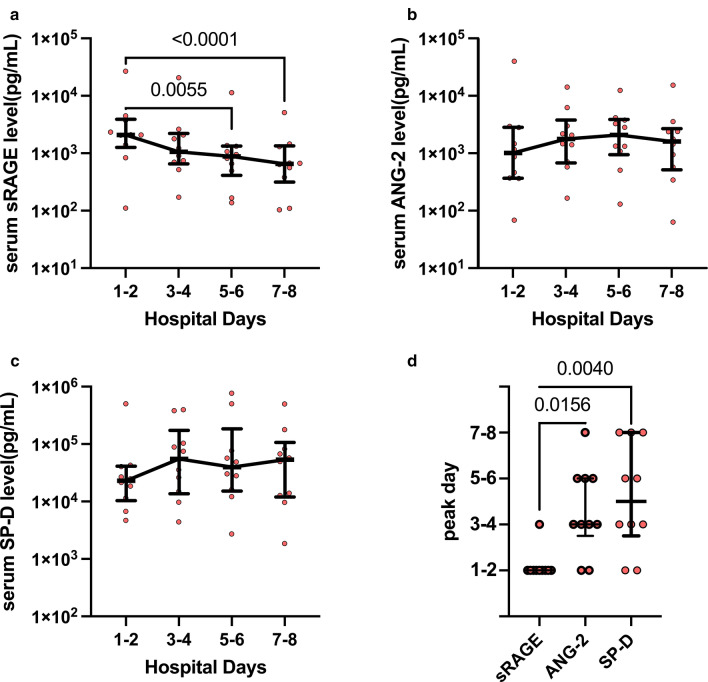
Analysis of temporal changes in these markers in 10 patients with ARDS, after excluding one patient with missing data, revealed that the serum sRAGE level peaked just after admission, and gradually decreased with hospital days (Fig. 1). Conversely, serum ANG-2 and SP-D levels did not significantly decrease during the first 8 hospital days and the peak timings of these markers were observed during a later disease stage (Fig. 1).
Fig. 1.
Temporal changes in a sRAGE, b ANG-2, and c SP-D levels. The examination was performed in patients with ARDS with COVID-19 during the first 8 days from admission. In cases where the values for every 2-days were available, the mean values were used; where only a single-day value was available, this value was used. d The peak day of each alveolar tissue injury marker is presented. Data are presented as medians ± IQRs. ARDS acute respiratory distress syndrome, ANG-2 angiopoietin-2, SP-D surfactant protein D, sRAGE soluble form of receptor for advanced glycation end-products
We showed that alveolar epithelial injury occurring at the very early disease stage, indicated by the increased sRAGE level, is a hallmark of COVID-19 ARDS. Conversely, the ANG-2 and SP-D levels peaked at later time points, suggesting that the endothelial injury and alveolar barrier disruption continued to exacerbate for several days after admission.
A study limitation was that the precise mechanism of sRAGE or ANG-2 release from the alveolar epithelial or endothelial cells remains unknown. However, the difference in the peak timing of these markers suggested distinct mechanisms for injury to each cell type. Additionally, it is possible that the initial alveolar epithelial injury might be a trigger of the subsequent exacerbation. Further investigations analysing temporal associations between these markers and inflammatory mediators could help identify the mechanisms underlying alveolar tissue injury. Moreover, the trajectory analysis of these markers linking clinical outcomes could help understand the detailed COVID-19 pathogenesis.
Acknowledgements
The authors would like to thank Department of Emergency Medicine (Prof. Ichiro Takeuchi), Department of Microbiology (Prof. Akihide Ryo), and Yokohama City University Centre for Novel and Exploratory Clinical Trials for collecting and providing the blood samples.
Abbreviations
- ARDS
acute respiratory distress syndrome
- COVID-19
coronavirus disease
- sRAGE
soluble receptor for advanced glycation end-products
- ANG-2
angiopoietin-2
- SP-D
surfactant protein D
- SARS-CoV-2
severe acute respiratory syndrome coronavirus-2
Authors' contributions
KT conducted the study, performed ELISA, analysed data, and wrote the manuscript. NY performed ELISA and reviewed the manuscript. TM supervised statistical data analysis and reviewed the manuscript. MA collected patients’ clinical data and reviewed the manuscript. TG supervised the study and reviewed the manuscript. All authors read and approved the final manuscript.
Funding
The authors have declared no specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study protocol was reviewed and approved by the institutional review board of Yokohama City University Hospital (Approval Number: B200700100). The need for informed consent was waived by the institutional review boards because of the retrospective observational design of the study.
Consent for publication
Not applicable.
Competing interests
The authors have disclosed that they do not have any potential competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Polak SB, Gool ICV, Cohen D, von der Thüsen JH, van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020;33:2128–2138. doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Zee P, Rietdijk W, Somhorst P, Endeman H, Gommers D. A systematic review of biomarkers multivariately associated with acute respiratory distress syndrome development and mortality. Crit Care. 2020;24:243. doi: 10.1186/s13054-020-02913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, et al. Receptor for advanced glycation end-products is a marker of Type I cell injury in acute lung injury. Am J Respir Crit Care. 2006;173:1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, van Hinsbergh VWM, Groeneveld ABJ. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax. 2008;63:903. doi: 10.1136/thx.2007.087387. [DOI] [PubMed] [Google Scholar]
- 5.Pan T, Nielsen LD, Allen MJ, Shannon KM, Shannon JM, Selman M, et al. Serum SP-D is a marker of lung injury in rats. Am J Physiol Lung C. 2002;282:L824–L832. doi: 10.1152/ajplung.00421.2000. [DOI] [PubMed] [Google Scholar]
- 6.Spadaro S, Fogagnolo A, Campo G, Zucchetti O, Verri M, Ottaviani I, et al. Markers of endothelial and epithelial pulmonary injury in mechanically ventilated COVID-19 ICU patients. Crit Care. 2021;25:74. doi: 10.1186/s13054-021-03499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.